Abstract
Background
Previous reports have implicated multiple genetic loci associated with atrial fibrillation (AF), but the contributions of genome-wide variation to AF susceptibility have not been quantified.
Methods and Results
We assessed the contribution of genome-wide single nucleotide polymorphism (SNP) variation to AF risk (SNP-heritability, h2g) using data from 120,286 unrelated individuals of European ancestry (2,987 with AF) in the population-based UK Biobank. We ascertained AF based on self-report, medical record billing codes, procedure codes, and death records. We estimated h2g using a variance components method with variants having a minor allele frequency (MAF) ≥1%. We evaluated h2g in age, sex, and genomic strata of interest. The h2g for AF was 22.1% (95% CI 15.6%–28.5%), and was similar for early- versus older-onset AF (≤65 versus >65 years of age) as well as for men and women. The proportion of AF variance explained by genetic variation was mainly accounted for by common (MAF ≥ 5%) variants (20.4%, 95% CI 15.1%–25.6%). Only 6.4% (95% CI 5.1–7.7%) of AF variance was attributed to variation within known AF susceptibility, cardiac arrhythmia, and cardiomyopathy gene regions.
Conclusions
Genetic variation contributes substantially to AF risk. The risk for AF conferred by genomic variation is similar to that observed for several other cardiovascular diseases. Established AF loci only explain a moderate proportion of disease risk, suggesting that further genetic discovery, with an emphasis on common variation, is warranted to understand the causal genetic basis of AF.
Keywords: atrial fibrillation, heritability, genome-wide association study, epidemiology
Journal Subject Terms: Atrial Fibrillation, Genetics, Genetic, Association Studies, Epidemiology, Arrhythmias
Introduction
Atrial fibrillation (AF) affects nearly 34 million individuals worldwide1 and imposes a major public health burden.2 Epidemiologic data underscore the heritable nature of AF.3–8 Both traditional genetic mapping approaches and genome-wide association studies (GWAS) have identified numerous loci implicated in the pathogenesis of AF.9–12 Despite progress identifying susceptibility loci for AF, the aggregate contributions of genetic variation to AF susceptibility remain unclear.
Since complex phenotypes may be caused by the aggregate effects of thousands of variants that are not associated with disease at genome-wide significance levels,13, 14 several approaches have been developed to assess the total contributions of genome-wide variation to disease susceptibility.15–18 Such methods can provide estimates of the proportion of phenotypic variance explained by additive genetic variation, otherwise referred to as narrow sense single nucleotide polymorphism (SNP)- heritability (h2g). Measurements of h2g estimated in unrelated subjects can minimize the potential inflation of traditional heritability estimates caused by shared familial environmental factors. Moreover, such methods enable partitioning of the contributions of variation in specific genomic regions, and according to variant characteristics and annotations, to disease susceptibility.17, 18 Currently, the contribution of genome-wide variation to AF susceptibility is not well-understood.
We therefore sought to assess the genetic architecture of AF by using genome-wide imputation data from the population-based UK Biobank, leveraging recently released data for over 120,000 individuals of predominantly European ancestry. We performed a genome-wide association study of AF in the participants, and specifically estimated h2g stratified by age of AF onset, sex, allele frequency, and variation within previously implicated AF susceptibility gene regions.
Methods
Study participants
The UK Biobank is a prospective, population-based, cohort study, which enrolled over 500,000 individuals aged 40 to 69 from the United Kingdom. Participants were invited to one of the 22 centers in the UK between 2006 and 2010 (the initial assessment visit). Blood, urine, and saliva samples, information from questionnaires on health and lifestyle, and physical measurements were collected. In the current study, we assessed 152,249 participants with available genome-wide genotyping information from an interim data release. Individuals of non-European ancestry and close relatives were removed. Unrelated individuals were chosen by omitting first, second, and third degree relatives with an empirical kinship coefficient >0.044 as estimated and defined by the KING software performed by UK Biobank.19 Individuals of European ancestry were identified from a principal component analysis of 40,538 common variants with low level of linkage disequilibrium followed by the application of an outlier detection algorithm20 to the two leading principal components performed by UK Biobank as well. All participants provided written informed consent to participate in research as previously described,21 and the UK Biobank was approved by the UK Biobank Research Ethics Committee (reference number 11/NW/0382). Use of UK Biobank data was approved by the local Partners Healthcare Institutional Review Board.
Atrial fibrillation ascertainment
We defined AF based on data ascertained from baseline interviews, procedure codes, billing codes, and death records. A detailed description of the AF definition we derived and utilized is provided in Supplemental Table 1. Briefly, we classified participants as having AF if they reported a history of AF, atrial flutter, or had undergone cardioversion, atrioventricular node ablation, pulmonary vein isolation procedure, atrial flutter ablation, or had received an International Classification of Diseases (ICD) billing code of 427.3 (ICD-9) or I48 (ICD-10).
