Abstract
NSAID analgesics may confound models that require inflammation to mimic disease development in humans. This effect presents a challenge for veterinary staff and investigators, because surgery is often necessary to create mouse models of disease and NSAID are first-line analgesics used to treat postoperative pain. We evaluated robenacoxib, a NSAID highly selective for cyclooxygenase 2, in a carrageenan paw edema (CPE) assay and surgical model of venous thrombosis (VT). We generated a mouse-specific dose–response curve by using the CPE assay for robenacoxib doses of 3.2, 10, 32 and 100 mg/kg SC. Electronic von Frey assay, calipers, and novel software for measuring open-field activity revealed that all robenacoxib doses provided, identified effective analgesia at 3 and 6 h, compared with saline. In addition, the 100-mg/kg dose had measurable antiinflammatory effects but yielded adverse clinical side effects. Because the 32-mg/kg dose was the highest analgesic dose that did not decrease paw swelling, we evaluated it further by using the same nociceptive and behavioral assays in addition to a novel nest-consolidation test, and assessment of blood clotting and hematologic parameters in the surgical VT model. A single preemptive dose of either 32 mg/kg SC robenacoxib or 5 mg/kg SC carprofen protected against secondary hyperalgesia at 24 and 48 h. Neither drug altered clot formation or hematology values in the VT model. The open-field activity software and our novel nest consolidation test both identified significant postoperative discomfort but did not differentiate between saline and analgesia groups. In light of these data, a single preemptive subcutaneous dose of 32 mg/kg of robenacoxib or 5 mg/kg of carprofen did not impede this VT mode but also failed to provide sufficient postoperative analgesia.
Abbreviations: CPE, carrageenan paw edema; eVF, electric von Frey; VT, venous thrombosis
Venous thrombosis (VT) affects 900,000 people and results in 300,000 deaths annually in the United States.43 The Surgeon General has called for research using translational animal models to elucidate the pathogenesis and molecular mechanisms of venous thromboembolism and to find therapeutic treatment or prevention options.50 At this time, preclinical studies using surgical models of VT frequently withhold postoperative analgesia due to concerns regarding the introduction of a confounding experimental variable.15 The most common analgesic options significantly affect thrombus weight and formation.15 In addition, unalleviated pain and surgery itself may serve as scientific variables, by suppressing the immune system.38,39 As IACUC-approved studies must consider alternatives to potentially painful or distressing procedures, the medical literature should be reviewed regularly to identify new means by which to refine research studies.47 Therefore, we sought to identify a novel analgesic that alleviated postoperative pain in a murine VT surgical model yet minimally affected clot parameters.
To find an appropriate analgesic option for mice undergoing this surgery, or other procedures that currently necessitate withholding pain medication, we assessed a novel NSAID, robenacoxib. Robenacoxib is highly selective for cyclooxygenase 2, (COX2:COX1, approximately 27:1)21 and is currently approved for the control of pain and inflammation associated with musculoskeletal disorders and soft-tissue injuries of cats and dogs in the United States, Canada, United Kingdom, and Australia.3,4,42,48 Although the analgesia provided by robenacoxib is similar to other commonly used NSAID, such as carprofen and meloxicam, in dogs,9,14 robenacoxib purportedly has fewer side effects because it is rapidly cleared from the plasma compartment and circulation and has high tissue selectivity, targeting the site of inflammation and thus increasing the safety profile for kidneys and other nontarget organs.21 Referring to a preclinical study in rats,21 we selected doses using a logarithmic scale whose range encompassed both the rat dose and an allometrically derived mouse dose of robenacoxib. We assessed the drug's antiinflammatory properties by using a well-validated assay for assessing pain and inflammation,44 the carrageenan paw edema test (CPE).
In accordance with our analgesic refinement goals, we assessed 2 novel indicators of pain: the MouseTrapp behavior-recording system and nest consolidation. Proxy indicators such as nest building,11 voluntary wheel running,5 and burrowing2 are indirect measures of an animal's normal behavior that are performed in the absence of an observer.8 The decrease or absence of these behaviors might indicate altered wellbeing for these animals, including potential pain or illness. Other indirect measures, such as the Mouse Grimace Scale, have been validated to detect spontaneous pain in mice accurately.23,25 These tools are critical in compensating for the spontaneous pain sensations that measurements of evoked pain, such as mechanical, thermal, and chemical assays, might miss. Evoked measures, although valuable, do not represent the resting level of pain but rather the nociceptors that are stimulated, and recent studies have demonstrated discrepancies between evoked and spontaneous measures of pain.7,24,33,49 In addition, indirect or proxy indicators can be used to demonstrate change or results when direct measures are not feasible. The behavioral recording system MouseTrapp has been validated for anxiety research, and uses an Android-based, touchscreen tablet as a floor space to monitor movements of mice within a specially designed observation cage.27 Hindpaw incisional pain induces anxiety-like behavior in rats26 and this response is linked to mechanical hypersensitivity during the early phase of postoperative pain,6 however parameters outside of traditional evaluation of thigmotaxis and distance traveled have not been evaluated. MouseTrapp enables measurements such as velocity or speed, total number of paw touches, total number of rears, and duration of rears in addition to the traditional evaluations used to evaluate postprocedural behavior in both CPE and VT surgical models. Nest consolidation is a novel, modified application of nest zone clearance11,37 and has been used to quantify pain-related nesting depression. In our study, only 4 squares of nesting material were used, and the nest could be built anywhere in the home cage, thus simplifying the scoring system. Nest consolidation was evaluated in our surgical model only. We compared both of the proxy indicators (nest consolidation and MouseTrapp analysis) with the traditional von Frey assay. Evoked pain was assessed by using electronic von Frey testing (eVF), a frequently used analgesiometric assay; this assay relies on mechanical sensitivity, which serves as a metric of tissue sensitivity to provide an indication of pain in rodent surgical models.28
The purposes of this preliminary study were: 1) to evaluate whether robenacoxib is appropriate as a single subcutaneous dose for the treatment of acute postsurgical pain; 2) to determine whether the unique properties of robenacoxib (such as its short half-life in the blood) can be used advantageously to prevent interference with surgical VT models in mice; and 3) to assess 2 new proxy indicators of pain in conjunction with conventional methods in an attempt to identify and further refine options for monitoring postoperative pain. We hypothesized that 1) robenacoxib administered subcutaneously 60 min prior to surgery would provide postsurgical analgesia equivalent to that of carprofen, a commonly used NSAID in laboratory animal medicine; 2) robenacoxib would not significantly interfere with thrombus formation or thrombus weight; and 3) robenacoxib would have a minimal effect on hematologic parameters.
