Abstract
Objective:
MRI is being used increasingly as a modality that can provide important information about breast cancer. Diffusion-weighted imaging (DWI) is an imaging technique from which apparent diffusion coefficient (ADC) values can be calculated in addition to obtaining important structural information which cannot be obtained from other imaging studies. We did not find any significant relationships between ADC values and prognostic factors, but did provide some explanations for conflicting results in the literature.
Methods:
The ADC results of 61 females with invasive ductal carcinomas were evaluated. DWI was performed and ADC values were calculated from the area in which restriction of diffusion was the highest in ADC mapping. B value was 500 and region of interest (ROI) was designated between 49 and 100 mm2. Calculations were performed automatically by the device. Tissue samples were obtained for prognostic factor evaluation. The relationships between ADC and prognostic factors were investigated. Comparisons between groups were made with one-way ANOVA and Kruskal Wallis test. Pairwise comparisons were made with Dunn’s test. Analyses of categorical variables were made with Chi-square test.
Results:
We found a weak negative correlation between ADC and Ki-67 values (r = −0.279; p = 0.029). When we compared ADC values in regard to tumour type, we found no significant differences for tumour grade, Ki-67 positivity, estrogen receptor positivity, progesterone receptor positivity, C-erb B2, lymphovascular invasion and ductal carcinoma in situ or lobular carcinoma in situ component. On a side note, we found that mean ADC values decreased as tumour grade increased; however, this was not statistically significant.
Conclusion:
The literature contains studies that report conflicting results which may be caused by differences in B values, ROI area and magnetic field strength. Multicentre studies and systematic reviews of these findings may produce crucial data for the use of DWI in breast cancer.
Advances in knowledge:
To determine if any significant relationship exists between DWI findings and prognostic factors of breast cancer.
Introduction
Currently, most of the imaging modalities available to physicians can be used –one way or another- in the investigation of breast cancer.1–7 MRI has been shown to be one of the best modalities in the detection and evaluation of breast cancer.8–10 MRI excels in areas in which mammography, ultrasonography and physical examination are weak, such as the detection of lesions in dense breast tissue, identification of invasive components, screening of high-risk individuals and evaluation of metastatic cancers.11–16
Determining the histological prognosis of breast cancer is important for both the determination of management and the patient. Currently, the routine method of prognosis determination is the Nottingham Grading System.17 This system is based on histological type and grade, lymph node status and tumour size.18, 19 The results of this approach are found to be significantly correlated with recurrence-free and overall survival.20 With the advance in molecular biomarker identification, various biomarkers from blood or biopsy material including Ki-67 and cerbB2 (HER2 and sHER2) have been found to determine prognosis at different reliability levels.21 Steroid hormone receptor (oestrogen and progesterone receptors) expressions are also important for prognosis due to their influence on hormone therapy efficacy.22, 23
Diffusion-weighted imaging (DWI), which is utilized widely in the evaluation of brain tissue, especially in stroke cases,24 has been also suggested to provide important diagnostic data on breast tumours with its ability to quantify the amount of water diffusion in tissue.25 This quantification results in the apparent diffusion coefficient (ADC) value. When a tissue or part of a tissue contains a higher number of cells, water diffusion is restricted by the cell membranes and extracellular fluid is reduced, which both lead to a lower amount of diffusion and lower ADC in DWI. Naturally, a tumour with uncontrolled cell multiplication should “light-up” in DWI. Another highlight of DWI is that ADC value can differentiate between benign and malignant tumours.26, 27 As expected, they report that ADC is lower in malignant lesions which indicates restriction of water diffusion. A meta-analysis including 12 previous studies showed that ADC value could distinguish between benign and malignant lesions with a sensitivity of 89% and specificity of 77%.28 While literature is almost unanimous on the usefulness of ADC in the determination of benign or malignant tumours, ADC’s correlation with prognostic factors and tumour characteristics is a lesser researched area.
We hypothesized that patients with lymphovascular invasion and/or higher grade tumours who should have higher mitosis resulting in decreased amount of extracellular fluid would show a more restricted diffusion pattern and thus have lower ADC values. Other factors indicative of poor prognosis were also evaluated.
Methods and materials
Patients with invasive ductal breast cancer who had been treated at Ankara Oncology Training and Research Hospital between 2015 and 2016 were prospectively evaluated. A total of 105 patients who presented with breast imaging and reporting data system (BIRADS)-4 and BIRADS-5 tumours (identified by breast ultrasonography or mammography) underwent diffusion-weighted MR imaging of the breast. Among these patients, those who were diagnosed with primary invasive breast tumours were included in the study. 18 patients who had ductal carcinoma in situ, 1 patient with phylloides tumour and 1 patient who had breast invasion of acute lymphocytic leukaemia were excluded from the study. A further 24 patients were excluded because their tumours were benign. Thus, a final group of 61 patients were enrolled. The ADC values of these patients were recorded. Patient characteristics, lab results and other factors (Ki-67 index, ER positivity, PR positivity, c-erb-B2 value, lymphovascular invasion and neoadjuvant chemotherapy) were also recorded.
