Abstract
Objective:
To determine the malignancy rate (defined in this study as stability or absence of malignancy developed on close imaging follow-up post-biopsy) of conservative management in patients with a vacuum-assisted breast biopsy (VAB) diagnosis of flat epithelial atypia (FEA), performed on single group of microcalcifications, completely removed during procedure.
Methods:
This is a retrospective, monocentric, observational study, approved by IRB. Inclusion criteria were: VAB performed on a single group of microcalcifications; the absence of residual calcifications post-VAB; diagnosis of isolated FEA as the most advanced proliferative lesion; radiological follow-up at least of 12 months. The personal history of breast cancer or other high-risk lesions was an exclusion criteria. The patients enrolled were conservatively managed, without surgical excision, through close follow-up: the first two mammographies performed with an interval of 6 months after biopsy, followed by annual mammographic and clinical checks.
Results:
48 consecutive patients were enrolled in the study, all females, with age range of 39–76 years (mean 53,3 years) and radiological follow-up range of 13–75 months (mean 41.5 months). All the lesions were classified as BI-RADS 4b. The diameter range of the group of calcifications was 3–10 mm (mean 5, 6 mm). In each patient, 7 to 15 samples (mean 11) were obtained. Among all the patients, there was only one case (2%) of new microcalcifications, developed in the same breast, 26 months after and 8 mm from the site of previous VAB, and interpreted as ADH at surgical excision. All the checks of the other patients were negative.
Conclusion:
Even with a limited follow-up, we found a malignancy rate lower than 2%, through a defined population. Further studies with bigger number of patients and extended follow-up are needed to reinforce this hypothesis.
Advances in knowledge:
Surgical excision may not be necessary in patients with VAB diagnosis of isolated FEA, without residual microcalcifications post-procedure and considered concordant with the mammographic presentation, considering the low rate of malignancy at subsequent follow-ups.
Introduction
Breast flat epithelial atypia (FEA) is an intraductal proliferative lesion,1,2 firstly described in late 1970s,3 characterized by replacement of normal ductal epithelium of terminal ductal lobular unit by 1–5 layers of columnar cells with low-grade atypia, without architectural atypia.4 It is classified as part of the uncertain malignant potential breast lesions, a heterogeneous group of abnormalities with a borderline histological spectrum and a variable risk of associated malignancy.5 They encompass a group of histological diagnoses that includes: atypical ductal hyperplasia (ADH), FEA, classical lobular neoplasia, papillary lesions, benign phyllodes tumours and radial scars. Each of these characterized by variable rates of upgrade to malignancy at surgical excision and long-term increased risk of breast cancer during the patients’ lifetime.6,7 The association between FEA, lobular neoplasia, low-grade ductal carcinoma in situ (DCIS) and tubular neoplasia has been described as “Rosen Triad”.8,9 FEA has been proposed as a precursor of ADH or lobular neoplasia,10–13 and described as a non-mandatory precursor to carcinogenesis, along with ADH and DCIS,14,15 to invasive ductal carcinoma. In the literature, we find papers focused on genetic abnormalities that can support this association,16 but the upgrade rate to malignancy at surgical excision is extremely variable, ranging from 13 to 67% in case of radiological–pathological discordance, and between 0 and 7% when there is radiological–pathological concordance, especially when the microcalcifications are completely removed.17–19
This uncertainty about the role of this type of lesion keeps the patients’ management indefinite. The hypothesis of favourable clinical outcome in isolated diagnosis of FEA is emerging,20–22 especially in patients with completely removed microcalcifications post-vacuum-assisted breast biopsy (VAB).23–25
The aim of this study was to determine the malignancy rate (defined in this study as stability or absence of malignancy developed on close imaging follow-up post-biopsy) of conservative management in patients with a diagnosis of pure FEA as the most advanced pathologic lesion, performed on a single group of microcalcifications biopsied with stereotactic VAB, without residual post-procedure. The purpose was to reach an underestimation rate (UR) lower than 2%, fitting the probably benign definition. The consequent aim is to propose to these patients a conservative management, avoiding surgical excision.
Methods and Materials
This is a retrospective, monocentric, observational study, approved by IRB. The clinical-radiological history of consecutive patients with a diagnosis of FEA, performed by VAB between 2011 and 2015 in our Institution, was collected by the authors, investigating the electronic database of radiological, surgical and pathologic anatomy units.
