Abstract
Objectives:
To comparein vitro differences in the apical filling regarding working length (WL) change and presence of voids and to validate optical coherence tomography (OCT) in comparison with computerized microtomography (µCT) for the detection of failures in the apical filling.
Methods:
Forty-five uniradicular teeth with round canals, divided into groups (n = 15) following the obturation protocols: LC (lateral condensation), TMC (thermomechanical compaction) and SC (single cone). Samples were scanned using µCT (parameters: 80 kV, 222 µA, and resolution of 11 µm), OCT (parameters: SSOCT, 1300 nm and axial resolution of 12 µm), and periapical digital radiography. The images were analyzsed by two blind and calibrated observers using ImageJ software to measure the boundary of the obturation WL and voids presence. Categorical and metric data were submitted to inferential analysis, and the validity of the OCT as a diagnostic test was assessed with performance and reliability tests.
Results:
The WL average remained constant for all obturation techniques and image methods. OCT showed adequate sensitivity and specificity to detect voids in the WL of apical obturations in vitro in comparison with µCT. Both image methods found a higher number of voids for LC technique (µCT p = 0.011/OCT p = 0.002).
Conclusions:
OCT can be used in apical obturation voids assessment and the LC technique revealed more voids with larger dimensions.
Keywords: optical coherence tomography, X-ray microtomography, root canal obturation
Introduction
The endodontic obturation promotes a tridimensional filling of the modelled portion of the canal. Filling can be done apically, coronally and laterally with inert or antiseptic material that either stimulate or have no effect on the restoration process.1 Clinically, obturation quality and working length is checked with the use of bidimensional periapical radiographies.
However, to compare apical filling resulting from obturation protocols of different techniques, in vivo and ex vivo micrometric methods seems to be essential. Computerized microtomography (µCT) represents a non-destructive method that generates root obturation images with histological correlation owing to its high 3D spatial resolution at the micrometric scale (~10to15µm) and minimum artefacts.2–4
Another non-destructive imaging method with similar micrometric resolution is optical coherence tomography (OCT). It consists of a non-radioactive method that uses a near infrared (780 to 1550 nm) and wideband (100 nm) light source, which results in a spatial resolution of the order of 10 µm or less, and real time 2D or 3D images with in vivo and in vitro application in dentistry.5–9 In endodontics, the use of OCT was described for the evaluation of intracanal anatomy, root perforations, vertical fractures, presence of smear layer on the root canal and analysis of the apical region.5,10–12
The apical filling consists of an important item of endodontic success, as it could be associated to apical sealing, and control of recontamination. The advent of NiTi instruments associated with single cone (SC) obturation technique, along with the obturation cement, avoids the use of accessory cones commonly used in lateral condensation (LC) technique. In addition, this technique is considered fast, simple, provides a better adaptation to the dentinary wall of the root canal and creates less stress to both patients and professionals.13,14 Other advantage of NiTi rotary instruments, is the gutta-percha cone thermomechanical plasticizing, initially proposed by McSpaden15 and allows a reduction of the working time and number of empty spaces with the heat generation by friction.16,17
Considering the micrometrical differences observed in apical filling, different obturation protocols were tested by both detection methods, with the following objectives: (i) to validate OCT in comparison with µCT for the detection of failures in the apical filling in vitro; (ii) to compare differences in the apical filling regarding working length change and the presence of voids.
Methods and materials
Sample preparation
After obtaining approval of the Ethics Committee of the State University of Paraíba (CAAE – 51498015.7.0000.5187), 45 inferior premolars with complete rhizogenesis single root canals tilted by ≤5° according to Schneider’s18 method. Teeth showing the presence of pulp nodules, internal resorption, previous endodontic treatment or root fracture were excluded from the samples. The teeth’s crowns were removed with an incision perpendicular to the root axis at the amelocemental junction using a diamond disc (KG Sorensen, Zenith Dental ApS, Agerskov, Denmark). The samples were stored in saline solution (NaCl 0.9%).