Ethical approval was obtained from the local ethics committee. Additional approval was obtained from the hospital board as our institution is considered as a reference hospital for breast cancer.
MR imaging
MR imaging results were acquired with a 1.5 Tesla scanner (Signa HDx, 1.5 T, GE Healthcare) prior to biopsy and treatment in all patients. All images were evaluated in the clinical routine according to breast imaging and reporting data system (BI-RADS)-MR imaging lexicon, by at least one of three radiologists with at least 5 years of experience in breast MRI evaluation. Images consisted of: Inversion recovery magnitude axial sequence [Repetition Time (TR) = 6500.0, Echo Time (TE) = 45.0, inversion time (TI) = 150 ms, Matrix 416 × 224, Thickness = 5.0 mm, Field of View (FOV) = 320 mm, Number of Excitations (NEX) = 2], T1 weighted fast spin echo axial sequence (TR = 400, TE = 8.80, NEX = 1, Thickness = 5.0 mm, Matrix 448 × 224, FOV = 320 mm) and pre- and post-contrast T1 weighted three-dimensional fat-suppressed axial sequence (TR = 4.00, TE = 1.50, FA = 10.0, Matrix 350 × 350, Thickness 2.80, FOV = 320 mm, NEX = 1). Images were taken once before contrast and five times after contrast injection with 80 s intervals. Contrast was gadobutrol/gadopentetate dimeglumine with a dose of 0.1 mmol kg–1. Diffusion-weighted images [TR/TE = 1000/83, FOV = 320 mm, Matrix 192 × 192, NEX = 4 and Slice Thickness = 5 mm] were obtained with single-shot echo planar imaging technique and ADC mappings were calculated. Additionally, re-evaluation of all imaging data (to ensure that ADC acquisition was objective) was performed by a single radiologist. During re-evaluation, ADC mapping was evaluated and the site which showed highest diffusion restriction was chosen for ADC measurement. Region of interest (ROI) was designated between 49 and 100 mm2 and calculations were performed automatically by the device. B 500 value was used for diffusion MRI.
Histopathology
Tumour, node and metastasis (TNM) staging was used in the histopathological evaluation of tumours. All assessments were performed by certified pathologists. Tissue obtained in surgery or biopsy was fixed in %10 formalin and was processed to paraffin blocks at 5 µm thickness, which was the routine protocol. Tumour type was determined by the WHO classification. The modified Bloom-Richardson protocol was used to grade tumours (Grade 1, 2 and 3). Classical guidelines were used in the determination of prognostic factors. Haematoxylin-eosin staining was used in the assessment of lymphovascular invasion. Standardized protocols were used in the staining of samples from the invasive part of the tumour for Ki-67, oestrogen, progesterone and c-erbb2 (Her2/neu) receptors, results were obtained by calculating the percentage of stained cells.
Statistical analysis
All analyses were performed with SPSS v. 21. Normality testing was done by the Shapiro-Wilk test. Normally distributed continuous variables are given as mean ± standard deviation and non-normally distributed variables are given as median (minimum–maximum). Normally distributed data groups were compared with one-way ANOVA while non-normally distributed data groups were compared with Mann–Whitney U and Kruskal–Wallis tests. Dunn’s test was used for pairwise comparisons. The relationship between two continuous variables was evaluated by the calculation of the Spearman Correlation Coefficient. All categorical variables are given as frequency (percentage). Categorical variable analyses were done with the Chi-square test. A p value below 0.05 was accepted to be statistically significant in all evaluations.
Results
We included 61 female patients into our study, mean age was 47.46 ± 12.72. 48 (78.7%) patients had invasive ductal carcinoma (IDC) while 6 (9.8%) patients had invasive lobular carcinoma (ILC). When we evaluated tumour grades there were 7 (12.5%) patients with Grade 1, 21 (37.5%) patients with Grade 2 and 28 (%50.0) patients with Grade 3 while data for 5 patients were missing. 37 (60.7%) patients had DCIS component while 8 (13.1%) patients had LCIS component. 21 (34.4%) patients had lymphovascular invasion and 4 (6.6%) patients received neoadjuvant chemotherapy.
Eight of our patients had non-mass enhancement (NME). Restriction of diffusion was found in three of these patients. Seven of the lesions were determined as segmental type NME and one was linear type NME. Two patients had accompanying masses; one patient had linear NME and a mass identified as invasive ductal carcinoma, while the other had segmental NME and a mass identified as DCIS + IDC. Among these eight patients with NME, four had lymphovascular invasion (LVI) positivity of which only one was found to have an ADC value consistent with diffusion restriction.
