Abstract
Objective:
This pilot study determined if the ultrasound texture feature “contrast” was associated with cardiovascular disease (CVD) risk factors and subclinical arterial disease.
Methods:
We evaluated ultrasound images of the right common carotid artery (CCA) from a convenience sample of 151 participants and examined relationships between contrast, CVD risk factors, carotid intima-media thickness (IMT) and coronary artery calcium (CAC). Grey level difference statistics algorithms were used to evaluate the texture feature “contrast” from carotid ultrasound images. Right CCA IMT measurements were made in triplicate in the distal 1 cm segment of the far wall of the artery and CAC score was measured using the Agatston scoring method.
Results:
In individual models that included age, sex and race, grey level difference statistics contrast (outcome) was associated independently with age [beta (standard error) = –0.87 (0.38) per year; p = 0.02], C-reactive protein [–2.22 (0.96) per mg dl–1; p = 0.02], high-density lipoprotein cholesterol [0.61 (0.24) per mg dl–1; p = 0.01] and CCA IMT [–0.06 (0.02) microns; p = 0.001]. Other CVD risk factors and CAC were not associated independently with contrast.
Conclusion:
These findings support the potential use of the ultrasound texture contrast for evaluating arterial injury and CVD risk.
Advances in knowledge:
This paper contributes to the literature in that it describes how the greyscale texture feature “contrast” is related to CVD risk factors.
INTRODUCTION
Image processing is used to characterize tissues and detect subtle differences associated with preclinical disease states.1 In ultrasound, image processing methods of texture analysis can characterize the arterial wall.2–4 Greyscale median (GSM) is the most frequently used greyscale parameter to describe the overall echodensity of the arterial wall and lower GSM values (darker walls) are associated with greater cardiovascular disease (CVD) risk and risk factors.5–7 GSM describes the echodensity of the arterial wall within a selected region of interest. However, it does not provide information about the distribution and spatial relationships of the greyscale values of the pixels,2, 4,8,9 which might be a better descriptor of arterial wall changes associated with early atherosclerosis.2, 3
The grey level difference statistics (GLDS) method overcomes this limitation by studying the distributions of grey levels and the spatial relationships of ultrasound pixels to each other.3,8,10–13 GLDS contrast also provides information regarding tissue heterogeneity.10, 11 In cardiac ultrasound, GLDS contrast (an ultrasound texture feature that describes spatial differences in greyscale brightness among image pixels)8 has shown differences between normal and myopathic myocardium in humans and myocardial contusion in animal models.10, 11 GLDS methods also have been used in vascular ultrasound imaging to assess plaque and arterial wall characteristics and to relate them to cerebrovascular symptoms and histopathology findings.3, 9,13,14 Plaque with low GSM and texture measures of homogeneity has been associated with features of plaque instability at histopathology examination.9 In unstable plaques, lipids and/or inflammatory cells may replace normal fibrous tissue, resulting in fewer specular reflectors on ultrasound imaging and the appearance of a more homogeneous plaque.9
The purpose of this study was to determine if the ultrasound texture feature “contrast” was associated with CVD risk factors and subclinical arterial disease. We hypothesized that this novel texture measure of contrast may represent one of the earliest ultrasound changes of the arterial wall, prior to wall thickening and plaque formation.
Methods and materials
Participants
This is a pilot study of images from 151 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) from 2000 to 2002. MESA is a large, prospective cohort study that investigated the prevalence and risk factors associated with subclinical CVD and its progression (Bild et al 2002).15 We chose a convenience sample—the first 151 readable images—from participants initially selected to be part of a case–cohort study to determine the feasibility of evaluating multiple features of carotid arterial stiffness and greyscale features.
At the time of recruitment, male and female participants were 45 to 84 years old and free of clinically known CVD.15–17 This specific study sample was obtained from four out of six MESA field centres (Baltimore City and Baltimore County, MD; Chicago, IL; Los Angeles County, CA; Northern Manhattan and the Bronx, NY) that used the same carotid ultrasound preset and greyscale map (as described below and in Table 1). All subjects provided written informed consent at their respective field centres. The MESA study objectives and design have been previously published.15 This study was approved by all participating field centres and the University of Wisconsin Institutional Review Boards.