The root canal was irrigated with 2 ml of sodium hypochlorite 2.5% (Ciclo farma, Serrana, SP, Brazil). K-type hand files #10 (DentsplyMaillefer, Ballaigues, Switzerland) were introduced up to the apical foramen and the length measured, referring to the tooth's length (TL), was defined as the working length (WL). The apical limit of instrumentation and obturation was defined as 0.0 mm (WL = TL).
The root canal preparation was carried out by a specialist with a single use NiTi Reciproc file (VDW, Munich, Germany) according to the manufacturer’s instructions, an R50 instrument (50.05) for wide canals or R40 (40.06) for medium canals . The reciprocal system was actuated with the electrical motor VDW Silver (VDW GmbH, Munich, Germany) with speed and torque automatically calibrated.
Following the instrumentation, the root canals were irrigated with 2 ml of ehtylenediaminotetraacetic acid (EDTA) 17% (Biodinâmica Química e Farmacêutica Ltda, Ibipora, PR, Brazil) for 3 min under stirring with a type k-15 hand file, followed by a second irrigation with 2 ml of sodium hypochloride 2.5% and drying on paper cone.
Obturation protocols
Samples were randomly divided into three groups (n = 15), as follows:
Group LC: lateral condensation technique
Insertion of the main gutta-percha cone with 40.02 or 50.02 of conicity smeared on the cement on the WL, and accessory cones (DentsplyMaillefer, Ballaigues, Switzerland) embedded in cement Ah Plus (DentsplyMaillefer, Ballaigues, Switzerland). The NiTi spacer (DentsplyMaillefer, Ballaigues, Switzerland) was used to provide adequate space for the accessory cones. The process was repeated until the complete filling of the canal. The excessive gutta-percha was removed with heat and the crown was compacted with an appropriate presser (Odous de Deus, Belo Horizonte, Brazil).
Group SC: single cone
Adaptation of the gutta-percha cone with identical size and conicity to those of the instrument used in the mechanical preparation (40.06 or 50.05), followed by the smearing of cement on the WL, removing the cervical excess with a heated presser and subsequent cold vertical compaction.
Group TMC: thermomechanical compaction
Adaptation of the cone with identical size and conicity to those of the instrument used in the mechanical preparation (40.06 or 50.05), smeared on the cement on the WL. The thermocompactor PacMac 45.04 of 21mm (SybronEndo Dental Specialties, Glendora, CA) mounted at the counter-angle with rotation to the right, was inserted beside the cone, actuated in back and forth movements to obtain the apical filling.
Computerized microtomography
Samples were scanned in the microtomogram NIKON (model XTEK XT-H 225 ST, 225 kV microfocus source,Brighton,MI,EUA), with the parameters: 80 kV voltage, 222 µA current. The resolution adopted in this protocol is 11 µm to adjust to the volumetric limits of the sample and to match OCT’s resolution. The volume of interest is the apical portion (1.5 × 3×3 mm). The samples were inserted in pairs into Styrofoam blocks of 14×14×15 cm given that its radiographic density close to the air. The blocks were then tied to the support for scanning.
The images obtained were reconstructed along the three planes (axial, sagittal and coronal) using the software XTEK-CT PRO 3D (Brighton, MI, EUA), and imported to the software VG Studio Max 2.2 (Volume Graphics Gmbh, Heidelberg, Germany). The assignment of values was established according to predetermined parameters based on the Hounsfield scale (HOUNSFIELD, 1973) for the Geosciences (Air = 0; water = 1000). In the sequence, a Gaussian filter (3 × 3×3 mm) was applied to smooth artefacts and noise. The images were finally exported in TIFF format.
Optical coherence tomography
Samples were scanned with Swept Source-Optical Coherence System SSOCS 1300 nm (Thorlabs Inc., New Jersey, EUA, Thorlabs GmbH, Dachau, Germany), with spectral domain near infrared source (1300 nm), lateral resolution of 25 µm, and axial resolution of 12 µm, in high speed. The tomographic scanning 3 × 3×1.5 mm took around 40 s, resulting in 540 axial images. The samples were positioned in a condensation silicone support (Perfil, Coltene, Rio de Janeiro, Brazil) placing the root canal opening perpendicular to the beam of light, enabling the detection of apical voids.
