Abstract
The injectable anesthetic mixture ketamine–xylazine is commonly used for electrophysiologic experiments in laboratory animals, especially rodents. General anesthesia can induce significant changes in systemic physiology, including those that compromise neural function, thus introducing research confounds. The extent of such concerns varies by agent. Here in mice, we compared the effects of ketamine–xylazine and urethane–xylazine anesthesia on systemic physiologic parameters and the vestibular sensory evoked potential (VsEP), a tool used commonly to assess peripheral vestibular function. Urethane–xylazine anesthesia provided longer anesthesia, prolonged survival times, and less compromised respiratory and cardiovascular function, compared with ketamine–xylazine. In the absence of countermeasures, mice anesthetized with either ketamine–xylazine or urethane–xylazine showed evidence of hypoxemia and fluctuations in brain temperature, heart rate, respiration rate, and VsEP response latency. The levels of hypoxemia had no effect on VsEP response parameters over the period of study (2 to 5 h). Hypoxemia was effectively countered with O2 supplementation, which stabilized respiratory rates and improved mean survival times by 160% in mice anesthetized with ketamine–xylazine. Monitoring and controlling brain temperature reduced variation in VsEP latency. VsEP thresholds, latencies, and amplitudes did not differ between mice under ketamine–xylazine compared with urethane–xylazine when the brain temperature was held at the same set point. These findings demonstrate that urethane–xylazine provides improved systemic physiologic conditions during anesthesia in mice and may be substituted for ketamine–xylazine in studies using the VsEP to evaluate peripheral vestibular function. Such advantages may prove useful to research in other neuroscience areas and might reduce the number of animals used to achieve adequate sample sizes.
Abbreviation: VsEP, vestibular sensory evoked potential
Injectable anesthetics are commonly used during in vivo electrophysiologic research in animals. However, general anesthesia can interfere with homeostasis and induce physiologic changes, including the depression of respiratory, cardiovascular, and neural function (for review, see references 30 and 31). Choosing an effective and appropriate agent from among the many general anesthetics available is an essential part of planning for animal experiments.
The anesthetic mixture of ketamine–xylazine is widely used in laboratory animals, especially rodents.8,9 Ketamine acts on glutamate N-methyl-D-aspartate receptors as an antagonist and inhibits neural synaptic transmission (for review, see reference 9). Because using ketamine as the sole anesthetic can cause muscle rigidity, marked mortality, and insufficient analgesia, the α2-adrenoceptor agonist xylazine—because of its additional sedative and analgesic properties— is often mixed with ketamine.8,30 Although general anesthesia with the ketamine–xylazine mixture is noted for rapid induction (within 2 to 3 min of injection), limitations of this mixture include cardiovascular dysfunction, such as bradycardia, hypotension, and respiratory depression, and a relatively short period (approximately 80 min) of surgical anesthesia without maintenance dosing.7,30 For the last decade, a ketamine–xylazine mixture has been the anesthetic of choice for electrophysiologic studies using recordings such as vestibular sensory evoked potentials (VsEP) in rodents.21,34
Urethane is another candidate anesthetic available for use in rodents. Its mechanism of action is not clearly understood, and urethane may act nonselectively to produce only modest effects on multiple neurotransmitter receptors, including glycine, N-methyl-D-aspartate, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, acetylcholine, and γ-aminobutyric acid type A receptors to produce anesthesia rather than producing major effects selectively on one or 2 primary targets.12 A urethane–xylazine mixture induces deep sedation, muscle relaxation, and prolonged anesthesia with minimal effects on cardiovascular and respiratory systems.24,25 Thus, urethane anesthesia may have several advantages for pharmacologic experiments involving the acetylcholine and γ-aminobutyric acid neurotransmitter systems. However, urethane anesthesia reportedly produces a decreased spontaneous release of glutamate in the rat cerebral cortex,28 and olivocochlear efferent response characteristics may be slightly different under urethane compared with barbiturate anesthesia.3 Despite these caveats, urethane seems to have only minor negative effects on anesthetized animals compared with other anesthetics including ketamine–xylazine.
The VsEP is a commonly used, effective test of peripheral vestibular function.16 In mammals, VsEP studies are completed in ketamine–xylazine anesthetized animals. The effects of urethane anesthesia on the VsEP and vestibular function have not been evaluated. Given the reported advantages of urethane anesthesia, we were interested in comparing the physiologic status of mice anesthetized with ketamine–xylazine with that of mice anesthetized with urethane–xylazine during standard VsEP testing conditions and in determining whether the urethane mixture altered the VsEP.
Our hypothesis was that in the absence of countermeasures, general anesthesia (ketamine–xylazine or urethane–xylazine) in mice changes systemic physiology, compromises pulmonary respiration, and reduces the arterial oxygen hemoglobin saturation level. In turn, these effects negatively alter neural function and change VsEP response parameters in anesthetized animals. In addition, general anesthesia prevents normal thermoregulation and can readily lead to core hypothermia. Controlling rectal core temperature is one common strategy taken to prevent hypothermia and associated changes in VsEP response parameters.10,29 In the present study, we tested the effectiveness of controlling brain compared with rectal core temperatures of animals during anesthesia. Hypothetically, this control will improve physiologic conditions and minimize changes in response parameters (for example, VsEP latencies, amplitudes, and thresholds). To test these hypotheses, we measured VsEP in C57BL/6 mice anesthetized with ketamine–xylazine or urethane–xylazine for extended periods of time with and without countermeasures. Whether hypoxemia typically accompanies routine urethane–xylazine and ketamine–xylazine anesthesia during VsEP testing has not previously been evaluated. Here we sought to estimate SaO2 levels, brain temperature as well as the status of other physiologic parameters in mice during anesthesia. Our overall aim in the present study was to better understand the physiologic conditions resulting from the administration of 2 different anesthetic mixtures (that is, ketamine–xylazine compared with urethane–xylazine) and determine how such conditions affect vestibular function.
Materials and Methods
Experiments were performed by using 29 female C57BL/6J mice (age, 8 to 15 wk; Jackson Laboratory, Bar Harbor, ME). All experimental procedures were approved by the IACUC at the University of Nebraska-Lincoln and were performed in accordance with NIH guidelines.
Anesthesia preparation.
Seventeen mice (weight, 22.9 ± 5.0 g [mean ± SEM]) were anesthetized ketamine–xylazine by using a single intraperitoneal dose comprising 90 mg/kg ketamine and 10 mg/kg xylazine (injection volume, approximately 0.1 mL; mixture concentrations: ketamine 18 mg/mL and xylazine 2 mg/mL). Maintenance doses of 0.05 mL ketamine–xylazine were administered on the reappearance of blinking or the withdrawal reflex (generally at intervals of 30 to 40 min or sooner). In addition, 12 mice (weight, 18.3 ± 2.3 g) were anesthetized by using a single intraperitoneal injection of urethane–xylazine mixture (injection volume, 0.25 to 0.45 mL; dose,1.2 g/kg urethane and 20 mg/kg xylazine; solution concentrations: urethane, 200 mg/mL; xylazine, 2 mg/mL). Supplementary injections of 0.05 mL urethane were administered as needed (every 2 to 3 h or sooner). The duration of experiments was 4 to 6 h, after which mice were euthanized by using a lethal dose of sodium pentobarbital (500 mg/kg; Vortech Pharmaceutical, Dearborn, MI).
