Abstract
Introduction
People living with HIV (PLWH) are at risk of both polypharmacy and unintentional overdose yet there are few data on whether polypharmacy increases risk of overdose. The study objective was to determine if the number and type of medication (e.g., sedating) were associated with non-fatal overdose (OD) among PLWH with past-year substance dependence or a lifetime history of injection drug use.
Materials and Methods
This was a longitudinal study of adults recruited from two urban, safety-net HIV clinics. Outcomes were i) lifetime and ii) past-year non-fatal OD assessed at baseline and a 12-month follow-up. We used logistic regression to examine the association between each outcome and the number of medications (identified from the electronic medical record) in the following categories: i) overall medications, ii) non-antiretroviral (non-ARV), iii) sedating, iv) non-sedating, as well as any vs. no opioid medication and any vs. no non-opioid sedating medication. Covariates included demographics, medical comorbidities, depressive and anxiety symptoms, and substance use.
Results
Among 250 participants, 80% were prescribed a sedating medication, 50% were prescribed an opioid; 51% exceeded risky drinking limits. In the past month, 23% reported illiict opioid use and 9% illicit opioid sedative use; 37% reported lifetime non-fatal OD and 7% past-year non-fatal OD. The median number (interquartile range) of total medications was 10 (7, 14) and 2 (1, 3) sedating. The odds of lifetime non-fatal OD were significantly higher with each additional sedating medication (OR 1.26, 95% CI 1.08, 1.46) and any opioid medication (OR 2.31; 95% CI 1.37, 3.90), but not with each overall, non-ARV, or non-sedating medication. The odds of past year non-fatal OD were greater with each additional sedating medication (OR 1.18; 95% CI 1.00, 1.39, p=0.049), each additional non-ARV medication (OR 1.07; 95% CI 1.00, 1.15, p=0.048), and non-significantly for any opioid medication (OR 2.23; 95% CI 0.93, 5.35).
Conclusions
In this sample of PLWH with substance dependence and/or injection drug use, number of sedating medications and any opioid were associated with non-fatal overdose; sedating medications were prescribed to the majority of patients. Polypharmacy among PLWH and substance dependence warrants further research to determine whether reducing sedating medications, including opioids, lowers overdose risk.
Keywords: HIV, overdose, polypharmacy, antiretroviral medication
1. Introduction
Treatment with effective antiretroviral medications has substantially extended the lives of people living with HIV (PLWH). Widespread use of single-tablet antiretroviral medications (ARV) with at least three active medications has led to prolonged viral suppression and management of HIV infection as a chronic disease (Greene, Justice, Lampiris, & Valcour, 2013; Deeks, Lewin, & Havlir, 2013). As a result, more than one-half of PLWH in the United States are over the age 50 (High et al., 2012). This epidemiologic shift has led to the accrual of age-related comorbidities such as cardiovascular disease, cancer, and neurocognitive disease in PLWH (Althoff et al., 2015; Greene et al., 2013; Robbins, Shiels, Pfeiffer, & Engels, 2014). In addition to older age, HIV-associated inflammation, frailty, and substance use (i.e. tobacco, alcohol, and other drugs) (Justice et al., 2016; Crothers et al., 2005; Brothers & Rockwood, 2014) contribute to a greater number of comorbidities complicating the management of HIV infection. Treatment guidelines, developed for people with one disease, but applied to people with multiple chronic conditions can lead to the prescription of multiple concomitant medications, often termed “polypharmacy,” resulting in a significant daily medication burden (Edelman et al., 2013; Moore, Mao, & Oramasionwu, 2015). For PLWH, a greater number of medications raises the risk of medication interactions (Holtzman et al., 2013), medication nonadherence (Monroe, Rowe, Moore, & Chander, 2013), and discontinuation of ARV (Krentz & Gill, 2016; Cantudo-Cuenca, Jiménez-Galán, Almeida-Gonzalez, & Morillo-Verdugo, 2014).
Polypharmacy may also contribute to overdose, the leading cause of accidental injury death in the United States (Centers for Disease Control and Prevention, 2015). Examining overdose risks specifically among HIV populations is important because PLWH have twice the risk of overdose death as people without HIV infection (Mathers et al., 2013; Green, McGowan, Yokell, Pouget, & Rich, 2012). The proportion of deaths due to overdose among PLWH has increased as AIDS-related causes have declined (Schwarcz, Vu, Hsu, & Hessol, 2014). Why PLWH have greater overdose risk is not clear, but proposed reasons include co-morbid liver dysfunction, pulmonary dysfunction, more illicit drug and heavy alcohol use, and social isolation (Edelman et al., 2013; Green et al., 2012). Prescribed medications, particularly sedating medications like opioids and benzodiazepines, commonly contribute to polysubstance use-related overdose. PLWH are more likely to be prescribed high-dose opioid medications (Becker et al., 2016). Among patients prescribed opioid medications, the risk of overdose risk is greater for patients with depressive disorders (Turner & Liang, 2015), a common comorbidity of HIV infection. Despite the risk of overdose and increasing number of medications among a population with high incidence of substance use disorders, there are few data about whether the number of medications, or “polypharmacy” is associated with a greater risk of overdose for PLWH.
The objective of this study was to determine if the number of medications prescribed and/or type of medications prescribed is associated with non-fatal overdose in PLWH. Rather than using the conventional definition of polypharmacy, (i.e., five or more medications) validated largely in elderly populations (Gnjidic et al., 2012), we also sought to determine an optimal discriminating number of medications associated with non-fatal overdose (both overall and for sedating medications specifically). We hypothesized that an association between the overall number of medications and non-fatal overdose would be driven by sedating medications, both from opioid and non-opioid sedating medications.
2. Material and methods
2.1 Study design
We used data from the Boston ARCH Cohort study, a longitudinal study of adults with HIV infection and substance dependence in the past year (as assessed by the Mini International Neuropsychiatric Interview Version 6.0 (MINI) (Sheehan et al., 1998) or ever injection drug use. Boston ARCH Cohort participants were recruited from the Center for Infectious Diseases at Boston Medical Center and the HIV program at Boston Healthcare for the Homeless Program.
Inclusion criteria were: documentation of HIV in any medical record, past 12-month substance dependence or ever injection drug use, ability to speak English, age 18 or older, and willingness to provide contact information for one other person to assist with follow up. Exclusion criteria were: pregnancy at time of enrollment, plans to leave the Boston area in the next year, or cognitive impairment such that the patient could not provide informed consent. Study enrollment occurred from December 2012 to November 2014. Past 12-month substance dependence or ever injection drug use will collectively be referred to as “substance dependence” given that PLWH with a lifetime history of injection drug use are likely to have a history of substance dependence)
Participants provided written informed consent and received compensation for each study assessment completed. The Boston University Medical Campus Institutional Review Board approved the study. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) further protected participants with a Certificate of Confidentiality and the US Department of Health and Human Services approved the performance of follow-up assessments with participants who were incarcerated.