Digital radiography
To improve the clinical correlation of the obturation analysis, the samples were subjected to bidimensional radiographies with the X-ray apparatus digital Heliodent with the parameters: 7mA, 70kV voltage and 0.05 s of exposure time. To ensure reproducible imaging geometry, the samples were inserted into Styrofoam cubes for fixation and arranged over the solid sensor Kodak RVG 6100 digital radiography system #2, with the distance between sample and tube given by the acrylic support.
Image analysis
All images were analysed and measured using an ImageJ software (National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/), employing the analysis of cuts (axial, frontal and sagittal) in the tridimensional images and the Straight Line tool to measure limits of working length and voids.
At first, images were analysed twice by a randomly assigned blind and calibrated observer (intraobserver Cohen κ 0.790) for the real working length. The apical limit of 0.0 mm was adopted for instrumentation and obturation, and alterations were considered normal if within 0.5 mm from the foramen.
Owing to the less resolution of digital radiography, this technique was not used as a reference to detect of voids in the apical filling. The presence, depth and width dimension of voids resulting of different obturation procedures were analysed between µCT and OCT tridimensional images by a examiner randomly assigned and masked (Interobserver Cohen κ 0.864). Each void dimension was also measured on the three axes by two calibrated and blind examiners and the intraclass correlation coefficient in each method of analysis was for number of voids (µCT 0.88/OCT 0.88), for depth (µCT 0.96/OCT 0.97) and for width (µCT 0.73/OCT 0.81).
Statistical analysis
Statistical analysis was both descriptive and inferential. Categorical data of WL and presence of voids were analysed using Fisher’s exact test, and sensitivity and specificity tests applied to evaluate OCT and digital radiography agreement to µCT.
Voids dimensions of depth and width, as numerical data, were submitted to Kolmogorov–Smirnov test, that indicated the applicability of non-parametric tests, such as Kruskal–Wallis test used owing to the observance of the alternative hypothesis of difference between groups (p < 0.05) and Mann–Whitney’s test was applied for the pairwise comparison between groups.
The analyses were conducted on SPSS for Windows v.20.0 (SPSS, Inc, Chicago, IL).
Results
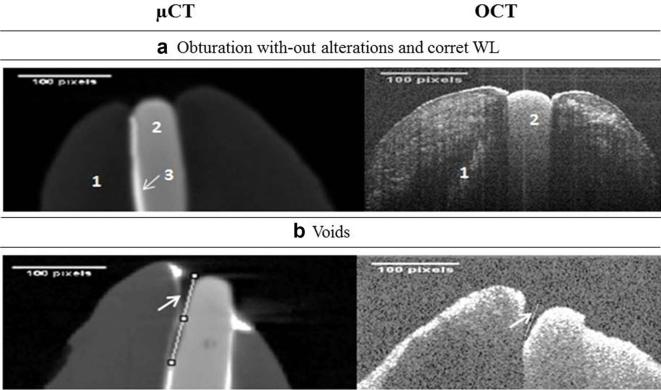
The millimetric measurement of the predetermined apical limit (0.0± 0.5 mm) showed that all obturation techniques were efficient considering WL, as observed in Table 1, and illustrated by Figure 1a.
Table 1.