Table 1.
MESA carotid ultrasound instrumentation settings
| Ultrasound system | Logiq 700 ultrasound system (General Electric Medical Systems, Waukesha, WI) |
| Transducer | M12L Linear transducer |
| Transducer frequency | 13 MHz |
| Overall gain setting | Optimized for each patient |
| Time gain compensation settings | Optimized for each patient |
| Dynamic range) | 66 |
| Edge enhance | E3 |
| Average settings | A2 |
| Greyscale map | MG |
Risk factor measurements
Laboratory, medical history and demographic data were obtained during MESA (July 2000–August 2002). MESA participants answered a standardized questionnaire in which data regarding age, sex, race/ethnicity, medication history and cigarette smoking status were collected. High-sensitivity C reactive protein (CRP), interleukin-6, glucose and total and high-density lipoprotein cholesterol (HDL-C) levels were measured from blood samples acquired after a 12-h fast and measured at a centralized laboratory.16–18 Impaired fasting glucose was defined as a fasting glucose level of 100–125 mg dl–116and diabetes mellitus was defined as a blood glucose level >126 mg dl–1 or use of an antidiabetes medication.17, 18
Blood pressure was measured in triplicate in the right arm using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon; Tampa, FL). All measurements were made with the participant seated in a quiet room for 5 minutes prior to blood pressure measurement and the average of the last two measurements were used for analysis.15, 17,18 Hypertension was defined as a systolic blood pressure greater than 140 mmHg, diastolic blood pressure greater than 90 mmHg or treatment with antihypertensive medication.17, 18 Cigarette smoking status was categorized as current, former or never.17, 18 Coronary artery calcium (CAC) score was measured using the Agatston scoring method and reported as a continuous variable.19, 20 CT scanning and reading methods for MESA have been previously reported.19
Carotid ultrasound imaging intima-media thickness measurements
High-resolution B-mode images of the right and left common carotid arteries (CCAs) were obtained from 2000–2002.15, 21 A Logiq 700 ultrasound system and an M12L linear transducer (General Electric Medical Systems, Waukesha, WI) were used to acquire all ultrasound images and record on videotape. Images were digitized from the video tape using the Medical Digital Recording (MDR) device (PACSGEAR, Pleasanton, CA). Once digitized, images were converted to DICOM digital records16, 21 (Table 1 for ultrasound instrumentation settings).
For intima-media thickness (IMT), digital images were imported into the Syngo Ultrasound Workplace reading stations and then measured using the Arterial Health Package software (Siemens Medical, Malvern, PA).21 Right CCA IMT measurements were made in triplicate in the distal 1 cm segment of the far wall of the artery. IMT was defined as the mean of the mean right far wall distal CCA thickness.21 If plaque was present in the distal segment of the CCA, it was included in the CCA IMT measurement.21 All IMT measurements were performed at the University of Wisconsin Atherosclerosis Imaging Research Program Laboratory (UW AIRP), MESA Carotid Ultrasound Reading Center (Madison, WI, PI: J. Stein).
Greyscale median and contrast measurement
Image viewing software (Access Point, Freeland Systems, Alpharetta, GA) was used to convert the carotid ultrasound DICOM files into BITMAP images that were used for greyscale analysis and measurement of GSM and GLDS contrast. Images were normalized to assign the blackest area of the blood a greyscale value of 0 and the whitest area of the middle two-fourths of the adventitia a greyscale value of 190 (LifeQ Medical, Cyprus).22, 23 Images were then standardized to a uniform pixel density of 20/mm.22, 23 The distal 1.0 cm of the far wall of the right CCA was traced utilizing an online ruler tool to define the length of 1.00 cm. GSM of the CCA intima-media complex was calculated by taking the median grey level value within the traced region of interest in the arterial wall using plaque texture analysis software (LifeQ Medical, Cyprus).