Monitoring SpO2 and Heart and Respiratory Rates.
SpO2 was measured by using a standalone animal pulse oximeter (model no. CANL-425SV-A, Med Associates, St Albans, VT). An oximeter clip sensor was placed on the upper thigh of each mouse after the surface of the skin was shaved. For maintaining reliable sensor outputs, the sensor was cleaned every 20 min by using premoistened alcohol wipes. Routine testing of the oximeter included a comparison of SpO2 levels with and without oxygen supplementation. The measured SpO2 response to changes in inspired O2 concentrations was rapid and consistent across all studies, although the absolute value of resultant blood saturation levels was not independently confirmed. ECG activity was monitored on an oscilloscope, and heart rate was calculated based on the QRS interval. Respiratory rate was assessed by counting the number of thoracic movements per unit of time. SpO2 (%), heart rate (bpm), and respiratory rate were monitored continuously and recorded every 5 min. Lactated Ringers solution (0.1 to 0.3 mL) was subcutaneously administered every hour (or as needed) to prevent dehydration.
Routine anesthesia with ketamine–xylazine is generally used without oxygen (O2) supplementation. Here we included an oxygen supplementation treatment group to evaluate whether these supportive efforts improved physiologic status in anesthetized mice. For O2 supplementation under ketamine–xylazine (n = 6) or urethane–xylazine (n = 6) anesthesia, an oxygen delivery tube and funnel provided a means to mix oxygen-rich gas (4% CO2, 39.5% N2, balance O2 or 50% N2, balance O2) with normal inspired room air. Thus, supplemented animals breathed an O2-rich mixture of gas and room air (Figure 1). A PaO2 of 60 mm Hg is considered to be the threshold for hypoxia, a level insufficient for normal function.5 This Pa O2 corresponds to an SaO2 level of approximately 80%, according to the oxyhemoglobin dissociation curve. For this reason, supplemental gas flow was adjusted (0.5 to 1.0 L/min) to achieve SaO2 levels above 80% during anesthesia. O2-nonsupplemented control mice (ketamine–xylazine, n = 6; urethane–xylazine, n = 6) breathed normal room air without oxygen supplementation, as is typical under standard VsEP testing conditions.
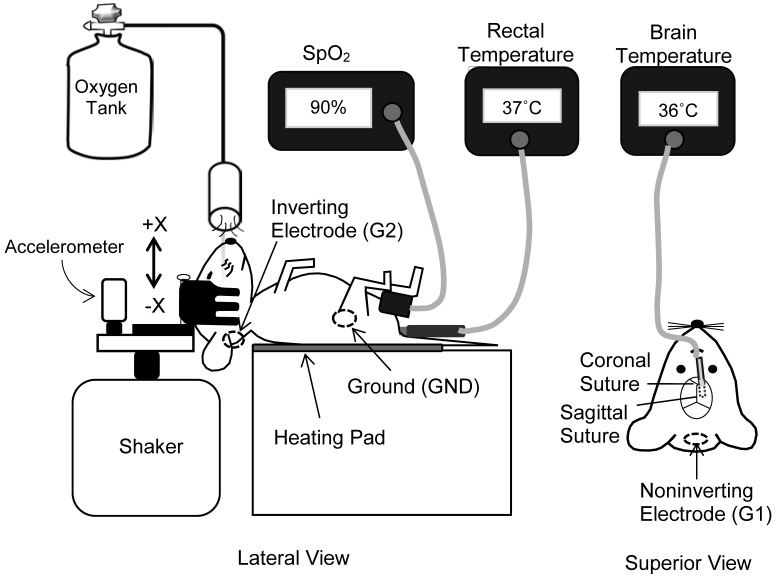
Figure 1.
VsEP recording set-up. The anesthetized mouse was placed on a homeothermic heating pad, and a noninvasive head clip was used to couple its head to an aluminum platform. The shaker platform produced a transient head translation in the nasooccipital axis. Oxygen-rich gas was presented to the animal's face. The oximeter clip was placed on the animal's thigh, and the SpO2 level was monitored by using pulse oximetry. Body core temperatures were monitored by noninvasive rectal and invasive brain probes. Three stainless steel electrodes were placed on the nuchal crest (noninverting, G1), below and behind the ear (inverting, G2), and on the hip (ground, GND).
Rectal and brain temperatures.
A noninvasive rectal temperature probe was used to monitor core temperature. A homeothermic heating pad (FHC, Bowdoin, ME) was used to maintain rectal (core) temperatures at 37.0 ± 0.2 °C in all mice. In addition, brain temperature was monitored and controlled in all urethane–xylazine-anesthetized mice, whereas brain temperature was monitored and controlled in one of the 2 ketamine–xylazine groups studied. Unless otherwise stated, brain temperatures were maintained at 36.0 ± 0.2 °C by using a laboratory heating lamp.2
After induction of anesthesia, a small hole was made in the skull to access the brain surface, and a thermocouple microwire (diameter, <1 mm; Cole Parmer, Vernon Hills, IL) was implanted 5 mm below the skull surface in the brain (posterior–parasagittal entry) for the measurement of brain temperature. The thermocouple wire was secured by using cyanoacrylate glue and surgical suture (Figure 1).
VsEP.
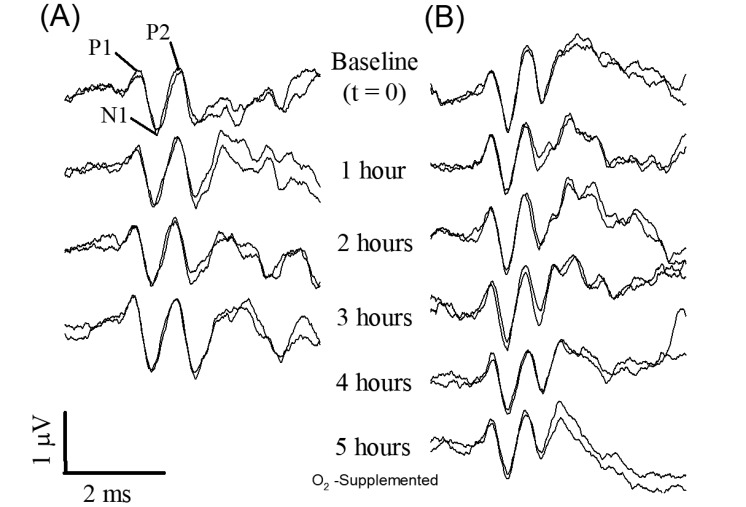
The VsEP response is a compound action potential generated by the peripheral vestibular nerve and central relays and, as such, is a noninvasive direct measurement of peripheral and central vestibular function (for review, see reference 16). The short-latency linear VsEP consists of a series of positive and negative electrical peaks (for example, P1, N1, and P2; Figure 2) generated by vestibular sensory receptors in response to a transient linear acceleration of the head. VsEP response parameters measured and analyzed here were VsEP response latencies (P1, N1, and P2 latencies; Figure 2), amplitudes (P1–N1 and P2–N1), and threshold. P1 and N1 peaks represent the compound action potential of the peripheral vestibular nerve innervating macular sensors.14,15 P2 and later peaks reflect the activity of central vestibular relays in the brainstem vestibular nuclei.17,29 VsEP threshold indicates the sensitivity of peripheral macular sensors to a transient linear acceleration of the head18 and is defined later.