When we made comparisons between tumour grades, we found Ki-67 percentages were significantly higher in patients with Grade 3 tumours (p < 0.001). ADC values were lower for Grade 3 Tumours than Grade 1 and Grade 2 tumours but this result was not significant. There were no significant differences between groups regarding age, ER (%) and PR (%). Also we found that 10 of the 11 (90.9%) patients with a C-erb-B2 score of 3 had Grade 3 tumours. This result was also found as significant (p = 0.003) (Table 1).
Table 1.
Patients’ characteristics regarding tumour grade. a and b, same letter denotes the lack of statistically significant difference between groups.
| Grade 1 (n = 7) | Grade 2 (n = 29) | Grade 3 (n = 34) | p | |
| Age, mean ± SD | 46.14 ± 6.26 | 51.38 ± 13.32 | 44.04 ± 12.93 | 0.134 |
| Type, n (%) | ||||
| IDC | 4 (9.3) | 15 (34.9) | 24 (55.8) | 0.271 |
| ILC | 1 (16.7) | 4 (66.7) | 1 (16.7) | |
| Other | 2 (28.6) | 2 (28.6) | 3 (42.9) | |
| ADC-B 500 (×10−3), median (min–max) | 1.190 (0.108–1.630) | 1.020 (0.155–1.410) | 0.979 (0.108–1.630) | 0.485 |
| Ki-67 (%), median (min–max) | 5.00 (2.00–20.00)a | 6.00 (2.00–70.00)a | 40.00 (5.00–95.00)b | <0.001** |
| ER (%), median (min–max) | 90.00 (0.00–100.00) | 90.00 (20.00–100.00) | 75.00 (0.00–100.00) | 0.077 |
| PR (%), median (min–max) | 90.00 (0.00–90.00) | 90.00 (0.00–100.00) | 70.00 (0.00–100.00) | 0.263 |
| C-erb-B2, n (%) | ||||
| 0 | 7 (21.2) | 13 (39.4) | 13 (39.4) | 0.003* |
| 1 | 0 (0.0) | 7 (77.8) | 2 (22.2) | |
| 2 | 0 (0.0) | 0 (0.0) | 3 (100.0) | |
| 3 | 0 (0.0) | 1 (9.1) | 10 (90.9) | |
| Lymphovascular Invasion, n (%) | ||||
| Negative | 6 (16.2) | 15 (40.5) | 16 (43.2) | 0.291 |
| Positive | 1 (5.3) | 6 (31.6) | 12 (63.2) | |
| Neoadjuvant Chemotherapy, n (%) | ||||
| Absent | 6 (11.3) | 19 (35.8) | 28 (52.8) | 0.182 |
| Present | 1 (33.3) | 2 (66.7) | 0 (0.0) | |
Same letter (a, b, c, * and **) denotes the lack of statistically significant difference between groups. ADC, apparent diffusion coefficient; ER, estrogen receptore; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; PR, progesteron receptore; SD, standard deviation.
There was a weak negative correlation between ADC and Ki-67 values (r = −0.279; p = 0.029) (Figure 1a,b). ADC values had no significant correlation with age, ER and PR. When we compared ADC values between patients regarding tumour type, tumour grade, Ki-67 positivity, ER positivity, PR positivity, C-erb B2, lymphovascular invasion and DCIS or LCIS component, we found no significant differences (Table 2). Mean ADC values decreased as tumour grade increased; however, this was not statistically significant.
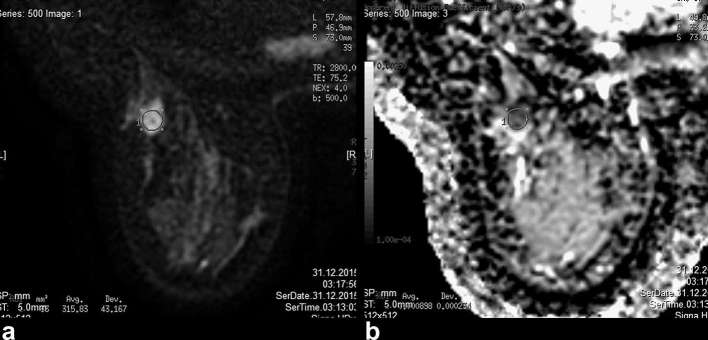
Figure 1. .
(a, b) Diffusion-weighted MRI and ADC values of a malignant mass in a 49-year-old female. Final pathology showed Grade 2 invasive ductal carcinoma. ADC value was 0.898 × 10−3, lymphovascular invasion was positive and Ki-67 value was 5%. ADC, apparent diffusion coefficient.
Table 2.
