Abstract
The combination of positron emission tomography (PET) and MRI has attracted the attention of researchers in the past approximately 20 years in small-animal imaging and more recently in clinical research. The combination of PET/MRI allows researchers to explore clinical and research questions in a wide number of fields, some of which are briefly mentioned here. An important number of groups have developed different concepts to tackle the problems that PET instrumentation poses to the exposition of electromagnetic fields. We have described most of these research developments in preclinical and clinical experiments, including the few commercial scanners available. From the software perspective, an important number of algorithms have been developed to address the attenuation correction issue and to exploit the possibility that MRI provides for motion correction and quantitative image reconstruction, especially parametric modelling of radiopharmaceutical kinetics. In this work, we give an overview of some exemplar applications of simultaneous PET/MRI, together with technological hardware and software developments.
INTRODUCTION
The combination of positron emission tomography (PET)1 and MRI offers a number of advantages that can be exploited in different ways.2–4 There are currently three different possibilities to design PET/MRI scanners.
Placing the PET and the MRI scanners close together in the same room was the first approach to be proposed to increase patient throughput and comfort in clinical5 and preclinical6 experiments, since it is technically the least challenging configuration. The second alternative is to insert a PET scanner in an existing MRI scanner. This option is technically challenging, since the PET insert needs to be insensitive and transparent to electromagnetic fields. Clinical PET inserts have not been around for too long,7 but there have been groups working in preclinical PET inserts for almost 20 years.8,9 The last option, and technically most challenging, is the combination of the PET and MRI in the same scanner. So far, only clinical scanners for whole-body scanners [Siemens Biograph mMR (Siemens Healthcare GmbH, Erlangen, Germany), GE SIGNA PET/MRI (GE Healthcare, Waukesha, WI)] and dedicated brain scanners (http://www.trimage.eu/) have been or are under development. In this work, we will focus on the last two approaches, where simultaneous imaging is performed.
PET/MRI offers new aspects compared with PET/CT. From the imaging perspective, MRI provides higher soft-tissue contrast than CT and accurate anatomical-functional image co-registration. In the case of PET/CT, image registration is performed after acquisition and image reconstruction and can be cumbersome in whole-body scans. In addition, truly simultaneous acquisitions can potentially provide relevant information from both modalities that is correlated in time. For PET kinetic analysis, MRI information can aid in localizing and extracting accurately the arterial input function (AIF). MRI can also be used to measure the motion of internal organs, especially cardiac and respiratory motions, to correct the PET data. From the safety perspective, MRI spares the significant dose that CT exposes to the patient, which is especially important in follow-up studies. From the economical perspective, even though a PET/MRI examination is generally longer than a PET/CT examination, in cases where a PET and an MRI scan are recommendable, simultaneous PET/MRI is more efficient and better for patient comfort than sequential PET and MRI examinations, increasing the patient throughput. To overcome the lower throughput of PET/MRI compared with PET/CT, there are efforts by some groups to optimize protocols in the PET/MRI workflow by identifying critical MRI sequences that are paramount for specific diseases.10,11
There are a range of clinical applications in oncology, cardiology, paediatrics and neurology where simultaneous PET/MRI can be beneficial. In oncology, perfect co-registration between PET and MRI can help in extracting regional information from within tumours to test drug effectiveness or in localizing functional brain regions together with tumour delineation before and after surgery. For better treatment, understanding of a tumour microenvironment can be improved by combining MRI perfusion-weighted imaging with hypoxic PET markers.12 In cardiology, myocardial blood flow from MRI can be simultaneously studied with 13N-ammonia-PET or 15O-water-PET to obtain accurate quantitative results.13 In the case of infarcted myocardium, the combination of high-resolution wall thickness, together with scar tissue, and plaque burden can be complemented with specific quantitative PET data of blood flow.14 In paediatrics, the two main driving forces of PET/MRI compared with PET/CT are the reduction of ionizing radiation exposure and the combination of two examinations in one single session, where anaesthesia or sedation is reduced. In neurology, MR perfusion from brain strokes can be complemented with simultaneous PET data.15 Functional information from PET and MRI can be used to study diffusion parameters with receptor binding,16 regional perfusion (blood-oxygen-level-dependent contrast imaging) with receptor activation or brain oxygenation with 15O-water-PET.17 The efficacy of dementia disorder classification can be increased by the simultaneous acquisition of brain metabolism, functional connectivity and anatomical structure.18
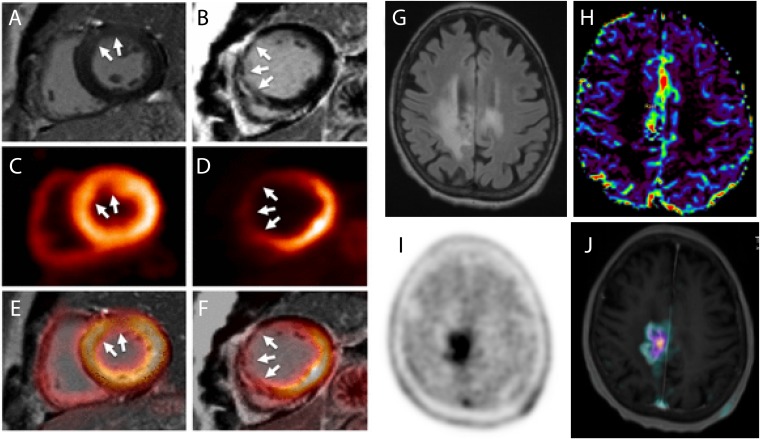
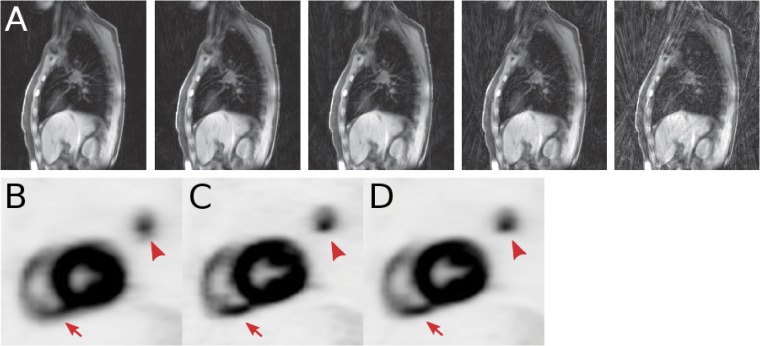
Figure 1 shows two exemplar applications: one from cardiology and one from neuro-oncology, where the combination of PET and MRI confirms a diagnosis and provides complementary information. Figure 1a,b show two cases with myocardial infarction of late gadolinium-enhanced MRI and fluorine-18 fludeoxyglucose (18F-FDG)-PET uptake, comparing extravascular space and dysfunctional myocardium tissue, respectively, produced in both cases by an infarct, confirming diagnostic agreement between both modalities.19 Figure 1c–f show MRI and fluorine-18 fluoroethyl-L-tyrosine (18F-FET)-PET images of the brain of a follow-up patient diagnosed with grade III anaplastic astrocytoma after radiotherapy. Information from a fluid-attenuated inversion-recovery image (Figure 1g) does not provide morphological differences compared with the same image sequence acquired before radiotherapy, while diffusion-weighted imaging (Figure 1h), 18F-FET-PET (Figure 1i) and T1 weighted imaging (Figure 1j) were combined to diagnose tumour recurrence.
Figure 1.
Cardiology example: two cases with myocardial infarction of late gadolinium-enhanced (LGE) MRI (a,b), fluorine-18 fludeoxyglucose-positron emission tomography (18F-FDG-PET) uptake (c,d) and fused images (e,f) showing the diagnostic agreement between LGE transmurality and 18F-FDG uptake. Neuro-oncology example: fluid-attenuated inversion recovery (g), diffusion-weighted imaging (h), fluorine-18 fluoroethyl-L-tyrosine (18F-FET)-PET (i) and fusion of 18F-FET-PET with T1 weighted imaging (j) of a patient with grade III anaplastic astrocytoma after radiotherapy are demonstrating tumour recurrence. Reproduced from Rischpler et al19 with permission from Oxford University Press.
The combination of PET/MRI technology and imaging is not exempt of challenges that need to be addressed in order to use quantitative accurate PET/MRI in clinical routine. The first of them is the technical challenge of combining PET and MR instrumentation without hindering the performance of each other. The second main problem to be addressed is the extraction of attenuation information from the MR data of the subject to obtain quantitative PET images.
The aim of this review was to give an overview of the different clinical and preclinical simultaneous PET/MRI systems, focusing on the different detector technologies and achieved performance. We also discuss the different benefits and challenges in the software developments that are required or can be exploited by combining PET and MRI modalities.
POSITRON EMISSION TOMOGRAPHY INSTRUMENTATION
Photomultiplier tubes (PMTs) were the standard choice for photodetectors in PET. After a γ-photon deposits energy in a scintillator crystal producing low-energy (visible) photons, these interact in the photocathode, producing electrons via the photoelectric effect. The electrons are guided by an electric field and subsequently multiplied as they interact in successive focusing dynodes contained in a vacuum tube. PMTs have a high gain, low noise and fast response. However, they are also fragile, bulky, require high voltages and are sensitive to magnetic fields. The presence of a magnetic field in PMT alters the trajectory of the electrons, hindering the interaction of the electrons in the dynodes.
The basic element of solid-state radiation detectors is the p–n junction from which, upon light detection, electron–hole pairs are generated in a depleted region and separated by a reverse bias applied in the p–n junction. During this process, electrons collide in the crystal lattice, producing an avalanche of secondary electrons. Owing to the short distance (in the order of a few micrometres) that these electrons travel, an external magnetic field has limited impact on their trajectory.20
Positron emission tomography/MRI-compatible photodetector technologies
The development of MR-insensitive photodetectors for low optical signals was triggered by high-energy physics experiments in the 1980s,21 and accelerated by the telecommunications industry for optical communications. Avalanche photodiodes (APDs) were originally developed to detect red and near-infrared electromagnetic radiation with small active areas (approximately 1 mm2). Later on, the technology expanded: the process became more feasible, manufacturing larger active areas sensitive to blue and near-ultraviolet wavelength regions.
APD detectors have a high quantum efficiency and are compact and insensitive to magnetic fields. The main problems that APD detectors present are the limited gain and low temporal performance. There are currently a number of manufacturers that produce APDs for experimental nuclear and particle physics and medical imaging applications (Hamamatsu Photonics, Advanced Photonics, Inc., Radiation Monitoring Devices, Inc., Perkin Elmer Optoelectronics). There are several PET/MRI scanners implemented using APD technology. The Siemens Biograph mMR is based on APD detectors. Besides, a number of research prototype small-animal PET inserts designed to work in combination with an MRI scanner are implemented with APD technology.22–25
More recently, APDs working in Geiger mode, known as silicon photomultipliers (SiPM), emerged as a candidate to be used in PET scanners and are nowadays provided by several manufacturers (Hamamatsu Photonics, AdvanSiD, Ketek GmbH, SensL Technologies Ltd, Photonic SA). Their application to PET was soon adopted owing to the high gain and timing properties that SiPMs have compared with APDs,26 still preserving the benefits of APD technology compared with the classical PMT detector. The GE SIGNA PET/MRI scanner27 and a number of small-animal PET inserts28–31 are based on SiPM detectors.
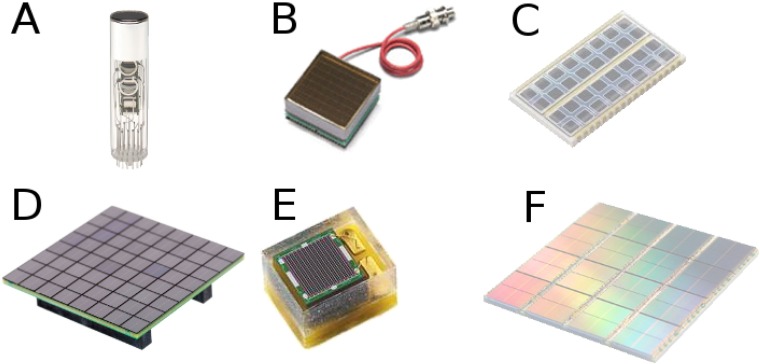
The latest development in PET detectors was the digital SiPM (dSiPM),32 presented by the Philips Digital Photon Counting Business in 2009. With dSiPMs, photons are detected and immediately converted to digital signals. Some of the benefits of dSiPMs are no after-pulsing and high timing performance, owing to the individual microcell readout and the possibility to trigger after the first electron(s) is (are) detected, respectively. In addition, an application specific integrated circuit (ASIC) to pre-process and digitize the signals after the photodetector is not necessary, which is especially relevant in systems with thousands of channels. An MR-compatible version of the Philips dSiPM was used for a small-animal PET insert.33 Following the same concept, in 2011, the University of Delft presented some of their developments on a research dSiPM,34,35 as part of the EndoTOFPET-US collaboration (http://endotofpet-us.web.cern.ch/endotofpet-us/).36 Figure 2 shows examples of several photodetectors: PMTs, APD, SiPM and dSiPM arrays.
Figure 2.
Photodetectors used for positron emission tomography detectors: a photomultiplier tube (PMT) for block detector (Hamamatsu Photonics) (a), flat-panel position-sensitive PMT (Hamamatsu Photonics) (b), avalanche photodiode array (Hamamatsu Photonics) (c), silicon photomultipliers (SiPM) array (SensL Technologies) (d), SiPM detector (Ketek) (e) and digital SiPM array (Philips) (f).
Scintillation crystals
Scintillator materials produce scintillation light after excitation by ionizing radiation owing to their crystalline structure. Inorganic scintillator crystals are commonly used in PET detectors to detect γ-photons. Scintillation crystals should have high detection efficiency, for which high atomic number elements and high density are required, to enhance the photoelectric and Compton scatter cross-sections. High light yield is important for crystal identification in block detectors and energy resolution. Short scintillation decay time is necessary for good timing properties and count rate performance. There are other important properties such as non-hygroscopicity, low self-absorption of optical photons, refractive index similar to that of the entrance window, emission wavelength match with the coupled photodetector, radiation hardness, ruggedness and reasonable cost.37 In the case of MR-compatible PET detectors, it is important that the scintillator does not contain materials with magnetic properties like Gd3Al2Ga3O12[Ce].38
Originally, continuous crystals of NaI[Tl] were coupled to PMT detectors as in γ-cameras. Bismuth germanate (BGO) was later manufactured, surpassing NaI[Tl] in γ-photon detection efficiency but with only 15% light output and longer decay time. The BGO block detector usually consisted of segmented crystals coupled to several PMTs. Other scintillation crystals were BaF2, YA1O3[Ce] (YAP) or Gd2SiO5[Ce] (GSO).37 Later, Lu2SiO5[Ce] (LSO) was introduced, offering an excellent combination of physical properties to be used in PET.39 Since LSO was covered by patent, many PET vendors looked for alternative lutetium-based scintillators. The development of Lu1.8Y0.2SiO5[Ce] (LYSO) with similar properties to LSO has been particularly successful. In LYSO, some lutetium is replaced by yttrium to enhance scintillation efficiency and to modify the decay time so that it could be used in a phoswich structure.40 LSO and LYSO contain a small amount of 176Lu, which is radioactive and introduces a low level of noise in the detection signal. This can be a problem in small-animal imaging and scans in the presence of low activity, but can potentially also be a benefit for some clinical applications, like in transmission scans using the radiation emitted by the radioactive component for attenuation correction (AC) with time-of-flight (ToF) PET.41 In 2001, LaBr3 was presented, showing excellent timing and energy resolution,42 which made it a suitable candidate for ToF PET.43
Alternatively to scintillation crystals, some groups are working on semiconductor detectors that convert γ-photons directly into electrical signals, circumventing the intermediate step of the optical photon generation. Some benefits of these detectors are the excellent energy resolution and the small pixels that can be manufactured with these materials. On the other hand, the stopping power and timing performance are inferior to that of scintillation crystals. Examples of these detectors are cadmium zinc telluride44 and cadmium telluride.45,46
Time of flight
The use of ToF information in the reconstruction process converts the uniform probability of an event occurring somewhere within a line between detectors (line of response) to a Gaussian probability with full width at half maximum given by the time resolution between detectors. The width of the Gaussian probability is related to a reduced region in space where the annihilation takes place.
