Guidelines from the American Thyroid Association and the American Association of Clinical Endocrinologists suggest monitoring thyroid-stimulating hormone and serum-free thyroxine to make clinical decisions for starting and monitoring therapy with their mainstay treatment of levothyroxine monotherapy. Although this monotherapy provides adequate control of symptoms for the majority of the population, a strong subset of the population continues to complain of hypothyroidism despite normal thyroid-stimulating hormone and serum-free thyroxine levels. Combination levothyroxine and liothyronine therapy to improve symptoms of hypothyroidism has been a topic of interest for several decades, but the data have been mixed. This study provides insight into the effects of combination therapy because there is no reported retrospective or prospective study in the literature.
Supplemental digital content is available in the text.
Key Words: combination therapy, desiccated thyroid extract, hypothyroidism, liothyronine
Abstract
Objectives
Hypothyroidism results in decreased mood and neurocognition, weight gain, fatigue, and many other undesirable symptoms. The American Association of Clinical Endocrinologists, the American Thyroid Association (ATA), and The Endocrine Society recommend levothyroxine (LT4) monotherapy as the treatment for hypothyroidism; however, after years of monotherapy, some patients continue to experience impaired quality of life. Combination LT4 and synthetic liothyronine (LT3) therapy or the use of desiccated thyroid extract (DTE), has not been suggested for this indication based on short-duration studies with no significant benefits. Our first observational study examined the role of combination therapy for 6 years in improving quality of life in a subset of a hypothyroid population without adverse effects and cardiac mortality.
Methods
An observational retrospective study examining patients prescribed thyroid replacements with serum triiodothyronine (FT3), LT4 with LT3 (synthetic therapy) or DTE (natural therapy), compared with LT4 alone in the United States from 2010 to 2016. Thyroid-stimulating hormone (TSH), serum thyroxine (FT4), and FT3 levels were documented for each patient in addition to any admissions of myxedema coma, thyrotoxicosis, or cardiovascular complications, such as arrhythmias, atrial fibrillation, and mortality. At the conclusion of the study, a cross-sectional interview assessed quality of life for each combination therapy through the Medical Outcomes Study Short Form-20 questionnaire.
Results
Compared with patients taking only LT4, 89.47% using synthetic therapy had therapeutic TSH (P < 0.05). Similarly, 96.49% using natural therapy had therapeutic TSH (P < 0.05). Less than 5% of patients had supratherapeutic FT3. None of the patients who had abnormally low TSH or elevated FT3 or FT4 levels had hospitalizations for arrhythmias or thyrotoxicosis. On the Medical Outcomes Study Short Form-20 questionnaire, >92% answered feeling “excellent, very good, or good” when questioned about their health while undergoing thyroid replacement compared with levothyroxine alone.
Conclusions
This is the only retrospective study reported to use long-term (mean 27 months) thyroid replacements with combination therapy and to compare between the two forms of therapy: synthetic and natural. For patients undergoing either therapy, we did not identify additional risks of atrial fibrillation, cardiovascular disease, or mortality in patients of all ages with hypothyroidism.
Key Points
Even after years of levothyroxine monotherapy, some patients believe that they still have an impaired quality of life.
The meta-analysis of combination therapy has been negative for combination therapy in managing hypothyroidism, and the data reported on some general well-being questionnaires also are inconclusive.
Low levels of 25[OH] vitamin D, vitamin B12, and hemoglobin were checked in our study to rule out other causes of fatigue and hypothyroidism before starting combination therapy because they have not been ruled out in previous literature.
In our population, the symptoms of hypothyroidism improved significantly as reported on follow-up encounters with subsequent improvement in laboratory thyroid function studies and questionnaires, and furthermore, not resulting in hyperthyroidism that caused hospitalizations for medication adverse effects, arrhythmias, or cardiac death.