ADC values regarding other prognostic factors
| N | ADC values (×10−3) | p | |
| Tumour type | |||
| IDC | 48 | 0.988 (0.108–1.520) | 0.867 |
| ILC | 6 | 1.081 (0.155–1.300) | |
| Other | 7 | 0.874 (0.686–1.630) | |
| Tumour grade | |||
| Grade 1 | 7 | 1.190 (0.108–1.630) | 0.485 |
| Grade 2 | 21 | 1.020 (0.155–1.410) | |
| Grade 3 | 28 | 0.979 (0.108–1.630) | |
| Ki-67 | |||
| Negative | 22 | 1.045 (0.108–1.630) | 0.080 |
| Positive | 39 | 0.965 (0.569–1.520) | |
| Oestrogen receptors | |||
| Negative | 11 | 0.970 (0.108–1.430) | 0.632 |
| Positive | 50 | 0.993 (0.155–1.630) | |
| Progesterone receptors | |||
| Negative | 18 | 0.968 (0.108–1.630) | 0.658 |
| Positive | 43 | 1.010 (0.155–1.520) | |
| C-erb B2 | |||
| 0 | 35 | 1.020 (0.108–1.630) | 0.859 |
| 1 | 9 | 0.940 (0.874–1.290) | |
| 2 | 3 | 0.850 (0.834–1.090) | |
| 3 | 14 | 0.980 (0.569–1.520) | |
| Lymphovascular Invasion | |||
| Negative | 40 | 1.001 (0.155–1.630) | 0.154 |
| Positive | 21 | 0.951 (0.108–1.360) | |
| Component | |||
| Absent | 16 | 0.893 (0.108–1.630) | 0.187 |
| DCIS | 37 | 0.993 (0.569–1.520) | |
| LCIS | 8 | 1.040 (0.155–1.300) | |
ADC values given as median (minimum–maximum). ADC, apparent diffusion coefficient; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma
Discussion
Our aim was to determine if ADC values had any relationship with prognostic factors, especially tumour grade and lymphovascular invasion. Tumour characteristics are crucial in the management of tumours and DWI is a very sensitive method in determining tissue structure; this structure may accurately represent the aggressiveness of the tumour, thus important data may be derived from DWI.
In our study, we did not find any relationships between ADC values and any of the factors analysed. However, several studies have reported relationships between ADC value and factors such as oestrogen receptor positivity,29–31 progesterone receptor positivity,29, 32 lymph node metastasis,33, 34 HER2/c-erb-B2 positivity,30 Ki-67 index,33 tumour grade,35–37 tumour type.27, 38,39 An almost equal number of studies also report contrasting results on the same factors.33, 40,41 Furthermore, some studies that simultaneously show a relationship with a specific factor, report drastically different results in other factors. If a consensus is to be identified, it is that tumour size is almost unanimously shown to not correlate with ADC values.29, 33,40,41 We believe these contrasting results may be explained by differences in B values, ROI area and device magnetic power. In Table 3, we have provided the results and technical parameters of various studies. Although suggested values in different tissues exist, ADC is usually calculated with a B value between 0 and 1000. However, in order to accurately determine slow diffusion, a B value of at least 500 s mm–2 should be chosen. However, optimal B values for each and every tissue have not been determined. Thus, while we believe that an ADC value of at least 500 should be used in ADC calculation, factors such as device magnetic power, mechanical artefacts, noise, patient pathology and the varying density of breast tissue should be taken into account when determining B values.
Table 3.
The technical parameters and results of various studies investigating the relationship between ADC and prognostic factors of IDC
| Author (year) | Field strength | B values (s mm–2) | Factors found to be associated with ADC |
| Kim et al (2009)42 | 1.5 T | 1000 | None |
| Costantini et al (2010)36 | 1.5 T | 0 and 100 | Grade |
| Jeh et al (2010)30 | 1.5 and 3 T | 750 and 1000 | ER and HER2 expressions |
| Razek et al (2010)35 | 1.5 T | 200 and 400 | Lymph node metastasis, histological grade, tumour size |
| Martincich et al (2012)31 | 1.5 T | 0 and 900 | HER2 expression |
| Kamitani et al (2013)40 | 1.5 T | 0,500 and 1000 | Lymph node metastasis, ER and PR expressions |
| Belli et al (2014)43 | 1.5 T | 0 and 1000 | Grade and lymph node metastasis |
| Park et al (2016)33 | 3 T | 0 and 1000 | Lymph node metastasis and Ki-67 expression |
| Rabasco et al (2017)29 | 3 T | 0 and 750 | Metastasis |
ADC, apparent diffusion coefficient; ER, estrogen receptor; IDC, invasive ductal carcinoma.