2.2 Data collection
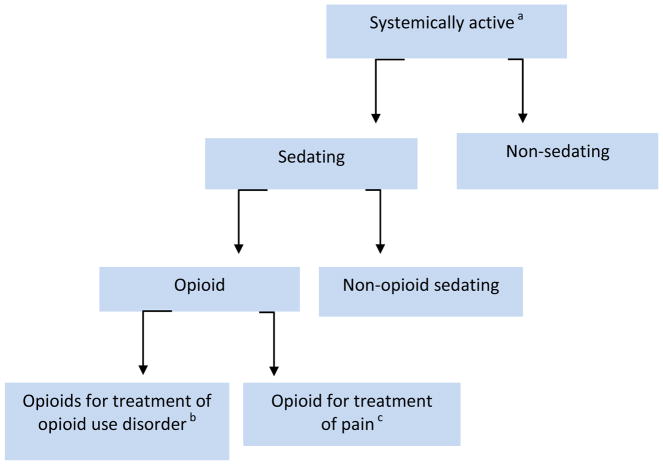
Medication data were extracted from a clinical data warehouse that included the electronic medical record (EMR) of both recruitment sites. We abstracted the medication list corresponding to date of study entry for each participant. In order to obtain an accurate count of medications, duplicate medications were identified and removed by cross-referencing with both the National Drug Code (NDC) number and with the generic name as defined in the National Drug File (U.S. Department of Veterans Affairs) (“VA National Formulary - Pharmacy Benefits Management Services,” 2016). Because multiple NDC numbers exist for the same medication, unique counts of the “generic name” were used as a proxy for total number of medications. Combination ARVs were recoded into their individual drug components. After a list of unique medications was determined for each study participant, we excluded medications that are not systemically active such as emollients, irrigation solutions, vitamins (except for vitamin D), peritoneal solutions, eye drops, rectal or vaginally administered medications, and complementary and alternative medications. We then further classified medications into the subcategories “sedating” and “non-sedating” (Figure 1). Sedating medications were further subcategorized as opioid or non-opioid. The medication exclusion and categorization decisions were made by two of the study investigators (TK and AW) guided by U.S. Department of Veterans Affairs Drug Classification (“VA National Formulary - Pharmacy Benefits Management Services,” 2016).
Figure 1.
Summary of Medication Categories Used in Analyses
aMedications not systemically active includes emollients, irrigation solutions, vitamins (except for vitamin D), peritoneal solutions, eye drops, rectal or vaginally administered medications, complementary and alternative medications.
bBuprenorphine, methadone
cAll other opioid medications
All other study data was collected by trained research associates who administered standardized in-person interviews at a study entry and a 12-month follow-up interview.
2.3 Measurements
2.3.1 Outcomes
Two separate outcomes were examined: 1) lifetime non-fatal overdose (“Have you ever overdosed?”) assessed at study entry; and 2) past year non-fatal overdose assessed at study entry and 12-month study interview, defined as responding ≥1 to the question, “How many times have you overdosed in the past year?” The following information was provided by research associates to participants before asking the overdose questions. “We are referring to ANY overdose you may have had including accidental and deliberate (on purpose) overdoses on illegal drugs, over the counter medications, prescription medications, or alcohol.”
2.3.2 Main independent variables and covariates
The main independent variables were (1) total number of (“systemically active”) medications, 2) total number of medications excluding ARVs, 3) total number of sedating medications and 4) total number of non-sedating medications. We also examined associations with (i) any opioid medication and (ii) any non-opioid sedating medication (Figure 1). Medication count and type were measured at study entry.
Covariates included demographic data (age, sex, and race/ethnicity); medical comorbidity (Charlson Comorbidity Index Score [Quan et al., 2005]); any recent (past 30 day)heavy alcohol use (> 14 drinks in a week or 5+ drinks in a day for men and >7 drinks in a week or 4+ drinks in a day for women) assessed by 30-day Timeline Follow-Back Interview (Vinson, Reidinger, & Wilcosky, 2003); and any recent illicit opioid use, illicit sedative use, and cocaine use assessed by the Addiction Severity Index (McLellan et al., 1992). We considered adjusting for mental health diagnosis (post-traumatic stress disorder, other anxiety disorders, depressive disorders, and bipolar or psychotic disorders as defined by Park et al. (Park, Saitz, Ganoczy, Ilgen, & Bohnert, 2015), but found that 85% of the study sample had at least one of these comorbid psychiatric illnesses. Therefore, we used binary measures of depressive symptoms (2-item Patient Health Questionnaire score ≥ 3) (Kroenke, Spitzer, & Williams, 2003) and anxiety symptoms (Overall Anxiety Severity and Impairment Scale (OASIS) score ≥8) (Campbell-Sills et al., 2009).
2.4 Statistical analysis
We used descriptive statistics to characterize the sample at study entry. Separate analyses were conducted for lifetime and past year non-fatal overdose. 1) Lifetime non-fatal overdose analyses used data collected at baseline for both medication counts and report of lifetime non-fatal overdose. Logistic regression models were used to examine the association between number of medications in each category (e.g., overall, non-ARV, sedating, non-sedating) and lifetime non-fatal overdose fitting separate models for each medication count and outcome. 2) The analysis of number of medications and past year overdose used two types of observations: i) medication data and past year non-fatal overdose from the baseline assessment and ii) medication data at baseline and past year non-fatal overdose at the 12-month follow up. Past year overdose was examined using separate generalized estimating equations (GEE) logistic regression models for each medication count and past year non-fatal overdose to account for non-independence of repeated measures.
To assess whether the association of the number of overall medications was due to sedating medications, we used a model with both the number of sedating medications and number of non-sedating medications as main predictors. To examine more closely the effect of sedating medications, we used a logistic regression model that included any opioid and any non-opioid sedating medication as the main predictors.
The modeling strategy is summarized below and in Figure 1.
Model 1 assessed the total number of medications.
Model 2 assessed the total number of medications excluding ARV.
Model 3 assessed the number of sedating and non-sedating medications.
Model 4 assessed “any opioid medication” and “any non-opioid sedating medication.”
To assess if associations were different for opioid agonists used for addiction treatment (i.e., buprenorphine or methadone) than for other opioids for pain, we ran additional models (Model 5) that replaced “any opioid medication” with two separate, mutually exclusive variables: i) any buprenorphine or methadone and ii) any other opioid medication. Because co-prescribed opioid and non-sedating medications are associated with overdose (Park et al., 2015; Collet et al., 2016), we also assessed the association of co-prescribed opioid and non-opioid sedating medications, opioid medication only, and non-opioid sedating medication only. All were dichotomous variables (Model 6). Given the number of past year non-fatal overdoses in the sample, only a limited number of covariates could be included in a multiple logistic regression model to control for confounding. Therefore, we fit models controlling separately for each of the following covariates: age, sex, race/ethnicity, Charlson Comorbidity Score, depressive symptoms, anxiety symptoms, and any recent heavy alcohol use, illicit opioid use, illicit sedative use and cocaine use. In a Pearson correlation matrix, no covariates had a correlation coefficient of 0.4 or greater.
To explore the best cutoff value for identifying the risk of each outcome, we used receiver operating characteristic (ROC) curve analyses to calculate the area under the curve for the number of medications, overall and sedating, without adjustment for covariates. We report the Youden Index (sensitivity + specificity − 1), a summary measure of the ROC curve, to determine the optimal cutoff point. ROC curves are presented for each medication count and overdose analyses.