Genotyping, imputation, and variant selection
Two-thirds of the samples in the interim UK Biobank data release were genotyped on the UK Biobank Axiom array. About 50,000 samples were genotyped by the UK BiLEVE Axiom array. Both the UK Biobank Axiom array and the UK BiLEVE Axiom array include ~800,000 SNPs, with over 95% marker overlap. All genotyping was performed at Affymetrix Research Services laboratory in Santa Clara, California, USA.
For different arrays, different quality control filters were applied to select variants for imputation. Genotypes were pre-phased to estimate the underlying haplotype of each individual by SHAPEIT-3, and the haplotype estimates were then used for imputation to infer unobserved genotypes using IMPUTE2, with the 1000 Genomes Phase 3 and UK10K reference panels. Phasing and imputation were centrally carried out at the Wellcome Trust Centre for Human Genetics in Oxford. Additionally, the first 15 principal components of ancestry were estimated by flashPCA.22 Detailed descriptions of the genotyping, imputation procedures, and principal component calculation can be found on the UK Biobank website (http://www.ukbiobank.ac.uk/).23, 24
All analyses were performed using the imputed dataset restricted to variants of high imputation quality (info ≥0.4) with low missingness rates (<5%), minor allele frequency (MAF) of at least 1%, and high genotype imputation probability (≥0.9) across at least 90% of samples. Variants that passed these quality control criteria were transformed to hard-called genotypes in PLINK version 1.90b25 using a probability threshold ≥ 0.9. In total, 8,325,606 genetic variants (SNPs and indels) with MAF ≥1% and low missingness rate (<5% in hard-called genotypes) were retained for association analysis.
To estimate h2g, additional quality control was applied as previously described.26 Specifically, we removed variants with a missingness rate of ≥0.5%, MAF <1%, and variants with differential missingness between cases and referents (p-value <0.05). In order to reduce suspicious LD bias from REML estimation15, 27–29 and due to computational capacity of the software, we further performed two rounds of linkage disequilibrium (LD) pruning at r2 =0.9 (PLINK 1.90b;25 i.e., using a --indep-pairwise 50 5 0.9 flag) and decreased the total number of variants to 811,488 (with a small fraction of biallelic indels).
Statistical analysis
To validate the definition of AF we developed, we performed a GWAS of AF in the UK Biobank, and then compared the associations of top variants to a prior independent analysis from the AFGen Consortium.11 The prior analysis included a GWAS in nearly 18,000 individuals with AF and over 100,000 without, as well as an exome-chip association analyses comprised about 22,000 individuals with AF and 150,000 without. In total, 25 independent loci were identified in this analysis (23 in the GWAS, and an additional 2 in the exome chip analysis). To perform the GWAS in the UK Biobank, each variant was tested for association with AF using logistic regression assuming an additive genetic model. We fit models adjusted for baseline age, sex, and one principal component of ancestry that was associated with AF. To account for array differences, we also adjusted for array type in all analyses.
We then examined associations between the top variants at the 25 established AF susceptibility loci from the AFGen analysis with those of the present analysis in the UK Biobank for concordance. Correlation between the log-odds ratios for the top variants tagging the 25 loci in the prior analysis and the present analysis in the UK Biobank was assessed using Spearman’s rho. We created a weighted linear genetic risk score for each individual in the UK Biobank using the 25 top AF variants. To create the score, we summed the product of the AF risk allele count (i.e., 0, 1, or 2) for the variant multiplied by the log-relative risk for the respective variant, which we derived from the prior analysis as implemented in PLINK 1.90b using the --score flag (Supplemental Table 2). If the hard-called variant was missing, PLINK used the mean genotype frequency in the sample instead. Thus, for each individual, we obtained a single continuous variable representing AF genetic risk, which we used in subsequent analyses.25 Scores were treated as continuous variables and tested for association with AF using multivariable logistic regression based on a two-sided p-value <0.05, adjusted for age, sex, array, and one principal component of ancestry as above. For the remainder of the genome-wide association study, we considered loci to be significantly associated with AF if a variant was associated with AF with a p-value < 5×10−8.
We calculated h2g based on LD-pruned markers using BOLT-REML as previously specified.15 In brief, BOLT-REML uses an efficient implementation of restricted maximum likelihood to estimate the genetic and residual variance components for a given phenotype using genetic data. In the analysis, we adjusted for baseline age, sex, array, and one AF related principal component of ancestry. We converted the estimate on the observed scale into a liability scale26 by setting the disease prevalence as the observed proportion of AF cases in the UK Biobank sample. For all h2g estimates, we provided the 95% confidence intervals (CI).
