Abstract
Focal incidental uptake, with or without CT abnormalities, is a common finding on fluorodeoxyglucose PET/CT and evidence-based management for this type of uptake is lacking. This article reviews the evidence on focal incidental uptake including the incidence of malignancy, differential diagnosis and imaging criteria which can be used to further characterize it. The article focusses on PET rather than CT criteria. The strength of the evidence base is highly variable ranging from systematic reviews and meta-analyses to a virtual absence of evidence. Caution needs to be used when using standardized uptake values (SUVs) reported in other studies due to interpatient and institution observed variation in SUVs. There is sufficient evidence to permit specific suggestions on how to interpret the foci and recommend further management in the: pituitary (investigate when SUVmax >4.1), thyroid (investigate all), breast (investigate all), lung parenchyma (if focus of fluorodeoxyglucose without a CT nodule, no further investigations), colon (investigate all foci with SUVmax >5.9, urgently if SUVmax >11.4), adrenals (criteria depend on if patient has cancer) and prostate gland (investigate in males aged >50 years or >40 years if peripheral uptake or patient has other risk factors). There is some evidence to guide further management for the parotid gland, naso-orophaynx, oesophagus, pancreas, uterus and ovaries. There is insufficient evidence to guide management for the liver, spleen, kidneys, gallbladder, testis and bone, for these organs patient characteristics and other guidelines will likely be of more use in determining further management.
INTRODUCTION
Incidental abnormalities on 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT have been reported in 6.71–12%2 of scans.
Accurate interpretation of incidental focal uptake and knowing when to suggest further investigations therefore requires knowledge of its differential diagnosis, the incidence of malignancy and any PET/CT criteria which can be used to further characterize it. In this article, we have, therefore, reviewed the evidence on incidental, focal uptake on FDG PET/CT and used it to suggest guidelines for further management. We have chosen not to discuss diffuse uptake as it is usually ascribed to physiological or inflammatory causes.
With some exceptions, we have chosen not to discuss the CT appearances associated with the incidental FDG focus. This is in the interest of article length and because useful diagnostic CT criteria of incidental findings have been described in multiple other publications and comprehensively covered in the American College of Radiology (ACR) white papers.
Where there were multiple studies examining the significance of an incidental focus, we have included the studies we consider to be most useful or accurate, e.g. those looking at the largest cohort of patients, those with the highest rates of follow up or meta-analyses. It should be noted that in almost all the studies which directly investigated the significance of incidental focal uptake, only a minority of foci have a final diagnosis. It is reasonable to assume that there was a selection bias with the most suspicious foci being further evaluated. Therefore, it is likely the incidence of malignancy reported in these papers is higher than the true incidence of malignancy of all foci. We considered ascribing a level of evidence to each recommendation. However the “question” being addressed (how to manage incidentally identified abnormalities) does not fit into classic evidence-based questions, as it is not a question specifically about diagnostic accuracy or of an intervention. Additionally, in general, the quality of the evidence was low (retrospective, observational studies with low rates of follow up and systematic reviews of these observational studies). Therefore, as an alternative, we have summarized the relevant articles in sufficient detail to allow readers to assess the quality of the evidence for themselves and understand the rationale behind the recommendation. It is important to be aware that multiple factors affect standardized uptake value (SUV) measurements. Under carefully controlled conditions, the SUVs in the same patient can normally vary by up to 10% but this increases to >20% in less well controlled conditions.3, 4 The introduction of newer scanners and reconstruction techniques such as point spread function, time of flight5 and algorithms which allow fully convergent PET image reconstruction but without excessive noise6 have been shown to increase SUVs and SUVmax. Despite this limitations, using evidence on published SUVs as a guide to managing incidental focal FDG uptake is preferable to non-evidence-based decisions.
For each organ, we have, therefore, summarized the relevant articles in sufficient detail to allow readers to assess the quality of the evidence for themselves and understand the rationale behind the suggested management. There are summary tables for each section for ease of future reference.
Finally, any suggestions for further management should be made in the context of the patient’s individual circumstances and choice. In particular, if the patient has known serious comorbidities which would limit life more than the pathology of any incidental findings, then the risks and benefits of further investigations should be carefully evaluated.
Head and Neck
Pituitary
A study of 13,145 consecutive subjects7 found that 0.8% demonstrated incidental pituitary uptake. In 29 of 71 subjects with the final diagnosis, the pituitary uptake was pathological: macroadenomas (n = 21), microadenomas (n = 5) and malignancy (n = 3). When SUVmax >4.1 was used as an optimal criterion for detecting pathologic uptake, the diagnostic sensitivity, specificity and accuracy were 97, 88, and 92%, respectively.
Summary and suggestion
Incidental, focal, pituitary uptake with SUVmax >4.1 should be further investigated with pituitary MRI and/or testing of hormonal levels or endocrinology review.
Naso- oropharynx
There is useful evidence on interpreting focal incidental uptake in the pirform fossa, palatine tonsils and nasopharynx which is described below. Evidence for interpreting potential incidental uptake elsewhere (e.g. the floor of mouth and base of tongue) is scarce and a single study without easily adaptable conclusions is outlined at the end of the section.
Within the piriform fossa, Cho et al8 found that 0.05% of 56,585 PET/CTs had incidental, focal uptake. There was a final diagnosis of malignancy in 10.3% (n = 29) and benign causes in 76.5% (n = 215). Malignant lesions had significantly higher SUVmax (9.1 ± 3.6 vs 3.5 ± 1.0) and ipsilateral to contralateral SUVmax ratio (SUVmaxI:SUVmaxC) (4.2 ± 1.8 vs 1.5 ± 0.4) compared to benign causes. Lesion SUVmax ≥ 4.2 identified malignancy with a sensitivity of 93% and specificity of 87%. SUVmaxI: SUVmaxC ≥1.8 had a sensitivity of 100% and specificity of 90% for the detection of malignancy.
Davison et al9 compared the SUVmax ratio in patients with and without (n = 26 each) palatine tonsillar squamous cell carcinoma (SCC). When an SUVmax ratio cut-off of 1.48 was used the area under the curve (AUC) of the receiver operating characteristic (ROC) analysis was 1.0, i.e. 100% sensitivity and specificity. Lee et al10 reported that the SUVmax ratio (mean, range) of patients with early pT stage tonsillar SCC was (2.5, 1.0–5.4),occult tonsillar SCC found at surgery (1.7, 0.85–3.8). in patients with cervical metastases of SCC of unknown primary without tonsillar primaries (1.1, 0.86–1.3) and in healthy controls (1.2, 1.0–1.5).
Lee et al11 compared patterns of benign (n = 177) and malignant (n = 48) nasopharyngeal FDG uptake and found that uptake was significantly more intense in the malignant group (SUVmax 10.4 ± 4.6) than the benign group (3.9 ± 1.4) p < 0.001; however, asymmetry of uptake was not: 68% in the benign group vs 90% in the malignant group. When SUVmax of 6.0 was used as cut-off for detection of malignant nasopharyngeal uptake, the AUC of the ROC was 0.93 [95% CI (0.88–0.98)] with a sensitivity of 88% and a specificity of 92%.
Heusner et al12 studied 590 patients to assess for qualitatively increased or asymmetric uptake within the head and neck without a corresponding CT abnormality with a mean follow up of 2.5 years. Of 87 patients with asymmetric uptake within Waldeyer’s ring, 1 developed palatine carcinoma (SUVmax 3.2); of 98 patients with asymmetric uptake in the oral floor one developed carcinoma (SUVmax 3.7) and of 72 with asymmetric laryngeal uptake none developed carcinoma.
Summary and suggestions
Intensity of FDG uptake is significantly associated with malignancy.8, 11 SUVmax ≥4.2 in the piriform fossa8 and SUVmax ≥6.0 in the nasopharynx11.
When comparing known benign and malignant uptake SUVmaxI:SUVmaxC >1.8 in the piriform fossa8 and SUVmaxI:SUVmaxC >1.48 in the palatine tonsils9 are highly suspicious for SCC.
Based on this, we suggest that patients with the above findings should be referred to ear, nose and throat (ENT) for direct visualization. In patients with incidental focal uptake not meeting the criteria above, there is inadequate evidence to formulate guidelines and decisions should be made in individually. Knowledge of if the patient has any important risk factors for head and neck cancer: smoking, alcohol, human papillomavirus and Epstein–Barr virus infection13 might be helpful. It should also be remembered that asymmetrical uptake is frequently observed in patients without head and neck cancer.12
Parotid
Mabray et al14 analysed 38,302 PET studies and identified 73 incidental foci of uptake within the parotid which had a follow up diagnosis. 33/73 were manifestations of the patient’s known malignancy (26 metastases, 7 lymphomas), 25/73 were benign primary parotid tumours (14 Warthins, 7 benign pleomorphic adenomas, 4 oncocytomas) and 15/73 non-neoplastic causes (lymphatic tissue or inflammation). No malignant primary parotid tumours were identified. In patients with head and neck pathology, there were higher odds ratios (ORs) that focal parotid uptake was a metastasis [OR = 24.6, p < 0.01 for cancer/melanoma, OR = 7.2, p = 0.02 for lymphoma and OR = 3.6, p = 0.07 for FDG-avid cervical lymph node(s)]. Mean SUVmax of the lesions which were manifestation of the patient’s known malignancy was 8.4 vs 10.3 for benign parotid lesions.
Makis et al15 found that, of 7,252 oncologic PET/CT studies, FDG incidental parotid uptake occurred in 0.4% (n = 29) of scans of which only 1 (4%) was malignant: follicular lymphoma (FL) in a patient with a previous history of follicular lymphoma. The commonest histology was Warthin’s tumour. PET/CT was unable to differentiate benign from malignant parotid lesions based on SUVmax alone.