Comparison of WL changes and the presence of voids according to three diagnostic imaging methods and tests of performance and reliability compared with µCT
| Diagnostic methods | Obturation technique | Diagnostic tests | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CL | SC | TMC | pa | κ concordance | Sensitivity | Specificity | Accuracy | |||
| WL | µCT | 1(6.7%) | 2(13.3%) | 2(13.3%) | 0.001 | – | – | – | – | |
| OCT | 0(0.0%) | 0(0.0%) | 1(6.7%) | 0.737 | 97% | 73% | 89% | |||
| Radiography | 1(6.7%) | 0(0.0%) | 4(26.7%) | 0.125 | 97% | 13% | 69% | |||
| Voids | µCT | 14(93.3%) | 6(40%) | 1(6.7%) | 0.000 | – | – | – | – | |
| OCT | 11(73.3%) | 4(26.6%) | 1(6.7%) | 0.684 | 96% | 71% | 84% | |||
μCT, computerized microtomography; CL, lateral condensation; OCT, optical coherence tomography; SC, single cone; TMC, thermomechanical compaction; WL, working length.
aFisher’s exact test.
Figure 1. (a) Efficent aspect of apical filling by μCT and OCT images 1,dentine; 2,gutta-percha cone; 3,endodotic cement. (b) Void on apical filling are demarcated by arrows.μCT,computerized microtomography;OCT, optical coherence tomography; WL,workinglength.
Regarding apical working length, the periapical digital radiography showed lower κ concordance when compared with µCT. OCT was in agreement with µCT also to voids detections with good sensitivity, specificity and accuracy, as demonstrated in Table 1.
The micrometric evaluation methods (µCT and OCT) showed voids on the apical filling, frequently between gutta-percha cone/dentine and gutta-percha cone/cement interfaces, as demonstrated in Figure 1b.
Regarding the size of voids, there are significant differences between obturation techniques in the pairwise comparison of samples subjected to LC (Table 2).
Table 2.
Comparison of mode of the number, meanandstandarddeviationofwidth and depth of voids by two observer, for each obturation type using two different methods of analysis.
| Number of voids | Width | Depth | |||||
|---|---|---|---|---|---|---|---|
| Obturation technique | N | Mode | Mean (SD) obs 1 | Mean (SD) obs 2 | Mean (SD) obs 1 | Mean (SD) obs 2 | |
| μCT | |||||||
| LC | 15 | 2b | 0.11b(±0.09) | 0.12b(±0,1) | 0.37b(±0.27) | 0.4b(±0,33) | |
| SC | 15 | 1b | 0.10b(±0.13) | 0.08b(±0.09) | 0.13b(±0.14) | 0.14b(±0.15) | |
| TMC | 15 | 0b | 0.09b(±0.25) | 0.13b(±0.29) | 0.16b(±0.22) | 0.16b(±0.22) | |
| pa | 0.011 | 0.027 | 0.005 | ||||
| OCT | |||||||
| LC | 15 | 1b | 0.13b(±0.17) | 0.14b(±0.18) | 0.04b(±0.05) | 0.3b(±0.05) | |
| SC | 15 | 0b | 0.00b(±0.02) | 0.00b(±0.01) | 0.00b(±0.01) | 0.01b(±0.04) | |
| TMC | 15 | 0b | 0.04b(±0.17) | 0.04b(±0.18) | 0.00b(±0.00) | 0.00b(±0.00) | |
| pa | 0.002 | 0.006 | 0.001 | ||||
LC,lateral condensation;SC,single cone; TMC,thermomechanical compaction.
aKruskal–Wallis’ test.
bPairwise comparison using Mann–Whitney’s test, with Bonferroni penalty (p < 0.01).
Discussion
The complex anatomy of the root canal apical region is a challenge to endodontics, mainly in apical obturation and filling.19–21 The presence of apical voids is associated to a higher risk of marginal apical infiltration and consequent endodontic failure. This is influenced by several variables, such as obturation techniques, apical filling limit, chemical and physical properties of the chosen material, and smear layer permanency.21 In vivo, the endodontic obturation is evaluated by apical Rx (X-rays), and in specific cases patients may be submitted to cone beam CT, although these are not indicated for the observation of voids.22
One of the criteria to evaluate the apical filling was by the WL, that was defined as adequate in the range between +0.5 mm, and results demonstrated that the three protocols tested performed similarly (p > 0.05). The real WL measured by µCT was considered the reference, and showed poor agreement with digital radiography, which represents a limit of the clinical technique more commonly used, with limitations of bidimensional image, as lacking coincidence of the anatomical apex with apical foramen.2,4,16,19,20
Beyond WL fails, the presence or combination of multiples voids intern and external can form a gap between the filing materials and the dentinal walls, which possibly results in percolation of periradicular tissue fluids, microorganisms and toxins, as defined by American Association of Endodontists.23 As the clinical detection of apical voids is limited, even through the use of the cone beam CT,22,23 the evaluation of endodontic obturation protocols considering the aspect of apical filling must be in vitro, by imaging methods of micrometric resolution, such as µCT, the standard technique, and OCT, an alternative method.
