Abstract
Objective:
To investigate whether the parameters derived from intravoxel incoherent motion (IVIM) MRI could differentiate phyllodes tumours (PTs) from fibroadenomas (FAs) by comparing the apparent diffusion coefficient (ADC) values.
Methods:
This retrospective study included 7 FAs, 10 benign PTs (BPTs), 4 borderline PTs, and one malignant PT. Biexponential analyses of IVIM were performed using a 3 T MRI scanner. Quantitative IVIM parameters [pure diffusion coefficient (D), perfusion-related diffusion coefficient (D*), and fraction (f)] were calculated. The ADC was also calculated using monoexponential fitting.
Results:
The D and ADC values showed an increasing tendency in the order of FA, BPT, and borderline or malignant PT (BMPT). No significant difference was found in the D value among the three groups. The ADC value of the BMPT group was significantly higher than that of the FA group (p = 0.048). The D* value showed an increasing tendency in the order of BMPT, BPT, and FA, and the D* value of the BMPT group was significantly lower than that of the FA group (p = 0.048).
Conclusion:
The D* derived from IVIM and the ADC were helpful for differentiating between FA and BMPT.
Advances in knowledge:
IVIM MRI examination showed that the perfusion-related diffusion coefficient is lower in borderline and malignant PTs than in FAs and the opposite is true for the ADC.
INTRODUCTION
Fibroadenomas (FAs) and phyllodes tumours (PTs) are relatively frequent breast tumours, which are targeted for enucleation when deemed of a large size. Both are pathologically classified as fibroepithelial tumours. Core needle biopsy often fails to distinguish between them.1 It is not uncommon for a tumour diagnosed as FA by needle biopsy to receive a final diagnosis of PT when resected. PT, unlike FA, has a high local recurrence rate.2 Moreover, there is malignant transformation of the stromal component in PT. Thus, in PT enucleation, it is necessary to secure wider margins than in FA. Therefore, the ability to differentiate between PT and FA using diagnostic imaging before the surgery is of major benefit to the physicians. Several reports have compared the findings of mammography, ultrasound, and dynamic contrast-enhanced MRI (DCE-MRI).3, 4 PT tends to have more irregular shape and margins and more heterogeneous internal properties than FA; however, most reports have concluded that the complete differentiation of the two based on imaging findings is difficult.3, 4
Diffusion-weighted imaging (DWI) can provide information on the microstructural characteristics, in addition to information about the blood flow, using DCE-MRI.5 An association between the apparent diffusion coefficient (ADC) from DWI and the biological characteristics of breast cancer has been evaluated and reported.6 Kamitani et al7 reported that no significant difference was found in the ADC value between PT and FA.
In addition, intravoxel incoherent motion (IVIM), first described by Le Bihan et al, is an imaging technique that separates perfusion and diffusion using multi-b-value DWI with biexponential curve fitting.8 IVIM can provide a pure diffusion coefficient (D) and the perfusion-related incoherent microcirculation (D*) separately. Several studies evaluating IVIM for breast lesions have been conducted.9, 10 These studies were aimed at the differentiation of benign and malignant breast lesions with IVIM.9, 10
The purpose of this study was to investigate whether the parameters derived from IVIM could differentiate PT from FA, by comparing them to the ADC values.
METHODS and Materials
Patients
The Institutional Review Board’s approval was obtained. The written informed consent requirement was waived as this was a retrospective study. Between August 2013 and March 2017, pre-operative breast MRI was performed on 20 patients with 22 lesions, histologically diagnosed with FA or PT by core needle biopsy. One patient had bilateral FAs, and one patient had two PTs in a unilateral breast. In Kanazawa University Hospital, we perform MRI to examine the presence of a second lesion before the enucleation of FA or PT. Furthermore, we confirm with MRI whether FA diagnosed by needle biopsy shows findings to strongly suspect PT.
All patients underwent surgery after the MRI investigation, and definitive diagnoses were provided histopathologically. Our 20 patients had a total of 7 FAs, 10 benign PTs (BPTs), 4 borderline PTs, and 1 malignant PT. Because there was only one malignant PT, we decided to include it in the group of the borderline PTs. We divided all cases into three groups, FA, BPT, and borderline or malignant PT (BMPT) and conducted comparative analyses. Age ranged from 20 to 42 years (mean age, 34.3 years) in the patients with FAs, from 20 to 62 years (mean age, 42.2 years) in the patients with BPTs, and from 37 to 50 years (mean age, 42.4 years) in the patients with BMPTs. The maximum diameter of the FAs ranged from 18 to 90 mm (mean, 36.4 mm), of the BPTs from 18 to 73 mm (mean, 49.5 mm), and of the BMPTs from 12 to 50 mm (mean, 33.4 mm). There was no significant difference among sizes for the three groups.