3.0 Results
3.1 Study participants
At study entry, the median age of the 250 participants (Table 1) was 50 (interquartile range [IQR] 44,56). The majority had both alcohol and drug dependence (51%) and 19% no substance dependence in the past year, but had a lifetime history of injection drug use. In the total sample, recent (past month) substance use was common. Specifically, 51% had heavy alcohol use; 30% reported cocaine use; and 23% and 9% had recent illicit/misused prescribed opioid and sedative use, respectively.
Table 1.
Baseline characteristics of participants with HIV infection and substance dependence (n=250)
| Characteristic | % (n) |
|---|---|
| Age, median (IQR) | 50 years (44, 56) |
| Female | 37% (93) |
| Race/ethnicity | |
| Hispanic | 24% (62) |
| Black | 50% (125) |
| White | 20% (51) |
| Employed | 16% (40) |
| Health insurance | 99% (248) |
| Depressive symptomsa | 30% (74) |
| Anxiety symptomsb | 45% (112) |
| Current tobacco | 78% (195) |
| Ever injected drugs | 19% (47) |
| DSM-IV Substance Dependence c, past year | |
| Both alcohol and drug dependence | 51% (127) |
| Drug dependence only | 21% (53) |
| Alcohol dependence only | 9% (23) |
| No dependence d | 19% (47) |
| Alcohol use,e past 30 days | |
| Heavy alcohol use | 51% (127) |
| Non-heavy alcohol use | 16% (40) |
| No alcohol use | 33% (83) |
| Past month drug use, past 30 days f | |
| Any illicit opioid use g | 23% (58) |
| Any illicit sedative use | 9% (22) |
| Any cocaine use | 30% (76) |
| Prescribed antiretroviral medications | 88% (220) |
| HIV viral load < 200 copies | 72% (178) |
| Prescribed medications | |
| Number of overall medications, median (IQR) | 10 (7,14) |
| Number of overall medications excluding ARV, median (IQR) | 8 (5, 11) |
| Five or more overall medications | 91% (227) |
| Sedating medication, any | 80% (201) |
| Number of sedating medications, median (IQR) | 2 (1,3) |
| Type of sedating medication: | |
| Opioid medication, any | 50% (124) |
| Non-opioid sedating medication, any | 72% (179) |
| Co-prescribed opioid and non-opioid sedating medications | 41% (102/250) |
| Opioid only (no non-opioid sedating), any | 9% (22) |
| Non-opioid sedating only (no opioid), any | 32% (77) |
| Type of opioid medication | |
| Buprenorphine or methadone, any | 27% (68) |
| Other opioid medications, any | 25% (62) |
| Overdose, any lifetime | 45% (111) |
| Overdose, past 12 months h | 7% (18) |
Patient Health Questionnaire-2 (PHQ-2) score ≥ 3
Overall Anxiety Severity and Impairment Scale (OASIS) score ≥ 8
Mini International Neuropsychiatric Interview (MINI) 6.0 DSM IV criteria
patients with no past year history of substance dependence were eligible for the study if s/he had a lifetime history of injection drug use
for women: more than 7 drinks on average in a week or 4+ drinks in a day; for men more than 14 drinks on average in a week or 5+ drinks in a day in the past 30 days
from the Addiction Severity Index
includes use of medications without a prescription or more than prescribed
Reported at study entry. At the 12-month follow-up study interview, 9 participants reported non-fatal overdose in the previous 12 months.
The median number of prescription medications was 10 (interquartile [IQR] 7,14) overall and 8 (IQR 5,11) non-ARV medications. Prescription of five or more medications (a common definition of polypharmacy) was almost universal (91%, 227/250). Among the study sample (n=250), 80% were prescribed at least one sedating medication. This category of medications included any opioid medication (50%) and any non-opioid sedating medication (72%). Co-prescribed opioid and non-opioid medications were common (41%). The most frequent non-opioid sedating medications were (in descending order of frequency): gabapentin, mirtazapine, trazodone, hydroxyzine, diphenhydramine, amitriptyline, doxepin, zolpidem, quetiapine, and clonazepam. (See Table 1a in Appendix for a complete list of sedating medications). At baseline, almost half (45%) reported lifetime non-fatal overdose and 7% past-year non-fatal overdose. An additional nine participants reported non-fatal past-year overdose at the 12-month study follow-up.
3.2 Main findings
3.2.1 Lifetime non-fatal overdose
Each additional medication overall (odds ratio [OR] 1.05, 95% Confidence Interval [CI] 1.00, 1.10, p=0.06) (Table 2) was not significantly associated with lifetime non-fatal overdose. Excluding ARV medications from the total number of medications yielded similar results (OR 1.05, 95% CI 1.00, 1.10, p=0.08).
Table 2.
Association of number (by type) of medications and risk of lifetime and past-year non-fatal overdosea
| Medication type | Lifetime Overdose OR (95%CI) |
p-value | Past-year Overdose OR (95%CI) |
p-value |
|---|---|---|---|---|
| Each additional overall medication | 1.05 (1.00, 1.10) | 0.06 | 1.06 (0.99, 1.14) | 0.12 |
| Each additional non-antiretroviral medication | 1.05 (1.00, 1.10) | 0.08 | 1.07 (1.00, 1.15) | 0.048 |
| Each additional sedating medication b | 1.26 (1.08, 1.46) | 0.003 | 1.18 (1.00, 1.39) | 0.049 |
| Each additional non-sedating medication b | 0.99 (0.94, 1.06) | 0.84 | 1.02 (0.92, 1.14) | 0.67 |
Results of separate unadjusted logistic regression models examining the association of the number of medications in each category and each type of overdose. Lifetime non-fatal overdose analyses included 249 observations. (One participant declined to respond to the question about overdose). Past-year overdose analyses were generalized estimating equations logistic regression models using 482 observations: 249 at baseline and 233 at 12 months. Results of adjusted models were not substantially different (Appendix).
