Abstract
Growing emphasis on precision medicine in oncology has led to increasing use of targeted therapies that encompass a spectrum of drug classes including angiogenesis inhibitors, immune modulators, signal transduction inhibitors, DNA damage modulators, hormonal agents etc. Immune therapeutic drugs constitute a unique group among the novel therapeutic agents that are transforming cancer treatment, and their use is rising. The imaging manifestations in patients on immune therapies appear to be distinct from those typically seen with conventional cytotoxic therapies. Patients on immune therapies may demonstrate a delayed response, transient tumour enlargement followed by shrinkage, stable size, or initial appearance of new lesions followed by stability or response. These newer patterns of response to treatment have rendered conventional criteria such as World Health Organization and response evaluation criteria in solid tumours suboptimal in monitoring changes in tumour burden. As a consequence, newer imaging response criteria such as immune-related response evaluation criteria in solid tumours and immune-related response criteria are being implemented in many trials to effectively monitor patients on immune therapies. In this review, we discuss the traditional and new imaging response criteria for evaluation of solid tumours, review the outcomes of various articles which compared traditional criteria with the new immune-related criteria and discuss pseudo-progression and immune-related adverse events.
Introduction
Response evaluation with diagnostic imaging has evolved substantially over the past three decades since the initial efforts to standardize and systematically define response assessment was done with the introduction of World Health Organization (WHO) criteria in 1979.1,2 WHO criteria introduced the concept of determining tumour response to systemic therapies by two-dimensional measurement of tumour burden and categorizing treatment efficacy based on percentage changes in tumour burden compared to baseline scans performed before treatment initiation.2 Although a pioneer approach for standardizing treatment response assessment, WHO criteria posed challenges to routine use including lack of definitions for minimum size of the lesion to be measured and total number of lesions to be considered in assessing tumour burden, as well as potential exaggeration of magnitude of changes in tumour burden due to consideration of product of perpendicular diameters, which in some cases resulted in early progression, denying patients’ continued access to the clinical drug trial.3
To overcome these limitations, Response Evaluation Criteria In Solid Tumours (RECIST) criteria was proposed in 2000 by the U.S. National Cancer Institute, European Organization for Research and Treatment of Cancer and WHO.4 RECIST addressed the shortcomings of WHO criteria and established specific guidelines for tumour response assessment including minimum lesion size, total number of measurable lesions and clear-cut guidelines for assessing response and determining progression.4 RECIST also simplified tumour measurements by allowing single long axis tumour diameter instead of two-dimensional measurements. A revised version of RECIST was established in 2009 as RECIST 1.1 based on the statistical analysis of a database with around 6500 patients to incorporate updated assessment of new lesions, lymph nodes, bone lesions and cystic and necrotic lesions.5, 6 These criteria consider therapeutic success as reduction in tumour burden without any new lesions, whereas early tumour growth and appearance of new lesions are considered as treatment failure.7 Ever since their introduction, clinical trials have confirmed the role of RECIST 1.0 and 1.1 for assessment of therapeutic effectiveness for a wide range of cytotoxic chemotherapeutic agents and their response criteria have been shown to correlate with patient outcome.8, 9 Despite the tremendous success of size-based criteria such as RECIST in assessing response to various solid tumours, their principal shortcoming is that they are primarily designed to estimate response to therapy based on decrease in tumour size following cytotoxic therapy and are not optimal to gauge antitumour activity other than shrinkage as seen with new cytostatic agents including immune therapeutic drugs. Additionally, the unidimensional approach to monitoring changes in tumour burden, which does not take into consideration other parameters such as tumour enhancement, has led to constant attempts at modifications to RECIST such as modified RECIST (mRECIST) in hepatocellular carcinoma.
Newer therapies–cancer immunotherapy
There has been a paradigm shift in oncology drug development in recent years with the rise in use of targeted therapeutic agents for cancer treatment.10 Targeted therapy includes a wide spectrum of drug classes including angiogenesis inhibitors, immune modulators, signal transduction inhibitors, DNA damage modulators and hormonal agents. Many targeted therapies induce a cytostatic effect by enhancing antitumour immune responses and are not cytocidal like conventional chemotherapeutic agents.11 Immuno-modulator or immune checkpoint inhibitor drugs act by inhibiting regulatory steps in the immune system, thereby promoting proliferation and activation of T-cells to induce tumour infiltration and regression.10
This field of oncological immunotherapy has rapidly expanded with the approval of a handful of medications and nearly 1,500 cancer immunotherapy trials listed on the U.S. National Institutes of Health ClinicalTrials.gov registry.12 Three main types of drugs – Cytotoxic T-Lymphocyte antigen-4 (CTLA-4) antibodies (Ipilimumab, Tremelimumab); Programmed cell Death (PD-1) antibodies; and Programmed cell Death Ligand (PD-L1, PD-L2) antibodies (Nivolumab, Pembrolizumab, Atezolizumab) are currently under study and have been approved by the US Food and Drug Administration (FDA)13 (Table 1). CTLA-4 is expressed completely on T cells where it principally regulates the amplitude of the early stages of T cell activation by counteracting the activity of the T cell co-stimulatory receptor, CD28. CD28 and CTLA-4 share identical ligands: CD80 and CD86.14 CTLA–4 expression on the surface of T cells decreases the activation of T cells by outcompeting CD28 in binding CD80 and CD86, besides actively distributing inhibitory signals to the T cell. PD–1 is a transmembrane inhibitory protein expressed not only on T-cells, but also on B-cells and natural killer cells, which binds to ligands PD-L1 and PD-L2. In contrast to CTLA-4, PD-1 limits the T cell activity in autoimmunity and in peripheral tissues at the time of an inflammatory response to infection. PD-1 blockade enhances the activity of effector T cells in tissues and in the tumour microenvironment.15
Table 1.