Figure 2.
VsEP traces from representative (A) ketamine–xylazine- and (B) urethane–xylazine-anesthetized mice. Both animals were provided with O2 supplementation. Brain temperature was not controlled in the case of the ketamine–xylazine mouse (A) but was controlled in urethane–xylazine mouse (panel B, 36.0 ± 0.2 °C). Times immediately before (baseline, t = –20 to 0 min) and during O2 provision over 3 to 5 h are shown. O2 supplementation began at t = 0 min. The first positive peak (P1), negative peak (N1), and second positive peak (P2) are labeled.
The VsEP recording method has been described in detail elsewhere.20,21 Briefly, subcutaneous electrodes were placed on the midline over the nuchal crest (noninverting, G1), below and behind the right pinna (inverting, G2), and on the hip (ground). EEG signals were amplified (200,000×) and filtered (0.3 to 3 kHz, –6 dB points). Analog-to-digital conversion of the EEG was triggered at the onset of each stimulus (conversion time, 10 µs/point; 1024 points). Signal averaging was used to resolve responses from background noise. An average of 256 individual responses was computed (128 normal stimulus polarity and 128 inverted stimulus polarity) to produce the final VsEP response trace. Two response traces were recorded for each stimulus level used. A noninvasive head clip was used to secure the mouse's head to a shaker for delivery of a transient head translation in the nasooccipital axis (Figure 1). VsEP stimuli were produced by applying a linear voltage ramp (digital-to-analog conversion time, 20 µs per point) to a commercial power amplifier (model no. CS-800, Peavey, Clovis, NM). The power amplifier, in turn, drove the motion of the shaker and coupling platform. The mechanical shaker (model no. ET132-203, Lab Works, Costa Mesa, CA) applied a linear acceleration ramp of 2 ms duration to the animal's head. This motion represented a 2-ms rectangular jerk stimulus.19,20 The jerk level was monitored on an oscilloscope and controlled by an attenuator. The amplitude of the jerk stimulus was measured and adjusted to 1.0 g/ms at 0 dB attenuation, where g = 9.81 m/s2. Jerk stimulus level was then expressed in dB relative to 1.0 g/ms. Stimuli were presented at a rate of 17/s. To confirm the absence of auditory responses during VsEP testing, a binaural forward masker (92 dB SPL; bandwidth, 50 to 50,000 Hz)18 was presented with the use of a free-field speaker (model no. FF1, Tucker Davis Technologies, Alachua, FL).
VsEPs were measured at approximately 5-min intervals at +6 dB re: 1.0 g/ms, and VsEP thresholds were determined at approximately 20-min intervals. A threshold-seeking protocol was used, where stimulus level was adjusted in 3-dB steps to cover a range of stimulus levels between +6 dB and –18 dB re: 1.0 g/ms. Response thresholds were defined as the stimulus level midway between the minimal level producing a response and the maximal level failing to produce a response.18
Data analysis and statistics.
Data were collected over a period of 120 to 300 min after a 20-min stable baseline period. We averaged 4 or 5 consecutive VsEP measurements to characterize each 20-min period. During experiments, the change in VsEP latencies (P1, N1, P2) and amplitudes (P1–N1, P2–N1) over time were evaluated. Repeated-measures multivariate ANOVA was used to evaluate response parameters at multiple trials, and the linear regression slope was used to evaluate the relationships between response parameters and time. Response parameters of VsEP were evaluated between control and treatment groups as fixed factors. Descriptive statistics were expressed as mean ± 1 SD unless otherwise stated. A P value less than 0.05 was considered significant. All statistical analyses were performed by using SPSS (version 22.0, SPSS, Chicago, IL).
Results
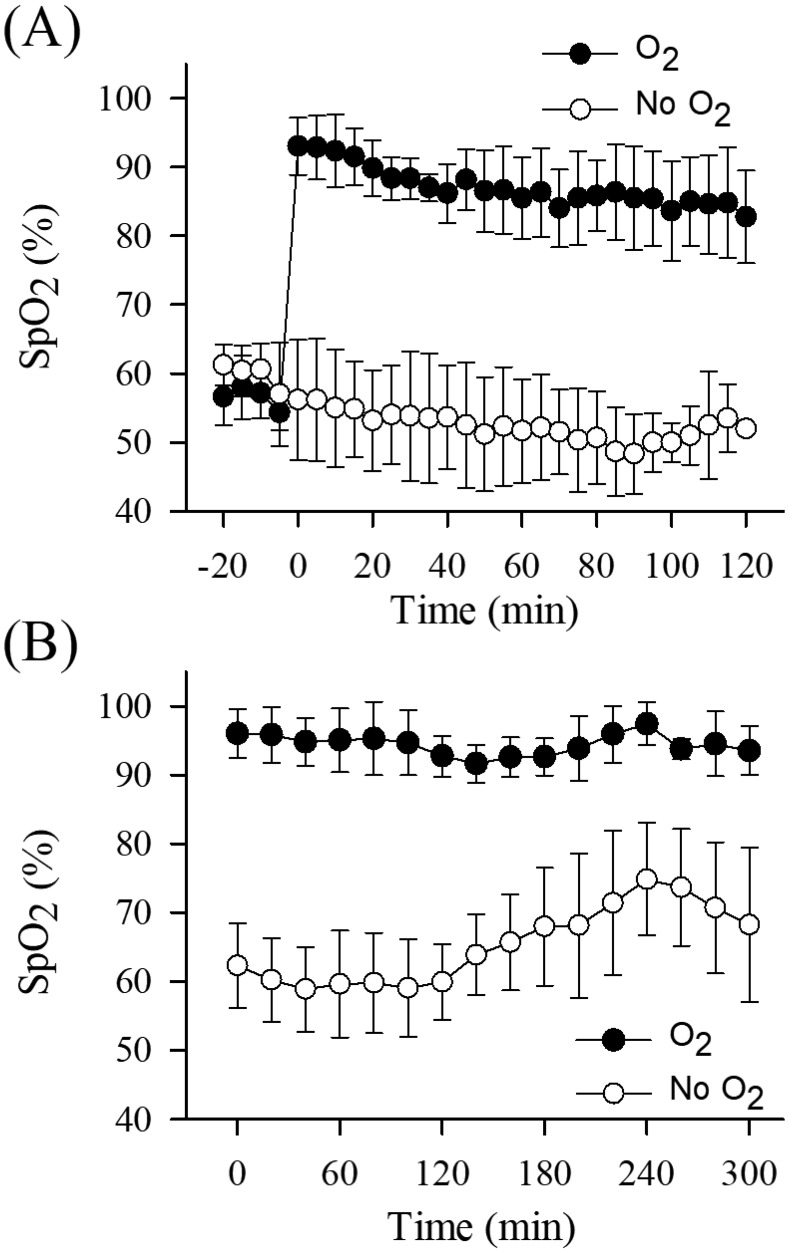
Effect of anesthesia on SpO2
Six mice were anesthetized with ketamine–xylazine, and VsEP were recorded without O2 supplementation over a stable baseline period of 20 min. SpO2 levels during the baseline period indicated that all mice experienced hypoxemia (Figure 3 A). O2 supplementation was initiated immediately after the baseline period. With the start of O2 supplementation in all 6 mice, SpO2 rose substantially (from approximately 60% to 90% on average) and rapidly stabilized and remained at higher levels throughout the remainder of the study (Figure 3 A). In the absence of O2 supplementation, SpO2 levels remained low but relatively stable throughout procedures (Figure 3 A and B). These findings provided evidence that the supplementation was effective in delivering additional O2 to the mice and that mice were hypoxic prior to O2 supplementation.