For each patient was recorded: demographic information, personal history of breast cancer, radiological findings (microcalcifications type, location and dimension based on maximum diameter of the group, residual post-VAB, BI-RADS),26 histological diagnosis and follow-up.
Inclusion criteria were: VAB performed on a single group of microcalcifications; the absence of residual calcifications post-VAB; diagnosis of isolated FEA as the most advanced proliferative lesion and radiological follow-up at least of 12 months. The personal history of previous diagnosis of breast cancer or other high-risk lesions was an exclusion criteria.
Imaging, percutaneous biopsy and pathological diagnosis
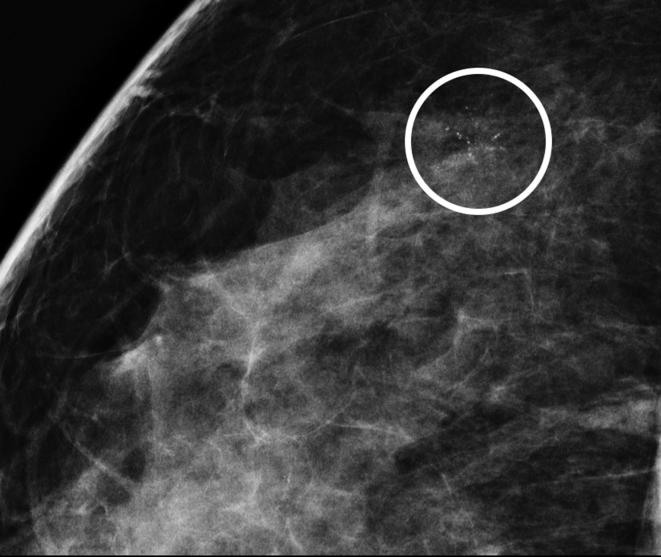
In the diagnostic work-up images acquisition was all performed in the craniocaudal and oblique projections, using a full field digital stereotactic unit (Selenia® Dimensions® digital mammography system with AffirmTM Breast Biopsy Guidance System, Hologic, Bedford, MA), followed by magnified views. The magnified views were reviewed on high-resolution digital mammographic screen by two radiologists with 10 and 25 years of breast radiology experience (Figure 1).
Figure 1.
Magnified view of suspicious group of fine pleomorphic microcalcifications of about 6 mm of extension, in the upper-external quadrant, classified as BI-RADS 4b.
All the patients were biopsied with 9-gauge stereotactically guided VAB (Suros ATEC, Suros Surgical Systems/Hologic, Indianapolis, IN), and a metallic marker placed in the biopsy site.
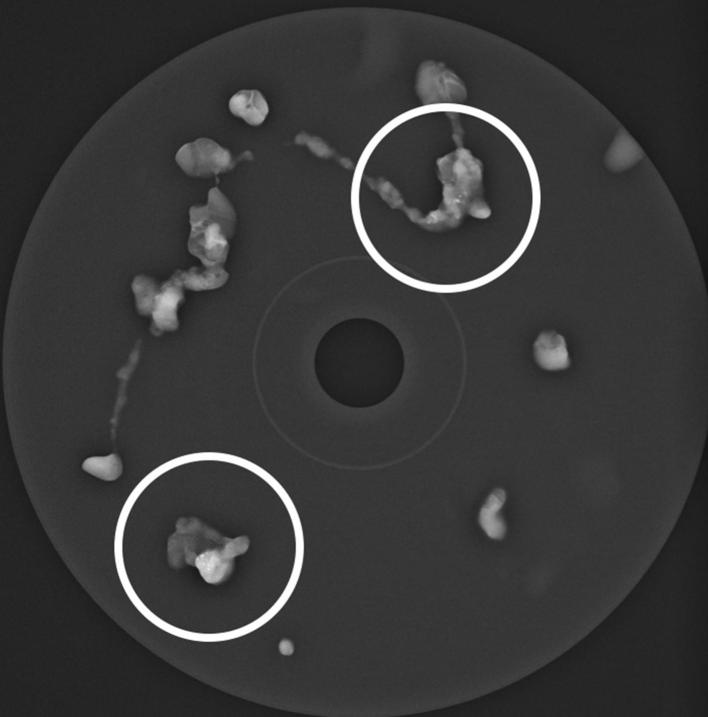
All the specimens were radiographically checked for the presence of microcalcifications in the cores (Figure 2). To rule out the presence of residual microcalcifications and confirm the correct position of the metallic marker, a mammogram was performed after the VAB procedure.
