Abstract
Aggression among mice remains a common undesirable problem in laboratory settings, and animal welfare and scientific outcomes may become compromised depending on the severity of aggression. This study evaluated the effect of cage enrichment comprising a bilevel, mounted ‘mezzanine’ compared with a cotton square or shelter on intracage male aggression over a 6-wk period. Our first study involved home-cage behavioral challenges to male mice from a high-aggression substrain (BALB/cJ) and low-aggression substrain (BALB/cByJ). Aggressive interactions and locomotor activity were scored manually and then compared with measures of activity obtained by using a continuous automated home-cage monitoring system, the Digital Ventilated Caging (DVC) system. BALB/cJ mice exhibited similar levels of aggression across housing conditions, whereas BALB/cByJ mice had lower aggression when housed with a mezzanine. In the second study, videorecordings and continuous DVC automated measures were collected over 24 h and divided into 12-h light and dark phases. BALB/cByJ mice—but not BALB/cJ—mice had increased aggressive behaviors during the dark phase. However, the DVC detected higher activity levels during the dark phase, compared with the light phase, in both substrains. Elevated activity levels recorded by the DVC correlated with fighting bouts and high levels of locomotion. These results show that a bilevel structural form of enrichment reduces aggression, depending on the BALB/c substrain, and confirms higher aggression levels in the BALB/cJ substrain. In addition, our findings provide evidence that the DVC is effective in identifying mouse cages with patterns of high activity levels, signaling possible aggression incidences, thus potentially allowing for early intervention and consequently improving animal welfare.
Abbreviations: DVC, digital ventilated caging system
Mice are the most commonly used laboratory animal species in research.3,23 In their natural habitat, a social patriarchal hierarchy is established in which the dominant male mouse defends its territory by scent marking, while other males become subordinates or escapees.15,14,30 The ability of mice to escape or become submissive in aggressive interactions makes incidences of severe injury and deaths rare.14 However, in laboratory settings, intermale aggression is an important animal welfare and experimental concern. When mice are unable to escape within their home cage, increased amounts of aggression can occur, some of which may result in severe wounds and even mortality.
Aggression is a behavior commonly observed among group-housed male laboratory mice and is defined as a violent or defensive behavior directed toward another conspecific. These agonistic behaviors consist of biting, chasing, pinning, threatening postures, and fighting and may lead to fight wounds that require medical treatment, separation leading to single housing, and even euthanasia in severe cases.14,32 In general, aggression is demonstrated predominantly among males, although breeding females display postpartum maternal aggression when protecting their litters from intruders. In addition, the effects of aggression can lead to altered experimental data, such as increased corticosterone levels, decreased immune responses, and premature experimental endpoints.7,22 In turn, the principle of animal reduction is compromised by the need to test additional subjects due to unanticipated losses.13,14,18 Furthermore, some strains, such as SJL and FVB mice, are characterized by particularly high levels of aggression; due to these undesirable traits, investigators may be inclined to select female mice or other, more compatible strains for research experimentation to avoid issues associated with aggression.1,14
Efforts to reduce aggression in group-housed male mice include transferring cotton squares during routine cage changes, decreasing housing density to 3 mice per cage,30 and cohousing littermates or familiar conspecifics.6,14,17,28 Similarly, various types of environmental enrichment items have been evaluated to mitigate agonistic interactions, including shelters, wheels, and toys.12,26,27 Even though cotton squares have positive effects on decreasing intermale aggression, their efficacy may diminish as a long-lasting form of enrichment.13 In addition, providing environment enhancements, such as shelters, may lower the incidence of aggression,25 but they have also been shown to augment these behaviors.10 Overall, the cited studies present conflicting evidence of whether particular practices or forms of environmental enrichment are effective in reducing aggression.
Common husbandry and experimental practices, such as cage changing and extracting animals from the home cage for procedures, affect physiologic responses.15,22 Although cage changes are an essential component of maintaining animal wellbeing, removing pheromones creates a stressful environment, provoking the reestablishment of a social hierarchy, demonstrated as attacks that occur only minutes after exposure to a clean cage. Although transferring bedding material into clean cages has been shown to mitigate aggression, other studies have shown that transferring mice into completely clean cages lessened the number of agonistic behaviors.5,15,22 In addition, some laboratory procedures, such as behavioral tests, involve temporarily removing and then returning test subjects to their home cage,2,11 a practice that can invoke an agonistic reestablishment of social dominance. Few studies have monitored aggressive behaviors specifically within the home cage after the reintroduction of a resident or familiar cage mate.4
In the current study, we assessed the effect of housing conditions on aggression by using a bilevel mezzanine, compared with a cotton square or mouse hut, as enrichment. As noted in the Guide for the Care and Use of Laboratory Animals,8 an elevated platform provides opportunities for mice to display species-specific behaviors, such as exploring, climbing, and locomotion. In addition, a mezzanine contributes to floor space, protects mice from flooded cages due to malfunctioning water reservoirs, and serves as a form of shelter. Elevated nonmobile forms have been evaluated for reducing anxiety behaviors in mice at various time points;19 however, the use of bilevel structures within the home cage has not been evaluated as a means to decrease aggression.
