Supplemental digital content is available in the text.
Key Words: istradefylline, lower urinary tract symptoms, overactive bladder, Parkinson disease
Abstract
Objectives
In addition to motor symptoms, bladder dysfunction is a major clinical issue in patients with Parkinson disease (PD). Istradefylline is adenosine A2A receptor antagonist approved for PD patients with wearing-off symptoms. The aim of this study was to determine the long-term effects of istradefylline on lower urinary tract symptoms (LUTSs) in PD patients.
Methods
We enrolled 14 male PD patients. The mean age of patients was 73 years (61–77 years), the Hoehn-Yahr stage was 2 (2–3), and disease duration was 9 years (3–28 years). The effects of istradefylline (20 mg/d) on LUTSs in PD patients with motor complications after 3, 6, and 12 months of therapy were evaluated based on the International Prostate Symptom Score and Overactive Bladder Symptom Score before and after its administration.
Results
Motor symptoms significantly improved at 12 months' administration (Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale part III: 30.0 ± 12.9 vs 13.8 ± 8.1; P < 0.01). Significant improvements were also observed in the answers provided on urinary questionnaires (International Prostate Symptom Score, 14.4 ± 7.6 vs 8.5 ± 6.8; Overactive Bladder Symptom Score, 6.9 ± 2.8 vs 5.5 ± 3.7; P < 0.05). Nighttime urinary frequency and the percentage of the nocturnal urine volume also improved significantly at 3 months' administration (P < 0.01).
Conclusions
Istradefylline effectively improved not only motor symptoms, but also LUTSs in patients with PD.
Bladder dysfunction is a major clinical issue in patients with Parkinson disease (PD). The prevalence of lower urinary tract symptoms (LUTSs) characterized by urinary urgency, frequency, and incontinence was previously reported to be between 27% and 40%.1–3 Bladder symptoms often appear once treatments for PD have been initiated.1 Anti-PD treatments may influence bladder control in an unpredictable manner.4 These symptoms have a severe impact on the quality of life (QOL) of PD patients.5 The adenosine receptor A2A is strongly expressed in the striatum, interacts with dopamine D2 receptors, and modulates dopamine transmission. Istradefylline is a novel nondopaminergic selective adenosine A2A receptor antagonist. The Japanese phase 3 trial showed that 20 mg of orally administered istradefylline decreased the off-time. A recent study shows that adding istradefylline to low doses of l-DOPA and dopamine agonists is superior to the later use in PD patients treated with high doses of l-DOPA. It may be effective to administer istradefylline before the off symptoms progress.6 We previously reported that istradefylline improved not only motor symptoms, but also LUTSs in patients with PD in a short-term period.7 However, it currently remains unclear whether istradefylline is useful for the treatment of PD patients with LUTSs in the long-term real-world clinical settings. Therefore, the aim of this study was to determine the effects of 1-year istradefylline treatment on LUTSs in PD patients.
METHODS
Study Participants
In this prospective study, patients were invited consecutively from the Department of Neurology to participate between March 2015 and July 2015, regardless of the presence of LUTSs. Study exclusion criteria were any pelvic or urological abnormalities, bladder surgery, or disease that may affect bladder function. During this study, no changes to the type of rehabilitation or PD medication were required. The present study was approved by the Scientific Ethics Committee of Hokkaido University (#014-0342), and patients provided informed consent.
Assessments
International Prostate Symptom Score, Overactive Bladder Symptom Score, King's Health Questionnaire
In order to analyze LUTSs and QOL, we used the International Prostate Symptom Score (IPSS), QOL score, and Overactive Bladder Symptom Score (OABSS) at baseline and 3 months, 6 months, and 1 year after the treatment (the data of 3 months were reported previously7). The IPSS is a 7-question questionnaire that assesses LUTSs in the storage (urgency episodes, nocturia, and increased daytime frequency) and voiding (incomplete emptying, a weak urinary stream, and straining at urination) phases. An additional question on the QOL score was evaluated. The IPSS is useful for detecting voiding dysfunctions in patients with PD.8 Total IPSS scores range from 0 to 35, with greater scores indicating increasing symptom severity. The OABSS quantifies daytime frequency, nocturia, urgency, and urinary incontinence over a 1-week recall period.9 Scores range from 0 to 15, with greater scores indicating increasing symptom severity. Question 3 of the OABSS measures urgency. The King's Health Questionnaire (KHQ-QOL) score was also calculated in the following 8 domains (Appendix 1, Supplemental Digital Content, http://links.lww.com/CNP/A5), using the formula shown in Appendix 2: General Health Perceptions, Impact on Life, Role Limitations, Physical Limitations, Social Limitations, Personal Relationships, Emotional Problems, and Sleep/Energy Disturbance, (Supplemental Digital Content, http://links.lww.com/CNP/A5). Symptom scores range from 0 to 100, with lower scores reflecting better KHQ-QOL.
