Abstract
Objective:
Mepitel Film significantly decreases acute radiation-induced skin reactions in breast cancer patients. Here we investigated the feasibility of using Mepitel Film in head and neck cancer patients (ACTRN12614000932662).
Methods:
Out of a total of 36 head and neck cancer patients from New Zealand (NZ) (n = 24) and China (n = 12) recruited between June 2015 and December 2016, 33 patients complied with protocol. Of these, 11 NZ patients followed a management protocol; 11 NZ patients and 11 Chinese patients followed a prophylactic protocol. An area of the neck receiving a homogenous radiation dose of > 35 Gy was divided into two equal halves; one half was randomized to Film and the other to either Sorbolene cream (NZ) or Biafine cream (China). Skin reaction severity was measured by Radiation Induced Skin Reaction Assessment Scale and expanded Radiation Therapy Oncology Group toxicity criteria. Skin dose was measured by thermoluminescent dosimeters or gafchromic film.
Results:
Film decreased overall skin reaction severity (combined Radiation Induced Skin Reaction Assessment Scale score) by 29% and moist desquamation rates by 37% in the Chinese cohort and by 27 and 28%, respectively in the NZ cohort. Mepitel Film did not affect head movements but did not adhere well to the skin, particularly in males with heavy beard stubble, and caused itchiness, particularly in Chinese patients.
Conclusion:
Mepitel Film reduced acute radiation-induced skin reactions in our head and neck cancer patients, particularly in patients without heavy stubble.
Advances in knowledge:
This is the first study to confirm the feasibility of using Mepitel Film in head and neck cancer patients.
Introduction
Acute radiation-induced skin reactions remain an issue for many breast cancer patients and head and neck cancer patients, their oncologists, oncology nurses and radiation therapists. With tumours relatively close to the surface, the skin of these patients receives a radiation dose high enough to cause symptoms of acute radiation dermatitis (erythema, dry desquamation, moist desquamation and, rarely, necrosis). Although several clinical studies have reported some success in decreasing skin reaction severity using a variety of topical interventions, systematic reviews and meta-analysis of data from clinical trials1–9 have shown a lack of evidence to support the use of any particular agent. The majority of these clinical studies have been focussed on breast cancer patients. However, a very recent meta-analysis by Ferreira and colleagues10 on pharmacological topical interventions for acute radiation-induced skin reactions in head and neck cancer patients also could not support any intervention studied in the clinical setting to date.
Our group has previously shown that soft silicone dressings decrease the severity of acute radiation-induced skin reactions in breast cancer patients.11–13 Soft silicone dressings are hypothesized to provide mechanical protection from friction, allowing the skin to repair the daily damage caused by radiation therapy. Few other topically applied products also protect the radiation-damaged skin from friction damage.14 When used in a management setting Mepilex Lite dressings decreased overall skin reactions by 30–40% with no statistically significant decrease in moist desquamation incidence.11,12 When used prophylactically, Mepitel Film prevented moist desquamation in 78 breast cancer patients (34 of whom had had a mastectomy) with an overall decrease in skin reaction severity of more than 90% (using the Radiation-Induced Skin Reaction Assessment Scale (RISRAS).13 Applying dressings to the head and neck area is more challenging than to the breast/chest area; patients need to be able to move their head in all directions and even although Mepitel Film is transparent, it would still be very visible, potentially affecting patient compliance. In addition, head and neck cancer patients receive a higher radiation dose than breast cancer patients, often concomitant with chemotherapy and are further challenged with significant oral mucositis, nutritional insufficiency and weight loss. It may well be that soft silicone dressings do not adhere well enough to provide protection, affect neck mobility and/or are not acceptable to these patients. We therefore explored the feasibility of using Mepitel Film in different head and neck patient cohorts in New Zealand (NZ) and China. We were particularly interested in these two cohorts because of the increasingly common influence of the human papillomavirus on the aetiology of head and neck cancer15 and its increasing prevalence in NZ16 and of the Epstein–Barr virus as the causative agent in nasopharyngeal cancer in China.17,18
We employed an intra-patient randomized controlled trial methodology with both prophylactic and management protocols; we measured skin reaction severity using RISRAS and modified Radiation Therapy Oncology Group (RTOG) and skin dose using thermoluminescent dosimeters (TLDs) and Gafchromic film. We used a moisturizing cream without sodium lauryl sulphate (SLS) as the control cream (Sorbolene in the NZ cohort and Biafine in the Chinese cohort) as SLS has previously been shown to affect skin barrier function.19
Methods and materials
This randomized, intrapatient controlled open label stage II clinical trial was approved by the University of Otago Ethics Committee for the New Zealand Cohort (H14/111), and the Drum Tower Hospital Ethics Committee (2016-019-12) for the Chinese cohort and is registered with the Australia New Zealand Clinical Trials Registry (ACTRN12614000932662). All participants gave written informed consent before the start of radiation therapy treatment. As this was a feasibility study no power calculation was performed. We initially planned to recruit 30 patients in NZ and 15 patients from China in the time we had available to run the trial. However, recruitment was slower than anticipated and recruitment ceased after 24 patients were recruited from NZ and 12 patients from China.
Trial outcomes
We ascertained the feasibility of using Mepitel Film in head and neck cancer patients with respect to (1) how well the Film adhered to the skin, (2) its acceptability to patients and (3) its superiority over Sorbolene cream in decreasing skin reaction severity and the incidence of moist desquamation.
Participants
New Zealand cohort. All patients receiving radiation therapy for mucosal squamous cell carcinoma of the head and neck region in Christchurch Hospital and Dunedin Hospital were screened for recruitment between June 2015 and July 2016 and July 2015 and August 2015, respectively.
Chinese cohort. All patients receiving radiation therapy for nasopharyngeal carcinoma were screened for recruitment between April 2016 and December 2016.