To assess the effect of various enrichment items on aggression, an automated home cage monitoring system using digital ventilated cages (DVC) was used. The DVC system enables researchers to record data, such as animal activity, while home cages are positioned on a conventional rack where mice spend a majority of their experimental life. With its ability to continuously gather comprehensive cage activity and compare levels to previous days, the DVC system might provide a new method to detect subtle shifts in activity, such as debilitated or immobile mice. It is possible the DVC system could enhance efficient monitoring of animal welfare and behavioral changes with minimal disruption to day-to-day activity.
The inbred murine strain BALB/c is frequently chosen for research experiments in a variety of research disciplines, including cardiology, immunology, oncology, and neurobiology.9,16,21,34 However, this common strain is particularly aggressive, although the level of hostility toward conspecifics varies among substrains.14,32 To evaluate whether housing condition influenced agonistic interactions, we compared the high-aggression BALB/cJ substrain with BALB/cByJ, a low-aggression substrain. Male mice from each strain were housed with either a nonmobile elevated homecage platform (or ‘mezzanine’), a cotton square, or a shelter. Mice were then evaluated in 2 types of behavioral challenge tests that reflected husbandry and laboratory practices known to instigate agonistic behaviors: placement in a clean cage and separation and reintroduction of a cagemate. We hypothesized that the mezzanine would decrease aggression between 2 BALB/c substrains. In addition, we hypothesized that the DVC system would detect increased animal activity that would correlate with observations of intermale aggression.
Materials and Methods
Animals.
All research was approved by the IACUC of the University of North Carolina at Chapel Hill, an AAALAC-accredited institution. Subjects were male BALB/cJ (n = 90) and BALB/cByJ (n = 90) mice (age, 3 wk at arrival; Jackson Laboratories, Bar Harbor, ME) housed according to recommendations in the Guide.8 Mice arrived in 2 cohort groups (n = 45 mice per substrain per cohort group) separated by 3 wk, due to limited space on the DVC housing rack. The disease status of sentinels housed within the housing room was screened quarterly, and all animals tested negative for common murine pathogens detected by serology and parasitology: epizootic diarrhea of infant mice, Theiler murine encephalomyelitis virus and the GDVII strain of Theiler murine encephalomyelitis virus, mouse hepatitis virus, Mycoplasma pulmonis, mouse parvoviruses, minute virus of mice, mouse cytomegalovirus, mouse adenovirus 1 and 2, polyoma virus, pneumonia virus, cilia-associated respiratory bacillus, and external and internal parasites.
Mice were observed by husbandry staff daily, and study investigators weighed each mouse once each week. In addition to the routine daily visual health checks, mice were inspected thoroughly for gross lesions characteristic of fight wounds or any other irregularities during the weighing sessions. After weights were recorded, the mouse was returned to its original home cage. Animals were treated with antibiotics and NSAID for moderate wounds or euthanized when they exhibited severe signs of injury or distress.
Husbandry.
On arrival, mice from each substrain were randomly allocated and housed 3 per cage in IVC (77.66 in2, model GM500 SealSafe, Tecniplast, Buguggiate, Italy) on irradiated 1/4-in. bedding (Bed-o-Cobs, Anderson, Maumee, OH). Animals had unrestricted access to irradiated feed (Teklad 2919, Envigo, Indianapolis, IN) and bottled reverse-osmosis–purified, chlorinated water. Environmental conditions in the animal housing rooms consisted of a 12:12-h light:dark photoperiod, temperature of 70 to 72 °F (21.1 to 22.2 °C), and relative humidity of 30% to 70%.
Every other week, mice were placed in clean, ventilated cages. The cages were sanitized, washed, and rinsed with 180 °F water prior to stocking them with clean bedding and fresh food and water. Subsequently, mice were either given a new cotton square or a clean, sanitized structural item (described later) according to their assigned enrichment condition. Each cage of mice was exposed to the same type of enrichment item throughout the entire study. After changing, cages were inserted into observation spots for direct visual monitoring of aggressive behaviors for 10 min, after which the cages were placed back in their assigned area on the DVC rack.
Enrichment.
Each cage was given 1 of 3 enrichment items: 1) a cotton square (Ancare, Bellmore, NY) used for nesting material; 2) a shelter (Mouse Hut, Bio-Serv, Flemington, NJ); or 3) a mezzanine (0.26 ft2, Tecniplast), an autoclavable, polysulfone ledge mounted from the wire bar that provided an additional level or extra floor space (Figure 1). Both structural forms of enrichment provided both entrance and exit points. Ten cages containing each enrichment type were used for each BALB/c substrain, totaling 30 mice from each substrain per enrichment condition. To reflect most institutional standard housing conditions, which require at least one type of enrichment item in mouse cages unless scientifically justified for exemption, we refrained from including a group free of enrichment.
Figure 1.

Environmental housing conditions for BALB/cJ and BALB/cByJ mice. Examples of cages with (A) cotton square, (B) shelter, and (C) bilevel mezzanine with staircase.
Behavioral challenge testing.