Park et al16 examined PET and CT criteria in 272 patients who exhibited focal parotid uptake, 68 had pathological confirmation. There were 32 malignant lesions: 11 metastatic carcinoma, 8 primary salivary malignancies/other. The benign lesions included 24 Warthins tumours, 4 pleomorphic adenomas and “other” in 8. There were no significant differences in SUVmax, size or Hounsfield Unit (HU) max between benign and malignant lesions. Malignant lesions were significantly more likely to have heterogeneous uptake and ill-defined margins on CT.
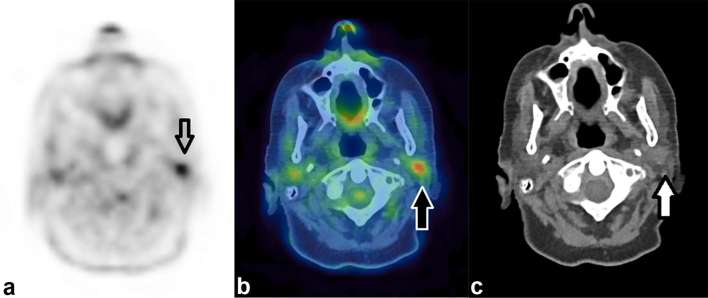
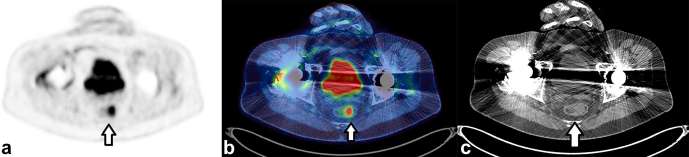
Figure 1 demonstrates an incidental focus of uptake within the parotid gland.
Figure 1.
Incidental focal uptake in the parotid gland. A 71-year-old female had a PET/CT for further characterization of CT abnormalities identified during follow up for previous TCC of the ureter. Focal uptake of FDG (SUVmax 6.0) demonstrated by the arrow on the axial PET only image (a) localizes to the left parotid gland. On the CT only image (c) a soft tissue density nodule is demonstrated in the parotid. This was thought to be incidental rather than related to the TCC and histology from ultrasound-guided biopsy demonstrated a pleomorphic adenoma. FDG, fluorodeoxyglucose; PET, positron emission tomography; TCC, transitional cell carcinoma.
Summary and suggestions
SUVmax cannot distinguish between benign and malignant causes of uptake. Warthin’s tumour followed by pleomorphic adenoma are the commonest incidental findings which, although benign, when they present with a mass or other symptoms, are typically managed surgically.17, 18 Some incidental malignant lesions were also detected. Therefore, it is reasonable to refer patients without a known malignancy with incidental focal parotid uptake for further evaluation, either to ENT or with imaging such as parotid ultrasound or MRI.
In patients with a known malignancy, particularly within the head and neck, the uptake is much more likely to represent either a metastasis or a benign lesion than a primary malignant parotid lesion. The benefit of further assessment should, therefore, be balanced against the prognosis of the known malignancy
Thyroid
Focal thyroidal uptake can represent benign or malignant pathologies, Barrio et al19 listed over 20 different histopathology descriptions following thyroidal FNA of focal hypermetabolic thyroid lesions. Benign lesions included benign colloid nodule, benign follicular nodule and adenomatoid nodule. Malignant pathologies included follicular cell neoplasm, Hurthle cell neoplasm and papillary carcinoma. Diffuse uptake is usually ascribed to Graves disease, chronic thyroiditis and hypothyroidism.20
Soelberg et al21 performed a systematic review on the risk of malignancy in incidental thyroidal uptake. Out of 125,754 subjects, 1994 (1.6%) had unexpected focal activity, 1051 of these had a final diagnosis of whom 366 (34.8%) had thyroid malignancy. In the eight studies reporting individual SUVmax, the mean SUVmax was 4.8 (standard deviation 3.1) in benign and 6.9 (standard deviation 4.7) in malignant lesions (p < 0.001). More recent, smaller, single site studies found a malignancy rate of 23% (7 patients),22 19% (9 patients)23 and 23% (19 patients)24 who received final diagnoses. All three studies found the mean SUVmax of the malignant group was higher than the benign group but with large overlap in the range between the groups.
Kim et al25 analysed the use of the unenhanced CT in further characterizing incidental thyroidal uptake in 82 patients. They found that the ratio of the HU of the thyroid nodule to the contralateral thyroid lobe (T/BHU) in predicting malignancy had an AUC of the ROC of 0.94 when a cut off T/BHU ≤ 0.68 was used. The sensitivity, specificity, and accuracy of T/BHU was 100, 80 and 87% respectively.
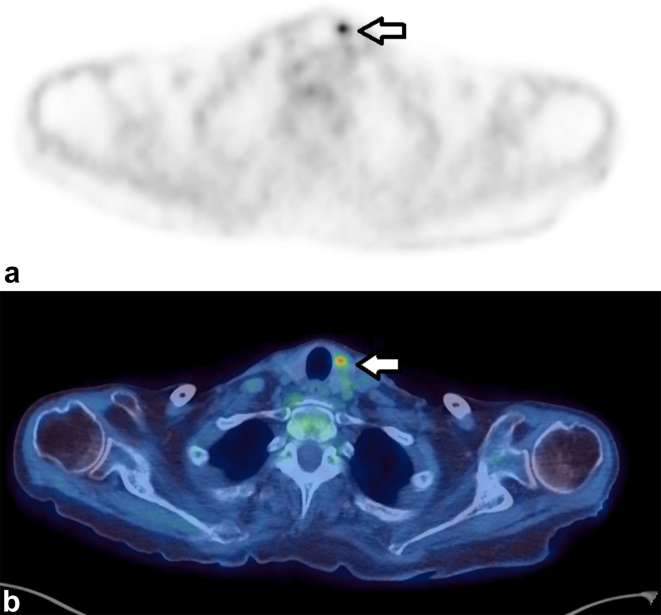
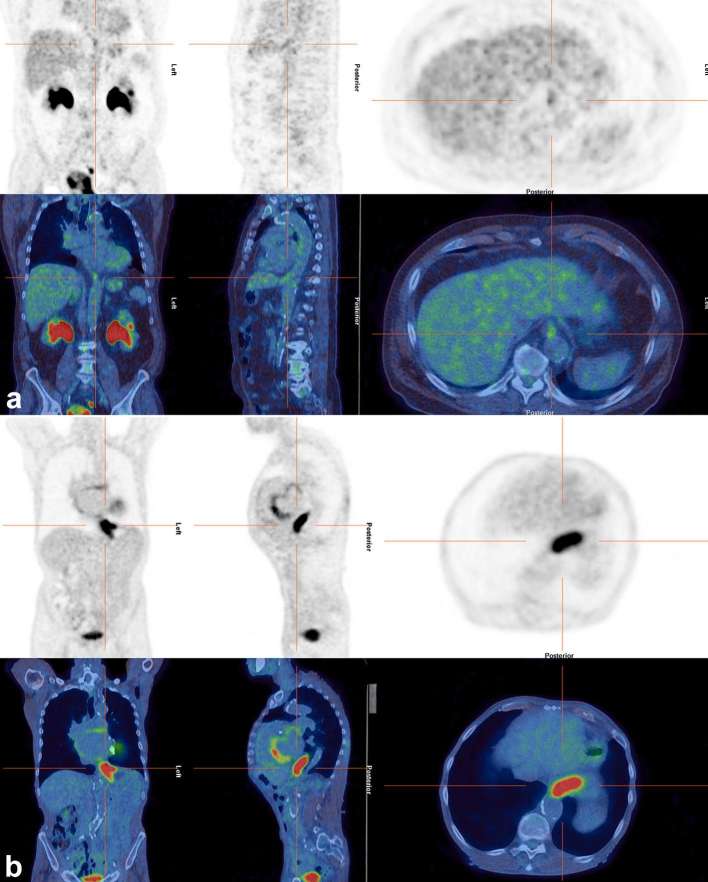
Figure 2 demonstrates an incidental focus in the thyroid gland.
Figure 2.
Axial images of a 71-year-old female who had a PET/CT to assess for possible malignancy as a paraneoplastic cause of her necrotizing autoimmune myopathy. A focus of increased uptake (SUVmax 6.1) (a) localized to the thyroid gland (b) Fine-needle aspiration showed blood and clumps of colloid and clusters of crowded epithelial cells with enlarged nuclei, the appearances were suspicious of papillary carcinoma. Although the study was performed to look for malignancy, this finding was not thought to be the cause of her myopathy. PET, positron emission tomography; SUV, standardized uptake value.
Summary and suggestions
Incidental, focal, thyroidal uptake should have ultrasound scan±fine-needle aspiration. The same recommendation was made by the American Thyroid Association26 and the ACR white paper.27 In future, the T/BHU may help differentiate which patients require further investigation.
Table 1 for a summary of the evidence and suggestions regarding incidental FDG uptake in the head and neck.
Thorax
Breast
Thorax Breast ThBShin et al28 identified incidental focal uptake in the breast in 1.0% of 21,224 females who underwent PET/CT.
91 lesions had sufficient histological or imaging follow-up for a diagnosis of which 30% were malignant, these pathologies included invasive ductal carcinoma, invasive lobular carcinoma, ductal carcinoma in situ and secondary deposits from other malignancies. In order of most frequent, the benign pathologies were fibroadenoma, intraductal papilloma, fibrocystic change, sclerosing adenosis and other rarer causes. Multivariate analysis found that none of patient's age, SUVmax or lesion size was useful in distinguishing between benign and malignant causes. Chae et al29 found that incidental focal FDG uptake within the breast occurred in 0.40% of 32,988 patients who had PET/CTs. Of these, 72 had either sufficient histological or imaging follow up to establish a diagnosis of which 45% had a malignant cause. SUVmax and lesion size was larger in the malignant than benign lesions but there was significant overlap in the confidence intervals for both.