Given the association between age and AF risk, we estimated h2g stratified by early- versus older-onset AF status to determine whether h2g varies by age. In the early-onset AF analyses, we included individuals in whom the last follow-up or AF onset occurred prior to or equal to 65 years of age. In the older-onset AF analyses, we included individuals in whom the last follow-up or AF onset was over 65 years of age. We also stratified h2g estimates by sex given the increased risk of AF among men, to assess whether additive genetic variation contributes to disease risk differentially in each stratum. Additionally, we computed estimates for low frequency variants (MAF between 1 to 5%), and common variants (MAF ≥5%), separately to investigate the contribution of variant frequency to the genetic architecture of AF.
To determine the extent to which previously identified AF susceptibility loci contributed to disease risk, we further assessed h2g stratified by variation at established GWAS loci for AF alone, with additional putative AF susceptibility genes, and with an expanded panel of AF, cardiac arrhythmia and cardiomyopathy genes given the associations between such conditions and AF. We defined established AF GWAS loci as the top SNP plus or minus 500 kilobases (kb) for each of 25 loci identified in a prior independent GWAS11 (Supplemental Table 2). We defined putative AF susceptibility genes (n=37) as those implicated through prior linkage, candidate gene sequencing, or large-scale association testing reports (Supplemental Table 3) as previously described.30 The expanded panel of AF, cardiac arrhythmia and cardiomyopathy genes (n=82) included all previously implicated putative AF genes, in addition to nonoverlapping clinically established cardiac arrhythmia and cardiomyopathy genes (n=53) (Supplemental Table 4). For the AF and expanded cardiac arrhythmia and cardiomyopathy gene sets, we included variants within 5 kb upstream and downstream of each included gene, to capture potential regulatory variation related to each gene. We then partitioned the variance in AF risk explained by genetic variation in these regions. For all stratified analyses of h2g, we used the observed prevalence of AF within each stratum to convert the measurement from the observed to the liability scale.
Statistical analyses were performed using PLINK version 1.90b,25 R 3.2.1, and BOLT-REML.15 Manhattan plots and regional association plots were created using R (qqman)31 and LocusZoom,32 respectively.
Results
In total, 120,286 unrelated individuals of European ancestry were included in our analysis, of whom 2,987 fulfilled our definition for AF. Individuals with AF were older than individuals without AF, and a greater proportion were men, in keeping with well-established epidemiologic observations (Table 1).33
Table 1.
Characteristics of the 120,286 subjects included in the analysis.
| AF cases | Referents | |
|---|---|---|
| N | 2987 | 117299 |
| Baseline age, y | 62.3 (5.9) | 56.8 (7.9) |
| Women, % | 31 | 53 |
| Systolic blood pressure, mm Hg | 140 (19) | 138 (19) |
| Diastolic blood pressure, mm Hg | 82 (11) | 82 (10) |
| Body mass index at baseline, kg/m2 | 29.1 (5.5) | 27.5 (4.8) |
| Hypertension, % | 50 | 46 |