GLDS contrast was measured from the same region of interest as the GSM. GLDS are computed by calculating the absolute difference in greyscale values between pixels at a given distance and direction and can be used to describe pixel–greyscale relationships.1,3,10–12,24–28 Images that have low GLDS contrast will have more pixels with the same greyscale value and images that have high GLDS contrast will have large differences in greyscale values between pixels (Figure 1). The LifeQ GLDS algorithm uses the probability density function pδ (i) to express the likelihood that two image pixels, separated by a distance δ = (Δχ, Δy), will have an absolute difference in greyscale value, i27, 29 (Supplementary Material 1, Supplementary material available online). For this study, we extracted the texture feature GLDS contrast using the LifeQ software [Equation (1)].27, 29
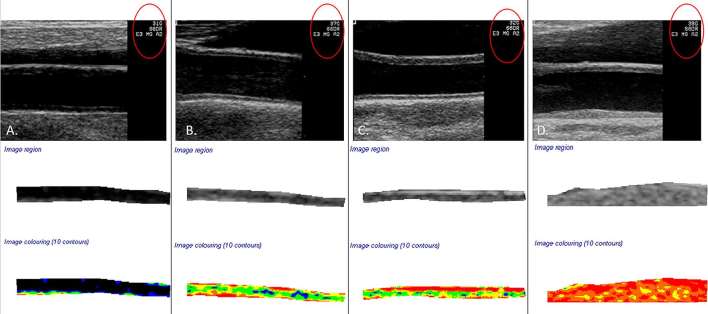
Figure 1.
(a) Demonstrates a normalized and standardized image of a common carotid artery with a far wall greyscale median (GSM) value of 8.1 and a grey level contrast value of 53.6, gain setting 31 (red circle). (b) Demonstrates an artery with a GSM value of 79.5 and contrast value of 121.6 (representative of the mean grey level difference statistics contrast value for this study), gain setting 37 (red circle). (c) Demonstrates an artery with a GSM value of 94.6 and a grey level difference statistics contrast value of 299.3, gain setting 32 (red circle). (d) Represents a GSM value of 120.9, a contrast of 68.7 and a gain setting of 38.
where CON is contrast, i represents the difference in greyscale value between two pixels and pδ(i) represents the individual probabilities.
Statistical analysis
Continuous variables are reported as mean (standard deviation) and categorical variables as counts and percentages. CAC Agatston values were log transformed after addition of 1 to account for zeros and skewed distributions. Pearson correlations and multivariable linear regression models (adjusted for age, sex and race/ethnicity) were used to examine relationships between GLDS contrast (outcome variable) and CVD risk factors [age, body mass index (BMI), total cholesterol, HDL-C, low-density lipoprotein cholesterol, triglycerides, hypertension, diabetes mellitus, cigarette smoking, glomerular filtration rate, CRP, interleukin-6, D-dimer, fibrinogen, alcohol consumption, education level, physical activity level, statin use], carotidIMT, CAC and 10-year American College of Cardiology/American Heart Association (ACC/AHA) risk score for CVD.30 Models with lipids and CRP included additional adjustment for statin use. All analyses were conducted using Stata/IC version 14.0 (College Station, Texas).
Results
Participants (Table 2)
Table 2.
Participant characteristics
| Variable | N (%) OR Mean (SD) |
| Age, years | 68.1 (9.2) |
| Female, n (%) | 82 (54.3) |
| Race/ethnicity | |
| White | 47 (31.1) |
| Chinese | 15 (9.9) |
| Black | 42 (27.8) |
| Hispanic | 47 (31.1) |
| Smoking status | |
| Never | 77 (51.0) |
| Former | 57 (37.7) |
| Current | 17 (11.3) |
| Body mass index (kg m–2) | 27.5 (4.8) |
| Total cholesterol (mg dl–1) | 199.5 (41.8) |
| High-density lipoprotein cholesterol (mg dl–1) | 53.7 (16.5) |
| Low-density lipoprotein cholesterol (mg dl–1) | 121.4 (36.9) |
| Triglycerides (mg dl–1) | 128.7 (82.2) |
| Estimated glomerular filtration rate (ml min–1/1.73 m2) | 76.8 (18.5) |
| Intentional exercise (MET-hours/week) | 24.8 (34.4) |
| C-reactive protein (mg l–1) | 3.52 (3.7) |
| Interleukin-6 (pg ml–1) | 1.76 (1.1) |
| D-dimer (µg l–1) | 0.44 (0.55) |
| Fibrinogen (mg dl–1) | 362.5 (73.8) |
| American College of Cardiology/American Heart Association Cardiovascular Risk Score (%) | 20.4 (16.5) |
| CAC Score [ln (Agatston + 1)] | 3.48 (2.62) |
| Common carotid artery intima-media thickness (microns) | 866.1 (210.4) |
| GLDS contrast (unitless) | 121.3 (42.6) |
| GSM (unitless) | 56.8 (22.4) |
CAC, coronary artery calcium; GLDS, grey level difference statistics; GSM, greyscale median; SD,standard deviation.
