Abstract
Objectives:
To develop and validate a new reproducible 3D upper airway analysis based on skeletal structures not involved in the modification, which occur during orthognathic surgery.
Methods:
From retrospective cohort of orthognathic surgically treated patients, pre- and postsurgical CBCT-scans of 10 post-pubertal patients were randomly selected. Two operators identified the landmarks, calculated the airway volumes, cross sections and linear measurements on the 10 scans twice at two different time intervals. Statistical analysis included test for normal distribution, technical error measurements, and intra- and inter-observers reliability.
Results:
Intra- and inter-observer reliability was excellent for volumes and cross sections. The entire data sets exhibited normal distribution. Technical error of measurements showed an error in the range of 1.6 to 10.2% for volume, 1.6 to 12.2% for cross-sectional measurements, and 0.3 to 2.5% for linear measurements. No systematic errors were detected.
Conclusions:
This new proposed definition of upper airway boundaries was shown to be technical feasible and tested to be reliable in measuring upper airway in patients undergoing orthognathic surgery.
Keywords: cone-beam computed tomography; orthognathic surgery; pharynx; imaging, three-dimensional
Introduction
The increased awareness on the detrimental effects generated by sleep disordered breathing has increased the interest on upper airway (UA) morphology alterations after orthognathic surgical procedures. Although sleep disturbances are not directly linked to the anatomical modification of the upper airway morphology following orthognathic surgery, they can arise in patients with predisposing risk factors.1 A way to visualize UA in 3D is offered by Magnetic Resonance Imaging (MRI) and Computed Tomography (CT); nevertheless their use is limited by high cost, scanning time for MRI, restricted accessibility, and exposure to high ionizing radiation for CT.2,3 The introduction of Cone Beam Computed Tomography (CBCT) in the dentistry has generated an increased interest in studying the changes in airway patency following maxillo-mandibular surgical modification in orthognathic patients.4,5
Nevertheless, despite the high number of publications studying UA in orthognathic patients based on CBCT scans, there is no standard procedure shared by all researchers in assessing UA morphology, including the definition of its boundaries.5 Beside the fact that a wide discrepancy between the general cephalometric definition of superior and inferior border of upper airway exists, this aspect is further complicated when a skeletal modification occurs after orthognathic surgery. Thus, to a greater extend the selection of anatomical structures which are not affected by surgery is essential.
A variety of anatomical landmarks have been proposed and used in previous studies assessing changes in UA following orthognathic surgery: Some studies have proposed analyses based on anatomical structures like the posterior nasal spine and the tip of epiglottis, which are generally displaced during the surgical procedures.5–9 In other analyses, cervical vertebrae have been used as well as reference points, although their position has been shown to be greatly affected by head posture, as already demonstrated in 2D cephalometric studies.8–11
The aim of this technical report is to propose a new 3D analysis to study UA in patients undergoing orthognathic surgery, based on stable, reproducible anatomical structures which are not involved in modifications, which may develop due to surgical corrections of the facial skeleton. This method will be tested for intra- and interobserver reliability and reproducibility.
Methods and materials
Permission was granted by the Danish Patient Safety Authority (3-3013-1379/1) and by the Danish Data Protection Agency (2008-58-0035, 31 August 2015). All data were anonymized. As the study comprised retrospective material, none of the patients were exposed to any extra radiation and no extra examination has been performed to acquire additional information.
Subjects
This study is based on 10 randomly selected (randomization performed on: random.org) pre- and postsurgical CBCT-scans among 124 post-pubertal patients diagnosed with maxillomandibular growth disturbances, who had undergone orthognathic surgical corrections at the Hospital of South West Jutland, Department of Oral and Maxillofacial Surgery, Esbjerg, Denmark.12
The mean time interval between the acquisition of pre- and postsurgical CBCT scans is 23.1 months (min: 12 months, max: 31 months).
The CBCT scans were from seven females and three males; nine patients underwent a combined bilateral sagittal split osteotomy and Le Fort 1 procedure; whilst one underwent a Le Fort 1 procedure.
The selected sample size was calculated in order to obtain a power equal to 0.99 with an effect size of 1 mm for linear measurements, 1 mm2 for surface and 1 mm3 for volumetric and α = 0.5. The exclusion criteria were: syndromes or detectable pathologies involving UA; CBCT scans not including all the craniofacial structures required for the cephalometric analysis. Particular attention was paid to patients’ positioning during CBCT scan: subjects without the mandible at maximum intercuspation were excluded as well as patients wearing a bite during scan acquisition. Patients with inappropriate head positioning, including major head extension, head flexion or head rotation, were excluded as well.13
The CBCT-images were acquired using an i-CAT scanner, version 17–19 (Imaging Sciences International, Hatfield, PA 19440, USA) with the following scan parameters: 120 kVp; X-ray tube current = 5 mA; exposure time = 7 s; number of slices = 576; slice thickness = 0.300 mm, producing isotropic voxel with a dimension of 0.30 mm. The patients were scanned in a seated upright position, with the clinical Frankfort horizontal plane parallel to the floor. The patients were instructed to breathe quietly. The CBCT original-data of all patients were exported via the DICOM format and imported into a specific software program (MIMICS 18.0, Materialise, Leuven, Belgium).