Figure 2.
Magnification radiograph of VAB specimens, checking the presence of microcalcifications in the cores. VAB, vacuum-assistedbreast biopsy.
Diagnoses were made by two different pathologists, both with over 20 years of experience in breast pathology, using the criteria defined by the WHO classification.4 The diagnosis of FEA was reasonably concordant with the radiological presentation in all cases.
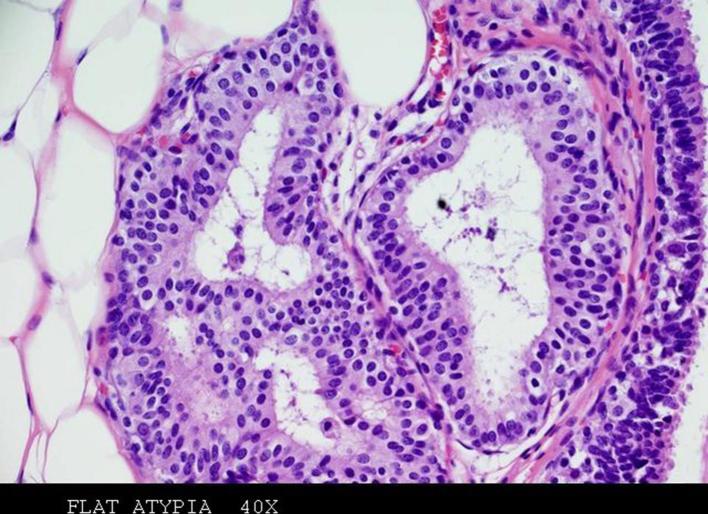
FEA was defined by replacement of normal ductal epithelium of the terminal ductal lobular unit by 1–5 layers of columnar cells with hyperchromatic nuclei and low-grade atypia (Figure 3). The absence of architectural atypia excluded the diagnosis of atypical hyperplasia. Only patients with diagnosis of pure FEA as the most advanced pathologic lesion were enrolled in the study; the association of FEA and atypical hyperplasia (both ADH and atypical lobular hyperplasia), DCIS, lobular carcinoma in situ or invasive carcinoma was an exclusion criteria.
Figure 3.
Haematoxylin and eosin (magnification 40×)—stained sections of specimen obtained with VAB show FEA. A few layers of columnar epithelial cells, with low-grade cytologic atypia, replace the normal ductal epithelium of the terminal duct lobular unit. FEA, flat epithelial atypia; VAB, vacuum-assistedbreast biopsy.
Patients’ management
The patients fitting inclusion criteria described above were conservatively managed, without surgical excision, through close follow-up. The close follow-up consisted in the first two mammography performed with an interval of 6 months after biopsy, followed by annual mammographic and clinical visit.
The mammographic checks were focused on the detection of new microcalcifications or other suspicious lesions.
The number of months from VAB to last programmed mammographic check or last contact (e.g. spontaneous check for symptoms) defined the duration of follow-up.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation; categorical variables are expressed as frequency and percentage.
Results
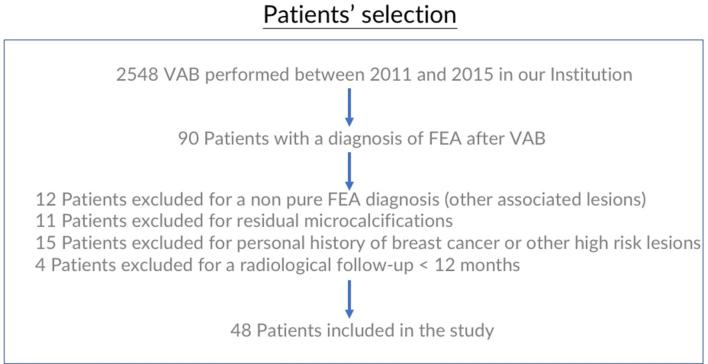
Among 2548 stereotactically VAB performed between 2011 and 2015, only 48 (1,9%) consecutive patients were enrolled in the study (Figure 4), all females, with age range of 39–76 years (mean 53, 3 years) and radiological follow-up range of 13–75 months (mean 41.5 months).