The 151 participants were of mean (standard deviation) age 68 (9) years (54% female; 31% Hispanic, 28% Black, 10% Chinese, and 31% White).
Greyscale median values
GSM was weakly correlated inversely with BMI (r = –0.18; p < 0.05) and high school education (–0.18; p < 0.05). GSM was also associated with race; Chinese and Hispanic participants had higher GSM values on average (p < 0.05). No significant correlations were noted between GSM and other CVD risk factors or with CCA IMT, CAC or CVD risk score (p values all > 0.05). In models that included age, sex and race, GSM was associated independently with BMI [beta (standard error) = –0.82 (0.38) kg m–2; p = 0.03]. Other risk factors were not significantly associated with GSM nor were IMT, CAC or CVD risk score (p-values all >0.05)
Grey level contrast (Table 3)
Table 3.
Age, sex and race-adjusted models for right common carotid artery GLDS contrasta
| Covariate | Beta coefficient | Standard error | p value | 95% confidence interval |
| Age | –0.87 | 0.38 | 0.02 | –1.62,–0.12 |
| Body mass index (kg m–2) | –0.02 | 0.73 | 0.98 | –1.50, 1.47 |
| Total cholesterol (mg dl–1) | 0.03 | 0.09 | 0.74 | –0.15, 0.20 |
| High-density cholesterol (mg dl–1) | 0.61 | 0.24 | 0.01 | 0.13, 1.09 |
| Low-density cholesterol (mg dl–1) | –0.003 | 0.099 | 0.98 | –0.19, 0.20 |
| Triglycerides (mg dl–1) | –0.003 | 0.05 | 0.96 | –0.10, 0.09 |
| Estimated glomerular filtration rate (ml min–1/1.73 m2) | –0.30 | 0.20 | 0.13 | –0.69, 0.09 |
| Intentional exercise (MET-hours/week) | 0.16 | 0.10 | 0.12 | –0.04, 0.36 |
| C-reactive protein (mg l–1) | –2.22 | 0.96 | 0.02 | –4.11,–0.33 |
| Interleukin-6 (pg ml–1) | 1.29 | 3.26 | 0.69 | –5.15, 7.72 |
| D-Dimer (ug ml–1) | 4.70 | 6.63 | 0.48 | –8.40, 17.81 |
| Fibrinogen (mg dl–1) | 0.01 | 0.05 | 0.78 | –0.08, 0.11 |
| Smoking status | ||||
| Former | 4.80 | 8.13 | 0.56 | –11.27, 20.86 |
| Current | –11.04 | 11.62 | 0.34 | –34.00, 11.93 |
| Alcohol use | ||||
| Yes | 3.96 | 8.75 | 0.65 | –13.34, 21.30 |
| Diabetes mellitus | ||||
| Yes | –1.01 | 9.35 | 0.91 | –19.50, 17.47 |
| Statin use | ||||
| Yes | 9.17 | 8.64 | 0.29 | –7.91, 26.25 |
| Hypertension | ||||
| Yes | –9.77 | 7.23 | 0.18 | –24.05, 4.52 |
| Common carotid artery IMT | –0.06 | 0.02 | 0.001 | –0.09,–0.02 |
| CAC: Ln(Agatston + 1) | –1.19 | 1.6 | 0.46 | –4.34, 1.97 |
| American College of Cardiology/American Heart Association Cardiovascular Risk Score (%) | 0.06 | 0.29 | 0.84 | –0.52, 0.64 |
CAC, coronary artery calcium; GLDS, grey level difference statistics; IMT, intima-media thickness; MET, metabolic equivalent task.
aAll data are from models adjusted for age, sex and race with addition of the parameter in each row above. Models for total cholesterol, HDL, LDL, triglycerides and CRP are additionally adjusted for use of statins.