| UK BiLEVE Axiom array, % | 39 | 34 |
Data shown as mean (standard deviation), unless otherwise indicated. AF: atrial fibrillation.
Genetic associations with AF
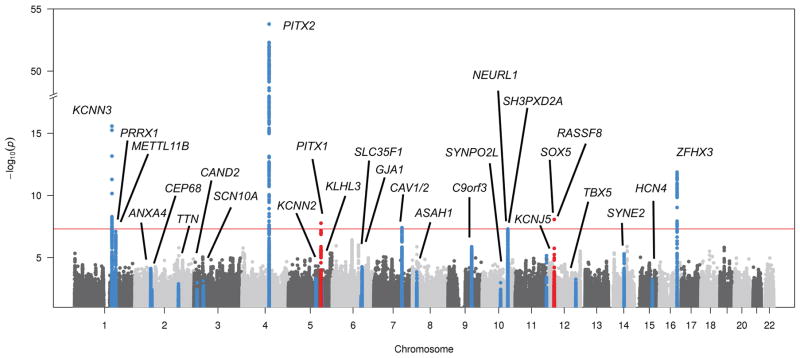
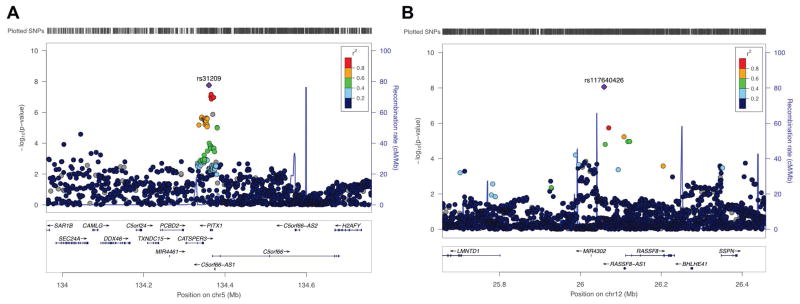
We tested about 8.5 million variants that passed quality control filters for association with AF. No substantive genomic inflation was observed (ƛ =1.026; Supplemental Figure 1) with additional PCs of ancestry. The results of the GWAS for AF are displayed in Figure 1. Seven loci exceeded the genome-wide significance threshold, five of which were previously known and two of which have not been previously reported (Figure 2 and Supplemental Table 5). Of the two loci not previously reported, one was located on chromosome 5q31 (rs31209, about 3kb downstream of PITX1, p-value = 1.7×10−8). SNP rs31209 is also an eQTL for PITX1 with reduced expression associated with the protective T allele in testis (p-value = 2.7×10−12).34 The second locus was identified on chromosome 12p12 (rs117640426, upstream of RASSF8, p-value = 8.7×10−9) (Supplemental Table 5). Given the relatively low frequency of the top variant at the RASSF8 locus, we repeated the analysis stratified by the genotyping array used to minimize the possibility of confounding by an unrecognized batch effect. We observed concordant directions of allelic effect and nominally significant associations between rs117640426 and AF, though the effect sizes differed between the two arrays (Supplemental Table 6).
Figure 1.
Manhattan plot demonstrating associations between tested variants and AF. Known AF loci are shown in blue, and loci uniquely associated with AF in the UK Biobank are shown in red.
Figure 2.
Regional association plots for loci uniquely associated with AF in the UK Biobank. Panel A displays associations at chromosome 5q31, and panel B at 12p12.
Twenty of 25 variants tagging established AF susceptibility loci from a prior independent analysis11 were associated with AF in the UK Biobank with a p-value <0.05 (Figure 1). The log-odds ratios for concordant risk alleles from the current analysis were highly correlated with the log-relative risks from the prior analysis11 (rho=0.904, p-value = 5.7×10−10). When the 25 established AF variants were combined using a weighted genetic risk score approach and tested for association with AF in the UK Biobank, a significant association was observed (p-value = 1.4×10−112, Supplemental Table 2). Based on our results, the AF definition utilized to ascertain AF in the UK Biobank was considered valid for further analysis.
SNP-heritability of AF
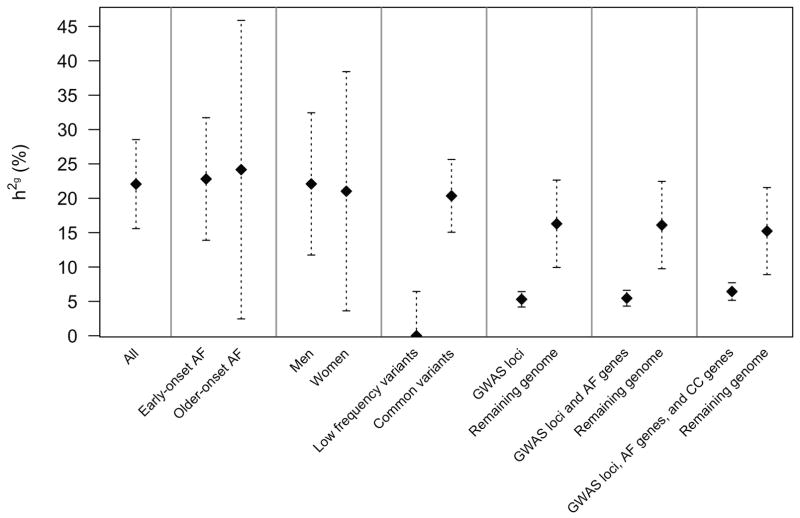
We estimated the overall h2g of AF attributable to additive common and low-frequency genetic variation to be 22.1% (95% CI 15.6%–28.5%; Figure 3). The h2g estimation was similar for early-onset AF (22.8%, 95% CI 13.9%–31.7%) and older-onset AF (24.2% 95%CI 2.5%–45.9%). The h2g estimates were similar for both sexes (21.0%, 95% CI 3.6%–38.4%, in women versus 22.1%, 95% CI 11.7%–32.4%, in men). We observed that common variants (MAF ≥5%) explained 20.4% (95% CI 15.1%–25.6%) of AF variance, whereas low frequency variants (MAF 1–5%) provided minimal contribution to AF susceptibility.
Figure 3.