FDA approved immune-checkpoint inhibitors
| Drug | Mechanism of action | Brand name/Pharma | Disease group | Approval |
| Pembrolizumab | PD-1 inhibitor | Keytruda/Merck | Melanoma, Lung, Head and Neck cancers, Hodgkin’s lymphoma, Urothelial tumours | FDA EMA |
| Ipilimumab | Anti–CTLA-4 Antibody | Yervoy/Bristol Myers Squibb | Melanoma, Urothelial tumours | FDA EMA |
| Nivolumab | PD-1 inhibitor | Opdivo/Bristol Myers Squibb | Melanoma, Lung, Renal, Head and Neck cancers and Hodgkin’s lymphoma | FDA EMA |
| Atezolizumab | PD-L1 inhibitor | Tecentriq/Genentech | Bladder and Lung cancers | FDA |
| Nivolumab + Ipilimumab | PD-1 inhibitor + Anti–CTLA-4 Antibody | Opdivo + Yervoy/ Bristol-Myers Squibb | Melanoma, Relapsed or Refractory Haematological Malignancies | FDA EMA |
| Avelumab | PD-L1 inhibitor | BAVENCIO/EMD Serono | Urothelial carcinoma, Merkle cell cancer | FDA EMA |
| Durvalumab | PD-L1 inhibitor | IMFINZI/AstraZeneca | Urothelial carcinoma | FDA |
CTLA-4, cytotoxic T-lymphocyte antigen-4; EMA, European Medicines Agency; FDA, food and drug administration; PD-1, programmed death-1; PD-L1, programmed death-ligand 1.
Multiple clinical trials are currently investigating combination regimens of immune modulator drugs as a few prior trials proved the synergistic effect of these drugs (Table 2).29 Randomized controlled trials in patients with advanced melanoma showed improved overall survival when ipilimumab (a CTLA-4 inhibitor) was used either alone or as an adjuvant therapy for high risk melanoma, as an alternative to interferon.30 In patients with advanced melanoma, Wolchok and colleagues showed that concurrent therapy with nivolumab and ipilimumab had a rapid and deep tumour regression in a substantial proportion of patients with a feasible safety profile and clinical activity, which is distinct from that in published data on monotherapy.24 Immune therapeutic agents have shown efficacy in other malignancies including non-small cell lung cancer (NSCLC).31 In a phase III trial of 305 patients with treatment-naïve metastatic NSCLC with PD-L1 expression on at least 50% of tumour cells, pembrolizumab was related with considerably longer progression-free survival (PFS) and overall survival and with fewer adverse events than was observed with platinum-based chemotherapy.31
Table 2. .
Trials using immunotherapeutic agents and immune-related response assessment criteria
| Author; journal and year | No. of patients/tumours and study design/disease group | Trial agent | Response criteria | Observations |
| Kim, Cancer Chemother Pharmacol, 201716 | 41 patients; retrospective; Non-small cell lung cancer | Immune checkpoint inhibitors | RECIST 1.1 and irRC | ORR was 29.2% [95% CI (17.6–44.5)] as assessed by RECIST and 34.1% [95% CI (21.6–49.4)] by irRC. Pseudo-progression was observed in RECIST 1.1 but not in irRC, which showed a durable response to immunotherapy. These atypical responses could be missed as PD by RECIST |
| Khoja L, Br J Cancer, 201617 | 37 patients; retrospective; metastatic melanoma | Pembrolizumab | RECIST 1.1, irRC | Delayed response post first scan was seen in 5% of RECIST PD cases and 14% of irRC PD cases. 5% (2 out of 37) of treated patients, who were initially characterized as PD by RECIST criteria, did go on to demonstrate some treatment benefit. |
| Rosenberg JE, Lancet. 201618 | 315 patients; multicentre phase II trial; urothelial cancer | Atezolizumab | RECIST 1.1 and irRECIST | Compared with a historical control overall response rate of 10%, treatment with atezolizumab resulted in a significantly improved RECIST v. 1.1 objective response rate for each pre-specified immune cell group {IC2/3: 27% [95% CI (19–37)], p < 0·0001; IC1/2/3: 18% (13–24), p = 0·0004} and in all patients [15% (11–20), p = 0·0058] |
| Wilgenhof S, J Clin Oncol. 201619 | 39 patients, phase II trial; melanoma | Autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab | irRC criteria | Six-month disease control rate according to the immune-related response criteria served as the primary end point. The 6-month disease control rate was 51% [95% CI (36–67%)], and the overall tumour response rate was 38% (including eight complete and seven partial responses) |
| McDermott DF, J Clin Oncol. 201620 | 70 patients, phase IA; renal cell cancer | Atezolizumab | RECIST 1.1 and irRC | Secondary end points assessed clinical activity per Response Evaluation Criteria in Solid Tumours v. 1.1 and immune-related response criteria |
| Hodi FS, J Clin Oncol. 201621 | 655 patients phase Ib; melanoma | Pembrolizumab | RECIST 1.1 and irRC criteria | Modified criteria that permit treatment beyond initial progression per RECIST v. 1.1 might prevent premature cessation of treatment, as conventional RECIST might underestimate the benefit of pembrolizumab in approximately 15% of patients |
| Chiarion Sileni V, J Exp Clin Cancer Res. 201422 | 188 patients retrospective; melanoma | Ipilimumab | irRC criteria | The immune-related disease control rate among 188 evaluable patients was 38%, including 4 for irCR, 24 with irPR and 44 with irSD |
| Bapsy PP, Cytotherapy. 201423 | 51 patients, multicentre, phase II; solid tumours | Autologous dendritic cell (DC) formulation | RECIST 1.1 and irRC criteria | Objective response rate by Response Evaluation Criteria In Solid Tumours was 28.9% (11/38) and immune-related response criteria was 42.1% (16/38); 90% confidence interval for objective response rate was (17.2, 43.3) and (28.5, 56.7) by Response Evaluation Criteria In Solid Tumours and immune-related response criteria, respectively |
| Wolchok JD, N Engl J Med. 201324 | 53 patients, phase I trial; melanoma | Nivolumab plus ipilimumab | irRC | Evidence of clinical activity (conventional, unconfirmed, or immune-related response or stable disease for ≥ 24 weeks) was observed in 65% of patients |
| Di Giacomo AM, Lancet Oncol. 201225 | 86 patients; open label, phase II trial; melanoma | Ipilimumab and fotemustine | irRC criteria | The primary endpoint was the proportion of patients with immune-related disease control as established with immune-related response criteria. 40 patients in the study population achieved disease control [46·5%, 95% CI (35·7–57·6)], as did 10 with brain metastases (50·0%, 27·2–72·8) |
| Lynch TJ, J Clin Oncol. 201226 | 204 patients, multicentre, phase II trial; non-small cell lung cancer | Ipilimumab with paclitaxel and carboplatin | irRC and modified WHO criteria | The study met its primary end point of improved irPFS for phased ipilimumab vs the control [hazard ratio (HR), 0.72; p = .05], but not for concurrent ipilimumab (HR, 0.81; p = 0.13). Phased ipilimumab also improved PFS according to modified WHO criteria (HR, 0.69; p = 0.02). Phased ipilimumab plus paclitaxel and carboplatin improved irPFS and PFS, which supports additional investigation of ipilimumab in NSCLC |
| Hamid O, J Transl Med. 201127 | 82 patients, phase II trial; melanoma | Ipilimumab | Modified WHO criteria | Baseline expression of immune-related tumour biomarkers and a post-treatment increase in tumour-infiltrating lymphocytes may be positively associated with ipilimumab clinical activity |
| O'Day SJ Ann Oncol. 201028 | 155 patients, multicentre, phase II trial; melanoma | Ipilimumab | irRC and modified WHO criteria | In patients with pre-treated advanced melanoma with primary endpoint of best overall response rate (BORR), irRC criteria showed a disease control rate of 35% as compared to 27% by WHO |
BORR, best overall response rate; CI, confidence interval; DC, dendritic cell; irPFS,immune-related PFS; irPR, immune-related Partial Response; irRC, immune-related response criteria; irRECIST, immune-related RECIST; irSD, immune-related Stable Disease; NSCLC, non-small cell lung cancer; ORR, overall response rate; PD, progressivedisease; PFS, progression free survival; RECIST, Response Evaluation CriteriaIn Solid Tumours; WHO, World Health Organization.
