Abstract
Objective:
In amino acid positron emission tomography brain tumour imaging, tumour-to-background uptake parameters are often used for treatment monitoring. We studied the effects of patients’ characteristics and anticancer treatments on 18F–fluoro-l–phenylalanine uptake of normal brain and tumour lesions, with particular emphasis on temozolomide (TMZ) chemotherapy.
Methods:
155 studies from 120 patients with glioma were analysed. Average uptake of normal background (standardized uptake value, SUVbckgr) and basal ganglia (SUVbg), as well as tumour-to-brain ratios (TBR) were compared between positron emission tomography/CT studies acquired before (Group A, n = 48), after (Group B, n = 50) or during (Group C, n = 57) TMZ treatment, using analysis of variance.
Results:
Overall, mean SUVbckgr and mean SUVbg were 1.06 ± 0.26 and 2.12 ± 0.47, respectively. Female had significantly higher SUVbckgr (p = 0.002) and SUVbg (p = 0.012) than male patients. Age showed a positive correlation with SUVbg (p = 0.001). In the overall cohort, there were significant effects of TMZ on SUVbckgr (p = 0.0237) and TBR (p = 0.0138). In particular, SUVbckgr was lower in Group C than in Group B (1.00 ± 0.25 vs 1.14 ± 0.31, p = 0.0173). Significant variations of SUVbckr could be observed in female only. TBR was significantly higher in Group C than in Group B (2.37 ± 0.54 vs 2.06 ± 0.38, p = 0.010). Variations of SUVbg between groups slightly missed significance (p = 0.0504).
Conclusion:
Temozolomide chemotherapy and patients’ characteristics, including gender and age, affect physiological [18F]–fluoro-l–phenylalanine uptake and, consequently, the calculation of TBRs.
Advances in knowledge:
For the first time, the effects of past or concurrent temozolomide chemotherapy on brain physiological amino acid uptake have been investigated. Such effects are relevant and should be taken into account when evaluating tumour-to-background ratios.
Introduction
Positron emission tomography (PET) with radiolabelled amino acids has become an important diagnostic tool in brain tumour imaging, and recent joint recommendations of the Response Assessment in Neuro-Oncology working group and the European Association of Neuro-Oncology advice its use in the management of gliomas in addition to MRI.1 Compelling evidence suggests that amino acid PET outperforms standard MRI, particularly in the definition of the true tumour extent in the post-therapy setting, competing with advanced MRI techniques in the differential diagnosis between treatment-induced changes and tumour progression.2, 3
The cellular uptake mechanisms of the most widely adopted amino acid PET tracers, namely 11C-methyl-L-methionine (MET), O-(2-[18F]fluoroethyl)-L-tyrosine (FET) and 3,4–dihydroxy–6–[18F]–fluoro-l–phenylalanine (18F-DOPA), are based on the increased expression of Na+-independent large neutral amino acid transporters on tumour cells.4–6 This mechanism is highly efficient, leading to favourable tumour-to-background uptake ratios, and does not depend on blood brain barrier (BBB) permeability. In fact, preclinical experimental studies have demonstrated that amino acid tumour uptake is not directly influenced by therapies reducing BBB permeability, such as steroids or bevacizumab.7, 8
The evaluation of amino acid PET images of brain tumours and, more specifically, the assessment of early response to therapy, is often based on tumour uptake parameters relative to background radioactivity of normal appearing contralateral hemispheres or cerebellum.9–11 In the case of PET with 18F-DOPA, physiological basal ganglia uptake represents an additional common reference for image interpretation and semi-quantitative evaluation, owing to the tracer metabolism by the aromatic amino acid decarboxylase in dopaminergic neurons.12–15
If concurrent medications have so far shown little influence on tumour uptake parameters,7, 8 yet this might not hold true for the uptake of normal structures, such as disease-free brain parenchyma or basal ganglia, with potential consequent impact on image interpretation and semi-quantitative evaluation. Changes of background amino acid uptake consequent to steroid treatment have already been observed in animal models of brain tumours.16 Furthermore, patient age and gender have known effects on physiological striatal uptake of 18F-DOPA,17–19 but their influence on the evaluation of patients with brain tumours has been not been assessed so far. A recently published study showed female gender and high body mass index to be associated with increased cerebral FET uptake, though the impact of such patients’ characteristics on tumour uptake was not specifically addressed.20
The primary objective of the present study was to investigate possible effects of patient characteristics and treatment history on the physiological uptake of 18F-DOPA, with a special focus on the oral alkylating agent Temozolomide (TMZ), the mainstream chemotherapy treatment of gliomas. Secondarily, the effects on tumour-to-normal brain ratio (TBR) were also assessed.
Methods and materials
Patients
A total of 187 consecutive 18F-DOPA PET/CT studies from 144 patients with primary brain tumours acquired at Sant'Andrea Hospital of Rome, Italy between May 2010 and February 2017 were retrospectively evaluated. After the exclusion of patients with non-glial primary brain tumours (n = 26) or with incomplete clinical data (n = 6), a data set of 155 studies from 120 patients with newly diagnosed or recurrent glioma were entered into the final analysis. According to our clinical protocols, 18F-DOPA PET is generally used for tumour target delineation, for the assessment of response to treatments [chemotherapy and/or radiation therapy (RT)] and for differential diagnosis between treatment-related changes and progression. Diagnoses were defined according to World Health Organization (WHO) classification 2007.21 Overall, there were 104 high-grade and 51 low-grade gliomas. Eight patients with histologically unverified tumours were classified as low-grade or high-grade gliomas based on clinical history and radiological findings. Five patients with a long history of a brain stem or thalamic lesion that did not show contrast enhancement at MRI were diagnosed as low-grade glioma. For the remaining three patients, a diagnosis of high-grade glioma was presumed based on the presence of a mass with marked enhancement after contrast administration and large necrotic areas at MRI. In two of them who received subsequent surgery, the diagnosis of glioblastoma was histologically confirmed after PET/CT examination. Tumour histologies are detailed in Table 1. None of the patients had history of Parkinson’s or Parkinson-like diseases.
