Abstract
Objective:
In diffuse Grade II–III gliomas, a high 3,4-dihydroxy-6-(18F)-fluoro-L-phenylalanine (18F-FDOPA) positron emission tomography (PET) uptake, with a standardized uptake value (SUVmax)/contralateral brain tissue ratio greater than 1.8, was previously found to be consistently associated with the presence of an isocitrate dehydrogenase (IDH) mutation, whereas this mutation is typically associated with a better prognosis. This pilot study was aimed to ascertain the prognostic value of this high 18F-FDOPA uptake in diffuse Grade II–III gliomas with regard to the velocity of diameter expansion (VDE), which represents an established landmark of better prognosis when below 4 mm per year.
Methods:
20 patients (42 ± 10 years, 10 female) with newly-diagnosed diffuse Grade II–III gliomas (17 with IDH mutation) were retrospectively included. All had a 18F-FDOPA PET, quantified with SUVmax ratio, along with a serial MRI enabling VDE determination.
Results:
SUVmax ratio was above 1.8 in 5 patients (25%) all of whom had a VDE <4 mm/year (100%) and IDH mutation (100%). Moreover, a SUVmax ratio above 1.8 was associated with higher rates of VDE <4 mm/year in the overall population (45 vs 0%, p = 0.04) and also in the subgroup of patients with IDH mutation (45 vs 0%, p = 0.10).
Conclusion:
This pilot study shows that in diffuse Grade II–III gliomas, a high 18F-FDOPA uptake would be predictive of low tumour growth, with a different prognostic significance than IDH mutation.
Advances in knowledge:
18F-FDOPA PET in a single session imaging could have prognostic value in initial diagnosis of diffuse Grade II–III gliomas.
Introduction
The prognosis of newly-diagnosed Grade II–III gliomas is difficult to determine, according to the classification of the World Health Organization (WHO).1 Nowadays, in clinical routine, most clinicians measure tumour growth by repeating MRI scans.2, 3
For patient monitoring, MRI is recommended to assess tumour volume and its evolution over time (growth kinetics). In this setting, even if experience is limited in heterogeneous patient samples, a velocity of diameter expansion (VDE) of less than 4 mm per year constitutes an established landmark of good prognosis, associated with a significantly longer overall survival and longer malignant progression-free survival.2, 4
PET using radiolabelled amino-acids was recently recommended by the Response Assessment in Neuro-Oncology working group as an additional tool in the diagnostic assessment of brain tumours.5 Among these amino-acid radiotracers, 3,4-dihydroxy-6-(18F)-fluoro-L-phenylalanine (18F-FDOPA) is useful for grading tumours in newly- diagnosed gliomas,6 although its prognostic value remains debated particularly for diffuse Grade II and III gliomas.7–9 In a recent report and related to a subsequent analysis of data, a rather high 18F-FDOPA Positron Emission Tomography (PET) uptake, with a maximal Standardized Uptake Value (SUVmax)/contralateral brain tissue ratio greater than 1.8 in diffuse Grade II–III gliomas has been shown to be consistently associated with the presence of isocitrate dehydrogenase (IDH) mutation,8 a factor of better prognosis according to the new classification of the WHO in 2016.10 However, the prognostic value of such high 18F-FDOPA uptake remains unknown.
In light of the above, the aim of this pilot study was to ascertain the predictive value of this elevated level of 18F-FDOPA PET uptake in histologically proven diffuse Grade II–III gliomas with regard to VDE.
Methods and materials
Patients
20 patients with histologically confirmed newly-diagnosed diffuse Grade II–III gliomas were retrospectively included. Patients had been referred to the Nuclear Medicine Department of the Nancy University Hospital (CHRU, Nancy) for an 18F-FDOPA PET. Serial MRIs were also conducted, with PET performed less than two months after the first MRI (MRI1) and before the second MRI (MRI2). The local ethics committee approved the retrospective evaluation of the data. There was no conflict with the Declaration of Helsinki. All patients from our institution are systematically informed that their medical data can be rendered anonymous and used for scientific purposes.
PET
18F-FDOPA PET examinations were performed on a Biograph 6 system (Siemens®, Erlangen, Germany) after injection of 3 MBq of 18F-FDOPA per kilogram of body weight. Acquisition and reconstruction parameters have already been detailed elsewhere.6
PET images were analysed and quantified by a single experienced observer as detailed previously.6 Briefly, ratios of tumour uptake to normal tissue uptake were generated by dividing Tumour SUVmax-derived indices with Normal contralateral brain tissue uptake (SUVmax T/N).