Immune-related criteria
Rationale
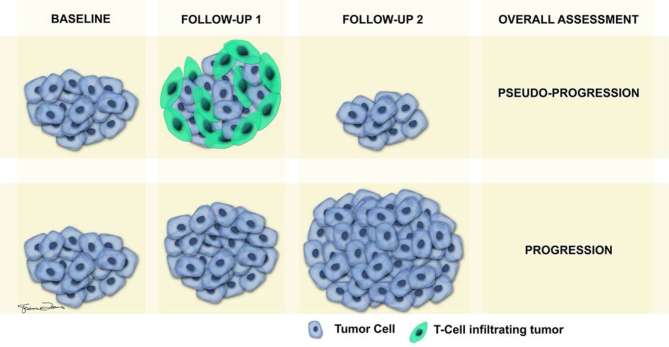
The emerging use of targeted immune therapies has led to the appearance of new patterns of treatment response, and conventional response assessment criteria such as RECIST might not be adequate in monitoring response to these therapies. RECIST relies on reduction in tumour size following cytotoxic therapy to assess response while the spectrum of manifestations of successful treatment response after immune therapeutic agents is varied and different. One of the major differences in tumour burden response to immune therapies compared to cytotoxic therapies is a longer lag time for suitable response, necessitating consideration of a durable stable disease (SD) to represent antitumour activity. Another unique non-conventional response associated with immune therapies is the enlargement of pre-existing lesions and development of new lesions during the initial phase of treatment, which would necessitate categorization as progressive disease (PD) with conventional criteria. However, in patients on immune therapeutic agents, therapeutic response (stabilization or improvement of tumour burden) can be observed in later follow-ups after initial enlargement and development of new lesions. The initial increase in tumour burden or development of new lesions during the initial phase of treatment with immunotherapies could be due to transient flare up and explained on a histological basis as either tumour growth until development of sufficient immune response or transient immune cell infiltrate.32, 33 The appearance of new lesions has been attributed to T cell infiltration into tumour deposits, which are undetectable radiologically at baseline scans (Figure 1).32, 34 Such a response should not be categorized as progression and the appropriate designation of those types of response is pseudo-progression. The patients manifesting with pseudo-progression are usually asymptomatic, whereas patients with true progression will show clinical worsening of symptoms. A short-term follow-up in 4–6 weeks generally confirms true vs pseudo-progression in these patients (Figures 1–2).
Figure 1.
Pictorial illustration depicting true vs pseudo-progression. Top row of images shows sequence of events during pseudo-progression at baseline, first follow-up and second follow-up scans. The apparent increase in size of tumour during first follow-up, which is typically obtained 4–6 weeks after baseline scan, is contributed by T-cells infiltrating the tumour. Second follow-up study performed ≥6 weeks later shows decrease in size of tumour compared to first follow-up, due to reduction of T-cells, leading to assessment of pseudo-progression. The bottom row of images shows gradual increase in size of tumour during the first and second follow-ups, due to infiltration by actual tumour cells, leading to the assessment of true progression.
Figure 2.
Pseudo-progression in a 65-year-old male with metastatic melanoma on a phase II trial with nivolumab and ipilimumab. Axial contrast enhanced CT image at baseline (a, b) shows normal mediastinal lymph node (black arrow) and liver lesions (white arrow). Axial contrast enhanced CT image (c, d) at first follow-up 12 weeks later shows new enlarged mediastinal node (black arrow) and significant increase in number and size of liver lesions (white arrow). Axial contrast enhanced CT image (e, f) performed 12 weeks later shows interval decrease in size of mediastinal node (black arrow) and decrease in size of liver lesions (white arrow).
To account for these differences in response to immune therapeutic drugs, a multidisciplinary group of oncologists, immunotherapists and regulatory experts participated in a series of workshops in 2004–2005 to discuss, propose and develop a response assessment criteria named the “immune-related Response Criteria (irRC)”.35 The proposed criteria was evaluated using data from a phase II clinical trial of 227 patients with advanced melanoma receiving CTLA-4 inhibitor (Ipilimumab) therapy.35 The analysis of the treatment response revealed four distinct patterns with favourable survival.35 These patterns included – (a) therapeutic response evident by week 12, with no new lesions (noted in 30% of patients), (b) stable disease as per WHO criteria followed in some patients by a slow, steady decline in total tumour burden (Figure 3), (c) a reduction in tumour burden after an initial increase and (d) reduction in total tumour burden during or after the appearance of new lesion(s) at time points later than week 12.35 The irRC took these factors into account and was developed from WHO criteria and therefore maintained the concept of bidimensional measurement of target lesions. The newly proposed irRC was able to identify an additional 10% of patients with favourable survival who were characterized as PD by WHO criteria35 and they helped explain the reason why low conventional response rates with ipilimumab of about 10% still translated into long-term survival in 20–25% of patients with metastatic melanoma,30 as new patterns of response not captured with standard WHO criteria contributed to the survival outcome of patients.35 Based on this analysis, the group decided to recommend “clinically insignificant” PD as part of the new criteria. “Clinically insignificant PD” refers to appearance of small new lesions in presence of other responding lesions.35 Subsequently, the immune-related RECIST (irRECIST) criteria was created based on irRC to assess tumour burden in patients treated with immunotherapies.36, 37 The irRECIST adapted the concept of unidimensional measurement similar to RECIST.38 In comparison to the bidimensional method used by irRC, unidimensional approach is more reproducible, shows fewer variability with measurements and results in lower misclassification rates for response assessment in clinical trials.39, 40
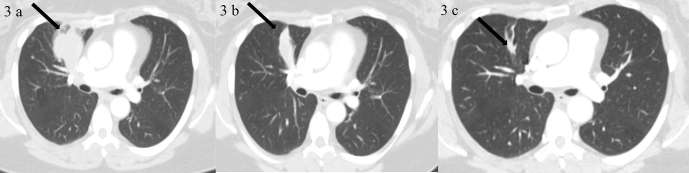
Figure 3.
