Abstract
The emotional state of domestic animals is an essential component of the assessment of their welfare. In addition, sensitivity to various rewards can be a valuable indicator when investigating these states. We aimed to design an exploration test and a contrast test that did not evoke fear and anxiety in C57BL/6N mice but that instead were perceived as positive experiences and that might be used to assess sensitivity to various rewards. The exploratory arena had a larger central area and 8 smaller sections containing various objects. Motivation (measured as latency to enter the arena under conditions of increasing weight of the entrance door), anticipation (measured as latency to enter the arena under conditions of increasing delay in opening the entrance door), and the numbers of visits to the different sections were evaluated during a 5-min session in the arena. In the contrast test, after traversing a runway, half of the mice received a tasty reward (hazelnut cream), whereas the others received a neutral reward (food pellet) at the far end. Latency to reach the reward was recorded. After baseline training, rewards were swapped for half of the mice from each category for 3 d, to establish a negative and positive contrast. Mice were both motivated and showed anticipation to enter the exploration arena; after entering, they were active and visited many sections. In the contrast test, latency during the baseline period was longer for mice given the neutral reward compared with the tasty reward. Compared with baseline, latency during the postshift phase decreased for the positive-contrast group (neutral–tasty reward pattern) but did not differ for the negative-contrast group (tasty–neutral reward pattern). Overall, both tests seemed to be positive experiences for the mice and showed potential for use to investigate reward sensitivity.
In recent years, the interest in developing methods to measure emotional states in domestic animals has increased.4 One of the aims of this approach is to be able to use the emotional state as an additional estimate when assessing the welfare of animals. The animal welfare science community generally agrees no single ‘gold standard’ assessment of animal welfare is available. Instead, several different parameters including health, physiology, and behavior need to be evaluated and compared to give a comprehensive picture of the state of the animals.6 Being able to assess emotional states would provide insight into positive dimensions of animal welfare and give indications of how animals themselves experience their situation when the other measures of welfare point in different directions.
The theoretical basis of many of the concepts that aim to measure emotional states is that an individual's background mood (that is, long-lasting emotional state) is affected by its environment and exposure to various situations. Depending on the nature of these situations, the background mood might be more positive or negative. The background mood has been suggested to influence how an animal perceives or appraises a short-term situation and, consequently, how it responds to that situation.35 Thus, an animal's response to brief exposure to an emotion-inducing situation can reflect its background mood.20
Concepts such as judgment bias and anticipation have been explored as measures of emotional state and have resulted in new test methods and observations.10,24,29 In addition, play behavior and exploration have been investigated as potential indicators of positive emotional states, relevant for animal welfare.4 However, sometimes the outcomes of the tests do not follow the hypothesis, such as when animals housed in a barren environment show a more optimistic response in the cognitive bias test, indicating that other factors such as boredom and fear perhaps influence reactions in the tests.36 In addition, tests and methods might need to be modified for particular species and applied settings (for example, on a farm or in a laboratory animal facility).
In the current study, our main aim was to adapt and further develop established tests and methods that can be used to assess primarily positive emotional states in mice by measuring reward sensitivity. We used mice as a model species to develop methods to assess positive emotional states in domestic animals as well as to find methods for assessing welfare in mice themselves. Most traditional behavioral tests for rodents are based on the approach–avoidance conflict and focus on negative emotional aspects such as anxiety.3 The focus of the method development that we present here was to design test arenas and test procedures that were free from negative elements that might affect animals’ willingness to explore. The goal was that the mice would experience the tests as positive so that they can be used as indicators of the mice's sensitivity to different rewards. Furthermore, we wanted to base the tests on free exploration (that is, being free to choose whether to explore or not), because the ability to choose to explore can affect both the quantity and quality of exploration.9,21 In both of the tests we developed, the mice were free to enter the test arena from a start box: being placed ‘by force’ into a novel arena to explore can induce neophobic responses in rodents.9,21 Our intention was to establish tests that we could later use to assess emotional state in mice. At that stage, we plan to induce different background moods in mice by keeping them in different housing environments.
The first test was based on exploration in a large arena, with the aim of investigating whether the set-up motivated the mice to repeatedly visit the arena. Exploration has been suggested to be an evolutionary important behavior for mice to perform, given that it provides them with information about their surroundings and might lead them to different resources.1 A major determinant of behavior in animals is a need to reduce environmental uncertainties.13 When an animal is satiated and does not need to search for food, then the drive to reduce environmental uncertainty is dominant, and the animal will perform information-gathering behavior (for example, patrolling).13 Therefore, exploration is likely a relevant behavior to observe when assessing reward sensitivity, because most species are motivated to perform it, that is, they are fulfilling a behavioral need.4
To test the rewarding magnitude of different resources, the animal's motivation or anticipation to get access to that reward (for example, palatable food, a preferred environment, the company of conspecifics) can be quantified. To assess whether mice experienced the set-up of our exploration test as positive, we included measures of motivation and anticipation to enter the arena to explore. Measuring elasticity of demand is a way of assessing how much an animal is willing to work or ‘pay’ to get access to a certain resource.18 The push-door has been used previously to measure motivation in mice, by increasing the resistance or weight that mice have to push open to get access to a resource.30 Push-doors have also been used in other species, including rats17 and chickens.23,37 In addition, anticipatory behavior can be influenced by previous experience of positive or negative experiences and thus be an indicator of reward sensitivity. For example, rats housed in standard environment performed more anticipatory behavior when expecting a reward compared with rats in an enriched housing.33 The authors suggested that the rats in the standard housing had a poorer welfare and were not able to fulfill their behavioral needs to the same extent as the rats in the enriched housing.33 A common method to measure anticipation in animals is by quantifying behaviors performed in expectation of certain events.29 This event can be either positive, such as receiving a food reward, or negative, such as being exposed to a squirt of water. Various species, such as rats, mink, and pigs perform more behavioral transitions, that is, changing from walking to running to grooming and so on,34 when expecting a positive reward. In mice, anticipation for a scheduled food reward has been measured as increased wheel running,11,14 and rats have been shown to perform an anticipatory response to getting access to an enriched cage.33
The contrast test was the second behavioral test we used in the current study. The principle of this test is based on findings that brief positive and negative emotional states can be induced by shifting the magnitude of an expected reward, so-called successive positive or negative contrast.7 These emotional states are said to correspond to the emotions of elation and joy compared with frustration and disappointment, respectively.4,5 Rats have been used frequently to assess contrast effects, commonly by measuring their latency to cover a distance (for example, a runway) to reach a reward of a certain magnitude. For example, rats that were deprived of an enriched environment displayed a stronger negative contrast effect than rats remaining in the enriched environment.5 These results indicate that the contrast test might be used to assess the background mood of animals.5 To our knowledge, contrast tests have not previously been performed in mice on runways. However, successive negative contrast has been measured in mice by using the intake of fluids of different sucrose concentrations.22
In the current study, our aim was to develop a contrast test and an exploration test that were based on free exploration and that the mice experienced as positive. The motivation and anticipation of male and female mice to get access to the exploration arena were measured, and contrast effects that occurred after a change in reward magnitude were investigated.