The use of OCT as an alternative method was validated for the visualization of voids in the WL of apical obturations in vitro, with good sensitivity, specificity and accuracy relative to the standard method, µCT. These results strengthen the correlation between micrometric methods of image analysis found by Majkut et al8 Minamino et al9 and Oliveira et al.10 It is worth stressing that OCT, a tomographic option free from ionizing radiation, offers high resolution and velocity of acquisition with a wide range of clinical applications in dentistry.9 It is believed that the lower specificity of OCT can be attributed to the interposition between anatomical structures resulting in less and short voids than what is observed with µCT.
LC technique showed more and larger voids in average in comparison with the other techniques, corroborating the studies of Kierklo et al3 and Ho etal1 It is also noticed that voids created by LC technique are deeper and wider in average, probably owinge to the protocol of lateral adaptation of several gutta-percha cones with ISO-sized gauge.24 However, as in the work of Zogheib et al25 the voids found can be results of root filling procedures.
The two other protocols-SC and thermomechanical techniques-recommend the adaptation of one gutta-percha cone that match the memory instrument of the biomechanical preparation procedures,17 assuring a more homogeneous filling with less cement.26,27 There is no statistical difference for frequency and size of voids between the SC and thermomechanical technique, and good results were also found for the last one by Antunes et al28 and Hwang et al29
The limitations of this study were related to the images acquisition processes. The OCT requires directly visualization of the region to be scanned to minimize the presence of shadows, superpositions or regions not well-reproduced. Considering resolution aspects of SS-OCTs, typical axial resolution in commercial products is limited to 5–15 µm owing to the optical source employed. Experimental laboratory systems can provide submicrometer resolution.30 As for the lateral resolution, although the work of Watanabe et al31 describes resolutions of ten’s of micrometers, with proper adjustments of the collecting optics, this value can be reduced to less than 10 µm.
Further developments in dental OCT, including a hand piece for practical clinical devices, are needed to improve more applications with good imaging depth and quality.32
Conclusion
OCT showed good results for the visualization of apical voids in obturation when compared with µCT in vitro, validating the use of the technique to this aim. The obturation techniques did not alter the working length, although the LC technique showed the largest number of voids.
Contributor Information
Fernanda Clotilde Mariz Suassuna, Email: fernandacosta3@hotmail.com.
Ana Marly Araújo Maia, Email: anamarlyamaia@gmail.com.
Daniela Pita Melo, Email: danipita@gmail.com.
Antônio Celso Dantas Antonino, Email: acdantonino@gmail.com.
Anderson Stevens Leônidas Gomes, Email: andersonslgomes@gmail.com.
Patrícia Meira Bento, Email: patmeira@uol.com.br.