A 52-year-old female with metastatic melanoma in a Phase 1 trial of MK-3475 (Pembrolizumab). Images 3a–c show the right upper lobe lung mass (shown with black arrow) at baseline, follow-up 1 (15 weeks after BL) and follow-up 6 (1 year after follow-up 1) respectively. Target right upper lobe lung mass shows slow response to treatment. BL, baseline.
The key distinguishing features between the proposed immune-related and conventional criteria are: (a) Due to potentially delayed response to immunotherapy, imaging assessment of disease progression or tumour response to therapy should be made with two consecutive assessments made at least 4 weeks apart (b) the appearance of “new lesions” does not necessarily constitute progression in patients receiving immunotherapy. To assess further changes to tumour burden, follow-up imaging should be performed at least after 4 weeks to assess the “new lesions” and (c) “new lesions” meeting size criteria are assessed as “new measurable lesions” and included in “total tumour burden”.36–38
Early this year, a consensus guideline immune RECIST (iRECIST) was developed by the RECIST working group for use in cancer immunotherapy trials, to ensure consistent design, data collection and facilitate the ongoing collection of trial data.41 iRECIST is based on RECIST 1.1 and the responses are assigned a prefix of “i” to signify “immune”, e.g. immune complete response (iCR), immune partial response (iPR), immune stable disease (iSD) and unconfirmed progressive disease (iUPD) or confirmed progressive disease (iCPD). New lesions are assessed and subcategorized as new target and new non-target lesions. Each time point assessment is based on the assessment of target, non-target and new lesions. An assessment of iUPD will be made if there is >/= 20% increase in tumour burden, or appearance of new target or non-target lesions. Confirmation of progression (iCPD) is done immediately in the next follow-up, provided there is a further increase of at least 5 mm of target tumour burden or new target lesion or any increase in non-target disease. If no change is detected in new lesions, or existing lesions, the response is categorized as iUPD. Many objective tumour response principles for iRECIST are unchanged from RECIST 1.1, with the major change being resetting the bar for progression if tumour progression in current time point is followed by response/stable disease in follow-up scans.
Key concepts
The immune response criteria resemble the conventional criteria for determination of overall tumour burden at baseline, which includes selection of measurable (target/index) or non-measurable (non-target/non-index) lesions (Table 3). The definitions and criteria for selection of target lesions and non-target lesions are similar. The target lesions included in the quantitative assessment should be representative of all involved organs and are identified based on their size (lesions with the longest diameter) and reproducibility. The overall tumour burden at baseline is determined as the sum of the product of the diameters for all index lesions (irSPD) according to irRC and estimated as the sum of the diameters (irSOD - long axis for non-nodal lesions and short axis for nodal lesions) for all index lesions according to irRECIST. Non-measurable tumour lesions encompass small lesions (longest diameter <10 mm or pathological lymph nodes with short axis >10 but <15 mm, by CT or MR scan, or no less than double the slice thickness), as well as truly non-measurable lesions such as leptomeningeal disease, ascites, bone lesions etc. Multiple lesions in the same organ can be assessed as a single non-target lesion group. For example, multiple liver lesions or multiple bone metastases can be selected as a single non-target lesion.
Table 3. .
Comparison between conventional and immune-related response criteria
| WHO | IrRC | RECIST 1.0 | RECIST 1.1 | IrRECIST | iRECIST | ||
| Definition of target | No minimum lesion size; should be measurable in two dimensions | No minimum lesion size; should be measurable in two dimensions | >10 mm in long-axis | >10 mm in long-axis for non-nodal lesions and >15 mm in short-axis for lymph nodes | >10 mm in long-axis for non-nodal lesions and >15 mm in short-axis for lymph nodes | >10 mm in long-axis for non-nodal lesions and >15 mm in short-axis for lymph nodes | |
| Max. no. of targets | N/A | 15 (10 visceral + 5 cutaneous) | 10 | 5 | 5 | 5 | |
| No. of targets per organ | N/A | 5 | 5 | 2 | 2 | 2 | |
| Tumour burden | SPD | SPD | SLD | SOD (short-axis for lymph nodes) | SOD (short-axis for lymph nodes) | SOD (short-axis for lymph nodes) | |
| New lesions | Number | N/A | 15 lesions in total (10 visceral + 5 cutaneous), 5 per organ | N/A | N/A | 5 lesions in total (2 per organ) | 5 lesions in total (2 per organ) |
| Size | N/A | ≥5 × 5 mm | N/A | N/A | ≥10 mm in long-axis (15 mm short-axis for lymph nodes) | ≥10 mm in long-axis (15 mm short-axis for lymph nodes) | |
irRC, immune-related response criteria; irRECIST, immune-related RECIST; Max, maximum; No, number; N/A, not applicable; RECIST, Response Valuation Criteria in Solid Tumours; SLD, sum of long-axis diameters; SOD, sum of diameters; SPD, sum of products of perpendicular diameters; WHO, World Health Organization.
The target lesion burden at baseline is compared with tumour measurements at subsequent time points during therapy for quantitative determination of treatment response (Table 4). The responses are quantitatively categorized as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). The responses of non-target lesions are assessed qualitatively and are categorized as CR, PD, or non-CR/non-PD. When new measurable lesions appear during therapy they are included in the overall tumour burden assessment. According to irRC, the definition for new measurable lesions include a size of ≥5 × 5 mm and up to 5 new lesions per organ with a maximum 10 visceral and 5 cutaneous lesions can be included for assessment. The updated total tumour burden on irRC includes the sum of the products of diameters (SPD) of the index lesions and the new, measurable lesions.