According to the National Health and Nutrition Examination Survey, approximately 4.6% of the US population has hypothyroidism.1 Physiologically, the hypothalamus releases thyroid-releasing hormone, which produces thyroid-stimulating hormone (TSH) that subsequently enhances the thyroid gland’s production of largely inactivated serum thyroxine (FT4) and some serum triiodothyronine (FT3). FT3 is the main active hormone at the cellular level, converted from FT4 by intracellular deiodinases. Synthetic liothyronine (LT3) is a synthetic drug that is identical to the hormone FT3. For years, the American Thyroid Association (ATA) and European guidelines suggested monitoring TSH and FT4 to make clinical decisions for starting and monitoring therapy of hypothyroidism.2,3 This classic monotherapy of levothyroxine (LT4) provides adequate control of symptoms for a majority of the population; however, approximately 5% to 10% of patients continue to have symptoms of hypothyroidism, despite normal TSH and FT4 levels.4 This may occur because of dysfunctional deiodinase in a subset of hypothyroid patients, which is an important rationale for including active FT3 to improve symptoms in those patients. A subset of patients with hypothyroidism who continue to be symptomatic on LT4 therapy with normal TSH and FT4 or with normal or subnormal FT3; therefore, they will benefit from the addition of FT3 therapy (synthetic or natural).
In this retrospective study, we sought to measure the clinical and biochemical effects of the addition of LT3 therapy to the standard of care (LT4) in hypothyroid patients and to discover the differences between two different forms of FT3 combination therapies: desiccated thyroid extract (DTE), containing FT4, (“natural therapy”) and LT4 with synthetic LT3 (“synthetic therapy”). A combination of LT4 and LT3 to improve the symptoms of hypothyroidism has been a topic of interest for several decades. Despite this interest, there remains a great deal of uncertainty regarding the best approach for the treatment of patients who do not respond to LT4 therapy alone, including the potential addition of FT3, whether synthetic or natural.
LT4 was initially found to be suboptimal in normalizing FT4 and FT3 in hypothyroid rats.5 Subsequently, researchers were able to restore euthyroidism with a combination of LT4 and synthetic LT3, resulting in lower doses of LT4 and a normalization of FT3.6 Furthermore, in patients undergoing LT4 alone, the doses needed to normalize serum TSH were supraphysiological to compensate for the FT3 levels secreted from the thyroid gland.7–10 Many patients reported feeling psychologically suboptimal on LT4.11 They also concluded that variable levels of circulating FT4 and TSH, even within normal ranges of LT4, may affect psychological well-being, as measured by the General Health Questionnaire score.12
Bunevicius et al published a randomized controlled trial (RCT) in which combined LT4 and synthetic LT3 (12.5 μg) were used in 33 patients, improving mood, well-being, and psychometric functionality.13 Another study in 2002 with a sample size of 10 thyroidectomized patients for the treatment of Graves disease showed similar positive effects of the synthetic therapy.14 Similarly, a study of 697 patients found slight improvements in hypothyroid symptoms during 3 months of synthetic therapy, but these results were nonreplicable 1 year later.15 Escobar-Morreale and colleagues reported that synthetic therapy appeared to have no beneficial effects on mood, quality of life, and psychometric performance of patients, as compared with LT4 alone.16 A few subsequent studies showed hyperthyroidism with the use of combination therapy. The findings are controversial at best.4,17–26
In the last 5 years, there has been renewed interest in unraveling the uncertainty of combination therapies–synthetic and natural. In 2013, an RCT of 70 patients for 16 weeks showed no significant difference in symptoms and neurocognitive measures between LT4 monotherapy and DTE, although patients in the latter therapy group reported 4 pounds of weight loss on average, and subjectively, 48.6% reported preferring the period they were taking the DTE.27 Despite the availability of natural therapy for >130 years, the Food and Drug Administration’s lack of monitoring of FT4-FT3 dosage levels in these preparations has limited their use.28 In 2014, the first observational study addressed the long-term (17 years) use of synthetic therapy in clinical practice.29 Patients were predominantly younger with previous confounding hypothyroid-like symptoms of fatigue from mental disorders and depression, however.