GLDS contrast correlated inversely with CCA IMT (r = –0.33; p < 0.05) and CRP (–0.18; p < 0.05) and positively with HDL-C (r = 0.23; p < 0.05). No significant correlations were noted between GLDS contrast and other CVD risk factors, CAC or American College of Cardiology/American Heart Association risk score (p values all > 0.05). In individual models that included age, sex and race, GLDS contrast (outcome) was associated independently with age [beta (standard error) = –0.87 (0.38) per year; p = 0.02], CRP [–2.22 (0.96) per mg dl–1; p = 0.02], HDL-C [0.61 (0.24) per mg dl–1; p = 0.01] and CCA IMT [–0.06 (0.02) microns; p = 0.001]. (Table 3). Other CVD risk factors and CAC were not associated independently with GLDS contrast (p-values all >0.05).
Discussion
In this study of individuals without known CVD, we demonstrated that low GSM was associated with greater BMI but no other CVD risk factors. However, lower greyscale texture contrast was associated with greater age, lower HDL-C, higher CRP and higher carotid IMT, observations that suggest that it may reflect a different aspect of arterial injury than GSM and may be useful for evaluating arterial injury and CVD risk.
Greyscale characteristics of the arterial wall may represent some of the earliest in vivo changes associated with arterial injury.2, 4 To date, the most frequently used greyscale ultrasound parameter used to study the arterial wall for assessment of CVD risk is the GSM value.4, 6,7,31 This is a simple measure that provides the overall median value of a segment of the arterial wall, but does not take into account the distribution and differences of grey levels within the wall.8 Low GSM (i.e. hypoechoic/echolucent/darker) arterial walls have been associated with markers of inflammation, dyslipidemia and increased CVD risk.5–7,32 Indeed, in our small study, lower GSM was associated with greater BMI.
Early changes in atherogenesis are associated with changes in the echo texture of the arterial wall that may occur prior to thickening detected by ultrasound.2, 4 We evaluated the GLDS texture feature, contrast, because we hypothesized that it might describe early greyscale changes associated with lipid infiltration and inflammation of the arterial wall that might not be detected by GSM. On ultrasound, fat appears hypoechoic and will have a lower GSM value than fibrous tissue or calcium.33 Therefore, as lipids infiltrate the arterial wall, it would be expected that the wall will become more hypoechoic with less contrast due to less difference in greyscale values between pixels. Indeed, plaques with increased echolucency (lower GSM) and that were more homogeneous were associated with higher histopathological plaque classification scores, suggesting that increasing lipid content may be associated with increasing homogeneity.9 Increasing homogeneity in a plaque represents instability based on an increased amount of lipid compared to echogenic fibrous structures.9 If a similar process occurs in plaque-free areas of the arterial wall, it may indicate that as lipid infiltrates the wall, it becomes more hypoechoic with less contrast as lipid replaces more echogenic fibrous structures.
Inflammation associated with arteritis has also been noted to have a hypoechoic appearance on ultrasound imaging,34 possibly due to oedema.35, 36 With treatment, the walls decrease in thickness and become more hyperechoic.35, 36 Homogeneous plaques with low GSM are associated with increased lipid content and additional features of plaque instability such as inflammation and less fibrous tissue.9 Our findings of low contrast being associated with higher values of CRP may represent inflammation in the arterial wall, similar to the findings of increased inflammation seen with plaques, in which plaques that were more hypoechoic and homogeneous were associated with larger lipid cores and higher scores of inflammation at histopathology examination.9
Low ultrasound texture contrast does not necessarily represent low GSM: Arterial walls may be bright or dark but have low contrast. Risk factors associated with GSM and IMT differ when measured in the same segment.32 We further demonstrated different patterns of CVD risk factor relationships between GSM and GLDS contrast, including a different relationship with IMT, suggesting that these ultrasound parameters measure different structural aspects of the arterial wall and that heterogeneity in specular reflectors vary with risk factors and wall thickening. As changes in the arterial wall structure occur with early injury, we hypothesize that texture contrast may change first, followed by overall echogenicity changes, such as GSM values, and then wall thickening and plaque formation.