Materials and Methods
Animals and housing.
The regional ethics committee for animals used for scientific purposes in Uppsala, Sweden, approved the study according to C133/12, and studies were performed in accordance with the European Directive 2010/63/EU and Swedish animal welfare legislation. C57BL/6NCrl mice (24 female and 27 male; age, 4 wk; Charles River, Sulzfeld, Germany) arrived at the animal facilities at National Veterinary Institute in Uppsala, Sweden. On arrival, the mice were housed in same-sex trios in polycarbonate cages (800 cm2). Mice were randomly (within sex) designated to trios and cages. All cages contained aspen bedding (Tapvei, Estonia) a cardboard house (mouse house, DesRes, Brogaarden, Denmark), and soft paper as nesting material, and mice had free access to water and standard feed (Lab for R3, Lantmännen, Sweden). The room temperature was controlled at 21 to 23 °C and the relative humidity at 45% to 65%. The animals were kept on a reversed light cycle with lights on from 1900 to 0700. After 1 wk of acclimation, all mice were weighed (females, 16.2 ± 0.8 g; males, 18.4 ± 1.4 g), and 2 mice per cage were marked with ear punching; the third animal in each cage remained unmarked. Weighing was repeated at 13, 21, and 24 wk of age. To reduce negative experiences of handling,12 we turned the cardboard house in the cage upside down and let the mice climb onto it instead of lifting the mice by the tail. This handling was performed at all times, whether at cage cleaning or moving mice to and from test arenas.
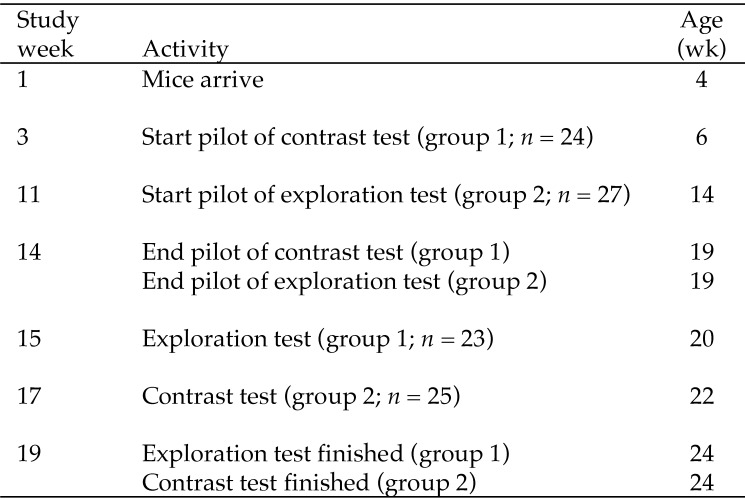
After 2 wk of acclimation, the mice were used for a pilot evaluation of the test equipment and test procedures. Half of the mice (both sexes) were used for the contrast test (group 1), and the other half were used for the exploration test (group 2; Figure 1). By using information from the pilot evaluation, we developed test protocols for each test, and mice participated in the test to which they were naïve (Figure 1). Only the methods and results from this second round are presented. One person performed the exploratory test, another person ran the contrast test, and a third person observed the behaviors present in video recordings of the exploratory test.
Figure 1.
Overview of test procedures for the exploration and contrast tests in mice.
Exploration test.
Exploratory arena.
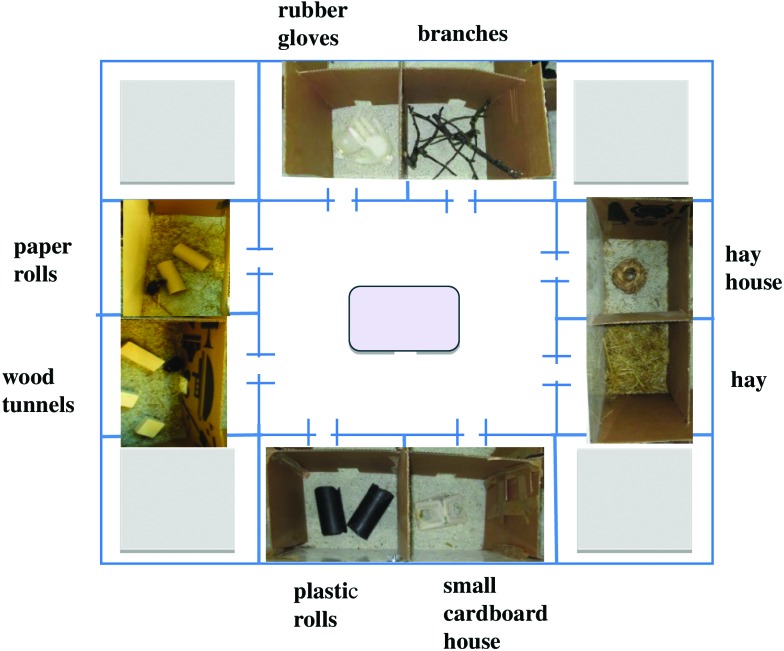
The exploratory arena was 100 × 100 × 50 cm (W × L × H) and divided into 9 sections by pieces of cardboard. One section was the larger central area (50 × 50 cm) where the start box was placed. It was surrounded by 8 smaller sections (25 × 25 cm) that could be entered from the central area (Figure 2). There were no connections between the smaller sections, so the mice had to return to the central area to enter another section. Each small section contained one type of object or objects (Figure 2). One object was a pair of rubber gloves which had been worn for around 5 min by the experimenter and then turned inside out and filled with water. The other objects used were branches, hay, a house made of straw, a cardboard house, 3 paper rolls, 2 larger plastic rolls, and wood tunnels (2 short and one long). The floor of the arena was covered with cat litter to minimize odor tracks and increase contrast (when the mice were to be filmed), as was done previously.2 Between test sessions, the cat litter was mixed to distribute any smells more evenly in the arena. The start box was a polycarbonate cage (370 cm2) supplied with aspen bedding and a small cardboard house, but no food or water was available. A piece of acrylic was used as a lid. To assess motivation, the start box was equipped with a push door, which was a short tunnel with an attached hinged door (modified from reference 30). To assess anticipation, the start box was equipped with a sliding door, at the same location as the push door. This start box setup was used for the control group also.