Specific exclusion criteria for both cohorts were: previous radiation therapy to the head and neck region, metastatic disease, facial hair in the research area and a Karnofsky performance status score of less than 70. After completion of treatment, participants had to be able to return to the department for weekly follow-up assessments for up to 4 weeks.
Randomization
At the start of radiation treatment, the research area was divided into two equal halves for randomization to either Mepitel Film (from here on referred to as “Film”) or Sorbolene (NZ)/Biafine (China) Cream (from here on referred to as “Cream”). Randomization was based on pre-prepared computer-generated randomization charts and conducted via randomization fax by the Principal Investigator (PMH), who had no patient involvement.
NZ cohort. The research area was at least 5 × 10 cm on one side of the neck, which was expected to receive a relatively uniform dose of > 35 Gy (with variation of less than 5 Gy over the area), based on the treatment plan. The research area was divided either into superior/inferior halves or in medial/lateral halves, depending on the shape of the research area.
Chinese cohort. Because patients received the same dose to both sides of the neck, covering the bilateral neck node areas, Mepitel Film was randomized to either the left or right side of the neck. This allowed for a much larger area to be included in the study.
Blinding
Because the Film was in place for days at a time; neither the research radiation therapist nor the patients were blinded to which skin area had been randomized to Film and which to Cream.
Radiation therapy and chemotherapy
Planning technique, dose, the use of bolus and chemotherapy information is presented for NZ patients in the demographics and Chinese patients in Table 1. All patients were treated in the supine position on a flat board that attached to the treatment couch. Each patient also had a thermoplastic mask made that reached down to their shoulders.
Table 1.
Demographics information for the NZ and Chinese cohorts
| NZ prophylactic cohort | NZ management cohort | Chinese prophylactic cohort | |
|---|---|---|---|
| Total enrolled | 12 | 12 | 12 |
| Total completed/analysed | 11 | 11 | 11 |
| Sex M-F | 8–3 | 9–2 | 10–1 |
| Average age: y (range) | 60 (52–69) | 55 (40–83) | 50 (37–66) |
| Ethnicity | |||
| NZ European | 10 | 9 | 0 |
| NZ Maori | 1 | 2 | 0 |
| Asian | 0 | 0 | 11 |
| Cancer type | |||
| SCC oral cavity | 10 | 11 | 0 |
| SCC naso-pharynx | 0 | 0 | 11 |
| SCC unknown primary | 1 | 0 | 0 |
| Disease stage | |||
| I | 0 | 2 | 0 |
| II | 2 | 1 | 2 |
| III | 1 | 2 | 7 |
| IV | 8 | 5 | 2 |
| Radiation therapy | |||
| Total dose to primary tumour | 60–66 Gy | 60–66 Gy | 74 Gy |
| Total dose to neck nodes | 50–54 Gy | 50–54 Gy | 50 Gy |
| IMRT | 3 | 6 | 11 |
| VMAT | 8 | 2 | 0 |
| 3DCRT | 0 | 2 | 0 |
| Chemo therapy | |||
| None | 1 | 4 | 0 |
| Concomitant | 10 | 7 | 11 |
| Fitzpatrick skin type | |||
| I | 0 | 0 | 0 |
| II | 0 | 1 | 0 |
| III | 7 | 7 | 10 |
| IV | 4 | 2 | 1 |
| V | 0 | 1 | 0 |
| VI | 0 | 0 | 0 |
| Viral infection | |||
| HPV positive | 9 | 4 | 0 |
| HPV negative | 1 | 1 | 0 |
| HPV status unknown | 1 | 6 | 0 |
| EBV positive | 0 | 0 | 11 |
| Smoker | |||
| Current | 1 | 1 | 2 |
| Previous | 4 | 5 | 1 |
| Never | 6 | 5 | 8 |
| Alcohol consumption per week | |||
| None | 3 | 1 | 6 |
| <1 | 2 | 2 | 1 |
| 1–3 | 4 | 4 | 0 |
| 3–10 | 1 | 2 | 3 |
| 10–20 | 1 | 1 | 1 |
| >20 | 0 | 1 | 0 |
| Sun exposure | |||
| Often | 7 | 7 | 3 |
| Rarely | 4 | 4 | 8 |
EBV, Epstein–Barr virus; HPV, human papillomavirus; SCC, squamous cell carcinoma.
NZ cohort. Radiation therapy was delivered using 3D-CRT, IMRT or VMAT with 6 MV photons with a total dose of 66 Gy in 30 fractions for definitive treatment (n = 20) and 60 Gy in 30 fractions for postoperative treatment (n = 4). Elective nodal regions were prescribed 50 Gy in 25 fractions (3D-CRT) or 54 Gy in 30 fractions (IMRT or VMAT). Chemotherapy was given concomitantly to stage III and IV patients (weekly IV cisplatin (40 mg m–2).
Chinese cohort. Patients received 74 Gy in 37 fractions to the primary tumour. The research area focussed on the electively treated neck node regions, which received 50 Gy in 25 fractions. Radiation was delivered using IMRT or tomotherapy with 6 MV photons. All patients received concurrent chemotherapy [weekly IV nedaplatin (25 mg m–2)].
Application of film and cream
Patients doubled as their own controls to eliminate confounding patient- and treatment-related factors. Patients on the prophylactic protocol started using Film and Cream from commencement of radiation therapy. Patients on the management protocol started using Film and Cream from the moment faint erythema was visible. Film was applied by the researcher on the skin area randomized to film whilst Cream was applied twice daily to the control area by the patients. Care was taken not to stretch the Film during application and not to overlap pieces of Film. Gentle digital pressure was used to ease the Film neatly into all skin folds. If small areas of Film curled up, these were carefully removed with scissors leaving the rest of the film in place. Film was replaced if it came off the skin overnight or if significant areas separated from the skin at the edges. Patients were asked about the effect of Film on the ease with which they could move their head in order to assess neck mobility. Because Film is vapour permeable but not moisture permeable it cannot be used to treat moist desquamation. Moist desquamation was treated according to standard departmental protocol. Mepitel Film was donated by Molnlycke Healthcare LTD (Gothenburg, Sweden). The control moisturizing creams did not contain SLS. The NZ cohort used Dermasoft® Sorbolene Cream (NZ), which contains deionized water, glycerin, cetearyl alcohol, mineral oil, Ceteth 20, Polysorbate 60, paraffin, benzyl alcohol, methyl paraben and propyl paraben. The Chinese cohort used Biafine from Johnson & Johnson (France) as a control cream, which contains contains purified water, liquid paraffin, ethylene glycol monostearate, stearic acid, propylene glycol, paraffin wax, squalane, avocado oil, trolamine/sodium alginate, triethanolamine, cetyl palmitate, methylparaben (sodium salt), sorbic acid (potassium salt), propylparaben (sodium salt) and fragrance.