CT appearances of breast lesions should also be considered: a spiculated and irregular margin (and rim enhancement for contrast enhanced) is the most accurate sign for malignancy.30 Due to lobular carcinoma’s more infiltrative pattern of growth CT findings may be non-specific: appearing as an asymmetric soft-tissue density with or without associated skin thickening or as a mass. A discrete mass is also often absent in inflammatory carcinoma, but with marked skin thickening. Benign breast fibroadenomas appear as well circumscribed round or oval masses which may have popcorn calcifications, fibroadenolipoma often contains fat and glandular tissue. Fat necrosis can have a range of appearances including mimicking malignancy. Despite these recognized CT appearances, a systematic review of incidental breast lesions detected on CT found that benign and malignant breast lesions cannot be safely distinguished from each other on standard chest CT.31 Additionally, many benign breast lesions, including those described above, can show increased FDG uptake.32
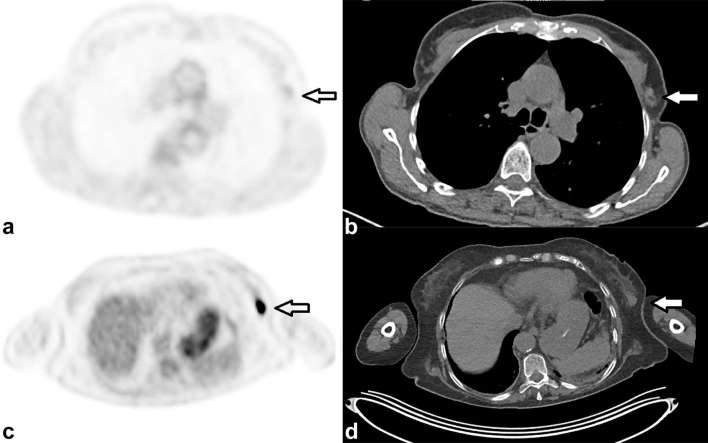
Figure 3 demonstrates two different incidental findings within the breast, their differing PET/CT appearances and histologies.
Figure 3.
Two cases demonstrating how incidental breast uptake, which were both later proven to be carcinoma, can have highly variable FDG uptake. (a, b) are axial PET and CT images respectively from a 69-year-old female who had a PET/CT as part of staging for a previously removed melanoma. No other sites of melanoma were identified; however; there was low-grade uptake (SUVmax 1.8) in the axillary tail of the left breast (arrow) in (a) which localized to a soft-tissue nodule, on the CT (b). This was confirmed as invasive ductal adenocarcinoma on histology. In contrast, there was very intense uptake (SUVmax 19.1), axial PET only image, (c) in the breast of a 73-year-old female who had a PET/CT for potential myeloma. This localized to a breast soft-tissue lesion demonstrated on the CT only image, (d) which was also later confirmed as primary breast carcinoma. FDG, fluodeoxyglucose; PET, positron emission tomography; SUV, standardized uptake value.
Summary and suggestions
Focal incidental breast uptake, irrespective of the CT appearances requires further evaluation, we recommend by referral to the breast team for triple assessment.
Lung
Focal uptake within the lung, without a corresponding nodule assuming there is no misregistration, was reported to occur in 0.15% of 10,500 patients.33 In this paper, and in another reported series,34 some of the patients were rescanned a few days later with FDG PET/CT and the foci had disappeared or they were followed up with contrast enhanced CT and no abnormality developed. A different paper reported focal pulmonary FDG without a corresponding CT abnormality in four patients who had all received a paravenous injection of FDG.
This finding has been described as the: “hot-clot artefact”, where there is agglutination of FDG by erythrocytes during FDG injection. This causes occlusive plugs/microemboli in the pulmonary arterial system and create focal FDG uptake with high SUVs. Microemboli can occur from blood aspiration into the injector, paravenous injection and possibly high speed injection. They are typically peripheral with visually and quantitatively high FDG uptake.35
Due to the nature of the hot-clot artefact, these papers have concluded these foci do not require further follow up.
FDG uptake within CT evident solitary pulmonary nodule (as defined on page ii5 of British Thoracic Society guidelines)36 has been discussed in published guidelines, with increasing intensity of uptake increasing the likelihood of malignancy.36, 37 The Herder risk prediction model38 could be used (e.g. with online tools)39 to further evaluate its risk of malignancy.
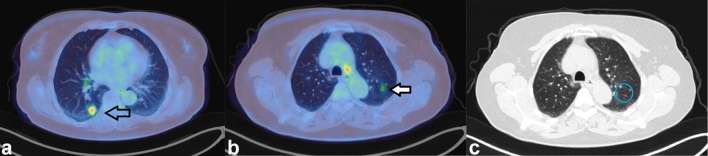
Figure 4 demonstrates an FDG avid lung nodule and a pulmonary focus of FDG without CT correlate.
Figure 4.
Axial-fused PET/CT images of a 66-year-old female who had a PET/CT for further characterization of a right lower lobe lung nodule. The nodule was intensely FDG avid (SUVmax 6.4) (a) and (b) and was shown to be lung cancer on biopsy. She had a further focus of FDG (SUVmax 4.0), arrow in (c) in the contralateral lung which was not associated with a nodule on CT (d) This was assumed to be a microembolus of FDG and chest X-ray 20 months later showed no abnormality at this site. FDG, fluorodeoxyglucose; PET, positron emission tomography; SUV, standardized uptake value.
Summary and suggestions
Incidental foci of FDG in the lungs without a CT correlate can be assumed to be a microembolus of FDG and do not require follow up.
An incidental (i.e. highly unlikely to be metastatic) pulmonary nodule associated with increased FDG uptake could be referred to a lung MDT or followed up with CT depending on its probability of malignancy as predicted by the Herder prediction model.
Oesophagus and Gastro-Oesophageal junction
There are no relevant studies looking at incidental uptake within the oesophagus; however; physiological increased uptake at the gastro-oesophageal junction (GOJ) is well-recognized and differentiating benign from malignant uptake at this site can be difficult.40 Knowledge of the normal range of uptake can be helpful which has been reported as SUVmax of 1.0–8.4 in 546 patients without CT abnormalities who did not develop GOJ cancer.41 It should be noted that patients in this paper were scanned from 2002 to 2004 and so the normal range of SUVmax may now be higher.
Stagg et al42 compared the uptake at the GOJ with the results of endoscopy performed within 6 months. They found the mean SUVmax in patients with normal findings at endoscopy (n = 151) was 2.1 vs 6.7 for those with oesophageal malignancy (n = 34), 2.5 for those with oesophagitis (n = 21), 2.4 for those with Barrett's oesophagus (n = 8) and 3.5 for those with other non-malignant disorders (n = 5). They determined that a SUVmax ≥3.5 predicted a necessity for endoscopy with a positive-predicted value (PPV) of 79% and a SUVmax ≤2.2 had a negative-predicted value (NPV) of 86%.
A different study43 compared the characteristics of early malignant (Barrett’s oesophagus, Tis, T1 and T2 adenocarcinomas) and benign oesophageal lesions. This found early malignant lesions had significantly higher FDG uptake on visual scoring, eccentricity and focality compared with benign lesions and that eccentricity and focality were better predictors than intensity.
Summary and suggestions
There is evidence that when uptake is more eccentric and focal43 and more intense at the GOJ (e.g. SUVmax >6)42 it is more likely to be malignant. Foci with these characteristics should, therefore, be routinely referred for endoscopy.
Figure 5 demonstrates presumed physiological uptake at the GOJ vs an oesophageal cancer extending to the GOJ.
Figure 5.
Axial images of a 74-year-old male who had a PET/CT for staging of melanoma with a presumed single metastasis to lung. A focus of uptake in the rectosigmoid junction (SUVmax 9.8) (a) appeared to localize to abnormal soft tissue on the fused (b) and CT-only (c) images. The patient went on to have a sigmoidoscopy which demonstrated an 18-mm polyp, he was referred for endoscopic mucosal resection. PET, positron emission tomography; SUV, standardized uptake value.
See Table 2 for a summary of the evidence and suggestions regarding incidental FDG uptake in the thorax.
Abdomen and Pelvis
Stomach
Similar to oesophagus, there were no studies directly analysing the significance of focal incidental gastric uptake. However, Le Roux et al44 assessed the diagnostic utility of gastric distension in FDG PET/CT in 39 patients with previously identified suspicious incidental, focal, gastric uptake. Of these, 14 patients had persistent suspicious uptake, of these 10 had cancer (6 lymphoma, 2 melanoma metastases, a pancreatic serosal metastasis and a primary gastric adenocarcinoma) and the remaining 4 had benign pathology. Incidental focal gastric uptake has, therefore, been shown to represent gastric cancer44 but the true incidence is unknown. Providing evidence-based suggestions for incidental uptake within the stomach is, therefore, not possible.
Colon
A systematic review and meta-analysis of incidental focal colonic uptake45 found that, of 89,061 patients evaluated by FDG PET or PET/CT, the pooled prevalence of incidental colonic uptake was 3.6%. Of these, approximately one-third (n = 1044) underwent colonoscopy or histological examination (a presumed selection bias of more suspicious appearing foci). The pooled risk of malignant or premalignant lesions was 68% [95% CI (60–75%)]. There was a significant overlap in average SUV between malignant, premalignant and benign focal uptake.
The largest single-site study published to date46 of 7300 patients found a prevalence of incidental focal colonic uptake of 5.5%. Of these, 60% went on to have colonoscopy. The authors listed reasons why patients did not have colonoscopy of which 14% were because the uptake was described as “probably physiological”. 53% of patients had a benign cause for the uptake, 37% had an adenoma and 10% adenocarcinoma. The median SUVmax (and interquartile range) for the benign group was 8.2 (5.9–10.1), for the advanced adenomas 9.7 (7.2–12.6), the non-advanced adenomas 8.3 (6.1–10.5) and the adenocarcinomas 16.6 (12–20.8). Using an SUVmax >11.4 as a cut off to distinguish adenocarcinoma from other aetiologies, the AUC of the ROC was 0.868 with a sensitivity of 80%, specificity of 82%, PPV 34% and NPV 98%.