Figure 2.
Outline of the exploratory arena (not to scale), with the central area containing the start box (gray square) and the 8 small sections containing the different objects. The entries into the different sections were open at all times.
Test procedure.
Exploration was evaluated for the level of motivation (opening a push door of increasing weight) and anticipation (behavioral transitions in a start box) the mice showed to get access to the exploration arena. Control mice were placed in the start box for comparison with the anticipation group to investigate differences in behaviors in the start box between animals that were let into the arena compared with moved directly back to the home cage. The control group was never allowed to enter the exploratory arena; the mice were kept in the start box for 3 min and then returned to their home cage.
In total, 4 cages with females (n = 11, one female had been euthanized due to a paw injury) and 4 cages with males (n = 12) were used in the test. The 3 mice in every cage were designated to each of the 3 test groups (motivation, anticipation, and control), and all mice were tested individually. The order of cages tested was balanced between days and the test order within every cage was balanced between test groups and test days. Every other day, females were tested first, and males were tested first on the alternate days. The test was performed from approximately 0830 until 1630 in the animal holding room. The arena was not cleaned between mice and so likely contained numerous odors. The start boxes were cleaned and cardboard houses changed between sexes. Each day, the arena was turned a quarter turn and the objects were randomly moved between the smaller sections, but there was never more than one type of object in each section.
Anticipation.
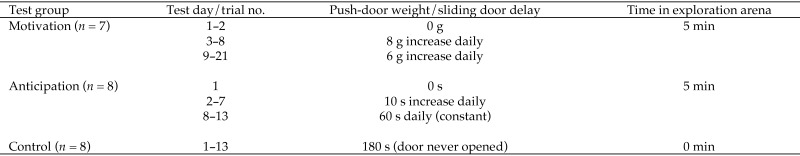
The mice in the anticipation group were released from the start box when the experimenter opened the sliding door. To create anticipation, the delay until door opening was increased progressively. During the first trial, the door was opened immediately after the mouse had been placed in the start box. In trials 2 through 6, the delay was increased by 10 s per trial until a delay of 60 s had been reached. From trial 7 onward, the delay was kept at 60 s (Figure 3). The mice in the control and anticipation groups performed one trial daily for 13 consecutive days, after which the testing was stopped. We assumed that the mice would have had enough time during 13 d to learn what to expect in the test situation.
Figure 3.
Overview of test protocol for assessing motivation and anticipation to reach an exploration arena.
Motivation.
For the motivation group, the push door was open on the first test day. Thereafter the weight of the door was increased for each test day by attaching metal weights to the outside of the door (Figure 3). If a mouse did not pass the push door within 3 min, it was returned to the home cage and retested the next day with the same weight. When the mouse failed to pass through the door when retested, it was excluded from further testing; but if the mouse passed the push door on retesting, it was allowed to continue according to the test protocol. The exception was one mouse that failed on the second day of testing. This mouse was tested the next day with the door completely open again, as it was considered not to have learned the task initially. Only one failure was accepted during the series, and so when a mouse did not pass the push door a second time, it was excluded from further testing. Due to practical issues, mice in the motivation group were tested in one trial daily for 16 consecutive days (trials 1 through 16), had a break for 5 d, and then were tested for another 5 consecutive days (trials 17 through 21).
Behavior observations.
Mice in the anticipation and motivation groups were allowed to explore the arena for 5 min after leaving the start box. The number of visits to different sections (including the start box for the anticipation group) in the exploratory arena was continuously scored. In the start box, the latency to pass the push door (motivation group) and the latency to leave the start box once the sliding door had been opened (anticipation group) were noted. In addition, behavioral differences between the anticipation and control groups while in the start box were observed, by continuously scoring the frequency of the behaviors listed in Figure 4 during the first 60 s in the start box during trials 7 through 13.
Figure 4.
Ethogram of behaviors displayed by mice allocated to the anticipation and control groups during the exploration test. The behaviors are mutually exclusive. ‘House’ refers to the cardboard house that remained in the start box throughout testing.
Contrast test.
The runway.
The runway used for the contrast test was made of white plastic, 140 cm long and 12 cm wide with walls 25 cm high. One end contained a cardboard house of the same type as in the home cage, to provide shelter for the mice in the start area. Along the runway, 2 wooden obstacles (height, 7 cm; length, 13 cm; width, 7 cm) were placed diagonally across the runway (Figure 5). At 30 cm from the other end, another wooden obstacle (height, 4 cm; width, 10 cm) was placed so that the mice had to pass it to enter the goal area, which contained the reward. The goal area was 21 cm long and the start area was 22 cm long, making the total runway distance 97 cm. The same runway and obstacles were used for both sexes, but different start boxes and cardboard screens (see later section) were used for males and females. The runway and obstacles were cleaned with 70% alcohol between cages, and between mice when a mouse had urinated or defecated in the arena and when the reward had been hazelnut cream (see later section).
Figure 5.
The contrast test arena, viewed from the start area.
Treatments and test procedure.
Ten female mice (4 cages, 2 females received the incorrect type of reward during baseline and were omitted from the analysis) and 15 males (5 cages) participated in this test. The test was performed from 0900 until approximately 1300 in the animal holding room. The day before the first test day, the mice were habituated to the runway by allowing them to freely explore the arena for 3 min with its designated reward in the goal area and all the obstacles in place. Half of the mice received a neutral reward (regular food pellet) and half of the mice a tasty reward (55 ± 10 mg hazelnut cream spread on a neutral piece of paper; Änglamark ekologisk hasselnötscreme, Coop, Sweden). The mice had been allowed to taste the hazelnut cream in their home cages 3 d before the habituation day. All mice were exposed to 7 d of baseline training in the test arena followed by 3 d of contrast effect, with a shift in (hypothesized) reward magnitude (see later section).
For the test procedure, each mouse was transferred to the start area and kept there for 10 s. At this point, the start area was screened off with a cardboard screen toward the rest of the runway. The cardboard screen was then removed, and the mouse was free to enter the runway. Timekeeping started when the mouse left the start area with all 4 paws and stopped when the mouse had stepped into the goal area with all 4 paws. This time was defined as the latency to reach the goal area. The goal area was then screened off with the cardboard screen. For the next trial, the mouse was picked up in the goal area (in its cardboard house) and transferred directly to the start area. The time in the goal area was 5 to 7 s. The maximal time allowed was 120 s in the runway and 60 s in the start area. If the mouse had not left the start area within this time, the trial was considered complete, and the mouse was picked up from the start area and then released again for a new trial. Each mouse was exposed to 3 trials daily, performed directly after one another without any intertrial interval.