Thresholding
The threshold used to segment and visualize the upper airway was determined for each CBCT data set individually.2,3 A profile line and the correspondent vertical intersecting lines were determined. Using the profile line it is possible to visualize a profile with the grey values. Based on the minimal and maximal threshold values a layer of the relevant structures is defined. This layer is called mask. From the masks, the corresponding 3D surfaces were generated.2,3
Airway analysis for orthognathic surgery
Bony structures not involved in any movement due to surgical procedures were included, and the 3D cephalometrics landmarks identified for the present analysis are reported in Table 1. Following a procedure similar to what normally performed with lateral cephalometric analysis, all mid-sagittal landmarks were first identified on the midsagittal plane, and then the position of every landmark was checked on the other two orthogonal planes to improve landmark positioning. Bilateral points were identified first on the 3D surface, then fine adjusted by checking and finely relocating them on the axial, coronal and sagittal views.2
Table 1.
Landmarks selected for airway analysis
| Ba | Basion, the anterior margin of the foramen magnum |
| N | Nasion, the intersection of the internasal and frontonasal sutures in the midsagittal plane |
| Or L | Orbitale left, the most inferior anterior point on left orbit’s margin |
| Or R | Orbitale right, the most inferior anterior point on right orbit’s margin |
| PoL | Porion left, the most upper point on left bony external auditory meatus |
| PoR | Porion right, the most upper point on right bony external auditory meatus |
| S | Sella turcica, the centroid of Sella turcica |
| So | Midpoint of the Sella-Basion line |
| H | Hyoid bone, upper most point of the hyoid bone |
| E | Epiglottis, tip of epiglottis |
| D100 | Point located at the 100% of the distance between E and Airway superior border plane, measured on the presurgical scan |
| D90 | Point located at the 90% of the distance between D100 and Airway superior border plane |
Based on these landmarks a series of planes have been constructed (Table 2): The first reference plane was the Frankfurt Plane; the other three reference planes constructed following the sequence described in Table 2. Based on these references planes, three additional planes were created to limit the superior border of the upper airway and to encompass the retro-palatal region of the upper airway (Table 2 and Figure 1a).
Table 2.
Planes used to encompass upper airway
| References planes | |
| Frankfurt plane | A plane passing through the inferior borders of the bony orbits, encompassed by OrR and OrL, and the upper margin of the auditory meatus encompassed by PoR and PoL. |
| Sagittal SN | Plane perpendicular to Frankfurt plane passing through S and N points |
| SN horizontal | Plane through S and N points, perpendicular to “Sagittal (SN)” |
| S Ba coronal | Plane through S and Ba points perpendicular to “Sagittal (SN)” |
| Retropalatal region | |
| Airway superior border | Plane passing through So and perpendicular to S-Ba coronal and sagittal (SN) planes |
| Airway superior border 2 | Plane passing through Ba and parallel to Airway Superior Border |
| Airway superior border 3 | Plane passing through Ba and parallel to Frankfurt |
| Oropharyngeal region | |
| D100 plane | Plane passing through D100 and parallel to Frankfurt |
| D90 plane | Plane passing through D90 and parallel to Frankfurt |
Figure 1.
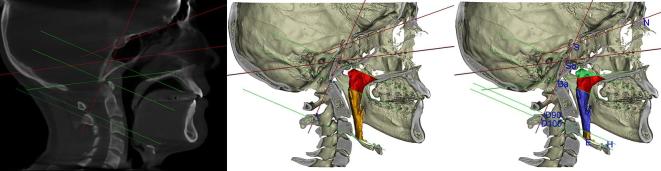
(a) In red the references planes, in green the planes used to delimit upper airway; (b) In red the retropalatal partial volume, in orange the oropharynx volume; (c) In green the Upper retropalatal volume, in red the lower retropalatal volume, in light blue the D90 volume, in orange part of the D100 volume.
In order to delimit the inferior border of the oropharyngeal region, one landmark (D100) was identified on the tip of the epiglottis on the midsagittal plane on the pre-surgical scan, and the distance from the Airway superior border plane to the tip of the Epiglottis was recorded. The 90% of the above-mentioned distance was used to create a landmark (D90) taken perpendicularly to the Airway superior border plane in the caudal direction (Figure 1b). Two planes parallel to the Airway Superior Border plane passing through the D100 and D90 landmarks were defined: These planes were used to delimit the inferior borders of the oropharynx, giving the Oropharynx 100 and the Oropharynx 90 volumes (Table 2, Figure 1b). Using the above described planes the Upper Airway could be divided into 5 partial volumes (Figure 1c and Table 3). At the intersection between the upper airway and the Airway Superior Border, Airway Superior Border 3, D90, and D100 planes, four cross sections have been identified and the relative cross sectional areas calculated. Six linear measurements were also performed (Table 3). The distances between the D100 & D90 to the Airway Superior Border measured on the pre-surgical scans were transferred to the post-surgical scans in order to define the position of the post-surgery D100 Plane and D90 Plane and thus to delimit the inferior borders of the upper airway in a consistent way. These distances were reported on the post-surgical scans starting from the Airway Superior Border following a perpendicular caudal direction. Two planes were generated at D90 and D100 distances. In other words, Epiglottis was not used as a reference point on the post-surgical scans since it could have moved during surgery.