MRI
MRI was performed using a 3 T magnet (Signa HDxt; GE Healthcare, Waukesha, WI) with an eight-channel phased-array breast coil (GE Healthcare, Waukesha, WI) in the prone position. Prior to DCE-MRI, axial DWI was performed using a single-shot echoplanar imaging technique with fat suppression (repetition time /echo time, 5025/89.2 ms; field of view, 360 mm; matrix, 128 × 128; slice thickness/gap, 6/1.5 mm; number of excitations, 2; b-values, 0, 20, 40, 80, 120, 200, 400, 600, 800, and 1000 s mm–2; total acquisition time, 7 min 37 s). Axial dynamic contrast-enhanced T1 weighted imaging with fat suppression (VIBRANT) was also performed (repetition time/echo time, 5.7/2.5 ms; a flip angle, 10°; field of view, 320 mm; matrix, 352 × 352; slice thickness/gap, 1.8/–0.9 mm; and an acquisition time, 1 min 5 s). An intravenous bolus injection of 0.1 mmol kg–1 gadopentetate dimeglumine (Magnevist; Bayer Healthcare, Berlin, Germany) was administered at a rate of 1 ml s–1, followed by a 20 ml saline flush. Acquisitions before and three times (1, 2, and 5 min) after the injection of contrast media were performed.
Diffusion data analysis
First, we determined the mean signal intensities in the regions of interest (ROIs) in every b-value image using the ImageJ software (National Institutes of Health, Bethesda, MD). One radiologist with 23 years of experience with breast MRI, manually placed an ROI on a representative slice that contained the largest dimension of the tumour, based on the post-contrast MR images. The largest possible ROI within the tumour was selected, which did not include the cystic or necrotic portions of the tumour, based on the T2 weighted image and postcontrast images. In every b-value image, an ROI of identical size and position was selected.
We used a segmental approach for the biexponential analysis. First, the perfusion-independent diffusion coefficient was determined using the monoexponential function in b-values over 200 s m–2, since the contribution of perfusion to the signal intensity becomes minor with b-values over 200 s m–211
where D is the perfusion-independent diffusion coefficient. Subsequently, D was applied to the following equation (biexponential function). Thereafter, with the D fixed, the perfusion-related diffusion coefficient (D*) and fraction (f) were derived using all the b-values:
where Sb is the signal intensity with diffusion gradient b and S0 is the signal intensity without a diffusion gradient.
Furthermore, using the following monoexponential function, we performed a fitting for all the measurement data that were the same as the analysis mentioned above and calculated the ADC.
All fitting procedures were performed with MATLAB (MathWorks, Natick, MA) using the Levenberg–Marquardt non-linear algorithm.
Histopathological analysis
All patients underwent surgery. The histological diagnosis was performed by a single pathologist with 15 years of experience in breast histological evaluation. PTs were classified as benign, borderline, or malignant according to the criteria proposed by Salvadori et al12.
Statistical analysis
A statistical analysis was performed using BellCurve for Excel for Windows, v. 2.02 (SSRI, Tokyo, Japan). The Steel–Dwass test was used. p values < 0.05 were considered to be statistically significant.
RESULTS
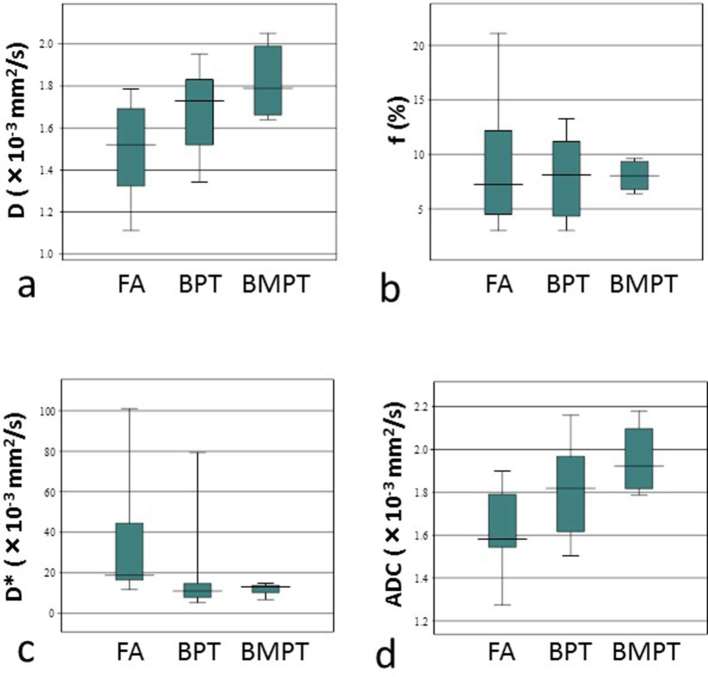
The D, f, and D* values from the IVIM biexponential fitting of FA, BPT, and BMPT are shown in Table 1 and Figure 1. The D-value showed an increasing tendency in the order of FA, BPT, and BMPT; however, no significant difference was found among the three groups. The D*-value showed an increasing tendency in the order of BMPT, BPT, and FA, and the D* value of the BMPT group was significantly lower than that of the FA group (p = 0.048).