Skin reaction severity
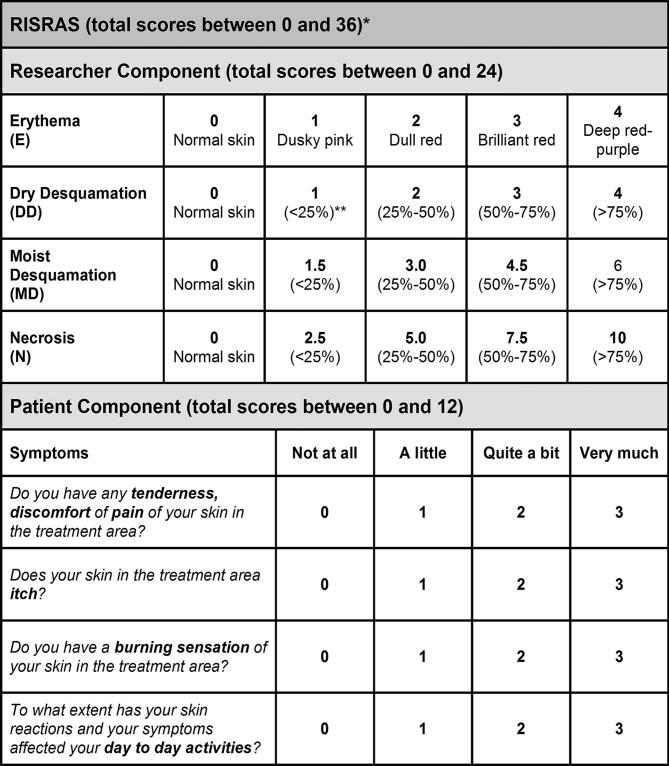
Both the modified RISRAS11,20–22 and the expanded RTOG scale23 were used to measure skin reactions severity. RISRAS has not been widely used in trials measuring radiation skin reactions but it has the advantage that it is much more sensitive than the RTOG and CTCAE grading systems. An exploratory study of its use found it to have reasonable reproducibility20 and in our previous study of Mepitel Film in 78 breast cancer patients, RTOG scores corresponded very well with the researcher component of RISRAS.13 We used the expanded RTOG scale in addition to RISRAS to allow comparison with other trials. RTOG scores were reported by the research radiation therapist as follows; Grade 0; no change; Grade IA: follicular faint or dull erythema; Grade IB: dry desquamation; Grade IIA: tender or bright erythema; Grade IIB: patchy moist desquamation; Grade III: confluent moist desquamation other than in skinfolds. For RISRAS (Figure 1), the radiation therapist/oncology nurse scored the visible extent of the skin reactions whilst the patient scored the level of pain, itchiness and burning as well as the effect on day-to-day life. Summation of these two scores gives the combined RISRAS score. Radiation therapists and oncology nurses responsible for measuring skin reaction severity had been trained to use RISRAS and RTOG. Scores were determined three times weekly from start to completion of radiation treatment, then once a week for 4 weeks after completion. RISRAS scores for each skin patch were added from the first assessment that recorded a score of 1 (for either Film or Cream) until the final assessment and divided by the number of assessments between these two time points, yielding an average RISRAS score for that area.
Figure 1.
RISRAS scale. The radiation therapist/oncology nurse scores the visible extent of the skin reactions whilst the patient scores the level of pain, itchiness and burning as well as the effect on day-to-day life. Summation of these two scores gives the combined RISRAS score. RISRAS, Radiation Induced Skin Reaction Assessment Scale.
Skin dose measurements
For the NZ cohort, a group of 3–5 TLDs were placed on the skin area to be covered in Film and the skin area to be covered in Cream of all patients to calculate the skin dose received to both skin patches. Gafchromic film was used to determine the skin dose to the Film-covered and Cream-covered skin patches of the Chinese patients.
Exit questionnaire
On completion of the trial, patients were given an exit questionnaire to comment on different aspects of participating in the trial and using the Film. Responses were subjected to a content analysis by two of the authors (HW and PMH) to provide an account of the participants’ experiences with using the Film.
Statistical analysis
SPSS 15.0 (IBM, Chicago, IL) was used for all statistical analyses. Average RISRAS scores were determined for skin reaction severity and skin dose for each skin area for each patient. The Wilcoxon signed-rank test and Wilcoxon sum rank tests were used to determine the statistical significance in dose and skin reaction severity (RISRAS) between Film and Cream covered patches and between treatment groups (IMRT vs VMAT, RT vs ChemoRT and management vs prophylactic protocol) respectively. In all cases, p < 0.05 was considered statistically significant.