Figure 3.
The pattern of SpO2 levels over time during (A) ketamine–xylazine anesthesia for 120 min and (B) urethane–xylazine anesthesia for 300 min. Black circles represent the O2 treatment group, and white circles represent the nonsupplemented control group in both anesthetic protocols. (A) In the O2 supplementation treatment group for ketamine–xylazine anesthesia, supplementation was initiated (at approximately –1 min) after a 20-min stable baseline period, and SpO2 level increased quickly above 80% (saturation increase by approximately 30% relative to baseline). (B) In the O2 supplementation group for urethane–xylazine anesthesia, supplementation was provided continuously and initiated immediately after the induction of anesthesia (time 0). We averaged 4 or 5 consecutive 5-min measurements of SpO2 to characterize each 20-min period.
General considerations of anesthesia.
In the initial ketamine–xylazine and urethane–xylazine anesthetic protocols, a total of 24 mice were deeply anesthetized without complications. Maintenance doses were prompted by increased muscle activity in the ongoing EEG or by a return of the withdrawal reflex. Half of the mice (that is, n = 6) in each anesthetic group were given supplemental O2, and the other half were not. Survival times of mice under anesthesia were different (P = 2.2 x 10–5) for the 2 anesthetic agents. The survival time after ketamine–xylazine anesthesia for the combined O2-supplemented and ‑nonsupplemented groups was about 3 h (3.1 ± 1.1 h, n = 12), whereas all mice under urethane–xylazine anesthesia (n = 12) survived the entire 4- to 6-h period of study, whether supplemented with O2 or not.
In addition, survival times were affected by O2 supplementation alone. O2 supplementation improved survival times for ketamine–xylazine anesthetized mice, in that the mean value for O2-supplemented survival was 3.6 h and that for nonsupplemented survival was 2.2 h, a significant difference (ANOVA, F1,10 = 8.891, P = 0.014). Indeed, one O2-supplemented ketamine–xylazine-anesthetized mouse survived 4.5 h. Supplementation groups under urethane–xylazine anesthesia did not differ because all mice survived the entire 5-h test period.
We then evaluated the time before the first maintenance dose was required for each anesthetic. For ketamine–xylazine anesthesia, the first maintenance dose was given after approximately 30 min (38 ± 18 min, n = 12) after induction. In contrast, a single dose of urethane–xylazine anesthesia was effective for approximately 2.5 h (156 ± 52 min, n = 12), a significantly longer time than for ketamine–xylazine (ANOVA, F1,22, P = 1.893 × 10−7).
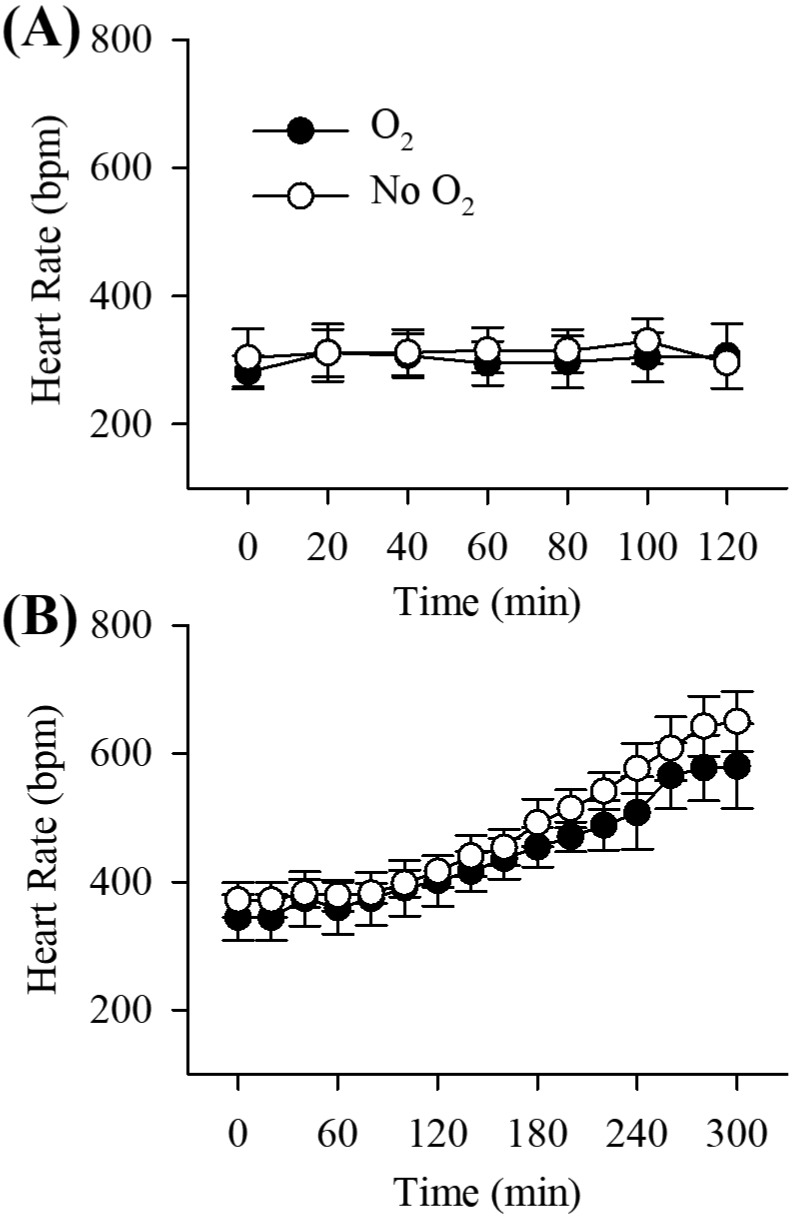
Effects of anesthesia on heart and respiratory rates.
Figure 4 shows the heart rates over time for O2-supplemented and -nonsupplemented mice in both ketamine–xylazine and urethane–xylazine protocols. In the ketamine–xylazine protocol, heart rates remained stable over a period of 2 h in both O2-supplemented (baseline, 281.2 ± 25.9 bpm [n = 6]; 120 min, 306.4 ± 50.3 bpm [n = 5]) and O2-nonsupplemented (baseline, 303.6 ± 45.0 bpm [n = 6]; 120 min, 326.5 ± 43.4 [n = 2]) groups (Figure 4 A). However, all urethane–xylazine-anesthetized mice showed relatively stable heart rates for the first 80 min; thereafter heart rate increased gradually (Figure 4 B; repeated-measures ANOVA, F2.8,28.4 = 113.589, P = 1.04 × 10−15). There were no significant differences in heart rate between O2 supplementation and nonsupplementation groups for either ketamine–xylazine or urethane–xylazine anesthesia. In contrast, urethane–xylazine mice demonstrated higher heart rates (baseline, 357.8 ± 33.3) bpm, 444.7 ± 29.9 (n = 12) bpm at 120 min [n = 12]) compared with ketamine–xylazine mice (baseline, 292.4 ± 36.9; 120 min 312.1 ± 45.8 [n = 7]) over a period of 2 h (repeated-measures ANOVA, F1,17 = 34.73, P = 1.77 × 10−5).