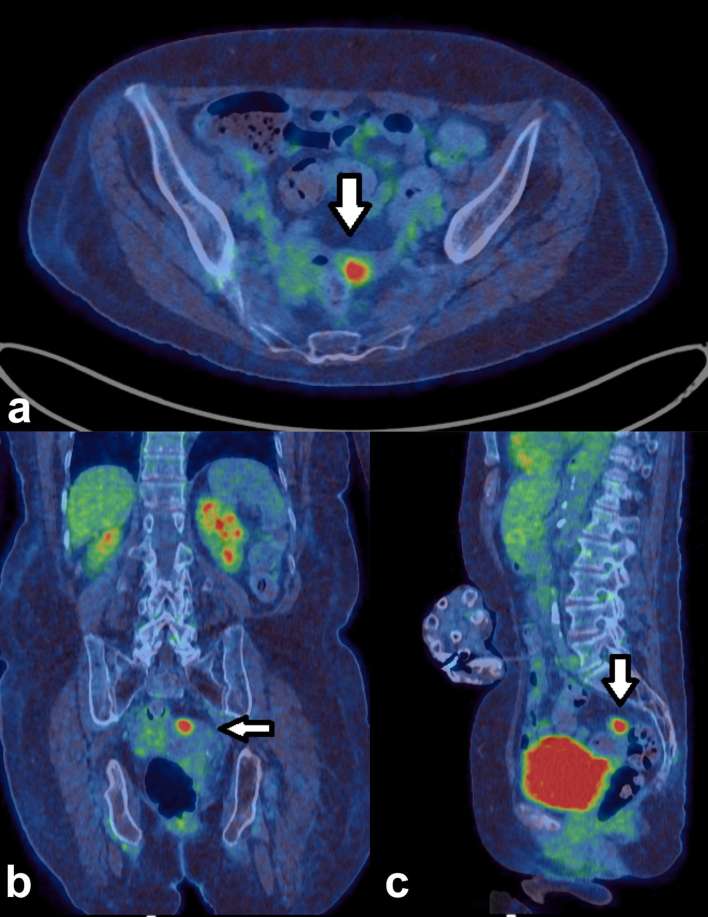
Figure 6 demonstrates an incidental focus of FDG within the sigmoid colon.
Figure 6.
Axial, coronal and sagittal PET/CT images (a) of a 73-year-old male with recurrent bladder cancer and faint foci at his GOJ which were presumed to be physiological. This contrasts with (b) which shows a known oesophageal tumour extending to the GOJ. GOJ, gastro-oesophageal junction; PET, positron emission tomography.
Summary and suggestions
Focal colonic uptake represents adenoma or adenocarcinoma in up to 4746–68%45 of patients with significant overlap in the range of SUVmax between the different aetiologies. Based on this, we recommend that all patients with focal colonic uptake and SUVmax >5.9 have colonoscopy or virtual colonoscopy unless there is sufficient reason not to such as advanced illness or recent colonoscopy. This should be performed urgently if SUVmax >11.4 as the likelihood of malignancy is higher. There is no good evidence on the significance of focal colonic uptake with SUVmax <5.9 and, for these foci, decisions should be made on a case by case basis.
Pancreas
We could find only one publication on incidental pancreatic uptake, a letter to the editor.47 This reported incidental pancreatic uptake in 14 of 2868 patients (0.49%), of whom 11 had widespread FDG avid lesions in liver, bone, lungs and lymph nodes from lymphoma or metastases. Pancreatic cancer was diagnosed in two and unclear in one patient. Within the pancreas, intensity of FDG uptake cannot reliably distinguish between different pancreatic pathologies.48
Summary and suggestions
From the limited data, we, therefore, recommend that foci which are not most likely part of a more widespread disease process (e.g. lymphoma or metastatic cancer) are further investigated, with either CT or MRI.
Liver
We could find no articles assessing the significance of focal incidental hepatic uptake, although there was an article on the use of FDG PET/CT to help characterize incidental hepatic abnormalities identified via other imaging.49 The intensity of FDG uptake is variable in both benign (e.g. adenomas, haemangioendotheliomas and abscesses) and malignant aetiologies (metastases and primary hepatic malignancies)50, 51 and cannot be used to distinguish between them. Hepatocellular carcinoma in particular frequently does not demonstrate significantly increased FDG uptake50 but should clearly be considered as a cause of the uptake if there are signs of chronic liver disease.
Gallbladder
We could find no papers discussing incidental focal uptake in the gallbladder. Gallbladder carcinoma52 and benign causes (focal adenomyomatosis53 and xanthogranulomatous cholecystitis)54 have all been shown to be FDG avid. A study of 34 patients with gallbladder malignancy52 reported a wide variation in the SUVmax of lesions (mean SUVmax 7.92 ± 6.25). In addition, a meta-analysis55 showed marked heterogeneity in different studies’ abilities to distinguish benign from malignant uptake.
Spleen
We could find no papers discussing incidental uptake in the spleen although temporary, focal, non-pathological FDG retention in the spleen has been described.56 There are multiple published case reports of FDG uptake in benign splenic lesions (e.g. hamartoma,57 epidermoid cyst with haemorrhage,58 lymphangioma59 and inflammatory pseudotumour-like follicular dendritic cell tumour60) Lymphomatous or metastatic splenic involvement is also possible.
Kidney
We could find no papers focussing on incidental renal uptake of FDG. However, Kochhar et al wrote a paper on the role of FDG PET/CT in imaging of renal lesions.61 Useful conclusions from this paper are that angiomyolipomas, oncocytomas and renal cell carcinoma (RCC) can all display variable FDG avidity (simple cysts are photopenic). Renal metastases are typically FDG avid.
Summary and suggestions for liver, gallbladder, spleen and kidney
There is inadequate evidence for these organs and decisions on when to further investigate will be have to be made individually depending on patient characteristics and the guidelines from the ACR white papers.62–64
Adrenal
Due to adrenal morphology and the resolution of PET, identifying incidental focal adrenal uptake without an associated CT nodule is unlikely and consequently, there were no studies examining this. However, the detection of incidental adrenal nodules is common (occurring in 1.5% of CT studies performed as part of screening)65, as is their frequency as a site of metastases. Therefore, using published evidence to help characterize, and so distinguish, incidental adrenal nodules from more significant pathology is useful. A meta-analysis revealed that FDG PET has 97% sensitivity and 91% specificity in the differentiation between malignant and benign adrenal disease (using a variety of diagnostic criteria).66 It also stated that the specificity of FDG PET for characterizing adrenal lesions smaller than 10 mm should be considered with caution.
Tessonnier et al67 evaluated FDG PET/CT in distinguishing benign from malignant uptake in patients with known adrenal nodules without evidence of hormonal hypersecretion or active cancer and when CT or MRI had been inconclusive. They reported that the use of 1.8 as the threshold for nodule/liver SUVmax ratio demonstrated 100% sensitivity and specificity in 41 adrenal masses of which there were 12 malignant tumours, 17 benign tumours and 12 tumours classified as benign on follow-up.
Boland et al68 reported that qualitative FDG uptake was the most accurate way to distinguish benign from malignant nodules in 165 adrenal lesions in 150 patients with known cancer when uptake moderately or significantly increased compared to liver was considered malignant and uptake less than or equal to liver was considered benign. They also incorporated CT appearances where an unenhanced HU of <10 (previously shown to be a useful cut-off)69 or the presence of macroscopic fat (to identify myelolipomas) was used to establish benignity. By combining unenhanced and qualitative CT and PET data, the analysis yielded a sensitivity of 100% for the detection of malignancy, a specificity of 99%, a PPV of 93%, a NPV of 100% and an accuracy of 99%. Conversely, for the detection of benignity, the sensitivity, specificity, PPV, NPV and accuracy were 99, 100, 100, 93 and 99%, respectively. However, some benign nodules are known to be moderately FDG avid and Brady et al70 investigated patients with known or suspected lung cancer and found that SUVmax ratio (adrenal:liver) of >2.5 excluded all FDG avid benign adrenal nodules.
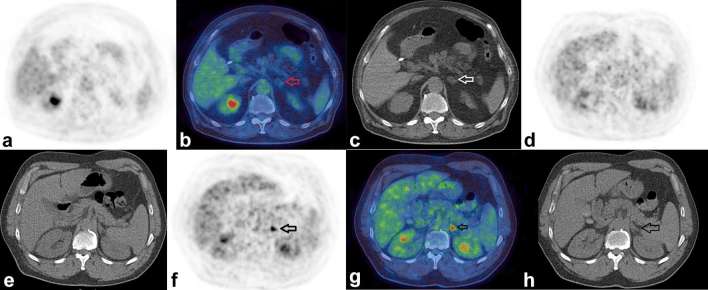
Figure 7 demonstrates a presumed benign small adrenal myelolipoma and a presumed early adrenal metastasis from lung cancer.
Figure 7.
Benign and malignant adrenal nodules. Figure shows the PET-only (a) fused (b) and CT-only (c) images respectively of a 72-year-old male who had a PET/CT to assess for possible large vessel vasculitis. There was no evidence for vasculitis, however there was a 1.5 cm low attenuation nodule (HU –30) in the left adrenal demonstrating only low-grade FDG uptake (SUVmax 1.5). Based on the presence of macroscopic fat, this was presumed to be an adrenal myelolipoma. Figures (d, e) show PET- and CT-only images respectively in a 68-year-old male who had a PET/CT to further characterize persistent consolidation in his lung, the adrenals look normal. A PET/CT repeated 9 months later (f, g, h) now shows an intense focus of FDG uptake in the left adrenal gland with SUVmax 7.3 (SUVmax of liver 4.1, HU of the adrenal 28). This was assumed to be an adrenal metastasis from his lung cancer which had also progressed elsewhere. FDG, fluorodeoxyglucose; HU, Hounsfield unit; PET, positron emission tomography; SUV, standardized uptake value.