PMT and (analogue and digital) SiPM detectors are the only technologies at the moment that are able to provide ToF information that can be exploited in clinical (and possibly preclinical) PET.
ToF has been demonstrated to increase the signal-to-noise ratio (SNR) in clinical PET scanners, improving lesion detection47–49 or reducing the injected activity in patients.50 There are currently several PET scanners from different manufactures with ToF capabilities, ranging from 316 to 585 ps.27,43,51–54 Of particular interest are the Philips Vereos system with a time resolution of 316 ps owing to the fully digital PET detectors,54 the prototype scanner developed at the University of Pennsylvania based on LaBr3 scintillators with 375-ps time resolution43 and the GE SIGNA PET/MRI scanner with a time resolution of 394 ps.27
Image reconstruction could potentially be not necessary in the future if the time resolution of a full PET system could be reduced to <10 ps. So, far a coincidence time resolution <100 ps has been measured with LaBr3[Ce]55 and with LSO[Ce],56 with short crystals and under special conditions in the laboratory.
The timing limitations of current scintillators and photodetectors have been identified and to reach a resolution <100 ps, the scintillation rise time, photon time resolution, photon travel spread and light yield have to be optimized,57 in combination with fast photodetectors, improved light transport across scintillator–photodetector optical coupling and acquisition electronics, including ASICs. Alternative light emission mechanisms such as Cherenkov photons have already been suggested to circumvent the current limitations in scintillation time that scintillators used in PET detectors have.58
Positron emission tomography/MR systems
Several classifications can be used to give an overview of the different available PET/MR systems: PET/MRI-integrated scanners vs PET insert, clinical vs preclinical, or PMT-based, APD-based or SiPM-based PET/MRI scanners. A complete review about the different challenges that each approach exhibits was recently published,59 and a thorough overview of the different available systems was presented in the study by Disselhorst et al.60 We will start briefly describing the integrated PET/MRI scanners, then we will separate them between clinical vs preclinical scanners and finally we will expand the overview depending on the scanner photodetector technology.
Positron emission tomography/MRI-integrated systems
There are currently two available commercial clinical whole-body integrated PET/MRI scanners: the Siemens Biograph mMR61 and GE SIGNA.27 Both MRI scanners have a 3.0-T magnet cooled with helium. The former is based on APD technology and the latter on SiPM technology. The use of SiPM technology in the GE scanner enables the possibility to exploit ToF information (<400 ps timing resolution) to include in the PET image reconstruction. The sensitivity in the PET GE scanner is higher than that in the Siemens PET scanner (21 cps kBq−1 vs 15 cps kBq−1), owing partly to the longer lutetium-based crystals used (25 mm vs 20 mm). There are currently over 80 clinical whole-body PET/MRI scanners installed in the field.
Regarding dedicated integrated scanners, TRIMAGE (www.trimage.eu) is a research project aimed at developing an integrated brain PET/MRI/electroencephalogram (EEG) scanner. The MRI has a compact 1.5-T cryogen-free magnet and the PET scanner is based on SiPM technology. The crystal arrays consist of two layers of 3.3 × 3.3-mm2 LYSO crystals and are 8 and 12-mm long for the first and second layers, respectively.62 The inner diameter is 31.2 cm and the axial length is 16.7 cm. MRI and PET information will be combined with EEG, providing excellent temporal resolution to combine with the anatomical-functional information from the PET/MRI scanner.
Regarding small-animal PET scanners, there is currently no project focused on the development of a small-animal integrated PET/MRI scanner. All the works are based on inserts.
Positron emission tomography inserts
Clinical positron emission tomography inserts
There is no whole-body clinical PET insert available. However, there are several dedicated MR-compatible dedicated brain PET inserts: one available as a research prototype (Siemens BrainPET)7 and a number of research projects at different stages.63–66
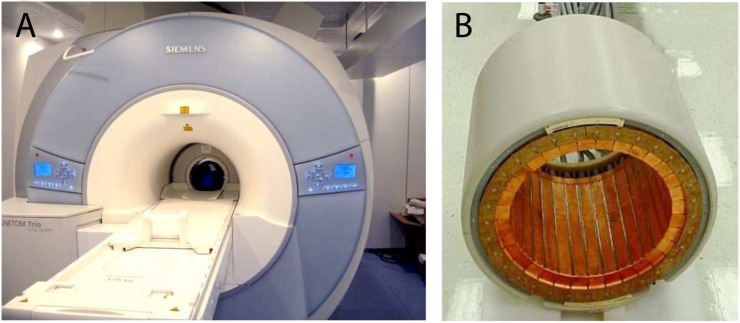
The Siemens BrainPET scanner (Figure 3) consists of 32 cassettes copper shielded with 12 × 12 matrices of 2.5 × 2.5 × 20-mm3 LSO crystals coupled to APD arrays. The field of view (FoV) has an inner diameter of 32 cm and an axial length of 19.2 cm. The system was demonstrated to work in a 3.0-T magnet,67 under strong magnetic fields in a 9.4-T MRI scanner,68 and in combination with EEG information.69 A wide range of clinical and research studies have been developed with the BrainPET scanner, demonstrating the variety of different measurements that can be performed with simultaneous PET/MRI scanners.70
Figure 3.
A 3.0-T MRI scanner MAGNETOM Tim-Trio with BrainPET insert within the MR bore (a) and MR-compatible BrainPET insert (b). Courtesy of Hans Herzog.
Minimizing the interferences of the PET insert in the MRI scanner is an issue that needs to be addressed carefully. With this aim, a radiofrequency (RF)-penetrable dedicated brain PET insert was developed at Stanford University. The insert consists of 16 shielded PET detector modules distributed around a 32-cm diameter bore. There are 1-mm gaps between the PET detectors, which by decoupling the ground of the PET detectors from the RF coil, allow the RF fields to pass through the gaps, minimizing RF attenuation. Ground decoupling is achieved by converting the electrical signals from the photodetectors into optical signals and by powering the PET detectors with independent batteries.65
An alternative approach was presented as an integrated PET insert with a birdcage RF coil, developed at the National Institute of Radiological Sciences (Chiba, Japan). To avoid blocking of the RF fields by the shielded PET detectors, each RF coil element is located in gaps between the PET detectors, within the shielding enclosure around the PET detectors. Special emphasis was given to depth of interaction (DoI), by using four layers of 6 × 6 LYSO crystal arrays coupled to SiPM arrays.71 Experiments were performed in a 3.0-T MRI scanner, demonstrating negligible interferences of the MRI scanner in the PET insert and no distortions or signal attenuation in the MRI signals.66
Placing the RF coil inside the PET insert was also suggested as an alternative, recently developed at Sogang University (Republic of Korea).64 Only the crystal arrays and SiPM arrays are placed between the gradient and the RF coil in a 3.0-T MRI scanner. The PET insert, with 39-cm diameter and 6-cm axial length, is shielded and connected by 4-m-long shielded flat cables to the nearby acquisition system. It has 18 detector modules, each comprised of 4 × 4 matrix modules. The crystal arrays are LYSO 4 × 4—3 × 3 × 20 mm3 and each crystal array is directly coupled to a SiPM array. Experiments showed slight degradations in sensitivity and SNR in the MR images. Scatter correction was especially important in this study owing to scatter in the RF coil. The final energy resolution was 20%, 3.8-ns timing resolution, 0.8% sensitivity and 3–4.8-mm spatial resolution.
The Multimodal Imaging of Neurological Disorders project (http://www.mindview.i3m.upv.es/) is currently developing a dedicated brain PET insert based on SiPM technology integrated with an RF coil, with the aim of being operated in any MRI scanner.63 Two crystal configurations are being evaluated, a three-layer staggered 1.5 × 1.5 × 6-mm3 LYSO crystal array and LYSO 50 × 50 × 20-mm3 monolithic crystals painted in black.72 Preliminary findings resulted in an average energy resolution of 17% and in an intrinsic spatial resolution of 1.5–3.5 mm using the monolithic crystals.
There is also growing interest in developing MR-compatible PET inserts dedicated to breast imaging.
The Brookhaven National Lab developed the first MR-compatible PET insert based on APD technology,73 given the extensive expertise this group acquired developing small-animal PET inserts based on the same technology. Recently, the Digital Hybrid Breast PET/MRI for Enhanced Diagnosis of Breast Cancer (https://www.institut3b.physik.rwth-aachen.de/) project was announced. The technical aim is to develop a dedicated breast hybrid PET/RF insert. The project, led by the University Hospital of Aachen (Germany), aims at early diagnosis and targeted treatment of breast cancer.
Preclinical positron emission tomography inserts
Small-animal PET inserts is where most PET/MR instrumentation projects are focused on. Novel concepts are usually first implemented in small-animal scanners, given the relatively low economical cost. Therefore, the small-animal arena is where we can find a significant variety of PET detector combinations. Most detectors consist of lutetium-based scintillators, with few exceptions based on BGO.
The first prototype of a simultaneous PET/MRI system was McPET, developed in a combined effort between the CRUMP Institute for Biological Sciences (USA) and the Guy's and St Thomas' Clinical PET Centre (UK) in 1997. There were two versions built and published almost simultaneously.8,74 The first version, McPET I, has 48 2 × 2 × 10-mm3 LSO crystals in a 38-mm diameter PET insert8 and the second version, McPET II, contains 72 2 × 2 × 5-mm3 LSO crystals in a 54-mm diameter PET insert.74 In both cases, the system consists of only one single ring and the crystals are coupled to individual pixels of a multichannel PMT via 2-mm-diameter and 4-m-long optical fibres. The poor coupling and light collection efficiency of the optical fibres are the main reason for the 45% energy resolution and 26-ns coincidence window obtained. The insert was simultaneously run in a 0.2-T MR system and in a 9.4-T nuclear magnetic resonance spectrometer without significant degradations. Further developments focused on increasing the sensitivity and the FoV, including a multilayer crystal layout to measure DoI, and improving the coupling between the optical fibres and multichannel PMTs.75,76
Earlier than the first works of combined PET/MRI systems, a cooperation between several institutes led by the Max Planck Institute for Physics (Germany) worked on the characterization of newly developed low-noise and stable APD detectors.77,78 In this work, single APD pixels coupled to LSO crystals were investigated as possible photodetectors for PET compatible with MRI scanners. Motivated by these studies, the cooperation between the Max Planck Institute and the Technische Universität München (Germany) focused their efforts on developing a high-resolution PET detector for a small-animal PET scanner: the Munich Avalanche Diode PET.79,80 Simultaneously, the group at Sherbrooke University (Canada) also investigated the performance of APD detectors for implementation in a small-animal PET scanner.81 However, in this case, MR compatibility was not investigated.
The group at the University of California Davis (USA) published their first tests with APD detectors to replace the MR-sensitive PMT detectors in 2004.82,83 This work resulted in the first PET block detector based on APD technology in 2006, simultaneously running in a 7.0-T MRI scanner.22 The PET detector consists of 10 × 10 LSO crystal arrays of 2 × 2 × 12 mm3 coupled to APD arrays via 3.5-mm-thick light guides. The measured energy resolution was 18.7% with the MRI scanner on, which was significantly better than that of previous designs based on optical fibres with PMTs. Further developments using APD technology within the same group resulted in complete PET systems based on 8 × 8 LSO crystal arrays of 1.43 × 1.43 × 6 mm3 coupled via 10-cm-long optical fibres to APD arrays23,84 and 12 × 12 LSO crystal arrays of 1.6 × 1.6 × 4.5 mm3 directly coupled to APD arrays.24
In 2004, the group at Brookhaven National Laboratory (USA) published their work with RatCAP: a 38-mm-diameter PET scanner based on 12 detector blocks, with 4 × 8 LSO crystal arrays of 2 × 2 × 5 mm3coupled to APD arrays. The original idea was to miniaturize the PET scanner, rather than working in simultaneous PET/MRI.85 Later, in 2006, the APD detectors of RatCAP were modified to exclude MR-sensitive materials for integration in a 4.0-T MRI scanner.86 Further developments of the same concept followed in RatCAP integrated in a 9.4-T MRI scanner, obtaining 14% energy resolution, 10-ns timing resolution, 0.31% sensitivity and a spatial resolution of 1.2 mm.25 In this work, the RF transceiver coil was shielded but not the PET insert since, owing to the large distance between the insert and the imaging FoV, image distortions were negligible.
Whilst the first systems using APD detectors were being developed, some groups continued working on PS-MPTs with crystal arrays connected via long optical fibres (0.8–2.5 m).87–90 The systems were all successfully operated in a magnet obtaining more or less degraded performance, partly depending on the optical fibre length. In parallel with these studies, in 2007, several groups started to evaluate SiPMs for PET detectors.91–93 Subsequently, some groups started to work on developing full PET inserts for simultaneous PET/MRI systems based on SiPM technology.
As early as in 2011, the first prototype of an MR-compatible full PET system based on SiPMs was published by the group at the Department of Nuclear Medicine of the Seoul National University (Republic of Korea),28 even though it was not tested in an MRI scanner. There have been different inserts produced by this group, improving the performance.29,94 The last insert was recently published with a geometry of 16 detector modules, comprised of 9 × 9—1.2 × 1.2 × 10-mm3 LYSO crystals, achieving a sensitivity of 3.4%, an energy resolution of 14.2% and a spatial resolution of 0.75 mm.29
Other PET inserts developed using SiPM technology were focused on optimizing the shielding structure to minimize interferences in the MRI scanner95 or circumventing the problem by placing all MR-sensitive electronic components outside the MR bore.64,96 However, in general, shielding with either carbon fibre or copper is the preferred choice. Among all the available designs, some of them included DoI in their designs by using multilayer structures.30,31 Other approaches focused on minimizing signal degradation from the SiPMs by digitizing the analogue signals directly after the SiPM arrays.97,98
Only 2 years after analogue SiPMs were suggested as a photodetector for PET detectors, Philips Digital Photon Counting Business, part of Philips Technology GmbH (Germany), introduced a fully digital SiPM detector.32,99 Coming from the experience of Hyperion I,97 a fully digital PET insert, Hyperion IID, was first presented in 2012100 and further characterized in the following years.33,101–103 In this case, compared with Hyperion I, the shielding was exchanged from copper housing to carbon fibre composites. An energy resolution of 12.6%, timing resolution of 565 ps (which could be reduced to 260 ps with the optimized timing scheme and cooled at −5 °C), sensitivity of 2.6% and spatial resolution of 0.9 mm at the centre of the FoV were measured within a 3.0-T MRI scanner.