Limitations
Imaging was performed with an ultrasound system that no longer is state of the art. At the time of acquisition, sonographers were allowed to optimize the overall gain and time gain compensation for each subject. This may have introduced variability into our data; however, such variability would be expected to null bias our findings. Our data are from a pilot study using a convenience sample from a large, prospective longitudinal study. These measures need to be evaluated in a larger sample with standardized time gain compensation and gain settings in order to better understand their relationships with CVD risk factors and markers of subclinical atherosclerosis. A longitudinal analysis may better describe associations with risk for CVD events. Serial evaluations may better describe the time course of arterial wall changes.
Conclusions
GLDS contrast is a novel greyscale ultrasound texture feature of the carotid arterial wall that has different CVD risk factor associations than GSM. Lower contrast is associated with increasing age, lower HDL-C, higher CRP and higher carotid IMT, supporting its potential use for evaluating arterial injury and CVD risk.
Footnotes
Conflicts of Interest: Carol C Mitchell, PhD: Davies Publishing Inc., authorship for two echocardiography textbooks, one published, one under review, may have future royalties. Elsevier, Wolters Kluwer, author textbook chapters, may have future royalties. James H Stein, MD: Wisconsin Alumni Research Foundation-patent related to carotid wall thickness and vascular age.
Contributor Information
Carol C Mitchell, Email: ccm@medicine.wisc.edu.
Claudia E Korcarz, Email: ck4@medicine.wisc.eud.
Matthew C Tattersall, Email: mtattersall@medicine.wisc.edu.
Adam D Gepner, Email: Adam.Gepner@va.gov.
Rebekah L Young, Email: rebekayoung@gmail.com.
Wendy S Post, Email: wpost@jhmi.edu.
Joel D Kaufman, Email: joelk@uw.edu.
Robyn L McClelland, Email: rmcclell@uw.edu.
James H Stein, Email: jhs@medicine.wisc.edu.
REFERENCES
- 1.García G, Maiora J, Tapia A, De Blas M. Evaluation of texture for classification of abdominal aortic aneurysm after endovascular repair. J Digit Imaging 2012; 25: 369–76. doi: 10.1007/s10278-011-9417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis SM, Sidhu PS. Granularity of the carotid artery intima-medial layer: reproducibility of quantification by a computer-based program. Br J Radiol 2000; 73: 595–600. doi: 10.1259/bjr.73.870.10911781 [DOI] [PubMed] [Google Scholar]
- 3.Niu L, Qian M, Yang W, Meng L, Xiao Y, Wong KKL, et al. Surface roughness detection of arteries via texture analysis of ultrasound images for early diagnosis of atherosclerosis. PLoS One 2016; 8: e76880. doi: 10.1371/journal.pone.0076880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loizou CP, Pantziaris M, Pattichis MS, Kyriacou E, Pattichis CS. Ultrasound image texture analysis of the intima and media layers of the common carotid artery and its correlation with age and gender. Comput Med Imaging Graph 2009; 33: 317–24. doi: 10.1016/j.compmedimag.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 5.Wohlin M, Sundström J, Andrén B, Larsson A, Lind L. An echolucent carotid artery intima-media complex is a new and independent predictor of mortality in an elderly male cohort. Atherosclerosis 2009; 205: 486–91. doi: 10.1016/j.atherosclerosis.2009.01.032 [DOI] [PubMed] [Google Scholar]
- 6.Andersson J, Sundström J, Gustavsson T, Hulthe J, Elmgren A, Zilmer K, et al. Echogenecity of the carotid intima-media complex is related to cardiovascular risk factors, dyslipidemia, oxidative stress and inflammation: the prospective investigation of the vasculature in uppsala seniors (PIVUS) study. Atherosclerosis 2009; 204: 612–8. doi: 10.1016/j.atherosclerosis.2008.10.038 [DOI] [PubMed] [Google Scholar]