Summary and suggestions for distinguishing benign and malignant adrenal nodules
Patients without known cancer or hormonal hypersecretion: a nodule:liver SUVmax ratio >1.8 should have further evaluation either with CT or MRI. Given the small sample size of the study from which this recommendation is taken,67 it would be reasonable to follow up other patients with interval imaging, e.g. if the SUVmax ratio is close to 1.8 and the CT features cannot diagnose an adenoma or myelolipoma.
Patients with cancer: the combination of PET (uptake less than or equal to liver) or CT criteria (HU <10 or macroscopic fat) to identify benign nodules had excellent diagnostic accuracy68 but not 100% sensitivity or PPV. An SUVmax ratio (adrenal:liver) of >2.570 excluded all benign nodules.
Using PET/CT to characterize nodules <1 cm should be done with caution.
Prostate
A systematic review and meta-analysis of incidental focal prostatic uptake included 47,925 patients.71 The prevalence of incidental prostatic uptake was 1.8%, of these 444 were further evaluated and 17% had malignancy. There was a trend to higher SUVmax in the malignant group but this was not statistically significant. Peripheral uptake but not presence or absence of calcification was a predictor of malignancy. The predictive nature of peripheral uptake is to be expected because prostate cancer is more likely to arise in the peripheral zone and because a central focus could represent urine in the urethra. A more recent study72 of 20,422 patients found 1.4% of scans had focal prostatic uptake. Only 22.5% of these patients had further investigations of which 55% had malignancy, again SUVmax between benign and malignant uptake was not statistically significantly different.
It should also be noted that prostate cancer is very strongly correlated with age, in the UK, the age-specific incidence rates were 3/100,000 for males aged 40–44 years, 17/100,000 for males aged 45–49 years and with a rapid increase in rates with increasing age over 50 years.73 Other important risk factors for prostate cancer are being of black ethnicity74, 75 and having one or more first degree relatives affected.76
Summary and suggestions
From the evidence above, males aged >50 years with focal prostatic uptake should have PSA testing and males aged 40–50 years should have PSA testing if the focus is peripheral, they are of black ethnicity or have affected first degree relatives.
Uterus and ovaries
Interpreting the significance of possible incidental ovarian and uterine uptake requires an understanding of normal physiological uptake. In pre-menopausal females, there is increased physiological ovarian and endometrial uptake during the late follicular and early luteal phase of the menstrual cycle and slightly more intense physiological endometrial uptake in the first few days of the menstrual cycle.77, 78 Increased physiological uptake does not occur in post-menopausal females,77, 78 the mean endometrial SUV in postmenopausal patients not receiving hormonal therapy was 1.7.78
Uterine leiomyomas (fibroids) are present in 25–30% of pre-menopausal females,79, 80 therefore an understanding of normal fibroid uptake is also required. They have been reported as showing increased FDG uptake in 10.4% of pre-menopausal females and in 1.2% of post-menopausal females.81 In females who had more than one PET scan, uptake within the leiomyoma disappeared or newly appeared, therefore newly emerging uptake does not necessarily mean malignant transformation. In pre-menopausal females, uptake is usually at a similar level to liver, however, in 3 out of 22 proven leiomyomas uptake was greater than liver.79 Kitajima et al reported the SUVmax of 61 leiomyomas as 2.34 (range 1.59–5.15)82 and that the SUVmax of degenerated leiomyomas (mean 2.89, range 1.59–5.15) was significantly higher than that of non-degenerated leiomyomas (mean 2.17, range 1.61–4.44). Zhao et al83 showed that FDG uptake is significantly higher in uterine sarcomas than leiomyomas with an SUVmean of 2.5 (range 1.0–5.8) in leiomyomas (n = 33) and 5.5 (range 1.8–12) in sarcomas (n = 14), they did not report on SUVmax. Their study only analysed females with suspicious uterine findings, either clinically or on MRI/ultrasound scan, which likely explains the higher SUV in the leiomyomas than that reported in the other study.
In terms of interpreting incidental uptake, we could find no studies that examined the significance of uptake which was neither physiological nor fibroid related. FDG PET/CT cannot accurately discriminate between benign and malignant ovarian and uterine pathologies.78, 84,85 It is also worth noting that that the incidence of uterine cancers significantly increases with age, occurring at a rate of less than 7/100,000 in females under 45 but rising relatively steeply after that.86
Figure 8 demonstrates incidental uptake within the endometrium of a post-menopausal female.
Figure 8.
Incidental uptake in the endometrium. An 80-year-old female had a PET/CT to investigate for a paraneoplastic cause of her large fibre sensory neuropathy. Intensely increased uptake (SUVmax 12.4) was present in the endometrial cavity, shown on the axial (a) coronal (b) and sagittal images (c) which was shown to be grade 1 endometrioid endometrial carcinoma on biopsy. Although this was thought unlikely to be inducing a paraneoplastic neuropathy, the patient had a hysterectomy but without any improvement in her symptoms, it was, therefore, an incidental finding. PET, positron emission tomography; SUV, standardized uptake value.
Summary and suggestions
Post-menopausal females: increased ovarian/adnexal uptake or uterine uptake which does not have PET/CT findings consistent with leiomyomas, should be further evaluated with pelvic ultrasound scan or MRI.
-
Pre-menopausal females:
Ovarian uptake which is not occurring around midcycle should be further imaged with ultrasound.
Endometrial uptake in a normal-sized uterus at midcycle or menstruation should be considered physiological. Leiomyomas are common and usually show intensity of FDG uptake similar to liver.79, 82 Uterine FDG uptake above this level or occurring outside of midcycle or menstruation should be considered for further imaging, although uterine malignancies in females under 45 are rare.
Testis
We could find no studies which examined the significance of incidental, non-physiological testicular uptake, although abnormal increased uptake within the testicles due to lymphoma has been shown to not always be associated with CT abnormalities.87 There is, therefore, insufficient data to make evidence-based suggestions. If testicular pathology is suspected it should be further evaluated with testicular ultrasound.
Bone
We could find no studies that analysed incidental, focal skeletal uptake without associated CT abnormalities. However, it should be noted that FDG PET/CT has a high sensitivity for the diagnosis of bone metastases, reported as 89.7%, similar to MRI and better than CT and bone scintigraphy.88 It has also been shown that, of 31 lesions with positive findings at PET and negative findings at CT, 19 were malignant giving a PPV of 61%.89
Summary and suggestions
Focal uptake within bone, without CT abnormalities, in patients with known cancer should be considered suspicious for a bone metastasis. Due to the lack of evidence, we are unable to suggest evidence-based guidelines for focal skeletal FDG uptake without a CT abnormality in patients without a known cancer. In this circumstance, MRI may be helpful to evaluate for the presence and nature of soft-tissue abnormalities within the bone. Bone single photon emission CT-CT is less likely to be helpful if there are no detectable abnormalities on CT to aid the interpretation of the aetiology of bone tracer uptake.
See Table 3 for a summary of the evidence and suggestions regarding incidental FDG uptake in the abdomen,pelvis and bone.
Table 3.
Summary of evidence-based suggestions for further evaluation of incidental FDG uptake in the abdomen and pelvis and bonea
| Site of focal uptake | Evidence/when to further evaluate | Recommended form of evaluation |
| Stomach | Insufficient evidence | |
| Colon | Refer focal colonic uptake with SUVmax > 5.9 Urgent referral if SUVmax > 11.446 No good evidence on the significance of focal colonic uptake with SUVmax < 5.9 |
Colonoscopy or virtual colonoscopy |
| Pancreas | Investigate foci which are not most likely part of a more widespread disease process, e.g. lymphoma or metastatic cancer | MRI or CT |
| Liver, gallbladder, spleen and kidney | Inadequate evidence, benign and malignant causes can be avid. Take into account patient characteristics, CT appearances and publications on CT features.62–64 | Ultrasound, CT or MRI |
| Adrenal: patients without known cancer or hormonal hypersecretionb | Evaluate nodules with a nodule:liver SUV max ratio > 1.8.67 Consider following up other patients, e.g. if the SUVmax ratio is close to 1.8 or the CT features are non diagnostic. | Adrenal CT or MRI |
| Adrenal: patients with cancerb | The combination of FDG uptake ≤ liver or CT criteria: HU < 10 or macroscopic fat is highly accurate in distinguishing benign nodules from metastases68 An SUVmax ratio (adrenal:liver) of > 2.5 had been shown to exclude all benign nodules.70 Nodules not characterized by the above criteria require further evaluation |
Adrenal CT or MRI |
| Prostate | Test all males aged > 50 Test males aged 40–50 years if the focus is peripheral, they are of black ethnicity or have affected first degree relatives. |
PSA testing |
| Ovaries | Post-menopausal females: refer increased ovarian/adnexal uptake. Pre-menopausal females: refer ovarian uptake which is not occurring around midcycleor uptake at these times which is associated with concerning CT features. |
Pelvic ultrasound |
| Uterus | Post-menopausal: refer endometrial uptake unless it has typical appearances of fibroids with low grade uptake. Pre-menopausal: Uptake occurring outside of midcycle or menstruation or fibroid related FDG uptake significantly above liver79, 82 should be considered for further evaluation |
Pelvic ultrasound |
| Testis | Insufficient evidence | |
| Bone | Foci without CT abnormalities: insufficient evidence for patients without cancer. Consider focus as suspicious for a metastasis in patients with cancer |
Consider MRI |
aLimitations in SUV reproducibility and individual patient characteristics, particularly other life-limiting comorbidities and patient choice, should be taken into account when using this evidence.
bUsing PET-CT to characterize nodules < 1 cm should be done with caution.
FDG, fluorodeoxyglucose; PSA, prostate specific antigen; SUV, standardized uptake value.