Table 3.
Airway volumes,cross-sections and linear measurements
| Volumes | |
| Retropalatal | The airway volume encompassed superiorly by airway superior border and, inferiorly by airway superior border 2 |
| Upper retropalatal | The airway volume encompassed superiorly by airway superior border and, inferiorly by airway superior border 3 |
| Lower retropalatal | The airway volume encompassed superiorly by airway superior border 3 and, inferiorly by airway superior border 2 |
| Oropharynx 90 | The airway volume encompassed superiorly by airway superior border 2 and, inferiorly by a D90 plane |
| Oropharynx 100 | The airway volume encompassed superiorly by airway superior border 2 and, inferiorly by a D100 plane |
| Cross-sections | |
| Cross-section 1 | Cross-sections at the boundary between upper retropalatal volume and lower retropalatal volume |
| Cross-section 2 | Cross-sections at the boundary between lower retropalatal volume and oropharynx |
| Cross-section 3 | Cross-sections at the boundary between oropharynx 90 vol and oropharynx 100 vol |
| Cross-section 4 | Cross-section located at the bottom of oropharynx 100 vol |
| Linear measurements | |
| E to airway superior border | Distance between epiglottis and airway superior border plane |
| E to Frankfurt | Distance between epiglottis to Frankfurt plane |
| E to SN | Distance between epiglottis to SN plane |
| H to Frankfurt | Distance between hyoid bone and Frankfurt |
| H to SN | Distance between hyoid bone and SN plane |
| Sella-Basion | Distance from Sella to Basion |
Statistical analysis
Two observers (GDC and SFG) measured twice, with a two-week period interval, and independently all the variables (on both the pre-surgery and post-surgery scans). The data were checked for normal distribution with the Kolmogorov Smirnov test (KS). In case the data exhibited a normal distribution, the intra- and inter-examiner comparison was carried out using the independent sample t-test; on the contrary, the Wilcoxon signed rank test was used. The Bland-Altman plot was applied to report intra- and inter-observer reliability.14 In order to quantify the technical error of measurements both at the inter- and intra-observers level, we taken into account the Dahlberg’s formula.15
The Kolmogorov Smirnov test showed that the data was normal distributed. The error of the method obtained using Dahlberg formula was in the range 1.6 to 10.2 (%) for the volume calculation; for linear measurements the range was 0.3 to 2.5 (%); for cross sectional the range 1.6 to 12.2 (%).The complete intra- and inter-observer(s) errors of the method are reported in Tables 4–6.
Table 4.
Intraobserver analysis for observer 1 (GDC)
| Presurgical | Post-surgical | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent samples t-test | Independent samples t-test | ||||||||||||||||||||||||||
| 1st measurement | 2nd measurement | Dahlberg’s d | %a | 95% CI | 1st measurement | 2nd measurement | Dahlberg’s d | %a | 95% CI | ||||||||||||||||||
| Mean | SD | Mean | SD | p-value | Lower | Upper | Mean | SD | Mean | SD | p-value | Lower | Upper | ||||||||||||||
| Retroapalatal | mm3 | 9135 | 2708 | 9375 | 2645 | 370 | 4.1 | 0.8 | −2755 | 2274 | 9446 | 3650 | 9620 | 3708 | 220 | 2.3 | 0.9 | −3631 | 3283 | ||||||||
| U-retropalatal | mm3 | 3625 | 1713 | 3940 | 1738 | 305 | 8.4 | 0.7 | −1936 | 1306 | 3771 | 2134 | 3808 | 2014 | 362 | 9.6 | 1.0 | −1986 | 1912 | ||||||||