Table 1.
Intravoxel incoherent motion parameters and ADC values of phyllodes tumours and fibroadenomas
| p-value | p-value | p-value | ||||
| FA | BPT | BMPT | FA vs BPT | FA vs BMPT | BPT vs BMPT | |
| D (×10–3 mm2 s–1) | 1.503 ± 0.241 | 1.670 ± 0.205 | 1.823 ± 0.181 | 0.305 | 0.103 | 0.435 |
| f (%) | 9.390 ± 6.241 | 8.184 ± 3.633 | 8.050 ± 1.406 | 0.979 | 0.967 | 0.992 |
| D* (×10–3 mm2 s–1) | 34.047 ± 32.506 | 18.224 ± 22.133 | 11.868 ± 3.110 | 0.079 | 0.048 | 0.928 |
| ADC (×10–3 mm2 s–1) | 1.624 ± 0.207 | 1.798 ± 0.215 | 1.957 ± 0.165 | 0.305 | 0.048 | 0.365 |
ADC, apparent diffusion coefficient; BPT, benign phyllodes tumour; BMPT, borderline or malignant phyllodes tumour; D, pure diffusion coefficient; D*, perfusion-related diffusion coefficient; f, fraction; FA, fibroadenoma.
Data are mean values ± standard deviations.
Figure 1.
(a–d) Box plots of pure diffusion coefficient (D) (a), fraction (f) (b), perfusion-related diffusion coefficient (D*) (c), and ADC (d) in FA, BPT, and BMPT. The top and bottom lines of the box represent the 25th–75th percentile values and the line in the box represents the median value. ADC, apparent diffusion coefficient; BPT, benign phyllodes tumour; BMPT, borderline or malignant phyllodes tumour; D, pure diffusion coefficient; D*, perfusion-related diffusion coefficient; FA, fibroadenoma.
The ADC value showed an increasing tendency in the order of FA, BPT, and BMPT, and the ADC value of the BMPT group was significantly higher than that of the FA group (p = 0.048) (Table 1 and Figure 1).
Representative cases of FA, BPT, borderline PT, and malignant PT are shown in Figure 2.
Figure 2.
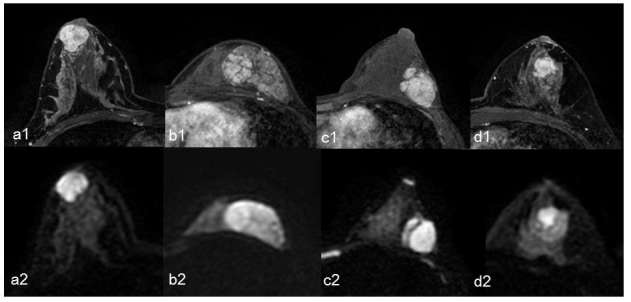
A representative case of fibroadenoma (a1, a2), benign phyllodes tumour (b1, b2), borderline phyllodes tumour (c1, c2), and malignant phyllodes tumour (d1, d2). Axial dynamic contrast-enhanced fat-suppressed T1 weighted images 1 min after administration of gadolinium (a1, b1, c1, and d1) and axial DWI with a b-value of 1000 s mm–2 (a2, b2, c2, and d2) are shown. (a) A 39-year-old female with fibroadenoma. The pure diffusion coefficient (D), fraction (f), perfusion-related diffusion coefficient (D*), and ADC values were 1.27 × 10–3 mm2 s–1, 21.10%, 20.14 × 10–3 mm2 s–1, and 1.55 × 10–3 mm2 s–1, respectively. (b) A 20-year-old female with benign phyllodes tumour. The D, f, D*, and ADC values were 1.55 × 10–3 mm2 s–1, 7.31%, 7.89 × 10–3 mm2 s–1, and 1.66 × 10–3 mm2 s–1, respectively. (c) A 37-year-old female with borderline phyllodes tumour. The D, f, D*, and ADC values were 1.67 × 10–3 mm2 s–1, 9.62%, 13.01 × 10–3 mm2 s–1, and 1.83 × 10–3 mm2 s–1, respectively. (d) A 49-year-old female with malignant phyllodes tumour. The D, f, D*, and ADC values were 1.97 × 10–3 mm2 s–1, 6.41%, 6.74 × 10–3 mm2 s–1, and 2.07 × 10–3 mm2 s–1, respectively. ADC, apparent diffusion coefficient; D, pure diffusion coefficient; D*, perfusion-related diffusion coefficient; DWI, diffusion-weighted imaging; f, fraction.