To establish a baseline in the latency for the mice to reach the goal area, the mice went through 7 d of training (day 1 through 7, trials 1 through 21) with their designated reward. Thereafter a shift in reward was implemented to investigate the contrast effect. The mice continued with the postshift treatment (that is, with the new reward magnitude) for 3 d (days 8 through 10, trials 22 through 30). The mice were divided into 4 treatment groups: negative contrast (downshift in reward from tasty during baseline to neutral in the postshift phase; n = 7; TN), positive contrast (upshift in reward from neutral to tasty; n = 6; NT), control neutral reward (the same reward during baseline and postshift phases; n = 7; NN), and control tasty reward (n = 5; TT). Every other day, females were tested first, and males were tested first on the alternate days. Within each sex, the start order was balanced between cages and between mice within cages.
Statistical analyses.
Data were analyzed by SPSS (version 22, IBM, Armonk, NY). Data for visits to different sections were normally distributed and analyzed by using a general linear model for comparisons between sexes. The difference over time in the number of visited sections was analyzed by using linear regression, with day as an independent variable. Correlations between sections visited and push-door weight or anticipatory behavior in the exploration test were analyzed by using Spearman rank correlation. The maximal weight of the passed push door and number of visits to different sections in the arena are presented as mean ± 1 SD.
Behavior differences between anticipation and control groups during the first minute in the start box during the exploration test of trials 7 through 13 were analyzed only for the behaviors that we considered to be most relevant for anticipation. These parameters included the number of total behavior transitions, and the specific behaviors of ‘rear door’ and ‘sniff door.’ The number of total behavior transitions was calculated as the number of times a mouse changed from one behavior to another. Data for the mean numbers of transitions and rear door were normally distributed and analyzed by univariate general linear methods, with treatments included as the factor. Sniff door was not normally distributed and therefore was analyzed by using the Mann–Whitney U test.
Effects of treatment on latency to reach the goal area during the contrast test were compared by using the means of different trials. As a measure of baseline, trials 16 through 21 (days 6 and 7 of baseline) were included in the analysis. For postshift phase, the first trial of day 8 (trial 22) was excluded because the mice were unaware of the change in reward at that point, so only trials 23 and 24 were included. In addition trials 25 through 27 and 28 through 30 were included and presented as days 9 and 10 postshift, respectively. Data were not normally distributed and therefore the Mann-Whitney U test was used for comparisons of the 2 baseline treatments and for pairwise comparisons of the contrast effects after the shift from NN to TN and from TT to NT. Differences were considered significant at P values less than 0.05.
Results
Exploration test.
No significant differences were found between sexes for the parameters of push-door weight (P = 0.731, F = 0.133, n = 7), the number of visits to different sections when exploring the arena (P = 0.366, F = 0.88, n = 15; motivation and anticipation group means of trials 1 through 13) and the number of total behavioral transitions in the start cage (P = 0.537, F = 0.40, n = 16, motivation and control group means of trials 1 through 13). Male and female data were pooled for all subsequent analyses.
Behaviors in the start box.
Compared with controls, mice in the anticipation group had more total behavior transitions (P = 0.000, F = 40.2) and showed more sniff-door behavior (P = 0.012) but not rear door (P = 0.324, F = 1.04). The mean frequencies of performance of the other behaviors are presented in Table 1, except for the behaviors ‘stretched attend posture’ and ‘freeze,’ which were never observed for either group, and for ‘escape,’ which was observed only once.
Table 1.
Frequency (mean ± 1 SD) of behaviors performed by mice (n = 8 per group) during the first 60 s in the start box for trials 7 through 13 of the exploration test
| Behavior | Control | Anticipation |
| Transitions | 54.98 ± 3.43 | 68.27 ± 4.84a |
| Sniff door | 5.86 ± 2.79 | 10.02 ± 2.23b |
| Rear door | 1.34 ± 0.57 | 1.79 ± 1.10 |
| In house | 2.57 ± 0 0.62 | 3.07 ± 0.82 |
| Exit house | 1.43 ± 0 0.55 | 1.82 ± 0.74 |
| Off house | 2.86 ± 0 0.53 | 4.30 ± 0.43 |
| Climb house | 3.48 ± 0 0.48 | 5.04 ± 0.67 |
| Rear house | 2.96 ± 1.36 | 4.25 ± 1.68 |
| Rear wall | 3.18 ± 0.45 | 3.34 ± 1.58 |
| Walk | 12.86 ± 1.41 | 14.85 ± 2.50 |
| Run | 2.93 ± 1.35 | 6.16 ± 2.39 |
| Sniff sit | 10.57 ± 1.46 | 8.91 ± 1.86 |
| Move house | 1.23 ± 0.42 | 0.64 ± 0.53 |
| Wall | 3.71 ± 0.48 | 4.18 ± 0.88 |
P < 0.001
P < 0.05
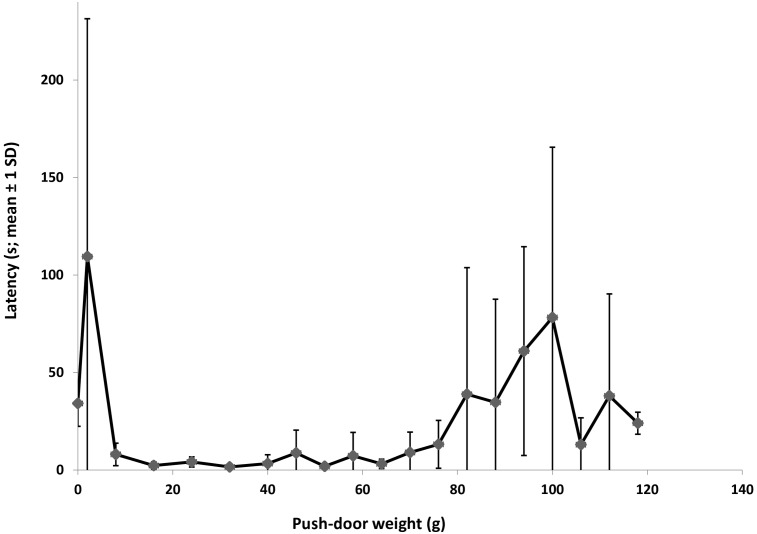
All mice in the anticipation treatment entered the exploratory arena on every trial, and the mean latency to leave the start box was 4.6 ± 5.2 s, once the door was opened. All mice in the motivation treatment entered the exploratory arena on at least 15 test days and managed to pass a weight of 88 g on the push door. The maximal weight passed was 118 g (2 mice), and the mean weight passed was 102.6 ± 11.9 g. This value corresponds to 424% of the mean body weight of females (24.2 ± 1.2 g) and 355% of that of males (28.9 ± 1.5 g). The latency data show that once the mice learned to push open the door, they did so quickly, usually within 10 s of entering the start box, until the door weighed approximately 80 g. As the door became heavier, the mice either failed to pass or latency to pass increased (Figure 6).