After 1 wk of acclimation to the new housing conditions, mice were evaluated in 2 challenge tests for aggressive interactions: placement in a completely clean cage without transfer of cotton square material, and removal and reintroduction of a cagemate. Visual observations were obtained after each cage change, starting when mice were 4 wk old, with 2 wk between clean cage tests. On alternate weeks, a modified resident-intruder behavioral test was used to measure offensive and defensive aggression in a removal and reintroduction test. One mouse was removed from each cage and held in a clean well-ventilated box for 5 min. Mice were identified by ear notching (that is, left, right, or no notch), so that a different subject was removed from the cage for each round of testing. This intruder mouse was then returned to the same home cage, and behaviors were manually recorded for 1-min periods across 10 min. Each aggressive encounter (tail bite, chasing, or fighting) was recorded and scored in 15-s intervals (4 intervals per minute), with scores ranging from 0 (no observations of aggression) to 4 (aggression occurring in each 15-s bin across 1 min). Tail bites were defined as a single mouse using its mouth and gripping the tail of a cagemate. Chasing consisted of cagemates aggressively pursuing one another, and a fighting bite was characterized by any of combination of wrestling, kicking, and rolling. Additional noted behaviors included sleeping and locomotion (taking 2 or more steps), and unusual behaviors such as stereotypies and vocalization. Each behavioral challenge was performed on alternating weeks for a total of 6 wk, 3 rounds per behavioral test (Figure 2). The observer was blinded to each mouse substrain during the duration of the experiment.
Figure 2.
Flow chart of experimental design.
DVC system.
Retrofitted standard IVC mouse cages were assigned and placed above touchless individual digital base plates connected to a conventional animal rack. The DVC board is composed of 12 electrodes evenly positioned underneath the cage. Activity was measured by each electrode and tracked over time, with the average activity collected from all electrodes. The DVC can provide information from activity collected as often as 4 times per second. However, for the analysis of activity in this experiment, an average activity was calculated during 1-min intervals. Therefore, the basic DVC activity metric measures an average of the activity in both space (over 12 locations in the cage) and time (in this study, 1 min). To correlate the DVC system's ability to detect increased activity congruent with aggressive behaviors, each cage was placed into one of 3 designated observation spots on the rack for behavioral coding located within the animal housing room. These allocated slots allowed sufficient visibility of the full length of the cages important for the observational measures of behavior. DVC measures were collected when cages were inserted into observation spots for 10 min or in specific locations to match 24-h video recordings.
A subset of 18 cages (3 cages per each of the 3 enrichment types for each of the 2 substrains) was videotaped for 24 h to investigate the DVC's ability to detect increased activity corresponding to observed aggression. Two-minute video records were taken of each cage for each hour across the 24-h period and analyzed by using Noldus Observer software (Leesburg, VA). The frequencies and durations of aggression (tail bites, fighting bouts, chasing), sleeping, locomotion and any other unusual behaviors were recorded to determine whether increased spikes in the automated DVC measures of activity corresponded with aggression or other behaviors.
Statistical analysis.
All graphs were prepared and statistical analysis was performed by using Statview (SAS, Cary, NC) or Prism 7.0 (GraphPad Software, La Jolla, CA). Except for body weight, each cage was evaluated as the unit of analysis for statistical comparison. Weight data were analyzed by using repeated-measures ANOVA, with factors of strain, enrichment condition, and week of testing. Data from the behavioral challenge tests were first analyzed by using 2-way or repeated-measures ANOVA, with the factors of substrain and housing condition. Recordings from the 24-h period were analyzed by using repeated-measures ANOVA, with the factors substrain and phase of the photoperiod. Fisher protected least-significant difference tests were used for comparing group means only when a significant F value was determined. A linear correlational test or paired t tests were used to compare scores from the human observer with scores from the automated DVC system. In addition, t tests were performed to ensure consistency in scoring across 2 observers for video data collection. For all comparisons, significance was set at a P value of < 0.05.
Results
Effect of housing condition on weight gain in BALB/cJ or BALB/cByJ substrains.
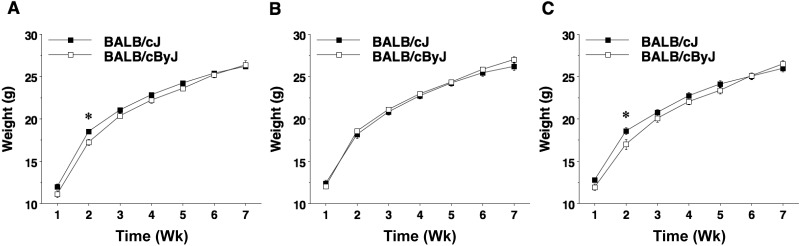
Mice were weighed each week of the study to determine whether housing conditions affected the weights of substrains over time (Figure 3). Repeated-measures ANOVA indicated a significant strain×week interaction (F6,996 = 6.04, P < 0.0001) but no effects of housing condition. Posthoc analyses revealed that BALB/cJ mice weighed more overall than BALB/cByJ during the first weeks of the study, but these differences were no longer present by the last weeks.
Figure 3.
Body weights across study. BALB/cJ and BALB/cByJ substrains housed with a (A) cotton square, (B) shelter, and (C) bilevel mezzanine were weighed weekly. Data are provided as mean ± SEM (n = 90 mice per strain). *, P < 0.05.
Effect of housing condition and substrain on aggressive behavior.