SNP-heritability of AF in clinical and genomic strata.Error bars represent the 95% confidence intervals for the h2g estimates. Early-onset AF was defined as that occurring prior to age 65, and older-onset AF as occurring after age 65. Low frequency variants were SNPs with minor allele frequencies between 1%–5%, and common variants with frequencies ≥ 5%. GWAS loci were comprised of the 23 top SNPs from a prior independent association study ± 500 kilobases (kb). AF genes included 37 putative AF susceptibility genes ± 5 kb. AF, cardiac arrhythmia, and cardiomyopathy genes included 82 putative susceptibility genes ± 5 kb. The numbers of individuals and variants included in each stratum are provided in Supplemental Table 7.
In aggregate, the 25 known AF susceptibility loci from a prior GWAS explained 5.3% (95%CI 4.2%–6.5%) of variance in AF risk, corresponding to nearly one-fourth of the AF h2g. Collectively, the 23 known loci and additional 37 putative AF susceptibility genes explained only 5.4% (95%CI 4.3%–6.6%) of total AF variance, whereas the additional inclusion of cardiac arrhythmia and cardiomyopathy genes regions (total n=82 genes) explained a total of 6.4% (95%CI 5.1%–7.7%) of AF variance. In aggregate, about 29% (=6.4%/22.1%) of AF h2g was explained by accounting for all known AF loci, putative cardiac arrhythmia, and cardiomyopathy gene regions.
Discussion
In our analysis of 120,286 individuals of European ancestry from the population-based UK Biobank, common and low-frequency genetic variation accounted for 22.1% of variance in AF risk. The proportion of variance in AF risk was similar for early-onset and for older-onset AF, and between men and women. Nearly all the observed variance in AF risk explained by genetic variation was attributed to common variants. Established AF susceptibility loci accounted for only one-fourth of the AF h2g, indicating that a substantial proportion of AF risk is driven by variation in regions that have not exceeded genome-wide significance in analyses to date.
Our population-based study supports and extends prior observations of AF heritability by providing quantitative and genetically-determined estimates. In the Framingham Heart Study, individuals with an affected first-degree relative had an approximately 40% increased hazard after accounting for clinical risk factors for disease.6 Similarly, an Icelandic study indicated that the relative risk of AF declined from 1.77 with an affected first-degree relative to 1.05 with an affected fifth-degree relative.3 A Danish twin study estimated the heritability of AF to be as high as 62%,4 though overestimation of heritability can occur in family-based studies.35 Our approach limits the potential confounding by shared environmental factors and provides an unbiased estimate of additive genetic contribution to AF variance from common genetic variants.
Our findings have three major implications. First, the fact that about one-fifth of variance in AF risk was explained by additive genetic variation underscores the substantial contribution of genome-wide variation to AF susceptibility. Common genetic variation accounted for most AF genetic risk, highlighting the complex polygenic architecture of AF. The complex genetic architecture of AF contrasts with some other inherited arrhythmia syndromes, such as long QT syndrome and catecholaminergic polymorphic ventricular tachycardia, which are often driven by rare and penetrant mutations.36 Our findings also indicate that variation at known AF susceptibility loci, as well as at putative arrhythmia and cardiomyopathy gene regions, accounts for only a modest proportion of AF risk observed in the community, thereby justifying future genetic discovery efforts in larger samples.
Second, the h2g of AF we observed is similar to that of other complex cardiovascular traits, including hypertension, dyslipidemia, and type 2 diabetes which range from 26–30%.15 A prior effort in a smaller subset of the UK Biobank demonstrated a heritability of AF of 11.3% using a definition of AF based on a single ICD10 code. 37 In contrast, our comprehensive AF definition included self-report, ICD9 and ICD10 codes, and AF-related procedures such as catheter ablation, which likely minimized misclassification of AF cases as referents. Genetic risk for AF has previously been associated with a variety of clinical outcomes including incident AF,38, 39 ischemic stroke,39, 40 and variably with catheter ablation success,41 though any potential clinical application of genetic risk information may be highly context-dependent. Whether the magnitude of AF risk explained by genome-wide variation, or perhaps family history of AF, will contribute meaningfully to clinical risk assessment warrants further evaluation.
Third, we identified two loci associated with AF risk, though neither was associated with AF in the prior independent meta-analysis of nearly 18,000 individuals with and over 100,000 without AF (Supplemental Table 5),11 which may warrant further evaluation. The first locus was about 3 kb downstream of PITX1 at chromosome 5q31. PITX1 belongs to the same RIEG/PITX homeobox family as a known AF susceptibility gene, PITX2. PITX2 is involved in specifying pulmonary venous myocardial sleeves, suppression of a default left-atrial sinus node, and conditional knockout of the Pitx2c transcript has been linked to increased AF susceptibility in mice.42–44 Activation of PITX1 was suggested to be associated with tumor suppression in various cancers,45, 46 but the function of PITX1 in cardiac function has not been well-understood. The second novel locus is at 53kb upstream of RASSF8, encoding a candidate tumor suppressor protein, RASSF8,47 but the association of RASSF8 and cardiac physiology remains unclear. These variants may represent spurious associations or, alternatively, may be sample-specific associations. Future examination with the upcoming release of the full 500,000-person UK Biobank dataset will help clarify the validity of these loci.