Figure 6.
Latency (s; mean ± 1 SD) to leave the start box relative to the weight of the push door for mice in the motivation group during 21 d of testing. Because mice were removed from further testing when they failed to pass the door twice, the number of animals that contributed to the different measuring points varied: 0–64 and 82–88 g, n = 7; 70–76 and 94 g, n = 6; 100 g, n = 4; 106 g, n = 3; and 112–118 g, n = 2.
Activity in the exploratory arena.
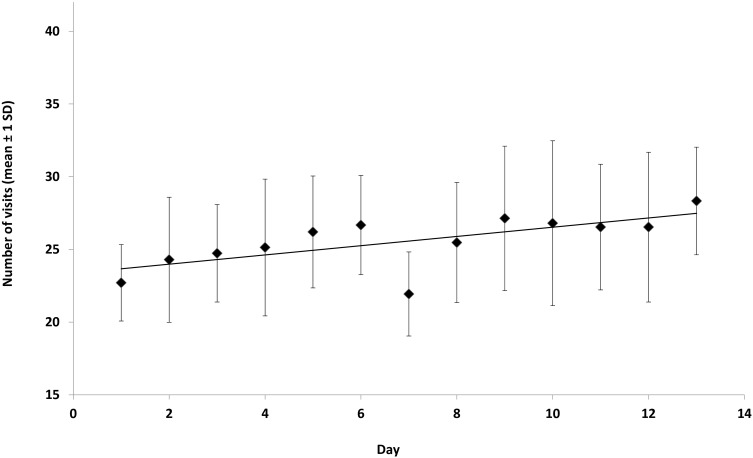
The mean number of visits (anticipation and motivation groups pooled) to the different sections in the exploratory arena during the first 13 d was 25.5 ± 2.8. The number of visits to different sections increased over the experimental period (P < 0.000, F = 15.1; Figure 7). For the motivation group, the weight of the passed push door and mean number of sections visited in the exploratory arena during day 1 to 13 showed no correlation (R = 0.31, P = 0.49). In addition, the anticipation group showed no correlation between the number of behavioral transitions in the start box and the number of sections visited in the exploratory arena during days 7 through 13 (R = 0.11 and P = 0.79).
Figure 7.
Number of visits (mean ± 1 SD) by mice in motivation (M) and anticipation (A) groups (n = 15) to sections in exploration arena (M, 8 sections; A, 9 sections [start box included]) during test days 1 through 13. The black line indicates the slope of the regression.
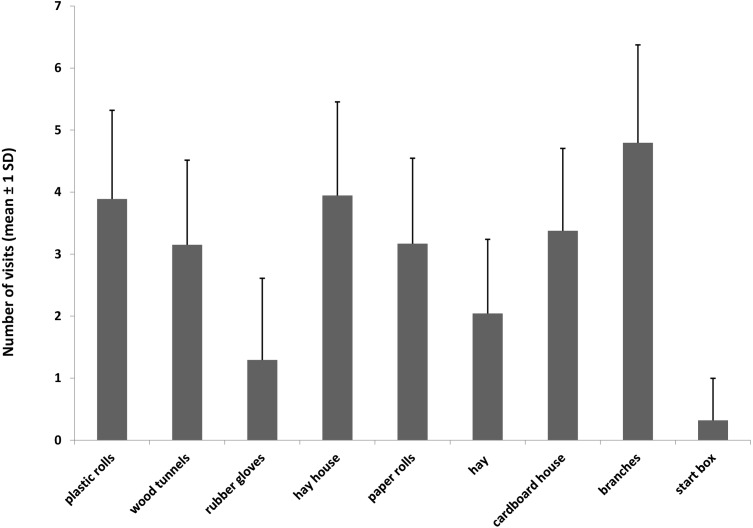
The section containing the branches was visited most frequently, and the least preferred sections of the exploratory arena were those containing the rubber gloves and the start box (Figure 8). However, only the anticipation group was able to enter the start box when exploring the arena because the push door (motivation group) could only be opened in one direction, whereas the sliding door had been removed for the anticipation group.
Figure 8.
Number of visits (mean ± 1 SD) of mice in motivation (M) and anticipation (A) groups (n = 15) to the different sections in the exploration arena according to the objects they contained (only A mice had access to the start box).
Contrast test.
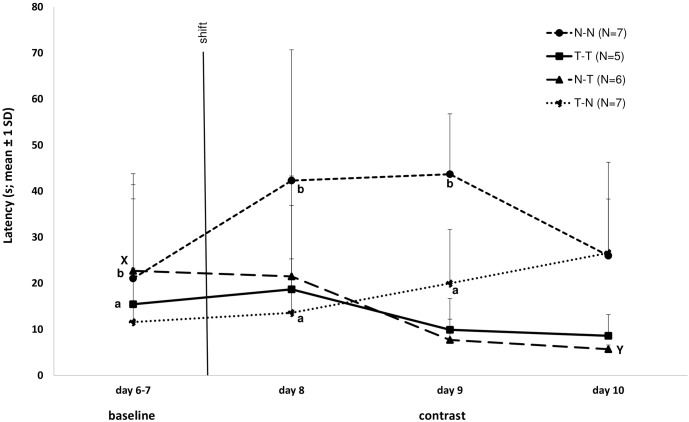
During baseline, latency to reach the goal area did not differ between sexes for either the mice that received the tasty (P = 0.268, 5 females and 7 males) reward or those that received the neutral reward (P = 0.833, 5 females and 8 males). Therefore, male and female data were pooled in subsequent analyses. Latency to reach the goal area during baseline (mean for trial 16 to 21, day 6 to 7) differed between groups, with mice receiving a neutral reward displaying a longer latency compared with mice given a tasty reward (P = 0.03; Figure 9). The negative contrast postshift phase showed a significant pairwise difference in latency between the NN and TN groups for day 8 (P < 0.05) and day 9 (P < 0.05; Figure 9). However, the difference was in the opposite direction to what we expected, and the NN mice showed a greater increase in latency during the postshift phase than the TN mice did (Figure 9). In the positive contrast treatment, NT mice had a numerically shorter latency to reach the goal area during the postshift phase compared with TT mice, but this difference did not reach significance (P = 0.2). The shortest latency was 3 s.
Figure 9.