Mice were evaluated by direct, cageside visual observations for aggressive activity in 2 assays for provoking social interaction: placement into a clean cage and the removal and reintroduction of one mouse. Mice underwent a total of 3 tests for each assay, one test per week across 6 wk. Because of the low occurrence of active aggression, data were summed across all 3 tests for each type of challenge assay. For the clean cage condition, the overall levels (mean ± SEM) of agonistic responses determined by direct visual observation after cage change were markedly low among both substrains (BALB/cJ, 1.17 ± 0.36; BALB/cByJ, 0.27 ± 0.12 bouts per minute). However, 2-way ANOVA indicated a significant main effect of strain (F1,2 = 5.83, P = 0.0192), without main effects or interactions with the factor housing condition. Posthoc analyses revealed that the BALB/cJ strain had significantly more aggressive encounters than BALB/cByJ after placement in a clean cage (Fisher protected least-significant difference test, P = 0.0204).
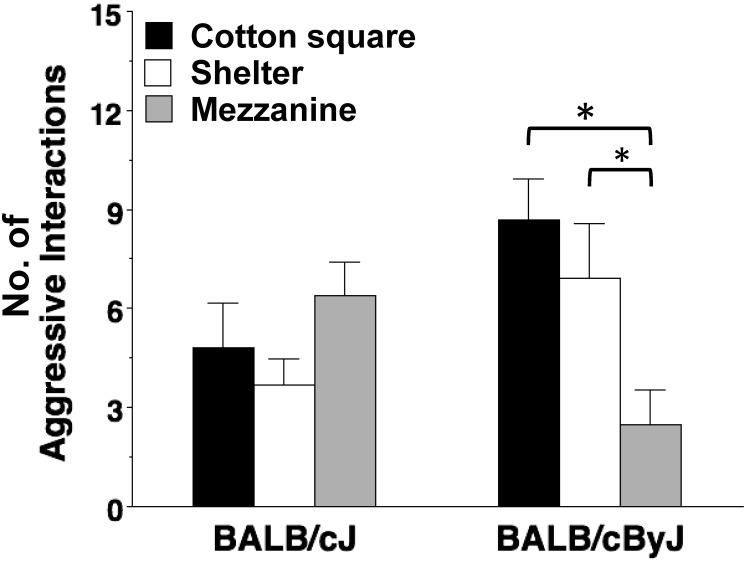
High levels of aggressive interactions occurred during the removal-and-reintroduction test (Figure 4). Two-way ANOVA revealed a highly significant strain × housing condition interaction (F2,54 = 6.29, P = 0.0035). Further analyses indicated that, in the BALB/cJ mice, enrichment did not have any effects on the amount of aggression. However, in the BALB/cByJ strain, mice housed with a mezzanine had significantly (P < 0.05) less fighting than both the cotton square and shelter groups.
Figure 4.
Strain-specific effects of enrichment on aggression. Mice underwent a removal test and reintroduction test and the resulting aggressive interactions were measured by manual live recordings. Data are means (+ SEM) of summed measures across 3 tests, with 2 wk between each test. n = 10 cages per strain for each housing condition. *, P < 0.05.
At 1 wk before the study concluded, one cage of BALB/cJ mice was euthanized due to severe wounds despite medical treatment; this cage of mice was the only one that required medical treatment. In addition, one mouse within another cage was found dead during the third week, with the cause of death indeterminate.
Effect of substrain on aggression and activity during different light levels.
Levels of homecage aggression, sleep, and locomotor activity were measured across a 24-h period by using video recording in a subset of cages containing either BALB/cJ or BALB/cByJ mice. Two-minute video records of each cage were taken at hourly time points, resulting in 12 measures per phase of the photoperiod (either light or dark). Because of the generally low incidence of active aggression, data across the 12 measures were summed for each phase.
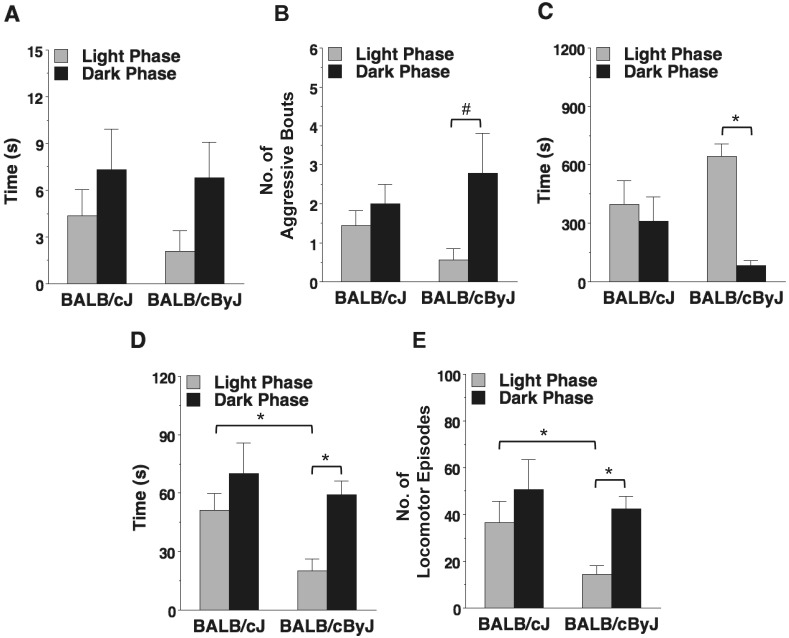
Mouse substrain did not significantly affect the time spent in aggressive encounters (F1,16 = 3.84, P = 0.0678; Figure 5 A), although BALB/cByJ mice appeared to have higher levels of aggressive behavior in the dark phase of the lighting cycle compared with the light phase. The phase of the lighting cycle had an overall significant effect on bouts, or episodes, of aggressive interaction (F1,16 = 5.92, P = 0.027 [repeated-measures ANOVA]; Figure 5 B), with the number of aggressive episodes increased during the dark phase. Within-strain comparisons indicated the effect of phase very closely approached significance in the BALB/cByJ mice (F1,8 = 5.26, P = 0.0509) but not BALB/cJ (F1,8 = 0.85, P = 0.3842). As shown in Figure 5 C, BALB/cByJ mice spent significantly more time sleeping during the light phase compared with the dark phase (F1,16 = 8.04, P = 0.012), whereas sleep in BALB/cJ mice was similar across the 2 phases (F1,16 = 4.32, P = 0.0542).
