Rising CO2 levels may induce nutritional deficits (protein, minerals, and vitamins) in the highest rice-consuming countries.
Abstract
Declines of protein and minerals essential for humans, including iron and zinc, have been reported for crops in response to rising atmospheric carbon dioxide concentration, [CO2]. For the current century, estimates of the potential human health impact of these declines range from 138 million to 1.4 billion, depending on the nutrient. However, changes in plant-based vitamin content in response to [CO2] have not been elucidated. Inclusion of vitamin information would substantially improve estimates of health risks. Among crop species, rice is the primary food source for more than 2 billion people. We used multiyear, multilocation in situ FACE (free-air CO2 enrichment) experiments for 18 genetically diverse rice lines, including Japonica, Indica, and hybrids currently grown throughout Asia. We report for the first time the integrated nutritional impact of those changes (protein, micronutrients, and vitamins) for the 10 countries that consume the most rice as part of their daily caloric supply. Whereas our results confirm the declines in protein, iron, and zinc, we also find consistent declines in vitamins B1, B2, B5, and B9 and, conversely, an increase in vitamin E. A strong correlation between the impacts of elevated [CO2] on vitamin content based on the molecular fraction of nitrogen within the vitamin was observed. Finally, potential health risks associated with anticipated CO2-induced deficits of protein, minerals, and vitamins in rice were correlated to the lowest overall gross domestic product per capita for the highest rice-consuming countries, suggesting potential consequences for a global population of approximately 600 million.
INTRODUCTION
One of the consequential impacts of rising carbon dioxide concentration ([CO2]) and climate change is expected to be on food security (1). This expected impact is due, in part, to the vulnerability of the global population to food supply: Depending on definition, up to 1 billion people are deemed food insecure (2). For example, harvests of staple cereal crops, such as rice and maize, could decline by 20 to 40% as a function of increased surface temperatures in tropical and subtropical regions by 2100 without considering the impacts of extreme weather and climate events (3). Overall, there has been a directed effort to understand the consequences of [CO2] and climate on agricultural production (4, 5).
However, the connection between food security and well-being extends beyond production per se; for example, dietary quality has a substantial influence on human health (6). Globally, insufficient micronutrients, protein, vitamins, etc. can contribute to nutritional deficiencies among 2 billion people in developing and developed countries (7). These deficiencies can directly (cognitive development, metabolism, and immune system) and indirectly (obesity, type 2 diabetes mellitus) affect human health on a panoptic scale (8).
The elemental chemical composition of a plant (that is, ionome) reflects a balance between carbon, obtained through atmospheric [CO2], and the remaining nutrients, obtained through the soil. As evidenced by over a hundred individual studies and several meta-analyses, projected increases in atmospheric [CO2] can result in an ionomic imbalance for most plant species whereby carbon increases disproportionally to soil-based nutrients (9–11). This imbalance, in turn, may have significant consequences for human nutrition (12, 13) including protein and micronutrients. However, at present, no information is available regarding a key constituent of nutrition, vitamin content; as a result, no integrated assessment (protein, micronutrients, and vitamins) is available.
The consequences of CO2-induced qualitative changes may be exacerbated where food diversity is limited, that is, where populations rely heavily on a single plant-based food source. In this regard, rice supplies approximately 25% of all global calories, with the percentage of rice consumed varying by socioeconomic status, particularly in Asia (14). Rice is considered among the most important caloric and nutritional sources particularly for low- and lower-middle–income Asian countries (15).
Therefore, for those populations that are highly rice-dependent, any CO2-induced change in the integrated nutritional value of rice grains could disproportionally affect health. We use a multiyear, multilocation, multivarietal evaluation of widely grown, genetically diverse rice lines at ambient and anticipated end-of-century [CO2] to (i) quantify varietal response to changes in dietary components, including protein, iron, calcium, zinc, vitamin E, and the vitamin B complex, and (ii) socioeconomically calculate any CO2-induced deficits in these nutritional parameters for the 10 most rice-centric countries globally, as a function of gross domestic product (GDP) per capita.
Although end-of-century [CO2] projections vary, it is very likely that actual atmospheric [CO2] will reach 570 μmol mol−1 before the end of this century (16). Global [CO2] is expected to reach these levels even as additional steps are taken to decrease emissions, due, in part, to the projected energy usage, the longevity of the CO2 molecule in the atmosphere, and the temporal delay in reducing [CO2] emissions before mid-century (17). Overall, the experimental concentrations used here for the elevated [CO2] treatment (568 to 590 μmol mol−1) reflect the reality that those born today will be eating rice grown at [CO2] of 550 μmol mol−1 (or higher) within their lifetimes.
RESULTS
When grown under field conditions at these anticipated [CO2] a significant reduction (an average of −10.3%) in protein relative to current [CO2] was observed for all rice cultivars (Fig. 1). Similarly, significant reductions in iron (Fe) and zinc (Zn) were also observed (−8.0 and −5.1%, respectively) among all rice cultivars tested (Fig. 2). On the basis of [CO2] assessment per se, there were no significant site difference effects on rice grain quality between Japan and China (P = 0.26, 0.17, and 0.10 for protein, iron, and zinc, respectively).
Fig. 1. Average reduction in grain protein at elevated relative to ambient [CO2] for 18 cultivated rice lines of contrasting genetic backgrounds grown in China and Japan using FACE technology.
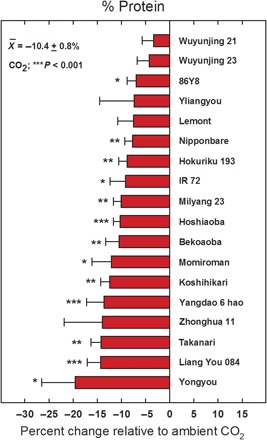
A country by [CO2] effect on protein reduction was not significant (P = 0.26). Bars are ±SE. *P < 0.05 and **P < 0.01 (see Methods for additional details).
Fig. 2. Average reduction in grain micronutrients, iron (Fe), and zinc (Zn) concentration at elevated relative to ambient [CO2] for 18 cultivated rice lines of contrasting genetic backgrounds grown in China and Japan using FACE technology.