| L-retropalatal | mm3 | 5456 | 1591 | 5378 | 1579 | 243 | 4.5 | 0.9 | −1411 | 1567 | 5621 | 2052 | 5758 | 2332 | 302 | 5.4 | 0.9 | −2201 | 1927 | ||||||||
| Oropharynx 90 | mm3 | 7775 | 2378 | 8093 | 2421 | 447 | 5.7 | 0.8 | −2573 | 1936 | 1.0072 | 4478 | 1.0092 | 4360 | 438 | 4.3 | 1.0 | −4172 | 4132 | ||||||||
| Oropharynx 100 | mm3 | 9334 | 3404 | 9647 | 3228 | 488 | 5.2 | 0.8 | −3430 | 2803 | 1.1630 | 5371 | 1.1565 | 5136 | 450 | 3.9 | 1.0 | −4872 | 5002 | ||||||||
| E to Airway Superior border | mm | 58.5 | 8.2 | 58.8 | 8.1 | 0.6 | 1.0 | 0.9 | −8.0 | 7.3 | 57.7 | 7.2 | 57.8 | 7.2 | 0.6 | 1.1 | 1.0 | −6.8 | 6.6 | ||||||||
| E to Frankfurt | mm | 73.5 | 9.2 | 73.7 | 9.2 | 0.3 | 0.4 | 1.0 | −8.8 | 8.5 | 75.0 | 8.7 | 74.8 | 8.7 | 0.7 | 0.9 | 1.0 | −8.0 | 8.4 | ||||||||
| E to SN | mm | 89.7 | 11.0 | 90.6 | 10.7 | 10 | 1.2 | 0.9 | −11.1 | 9.3 | 92.0 | 9.9 | 92.3 | 9.6 | 0.7 | 0.8 | 1.0 | −9.4 | 8.9 | ||||||||
| H to Frankfurt | mm | 82.5 | 10.8 | 82.3 | 10.5 | 06 | 0.8 | 1.0 | −9.9 | 10.2 | 82.4 | 103 | 82.6 | 10.1 | 0.3 | 0.4 | 1.0 | −9.8 | 9.4 | ||||||||
| H to SN | mm | 101.8 | 12.2 | 101.9 | 12.0 | 1.1 | 1.1 | 1.0 | −11.5 | 11.3 | 102.7 | 11.8 | 103.3 | 11.4 | 0.6 | 0.6 | 0.9 | −11.5 | 10.4 | ||||||||
| Sella-Basion | mm | 43.7 | 4.9 | 44.0 | 4.6 | 0.5 | 1.0 | 0.9 | −4.8 | 4.1 | 43.5 | 4.8 | 43.8 | 4.5 | 0.4 | 1.0 | 0.9 | −4.7 | 4.0 | ||||||||
| Surface area 1 | mm2 | 528.8 | 145.3 | 546.3 | 147.5 | 23.3 | 4.4 | 0.8 | −155.0 | 120.0 | 536.6 | 187.7 | 532.7 | 180.7 | 23.4 | 4.4 | 1.0 | −169.2 | 176.9 | ||||||||
| Surface area 2 | mm2 | 339.9 | 90.6 | 345.6 | 100.1 | 12.3 | 3.6 | 0.9 | −95.4 | 84.0 | 378.9 | 140.2 | 379.6 | 140.9 | 5.9 | 1.6 | 1.0 | −132.8 | 131.4 | ||||||||
| Surface area 3 | mm2 | 241.2 | 134.8 | 241.9 | 130.3 | 8.5 | 3.5 | 1.0 | −125.2 | 123.9 | 258.6 | 150.7 | 263.1 | 154.9 | 19.9 | 7.7 | 0.9 | −148.0 | 139.1 | ||||||||
| Surface area 4 | mm2 | 262.7 | 149.6 | 259.9 | 138.4 | 19.2 | 7.3 | 1.0 | −132.6 | 138.2 | 230.3 | 116.9 | 218.9 | 99.3 | 20.0 | 8.7 | 0.8 | −90.5 | 113.3 | ||||||||
Calculated in respect to the first measurements.
Table 6.
Interobserver analysis
| Interobserver analysis at presurgical stage | Interobserver analysis at postsurgical stage | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| obs 1 vs obs 2 at the first measurement | obs 1 vs obs 2 at the second measurement | obs 1 vs obs 2 at the first measurement | obs 1 vs obs 2 at the second measurement | ||||||||||||||
| Independent samples t-test | 95% CI | Dahlberg’s % | Independent samples t-test | 95% CI | Dahlberg’s % | Independent samples t-test | 95% CI | Dahlberg’s % | Independent samples t-test | 95% CI | Dahlberg’s % | ||||||
| p-value | Lower | Upper | p-value | Lower | Upper | p-value | Lower | Upper | p-value | Lower | Upper | ||||||
| Retropalatal | mm3 | 0.8 | −2843 | 2349 | 6.1 | 1.0 | −2366 | 2444 | 2.2 | 0.9 | −3675 | 3375 | 3.6 | 1.0 | −3376 | 3553 | 1.6 |
| U-retropalatal | mm3 | 0.9 | −1809 | 1614 | 8.4 | 0.8 | −1432 | 1898 | 6.6 | 0.9 | −1911 | 2079 | 6.3 | 0.8 | −1649 | 2137 | 6.5 |
| L-retropalatal | mm3 | 0.8 | −1686 | 1371 | 7.1 | 0.7 | −1694 | 1204 | 5.0 | 0.8 | −2403 | 1829 | 8.0 | 0.8 | −2415 | 1997 | 4.0 |
| Oropharynx 90 | mm3 | 0.8 | −2972 | 2200 | 10.2 | 0.9 | −2574 | 2281 | 8.4 | 1.0 | −4031 | 4225 | 2.8 | 1.0 | −3898 | 4033 | 3.9 |