Figure 5.
Strain-dependent circadian rhythmicity for sleep time and locomotor activity. Mice were evaluated for (A) duration of aggression, (B) no. of aggressive episodes, (C) duration of sleep, (D) duration of locomotion, and (E) no. of locomotor episodes by using videorecording during the light (gray bars) and dark (black bars) phases of the photoperiod. Data are shown as mean ± SEM (n = 9 cages per strain) of summed measures across 12 time points per phase. *, P < 0.05; #, P = 0.0509.
Significant effects of both strain (F1,16 = 5.29, P = 0.0353) and phase (F1,16 = 7.05, P = 0.0173) were found for time spent in active locomotion (Figure 5 D). In particular, BALB/cByJ mice had higher levels of activity in the dark phase compared with the light phase; in contrast, BALB/cJ showed no circadian patterns in activity. A similar pattern was observed for bouts of locomotor activity, with significant effects of both strain (F1,16 = 4.56, P = 0.0486) and phase (F1,16 = 4.73, P = 0.045; Figure 5 E). During the light phase, BALB/cJ mice had more locomotor episodes than BALB/cByJ mice.
DVC activity levels in BALB/cJ or BALB/cByJ substrains during 24-h recordings.
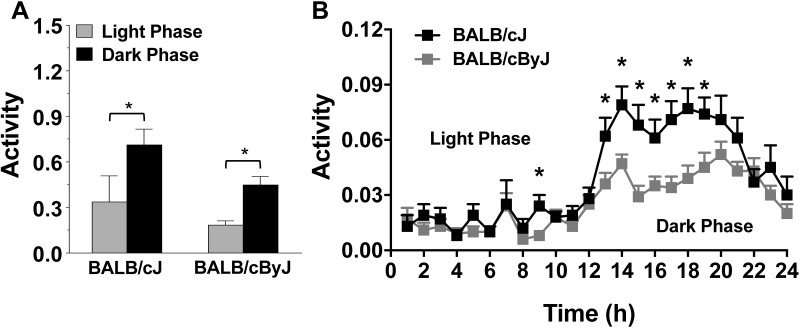
We next used the novel automated DVC system to examine activity levels of BALB/cJ and BALB/cByJ mice in their home cages. As shown in Figure 6 A, both BALB/cJ and BALB/cByJ mice had more activity during the dark phase compared with the light phase (paired t tests after repeated-measures ANOVA, F1,17 = 15.76, P = 0.001). Examination of average DVC activity across the 24-h period confirmed the clear differences between light- and dark-phase activity (Figure 6 B). In addition, the automated system revealed a striking strain-associated difference, with BALB/cJ mice having higher activity than BALB/cByJ across the first 7 h of the dark phase (posthoc comparisons after repeated-measures ANOVA; main effect of strain, F1,16 = 10.71, P = 0.0048; strain×hour interaction, F23,368 = 2.49, P = 0.0002).
Figure 6.
DVC activity levels of BALB/cJ and BALB/cByJ during the light and dark phases. (A) Mice were evaluated for activity by DVC during the light (gray bars) and dark (black bars) phases of the photoperiod. (B) DVC activity of BALB/cJ and BALB/cByJ mice was scored at each hour over 24 h. Data are shown as mean ± SEM (n = 9 cages per strain) of average DVC activity scores across 12 time points per phase. *, P < 0.05.
Comparison of intracage aggression and locomotion with DVC activity scores.
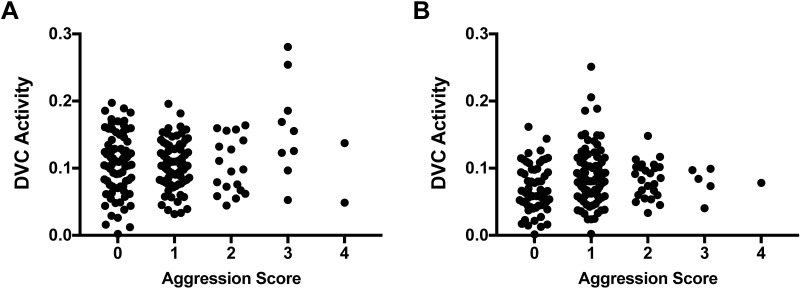
Because we saw higher aggression scores more frequently after the removal-and-reintroduction test than the cage change challenge, we retrospectively examined whether the automated DVC activity scores correlated with the severity of aggression according to a 4-point aggression scoring system during 3 rounds of 10-min observations. A correlational analysis was conducted between the coded observations of aggressive interactions per minute and the corresponding DVC activity measures (Figure 7). The resulting value for R (0.101) was not significant, indicating that DVC activity did not correlate with severity of aggression.