Table 4. .
Comparison of response categories between conventional and immune-related criteria
| WHO | IrRC | RECIST 1.0 | RECIST 1.1 | irRECIST | iRECIST | |
| CR | Complete resolution of lesions (confirmed at 4 weeks) | Complete resolution of lesions (confirmed at 4 weeks) from first irCR scan | Complete resolution of lesions | Complete resolution of non-nodal lesions and < 10 mm short-axis for lymph nodes | Complete resolution of non-nodal lesions and < 10 mm short-axis for lymph nodes. No confirmation necessary | Complete resolution of non-nodal lesions and < 10 mm short-axis for lymph nodes. No new lesions |
| PR | ≥50% decrease in tumour burden (confirmed at 4 weeks) | ≥50% decrease in tumour burden (confirmed at 4 weeks) | ≥30% decrease in tumour burden | ≥30% decrease in tumour burden | ≥30% decrease in tumour burden | ≥30% decrease in tumour burden |
| SD | Does not meet criteria for CR/PR/PD | Does not meet criteria for irCR/irPR/irPD | Does not meet criteria for CR/PR/PD | Does not meet criteria for CR/PR/PD | Does not meet criteria for irCR/irPR/irPD | Does not meet criteria for iCR/iPR/iUPD/iCPD |
| PD | ≥25% increase in tumour burden relative to nadir; new lesions | ≥25% increase in tumour burden relative to nadir; new lesions. Confirmation of PD via a subsequent scan ≥ 4 weeks later is required | ≥20% increase in tumour burden relative to nadir; new lesions | ≥20% increase in tumour burden relative to nadir and a minimum absolute increase of 5 mm; new lesions | ≥20% increase in tumour burden relative to nadir and a minimum absolute increase of 5 mm; new lesions. Confirmation of PD via a subsequent scan ≥ 4 weeks later to detect delayed responses is required | iUPD—presence of new measurable/non- measurable lesions, or ≥ 20% increase in tumour burden relative to nadir iCPD-confirmation of IUPD with >/= 5 mm increase in size of target or new target lesions, increase in non-target or new non-target or increase in number of new lesions. |
CR, complete response; iCPD, immune-confirmed progressive disease; iCR, immune complete response; iPR, immune partial response; irCR, immune-related complete response; irPR, immune-related partial response; irPD, immune-related progressive disease; irRC, immune-related response criteria; irRECIST, immune-related Response Evaluation Criteria in Solid Tumours; iUPD, immune–unconfirmed progressive disease; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumours; SD, stable disease; WHO, World Health Organization.
irRC tumour burden (irSPD) = SPD of index lesions + SPD of new, measurable lesions
The definitions for size and number of new lesions for irRECIST are different in comparison to irRC. According to irRECIST, new measurable non-nodal lesions should measure ≥10 mm in long axis while new nodal lesions should measure ≥15 mm in short axis. Additionally, up to 2 new lesions per organ and 5 lesions in total are allowed for quantitative evaluation. Updated total tumour burden on irRECIST includes the sum of long-axis dimensions of the target non-nodal lesions/short-axis dimensions of the target nodal lesions and similar measurements of new lesions.
irRECIST tumour burden (SOD) = SOD of target lesions + SOD of new, measurable lesions
Confirmation of CR, PR and PD by a consecutive imaging (CT/MR) assessment at least 4 weeks from the date of first documentation is required for immune-related criteria.
Current research and validation of irRC & irRECIST
Since its inception, immune-related response criteria have been used in several clinical trials in patients receiving immune therapies and have potentially demonstrated some advantages over conventional criteria for characterization of treatment response; however, robust validation is needed (Table 2). Immune criteria have undergone modifications (irRC, irRECIST, iRECIST) since their initial introduction; however, the basic concept has not vastly changed. irRC concepts have been included in regulatory guidance documents of the US FDA and European Medicines Agency (EMA), and their application was extended beyond melanoma to several cancers including lung, renal and lymphomas.42–44 While these criteria are the mainstay in the early phases of drug development trials, they have yet to be implemented for use in phase III trials necessary for final marketing approval by regulatory agencies (e.g. FDA).45
In a multicentre phase II study investigating the efficacy and safety of ipilimumab monotherapy in patients with pre-treated advanced melanoma with primary endpoint of best overall response rate, using irRC a disease control rate of 35% was achieved as compared to 27% using WHO criteria.27 In a randomized trial involving pembrolizumab in ipilimumab–refractory and ipilimumab-naïve melanoma patients, application of irRC resulted in a higher 24-week PFSas compared to RECIST 1.1.44 In a phase Ib KEYNOTE-001 study done by Hodi et al, involving 655 patients, 327 had ≥28 weeks of imaging follow-up where 7% of them (24/327) had atypical responses (5% early and 3% delayed pseudo-progression). Based on survival analysis, it was recommended that modified criteria that permit treatment beyond initial progression per RECIST 1.1 might prevent premature cessation of treatment, as conventional RECIST might underestimate the benefit of pembrolizumab in approximately 15% of patients.21 Chiou et al, studied the incidence of distinct immune responses from RECIST across different solid tumour types, which was reported in multiple patients with melanoma (6.6%; 31/471), bladder cancer (1.5%; 1/65), renal cell cancer (1.8%; 3/168) and lung cancer (unquantified; reported in a study with multiple malignancies).46
Despite the potential benefits of the immune response criteria, RECIST remains a highly validated and reproducible tool in practice and a majority of trials continue to use RECIST 1.1 for assessment of treatment response. However, the trend has been to provide more flexibility to the investigators to use their discretion to continue treatment beyond the conventional definition of “progression” by using modifications to the RECIST criteria. In those patients where disease progression is identified according to RECIST, treatment is continued in those patients who are clinically stable prior to a repeat scan in 4–6 weeks. In those patients in whom a repeat scan confirms progression, the treatment is discontinued. In certain immunotherapy trials, despite repeat imaging showing progression, treatment can be continued based on the assessment by the investigator and medical monitor as long as there is perceived clinically meaningful benefit.