Results of one model “number of sedating medications” and ”number of non-sedating medications”
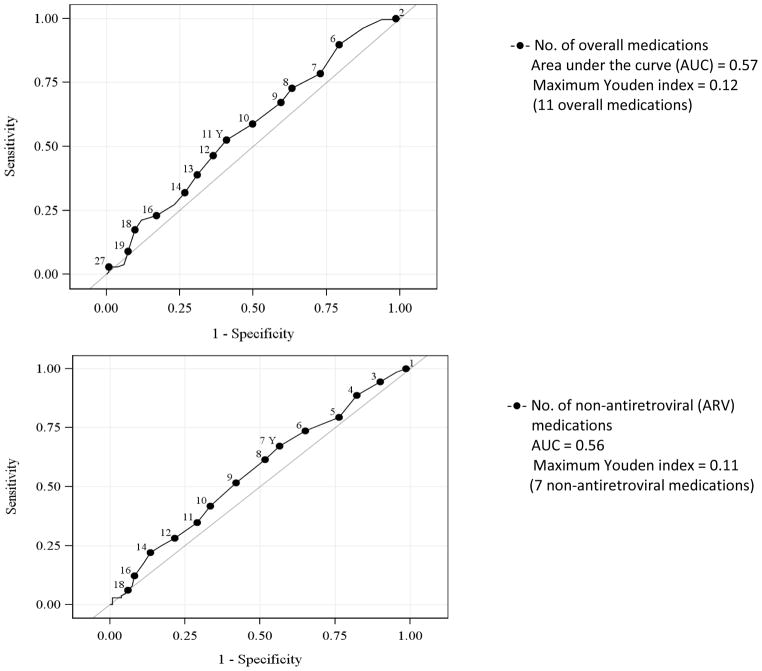
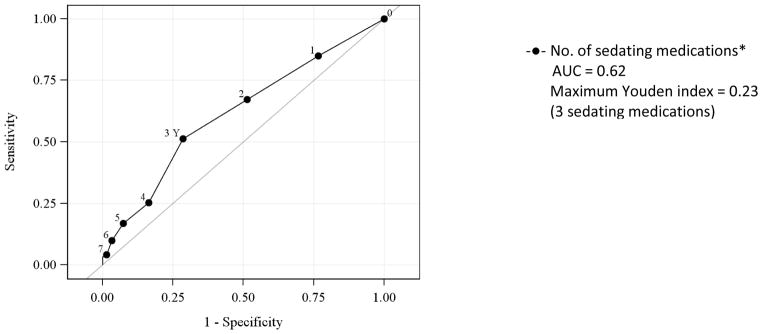
In a model that included the number of sedating and non-sedating medications, each additional sedating medication was significantly associated with greater odds of lifetime non-fatal overdose (OR 1.26, 95% CI 1.08, 1.46) whereas each additional non-sedating medication was not (OR 0.99, 95% CI 0.94, 1.06). ROC curve analyses indicated the optimal cutoff for identifying lifetime non-fatal OD was ≥ 3 sedating medications (Youden index 0.23, area under the curve [AUC] 0.62) (Figure 2).
Figure 2.
Receiver operating characteristic curves for the association of the number of medications overall and risk of lifetime overdose
* p< 0.05 statistically significant association
Prescription of an opioid medication (OR 2.31, 95% CI 1.37, 3.90) but not of a non-opioid sedating medication (OR 1.18, 95% CI 0.66, 2.13) was significantly associated with odds of a lifetime non-fatal overdose (Table 3). Opioid and non-opioid sedating medications were examined in one model. Because we found an effect of “any opioid medication”, we explored the association of opioid agonists for addiction treatment (i.e., buprenorphine or methadone) and other opioids for pain in the same model (Table 3). We found that opioid agonists for addiction treatment were signifcantly associated with lifetime non-fatal overdose (OR 4.79, 95% CI 2.53, 9.08) but that prescription of other opioid medications (e.g. for pain) was not (OR 1.11, 95%CI 0.59, 2.10).
Table 3.
Association of type of sedating medication and risk of lifetime and past-year non-fatal overdose
| Medication type | Lifetime Overdose OR (95%CI) |
p-value | Past-year Overdose OR (95%CI) |
p-value |
|---|---|---|---|---|
| Any opioid medication a | 2.31 (1.37, 3.90) | 0.002 | 2.23 (0.93, 5.35) | 0.07 |
| Any non-opioid sedating medication a | 1.18 (0.66, 2.13) | 0.57 | 1.99 (0.66, 5.94) | 0.22 |
| Any opioid agonist treatment medication b | 4.79 (2.53, 9.08) | <0.001 | 2.73 (1.20, 6.19) | 0.01 |
| Any opioid medication for pain b | 1.11 (0.59, 2.10) | 0.74 | 0.81 (0.27, 2.45) | 0.71 |
| Any non-opioid sedating medication b | 1.18 (0.66, 2.13) | 0.57 | 1.99 (0.66, 5.94) | 0.22 |
Results of one unadjusted logistic regression model that included ”any opioid medication” and “any non-opioid sedating medication”
Results of one unadjusted logistic regression model that included ”any opioid agonist treatment medication” (buprenorphine or methadone), “any opioid medication for pain (all other opioid medications) and any non-opioid sedating medication.
Although non-opioid sedating medications alone were not associated with overdose (OR 1.18, 95% CI 0.66, 2.13), co-prescribed opioid and non-opioid sedating medications were associated with non-fatal lifetime overdose (global p-value 0.01) (OR 2.54, 95%CI 1.25, 5.16) (Table 4). This logistic regression model included opioid medication only (no non-opioid sedating medication) and non-opioid sedating medication only (no opioid sedating medication); the latter 2 were not significantly associated with non-fatal lifetime overdose.
Table 4.
Association of co-prescribed sedating medications and risk of lifetime and past-year non-fatal overdosea
| Type of sedating medication | Lifetime Overdose OR (95%CI) |
Global p-value | Past-year Overdose OR (95%CI) |
Global p-value |
|---|---|---|---|---|
| Co-prescribed opioid and non-opioid sedating medications | 2.54 (1.25, 5.16) | 0.01 | 0.81 (0.27, 2.45) | 0.14 |
| Opioid only | 1.57 (0.56, 4.37) | --- | 2.23 (0.93, 5.35) | --- |
| Non-opioid sedating medication only | 0.96 (0.45, 2.04) | --- | 4.42 (0.98, 19.99) | --- |
Results of one logistic regression model. Referent group is no sedating medication.
3.2.2 Past year non-fatal overdose
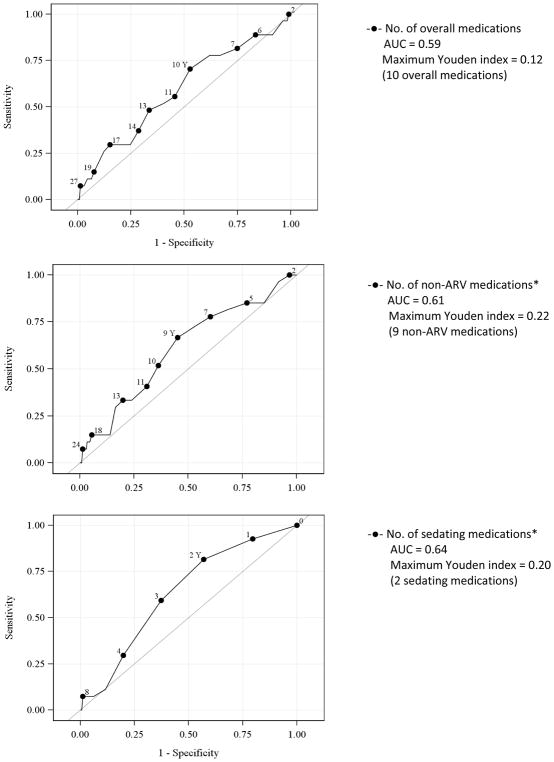
Each additional non-ARV medication (OR 1.07, 95% CI 1.00, 1.15, p=0.048) was significantly associated with an increase in the odds of past year non-fatal overdose (Table 2). In a model that further characterized medications as sedating and non-sedating, each additional sedating medication was associated with past year non-fatal OD (OR 1.81, 95% CI 1.00, 1.39, p=0.049) but non-sedating medications were not (OR 1.02, 95% CI 0.92, 1.14). Optimal cutoffs for identifying non-fatal overdose were: ≥ 9 non-ARV medications (Youden index 0.22, AUC 0.61) and ≥ 2 sedating medications (Youden index 0.20, AUC 0.64) (Figure 3).