Results
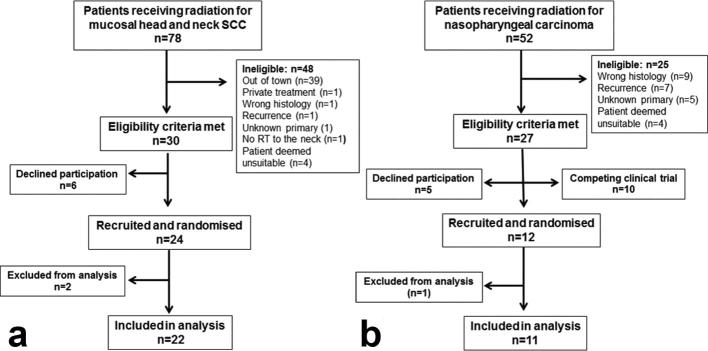
A total of 36 head and neck patients were recruited for this trial. Figure 2 shows the flow of patients through the trial for the NZ and Chinese cohorts. A total of 24 patients were enrolled in NZ but the data from two patients were excluded from analysis because they did not follow the protocol. Out of the remaining 22 patients, 20 patients were recruited from Christchurch Hospital. 11 of these patients followed the prophylactic protocol and nine patients followed the management protocol. Only two patients were recruited from Dunedin due to resource constraints, both patients were on the management protocol. Table 1 shows the distribution over the cohorts of demographic data. The NZ cohort consisted of 17 males and 5 females. The number of patients was evenly distributed between management and prophylactic protocols with respect to age, ethnicity, cancer type, Fitzpatrick skin type and sun exposure. Alcohol consumption was low in both cohorts and only one patient in each cohort was a current smoker. More patients on the management protocol were treated with VMAT, received concurrent cisplatin chemotherapy and were known to be HPV positive. A total of 12 patients were enrolled in China but one of these patients did not follow the protocol. The 11 remaining Chinese patients, one female and ten males, all followed the prophylactic protocol. The Chinese cohort was slightly younger than the NZ cohort; all were EBV positive, had nasopharyngeal cancer and all but one patient had Fitzpatrick skin Type III. Radiation therapy was delivered via Tomotherapy for nine patients and IMRT for the two remaining patients. All patients received concomitant chemotherapy with nedaplatin. Most Chinese patients had never smoked, didn’t consume much alcohol and rarely exposed their skin to sunlight.
Figure 2.
Consort diagrams. Flow of patients through the trial for the NZ cohort (a) and the Chinese cohort (b). Patients who were randomized but excluded from analysis did not follow protocol.
Comparison of skin doses
Although our intra-patient controlled design minimized the potentially confounding patient-related and treatment-related factors, we wanted to ensure that the total dose received by Film-covered skin patches was similar to that received by Cream-covered skin patches. Extrapolating skin dose from treatment plans is not accurate; therefore we measured the actual dose received by both Film-covered and Cream-covered skin patches of all patients using either TLDs (NZ) or Gafchromic film (China). The average dose (±SEM) received by NZ patients was 49.8 ± 1.2 Gy for Film-covered patches and 49.2 ± 1.4 Gy for Cream-covered patches (p = 0.760, Wilcoxon signed-rank test). Chinese patients received 42.9 ± 3.2 and 43.0 ± 3.2 Gy to their Film-covered and Cream-covered skin patches, respectively (p = 0.965, Wilcoxon signed-rank test). There was no statistically significant difference between patches covered in Film or Cream in either cohort, making it unlikely that the differences in skin severity between Film and Cream were due to differences in skin dose received. The skin dose received by NZ patients was significantly higher than that received by Chinese patients (p < 0.001, Wilcoxon rank sum test).
Comparison of skin reaction severity
Based on our very successful prophylactic breast cancer trial,13 we initially enrolled all of our patients on the prophylactic protocol where Film was applied from day one of radiation treatment. However, the Film did not adhere well enough to the skin of males whose beard stubble would push the film away from the skin during the night. We therefore put the remaining patients on the management protocol. Because the protective effect of the Film on the skin was very similar for both protocols, we combined the results for both protocols for comparison with the Chinese cohort. The Chinese patients all followed the prophylactic protocol. Chinese males do not have heavy beard growth in the neck area and adhesion of film to the skin was not a problem in this cohort.
Skin reaction severity was scored according to the extended RTOG grading system and the results are shown in Table 2. In both the NZ and Chinese cohorts, Film performed better than Cream, with 73% of NZ patients and 64% of Chinese patients developing moist desquamation (Grades IIB and III) in their Cream-covered skin patches but only 45% of NZ and 27% of Chinese patients developing moist desquamation in their Film-covered skin patches. Using film therefore resulted in a 28% and 37% decrease in moist desquamation rates in the NZ and Chinese cohorts respectively.
Table 2.
Skin reaction severity as measured by the extended RTOG grading scale
| RTOG grades | Moist desquamation | |||||||
|---|---|---|---|---|---|---|---|---|
| # Patients | 0 | IA | IB | IIA | IIB | III | ||
| New Zealand n = 22 | Film | 0 | 1 (5%) | 1 (5%) | 10 (45%) | 8 (36%) | 2 (9%) | 10 (45%) |
| Cream | 0 | 0 | 1 (5%) | 5 (23%) | 15 (68%) | 1 (5%) | 16 (73%) | |
| 28% decrease | ||||||||
| China n = 11 | Film | 0 | 0 | 4 (36%) | 4 (36%) | 2 (18%) | 1 (9%) | 3 (27%) |
| Cream | 0 | 0 | 1 (9%) | 3 (27%) | 6 (55%) | 1 (9%) | 7 (64%) | |
| 37% decrease | ||||||||
RTOG, RadiationTherapy Oncology Group.
Skin reaction severity was also measured by the more sensitive RISRAS, which also contains a patient-focused component. Figure 3a,b show the mean RISRAS scores for combined, researcher and patient components of the NZ and Chinese cohorts respectively. Figure 3c shows that the difference in skin reaction severity between Film and Cream skin patches was statistically significant for combined, researcher and patient components of RISRAS for the NZ cohort (p-values of <0.001, 0.001 and <0.001 respectively). However, in the Chinese cohort only the differences in reaction severity of the combined and researcher components of RISRAS were statistically significant (p = 0.003 for both), whilst the patient component of the RISRAS showed no statistically significant difference in skin reaction severity between Film and Cream covered skin patches (p = 0.185). Interestingly, although the combined scores for NZ and Chinese cohorts were similar (see also Table 3), the contribution of the researcher component was much larger in the Chinese cohort and the patient component was much larger in the NZ cohort. Five of the 11 Chinese patients found the Film very itchy and the remaining 6 patients found the Film a little bit itchy. This was less of an issue for the 22 NZ patients, three of whom found the Film quite itchy and six of whom found the film a little bit itchy. Even although the NZ patients received a higher skin dose, the severity of skin reactions in the Cream-covered skin patches was similar to that of the Chinese cohort.