MRI
Two MRIs were performed in each patient on a GE Healthcare 1.5 or 3T magnet. All MRI examinations were analysed in this study by a single experienced radiologist in two blinded sessions to assess reproducibility in size assessment. Mean tumour diameters were determined on fluid attenuation inversion recovery (FLAIR) weighted MR images as recommended,2 after semi-automated tumour volume contouring (ADW, GE®). VDE was calculated as the difference in mean tumour diameter measurement between the two MRI examinations divided by the interval-time between the two examinations. A VDE of less than 4 mm per year was considered to be indicative of a better prognosis.2
Histological and molecular analysis
All cases were reviewed and classified according to the 2016 “WHO Classification of Tumours of the Central Nervous System”. Pathological confirmation was conducted by resection (n = 15) or biopsy (n = 5). IDH mutation status was assessed by immunohistochemistry with IDH1 R132H protein expression (Dianova, clone H09), or Sanger sequencing in case of ATRX loss without IDH1 R132H staining. For oligodendroglial morphology, tumours were tested for 1p 19q codeletion using multiplex PCR analysis (loss of heterozygosity), or comparative genomic hybridization.
Statistical analysis
Quantitative variables are expressed as means ± standard deviations, and categorical variables as percentages. Two-group unpaired comparisons were performed with Mann-Whitney tests for quantitative variables and with Fischer exact tests for categorical variables. A p-value < 0.05 was determined as significant. All analyses were performed with SPSS® 20.0 software.
Results
The final study population consisted of 10 males and 10 females with a mean age of 41.8 ± 10.3 years (24–62 years). According to the WHO 2016 classification, the 20 gliomas involved 3 oligodendrogliomas, IDH-mutant and 1p 19q codeleted; 1 anaplastic oligodendroglioma, IDH-mutant and 1p 19q codeleted; 8 diffuse astrocytomas, IDH-mutant; 2 diffuse astrocytomas, IDH-wild type; 5 anaplastic astrocytomas, IDH-mutant; and 1 anaplastic astrocytoma, IDH-wildtype. On average, the mean interval-time separating the first MRI from the 18F-FDOPA PET was 40.9 ± 20.9 days and between the two MRI examinations was 82 ± 29 days with a minimum of 40 days. Mean delay between 18F-FDOPA PET and histological diagnosis was 99.2 days ± 67.3.
A low growth rate, with a VDE <4 mm per year, was documented in 11 of the 20 patients (55%) and an IDH mutation documented in 17 (85%). Patients with VDE <4 mm showed a trend toward higher rates of IDH mutations compared to those with VDE ≥4 mm (p = 0.07, Table 1).
Table 1.
Patient and tumour characteristics
| Velocity of diameter expansion <4 mm/year n = 11 (55%) | Velocity of diameter expansion ≥4 mm/year n = 9 (45%) | p | |
| Age (years) | 42.4 ± 11.4 | 41.1 ± 10.3 | 0.80 |
| Female gender | 4 (36%) | 6 (67%) | 0.37 |
| Time between MRI 1 and PET (days) | 41.0 ± 23.2 | 40.8 ± 18.9 | 0.98 |
| Time between PET and histology (days) | 106.1 ± 77.6 | 90.7 ± 55.5 | 0.61 |
| Time between MRI 1 and MRI 2 (days) | 81.9 ± 24.5 | 82.4 ± 36.3 | 0.97 |
| WHO 2016 Classification | |||
| Diffuse astrocytoma, IDH-mutant | 6 (55%) | 2 (22%) | 0.20 |
| Anaplastic astrocytoma, IDH-mutant | 3 (27%) | 2 (22%) | 1 |
| Diffuse astrocytoma, IDH-wildtype | 0 | 2 (22%) | 0.19 |
| Anaplastic astrocytoma, IDH-wildtype | 0 | 1 (11%) | 0.45 |
| Oligodendroglioma, IDH-mutant and 1p 19q codeleted | 2 (18%) | 1 (11%) | 1 |
| Anaplastic oligodendroglioma, IDH-mutant and 1p 19q codeleted | 0 | 1 (11%) | 0.45 |
| IDH mutation | 11 (100%) | 6 (66%) | 0.07 |
| Anaplastic | 3 (27%) | 4 (36%) | 0.64 |
| SUVmax T/N | 1.6 ± 0.9 | 1.0 ± 0.3 | 0.06 |
| SUVmax T/N > 1.8 | 5 (45%) | 0 (0%) | 0.04 |
IDH mutation,isocitrate deshydrogenase mutation; PET, positron emission tomography; SUV, standardized uptake value; T/N, tumour/contralateral normal brain tissue uptake; WHO, World Health Organization.