Conclusion
Interpreting incidental FDG uptake on PET/CT can be challenging, However there is published evidence about its aetiology; parameters to distinguish different causes of uptake and the CT can provide further useful diagnostic information. These findings, together with knowledge of the patient’s risk factors, allows evidence-based suggestions to be made and is likely to lead to improved patient management. However, SUVs can vary between patient, institution and over time and this needs to be taken into account when using the evidence. Finally, for some organs, there is very little evidence on the significance of incidental uptake and these areas would benefit from further research.
Table 1.
Summary of evidence-based suggestions for further evaluation of incidental FDG uptake in the head and necka
| Site of focal uptake | Evidence/when to further evaluate | Recommended form of evaluation |
| Pituitary gland | Foci with SUVmax > 4.17 | Pituitary MRI and/or hormonal testing |
| Piriform fossa | Foci with SUVmax ≥ 4.28 | Referral to ENT for direct visualization |
| Foci with SUVmaxI : SUVmaxC ≥ 1.8 | ||
| Palatine tonsils | Foci with SUVmax ratio between tonsils > 1.59, 10 | |
| Nasopharynx | Foci with SUVmax > 6.011 | |
| Oro and nasopharynx not specified above | Inadequate evidence for foci not meeting the above criteria. | |
| Parotid: patients without cancer | Consider referring all foci, as common benign causes usually require further evaluation and some incidental malignancies occur. | Ultrasound, MRI or referral to ENT |
| Parotid: patients with head and neck malignancy | Focus is much more likely to be either a metastasis from the known cancer or benign.14 Further evaluation may well be unnecessary | |
| Thyroid | All foci | Ultrasound ± FNA |
aLimitations in SUV reproducibility and individual patient characteristics, particularly other life-limiting comorbidities and patient choice, should be taken into account when using this evidence.
ENT, ear, nose and throat; FDG, fluorodeoxyglucose; FNA, fine-needle aspiration; SUV, standardized uptake value.
Table 2.
Summary of evidence-based suggestions for further evaluation of incidental FDG uptake in the thoraxa
| Site of focal uptake | Evidence/when to further evaluate | Recommended form of evaluation |
| Breast | All foci | Referral to the breast team |
| Lung | Incidental foci without a CT correlate can be assumed to be a microembolus of FDG33 | No follow up required |
| CT nodules demonstrating increased FDG uptake have a higher risk of malignancy37 | Refer to lung MDT/lung cancer specialist or follow up with CT depending on the risk of malignancy | |
| Oesophagus and GOJ | Foci with the following characteristics are associated with malignancy: increased focality, eccentricity43 and increased intensity of uptake (SUVmax > 6)42 | Routinely refer foci with these characteristics for endoscopy |
aLimitations in SUV reproducibility and individual patient characteristics, particularly other life-limiting comorbidities and patient choice, should be taken into account when using this evidence.
FDG, fluorodeoxyglucose; GOJ, gastro-oesophageal junction; MDT, multidisciplinary team; SUV, standardized uptake value.
Contributor Information
Deborah Pencharz, Email: deborah.pencharz@bsuh.nhs.uk.
Malavika Nathan, Email: malavika.nathan@nhs.net.
Thomas L Wagner, Email: thomas.wagner@nhs.net.
REFERENCES
- 1.Sone Y, Sobajima A, Kawachi T, Kohara S, Kato K, Naganawa S. Ability of 18-fludeoxyglucose positron emission tomography/CT to detect incidental cancer. Br J Radiol 2014; 87: 20140030. doi: 10.1259/bjr.20140030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G, Lau EW, Shakher R, Rischin D, Ware RE, Hong E, et al. How do oncologists deal with incidental abnormalities on whole-body fluorine-18 fluorodeoxyglucose PET/CT? Cancer 2007; 109: 117–24. doi: 10.1002/cncr.22370 [DOI] [PubMed] [Google Scholar]
- 3.Kinahan PE, Fletcher JW. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR 2010; 31: 496–505. doi: 10.1053/j.sult.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am J Roentgenol 2010; 195: 310–20. doi: 10.2214/AJR.10.4923 [DOI] [PubMed] [Google Scholar]
- 5.Akamatsu G, Mitsumoto K, Taniguchi T, Tsutsui Y, Baba S, Sasaki M. Influences of point-spread function and time-of-flight reconstructions on standardized uptake value of lymph node metastases in FDG-PET. Eur J Radiol 2014; 83: 226–30. doi: 10.1016/j.ejrad.2013.09.030 [DOI] [PubMed] [Google Scholar]
- 6.Reynés-Llompart G, Gámez-Cenzano C, Romero-Zayas I, Rodríguez-Bel L, Vercher-Conejero JL, Martí-Climent JM. Performance characteristics of the whole-body discovery IQ PET/CT system. J Nucl Med 2017; 58: 1155–61. doi: 10.2967/jnumed.116.185561 [DOI] [PubMed] [Google Scholar]
- 7.Hyun SH, Choi JY, Lee KH, Choe YS, Kim BT. Incidental focal 18F-FDG uptake in the pituitary gland: clinical significance and differential diagnostic criteria. J Nucl Med 2011; 52: 547–50. doi: 10.2967/jnumed.110.083733 [DOI] [PubMed] [Google Scholar]
- 8.Cho YS, Moon SH, Choi JY, Choe YS, Kim BT, Lee KH. Clinical significance of incidental 18F-FDG uptake in the pyriform sinus detected by PET/CT. Clin Nucl Med 2016; 41: e82–e86. doi: 10.1097/RLU.0000000000000992 [DOI] [PubMed] [Google Scholar]
- 9.Davison JM, Ozonoff A, Imsande HM, Grillone GA, Subramaniam RM. Squamous cell carcinoma of the palatine tonsils: FDG standardized uptake value ratio as a biomarker to differentiate tonsillar carcinoma from physiologic uptake. Radiology 2010; 255: 578–85. doi: 10.1148/radiol.10091479 [DOI] [PubMed] [Google Scholar]
- 10.Lee HJ, Kim JS, Roh JL, Lee JH, Cho KJ, Park GC, et al. Utility of quantitative 18 F-fluorodeoxyglucose uptake measurement to identify occult tonsillar carcinoma in patients with cervical metastasis of unknown primary tumours: a retrospective case-control study. Clin Otolaryngol 2013; 38: 30–8. doi: 10.1111/coa.12055 [DOI] [PubMed] [Google Scholar]
- 11.Lee N, Yoo I, Park SY, Yoon H, Lee Y, Oh JK. Significance of incidental nasopharyngeal uptake on 18F-FDG PET/CT: patterns of benign/physiologic uptake and differentiation from malignancy. Nucl Med Mol Imaging 2015; 49: 11–18. doi: 10.1007/s13139-014-0299-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heusner TA, Hahn S, Hamami ME, Kögel S, Forsting M, Bockisch A, et al. Incidental head and neck 18F-FDG uptake on PET/CT without corresponding morphological lesion: early predictor of cancer development? Eur J Nucl Med Mol Imaging 2009; 36: 1397–406. doi: 10.1007/s00259-009-1113-1 [DOI] [PubMed] [Google Scholar]
- 13.Sankaranarayanan R, Masuyer E, Swaminathan R, Ferlay J, Whelan S. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res 1998; 18: 4779–86. [PubMed] [Google Scholar]
- 14.Mabray MC, Behr SC, Naeger DM, Flavell RR, Glastonbury CM. Predictors of pathologic outcome of focal FDG uptake in the parotid gland identified on whole-body FDG PET imaging. Clin Imaging 2015; 39: 1073–9. doi: 10.1016/j.clinimag.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makis W, Ciarallo A, Gotra A. Clinical significance of parotid gland incidentalomas on 18F-FDG PET/CT. Clin Imaging 2015; 39: 667–71. doi: 10.1016/j.clinimag.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 16.Park SB, Choi JY, Lee EJ, Yoo J, Cheon M, Cho SK, et al. Diagnostic criteria on 18F-FDG PET/CT for differentiating benign from malignant focal hypermetabolic lesions of parotid gland. Nucl Med Mol Imaging 2012; 46: 95–101. doi: 10.1007/s13139-012-0135-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emodi O, El-Naaj IA, Gordin A, Akrish S, Peled M. Superficial parotidectomy versus retrograde partial superficial parotidectomy in treating benign salivary gland tumor (pleomorphic adenoma). J Oral Maxillofac Surg 2010; 68: 2092–8. doi: 10.1016/j.joms.2009.09.075 [DOI] [PubMed] [Google Scholar]
- 18.O'Brien CJ. Current management of benign parotid tumors-the role of limited superficial parotidectomy. Head Neck 2003; 25: 946–52. doi: 10.1002/hed.10312 [DOI] [PubMed] [Google Scholar]
- 19.Barrio M, Czernin J, Yeh MW, Palma Diaz MF, Gupta P, Allen-Auerbach M, et al. The incidence of thyroid cancer in focal hypermetabolic thyroid lesions: an 18F-FDG PET/CT study in more than 6000 patients. Nucl Med Commun 2016; 37: 1290–6. doi: 10.1097/MNM.0000000000000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agrawal K, Weaver J, Ngu R, Krishnamurthy Mohan H. Clinical significance of patterns of incidental thyroid uptake at 18F-FDG PET/CT. Clin Radiol 2015; 70: 536–43. doi: 10.1016/j.crad.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 21.Soelberg KK, Bonnema SJ, Brix TH, Hegedüs L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid 2012; 22: 918–25. doi: 10.1089/thy.2012.0005 [DOI] [PubMed] [Google Scholar]