DISCUSSION
In this study, we aimed to determine whether the parameters derived from IVIM could differentiate PT from FA, by comparing them to the ADC values. We found that the D* value of the BMPT was significantly lower than that of the FA group, and the ADC value of the BMPT was significantly higher than that of the FA group.
Regarding the ADC value, Yabuuchi et al reported that a low ADC value is correlated with a high histologic grade in PT.13 They determined that a low ADC value in malignant PT was to be attributable to stromal hypercellularity. Whereas, a study by Kamitani et al reported that PT tended to show higher ADC values than FA; however, no significant difference was found between the two.7 They indicated that the proportion of the epithelium is higher in FA than in PT, and epithelial hyperplasia is more often seen in FA than in PT. The results of these two studies appear contradictory. In our study, we compared FA, BPT, and BMPT. As a result, the ADC value showed an increasing tendency in the order of FA, BPT, and BMPT, and the ADC value of the BMPT was significantly higher than that of the FA group. These findings correspond to the results reported by Kamitani et al. The ADC value of epithelial breast tumours is usually inversely correlated with tumour cellularity and shows a negative correlation with the grade of malignancy. In FA and PT, which are fibroepithelial tumours, however, the ADC value showed a tendency toward a positive correlation with the grade of malignancy.
A study by Liu et al showed that D and ADC values were significantly lower in malignant than in benign tumours.10 They also reported that the D values showed a higher area under the receiver operator characteristic curve and higher specificity than the ADC values for comparisons between malignant and benign lesions. It is thought that cellularity and microcirculation may influence ADC measurement in diametrically opposite ways. To wit, a study by Sigmund et al concluded that the D values provided better differentiation between benign and malignant lesions than the ADC values by avoiding microcirculation contributions.9 In the present study, the D value showed an increasing tendency in the order of FA, BPT, and BMPT; although there were no significant differences among the groups. It should be noted that the D value showed the same tendency with the ADC and significant differences may arise if a larger sample is examined.
The D*-value showed an increasing tendency in the order of BMPT, BPT, and FA, and the D*-value of the BMPT was significantly lower than that of the FA group. The D*-value was considered to be proportional to the mean capillary segment length and average blood velocity.8 The past reports that compared the D*-value of benign and malignant breast tumours have produced inconsistent results.14, 15 In this study, BMPT showed a slow blood velocity compared to FA. The quantity of stroma may be associated with this.
In summary, it is expected that the association between the ADC value and the grade of malignancy in fibroepithelial tumours differs from that in epithelial tumours. The FAs that we targeted in this study were of large size and rapid growth. In this situation, FA, when analysed as a whole gross tumour, tends to show higher blood velocity and tumour cellularity than BMPT. We propose that these attributes were reflected in the results of the D*, D, and ADC values.
There are several limitations to our study. First, this study enrolled a small number of patients; for instance, only one malignant PT was included, which may have affected the credibility of the results presented in the current work. Second, this study was retrospectively conducted at a single institution.
CONCLUSIONS
The D* derived from IVIM and the ADC were helpful for differentiating between FA and BMPT. The D value may also prove helpful should a larger sample be examined. Further study is required to determine whether IVIM-DWI can predict FA or BMPT in the clinical setting.
Contributor Information
Hiroko Kawashima, Email: hirokok@med.kanazawa-u.ac.jp.
Tosiaki Miyati, Email: ramiyati@mhs.mp.kanazawa-u.ac.jp.
Naoki Ohno, Email: nohno@med.kanazawa-u.ac.jp.