SUVmax ratio was above 1.8 in 5 patients (25%) all of whom had a VDE <4 mm/year (100%) and IDH mutation (100%). In contrast, patients with a SUVmax ratio below 1.8 had a VDE <4 mm/year in 40% of cases (6/15) and IDH mutation in 80% of cases (12/15). Examples of PET images of gliomas with SUVmax T/N > 1.8 and < 1.8 are shown in Figures 1–3.
Figure 1.
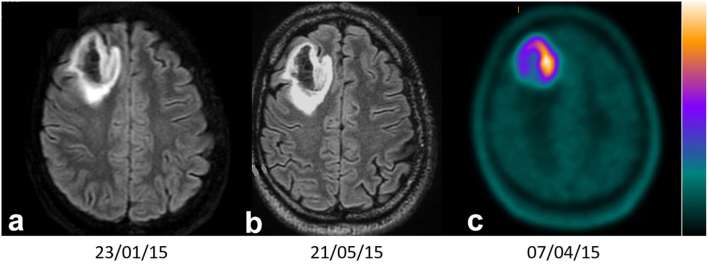
Axial FLAIR MRI1 (a), MRI2 (b) and PET 18F-FDOPA (c) imaging of a diffuse IDH-mutant (WHO 2016) astrocytoma, with a SUVmax T/N (Tumour/contralateral Normal brain tissue uptake) ratio = 5.8 (>1.8) and velocity of diameter expansion between MRI1 and MRI2 of 1.2 mm (<4 mm per year). F-FDOPA, 3,4-dihydroxy-6-(18F)-fluoro-L-phenylalanine; FLAIR, fluid attenuation inversion recovery; PET, positron emission tomography; SUV, standardized uptake value.
Figure 2.
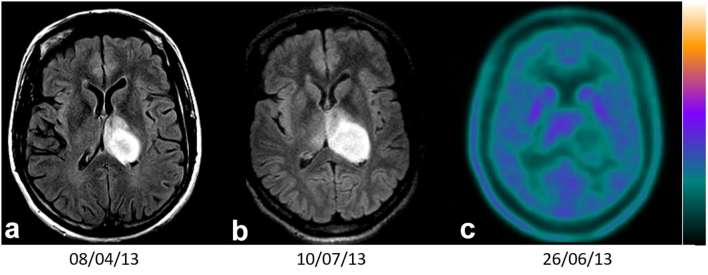
Axial FLAIR MRI1 (a), MRI2 (b) and PET 18F-FDOPA (c) imaging of a diffuse IDH wild type (WHO 2016) astrocytoma, with a SUVmax T/N (Tumour/contralateral Normal brain tissue uptake) ratio = 1.1 (<1.8) and velocity of diameter expansion between MRI1 and MRI2 of 7.7 mm (>4 mm per year). F-FDOPA, 3,4-dihydroxy-6-(18F)-fluoro-L-phenylalanine; FLAIR, fluid attenuation inversion recovery; IDH, isocitrate deshydrogenase; PET, positron emission tomography; SUV, standardized uptake value; WHO, World Health Organization.
Figure 3.
Axial FLAIR MRI1 (a), MRI2 (b) and PET 18F-FDOPA (c) imaging of an anaplastic IDH-mutant (WHO 2016) astrocytoma, with a SUVmax T/N (tumour/contralateral normal brain tissue uptake) ratio = 1.7 (<1.8) and velocity of diameter expansion between MRI1 and MRI2 of 21 mm (>4 mm per year).
As detailed in Table 1, the rates of high SUVmax ratio (>1.8) were significantly higher in patients with a VDE <4 mm comparatively to those with a VDE ≥4 mm (45 vs 0%, p = 0.04) as well as a trend toward higher SUVmax ratio, analysed as a continuous variable, in patients with a VDE <4 mm than in those with VDE ≥4 mm (1.6 ± 0.9 vs 1.0 ± 0.3, p = 0.06). The remaining demographic and histological variables were not significantly different between the two groups (Table 1).
Of note, when the analysis was restricted to the vast majority of patients showing an IDH mutation (n = 17), the rates of high SUVmax ratio (>1.8) remained higher in those with a VDE <4 mm than in those with a VDE ≥4 mm although this relationship was slightly above the level of statistical significance (45 vs 0%, p = 0.10).
Discussion
In diffuse Grade II–III gliomas, a high 18F-FDOPA uptake, with a SUVmax ratio above 1.8, was previously found to be consistently associated with the presence of an IDH mutation. The present pilot study shows that this criterion is additionally predictive of a low tumour growth (<4 mm per year) and thus, presumably of a better prognosis.