Materials and Methods
Animals.
All animal procedures were approved by the University of Michigan IACUC. The study involved 88 male Swiss Webster (CFW) mice (Charles River Laboratories, Raleigh, NC; age, 5 to 8 wk; mean body weight at baseline [time 0], 28.9 g). Outbred male mice were chosen because the study was designed for proof-of-concept. Mice were housed with nesting material enrichment (Cotton squares, Ancare, Bellmore, NY) on a ventilated rack (Allentown Caging, Allentown, NY) in temperature- and humidity-controlled rooms on a 12:12-h light:dark cycle (lights on, 0500 to 1700 [0600 to 1800 during Daylight Savings Time]) in compliance with the Guide.18 Mice were fed irradiated chow (LabDiet 5LOD, PMI Nutrition International, Brentwood, MO) without restriction, and had continuous access to reverse-osmosis–purified water through auto-watering systems. All mice used in this study were in good health and were SPF36 for pathogens according to inhouse rodent surveillance guidelines and as described previously.15 All research is presented in accordance with the ARRIVE guidelines.36 Mice were randomly assigned to treatment groups, and all data were collected by the same female lab member who was blinded to the mice's treatment.
Experimental design.
Dose–response study.
On arrival, mice were housed in groups of 3 or 4 according to date of birth and weight and acclimated to their new housing for at least 3 d before beginning 2 d of acclimation to the analgesic and inflammation assays. Acclimation to assays occurred between 1500 and 1800. Assay acclimation entailed transportation of mouse cages to the procedure space for the day, handling, and introduction to the assays. During this process, individual mice were weighed, and placed into MouseTrapp behavior-recording units for 10 min.13 Mice were subsequently transferred to the von Frey platform for 10 min of acclimation to the testing space before brief exposure to the von Frey probe 3 times, in the same way as for data collection. Mice were then restrained for measurement of sagittal pawpad thickness (iGaging Absolute Origin 0 to 6 in. digital electronic caliper, San Clemente, CA). Three measurements were taken on each hindpaw. Assays were completed on each cage of mice as a group. The day after acclimation was complete, animals underwent this same order of testing for collection of data at baseline and after injection of robenacoxib, except for the addition of electronic von Frey (eVF) measurements, which were collected by using an anesthesiometer (IITC Life Science, Woodland Hills, CA) with a 90-g probe with rigid tips on both the left and right hind paws. A total of 3 paired measurements were collected, with 3 min between data collection sessions. Baseline data were collected for 2 d before paws were injected and postinjection data were collected. Afternoon baseline data were collected between 1200 and 1500 and evening baseline data between 1800 and 2100, to capture any behavioral differences that might occur between light and dark phases.22,40,41
All of the mice in a cage were anesthetized together in an induction box by using isoflurane (Fluriso, MWI, Boise, ID) in 100% oxygen as the carrier gas, and their eyes were lubricated; and they then were placed in sternal recumbency, and anesthesia during injections was maintained by using a nosecone. Mice received approximately 0.2 mL subcutaneously of robenacoxib (3.2, 10, or 32 mg/kg) or saline over the dorsum, as randomly assigned; they then were turned to dorsal recumbency and a line was drawn between the metatarsophalangeal joints of the first and fifth digits to ensure consistency of caliper measurements. As done during acclimation and baseline exposure, true baseline caliper measurements for each paw were collected under anesthesia. The left hindpaw was prepped by using a chlorohexidine solution before the injection of 30 μL λ-carrageenan (1% [w/v] in 0.9% saline Sigma, St Louis, MO) into the paw pad. All carrageenan solutions were made as described previously,37 stored in sterile vials, and used within 24 h of production. The same batch of carrageenan was used for all assays. Postinjection measurements were taken immediately on the injected paw. To ensure consistent anesthesia exposure time, all mice remained anesthetized until every one of the procedures were completed on all animals, after which they were recovered under a heat lamp. Mice were returned to their home cage once they were alert and mobile. MouseTrapp, eVF, and caliper data were collected at 3, 6, 12, 24, and 48 h after injection, as described earlier. After the 48-h MouseTrapp and eVF data collection, mice were anesthetized as for injections, and euthanized by means of bilateral pneumothorax. After euthanasia was confirmed, final caliper measurements were taken, and the hindpaws were harvested and placed into formalin.
Nine animals were removed from this part of the study due to concerns regarding variations in analgesic dosing. These animals (2 to 4 from each dose assessed) were replaced 1:1 and tested after the initial 10 groups of 4 and assessed in addition to 2 control animals to ensure appropriate blinding and that appropriate randomization was maintained. Even distribution of treatment groups and analysis comparing the saline-treated animals in both groups revealed no significant difference between results collected (data not shown), and we deemed it appropriate to combine with the originally collected data. Because analysis of the mice that received 3.2, 10, or 32 mg/kg or saline did not result in any significant antiinflammatory effects, we tested a higher dose of robenacoxib (100 mg/kg SC) in an additional 10 animals and compared them with the 2 saline-treated animals.
Murine VT surgical model.
Mice were acclimated to individual housing for at least 3 d before beginning a 1-d baseline measurement collection to allow for accurate interpretation of the nesting assay being used. Animals were processed as described earlier, with the addition of a novel nest-consolidation test and postmortem clot and hematologic assessments. Baseline nest consolidation data were collected after completion of eVF data in lieu of caliper measurements. Baseline measurements were collected between 1000 and 1200.
The 32-mg/kg robenacoxib dose was the highest analgesic dose that did not decrease paw swelling in the part I dose discovery study, and therefore was chosen for evaluation in part II. For the surgical model, we compared robenacoxib (32 mg/kg) with carprofen (5 mg/kg, the recommended dose in mice)10 and saline (to represent the current standard of withholding analgesia for a surgical VT model, by using complete ligation of the inferior vena cava [IVC] in adult male mice). Mice were randomly assigned to receive a subcutaneous dose of robenacoxib, carprofen, or saline 60 min prior to the start of surgery. At the 60-min mark, mice were anesthetized with isoflurane gas anesthesia in 100% oxygen in an induction box, their eyes were lubricated, and they were placed in dorsal recumbency and maintained using a nosecone. A midline laparotomy for a model of IVC ligation was performed as previously described.51 Mice recovered from anesthesia and were replaced into their home cage, where any existing nest was removed and replaced with new nesting material (described below) to begin nest consolidation. The same female laboratory member performed all surgeries.