Figure 4.
The pattern of heart rate over time in (A) ketamine–xylazine anesthesia and (B) urethane–xylazine anesthesia. Brain temperature was not controlled in ketamine–xylazine-anesthetized mice but was controlled in urethane–xylazine-anesthetized mice. Rectal temperature was maintained at 37.0 ± 0.2 °C. Black circles represent the O2 treatment group, and white circles represent the nonsupplemented control group in both anesthetic protocols. Note differences in the time scales between panels A and B. Time 0 represents the end of the 20-min baseline period before O2 supplementation. Data are presented as mean ± 1 SD of 6 mice for each successive 20-min period.
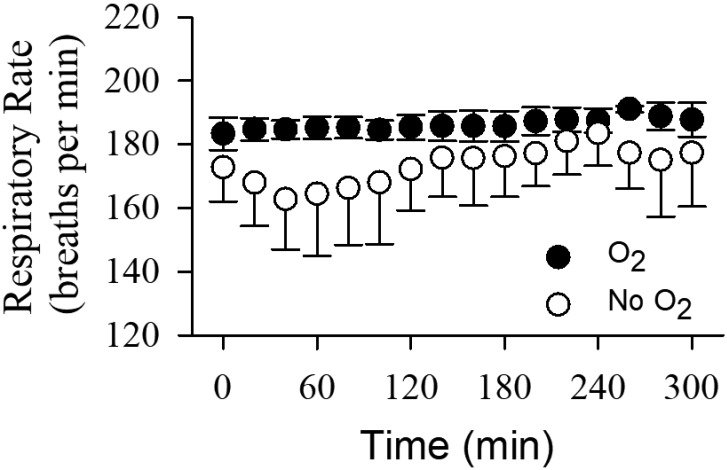
Respiration rates were monitored only in the urethane–xylazine-anesthetized mice, and results over a period of 5 h are shown in Figure 5. Respiration rates increased slightly over time in both O2-supplemented (from 183.3 ± 5.1 to 191.1 ± 4.3 breaths per minute, n = 6) and nonsupplemented (from 162.8 ± 15.8 to 183.5 ± 10.3 breaths per minute, n = 6) groups (repeated-measures ANOVA, F2.7,27 = 3.187, P = 0.044). Respiration rates in the O2-supplemented group were generally higher than in the nonsupplemented group (repeated-measures ANOVA, F(1,10) = 6.744, P = 0.027), but most importantly, respiratory rates were stabilized by O2 supplementation.
Figure 5.
Respiratory rate over 5 h during urethane–xylazine anesthesia. Black circles represent the O2 treatment group, and white circles represent the nonsupplemented control group (n = 6 each group). Brain and rectal temperatures were controlled in all animals. Time 0 represents the 20-min baseline period before O2 supplementation. Data are presented as mean ± 1 SD of 6 mice for every 20-min period.
Effects of O2 supplementation on VsEP.
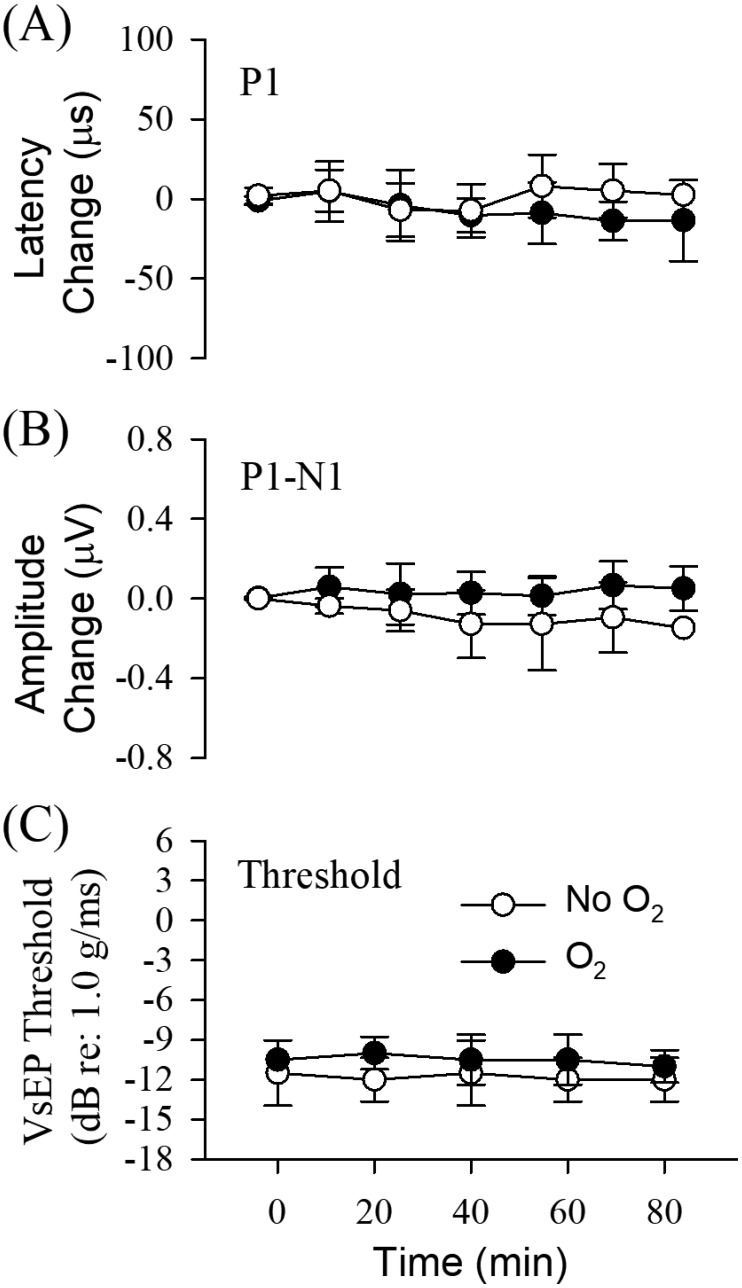
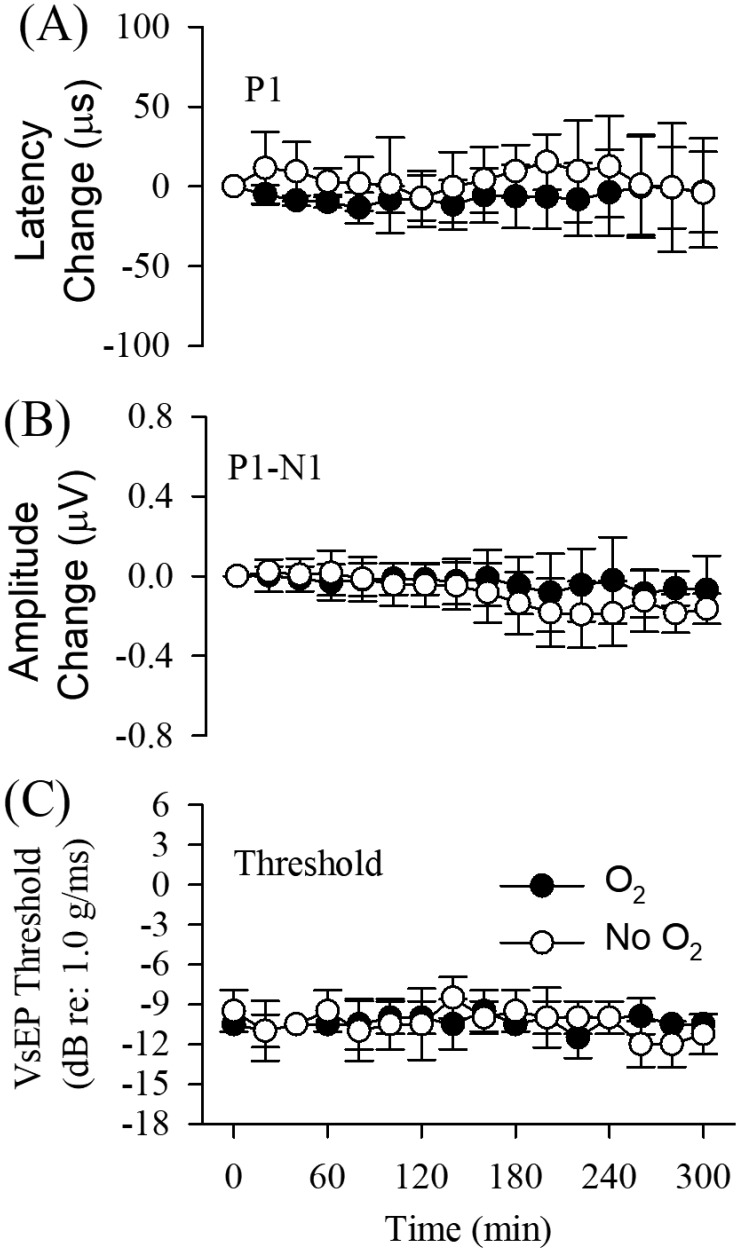
Figure 2 illustrates VsEP response waveforms obtained at 1-h intervals for 2 representative mice receiving O2 supplementation, where one was anesthetized with ketamine–xylazine (Figure 2 A) and the other with urethane–xylazine (Figure 2 B). The mouse anesthetized with ketamine–xylazine survived for only 3.2 h after O2 supplementation, whereas the animal under urethane–xylazine survived the entire duration of the study. Notice that responses are stable over the entire survival period in both cases. Mean values of VsEP response parameters (latencies, amplitudes, and thresholds) across animals in each treatment group were quantified and compared between the O2-supplement treatment and nonsupplemented control groups for both anesthetics (Figures 6 and 7, Tables 1 through 3). Generally, VsEP waveforms remained relatively stable over time in all mice for both anesthetics and with or without O2 supplementation. VsEP parameters (latencies, amplitudes and thresholds) did not differ significantly between O2 supplemented and nonsupplemented groups for either anesthetic. Therefore, the level of hypoxemia produced by these anesthetics alone had no direct effect on VsEP responses.
Figure 6.