Figure 7.
Scatter plots for DVC activity levels across aggression scores in the removal-and-reintroduction assay. Data were combined for three 10-min tests, with 2 wk between tests. Aggression was manually scored for 1-min periods across each 10-min test, with scores ranging from 0 (no observations of aggression) to 4 (aggression during each 15-s bin across 1 min).
To investigate whether the DVC activity measures differentiated between the 2 forms of high activity, measurements recorded by the DVC system were matched with fighting episodes observed during 10-min direct visual observations among BALB/cJ mice. In the same manner, DVC activity measurements were matched with cages containing BALB/cJ mice that did not display aggressive interactions at any point during 10-min observations but instead demonstrated high amounts of locomotion. Analysis of the scores revealed that DVC activity measures were very similar for the intervals with aggression and locomotion (aggression, 0.32 ± 0.02; locomotion, 0.31 ± 0.01; t22 = 0.299, P = 0.7677 [paired t test]).
Discussion
Intermale aggression remains an issue in laboratory settings and affects animal welfare. In this study, we evaluated 3 forms of environmental enrichment as ways to mitigate these unwanted behaviors in mice. According to the Guide,8 environmental enrichment should be designed to encourage species-specific behaviors and create an intracage atmosphere conducive for coping with secondary stressors, such as routine husbandry and experimental procedures.20 We chose specific behavioral tests that reflected laboratory activities known to provoke aggression. Cage changing and removal-and-reintroduction testing were conducted to assess the effect of housing condition on aggression in each substrain. Enrichment items included cotton squares (a source of nesting material), which provide heat support to aid in thermoregulation and decrease cold stress,12 and a shelter to enable animals to hide during external disruptions. These items were tested in comparison to a bilevel, nonmobile mezzanine, which provides additional floor space, permitting species-specific behaviors including locomotion and exploration and providing a possible area for escape when aggression occurs.
Regardless of the type of environmental enrichment, after cage changing, which completely removes pheromones in scent-marked areas and creates a disturbance in male dominance, BALB/cJ mice demonstrated higher levels of aggressive interactions than BALB/cByJ mice. Although fighting bouts were seen only infrequently after cage changing, these behaviors might have continued beyond the 10-min period of visual recording, as reported in a previous study.5 In the present study, we observed a minimal influence of cage cleaning on aggressive interactions.5,15,25
Interestingly, high levels of aggressive interactions occurred after the removal-and-reintroduction test. In a laboratory setting, extracting a single mouse for experimental purposes might interrupt conformity between submissive and dominant mice, even though scent-marking and other aspects of the primary enclosure remain undisturbed. Although the housing conditions were ineffective at mitigating aggression among BALB/cJ mice, the BALB/cByJ substrain showed significantly less aggressive behaviors after the removal-and-reintroduction test when housed with the mezzanine compared with the cotton square or shelter. Future studies evaluating the use of mezzanines compared with cotton squares and shelters and their effects on social ranking may reveal whether the mezzanine was used territorially or as a refuge to escape.
To evaluate intracage aggression within a 24-h time period, videorecordings were scored for 2 min of each hour within a subset of cages. Scoring specifically consisted of the duration and quantity of aggression, locomotion, sleeping, and any unusual activity, such as stereotypies. Because mice are a naturally nocturnal species, activity scores increased during the dark cycle. Although BALB/cByJ mice slept more during the light phase and displayed locomotion in the dark phase, BALB/cJ mice showed varying amounts of daily activity regardless of light or dark phase. These findings are in line with a previous report indicating that BALB/c mice have highly variable patterns of circadian activity.24
The present study confirmed the ability of the DVC system to detect significant differences in activity during both the light and dark phases in both the BALB/cJ and BALB/cByJ substrains. Some forms of activity, such as grooming or single steps, were not recorded during video observations. However, these movements were likely incorporated in the DVC activity measurements.
We compared visual observations of aggression with DVC activity measurements. Because aggression was observed infrequently after cage changes, we analyzed recorded scores from the removal-and-reintroduction test. Correlation was nonsignificant, perhaps because more severe aggression (3 or 4 fighting bouts within 1 min) was observed only rarely in both substrains. Our comparison between the results of video scoring and the automated activity measures showed that the DVC system did not differentiate between aggression and high levels of locomotion. Although the DVC recorded the overall animal activity within a cage, animals must be in direct contact with bedding on the cage floor for the DVC system to detect movement. In line with this limitation, animals housed with the second-floor mezzanine rather than the cotton square or shelter had lower overall activity level recordings from the DVC system. Furthermore, automated continuous scoring systems are commonly used to assess the frequency and duration of specific behaviors.15,20 Future studies may incorporate manual zero–one scoring for video analysis of the rapid and infrequent behaviors associated with aggression to further compare with data from direct visual observations using the DVC.