There are other projects which are under development, mainly based on lutetium-based crystals directly coupled to SiPM arrays with or without shielding.104–106
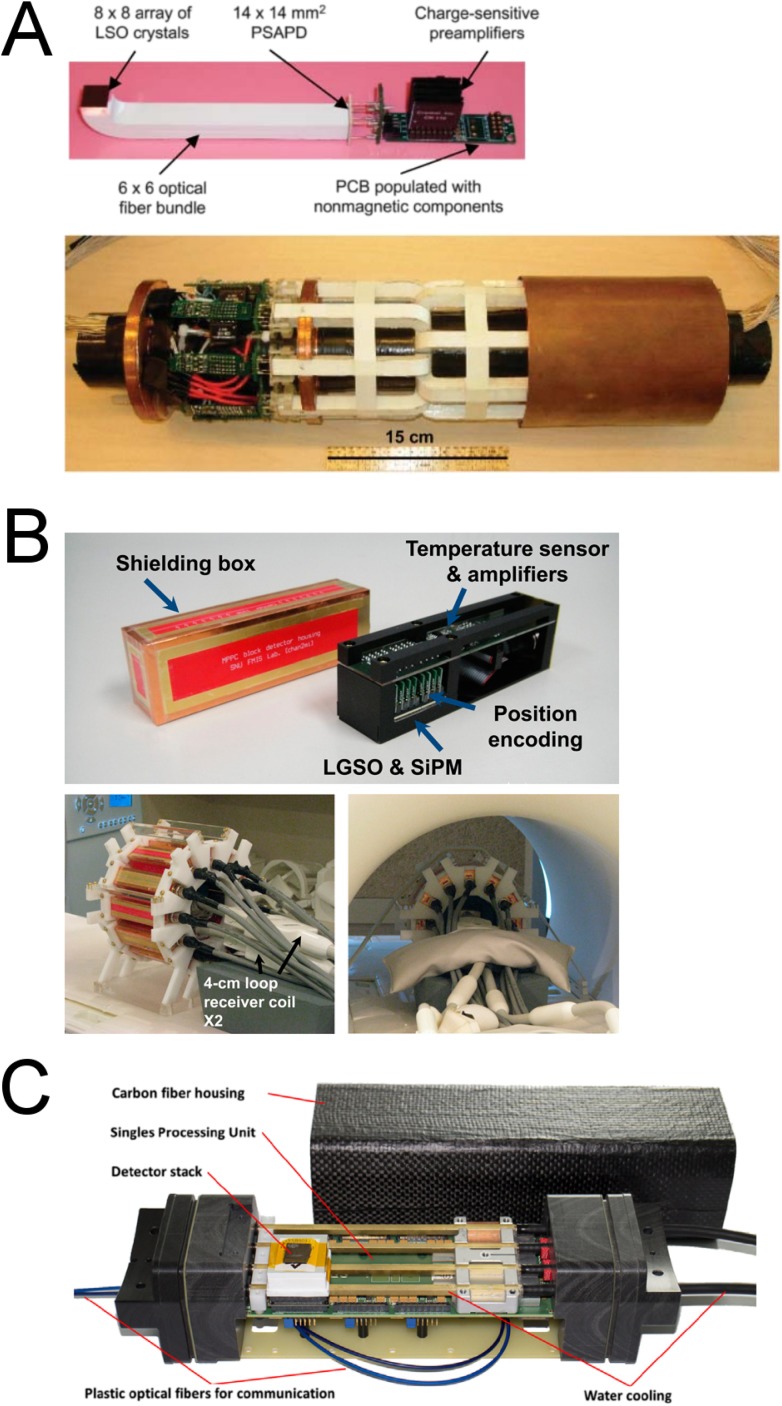
In summary, the development of new small-animal PET/MRI systems and concepts is steadily growing, especially in the past 2 years. The trend is clearly moving away from PMT detectors and optical fibre coupling, while solid-state detectors, especially SiPM detectors, are gaining relevance. Even though SiPM detectors are MR compatible and do not contain magnetic components, the associated electronics (amplifiers and ASICs), usually placed nearby, and connectors are not MR compatible. Therefore, a good shielding is a key feature of the system. Alternatively, a few systems have circumvented this issue, by placing either the PET detectors and electronics far from the PET imaging FoV or the MR-sensitive electronic components out of range of the strong electromagnetic fields of the MRI scanner.96,106 Figure 4 shows several examples of PET inserts using APD, SiPM and dSiPM detectors.23,94,107
Figure 4.
A positron emission tomography (PET) detector module based on position-sensitive avalanche photodiode (PSAPD) technology, including crystals, optical fibers and electronics on the printed circuit board (PCB), and complete PET insert (originally published in JNM23 reproduced with permission from the Society of Nuclear Medicine and Molecular Imaging, Inc.) (a). Silicon photomultipliers (SiPM) detector cassette with shielding box and complete PET insert outside and inside the MRI scanner (originally published in JNM94 reproduced with permission from Society of Nuclear Medicine and Molecular Imaging, Inc.) (b). Digital SiPM detector cassette with shielding box from Hyperion IID (Reproduced from Wehner et al107 published under terms of the Creative Commons Attribution License CC-BY, http://creativecommons.org/licences/by/4.0/ (c).
Comparing the performance between scanners is not a trivial issue. In most published works, the description of the method used to measure the sensitivity, timing resolution, energy resolution and spatial resolution are different or omitted. Results are affected by not only the technical performance of the hardware under assessment but also the software tool and procedure used for measurement. In this case, following the National Electrical Manufacturers Association protocol108 would help to provide a fair comparison among all the scanners. By objectively looking at the published performance in each study, the best timing resolution is 565 ps from the system using digital SiPM detectors.102 The best spatial resolution and sensitivity are 0.75 mm and 3.4%, respectively,29 and the best mean energy resolution for the entire scanner is 10%,30 using analogue SiPM arrays. These can be used as target performance for future developments.
CORRECTION METHODS AND RECONSTRUCTION
Motion correction
Cardiac and respiratory motions are one of the major sources of image degradation in PET imaging, causing image blurring, misregistration with the MR data and mismatches between attenuation maps and emission data. For accurate lesion identification and quantification, physiological motion needs to be corrected. Gating is nowadays a common method in the clinic, whereby emission data are binned in time frames and independently reconstructed. The number of frames used implies a trade-off between image SNR and motion correction accuracy.
A number of approaches aim to measure motion using external sensors (pressure sensors or optical tracking)109 or signal from PET data,110,111 acting as a surrogate for the internal organ motion. With PET/MRI scanners, the simultaneous acquisition of high-temporal-resolution MRI images together with PET data has been exploited in several ways to include the motion information in the PET reconstruction after or during reconstruction.112–115 Most techniques are divided into methods that obtain the motion model at the beginning of the PET acquisition, and then assume the same model all along the PET scan, or methods that measure the motion model throughout the PET scan. In the former case, a signal obtained with external sensors or MR monitoring is required to correlate the motion model with the motion during the entire scan. A belt can be used to extract the respiratory signal to correlate with the original motion model obtained with an MRI acquisition. However, the quality of the motion correction substantially relies on an appropriate positioning of the belt on the patient, which in clinical routine may not be feasible.
A way to monitor motion using MRI is with especially quick encoded navigator echoes (spin echoes or gradient echoes) interleaved between or integrated in the imaging sequences. These navigators can use one-dimensional, two-dimensional116,117 or three-dimensional118 k-space trajectories, depending on the levels of freedom and accuracy required. Alternatively, motion can be extracted from MR data without navigators (self-navigation) using radial or spherical k-space sampling sequences.119 MRI tagging has also been proposed to measure motion, where a specific tissue is tagged with selective frequencies or spatial modulation. By superimposing a grid pattern on the tissue of interest, the grid works as markers that deform during motion.120 However, this approach has been shown to be difficult to realize for human studies.
Accurate internal organ motion measured with MRI produces accurate motion fields for PET motion correction.121 However, continuous motion measurement with such an approach blocks the MRI scanner, not being possible to simultaneously acquire clinical sequences of interest to physicians. To circumvent the problem, k-space undersampling has been effectively implemented reducing the motion acquisition time to 7–8 min. However, excessive undersampling can produce severe image degradation. Figure 5 shows an example of the degradation level of gated MRI reconstructions with shortened scan times (10, 7, 5, 3 and 1 min) and different levels of k-space undersampling. Figure 5 also shows an example of 18F-FDG uptake in the heart and in a lesion, comparing an ungated reconstruction with a post-reconstruction registration, using the motion fields extracted from slightly undersampled MR data, demonstrating that less blurring and crisper images are produced accounting for the cardiac motion correction.122
Figure 5.
Gated MRI reconstructions with shortened scan times (10, 7, 5, 3 and 1 min) and different levels of k-space undersampling (a). Sagittal slice of fluorine-18 fludeoxyglucose uptake in the heart and in a lesion, as a result of an ungated reconstruction using all the positron emission tomography (PET) data (b), gated reconstruction using 40% of the PET data (c) and gated reconstruction using post-reconstruction registration (d). Reproduced from Grimm et al122 with permission from Elsevier.
Alternatively, studies comparing the extraction of respiratory information from only PET data, with MR-based and sensor-based methods, resulted in comparable motion-corrected PET image quality.123 This approach is interesting from the clinical perspective, since it allows the MRI scanner to be used exclusively for clinically relevant sequences. However, PET data-only motion correction methods can be difficult to implement for areas with low uptake.
Attenuation correction
AC is a required PET data correction for quantitative PET, to account for tissue attenuation of the γ-photons, depending on the tissue electron density. AC in small-animal PET is not as important as for clinical PET, but is still required for quantitative studies.124 The accuracy of AC depends on the accuracy of the attenuation map (μ-map), which is the tissue classification in the subject with attenuation coefficients for 511-keV γ-photons. The different tissues considered in the μ-map, their classification and their linear attenuation coefficients are key parameters to define an accurate μ-map.
In the case of PET/CT scanners, the μ-map is directly calculated from a low-dose CT scan, downsampled to match the PET spatial resolution. However, in the case of PET/MRI scanners, since MR information is not directly related to electron tissue density rather than to proton density, special MR sequences are being explored for this purpose. The whole-body μ-map used for clinical PET/MR studies is usually based on the two-point Dixon sequence to classify between the background, fat and soft tissue.125 Such an approach provides errors <10% for whole-body studies, with the exception of bone lesions. However, the two-point Dixon-based μ-map, where the bone is ignored, has been shown to produce errors between 10 and 30% in brain studies.126 Moreover, increased accuracy is required for head studies compared with whole-body studies, and errors >5% are considered unreliable for clinical routine examinations.
Since PET/MRI scanners became available, a number of methods to calculate the μ-map have been developed for head and neck studies. The Siemens Biograph mMR provides two possible μ-maps to apply AC: one based on the Dixon sequence used for whole-body PET scans and another based on the ultrashort time-echo sequence, which is able to obtain bone information and is only used for brain PET studies. Both methods result in errors of 10–20% in brain studies,127 which has triggered the investigation of new methods to calculate an accurate μ-map including bone information based on atlas,128,129 template,130 segmentation,131,132 machine learning133,134 and new MR sequences devised to extract accurate bone information, such as ultrashort time-echo triple echo,135 zero time echo136 and pointwise encoding time reduction with radial acquisition.137 Another approach that was suggested for AC in PET/MRI scanners is maximum likelihood reconstruction of attenuation and activity (MLAA),138 under the idea that wherever there is a γ-emission, there must be a tissue for the positron annihilation to occur and requires no information from the MRI scanner. MLAA was later improved by eliminating the cross-talk between the emission and transmission map that MLAA suffered, when ToF information was included in the reconstruction process.139 The accuracy of MLAA depends on the ToF resolution, counts statistics and biodistribution of the used radioligand.
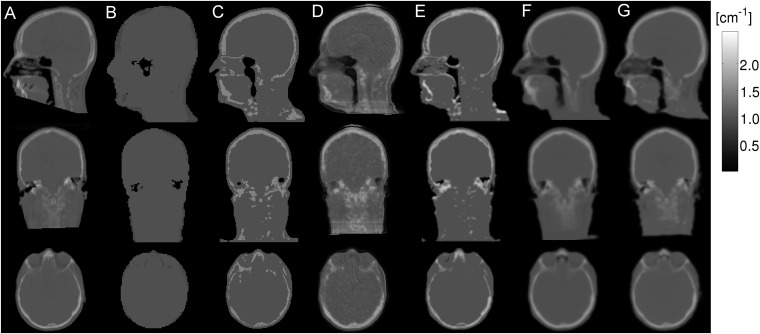
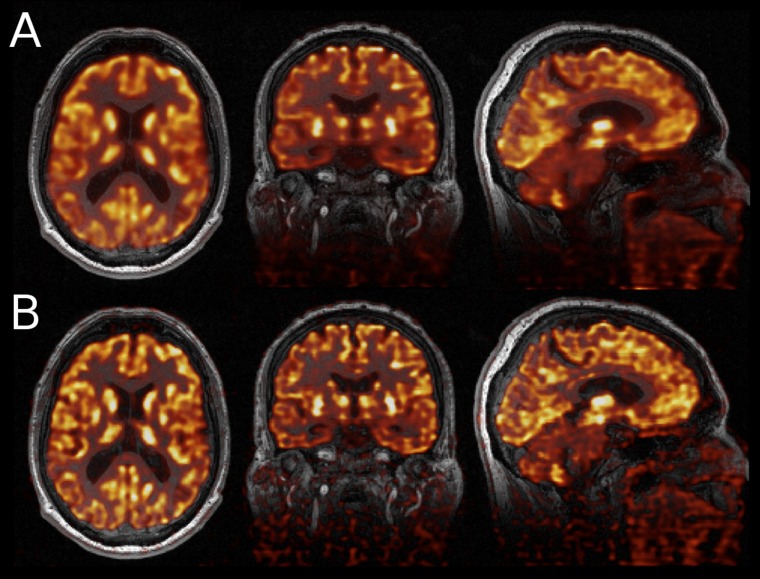
Novel AC methods developed for the Siemens Biograph mMR achieved errors of ±5.0%.128,130,133,134,140 A recent comparison among methods128,130,134,140 showed no substantial differences between them, producing similar quantitative results. Figure 6 shows the μ-maps of an exemplar patient obtained with CT and several methods from MR sequences.141 In comparison, the GE SIGNA PET/MR provides brain AC using a μ-map based on an atlas method (Dixon for the rest of the body),142 resulting in errors of −3.0% to 7.3% for brain studies.
Figure 6.
Sagittal, coronal and axial exemplar µ-maps obtained from CT (a), Dixon (b), dual ultrashort time echo (UTE) (c), Artificial neural network-based atlas (d), dual UTE-based R2 (e), magnetization-prepared rapid acquisition gradient echo (MPRAGE)-based atlas (f) and MPRAGE-based template (g). Reproduced from Cabello et al141 with permission from Springer.
The aforementioned methods provided excellent performance for brain examinations. However, there are still a few areas where improved μ-maps or alternative approaches are required for AC, like bone lesions, paediatric examinations and flexible parts like the neck.