In the present study, no relationship was found between LVI and ADC with 1.5T imaging. Similarly, in a very recent study, Shin et al also found no relationships between quantitative MRI parameters and LVI with 3.0T imaging. However, it should be noted that they found significantly lower ADC values in tumours with larger size, higher histological grade and axillary lymph node metastasis, contrary to our results.44 On the other hand, two studies, both done with 3.0T strength, reported that ADC values were significantly lower in LVI-positive patients.45, 46 Although various studies (at 1.5T and 3.0T) report significant differences in ADC values when IDC, DCIS and ILC are compared,36, 47,48 the same cannot be said when the relationship between ADC values and parameters such as lymph node involvement and vascular invasion are evaluated.47, 49, 50
The Ki-67 index is accepted to accurately define cell proliferation. Thus, a strong negative correlation between ADC value and Ki-67 values may be expected. Although various studies have found Ki-67 and ADC to be correlated,32, 34 the correlation coefficients are low and several studies show conflicting findings.37, 41 This may be because the ADC value is derived from a larger portion of tissue, while the Ki-67 index is a very local representation of the tumour tissue. Another explanation may be that, although increased Ki-67 index represents an increase in cell multiplication, this may not directly translate to increased density of cells due to the varying density of breast tissue among patients.
In the light of greatly contrasting results, a study by Rabasco et al29 drew our interest due to a 3-year follow up of 60 breast cancer patients. They found ADC values were correlated with patients’ clinical condition in the third year. After regression analysis of factors, they reported ADC value, ER and PR positivity and presence of lymph nodes to be the only statistically significant differences. Thus, it may be suggested that ADC values should not only be compared with prognostic factors, but also the actual prognosis of patients. A large study comprised of 289 patients found that Grade 1 lesions, in situ carcinomas and tumours without lymphovascular invasion had significantly higher ADC values compared to Grade 2–3 tumours, invasive carcinomas and tumours with lymphovascular invasion, respectively.43
Kim et al,42 consistent with our findings, found no relationship between ADC values and prognostic factors. Another finding that was similar to our findings was that they also reported ADC values decreased with the increase in grade; however, they also found that the differences were not significant.
In our study, eight patients were found to have NME. Among these patients, only 1 had an ADC value consistent with restriction of diffusion. A study by Yabuuchi and colleagues found that malignant tumours had significantly lower ADC values compared to benign tumours. They also reported high sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) when other factors were included in the analysis.50 However, in a study by Imamura et al, although ADC values were again lower in the malignant group, the difference was not statistically significant. When they combined ADC findings with dynamic contrast-enhanced MRI pattern, they reported better negative predictive value and accuracy, but lower specificity.51 Thus, we may conclude that the evaluation of NME lesions via ADC is also a controversial matter. We believe these differences may be due to the presence of normal tissue within tumour sites which complicates evaluation.
A strength of our study is that most of the major prognostic factors of invasive breast cancers were assessed; thus comparisons with DWI findings were done for all of these factors. Another strength is that the distribution of patients among our major points (grade and lymphovascular invasion) can be considered diffuse. Finally, the site for ADC measurement was determined as the area in which diffusion restriction was highest on ADC mapping. Thus, objective ADC sampling was ensured; however, it is likely that this area was not the same area from which pathological samples were obtained, which brought an inherent limitation. There are several other limitations to our study. First, the number of patients can be considered low. Second, as the study is comprised of patients from a single centre, homogeneity may also be considered low. However, the regional reference status of our hospital for breast cancer causes a high number of referrals from other hospitals. Nevertheless, this is still a limitation. Third, other results obtained from MRI were not evaluated in our study, whereas some studies included these findings in their analyses. This may be seen as a limitation; however, our study was focused on the ADC values and their correlation with prognostic factors, thus the exclusion of other MRI results should not directly affect our evaluation. A final limitation may be the B value (500) we used in ADC determination. Although higher B values may identify restriction better, we decided to use B 500 as it was available in all patients.
Conclusions
Although some literature on this topic reports significant correlations with various factors, none of the prognostic factors have been unanimously deemed related to ADC value. And several studies, such as ours, found no relationship between ADC values and prognostic factors. Further studies, especially multicentre studies and systematic reviews of contemporary literature are required to identify relationships. Another important point is that, DWI of the breast may not be practiced consistently in different centres. Small differences in factors such as B values, ROI area, device magnetic power and the location of ADC measurement may result in conflicting results; thus studies may have to account for these differences when presenting their findings.
Footnotes
IRB Statement: Institutional review board approval was obtained for this prospective study.
Contributor Information
Hale Aydin, Email: halemaydin@gmail.com, hmusapasaoglu@yahoo.com.
Bahar Guner, Email: baharshahin@gmail.com.
Isil Esen Bostanci, Email: isilesenbostanci@yahoo.com.
Bilgin Kadri Aribas, Email: bilginaribas@hotmail.com.
Lutfi Dogan, Email: lutfidogan1@yahoo.com.
Mehmet Ali Gulcelik, Email: mgulcelik@yahoo.com.