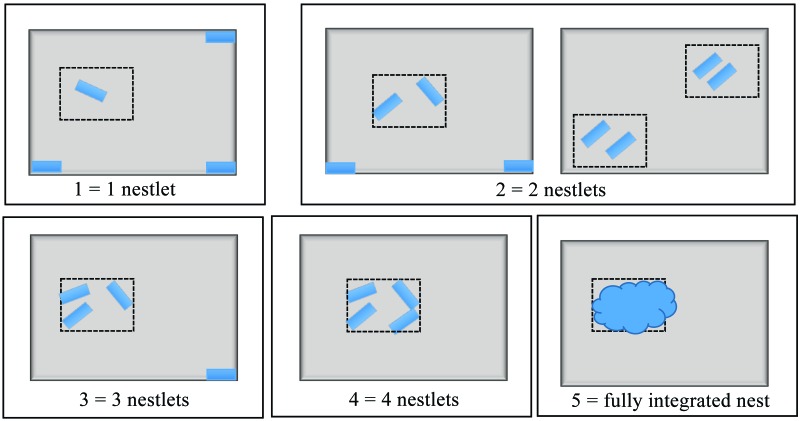
For the nest consolidation assay, one quarter of a cotton square (Nestlet, Ancare, Bellmore, NY) was placed in each of the 4 corners of the cage; that is, 4 pieces of nesting material total. The mouse then was placed in the center of the cage, and nest consolidation was scored (scale, 1 to 5; Figure 1) 15 min after provision of the nesting material, at baseline and immediately after recovery from surgery. In addition, nest consolidation of the 4 cotton pieces supplied at surgery was scored at the 4-, 10-, 24-, and 48-h postsurgical time points.
Figure 1.
Nest consolidation scoring. Scoring system for nesting consolidation had a scale of 1 to 5. Four pieces of nesting material were placed in the corners of the animal's home cage. Animals were placed in the center of the cage, and scores were assed 15 min after placement for both baseline and surgery. After surgery, additional data were collected at each of the postoperative behavioral assessment time points (4, 10, 24, and 48 h), and scoring was performed by using a 2.5×3.5-in. card. Scores: 1, one piece moved away from the corner of the cage; 2, 2 pieces moved from the corners of the cage and touching or within the area of the measuring card, or 2 groups of 2 pieces touching or within the area of the measuring card; 3, 3 pieces moved from the corners of the cage and touching or within the area of the measuring card; 4, 4 pieces moved from the corners of the cage and touching or within the area of the measuring card; and 5, a fully integrated nest including all 4 pieces of nesting material.
In addition, mice underwent MouseTrapp and eVF assessments as described earlier. To assess the effects on hematologic parameters, terminal cardiac blood samples were collected in EDTA containers for CBC at the time of tissue harvest. Postmortem examination and histology on collected tissues (thrombi and associated vein wall) were performed on each animal after the final assessment at 48 h after surgery, to assess any changes in clot formation or size and healing between test groups. Postmortem examination included measurement of the length of clot while still in the mouse and removal of the entire clot with the associated vein wall. The removed IVC and thrombus were carefully dissected to remove overlying connective tissue and then were weighed together and as individual components.
We initially planned to use 30 mice to assess robenacoxib, carprofen and saline in the VT surgical model (n = 10 per group). Equipment failure of the eVF probe prompted analysis of an additional 4 mice, included in a second shipment of animals, which were merged into the study timeline to ensure collection of sufficient eVF data per group. Of the 34 animals assessed in the VT surgical model, an additional 2 died under anesthesia. Complications were not evenly distributed among the groups, and complete data were not collected from all mice, due to a variety of reasons (Table 1). Consequently, all data from each assay were assessed, but not all animals in a group were represented in each dataset. Rather than completely excluding all mice with incomplete data, we decided to evaluate any and all viable data. This decision was most noteworthy for mice from which we were unable to collect eVF data because of equipment failure but from which MouseTrapp, nesting, blood, and clot data were available.
Table 1.
Animal numbers for surgical model
| Measurement | Saline | Carprofen | Robenacoxib |
| eVF | 8a | 9a | 8 |
| MouseTrapp | 9 | 11 | 8 |
| Bloodwork | 8b | 11 | 8 |
| Clot and IVC data | 9 | 9c | 8 |
| Nest consolidation | 9 | 11 | 8 |
| Histology | 9 | 9c | 8 |
Total number of mice assessed in each group after the removal of animals with incomplete data due to eVF probe failure (a), clot in blood sample (b), or no thrombus formed (c).
Statistical analysis.
Statistical consultation was obtained from the Center for Statistics, Computing, and Analytics Research at the University of Michigan. Statistical analysis was performed by using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA). Data were normally distributed, and eVF, caliper, MouseTrapp, and nest consolidation data were evaluated by using both 1- and 2-way repeated-measure ANOVA with Dunnet or Tukey posthoc analysis. Two-way repeated-measure ANOVA with Tukey multiple comparison for posthoc testing was performed to compare data for caliper measurements, mechanical threshold data, MouseTrapp activities, and nesting consolidation testing either within a dose or within an analgesic treatment across multiple time points. In addition, differences between doses or analgesic treatments were calculated for each time point and used to compare analgesic treatments or doses by using one-way ANOVA. CBC, thrombus, and IVC data, which were normally distributed, were evaluated by using 1-way ANOVA with Dunnet posthoc analysis. For all analyses, a P value less than 0.05 was considered significant.
Results
Dose–response study.
eVF data.
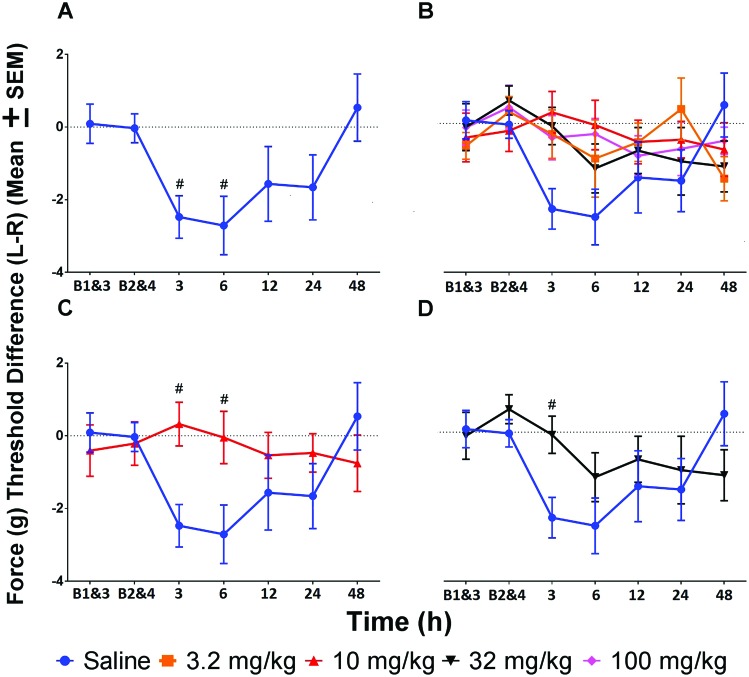
Data were evaluated both as a comparison between saline and various robenacoxib doses at each time point as well as within each treatment over time. Analysis of the effect of saline over time showed decreased mechanical threshold at 3 and 6 h. All robenacoxib doses, when assessed for change over time from baseline, showed no difference in mechanical threshold at any time point. Comparison of robenacoxib doses with saline at various time points showed significantly (P < 0.05) increased mechanical threshold for 10 mg/kg robenacoxib at 3 and 6 h and for 32 mg/kg at 3 h (Figure 2 A through D).