The meta-analysis of combination therapy also has shown conflicting results. The first, a 2006 meta-analysis analyzed 1216 patients in 11 RCTs resulting in no advantage of synthetic therapy in bodily pain, depression, anxiety, fatigue, quality of life, body weight, and serum cholesterol.30 A second meta-analysis in 2009 found some benefits in synthetic therapy for psychological and physical well-being and quality of life in 1243 patients; however, data were not statistically significant.31 A third meta-analysis concluded that there was no significant difference in psychiatric symptoms among synthetic therapies.32 A 2015 review summarized previous literature and data on LT4 and synthetic therapy, concluding use of LT4 monotherapy as the principal treatment of hypothyroidism and not combination therapy, unless future studies show clear benefits.7
The data on general well-being in hypothyroid patients on LT4 monotherapy are likewise inconclusive. Samuels et al demonstrated no clear association among TSH levels, cognition, and psychological performance while titrating on LT4 monotherapy.33 For synthetic therapy, a randomized parallel design trial to evaluate FT3 substitution did not show any beneficial effects on psychometric performance, quality of life, and mood.22 Nygaard and colleagues reported improvement in 7 of 11 scores pertaining to quality of life and depression with FT3 substitution compared with LT4 monotherapy.26
To summarize, there is no consensus, even among different international guidelines. In contrast to the American Association of Clinical Endocrinologists (AACE), the European consensus group suggests that LT4 monotherapy should serve as standard for hypothyroidism management, but they have suggested further researching LT4 and LT3 combinations in subsets of patients with symptoms.34 The Italian Society of Endocrinology does not have evidence-based data, but recognizes that LT4 and LT3 therapy may be considered an experimental approach in overtly hypothyroid patients who have persistent symptoms despite adequate LT4 doses resulting in biochemical euthyroidism after exclusion of other specific causes for persistent symptoms.35
Methods
We conducted a 6-year (2010–2016), retrospective chart review of 100 patients undergoing combination therapies from the 2400 hypothyroid patients receiving LT4 monotherapy in an endocrinology clinic in Pennsylvania. This study was approved by the Pinnacle Health System institutional review board. All of the patients were hypothyroid as defined by the ATA criteria, with elevated TSH and normal or subnormal FT4 at the beginning of LT4 therapy.2
Serum Assays
TSH was measured by a third-generation immunochemiluminescent assay having a functional detection limit of 0.01 mIU/L and a normal range of 0.3 to 5.1 mIU/L. FT4 and FT3 were measured by enzyme immunoassay. Laboratory collection was from 8:00 am to 4:00 pm. The following are accepted normal ranges by the ATA and AACE2,3,32,36:
Euthyroid (TSH level within normal range, 0.35–5.5 μIU/mL)
Hypothyroid (TSH level >5.5 μIU/mL and FT4 level <0.56 μIU/mL)
Hyperthyroid (TSH level <0.01 μIU/mL and FT4 level >1.64 μIU/mL)
FT3 (normal range 2.5–4 μIU/mL)
25-hydroxyvitamin D (25[OH]D; normal range >30 ng/mL)
Vitamin B12 (normal range >240 pg/mL)
Hemoglobin (Hb; normal range 12–17 g/dL)
Data Collection
Data were collected for clinical signs and symptoms of hypothyroidism (eg, fatigue, poor memory, depressed mood, amenorrhea, dry skin, cold intolerance, weight gain, myxedema coma) and hyperthyroidism (eg, palpitations, tremor, arrhythmia, anxiety) using a standard baseline “Hypothyroid Office Note” as an objective clinical measure (Appendix, http://links.lww.com/SMJ/A93). The laboratory tests included TSH, FT4, and FT3 while undergoing LT4 monotherapy (baseline) and combination therapy, either synthetic or natural. Monotherapy doses were started and titrated to physiological thyroid levels as recommended by ATA guidelines and maximizing control of hypothyroid symptoms. Levels before initiation of FT3 therapies served as baseline for our comparison. Levels of 25[OH]D, vitamin B12, and Hb also were collected on LT4 and combination therapy.
In 2015, the patients were contacted via telephone by the primary author in a blinded fashion. Patients were questioned about their symptoms via the Medical Outcomes Study Short Form-20 questionnaire (SF-20). This 20-question survey developed by the RAND Corporation has been shown to be effective in the evaluation of daily symptoms of hypothyroidism. The patients also were questioned about their opinions of “feeling better” on combination therapy versus monotherapy.