Figure 4.
Flow chart of patient enrollment.
In accordance with the last version of BI-RADS, all the lesions (100%) were classified as BI-RADS 4b, and divided in two types of microcalcifications: 22 (46%) cases with fine pleomorphic calcifications and 26 (54%) cases with amorphous calcifications (Table 1).
Table 1.
Patients demographics and mammographic data
| No. of patients. | 48 |
| Female (%) | 48 (100%) |
| Age range at diagnosis (mean) | 39–76 years (53, 3 years) |
| Radiological follow-up range (mean) | 13–75 months (41.5 months) |
| BI-RADS 4b (%) | 48 (100%) |
| Type of microcalficiations | 22 (46%) Fine pleomorphic 26 (54%) Amorphous |
| Diameter range of the group of calcifications (mean) | 3–10 mm (5, 6 mm) |
| Number range of specimens obtained in each patient (mean) | 7–15 (11) |
The diameter range of the group of microcalcifications was 3–10 mm (mean 5, 6 mm). In each patient, 7 to 15 samples (mean 11) were obtained.
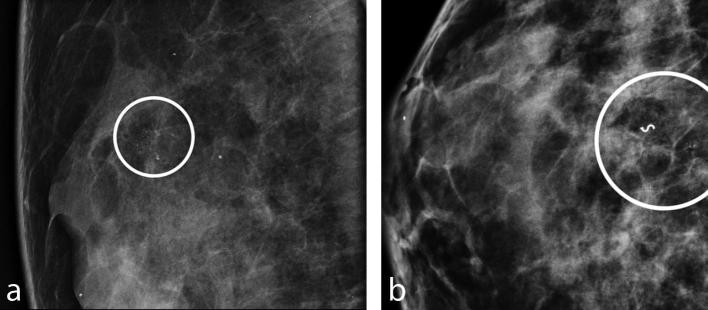
Among all the patients, there was only one case (2%) of new microcalcifications, developed in the same breast, 26 months after and 8 mm from the site of the previous VAB (Figure 5a,b). Histopathology revealed ADH, confirmed by the pathologist after surgical excision. Mammographic follow-up in the remaining patients was negative for the presence of new microcalcifications or other suspicious lesions.
Figure 5.
First (a) and second (b) group of microcalcifications detected in the same patient, with 2 years of interval, interpreted, respectively as FEA and ADH. (a) Magnified view of suspicious group of fine pleomorphic microcalcifications of about 7 mm of extension, in the upper quadrants, classified as BI-RADS 4b. (b) Programmed mammographic check detected a new group of microcalcifications 8 mm from the metallic marker of the previous VAB, performed 2 years before, and interpreted as ADH at surgical excision. ADH, atypical ductal hyperplasia; FEA, flat epithelial atypia; VAB, vacuum-assisted breast biopsy.
Discussion
The management of patients with lesions of uncertain malignant potential is evolving over the last few years, focusing on patient’s tailored therapeutic strategy. The purpose of many studies is to try to predict malignancy upgrade and to avoid unnecessary surgery.
Publications dating back to the beginning of 2000s still consider mandatory surgical excision in these patients,27,28 while the most recent papers are more about conservative therapy.
Breast cancer risk attributable to FEA is still undefined in the literature, although de Mascarel found no development of subsequent invasive cancer after a median follow-up of 13 years, after pure FEA surgically biopsied.29 Boulos17 revealed no increased risk in patients with FEA compared with patients with other types of columnar cell lesions, and Said shared these results.20 We could question the development of breast carcinoma in the FEA group of the paper published by Martel10 as possible missed breast cancers, but in a multicentric study with 190 cases of pure FEA diagnosed at micro-histology, Bianchi30 did not exclude the presence of associated malignancy at surgical excision, even in absence of residual microcalcifications on post-VAB mammography.
The conservative management with imaging follow-up of patients with pure FEA and without residual has been proposed and hypothesized by several authors in the last 20 years.22,23,30,31 This kind of management, without development of invasive ductal carcinoma, has been published more than 20 years ago, in the series published by Eusebi in 1994,13 where 25 patients were followed for 19 years, as average, without upgrading to malignancy. However, the authors didn’t focus on the absence of residual microcalcifications post-VAB that is, in our opinion, one of the most important factor for the conservative option. In their papers, Villa23 and Dialani32 found no upgrade to malignancy at surgical excision, in absence of residual microcalcifications.