Voiding Diary
Patients also completed a 3-day voiding diary at baseline and 3 months and 1 year after the treatment. Patients recorded daytime and nighttime urinary frequencies, as well as voided volumes. The nocturnal polyuria index was defined as the nocturnal urine volume divided by the 24-hour volume. Nocturnal polyuria was also defined as a nocturnal urine volume exceeding 33% of the 24-hour urine output.
Uroflowmetry and Residual Urine Volume
Measurements of the urinary flow rate and postvoiding residual urine volume using ultrasound were performed at baseline and 3 months and 1 year after the treatment.
Scales for Outcomes in PD
Motor symptoms were assessed using the Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS) at baseline and 3 months, 6 months, and 1 year after the treatment. The motor symptom test was performed at nearly the same time period with every clinic visit.
Statistical Analyses
All data are shown as the mean ± SD. GraphPad Prism (GraphPad Software, La Jolla, CA) was used for statistical analyses, which were conducted using the paired t test (parametric) or Wilcoxon signed rank test (nonparametric), with P < 0.05 being considered significant.
RESULTS
Fourteen male patients were included, and 12 patients completed the study. One patient was unable to complete this study because of the adverse effects of istradefylline (the deterioration of dyskinesia), and the other patient was changed to another anti-PD drug because of the clinical condition. The mean age of our patients was 66 years (61–80 years), the Hoehn-Yahr stage was 2 (2–3), and disease duration was 9 years (4–26 years). The MDS-UPDRS parts I, II, and III were 9.2 ± 5.4, 13.8 ± 7.3, and 30.0 ± 12.9, respectively.
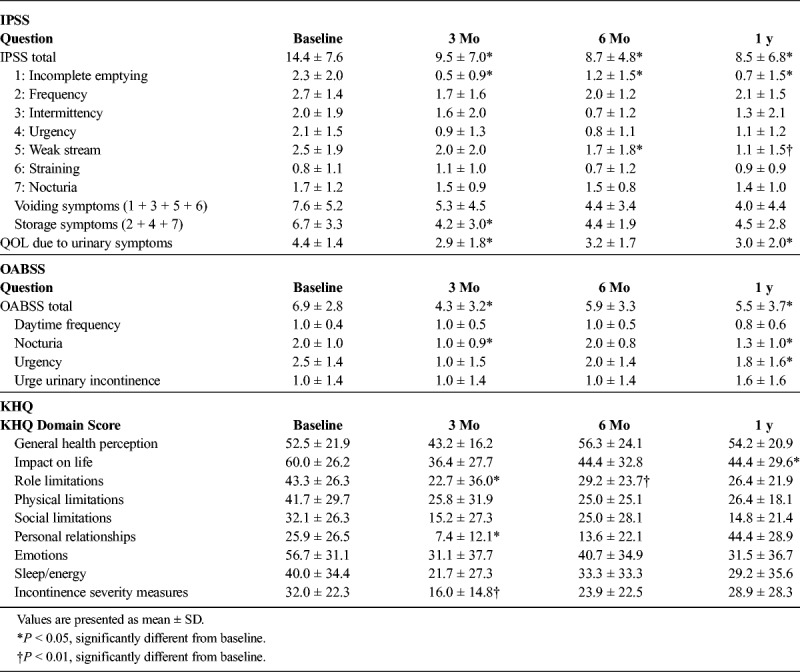
IPSS, QOL, and OABSS
In all patients (including those with the absence of LUTSs), statistical analyses revealed significant decreases in the IPSS total number (at 3 months, 6 months, and 1 year), QOL (at 3 months and 1 year), and OABSS total number (at 3 months and 1 year) after the treatment with istradefylline (Table 1). In patients with no or mild LUTSs or no OAB symptom (IPSS of <8 and OABSS urgency score [for question 3] of <2), no significant changes were noted in IPSS or OABSS total numbers after 1-year treatment (IPSS: 5.3 ± 2.9 to 4.7 ± 4.6, OABSS: 4.3 ± 2.1 to 6.0 ± 5.6).