Our study should be interpreted in the context of the study design. First, our study is comprised of individuals of European ancestry, so the findings may not be generalizable to other ancestral groups. Genetic association studies in diverse populations are warranted. Second, we did not observe a difference in h2g according to age, in contrast to epidemiologic evidence supporting greater heritability for early-onset AF.6 It is likely that older onset forms of AF are less heritable than earlier onset forms,6 though our analysis was underpowered to precisely quantify h2g values in the older age stratum. Larger samples will be necessary to fully address the contributions of genetic variation across a broad spectrum of ages. Our power calculations48, 49 indicated that the minimum h2g that could be detected with 80% power in the older-onset group was 18.9%, in contrast to 5.3% in the overall sample (Supplemental Table 8). Larger analyses are warranted to quantify the relative contributions of genetic variation to AF risk in different age and important clinical risk factor strata. Third, potential misclassification of some referent individuals may have created a negative bias in the h2g estimates we observed, particularly since AF may be subclinical. Fourth, we assessed variants with MAF ≥1% and therefore the contribution of rare or loss of function variants to total AF variance was not assessed. Whole genome-sequencing results may provide insights into the contribution of rare variation to AF susceptibility. We acknowledge that our approach of using hard-called genotypes may have reduced power for discovery as compared to an approach using dosages and estimated genotype probabilities. However, we submit that our computationally efficient approach was balanced by the use of robust high-confidence genotype information.
Conclusion
In a population-based sample of European ancestry, we observed that approximately 22% of AF susceptibility was attributable to additive genetic variation, with a predominant enrichment for common genetic variants. Genetic variants located at known AF loci contributed modestly to AF variance, indicating that substantial additional variation exists that is not associated at genome-wide significance thresholds in current studies. Further analyses to explore AF genetic architecture in larger datasets, including partitioning by genomic annotation and by variation underlying clinical AF risk factors, are warranted.
Supplementary Material
Clinical Perspective.
Atrial fibrillation affects about 34 million individuals worldwide. Recently, a widespread heritable component underlying atrial fibrillation has been appreciated. Genetic association studies have identified multiple genetic loci related to atrial fibrillation. Yet the aggregate contributions of genetic variation to atrial fibrillation risk remain undefined. We therefore estimated atrial fibrillation heritability in the population-based UK Biobank study using genome-wide single nucleotide polymorphism information in over 120,000 individuals, of whom about 3,000 had atrial fibrillation. We observed that about 22% of the variance in atrial fibrillation risk was accounted for by additive genetic variation. The proportion of variance explained by genetic factors was similar for both men and women. We further observed that nearly one-third of the additive genetic variance of atrial fibrillation was explained by genomic variation at known atrial fibrillation loci, putative cardiac arrhythmia, and putative cardiomyopathy gene regions. Our findings underscore the notion that genetic variation contributes substantially to atrial fibrillation risk. Nevertheless, established disease loci for atrial fibrillation, arrhythmias, and cardiomyopathies do not fully account for genetic susceptibility to atrial fibrillation. Future genetic discovery efforts in large data sets and using whole genome-sequencing may help elucidate the genetic basis of this complex and morbid condition.
Acknowledgments
The authors would like to thank all UK Biobank participants, staff, and investigators. This research has been conducted using the UK Biobank Resource under Application Number 15293.
Sources of Funding: This work was supported by NIH grants K23HL114724 (Lubitz), 2R01HL092577 (Ellinor, Benjamin, Lunetta), 1R01HL128914 (Ellinor and Benjamin), R01HL104156 and K24HL105780 (Ellinor), NHLBI contract HHSN268201500001I and N01-HC-25195 (FHS), a Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105 (Lubitz), Clinical Research Mentorship Grant 2016077 from the Doris Duke Charitable Foundation (Hulme/Lubitz), the American Heart Association Established Investigator Award 13EIA14220013 (Ellinor) and by the Fondation Leducq 14CVD01 (Ellinor). Dr Klarin is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (T32 HL007734). Dr Smith was supported by the Swedish Heart-Lung Foundation, the Swedish Research Council, the Wallenberg Centre for Molecular Medicine at Lund University, the Crafoord Foundation, governmental funding of clinical research within the Swedish National Health Service, Skåne University Hospital in Lund, and the European Research Council. Dr. Kathiresan is supported by a Research Scholar award from the Massachusetts General Hospital (MGH), the Donovan Family Foundation, and the National Institutes of Health (R01HL127564).