| Oropharynx 100 | mm3 | 0.9 | −3442 | 3040 | 4.9 | 1.0 | −2977 | 3032 | 3.6 | 1.0 | −4895 | 5055 | 2.6 | 1.0 | −4766 | 4710 | 2.8 |
| E to airway superior border | mm | 0.7 | −9.2 | 6.1 | 2.5 | 0.7 | −8.8 | 6.3 | 2.1 | 0.8 | −7.6 | 5.7 | 2.0 | 0.8 | −7.5 | 5.6 | 1.9 |
| E to Frankfurt | mm | 0.7 | −10.0 | 7.2 | 1.5 | 0.7 | −10.1 | 7.3 | 1.6 | 0.8 | −9.4 | 7.0 | 1.3 | 0.7 | −9.9 | 6.5 | 1.7 |
| E to SN | mm | 0.8 | −11.5 | 9.1 | 1.5 | 0.9 | −10.5 | 9.6 | 1.6 | 0.8 | −10.5 | 8.1 | 1.3 | 0.8 | −10.0 | 8.1 | 1.5 |
| H to Frankfurt | mm | 0.9 | −10.6 | 9.1 | 1.0 | 0.8 | −11.1 | 8.7 | 1.5 | 0.7 | −11.0 | 8.0 | 1.5 | 0.8 | −10.8 | 7.9 | 1.4 |
| H to SN | mm | 1.0 | −11.6 | 11.1 | 1.2 | 1.0 | −11.5 | 10.9 | 1.4 | 0.9 | −11.9 | 9.9 | 1.0 | 0.9 | −10.8 | 10.1 | 0.7 |
| Sella-Basion | mm | 1.0 | −4.6 | 4.4 | 2.1 | 1.0 | −4.4 | 4.6 | 2.5 | 0.9 | −4.8 | 4.3 | 1.2 | 0.8 | −3.9 | 4.6 | 1.7 |
| Surface area 1 | mm2 | 0.9 | −138.2 | 125.2 | 5.1 | 0.8 | −117.2 | 148.0 | 3.2 | 0.8 | −159.8 | 198.1 | 4.9 | 0.9 | −160.1 | 186.4 | 4.5 |
| Surface area 2 | mm2 | 1.0 | −85.6 | 88.4 | 4.6 | 0.9 | −83.8 | 93.8 | 3.2 | 1.0 | −133.3 | 136.2 | 1.9 | 1.0 | −138.6 | 135.4 | 2.5 |
| Surface area 3 | mm2 | 1.0 | −127.6 | 130.4 | 4.0 | 1.0 | −125.4 | 124.4 | 2.7 | 1.0 | −148.0 | 142.1 | 3.2 | 1.0 | −144.1 | 146.1 | 6.8 |
| Surface area 4 | mm2 | 1.0 | −133.9 | 138.2 | 9.4 | 0.9 | −135.1 | 125.0 | 6.0 | 1.0 | −116.7 | 118.8 | 6.6 | 0.9 | −114.5 | 96.5 | 12.2 |
obs1, observer 1; obs 2, observer 2.
Table 5.
Intraobserver analysis for observer 2 (SFG)
| Presurgical | Post-surgical | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Independent samples t-test | Independent samples t-test | ||||||||||||||||||||||||||
| 1st measurement | 2nd measurement | Dahlberg’s d | %a | 95% CI | 1st measurement | 2nd measurement | Dahlberg’s d | %a | 95% CI | ||||||||||||||||||
| Mean | SD | Mean | SD | p-value | Lower | Upper | Mean | SD | Mean | SD | p-value | Lower | Upper | ||||||||||||||
| Retropalatal | mm3 | 9382 | 2818 | 9336 | 2471 | 443 | 4.7 | 1.0 | −2444 | 2536 | 9596 | 3851 | 9531 | 3666 | 275 | 2.9 | 1.0 | −3468 | 3597 | ||||||||
| U-retropalatal | mm3 | 3722 | 1924 | 3706 | 1806 | 338 | 9.1 | 1.0 | −1737 | 1769 | 3687 | 2112 | 3564 | 2015 | 249 | 6.8 | 0.9 | −1816 | 2063 | ||||||||
| L-retropalatal | mm3 | 5613 | 1663 | 5624 | 1504 | 214 | 3.8 | 1.0 | −1500 | 1479 | 5908 | 2435 | 5967 | 2364 | 148 | 2.5 | 1.0 | −2314 | 2196 | ||||||||
| Oropharynx 90 | mm3 | 8161 | 3081 | 8240 | 2737 | 403 | 4.9 | 1.0 | −2817 | 2658 | 9975 | 4308 | 1.0025 | 4076 | 408 | 4.1 | 1.0 | −3990 | 3890 | ||||||||
| Oropharynx 100 | mm3 | 9535 | 3494 | 9620 | 3167 | 459 | 4.8 | 1.0 | −3219 | 3048 | 1.1550 | 5219 | 1.1593 | 4947 | 445 | 3.9 | 1.0 | −4820 | 4734 | ||||||||
| E to airway superior border | mm | 60.0 | 8.1 | 60.1 | 7.9 | 0.91 | 1.5 | 1.0 | −7.6 | 7.4 | 58.7 | 6.9 | 58.8 | 6.7 | 09 | 1.6 | 1.0 | −6.5 | 6.3 | ||||||||
| E to Frankfurt | mm | 74.9 | 9.1 | 75.1 | 9.4 | 0.38 | 0.5 | 1.0 | −8.9 | 8.5 | 76.3 | 8.7 | 76.5 | 8.7 | 0.4 | 0.5 | 1.0 | −8.4 | 7.9 | ||||||||
| E to SN | mm | 90.9 | 11.0 | 91.0 | 10.7 | 0.89 | 1.0 | 1.0 | −10.3 | 10.0 | 93.2 | 9.8 | 93.2 | 9.7 | 1.0 | 1.0 | 1.0 | −9.2 | 9.2 | ||||||||
| H to Frankfurt | mm | 83.2 | 10.2 | 83.5 | 10.6 | 0.49 | 0.6 | 0.9 | −10.1 | 9.5 | 83.9 | 9.9 | 84.1 | 9.8 | 0.4 | 0.5 | 1.0 | −9.4 | 9.1 | ||||||||