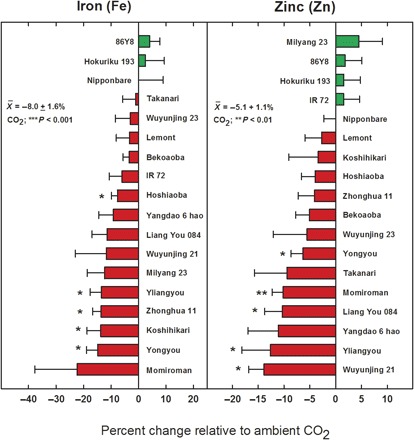
A country by [CO2] effect was not significant for either micronutrient [P = 0.17 and 0.10 for iron (Fe) and zinc (Zn), respectively] so data from both locations are shown. Bars are ±SE. *P < 0.05 and **P < 0.01 for a given cultivar. CO2; **P < 0.01 is based on all cultivars (see Methods for additional details).
The rice lines chosen reflect a wide genotypic and phenological range, suggesting that the declines in nutrient parameters observed here are representative of rice in toto. However, a larger sample size would be of benefit both to confirm these findings and, if possible, to determine whether any lines may be preferred for improving protein or micronutrient availability as [CO2] increases.
Regarding the B vitamin complex, significant reductions in vitamins B1 (thiamine), B2 (riboflavin), B5 (pantothenic acid), and B9 (folate) were observed in response to projected CO2 levels with average declines among cultivars of −17.1, −16.6, −12.7, and −30.3%, respectively (Fig. 3). As observed for protein and minerals, no increase in these parameters was detected for any of the 18 rice lines evaluated; in addition, no significant [CO2] by cultivar interactions were noted (Fig. 3). In contrast, increases were observed on average for vitamin E (α-tocopherol) (fig. S1).
Fig. 3. CO2-induced reductions in vitamins B1 (thiamine), B2 (riboflavin), B5 (pantothenic acid), and B9 (folate) by cultivar.
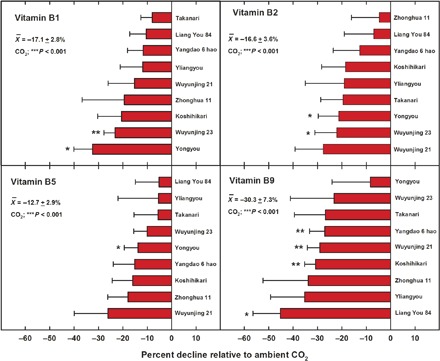
No significant effect was observed for vitamin B6 (pyridoxine), and results are not shown. Analysis was conducted only for the China FACE location. Bars are ±SE. *P < 0.05 and **P < 0.01 for a given cultivar. CO2; **P < 0.01 is based on all cultivars (see Methods for additional details).
Although these data indicate that [CO2] affects nutrient composition, the impact of these qualitative changes on health will vary as a function of rice consumed relative to the total caloric intake. Previous calculations of the impact of rising CO2 on human nutrition relied on Food and Agriculture Organization (FAO) food balance sheets combined with Monte Carlo simulations run on the range of projected declines of zinc, protein, and iron (12, 13). Here, we also rely on FAO food balance sheets but use an economic approach whereby average qualitative changes observed with [CO2] as a function of rice consumption for the top 10 rice-consuming countries as of 2013 are compared with GDP per capita of that country. In this context, any protein and mineral deficits (Fe + Zn), associated with higher CO2 values, are observed to be greater for those countries with the lowest overall GDP per capita (for example, Bangladesh and Cambodia) (Fig. 4). The reductions in vitamin B (B1, B2, B5, and B9) availability were greatest for these same countries (Fig. 4). Similarly, the increase in vitamin E with higher CO2 levels and the subsequent consumption is proportionally greater for those poorer countries that ingest greater quantities of rice (Fig. 4).
Fig. 4. Projected [CO2]-induced deficits in protein and minerals (Fe and Zn) and cumulative changes in vitamin B and cumulative changes in vitamin E derived from rice as a function of GDP per capita.
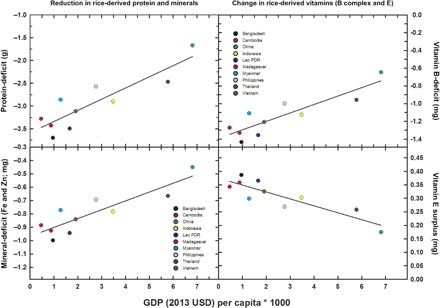
Data are based on 2011/2013 FAO food balance sheets for rice consumption and 2011/2013 World Bank estimates of GDP per capita per country.
There is growing evidence demonstrating a clear link between crop growth at projected increases in [CO2] and changes in nutritional quality including, but not limited to, protein, secondary compounds, and minerals (for example, Zn) (9–11, 18, 19). The basis for the CO2-induced changes in crop quality is still being elucidated, in part, because increasing [CO2] influences several biophysical processes (20). However, for near-term projections of [CO2], the qualitative decline can be reasonably (given the accuracy of the current data) approximated as linear (for example, protein) (21).
The nutritional data reported here for elevated [CO2] confirm that deficits in protein, zinc, and iron may occur even among genetically diverse rice lines grown in different countries (11, 22). In addition, the current data indicate, for the first time, a pattern in the changes in vitamin content, that is, the extent of observed variation between vitamin B (B1, B2, B5, B6, and B9) and vitamin E (α-tocopherol).
Variation among [CO2]-induced changes in secondary compounds, such as vitamins, may relate to the well-established decline of nitrogen in plants exposed to elevated [CO2] [for example, see the study of Taub et al. (9)]. The effect of increasing levels of [CO2] on vitamin levels could therefore be inversely correlated with the molecular fraction of nitrogen within the vitamin. This was observed for rice in the current study (r2 = 0.82) (Fig. 5), consistent with the carbon-nutrient balance hypothesis (23); at least in the context of rapid increases in atmospheric [CO2] and carbon availability [but see the study of Hamilton et al. (24)], that is, the levels of nitrogen containing vitamins decreased (B vitamin group), whereas the level of carbon-based compounds (vitamin E) increased. Additional information regarding the effects of [CO2] on nutritional quality is obviously desired; however, this relationship could provide initial guidance as to the aspects of rice grain chemistry affected by increasing atmospheric [CO2].