Immune-related adverse events
Unintended auto-immune complications can also occur when the immune system is enhanced to fight cancer. Radiological manifestations of immune-therapy-related adverse events are seen in both clinically symptomatic and asymptomatic patients. Some of these adverse events include colitis (diffuse colitis and segmental colitis with diverticulosis), endocrinopathies such as hypophysitis and thyroid disorders, hepatitis, pancreatitis, pneumonitis, dermatitis and/or sarcoid-like reaction.47 These immune-related adverse events can occur at any stage of therapy. The median onset of events typically ensues during the following time periods: varies by organ-system affected with - skin-related events at 3 weeks, hepatitis at 3–9 weeks, gastrointestinal manifestations at 8 weeks and endocrinopathies at 7–20 weeks.48, 49 Almost all of these immune-related adverse events can be treated by stopping the immune-therapy and administering steroids.
The expanding spectrum of immuno-oncology drugs under investigation has further increased the complexity of clinical activity patterns. Every new drug will likely carry its own clinical activity profile, which can only be understood with image assessment tools able to capture them, such as irRC. The challenge is to identify the normal and abnormal response patterns, which can present as treatment response or adverse effect. Often, new imaging findings may reflect inflammatory response rather than new sites of metastatic disease. An example of this effect is drug-induced sarcoid-like distribution of lymph nodes which resemble nodal metastases. Radiologists should be cognizant of these manifestations and be aware of findings that allow identification of adverse effects ; for example, treatment response at other sites, and absence of infection that favour sarcoid-like reaction. In such situations, the patient would benefit from continued treatment as enlarged and new nodes might represent an initial flare reaction to immunetherapy and might revert to normal spontaneously.50, 51 Several studies have shown that radiological manifestations of immune-related adverse events have been associated with improved tumour response and disease control.52, 53 A study done by Howard et al, in 119 patients with metastatic melanoma on Ipilimumab therapy showed 17% of patients showing immune-related adverse events, but most (55%) showed disease control and response to therapy.54 An increasingly recognized adverse event is termed “hyperprogression”, which is characterized by rapid increase in tumour burden in patients treated with immune-therapy with characteristics of progression including Time to Treatment Failure <2 months, >50% increase in tumour burden compared to pre-baseline levels and >2 fold increase in pace of tumour growth. The patients usually have a deteriorating clinical condition, which leads to treatment failure.55 Further research will give a clearer picture of these adverse effects, but meanwhile, radiologists should be aware of the immune-related patterns of response and adverse effects as immunetherapy drugs are increasingly used in treating a growing number of cancers.
Functional criteria and future developments
An emerging and exciting field in monitoring response to novel therapies includes the functional imaging realm, such as perfusion imaging (CT/MR/US), 18-fluorodeoxy glucose PET imaging and diffusion-weighted imaging (DWI). There are no currently recognized functional response criteria for use in patients treated with immuno-oncology drugs. PET-CT imaging, which combines the functional information provided by PET with anatomic information provided by CT, is accepted for staging and post-treatment follow-up of several malignancies such as melanoma and lung cancer.56 Immuno-PET studies explore the possibility of non-FDG radiotracers using labelled monoclonal antibodies that can evaluate CTLA-4, PD-1, PD-L1 cellular expression status. These non-invasive immune-diagnostic approaches can provide novel insights into pathophysiology of immuno-oncology drugs, although further studies and investigations are needed before their introduction into clinical setting.57 Radiomics, an exciting field involving different predictive modelling techniques and feature selections comprised of tumour features such as size, shape, texture, wavelets are being explored to determine the optimal configuration of prognosis analysis.58 Radiomics-based models can have a complimentary role in predicting survival and other clinical outcomes in various cancers including early or advanced lung cancer and head and neck cancer patients.45, 59 Initial studies have demonstrated the potential of this discipline in the identification of general imaging phenotype features that exist in various cancers, and might have the ability to identify the same in patients treated with immuno-oncology drugs.
Conclusion
Immunotherapy agents represent a new breed of anticancer drugs being tested and approved for treatment for various malignancies including solid tumours, lymphomas and leukaemias. Their treatment response patterns and treatment-related complications are different from conventional chemotherapeutic regimens. While applying standardized response assessment criteria in patients receiving immune therapies, it is important to be cognizant of the differences in treatment response to these therapies and to apply the recently introduced immune-related response criteria. In the same way, awareness and early diagnosis of immune-related adverse events is critical to successful management of complications and for allowing patients to continue on trial when appropriate, thereby continuing access to life-prolonging drugs.
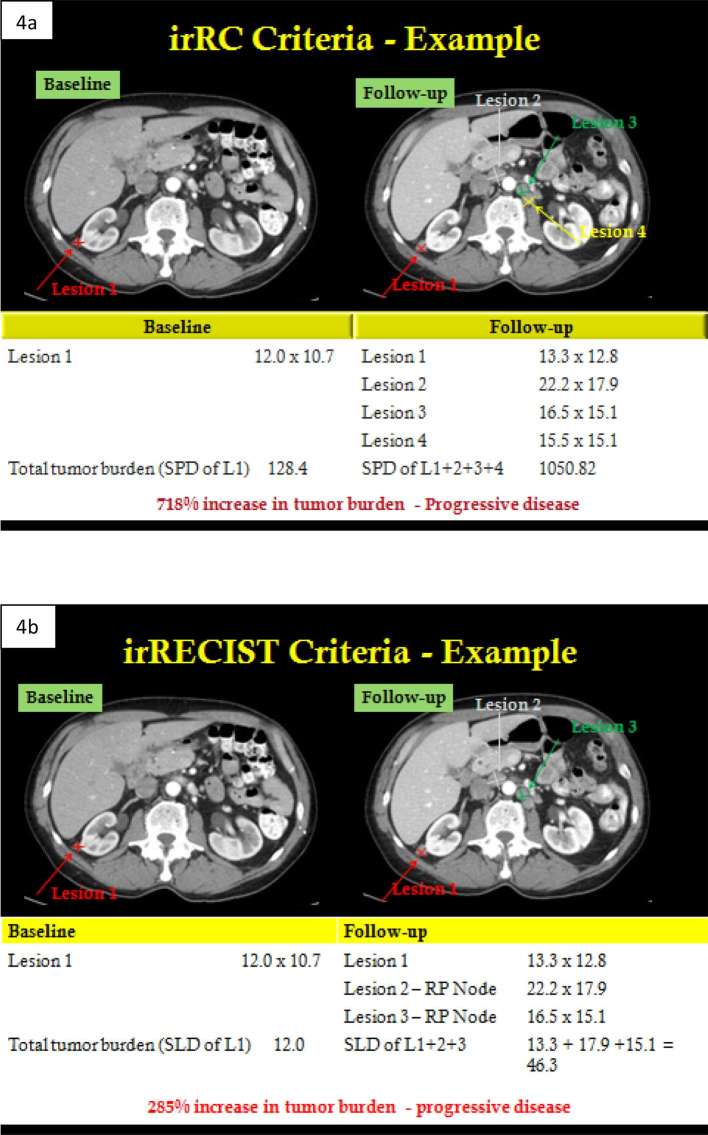
Figure 4. .