- 7.Lind L, Andersson J, Rönn M, Gustavsson T. The echogenecity of the intima-media complex in the common carotid artery is closely related to the echogenecity in plaques. Atherosclerosis 2007; 195: 411–4. doi: 10.1016/j.atherosclerosis.2007.03.029 [DOI] [PubMed] [Google Scholar]
- 8.Griffin M, Kyriacou E, Kakkos SK, Beach KW, Nicolaides A. Image normalization, plaque typing, and texture feature extraction : Nicolaides A, Beach K. W, Kyriacou E, Pattichis C. S, Ultrasound and carotid bifurction atherosclerosis. London: The British Institute of Radiology.; 2012. 207. [Google Scholar]
- 9.Doonan RJ, Gorgui J, Veinot JP, Lai C, Kyriacou E, Corriveau MM, et al. Plaque echodensity and textural features are associated with histologic carotid plaque instability. J Vasc Surg 2016; 64: 671–7. doi: 10.1016/j.jvs.2016.03.423 [DOI] [PubMed] [Google Scholar]
- 10.Skorton DJ, Collins SM, Nichols J, Pandian NG, Bean JA, Kerber RE. Quantitative texture analysis in two-dimensional echocardiography: application to the diagnosis of experimental myocardial contusion. Circulation 1983; 68: 217–23. doi: 10.1161/01.CIR.68.1.217 [DOI] [PubMed] [Google Scholar]
- 11.Chandrasekaran K, Aylward PE, Fleagle SR, Burns TL, Seward JB, Tajik AJ, et al. Feasibility of identifying amyloid and hypertrophic cardiomyopathy with the use of computerized quantitative texture analysis of clinical echocardiographic data. J Am Coll Cardiol 1989; 13: 832–40. doi: 10.1016/0735-1097(89)90225-8 [DOI] [PubMed] [Google Scholar]
- 12.Stoitsis J, Golemati S, Nikita KS. A modular software system to assist interpretation of medical images—application to vascular ultrasound images. IEEE Trans Instrum Meas 2006; 55: 1944–52. doi: 10.1109/TIM.2006.884348 [DOI] [Google Scholar]
- 13.Christodoulou CI, Pattichis CS, Pantziaris M, Nicolaides A. Texture-based classification of atherosclerotic carotid plaques. IEEE Trans Med Imaging 2003; 22: 902–12. doi: 10.1109/TMI.2003.815066 [DOI] [PubMed] [Google Scholar]
- 14.Wilhjelm JE, Grønholdt ML, Wiebe B, Jespersen SK, Hansen LK, Sillesen H. Quantitative analysis of ultrasound B-mode images of carotid atherosclerotic plaque: correlation with visual classification and histological examination. IEEE Trans Med Imaging 1998; 17: 910–22. doi: 10.1109/42.746624 [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002; 156: 871–81. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 16.Gepner AD, Colangelo LA, Reilly N, Korcarz CE, Kaufman JD, Stein JH. Carotid artery longitudinal displacement, cardiovascular disease and risk factors: the multi-ethnic study of atherosclerosis. PLoS One 2015; 10: e0142138. doi: 10.1371/journal.pone.0142138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasserman BA, Sharrett AR, Lai S, Gomes AS, Cushman M, Folsom AR, et al. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA). Stroke 2008; 39: 329–35. doi: 10.1161/STROKEAHA.107.498634 [DOI] [PubMed] [Google Scholar]
- 18.Jain A, McClelland RL, Polak JF, Shea S, Burke GL, Bild DE, et al. Cardiovascular imaging for assessing cardiovascular risk in asymptomatic men versus women: the multi-ethnic study of atherosclerosis (MESA). Circ Cardiovasc Imaging 2011; 4: 8–15. doi: 10.1161/CIRCIMAGING.110.959403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of multi-ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology 2005; 234: 35–43. doi: 10.1148/radiol.2341040439 [DOI] [PubMed] [Google Scholar]