Masako Ohno, Email: takanaga@med.kanazawa-u.ac.jp.
Masafumi Inokuchi, Email: inokuchi@staff.kanazawa-u.ac.jp.
Hiroko Ikeda, Email: h-ikeda@med.kanazawa-u.ac.jp.
Toshifumi Gabata, Email: gabata@med.kanazawa-u.ac.jp.
REFERENCES
- 1.Foxcroft LM, Evans EB, Porter AJ. Difficulties in the pre-operative diagnosis of phyllodes tumours of the breast: a study of 84 cases. Breast 2007; 16: 27–37. doi: 10.1016/j.breast.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz E, Sal S, Lebe B. Differentiation of phyllodes tumors versus fibroadenomas. Acta Radiol 2002; 43: 34–9. [DOI] [PubMed] [Google Scholar]
- 3.Jorge Blanco A, Vargas Serrano B, Rodríguez Romero R, Martínez Cendejas E, Blanco AJ, Serrano BV. Phyllodes tumors of the breast. Eur Radiol 1999; 9: 356–60. doi: 10.1007/s003300050680 [DOI] [PubMed] [Google Scholar]
- 4.Wurdinger S, Herzog AB, Fischer DR, Marx C, Raabe G, Schneider A, et al. Differentiation of phyllodes breast tumors from fibroadenomas on MRI. AJR Am J Roentgenol 2005; 185: 1317–21. doi: 10.2214/AJR.04.1620 [DOI] [PubMed] [Google Scholar]
- 5.Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging 2002; 16: 172–8. doi: 10.1002/jmri.10140 [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging 2009; 30: 615–20. doi: 10.1002/jmri.21884 [DOI] [PubMed] [Google Scholar]
- 7.Kamitani T, Matsuo Y, Yabuuchi H, Fujita N, Nagao M, Kawanami S, et al. Differentiation between benign phyllodes tumors and fibroadenomas of the breast on MR imaging. Eur J Radiol 2014; 83: 1344–9. doi: 10.1016/j.ejrad.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988; 168: 497–505. doi: 10.1148/radiology.168.2.3393671 [DOI] [PubMed] [Google Scholar]
- 9.Sigmund EE, Cho GY, Kim S, Finn M, Moccaldi M, Jensen JH, et al. Intravoxel incoherent motion imaging of tumor microenvironment in locally advanced breast cancer. Magn Reson Med 2011; 65: 1437–47. doi: 10.1002/mrm.22740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Liang C, Liu Z, Zhang S, Huang B. Intravoxel incoherent motion (IVIM) in evaluation of breast lesions: comparison with conventional DWI. Eur J Radiol 2013; 82: e782–e789. doi: 10.1016/j.ejrad.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Patel J, Sigmund EE, Rusinek H, Oei M, Babb JS, Taouli B. Diagnosis of cirrhosis with intravoxel incoherent motion diffusion MRI and dynamic contrast-enhanced MRI alone and in combination: preliminary experience. J Magn Reson Imaging 2010; 31: 589–600. doi: 10.1002/jmri.22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salvadori B, Cusumano F, Del Bo R, Delledonne V, Grassi M, Rovini D, et al. Surgical treatment of phyllodes tumors of the breast. Cancer 1989; 63: 2532–6. doi: [DOI] [PubMed] [Google Scholar]
- 13.Yabuuchi H, Soeda H, Matsuo Y, Okafuji T, Eguchi T, Sakai S, et al. Phyllodes tumor of the breast: correlation between MR findings and histologic grade. Radiology 2006; 241: 702–9. doi: 10.1148/radiol.2413051470 [DOI] [PubMed] [Google Scholar]
- 14.Liu C, Wang K, Chan Q, Liu Z, Zhang J, He H, et al. Intravoxel incoherent motion MR imaging for breast lesions: comparison and correlation with pharmacokinetic evaluation from dynamic contrast-enhanced MR imaging. Eur Radiol 2016; 26: 3888–98. doi: 10.1007/s00330-016-4241-6 [DOI] [PubMed] [Google Scholar]
- 15.Ma D, Lu F, Zou X, Zhang H, Li Y, Zhang L, et al. Intravoxel incoherent motion diffusion-weighted imaging as an adjunct to dynamic contrast-enhanced MRI to improve accuracy of the differential diagnosis of benign and malignant breast lesions. Magn Reson Imaging 2017; 36: 175–9. doi: 10.1016/j.mri.2016.10.005 [DOI] [PubMed] [Google Scholar]