Figure 2.
Force threshold (g) difference (mean ± SEM) between the left (injected paw), and right (control paw). Afternoon baselines (B1 and B3) were averaged, and evening baselines (B2 and B4) were averaged. (A) Tolerance at 3 and 6 h was decreased compared with averaged afternoon and evening baseline measurements. (B) Tolerance did not differ significantly compared with averaged afternoon and evening baseline measurements for any dose of robenacoxib. (C) Mice treated with 10 mg/kg robenacoxib showed significantly increased tolerance at 3 and 6 h when compared with saline-treated animals. (D) Animals given 32 mg/kg robenacoxib showed significantly increased tolerance at 3 h when compared with saline-treated animals. #, P < 0.05 compared with baseline; *, P < 0.05 compared with saline at a given time point.
MouseTrapp analysis.
Analysis of the total number of touches revealed significant (P < 0.05) differences over time from baseline within individual treatment groups. Other measures recorded by using MouseTrapp were less sensitive, showing no treatment effect. Data were evaluated as change over time from baseline within a treatment group and as difference between robenacoxib dose and saline at a given time point. Although the number of total touches did not identify differences between saline and any dose at any time point, it revealed significant (P < 0.05) decreases from baseline measurements in the nonanalgesia control group at 6 and 12 h after paw injection, whereas the 3.2- and 32-mg/kg drug groups showed no decrease over time. The 10-mg/kg group had a significant decrease at 12 h from baseline, and the 100-mg/kg had a significant decrease from baseline at all time points except 24 h. Total distance data showed the same pattern as total touches for the 100-mg/kg group, but significant findings over time were not identified for any other dose. The rest of the measures collected by MouseTrapp software (time to take a step, speed, percentage of touches in the outer 50% of the screen, percentage of touches in the inner 50% of the screen, number of rears, and duration of rears) showed only a single significant postinjection change between saline and any dose: at 48 h, saline-treated mice took significantly (P < 0.05) less time to take a step than mice treated with 10 mg/kg. Significant (P < 0.05) differences emerged within groups when change over time from baseline was assessed, showing decreased speed at 48 h in mice treated with 100 mg/kg robenacoxib, decreased time in the inner 50% of the tablet at 48 h for the 3.2-mg/kg group, and decreased time in the outer 50% of the tablet at 12 and 48 h for mice given 32 mg/kg robenacoxib.
Caliper measurements.
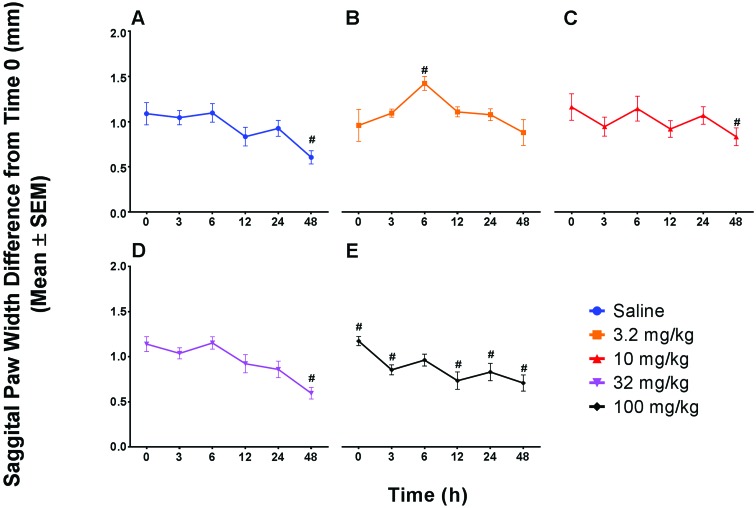
Data were evaluated both as a comparison between saline and robenacoxib dose at each time point as well as within each treatment group over time. Both percentage inhibition and swelling were analyzed. Percentage inhibition did not differ between any robenacoxib dose and saline or change over time within any of the treatment groups. Swelling alone yielded only a single significant (P < 0.05) difference between saline and any dose, with mice given 3.2 mg/kg robenacoxib showing increased paw swelling at 6 h compared with saline-treated animals. Change over time for saline-treated animals showed a significant (P < 0.05) decrease in paw thickness at 48 h when compared with baseline. In addition, significant (P < 0.05) changes from baseline emerged, with an increase at 6 h in mice treated with 3.2 mg/kg robenacoxib and decreases at 48 h for mice treated with either 10 or 32 mg/kg. Animals treated with 100 mg/kg robenacoxib showed a significant (P < 0.05) decrease in swelling compared with baseline at all time points except 6 h. Animals that received a 100-mg/kg dose of robenacoxib were subjectively noted to be ill-thrift, with marked hunching and an unkempt appearance at 3 and 6 h after surgery. In addition, 5 of the 10 mice that received the 100-mg/kg dose had matted fur and potential drainage at the robenacoxib injection site, with one animal also having subcutaneous edema on the right flank, which resolved by the 48-h time point (Figure 3 A through E).
Figure 3.
Sagittal paw width difference (mm; mean ± SEM) between the left paw before and after injection of carrageenan (30 μL) for all doses and control. Change over time within a dose group was significantly different from baseline in the (A) saline-treated mice at 48 h (mean difference, 0.48 mm); (B) the 3.2 mg/kg robenacoxib group at 6 h (shows increased swelling compared with saline); (C) 10 mg/kg robenacoxib at 48 h (mean difference, 0.33 mm; with a 0.15-mm difference from the saline mean difference in the direction of increased swelling); (D) 32 mg/kg robenacoxib at 24 and 48 h (mean difference, 0.54 mm; with a 0.06-mm difference from the saline mean difference in the direction of decreased swelling, and most similar to saline); (E) 100 mg/kg robenacoxib at all time points except 6 h, and overall showed less swelling over time. #, P < 0.05 relative to baseline.