Regarding other parts of the body, the literature is not as prolific as it is for head studies. Few works have addressed the issue of too simplistic constant attenuation coefficients for the lungs143,144 or compared the validity of such values with CT scans as reference.145 In the case of cardiac PET/MRI scans, the μ-map based on the Dixon sequence has been compared with CT scans and validated, obtaining excellent PET standardized uptake value correlation between CT-based and MRI-based AC.146
An additional relevant issue is the motion which is necessary to apply to the μ-map for image reconstruction, not to incur into inaccuracies close to moving organs. This point can be of special importance for lesions in the lungs, whose uptake can be greatly degraded if the μ-map is not registered according to the breathing motion for lesions moving close to the boundary between soft tissue and air (regions with very different attenuation coefficients).147
Another aspect of PET/MRI which has been investigated is the effect of incomplete arms in the patient μ-map, since the FoV of the MRI is smaller than the FoV of the PET scanner.148 Several alternatives have been proposed, from simultaneous estimation of attenuation and activity information within the image reconstruction framework (MLAA)138,139 to extending the MRI FoV by B0 homogenization using gradient enhancement.149
MR-guided positron emission tomography reconstruction
PET image resolution, noise and quantification accuracy are mainly hindered by the statistical noise of the data owing to several interrelated factors. Some of these are the limited scanner sensitivity, amount of injected activity and scan time.
Statistical iterative methods are currently the gold standard in PET image reconstruction to achieve high image quality.150,151 Compared with analytical methods, they can accommodate in the reconstruction process the random nature inherent from the radiodistribution and the different physical effects occurring in a PET measurement. Most algorithms aim at minimizing iteratively the distance between the estimate and acquired data via a cost function. Popular cost functions are maximum likelihood (ML)152 and penalized least squares.153 Using ML-based methods, as new image estimates are calculated, the new estimates approach to the most likely result, improving image quantification and spatial resolution at the cost of amplifying the image noise. A number of approaches have been suggested to reduce the typical increased image noise that PET images contain, as more iterations are calculated. Early stop of the reconstruction process and/or post-reconstruction filters are the two most common alternatives for clinical and preclinical research, at the cost of hindering the spatial resolution and the quantitative accuracy. Using alternative basis functions to the traditional voxels, such as smooth spherical basis functions154,155 or tetrahedral meshes,156 can produce a better image quality than the aforementioned approaches. Another alternative is to impose constrains during the optimization process in the image domain. The combination of PET and anatomical information using maximum a posteriori (MAP) can outperform the other methods discussed above.157
Combining perfectly registered anatomical information from other imaging modalities can be used to guide the PET reconstruction.157 It is important that the accuracy of the multimodality registration does not deteriorate the resulting image quality, especially at the boundaries between different tissues. Therefore, simultaneous acquisition is preferred to sequential acquisition to minimize misregistration errors. In the following, we will consider MRI as the source of anatomical information.
Combining anatomical information with PET data is based on the assumption that the functional information is spatially related to the underlying anatomy and can be basically performed in two ways: post-reconstruction and integration in the reconstruction process.158 Partial volumes is a typical effect in PET images due to the limited resolution of the imaging system. PET image voxels are usually a few millimetres; hence, spread of activity from one voxel to the neighbouring voxels and the inverse effect, spill over of activity from neighbouring voxels into one voxel, occur.
Post-reconstruction methods typically rely on a segmentation step of the anatomical data, previous to filtering the PET image using region spread functions.159 Image segmentation has been shown to be a critical step that can be optimized by adaptively defining the anatomical regions.160,161 Alternative methods avoid the segmentation process by assuming the number of tissues present in the image and modelling the impact of each tissue in each voxel.162 Post-reconstruction deconvolution approaches usually tend to control the image noise, but they can hardly obtain full resolution recovery.163–165
Even though post-reconstruction methods can produce high image quality, an important number of studies are focused on integrating the anatomical information in the cost function used in the reconstruction process (regularization). This process is commonly implemented using the Markov random function prior within a Bayesian framework (MAP). Using such approaches, convergence can potentially be reached, avoiding noise amplification. The Markov random function is a probabilistic image model that describes the interaction between neighbouring voxels based on some properties: prior function. To use the MR anatomical information, to define the extension of neighbouring voxels to consider (anatomical boundaries), and their interaction properties is a natural way of integrating anatomical and function information. The prior function can be included in the reconstruction process using a simple approach such as the one-step-late function,166 or more elaborately by using (concave and continuously differentiable) surrogate functions.167,168
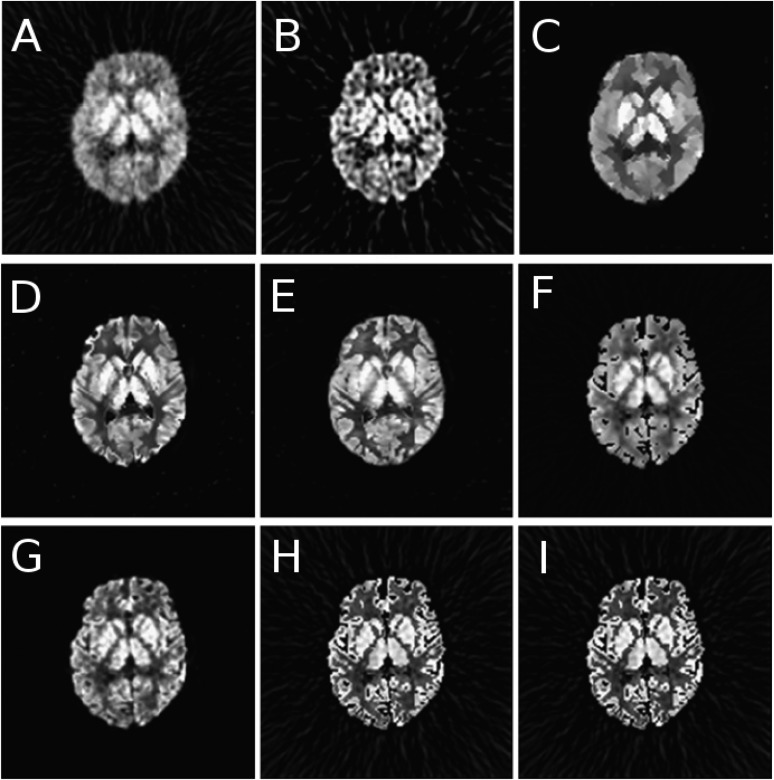
The different algorithms used to combine anatomical information within the reconstruction process basically vary in the way the relation between neighbouring voxels is calculated and in the weight associated with each specific voxel pair. As in the case of post-reconstruction methods, the anatomical information can be segmented and subsequently included in the reconstruction process.169 However, such an approach is exposed to segmentation errors. Alternatively, other methods avoid the segmentation step, like the Bowsher function170 and modifications,171–173 or approaches based on joint entropy between PET and MR images.174,175 A comparison among some of the above-described approaches, using a simulated brain phantom with 18F-FDG, is shown in Figure 7.158
Figure 7.
Sample transaxial slices for fluorine-18 fludeoxyglucose simulations, corrected for partial volume using various approaches: no correction (a), reblurred van Cittert (b), anisotropic diffusion prior (c), modified Bowsher prior (d), semi-parametric joint entropy (e), Gaussian mixture deconvolution (f), iterative projection (g), iterative Yang (h) and region-based voxelwise (i). Reproduced from Hutton et al158 published under the Creative Commons Attribution License CC-BY, http://creativecommons.org/licenses/by/4.0.
One problem that can arise from combining anatomical and functional information is when they are not fully related. This can happen under two circumstances: misregistration between both images or lesion boundary mismatches with the anatomical boundaries. The former is implicitly avoided in simultaneous PET/MRI scanners as long as there is no patient motion or it is corrected. However, the latter can occur in a number of cases such as in small lesions localized in organs or in tumour heterogeneity studies, among others. In both cases, the methods to combine anatomical and functional information need to have mechanisms to relax the constrains, to account for such cases.176
Positron emission tomography kinetics parametric reconstruction
The quality of the data measured in PET can be substantially improved when the radiopharmaceutical kinetics and biodistribution are modelled and parameterized. This area of research, also known as four-dimensional imaging or parametric reconstruction, is especially important in the development of novel drugs. Some biological parameters that can be quantified are blood flow, receptor density or metabolism. It is important the these methods are accurate enough to correctly model the underlying physiological function under study, without increasing the complexity of the experiment protocol substantially, so that they can be integrated in clinical routine.
PET scanner sensitivity, image reconstruction algorithm, radiopharmaceutical dynamics and kinetic model among others are some of the influential factors in parametric imaging. In indirect four-dimensional image reconstruction, acquired PET data for dynamic studies (sinograms or list-mode format) are framed to reconstruct individual images at specific time points. These images are calculated with limited statistics (compared with the full acquired data set), resulting in potentially low-statistical-quality images and low SNR images. Kinetic parameter estimation from low count frames results in noisy and biased estimates, especially if the analysis is performed voxelwise rather than on regions of interest. In addition, to estimate the kinetic parameters, the AIF (or an estimate) is mandatory. There are different methods to extract the AIF: invasive arterial sampling (considered the gold standard), image-derived, reference tissue model,177 mathematical estimation178 or dual pharmacokinetic modelling with gadolinium.179 In the case of image-derived AIF, a section of an artery needs to be segmented (in the absence of the heart in the PET FoV) to extract the time–activity curve of the approximated AIF, which needs to be later corrected for metabolites. This task is usually performed using the reconstructed PET data, which are known to suffer from partial volume effects. MRI high-resolution information can be used in this scenario to avoid oversegmentation/undersegmentation by applying partial volume correction to the PET data.180
Estimation of the kinetic parameters after reconstruction using a set of images obtained with PET data acquired in specific time frames can be considered as the conventional approach (indirect reconstruction).181,182 However, indirect kinetic parameter estimation leads to inaccuracies and low precision estimates.183 Alternatively, the mathematical model of the radiopharmaceutical dynamics can be included in the reconstruction process as a priori information (direct reconstruction). Direct estimation of the kinetic parameters from the PET data has gained interest owing to the more accurate and less noisy kinetic parameters estimates compared with indirect reconstruction.184–187 The three-dimensional radioactive biodistribution can be represented as a linear combination of spatial or/and temporal basis functions. Spatial basis functions are typically modelled as non-overlapping cubic functions (voxels); hence, most research is focused on the study of different linear or non-linear temporal basis functions.188 Examples of linear basis functions are wavelets,189 B-splines,190 Patlak191 or spectral192 among others. In comparison with MAP static image reconstruction, temporal penalties have not been the focus of many research studies, but rather investigations have mainly focused on temporal basis functions. Therefore, the combination of spatiotemporal penalties represents an interesting approach to further obtain low noise and accurate kinetic parameterization.
Combining anatomical information (CT or MRI) in this framework has proven to improve the accuracy compared with approaches with PET data only.171 The increased tissue contrast of MRI data compared with CT data can potentially lead to less noisy estimates of the kinetic parameters in regions where CT does not distinguish between soft tissues.171,193,194 Noticeably, perfect registration between PET data and any underlying anatomical information is of paramount importance. The work published by Löb et al171 exploits perfectly the co-registered PET/MRI information to apply spatial regularization, jointly with temporal regularization. Figure 8 shows the estimated k3 parameter of a patient injected with 18F-FET diagnosed with a glioblastoma (from compartmental analysis, k3 represents the rate of 18F-FET trapped in the tumour in a two-tissue compartment model), reconstructed with three different methods: one indirect method where reconstruction was performed using MAP (one-step late), one direct method using MAP, applying regularization only to the activity images without anatomical guidance (spatial penalty), and another direct method using MAP applying regularization to the activity images using a Bowsher function in combination with anatomical information and regularization applied to the microkinetic parameters. The latter, using spatiotemporal penalties, visibly obtained the cleanest and crisper results.171 These findings were quantitatively confirmed through bias vs variance analysis.
Figure 8.
Axial, sagittal and coronal slices of a patient with glioblastoma injected with fluorine-18 fluoroethyl-L-tyrosine, showing the reconstructed map of the k3 microparameter obtained from indirect reconstruction (maximum a posteriori-expectation maximization using Green's one-step-late approach) (a), direct methods using distance weighting in the activity images (b) and Bowsher weighting with simultaneous regularization of activity images and kinetic parameters (c). Reproduced from Loeb et al171 with permission from the IEEE.
Joint positron emission tomography/MR reconstruction
As opposed to MR-guided PET reconstruction, where the MRI data already reconstructed are integrated in the PET reconstruction as a prior functional, there is a novel approach where raw MR data (k-space samples) are combined with the raw PET data (in projection space) in the reconstruction process. With this alternative approach, both images are simultaneously reconstructed, exploiting the combination of information to produce better PET and MR image quality. Under the assumption that both modalities, even though they show different information, the functional and anatomical structure of the subject under study is somehow related.157
From PET perspective, it is intuitive that the PET image quality can be improved by including the MRI data in the reconstruction. However, from the MRI perspective, it is not clear how the PET data could improve the MR image quality. MRI acquisitions are currently longer than PET acquisitions for an important number of studies. Reducing the length of MRI scans is currently an active area of research, not only for motion correction as mentioned above, but to have short PET/MRI protocols that allow increasing the scanning throughput. An alternative to reduce the MRI scan length is to undersample the k-space combined with compressed sensing techniques to retain the high image quality with reduced number of k-samples.195 If too much k-space oversampling is used, even using compressed sensing techniques, the MR image quality can be degraded compared with a fully k-sampled case. In such a scenario, it makes sense, from the MRI perspective, to combine the PET and MR raw data in the reconstruction process, so that the image quality from MRI could be improved by the PET data.
There are currently a scant number of studies combining simultaneously the PET and MRI data in the reconstruction process. The first study was published by Ehrhardt et al196 in an attempt to exploit the structural similarity. The data from both modalities were combined by using a prior function, assuming that the information from both modalities is somehow related. Several prior functions were compared: joint total variation and parallel level sets. The work relied on similarities between modalities measured in the image gradients, where the edges from the structural information showed parallelism. In the case of MRI, different levels of radial, spiral and linear undersampling were analyzed. Results using numerical examples showed that linear parallel level sets always performed better than total variation or quadratic parallel level sets for the PET and MR images.
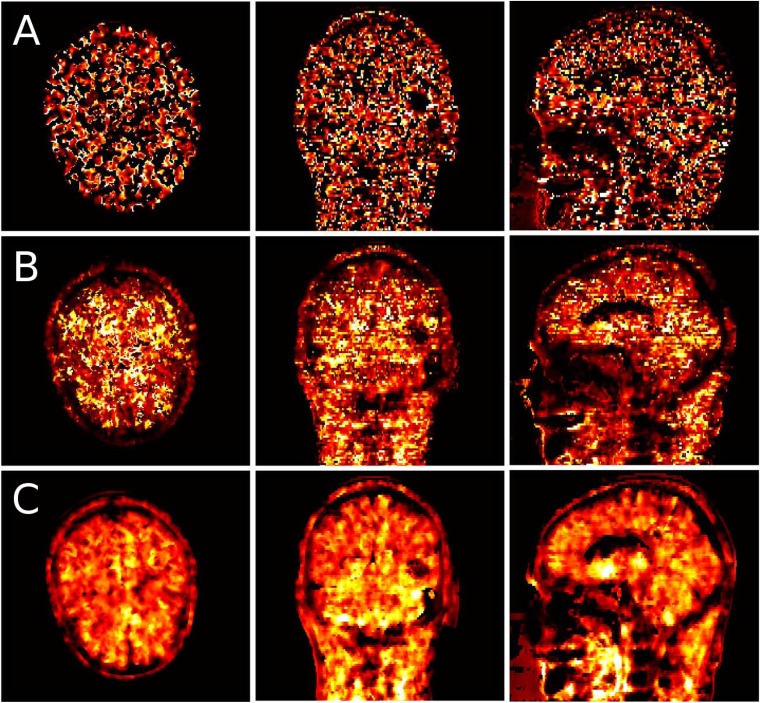
The second published study combining PET and MR raw data in the reconstruction process up to date is the study of Knoll et al197 with a similar idea to that of Ehrhardt et al.196 In this case, MR and PET data were combined using a joint sparsity constraint. Results using numerical examples and real data acquired with the Siemens Biograph mMR scanner demonstrated reduced noise in the MR and PET images and increased spatial resolution in PET images compared with the individual reconstruction of both modalities. Figure 9 shows reconstructed 18F-FDG-PET data, overlaid on conjugate gradient sensitivity encoding MRI images for anatomical correspondence, comparing the results of ML-expectation maximization reconstruction and the proposed joint reconstruction, demonstrating how the latter produces crisper images.198
Figure 9.