TABLE 1.
International Prostate Symptom Score, OABSS, and KHQ Before and After the Treatment With Istradefylline
Voiding Diary
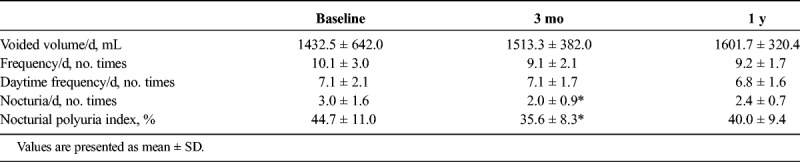
Table 2 shows significant decreases in the nighttime voiding frequency (nocturia) in the 3-day voiding diary after 3 months of the treatment (3.0 ± 1.6 to 2.0 ± 0.9, P < 0.05). Significant decreases were also observed in nocturnal polyuria index (44.7 ± 11.0 to 35.6 ± 8.3%, P < 0.05). However, 1 year after the treatment, these changes were not significant.
TABLE 2.
Three Days Voiding Diary Before and After the Treatment With Istradefylline
Uroflowmetry and Residual Urine Volume
No significant changes were observed in the urinary flow rate or postvoiding residual urine volume between before and after the administration of istradefylline. The mean maximum urinary flow rates at baseline and 3 months and 1 year after the treatment were 9.5 ± 5.5, 10.4 ± 4.0, and 9.4 ± 4.5 mL/s, respectively, whereas residual urine volumes were 23.0 ± 47.2, 21.5 ± 34.2, and 45.4 ± 31.0 mL, respectively.
King's Health Questionnaire
Data from the KHQ revealed that the 2 domains of personal relationships and incontinence severity measures had significantly improved at 3 months (P < 0.05 and P < 0.01, respectively). Role limitation at 3 and 6 months (P < 0.01 and P < 0.05, respectively) had significantly improved, whereas impact on life scores improved only after 1 year (P < 0.05) (Table 1).
Movement Disorder Society–Sponsored Revision of the Unified Parkinson's Disease Rating Scale
Motor symptoms were significantly better at 3 and 6 months and 1 year than at baseline (MDS-UPDRS part III: 30.0 ± 12.9 vs 10.9 ± 7.1, 13.8 ± 8.1, and 13.5 ± 7.0; P < 0.01).
During this study, 2 patients developed slight dyskinesia, and 1 patient exhibited visual hallucination, which was so mild that the istradefylline administration was not discontinued. No adverse urological effects (such as urinary retention and urinary tract infection) were observed in any patient.
DISCUSSION
The results of the present study demonstrate that long-term administration of istradefylline improves LUTSs in PD patients. Moreover, istradefylline did not aggravate LUTSs in PD patients without LUTSs. During this study, no adverse urological effects (such as urinary retention and urinary tract infection) were observed in any patient. Central LUTSs in patients with PD are overactive bladder (OAB) symptoms, characterized by urinary urgency and increases in daytime and nighttime urinary frequency.2 Lower urinary tract symptoms are more likely to occur at more advanced stages of PD and progressively deteriorate with the disease duration. Furthermore, LUTSs become “unmasked” in patients with advanced PD. Prior to the treatment of motor symptoms, patients may be preoccupied with coping with motor symptoms and thus be less aware of bladder symptoms. When the severity of motor symptoms decreases with treatments, patients become more aware of LUTSs. In the present study, only 9 patients (64.3%) consulted a urologist prior to the administration of istradefylline, even among patients with moderate or severe LUTSs (IPSS of ≧8 and OABSS urgency score [for question 3] of ≧2 and a total OABSS of ≧3).
Previous studies reported that in contrast to motor dysfunction LUTSs are often nonresponsive to levodopa, suggesting that they occur through complex mechanisms. Therefore, add-on therapy is required in order to prioritize improvements in QOL.4,10 Istradefylline, a nondopaminergic selective adenosine A2A receptor antagonist, does not have direct effects on dopaminergic mechanisms, which may provide anti-PD effects on motor and lower urinary tract functions without adverse events.