Figure 3.
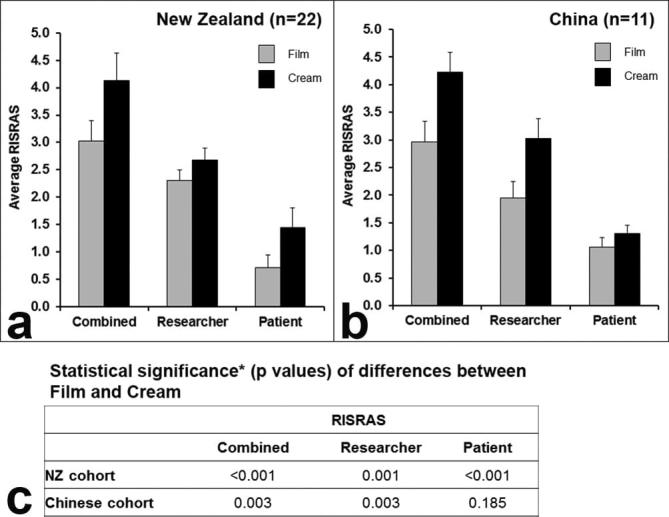
Skin reaction severity as measured by RISRAS in the NZ cohort (a) and the Chinese cohort (b). Grey bars represent skin patches covered in Film and black bars represent skin patched covered in Cream. Skin reaction severity as measured by the mean of the average RISRAS scores for each patient (error bars: SEM) for the combined, researcher and patient RISRAS components. (c) Statistical analysis of RISRAS results using Wilcoxon signed-rank test. RISRAS, Radiation Induced Skin Reaction Assessment Scale.
Table 3.
The improvement of skin reaction severity by Film over Cream in different cohorts and subcohorts
| RISRAS scoresa | ||||||
|---|---|---|---|---|---|---|
| Film | Cream | |||||
| Combined | Researcher | Patient | Combined | Researcher | Patient | |
| China prophylactic (n = 11) % improvement p valueb |
2.97 ± 0.37 29% <0.003 |
1.95 ± 0.30 35% <0.003 |
1.06 ± 0.16 15% 0.185 |
4.23 ± 0.35 | 3.02 ± 0.36 | 1.30 ± 0.15 |
| NZ both protocols (n = 22) % improvement p value |
3.03 ± 0.26 27% <0.001 |
2.31 ± 0.14 14% 0.001 |
0.71 ± 0.17 51% <0.001 |
4.13 ± 0.36 | 2.68 ± 0.16 | 1.44 ± 0.26 |
| NZ management (n = 11) % improvement p value |
3.23 ± 0.41 25% 0.005 |
2.41 ± 0.25 15% 0.019 |
0.81 ± 0.21 46% 0.005 |
4.32 ± 0.48 | 2.81 ± 0.28 | 1.50 ± 0.26 |
| NZ prophylactic (n = 11) % improvement p value |
2.62 ± 0.35 29% 0.008 |
2.11 ± 0.11 12% 0.022 |
0.51 ± 0.27 60% 0.008 |
3.68 ± 0.56 | 2.41 ± 0.15 | 1.26 ± 0.48 |
| NZ RT only (n = 8) % improvement p value |
2.62 ± 0.12 30% 0.012 |
2.15 ± 0.27 14% 0.018 |
0.45 ± 0.14 63% 0.012 |
3.73 ± 0.46 | 2.50 ± 0.24 | 1.22 ± 0.25 |
| NZ chemoRT (n = 14) % improvement p value |
3.10 ± 0.43 25% 0.004 |
2.15 ± 0.21 13% 0.028 |
1.09 ± 0.29 47% 0.003 |
4.15 ± 0.59 | 2.67 ± 0.25 | 1.47 ± 0.45 |
| NZ IMRT (n = 11) % improvement p value |
3.53 ± 0.47 30% 0.003 |
2.38 ± 0.26 17% 0.003 |
1.16 ± 0.27 51% 0.003 |
5.05 ± 0.57 | 2.86 ± 0.29 | 2.18 ± 0.40 |
| NZ VMAT (n = 11) % improvement p value |
2.62 ± 0.35 25% 0.008 |
2.11 ± 0.11 11% 0.022 |
0.51 ± 0.27 58% 0.008 |
3.86 ± 0.56 | 2.41 ± 0.56 | 1.26 ± 0.48 |
| NZ females (n = 5) % improvement p value |
2.37 ± 0.33 44% 0.043 |
1.78 ± 0.17 24% 0.043 |
0.55 ± 0.18 71% 0.043 |
4.25 ± 0.59 | 2.34 ± 0.13 | 1.91 ± 0.58 |
| NZ males (n = 17) % improvement p value |
3.09 ± 0.33 21% 0.001 |
2.40 ± 0.16 11% 0.012 |
0.69 ± 0.21 44% 0.001 |
3.93 ± 0.44 | 2.69 ± 0.20 | 1.22 ± 0.29 |
Values are averages ± SEM.
Wilcoxon signed-rank test for differences in skin reaction severity between Film and Cream.