Table 1.
Histological classification of the overall cohort
| Tumour histology | N° patients (%) | N° studies (%) |
| Low grade (WHO Grade II) | ||
| Astrocytoma | 12 (10%) | 16 (10.3%) |
| Oligodendroglioma | 20 (16.7%) | 26 (16.8%) |
| Oligoastrocytoma | 2 (1.7%) | 4 (2.6%) |
| Histologically unverifieda | 5 (4.2%) | 5 (3.2%) |
| High grade (WHO Grade III/IV) | ||
| Astrocytoma | 25 (20.8%) | 35 (22.5%) |
| Oligodendroglioma | 12 (10%) | 16 (10.3%) |
| Oligoastrocytoma | 4 (3.3%) | 5 (3.2%) |
| Glioblastoma | 37 (30.8%) | 45 (29%) |
| Histologically unverifieda | 3 (2.5%) | 3 (1.9%) |
| Total | 120 | 155 |
WHO, World Health Organization.
Note: aHistologically unverified tumours were classified as low- or high-grade based on clinical history and radiological appearance at MRI.
Treatments
Four (2.5%) PET/CT studies were acquired before any treatment or invasive diagnostic procedure was undertaken. The remaining 151 (97.5%) PET/CT studies were acquired after patients had received previous treatments, including surgery, RT and TMZ chemotherapy given alone or in combination. In general, patients with high-grade gliomas received RT at a total dose of 59.4–60 Gy in 30–33 fractions of 1.8–2.0 Gy given concomitantly to TMZ (75 mg m–2 daily), followed by adjuvant TMZ (150–200 mg m–2 for 5 days every 28 days) up to 12 cycles. Patients with low-grade gliomas received RT (54 Gy in 30 fractions of 1.8 Gy) followed or not by adjuvant TMZ.
107 (69%) studies were acquired in previously irradiated patients; in the remaining 48 (31%) cases, patients had not received prior RT. None of the patients has previously received antiangiogenic treatment. At the time of PET/CT, only eight (5.1%) patients were assuming low-dose steroids (Dexamethasone 1–4 mg/daily).
18F-DOPA PET protocol and image analysis
A 20-min static image of the brain was acquired on a Philips Gemini PET/CT Camera (Philips Medical Systems, the Netherlands) starting 15 ± 5 min after intravenous injection of 185 MBq (±10%) of 18F-DOPA. Patients were required to fast for at least 6 h before the scan and no carbidopa premedication was given. A low-protein diet was not requested before the study. A low-dose CT scan (120 kV, 60 mA) without contrast-enhancement was acquired for attenuation correction. Images were reconstructed using a three-dimensional RAMLA algorithm on a 128 × 128 matrix and a square field of view of 256 mm side, yielding a final voxel of 8 mm3.
Image data analysis was performed on a Hermes workstation (Hermes Medical Solutions, Stockholm, Sweden). Physiological 18F-DOPA uptake parameters were calculated in the normal appearing brain tissue and in the basal ganglia. In order to obtain the average background uptake (standardized uptake value, SUVbckgr), a large hemispheric ROI including both grey and white matter was drawn contralaterally to the lesion, on a plane above the lateral ventricles. SUVmean of the basal ganglia (SUVbg) was obtained using a threshold-based volume of interest (VOI) semi-automatic segmentation tool. For this purpose, a threshold of 2.0 over the background activity was used; when needed, VOIs were manually adjusted by two experienced operators in consensus (LC and MM). In case of bilateral tumours, SUVbckgr and SUVbg were calculated by averaging the uptake values obtained in both hemispheres and basal ganglia, respectively. There were no cases of tumours infiltrating supraventricular portions of frontoparietal lobes, bilaterally.
In PET-positive studies (n = 99), tumour volumes were contoured semi-automatically by taking a threshold value of 1.6 over the background uptake as a reference.22 However, such a threshold did not allow a precise delineation of the tumour extent in all cases. In most cases, tumour delineation was visually considered adequate after changing the threshold in the range 1.4–2. Further adjustment was required in case of signal interference between tumour and basal ganglia. Patterns of interference between physiological basal ganglia uptake and tumour delineation were classified into Pattern A and Pattern B, as previously described.22 In particular, Pattern A was defined as tumour signal touching but not infiltrating the striatum; Pattern B was defined as tumour displacing and infiltrating the basal ganglia, preventing a clear distinction between the two. Manual adjustment of the VOI was sufficient in case of 15 (15.1%) studies showing Pattern A signal interference. In contrast, in case of 13 (13.1%) studies with Pattern B interference, the most recent MRI was co-registered to the PET/CT scan and used as a reference for contouring the striatum.
TBR was defined as the mean SUV calculated within the tumour VOI, divided by SUVbckgr.
Statistical analysis
For statistical analysis, PET/CT studies were divided into the following three groups (referred as “study groups” all throughout the manuscript) according to chemotherapy treatment history: (i) no prior TMZ treatment (Group A, n = 48), (ii) TMZ treatment concluded >1 month before PET/CT (Group B, n = 50) and (iii) ongoing TMZ treatment (Group C, n = 57) (Table 2). Comparison of analysed variables between groups was made by analysis of variance (ANOVA), with Turkey’s test for multiple comparisons (GraphPad Prism 7.03).
Table 2.
Characteristics of the patient cohort
| Tumour grade | Chemotherapy | Radiotherapy | |||
| Group A | Group B | Group C | Y | N | |
| Low-grade | 27 | 11 | 13 | 21 | 30 |
| High-grade | 21 | 39 | 44 | 86 | 18 |
| Total | 48 | 50 | 57 | 107 | 48 |
Note. Previous chemotherapy and radiotherapy treatments. Data are reported based on single studies, not on patients.