Fig. 5. Average change in vitamin concentration (as percentage) in response to anticipated, relative to current, [CO2] ±SE as a function of the ratio of the molecular weight of nitrogen (N) to the molecular weight of the vitamin.
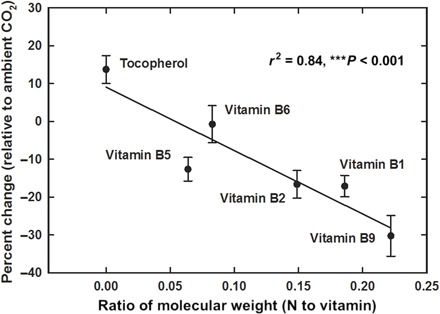
There was a highly significant correlation between the amount of N present in the vitamin and the overall decrease or increase in response to higher [CO2].
DISCUSSION
As of 2013, approximately 600 million individuals, primarily in Southeast Asia [the countries of Bangladesh, Cambodia, Indonesia, Lao People’s Democratic Republic (PDR), Madagascar, Myanmar, and Vietnam], consume ≥50% of their per capita dietary energy and/or protein directly from rice (25, 26). The data shown here provide the first integrated assessment of [CO2]-induced changes in nutritional quality (protein, minerals, and vitamins) for many of the most widely grown rice lines; as such, they indicate that, for key dietary parameters, the [CO2] likely to occur this century will add to nutritional deficits for a large segment of the global population.
In assessing the outcome of the [CO2]-induced dietary changes for rice in the current study, it is evident (Fig. 4) that the bulk of these changes, and the greatest degree of risk, will occur among the highest rice-consuming countries with the lowest GDP. However, as income increases, consumers prefer more diverse caloric sources, with a greater emphasis on protein from fish, dairy, and meat as per western foods (27). Therefore, future economic development could potentially limit future CO2-induced changes in rice nutrition. For example, in Japan, rice accounted for 62% of total food energy consumption in 1959, but that share fell to 40% by 1976 and, in recent years, is <20% (28); in South Korea, per capita rice consumption almost halved since 1975 (29). However, strong, sustained economic growth cannot be assumed for all rice-consuming countries. For example, in Bangladesh, 75% of the total caloric supply per capita came from rice in 1990; 23 years later, in 2013, it was 70% (http://faostat.fao.org/beta/en/#data/FBS); in Madagascar, the percentage of rice consumption has increased since 1990 (25). In addition, other countries, such as Guinea, Senegal, and Côte d’Ivoire, have become more reliant on rice as a percentage of their caloric supply (20 to 40% as of 2011) (30). Overall, although the top rice-consuming countries are likely to change in the coming decades, the reliance on rice globally as a dietary staple will continue.
Specific health outcomes of consuming rice with reduced nutritional quality are also difficult to forecast. Staple foods, such as rice, are widely available and affordable for most of the world’s population, particularly the poor. It is understood that undernutrition can put people at risk in low-income countries for a wide range of other adverse health outcomes, particularly stunting, diarrheal disease, and malaria (31). For example, Kennedy et al. (15) found that the percentages of children under 5 years of age who suffer from stunting, wasting, or are underweight are generally high in countries with very high per capita rice consumption. Overall, the current data suggest that, for these countries, any [CO2]-induced change in nutritional quality would likely exacerbate the overall burden of disease and could affect early childhood development.
It is difficult, without a great deal of additional socioeconomic data at the country level (which is often unavailable), to provide exact estimates of nutritional deficits (protein, minerals, and vitamins) and associated health consequences likely to incur for rice-dependent populations. Yet, CO2-induced reductions in these qualities and associated risks of undernutrition or malnutrition are likely to transcend the entire food chain, from harvest to consumption, especially for the poorest people within a country or region.
Is there a way then to reduce—or negate—this risk? Cultivar selection, either through traditional breeding or genetic modification, to provide nutritionally superior rice with additional CO2 is an obvious strategy. The current data for a genetically diverse set of rice lines suggest that, at least for some characteristics (for example, protein and vitamin B2), many additional lines would need to be screened; furthermore, at present, it can take many years, even decades, to identify, cultivate, and distribute new cereal lines that are adapted to a changing climate (32). In addition, other aspects of climate change, especially temperature, would need to be considered. For example, previous work indicated that rising temperature per se can also reduce protein concentration in rice (33). Although the extent of future surface temperatures would vary depending on location, temperature and [CO2] should also be evaluated concurrently regarding rice nutritional impacts in future assessments.
In addition, management could include application of mineral fertilizers or postharvest biofortification. On the consumer side, education about the role of rising [CO2] on nutrition, including opportunities to implement favorable nutrition practices and food fortification, may also provide opportunities to maintain nutritional integrity. Finally, there is an obvious need for the research community, including agronomists, physiologists, nutritionists, and health care providers, to accurately quantify the exact nature of the [CO2]-induced changes in human nutritional status and their associated health outcomes.
Whereas much remains to be done, the current study provides the first evidence that anticipated [CO2] will result in significant reductions in integrated rice quality, including protein, minerals, and vitamin B, for a genetically diverse and widely grown set of rice lines. Occurrence of these nutritional deficits will most likely affect the poorest countries that are the most rice-dependent. Overall, these results indicate that the role of rising [CO2] on reducing rice quality may represent a fundamental, but underappreciated, human health effect associated with anthropogenic climate change.
METHODS
Free-air CO2 enrichment sites
The multiyear study was conducted at free-air CO2 enrichment (FACE) facilities in two countries: (i) China [at Zhongcun Village (119°42′0″E, 32°35′5″N), Yangzhou City, Jiangsu Province; as part of the Yangtze River Delta region, a typical rice growing region (34)] and (ii) Japan [at Tsukuba (35°58°N, 139°60′E), in Ibaraki Prefecture within farmer’s fields (35)]. Eighteen rice lines representing varietal groups of cultivated rice (Indica and Japonica) and new hybrid lines were chosen. These lines were, for the most part, representative and widely grown in the geographical regions where the FACE facilities were located (Table 1).