REFERENCES
- 1.Aminololama-Shakeri S, Khatri VP. Emerging modalities in breast cancer imaging. Surg Oncol Clin N Am 2014; 23: 735–49. doi: 10.1016/j.soc.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 2.Vashi R, Hooley R, Butler R, Geisel J, Philpotts L. Breast imaging of the pregnant and lactating patient: imaging modalities and pregnancy-associated breast cancer. AJR Am J Roentgenol 2013; 200: 321–8. doi: 10.2214/AJR.12.9814 [DOI] [PubMed] [Google Scholar]
- 3.Crivello ML, Ruth K, Sigurdson ER, Egleston BL, Evers K, Wong YN, et al. Advanced imaging modalities in early stage breast cancer: preoperative use in the United States medicare population. Ann Surg Oncol 2013; 20: 102–10. doi: 10.1245/s10434-012-2571-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filippi V, Malamitsi J, Vlachou F, Laspas F, Georgiou E, Prassopoulos V, et al. The impact of FDG-PET/CT on the management of breast cancer patients with elevated tumor markers and negative or equivocal conventional imaging modalities. Nucl Med Commun 2011; 32: 85–90. doi: 10.1097/MNM.0b013e328341c898 [DOI] [PubMed] [Google Scholar]
- 5.Podo F, Sardanelli F, Canese R, D'Agnolo G, Natali PG, Crecco M, et al. The Italian multi-centre project on evaluation of MRI and other imaging modalities in early detection of breast cancer in subjects at high genetic risk. J Exp Clin Cancer Res 2002; 21(3 Suppl): 115–24. [PubMed] [Google Scholar]
- 6.Douek M, Davidson T, Taylor I. Breast cancer imaging-what are the optimal modalities? Eur J Surg Oncol 1998; 24: 573–82. doi: 10.1016/S0748-7983(98)93824-0 [DOI] [PubMed] [Google Scholar]
- 7.Feig SA. The role of new imaging modalities in staging and follow-up of breast cancer. Semin Oncol 1986; 13: 402–14. [PubMed] [Google Scholar]
- 8.Berg WA, Gutierrez L, NessAiver MS, Carter WB, Bhargavan M, Lewis RS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004; 233: 830–49. doi: 10.1148/radiol.2333031484 [DOI] [PubMed] [Google Scholar]
- 9.Menezes GL, Knuttel FM, Stehouwer BL, Pijnappel RM, van den Bosch MA. Magnetic resonance imaging in breast cancer: a literature review and future perspectives. World J Clin Oncol 2014; 5: 61–70. doi: 10.5306/wjco.v5.i2.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 2008; 246: 116–24. doi: 10.1148/radiol.2461061298 [DOI] [PubMed] [Google Scholar]
- 11.Huzarski T, Górecka-Szyld B, Huzarska J, Psut-Muszyńska G, Wilk G, Sibilski R, et al. Screening with magnetic resonance imaging, mammography and ultrasound in women at average and intermediate risk of breast cancer. Hered Cancer Clin Pract 2017; 15: 4. doi: 10.1186/s13053-017-0064-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasanzadeh F, Faeghi F, Valizadeh A, Bayani L. Diagnostic value of diffusion weighted magnetic resonance imaging in evaluation of metastatic axillary lymph nodes in a sample of iranian women with breast cancer. Asian Pac J Cancer Prev 2017; 18: 1265–70. doi: https://doi.org/10.22034/APJCP.2017.18.5.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang ES, Kinkel K, Esserman LJ, Lu Y, Weidner N, Hylton NM. Magnetic resonance imaging in patients diagnosed with ductal carcinoma-in-situ: value in the diagnosis of residual disease, occult invasion, and multicentricity. Ann Surg Oncol 2003; 10: 381–8. doi: 10.1245/ASO.2003.03.085 [DOI] [PubMed] [Google Scholar]
- 14.Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Application of abbreviated protocol of magnetic resonance imaging for breast cancer screening in dense breast tissue. Acad Radiol 2017; 24: 316–20. doi: 10.1016/j.acra.2016.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD, et al. ACR appropriateness criteria breast cancer screening. J Am Coll Radiol 2016; 13(11 Suppl): R45–R49. doi: 10.1016/j.jacr.2016.09.021 [DOI] [PubMed] [Google Scholar]
- 16.Covington MF, Rhodes DJ, Pizzitola VJ. Molecular breast imaging and the 2016 update to the ACR appropriateness criteria for breast cancer screening. J Am Coll Radiol 2016; 13: 1408. doi: 10.1016/j.jacr.2016.09.017 [DOI] [PubMed] [Google Scholar]
- 17.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991; 19: 403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x [DOI] [PubMed] [Google Scholar]