Study Population
Patients were started on a combination therapy if they complained of hypothyroid signs and symptoms despite optimal trials of LT4 monotherapy for at least 1 year, to achieve optimal normal TSH levels, preferably <2.5 μIU/mL based on recent evidence,37 and if they continued to have low normal FT3 (2.5–3 μIU/mL). Then natural or synthetic therapy was chosen based on physician and patient preferences. Some patients were switched from one combination therapy to the other, depending upon thyroid levels and the patient’s signs and symptoms. The starting dose of LT3 was 5 μg in conjunction with an appropriate decrease of 12.5 μg in LT4 to achieve the standard physiological circulating FT4:FT3 ratio of nearly 14:1.38 LT3 was titrated with the maximum 12.5 μg dosage to achieve physiologic and therapeutic FT3 levels along with symptom relief. None of the patients were treated with LT3 alone.
The starting dose for DTE, with a known FT4:FT3 ratio of 4:1,39,40 was 15 mg and was titrated to obtain physiologic and therapeutic TSH, FT4, and FT3 levels along with symptom relief. Individual endocrinologists chose the smaller dosage when switching from LT4 to DTE and to prevent adverse effects of hyperthyroidism. All of the patients receiving either therapy were studied every 3 to 6 months to achieve therapeutic thyroid levels.
Exclusion Criteria
The exclusion criteria were as follows: having other cofactors that mimicked symptoms of hypothyroidism, including low Hb, 25[OH]D deficiency, vitamin B12 deficiency, and depression; having long-standing psychiatric disorders or fibromyalgia because those can mask some of the hypothyroid symptoms (eg, fatigue, arthralgia, depression, cognitive slowing); and having a primary care physician instead of an endocrinologist studying them because those patients were not monitored per our protocol (and to prevent physician bias).
Outcomes
The primary outcomes of the study were whether combination therapy was effective in improving clinical signs and symptoms of hypothyroidism as documented in the clinic note; improvement in hypothyroidism symptoms via the SF-20 questionnaire; and adverse effects of clinical or biochemical hyperthyroidism, as measured by TSH, FT4, and FT3.
Statistical Analyses
Proportions were computed for all of the categorical variables. We reported continuous variables as means and medians and categorical variables as proportions. The Student t test was used to analyze between-group differences and the paired t test was used to conduct the before and after treatment comparisons. The χ2 and Fisher exact tests were used for categorical variable comparisons. P < 0.05 was considered significant. All of the statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Of the 100 patients with a mean age of 54 years (range 20–81 years), 95 were women and 5 were men (57 women and 3 men receiving DTE and 38 women and 2 men receiving LT4/LT3). Six patients were excluded because they had ceased therapy. Discontinuation of DTE was caused by pregnancy, minimal hyperthyroid adverse effects, and lack of improvement with continued signs and symptoms of hypothyroidism. Discontinuation of LT4/LT3 therapy was caused by adverse effects, preference, and being lost to follow-up.
The mean follow-up duration was 27 months (range 1–111 months, median 22 months). Only 1 patient had a range of 111 months duration because she had come to the clinic while undergoing combination therapy after failing LT4 monotherapy for several years. The average dose of DTE was 30 mg. The average LT4/LT3 dose was 75 μg/5 μg to obtain physiologic thyroid levels. Fifty-two percent had Hashimoto disease, 22% had surgical hypothyroidism, 10% had ablation for either Graves disease or thyroid cancer, and 16% had miscellaneous etiologies.
Baseline laboratory values for 25[OH]D on LT4 monotherapy were normal in 69% of the DTE population and in 75% of the LT4/LT3 population. For patients with abnormal 25[OH]D levels, appropriate treatment was provided with supplementation per AACE guidelines to levels >30 ng/mL before starting on combination therapy.
The Table compares thyroid function tests between LT4 monotherapy and combination therapies, natural and synthetic. The average TSH remained normal in 89.47% after LT4/LT3 compared with monotherapy alone (P < 0.05), and the average FT3 remained normal in 90% compared with monotherapy (P < 0.05). The average FT4 remained normal in 92.5% after LT4/LT3 compared with LT4 (P > 0.05).
Table.