Footnotes
Disclosures: Drs. Ellinor and Kathiresan are the principal investigators on a grant from Bayer HealthCare grant to the Broad Institute related to the development of therapeutics for atrial fibrillation and myocardial infarction. Dr. Lubitz receives sponsored research support from Bayer HealthCare, Biotronik, and Boehringer Ingelheim, and has consulted for St. Jude Medical and Quest Diagnostics.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation. 2013;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 3.Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H, et al. Familial aggregation of atrial fibrillation in iceland. Eur Heart J. 2006;27:708–712. doi: 10.1093/eurheartj/ehi727. [DOI] [PubMed] [Google Scholar]
- 4.Christophersen IE, Ravn LS, Budtz-Joergensen E, Skytthe A, Haunsoe S, Svendsen JH, et al. Familial aggregation of atrial fibrillation: A study in danish twins. Circ Arrhythm Electrophysiol. 2009;2:378–383. doi: 10.1161/CIRCEP.108.786665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 6.Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. 2010;304:2263–2269. doi: 10.1001/jama.2010.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oyen N, Ranthe MF, Carstensen L, Boyd HA, Olesen MS, Olesen SP, et al. Familial aggregation of lone atrial fibrillation in young persons. J Am Coll Cardiol. 2012;60:917–921. doi: 10.1016/j.jacc.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 8.Yang YQ, Zhang XL, Wang XH, Tan HW, Shi HF, Fang WY, et al. Familial aggregation of lone atrial fibrillation in the chinese population. Intern Med. 2010;49:2385–2391. doi: 10.2169/internalmedicine.49.4130. [DOI] [PubMed] [Google Scholar]
- 9.Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinner MF, Tucker NR, Lunetta KL, Ozaki K, Smith JG, Trompet S, et al. Integrating genetic, transcriptional, and functional analyses to identify five novel genes for atrial fibrillation. Circulation. 2014;130:1225–1235. doi: 10.1161/CIRCULATIONAHA.114.009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christophersen IE, Rienstra M, Roselli C, Yin X, Geelhoed B, Barnard J, et al. Large-scale analyses of common and rare variants identify 12 new loci associated with atrial fibrillation. Nat Genet. 2017;49:946–952. doi: 10.1038/ng.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low SK, Takahashi A, Ebana Y, Ozaki K, Christophersen IE, Ellinor PT, et al. Identification of six new genetic loci associated with atrial fibrillation in the japanese population. Nat Genet. 2017 doi: 10.1038/ng.3842. [DOI] [PubMed] [Google Scholar]
- 13.Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loh PR, Bhatia G, Gusev A, Finucane HK, Bulik-Sullivan BK, Pollack SJ, et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat Genet. 2015;47:1385–1392. doi: 10.1038/ng.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Lee SH, Goddard ME, Visscher PM. Gcta: A tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Manolio TA, Pasquale LR, Boerwinkle E, Caporaso N, Cunningham JM, et al. Genome partitioning of genetic variation for complex traits using common snps. Nat Genet. 2011;43:519–525. doi: 10.1038/ng.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finucane HK, Bulik-Sullivan B, Gusev A, Trynka G, Reshef Y, Loh PR, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet. 2015;47:1228–1235. doi: 10.1038/ng.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen W-M. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellenguez C, Strange A, Freeman C, Donnelly P, Spencer CCA, Consor WTCC. A robust clustering algorithm for identifying problematic samples in genome-wide association studies. Bioinformatics. 2012;28:134–135. doi: 10.1093/bioinformatics/btr599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. Uk biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham G, Inouye M. Fast principal component analysis of large-scale genome-wide data. PLoS One. 2014;9:e93766. doi: 10.1371/journal.pone.0093766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.UK Biobank Analysis Team. [Accessed July 5, 2016];Genotyping and quality control for uk biobank. http://biobank.ctsu.ox.ac.uk/showcase/docs/genotyping_qc.pdf.
- 24.UK Biobank Analysis Team. [Accessed July 5, 2016];Genotype imputation and genetic association studies using uk biobank data. http://biobank.ctsu.ox.ac.uk/crystal/docs/impute_ukb_v1.pdf.