We provided PD patients with detailed urinary questionnaires in order to survey LUTSs. A large number of studies have demonstrated that LUTSs have a significant impact on QOL measurements.5,11 In the present study, both IPSS and OABSS significantly improved. This result is consistent with previous findings. Storage and voiding symptoms both significantly improved, with the latter showing early improvements following the administration of the treatment. The mechanism responsible for this early improvement and lasting effect has not yet been elucidated but may be related to the mechanism underlying the indirect impact of adenosine A2A receptor antagonists. Further studies are needed in order to clarify this background mechanism. Previous studies on PD patients reported that the degeneration of dopaminergic neurons in the substantia nigra and subsequent loss of striatal dopamine led to detrusor overactivity through an inability to activate D1-mediated tonic inhibition. In patients with PD, other mechanisms may include selective damage to inhibitory dopaminergic neurons.2 We previously used a rat model of PD to examine the effects of an adenosine A2A receptor antagonist on the micturition reflex and found that its intravenous administration increased the intercontraction interval (inhibited the micturition reflex) in PD and sham rats, with inhibitory effects being stronger in PD rats.12 These findings indicate that an adenosine A2A receptor–mediated excitatory mechanism is enhanced in the brain, thereby inducing bladder overactivity, and also that the inhibition of A2A receptors effectively suppressed bladder overactivity in the rat model of PD12–14 (Fig. 1). As we represented in the hypothetical diagram (Fig. 1), dopaminergic neurons in the substantia nigra pars compacta are lost, which leads to the loss of dopamine D1 receptor activation and facilitation of the spinobulbospinal pathway controlling the micturition reflex. The administration of adenosine A2A receptor antagonists may suppress the A2A receptor–mediated activation of the micturition reflex in order to reduce bladder overactivity in PD patients.
FIGURE 1.
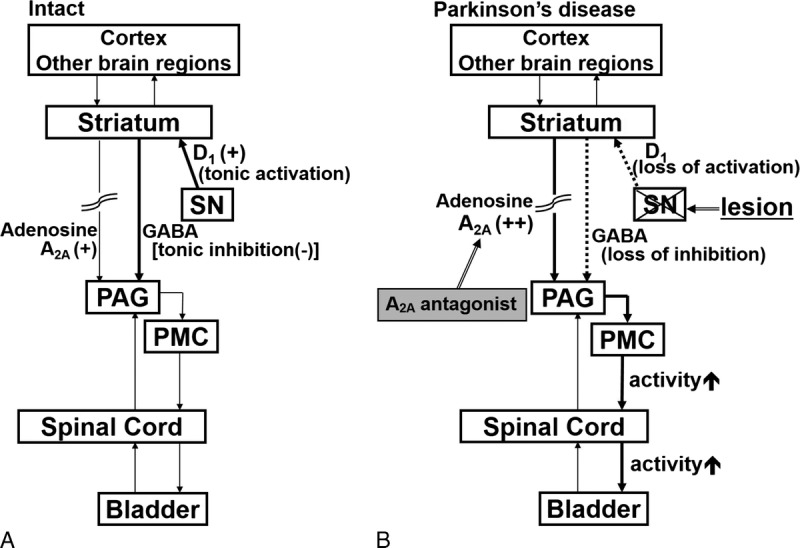
Hypothetical diagram showing neural mechanisms of bladder function in normal (intact) and PD. The micturition reflex is controlled by the spinobulbospinal pathway passing through the periaqueductal gray matter (PAG) in the midbrain and the pontine micturition center (PMC). This neural circuit is under the control of higher centers. A, In intact conditions, tonic activation (+) of dopaminergic neurons in the substantia nigra pars compacta (SN) activates dopamine D1 receptors expressed on GABAergic inhibitory neurons in the striatum in order to inhibit the micturition reflex. At the same time, D1 receptor stimulation suppresses the activity of adenosinergic neurons, which exert an excitatory effect on micturition via adenosine A2A receptors. B, In PD, dopaminergic neurons in the SN are lost, leading to the loss of dopamine D1 receptor activation, which results in reduced activation of inhibitory GABAergic neurons in the striatum. At the same time, reduced D1 receptor stimulation enhances the adenosinergic mechanism to stimulate adenosine A2A receptors, leading to facilitation of the spinobulbospinal pathway controlling the micturition reflex. Administration of adenosine A2A receptor antagonists could suppress A2A receptor–mediated activation of the micturition reflex to reduce bladder overactivity in PD. D1 indicates dopamine D1 receptor; GABA, γ-aminobutyric acid. Modified with permission from Yoshimura et al.14