References
- 1.Chang JWWJW, Cheung GSPGS. Quality of root canal fillings using three gutta-percha obturation techniques. RestorDentEndod 2016; 41: 22–8.doi: 10.5395/rde.2016.41.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortman RE. Technologic advances in endodontics. DentClin North Am 2011; 55: 461–80. doi: 10.1016/j.cden.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 3.Kierklo A, Tabor Z, Pawińska M, Jaworska M. A microcomputed tomography-based comparison of root canal filling quality following different instrumentation and obturation techniques. MedPrincPract 2015; 24: 84–91. doi: 10.1159/000368307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kierklo A, Tabor Z, Petryniak R, Dohnalik M, Jaworska M. Application of microcomputed tomography for quantitative analysis of dental root canal obturations. Postepy Hig Med Dosw 2014; 68: 310–5. doi: 10.5604/17322693.1095271 [DOI] [PubMed] [Google Scholar]
- 5.Shemesh H, Vanvan Soest G, van der Sluis LW, Wesselink PR. The ability of optical coherence tomography to characterize the root canal walls. J Endod 2007; 33: 1369–73. doi: 10.1016/j.joen.2007.06.022 [DOI] [PubMed] [Google Scholar]
- 6.Maia AAM, FonsecaFonsêca DD, Kyotoku BB, Gomes AS. Characterization of enamel in primary teeth by optical coherence tomography for assessment of dental caries. IntJPaediatrDent 2010; 20: 158–64. doi: 10.1111/j.1365-263X.2009.01025.x [DOI] [PubMed] [Google Scholar]
- 7.Shimada Y, Sadr A, Burrow MF, Tagami J, Ozawa N, Sumi Y. Validation of swept-source optical coherence tomography (SS-OCT) for the diagnosis of occlusal caries. JDent 2010; 38: 655–65. doi: 10.1016/j.jdent.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 8.Majkut P, Sadr A, Shimada Y, Sumi Y, Tagami J. validation of optical coherence tomography against micro-computed tomography for evaluation of remaining coronal dentin thickness. JEndod 2015; 41: 1349–52. doi: 10.1016/j.joen.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 9.Minamino T, Mine A, Matsumoto M, Sugawa Y, Kabetani T, Higashi M, et al. Nondestructive observation of teeth post core-space using optical coherence tomography: comparison with microcomputed tomography and live images. J Biomed Opt 2015; 20: 107001. doi: 10.1117/1.JBO.20.10.107001 [DOI] [PubMed] [Google Scholar]
- 10.de Oliveira BP, Câmara AC, Duarte DA, Gomes ASL, Heck RJ, Antonino AACD, et al. Detection of apical root cracks using spectral domain and swept-source optical coherence tomography. JEndod 2017; 43: 1148–51. doi: 10.1016/j.joen.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 11.Shemesh H, Vanvan Soest G, Wesselink PR. Diagnosis of vertical root fractures with optical coherence tomography. JEndod 2008; 34: 739–42. doi: 10.1016/j.joen.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 12.Negrutiu ML, Nica L, Sinescu C, Topala F, Ionita C, Bradu A. SEM and microCT validation for en face OCT imagistic evaluation of endodontically treated human teeth. SPIE Medical Imaging 2011: 79614W. [Google Scholar]
- 13.Berutti E, Chiandussi G, Paolino DS, Scotti N, Cantatore G, Castellucci A, et al. Effect of canal length and curvature on working length alteration with WaveOne reciprocating files. JEndod 2011; 37: 1687–90. doi: 10.1016/j.joen.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 14.Cavenago BC, Duarte MAHMA, Ordinola-Zapata R, Marciano MA, del Carpio-Perochena AE, Bramante CM. Interfacial adaptation of an epoxy-resin sealer and a self-etch sealer to root canal dentin using the System B or the single cone technique. Braz Dent J 2012; 23: 205–11. doi: 10.1590/S0103-64402012000300004 [DOI] [PubMed] [Google Scholar]
- 15.McSpadden JT. Self study course for the thermatic condensation of gutta-percha. Toledo: Ransom and Randolph; 1980. [Google Scholar]
- 16.Malagnino VA. Hybrid microseal/pacmac obturation. Dent Update 2011; 38: 477–84. [DOI] [PubMed] [Google Scholar]