Axial, coronal and sagittal images of fluorine-18 fludeoxyglucose-positron emission tomography (PET) acquired with the Siemens mMR Biograph, reconstructed with maximum likelihood-expectation maximization (a) and multichannel MR-PET total generalized variation reconstruction (b) overlaid on MR images (conjugate gradient sensitivity encoding) as reference. Reproduced from Knoll et al198 from Springer. under the terms of Creative Commons Attribution License CC-BY, http://creativecommons.org/by/4.0/.
DISCUSSION
There have been great advances in photodetector technologies for PET in the last approximately 20 years. The advances mentioned in this work have circumvented most of the problems that arose when PET and MRI instrumentation were first combined. New developments are focusing on reducing interferences with the electromagnetic fields by completely eliminating traces of MR-sensitive materials from not only the photodetectors, but also the connectors. Better shielding strategies is an active area of research where Monte Carlo simulations play an important role. There are new studies presenting possibilities to shield the PET instrumentation from the electromagnetic fields at different frequencies, mitigating Eddy currents evoked on the electronic components of the PET system, and also shielding the MRI scanner from electromagnetic interferences emitted from the electronic components of the PET system.
AC has been largely discussed, and a number of approaches have been presented and validated against CT data, obtaining low quantitative errors in global and regional analyses. Some of these methods are being introduced in the commercial PET/MRI scanners; hence, AC is perceived as a problem potentially solved.
Regarding motion correction, MRI can be of great benefit for PET imaging. The accuracy and temporal resolution that MRI provides are of great value and it has proved the important image quality increase obtained when both modalities are combined. However, in clinical practice, it can be very difficult to justify the use of a simultaneous PET/MRI system exclusively for motion correction in some examinations, since the clinical value of MRI is lost. Therefore, to measure motion, either sequences have to be greatly reduced in time or there must be other alternatives to measure motion, from either external devices or the PET data themselves, hence allowing the PET and MRI systems to provide clinically valuable information.
In the image reconstruction area, the combination of PET/MRI can be much further exploited. Static reconstructions can definitely benefit from combining the raw information of PET and MRI within the cost function. Reconstructed PET data can benefit from the higher spatial resolution of MRI data. In the case of MRI data, PET data can be potentially a benefit in the case of using undersampled k-space. However, combining PET/MRI data can be of great help in parametric reconstruction. In this area of research, image reconstruction especially suffers from statistical noise, and we foresee new approaches where simultaneous PET/MRI will show great benefits. As an example, a collaboration project between several groups in the UK has been recently funded to bring the knowledge of all these groups together and develop a common PET/MRI software framework (http://www.ccppetmr.ac.uk/).
PET/MRI is a rapidly changing field where a number of methodologies are maturing. There is increased emphasis on quantitative imaging for personalized cancer therapy to understand tissue microenvironment with multiparametric imaging,199 tumour phenotype assessment and response to targeted therapies, using regional imaging.200 A number of applications in cardiology, oncology, paediatrics and neurology have been identified, where PET/MRI can play an important role. The combined use of PET/MRI requires the identification of appropriate MR sequences to be used in combination with PET to produce complementary information. To further increase the use of PET/MRI in the clinic, protocols need to be standardized across centres, shortened to increase patient comfort and throughput, and initial cost needs to be reduced. One important point already made by other authors is that the role of PET/MRI in the clinic is not to replace PET/CT, but to increase the available imaging portfolio in nuclear medicine and radiology.200
CONCLUSION
In this review, we covered the advances in simultaneous PET/MRI instrumentation starting 20 years ago with the first PET inserts, indicating the different photodetectors that were proposed along the way until now. Different concepts have been suggested to address several problems that arise from exposing PET detectors to strong magnetic fields, avoiding interferences between the PET and MRI systems. More recently, especially since the advent of clinical PET/MRI scanners, a number of software applications for AC, motion correction and image reconstruction have been developed. We gave a short overview of some of the most important and novel approaches on software. The number of groups working on all these areas is steadily increasing, more clinical PET/MRI systems are being installed and more research projects to develop dedicated clinical and preclinical systems are being funded. New developments will come, not only from the instrumentation and software research fields, but also applications from the different medical fields in nuclear medicine and radiology.
Acknowledgments
ACKNOWLEDGMENTS
We would like to acknowledge Stephan Nekolla, Thomas Pyka, Florian Knoll, Jae Sung Lee and Hans Herzog for their material and information.
Contributor Information
Jorge Cabello, Email: jorge.cabello@tum.de.
Sibylle I Ziegler, Email: sibylle.ziegler@tum.de.
REFERENCES
- 1.Del Guerra A, Belcari N, Bisogni M. Positron emission tomography: its 65 years. Riv Nuovo Cimento 2016; 39: 155–223. [Google Scholar]
- 2.Zaidi H, Del Guerra A. An outlook on future design of hybrid PET/MRI systems. Med Phys 2011; 38: 5667–89. doi: 10.1118/1.3633909 [DOI] [PubMed] [Google Scholar]
- 3.Zaidi H, Becker M. The Promise of hybrid PET/MRI: technical advances and clinical applications. IEEE Signal Process Mag 2016; 33: 67–85. doi: 10.1109/MSP.2015.2482225 [DOI] [Google Scholar]
- 4.Zaidi H. PET/MRI: advances in instrumentation and quantitative procedures. PET Clin 2016; 11: 95–202. [DOI] [PubMed] [Google Scholar]
- 5.Zaidi H, Ojha N, Morich M, Griesmer J, Hu Z, Maniawski P, et al. Design and performance evaluation of a whole-body ingenuity TF PET-MRI system. Phys Med Biol 2011; 56: 3091–106. doi: 10.1088/0031-9155/56/10/013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy K, Toth M, Major P, Patay G, Egri G, Haggkvist J, et al. Performance evaluation of the small-animal nanoScan PET/MRI system. J Nucl Med 2013; 54: 1825–32. doi: 10.2967/jnumed.112.119065 [DOI] [PubMed] [Google Scholar]
- 7.Herzog H, Langen KJ, Weirich C, Rota Kops E, Kaffanke J, Tellmann L, et al. High resolution BrainPET combined with simultaneous MRI. Nuklearmedizin 2011; 50: 74–82. doi: 10.3413/Nukmed-0347-10-09 [DOI] [PubMed] [Google Scholar]
- 8.Shao Y, Cherry SR, Farahani K, Meadors K, Siegel S, Silverman RW, et al. Simultaneous PET and MR imaging. Phys Med Biol 1997; 42: 1965–70. doi: 10.1088/0031-9155/42/10/010 [DOI] [PubMed] [Google Scholar]
- 9.Pichler BJ, Boning G, Rafecas M, Schlosshauer M, Lorenz E, Ziegler SI. LGSO scintillation crystals coupled to new large area APDs compared to LSO and BGO. IEEE Trans Nucl Sci 1999; 46: 289–91. doi: 10.1109/23.775530 [DOI] [Google Scholar]
- 10.Martinez-Moller A, Eiber M, Nekolla SG, Souvatzoglou M, Drzezga A, Ziegler S, et al. Workflow and scan protocol considerations for integrated whole-body PET/MRI in oncology. J Nucl Med 2012; 53: 1415–26. doi: 10.2967/jnumed.112.109348 [DOI] [PubMed] [Google Scholar]
- 11.Barbosa FD, von Schulthess G, Veit-Haibach P. Workflow in simultaneous PET/MRI. Semin Nucl Med 2015; 45: 332–44. doi: 10.1053/j.semnuclmed.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 12.Lee N, Jensen J, Schoder H, Wang Y, Fury M, Pfister DG, et al. Correlation of dynamic contrast enhanced magnetic resonance imaging (DCE MRI) with (18)f-fluoromisonidazole positron emission and computed tomography (F-18-FMISO PET/CT) in assessing tumor hypoxia in a series of head and neck cancer (HNC) patients with nodal metastases. J Clin Oncol 2009; 27. [Google Scholar]
- 13.Waller AH, Blankstein R, Kwong RY, Di Carli MF. Myocardial blood flow quantification for evaluation of coronary artery disease by positron emission tomography, cardiac magnetic resonance imaging, and computed tomography. Curr Cardiol Rep 2014; 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rischpler C, Nekolla SG, Kunze KP, Schwaiger M. PET/MRI of the heart. Semin Nucl Med 2015; 45: 234–47. doi: 10.1053/j.semnuclmed.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 15.Catana C, Drzezga A, Heiss WD, Rosen BR. PET/MRI for neurologic applications. J Nucl Med 2012; 53: 1916–25. doi: 10.2967/jnumed.112.105346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen-Berg H, Rushworth MF. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci 2009; 32: 75–94. doi: 10.1146/annurev.neuro.051508.135735 [DOI] [PubMed] [Google Scholar]
- 17.Pichler BJ, Wehrl HF, Kolb A, Judenhofer MS. Positron emission tomography/magnetic resonance imaging: the next generation of multimodality imaging? Semin Nucl Med 2008; 38: 199–208. doi: 10.1053/j.semnuclmed.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drzezga A, Barthel H, Minoshima S, Sabri O. Potential clinical applications of PET/MR imaging in neurodegenerative diseases. J Nucl Med 2014; 55: 47s–55. doi: 10.2967/jnumed.113.129254 [DOI] [PubMed] [Google Scholar]
- 19.Rischpler C, Langwieser N, Souvatzoglou M, Batrice A, van Marwick S, Snajberk J, et al. PET/MRI early after myocardial infarction: evaluation of viability with late gadolinium enhancement transmurality vs 18F-FDG uptake. Eur Heart J Cardiovasc Imaging 2015; 16: 661–9. doi: 10.1093/ehjci/jeu317 [DOI] [PubMed] [Google Scholar]
- 20.Knoll G. Radiation detection and measurement. Hoboken, NJ: Wiley; 1979. [Google Scholar]
- 21.Lorenz E, Natkaniec S, Renker D, Schwartz B. Fast readout of plastic and crystal scintillators by avalanche photodiodes. Nucl Instr Methods Phys Res 1994; 344: 64–72. doi: 10.1016/0168-9002(94)90651-3 [DOI] [Google Scholar]
- 22.Pichler BJ, Judenhofer MS, Catana C, Walton JH, Kneilling M, Nutt RE, et al. Performance test of an LSO-APD detector in a 7-T MRI scanner for simultaneous PET/MRI. J Nucl Med 2006; 47: 639–47. [PubMed] [Google Scholar]
- 23.Catana C, Wu Y, Judenhofer MS, Qi J, Pichler BJ, Cherry SR. Simultaneous acquisition of multislice PET and MR images: initial results with a MR-compatible PET scanner. J Nucl Med 2006; 47: 1968–76. [PubMed] [Google Scholar]
- 24.Judenhofer MS, Catana C, Swann BK, Siegel SB, Jung WI, Nutt RE, et al. PET/MR images acquired with a compact MR-compatible PET detector in a 7-T magnet. Radiology 2007; 244: 807–14. doi: 10.1148/radiol.2443061756 [DOI] [PubMed] [Google Scholar]
- 25.Maramraju SH, Smith SD, Rescia S, Stoll S, Budassi M, Vaska P, et al. Electromagnetic interactions in a shielded PET/MRI system for simultaneous PET/MR imaging in 9.4 T: evaluation and results. IEEE Trans Nucl Sci 2012; 59: 1892–9. doi: 10.1109/TNS.2012.2205705 [DOI] [Google Scholar]
- 26.Roncali E, Cherry SR. Application of silicon photomultipliers to positron emission tomography. Ann Biomed Eng 2011; 39: 1358–77. doi: 10.1007/s10439-011-0266-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levin CS, Maramraju SH, Khalighi MM, Deller TW, Delso G, Jansen F. Design features and mutual compatibility studies of the time-of-flight PET capable GE SIGNA PET/MR system. IEEE Trans Med Imaging 2016. [DOI] [PubMed] [Google Scholar]
- 28.Kwon SI, Lee JS, Yoon HS, Ito M, Ko GB, Choi JY, et al. Development of small-animal PET prototype using silicon photomultiplier (SiPM): initial results of phantom and animal imaging studies. J Nucl Med 2011; 52: 572–9. doi: 10.2967/jnumed.110.079707 [DOI] [PubMed] [Google Scholar]
- 29.Ko GB, Kim KY, Yoon HS, Lee MS, Son JW, Im HJ, et al. Evaluation of a silicon photomultiplier PET insert for simultaneous PET and MR imaging. Med Phys 2016; 43: 72. doi: 10.1118/1.4937784 [DOI] [PubMed] [Google Scholar]
- 30.Thompson CJ, Goertzen AL, Thiessen JD, Bishop D, Stortz G, Kozlowski P, et al. Development of a PET scanner for simultaneously imaging small animals with MRI and PET. Sensors (Basel) 2014; 14: 14654–71. doi: 10.3390/s140814654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto S, Watabe T, Watabe H, Aoki M, Sugiyama E, Imaizumi M, et al. Simultaneous imaging using Si-PM-based PET and MRI for development of an integrated PET/MRI system. Phys Med Biol 2012; 57: N1–13. doi: 10.1088/0031-9155/57/2/N1 [DOI] [PubMed] [Google Scholar]
- 32.Frach T, Prescher G, Degenhardt C, Gruyter RD, Schmitz A, Ballizany R, eds. The digital silicon photomultiplier—Principle of operation and intrinsic detector performance. Nuclear Science Symposium Conference Record (NSS/MIC), 2009 IEEE; 2009. [Google Scholar]