(A) VsEP latency (in µs), (B) amplitude (in µV), and (C) threshold (in dB) during ketamine–xylazine anesthesia. Normalized VsEP values are represented (difference from mean baseline) over time (minutes) for both O2 supplementation treatment (black circles; n = 6) and nonsupplementation control (white circles; n = 6) groups. Actual mean baseline values for VsEP response parameters are presented in Table 1. Values are presented as mean ± 1 SD for each successive 20-min period after the baseline period (t = –20 to 0 min). Brain temperatures were not controlled, whereas rectal was maintained at 37.0 ± 0.2 °C.
Figure 7.
VsEP response parameters during urethane–xylazine anesthesia. Normalized VsEP values (difference from mean baseline) for (A) latencies (µs) and (B) amplitudes (µV) over time (minutes) for both O2 supplementation (black circles; n = 6) and nonsupplementation (white circles; n = 6) groups. Actual mean baseline values for VsEP response parameters are presented in Table 2. Values are presented as mean ± 1 SD for successive 20-min periods after baseline (t = –20 to 0 min). (C) VsEP thresholds as a function of time for both groups. Brain temperature was controlled at 36.0 ± 0.2 °C and rectal temperature at 37.0 ± 0.2 °C in all animals.
Table 1.
Effects of ketamine–xylazine anesthesia over 120 min after a 20-min baseline period
| Baseline | 1–20 min | 21–40 min | 41–60 min | 61–80 min | 81–100 min | 101-120 mins | ||
| P1 latency | Oxygen | 1447 ± 51 | 1457 ± 79 | 1443 ± 70 | 1437 ± 77 | 1440 ± 85 | 1439 ± 71 | 1435 ± 96d |
| No Oxygen | 1410 ± 24 | 1417 ± 36 | 1403 ± 34 | 1407 ± 36 | 1423 ± 47 | 1403 ± 48b | 1378 ± 0a | |
| N1 latency | Oxygen | 1782 ± 91 | 1792 ± 111 | 1771 ± 95 | 1752 ± 91 | 1760 ± 105 | 1754 ± 89b | 1741 ± 113d |
| No Oxygen | 1735 ± 45 | 1728 ± 51 | 1700 ± 45 | 1705 ± 38 | 1719 ± 56 | 1688 ± 52b | 1674 ± 0a | |
| P2 latency | Oxygen | 2212 ± 64 | 2210 ± 73 | 2212 ± 66 | 2199 ± 74 | 2186 ± 72 | 2185 ± 76 | 2183 ± 92d |
| No Oxygen | 2107 ± 59 | 2117 ± 69 | 2114 ± 68 | 2117 ± 49 | 2133 ± 79 | 2104 ± 38c | 2055 ± 0a | |
| P1-N1 amplitude | Oxygen | 0.82 ± 0.23 | 0.82 ± 0.29 | 0.81 ± 0.25 | 0.83 ± 0.22 | 0.85 ± 0.19 | 0.83 ± 0.22 | 0.94 ± 0.19d |
| No Oxygen | 1.13 ± 0.39 | 1.09 ± 0.40 | 1.01 ± 0.37 | 0.96 ± 0.27 | 0.94 ± 0.28 | 1.15 ± 0.08c | 1.35 ± 0a | |
| P2-N1 amplitude | Oxygen | 0.82 ± 0.23 | 0.82 ± 0.29 | 0.81 ± 0.25 | 0.83 ± 0.22 | 0.85 ± 0.19 | 0.83 ± 0.22 | 0.94 ± 0.19d |
| No Oxygen | 1.13 ± 0.39 | 1.09 ± 0.40 | 1.01 ± 0.37 | 0.96 ± 0.27 | 0.94 ± 0.28 | 1.15 ± 0.08c | 1.35 ± 0a | |
| Threshold | Oxygen | −10.5 ± 0.0 | −10.5 ± 0.0 | −10.0 ± 1.2 | −10.5 ± 1.9 | −10.5 ± 1.9 | −11.0 ± 1.2 | −10.5 ± 0d |
| No Oxygen | −11.5 ± 2.5 | −11.5 ± 2.5 | −12.0 ± 1.6 | −11.5 ± 2.5 | −12.0 ± 1.6 | −12.5 ± 1.7c | — |
VsEP latencies (µs) and amplitudes (µV) obtained at a stimulus level of +6 dB at 1.0 g/ms and response thresholds are shown as mean ± 1 SD for O2-supplemented and nonsupplemented groups under ketamine–xylazine anesthesia. Each time period was characterized by averaging 4 to 5 consecutive measurements at approximately 5-min intervals. Thresholds were obtained at 20-min intervals. Brain temperature was not controlled, although rectal temperatures were held at 37.0 ± 0.2 °C. All groups contained 6 mice, except where noted. Numbers for the periods longer than 80 min reflect the reduction in sample size associated with animal deaths in the nonsupplemented group.
n = 1.
n = 2.
n = 3.
n = 5.