- 22.Brindle R, Mullan D, Yap BK, Gandhi A. Thyroid incidentalomas discovered on positron emission tomography CT scanning - malignancy rate and significance of standardised uptake values. Eur J Surg Oncol 2014; 40: 1528–32. doi: 10.1016/j.ejso.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 23.Agrawal K, Weaver J, Ul-Hassan F, Jeannon JP, Simo R, Carroll P, et al. Incidence and significance of incidental focal thyroid uptake on (18F-FDG PET study in a large patient cohort: retrospective single-centre experience in the United Kingdom. Eur Thyroid J 2015; 4: 115: 115–22. doi: 10.1159/000431319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stangierski A, Woliński K, Czepczyński R, Czarnywojtek A, Lodyga M, Wyszomirska A, et al. The usefulness of standardized uptake value in differentiation between benign and malignant thyroid lesions detected incidentally in 18F-FDG PET/CT examination. PLoS One 2014; 9: e109612. doi: 10.1371/journal.pone.0109612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Hwang SH, Cha J, Jo K, Lee N, Yun M. Risk stratification of thyroid incidentalomas found on PET/CT: the value of iodine content on noncontrast computed tomography. Thyroid 2015; 25: 1249–54. doi: 10.1089/thy.2015.0200 [DOI] [PubMed] [Google Scholar]
- 26.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26: 1–133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoang JK, Langer JE, Middleton WD, Wu CC, Hammers LW, Cronan JJ, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR incidental thyroid findings committee. J Am Coll Radiol 2015; 12: 143–50. doi: 10.1016/j.jacr.2014.09.038 [DOI] [PubMed] [Google Scholar]
- 28.Shin KM, Kim HJ, Jung SJ, Lim HS, Lee SW, Cho SH, et al. Incidental breast lesions identified by 18F-FDG PET/CT: which clinical variables differentiate between benign and malignant breast lesions? J Breast Cancer 2015; 18: 73–9. doi: 10.4048/jbc.2015.18.1.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chae EY, Cha JH, Kim HH, Shin HJ, Kim HJ, Oh HY, et al. Analysis of incidental focal hypermetabolic uptake in the breast as detected by 18F-FDG PET/CT: clinical significance and differential diagnosis. Acta Radiol 2012; 53: 530–5. doi: 10.1258/ar.2012.120015 [DOI] [PubMed] [Google Scholar]
- 30.Inoue M, Sano T, Watai R, Ashikaga R, Ueda K, Watatani M, et al. Dynamic multidetector CT of breast tumors: diagnostic features and comparison with conventional techniques. AJR Am J Roentgenol 2003; 181: 679–86. doi: 10.2214/ajr.181.3.1810679 [DOI] [PubMed] [Google Scholar]
- 31.Bach AG, Abbas J, Jasaabuu C, Schramm D, Wienke A, Surov A. Comparison between incidental malignant and benign breast lesions detected by computed tomography: a systematic review. J Med Imaging Radiat Oncol 2013; 57: 529–33. doi: 10.1111/1754-9485.12046 [DOI] [PubMed] [Google Scholar]
- 32.Adejolu M, Huo L, Rohren E, Santiago L, Yang WT. False-positive lesions mimicking breast cancer on FDG PET and PET/CT. AJR Am J Roentgenol 2012; 198: W304–W314. doi: 10.2214/AJR.11.7130 [DOI] [PubMed] [Google Scholar]
- 33.Chondrogiannis S, Marzola MC, Grassetto G, Zorzi A, Milan E, Rampin L, et al. 18F-FDG PET/CT lung 'focalities' without coregistered CT findings: an interpretative clinical dilemma. Nucl Med Commun 2015; 36: 334–9. doi: 10.1097/MNM.0000000000000261 [DOI] [PubMed] [Google Scholar]
- 34.Farsad M, Ambrosini V, Nanni C, Castellucci P, Boschi S, Rubello D, et al. Focal lung uptake of 18F-fluorodeoxyglucose (18F-FDG) without computed tomography findings. Nucl Med Commun 2005; 26: 827–30. doi: 10.1097/01.mnm.0000175786.27423.42 [DOI] [PubMed] [Google Scholar]
- 35.Ozdemir E, Poyraz NY, Keskin M, Kandemir Z, Turkolmez S. Hot-clot artifacts in the lung parenchyma on F-18 fluorodeoxyglucose positron emission tomography/CT due to faulty injection techniques: two case reports. Korean J Radiol 2014; 15: 530–3. doi: 10.3348/kjr.2014.15.4.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British thoracic society guidelines for the investigation and management of pulmonary nodules. Thorax 2015; 70(Suppl 2): ii1–ii54. doi: 10.1136/thoraxjnl-2015-207168 [DOI] [PubMed] [Google Scholar]
- 37.Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, Naidich DP, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2013; 143(Suppl 5): e93S–120. doi: 10.1378/chest.12-2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herder GJ, van Tinteren H, Golding RP, Kostense PJ, Comans EF, Smit EF, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005; 128: 2490–6. doi: 10.1378/chest.128.4.2490 [DOI] [PubMed] [Google Scholar]
- 39.Society BT. 2017. https://www.britthoracic.org.uk/standards-of-care/guidelines/bts-guidelines-for-the-investigation-and-managementof-pulmonary-nodules/bts-pulmonary-nodule-risk-prediction-calculator/ [cited 2017 19th November].
- 40.Kostakoglu L, Agress H, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics 2003; 23: 315–40. doi: 10.1148/rg.232025705 [DOI] [PubMed] [Google Scholar]
- 41.Heusner TA, Hahn S, Hamami ME, Kim UH, Baumeister R, Forsting M, et al. Gastrointestinal 18F-FDG accumulation on PET without a corresponding CT abnormality is not an early indicator of cancer development. Eur Radiol 2009; 19: 2171–9. doi: 10.1007/s00330-009-1405-7 [DOI] [PubMed] [Google Scholar]
- 42.Stagg J, Farukhi I, Lazaga F, Thompson C, Bradshaw L, Kaif M, et al. Significance of 18F-Fluorodeoxyglucose uptake at the gastroesophageal junction: comparison of PET to esophagogastroduodenoscopy. Dig Dis Sci 2015; 60: 1335–42. doi: 10.1007/s10620-014-3456-0 [DOI] [PubMed] [Google Scholar]
- 43.Roedl JB, Colen RR, King K, Fischman AJ, Mueller PR, Blake MA. Visual PET/CT scoring for nonspecific 18F-FDG uptake in the differentiation of early malignant and benign esophageal lesions. AJR Am J Roentgenol 2008; 191: 515–21. doi: 10.2214/AJR.07.3320 [DOI] [PubMed] [Google Scholar]
- 44.Le Roux PY, Duong CP, Cabalag CS, Parameswaran BK, Callahan J, Hicks RJ. Incremental diagnostic utility of gastric distension FDG PET/CT. Eur J Nucl Med Mol Imaging 2016; 43: 644–53. doi: 10.1007/s00259-015-3211-6 [DOI] [PubMed] [Google Scholar]
- 45.Treglia G, Taralli S, Salsano M, Muoio B, Sadeghi R, Giovanella L. Prevalence and malignancy risk of focal colorectal incidental uptake detected by 18F-FDG-PET or PET/CT: a meta-analysis. Radiol Oncol 2014; 48: 99–104. doi: 10.2478/raon-2013-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Hoeij FB, Keijsers RG, Loffeld BC, Dun G, Stadhouders PH, Weusten BL. Incidental colonic focal FDG uptake on PET/CT: can the maximum standardized uptake value (SUVmax) guide us in the timing of colonoscopy? Eur J Nucl Med Mol Imaging 2015; 42: 66–71. doi: 10.1007/s00259-014-2887-3 [DOI] [PubMed] [Google Scholar]
- 47.Pitts A, Nissen NN, Waxman A, Yu R. Unsuspected fluorodeoxyglucose positron emission tomography (FDG-PET)-positive pancreatic lesions: prevalence and significance. Pancreas 2013; 42: 1191–3. doi: 10.1097/MPA.0b013e318287d06e [DOI] [PubMed] [Google Scholar]
- 48.Sahani DV, Bonaffini PA, Catalano OA, Guimaraes AR, Blake MA. State-of-the-art PET/CT of the pancreas: current role and emerging indications. Radiographics 2012; 32: 1133–58. doi: 10.1148/rg.324115143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grassetto G, Marzola MC, Chondrogiannis S, Maffione AM, Rampin L, Galeotti F, et al. Potential role of FDG PET/CT in evaluating patients with hepatic incidentalomas. Clin Nucl Med 2014; 39: 156–9. doi: 10.1097/RLU.0000000000000312 [DOI] [PubMed] [Google Scholar]
- 50.Delbeke D, Martin WH, Sandler MP, Chapman WC, Wright JK, Pinson CW. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch Surg 1998; 133: 510–5. doi: 10.1001/archsurg.133.5.510 [DOI] [PubMed] [Google Scholar]
- 51.Tan GJ, Berlangieri SU, Lee ST, Scott AM. FDG PET/CT in the liver: lesions mimicking malignancies. Abdom Imaging 2014; 39: 187–95. doi: 10.1007/s00261-013-0043-3 [DOI] [PubMed] [Google Scholar]
- 52.Ramos-Font C, Gómez-Rio M, Rodríguez-Fernández A, Jiménez-Heffernan A, Sánchez Sánchez R, Llamas-Elvira JM. Ability of FDG-PET/CT in the detection of gallbladder cancer. J Surg Oncol 2014; 109: 218–24. doi: 10.1002/jso.23476 [DOI] [PubMed] [Google Scholar]
- 53.Maldjian PD, Ghesani N, Ahmed S, Liu Y. Adenomyomatosis of the gallbladder: another cause for a "hot" gallbladder on 18F-FDG PET. AJR Am J Roentgenol 2007; 189: W36–W38. doi: 10.2214/AJR.05.1284 [DOI] [PubMed] [Google Scholar]