- 33.Wehner J, Weissler B, Dueppenbecker PM, Gebhardt P, Goldschmidt B, Schug D, et al. MR-compatibility assessment of the first preclinical PET-MRI insert equipped with digital silicon photomultipliers. Phys Med Biol 2015; 60: 2231–55. doi: 10.1088/0031-9155/60/6/2231 [DOI] [PubMed] [Google Scholar]
- 34.Maruyama Y, Charbon E, eds. An all-digital 128×128 CMOS optical/electrical image sensor. Proceedings International Symposium on Microchemistry and Microsystems (ISMM); 2011. [Google Scholar]
- 35.Mandai S, Jain V, Charbon EA. 780×800 {\mu} m^2 multichannel digital silicon photomultiplier with column-parallel time-to-digital converter and basic characterization. IEEE Trans Nucl Sci 2014; 61: 44–52. doi: 10.1109/TNS.2013.2294022 [DOI] [Google Scholar]
- 36.Aubry N, Auffray E, Mimoun FB, Brillouet N, Bugalho R, Charbon E, et al. EndoTOFPET-US: a novel multimodal tool for endoscopy and positron emission tomography. J Instrumentation 2013; 8: C04002. [Google Scholar]
- 37.Melcher CL. Scintillation crystals for PET. J Nucl Med 2000; 41: 1051–5. [PubMed] [Google Scholar]
- 38.Kamada K, Shimazoe K, Ito S, Yoshino M, Endo T, Tsutsumi K, et al. Development of a prototype detector using APD-arrays coupled with pixelized Ce: GAGG scintillator for high resolution radiation imaging. IEEE Trans Nucl Sci 2014; 61: 348–52. doi: 10.1109/TNS.2013.2290319 [DOI] [Google Scholar]
- 39.Melcher CL, Schweitzer JS. A promising new scintillator: cerium-doped lutetium oxyorthosilicate. Nucl Instr Methods Phys Res 1992; 314: 212–14. doi: 10.1016/0168-9002(92)90517-8 [DOI] [Google Scholar]
- 40.Pepin CM, Berard P, Perrot AL, Pepin C, Houde D, Lecomte R, et al. Properties of LYSO and recent LSO scintillators for phoswich PET detectors. IEEE Trans Nucl Sci 2004; 51: 789–95. doi: 10.1109/TNS.2004.829781 [DOI] [Google Scholar]
- 41.Rothfuss H, Panin V, Moor A, Young J, Hong I, Michel C, et al. LSO background radiation as a transmission source using time of flight. Phys Med Biol 2014; 59: 5483–500. doi: 10.1088/0031-9155/59/18/5483 [DOI] [PubMed] [Google Scholar]
- 42.van Loef EV, Dorenbos P, van Eijk CW, Krämer K, Güdel HU. High-energy-resolution scintillator: Ce3+ activated LaBr3. Appl Phys Lett 2001; 79: 1573–5. doi: 10.1063/1.1385342 [DOI] [Google Scholar]
- 43.Daube-Witherspoon ME, Surti S, Perkins A, Kyba CC, Wiener R, Werner ME, et al. The imaging performance of a LaBr3-based PET scanner. Phys Med Biol 2010; 55: 45–64. doi: 10.1088/0031-9155/55/1/004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu Y, Matteson JL, Skelton RT, Deal AC, Stephan EA, Duttweiler F, et al. Study of a high-resolution, 3D positioning cadmium zinc telluride detector for PET. Phys Med Biol 2011; 56: 1563–84. doi: 10.1088/0031-9155/56/6/004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morimoto Y, Ueno Y, Takeuchi W, Kojima S, Matsuzaki K, Ishitsu T, et al. Development of a 3D brain PET scanner using CdTe semiconductor detectors and its first clinical application. IEEE Trans Nucl Sci 2011; 58: 2181–9. doi: 10.1109/TNS.2011.2146790 [DOI] [Google Scholar]
- 46.Mikhaylova E, Lorenzo GD, Chmeissani M, Kolstein M, Canadas M, Arce P, et al. Simulation of the expected performance of a seamless scanner for brain PET based on highly pixelated CdTe detectors. IEEE Trans Med Imaging 2014; 33: 332–9. doi: 10.1109/TMI.2013.2284657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lois C, Jakoby BW, Long MJ, Hubner KF, Barker DW, Casey ME, et al. An assessment of the impact of incorporating time-of-flight information into clinical PET/CT imaging. J Nucl Med 2010; 51: 237–45. doi: 10.2967/jnumed.109.068098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surti S. Update on time-of-flight PET imaging. J Nucl Med 2015; 56: 98–105. doi: 10.2967/jnumed.114.145029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vandenberghe S, Mikhaylova E, D'Hoe E, Mollet P, Karp JS. Recent developments in time-of-flight PET. EJNMMI Phys 2016; 3: 1–30. doi: 10.1186/s40658-016-0138-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Queiroz MA, Delso G, Wollenweber S, Deller T, Zeimpekis K, Huellner M, et al. Dose optimization in TOF-PET/MR compared to TOF-PET/CT. PLoS One 2015; 10: e0128842. doi: 10.1371/journal.pone.0128842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med 2007; 48: 471–80. [PubMed] [Google Scholar]
- 52.Jakoby BW, Bercier Y, Conti M, Casey ME, Bendriem B, Townsend DW. Physical and clinical performance of the mCT time-of-flight PET/CT scanner. Phys Med Biol 2011; 56: 2375–89. doi: 10.1088/0031-9155/56/8/004 [DOI] [PubMed] [Google Scholar]
- 53.Bettinardi V, Presotto L, Rapisarda E, Picchio M, Gianolli L, Gilardi MC. Physical performance of the new hybrid PET/CT Discovery-690. Med Phys 2011; 38: 5394–411. doi: 10.1118/1.3635220 [DOI] [PubMed] [Google Scholar]
- 54.Miller M, Zhang J, Binzel K, Griesmer J, Laurence T, Narayanan M, et al. Characterization of the vereos digital photon counting PET system. J Nucl Med 2015; 56(Suppl. 3): 434. [Google Scholar]
- 55.Schaart DR, Seifert S, Vinke R, van Dam HT, Dendooven P, Lohner H, et al. LaBr(3): Ce and SiPMs for time-of-flight PET: achieving 100 ps coincidence resolving time. Phys Med Biol 2010; 55: N179–89. doi: 10.1088/0031-9155/55/7/N02 [DOI] [PubMed] [Google Scholar]
- 56.Nemallapudi MV, Gundacker S, Lecoq P, Auffray E, Ferri A, Gola A, et al. Sub-100 ps coincidence time resolution for positron emission tomography with LSO: Ce codoped with Ca. Phys Med Biol 2015; 60: 4635–49. doi: 10.1088/0031-9155/60/12/4635 [DOI] [PubMed] [Google Scholar]
- 57.Gundacker S, Auffray E, Pauwels K, Lecoq P. Measurement of intrinsic rise times for various L(Y)SO and LuAG scintillators with a general study of prompt photons to achieve 10 ps in TOF-PET. Phys Med Biol 2016; 61: 2802. doi: 10.1088/0031-9155/61/7/2802 [DOI] [PubMed] [Google Scholar]
- 58.Lecoq P, Auffray E, Brunner S, Hillemanns H, Jarron P, Knapitsch A, et al. Factors influencing time resolution of scintillators and ways to improve them. IEEE Trans Nucl Sci 2010; 57: 2411–16. doi: 10.1109/TNS.2010.2049860 [DOI] [Google Scholar]
- 59.Vandenberghe S, Marsden PK. PET-MRI: a review of challenges and solutions in the development of integrated multimodality imaging. Phys Med Biol 2015; 60: R115–54. doi: 10.1088/0031-9155/60/4/R115 [DOI] [PubMed] [Google Scholar]
- 60.Disselhorst JA, Bezrukov I, Kolb A, Parl C, Pichler BJ. Principles of PET/MR imaging. J Nucl Med 2014; 55(Suppl. 2): 2S–10. doi: 10.2967/jnumed.113.129098 [DOI] [PubMed] [Google Scholar]
- 61.Delso G, Furst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med 2011; 52: 1914–22. doi: 10.2967/jnumed.111.092726 [DOI] [PubMed] [Google Scholar]
- 62.Papadimitroulas P, Loudos G, Cabello J, Scheins J, Belcari N, Del Guerra A, eds. Investigation and evaluation of a simulated dedicated PET prototype for brain studies: applicability to a trimodal PET/MR/EEG system. Nuclear Science Symposium Conference Record (NSS/MIC), 2014 IEEE; 2014. [Google Scholar]
- 63.González AJ, Majewski S, Sánchez F, Aussenhofer S, Aguilar A, Conde P, et al. The MINDView brain PET detector, feasibility study based on SiPM arrays. Nucl Instr Methods Phys Res 2016; 818: 82–90. doi: 10.1016/j.nima.2016.02.046 [DOI] [Google Scholar]
- 64.Jung JH, Choi Y, Jung J, Kim S, Lim HK, Im KC, et al. Development of PET/MRI with insertable PET for simultaneous PET and MR imaging of human brain. Med Phys 2015; 42: 2354–63. doi: 10.1118/1.4918321 [DOI] [PubMed] [Google Scholar]
- 65.Lee B, Grant A, Chang CM, Glover G, Levin C. Successful demonstration of simultaneous PET/MR imaging with a RF-penetrable PET insert. EJNMMI Phys 2015; 2(Suppl. 1): A17. doi: 10.1186/2197-7364-2-S1-A17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishikido F, Tashima H, Suga M, Inadama N, Eiji Y, Obata T, et al. Imaging performance of a full-ring prototype PET-MRI system based on four-layer DOI-PET detectors integrated with a RF coil. EJNMMI Phys 2015; 2(Suppl. 1): A18. doi: 10.1186/2197-7364-2-S1-A18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kolb A, Wehrl HF, Hofmann M, Judenhofer MS, Eriksson L, Ladebeck R, et al. Technical performance evaluation of a human brain PET/MRI system. Eur Radiol 2012; 22: 1776–88. doi: 10.1007/s00330-012-2415-4 [DOI] [PubMed] [Google Scholar]
- 68.Shah NJ, Herzog H, Weirich C, Tellmann L, Kaffanke J, Caldeira L, et al. Effects of magnetic fields of up to 9.4 T on resolution and contrast of PET images as measured with an MR-BrainPET. PLoS One 2014; 9: e95250. doi: 10.1371/journal.pone.0095250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shah NJ, Oros-Peusquens AM, Arrubla J, Zhang K, Warbrick T, Mauler J, et al. Advances in multimodal neuroimaging: hybrid MR-PET and MR-PET-EEG at 3 T and 9.4 T. J Magn Reson 2013; 229: 101–15. doi: 10.1016/j.jmr.2012.11.027 [DOI] [PubMed] [Google Scholar]
- 70.Neuner I, Kaffanke JB, Langen KJ, Kops ER, Tellmann L, Stoffels G, et al. Multimodal imaging utilising integrated MR-PET for human brain tumour assessment. Eur Radiol 2012; 22: 2568–80. doi: 10.1007/s00330-012-2543-x [DOI] [PubMed] [Google Scholar]
- 71.Nishikido F, Tachibana A, Obata T, Inadama N, Yoshida E, Suga M, et al. Development of 1.45-mm resolution four-layer DOI-PET detector for simultaneous measurement in 3T MRI. Radiol Phys Technol 2015; 8: 111–19. doi: 10.1007/s12194-014-0298-6 [DOI] [PubMed] [Google Scholar]
- 72.Conde P, González AJ, Hernández L, Bellido P, Iborra A, Crespo E, et al. Results of a combined monolithic crystal and an array of ASICs controlled SiPMs. Nucl Instr Methods Phys Res 2014; 734: 132–6. [Google Scholar]
- 73.Ravindranath B, Junnarkar S, Purschke M, Hong X, Bennett D, Maramraju SH, et al. Simultaneous PET-MRI results from the BNL dedicated breast imaging system prototype. J Nucl Med 2010; 51(Suppl. 2): 410. [Google Scholar]
- 74.Shao Y, Cherry SR, Farahani K, Slates R, Silverman RW, Meadors K, et al. Development of a PET detector system compatible with MRI/NMR systems. IEEE Trans Nucl Sci 1997; 44: 1167–71. doi: 10.1109/23.596982 [DOI] [Google Scholar]
- 75.Mackewn JE, Halsted P, Charles-Edwards G, Page R, Totman JJ, Sunassee K, et al. Performance evaluation of an MRI-compatible pre-clinical PET system using long optical fibers. IEEE Trans Nucl Sci 2010; 57: 1052–62. doi: 10.1109/TNS.2010.2044891 [DOI] [Google Scholar]
- 76.Mackewn JE, Strul D, Hallett WA, Halsted P, Page RA, Keevil SF, et al. Design and development of an MR-compatible PET scanner for imaging small animals. IEEE Trans Nucl Sci 2005; 52: 1376–80. doi: 10.1109/TNS.2005.858260 [DOI] [Google Scholar]
- 77.Schmelz C, Bradbury SM, Holl I, Lorenz E, Renker D, Ziegler S. Feasibility study of an avalanche photodiode readout for a high resolution PET with nsec time resolution. IEEE Trans Nucl Sci 1995; 42: 1080–4. doi: 10.1109/23.467745 [DOI] [Google Scholar]
- 78.Pichler B, Lorenz E, Mirzoyan R, Pimpl W, Roder F, Schwaiger M, et al. , eds. Performance test of a LSO-APD PET module in a 9.4 Tesla magnet. Nuclear Science Symposium, 1997 IEEE; 1997. [Google Scholar]
- 79.Ziegler SI, Pichler BJ, Boening G, Rafecas M, Pimpl W, Lorenz E, et al. A prototype high-resolution animal positron tomograph with avalanche photodiode arrays and LSO crystals. Eur J Nucl Med 2001; 28: 136–43. doi: 10.1007/s002590000438 [DOI] [PubMed] [Google Scholar]
- 80.Pichler BJ, Pimpl W, Buttler W, Kotoulas L, Boning G, Rafecas M, et al. Integrated low-noise low-power fast charge-sensitive preamplifier for avalanche photodiodes in JFET-CMOS technology. IEEE Trans Nucl Sci 2001; 48: 2370–4. doi: 10.1109/23.983270 [DOI] [Google Scholar]
- 81.Lecomte R, Cadorette J, Rodrigue S, Lapointe D, Rouleau D, Bentourkia M, et al. Initial results from the Sherbrooke avalanche photodiode positron tomograph. IEEE Trans Nucl Sci 1996; 43: 1952–7. doi: 10.1109/23.507252 [DOI] [Google Scholar]
- 82.Shah KS, Grazioso R, Farrell R, Glodo J, McClish M, Entine G, et al. Position sensitive APDs for small animal PET imaging. IEEE Trans Nucl Sci 2004; 51: 91–5. doi: 10.1109/TNS.2003.823012 [DOI] [Google Scholar]
- 83.Pichler BJ, Swann BK, Rochelle J, Nutt RE, Cherry SR, Siegel SB. Lutetium oxyorthosilicate block detector readout by avalanche photodiode arrays for high resolution animal PET. Phys Med Biol 2004; 49: 4305–19. doi: 10.1088/0031-9155/49/18/008 [DOI] [PubMed] [Google Scholar]
- 84.Wu Y, Catana C, Farrell R, Dokhale PA, Shah KS, Qi J, et al. PET performance evaluation of an MR-compatible PET insert. IEEE Trans Nucl Sci 2009; 56: 574–80. doi: 10.1109/TNS.2009.2015448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vaska P, Woody CL, Schlyer DJ, Shokouhi S, Stoll SP, Pratte JF, et al. RatCAP: miniaturized head-mounted PET for conscious rodent brain imaging. IEEE Trans Nucl Sci 2004; 51: 2718–22. doi: 10.1109/TNS.2004.835740 [DOI] [Google Scholar]
- 86.Schlyer D, Vaska P, Tomasi D, Woody C, Solis-Najera S, Southekal S, et al. , eds. Preliminary studies of a simultaneous PET/MRI scanner based on the RatCAP small animal tomograph. Nuclear Science Symposium Conference Record, 2006 IEEE; 2006. [Google Scholar]
- 87.Lucas AJ, Hawkes RC, Ansorge RE, Williams GB, Nutt RE, Clark JC, et al. Development of a combined microPET-MR system. Technol Cancer Res Treat 2006; 5: 337–41. doi: 10.1177/153303460600500405 [DOI] [PubMed] [Google Scholar]