A 72-year-old male with metastatic melanoma in a phase 1 trial of Pembrolizumab. Images a, b show case examples of baseline and follow-up 1 study (13 weeks later) assessed in irRC and irRECIST criteria, respectively. Patient had only 1 target lesion at baseline\, but developed several new lesions (retroperitoneal lymph nodes) on follow-up. In irRC criteria (a), a total of 10 new lesions (5 per organ) can be assessed as “new target lesions”. Hence, all three new retroperitoneal lymph nodes were assessed as “new lesions” and total tumour burden was calculated from SPD of old and new target lesions. In irRECIST criteria (b), a total of 5 new lesions (2 per organ) can be assessed as “new target lesions”. Hence, only 2 new retroperitoneal lymph nodes (from among 3 new lesions) were assessed as “new lesions” and total tumour burden was calculated from SLD (or short-axis for lymph nodes) of old and new target lesions. irRC, immune-related response criteria; irRECIST, immune-related Response Evaluation Criteria in Solid Tumours; SLD, sum of long axis diameters; SPD, sum of product of diameters.
Contributor Information
Bhanusupriya Somarouthu, Email: bsomarouthu@mgh.harvard.edu.
Susanna I Lee, Email: slee0@partners.org.
Trinity Urban, Email: turban@partners.org.
Cheryl A Sadow, Email: csadow@bwh.harvard.edu.
Gordon J Harris, Email: HARRIS@HELIX.MGH.HARVARD.EDU.
Avinash Kambadakone, Email: akambadakone@mgh.harvard.edu.
REFERENCES
- 1. WHO. Handbook for reporting results of cancer treatment. 48th ed Geneva, Switzerland: The British Institute of Radiology.; 1979. [Google Scholar]
- 2.Miller AB, Winkler A, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14. [DOI] [PubMed] [Google Scholar]
- 3.James K, Eisenhauer E, Christian M, Terenziani M, Vena D, Muldal A, et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst 1999; 91: 523–8. doi: 10.1093/jnci/91.6.523 [DOI] [PubMed] [Google Scholar]
- 4.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92: 205–16. doi: 10.1093/jnci/92.3.205 [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 6.Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K. Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics 2013; 33: 1323–41. doi: 10.1148/rg.335125214 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki C, Jacobsson H, Hatschek T, Torkzad MR, Bodén K, Eriksson-Alm Y, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics 2008; 28: 329–44. doi: 10.1148/rg.282075068 [DOI] [PubMed] [Google Scholar]
- 8.Nishino M, Jackman DM, Hatabu H, Yeap BY, Cioffredi LA, Yap JT, et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 2010; 195: W221–W228. doi: 10.2214/AJR.09.3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain RK, Lee JJ, Ng C, Hong D, Gong J, Naing A, et al. Change in tumor size by RECIST correlates linearly with overall survival in phase I oncology studies. J Clin Oncol 2012; 30: 2684–90. doi: 10.1200/JCO.2011.36.4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016; 15: 235–47. doi: 10.1038/nrd.2015.35 [DOI] [PubMed] [Google Scholar]
- 11.Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol 2004; 22: 4442: 4442–5. doi: 10.1200/JCO.2004.07.960 [DOI] [PubMed] [Google Scholar]
- 12.U.S. National Institutes of Health ClinicalTrials. gov registry. 2017. . Available from: https://clinicaltrials.gov/ct2/resultsterm=cancer+immuno+therapy&Search=Search
- 13.U.S. Department of Health and Human Services. Hematology/Oncology (Cancer) Approvals & Safety Notifications. 2017. Available from: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm
- 14.Champiat S, Ileana E, Giaccone G, Besse B, Mountzios G, Eggermont A, et al. Incorporating immune-checkpoint inhibitors into systemic therapy of NSCLC. J Thorac Oncol 2014; 9: 144–53. doi: 10.1097/JTO.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 15.Pardoll DM, Drew M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HK, Heo MH, Lee HS, Sun JM, Lee SH, Ahn JS, et al. Comparison of RECIST to immune-related response criteria in patients with non-small cell lung cancer treated with immune-checkpoint inhibitors. Cancer Chemother Pharmacol 2017; 80: 591–8. doi: 10.1007/s00280-017-3396-4 [DOI] [PubMed] [Google Scholar]
- 17.Khoja L, Kibiro M, Metser U, Gedye C, Hogg D, Butler MO, et al. Patterns of response to anti-PD-1 treatment: an exploratory comparison of four radiological response criteria and associations with overall survival in metastatic melanoma patients. Br J Cancer 2016; 115: 1186–92. doi: 10.1038/bjc.2016.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387: 1909–20. doi: 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced melanoma. J Clin Oncol 2016; 34: 1330–8. doi: 10.1200/JCO.2015.63.4121 [DOI] [PubMed] [Google Scholar]
- 20.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, et al. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol 2016; 34: 833–42. doi: 10.1200/JCO.2015.63.7421 [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol 2016; 34: 1510–7. doi: 10.1200/JCO.2015.64.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiarion Sileni V, Pigozzo J, Ascierto PA, Grimaldi AM, Maio M, Di Guardo L, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. J Exp Clin Cancer Res 2014; 33: 30. doi: 10.1186/1756-9966-33-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bapsy PP, Sharan B, Kumar C, Das RP, Rangarajan B, Jain M, et al. Open-label, multi-center, non-randomized, single-arm study to evaluate the safety and efficacy of dendritic cell immunotherapy in patients with refractory solid malignancies, on supportive care. Cytotherapy 2014; 16: 234–44. doi: 10.1016/j.jcyt.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 24.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369: 122–33. doi: 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Giacomo AM, Ascierto PA, Pilla L, Santinami M, Ferrucci PF, Giannarelli D, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol 2012; 13: 879–86. doi: 10.1016/S1470-2045(12)70324-8 [DOI] [PubMed] [Google Scholar]
- 26.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012; 30: 2046–54. doi: 10.1200/JCO.2011.38.4032 [DOI] [PubMed] [Google Scholar]
- 27.Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 2011; 9: 204: 204. doi: 10.1186/1479-5876-9-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol 2010; 21: 1712–7. doi: 10.1093/annonc/mdq013 [DOI] [PubMed] [Google Scholar]