It has yet to be established whether adenosinergic mechanisms regulate LUTSs in patients with PD. In the present study, not only storage symptoms but also voiding symptoms were improved by the treatment with istradefylline. We also showed that objective urodynamic parameters were not significantly affected by this treatment even in long-term administration of adenosine A2A receptor antagonist. Discrepancies have been reported in LUTS studies. For example, objective findings such as the peak flow rate and residual urine volume did not correlate with IPSS and QOL scores, whereas each objective and subjective symptoms significantly improved after transurethral resection of the prostate.15 Istradefylline directly affects supraspinal lesions, which may ameliorate LUTSs without changing lower urinary tract functions. This function is preferable for other nonneurogenic OAB patients such as those with ischemic disease and multiple sclerosis. Moreover, the findings of an animal study12 showed that istradefylline may suppress the adenosine A2A receptor–mediated excitatory adenosinergic mechanism in the spinal cord, caused by OAB in PD patients. Further studies are needed to examine how the adenosine A2A receptor modulates and ameliorates bladder overactivity in PD patients.
Quality of life is severely affected by nocturia.16–19 Cornu et al20 identified nocturnal polyuria as a cause of LUTSs (nighttime urinary frequency). Furthermore, a recent study21 demonstrated that nocturnal polyuria developed in PD patients and deteriorated LUTSs. However, the pathophysiology of nocturnal polyuria in PD patients currently remains unclear. Nocturnal polyuria is associated with decreases in daily changes in the secretion of antidiuretic hormone (ADH).22 The secretion of pituitary hormones is controlled by hypothalamic hormones, which are directly and indirectly modulated by neurotransmitters such as dopamine, noradrenaline, and serotonin.23 Tateno et al24 reported that amantadine ameliorated nocturnal polyuria in patients with PD. They speculated that the effects of amantadine on dopamine, noradrenaline, and serotonin modulated the circadian secretion of ADH and ameliorated nocturnal polyuria in patients with PD. Istradefylline may also modulate the circadian secretion of ADH. However, long-term effect is not clear in the current study; further studies are needed in order to confirm this.
Anticholinergics are used to treat LUTSs in PD patients; however, many practitioners are well versed in the common adverse effects of dry mouth and constipation, other more serious problems of cognitive impairment. Moreover, polypharmacy in aged patient has increased in the past decade. From this aspect, adenosine A2A receptor antagonists are suitable agent as an “add-on” therapy for LUTSs in patients with PD.
The limitations of this study include the small number of patients treated and the absence of a placebo control. In the present study, we did not evaluate cognitive function at baseline or after the treatment, and thus, this warrants further examination.
CONCLUSIONS
The results of the present study confirmed that adenosine A2A receptor antagonists are useful as a new pharmacological treatment for OAB in patients with PD in a long-term period.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the medical specialists, continence nurses, nursing, and administrative staff of Hokkaido University Hospital. They also thank Ms Sachiyo Murai for her excellent data management during this study.
Footnotes
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest to declare.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.clinicalneuropharm.com).