- 88.Hawkes RC, Fryer TD, Siegel S, Ansorge RE, Carpenter TA. Preliminary evaluation of a combined microPET-MR system. Technol Cancer Res Treat 2010; 9: 53–60. doi: 10.1177/153303461000900106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raylman RR, Majewski S, Lemieux SK, Velan SS, Kross B, Popov V, et al. Simultaneous MRI and PET imaging of a rat brain. Phys Med Biol 2006; 51: 6371–9. doi: 10.1088/0031-9155/51/24/006 [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto S, Imaizumi M, Kanai Y, Tatsumi M, Aoki M, Sugiyama E, et al. Design and performance from an integrated PET/MRI system for small animals. Ann Nucl Med 2010; 24: 89–98. doi: 10.1007/s12149-009-0333-6 [DOI] [PubMed] [Google Scholar]
- 91.Sascha M, Alberto Del G, Deborah JH, Mark AM. A detector head design for small-animal PET with silicon photomultipliers (SiPM). Phys Med Biol 2006; 51: 1113. [DOI] [PubMed] [Google Scholar]
- 92.Spanoudaki VC, Mann AB, Otte AN, Konorov I, Torres-Espallardo I, Paul S, et al. Use of single photon counting detector arrays in combined PET/MR: characterization of LYSO-SiPM detector modules and comparison with a LSO-APD detector. J Instrumentation 2007; 2: P12002. doi: 10.1088/1748-0221/2/12/P12002 [DOI] [Google Scholar]
- 93.Britvitch I, Renker D. Measurements of the recovery time of Geiger-mode avalanche photodiodes. Nucl Instr Methods Phys Res 2006; 567: 260–3. doi: 10.1016/j.nima.2006.05.109 [DOI] [Google Scholar]
- 94.Yoon HS, Ko GB, Kwon SI, Lee CM, Ito M, Chan Song I, et al. Initial results of simultaneous PET/MRI experiments with an MRI-compatible silicon photomultiplier PET scanner. J Nucl Med 2012; 53: 608–14. doi: 10.2967/jnumed.111.097501 [DOI] [PubMed] [Google Scholar]
- 95.Hong SJ, Kang HG, Ko GB, Song IC, Rhee JT, Lee JS. SiPM-PET with a short optical fiber bundle for simultaneous PET-MR imaging. Phys Med Biol 2012; 57: 3869–83. doi: 10.1088/0031-9155/57/12/3869 [DOI] [PubMed] [Google Scholar]
- 96.Kang J, Choi Y, Hong KJ, Hu W, Jung JH, Huh Y, et al. A small animal PET based on GAPDs and charge signal transmission approach for hybrid PET-MR imaging. J Instrumentation 2011; 6: P08012. [Google Scholar]
- 97.Schulz V, Weissler B, Gebhardt P, Solf T, Lerche CW, Fischer P, et al. , eds. SiPM based preclinical PET/MR insert for a human 3T MR: first imaging experiments. Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), 2011 IEEE; 2011. pp. 23–9. [Google Scholar]
- 98.Weissler B, Gebhardt P, Lerche CW, Wehner J, Solf T, Goldschmidt B, et al. MR compatibility aspects of a silicon photomultiplier-based PET/RF insert with integrated digitisation. Phys Med Biol 2014; 59: 5119–39. doi: 10.1088/0031-9155/59/17/5119 [DOI] [PubMed] [Google Scholar]
- 99.Degenhardt C, Prescher G, Frach T, Thon A, Gruyter RD, Schmitz A, et al. , eds. The digital silicon photomultiplier—a novel sensor for the detection of scintillation light. Nuclear Science Symposium Conference Record (NSS/MIC), 2009 IEEE; 2009. [Google Scholar]
- 100.Weissler B, Gebhardt P, Goldschmidt B, eds. Design concept of world's first preclinical PET/MR insert with fully digital silicon photomultiplier technology. Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC); 2012. [Google Scholar]
- 101.Wehner J, Weissler B, Dueppenbecker P, Gebhardt P, Schug D, Ruetten W, et al. PET/MRI insert using digital SiPMs: Investigation of MR-compatibility. Nucl Instr Methods Phys Res 2014; 734: 116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weissler B, Gebhardt P, Dueppenbecker PM, Wehner J, Schug D, Lerche CW, et al. A digital preclinical PET/MRI insert and initial results. IEEE Trans Med Imaging 2015; 34: 2258–70. doi: 10.1109/TMI.2015.2427993 [DOI] [PubMed] [Google Scholar]
- 103.Schug D, Wehner J, Dueppenbecker PM, Weissler B, Gebhardt P, Goldschmidt B, et al. PET performance and MRI compatibility evaluation of a digital, ToF-capable PET/MRI insert equipped with clinical scintillators. Phys Med Biol 2015; 60: 7045–67. doi: 10.1088/0031-9155/60/18/7045 [DOI] [PubMed] [Google Scholar]
- 104.Goertzen A, Stortz G, Thiessen J, Bishop D, Khan MS, Kozlowski P, et al. First results from a high-resolution small animal PET insert for PET/MRI imaging. EJNMMI Phys 2015; 2(Suppl. 1): A54. doi: 10.1186/2197-7364-2-S1-A54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y, Zhang Z, Li D, Liu S, Wang P, Feng B, et al. Development of a PET insert for simultaneously small animal PET/MRI. EJNMMI Phys 2015; 2(Suppl. 1): A21. doi: 10.1186/2197-7364-2-S1-A21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schneider F, Seker G, Cabello J, Sharma R, Shimazoe K, Orita T, et al. , eds. MADPET4-a MRI compatible dual layer PET insert: first tests with two modules Nuclear Science Symposium Conference Record (NSS/MIC), 2014 IEEE; 2014. [Google Scholar]
- 107.Wehner J, Weissler B, Dueppenbecker P, Gebhardt P, Schug D, Ruetten W, et al. PET/MRI insert using digital SiPMs: investigation of MR-compatibility. Nucl Instr Methods Phys Res 2014; 734: 116–21. doi: 10.1016/j.nima.2013.08.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.National Electrical Manufacturers Association. NEMA Standard Publication NU 4–2008: performance measurements of small animal positron emission tomographs; 2008. [Google Scholar]
- 109.Rahmim A, Rousset O, Zaidi H. Strategies for motion tracking and correction in PET. PET Clin 2007; 2: 251–66. doi: 10.1016/j.cpet.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 110.Dawood M, Buther F, Jiang X, Schafers KP. Respiratory motion correction in 3-D PET data with advanced optical flow algorithms. IEEE Trans Med Imaging 2008; 27: 1164–75. doi: 10.1109/TMI.2008.918321 [DOI] [PubMed] [Google Scholar]
- 111.Woo J, Tamarappoo B, Dey D, Nakazato R, Le Meunier L, Ramesh A, et al. Automatic 3D registration of dynamic stress and rest (82)Rb and flurpiridaz F18 myocardial perfusion PET data for patient motion detection and correction. Med Phys 2011; 38: 6313–26. doi: 10.1118/1.3656951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsoumpas C, Mackewn JE, Halsted P, King AP, Buerger C, Totman JJ, et al. Simultaneous PET-MR acquisition and MR-derived motion fields for correction of non-rigid motion in PET. Ann Nucl Med 2010; 24: 745–50. doi: 10.1007/s12149-010-0418-2 [DOI] [PubMed] [Google Scholar]
- 113.Catana C. Motion correction options in PET/MRI. Semin Nucl Med 2015; 45: 212–23. doi: 10.1053/j.semnuclmed.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chun SY, Reese TG, Ouyang J, Guerin B, Catana C, Zhu X, et al. MRI-based nonrigid motion correction in simultaneous PET/MRI. J Nucl Med 2012. doi: 10.2967/jnumed.111.092353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Polycarpou I, Tsoumpas C, King AP, Marsden PK. Impact of respiratory motion correction and spatial resolution on lesion detection in PET: a simulation study based on real MR dynamic data. Phys Med Biol 2014; 59: 697. doi: 10.1088/0031-9155/59/3/697 [DOI] [PubMed] [Google Scholar]
- 116.King AP, Buerger C, Tsoumpas C, Marsden PK, Schaeffter T. Thoracic respiratory motion estimation from MRI using a statistical model and a 2-D image navigator. Med Image Anal 2012; 16: 252–64. doi: 10.1016/j.media.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 117.Bonanno G, Puy G, Wiaux Y, van Heeswijk RB, Piccini D, Stuber M. Self-navigation with compressed sensing for 2D translational motion correction in free-breathing coronary MRI: a feasibility study. PLoS One 2014; 9: e105523. doi: 10.1371/journal.pone.0105523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nguyen TD, Spincemaille P, Prince MR, Wang Y. Cardiac fat navigator-gated steady-state free precession 3D magnetic resonance angiography of coronary arteries. Magn Reson Med 2006; 56: 210–15. doi: 10.1002/mrm.20938 [DOI] [PubMed] [Google Scholar]
- 119.Wuerslin C, Schmidt H, Schwenzer N, Martirosian P, Claussen C, Stegger L. MR-based correction of respiratory motion in oncological PET imaging using an integrated PET/MR system. J Nucl Med 2012; 53(Suppl. 1): 370. [Google Scholar]
- 120.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology 1989; 171: 841–5. doi: 10.1148/radiology.171.3.2717762 [DOI] [PubMed] [Google Scholar]
- 121.Fayad H, Schmidt H, Wuerslin C, Visvikis D. Reconstruction-incorporated respiratory motion correction in clinical simultaneous PET/MR imaging for oncology applications. J Nucl Med 2015; 56: 884–9. doi: 10.2967/jnumed.114.153007 [DOI] [PubMed] [Google Scholar]
- 122.Grimm R, Fürst S, Souvatzoglou M, Forman C, Hutter J, Dregely I, et al. Self-gated MRI motion modeling for respiratory motion compensation in integrated PET/MRI. Med Image Anal 2015; 19: 110–20. doi: 10.1016/j.media.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 123.Fürst S, Grimm R, Hong I, Souvatzoglou M, Casey ME, Schwaiger M, et al. Motion correction strategies for integrated PET/MR. J Nucl Med 2015; 56: 261–9. [DOI] [PubMed] [Google Scholar]
- 124.Bini J, Izquierdo-Garcia D, Mateo J, Machac J, Narula J, Fuster V, et al. Preclinical evaluation of MR attenuation correction versus CT attenuation correction on a sequential whole-body MR/PET scanner. Invest Radiol 2013; 48: 313–22. doi: 10.1097/RLI.0b013e31827a49ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martinez-Moller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd'hotel C, Ziegler SI, et al. Tissue classification as a potential approach for attenuation correction in whole-body PET/MRI: evaluation with PET/CT data. J Nucl Med 2009; 50: 520–6. doi: 10.2967/jnumed.108.054726 [DOI] [PubMed] [Google Scholar]
- 126.Catana C, van der Kouwe A, Benner T, Hamm M, Michel C, Fischl B, et al. , eds. Is accurate bone segmentation required for MR-based PET attenuation correction? International Society Magnetic Resonance Medicine; 2009. [Google Scholar]
- 127.Andersen FL, Ladefoged CN, Beyer T, Keller SH, Hansen AE, Højgaard L, et al. Combined PET/MR imaging in neurology: MR-based attenuation correction implies a strong spatial bias when ignoring bone. Neuroimage 2014; 84: 206–16. doi: 10.1016/j.neuroimage.2013.08.042 [DOI] [PubMed] [Google Scholar]
- 128.Burgos N, Cardoso MJ, Thielemans K, Modat M, Pedemonte S, Dickson J, et al. Attenuation correction synthesis for hybrid PET-MR scanners: application to brain studies. IEEE Trans Med Imaging 2014; 33: 2332–41. doi: 10.1109/TMI.2014.2340135 [DOI] [PubMed] [Google Scholar]
- 129.Hofmann M, Steinke F, Scheel V, Charpiat G, Farquhar J, Aschoff P, et al. MRI-based attenuation correction for PET/MRI: a novel approach combining pattern recognition and atlas registration. J Nucl Med 2008; 49: 1875–83. doi: 10.2967/jnumed.107.049353 [DOI] [PubMed] [Google Scholar]
- 130.Izquierdo-Garcia D, Hansen AE, Förster S, Benoit D, Schachoff S, Fürst S, et al. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J Nucl Med 2014; 55: 1825–30. doi: 10.2967/jnumed.113.136341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schulz V, Torres-Espallardo I, Renisch S, Hu Z, Ojha N, Börnert P, et al. Automatic, three-segment, MR-based attenuation correction for whole-body PET/MR data. Eur J Nucl Med Mol Imaging 2010; 38: 138–52. doi: 10.1007/s00259-010-1603-1 [DOI] [PubMed] [Google Scholar]
- 132.Keereman V, Fierens Y, Broux T, De Deene Y, Lonneux M, Vandenberghe S. MRI-based attenuation correction for PET/MRI using ultrashort echo time sequences. J Nucl Med 2010; 51: 812–18. doi: 10.2967/jnumed.109.065425 [DOI] [PubMed] [Google Scholar]
- 133.Navalpakkam BK, Braun H, Kuwert T, Quick HH. Magnetic resonance-based attenuation correction for PET/MR hybrid imaging using continuous valued attenuation maps. Invest Radiol 2013; 48: 323–32. doi: 10.1097/RLI.0b013e318283292f [DOI] [PubMed] [Google Scholar]
- 134.Santos Ribeiro A, Rota Kops E, Herzog H, Almeida P. Hybrid approach for attenuation correction in PET/MR scanners. Nucl Instr Methods Phys Res 2014; 734: 166–70. [Google Scholar]
- 135.Berker Y, Franke J, Salomon A, Palmowski M, Donker HCW, Temur Y, et al. MRI-based attenuation correction for hybrid PET/MRI systems: a 4-class tissue segmentation technique using a combined ultrashort-echo-time/Dixon MRI sequence. J Nucl Med 2012. [DOI] [PubMed] [Google Scholar]
- 136.Delso G, Wiesinger F, Sacolick L, Kaushik S, Shanbhag D, Hüllner M, et al. Clinical evaluation of zero echo time MRI for the segmentation of the skull. J Nucl Med 2015. [DOI] [PubMed] [Google Scholar]
- 137.Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med 2012; 67: 510–18. doi: 10.1002/mrm.23017 [DOI] [PubMed] [Google Scholar]
- 138.Nuyts J, Bal G, Kehren F, Fenchel M, Michel C, Watson C. Completion of a truncated attenuation image from the attenuated PET emission data. IEEE Trans Med Imaging 2013; 32: 237–46. doi: 10.1109/TMI.2012.2220376 [DOI] [PubMed] [Google Scholar]
- 139.Rezaei A, Defrise M, Nuyts J. ML-reconstruction for TOF-PET with simultaneous estimation of the attenuation factors. IEEE Trans Med Imaging 2014; 33: 1563–72. doi: 10.1109/TMI.2014.2318175 [DOI] [PubMed] [Google Scholar]
- 140.Cabello J, Lukas M, Forster S, Pyka T, Nekolla SG, Ziegler SI. MR-based attenuation correction using ultrashort-echo-time pulse sequences in dementia patients. J Nucl Med 2015; 56: 423–9. doi: 10.2967/jnumed.114.146308 [DOI] [PubMed] [Google Scholar]
- 141.Cabello J, Lukas M, Rota Kops E, Ribeiro A, Shah NJ, Yakushev I, et al. Comparison between MRI-based attenuation correction methods for brain PET in dementia patients. Eur J Nucl Med Mol Imaging 2016: 1–11. [DOI] [PubMed] [Google Scholar]