Thyroid function results when comparing LT4 monotherapy with combination therapy, synthetic LT4/LT3 therapy, or natural desiccated thyroid extract
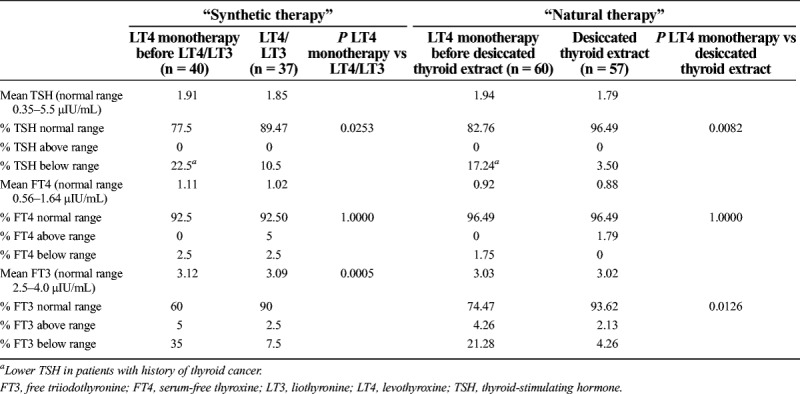
For the DTE population, the average TSH postextract remained normal in 96.49% of patients (P < 0.05). The average FT3 remained normal in 93.62% (P < 0.005). The average FT4 remained normal in 96.49% of patients (P > 0.05).
Although some patients undergoing either combination therapy had abnormal TSH for a short duration, the data were not statistically significant (P > 0.05). The ones who had abnormally low TSH were the patients with thyroid cancer requiring a lower TSH.
Neither natural nor synthetic therapies produced higher TSH levels than the normal values. None of the patients with low TSH or high FT4 and FT3 were hospitalized for adverse effects or arrhythmias. We also compared natural therapy with synthetic therapy and did not find one to be of a better value with TSH, FT3, and FT4.
Fifty-one patients receiving DTE and 26 patients receiving LT4/LT3 participated in the SF-20 questionnaire; 92% of patients receiving DTE and 100% of patients receiving LT4/LT3 answered feeling “excellent, very good, or good” when questioned about self-health; in addition, 80% to 100% on both FT3 therapies were not limited at all in their daily activities (ie, eating, dressing, bathing, carrying groceries, climbing stairs, running, walking, strenuous sports, housework). More than 70% receiving either DTE or LT4/LT3 reported being as “healthy as anybody [they knew]” (Fig.). On DTE, 86.8% reported “feeling calm and peaceful,” and 88.2% reported “being a happy person.” With LT4/LT3, 76.9% reported “feeling calm and peaceful,” and 92.31% reported “being a happy person.” Approximately 84.6% receiving LT4/LT3 and 100% receiving DTE denied any sense of “hopelessness.”
Fig.
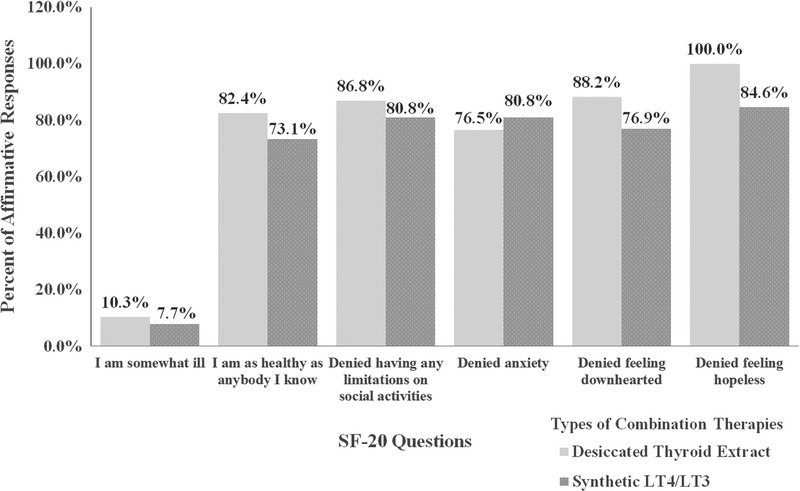
Survey results of the combination therapies. SF-20 Questions, Medical Outcomes Study Short Form-20 questionnaire.
Discussion
Our study is one of the few in the United States to analyze the long-term safety and effects of combination therapy. Most other studies had an average duration of 10 to 16 weeks, with a maximum of 52 weeks.