Table 1. Characteristics of rice lines used.
| Cultivar | Origin | Subgroup | Comments |
| 86Y8 | China | Hybrid | Bred for disease-resistance; high ripening rate |
| Bekoaoba | Japan | Japonica | Bred for lodging resistance, used in silage |
| Hokuriku 193 | Japan | Indica | High-yielding, blast-resistant |
| Hoshiaoba | Japan | Japonica | Cultivar used for silage and bioenergy |
| IR72 | Philippines | Indica | Semi-dwarf, often used as check cultivar |
| Koshihikari | Japan | Japonica | Widely grown in Japan |
| Lemont | United States | Japonica | Semi-dwarf grown in Mississippi Delta |
| Milyang 23 | Korea | Indica | High-yielding, cadmium accumulator |
| Momiroman | Japan | Japonica | Medium grain, high-yielding variety |
| Nipponbare | Japan | Japonica | Genome-sequenced |
| Liang You 084 | China | Hybrid | Grown extensively in southeast China |
| Takanari | Japan | Indica | Widely grown in Japan |
| Wuyunjing 21 | China | Japonica | Grown extensively in East China |
| Wuyunjing 23 | China | Japonica | Grown extensively in East China |
| Yangdao 6 hao | China | Indica | Grown extensively in East and Central China |
| Yliangyou | China | Hybrid | Recently introduced (2008) hybrid line |
| Yongyou 2640 | China | Hybrid | Widely planted in lower Yangtze River |
| Zhonghua 11 | China | Japonica | Disease-resistant line used in breeding |
CO2 and environmental parameters
A complete description of CO2 control for the China and Japan locations can be found in the studies of Zhu et al. (34) and Hasegawa et al. (35), respectively. The operation and control systems for the China FACE facilities were the same as those at the Japan FACE site. Briefly, each site consisted of identical octagonal rings imposed on farmer’s fields with three rings (China) or four rings (Japan) receiving pure CO2 supplied from polyethylene tubing installed horizontally on the periphery of the FACE ring at 30 cm above the rice canopy (elevated CO2 treatment), with additional rings (three and four, respectively) that did not receive supplemental CO2 (ambient CO2 treatment). The concentration of CO2 was monitored at the center of each ring, and using the ambient [CO2] as the control, a proportional-integral-derivative algorithm was used (relative to the ambient control) to regulate the injection and direction of CO2 in the elevated ring. Rings were spaced at 90-m intervals to prevent CO2 contamination between plots. Ring diameters varied between locations (14 and 17 m for the Tsukuba and Zongcun sites, respectively); [CO2] was controlled to within 80% of the set point for >90% of the time during the growing season for each location and year. For the China location, the average daytime [CO2] levels at canopy height for the elevated treatment were 571, 588, and 590 μmol mol−1 for 2012, 2013, and 2014, respectively; for the Japan location, the season-long daytime average CO2 was 584 μmol mol−1 (2010, Tsukuba); ambient [CO2] varied from 374 to 386 regardless of location.
Rice fields in all locations were flood-irrigated and grown as “paddy” rice, as consistent with local practices. For the China location, the average growing season temperature was 24.4°, 24.8°, and 22.1°C for 2012, 2013, and 2014, respectively; for Japan, the growing season temperature was 24.6°C for the Tsukuba location in 2010. The soil type in the China location was classified as Shajiang-Aquic Cambiosol with a sandy loam texture. The soil type at Tsukuba, Japan is Fluvisols, typical of alluvial areas. Fertilizer was applied at rates to maximize commercial yield, consistent with location; any additional pesticides were consistent with cultural agronomic practices for the given region. Sowing and transplanting methods are described elsewhere (34, 35). At seed maturity, 1 to 2 m2 per CO2 ring, per cultivar, per year, and per location were harvested for yield assessment.
Nutrient analysis
For the China FACE, a subsample (500 g) of grain was frozen before analysis. Dehusked (unpolished) brown (raw and uncooked) rice (100 g) was homogenized to a fine powder using a Mix/Mill Grinder, sifted through a 100-mesh sieve, and then dried to a constant weight at 70°C. A 0·5-g sample was added to a graphite tube for digestion, 0.2 ml of pure deionized (DI) water was added, followed by 8 ml of HNO3, and digested for 24 hours. An additional 2 ml of HClO4 was then added. Digestion temperature was regulated until clear color was obtained. Finally, DI water was added to increase any remaining solution to 50 ml. Inductively coupled plasma (ICP) atomic emission spectrometry (AES) (Optima 8000, PerkinElmer) was used to determine Ca content, whereas ICP–mass spectrometry (MS) (7700, Agilent) was used to determine Fe and Zn content. Elemental analyses for the samples from the Tsukuba FACE location are described by Dietterich et al. (36). Briefly, the air-dried husked (but unpolished) brown rice grains were air-dried and ground as described previously. Nitrogen was analyzed with a Leco TruSpec CN analyzer. Fe, Zn, and Ca were determined with an ICP optical emission spectrophotometer. Note that brown rice was analyzed because previous publications [for example, the study of Myers et al. (11)] had used brown rice as the standard for CO2 effects on nutrition.
Elemental concentrations of carbon and nitrogen were determined for an additional 30 mg of harvest sample using an elemental analyzer (Vario, MAX CN, Element). Nitrogen content and carbon content were determined as a percentage of the dry weight of the sample. A factor of 5·61 was used for converting nitrogen to protein concentration in rice, consistent with previous studies (37).
Vitamin extraction and analysis
Although rice does not supply the complete vitamin B complex, it is known to provide B1, B2, B5, B6, and B9, as well as vitamin E. These were extracted from dehusked, unpolished brown rice seed for the nine rice cultivars at the China FACE location. Brown rice (100 g) was homogenized to a fine powder using the previously described method; then, frozen sample was lyophilized using a VFD-1000 freeze dryer (Bilon). Lyophilization occurred in two cycles; drying at −20°C for 48 hours, followed by secondary drying at 0°C for 3 hours.