- 18.Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 1992; 20: 479–89. doi: 10.1111/j.1365-2559.1992.tb01032.x [DOI] [PubMed] [Google Scholar]
- 19.Shen J, Hunt KK, Mirza NQ, Krishnamurthy S, Singletary SE, Kuerer HM, et al. Intramammary lymph node metastases are an independent predictor of poor outcome in patients with breast carcinoma. Cancer 2004; 101: 1330–7. doi: 10.1002/cncr.20515 [DOI] [PubMed] [Google Scholar]
- 20.Muftah AA. Molecular-based diagnostic, prognostic and predictive tests in breast cancer : precision molecular pathology of breast cancer: The British Institute of Radiology.; 2015. 177–95. [Google Scholar]
- 21.Kutomi G, Mizuguchi T, Satomi F, Maeda H, Shima H, Kimura Y, et al. Current status of the prognostic molecular biomarkers in breast cancer: a systematic review. Oncol Lett 2017; 13: 1491–8. doi: 10.3892/ol.2017.5609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brouckaert O, Paridaens R, Floris G, Rakha E, Osborne K, Neven P. A critical review why assessment of steroid hormone receptors in breast cancer should be quantitative. Ann Oncol 2013; 24: 46–53. doi: 10.1093/annonc/mds238 [DOI] [PubMed] [Google Scholar]
- 23.Rastelli F, Crispino S. Factors predictive of response to hormone therapy in breast cancer. Tumori 2008; 94: 370–83. [DOI] [PubMed] [Google Scholar]
- 24.Horsfield MA, Jones DK. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases - a review. NMR Biomed 2002; 15: 570–7. doi: 10.1002/nbm.787 [DOI] [PubMed] [Google Scholar]
- 25.Partridge SC, Nissan N, Rahbar H, Kitsch AE, Sigmund EE. Diffusion-weighted breast MRI: clinical applications and emerging techniques. J Magn Reson Imaging 2017; 45: 337–55. doi: 10.1002/jmri.25479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partridge SC, DeMartini WB, Kurland BF, Eby PR, White SW, Lehman CD. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol 2009; 193: 1716–22. doi: 10.2214/AJR.08.2139 [DOI] [PubMed] [Google Scholar]
- 27.Woodhams R, Matsunaga K, Kan S, Hata H, Ozaki M, Iwabuchi K, et al. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci 2005; 4: 35–42. doi: 10.2463/mrms.4.35 [DOI] [PubMed] [Google Scholar]
- 28.Tsushima Y, Takahashi-Taketomi A, Endo K, resonance M. Magnetic resonance (MR) differential diagnosis of breast tumors using apparent diffusion coefficient (ADC) on 1.5-T. J Magn Reson Imaging 2009; 30: 249–55. doi: 10.1002/jmri.21854 [DOI] [PubMed] [Google Scholar]
- 29.Rabasco P, Caivano R, Simeon V, Dinardo G, Lotumolo A, Gioioso M, et al. Can diffusion-weighted imaging and related apparent diffusion coefficient be a prognostic value in women with breast cancer? Cancer Invest 2017; 35: 92–9. doi: 10.1080/07357907.2016.1267740 [DOI] [PubMed] [Google Scholar]
- 30.Jeh SK, Kim SH, Kim HS, Kang BJ, Jeong SH, Yim HW, et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging 2011; 33: 102–9. doi: 10.1002/jmri.22400 [DOI] [PubMed] [Google Scholar]
- 31.Martincich L, Deantoni V, Bertotto I, Redana S, Kubatzki F, Sarotto I, et al. Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol 2012; 22: 1519–28. doi: 10.1007/s00330-012-2403-8 [DOI] [PubMed] [Google Scholar]
- 32.Shao G, Fan L, Zhang J, Dai G, Xie T. Association of DW/DCE-MRI features with prognostic factors in breast cancer. Int J Biol Markers 2017; 32: e118–e125. doi: 10.5301/jbm.5000230 [DOI] [PubMed] [Google Scholar]
- 33.Park EK, Cho KR, Seo BK, Woo OH, Cho SB, Bae JW. Additional value of diffusion-weighted imaging to evaluate prognostic factors of breast cancer: correlation with the apparent diffusion coefficient. Iran J Radiol 2016; 13: e33133. doi: 10.5812/iranjradiol.33133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EJ, Kim SH, Park GE, Kang BJ, Song BJ, Kim YJ, et al. Histogram analysis of apparent diffusion coefficient at 3.0t: correlation with prognostic factors and subtypes of invasive ductal carcinoma. J Magn Reson Imaging 2015; 42: 1666–78. doi: 10.1002/jmri.24934 [DOI] [PubMed] [Google Scholar]
- 35.Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed 2010; 23: 619–23. doi: 10.1002/nbm.1503 [DOI] [PubMed] [Google Scholar]