Latency (s, mean ± 1 SD) to reach the reward for the treatment groups in the contrast test for the 2 last days of the baseline period (days 6 and 7, trials 16–21) and the postshift period (days 8–10, trials 23–30). The black line indicates the shift at trial 22 (the actual latency times for that trial are not included). Letters a and b indicate significant (P < 0.05) differences in latency between groups NN and TN on the same day. Letters X and Y indicate a significant(P < 0.05) difference in latency between days 6–7 and day 10 for the NT group.
Comparing differences within groups revealed an effect over time for NT group, in which latency to reach the reward on day 10 (postshift) was shorter than on days 6 and 7 (baseline; P < 0.05; Figure 9). The TN group showed a statistical trend toward an increase in latency over the same time period (P = 0.063; Figure 9). The NN and TT groups showed no differences in latency between the baseline and postshift periods.
Discussion
The aim of this study was to adapt and further develop previously established tests and methods that have the potential to assess reward sensitivity in mice. Both the exploration and contrast tests demonstrated promise for further use. The mice showed consistent interest in entering the exploration arena, displaying an increased number of behavioral transitions in the start box and passing a push door weighing more than 3 times their own weight. The contrast test revealed a difference in latency to reach the neutral compared with tasty reward during the baseline phase. No significant contrast effects were found, but the latencies changed in the expected direction after the reward was shifted, that is, mice exposed to a positive contrast ran faster and those exposed to a negative contrast ran slower to the reward after the shift. We suggest various modifications for improvement of the contrast test.
Results from the exploration test show that the mice were motivated to enter the arena, as represented by short latencies to leave the start box for mice in motivation and anticipation groups and increased number of behavioral transitions and sniffs of the door in the anticipation group compared with control mice. In addition, mice in the motivation group passed the push door even when it weighed more than 300% of their body weight. This finding indicates that the mice found exploring the arena to be a rewarding and positive experience. An alternative explanation for the short latency times is that the mice found the start box aversive. However, we observed no freezing or risk assessment behaviors in the start box, and it therefore seems more likely that the opportunity to enter the exploration arena motivated the mice to leave the start box. This explanation is supported by previous findings in rats:8 rats that could leave a safe den to explore a complex environment with different compartments had a substantially shorter latency to leave the den than rats that had access to only an open field. In addition, the higher number of behavior transitions performed in the start box by the anticipation group is in accordance with observations in rats and has been interpreted as a sign of positive anticipation.31,33
The limit to the push-door weight in the motivation group might have been decided by the physical capacity of the mice or by insufficient motivation for them to enter the arena. In a previous study,15 12-mo-old CD1 mice (both sexes) pushed a maximum of approximately 100 g to reach enrichment. According to standard weight curves from breeders (for example, Taconic, Envigo), adult CD1 female mice weigh 30 to 35 g whereas males weigh 37 to 45 g (body weight not stated in the paper); consequently the mice in the cited study15 pushed at least 200% of their body weight. To test for physical strength rather than motivation, the previous authors15 measured the maximal weight the mice pushed to reach food (the only food available to them) and found that the mice pushed a maximum of approximately 110 g to reach food. Taken together, mice move weights far greater their own body weight to reach desired resources. Although data are not strictly comparable between different studies using different door designs and different mouse strains, it seems plausible that the mice in our study reached, or were close to, the limit for what they were physically able to push open. This conclusion needs to be verified, however; for example, by giving mice access to food only if they pass the push door (that is, a closed economy [such that the resources are not available anywhere else, only in this setting]).
One main aim of dividing the arena into different sections was to provide an easy measure of activity, that is, the numbers of visits to different sections. The variation in preference (as indicated by the numbers of visits to the different objects) and increase in visit numbers over time suggests that this method yields a valid measure of activity. However, this method does not provide information regarding whether mice were hesitant during early visits or if they explored each section longer in the beginning, when the objects were novel. Some authors13 have suggested that mice might have a bias toward exploring areas that have been visited less frequently recently, to reduce uncertainty about their environment. Repeated visits to the exploratory arena might have rendered it a familiar environment, but the relatively short bouts in the arena, turning the arena, and reorganization of the items in the small sections for every session might have resulted in the mice still perceiving it as an unfamiliar environment that required further exploration. The objects in the arena were chosen for their diversity in biologic relevance to the mice, a feature that might also be reflected in the results. The branches, which might stimulate species-specific behaviors such as climbing, were visited the most frequently. The rubber gloves were the least-visited items, possibly because they did not fulfill any behavioral needs for the mice.
The increase in the number of visits to the different sections over the test period indicates that the mice experienced the arena as positive. This assessment might be supported by the theory that work for a resource that induces a positive effect will increase over time as familiarity and enjoyment increase, whereas work for a resource that only reduces negative effect will be more stable over time.16 Mice seem to have high motivation to visit areas accessible to them, even when a cost is imposed on performing the behavior.26,27 Furthermore, other authors28 suggest that mice find exploratory activity highly motivational and, therefore, will pay a price for an activity that is completely unrelated to the resource they reach. In line with this reasoning, our arena offers possibilities for exploratory activity that are likely rewarding to the mice.
In studies of elasticity of demand for different resources, some aspects are important to consider: the possibility for animals to choose their own bout lengths in the test, and that real elasticity of demand requires a closed economy.18 One reason for the importance of a closed economy is that mice tend to have a relaxed attitude toward resources that they have learned they can access elsewhere (for example, food in the home cage as well as in a test arena).18 Our animals’ resource (a large enriched arena) was accessible only through the test setup and not in the home cage. In addition, we controlled the maximal time in the start box (3 min) and in the test arena (5 min). Bout length seems to be particularly important when the behavior to be performed (after paying the price) has to be completed within a specific period (for example, mating) or of a specific duration to be rewarding (for example, sleep).18 But if mice have an innate need for exploring or revisiting known areas,26,27 perhaps repeated short bouts of opportunities for patrolling or exploring is as rewarding as a single prolonged bout.
Whether it is better to measure motivation or anticipation for assessing the reward sensitivity of mice can be debated. Both measures are influenced by the animals’ previous experiences and their current situation. One assumption is that the animals’ motivation or anticipation at the prospect of gaining access to valuable resources provides a similar interpretation of their emotional state. Mice living in an environment that satisfies their behavioral needs might show less response toward the possibility to explore a new environment, whereas animals that come from a poorer environment might be more motivated and show higher expectation of possible access to other resources, such as a larger area or tasty food.32 Which set up is more sensitive to and valid for assessing reward sensitivity as a result of the animals’ previous experiences can be debated. From a practical point of view, the push-door method requires more learning from the animals and might be more sensitive to failure to solve the task (that is, passing the door) on a single occasion. Furthermore, as already mentioned, testing a physical task benefits from calibration against physical strength. In contrast, the benefit of assessing anticipatory behavior is that the factors just mentioned are of less importance. However, assessment of anticipatory behavior requires more detailed behavioral observations and lacks the definite grading of motivation that is gained from, for example, passing specific weights. In addition, anticipatory behavior might be difficult to assess when positive anticipation to get access to the expected reward transforms into frustration when the animal has to wait a long time for the expected reward.