| H to SN | mm | 102.0 | 11.9 | 102.2 | 11.8 | 0.61 | 0.6 | 1.0 | −11.3 | 11.0 | 103.7 | 11.4 | 103.6 | 10.8 | 0.8 | 0.8 | 1.0 | −10.4 | 10.5 | ||||||||
| Sella-Basion | mm | 43.8 | 4.7 | 43.9 | 5.0 | 0.78 | 1.8 | 0.9 | −4.7 | 4.4 | 43.7 | 5.0 | 43.4 | 4.6 | 0.7 | 1.5 | 0.9 | −4.2 | 4.8 | ||||||||
| Surface area 1 | mm2 | 535.3 | 134.9 | 530.9 | 134.5 | 25.68 | 4.8 | 0.9 | −122.1 | 131.0 | 517.4 | 193.3 | 519.5 | 188.1 | 16.9 | 3.3 | 1.0 | −181.3 | 177.1 | ||||||||
| Surface area 2 | mm2 | 338.5 | 94.6 | 340.6 | 88.5 | 10.49 | 3.1 | 1.0 | −88.2 | 84.0 | 377.4 | 146.5 | 381.2 | 150.6 | 9.4 | 2.5 | 1.0 | −143.3 | 135.8 | ||||||||
| Surface area 3 | mm2 | 239.8 | 139.8 | 242.4 | 135.5 | 5.63 | 2.3 | 1.0 | −131.9 | 126.8 | 261.6 | 158.0 | 262.1 | 153.9 | 5.1 | 2.0 | 1.0 | −147.0 | 146.0 | ||||||||
| Surface area 4 | mm2 | 260.5 | 139.9 | 264.9 | 138.5 | 9.03 | 3.5 | 0.9 | −135.1 | 126.4 | 229.3 | 133.3 | 227.9 | 123.9 | 11.8 | 5.1 | 1.0 | −119.5 | 122.3 | ||||||||
Calculated in respect to the first measurements.
In order to delimit the inferior border of the oropharyngeal region, one landmark (D100) was identified on the tip of the epiglottis on the midsagittal plane on the pre-surgical scan, and the distance from the airway superior border plane to the tip of the epiglottis was recorded. The 90% of the above-mentioned distance was used to create a landmark (D90) taken perpendicularly to the airway superior border plane in the caudal direction (Figure 1b). Two planes parallel to the airway superior border plane passing through the D100 and D90 landmarks were defined: These planes were used to delimit the inferior borders of the oropharynx, giving the Oropharynx 100 and the Oropharynx 90 volumes (Table 2 and Figure 1b).
Using the above described planes the upper airway could be divided into five partial volumes (Figure 1c and Table 3).
At the intersection between the upper airway and the airway superior border, airway superior border 3, D90, and D100 planes, four cross-sections have been identified and the relative cross-sectional areas calculated. Six linear measurements were also performed (Table 3).
The distances between the D100 and D90 to the airway superior border measured on the pre-surgical scans were transferred to the post-surgical scans in order to define the position of the post-surgery D100 plane and D90 plane and thus to delimit the inferior borders of the upper airway in a consistent way. These distances were reported on the post-surgical scans starting from the airway superior border following a perpendicular caudal direction. Two planes were generated at D90 and D100 distances. In other words, epiglottis was not used as a reference point on the post-surgical scans since it could have moved during surgery.
Results
The Kolmogorov-Smirnov test showed that the data was normal distributed. The error of the method obtained using Dahlberg formula was in the range 1.6 to 10.2% for the volume calculation; for linear measurements the range was 0.3 to 2.5%; for cross sectional the range 1.6 to 12.2%.The complete intra- and interobserver(s) errors of the method are reported in Tables 4–6.
The Bland-Altman plots showed good reliability of the methods and no systematic errors both for the intra- and interobservers comparison. This was confirmed by the results of the independent sample t-test (Tables 4–6).