In our subset population, the symptoms of hypothyroidism improved significantly with no increase in hyperthyroidism. The abnormally low TSH and elevated FT4 and FT3 levels persisted only for a short duration because of dosage adjustments and varied among patients. We did not find any difference in levels in patients according to the etiology of hypothyroidism.
We believe one of the reasons for our success is diligent follow-up by the endocrinologists, who see a large volume of patients with hypothyroidism in central Pennsylvania. Those with abnormal laboratory values or with symptoms indicating thyrotoxicosis were counseled to stop, adjust dosages, or switch to the other combination therapy. None of the patients in either FT3 therapy group were hospitalized because of medication adverse effects, most notably, atrial fibrillations, arrhythmias, and cardiac death.
Our study has several strengths. The principal strength is the longer duration of FT3 therapy (mean 27 months, median 22 months). The second strength is the maintenance of a physiological FT4:FT3 ratio of approximately 14:1, especially with synthetic therapy, for which the therapeutic dose was 75 μg/5 μg; this is equivalent to an FT4:FT3 ratio of approximately 15:1. Although a significant number of our patients were undergoing DTE, the ratio of FT4:FT3 was approximately 4:1, and dynamic, significant adverse effects were not pronounced, perhaps because of the lower dosages prescribed. Unlike the observational study from the United Kingdom that showed some benefits from FT3 supplementation,29 our retrospective study excluded other causes of hypothyroid-like symptoms (eg, fibromyalgia, depression, chronic pain syndrome) to mitigate any confounding results before starting combination therapy. Another important aspect of this study is the SF-20 questionnaire used for the subjective analysis. DTE and LT4/LT3 populations, 92.7% and 88.6%, respectively, claimed to have improved quality of life, as assessed by the questionnaire. A convincing argument could be made that the increased rate of patient satisfaction from combination therapy may have been because some of our patients may possess underlying genetic polymorphisms that cannot be treated with levothyroxine alone. This conception of FT3 supplementation benefiting those with polymorphisms was suggested in Wiersinga’s research41 but not thoroughly investigated.
An important criticism of combination therapy is hyperthyroidism. In our study, at least 6.7% of the 100 patients complained of palpitations and anxiety and had confirmed TSH <0.35 μIU/mL but without atrial arrhythmias. This is hard to compare with other studies, particularly because many studies have not been conducted for an equal duration. From previous meta-analyses, only two studies reported significant atrial arrhythmias.17,42 It is important to point out that our study did not titrate LT4 to supraphysiologic levels for which thyrotoxicosis was a concern before starting any combination therapy.
Our study had several limitations. The biggest limitation of this study is the retrospective nature. There was no preintervention data to compare before the initiation of FT3 therapies, mainly because many of these patients were referrals and we were unable to obtain all of their previous records. Similarly, there is no simultaneous comparison to patients who were receiving LT4 alone. We also did not compare the SF-20 questionnaire pretherapy to see whether the benefits postcombination therapy were statistically significant; however, as documented in the Methods section, patients were asked via the telephone whether they felt better on combination therapy when compared with previous monotherapy. Many patients did emphasize “feeling better on combination therapy.” In addition, depending on the time of the day when laboratory tests for FT3 and FT4 were collected, thyroid function levels could vary because of the timing of thyroid medication ingestion. Most endocrinologists understand that physiologic FT4 increases to 16% for 4 to 6 hours postmedication and there are far greater increases in FT3 levels post-LT3 ingestion. Another interesting observation is the close equalization of FT3 and FT4 on either T3 therapy. We do not have an explanation for the last observation.
Conclusions
Combination therapy of LT4 and LT3 has remained an experimental treatment that can be used at the physician’s discretion. Our observational study concludes that for a subset of patients who feels suboptimal on LT4 monotherapy, synthetic therapy is beneficial and safe in controlling hypothyroid symptoms and improving quality of life. We are hopeful that our analysis will raise awareness and promote further RCTs focusing on appropriate dosages and populations. An interesting future study should be to compare the practices of endocrinologists and primary care physicians in their management of patients undergoing combination therapy.
Supplementary Material
Acknowledgments
The authors thank Helen Houpt, Laurie Schwing, and Azka Tariq for preparation of the manuscript.