In addition, analyses of covariance (ANCOVA) were performed to investigate the strength of the association between SUVbckgr, SUVbg and TBR (dependent variables), the chemotherapy history (variable of interest) and the covariates age, gender and patient weight (IBM SPSS Statistics for Windows, v. 22.0. Armonk, NY: IBM Corp). Tumour volume, SUVmax and WHO grade were entered as additional covariates in the ANCOVA for TBR. Two-tailed Student’s t-test and χ2 test were used to compare parameter means and frequency distributions, respectively. Pearson’s r was used for correlation analysis. Probability values of less than 0.05 were considered significant. For all analyses in this study, paired imaging data from the same patient were considered as unpaired and were entered as single entities.
Results
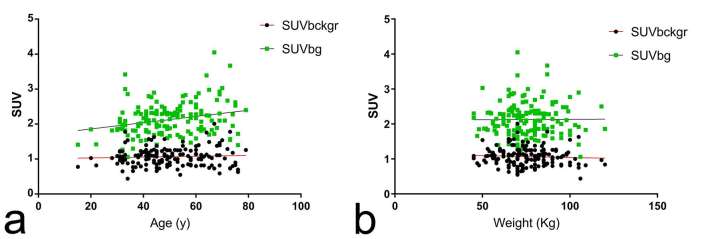
In the overall cohort, mean SUVbckgr and mean SUVbg were 1.06 ± 0.26 and 2.12 ± 0.47, respectively. Female had significantly higher SUVbckr and SUVbg than male patients (SUVbckr = 1.14 ± 0.30 vs 1.01 ± 0.21, p = 0.002; SUVbg = 2.23 ± 0.52 vs 2.04 ± 0.41, p = 0.012, Student’s t-test). Mean age was 50 ± 13 years at the time of PET/CT scan. Age did not correlate with SUVbckgr (Pearson’s r = 0.063, p = 0.435) but showed a significant positive correlation with SUVbg (Pearson’s r = 0.258, p = 0.001) (Figure 1a). Average patient weight, which may have an effect on SUV calculations,23 was significantly higher in male than in female patients (81.7 ± 13.1 vs 67.6 ± 13 kg, p < 0.0001, Student’s t-test). However, in the overall cohort, no significant correlation was found between weight and SUVbckgr (Pearson’s r = −0.059, p = 0.464) or SUVbg (Pearson’s r = 0.006, p = 0.935) (Figure 1b).
Figure 1.
Correlation of patients’ age and weight with physiological 18F-DOPA uptake. (a) Scatter plot of SUVbckgr (black dots) and SUVbg (green squares) data against patients’ age. There was a significant correlation between age and SUVbg (Pearson’s r = 0.258, p = 0.001) while no significant correlation was found between age and SUVbckgr (Pearson’s r = 0.063, p = 0.435). (b) Scatter plot of SUVbckgr (black dots) and SUVbg (green squares) data showing no significant correlation with patients’ weight (Pearson’s r = −0.059, p = 0.464 and Pearson’s r = 0.006, p = 0.935 for SUVbckgr and SUVbg, respectively). 18F-DOPA, 18F–fluoro-l–phenylalanine; SUV, standardized uptake value.
Appreciable tumour uptake was observed in 99 studies (Group A: n = 28; Group B: n = 30; Group C: n = 41), whereas 56 studies were PET-negative. TBR were significantly higher in GBMs than in Grade III or in Grade II gliomas (2.40 ± 0.43, 2.15 ± 0.43, 2.11 ± 0.44 in GBM, Grade III and Grade II gliomas, respectively, p = 0.0177, ANOVA). In contrast, SUVmax and tumour volumes were not significantly different across various tumour grades (p = 0.3436 and p = 0.6927, respectively, ANOVA). There were no significant differences in tumour uptake between astrocytomas and oligodendrogliomas, either Grade III or Grade II tumours (all p > 0.5, Student’s t-test).
Analysis of semi-quantitative normal brain parameters: SUVbckr and SUVbg
Results of semi-quantitative 18F-DOPA PET/CT analysis in the overall population and in different study groups are summarized in Table 3. Gender and weight differences were not significant between the three study groups (p = 0.2827 and 0.0743, χ2 test and ANOVA, respectively), while a significant heterogeneity of age was observed (p = 0.0059, ANOVA).
Table 3.
Summary of the results of semiquantitative 18F-DOPA PET/CT analysis in the overall population and in different study groups
| Variables | Temozolomide chemotherapy | ||||
| Overall cohort | Group A | Group B | Group C | p-values | |
| Age (years) | 50 ± 13 | 45 ± 13 | 54 ± 12 | 50 ± 13 | 0.0059a |
| Gender (F/M) | 69/86 | 22/26 | 26/24 | 21/36 | 0.2827 |
| Weight (kg) | 75.5 ± 14.8 | 72.1 ± 12.9 | 75 ± 15.47 | 78.7 ± 15.24 | 0.0743 |
| SUVbckgr | 1.06 ± 0.26 | 1.06 ± 0.21 | 1.14 ± 0.31 | 1.00 ± 0.25 | 0.0237a |
| Female | 1.14 ± 0.30 | 1.14 ± 0.20 | 1.23 ± 0.34 | 1.02 ± 0.30 | 0.0539 |
| Male | 1.01 ± 0.21 | 1.00 ± 0.19 | 1.04 ± 0.23 | 0.99 ± 0.20 | 0.6635 |
| SUVbg | 2.12 ± 0.47 | 2.06 ± 0.39 | 2.26 ± 0.57 | 2.07 ± 0.43 | 0.0504 |
| Female | 2.23 ± 0.52 | 2.14 ± 0.36 | 2.42 ± 0.61 | 2.08 ± 0.49 | 0.0552 |
| Male | 2.04 ± 0.41 | 1.97 ± 0.40 | 2.08 ± 0.46 | 2.06 ± 0.38 | 0.5995 |
| PET result (+/−) | 99/56 | 28/20 | 30/20 | 41/16 | 0.2771 |
| Tumour volume (cm3) | 8.42 ± 11.29 | 13.15 ± 15.26 | 5.68 ± 6.37 | 7.19 ± 10.16 | 0.0263a |
| SUVmax | 3.31 ± 1.14 | 3.63 ± 1.23 | 3.35 ± 1.20 | 3.07 ± 1.00 | 0.1305 |
| TBR | 2.22 ± 0.45 | 2.18 ± 0.30 | 2.06 ± 0.38 | 2.37 ± 0.54 | 0.0138a |
| Female | 2.20 ± 0.55 | 2.25 ± 0.35 | 1.99 ± 0.44 | 2.39 ± 0.68 | 0.0992 |
| Male | 2.23 ± 0.36 | 2.13 ± 0.26 | 2.14 ± 0.26 | 2.36 ± 0.42 | 0.0703 |
18F-DOPA, 18F–fluoro-l–phenylalanine; PET, positron emission tomography; SUV, standardized uptake value; TBR, tumour-to-brain ratios.