Surgical VT model.
eVF data.
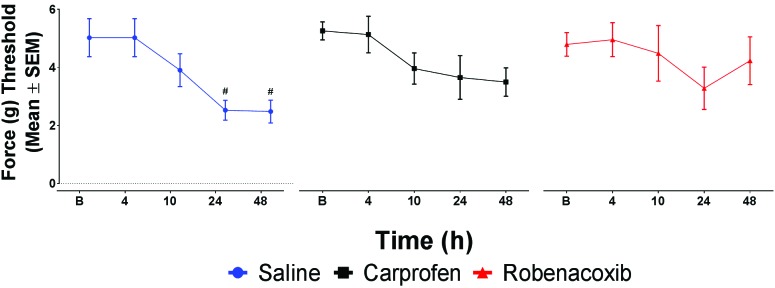
No treatment groups differed significantly at any time point. In assessing change over time within treatment groups, saline showed a significant (P < 0.05) drop from the baseline force-gram threshold at 24 h and 48 h (secondary hyperalgesia). Neither robenacoxib nor carprofen showed a significant decrease in the force-gram threshold from baseline, thus indicating protection from secondary hyperalgesia (Figure 4).
Figure 4.
Force threshold (g) of combined left and right paw measurements (mean ± SEM) shows that analgesics protected mice from developing a secondary hyperalgesia after surgery, because saline-treated mice demonstrated significantly reduced force threshold at 24 and 48 h. #, P < 0.05.
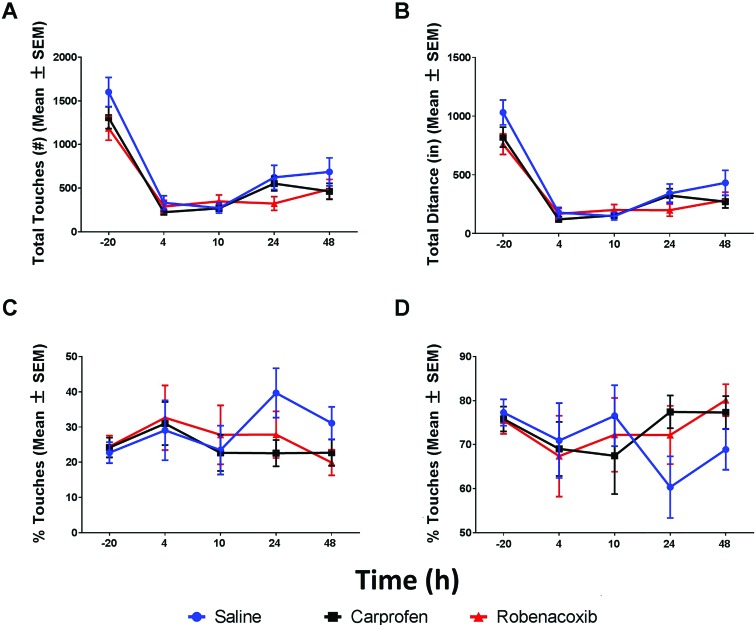
MouseTrapp data.
Analysis of total touches and total distance identified significant (P < 0.05) decreases in spontaneous movement after surgery when comparing baseline with all postoperative time points in all treatment groups (Figure 5 A and B). The only difference between saline and any dose was for robenacoxib at baseline for total touches and total distance. Evaluation of other MouseTrapp measures showed significant differences compared with saline-treated amimals, with robenacoxib-treated animals moving more rapidly at 4 h and with carprofen-treated mice showing an increase in time spent in the outer 50% of tablet at 24 h (P < 0.05). In comparison to baseline, time to take a step was increased at 48 h for saline-treated mice, at 24 h with robenacoxib mice, and at 4, 24, and 48 h with carprofen groups. Time spent in the inner 50% of the tablet was increased at 24 h in the saline group. Finally, increases from baseline were seen in number of rears at 48 h for carprofen and in the duration of rears at 4 and 10 h with saline, at 4 h for robenacoxib, and at 4 and 10 h with carprofen. Overall, calculation of the amount of time spent in the inner or outer 50% of the tablet floor space showed no evidence of increased or decreased thigmotaxis in any treatment group (Figure 5 C and D). The rest of the measures collected by using MouseTrapp software did not consistently identify significant changes after surgery between groups or within groups over time
Figure 5.
(A) Total touches (no., mean ± SEM) showing change from baseline for saline and all doses of robenacoxib. All doses show a significant decrease from baseline at all postsurgery time points. (B) Total distance (in., mean ± SEM) showing change from baseline for saline and all doses of robenacoxib. All doses show a significant decrease from baseline at all postsurgery time points. (C) Percentage movement in the inner 50% of the tablet (%; mean ± SEM) shows no significant change from baseline for saline and all dosesof robenacoxib. (D) Percentage of movement in the outer 50% of the table (%; mean ± SEM) shows no significant change from baseline for saline and all doses.
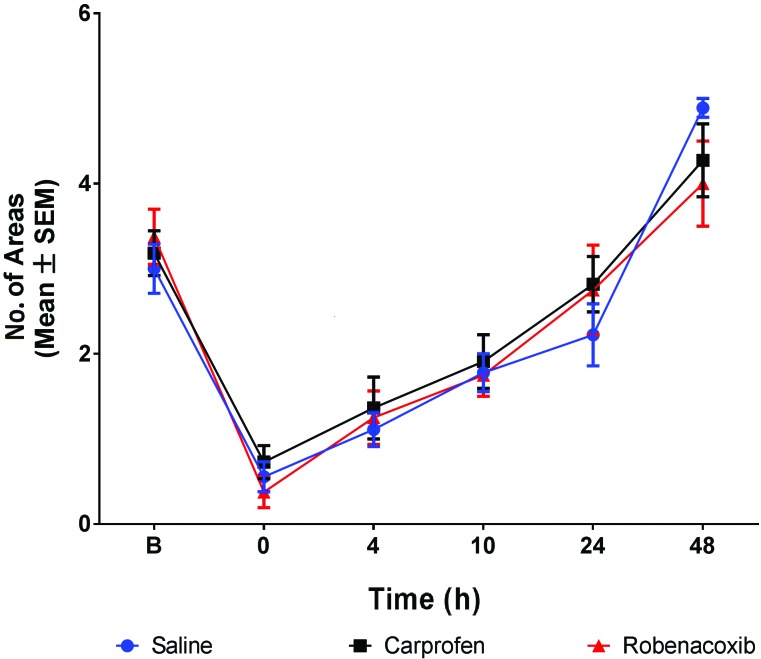
Nest consolidation score.