During the 10-min observations, we were able to determine that fighting bouts and cages with high amounts of locomotion led to elevated—yet indistinguishable—levels of activity according to the DVC. These high levels of locomotion predominantly occurred immediately after challenge testing and at the initiation of behavioral recordings. More severe forms of aggression might have been recorded with longer direct visual observations, to establish a pattern different from cages displaying high locomotion. Furthermore, the time intervals for DVC activity in this study were set to 1 min, meaning that all activity events were averaged across a time interval of 1 min. It is possible that very brief aggressive behaviors went undetected due to the averaging of activity over time. Future investigations using the DVC might explore whether reducing the time intervals for DVC activity averaging might help in identifying brief aggressive behaviors. Overall, further examination of the DVC's ability to detect an aggressive pattern is necessary, due to the infrequent occurrence of fighting bouts.
Body weight and health were assessed on a weekly basis in addition to standard daily examinations. Weights were used as a relatively noninvasive, benign marker to assess overall stress (occurring as weight loss and decreased feed intake). Mice were received and weighed starting at 3 wk of age, and BALB/cJ mice weighed more than BALB/cByJ mice at 5 wk. Although body weights continued to steadily increase with age regardless of substrain or enrichment item, indirectly indicating that stress levels were acceptable, physiologic parameters such as corticosterone would have been a more direct measure of stress levels. Furthermore, these findings indicate that any differences between substrains in aggression or activity level were not attributable to overt differences in body size. Only one cage, which contained BALB/cJ mice in the shelter enrichment group, was removed from the study due to wounds, most likely from fighting, refractive to medical treatment. It is notable this particular cage showed no inconsistencies in weight gain leading up to its endpoint; therefore, it appears the fighting or stressful event was more of an acute event rather than a chronic one.
In conclusion, this study demonstrated different effects of a bilevel form of enrichment between 2 substrains of mice. Introducing the mezzanine reduced aggression after a removal-and-reintroduction challenge in the more docile substrain that we tested. However, alternative forms of enrichment may be necessary to mitigate antagonistic behaviors among aggressive substrains. Furthermore, the DVC system may serve as a multipurpose research and clinical tool by enabling assessment of generalized cage activity over time and identifying ‘high-activity’ cages for further evaluation for evidence of aggression, thus supporting early intervention and benefitting animal welfare.
Acknowledgments
We thank Fabio Iannello (Tecniplast) for his time and effort in providing information regarding the DVC system. We thank Jacob Lott for his work in data collection and the UNC Mouse Behavioral Phenotyping Laboratory (particularly Viktoriya Nikolova and Natallia Riddick) for their expertise and assistance in conducting challenge tests. We thank Dr Victoria Baxter for her assistance with manuscript preparation. We thank the husbandry staff of the University of North Carolina at Chapel Hill for ensuring clean cages with appropriate enrichment items were readily available at all times. This project was supported by funds from the Department of Comparative Medicine (University of North Carolina–Chapel Hill) and from NIH grant NICHD U54HD079124 (for the Mouse Behavioral Phenotyping Laboratory).
References
- 1.Barthold SW, Griffey SM, Percy DH. 2016. Pathology of laboratory rodents and rabbits. Ames (IA): Wiley Blackwell. [Google Scholar]
- 2.Clipperton-Allen AE, Cragg CL, Wood AJ, Pfaff DW, Choleris E. 2010. Agonsitic behavior in males and females: effects of an estrogen receptor β agonist in gonadectomized and gonadally intact mice. Psychoneuroendorinology 35:1008–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT. 2015. Laboratory animal medicine, 3rd ed. Cambridge (MA): Elsevier. [Google Scholar]
- 4.Gaskill BN, Stottler AM, Garner JP, Winnicker CW, Mulder GB, Pritchett-Corning KR. 2017. The effect of early life experience, environment, and genetic factors on spontaneous home-cage aggression-related wounding in male C57BL/6 mice. Lab Anim (NY) 46:176–184. [DOI] [PubMed] [Google Scholar]
- 5.Gray S, Hurst J. 1995. The effects of cage cleaning on aggression within groups of male laboratory mice. Anim Behav 49:821–826. [Google Scholar]
- 6.Haemisch A, Gartner K. 1994. The cage design affects intermale aggression in small groups of male laboratory mice: strain-specific consequences on social organization and endocrine activations in 2 inbred strains (DBA/2J and CBA/J). J Exp Anim Sci 36:101–116. [PubMed] [Google Scholar]
- 7.Hutchinson EK, Avery AC, Vandewoude S. 2012. Environmental enrichment during rearing alters corticosterone levels, thymocyte numbers, and aggression in female BALB/c mice. J Am Assoc Lab Anim Sci 51:18–24. [PMC free article] [PubMed] [Google Scholar]