Note. If not indicated otherwise, data are reported as mean ± standard deviation. Comparisons between groups were obtained by analysis of variance or χ2 test, when appropriate. aindicates that differences between groups reached statistical significance.
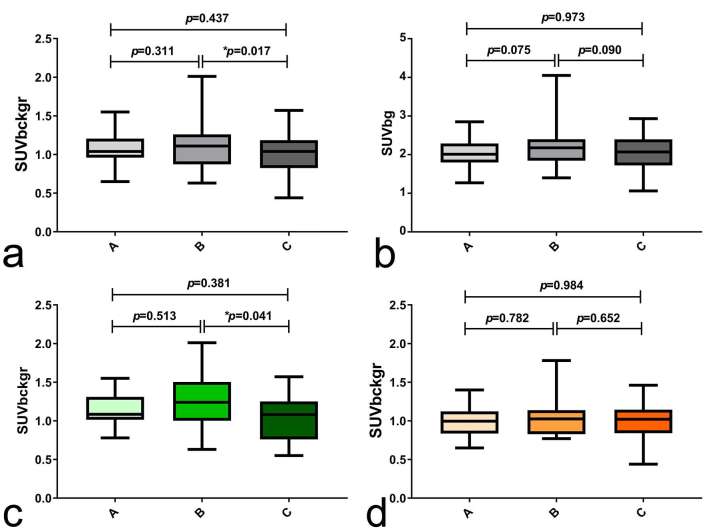
SUVbckgr was significantly different between groups (p = 0.0237, ANOVA). In particular, SUVbckgr was lower in patients with ongoing TMZ treatment (Group C) than in patients who concluded TMZ treatment >1 month before PET/CT (Group B) (1.00 ± 0.25 vs 1.14 ± 0.31, p = 0.0173, Turkey’s test) (Figure 2a). Interestingly, variations of SUVbckgr between groups mostly reflected the influence of TMZ treatment on female subjects. In fact, in female patients, SUVbckgr was significantly higher in Group B than in Group C (1.23 ± 0.34 vs 1.02 ± 0.30, p = 0.041, Turkey’s test) (Table 3 and Figure 2c); in contrast, normal brain metabolism of male patients did not significantly change with TMZ treatment (Table 3 and Figure 2d).
Figure 2.
Effects of TMZ chemotherapy on physiological 18F-DOPA uptake. 155 PET/CT studies were divided into the following three groups according to chemotherapy treatment history: (i) no prior TMZ treatment (Group A, n = 48), (ii) TMZ treatment concluded >1 month before PET/CT (Group B, n = 50) and (iii) ongoing TMZ treatment (Group C, n = 57). Panel (a) shows significant differences of SUVbckgr between the three study groups; in particular, Group C has significantly lower SUVbckgr than Group B. Panel (b) shows variations of SUVbg between the three groups, which slightly missed the significance level. Panel (c) shows significant differences of SUVbckgr uptake among female patients (green colour scale); in particular, group C female patients have significantly higher SUVbckgr than group B female patients. Panel (d) shows fairly stable SUVbckgr in male patients (orange colour scale) grouped according to TMZ treatment history. In the figure, p-values refer to Turkey’s test for multiple comparisons and * indicates that differences between groups reached statistical significance (p < 0.05). 18F-DOPA, 18F–fluoro-l–phenylalanine; PET, positron emission tomography; SUV, standardized uptake value; TMZ, temozolomide.
In the ANCOVA model, only the gender retained a significant effect on SUVbckgr, whereas the effect of chemotherapy slightly missed the significance level (Gender: F = 9.375, p = 0.003; chemotherapy history: F = 2.759, p = 0.067; Age: F = 0.919, p = 0.339; Weight: F = 1.034, p = 0.311). In the subgroup of female patients, chemotherapy history retained a significant effect on SUVbckgr after co-varying for age and weight (F = 3.439, p = 0.038).
Differences of SUVbg between groups slightly missed the significance level (p = 0.0504, ANOVA). Group B showed a trend for higher SUVbg values compared with other groups (p = 0.075 and p = 0.090 for A vs B and B vs C, respectively, Turkey’s test) (Table 3 and Figure 2b). Similarly to what observed for SUVbckgr, an apparent effect of chemotherapy on SUVbg was shown in female only, albeit none of the differences between groups reached statistical significance (Table 3, full results of multiple comparisons not shown). In the ANCOVA model, age and gender proved to be significant predictors of SUVbg (Gender: F = 11.004, p = 0.001; chemotherapy history: F = 1.065, p = 0.347; Age: F = 11.649, p = 0.001; Weight: F = 2.659, p = 0.105).