Nest consolidation scores did not differ significantly between robenacoxib and saline groups at any time point. All groups showed similar ability for nest consolidation over time. In particular, mice regained their baseline 15-min nest consolidation score by 24 h after surgery (Figure 6).
Figure 6.
Nest consolidation (no. of areas cleared; mean ± SEM). Significant change from baseline can be seen for all nest consolidation treatment groups at all time points except 24 h (P < 0.05). Nest consolidation did not differ significantly between saline and either robenacoxib or carprofen at any time point.
IVC and clot data.
No measurement of thrombus weight, IVC weight, combined IVC and thrombus weight, or total clot length differed significantly between any treatment groups.
CBC analysis.
There were no significant differences or biologic effects noted between saline-, robenacoxib- or carprofen-treated groups for any of the following measurements: RBC count, Hgb, Hct, MCV, MCH, MCHC, RBC distribution width, platelet count, MPV, WBC count, neutrophils (percentage and count), lymphocytes (percentage and count), monocytes (percentage and count), eosinophils (percentage and count), and basophils (percentage and count).
Venous thrombus histology.
At 48 h after thrombus formation, a board-certified veterinary pathologist evaluated venous thrombi from experimental groups for surface organization of fibroblasts and presence of WBC and endothelial cells. Thrombus structure did not differ between groups (data not shown).
Discussion
This study was as an initial evaluation of the therapeutic analgesic efficacy of robenacoxib in mice. The ultimate goals were to 1) determine a dose of robenacoxib that provided effective analgesia in mice, 2) compare this dose with carprofen, and 3) assess whether the high tissue selectivity and reported short circulatory half-life of robenacoxib21 might be exploited by administering a single subcutaneous dose to provide relief from surgical pain without interference with surgical VT models in mice. To refine pain assessment in mice, we assessed proxy indicators of pain in conjunction with well-established methods.
Using the traditional eVF assay, we found that all doses of robenacoxib showed antinociceptive properties when compared with saline in the CPE model. Data collected from MouseTrapp, the new proxy indicator method assessed in this study, supported eVF results, identifying the efficacy of treatment. In addition, MouseTrapp data for all robenacoxib doses less than 100 mg/kg paralleled previously published findings, which showed no change in distance traveled in a rat paw incision model.26
Caliper data indicated no inhibition of paw swelling for 3 of the 4 doses assessed in this study. Only the 100-mg/kg robenacoxib dose reduced paw edema. Data from the doses we tested for alleviation of pain and inflammation associated with the CPE model indicated that robenacoxib is not a potent antiinflammatory agent in mice, although it did provide some level of analgesia. This finding was unexpected given the robust inhibition of swelling shown by robenacoxib in preclinical research involving rats.21 In assessing this difference, we present 2 possible reasons for our divergent findings. First, despite the validation of calipers for assessing paw edema for the CPE model in mice,31,35 perhaps this assay was not sufficiently sensitive to fully detect the change in edema between our treatment groups; the assay was only able to detect changes in paw swelling for the highest dose (100 mg/kg). A more sensitive assay, like plethysmometry,35 might be required in future research on robenacoxib in mice, given that this method would account for changes in the entire volume of the paw. Second, although we commonly use the same dose of NSAID in both mice and rats, dose extrapolation between these 2 species might be inappropriate for robenacoxib. This explanation is plausible in light of the studies that have illustrated differences in drug processing between rats and mice, including significant pharmacokinetic differences with carprofen, another COX2-selective NSAID, which showed an increased terminal half-life and sensitivity to toxicity in lactating animals.17,30 In addition, conflicting findings regarding the efficacy of several NSAIDs in mice, including meloxicam45,46 and carprofen,1,20,29 have been reported. If robenacoxib is a weak antiinflammatory agent in mice, this drug may prove to be a valuable analgesic option for murine studies in which the presence of inflammation is critical, such as VT models.
Given our eVF, caliper, and MouseTrapp data combined with clinical presentation, we chose the 32-mg/kg dose of robenacoxib for further assessment in the VT model. Although the 10-mg/kg dose also demonstrated analgesic efficacy in the CPE model, the VT model requires surgery, which causes considerably more pain than paw injection, so we selected the higher dose. In addition, we deemed the 32-mg/kg dose the better choice for the surgical model because it was the highest dose of robenacoxib that provided analgesia with no measurable effect on inflammation, and it did not produce the negative side effects of the 100-mg/kg dose (ruffled hair coat, hunching, and depressed activity as measured by decreased total touches).
Assessment of the 32-mg/kg dose of robenacoxib in the surgical VT model by using the eVF assay showed that a single, subcutaneous dose prevented secondary hyperalgesia after surgery. However, the single dose provided insufficient analgesia after the surgical VT procedure, as reflected by nesting and ambulation parameters. Data from the proxy indicators supported the eVF data. Both MouseTrapp and nest consolidation evaluation discriminated healthy, unmanipulated mice from postsurgical animals. In addition to a decrease in the total number steps in the CPE model, postsurgical pain resulted in a decrease in the total distance traveled by all treatment groups. This difference between procedures most likely corresponds to a general unwillingness to move associated with the greater pain produced by a surgical procedure compared with carrageenan paw injection. This interpretation of data seems plausible, because decreased total distance also occurred in the CPE animals that appeared clinically unwell, specifically the group treated with the highest dose of robenacoxib assessed (100 mg/kg).
Comparison of robenacoxib with carprofen in the eVF, MouseTrapp, and nest consolidation assays yielded no significant differences. As with robenacoxib, a single subcutaneous dose of carprofen prevented secondary hyperalgesia but provided insufficient analgesia after the surgical VT procedure, according to nesting and ambulation parameters.
Assessment of the hematologic effects measured in this study showed that neither robenacoxib nor carprofen had any effect on clot formation, as assessed by the presence, weight, size, or histologic characteristics of IVC thrombi. In addition, neither robenacoxib nor carprofen altered CBC values. We had hoped that robenacoxib would have these effects, in light of the drug's brief time in the bloodstream in other species,12,21 however we were surprised to see lack of effect with carprofen given the previous findings with this drug in this same surgical VT model.15 A previous study revealed a significant decrease in thrombus weight and increased numbers of circulating monocytes in C57BL/6 mice at 48 h after surgery.15 The inconsistencies found between the previous study15 and our current one might be due to well-documented differences between strains of mice16,32,34 or, more likely, dosing regimens. In comparison to the single, preemptive 5-mg/kg dose of carprofen that we evaluated here, the mice in the earlier study15 received 5 mg/kg carprofen twice daily for 48 h. This use of multiple doses meant that carprofen was present in the bloodstream longer, allowing for increased exposure of the thrombus and surrounding IVC to the drug and likely translating into increased antiinflammatory effect on the thrombus. Taking together these findings, we consider that, despite previous findings, the use of carprofen in the VT surgical model should not be discounted.