Acott and colleagues still recommend surgical excision, but only in cases of residual microcalcifications,33 as we do in our everyday clinical practice. The association of ADH and FEA leads to UR incompatible with conservative therapy,16 so surgical excisional biopsy is still mandatory in this groups of patients.
In this setting, VAB can be considered as surrogate of surgical excision (or vacuum-assisted excision as referred in some countries, as United Kingdom), playing a therapeutic role in these patients, as introduced for ADH by several authors,34–39 and recommended by the First International Consensus Conference on lesions of uncertain malignant potential in the breast, that took place in Zurich (Switzerland) in January 2016.40
Our group focused on cases of isolated diagnosis of pure FEA, followed-up with imaging after VAB on single group of microcalcifications without residual post-procedure. We followed-up 48 patients with at least two mammographies, 6 and 12 months after the biopsy, without malignancy detected during this period, with the longest follow-up of 75 months in one patient.
We enrolled a limited number of patients, but the inclusion criteria were very selective. The mean diameter of the microcalcifications was quite small, but we must consider that only patients with complete removal of the lesion were included in the study.
The work presents some other limitations: data come from single centre experience, so bias derived from the centre cannot be excluded. The follow-up length is limited, but comparable to previous published paper on this topic, considering the low incidence of pure FEA, as it is often associated with other lesions (atypical hyperplasia). The MR is only based on imaging follow-up, without a histological validation. The pathologic diagnosis was withdrawn from the report, without a revision from a pathologist. Unfortunately, we were not able to consider the family history because the information from most patients were not available, but the absence of events (development of malignancy during follow-up) wouldn’t have permitted any speculation.
During our follow-up, we observed that the isolated diagnosis of pure FEA is not associated with independent increased long-term risk of breast cancer. The strength of this paper lies under a very reproducible protocol, characterized by pure FEA population, with diagnosis made by stereotactic VAB on single group of microcalcifications, in patients without personal history of breast cancer or other high-risk lesions.
In our opinion, the best management of these patients requires a close mammography 6 and 12 months after VAB and, in case of negative controls, the return to the normal screening program. The purpose of close controls is to identify both the presence of residual disease both the appearance of a new pathology because of increased risk. The possibility of avoiding the use of surgery allows the female to avoid the risks associated with the pre-, intra- and post-procedural, as well as the related psychophysical stress.
Even with a mean follow-up of 41.5 months, we confirmed the hypothesis proposed in 2013,23 with an UR lower than 2%, through a circumscribed population. Our mean follow-up is limited, but we are still following-up our patients. Further studies with bigger number of patients and extended follow-up are needed to reinforce this hypothesis.
Contributor Information
Simone Schiaffino, Email: schiaffino.simone@gmail.com.
Licia Gristina, Email: licia.gristina@virgilio.it.
Alessandro Villa, Email: dr.willaav@gmail.com.
Simona Tosto, Email: simona.tosto@hsanmartino.it.
Franca Carli, Email: franca.carli@hsanmartino.it.
Massimo Calabrese, Email: massimo.calabrese@hsanmartino.it.