For thiamine, riboflavin, pantothenic acid, and pyridoxine determination, 0·05 g of ascorbic acid was added to homogenized samples (0·5 g) as an antioxidant and then followed by 10 ml of extracting solution (methanol/water/phosphoric acid = 100:400:0·5, v/v/v). After the suspension was vortexed, it was autoclaved at 100°C for 20 min and then incubated under ultrasonic conditions for 30 min. The solution was allowed to cool to room temperature and then centrifuged at 11,945g for 15 min. Blank controls were generated following the same process without rice samples. The final supernatant was filtered through a 0·22-μm filter before high-performance liquid chromatography (HPLC)–MS analysis.
Folate determination was per Blancquaert et al. (38): 4 ml of extraction buffer was added to 0.5 g of homogenized samples, and the capped tube was placed at 100°C for 10 min. A tri-enzyme treatment with 80 μl of α-amylase (20 min), 350 μl of protease (1 hour at 37°C), and 250 μl of conjugase (2 hours at 37°C) was used to degrade the starch matrix, to release protein-bound folates, and to deconjugate polyglutamylated folates. To stop protease and conjugase activity, additional heat treatments were carried out, followed by cooling on ice. The resulting solution was ultrafiltrated at 11,958g for 15 min. The final solution was filtered through a 0·22-μm filter before analysis.
Vitamin E (α-tocopherol) was extracted using an improved method, as described by Zhang et al. (39). One gram of the homogenized fine powder was saponified under nitrogen in a screw-capped tube with 1 ml of potassium hydroxide (600 g/liter), 5 ml of ethanol, 1 ml of sodium chloride (10 g/liter), and 2.5 ml of ethanolic pyrogallol (60 g/liter) added as antioxidants. Tubes were placed in a 70°C water bath and mixed at 5-min intervals during saponification. Following alkaline digestion at 70°C for 30 min, the tubes were cooled in an ice bath, and 5 ml of sodium chloride (10 g/liter) was added. The suspension was extracted twice with 8 ml of n-hexane/ethyl acetate (4:1, v/v). The organic layer was collected and was dried using pure nitrogen (EVA 30A, Polytech Co.) and then dissolved in n-hexane/methanol (20:80, v/v; 1·0 ml). A similar procedure was used to generate a blank control. The final solution was filtered through a 0·22-μm filter before analysis.
HPLC–tandem MS (Thermo Finnigan TSQ) was used to quantify vitamin content. Column oven temperature was maintained at 25°C, and the autosampler was maintained at 4°C. Two separate Phenomenex Kinetex C18 columns (4.6 mm × 100 mm × 2.6 μm and 4.6 mm × 30 mm × 5 μm) were used for vitamins B and E, respectively. Injection volume was 20 μl. For gradient elution, the mobile phase consisted of eluent A (methyl alcohol) and eluent B (0·1% formic acid in water), with each eluent pumped at a flow rate of 0.6 ml min−1. The mobile phase was linearly adjusted to separate the different vitamins (table S1).
For the MS setting, source conditions were optimized for vitamin B as follows: ion source, electrospray ionization; spay voltage, 3500 V; vaporizer temperature, 400°C; capillary temperature, 350°C; sheath gas pressure, 50; auxillary gas pressure, 10; scan type, selected reaction monitoring (SRM); collision pressure, 1.0-mtorr Ar. For vitamin E, the source conditions were optimized as follows: ion source, atmospheric pressure chemical ionization; discharge current, 10 μA; vaporizer temperature, 300°C; capillary temperature, 350°C; sheath and auxiliary gas pressure, 50 and 10, respectively; scan type, SRM; collision pressure, 1.0-mtorr Ar (table S2). Known standards for vitamin B1 (thiamine hydrochloride), vitamin B2 (riboflavin), vitamin B5 (calcium-d-pantothenate), vitamin B6 (pyridoxine hydrochloride), vitamin B9 (folic acid), and vitamin E (α-tocopherol) were purchased from Sigma-Aldrich Co. All vitamin analyses were performed in duplicate. Before sample analysis, the instrument was calibrated using seven standards (six standards and the blank control).
Estimate of nutritional deficits
The 10 most rice-dependent countries were determined on the basis of the largest consumption of rice as a fraction of total available calories [Bangladesh, Cambodia, China, Indonesia, Lao PDR, Madagascar, Myanmar, Philippines, Thailand, and Vietnam (23)]. FAO food balance sheets (http://faostat.fao.org/beta/en/#data/FBS; food supply quantity, kilogram per capita per year and food supply, and kilocalorie per capita per day) from either 2011 (Cambodia and Lao PDR) or 2013 (all other countries) were used to determine rice consumption along with the U.S. Department of Agriculture (USDA) National Nutrient Database for Standard Reference data for raw brown long-grain rice (https://ndb.nal.usda.gov/ndb/foods/show/305240?manu=&fgcd=&ds=SR) to quantify any CO2-induced differences in qualitative nutritional characteristics by individual country.
With respect to nutritional characteristics, we used a holistic approach to assess changes in a number of qualitative parameters including protein, minerals (Fe, Ca, and Zn), and vitamins B1 (thiamine), B2 (riboflavin), B5 (pantothenic acid), B6 (pyridoxine), B9 (folic acid), and E (α-tocopherol). Inadequate intake of the vitamins and minerals assessed were associated with specific physiological conditions and clinical manifestations (40). Data for protein and minerals were available for all three experimental locations; however, vitamin analysis was only conducted for the rice lines from the China location. Because income level is the most important determinant of per capita rice consumption (25), and because of the wide range of per capita incomes of the countries assessed, any significant CO2-induced change in a nutritional characteristic was characterized with respect to GDP per capita (from 2013) for the 10 countries examined (https://data.worldbank.org/indicator/NY.GDP.PCAP.CD).