Discussion
The introduction of CBCT determined, especially in the last decade, a surge in the interest to study the existence of the possible relationship between UA and the craniofacial structures (e.g. craniofacial morphology and head posture) and focusing the attention on the effects of treatment interventions on the skeletal basis that may influence airway patency.5,6,16,17 Nevertheless, some of the issues mentioned in these studies remain not completely answered due to the presence of technical drawbacks that hinder the reduction of the systematic errors. In the present study we presented a technical analysis able to overcome these limitations with the aim to characterize three-dimensionally the upper airway by using as references only stable, reproducible anatomical landmarks, which will not be affected by orthognathic surgical procedures.
Two recent review concluded that it would be useful to follow a standard protocol for CBCT imaging and use standard reference planes.5,6 Thus, even though it is now possible to depict three-dimensionally the UA, the reliability of some previous studies might be questioned. Regarding the first issue (“standard protocol for CBCT imaging”), the respiration phase, although thanks to the gradual reduction in CBCT scanning acquisition time this issue is expected to be minor in the future, should not be overlooked when assessing UA. Another problem is the maximum intercuspation, which usually is not seriously considered in the research design of many airways studies.13 An open mouth may influence the airway morphology by mandibular retroposition. Finally, head posture and tongue position during CBCT-acquisition has also been advocated to be an important factor for UA morphology, yet often underestimated as reported in a previous systematic review.16
Regarding the second issue (“standard reference planes”), it has to be underlined that, the selection of reliable landmarks for upper airway analysis is of the outmost importance, even more when the changes are the result of orthognathic surgery. A variety of anatomic landmarks have been proposed, however, some refer to anatomical structures (e.g. the posterior nasal spine and the tip of epiglottis) that are most likely to be displaced during surgical procedures, while others (e.g. cervical vertebrae) are known for changing position as a consequence of head posture.10,18
In an attempt to solve the problem of the anatomic landmarks, the Frankfurt plane was chosen as the first reference plane, since it is not affected by surgical procedures. The other reference planes, created based on the Frankfurt plane and other structures in the cranial base, allowed to divide the retropalatal volumes into different compartments, all not influenced by the surgical procedures. Moreover, we used the tip of the epiglottis as a reference to delimit inferiorly the oropharynx and we introduced the distances from the Airway superior border plane (D100, D90). Although the epiglottis may move post-surgically, and even during the respiration phase, we transferred the same distances obtained at the presurgical scan to the postsurgical scan. In this way, we avoid a possible systematic error produced by movement of the epiglottis. This makes it possible to perform a consistent comparison of upper airway modification after surgery. Another major issue when measuring the upper airways on CBCT is the thresholding. The automatic thresholding did not show appropriate validity when compared to manual thresholding.2,3,19 In this study, a manual threshold value was individually determined for each CBCT scan. Our methodology shown appropriate reproducibility according to the results obtained, while a minor issue is that this approach is more time consuming.
Another problem is represented by the existence of different airway volumetric subdivisions among studies. Necessarily, this generates subsequent difficulties when comparing between studies. Schwab, claimed that two subdivisions are essential in describing the upper airway segment.20 Our results for all the proposed five volumes show good reliability. Nevertheless, the present study demonstrates that the use of only two volumes (i.e. the retropalatal volume, and the oropharynx 100 vol) is sufficient to characterize the morphology of UA, therefore the analysis is simplified.(Figure 1b)
In this technical note the adoption of the above mentioned procedures resulted in a reliable and reproducible approach. This was successfully applied to study the airway in both pre- and post-orthognathic surgery. We believe that the adoption of this novel method will contribute to overcome the limitations of the previously proposed analyses and may increase the consistency in measuring the airway among future studies.
Acknowledgement
We thank Dr Jens Jørgen Thorn and Dr Janne Ingerslev who performed the surgeries and Dr Kim Carlsson for the comprehensive Orthodontic treatment of the subjects involved in this technical note.
Contributor Information
Gabriele Di Carlo, Email: gabriele.dicarlo@uniroma1.it.
Sirwan Fernandez Gurani, Email: sirwan.fernandez.gurani@rsyd.dk.
Paolo Maria Cattaneo, Email: paolo.cattaneo@dent.au.dk.