References
- 1.Schnitt SJ. The diagnosis and management of pre-invasive breast disease: flat epithelial atypia – classification, pathologic features and clinical significance. Breast Cancer Res 2003; 5: 263–e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnitt SJ, Vincent-Salomon A. Columnar cell lesions of the breast. Adv Anat Pathol 2003; 10: 113–24. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi J. G, Ahmed A, Millis R. R. Problems in breast pathology. Philadelphia: WB Saunders; 1979. [PubMed] [Google Scholar]
- 4.Tavassoli F, Millis R, Boecker W. Pathology of the breast. Standford, CT: Appleton Lange; 2003. 60–76. [Google Scholar]
- 5.Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L. European guidelines for quality assurance in breast cancer screening and diagnosis. Fourth edition—summary document. Ann Oncol 2008; 19: 614–22. [DOI] [PubMed] [Google Scholar]
- 6.Lakhani S, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumors of the breast. Lyon: International Agency for Research on Cancer; 2012. [Google Scholar]
- 7.Fitzgibbons PL, Henson DE, Hutter RV. Benign breast changes and the risk for subsequent breast cancer: an update of the 1985 consensus statement. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med 1998; 122: 1053–5. [PubMed] [Google Scholar]
- 8.Abdel-Fatah TM, Powe DG, Hodi Z, Lee AH, Reis-Filho JS, Ellis IO. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. Am J Surg Pathol 2007; 31: 417–26. [DOI] [PubMed] [Google Scholar]
- 9.Brandt SM, Young GQ, Hoda SA. The “Rosen triad”: tubular carcinoma, lobular carcinoma in situ, and columnar cell lesions. Adv Anat Pathol 2008; 15: 140–6. [DOI] [PubMed] [Google Scholar]
- 10.Martel M, Barron-Rodriguez P, Tolgay Ocal I, Dotto J, Tavassoli FA. Flat DIN 1 (flat epithelial atypia) on core needle biopsy: 63 cases identified retrospectively among 1,751 core biopsies performed over an 8-year period (1992–1999). Virchows Arch 2007; 451: 883–91. [DOI] [PubMed] [Google Scholar]
- 11.Lerwill MF. Flat epithelial atypia of the breast. Arch Pathol Lab Med 2008; 132: 615–21. [DOI] [PubMed] [Google Scholar]
- 12.Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol 2005; 205: 248–54. [DOI] [PubMed] [Google Scholar]
- 13.Eusebi V, Feudale E, Foschini MP, Micheli A, Conti A, Riva C, et al. Long-term follow-up of in situ carcinoma of the breast. Semin Diagn Pathol 1994; 11: 223–35. [PubMed] [Google Scholar]
- 14.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A 2003; 100: 10393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol 2011; 223: 308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzoaru I, Morgan BR, Liu ZG, Bellafiore FJ, Gaudier FS, Lo JV, et al. Flat epithelial atypia with and without atypical ductal hyperplasia: to re-excise or not. Results of a 5-year prospective study. Virchows Arch 2012; 461: 419–23. [DOI] [PubMed] [Google Scholar]
- 17.Boulos FI, Dupont WD, Simpson JF, Schuyler PA, Sanders ME, Freudenthal ME, et al. Histologic associations and long-term cancer risk in columnar cell lesions of the breast: a retrospective cohort and a nested case-control study. Cancer 2008; 113: 2415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulos FI, Dupont WD, Schuyler PA, Sanders ME, Page DL, Fedda FA, et al. Clinicopathologic characteristics of carcinomas that develop after a biopsy containing columnar cell lesions: evidence against a precursor role. Cancer 2012; 118: 2372–7. [DOI] [PubMed] [Google Scholar]
- 19.El Khoury M, Sanchez LM, Lalonde L, Trop I, David J, Mesurolle B. Is the outcome at surgery different when flat epithelial atypia and lobular neoplasia are found in association at biopsy? Br J Radiol 2017; 90: 20160750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said SM, Visscher DW, Nassar A, Frank RD, Vierkant RA, Frost MH, et al. Flat epithelial atypia and risk of breast cancer: a Mayo cohort study. Cancer 2015; 121: 1548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aroner SA, Collins LC, Schnitt SJ, Connolly JL, Colditz GA, Tamimi RM. Columnar cell lesions and subsequent breast cancer risk: a nested case-control study. Breast Cancer Res 2010; 12: R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piubello Q, Parisi A, Eccher A, Barbazeni G, Franchini Z, Iannucci A. Flat epithelial atypia on core needle biopsy: which is the right management? Am J Surg Pathol 2009; 33: 1078–84. [DOI] [PubMed] [Google Scholar]