Previous treatment with stereotactic radiotherapy had no effect on normal brain uptake parameters. In particular, average SUVbckgr and SUVbg were 1.06 ± 0.28 and 2.16 ± 0.5 in patients with prior radiotherapy vs 1.07 ± 0.21 and 2.05 ± 0.5 in patients without prior radiotherapy, respectively (p = 0.86 and p = 0.19 for SUVbckgr and SUVbg, respectively, Student’s t-test).
Analysis of tumour uptake parameters: TBR
TBRs were significantly different between the groups (p = 0.0138, ANOVA). In particular, Group C had higher TBR values than Group B (2.37 ± 0.54 vs 2.06 ± 0.38, p = 0.010, Turkey’s test) (Table 3 and Figure 3a). In contrast, SUVmax did not significantly differ between the three study groups (p = 0.1305, ANOVA) (Table 3 and Figure 3c). Figure 3b shows significant variations of TBR according to chemotherapy history in the most numerous subgroup (n = 37 studies) of WHO Grade III tumours (TBR = 2.29 ± 0.32, 1.86 ± 0.23 and 2.34 ± 0.57 in Group A, B and C, respectively p = 0.0077, ANOVA). In the ANCOVA model, chemotherapy history retained its statistical significance in predicting TBR after co-varying for age, gender weight, SUVmax, tumour volume and WHO grade (F = 7.328, p = 0.001). An illustrative patient case is shown in Figure 4.
Figure 3.
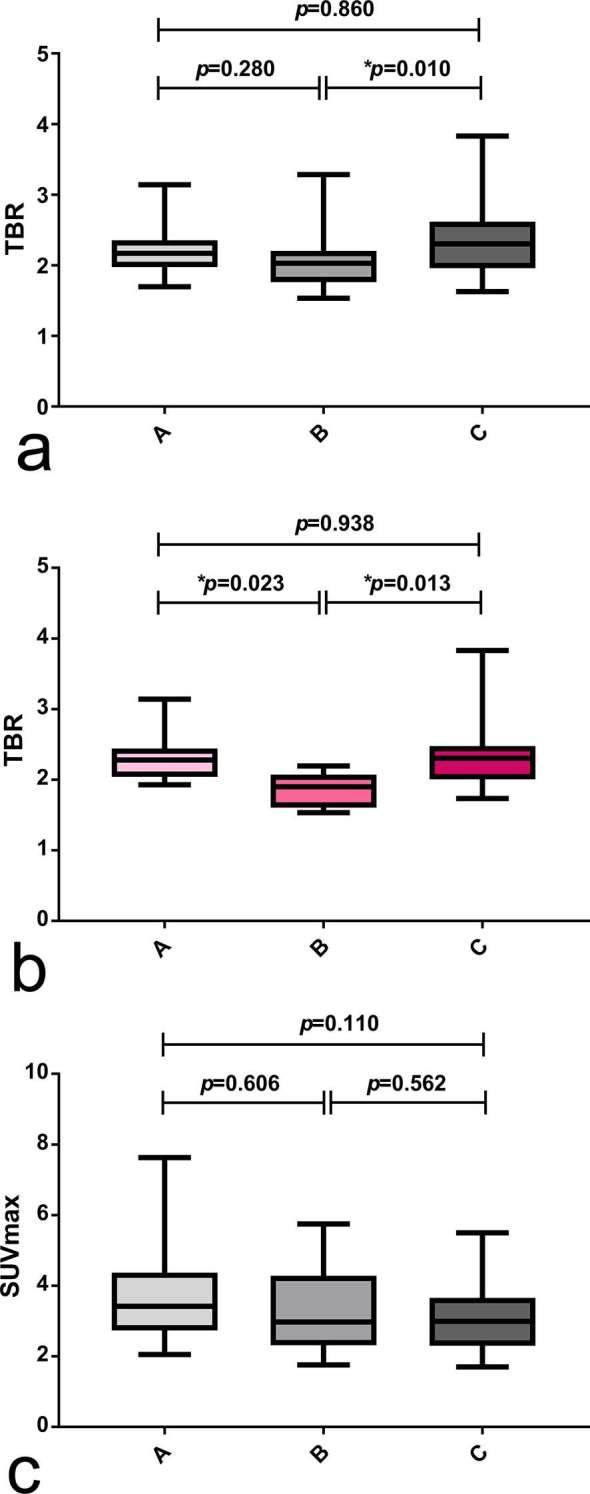
Effects of TMZ chemotherapy on tumour uptake. 99 PET/CT scans showing positive tumour signal were divided into the following three groups according to chemotherapy treatment history: (i) no prior TMZ treatment (Group A, n = 28), (ii) TMZ treatment concluded >1 month before PET/CT (Group B, n = 30) and (iii) ongoing TMZ treatment (Group C, n = 41). Panel (a) shows significant differences of TBR between the three study groups; in particular, TBR is significantly higher in Group C than in Group B. Panel (b) shows significant differences of TBR in the subgroup of WHO Grade III astrocytic tumours (n = 37); in particular, both Group A and Group C showed higher TBR than Group B. Panel (c) shows no significant differences of SUVmax between study groups. In the figure, p-values refer to Turkey’s test for multiple comparisons and * indicates that differences between groups reached statistical significance (p < 0.05).
Figure 4.