Table 3.
Effects of brain temperature control on VsEP response latencies (µs) and amplitudes (µV)
| Passive variation of brain temperature |
Active control of brain temperature |
No control of brain temperature |
|||||
| 31 °C | 32 °C | 33 °C | 34 °C | 35 °C | 36 °C | ||
| Latency (µs) | |||||||
| P1 | 1361 ± 41 | 1360 ± 58 | 1326 ± 55 | 1342 ± 57 | 1308 ± 61 | 1291 ± 62 | 1432 ± 51 |
| N1 | 1684 ± 26 | 1722 ± 80 | 1668 ± 66 | 1686 ± 84 | 1618 ± 80 | 1614 ± 88 | 1756 ± 67 |
| P2 | 2215 ± 68 | 2237 ± 90 | 2163 ± 81 | 2149 ± 101 | 2063 ± 86 | 1990 ± 38 | 2157 ± 75 |
| Amplitude (µV) | |||||||
| P1-N1 | 1.42 ± 0.13 | 1.38 ± 0.11 | 1.32 ± 0.14 | 1.22 ± 0.14 | 1.12 ± 0.20 | 1.04 ± 0.17 | 0.94 ± 0.32 |
| P2-N1 | 1.33 ± 0.23 | 1.10 ± 0.26 | 1.13 ± 0.26 | 1.14 ± 0.18 | 0.98 ± 0.20 | 1.00 ± 0.16 | 0.86 ± 0.24 |
Brain temperature was allowed to passively fluctuate (range, 30.9–33.4 °C, passive variation) while rectal temperature was controlled at 37.0 ± 0.2 °C. Subsequently, brain temperature was actively controlled at 36.0 ± 0.2 °C by using a laboratory heating lamp (range, 33.5–36.2 °C, active control). Data from the standard noninvasive preparation (no control of brain temperature; rectal temperature at 37.0 ± 0.2 °C) is also included. All mice were anesthetized with ketamine–xylazine. Animals in which brain temperature was controlled (n = 5) were administered oxygen supplementation. Animals in which brain temperature was not controlled represent the Table 1 baseline condition, half of which (n = 6) were provided O2 supplementation. Response data are shown as mean ± 1 SD. Temperatures indicated for each column represent central tendencies, with actual values remaining within 0.5 °C, except for the 36 °C condition, which ranged from 35.6 to 36.2 °C. Rectal temperature (n = 12) was held at 37 ± 0.2 °C. Response metrics for 36 °C represent means over a stable 20-min period.
Effects of brain temperature and anesthetic protocol on VsEP.
Tables 1 and 2 summarize results for VsEP response parameters under ketamine–xylazine and urethane–xylazine anesthetic conditions, respectively. VsEP latencies were shorter under urethane–xylazine anesthesia (for example, mean P1 latency: ketamine–xylazine, 1428 µs; urethane–xylazine, 1263 µs; VsEP latencies: repeated-measures MANOVA, F3,20 = 24.772, P = 6.11 × 10−7), whereas VsEP amplitudes were comparable. This difference in latency could result from either an effect of the anesthetic on the VsEP or the effect of differing brain temperatures between the 2 anesthetic groups. Brain and rectal temperatures were monitored and controlled in the urethane–xylazine anesthetic protocol. In contrast, brain temperature in the ketamine–xylazine group was allowed to vary as rectal temperatures were controlled. To rule out an effect of the anesthetic on VsEP latencies, an additional 5 mice were anesthetized with ketamine–xylazine, and their brain temperatures were monitored and controlled during measurement of VsEP throughout each experiment.
Table 2.
Effects of urethane–xylazine anesthesia after a 20-min baseline (0 min) period
| Time (min) |
||||||||||||||||
| 0 | 1–20 | 21–40 | 41–60 | 61–80 | 81–100 | 101–120 | 121–140 | 141–160 | 161–180 | 181–200 | 201–220 | 221–240 | 241–260 | 261–280 | 281–300 | |
| P1 latency | ||||||||||||||||
| O2 | 1253 ± 91 | 1249 ± 86 | 1245 ± 89 | 1244 ± 90 | 1240 ± 90 | 1245 ± 85 | 1243 ± 78 | 1242 ± 78 | 1248 ± 78 | 1247 ± 79 | 1247 ± 81 | 1245 ± 81 | 1249 ± 87 | 1262 ± 95a | 1261 ± 92a | 1258 ± 89a |
| No O2 | 1274 ± 34 | 1287 ± 38 | 1283 ± 41 | 1277 ± 37 | 1275 ± 38 | 1275 ± 38 | 1279 ± 48 | 1275 ± 41 | 1288 ± 35 | 1300 ± 42 | 1299 ± 25 | 1293 ± 22 | 1297 ± 26 | 1280 ± 30b | 1278 ± 36b | 1275 ± 35b |
| N1 latency | ||||||||||||||||
| O2 | 1551± 101 | 1540 ± 97 | 1532 ± 95 | 1524 ± 100 | 1523 ± 99 | 1525 ± 93 | 1524 ± 93 | 1526 ± 82 | 1529 ± 83 | 1527 ± 80 | 1523 ± 84 | 1531 ± 81 | 1529 ± 84 | 1533 ± 90a | 1538 ± 94a | 1537 ± 98a |
| No O2 | 1553 ± 47 | 1552 ± 45 | 1545 ± 40 | 1544 ± 37 | 1542 ± 40 | 1549 ± 49 | 1555 ± 64 | 1549 ± 35 | 1564 ± 39 | 1570 ± 36 | 1562 ± 25 | 1568 ± 32 | 1563 ± 27 | 1569 ± 33b | 1570 ± 39b | 1563 ± 33b |
| P2 latency | ||||||||||||||||
| O2 | 1932 ± 92 | 1915 ± 87 | 1918 ± 86 | 1906 ± 85 | 1903 ± 92 | 1898 ± 92 | 1893 ± 97 | 1900 ± 84 | 1901 ± 80 | 1903 ± 82 | 1896 ± 84 | 1886 ± 82 | 1889 ± 89 | 1907 ± 84a | 1905 ± 96a | 1904 ± 94a |
| No O2 | 1935 ± 41 | 1930 ± 36 | 1925 ± 26 | 1925 ± 43 | 1921 ± 41 | 1925 ± 49 | 1920 ± 41 | 1918 ± 36 | 1919 ± 29 | 1921 ± 29 | 1913 ± 29 | 1915 ± 24 | 1911 ± 28 | 1913 ± 35b | 1906 ± 35b | 1893 ± 9b |
| P1–N1 amplitude | ||||||||||||||||
| O2 | 1.10 ±0.27 | 1.11 ±0.26 | 1.14 ± 0.28 | 1.14 ± 0.27 | 1.15 ±0.24 | 1.17 ± 0.30 | 1.13 ± 0.28 | 1.15 ± 0.28 | 1.18 ± 0.26 | 1.14 ± 0.32 | 1.19 ± 0.29 | 1.17 ± 0.30 | 1.14 ± 0.30 | 1.00 ± 0.07a | 1.06 ± 0.10a | 1.07 ± 0.10a |
| No O2 | 0.97 ±0.30 | 0.97 ±0.25 | 0.96 ± 0.30 | 0.94 ± 0.34 | 0.95 ±0.34 | 0.95 ± 0.38 | 0.95 ± 0.36 | 0.95 ± 0.34 | 0.96 ± 0.30 | 0.89 ± 0.31 | 0.88 ± 0.32 | 0.91 ± 0.32 | 0.95 ± 0.38 | 0.79 ± 0.18b | 0.85 ± 0.26b | 0.84 ± 0.25b |
| P2–N1 amplitude | ||||||||||||||||
| O2 | 0.94 ±0.40 | 0.97 ±0.42 | 1.06 ± 0.35 | 1.00 ± 0.36 | 1.01 ±0.33 | 0.99 ± 0.31 | 1.02 ± 0.38 | 1.02 ± 0.38 | 0.97 ± 0.37 | 0.94 ± 0.33 | 0.90 ± 0.30 | 0.85 ± 0.32 | 0.85 ± 0.26 | 0.82 ± 0.20a | 0.83 ± 0.18a | 0.84 ± 0.18a |
| No O2 | 0.91 ±0.42 | 0.93 ±0.43 | 0.91 ± 0.40 | 0.92 ± 0.36 | 0.89 ±0.39 | 0.85 ± 0.47 | 0.83 ± 0.46 | 0.85 ± 0.41 | 0.82 ± 0.37 | 0.75 ± 0.35 | 0.72 ± 0.31 | 0.71 ± 0.31 | 0.71 ± 0.30 | 0.61 ± 0.24b | 0.60 ± 0.29b | 0.65 ± 0.34b |