The malignant progression of diffuse Grade II/III gliomas is variable and difficult to predict non-invasively given that these gliomas represent a very heterogeneous population. As evidenced by current MRI monitoring, these tumours grow inexorably although at an unpredictable rate. Pallud and al. reported a median interval of 21 months between the radiological discovery and oncological treatment of low-grade gliomas.2 An early non-invasive identification of prognostic factors would hence be useful in tailoring therapeutic strategy. Advanced MRI methods (perfusion, diffusion and spectroscopy) have been proposed to enhance prognostic information in this setting. However, PET imaging, especially with 18F-FDOPA, was recently found to be somewhat more efficient than the aforementioned advanced MRI techniques for glioma grading and prognosis.11, 12 Moreover, these studies have confirmed that information provided by MRI and 18F-FDOPA PET clearly differ.
As an illustration of this difference, gliomas with a high growth rate (VDE ≥4 mm) had a lower 18F-FDOPA uptake in the current study. This observation is in agreement with the previous observation that 18F-FDOPA uptake was higher in gliomas harbouring an IDH mutation, whereas this mutation is known to confer a better prognosis.8 Finally, these results strengthen the hypothesis that a rather high 18F-FDOPA uptake may confer a better prognosis in this setting. Possible explanation to this higher 18F-FDOPA uptake could be the specific accumulation of 2-hydroxyglutarate in IDH mutated gliomas, which may act as an oncometabolite with alternative molecular pathways.13 On the other hand, IDH mutation status is also associated with an elevation of intracellular free amino-acids including tyrosine,14 which could potentially facilitate the uptake of 18F-FDOPA via amino-acid transporters which act as exchangers. Another potential explanation is that 18F-FDOPA uptake in IDH-mutated gliomas could also be influenced by an impairment in dopamine catabolism due to a mitochondrial dysfunction.15
It should also be pointed out that, when the analysis was restricted to patients with IDH mutation, the rate of SUVmax ratio >1.8 remained higher in patients with low growth tumour rate, even if this relationship was only at the limit of the statistical significance, presumably due to the limited sample size. This suggests that this SUVmax ratio provides a very different prognostic information than that provided by IDH mutation, as illustrated by the example in Figure 3.
In more practical terms, our results suggest that the criterion of a SUVmax ratio >1.8, in case of suspicion of low-grade glioma on standard MRI, may allow identifying a subgroup of patients with a low growth rate, independently of IDH status. For this subgroup of patients, the treatment may be delayed allowing time to tailor their management as opposed to patients for whom treatment (such as surgical removal) should not be delayed.
Interesting, the oligodendroglial component didn’t influence nor MRI VDE nor 18F-FDOPA PET uptake since only 1 oligodendroglioma exhibited a SUVmax T/N > 1.8. Indeed, this component may positively affect the substrate metabolism of gliomas for certain amino-acid uptake.16
The most important limitations of the present study are its limited sample size and its retrospective and single-centre nature. Further larger-scale studies are thus required, especially for the subgroups of patients with IDH-mutant 1p/19q co-deleted oligodendrogliomas or wild-type IDH astrocytomas, in order to confirm the low growth rate as well as the good prognosis of patients showing a SUVmax ratio >1.8. Secondly, the average time between MRI 1 and MRI 2 (82 days ± 29) could be sometimes short to define precisely tumour growth rate. However, a minimum delay of 40 days was observed between the two investigations.2 Finally, the growth rate of tumour was chosen as a surrogate for clinical course in this study since no overall survival could be obtained in our population owing to the long clinical course duration of diffuse low-grade gliomas.
Overall, the present pilot study shows that in histologically proven diffuse Grade II–III gliomas, a high 18F-FDOPA uptake, with a SUVmax ratio above 1.8, would be predictive not only of an IDH mutation but also of a low tumour growth and thus, presumably of a better prognosis. This might be helpful to optimize the individualized therapeutic approach for these patients.
ACKNOWLEDGMENTS
The authors thank Pierre Pothier for his critical review of the manuscript.
Compliance with ethical standards
There was no conflict with the Declaration of Helsinki. The research procedures were approved by the institutional committee on human experimentation, and Informed consent was obtained for human subjects.
Contributor Information
Sibel Isal, Email: sibelisal@hotmail.com.
Guillaume Gauchotte, Email: g.gauchotte@chru-nancy.fr.
Fabien Rech, Email: f.rech@chru-nancy.fr.
Marie Blonski, Email: m.blonski@chru-nancy.fr.