- 17.Versiani MA, Leoni GB, Steier L, De-Deus G, Tassani S, Pécora JD, et al. Micro-computed tomography study of oval-shaped canals prepared with the self-adjusting file, Reciproc, WaveOne, and ProTaper universal systems. JEndod 2013; 39: 1060–6. doi: 10.1016/j.joen.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 18.Schneider SW. A comparison of canal preparations in straight and curved root canals. Oral Surg Oral Med Oral Pathol 1971; 32: 271–5. doi: 10.1016/0030-4220(71)90230-1 [DOI] [PubMed] [Google Scholar]
- 19.Vertucci FJ. Root canal morphology and its relationship to endodontic procedures. Endod Topics 2005; 10: 3–29. doi: 10.1111/j.1601-1546.2005.00129.x [Google Scholar]
- 20.Estevez R, Heilborn C, Cohenca N. Root anatomy and canalconfiguration of the permanentmandibularfirstmolar: asystematicreview. JEndod 2010; 36: 1919–31. doi: 10.1016/j.joen.2010.08.05521092807 [Google Scholar]
- 21.Muliyar S, Shameem KA, Thankachan RP, Francis PG, Jayapalan CS, Hafiz KAA. Microleakage in endodontics. J Int Oral Health 2013; 6: 99–104. [PMC free article] [PubMed] [Google Scholar]
- 22.Song D, Zhang L, Zhou W, Zheng Q, Duan X, Zhou X, et al. Comparing cone-beam computed tomography with periapical radiography for assessing root canal obturation in vivo using microsurgical findings as validation. DentomaxillofacRadiol 2017; 46: 20160463. doi: 10.1259/dmfr.20160463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Association of Endodontists. Quality Assurance Guidelines. Chicago, IL, USA: AAE; 1994. [Google Scholar]
- 24.Celikten B, F Uzuntas C, I Orhan AIA, Tufenkci P, Misirli M, DemiralO Demiralp KOK, et al. Micro-CT assessment of the sealing ability of three root canal filling techniques. J Oral Sci 2015; 57: 361–6. doi: 10.2334/josnusd.57.361 [DOI] [PubMed] [Google Scholar]
- 25.Zogheib C, Hanna M, Pasqualini D, Naaman A. Quantitative volumetric analysis of cross-linked gutta-percha obturators. AnnStomatol 2016; 7: 46. doi: 10.11138/ads/2016.7.3.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De‐-Deus G, Gurgel‐-Filho ED, MagalhaesMagalhães KM, Coutinho‐-Filho T. A laboratory analysis of gutta-percha-filled area obtained using Thermafil, System B and lateral condensation. IntEndodJ 2006; 39: 378–83. doi: 10.1111/j.1365-2591.2006.01082.x [DOI] [PubMed] [Google Scholar]
- 27.Pane ES, Palamara JEAJE, Messer HH. Behavior of resin-based endodontic sealer cements in thin and thick films. Dent Mater 2012; 28: e150–e159. doi: 10.1016/j.dental.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 28.Antunes HS, Gominho LF, Andrade‐Junior CV, Dessaune–Neto N, Alves FRF, Rôças IN. Sealing ability of two root–end filling materials in a bacterial nutrient leakage model. Int Endod J 2015; 49: 1–6. [DOI] [PubMed] [Google Scholar]
- 29.Hwang JH, Chung J, Park E, Kwak S, Kim HC. Comparison of bacterial leakage resistance of various root canal filling materials and methods: confocal laser-scanning microscope study. Scanning 2015; 37: 422–8. doi: 10.1002/sca.21231 [DOI] [PubMed] [Google Scholar]
- 30.Povazay B, Bizheva K, Unterhuber A, Hermann B, Sattmann H, Fercher AF, et al. Submicrometer axial resolution optical coherence tomography. OptLett 2002; 27: 1800–2. doi: 10.1364/OL.27.001800 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe H, Kuribayashi A, Sumi Y, Kurabayashi T. Resolution characteristics of optical coherence tomography for dental use. Dentomaxillofac Radiol 2017; 46: 20160358. doi: 10.1259/dmfr.20160358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JY, Chung JH, Lee JS, Kim HJ, Choi SH, Jung UW. Comparisons of the diagnostic accuracies of optical coherence tomography, micro-computed tomography, and histology in periodontal disease: an ex vivo study. J Periodontal Implant Sci 2017; 47: 30–40. doi: 10.5051/jpis.2017.47.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]