- 54.Ewelukwa O, Ali O, Akram S. Xanthogranulomatous cholecystitis mimicking gallbladder cancer. BMJ Case Rep 2014; 2014: bcr2013200530. doi: 10.1136/bcr-2013-200530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Annunziata S, Pizzuto DA, Caldarella C, Galiandro F, Sadeghi R, Treglia G. Diagnostic accuracy of fluorine-18-fluorodeoxyglucose positron emission tomography in gallbladder cancer: a meta-analysis. World J Gastroenterol 2015; 21: 11481–8. doi: 10.3748/wjg.v21.i40.11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park YJ, Lee JH, Jee KN, Namgung H. Incidental detection of temporary focal FDG retention in the spleen. Nucl Med Mol Imaging 2011; 45: 158–60. doi: 10.1007/s13139-011-0078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong A, Wang Y, Lu J, Zuo C, Enhanced CT. Enhanced CT and FDG PET/CT findings of splenic hamartoma. Clin Nucl Med 2014; 39: 968–71. doi: 10.1097/RLU.0b013e3182a75ccc [DOI] [PubMed] [Google Scholar]
- 58.Dong A, Wang Y, Lu J, Zuo C. FDG uptake in splenic epidermoid cyst with hemorrhage. Clin Nucl Med 2014; 39: 339–41. doi: 10.1097/RLU.0b013e318281652c [DOI] [PubMed] [Google Scholar]
- 59.Ji T, Kuang A. 18F-FDG PET/CT findings in a splenic lymphangioma. Clin Nucl Med 2015; 40: e375–e377. doi: 10.1097/RLU.0000000000000762 [DOI] [PubMed] [Google Scholar]
- 60.Rao L, Yang Z, Wang X, Zhang X, Shen B. Imaging findings of inflammatory pseudotumor-like follicular dendritic cell tumor of spleen. Clin Nucl Med 2014; 39: e286–e289. doi: 10.1097/RLU.0b013e3182952bfe [DOI] [PubMed] [Google Scholar]
- 61.Kochhar R, Brown RK, Wong CO, Dunnick NR, Frey KA, Manoharan P. Role of FDG PET/CT in imaging of renal lesions. J Med Imaging Radiat Oncol 2010; 54: 347–57. doi: 10.1111/j.1754-9485.2010.02181.x [DOI] [PubMed] [Google Scholar]
- 62.Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, et al. Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol 2010; 7: 754–73. doi: 10.1016/j.jacr.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 63.Sebastian S, Araujo C, Neitlich JD, Berland LL. Managing incidental findings on abdominal and pelvic CT and MRI, part 4: white paper of the ACR incidental findings committee II on gallbladder and biliary findings. J Am Coll Radiol 2013; 10: 953–6. doi: 10.1016/j.jacr.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 64.Heller MT, Harisinghani M, Neitlich JD, Yeghiayan P, Berland LL. Managing incidental findings on abdominal and pelvic CT and MRI, part 3: white paper of the ACR incidental findings committee II on splenic and nodal findings. J Am Coll Radiol 2013; 10: 833–9. doi: 10.1016/j.jacr.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 65.Furtado CD, Aguirre DA, Sirlin CB, Dang D, Stamato SK, Lee P, et al. Whole-body CT screening: spectrum of findings and recommendations in 1192 patients. Radiology 2005; 237: 385–94. doi: 10.1148/radiol.2372041741 [DOI] [PubMed] [Google Scholar]
- 66.Boland GW, Dwamena BA, Jagtiani Sangwaiya M, Goehler AG, Blake MA, Hahn PF, et al. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic test performance. Radiology 2011; 259: 117–26. doi: 10.1148/radiol.11100569 [DOI] [PubMed] [Google Scholar]
- 67.Tessonnier L, Sebag F, Palazzo FF, Colavolpe C, De Micco C, Mancini J, et al. Does 18F-FDG PET/CT add diagnostic accuracy in incidentally identified non-secreting adrenal tumours? Eur J Nucl Med Mol Imaging 2008; 35: 2018–25. doi: 10.1007/s00259-008-0849-3 [DOI] [PubMed] [Google Scholar]
- 68.Boland GW, Blake MA, Holalkere NS, Hahn PF. PET/CT for the characterization of adrenal masses in patients with cancer: qualitative versus quantitative accuracy in 150 consecutive patients. AJR Am J Roentgenol 2009; 192: 956–62. doi: 10.2214/AJR.08.1431 [DOI] [PubMed] [Google Scholar]
- 69.Boland GW, Lee MJ, Gazelle GS, Halpern EF, McNicholas MM, Mueller PR. Characterization of adrenal masses using unenhanced CT: an analysis of the CT literature. AJR Am J Roentgenol 1998; 171: 201–4. doi: 10.2214/ajr.171.1.9648789 [DOI] [PubMed] [Google Scholar]
- 70.Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: a proposal for an efficient diagnostic algorithm. Radiology 2009; 250: 523–30. doi: 10.1148/radiol.2502080219 [DOI] [PubMed] [Google Scholar]
- 71.Bertagna F, Sadeghi R, Giovanella L, Treglia G. Incidental uptake of 18F-fluorodeoxyglucose in the prostate gland. Systematic review and meta-analysis on prevalence and risk of malignancy. Nuklearmedizin 2014; 53: 249–58. doi: 10.3413/Nukmed-0668-14-05 [DOI] [PubMed] [Google Scholar]
- 72.Bertagna F, Piccardo A, Dib B, Bertoli M, Fracassi F, Bosio G, et al. Multicentre study of 18F-FDG-PET/CT prostate incidental uptake. Jpn J Radiol 2015; 33: 538–46. doi: 10.1007/s11604-015-0453-y [DOI] [PubMed] [Google Scholar]
- 73.Cancer research UK. Prostate cancer incidence statistics. 2016. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence [cited 2016 7th June]
- 74.Parker PM, Rice KR, Sterbis JR, Chen Y, Cullen J, McLeod DG, et al. Prostate cancer in men less than the age of 50: a comparison of race and outcomes. Urology 2011; 78: 110–5. doi: 10.1016/j.urology.2010.12.046 [DOI] [PubMed] [Google Scholar]
- 75.Lloyd T, Hounsome L, Mehay A, Mee S, Verne J, Cooper A. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008–2010. BMC Med 2015; 13: 171. doi: 10.1186/s12916-015-0405-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeegers MP, Jellema A, Ostrer H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma: a meta-analysis. Cancer 2003; 97: 1894–903. doi: 10.1002/cncr.11262 [DOI] [PubMed] [Google Scholar]
- 77.Nishizawa S, Inubushi M, Okada H. Physiological 18F-FDG uptake in the ovaries and uterus of healthy female volunteers. Eur J Nucl Med Mol Imaging 2005; 32: 549–56. doi: 10.1007/s00259-004-1703-x [DOI] [PubMed] [Google Scholar]
- 78.Lerman H, Metser U, Grisaru D, Fishman A, Lievshitz G, Even-Sapir E. Normal and abnormal 18F-FDG endometrial and ovarian uptake in pre- and postmenopausal patients: assessment by PET/CT. J Nucl Med 2004; 45: 266–71. [PubMed] [Google Scholar]
- 79.Lin CY, Ding HJ, Chen YK, Liu CS, Lin CC, Kao CH. F-18 FDG PET in detecting uterine leiomyoma. Clin Imaging 2008; 32: 38–41. doi: 10.1016/j.clinimag.2007.07.006 [DOI] [PubMed] [Google Scholar]
- 80.Kohan A, Avril NE. Pelvis: normal variants and benign findings in FDG-PET/CT imaging. PET Clin 2014; 9: 185–93. doi: 10.1016/j.cpet.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 81.Nishizawa S, Inubushi M, Kido A, Miyagawa M, Inoue T, Shinohara K, et al. Incidence and characteristics of uterine leiomyomas with FDG uptake. Ann Nucl Med 2008; 22: 803–10. doi: 10.1007/s12149-008-0184-6 [DOI] [PubMed] [Google Scholar]
- 82.Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Standardized uptake values of uterine leiomyoma with 18F-FDG PET/CT: variation with age, size, degeneration, and contrast enhancement on MRI. Ann Nucl Med 2008; 22: 505–12. doi: 10.1007/s12149-008-0135-2 [DOI] [PubMed] [Google Scholar]
- 83.Zhao Z, Yoshida Y, Kurokawa T, Kiyono Y, Mori T, Okazawa H. 18F-FES and 18F-FDG PET for differential diagnosis and quantitative evaluation of mesenchymal uterine tumors: correlation with immunohistochemical analysis. J Nucl Med 2013; 54: 499–506. doi: 10.2967/jnumed.112.113472 [DOI] [PubMed] [Google Scholar]
- 84.Kitajima K, Murakami K, Kaji Y, Sugimura K. Spectrum of FDG PET/CT findings of uterine tumors. AJR Am J Roentgenol 2010; 195: 737–43. doi: 10.2214/AJR.09.4074 [DOI] [PubMed] [Google Scholar]
- 85.Fenchel S, Grab D, Nuessle K, Kotzerke J, Rieber A, Kreienberg R, et al. Asymptomatic adnexal masses: correlation of FDG PET and histopathologic findings. Radiology 2002; 223: 780–8. doi: 10.1148/radiol.2233001850 [DOI] [PubMed] [Google Scholar]
- 86.Cancer Research UK. 2017. cruk.org/cancerstats. http://www.cancerresearchuk.org/sites/default/files/cstream-node/cases_crude_uterus_I14.pdf [cited 2017 9th October].
- 87.Sidhu P, Lin P, Son H, Rosenfeld D, Lin M. Testicular fluorine-18 fludeoxyglucose uptake on positron emission tomography CT in patients with lymphoma: clinical significance and management impact. Br J Radiol 2014; 87: 20140472. doi: 10.1259/bjr.20140472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol 2011; 21: 2604–17. doi: 10.1007/s00330-011-2221-4 [DOI] [PubMed] [Google Scholar]
- 89.Taira AV, Herfkens RJ, Gambhir SS, Quon A. Detection of bone metastases: assessment of integrated FDG PET/CT imaging. Radiology 2007; 243: 204–11. doi: 10.1148/radiol.2431052104 [DOI] [PubMed] [Google Scholar]