- 23.Villa A, Chiesa F, Massa T, Friedman D, Canavese G, Baccini P, et al. Flat epithelial atypia: comparison between 9-gauge and 11-gauge devices. Clin Breast Cancer 2013; 13: 450–4. [DOI] [PubMed] [Google Scholar]
- 24.Piubello Q, Parisi A, Eccher A, Barbazeni G, Franchini Z, Iannucci A. Flat epithelial atypia on core needle biopsy: which is the right management? Am J Surg Pathol 2009; 33: 1078–84. [DOI] [PubMed] [Google Scholar]
- 25.Lavoué V, Roger CM, Poilblanc M, Proust N, Monghal-Verge C, Sagan C, et al. Pure flat epithelial atypia (DIN 1a) on core needle biopsy: study of 60 biopsies with follow-up surgical excision. Breast Cancer Res Treat 2011; 125: 121–6. [DOI] [PubMed] [Google Scholar]
- 26.D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA. American College of Radiology, Bi-Rads committee, ACR BI-RADS Atlas: Breast imaging reporting and data system. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 27.Jacobs TW, Connolly JL, Schnitt SJ. Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol 2002; 26: 1095–110. [DOI] [PubMed] [Google Scholar]
- 28.Nasser SM. Columnar cell lesions: current classification and controversies. Semin Diagn Pathol 2004; 21: 18–24. [DOI] [PubMed] [Google Scholar]
- 29.de Mascarel I, MacGrogan G, Mathoulin-Pélissier S, Vincent-Salomon A, Soubeyran I, Picot V, et al. Epithelial atypia in biopsies performed for microcalcifications. practical considerations about 2,833 serially sectioned surgical biopsies with a long follow-up. Virchows Arch 2007; 451: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bianchi S, Bendinelli B, Castellano I, Piubello Q, Renne G, Cattani MG, et al. Morphological parameters of flat epithelial atypia (FEA) in stereotactic vacuum-assisted needle core biopsies do not predict the presence of malignancy on subsequent surgical excision. Virchows Arch 2012; 461: 405–17. [DOI] [PubMed] [Google Scholar]
- 31.Kunju LP, Kleer CG. Significance of flat epithelial atypia on mammotome core needle biopsy: should it be excised? Hum Pathol 2007; 38: 35–41. [DOI] [PubMed] [Google Scholar]
- 32.Dialani V, Venkataraman S, Frieling G, Schnitt SJ, Mehta TS. Does isolated flat epithelial atypia on vacuum-assisted breast core biopsy require surgical excision? Breast J 2014; 20: 606–14. [DOI] [PubMed] [Google Scholar]
- 33.Acott AA, Mancino AT. Flat epithelial atypia on core needle biopsy, must we surgically excise? Am J Surg 2016; 212: 1211–3. [DOI] [PubMed] [Google Scholar]
- 34.Jackman RJ, Birdwell RL, Ikeda DM. Atypical ductal hyperplasia: can some lesions be defined as probably benign after stereotactic 11-gauge vacuum-assisted biopsy, eliminating the recommendation for surgical excision? Radiology 2002; 224: 548–54. [DOI] [PubMed] [Google Scholar]
- 35.Plantade R, Hammou JC, Fighiera M, Aubanel D, Scotto A, Gueret S. Underestimation of breast carcinoma with 11-gauge stereotactically guided directional vacuum-assisted biopsy. J Radiol 2004; 85: 391–401. [DOI] [PubMed] [Google Scholar]
- 36.Travade A, Isnard A, Bouchet F, Bagard C. Non-palpable breast lesions and core needle biopsy with Mammotome 11G: is surgery required in patients with atypical ductal hyperplasia? J Radiol 2006; 87: 307–10. [DOI] [PubMed] [Google Scholar]
- 37.Forgeard C, Benchaib M, Guerin N, Thiesse P, Mignotte H, Faure C, et al. Is surgical biopsy mandatory in case of atypical ductal hyperplasia on 11-gauge core needle biopsy? A retrospective study of 300 patients. Am J Surg 2008; 196: 339–45. [DOI] [PubMed] [Google Scholar]
- 38.Ancona A, Capodieci M, Galiano A, Mangieri F, Lorusso V, Gatta G. Vacuum-assisted biopsy diagnosis of atypical ductal hyperplasia and patient management. Radiol Med 2011; 116: 276–91. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen CV, Albarracin CT, Whitman GJ, Lopez A, Sneige N. Atypical ductal hyperplasia in directional vacuum-assisted biopsy of breast microcalcifications: considerations for surgical excision. Ann Surg Oncol 2011; 18: 752–61. [DOI] [PubMed] [Google Scholar]
- 40.Rageth CJ, O'Flynn EA, Comstock C, Kurtz C, Kubik R, Madjar H, et al. First International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat 2016; 159: 203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]