Figure 3.
Receiver operating characteristic curves for the association of the number of medications and risk of past-year overdose
* p< 0.05 statistically significant association
Although not statistically significant, the odds of past year non-fatal OD were greater with prescription of an opioid medication (OR 2.23, 95%CI 0.93, 5.35) and a non-opioid sedating medication (OR 1.99, 95%CI 0.66, 5.94) (Table 3). Co-prescribed opioid and non-opioid sedating medications were not associated with past year non-fatal OD (Table 4). Buprenorphine and methadone were significantly associated with past year non-fatal overdose (OR 2.73, 95%CI 1.20, 6.19). Again, we did not find an association with other opioid medications (OR 0.81, 95%CI 0.27, 2.45).
3.2.3 Adjusted Models
Results of adjusted models for all regression models were not substantially different (Appendix). Other predictors of greater lifetime and past year non-fatal overdose included depressive and anxiety symptoms, and recent illicit opioid use and illicit sedative use.
4. Discussion
We examined the association of the type and number of prescribed medications with lifetime and past year nonfatal overdose in this study of individuals with HIV infection and substance dependence. The increasing odds of non-fatal overdose that we observed with our medication categories (systemically active < sedating < opioid) is consistent with our hypothesis that for non-fatal overdose, the risk from polypharmacy is attributable to additional sedating medications. Each additional sedating medication was associated with an approximately 25% increase in odds of lifetime non-fatal overdose. We also found that the lifetime non-fatal overdose risk from sedating medications was mostly driven by prescribed opioid medications. Co-prescribed opioid and non-opioid sedating medications were also associated with lifetime non-fatal overdose. Optimal cutoffs for identifying lifetime non-fatal overdose was ≥ 3 sedating medications and for past year non-fatal overdose ≥ 9 non-ARV medications and ≥ 2 sedating medications.
The current study extends our knowledge of overdose in PLWH with substance dependence by examining the association of polypharmacy and overdose. This is important because PLWH and substance dependence are at higher risk of developing non-AIDS-defining chronic medical conditions, also called multimorbidity (Salter et al., 2011), with an attendant higher number of prescribed medications. The study findings are consistent with literature on sedatives and risk of overdose. Patients co-prescribed opioid and benzodiazepine medications have greater mortality risk (Park et al., 2015) especially PLWH (Weisberg et al., 2015). Although the literature is less well-established for overdose and any sedating medication (Bernardy, Lund, Alexander, & Friedman, 2014; Turner & Liang, 2015), there are even fewer data on the risk with the number of sedating medications. One study found that the risk of overdose is associated with 5 or more sedating medications (which they termed “CNS polypharmacy”) among a high-risk population (Collett et al., 2016). Consistent with this study, we did not find any sedating medication to be a useful measure given that more than 80% were prescribed at least one sedating medication.
This study suggests that there is limited value to defining polypharmacy with strict cut-offs for identifying risks without regard for drug type (Gnjidic et al., 2012; Laflamme, Monárrez-Espino, Johnell, Elling, & Möller, 2015; Steinman, 2016). Conventional definitions of polypharmacy, such as five or more medications, have been validated largely in elderly populations (Gnjidic et al., 2012). It may be important to establish clinically relevant polypharmacy cut-offs for different age groups and populations (Kouladjian, Hilmer, Chen, Le Couteur, & Gnjidic, 2014). While we identified the best cutoff value in our cohort, we also demonstrated that the risk of overdose exists over a range of cutoffs. Given that almost all participants were prescribed five or more medications, we found that using this commonly used definition of polypharmacy was not appropriate in this sample. The median number of medications in this sample is somewhat higher than others in the literature (Moore, et al., 2015; Holtzman et al., 2013) but similar in another (Zhou et al., 2014). Variability in the number of prescribed medications cited by other studies stems in part from differences in age, regions of country, and study time period. The somewhat higher number of prescribed medications in this study likely reflects an insured, older (median age of 50), multi-morbid population with a high prevalence of psychiatric illness (85%), all recruited from medical clinics.
There are several potential reasons for the associations between polypharmacy and overdose. More medications contribute to the complexity of one’s treatment regimen with the potential for a less careful inspection of the medication list by providers resulting in prescriber errors and even patient confusion about medication instructions (Patel, Zimmerman, Fonda, & Linsky, 2016). Safer management of opioid and other sedating medications in clinic visits may compete for attention with HIV-related medical, psychiatric, and addiction problems (Rose et al., 2009). Although this was not a study of inappropriate prescribing, medication interactions, or pill burden, these may have been factors that contributed to the findings.
Although we adjusted for any recent illicit opioid use and benzodiazepine medication use, it is possible that misuse of prescribed sedative medications was a contributing factor. Vijayaraghavan et al. found patients with HIV prescribed opioid medications, benzodiazepines, and muscle relaxants are at greater risk of sedative misuse (Vijayaraghavan et al., 2014).
The reason that we did not find an association of opioid medications prescribed for pain and non-fatal overdose is unclear. Because our medication review was a one-time look at the EMR, we did not have information about length of time that participants were prescribed opioid medications. In many cases, opioids may have been prescribed for a brief time (e.g., after a surgical procedure) with minimal overdose risk.
The association of buprenorphine and methadone with both lifetime and past year non-fatal overdose is likely due, at least in part, the result of a participant’s history of severe opioid use disorder rather than the medication itself. A meta-analysis of prospective and retrospective cohort studies of the relationship between opioid agonist medication and fatal overdose demonstrated substantially lower overdose and all-cause mortality among people taking opioid agonists (Sordo et al., 2017). We would expect that people who have previously overdosed would be more likely to have a severe opioid use disorder and thus be appropriately treated with opioid agonist treatment. In the lifetime analysis, we do not know whether the medications were started before or after the overdose occurred. In the past year analysis, two thirds of the reported non-fatal overdoses occurred prior to study entry. Despite adjustment for psychiatric symptoms, the number of sedating medications could be a proxy for psychiatric comorbidity due to residual confounding. To fully sort out the attribution of the number and categories of medications to overdose risk. additional studies with larger cohorts and longer prospective observation periods are warranted to better address this confounding.
Another limitation relates to the absence of a validated method of assessing a history of overdose, a major gap in the literature (Green et al., 2012). Absent that, asking the question, “Have you had an overdose?” as we did in this study has face value. The limited number of past year overdoses may have affected the ability to detect associations between medication counts and overdose. Another limitation is the examination of non-fatal overdose rather than fatal overdose. However, sequelae of non-fatal overdose (e.g., aspiration pneumonia, cognitive impairments, renal failure) can be devastating. Also, given that non-fatal overdose is a risk factor for fatal overdose (Caudarella et al., 2016), interventions to decrease risk of non-fatal overdose could reasonably be expected to reduce fatal overdoses.
We did not have data on alternative and over the counter medications that were not listed in the EMR, however these medications are unlikely to have a strong impact on overdose and are more difficult to assess reliably. We also did not examine data on the number of tablets (pill burden) or frequency of dosing. Finally, generalizability is another consideration given that the study sample was recruited from two urban HIV clinics in a northeastern city (where the ecology of the availability of different types of illicit drugs and prescription drugs may directly affect personal risks).