REFERENCES
- 1.Araki I, Kuno S. Assessment of voiding dysfunction in Parkinson's disease by the international prostate symptom score. 2000;68:429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winge K, Fowler CJ. Bladder dysfunction in Parkinsonism: mechanisms, prevalence, symptoms, and management. 2006;21:737–745. [DOI] [PubMed] [Google Scholar]
- 3.Winge K, Skau AM, Stimpel H, et al. Prevalence of bladder dysfunction in Parkinsons disease. 2006;25:116–122. [DOI] [PubMed] [Google Scholar]
- 4.Winge K, Werdelin LM, Nielsen KK, et al. Effects of dopaminergic treatment on bladder function in Parkinson's disease. 2004;23:689–696. [DOI] [PubMed] [Google Scholar]
- 5.Sakakibara R, Shinotoh H, Uchiyama T, et al. Questionnaire-based assessment of pelvic organ dysfunction in Parkinson's disease. 2001;92:76–85. [DOI] [PubMed] [Google Scholar]
- 6.Yabe I, Kitagawa M, Takahashi I, et al. The efficacy of istradefylline for treating mild wearing-off in Parkinson disease. 2017;40(6):261–263. [DOI] [PubMed] [Google Scholar]
- 7.Kitta T, Yabe I, Takahashi I, et al. Clinical efficacy of istradefylline on lower urinary tract symptoms in Parkinson's disease. 2016;23:893–894. [DOI] [PubMed] [Google Scholar]
- 8.Brusa L, Petta F, Pisani A, et al. Acute vs chronic effects of l-DOPA on bladder function in patients with mild Parkinson disease. 2007;68:1455–1459. [DOI] [PubMed] [Google Scholar]
- 9.Homma Y, Yoshida M, Seki N, et al. Symptom assessment tool for overactive bladder syndrome—overactive bladder symptom score. 2006;68:318–323. [DOI] [PubMed] [Google Scholar]
- 10.Uchiyama T, Sakakibara R, Hattori T, et al. Short-term effect of a single levodopa dose on micturition disturbance in Parkinson's disease patients with the wearing-off phenomenon. 2003;18:573–578. [DOI] [PubMed] [Google Scholar]
- 11.Kubota Y, Kojima Y, Shibata Y, et al. Correlation between improvements in Overactive Bladder Symptom Score and Health-Related Quality of Life questionnaires in overactive bladder patients treated with an antimuscarinic drug. 2011;30:1309–1314. [DOI] [PubMed] [Google Scholar]
- 12.Kitta T, Chancellor MB, de Groat WC, et al. Roles of adenosine A1 and A2A receptors in the control of micturition in rats. 2014;33:1259–1265. [DOI] [PubMed] [Google Scholar]
- 13.Kitta T, Chancellor MB, de Groat WC, et al. Suppression of bladder overactivity by adenosine A2A receptor antagonist in a rat model of Parkinson disease. 2012;187:1890–1897. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura N, Miyazato M, Kitta T, et al. Central nervous targets for the treatment of bladder dysfunction. 2014;33:59–66. [DOI] [PubMed] [Google Scholar]
- 15.Machino R, Kakizaki H, Ameda K, et al. Detrusor instability with equivocal obstruction: a predictor of unfavorable symptomatic outcomes after transurethral prostatectomy. 2002;21:444–449. [DOI] [PubMed] [Google Scholar]
- 16.Appell RA, Sand PK. Nocturia: etiology, diagnosis, and treatment. 2008;27:34–39. [DOI] [PubMed] [Google Scholar]
- 17.Tikkinen KA, Johnson TM, 2nd, Tammela TL, et al. Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. 2010;57:488–496. [DOI] [PubMed] [Google Scholar]
- 18.Malmsten UG, Molander U, Peeker R, et al. Urinary incontinence, overactive bladder, and other lower urinary tract symptoms: a longitudinal population-based survey in men aged 45–103 years. 2010;58:149–156. [DOI] [PubMed] [Google Scholar]
- 19.Sakushima K, Yamazaki S, Fukuma S, et al. Influence of urinary urgency and other urinary disturbances on falls in Parkinson's disease. 2016;360:153–157. [DOI] [PubMed] [Google Scholar]
- 20.Cornu JN, Abrams P, Chapple CR, et al. A contemporary assessment of nocturia: definition, epidemiology, pathophysiology, and management—a systematic review and meta-analysis. 2012;62:877–890. [DOI] [PubMed] [Google Scholar]
- 21.Uchiyama T, Yamamoto T, Yanagisawa M, et al. Influence of nocturnal polyuria to nocturia in Parkinson's disease. 2012;31:985. [Google Scholar]
- 22.Sakakibara R, Uchiyama T, Liu Z, et al. Nocturnal polyuria with abnormal circadian rhythm of plasma arginine vasopressin in post-stroke patients. 2005;44:281–284. [DOI] [PubMed] [Google Scholar]
- 23.De Wied D, Diamant M, Fodor M. Central nervous system effects of the neurohypophyseal hormones and related peptides. 1993;14:251–302. [DOI] [PubMed] [Google Scholar]
- 24.Tateno H, Uchiyama T, Yamamoto T, et al. Amantadine can ameliorate lower urinary tract dysfunction and nocturnal polyuria in patients with Parkinson disease and vascular parkinsonism. 2015;42:21–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