- 142.Sekine T, Buck A, Delso G, ter Voert E, Huellner M, Veit-Haibach P, et al. Evaluation of atlas-based attenuation correction for integrated PET/MR in human brain—application of a head atlas and comparison to true CT-based attenuation correction. J Nucl Med 2015. [DOI] [PubMed] [Google Scholar]
- 143.Marshall HR, Prato FS, Deans L, Théberge J, Thompson RT, Stodilka RZ. Variable lung density consideration in attenuation correction of whole-body PET/MRI. J Nucl Med 2012. doi: 10.2967/jnumed.111.098350 [DOI] [PubMed] [Google Scholar]
- 144.Mehranian A, Zaidi H. Emission-based estimation of lung attenuation coefficients for attenuation correction in time-of-flight PET/MR. Phys Med Biol 2015; 60: 4813–33. doi: 10.1088/0031-9155/60/12/4813 [DOI] [PubMed] [Google Scholar]
- 145.Rauscher I, Eiber M, Furst S, Souvatzoglou M, Nekolla SG, Ziegler SI, et al. PET/MR imaging in the detection and characterization of pulmonary lesions: technical and diagnostic evaluation in comparison to PET/CT. J Nucl Med 2014; 55: 724–9. doi: 10.2967/jnumed.113.129247 [DOI] [PubMed] [Google Scholar]
- 146.Lau J, Laforest R, Sharma S, Priatna A, McConathy J, Amado L, et al. Feasibility of MRI attenuation correction in cardiac-gated FDG-PET. J Nucl Med 2013; 54(Suppl. 2): 27.23236018 [Google Scholar]
- 147.Fieseler M, Gigengack F, Jiang X, Schäfers KP. Motion correction of whole-body PET data with a joint PET-MRI registration functional. Biomed Eng 2014; 13: 1–9. doi: 10.1186/1475-925X-13-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Delso G, Martinez-Moller A, Bundschuh RA, Nekolla SG, Ziegler SI. The effect of limited MR field of view in MR/PET attenuation correction. Med Phys 2010; 37: 2804–12. doi: 10.1118/1.3431576 [DOI] [PubMed] [Google Scholar]
- 149.Blumhagen JO, Ladebeck R, Fenchel M, Scheffler K. MR-based field-of-view extension in MR/PET: B0 homogenization using gradient enhancement (HUGE). Magn Reson Med 2013; 70: 1047–57. doi: 10.1002/mrm.24555 [DOI] [PubMed] [Google Scholar]
- 150.Qi J, Huesman RH. Penalized maximum-likelihood image reconstruction for lesion detection. Phys Med Biol 2006; 51: 4017. doi: 10.1088/0031-9155/51/16/009 [DOI] [PubMed] [Google Scholar]
- 151.Reader AJ, Zaidi H. Advances in PET image reconstruction. PET Clin 2007; 2: 173–90. doi: 10.1016/j.cpet.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 152.Shepp LA, Vardi Y. Maximum likelihood reconstruction for emission tomography. IEEE Trans Med Imaging 1982; 1: 113–22. doi: 10.1109/TMI.1982.4307558 [DOI] [PubMed] [Google Scholar]
- 153.Fessler JA. Penalized weighted least-squares image reconstruction for positron emission tomography. IEEE Trans Med Imaging 1994; 13: 290–300. doi: 10.1109/42.293921 [DOI] [PubMed] [Google Scholar]
- 154.Daube-Witherspoon ME, Matej S, Karp JS, Lewitt RM. Application of the row action maximum likelihood algorithm with spherical basis functions to clinical PET imaging. IEEE Trans Nucl Sci 2001; 48: 24–30. doi: 10.1109/23.910827 [DOI] [Google Scholar]
- 155.Cabello J, Rafecas M. Comparison of basis functions for 3D PET reconstruction using a Monte Carlo system matrix. Phys Med Biol 2012; 57: 1759–77. doi: 10.1088/0031-9155/57/7/1759 [DOI] [PubMed] [Google Scholar]
- 156.Brankov JG, Yongyi Y, Wernick MN. Tomographic image reconstruction based on a content-adaptive mesh model. IEEE Trans Med Imaging 2004; 23: 202–12. doi: 10.1109/TMI.2003.822822 [DOI] [PubMed] [Google Scholar]
- 157.Bai B, Li Q, Leahy RM. Magnetic resonance-guided positron emission tomography image reconstruction. Semin Nucl Med 2013; 43: 30–44. doi: 10.1053/j.semnuclmed.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hutton BF, Thomas BA, Erlandsson K, Bousse A, Reilhac-Laborde A, Kazantsev D, et al. What approach to brain partial volume correction is best for PET/MRI? Nucl Instr Methods Phys Res 2013; 702: 29–33. doi: 10.1016/j.nima.2012.07.059 [DOI] [Google Scholar]
- 159.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. The J Nucl Med 1998; 39: 904. [PubMed] [Google Scholar]
- 160.Yang J, Huang SC, Lin KP, Small G, Phelps ME, eds. MRI-based elastic-mapping method for inter-subject comparison of brain FDG-PET images. Nuclear Science Symposium, 1996 Conference Record, IEEE; 1996. pp. 2–9. [Google Scholar]
- 161.Erlandsson K, Thomas B, Dickson J, Hutton BF. Partial volume correction in SPECT reconstruction with OSEM. Nucl Instr Methods Phys Res 2011; 648(Suppl. 1): S85–8. [Google Scholar]
- 162.Meltzer CC, Leal JP, Mayberg HS, Wagner HN, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr 1990; 14: 561–70. doi: 10.1097/00004728-199007000-00011 [DOI] [PubMed] [Google Scholar]
- 163.Kirov AS, Piao JZ, Schmidtlein CR. Partial volume effect correction in PET using regularized iterative deconvolution with variance control based on local topology. Phys Med Biol 2008; 53: 2577–91. doi: 10.1088/0031-9155/53/10/009 [DOI] [PubMed] [Google Scholar]
- 164.Boussion N, Cheze Le Rest C, Hatt M, Visvikis D. Incorporation of wavelet-based denoising in iterative deconvolution for partial volume correction in whole-body PET imaging. Eur J Nucl Med Mol Imaging 2009; 36: 1064–75. doi: 10.1007/s00259-009-1065-5 [DOI] [PubMed] [Google Scholar]
- 165.Nuyts J, Baete K, Beque D, Dupont P. Comparison between MAP and postprocessed ML for image reconstruction in emission tomography when anatomical knowledge is available. IEEE Trans Med Imaging 2005; 24: 667–75. doi: 10.1109/TMI.2005.846850 [DOI] [PubMed] [Google Scholar]
- 166.Green PJ. Bayesian reconstructions from emission tomography data using a modified EM algorithm. IEEE Trans Med Imaging 1990; 9: 84–93. doi: 10.1109/42.52985 [DOI] [PubMed] [Google Scholar]
- 167.Pierro AR. A modified expectation maximization algorithm for penalized likelihood estimation in emission tomography. IEEE Trans Med Imaging 1995; 14: 132–7. [DOI] [PubMed] [Google Scholar]
- 168.Lange K, Hunter DR, Yang I. Optimization transfer using surrogate objective functions. J Comput Graphical Stat 2000; 9: 1–20. [Google Scholar]
- 169.Baete K, Nuyts J, Van Laere K, Van Paesschen W, Ceyssens S, De Ceuninck L, et al. Evaluation of anatomy based reconstruction for partial volume correction in brain FDG-PET. Neuroimage 2004; 23: 305–17. doi: 10.1016/j.neuroimage.2004.04.041 [DOI] [PubMed] [Google Scholar]
- 170.Bowsher JE, Hong Y, Hedlund LW, Turkington TG, Akabani G, Badea A, et al. , eds. Utilizing MRI information to estimate F18-FDG distributions in rat flank tumors. Nuclear Science Symposium Conference Record, 2004 IEEE; 2004. pp. 16–22. [Google Scholar]
- 171.Loeb R, Navab N, Ziegler SI. Direct parametric reconstruction using anatomical regularization for simultaneous PET/MRI Data. IEEE Trans Med Imaging 2015; 34: 2233–47. [DOI] [PubMed] [Google Scholar]
- 172.Daniil K, Sébastien O, Brian FH, Katherine JD, Anders PK, William RBL, et al. A novel technique to incorporate structural prior information into multi-modal tomographic reconstruction. Inverse Probl 2014; 30: 065004. [Google Scholar]
- 173.Vunckx K, Dupont P, Goffin K, Van Paesschen W, Van Laere K, Nuyts J. Voxel-based comparison of state-of-the-art reconstruction algorithms for 18F-FDG PET brain imaging using simulated and clinical data. Neuroimage 2014; 102(Pt 2): 875–84. doi: 10.1016/j.neuroimage.2014.06.068 [DOI] [PubMed] [Google Scholar]
- 174.Tang J, Rahmim A. Anatomy assisted PET image reconstruction incorporating multi-resolution joint entropy. Phys Med Biol 2015; 60: 31–48. doi: 10.1088/0031-9155/60/1/31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Somayajula S, Panagiotou C, Rangarajan A, Li Q, Arridge SR, Leahy RM. PET image reconstruction using information theoretic anatomical priors. IEEE Trans Med Imaging 2011; 30: 537–49. doi: 10.1109/TMI.2010.2076827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Battle XL, Cunningham GS, Hanson KM. Tomographic reconstruction using 3D deformable models. Phys Med Biol 1998; 43: 983–90. doi: 10.1088/0031-9155/43/4/025 [DOI] [PubMed] [Google Scholar]
- 177.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage 1996; 4(3 Pt 1): 153–8. doi: 10.1006/nimg.1996.0066 [DOI] [PubMed] [Google Scholar]
- 178.Burger C, Buck A. Tracer kinetic modelling of receptor data with mathematical metabolite correction. Eur J Nucl Med 1996; 23: 539–45. [DOI] [PubMed] [Google Scholar]
- 179.Poulin E, Lebel R, Croteau E, Blanchette M, Tremblay L, Lecomte R, et al. Conversion of arterial input functions for dual pharmacokinetic modeling using Gd-DTPA/MRI and 18F-FDG/PET. Magn Reson Med 2013; 69: 781–92. doi: 10.1002/mrm.24318 [DOI] [PubMed] [Google Scholar]
- 180.Muller-Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 1992; 12: 571–83. doi: 10.1038/jcbfm.1992.81 [DOI] [PubMed] [Google Scholar]
- 181.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time—activity measurements applied to [N-11C-Methyl]-(−)-Cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990; 10: 740–7. doi: 10.1038/jcbfm.1990.127 [DOI] [PubMed] [Google Scholar]
- 182.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 1985; 5: 584–90. doi: 10.1038/jcbfm.1985.87 [DOI] [PubMed] [Google Scholar]
- 183.Yan J, Planeta-Wilson B, Carson RE. Direct 4-D PET list mode parametric reconstruction with a novel EM algorithm. IEEE Trans Med Imaging 2012; 31: 2213–23. doi: 10.1109/TMI.2012.2212451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rahmim A, Tang J, Zaidi H. Four-dimensional (4D) image reconstruction strategies in dynamic PET: beyond conventional independent frame reconstruction. Med Phys 2009; 36: 3654–70. doi: 10.1118/1.3160108 [DOI] [PubMed] [Google Scholar]
- 185.Reader AJ, Sureau FC, Comtat C, Trebossen R, Buvat I. Joint estimation of dynamic PET images and temporal basis functions using fully 4D ML-EM. Phys Med Biol 2006; 51: 5455–74. doi: 10.1088/0031-9155/51/21/005 [DOI] [PubMed] [Google Scholar]
- 186.Kamasak ME, Bouman CA, Morris ED, Sauer K. Direct reconstruction of kinetic parameter images from dynamic PET data. IEEE Trans Med Imaging 2005; 24: 636–50. doi: 10.1109/TMI.2005.845317 [DOI] [PubMed] [Google Scholar]
- 187.Tsoumpas C, Turkheimer FE, Thielemans K. A survey of approaches for direct parametric image reconstruction in emission tomography. Med Phys 2008; 35: 3963–71. doi: 10.1118/1.2966349 [DOI] [PubMed] [Google Scholar]
- 188.Reader AJ, Verhaeghe J. 4D image reconstruction for emission tomography. Phys Med Biol 2014; 59: R371–418. doi: 10.1088/0031-9155/59/22/R371 [DOI] [PubMed] [Google Scholar]
- 189.Verhaeghe J, Ville DV, Khalidov I, Asseler YD, Lemahieu I, Unser M. Dynamic PET reconstruction using wavelet regularization with adapted basis functions. IEEE Trans Med Imaging 2008; 27: 943–59. doi: 10.1109/TMI.2008.923698 [DOI] [PubMed] [Google Scholar]
- 190.Nichols TE, Jinyi Q, Asma E, Leahy RM. Spatiotemporal reconstruction of list-mode PET data. IEEE Trans Med Imaging 2002; 21: 396–404. doi: 10.1109/TMI.2002.1000263 [DOI] [PubMed] [Google Scholar]
- 191.Tsoumpas C, Turkheimer FE, Thielemans K. Study of direct and indirect parametric estimation methods of linear models in dynamic positron emission tomography. Med Phys 2008; 35: 1299–309. doi: 10.1118/1.2885369 [DOI] [PubMed] [Google Scholar]
- 192.Meikle SR, Matthews JC, Cunningham VJ, Bailey DL, Livieratos L, Jones T, et al. Parametric image reconstruction using spectral analysis of PET projection data. Phys Med Biol 1998; 43: 651–66. doi: 10.1088/0031-9155/43/3/016 [DOI] [PubMed] [Google Scholar]
- 193.Tang J, Kuwabara H, Wong DF, Rahmim A. Direct 4D reconstruction of parametric images incorporating anato-functional joint entropy. Phys Med Biol 2010; 55: 4261. doi: 10.1088/0031-9155/55/15/005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang G, Qi J. Direct estimation of kinetic parametric images for dynamic PET. Theranostics 2013; 3: 802–15. doi: 10.7150/thno.5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hollingsworth KG. Reducing acquisition time in clinical MRI by data undersampling and compressed sensing reconstruction. Phys Med Biol 2015; 60: R297–322. doi: 10.1088/0031-9155/60/21/R297 [DOI] [PubMed] [Google Scholar]
- 196.Ehrhardt MJ, Thielemans K, Pizarro L, Atkinson D, Ourselin S, Hutton BF, et al. Joint reconstruction of PET-MRI by exploiting structural similarity. Inverse Probl 2015; 31: 015001. [Google Scholar]
- 197.Knoll F, Holler M, Koesters T, Otazo R, Bredies K, Sodickson D. Joint MR-PET reconstruction using a multi-channel image regularizer. IEEE Trans Med Imaging 2016: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Knoll F, Koesters T, Otazo R, Block T, Feng L, Vunckx K, et al. Joint reconstruction of simultaneously acquired MR-PET data with multi sensor compressed sensing based on a joint sparsity constraint. EJNMMI Physics. 2014;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nensa F, Poeppel TD, Beiderwellen K, Schelhorn J, Mahabadi AA, Erbel R, et al. Hybrid PET/MR imaging of the heart: feasibility and initial results. Radiology 2013; 268: 366–73. doi: 10.1148/radiol.13130231 [DOI] [PubMed] [Google Scholar]
- 200.Bailey DL, Pichler BJ, Gückel B, Barthel H, Beer AJ, Bremerich J, et al. Combined PET/MRI: multi-modality multi-parametric imaging is here. Mol Imaging Biol 2015; 17: 595–608. doi: 10.1007/s11307-015-0886-9 [DOI] [PubMed] [Google Scholar]