In this sample of individuals with HIV infection and substance dependence, the number of sedating medications and any opioid were associated with non-fatal overdose. Sedating medications were prescribed to over three quarters of patients. Polypharmacy among HIV patients with substance dependence warrants further research to determine whether reducing sedating medications lowers overdose risk.
Highlights.
People living with HIV are at risk of polypharmacy and overdose (OD)
We assessed the effect of the number and type of medication on non-fatal OD
The risk from polypharmacy was attributable to the number of sedating medications
Research is needed whether reducing number of sedating medications lowers OD risk
Acknowledgments
Sources of Funding: This study was funded by the National Institute on Alcohol Abuse and Alcoholism [award numbers U01AA020784, U24AA020779, U24AA020778] and by the National Center for Advancing Translational Science (UL1TR001430). The funding sources did not have any role in the study design; in the collection, analysis, or data interpretation; writing of the report; or decision to submit the article for publication.
The authors would like to thank the Boston ARCH Cohort study participants for the time and effort they have dedicated to this research. We also wish to thank all staff at Boston University and Boston Medical Center who supported this project. Specifically, we want to acknowledge Margo Godersky, Kate Haworth, Keshia Toussaint and Laura Vercammen for their work in recruitment, data collection and participant retention; and Seville Meli for her leadership and assistance with study management.
Appendix
Table 1a.
Non-opioid sedating medications prescribed to a cohort of HIV-infected participants with past year substance dependence or lifetime history of injection drug use (n=250)
| Generic name | Number of participants n (%) |
|---|---|
| Gabapentin | 56 (22%) |
| Mirtazapine | 39 (16%) |
| Trazodone | 36 (14%) |
| Hydroxyzine | 29 (12%) |
| Diphenhydramine | 25 (10%) |
| Amitriptyline | 20 (8%) |
| Doxepin | 20 (8%) |
| Zolpidem | 20 (8%) |
| Quetiapine | 19 (8%) |
| Clonazepam | 18 (7%) |
| Butabarbital | 15 (6%) |
| Prazosin | 14 (6%) |
| Cyclobenzaprine | 13 (5%) |
| Clonidine | 12 (5%) |
| Lorazepam | 10 (4%) |
| Chlorpromazine | 6 (2%) |
| Aripirazole | 5 (2%) |
| Dronabinol | 5 (2%) |
| Nortripyline | 5 (2%) |
| Perphenazine | 5 (2%) |
| Benztropine | 4 (2% |
| Buspirone | 4 (2%) |
| Pregabalin | 4 (2%) |
| Risperidone | 4 (2%) |
| Haloperidol | 3 (1%) |
| Ziprasizone | 3 (1%) |
| Promethazine | 2 (1%) |
| Alprazolam | 2 (1%) |
| Tizanidine | 1 (<1%) |
| Chlordiazepoxide | 1 (<1%) |
| Fluphenazine | 1 (<1%) |
| Methocarbamol | 1 (<1%) |
| Paliperidone | 1 (<1%) |
| Phenobarbital | 1 (<1%) |
Table 2a.
Adjusted associations between number of medications and lifetime non-fatal overdosea
| All medications b OR (95%CI) |
Non-antiretroviral medications OR (95%CI) |
Sedating Medications c OR (95%CI) |
Non-sedating medications c OR (95%CI) |
|
|---|---|---|---|---|
| Each additional medication (unadjusted) | 1.05 (1.00, 1.10) | 1.05 (1.00, 1.10) | 1.26 (1.08, 1.46) | 0.99 (0.94, 1.06) |
|
| ||||
| Age | 1.02 (0.99, 1.05) | 1.02 (1.00, 1.05) | 1.04 (1.01, 1.07) | ---- |
| Each additional medication | 1.04 (0.99, 1.09) | 1.04 (0.99, 1.09) | 1.31 (1.12, 1.53) | 0.96 (0.90, 1.03) |
|
| ||||
| Sex (female vs male) | 0.92 (0.54, 1.56) | 0.91 (0.54, 1.55) | 0.87 (0.51, 1.49) | --- |
| Each additional medication | 1.05 (1.00, 1.10) | 1.05 (1.00, 1.10) | 1.26 (1.09, 1.46) | 1.00 (0.94, 1.06) |
|
| ||||
| Race | ||||
| Hispanic vs Black | 1.63 (0.87, 3.05) | 1.63 (0.87, 3.04) | 1.50 (0.79, 2.83) | --- |
| White vs Black | 2.74 (1.46, 5.13) | 2.69 (1.44, 5.04) | 2.29 (1.19, 4.43) | --- |
| Each additional medication | 1.04 (1.00, 1.10) | 1.04 (0.99, 1.10) | 1.19 (1.02, 1.39) | 1.01 (0.95, 1.07) |
|
| ||||
| Charlson Comorbidity Index | 1.07 (0.96, 1.20) | 1.08 (0.96, 1.21) | 1.11 (0.98, 1.25) | --- |
| Each additional medication | 1.03 (0.98, 1.09) | 1.03 (0.98, 1.09) | 1.25 (1.08, 1.45) | 0.97 (0.91, 1.04) |
|
| ||||
| Depressive symptoms | 2.08 (1.19, 3.65) | 2.07 (1.18, 3.64) | 2.02 (1.14, 3.57) | --- |
| Each additional medication | 1.04 (0.99, 1.09) | 1.03 (0.98, 1.09) | 1.24 (1.06, 1.44) | 0.99 (0.93, 1.05) |
|
| ||||
| Anxiety symptoms | 1.75 (1.05, 2.92) | 1.74 (1.04, 2.90) | 1.53 (0.90, 2.59) | --- |
| Each additional medication | 1.04 (0.99, 1.09) | 1.04 (0.99, 1.09) | 1.22 (1.05, 1.42) | 1.00 (0.94, 1.06) |
|
| ||||
| Alcohol use,d e | ||||
| Did not exceed daily/weekly limits vs no alcohol | 0.42 (0.19, 0.93) | 0.43 (0.19, 0.94) | 0.48 (0.22, 1.08) | --- |
| Exceeded daily/weekly limits vs no alcohol | 0.55 (0.31, 0.96) | 0.54 (0.31, 0.95) | 0.53 (0.30, 0.95) | --- |
| Each additional medication | 1.04 (1.00, 1.09) | 1.04 (0.99, 1.10) | 1.24 (1.07, 1.44) | 0.99 (0.93, 1.06) |
|
| ||||
| Illicit or misused prescription opioid, anye | 3.22 (1.72, 6.00) | 3.18 (1.71, 5.93) | 3.08 (1.64, 5.77) | --- |
| Each additional medication | 1.05 (1.00, 1.10) | 1.05 (1.00, 1.11) | 1.25 (1.07, 1.46) | 1.00 (0.94, 1.07) |
|
| ||||
| Non-prescribed sedative medication use, anye | 3.17 (1.23, 8.16) | 3.10 (1.21, 7.96) | 2.92 (1.12, 7.58) | --- |
| Each additional medication | 1.05 (1.00, 1.10) | 1.05 (1.00, 1.10) | 1.25 (1.07, 1.45) | 1.00 (0.94, 1.06) |
|
| ||||
| Cocaine, any e | 0.85 (0.49, 1.46) | 0.84 (0.48, 1.45) | 0.81 (0.46, 1.42) | --- |
| Each additional medication | 1.05 (1.00, 1.10) | 1.05 (1.00, 1.10) | 1.26 (1.09, 1.46) | 0.99 (0.94, 1.06) |
Results of separate logistic regression models examining the association of the number of medications in each category and overdose controlling for each covariate listed above. Each regression model included the number of medications and one covariate. Analyses used study entry data. The first odds ratio (95% CI) in each row is the parameter estimate for the covariate. The second OR is the parameter estimate for the number of medications in each category.