Because we set out to determine the feasibility of using Film in different situations, we performed an exploratory subgroup analysis on the effect of Film in different NZ subcohorts (Table 3). The percentage improvement listed in Table 3 for the different cohorts refers to the average decrease in skin reaction severity (improvement) for skin covered by Film compared with skin covered in Cream. Although numbers within cohorts and subcohorts were low, Film performed significantly better than Cream in a number of scenarios with the improvement in combined RISRAS scores ranging from 21–44%. The benefit of Film was greatest in the patient component (range 15–71%) and lowest in the researcher component (range 11–35%) of RISRAS. NZ females seemed to benefit most from having Film applied to their skin, with an improvement of 44, 24 and 71% in the combined, researcher and patient components of RISRAS respectively. There was little difference between the effects of Film on skin severity between management and prophylactic protocols, delivery using IMRT and VMAT, RT and ChemoRT regimen. Although skin reaction severity was worse for the management protocol over the prophylactic protocol (18% higher for combined Cream RISRAS), ChemoRT over RT (11% higher for combined Cream RISRAS) and IMRT over VMAT (37% higher for combined Cream RISRAS), these differences were not statistically significant (p = 0.243, 0.87 and 0.133 respectively).
Acceptability of film to patients
NZ patients rated the Film higher than the Chinese patients as evidenced by the patient RISRAS scores in the NZ cohort. Almost half of the Chinese patients found the Film very itchy. None of the 22 NZ patients rated the Film as very itchy in the patient component of the RISRAS. A content analysis of the exit questionnaires corroborated these findings. The Chinese patients all reported that taking part in this trial was a positive experience and that they would do it again if a suitable trial became available. Interestingly, all 11 patients preferred Film over Cream, seven patients specifically said Film improved their symptoms. With respect to disadvantages of using film, five Chinese patients mentioned the Film came off too easily, particularly in the shower and the bath, three patients found the Film uncomfortable and one patient found the Film a bit tight on the skin.
A total of 16 NZ patients returned the exit questionnaire (73%) and 15 of these patients found that being on the trial was a positive experience. Film was preferred by 13 patients and one patient found that Cream seemed to be better for healing. Positive aspects of the Film were that it decreased pain/burning/stinging sensations (n = 5), was more comfortable on the skin (n = 3) and was easy (e.g.it didn’t require the patient rubbing in Cream) (n = 5). The main disadvantage of the Film was poor adherence (n = 6). One patient said Film adhered better towards the end and one person reported that at the start Film helped with the burning sensation but towards the end it was too itchy. Itchiness was not mentioned by anyone else in the NZ cohort.
Discussion
After the success of Mepitel Film in breast cancer patients,13 we set out to determine how feasible it was to use Film in head and neck cancer patients. To our knowledge this is the first trial to use Mepitel Film for acute radiation-induced skin reactions in this patient cohort. Another soft silicone dressing, Mepilex Lite, similar to Film but less strongly adherent and with a foam layer to absorb exudate, was shown to decrease healing time of moist desquamation in 88 patients with nasopharyngeal carcinoma, treated with 60–66 Gy to the neck area and concomitant cisplatin (40 mg m–2).24 The authors also mentioned that Mepilex Lite improved patient sleep quality and did not affect neck mobility; they measured healing time but not skin reaction severity or incidence of moist desquamation.
Before embarking on a large randomized controlled trial, we decided to first complete a feasibility study to see if the Film would adhere well enough to the neck area, which is subject to a lot of movement and friction by clothing as well as exposure to the elements. We wanted to see if the Film interfered with neck mobility and how well different cohorts of head and neck cancer patients would tolerate the Film on their skin. We investigated the use of Film in a prophylactic and a management setting and in NZ and Chinese cohorts. Finally we wanted to know whether or not Film decreased skin reaction severity and the incidence of moist desquamation.
We found that Film did not affect patients’ assessment of neck mobility, similar to the study with Mepilex Lite.24 Although Mepitel Film is more adherent than Mepilex Lite, many patients mentioned that the Film came off too easily, particularly in the shower and when bathing. Early on in the trial it became obvious that the Film was pushed out of the folds in the skin of males with heavy beard stubble; the Film often fell off during the night and had to be replaced almost daily. If this had been part of normal practice, this would have been a time-consuming, disruptive and costly exercise. As it was, we decided to obtain ethical approval for putting patients on a management protocol. By the time erythema was visible, stubble growth had slowed or stopped altogether and the Film adhered better to the skin. Film cannot protect the skin from friction if it doesn’t adhere properly or if it isn’t applied properly. Therefore, it came as no surprise that the Film was equally effective in both the prophylactic and management settings in the NZ cohort. Skin reaction severity was similar between males and females but Film performed better in females (n = 5) than in males (n = 17). Whether or not the superior performance of Film in females is caused by better adherence to the skin remains to be seen. Six NZ patients and five Chinese patients mentioned poor adherence of Film in the exit questionnaire. Itchiness was another issue with the Film; particularly for Chinese patients even although they all preferred Film over Cream; they didn’t rate the Film as highly as the NZ patients. In comparison, slight itching was only mentioned by three of the 60 breast cancer patients who returned the exit questionnaire as part of the Mepitel Film breast cancer trial.13
The Chinese patient RISRAS score only showed a 15% improvement for Film compared with 51% for the NZ patients. The researcher component scores the extent of the visible skin reactions and could be considered to be less subjective than the patient component, even though a certain amount of researcher bias cannot be excluded. Interestingly, the researcher RISRAS component of the Chinese cohort favoured Film higher (35% improvement) than the NZ cohort (14% improvement). As the combined score reflects both the patient and researcher components, it was similar in both cohorts: 29 and 27% for the Chinese and NZ cohorts respectively but for different reasons.
It could be argued that as the NZ males on the prophylactic protocol did not get the protective benefit of the Film until their beard stubble growth stopped, effectively they followed the management protocol. Indeed, Film performance in both NZ protocols was very similar. Film performed better in Chinese patients who followed the prophylactic protocol successfully with a greater decrease in researcher RISRAS component (35% compared with 14% in the NZ cohort) as well as in a decrease in moist desquamation rates (37% compared with 28% in the NZ cohort). The Chinese males did not have heavy stubble growth in the neck area and the Film stayed on their skin without the problems seen in males of the NZ cohort who did have beard stubble that was heavy enough to push the Film away from the skin overnight. We believe therefore that, in the absence of heavy stubble, applying Film from the start of radiation therapy is more protective and thus more effective in decreasing skin reaction severity than applying Film when erythema is already visible.