Footnotes
Combination therapy of LT4 and LT3 is an experimental treatment for hypothyroidism and has not been approved for use by the US Food and Drug Administration.
The authors did not report any financial relationships or conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (sma.org/smj-home).
References
- 1.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). 2002;87:489–499. [DOI] [PubMed] [Google Scholar]
- 2.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. 2012;18:988–1028. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WM, Duntas L, Fadeyev V, et al. 2012 ETA guidelines: the use of L-T4 + L-T3 in the treatment of hypothyroidism. 2012;1:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biondi B, Wartofsky L. Combination treatment with T4 and T3: toward personalized replacement therapy in hypothyroidism? 2012;97:2256–2271. [DOI] [PubMed] [Google Scholar]
- 5.Escobar-Morreale HF, Obregon MJ, Escobar del Ray F, et al. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. 1995;96:2828–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escobar-Morreale HF, del Rey FE, Obregon MJ, et al. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. 1996;137:2490–2502. [DOI] [PubMed] [Google Scholar]
- 7.Escobar-Morreale HF, Botella-Carretero JI, Morreale de Escobar G. Treatment of hypothyroidism with levothyroxine or a combination of levothyroxine plus L-triiodothyronine. 2015;29:57–75. [DOI] [PubMed] [Google Scholar]
- 8.Utiger RD. Hypothyroidism. In: De Groot LJ, ed. . 3rd ed. Philadelphia: WB Saunders; 1995:752–768. [Google Scholar]
- 9.Hennessey JV, Evaul JE, Tseng YC, et al. L-thyroxine dosage: a reevaluation of therapy with contemporary preparations. 1986;105:11–15. [DOI] [PubMed] [Google Scholar]
- 10.Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. 1987;316:764–770. [DOI] [PubMed] [Google Scholar]
- 11.Saravanan P, Chau WF, Roberts N, et al. Psychological well-being in patients on ‘adequate’ doses of l-thyroxine: results of a large, controlled community-based questionnaire study. 2002;57:577–585. [DOI] [PubMed] [Google Scholar]
- 12.Saravanan P, Visser TJ, Dayan CM. Psychological well-being correlates with free thyroxine but not free 3,5,3′-triiodothyronine levels in patients on thyroid hormone replacement. 2006;91:3389–3393. [DOI] [PubMed] [Google Scholar]
- 13.Bunevicius R, Kazanavicius G, Zalinkevicius R, et al. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. 1999;340:424–429. [DOI] [PubMed] [Google Scholar]
- 14.Bunevicius R, Jakuboniene N, Jurkevicius R, et al. Thyroxine vs thyroxine plus triiodothyronine in treatment of hypothyroidism after thyroidectomy for Graves’ disease. 2002;18:129–133. [DOI] [PubMed] [Google Scholar]
- 15.Saravanan P, Simmons DJ, Greenwood R, et al. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. 2005;90:805–812. [DOI] [PubMed] [Google Scholar]
- 16.Escobar-Morreale HF, Botella-Carretero JI, Gomez-Bueno M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. 2005;142:412–424. [DOI] [PubMed] [Google Scholar]
- 17.Siegmund W, Spieker K, Weike AI, et al. Replacement therapy with levothyroxine plus triiodothyronine (bioavailable molar ratio 14:1) is not superior to thyroxine alone to improve well-being and cognitive performance in hypothyroidism. 2004;60:750–757. [DOI] [PubMed] [Google Scholar]
- 18.Regalbuto C, Maiorana R, Alagona C, et al. Effects of either LT4 monotherapy or LT4/LT3 combined therapy in patients totally thyroidectomized for thyroid cancer. 2007;17:323–331. [DOI] [PubMed] [Google Scholar]
- 19.Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. 2005;90:2666–2674. [DOI] [PubMed] [Google Scholar]
- 20.Walsh JP, Shiels L, Lim EM, et al. Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. 2003;88:4543–4550. [DOI] [PubMed] [Google Scholar]
- 21.Sawka AM, Gerstein HC, Marriot MJ, et al. Does a combination regimen of thyroxine (T4) and 3,5,3′-triiodothyronine improve depressive symptoms better than T4 alone in patients with hypothyroidism? Results of a double-blind, randomized, controlled trial. 2003;88:4551–4555. [DOI] [PubMed] [Google Scholar]