Includes only systemically active medications
Results of one model examining the association of the number of sedating medications and number of non-sedating medications with overdose controlling for one covariate. There is no separate parameter estimate for the covariate and non-sedating medications (as indicated by “---“).
NIAAA defined drinking limits (> 14 drinks in a week or 5+ drinks in a day, for men; or >7 drinks in a week or 4+ drinks in a day, for women)
Past 30 days
Table 3a.
Adjusted associations between number of medications and past year non-fatal overdosea
| All medicationsb OR (95%CI) |
Non-antiretroviral medications OR (95%CI) |
Sedating medications c OR (95%CI) |
Non-sedating medications c OR (95%CI) |
|
|---|---|---|---|---|
| Each additional medication (unadjusted) | 1.06 (0.99, 1.14) | 1.07 (1.00, 1.15) | 1.18 (1.00, 1.39) | 1.02 (0.92, 1.14) |
|
| ||||
| Age | 0.97 (0.92, 1.01) | 0.97 (0.92, 1.01) | 0.97 (0.93, 1.02) | --- |
| Each additional medication | 1.07 (1.00, 1.15) | 1.09 (1.01, 1.17) | 1.15 (0.98, 1.35) | 1.05 (0.94, 1.17) |
|
| ||||
| Sex (female vs male) | 1.73 (0.77, 3.91) | 1.68 (0.74, 3.80) | 1.69 (0.75, 3.83) | --- |
| Each additional medication | 1.05 (0.98, 1.13) | 1.06 (0.99, 1.14) | 1.17 (0.99, 1.37) | 1.02 (0.92, 1.13) |
|
| ||||
| Race | ||||
| Hispanic vs Black | 1.54 (0.60, 3.92) | 1.54 (0.60, 3.93) | 1.41 (0.54, 3.69) | --- |
| White vs Black | 1.49 (0.49, 4.48) | 1.43 (0.47, 4.38) | 1.27 (0.40, 4.03) | --- |
| Each additional medication | 1.06 (0.99, 1.14) | 1.07 (1.00, 1.15) | 1.16 (0.97, 1.39) | 1.03 (0.93, 1.14) |
|
| ||||
| Charlson Comorbidity Index | 0.99 (0.85,1.15) | 0.98 (0.85, 1.14) | 1.01 (0.86, 1.19) | --- |
| Each additional medication | 1.06 (0.97, 1.16) | 1.08 (0.99, 1.17) | 1.18 (1.00, 1.40) | 1.02 (0.90, 1.16) |
|
| ||||
| Depressive symptoms | 3.95 (1.79, 8.71) | 3.84 (1.75, 8.41) | 3.84 (1.74, 8.48) | --- |
| Each additional medication | 1.04 (0.97, 1.11) | 1.05 (0.98, 1.12) | 1.14 (0.98, 1.32) | 1.01 (0.92, 1.12) |
|
| ||||
| Anxiety symptoms | 2.81 (1.16, 6.80) | 2.72 (1.11, 6.65) | 2.65 (1.12, 6.25) | --- |
| Each additional medication | 1.04 (0.97, 1.12) | 1.05 (0.98, 1.13) | 1.11 (0.94, 1.32) | 1.02 (0.93, 1.13) |
|
| ||||
| Alcohol use d,e | ||||
| Did not exceed daily/weekly limits vs no alcohol | 1.12 (0.36, 3.50) | 1.12 (0.36, 3.48) | 1.23 (0.40, 3.80) | --- |
| Exceeded daily/weekly limits vs no alcohol | 1.16 (0.47, 2.86) | 1.15 (0.47, 2.84) | 1.16 (0.47, 2.88) | --- |
| Each additional medication | 1.06 (0.99, 1.14) | 1.07 (1.00, 1.15) | 1.19 (1.02, 1.39) | 1.02 (0.92, 1.14) |
|
| ||||
| Ilicit or misused prescription opioid, any e | 5.53 (2.56, 11.93) | 5.50 (2.54, 11.93) | 5.37 (2.51, 11.51) | --- |
| Each additional medication | 1.06 (0.99, 1.14) | 1.07 (1.00, 1.15) | 1.14 (0.98, 1.34) | 1.03 (0.94, 1.14) |
|
| ||||
| Non-prescribed sedative medication use, anye | 3.23 (1.07, 9.75) | 3.13 (1.02, 9.56) | 3.03 (1.01, 9.12) | --- |
| Each additional medication | 1.06 (0.99, 1.14) | 1.07 (1.00, 1.15) | 1.16 (0.99, 1.37) | 1.03 (0.93, 1.14) |
|
| ||||
| Cocaine, any e | 1.32 (0.61, 2.87) | 1.28 (0.59, 2.77) | 1.27 (0.58, 2.76) | --- |
| Each additional medication | 1.06 (0.99, 1.14) | 1.07 (1.00, 1.15) | 1.18 (1.00, 1.38) | 1.03 (0.92, 1.14) |
Results of separate logistic regression models examining the association of the number of medications in each category and overdose controlling for each covariate listed above. Each regression model included the number of medications and one covariate. The first odds ratio (95% CI) in each row is the parameter estimate for the covariate. The second odds ratio is the parameter estimate for each additional medication in each category
Includes only systemically active medications
Results of one model examining the association of the number of sedating medications and number of non-sedating medications with overdose controlling for one covariate. There is no separate parameter estimate for the covariate and non-sedating medications (as indicated by “---“).
National Institute on Alcohol Abuse and Alcoholism defined drinking limits (> 14 drinks in a week or 5+ drinks in a day, for men; or >7 drinks in a week or 4+ drinks in a day, for women)
Past 30 days
Footnotes
Prior presentations: Portions of the work were previously presented in abstract form at the 78th Annual Meeting of the College Problems Drug Dependence on June 16, 2016.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, … Justice AC. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clinical Infectious Diseases. 2015;60:627–38. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker WC, Gordon K, Edelman EJ, Kerns RD, Crystal S, Dziura JD, … Fiellin DA. Trends in any and high-dose opioid analgesic receipt among patients aging with and without HIV infection. AIDS Behavior. 2016;20:679–686. doi: 10.1007/s10461-015-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardy NC, Lund BC, Alexander B, Friedman MJ. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Medicine. 2014;15:1083–90. doi: 10.1111/pme.12321. [DOI] [PubMed] [Google Scholar]
- Brothers TD, Rockwood K. Biologic aging, frailty, and age-related disease in chronic HIV infection. Current Opinion HIV and AIDS. 2014;9:412–18. doi: 10.1097/COH.0000000000000070. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, Bystritsky A, Sherbourne C, Roy-Byrne P, Stein MB. Validation of a brief measure of anxiety-related severity and impairment: The Overall Anxiety Severity and Impairment Scale (OASIS) Journal of Affective Disorders. 2009;112:92–101. doi: 10.1016/j.jad.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantudo-Cuenca MR, Jiménez-Galán R, Almeida-Gonzalez CV, Morillo-Verdugo R. Concurrent use of comedications reduces adherence to antiretroviral therapy among HIV-infected patients. Journal of Managed Care & Specialty Pharmacy. 2014;20:844–50. doi: 10.18553/jmcp.2014.20.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, Hayashi K. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug and Alcohol Dependence. 2016;162:51–55. doi: 10.1016/j.drugalcdep.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control, National Center for Injury Prevention Control. [Accessed May 7, 2017];Leading cause of injury deaths in the United States. 2015 https://www.cdc.gov/injury/wisqars/LeadingCauses.html.
- Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ. Prevalence of central nervous system polypharmacy and associations with overdose and suicide-related behaviors in Iraq and Afghanistan war veterans in VA care 2010–2011. Drugs- Real World Outcomes. 2016;3:45–52. doi: 10.1007/s40801-015-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers K, Griffith TA, McGinnis KA, Rodriguez-Barradas MC, Leaf DA, Weissman S, … Justice Amy C. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. Journal of General Internal Medicine. 2005;20:1142–45. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–33. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JE, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: Polypharmacy. Drugs & Aging. 2013;30:613–28. doi: 10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnjidic D, Hilmer SN, Blyth FM, Naganathan V, Waite L, Seibel MJ, Le Couteur DG. Polypharmacy cutoff and outcomes: Five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. Journal of Clinical Epidemiology. 2012;65:989–95. doi: 10.1016/j.jclinepi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Green TC, McGowan SK, Yokell MA, Pouget ER, Rich JD. HIV infection and risk of overdose: A systematic review and meta-analysis. AIDS. 2012;26:403–17. doi: 10.1097/QAD.0b013e32834f19b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M, Justice AC, Lampiris HW, Valcour V. Management of Human Immunodeficiency Virus infection in advanced age. JAMA. 2013;309:1397–1405. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, … Volberding P. HIV and aging: State of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. Journal of Acquired Immune Deficiency Syndromes. 2012;60:S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman C, Armon C, Tedaldi E, Chmiel JS, Buchacz K, Wood K, Brooks JT. Polypharmacy and risk of antiretroviral drug interactions among the aging HIV-infected population. Journal of General Internal Medicine. 2013;28:1302–10. doi: 10.1007/s11606-013-2449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, … Fiellin DA. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug and Alcohol Dependence. 2016;161:95–103. doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krentz HB, Gill MJ. The impact of non-antiretroviral polypharmacy on the continuity of antiretroviral therapy (ART) among HIV patients. AIDS Patient Care and STDs. 2016;30:11–17. doi: 10.1089/apc.2015.0199. [DOI] [PubMed] [Google Scholar]
- Kouladjian L, Hilmer SN, Chen TF, Le Couteur DG, Gnjidic D. Assessing the harms of polypharmacy requires careful interpretation and consistent definitions. British Pharmacological Society. 2014;78:670–671. doi: 10.1111/bcp.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RS, Williams JBW. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Laflamme L, Monárrez-Espino J, Johnell K, Elling B, Möller J. Type, number or both? A population-based matched case-control study on the risk of fall injuries among older people and number of medications beyond fall-inducing drugs. PloS One. 2015;10:e0123390. doi: 10.1371/journal.pone.0123390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Bucello C, Lemon J, Wiessing L, Hickman M. Mortality among people who inject drugs: A systematic review and meta-analysis. Bulletin of the World Health Organization. 2013;91:102–23. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, … Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Monroe AK, Rowe TL, Moore RD, Chander G. Medication adherence in HIV-positive patients with diabetes or hypertension: A focus group study. BMC Health Services Research. 2013;13:488. doi: 10.1186/1472-6963-13-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HN, Mao L, Oramasionwu CU. Factors associated with polypharmacy and the prescription of multiple medications among persons living with HIV (PLWH) compared to non-PLWH. AIDS Care. 2015;27:1443–48. doi: 10.1080/09540121.2015.1109583. [DOI] [PubMed] [Google Scholar]
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert ASB. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: Case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CH, Zimmerman KM, Fonda JR, Linsky A. Medication complexity, medication number, and their relationships to medication discrepancies. Annals of Pharmacotherapy. 2016;50:534–40. doi: 10.1177/1060028016647067. [DOI] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, … Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care. 2005;43:1130–39. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-Infected people in the United States. AIDS. 2014;28:881–90. doi: 10.1097/QAD.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Hermos JA, Frayne SM, Pogach LM, Berlowitz DR, Miller DR. Does opioid therapy affect quality of care for diabetes mellitus? American Journal of Managed Care. 2009;15:217–24. [PubMed] [Google Scholar]
- Salter M, Lau B, Mehta SH, Kirk GD. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clinical Infectious Diseases. 2011;53:1256–1264. doi: 10.1093/cid/cir673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz SK, Vu A, Hsu LC, Hessol NA. Changes in causes of death among persons with AIDS: San Francisco, California, 1996–2011. AIDS Patient Care and STDS. 2014;28:517–23. doi: 10.1089/apc.2014.0079. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, … Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychology. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:1550. doi: 10.1136/bmj.j1550. www.bmj.com/content/357/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MA. Polypharmacy-time to get beyond numbers. JAMA Internal Medicine. 2016;176:482–83. doi: 10.1001/jamainternmed.2015.8597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Liang Y. Drug overdose in a retrospective cohort with non-cancer pain treated with opioids, antidepressants, and/or sedative-hypnotics: Interactions with mental health disorders. Journal of General Internal Medicine. 2015;30:1081–96. doi: 10.1007/s11606-015-3199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Accessed August 1, 2016];VA National Formulary - Pharmacy Benefits Management Services. http://www.pbm.va.gov/nationalformulary.asp.
- Vijayaraghavan M, Freitas D, Bangsberg DR, Miaskowski C, Kushel MB. Non-medical use of non-opioid psychotherapeutic medications in a community-based cohort of HIV-infected indigent adults. Drug and Alcohol Dependence. 2014;143:263–67. doi: 10.1016/j.drugalcdep.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson DC, Reidinger C, Wilcosky T. Factors affecting the validity of a Timeline Follow-Back Interview. Journal of Studies on Alcohol Drugs. 2003;64:733–40. doi: 10.15288/jsa.2003.64.733. [DOI] [PubMed] [Google Scholar]
- Weisberg DF, Gordon KS, Barry DT, Becker WC, Crystal S, Edelman EJ, … Fiellin DA. Long-term prescription of opioids and/or benzodiazepines and mortality among HIV-Infected and uninfected patients. Journal Acquired Immune Deficiency Syndromes. 2015;69:223–33. doi: 10.1097/QAI.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Martin K, Corbett A, Napravnik S, Eron J, Zhu Y, … Wohl DA. Total daily pill burden in HIV-Infected patients in the southern United States. AIDS Patient Care and STDs. 2014;28:311–17. doi: 10.1089/apc.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]