- 36.Costantini M, Belli P, Rinaldi P, Bufi E, Giardina G, Franceschini G, et al. Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clin Radiol 2010; 65: 1005–12. doi: 10.1016/j.crad.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 37.De Felice C, Cipolla V, Guerrieri D, Santucci D, Musella A, Porfiri LM, et al. Apparent diffusion coefficient on 3.0 Tesla magnetic resonance imaging and prognostic factors in breast cancer. Eur J Gynaecol Oncol 2014; 35: 408–14. [PubMed] [Google Scholar]
- 38.Iima M, Le Bihan D, Okumura R, Okada T, Fujimoto K, Kanao S, et al. Apparent diffusion coefficient as an MR imaging biomarker of low-risk ductal carcinoma in situ: a pilot study. Radiology 2011; 260: 364–72. doi: 10.1148/radiol.11101892 [DOI] [PubMed] [Google Scholar]
- 39.Rahbar H, Partridge SC, Demartini WB, Gutierrez RL, Allison KH, Peacock S, et al. In vivo assessment of ductal carcinoma in situ grade: a model incorporating dynamic contrast-enhanced and diffusion-weighted breast MR imaging parameters. Radiology 2012; 263: 374–82. doi: 10.1148/radiol.12111368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamitani T, Matsuo Y, Yabuuchi H, Fujita N, Nagao M, Jinnouchi M, et al. Correlations between apparent diffusion coefficient values and prognostic factors of breast cancer. Magn Reson Med Sci 2013; 12: 193–9. doi: 10.2463/mrms.2012-0095 [DOI] [PubMed] [Google Scholar]
- 41.Kızıldağ Yırgın İ, Arslan G, Öztürk E, Yırgın H, Taşdemir N, Gemici AA, et al. Diffusion weighted MR imaging of breast and correlation of prognostic factors in breast cancer. Balkan Med J 2016; 33: 301–7. doi: 10.5152/balkanmedj.2016.140555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging 2009; 30: 615–20. doi: 10.1002/jmri.21884 [DOI] [PubMed] [Google Scholar]
- 43.Belli P, Costantini M, Bufi E, Giardina GG, Rinaldi P, Franceschini G, et al. Diffusion magnetic resonance imaging in breast cancer characterisation: correlations between the apparent diffusion coefficient and major prognostic factors. Radiol Med 2015; 120: 268–76. doi: 10.1007/s11547-014-0442-8 [DOI] [PubMed] [Google Scholar]
- 44.Shin JK, Kim JY. Dynamic contrast-enhanced and diffusion-weighted MRI of estrogen receptor-positive invasive breast cancers: associations between quantitative MR parameters and Ki-67 proliferation status. J Magn Reson Imaging 2017; 45: 94–102. doi: 10.1002/jmri.25348 [DOI] [PubMed] [Google Scholar]
- 45.Mori N, Mugikura S, Takasawa C, Miyashita M, Shimauchi A, Ota H, et al. Erratum to: peritumoral apparent diffusion coefficients for prediction of lymphovascular invasion in clinically node-negative invasive breast cancer. Eur Radiol 2016; 26: 340–1. doi: 10.1007/s00330-015-3888-8 [DOI] [PubMed] [Google Scholar]
- 46.Durando M, Gennaro L, Cho GY, Giri DD, Gnanasigamani MM, Patil S, et al. Quantitative apparent diffusion coefficient measurement obtained by 3.0Tesla MRI as a potential noninvasive marker of tumor aggressiveness in breast cancer. Eur J Radiol 2016; 85: 1651–8. doi: 10.1016/j.ejrad.2016.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi SY, Chang YW, Park HJ, Kim HJ, Hong SS, Seo DY. Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br J Radiol 2012; 85: e474–e479. doi: 10.1259/bjr/79381464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bickel H, Pinker-Domenig K, Bogner W, Spick C, Bagó-Horváth Z, Weber M, et al. Quantitative apparent diffusion coefficient as a noninvasive imaging biomarker for the differentiation of invasive breast cancer and ductal carcinoma in situ. Invest Radiol 2015; 50: 95–100. doi: 10.1097/RLI.0000000000000104 [DOI] [PubMed] [Google Scholar]
- 49.Park SH, Choi HY, Hahn SY. Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 Tesla. J Magn Reson Imaging 2015; 41: 175–82. doi: 10.1002/jmri.24519 [DOI] [PubMed] [Google Scholar]
- 50.Yabuuchi H, Matsuo Y, Kamitani T, Setoguchi T, Okafuji T, Soeda H, et al. Non-mass-like enhancement on contrast-enhanced breast MR imaging: lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. Eur J Radiol 2010; 75: e126–e132. doi: 10.1016/j.ejrad.2009.09.013 [DOI] [PubMed] [Google Scholar]
- 51.Imamura T, Isomoto I, Sueyoshi E, Yano H, Uga T, Abe K, et al. Diagnostic performance of ADC for Non-mass-like breast lesions on MR imaging. Magn Reson Med Sci 2010; 9: 217–25. doi: 10.2463/mrms.9.217 [DOI] [PubMed] [Google Scholar]