In the contrast test, 7 d of 3 trials each day successfully revealed a significant difference in baseline latency between groups receiving the neutral reward compared with the tasty reward. Previous studies in rats have used 6 trials per day,5,25 but a more time-efficient test protocol seems possible, at least in mice. In the pilot evaluation of the arenas, we used 5 trials per day but found that this schedule resulted in marked variation between trials, especially between trials 4 and 5. During those trials, the mice performed more exploration of the arena (and occasionally attempted to leave the arena by jumping toward the edge of the walls) than goal-directed behavior to reach the reward. We, therefore, decided to use 3 trials each day; this schedule still resulted in some intertrial variation but to a lesser extent than with 5 trials. The greatest intraindividual variation in latency between trials of the same training or test day occurred in the groups given the neutral reward. Intertrial intervals are known to influence the contrast effect.7 Some trials might be more representative of a subject than others (for example, trial 2 might show the actual motivation to run to the reward, whereas trial 3 is more representative of patrolling behavior), but the sample sizes in our study were too small to reveal any clear pattern. Therefore the mean of all 3 trials was used for the statistical analyses.
Mice that experienced a shift in reward changed their latencies in the expected direction in the postshift phase—that is, the positive contrast group (NT) ran faster whereas the negative contrast group (TN) ran slower—but the change was insufficient to achieve a significant difference compared with the control groups (TT and NN, respectively). The positive contrast group (NT) had a slightly shorter latency than the positive control (TT) after the reward shift, but the difference was too small to be significant. This result could be due to a ceiling effect: the mice were running at their top speed to the tasty reward, such that subjects that experienced a positive contrast could not run faster than the control group when they shifted to the tasty reward.7,19 Delay in reward presentation (for example, by increasing the complexity or length of the runway) may be a way to obviate the ceiling effect and enhance the successive positive contrast effect.7 Although we placed obstacles in the runway to counteract ceiling effects, several mice ran the distance in just 4 or 5 s, and one trial was performed in 3 s.
In future use of the contrast test, we will extend the postshift period to 5 d given that the contrast effect seemed to stabilize later and because other authors5 found an effect on negative contrast in rats after 3 d. We will keep the protocol of 3 trials per day and analyze intertrial variation within subjects. The arena will be modified by upending the obstacle fencing off the goal area, so that the mice will have to jump over it to reach the reward. With the present setup, the mice could stop on top of this obstacle, peer down into the goal area (without entering it) before they learned which reward was present, and thus choose whether to go into the goal area (or not). This situation could result in the decision to return to the runway (which happened occasionally) and likely affected the intertrial variation.
In conclusion, the performance of the mice in the tests suggests that they experienced the set ups as positive and free from negative components; with some modifications of the methodology, these tests might be further evaluated for their potential as tools to assess reward sensitivity in mice. A point to consider is evaluation of the maximal weight mice are physically able to push open, calibrating that against the weight they are motivated to pass to enter the exploration arena. Furthermore, a few modifications to avoid ceiling effects in the contrast test arena should be considered. The next phase of research is to use the tests on mice with potentially different background moods to evaluate the efficiency of the tests as indicators of positive emotional states in mice.
Acknowledgments
This work was financed by the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (in collaboration with the Centre of Excellence in Animal Welfare Science, a Swedish collaborative research platform). We thank Peta Hitchens and Claes Andersson for comments on the manuscript and the animal caretakers at the National Veterinary Institute (Uppsala, Sweden) for assisting with the mice during the study.
References
- 1.Augustsson H. 2004. Ethoexperimental studies of behaviour in wild and laboratory mice. Risk assessment, emotional reactivity and animal welfare. Acta Uni Agri Suec Vet 174: 7–62. [Google Scholar]
- 2.Augustsson H, Dahlborn K, Meyerson BJ. 2005. Exploration and risk assessment in female wild house mice (Mus musculus musculus) and 2 laboratory strains. Physiol Behav 84:265–277. https://doi.org/10.1016/j.physbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Belzung C, Griebel G. 2001. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res 125:141–149. https://doi.org/10.1016/S0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 4.Boissy A, Manteuffel G, Jensen MB, Moe RO, Spruijt B, Keeling LJ, Winckler C, Forkman B, Dimitrov I, Langbein J, Bakken M, Veissier I, Aubert A. 2007. Assessment of positive emotions in animals to improve their welfare. Physiol Behav 92:375–397. https://doi.org/10.1016/j.physbeh.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Burman OHP, Parker RMA, Paul ES, Mendl M. 2008. Sensitivity to reward loss as an indicator of animal emotion and welfare. Biol Lett 4:330–333. https://doi.org/10.1098/rsbl.2008.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dawkins MS. 2003. Behaviour as a tool in the assessment of animal welfare. Zoology (Jena) 106:383–387. https://doi.org/10.1078/0944-2006-00122. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty C. 1982. Incentive contrast: a review of behavioral changes following shifts in reward. Anim Learn Behav 10:409–440. https://doi.org/10.3758/BF03212282. [Google Scholar]
- 8.Genaro G, Schmidek WR. 2000. Exploratory activity of rats in 3 different environments. Ethology 106:849–859. https://doi.org/10.1046/j.1439-0310.2000.00605.x. [Google Scholar]
- 9.Griebel G, Belzung C, Misslin R, Vogel E. 1993. The free-exploratory paradigm: an effective method for measuring neophobic behavior in mice and testing potential neophobia-reducing drugs. Behav Pharmacol 4:637–644. [PubMed] [Google Scholar]