This was not a definitive randomized controlled trial set out to enrol large numbers of patients to provide compelling evidence for or against the use of Mepitel Film in head and neck cancer patients. There are many potentially confounding factors in any trial that investigates the efficacy of skin care interventions; most of them can be attributed to patient-related factors and treatment-related factors (reviewed in9). A strength of our intra-patient controlled design is the minimization of those factors. As with our previous trials, we measured the actual skin dose to both Film-covered and Cream-covered patches and showed that our randomization procedure did not cause a significant difference in total dose to the Film- and Cream-covered skin patches. A limitation of this design is that we were unable to blind the trial; it was obvious to patients and investigators where the Film was applied. A further limitation of our study is the use of subjective scales for measuring the severity of skin reactions, so we cannot exclude researcher and patient bias. Skin reaction assessment was done mostly by one scorer in each hospital, with several back-up scorers (to accommodate the 3-weekly assessments). We attempted to minimize observer bias by training all scorers in the protocol and use of RISRAS in workshops held in NZ and China, and through using a slide presentation with many examples of skin reactions, which needed to be scored correctly before a scorer was accredited. However, a certain amount of inter-scorer variability cannot be excluded and may have attributed to some extent to the bigger improvement in researcher RISRAS scores and moist desquamation rates in the Chinese cohort.
We used the expanded RTOG scale in addition to RISRAS to allow comparison with other trials RTOG scores of 78 breast cancer patients corresponded very well with the researcher component of RISRAS in our previous Mepitel Film trial.13 Not many other researchers are using RISRAS, even although this scale is a lot more sensitive than the RTOG and CTCAE grading systems. RTOG scales initially comprised only 4 Grades,25 which can be extended to include Grade IA and B (to distinguish dry desquamation from follicular/faint/faint erythema) and Grade IIA and IIB (to distinguish moist desquamation from tender/brisk erythema). RISRAS was specifically designed for measuring skin reaction severity due to radiation therapy, and it distinguishes between different areas of skin affected by erythema, dry desquamation and moist desquamation. The increase in scale sensitivity allows us to tease out small differences in skin reaction severity, which is important in a field where after decades of research, a topical pharmacological agent that prevents skin reactions is still elusive. In addition, RISRAS has a patient component that allows us to gauge how the intervention has affected the patient and the impact on day to day life. It seems these are important issues to measure as we do this research to improve patient quality of life. In the current trial, Film performed better in Chinese patients with respect to their visible skin reactions and moist desquamation incidence but the patients did not score it very favourably and reported significant problems with itchiness. NZ patients scored the effect of the Film much higher and seemed to derive more symptom relief than the visible skin reaction improvement seemed to suggest. Even although we did not set out to prove this, the differences in RISRAS scores between Film and Cream were statistically significant for both cohorts and within all subcohorts.
Overall, Mepitel Film was less effective in head and neck cancer patients than in breast cancer patients. This is likely due to a number of factors unique to head and neck cancer patients, including skin dose, level of friction, exposure to the elements and the presence of skinfolds. Although head and neck patients are prescribed a much higher dose to the primary tumour than breast cancer patients, the actual skin dose revealed a smaller difference than expected. The average skin dose of NZ patients was 49–50 Gy, resulting in moist desquamation under Cream in 15 patients and under Film in 10 patients, an overall improvement in 22 patients of 28%. The average skin dose of Chinese patients was significantly lower at 42 Gy, resulting in moist desquamation under Cream in 7 patients and under Film in 3 patients, an overall improvement in 11 patients of 37%. In comparison, the skin dose of breast cancer patients in the Mepitel Film trial was 30–40 Gy with a moist desquamation rate of 26% under Cream and none under Film.13 Although Mepitel Film protects the radiation damaged skin from additional damage caused by friction, these protective effects are likely compromised at increasing skin doses. However, factors other than dose are likely to have contributed to the higher moist desquamation rates and poorer Film performance in head and neck cancer patients. The skin in the neck area may be more prone to moist desquamation because of the stretching of the skin to accompany movement of the head, leading to creasing and wrinkling (in older patients). Creasing also challenged the correct application of Film, as previously suggested by Russi and colleagues.9 In the previous breast trial, most moist desquamation occurred in areas of increased moisture and friction such as the axilla and inframammary skin folds.13 Skin of the head and neck area is also exposed to friction of clothing, the weather and previous sun exposure than the breast, making it more fragile and prone to moist desquamation. It is possible that the mechanical protection provided by Film is not always enough to prevent moist desquamation. However the decrease in the incidence in moist desquamation of 37% (Chinese cohort) and 28% (NZ cohort) achieved by Mepitel Film is better than that reported for any topical pharmacological intervention to date9,10 and it is worth exploring further in different head and neck patient cohorts, such as in female patients, who seemed to benefit most from Film.
In conclusion, Mepitel Film decreased overall skin reaction severity by 29% and moist desquamation rates by 37% in the Chinese cohort and by 27 and 28% respectively in the NZ cohort. The main issues with Mepitel Film were poor adherence to the skin, particularly in males with heavy beard stubble and itchiness of the skin underneath the Film. This feasibility study showed that Mepitel Film is most effective in head and neck cancer patients who do not have heavy stubble and when used in the prophylactic setting.
Acknowledgments
The authors wish to acknowledge the contributions to various aspects of the trial by Kate Dekker, Yasmin McQuinlan, Jo Tuaine, Kylie Hewitt, Dalice Sim, Ligui Wu, Juan Wang, Yu Zhuang, Danruo Wang, Juan Liu, Shuangshuang Li Ju Yang and Jie Shen. We further wish to acknowledge Molnlycke Healthcare LTD for providing Mepitel Film free of charge. This trial was also supported by the University of Otago, Canterbury District Health Board, Southern District Health Board and Drum Tower Hospital.