In reviewing our findings overall, we are better able to assess the value of our proxy indicators. The data collected show definitively that both MouseTrapp and nest consolidation evaluation can differentiate normal from unhealthy animals. These findings are consistent with previous rodent studies.11,19,26 Given that we have interpreted the correlating data obtained by using eVF to be indicative of pain, we consider that these assays have great potential to be useful indicators of pain,20 pending further studies with more efficacious analgesics or in a less invasive surgical model.
Overall, we conclude that among the doses we evaluated, 32 mg/kg robenacoxib is the most appropriate for subcutaneous dosing of mice in studies in which the antithrombotic properties of NSAID must be minimized. Furthermore, 32 mg/kg robenacoxib is comparable to carprofen in providing protection against secondary hyperalgesia in the surgical VT model, presenting the novel finding that neither drug—as a single subcutaneous dose—negatively affected the assessed hematologic parameters. On the basis of these conclusions, we consider that robenacoxib—and perhaps other NSAID—have potential use in thrombosis research. We further suggest that robenacoxib shows great promise as a single agent for less-invasive procedures or as a component of a multimodal analgesic plan for mice undergoing more invasive studies. Finally, we recommend future studies to evaluate robenacoxib doses between 32 and 100 mg/kg and to assess multiple, repeated doses of robenacoxib, to better characterize this drug and optimize dose recommendations for clinical pain management in mice.
Acknowledgments
We acknowledge Dr Robert E Sigler and the University of Michigan animal pathology cores for their preparation and analysis of histopathology samples; Dr Chris Andrews and the University of Michigan Consulting for Statistics, Computing, and Analytics Research core for their guidance and data analysis; and the comparative medicine training program of the University of Michigan Unit for Laboratory Animal Medicine.
This research was supported by the Ben and Alice Cohen Comparative Medicine Research Award 2015 (University of Michigan, Unit for Laboratory Animal Medicine).
References
- 1.Adamson TW, Kendall LV, Goss S, Grayson K, Touma C, Palme R, Chen JQ, Borowsky AD. 2010. Assessment of carprofen and buprenorphine on recovery of mice after surgical removal of the mammary fat pad. J Am Assoc Lab Anim Sci 49:610–616. [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice AS. 2012. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain 16:485–495. [DOI] [PubMed] [Google Scholar]
- 3.Australian Government. [Internet] 2018. Commonwealth of Australia Gazette APVMA Australian Pesticides and Veterinary Medicines Authority. [Cited 10 June 2016]. Available at: https://apvma.gov.au/sites/default/files/gazette_2010-06-22.pdf.
- 4.Canada Health. [Internet] 2016. Products for veterinary use—active data protection period. [Cited 10 June 2016]. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/register-innovative-drugs/register.html#a3.
- 5.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. 2012. Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153:876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai RP, Li CQ, Zhang JW, Li F, Shi XD, Zhang JY, Zhou XF. 2011. Biphasic activation of extracellular signal-regulated kinase in anterior cingulate cortex distinctly regulates the development of pain-related anxiety and mechanical hypersensitivity in rats after incision. Anesthesiology 115:604–613. [DOI] [PubMed] [Google Scholar]
- 7.De Rantere D, Schuster CJ, Reimer JN, Pang DS. 2015. The relationship between the rat grimace scale and mechanical hypersensitivity testing in 3 experimental pain models. Eur J Pain 20:417–426. [DOI] [PubMed] [Google Scholar]
- 8.Dunbar ML, David EM, Aline MR, Lofgren JL. 2016. Validation of a behavioral ethogram for assessing postoperative pain in guinea pigs (Cavia porcellus). J Am Assoc Lab Anim Sci 55:29–34. [PMC free article] [PubMed] [Google Scholar]
- 9.Edamura K, King JN, Seewald W, Sakakibara N, Okumura M. 2012. Comparison of oral robenacoxib and carprofen for the treatment of osteoarthritis in dogs: a randomized clinical trial. J Vet Med Sci 74:1121–1131. [DOI] [PubMed] [Google Scholar]
- 10.Flecknell PA. 2009. Analgesia and postoperative care, p 139–179. In: Laboratory animal anaesthesia, 3rd ed. San Diego (CA): Academic Press.
- 11.Gaskill BN, Karas AZ, Garner JP, Pritchett-Corning KR. 2013. Nest building as an indicator of health and welfare in laboratory mice. J Vis Exp e51012:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giraudel JM, King JN, Jeunesse EC, Lees P, Toutain PL. 2009. Use of a pharmacokinetic–pharmacodynamic approach in the cat to determine a dosage regimen for the COX2-selective drug robenacoxib. J Vet Pharmacol Ther 32:18–30. [DOI] [PubMed] [Google Scholar]
- 13.Gould TD, Dao DT, Kovacsics CE. 2009. The open field test, p 1–20. In: Gould TD. editor. Mood- and anxiety-related phenotypes in mice: characterization using behavioral tests. Totowa (NJ): Humana Press. [Google Scholar]
- 14.Gruet P, Seewald W, King JN. 2013. Robenacoxib versus meloxicam for the management of pain and inflammation associated with soft tissue surgery in dogs: a randomized, noninferiority clinical trial. BMC Vet Res 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hish GA, Jr, Diaz JA, Hawley AE, Myers DD, Jr, Lester PA. 2014. Effects of analgesic use on inflammation and hematology in a murine model of venous thrombosis. J Am Assoc Lab Anim Sci 53:485–493. [PMC free article] [PubMed] [Google Scholar]
- 16.Ho IK, Loh HH, Way EL. 1977. Morphine analgesia, tolerance, and dependence in mice from different strains and vendors. J Pharm Pharmacol 29:583–584. [DOI] [PubMed] [Google Scholar]
- 17.Ingrao JC, Johnson R, Tor E, Gu Y, Litman M, Turner PV. 2013. Aqueous stability and oral pharmacokinetics of meloxicam and carprofen in male C57BL/6 mice. J Am Assoc Lab Anim Sci 52:553–559. [PMC free article] [PubMed] [Google Scholar]
- 18.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 19.Jirkof P. 2014. Burrowing and nest building behavior as indicators of wellbeing in mice. J Neurosci Methods 234:139–146. [DOI] [PubMed] [Google Scholar]