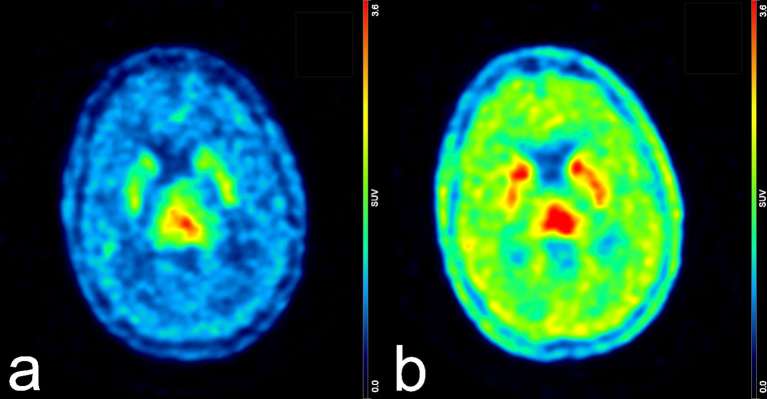
Example of variations of 18F-DOPA uptake induced by TMZ chemotherapy. The left panel (a) represents the brain PET scan of a female patient with a high-grade astrocytoma acquired during the course of TMZ treatment. SUVbckgr, SUVbg and TBR were 0.87, 1.86 and 2.91, respectively. The right panel (b) shows the same patient imaged 3 years later, after conclusion of chemotherapy. Notably, a “rebound effect” can be seen at sites of physiological uptake, whilst the opposite effect is observed for TBR. In fact, SUVbckgr and SUVbg increased to 1.78 and 3.42, respectively, whereas TBR dropped to 2.19 despite an increase of SUVmax from 3.62 to 5.47. Both images were scaled to the same maximum signal intensity. 18F-DOPA, 18F–fluoro-l–phenylalanine; PET, positron emission tomography; SUV, standardized uptake value; TBR, tumour-to-brain ratio; TMZ, temozolomide.
Discussion
Amino acid PET is increasingly used in the work-up of primary and secondary brain tumours, as it showed several advantages over standard MRI and advanced MRI techniques. Most of the convenience lies in the better ability of amino acid PET to discriminate between recurrent tumour and treatment-induced changes.1–3 In this setting, measures of tumour uptake relative to that of normal structures have often been successfully used to define the presence of disease and tumour extent.9–15 Quantitative estimations of tumour volume changes have shown to reliably stratify responses to a given treatment or to predict outcome.9–11,14 We speculated that the heterogeneity of patients’ characteristics, or specific anticancer treatments might significantly interfere with the reproducibility of these semi-quantitative measures, potentially affecting conclusions regarding differential diagnosis or outcome stratification. Steroids and antiangiogenic treatments have shown no significant impact on tumour uptake in preclinical models;7, 8 however, to our knowledge, no data have been produced on the effects of these and other compounds on the amino acid uptake of normal brain structures. Hence, this retrospective study was primarily designed to address the effects of several variables on the physiological uptake of brain structures used as a reference for semi-quantitative tumour evaluation, with a particular emphasis on TMZ treatment.
First finding of our study was the confirmation that age and gender have an impact on 18F-DOPA physiological uptake. In particular, age showed a significant positive correlation with SUVbg, consistently with previous observations,17 and with the hypothesis of age-related differences in dopamine turnover.17, 18 Overall, female showed higher background and basal ganglia uptake. Increased striatal and cortical dopaminergic activity had already been observed in female patients with Parkinson’s disease, suggesting an implication of oestrogens in the regulation of dopamine metabolism.19, 24,25 This is in line with the recent findings of Verger and co-authors, who showed a uniformly higher cortical brain FET uptake in females than in males, indicating that other amino acids might be characterized by a sex-dependent metabolism.20 Unfortunately, information on oestrogen levels or menopausal status in our female cohort was not retrospectively available.
Most importantly, we were able to show that TMZ chemotherapy induces prominent changes in physiological 18F-DOPA uptake. In particular, the uptake of cortical structures, including both grey and white matter, is significantly reduced in patients under TMZ treatment compared to patients who discontinued TMZ more than 1 month before the PET/CT examination. These effects of TMZ treatment on physiological uptake were significant in female subjects only. We do not have an explanation for that; a previous pharmacokinetic study of 11C-labelled TMZ enrolled only male patients and, unfortunately, cannot provide sufficient enlightenment on the reason of such differences.26 We suppose that the lower baseline uptake values found in males might have masked the effects of TMZ compared to female patients.
Though not surprising—virtually all chemo- and radiotherapy treatments are indeed associated with some degree of neurotoxicity27—the finding of a significant effect of TMZ treatment on cerebral physiological 18F-DOPA uptake is original and might have relevant implications in the interpretation of amino acid PET imaging, which have been probably overlooked so far. TMZ is recently raising the interest of neuroscientists as it demonstrated disruptive effects on neurogenesis and cognition in rodent models.28 More specifically, TMZ has proven to deplete hippocampal neurogenesis in mice and to produce behavioural changes, such as hyponeophagia and anhedonia, which represent an experimental model of depression.29 An impairment of brain dopaminergic activity might underlie these behavioural changes,30 explaining the reduced 18F-DOPA uptake observed in our study under TMZ treatment. In this case, other amino acid tracers that are not precursors of dopamine, such as FET or MET, might not uncover the same effect of TMZ on brain metabolism.
Interestingly, neurogenesis ablation and behavioural changes observed experimentally in laboratory animals were transient.29 One might therefore speculate that the relative increase of uptake parameters observed in patients who concluded TMZ treatment before PET examination represents a compensatory “rebound” response to the suppressive effects of TMZ. A potential confounding factor might be the levels of endogenous cortisol produced under TMZ treatment. An abnormal increase of corticosterone blood levels in response to stress was shown in mice treated with TMZ compared to untreated mice, although baseline levels were similar between the two groups.29 If high cortisol blood levels were present in our patients under TMZ treatment, this might have had an impact on amino acid brain metabolism, as suggested by Stegmayr and co-workers in a rat brain tumour model.16 In that work, they found an increase of background brain FET metabolism following the administration of dexamethasone, with consequent decrease of TBR.16 In our study, endogenous cortisol levels and their potential impact on 18F-DOPA uptake have not been assessed; however, we can at least reasonably exclude a significant interference of exogenous steroids, as only eight PET/CT studies (5.1% of the total) were performed on patients who were receiving low-dose dexamethasone.