Statistics
All field experiments at each location represented a completely randomized design with either three (China) or four (Japan) replicates. All measured and calculated parameters were analyzed using a two-way analysis of variance (ANOVA) with [CO2] and cultivar as fixed effects (Statview Software). Coefficient of determination (r2) was calculated for protein, mineral (Fe and Zn), and vitamin (B1, B2, B5, B6, B9, and E) deficits as a function of [CO2] and GDP per capita. Each value is the mean ± SE. **P < 0.01; *0.01 ≤ P < 0.05; †*0.05 ≤ P < 0.1; ns, not significant (P ≤ 0.1). The figures were generated using Systat Software (SigmaPlot 10.0, Systat Software Inc.). No significant differences for [CO2] by cultivar interaction were found for calcium (Ca) or vitamin B6; consequently, these data are not shown separately. Every cultivar was grown only at a single site, which does not allow separation of cultivar effects from site effects. However, when averaged for all cultivars within a single location (Japan or China), no significant country interaction was observed for [CO2] impacts on noted reductions in protein, iron, or zinc (P = 0.26, 0.17, and 0.1 for protein, iron, and zinc, respectively). Because our purpose was to elucidate the effect of [CO2] on rice, but not on geographic area, cultivar effects are inclusive for the figures. Seasonal (yearly) variation was not significant for a given location and, consequently, was averaged across years for each FACE site. Original data are available at https://doi.org/10.6084/m9.figshare.6179069.
Supplementary Material
Acknowledgments
We thank G. Kordzakhia of the U.S. Food and Drug Administration for his contributions. Funding: This work was supported by the National Basic Research Program of China (973 Program, 2014CB954500), Natural Science Foundation of Jiangsu Province in China (BK20140063), Youth Innovation Promotion Association of Chinese Academy of Sciences (CAS; member no. 2015248), and the frontier projects for 13th 5-year plan of CAS (Y613890000 to C.Z.). Author contributions: C.Z., L.H.Z., and K.K. designed the study. I.L., S.S., and K.L.E. did the literature review. I.L., C.Z., and L.H.Z. did the analysis with contributions from K.L.E., N.K.F., and A.D. J.Z., Q.J., X.X., and G.L. contributed data. L.H.Z. wrote the report. All authors interpreted the results, commented on the draft version of the report, and approved the submission draft. Competing interests: A.D. has received grants, honoraria, and consulting fees from numerous food, beverage, and ingredient companies and other commercial and nonprofit entities with an interest in diet quality and nutrient content of foods. The University of Washington receives research funding from public and private sectors. N.K.F. is the Editor-in-Chief of Nutrition Reviews, an International Life Sciences Institute publication, and has received honoraria from Monsanto and the National Dairy Council before employment by the USDA. The other authors declare that they have no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Original data are available at https://doi.org/10.6084/m9.figshare.6179069.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/5/eaaq1012/DC1
table S1. Elution procedures for vitamin B and vitamin E.
table S2. Compound parameters for vitamins B1, B2, B5, B6, B9 and E.
fig. S1. As for Fig. 3, but for vitamin E (α-tocopherol) (see Methods for additional details).
REFERENCES AND NOTES
- 1.K. R. Smith, A. Woodward, D. Campbell-Lendrum, D. D. Chadee, Y. Honda, Q. Liu, J. M. Olwoch, B. Revich, R. Sauerborn, 2014: Human health: Impacts, adaptation, and co-benefits, in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastradrea, Eds. (Cambridge Univ. Press, 2014); www.ipcc.ch/pdf/assessment-report/ar5/wg2/WGIIAR5-Chap11_FINAL.pdf.
- 2.Barrett C. B., Measuring food insecurity. Science 327, 825–828 (2010). [DOI] [PubMed] [Google Scholar]
- 3. Battisti D. S., Naylor R. L., Historical warnings of future food insecurity with unprecedented seasonal heat. Science 323, 240–244 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Schlenker W., Roberts M. J., Nonlinear temperature effects indicate severe damages to US crop yields under climate change. Proc. Natl. Acad. Sci. U.S.A. 106, 15594–15598 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobell D. B., Burke M. B., Tebaldi C., Mastrandrea M. D., Falcon W. P., Naylor R. L., Prioritizing climate change adaptation needs for food security in 2030. Science 319, 607–610 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Murray C. J., Vos T., Lozano R., Naghavi M., Flaxman A. D., Michaud C., Ezzati M., Shibuya K., Salomon J. A., Abdalla S., Aboyans V., Abraham J., Ackerman I., Aggarwal R., Ahn S. Y., Ali M. K., Alvarado M., Anderson H. R., Anderson L. M., Andrews K. G., Atkinson C., Baddour L. M., Bahalim A. N., Barker-Collo S., Barrero L. H., Bartels D. H., Basáñez M. G., Baxter A., Bell M. L., Benjamin E. J., Bennett D., Bernabé E., Bhalla K., Bhandari B., Bikbov B., Bin Abdulhak A., Birbeck G., Black J. A., Blencowe H., Blore J. D., Blyth F., Bolliger I., Bonaventure A., Boufous S., Bourne R., Boussinesq M., Braithwaite T., Brayne C., Bridgett L., Brooker S., Brooks P., Brugha T. S., Bryan-Hancock C., Bucello C., Buchbinder R., Buckle G., Budke C. M., Burch M., Burney P., Burstein R., Calabria B., Campbell B., Canter C. E., Carabin H., Carapetis J., Carmona L., Cella C., Charlson F., Chen H., Cheng A. T., Chou D., Chugh S. S., Coffeng L. E., Colan S. D., Colquhoun S., Colson K. E., Condon J., Connor M. D., Cooper L. T., Corriere M., Cortinovis M., de Vaccaro K. C., Couser W., Cowie B. C., Criqui