- 22.Clyde PW, Harari AE, Getka EJ, et al. Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. 2003;290:2952–2958. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez T, Lavis VR, Meininger JC, et al. Substitution of liothyronine at a 1:5 ratio for a portion of levothyroxine: effect on fatigue, symptoms of depression, and working memory versus treatment with levothyroxine alone. 2005;11:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fadeyev VV, Morgunova TB, Sytch JP, et al. TSH and thyroid hormones concentrations in patients with hypothyroidism receiving replacement therapy with L-thyroxine alone or in combination with L-triiodothyronine. 2005;4:101–107. [PubMed] [Google Scholar]
- 25.Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. 2009;34:80–89. [DOI] [PubMed] [Google Scholar]
- 26.Nygaard B, Jensen EW, Kvetny J, et al. Effect of combination therapy with thyroxine (T4) and 3,5,3′-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. 2009;161:895–902. [DOI] [PubMed] [Google Scholar]
- 27.Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. 2013;98:1982–1990. [DOI] [PubMed] [Google Scholar]
- 28.Hennessey JV. Historical and current perspective in the use of thyroid extracts for the treatment of hypothyroidism. 2015;21:1161–1170. [DOI] [PubMed] [Google Scholar]
- 29.Leese GP, Soto-Pedre E, Donnelly LA. Liothyronine use in a 17 year observational, population-based study—the tears study. 2016;85:918–925. [DOI] [PubMed] [Google Scholar]
- 30.Grozinsky-Glasberg S, Fraser A, Nahshoni E, et al. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. 2006;91:2592–2599. [DOI] [PubMed] [Google Scholar]
- 31.Ma C, Xie J, Huang X, et al. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. 2009;30:586–593. [DOI] [PubMed] [Google Scholar]
- 32.Joffe RT, Brimacombe M, Levitt AJ, et al. Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and metaanalysis. 2007;48:379–384. [DOI] [PubMed] [Google Scholar]
- 33.Samuels MH, Schuff KG, Carlson NE, et al. Health status, psychological symptoms, mood, and cognition in L-thyroxine-treated hypothyroid subjects. 2007;17:249–258. [DOI] [PubMed] [Google Scholar]
- 34.Michaelsson LF, Medici BB, la Cour JL, et al. Treating hypothyroidism with thyroxine/triiodothyronine combination therapy in Denmark: following guidelines or following trends? 2015;4:174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biondi B, Bartalena L, Chiovato L, et al. Recommendations for treatment of hypothyroidism with levothyroxine and levotriiodothyronine: a 2016 position statement of the Italian Society of Endocrinology and the Italian Thyroid Association. 2016;39:1465–1474. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Campoy JM, St Jeor ST, Castorino K, et al. Clinical practice guidelines for healthy eating for the prevention and treatment of metabolic and endocrine diseases in adults: cosponsored by the American Association of Clinical Endocrinologists/the American College of Endocrinology and the Obesity Society. 2013;19(Suppl 3):1–82. [DOI] [PubMed] [Google Scholar]
- 37.Razvi S, Weaver JU, Pearce SH. Subclinical thyroid disorders: significance and clinical impact. 2010;63:379–386. [DOI] [PubMed] [Google Scholar]
- 38.Butler CC, Vidal-Alaball J, Cannings-John R, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. 2006;23:279–285. [DOI] [PubMed] [Google Scholar]
- 39.Pilo A, Iervasi G, Vitek F, et al. Thyroidal and peripheral production of 3,5,3′-triiodothyronine in humans by multicompartmental analysis. 1990;258(4 Pt 1):E715–E726. [DOI] [PubMed] [Google Scholar]
- 40.Rees-Jones RW, Larsen PR. Triiodothyronine and thyroxine content of desiccated thyroid tablets. 1977;26:1213–1218. [DOI] [PubMed] [Google Scholar]
- 41.Wiersinga WM. Paradigm shifts in thyroid homone replacement therapies for hypothyroidism. 2014;10:164–174. [DOI] [PubMed] [Google Scholar]
- 42.Smith RN, Taylor SA, Massey JC. Controlled clinical trial of combined triiodothyronine and thyroxine in the treatment of hypothyroidism. 1970;4:145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