Additional mechanisms having a potential strong impact on 18F-DOPA brain uptake pertain to competition with other amino acids for transport across the BBB. As a matter of fact, a reduction of the plasmatic concentration of large neutral amino acid with consequent increased brain extraction of levodopa has been demonstrated under treatment with β-adrenergics.31 Ongoing anticancer treatments might change the patient’s nutritional conditions and the peripheral metabolism of competing large neutral amino acids, potentially affecting 18F-DOPA uptake of normal brain structures. Unfortunately, the impact of such variables could not be retrospectively assessed in our cohort. However, we believe that such uncontrolled biochemical mechanisms are unlikely to compromise the interpretation of our results, as we found no significant differences of patient weight between the study groups, suggesting a fairly homogeneous nutritional status in our population. Moreover, the absence of a strict regulation of all metabolic variables allows the extension of our results to the daily clinical practice, in which these are hardly accounted for.
A secondary objective of the study was to assess whether the observed effects of TMZ on physiological background metabolism translate into significant changes of the frequently used tumour uptake parameter TBR. In the subgroup of PET-positive tumours, for which a TBR could be calculated, we found higher TBR values in patients with ongoing TMZ treatment compared to patients who had discontinued TMZ. This finding is counterintuitive and can be attributed to variations of background physiological uptake, especially given the similar absolute tumour uptake parameters (i.e. SUVmax) found across different groups. Indeed, one might have expected that tumours receiving active treatment had lower amino acid metabolism than those receiving no treatment. In contrast, according to our results, ongoing TMZ treatment would reduce SUVbckgr, leading to an increase of TBR.
Our study has several limitations due to its retrospective nature. Heterogeneities between groups somehow weakened our results and do not allow to undoubtedly claim an independent effect of TMZ on TBR. Nevertheless, the grouping according to TMZ treatment history remained a significant predictor of TBR after co-varying for all potential confounders. This, in our opinion, is a fairly strong argument in favour of an indirect interference of TMZ on the calculation of TBR.
Finally, we did not observe significant effects of prior stereotactic RT on 18F-DOPA uptake. In a previous study carried on patients with brain metastases, Lizarraga et al found lower normal brain and basal ganglia 18F-DOPA uptake in patients treated with whole brain RT (WBRT) compared to those treated with stereotactic irradiation.12 None of our patients was previously treated with whole brain RT, therefore our results provide further support to the hypothesis that stereotactic irradiation has limited impact on brain amino acid metabolism.
In summary, we have shown that TMZ chemotherapy and patients’ characteristics including gender and age have a significant impact on physiological 18F-DOPA uptake parameters. As a consequence, the calculation of TBR is also affected, leading to possible pitfalls in image interpretation, which might be particularly relevant in case of longitudinal, intrapatient evaluations. The biological underpinnings and the clinical impact of these findings warrant further elucidation by future studies.
ACKNOWLEDGMENTS
Preliminary results of this paper were reported as an oral presentation at the Annual congress of the European Association of Nuclear Medicine (EANM 2017) and published in an abstract form: Carideo et al Impact of chemotherapy with Temozolomide on physiological brain 18F-DOPA uptake in patients with glioma. Eur J Nucl Med Mol Imaging; 44 (Suppl 2): S298, OP–501.
Contributor Information
Luciano Carideo, Email: lu.carideo@gmail.com.
Giuseppe Minniti, Email: gminniti@ospedalesantandrea.it.
Marcelo Mamede, Email: mamede.mm@gmail.com.
Claudia Scaringi, Email: clascaringi@gmail.com.
Ivana Russo, Email: ivana.russo@gmail.com.
Francesco Scopinaro, Email: francesco.scopinaro@uniroma1.it.
Francesco Cicone, Email: f.cicone@iol.it.
Compliance with ethical standards
All procedures described in this study were performed in accordance with the standards of the institutional ethical committee and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all patients.
REFERENCES
- 1.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 2016; 18: 1199–208. doi: 10.1093/neuonc/now058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filss CP, Cicone F, Shah NJ, Galldiks N, Langen KJ. Amino acid PET and MR perfusion imaging in brain tumours. Clin Transl Imaging 2017; 5: 209–23. doi: 10.1007/s40336-017-0225-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol 2017; 13: 279–89. doi: 10.1038/nrneurol.2017.44 [DOI] [PubMed] [Google Scholar]
- 4.Okubo S, Zhen HN, Kawai N, Nishiyama Y, Haba R, Tamiya T. Correlation of L-methyl-11C-methionine (MET) uptake with L-type amino acid transporter 1 in human gliomas. J Neurooncol 2010; 99: 217–25. doi: 10.1007/s11060-010-0117-9 [DOI] [PubMed] [Google Scholar]
- 5.Youland RS, Kitange GJ, Peterson TE, Pafundi DH, Ramiscal JA, Pokorny JL, et al. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J Neurooncol 2013; 111: 11–18. doi: 10.1007/s11060-012-0986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habermeier A, Graf J, Sandhöfer BF, Boissel JP, Roesch F, Closs EI. System L amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET. Amino Acids 2015; 47: 335–44. doi: 10.1007/s00726-014-1863-3 [DOI] [PubMed] [Google Scholar]