M. H., Cross M., Dabhadkar K. C., Dahiya M., Dahodwala N., Damsere-Derry J., Danaei G., Davis A., De Leo D., Degenhardt L., Dellavalle R., Delossantos A., Denenberg J., Derrett S., Des Jarlais D. C., Dharmaratne S. D., Dherani M., Diaz-Torne C., Dolk H., Dorsey E. R., Driscoll T., Duber H., Ebel B., Edmond K., Elbaz A., Ali S. E., Erskine H., Erwin P. J., Espindola P., Ewoigbokhan S. E., Farzadfar F., Feigin V., Felson D. T., Ferrari A., Ferri C. P., Fèvre E. M., Finucane M. M., Flaxman S., Flood L., Foreman K., Forouzanfar M. H., Fowkes F. G., Fransen M., Freeman M. K., Gabbe B. J., Gabriel S. E., Gakidou E., Ganatra H. A., Garcia B., Gaspari F., Gillum R. F., Gmel G., Gonzalez-Medina D., Gosselin R., Grainger R., Grant B., Groeger J., Guillemin F., Gunnell D., Gupta R., Haagsma J., Hagan H., Halasa Y. A., Hall W., Haring D., Haro J. M., Harrison J. E., Havmoeller R., Hay R. J., Higashi H., Hill C., Hoen B., Hoffman H., Hotez P. J., Hoy D., Huang J. J., Ibeanusi S. E., Jacobsen K. H., James S. L., Jarvis D., Jasrasaria R., Jayaraman S., Johns N., Jonas J. B., Karthikeyan G., Kassebaum N., Kawakami N., Keren A., Khoo J. P., King C. H., Knowlton L. M., Kobusingye O., Koranteng A., Krishnamurthi R., Laden F., Lalloo R., Laslett L. L., Lathlean T., Leasher J. L., Lee Y. Y., Leigh J., Levinson D., Lim S. S., Limb E., Lin J. K., Lipnick M., Lipshultz S. E., Liu W., Loane M., Ohno S. L., Lyons R., Mabweijano J., MacIntyre M. F., Malekzadeh R., Mallinger L., Manivannan S., Marcenes W., March L., Margolis D. J., Marks G. B., Marks R., Matsumori A., Matzopoulos R., Mayosi B. M., McAnulty J. H., McDermott M. M., McGill N., McGrath J., Medina-Mora M. E., Meltzer M., Mensah G. A., Merriman T. R., Meyer A. C., Miglioli V., Miller M., Miller T. R., Mitchell P. B., Mock C., Mocumbi A. O., Moffitt T. E., Mokdad A. A., Monasta L., Montico M., Moradi-Lakeh M., Moran A., Morawska L., Mori R., Murdoch M. E., Mwaniki M. K., Naidoo K., Nair M. N., Naldi L., Narayan K. M., Nelson P. K., Nelson R. G., Nevitt M. C., Newton C. R., Nolte S., Norman P., Norman R., O’Donnell M., O’Hanlon S., Olives C., Omer S. B., Ortblad K., Osborne R., Ozgediz D., Page A., Pahari B., Pandian J. D., Rivero A. P., Patten S. B., Pearce N., Padilla R. P., Perez-Ruiz F., Perico N., Pesudovs K., Phillips D., Phillips M. R., Pierce K., Pion S., Polanczyk G. V., Polinder S., Pope C. A. III, Popova S., Porrini E., Pourmalek F., Prince M., Pullan R. L., Ramaiah K. D., Ranganathan D., Razavi H., Regan M., Rehm J. T., Rein D. B., Remuzzi G., Richardson K., Rivara F. P., Roberts T., Robinson C., De Leòn F. R., Ronfani L., Room R., Rosenfeld L. C., Rushton L., Sacco R. L., Saha S., Sampson U., Sanchez-Riera L., Sanman E., Schwebel D. C., Scott J. G., Segui-Gomez M., Shahraz S., Shepard D. S., Shin H., Shivakoti R., Singh D., Singh G. M., Singh J. A., Singleton J., Sleet D. A., Sliwa K., Smith E., Smith J. L., Stapelberg N. J., Steer A., Steiner T., Stolk W. A., Stovner L. J., Sudfeld C., Syed S., Tamburlini G., Tavakkoli M., Taylor H. R., Taylor J. A., Taylor W. J., Thomas B., Thomson W. M., Thurston G. D., Tleyjeh I. M., Tonelli M., Towbin J. A., Truelsen T., Tsilimbaris M. K., Ubeda C., Undurraga E. A., van der Werf M. J., van Os J., Vavilala M. S., Venketasubramanian N., Wang M., Wang W., Watt K., Weatherall D. J., Weinstock M. A., Weintraub R., Weisskopf M. G., Weissman M. M., White R. A., Whiteford H., Wiebe N., Wiersma S. T., Wilkinson J. D., Williams H. C., Williams S. R., Witt E., Wolfe F., Woolf A. D., Wulf S., Yeh P. H., Zaidi A. K., Zheng Z. J., Zonies D., Lopez A. D., AlMazroa M. A., Memish Z. A., Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2197–2223 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Bailey R. L., West K. P. Jr, Black R. E., The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 66, 22–33 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Stein A. J., Global impacts of human mineral malnutrition. Plant Soil 335, 133–154 (2009). [Google Scholar]
- 9.Taub D. R., Miller B., Allen H., Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Global Change Biol. 14, 565–575 (2008). [Google Scholar]
- 10.Loladze I., Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 3, e02245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers S. S., Zanobetti A., Kloog I., Huybers P., Leakey A. D. B., Bloom A., Carlisle E., Dietterich L. H., Fitzgerald G., Hasegawa T., Michele Holbrook N., Nelson R. L., Ottman M. J., Raboy V., Sakai H., Sartor K. A., Schwartz J., Seneweera S., Tausz M., Usui Y., Rising CO2 threatens human nutrition. Nature 510, 139–142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers S. S., Wessells K. R., Kloog I., Zanobetti A., Schwartz J., Effect of increased concentrations of atmospheric carbon dioxide on the global threat of zinc deficiency: A modeling study. Lancet Glob. Health 3, e639–e645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith M. R., Golden C. D., Myers S. S., Potential rise in iron deficiency due to future anthropogenic carbon dioxide emissions. GeoHealth 1, 248–257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.J. L. McLean, D. C. Dawe, B. Hardy, G. P. Hettel, Rice Almanac (IRRI, 2002), 253 pp. [Google Scholar]
- 15.G. Kennedy, B. Burlingame, V. N. Nguyen, Nutritional contribution of rice an impact of biotechnology and biodiversity in rice-consuming countries, in Proceedings of the 20th Session of the International Rice Commission, 23 to 26 July 2002 (FAO, 2013). [Google Scholar]
- 16.IPCC, Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri, L.A. Meyer, Eds.] (IPCC, 2014), 151 pp. [Google Scholar]