- 8.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 9.Joanny P, Chouvet G, Giannellini F, Vial M. 1984. Brain diurnal levels of adenosine 3′,5′ cyclic monophosphate in C57BL/6 and BALB/C mice. Chronobiol Int 1:37–40. [DOI] [PubMed] [Google Scholar]
- 10.Kaliste EK, Mering SM, Huuskonen HK. 2006. Environmental modification and agonistic behavior in NIH/s male mice: nesting material enhances fighting but shelters prevent it. Comp Med 56:202–208. [PubMed] [Google Scholar]
- 11.Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ. 2013. The resident–intruder paradigm: a standardized test for aggression, violence, and social stress. J Vis Exp 77:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lockworth CR, Kim SJ, Liu J, Palla SL, Craig SL. 2015. Effect of enrichment devices on aggression in manipulated nude mice. J Am Assoc Lab Anim Sci 54:731–736. [PMC free article] [PubMed] [Google Scholar]
- 13.Maestripieri D, Alleva E. 1991. Litter defense and parental investment allocation in house mice. Behav Processes 23:223–230. [DOI] [PubMed] [Google Scholar]
- 14.Miczek KA, Maxson SC, Fish EW, Faccidomo S. 2001. Aggressive behavioral phenotypes in mice. Behav Brain Res 125:167–181. [DOI] [PubMed] [Google Scholar]
- 15.Miller AL, Kitson GL, Skalkoyannis B, Flecknell PA, Leach MC. 2016. Using the mouse grimace scale and behaviour to assess pain in CBA mice following vasectomy. Appl Anim Behav Sci 181:160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morahan PS, Breinig MC, McGeorge MB. 1977. Immune responses to vaginal or systemic infection of BALB/c mice with herpes simplex virus type 2. J Immunol 119:2030–2036. [PubMed] [Google Scholar]
- 17.Nicholson A, Malcolm RD, Russ PL, Cough K, Touma C, Palme R, Wiles MV. 2009. The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci 48:740–753. [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa S, Makino J. 1984. Aggressive behavior in inbred strains of mice during pregnancy. Behav Neural Biol 40:195–204. [DOI] [PubMed] [Google Scholar]
- 19.Õkva K, Nevalainen T, Pokk P. 2013. The effect of cage shelf on the behaviour of male C57BL/6 and BALB/c mice in the elevated plus-maze test. Lab Anim 47:220–222. [DOI] [PubMed] [Google Scholar]
- 20.Patel TP, Gullotti DM, Hernandez P, O'Brien WT, Capehart BP, Morrison B, 3rd, Bass C, Eberwine JE, Abel T, Meaney DF. 2014. An open-source toolbox for automated phenotyping of mice in behavioral tasks. Front Behav Neurosci 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng H, Yang XP, Carretero OA, Nakagawa P, D'Ambrosio M, Leung P, Xu J, Peterson EL, González GE, Harding P, Rhaleb NE. 2011. Angiotensin II-induced dilated cardiomyopathy in BALB/c but not C57BL/6J mice. Exp Physiol 96:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rasmussen S, Miller MM, Filipski SB, Tolwani RJ. 2011. Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. J Am Assoc Lab Anim Sci 50:479–483. [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenthal N, Brown S. 2007. The mouse ascending: perspectives for human-disease models. Nat Cell Biol 9:993–999. [DOI] [PubMed] [Google Scholar]
- 24.Rosenwasser AM. 1990. Circadian activity rhythms in BALB/c mice: a weakly coupled circadian system? J Interdiscipl Cycle Res 21:91–96. [Google Scholar]
- 25.Swetter BJ, Karpiak CP, Cannon JT. 2011. Separating the effects of shelter from additional cage enhancements for group-housed BALB/cJ mice. Neurosci Lett 495:205–209. [DOI] [PubMed] [Google Scholar]
- 26.Sztainberg Y, Chen A. 2010. An environmental enrichment model for mice. Nat Protoc 5:1535–1539. [DOI] [PubMed] [Google Scholar]
- 27.Toth LA. 2015. The influence of the cage environment on rodent physiology and behavior: implication for reproducibility of preclinical rodent research. Exp Neurol 270:72–77. [DOI] [PubMed] [Google Scholar]
- 28.Van de Weerd HA, Van Loo PL, Van Zutphen LF, Koolhaas JM, Baumans V. 1997. Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim 31:133–143. [DOI] [PubMed] [Google Scholar]
- 29.Van Loo PLP, Kruitwagen CLJJ, van Zutphen LFM, Koolhaas JM, Baumans V. 2000. Modulation of aggression in male mice: infuence of cage-cleaning regimen and scent marks. Anim Welf 9:281–295. [Google Scholar]
- 30.Van Loo PLP, Mol JA, Koolhaas JM, Van Zutphen BF, Baumans V. 2001. Modulation of aggression in male mice: influence of group size and cage size. Physiol Behav 72:675–683. [DOI] [PubMed] [Google Scholar]
- 31.Van Loo PLP, Van der Meer E, Kruitwagen CLJJ, Koolhaas JM, Van Zutphen LFM, Baumans V. 2003. Strain-specific aggressive behavior of male mice submitted to different husbandry procedures. Aggress Behav 29:69–80. [Google Scholar]
- 32.Van Loo PL, Van de Weerd HA, Van Zutphen LF, Baumans V. 2004. Preference for social contact versus environmental enrichment in male laboratory mice. Lab Anim 38:178–188. [DOI] [PubMed] [Google Scholar]
- 33.Van Loo PLP, Zutphen LFM, Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab Anim 37:300–313. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Ding W, Sun B, Jing R, Huang H, Shi G, Wang H. 2011. Targeting of colorectal cancer growth, metastasis, and antiapoptosis in BALB/c nude mice via APRIL siRNA. Mol Cell Biochem 363:1–10. [DOI] [PubMed] [Google Scholar]