Sophie Planel, Email: s.planel@chru-nancy.fr.
Mohammad B Chawki, Email: momodestains@yahoo.fr.
Gilles Karcher, Email: g.karcher@chru-nancy.fr.
Pierre-Yves Marie, Email: pierre.y.marie@gmail.com.
Luc Taillandier, Email: l.taillandier@chru-nancy.fr.
Antoine Verger, Email: a.verger@chru-nancy.fr.
REFERENCES
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109. doi: 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pallud J, Blonski M, Mandonnet E, Audureau E, Fontaine D, Sanai N, et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol 2013; 15: 595–606. doi: 10.1093/neuonc/nos331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffau H, Taillandier L. New concepts in the management of diffuse low-grade glioma: Proposal of a multistage and individualized therapeutic approach. Neuro Oncol 2015; 17: 332–42. doi: 10.1093/neuonc/nou153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandonnet E, Wait S, Choi L, Teo C. The importance of measuring the velocity of diameter expansion on MRI in upfront management of suspected WHO grade II glioma - case report. Neurochirurgie 2013; 59: 89–92. doi: 10.1016/j.neuchi.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 5.Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response assessment in neuro-oncology working group and European association for neuro-oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol 2016; 18: 1199–208. doi: 10.1093/neuonc/now058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janvier L, Olivier P, Blonski M, Morel O, Vignaud JM, Karcher G, et al. Correlation of SUV-derived indices with tumoral aggressiveness of gliomas in static 18F-FDOPA PET: use in clinical practice. Clin Nucl Med 2015; 40: e429–e435. doi: 10.1097/RLU.0000000000000897 [DOI] [PubMed] [Google Scholar]
- 7.Villani V, Carapella CM, Chiaravalloti A, Terrenato I, Piludu F, Vidiri A, et al. The role of PET [18F]FDOPA in evaluating low-grade glioma. Anticancer Res 2015; 35: 5117–22. [PubMed] [Google Scholar]
- 8.Verger A, Metellus P, Sala Q, Colin C, Bialecki E, Taieb D, et al. IDH mutation is paradoxically associated with higher 18F-FDOPA PET uptake in diffuse grade II and grade III gliomas. Eur J Nucl Med Mol Imaging 2017; 44: 1306–11. doi: 10.1007/s00259-017-3668-6 [DOI] [PubMed] [Google Scholar]
- 9.Verger A, Taieb D, Guedj E. Is the information provided by amino acid PET radiopharmaceuticals clinically equivalent in gliomas? Eur J Nucl Med Mol Imaging 2017; 44: 1408–10. doi: 10.1007/s00259-017-3710-8 [DOI] [PubMed] [Google Scholar]
- 10.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131: 803–20. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 11.Rossi Espagnet MC, Romano A, Mancuso V, Cicone F, Napolitano A, Scaringi C, et al. Multiparametric evaluation of low grade gliomas at follow-up: comparison between diffusion and perfusion MR with (18)F-FDOPA PET. Br J Radiol 2016; 89: 20160476. doi: 10.1259/bjr.20160476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bund C, Heimburger C, Imperiale A, Lhermitte B, Chenard MP, Lefebvre F, et al. FDOPA PET-CT of nonenhancing brain tumors. Clin Nucl Med 2017; 42: 250–7. doi: 10.1097/RLU.0000000000001540 [DOI] [PubMed] [Google Scholar]
- 13.Metellus P, Colin C, Taieb D, Guedj E, Nanni-Metellus I, de Paula AM, et al. IDH mutation status impact on in vivo hypoxia biomarkers expression: new insights from a clinical, nuclear imaging and immunohistochemical study in 33 glioma patients. J Neurooncol 2011; 105: 591–600. doi: 10.1007/s11060-011-0625-2 [DOI] [PubMed] [Google Scholar]
- 14.Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, et al. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc Natl Acad Sci U S A 2011; 108: 3270–5. doi: 10.1073/pnas.1019393108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oizel K, Gratas C, Nadaradjane A, Oliver L, Vallette FM, Pecqueur C. D-2-Hydroxyglutarate does not mimic all the IDH mutation effects, in particular the reduced etoposide-triggered apoptosis mediated by an alteration in mitochondrial NADH. Cell Death Dis 2015; 6: e1704. doi: 10.1038/cddis.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manabe O, Hattori N, Yamaguchi S, Hirata K, Kobayashi K, Terasaka S, et al. Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging. Eur J Nucl Med Mol Imaging 2015; 42: 896–904. doi: 10.1007/s00259-015-2996-7 [DOI] [PubMed] [Google Scholar]