- 17.B. S. Fisher, N. Nakicenovic, K. Alfsen, J. Corfee Morlot, F. de la Chesnaye, J.-Ch. Hourcade, K. Jiang, M. Kainuma, E. La Rovere, A. Matysek, A. Rana, K. Riahi, R. Richels, S. Rose, D. van Vuuren, R. Warren, Issues related to mitigation in the long term context, in Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Inter-governmental Panel on Climate Change, B. Metz, O. R. Davidson, P. R. Bosch, R. Dave, L. A. Meyer, Eds. (Cambridge Univ. Press, 2007), pp. 169–250. [Google Scholar]
- 18.Cotrufo M. F., Ineson P., Scott A., Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biol. 4, 43–54 (1998). [Google Scholar]
- 19.Ziska L. H., Emche S. D., Johnson E. L., George K., Reed D. R., Sicher R. C., Alterations in the production and concentration of selected alkaloids as a function of rising atmospheric carbon dioxide and air temperature: Implications for ethno-pharmacology. Global Change Biol. 11, 1798–1807 (2005). [Google Scholar]
- 20.McGrath J. M., Lobell D. B., Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 36, 697–705 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Ziska L. H., Pettis J. S., Edwards J., Hancock J. E., Tomecek M. B., Clark A., Dukes J. S., Loladze I., Polley H. W., Rising atmospheric CO2 is reducing the protein concentration of a floral pollen source essential for North American bees. Proc. R. Soc. B 283, 20160414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seneweera S. P., Conroy J. P., Growth, grain yield and quality of rice (Oryza sativa L.) in response to elevated CO2 and phosphorus nutrition. Soil Sci. Plant Nutr. 43, 1131–1136 (1997). [Google Scholar]
- 23.Bryant J. P., Chapin F. S. III, Klein D. R., Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40, 357–368 (1983). [Google Scholar]
- 24.Hamilton J. G., Zangerl A. R., DeLucia E. H., Berenbaum M. R., The carbon nutrient balance hypothesis: Its rise and fall. Ecol. Lett. 4, 86–95 (2001). [Google Scholar]
- 25.Food and Agriculture Organization (FAO) Food Balance Sheets; http://faostat.fao.org/beta/en/#data/FBS (2013) [accessed 21 October 2016].
- 26.Seck P. A., Diagne A., Mohanty S.,Wopereis M. C. S., Crops that feed the world 7: Rice. Food Sec. 4, 7–24 (2012). [Google Scholar]
- 27.Drewnowski A., Popkin B. M., The nutrition transition: New trends in the global diet. Nutr. Rev. 55, 31–43 (1997). [DOI] [PubMed] [Google Scholar]
- 28.V. Smil, K. Kobayashi, Japan’s Dietary Transition and Its Impacts (The MIT Press, 2012). [Google Scholar]
- 29.S. Choi, J. Dyck, N. Childs, A Report from the Economic Research Service: The Rice Market in South Korea (Washington, DC, 2016); www.ers.usda.gov/webdocs/publications/79794/rcs-161-01.pdf?v=42636.
- 30.GRiSP (Global Rice Science Partnership), Rice Almanac (International Rice Research Institute, ed. 4, 2013), 283 pp. [Google Scholar]
- 31.F. S. King, A. Burgess, V. J. Quinn, A. K. Osei, Eds., Nutrition for Developing Countries (Oxford Univ. Press, 2015). [Google Scholar]
- 32.Challinor A. J., Koehler A.-K., Ramirez-Villegas J., Whitfield S., Das B., Current warming will reduce yields unless maize breeding and seed systems adapt immediately. Nat. Clim. Change 6, 954–958 (2016). [Google Scholar]
- 33.Ziska L. H., Namuco O., Moya T., Quilang J., Growth and yield response of field-grown tropical rice to increasing carbon dioxide and air temperature. Agron. J. 89, 45–53 (1997). [Google Scholar]
- 34.Zhu C., Ziska L., Zhu J., Zeng Q., Xie Z., Tang H., Jia X., Hasegawa T., The temporal and species dynamics of photosynthetic acclimation in flag leaves of rice (Oryza sativa) and wheat (Triticum aestivum) under elevated carbon dioxide. Physiol. Plant. 145, 395–405 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa T., Sakai H., Tokida T., Nakamura H., Zhu C., Usui Y., Yoshimoto M., Fukuoka M., Wakatsuki H., Katayanagi N., Matsunami T., Kaneta Y., Sato T., Takakai F., Sameshima R., Okada M., Mae T., Makino A., Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct. Plant Biol. 40, 148–159 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Dietterich L. H., Zanobetti A., Kloog I., Huybers P., Leakey A. D., Bloom A. J., Carlisle E., Fernando N., Fitzgerald G., Hasegawa T., Holbrook N. M., Nelson R. L., Norton R., Ottman M. J., Raboy V., Sakai H., Sartor K. A., Schwartz J., Seneweera S., Usui Y., Yoshinaga S., Myers S. S., Impacts of elevated atmospheric CO2 on nutrient content of important food crops. Sci. Data 2, 150036 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sosulski F. W., Imafidon G. I., Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. J. Agric. Food Chem. 38, 1351–1356 (1990). [Google Scholar]
- 38.Blancquaert D., Van Daele J., Storozhenko S., Stove C., Lambert W., Van Der Straeten D., Rice folate enhancement through metabolic engineering has an impact on rice seed metabolism, but does not affect the expression of the endogenous folate biosynthesis genes. Plant Mol. Biol. 83, 329–349 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Zhang G.-Y., Liu R.-R., Xu G., Zhang P., Li Y., Tang K.-X., Liang G.-H., Liu Q.-Q., Increased α-tocotrienol content in seeds of transgenic rice overexpressing Arabidopsis γ-tocopherol methyltransferase. Transgenic Res. 22, 89–99 (2013). [DOI] [PubMed] [Google Scholar]
- 40.A. C. Ross, B. Caballero, R. J. Cousins, K. L. Tucker, T. R. Ziegler, Modern Nutrition in Health and Disease (Wolters Kluwer Publishing, ed. 11, 2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/4/5/eaaq1012/DC1
table S1. Elution procedures for vitamin B and vitamin E.
table S2. Compound parameters for vitamins B1, B2, B5, B6, B9 and E.
fig. S1. As for Fig. 3, but for vitamin E (α-tocopherol) (see Methods for additional details).