- 10.Harding EJ, Paul ES, Mendl M. 2004. Animal behavior: cognitive bias and affective state. Nature 427:312–312. https://doi.org/10.1038/427312a. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CT, Patton DF, Mistlberger RE, Steele AD. 2010. Palatable meal anticipation in mice. PLoS One 5:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst JL, West RS. 2010. Taming anxiety in laboratory mice. Nat Methods 7:825–826. https://doi.org/10.1038/nmeth.1500. [DOI] [PubMed] [Google Scholar]
- 13.Inglis IR, Langton S, Forkman B, Lazarus J. 2001. An information primacy model of exploratory and foraging behaviour. Anim Behav 62:543–557. https://doi.org/10.1006/anbe.2001.1780. [Google Scholar]
- 14.Kas MJH, van den Bos R, Baars AM, Lubbers M, Lesscher HMB, Hillebrand JJG, Schuller AG, Pintar JE, Spruijt BM. 2004. μ-Opioid receptor knockout mice show diminished food-anticipatory activity. Eur J Neurosci 20:1624–1632. https://doi.org/10.1111/j.1460-9568.2004.03581.x. [DOI] [PubMed] [Google Scholar]
- 15.Latham N, Mason G. 2010. Frustration and perseveration in stereotypic captive animals: is a taste of enrichment worse than none at all? Behav Brain Res 211:96–104. https://doi.org/10.1016/j.bbr.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Makowska IJ, Weary DM. 2013. Assessing the emotions of laboratory rats. Appl Anim Behav Sci 148:1–12. https://doi.org/10.1016/j.applanim.2013.07.017. [Google Scholar]
- 17.Manser CE, Elliott H, Morris TH, Broom DM. 1996. The use of a novel operant test to determine the strength of preference for flooring in laboratory rats. Lab Anim 30:1–6. https://doi.org/10.1258/002367796780744974. [DOI] [PubMed] [Google Scholar]
- 18.Mason G, McFarland D, Garner J. 1998. A demanding task: using economic techniques to assess animal priorities. Anim Behav 55:1071–1075. https://doi.org/10.1006/anbe.1997.0692. [DOI] [PubMed] [Google Scholar]
- 19.Mendl M, Burman OHP, Parker RMA, Paul ES. 2009. Cognitive bias as an indicator of animal emotion and welfare: emerging evidence and underlying mechanisms. Appl Anim Behav Sci 118:161–181. https://doi.org/10.1016/j.applanim.2009.02.023. [Google Scholar]
- 20.Mendl M, Burman OHP, Paul ES. 2010. An integrative and functional framework for the study of animal emotion and mood. Proc Biol Sci 277:2895–2904. https://doi.org/10.1098/rspb.2010.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misslin R, Cigrang M. 1986. Does neophobia necessarily imply fear or anxiety? Behav Processes 12:45–50. https://doi.org/10.1016/0376-6357(86)90069-0. [DOI] [PubMed] [Google Scholar]
- 22.Mustaca AE, Bentosela M, Papini MR. 2000. Consummatory successive negative contrast in mice. Learn Motiv 31:272–282. https://doi.org/10.1006/lmot.2000.1055. [Google Scholar]
- 23.Olsson I, Keeling L. 2002. The push-door for measuring motivation in hens: laying hens are motivated to perch at night. Anim Welf 11:11–19. [Google Scholar]
- 24.Roelofs S, Boleij H, Nordquist RE, van der Staay FJ. 2016. Making decisions under ambiguity: judgment bias tasks for assessing emotional state in animals. Front Behav Neurosci 10:1–16. https://doi.org/10.3389/fnbeh.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosas JM, Callejas-Aguilera JE, Escarabajal MD, Gómez MJ, de la Torre L, Agüero >Á, Tobeña A, Fern ndez-Teruel A, Torres C. 2007. Successive negative contrast effect in instrumental runway behaviour: a study with Roman high- (RHA) and Roman low- (RLA) avoidance rats. Behav Brain Res 185:1–8. https://doi.org/10.1016/j.bbr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Sherwin CM. 1996. Laboratory mice persist in gaining access to resources: a method of assessing the importance of environmental features. Appl Anim Behav Sci 48:203–213. https://doi.org/10.1016/0168-1591(96)01027-1. [Google Scholar]
- 27.Sherwin CM, Nicol CJ. 1996. Reorganization of behaviour in laboratory mice, Mus musculus, with varying cost of access to resources. Anim Behav 51:1087–1093. https://doi.org/10.1006/anbe.1996.0110. [Google Scholar]
- 28.Sherwin CM, Nicol CJ. 1998. A demanding task: using economic techniques to assess animal priorities. A reply to Mason et al. Anim Behav 55:1079–1081. https://doi.org/10.1006/anbe.1997.0694. [DOI] [PubMed] [Google Scholar]
- 29.Spruijt BM, van den Bos R, Pijlman FTA. 2001. A concept of welfare based on reward evaluating mechanisms in the brain: anticipatory behaviour as an indicator for the state of reward systems. Appl Anim Behav Sci 72:145–171. https://doi.org/10.1016/S0168-1591(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 30.Tilly SLC, Dallaire J, Mason GJ. 2010. Middle-aged mice with enrichment-resistant stereotypic behaviour show reduced motivation for enrichment. Anim Behav 80:363–373. https://doi.org/10.1016/j.anbehav.2010.06.008. [Google Scholar]
- 31.van den Bos R, Meijer MK, van Renselaar JP, van der Harst JE, Spruijt BM. 2003. Anticipation is differently expressed in rats (Rattus norvegicus) and domestic cats (Felis silvestris catus) in the same Pavlovian conditioning paradigm. Behav Brain Res 141:83–89. https://doi.org/10.1016/S0166-4328(02)00318-2. [DOI] [PubMed] [Google Scholar]
- 32.van der Harst JE, Baars AM, Spruijt BM. 2003. Standard housed rats are more sensitive to rewards than enriched housed rats as reflected by their anticipatory behaviour. Behav Brain Res 142:151–156. https://doi.org/10.1016/S0166-4328(02)00403-5. [DOI] [PubMed] [Google Scholar]
- 33.van der Harst JE, Fermont PCJ, Bilstra AE, Spruijt BM. 2003. Access to enriched housing is rewarding to rats as reflected by their anticipatory behaviour. Anim Behav 66:493–504. https://doi.org/10.1006/anbe.2003.2201. [Google Scholar]
- 34.van der Harst JE, Spruijt BM. 2007. Tools to measure and improve animal welfare: reward-related behaviour. Anim Welf 16:67–73. [Google Scholar]
- 35.Veissier I, Boissy A, Desire L, Greiveldinger L. 2009. Animals’ emotions: studies in sheep using appraisal theories. Anim Welf 18:347–354. [Google Scholar]
- 36.Wichman A, Keeling LJ, Forkman B. 2012. Cognitive bias and anticipatory behaviour of laying hens housed in basic and enriched pens. Appl Anim Behav Sci 140:62–69. https://doi.org/10.1016/j.applanim.2012.05.006. [Google Scholar]
- 37.Widowski TM, Duncan IJ. 2000. Working for a dustbath: are hens increasing pleasure rather than reducing suffering? Appl Anim Behav Sci 68:39–53. https://doi.org/10.1016/S0168-1591(00)00088-5. [DOI] [PubMed] [Google Scholar]