- 7.Stegmayr C, Bandelow U, Oliveira D, Lohmann P, Willuweit A, Filss C, et al. Influence of blood-brain barrier permeability on O-(2-18F-fluoroethyl)-L-tyrosine uptake in rat gliomas. Eur J Nucl Med Mol Imaging 2017; 44: 408–16. doi: 10.1007/s00259-016-3508-0 [DOI] [PubMed] [Google Scholar]
- 8.Stegmayr C, Oliveira D, Niemietz N, Willuweit A, Lohmann P, Galldiks N, et al. Influence of bevacizumab on blood-brain barrier permeability and O-(2-18F-fluoroethyl)-l-tyrosine uptake in rat gliomas. J Nucl Med 2017; 58: 700–5. doi: 10.2967/jnumed.116.187047 [DOI] [PubMed] [Google Scholar]
- 9.Hutterer M, Nowosielski M, Putzer D, Waitz D, Tinkhauser G, Kostron H, et al. O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med 2011; 52: 856–64. doi: 10.2967/jnumed.110.086645 [DOI] [PubMed] [Google Scholar]
- 10.Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging 2015; 42: 685–95. doi: 10.1007/s00259-014-2959-4 [DOI] [PubMed] [Google Scholar]
- 11.Roelcke U, Wyss MT, Nowosielski M, Rudà R, Roth P, Hofer S, et al. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro Oncol 2016; 18: 744–51. doi: 10.1093/neuonc/nov282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lizarraga KJ, Allen-Auerbach M, Czernin J, DeSalles AA, Yong WH, Phelps ME, et al. 18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J Nucl Med 2014; 55: 30–6. doi: 10.2967/jnumed.113.121418 [DOI] [PubMed] [Google Scholar]
- 13.Herrmann K, Czernin J, Cloughesy T, Lai A, Pomykala KL, Benz MR, et al. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol 2014; 16: 603–9. doi: 10.1093/neuonc/not166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarzenberg J, Czernin J, Cloughesy TF, Ellingson BM, Pope WB, Grogan T, et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin Cancer Res 2014; 20: 3550–9. doi: 10.1158/1078-0432.CCR-13-1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicone F, Minniti G, Romano A, Papa A, Scaringi C, Tavanti F, et al. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur J Nucl Med Mol Imaging 2015; 42: 103–11. doi: 10.1007/s00259-014-2886-4 [DOI] [PubMed] [Google Scholar]
- 16.Stegmayr C, Schöneck M, Oliveira D, Willuweit A, Filss C, Galldiks N, et al. Reproducibility of O-(2-(18)F-fluoroethyl)-L-tyrosine uptake kinetics in brain tumors and influence of corticoid therapy: an experimental study in rat gliomas. Eur J Nucl Med Mol Imaging 2016; 43: 1115–23. doi: 10.1007/s00259-015-3274-4 [DOI] [PubMed] [Google Scholar]
- 17.De La Fuente-Fernández R, Lim AS, Sossi V, Adam MJ, Ruth TJ, Calne DB, et al. Age and severity of nigrostriatal damage at onset of Parkinson's disease. Synapse 2003; 47: 152–8. doi: 10.1002/syn.10160 [DOI] [PubMed] [Google Scholar]
- 18.Kumakura Y, Vernaleken I, Gründer G, Bartenstein P, Gjedde A, Cumming P. PET studies of net blood-brain clearance of FDOPA to human brain: age-dependent decline of [18F]fluorodopamine storage capacity. J Cereb Blood Flow Metab 2005; 25: 807–19. doi: 10.1038/sj.jcbfm.9600079 [DOI] [PubMed] [Google Scholar]
- 19.Gallagher CL, Oakes TR, Johnson SC, Chung MK, Holden JE, Bendlin BB, et al. Rate of 6-[18F]fluorodopa uptake decline in striatal subregions in Parkinson's disease. Mov Disord 2011; 26: 614–20. doi: 10.1002/mds.23503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verger A, Stegmayr C, Galldiks N, Van Der Gucht A, Lohmann P, Stoffels G, et al. Evaluation of factors influencing 18F-FET uptake in the brain. Neuroimage Clin 2018; 17: 491–7. doi: 10.1016/j.nicl.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109. doi: 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cicone F, Filss CP, Minniti G, Rossi-Espagnet C, Papa A, Scaringi C, et al. Volumetric assessment of recurrent or progressive gliomas: comparison between F-DOPA PET and perfusion-weighted MRI. Eur J Nucl Med Mol Imaging 2015; 42: 905–15. doi: 10.1007/s00259-015-3018-5 [DOI] [PubMed] [Google Scholar]
- 23.Tahari AK, Chien D, Azadi JR, Wahl RL. Optimum lean body formulation for correction of standardized uptake value in PET imaging. J Nucl Med 2014; 55: 1481–4. doi: 10.2967/jnumed.113.136986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry 2007; 78: 819–24. doi: 10.1136/jnnp.2006.103788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaasinen V, Nurmi E, Brück A, Eskola O, Bergman J, Solin O, et al. Increased frontal [(18)F]fluorodopa uptake in early Parkinson's disease: sex differences in the prefrontal cortex. Brain 2001; 124(Pt 6): 1125–30. doi: 10.1093/brain/124.6.1125 [DOI] [PubMed] [Google Scholar]
- 26.Rosso L, Brock CS, Gallo JM, Saleem A, Price PM, Turkheimer FE, et al. A new model for prediction of drug distribution in tumor and normal tissues: pharmacokinetics of temozolomide in glioma patients. Cancer Res 2009; 69: 120–7. doi: 10.1158/0008-5472.CAN-08-2356 [DOI] [PubMed] [Google Scholar]
- 27.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 2008; 13: 1285–95. doi: 10.1634/theoncologist.2008-0130 [DOI] [PubMed] [Google Scholar]
- 28.Nokia MS, Anderson ML, Shors TJ. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur J Neurosci 2012; 36: 3521–30. doi: 10.1111/ejn.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egeland M, Guinaudie C, Du Preez A, Musaelyan K, Zunszain PA, Fernandes C, et al. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl Psychiatry 2017; 7: e1101. doi: 10.1038/tp.2017.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong TT, Husain M. The role of dopamine in the pathophysiology and treatment of apathy. Prog Brain Res 2016; 229: 389–426. doi: 10.1016/bs.pbr.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 31.EY U, Dienel GA, Cruz NF, Harik SI. beta-adrenergics enhance brain extraction of levodopa. Mov Disord 2002; 17: 54–9. [DOI] [PubMed] [Google Scholar]