- 20.Gepner AD, Young R, Delaney JA, Tattersall MC, Blaha MJ, Post WS, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2015; 8: e002262. doi: 10.1161/CIRCIMAGING.114.002262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, et al. Predictors of carotid thickness and plaque progression during a decade: the multi-ethnic study of atherosclerosis. Stroke 2014; 45: 3257–62. doi: 10.1161/STROKEAHA.114.005669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, et al. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010; 52: 1486–96. doi: 10.1016/j.jvs.2010.07.021 [DOI] [PubMed] [Google Scholar]
- 23.Mitchell CC, Stein JH, Cook TD, Salamat S, Wang X, Varghese T, et al. Histopathologic validation of grayscale carotid plaque characteristics related to plaque vulnerability. Ultrasound Med Biol 2017; 43: 129–37. doi: 10.1016/j.ultrasmedbio.2016.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakkos SK, Stevens JM, Nicolaides AN, Kyriacou E, Pattichis CS, Geroulakos G, et al. Texture analysis of ultrasonic images of symptomatic carotid plaques can identify those plaques associated with ipsilateral embolic brain infarction. Eur J Vasc Endovasc Surg 2007; 33: 422–9. doi: 10.1016/j.ejvs.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 25.Kim JK, Park HW. Statistical textural features for detection of microcalcifications in digitized mammograms. IEEE Trans Med Imaging 1999; 18: 231–8. doi: 10.1109/42.764896 [DOI] [PubMed] [Google Scholar]
- 26.Rathore S, Iftikhar MA, Hussain M, Jalil A. Texture analysis for liver segmentation and classification: a survey. IEEE 2011: 121–6. [Google Scholar]
- 27.LifeQMedical Version 4.5. 2013. Carotid plaque texture analysis research software for ultrasonic arterial wall and atherosclerotic plaques measurements. Operation manual version 4.5.
- 28.Weszka JS, Dyer CR, Rosenfeld A. A comparative study of texture measures for terrain classification. IEEE Trans Syst Man Cybern 1976; SMC-6: 17–285. doi: 10.1109/TSMC.1976.5408777 [DOI] [Google Scholar]
- 29.Christodoulou CI, Kyriacou E, Pattichis MS, Pattichis CS. Plaque feature extraction : Nicolaides A, Beach K. W, Kyriacoou E, Pattichis C. S, Ultrasound and carotid bifurcation atherosclerosis. London: The British Institute of Radiology.; 2012. 233–46. [Google Scholar]
- 30.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 Suppl 2): S49–S73. doi: 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 31.Loizou CP, Georgiou N, Griffin M, Kyriacou E, Nicolaides A, Pattichis CS. 2014. Texture analysis of the media-layer of the left and right common carotid artery. Valencia, Spain IEEE-EMBS International Conference on Biomedical and Health Informatics 684–7. [Google Scholar]
- 32.Jung M, Parrinello CM, Xue X, Mack WJ, Anastos K, Lazar JM, et al. Echolucency of the carotid artery intima-media complex and intima-media thickness have different cardiovascular risk factor relationships: the women’s interagency HIV study. J Am Heart Assoc 2015; 4: e001405. doi: 10.1161/JAHA.114.001405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lal BK, Hobson RW, Pappas PJ, Kubicka R, Hameed M, Chakhtoura EY, et al. Pixel distribution analysis of B-mode ultrasound scan images predicts histologic features of atherosclerotic carotid plaques. J Vasc Surg 2002; 35: 1210–7. doi: 10.1067/mva.2002.122888 [DOI] [PubMed] [Google Scholar]
- 34.Ammirati E, Moroni F, Pedrotti P, Scotti I, Magnoni M, Bozzolo EP, et al. Non-invasive imaging of vascular inflammation. Front Immunol 2014; 5: 399. doi: 10.3389/fimmu.2014.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romera-Villegas A, Vila-Coll R, Poca-Dias V, Cairols-Castellote MA. The role of color duplex sonography in the diagnosis of giant cell arteritis. J Ultrasound Med 2004; 23: 1493–8. doi: 10.7863/jum.2004.23.11.1493 [DOI] [PubMed] [Google Scholar]
- 36.Schmidt WA, Kraft HE, Vorpahl K, Völker L, Gromnica-Ihle EJ. Color duplex ultrasonography in the diagnosis of temporal arteritis. N Engl J Med 1997; 337: 1336–42. doi: 10.1056/NEJM199711063371902 [DOI] [PubMed] [Google Scholar]