- 25.Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation plink: Rising to the challenge of larger and richer datasets. Gigascience. 2015:4. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SH, Wray NR, Goddard ME, Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet. 2011;88:294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. Common snps explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gusev A, Bhatia G, Zaitlen N, Vilhjalmsson BJ, Diogo D, Stahl EA, et al. Quantifying missing heritability at known gwas loci. PLoS Genet. 2013;9:e1003993. doi: 10.1371/journal.pgen.1003993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SH, Yang J, Chen G-B, Ripke S, Stahl EA, Hultman CM, et al. Estimation of snp heritability from dense genotype data. Am J Hum Genet. 2013;93:1151–1155. doi: 10.1016/j.ajhg.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubitz SA, Brody JA, Bihlmeyer NA, Roselli C, Weng LC, Christophersen IE, et al. Whole exome sequencing in atrial fibrillation. PLoS Genet. 2016;12:e1006284. doi: 10.1371/journal.pgen.1006284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner SD. Qqman: An r package for visualizing gwas results using qq and manhattan plots. bioRxiv. 2014:005165. [Google Scholar]
- 32.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. Locuszoom: Regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 34.Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, et al. The genotype-tissue expression (gtex) project. Nature genetics. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, Mass: Sinauer; 1998. [Google Scholar]
- 36.Hofman N, Tan HL, Alders M, Kolder I, de Haij S, Mannens MM, et al. Yield of molecular and clinical testing for arrhythmia syndromes: Report of 15 years’ experience. Circulation. 2013;128:1513–1521. doi: 10.1161/CIRCULATIONAHA.112.000091. [DOI] [PubMed] [Google Scholar]
- 37.Ge T, Chen CY, Neale BM, Sabuncu MR, Smoller JW. Phenome-wide heritability analysis of the uk biobank. PLoS Genet. 2017;13:e1006711. doi: 10.1371/journal.pgen.1006711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013;34:2243–2251. doi: 10.1093/eurheartj/eht033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubitz SA, Yin X, Lin HJ, Kolek M, Smith JG, Trompet S, et al. Genetic risk prediction of atrial fibrillation. Circulation. 2017;135:1311–1320. doi: 10.1161/CIRCULATIONAHA.116.024143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tada H, Shiffman D, Smith JG, Sjogren M, Lubitz SA, Ellinor PT, et al. Twelve-single nucleotide polymorphism genetic risk score identifies individuals at increased risk for future atrial fibrillation and stroke. Stroke. 2014;45:2856–2862. doi: 10.1161/STROKEAHA.114.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoemaker MB, Bollmann A, Lubitz SA, Ueberham L, Saini H, Montgomery J, et al. Common genetic variants and response to atrial fibrillation ablation. Circ Arrhythm Electrophysiol. 2015;8:296–302. doi: 10.1161/CIRCEP.114.001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mommersteeg MTM, Brown NA, Prall OWJ, de Gier-de Vries C, Harvey RP, Moorman AFM, et al. Pitx2c and nkx2–5 are required for the formation and identity of the pulmonary myocardium. Circ Res. 2007;101:902–909. doi: 10.1161/CIRCRESAHA.107.161182. [DOI] [PubMed] [Google Scholar]
- 43.Mommersteeg MTM, Hoogaars WMH, Prall OWJ, de Gier-de Vries C, Wiese C, Clout DEW, et al. Molecular pathway for the localized formation of the sinoatrial node. Circ Res. 2007;100:354–362. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- 44.Chinchilla A, Daimi H, Lozano-Velasco E, Dominguez JN, Caballero R, Delpón E, et al. Pitx2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circ Cardiovasc Genet. 2011;4:269–279. doi: 10.1161/CIRCGENETICS.110.958116. [DOI] [PubMed] [Google Scholar]
- 45.Kolfschoten IGM, van Leeuwen B, Berns K, Mullenders J, Beijersbergen RL, Bernards R, et al. A genetic screen identifies pitx1 as a suppressor of ras activity and tumorigenicity. Cell. 2005;121:849–858. doi: 10.1016/j.cell.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Kong GB, Liu ZY, Wu KZ, Zhang Y, Deng ZH, Feng WL, et al. Strong expression of paired-like homeodomain transcription factor 1 (pitx1) is associated with a favorable outcome in human osteosarcoma. Tumor Biology. 2015;36:7735–7741. doi: 10.1007/s13277-015-3512-1. [DOI] [PubMed] [Google Scholar]
- 47.Volodko N, Gordon M, Salla M, Abu Ghazaleh H, Baksh S. Rassf tumor suppressor gene family: Biological functions and regulation. Febs Letters. 2014;588:2671–2684. doi: 10.1016/j.febslet.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 48.Visscher PM, Hemani G, Vinkhuyzen AA, Chen GB, Lee SH, Wray NR, et al. Statistical power to detect genetic (co)variance of complex traits using snp data in unrelated samples. PLoS Genet. 2014;10:e1004269. doi: 10.1371/journal.pgen.1004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. [Accessed June 12, 2017];Gcta-greml power calculator. http://cnsgenomics.com/shiny/gctaPower/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.