- 20.Jirkof P, Fleischmann T, Cesarovic N, Rettich A, Vogel J, Arras M. 2013. Assessment of postsurgical distress and pain in laboratory mice by nest complexity scoring. Lab Anim 47:153–161. [DOI] [PubMed] [Google Scholar]
- 21.King JN, Dawson J, Esser RE, Fujimoto R, Kimble EF, Maniara W, Marshall PJ, O'Byrne L, Quadros E, Toutain PL, Lees P. 2009. Preclinical pharmacology of robenacoxib: a novel selective inhibitor of cyclooxygenase 2. J Vet Pharmacol Ther 32:1–17. [DOI] [PubMed] [Google Scholar]
- 22.Konecka AM, Sroczynska I. 1998. Circadian rhythm of pain in male mice. Gen Pharmacol 31:809–810. [DOI] [PubMed] [Google Scholar]
- 23.Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, LaCroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7:447–449. [DOI] [PubMed] [Google Scholar]
- 24.Le Bars D, Gozariu M, Cadden SW. 2001. Animal models of nociception. Pharmacol Rev 53:597–652. [PubMed] [Google Scholar]
- 25.Leach MC, Klaus K, Miller AL, Scotto di Perrotolo M, Sotocinal SG, Flecknell PA. 2012. The assessment of postvasectomy pain in mice using behaviour and the Mouse Grimace Scale. PLoS One 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li CQ, Zhang JW, Dai RP, Wang J, Luo XG, Zhou XF. 2010. Surgical incision induces anxiety-like behavior and amygdala sensitization: effects of morphine and gabapentin. Pain Res Treat 2010:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mabrouk OS, Dripps IJ, Ramani S, Chang C, Han JL, Rice KC, Jutkiewicz EM. 2014. Automated touch screen device for recording complex rodent behaviors. J Neurosci Methods 233:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinov T, Mack M, Sykes A, Chatterjea D. 2013. Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von Frey apparatus. J Vis Exp 82:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the Mouse Grimace Scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49. [PMC free article] [PubMed] [Google Scholar]
- 30.McClain RM, Rubio F, Dairman W, Koechlin B. 1980. Factors related to increased susceptibility to intestinal lesions with nonsteroidal antiinflammatory agents in the lactating rat. Toxicol Appl Pharmacol 56:383–391.7222022 [Google Scholar]
- 31.Mizokami SS, Hohmann MS, Staurengo-Ferrari L, Carvalho TT, Zarpelon AC, Possebon MI, de Souza AR, Veneziani RC, Arakawa NS, Casagrande R, Verri WA., Jr 2016. Pimaradienoic acid inhibits carrageenan-induced inflammatory leukocyte recruitment and edema in mice: inhibition of oxidative stress, nitric oxide and cytokine production. PLoS One 11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. 2000. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev 24:375–389. [DOI] [PubMed] [Google Scholar]
- 33.Mogil JS, Crager SE. 2004. What should we be measuring in behavioral studies of chronic pain in animals? Pain 112:12–15. [DOI] [PubMed] [Google Scholar]
- 34.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. 1999. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80:67–82. [DOI] [PubMed] [Google Scholar]
- 35.Morris CJ. 2003. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol 225:115–121. [DOI] [PubMed] [Google Scholar]
- 36.NC3Rs Reporting Guidelines Working Group 2010. Animal research. Reporting in vivo experiments: the ARRIVE guidelines. J Physiol 588:2519–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. 2015. Effects of ketoprofen, morphine, and κ opioids on pain-related depression of nesting in mice. Pain 156:1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Page GG. 2005. Surgery-induced immunosuppression and postoperative pain management. AACN Clin Issues 16:302–309. [DOI] [PubMed] [Google Scholar]
- 39.Page GG, Blakely WP, Ben-Eliyahu S. 2001. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain 90:191–199. [DOI] [PubMed] [Google Scholar]
- 40.Perissin L, Boccalon S, Scaggiante B, Petrelli L, Ortolani F, Porro CA. 2004. Diurnal changes of tonic nociceptive responses in mice: evidence for a proalgesic role of melatonin. Pain 110:250–258. [DOI] [PubMed] [Google Scholar]
- 41.Perissin L, Facchin P, Porro CA. 2000. Diurnal variations in tonic pain reactions in mice. Life Sci 67:1477–1488. [DOI] [PubMed] [Google Scholar]
- 42.Plumb DC. 2015. Plumb's veterinary drug handbook, 8th ed. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 43.Raskob GE, Silverstein R, Bratzler DW, Heit JA, White RH. 2010. Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med 38 4 Suppl: S502–S509. [DOI] [PubMed] [Google Scholar]
- 44.Ren K, Dubner R. 1999. Inflammatory models of pain and hyperalgesia. ILAR J 40:111–118. [DOI] [PubMed] [Google Scholar]
- 45.Roughan JV, Bertrand HG, Isles HM. 2015. Meloxicam prevents COX2-mediated postsurgical inflammation but not pain following laparotomy in mice. Eur J Pain 20:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tubbs JT, Kissling GE, Travlos GS, Goulding DR, Clark JA, King-Herbert AP, Blankenship-Paris TL. 2011. Effects of buprenorphine, meloxicam, and flunixin meglumine as postoperative analgesia in mice. J Am Assoc Lab Anim Sci 50:185–191. [PMC free article] [PubMed] [Google Scholar]
- 47.United States Department of Agriculture, Animal and Plant Health Inspection Service, Animal Care 2011. Policy no. 12: consideration of alternatives to painful/distressful procedures. In: USDA/APHIS/AC animal care policies. Riverdale (MD): US Department of Agriculture. [Google Scholar]
- 48.US Food and Drug Administration 2018. Actions taken by FDA Center for Veterinary Medicine. 21 CFR Parts 500. [Cited 22 February 2018]. Available at: https://animaldrugsatfda.fda.gov/adafda/views/#/home/searchResult
- 49.Vierck CJ, Hansson PT, Yezierski RP. 2008. Clinical and preclinical pain assessment: are we measuring the same thing? Pain 135:7–10. [DOI] [PubMed] [Google Scholar]
- 50.Wakefield TW, McLafferty RB, Lohr JM, Caprini JA, Gillespie DL, Passman MA, Executive Committee of the American Venous Forum 2009. Call to action to prevent venous thromboembolism. J Vasc Surg 49:1620–1623.PubMed. [DOI] [PubMed] [Google Scholar]
- 51.Wojcik BM, Wrobleski SK, Hawley AE, Wakefield TW, Myers DD, Jr, Diaz JA. 2011. Interleukin 6: a potential target for postthrombotic syndrome. Ann Vasc Surg 25:229–239. [DOI] [PubMed] [Google Scholar]