Contributor Information
Hayley Wooding, Email: hayley.wooding@cdhb.health.nz.
Jing Yan, Email: yj20030610@126.com.
Ling Yuan, Email: 2560703582@qq.com.
Te-Yu Chyou, Email: Te-Yu.Chyou@southerndhb.govt.nz.
Shanbao Gao, Email: gbsource@sina.com.
Iain Ward, Email: Iain.Ward@cdhb.health.nz.
Patries M Herst, Email: patries.herst@otago.ac.nz.
References
- 1.Bolderston A, Lloyd NS, Wong RK, Holden L, Robb-Blenderman L. Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence-Based Care. The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer 2006; 14: 802–17. cited 2012 May 24. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Juresic E, Barton M, Shafiq J. Management of skin toxicity during radiation therapy: a review of the evidence. J Med Imaging Radiat Oncol 2010; 54: 264–79. cited 2012 Apr 10. [DOI] [PubMed] [Google Scholar]
- 3.Koukourakis GV, Kelekis N, Kouvaris J, Beli IK, Kouloulias VE. Therapeutics interventions with anti-inflammatory creams in post radiation acute skin reactions: a systematic review of most important clinical trials. Recent Pat Inflamm Allergy Drug Discov 2010; 4: 149–58. [DOI] [PubMed] [Google Scholar]
- 4.Salvo N, Barnes E, van Draanen J, Stacey E, Mitera G, Breen D, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol 2010; 17: 94–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butcher K, Williamson K. Management of erythema and skin preservation; advice for patients receiving radical radiotherapy to the breast: a systematic literature review. J Radiother Pract 2012; 11: 44–54. [Google Scholar]
- 6.Wong RK, Bensadoun RJ, Boers-Doets CB, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC skin toxicity study group. Support Care Cancer 2013; 21: 2933–48. . [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhang S, Shao X. Topical agent therapy for prevention and treatment of radiodermatitis: a meta-analysis. Support Care Cancer 2013; 21: 1025–31. [DOI] [PubMed] [Google Scholar]
- 8.Chan RJ, Webster J, Chung B, Marquart L, Ahmed M, Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta-analysis of randomized controlled trials. BMC Cancer 2014; 14: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russi EG, Moretto F, Rampino M, Benasso M, Bacigalupo A, De Sanctis V, et al. Acute skin toxicity management in head and neck cancer patients treated with radiotherapy and chemotherapy or EGFR inhibitors: literature review and consensus. Crit Rev Oncol Hematol 2015; 96: 167–82. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira EB, Vasques CI, Gadia R, Chan RJ, Guerra EN, Mezzomo LA, et al. Topical interventions to prevent acute radiation dermatitis in head and neck cancer patients: a systematic review. Support Care Cancer 2017; 25: 1001–11. [DOI] [PubMed] [Google Scholar]
- 11.Diggelmann KV, Zytkovicz AE, Tuaine JM, Bennett NC, Kelly LE, Herst PM. Mepilex Lite dressings for the management of radiation-induced erythema: a systematic inpatient controlled clinical trial. Br J Radiol 2010; 83: 971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson D. Randomized intra-patient controlled trial of mepilex lite dressings versus aqueous cream in managing radiation-induced skin reactions postmastectomy. J Cancer Sci Ther 2012; 04: 347–56. cited 2013 Jul 30. [Google Scholar]
- 13.Herst P, Bennet N, Sutherland A, Peszynski R, Paterson D, Jasperse M. Prophylactic use of mepitel film completely prevents radiation-induced moist desquamation in an intra-patient controlled RCT of 78 breast cancer patients in New Zealand. Radiother Oncol 2014; 110: 137–43. [DOI] [PubMed] [Google Scholar]
- 14.Herst PM. Protecting the radiation-damaged skin from friction: a mini review. J Med Radiat Sci 2014; 61: 119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015; 33: 3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas-Roxburgh R, Benschop J, Dunowska M, Perrott M. Prevalence of human papillomaviruses in the mouths of New Zealand women. N Z Med J 2015; 128: 45–52. [PubMed] [Google Scholar]
- 17.Tsang CM, Tsao SW. The role of Epstein-Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Virol Sin 2015; 30: 107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raab-Traub N. Nasopharyngeal carcinoma: an evolving role for the Epstein-Barr virus. Curr Top Microbiol Immunol 2015; 390(Pt 1): 339–63. [DOI] [PubMed] [Google Scholar]
- 19.Cork MJ, Danby S. Aqueous cream damages the skin barrier. Br J Dermatol 2011; 164: 1179–80. [DOI] [PubMed] [Google Scholar]
- 20.Noble-Adams R. Radiation-induced skin reactions. 3: evaluating the RISRAS. Br J Nurs 1999; 8: 1305–12. [DOI] [PubMed] [Google Scholar]
- 21.MacBride SK, Wells ME, Hornsby C, Sharp L, Finnila K, Downie L. A case study to evaluate a new soft silicone dressing, mepilex lite, for patients with radiation skin reactions. Cancer Nurs 2008; 31: E8–E14. [DOI] [PubMed] [Google Scholar]
- 22.Paterson D, Poonam P, Bennett NC, Peszynski RI, Van Beekhuizen MJ. Randomized intra-patient controlled trial of mepilex lite dressings versus aqueous cream in managing radiation-induced skin reactions postmastectomy. J Cancer Sci Ther 2012; 4: 347–56. [Google Scholar]
- 23.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6. [DOI] [PubMed] [Google Scholar]
- 24.Zhong WH, Tang QF, Hu LY, Feng HX. Mepilex lite dressings for managing acute radiation dermatitis in nasopharyngeal carcinoma patients: a systematic controlled clinical trial. Med Oncol 2013; 30 cited 2014 Jan 4. [DOI] [PubMed] [Google Scholar]
- 25.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–6. [DOI] [PubMed] [Google Scholar]