References
- 1.Susarla SM, Thomas RJ, Abramson ZR, Kaban LB. Biomechanics of the upper airway: changing concepts in the pathogenesis of obstructive sleep apnea. Int J Oral Maxillofac Surg 2010; 39: 1149–59.https://doi.org/10.1016/j.ijom.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 2.Lenza MG, Lenza MM, Dalstra M, Melsen B, Cattaneo PM. An analysis of different approaches to the assessment of upper airway morphology: a CBCT study. Orthod Craniofac Res 2010; 13: 96–105.https://doi.org/10.1111/j.1601-6343.2010.01482.x [DOI] [PubMed] [Google Scholar]
- 3.Di Carlo G, Polimeni A, Melsen B, Cattaneo PM. The relationship between upper airways and craniofacial morphology studied in 3D. A CBCT study. Orthod Craniofac Res 2015; 18: 1–11.https://doi.org/10.1111/ocr.12053 [DOI] [PubMed] [Google Scholar]
- 4.Scarfe WC, Farman AG. What is cone-beam CT and how does it work? Dent Clin North Am 2008; 52: 707–30.https://doi.org/10.1016/j.cden.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 5.Christovam IO, Lisboa CO, Ferreira DM, Cury-Saramago AA, Mattos CT. Upper airway dimensions in patients undergoing orthognathic surgery: a systematic review and meta-analysis. Int J Oral Maxillofac Surg 2016; 45: 460–71.https://doi.org/10.1016/j.ijom.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 6.Fernández-Ferrer L, Montiel-Company JM, Pinho T, Almerich-Silla JM, Bellot-Arcís C. Effects of mandibular setback surgery on upper airway dimensions and their influence on obstructive sleep apnoea - a systematic review. J Craniomaxillofac Surg 2015; 43: 248–53.https://doi.org/10.1016/j.jcms.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 7.Gonçales ES, Duarte MA, Palmieri C Jr, Zakhary GM, Ghali GE. Retrospective analysis of the effects of orthognathic surgery on the pharyngeal airway space. J Oral Maxillofac Surg 2014; 72: 2227–40.https://doi.org/10.1016/j.joms.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Kim YI, Hwang DS, Park SB. Effect of maxillary setback movement on upper airway in patients with class III skeletal deformities: cone beam computed tomographic evaluation. J Craniofac Surg 2013; 24: 387–91.https://doi.org/10.1097/SCS.0b013e31827fef0f [DOI] [PubMed] [Google Scholar]
- 9.Park JW, Kim NK, Kim JW, Kim MJ, Chang YI. Volumetric, planar, and linear analyses of pharyngeal airway change on computed tomography and cephalometry after mandibular setback surgery. Am J Orthod Dentofacial Orthop 2010; 138: 292–9.https://doi.org/10.1016/j.ajodo.2009.10.036 [DOI] [PubMed] [Google Scholar]
- 10.Muto T, Takeda S, Kanazawa M, Yamazaki A, Fujiwara Y, Mizoguchi I. The effect of head posture on the pharyngeal airway space (PAS). Int J Oral Maxillofac Surg 2002; 31: 579–83.https://doi.org/10.1054/ijom.2002.0279 [DOI] [PubMed] [Google Scholar]
- 11.Jakobsone G, Neimane L, Krumina G. Two- and three-dimensional evaluation of the upper airway after bimaxillary correction of Class III malocclusion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 110: 234–42.https://doi.org/10.1016/j.tripleo.2010.03.026 [DOI] [PubMed] [Google Scholar]
- 12.Random.org. Dublin: Randomness and Integrity Services Ltd. Available at: http://www.random.org/
- 13.Guijarro-Martínez R, Swennen GR. Three-dimensional cone beam computed tomography definition of the anatomical subregions of the upper airway: a validation study. Int J Oral Maxillofac Surg 2013; 42: 1140–9.https://doi.org/10.1016/j.ijom.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 14.Donatelli RE, Lee SJ. How to report reliability in orthodontic research: part 2. Am J Orthod Dentofacial Orthop 2013; 144: 315–8.https://doi.org/10.1016/j.ajodo.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 15.Springate SD. The effect of sample size and bias on the reliability of estimates of error: a comparative study of Dahlberg's formula. Eur J Orthod 2012; 34: 158–63.https://doi.org/10.1093/ejo/cjr010 [DOI] [PubMed] [Google Scholar]
- 16.Gurani SF, Di Carlo G, Cattaneo PM, Thorn JJ, Pinholt EM. Effect of head and tongue posture on the pharyngeal airway dimensions and morphology in three-dimensional imaging: a systematic review. J Oral Maxillofac Res 2016; 7: 1–12.https://doi.org/10.5037/jomr.2016.7101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El H, Palomo JM. Airway volume for different dentofacial skeletal patterns. Am J Orthod Dentofacial Orthop 2011; 139: e511–e521.https://doi.org/10.1016/j.ajodo.2011.02.015 [DOI] [PubMed] [Google Scholar]
- 18.Muto T, Yamazaki A, Takeda S, Kawakami J, Tsuji Y, Shibata T, et al. Relationship between the pharyngeal airway space and craniofacial morphology, taking into account head posture. Int J Oral Maxillofac Surg 2006; 35: 132–6.https://doi.org/10.1016/j.ijom.2005.04.022 [DOI] [PubMed] [Google Scholar]
- 19.El H, Palomo JM. Measuring the airway in 3 dimensions: a reliability and accuracy study. Am J Orthod Dentofac Orthop 2009; 137: S50.e1–9. [DOI] [PubMed] [Google Scholar]
- 20.Schwab RJ. Upper airway imaging. Clin Chest Med 1998; 19: 33–54.https://doi.org/10.1016/S0272-5231(05)70430-5 [DOI] [PubMed] [Google Scholar]