- 29.Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016; 17: 1558–68. doi: 10.1016/S1470-2045(16)30366-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016; 375: 1823–33. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 32.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA 2008; 105: 3005–10. doi: 10.1073/pnas.0712237105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodi FS, Oble DA, Drappatz J, Velazquez EF, Ramaiya N, Ramakrishna N, et al. CTLA-4 blockade with ipilimumab induces significant clinical benefit in a female with melanoma metastases to the CNS. Nat Clin Pract Oncol 2008; 5: 557–61. doi: 10.1038/ncponc1183 [DOI] [PubMed] [Google Scholar]
- 34.Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother 2007; 30: 1–15. doi: 10.1097/01.cji.0000211341.88835.ae [DOI] [PubMed] [Google Scholar]
- 35.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15: 7412–20. doi: 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 36.Nishino M, Jagannathan JP, Krajewski KM, O'Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 2012; 198: 737–45. doi: 10.2214/AJR.11.7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishino M, Gargano M, Suda M, Ramaiya NH, Hodi FS. Optimizing immune-related tumor response assessment: does reducing the number of lesions impact response assessment in melanoma patients treated with ipilimumab? J Immunother Cancer 2014; 2: 17: 17. doi: 10.1186/2051-1426-2-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013; 19: 3936–43. doi: 10.1158/1078-0432.CCR-13-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erasmus JJ, Gladish GW, Broemeling L, Sabloff BS, Truong MT, Herbst RS, et al. Interobserver and intraobserver variability in measurement of non-small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol 2003; 21: 2574–82. doi: 10.1200/JCO.2003.01.144 [DOI] [PubMed] [Google Scholar]
- 40.Zhao B, James LP, Moskowitz CS, Guo P, Ginsberg MS, Lefkowitz RA, et al. Evaluating variability in tumor measurements from same-day repeat CT scans of patients with non-small cell lung cancer. Radiology 2009; 252: 263–72. doi: 10.1148/radiol.2522081593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017; 18: e143–e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. U.S. Food and Drug Administration. Guidance for industry: clinical considerations for therapeutic cancer vaccines. Silver Spring, MD; 2011. Available from: http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm278673.pdf.. [Google Scholar]
- 43. European Medicines Agency. Guideline on the evaluation of anticancer medicinal products in man. London, UK; 2012. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/01/WC500137128.pdf. [Google Scholar]
- 44.Hamid O, Robert C, Ribas A, Wolchok JD. Randomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and IPI-naive (IPI-N) melanoma (MEL). J Clin Oncol 2014; 32(suppl; abstr 3000): 5s. [Google Scholar]
- 45.Hoos A, Wolchok JD, Humphrey RW, Hodi FS. CCR 20th Anniversary Commentary: immune-related response criteria-capturing clinical activity in immuno-oncology. Clin Cancer Res 2015; 21: 4989–91. doi: 10.1158/1078-0432.CCR-14-3128 [DOI] [PubMed] [Google Scholar]
- 46.Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumession and Immune-Related Response in Solid Tumors. J Clin Oncol 2015; 33: 3541–3. doi: 10.1200/JCO.2015.61.6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Regan KN, Jagannathan JP, Ramaiya N, Hodi FS. Radiologic aspects of immune-related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapy. AJR Am J Roentgenol 2011; 197: W241–W246. doi: 10.2214/AJR.10.6032 [DOI] [PubMed] [Google Scholar]
- 48.Weber JS, Dummer R, de Pril V, Lebbé C, Hodi FS. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013; 119: 1675–82. doi: 10.1002/cncr.27969 [DOI] [PubMed] [Google Scholar]
- 49.Kwak JJ, Tirumani SH, Van den Abbeele AD, Koo PJ, Jacene HA. Cancer immunotherapy: imaging assessment of novel treatment response patterns and immune-related adverse events. Radiographics 2015; 35: 424–37. doi: 10.1148/rg.352140121 [DOI] [PubMed] [Google Scholar]
- 50.Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune-related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2015; 3: 1185–92. doi: 10.1158/2326-6066.CIR-15-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braschi-Amirfarzan M, Tirumani SH, Hodi FS, Nishino M. Immune-checkpoint inhibitors in the era of precision medicine: what radiologists should know. Korean J Radiol 2017; 18: 42–53. doi: 10.3348/kjr.2017.18.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bronstein Y, Ng CS, Hwu P, Hwu WJ. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol 2011; 197: W992–W1000. doi: 10.2214/AJR.10.6198 [DOI] [PubMed] [Google Scholar]
- 53.Abramson RG, Abramson VG, Chan E, Horn L, Keedy VL, Pao W, et al. Complications of targeted drug therapies for solid malignancies: manifestations and mechanisms. AJR Am J Roentgenol 2013; 200: 475–83. doi: 10.2214/AJR.12.9049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howard SA, Krajewski KM, Jagannathan JP, Braschi-Amirfarzan M, Tirumani SH, Shinagare AB, et al. A new look at toxicity in the era of precision oncology: imaging findings, their relationship with tumor response, and effect on metastasectomy. AJR Am J Roentgenol 2016; 207: 4–14. doi: 10.2214/AJR.15.15480 [DOI] [PubMed] [Google Scholar]
- 55.Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23: 4242–50. doi: 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50(Suppl 1): 122S–50. doi: 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauckneht M, Piva R, Sambuceti G, Grossi F, Morbelli S. Evaluation of response to immune checkpoint inhibitors: Is there a role for positron emission tomography? World J Radiol 2017; 9: 27–33. doi: 10.4329/wjr.v9.i2.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pyka T, Bundschuh RA, Andratschke N, Mayer B, Specht HM, Papp L, et al. Textural features in pre-treatment [F18]-FDG-PET/CT are correlated with risk of local recurrence and disease-specific survival in early stage NSCLC patients receiving primary stereotactic radiation therapy. Radiat Oncol 2015; 10: 100. doi: 10.1186/s13014-015-0407-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parmar C, Leijenaar RT, Grossmann P, Rios Velazquez E, Bussink J, Rietveld D, et al. Radiomic feature clusters and prognostic signatures specific for lung and head & neck cancer. Sci Rep 2015; 5: 11044. doi: 10.1038/srep11044 [DOI] [PMC free article] [PubMed] [Google Scholar]