| Threshold | ||||||||||||||||
| O2 | −10.5 ± 0.0 | −11.0 ± 1.2 | −10.5 ± 0.0 | −10.5 ± 0.0 | −10.5 ± 1.9 | −10.0 ± 1.2 | −10.0 ± 1.2 | −10.5 ± 1.9 | −9.5 ± 1.6 | −10.5 ± 0.0 | −10.0 ± 1.2 | −11.5 ± 1.6 | −10.0 ± 1.2 | −9.9 ± 1.3a | −10.5 ± 0.0a | −10.5 ± 0.0a |
| No O2 | −9.5 ± 1.6 | −11.0 ± 2.3 | −10.5 ± 0.0 | −9.5 ± 1.6 | −11.0 ± 2.3 | −10.5 ± 2.7 | −8.5 ± 1.6 | −8.5 ± 1.6 | −10.0 ± 1.2 | −9.5 ± 1.6 | −10.0 ± 2.3 | −10.0 ± 1.2 | −10.0 ± 1.2 | −12.0 ± 1.7b | −12.0 ± 1.7b | −11.3 ± 1.5b |
VsEP latencies (µs) and amplitudes (µV) obtained at +6dB and 1.0 g/ms and response thresholds over time are shown. Results from O2-supplemented and nonsupplemented groups under urethane–xylazine anesthesia are represented. Each time period was characterized by averaging 4 or 5 consecutive measurements at approximately 5-min intervals. Brain temperature was controlled at 36.0 ± 0.2 °C, and rectal temperatures were maintained at 37.0 ± 0.2 °C in all animals. Data are shown as mean ± 1 SD of 6 animals, except where noted. Sample size decreased after 240 minutes because some animals were euthanized.
n = 5.
n = 4.
Table 3 summarizes VsEP latencies and amplitudes as a function of brain temperature. Immediately after induction of anesthesia, both rectal and brain temperatures were allowed to passively fluctuate. In all mice, temperatures fell within 15 min to about 31 °C in the brain and to approximately 32 °C in the rectal core, and corresponding mean P1 latency was 1376 µs. Subsequently, rectal temperature was raised to 37.0 ± 0.2 °C over approximately 15 min (14 ± 3 min, n = 6) by using a homeothermic heating pad. Once the rectal temperature was stable at 37.0 ± 0.2 °C, brain temperature was approximately 33 to 34 °C in all animals, and corresponding mean P1 latency was 1331.4 ± 43.2 µs (n = 6). After bringing brain temperature under control at 36.0 ± 0.2 °C by using a laboratory heating lamp, mean P1 latency decreased to 1291.3 ± 62.8 µs (n = 6) under ketamine–xylazine anesthesia.
After brain temperature control at 36 °C, neither VsEP latencies nor amplitudes differed between mice anesthetized with ketamine–xylazine and those under urethane–xylazine anesthesia. These findings indicate that the latency differences were associated with brain temperature variation rather than due to direct anesthetic effects on the VsEP.
Discussion
Our present findings demonstrate that, in the absence of countermeasures, mice anesthetized with ketamine–xylazine or urethane–xylazine showed evidence of hypoxemia. The results suggested further that the potential for hypoxemia can be countered effectively by using a simple noninvasive process of O2 supplementation. Supplementation improved the general physiologic conditions of anesthetized mice, as evidenced by increased survival times (ketamine group) and stabilized respiratory rates. This approach may be a suitable option for preventing unexpected moderate or catastrophic physiologic deterioration in complex experimental preparations studied under anesthesia.
Urethane–xylazine anesthesia acted longer, improved heart rates, and prolonged survival times, indicating better physiologic conditions with urethane than ketamine. These improvements are remarkable and suggest that the use of urethane–xylazine could reduce the mortality rate during experiments and thus reduce the number of animals needed to meet sample size requirement for studies.
The advantages of using urethane–xylazine have been underscored previously.12,24-27 The disadvantages of using ketamine–xylazine for anesthesia have also been reported and include the accompanying significant hypotension and reduction in heart rate4,30,33—features consistent with the observations we report here. One group33 noted that the heart rate of mice under ketamine–xylazine anesthesia was unstable and lower (that is, approximately 300 bpm during the first 20 to 40 min) than under other injectable anesthetics, including pentobarbital and the combination of medetomidine, midazolam, and butorphanol (ranging from 400 to 600 bpm). In the present study, ketamine–xylazine-anesthetized mice had relatively stable but lower heart rates on average (that is, approximately 300 bpm) compared with mice anesthetized with urethane–xylazine (that is, approximately 400 bpm over the initial 120 min).
Little published information is available concerning the effects of prolonged urethane anesthesia. During long-term studies (4 to 6 h) in the present work, heart rate in urethane–xylazine mice was stable for as long as 2 h (that is, approximately 350 to 450 bpm) and then increased gradually by approximately 48% on average (range, 454.0 ± 30.8 compared with 670.6 ± 28.0 bpm) over the next 3 h (Figure 4 B). Some authors11 reported heart rates in urethane-anesthetized dogs (1.5 to 2.0 g/kg IP) that increased by 25% over a period of 3.5 h (mean, 136 bpm at 1 h compared with 170 bpm at 4.5 h, n = 5). The heart rate increase was time-dependent and did not return to lower rates throughout the period of observation; these findings are consistent with our present results. The different magnitudes of change in heart rate between the previous11 and present studies (that is, 25% compared with 64%) is likely due to species-associated differences. Increasing heart rate during urethane anesthesia might be attributed to urethane's action on the postganglionic fibers of the sympathetic nervous system and the adrenal medulla.26 In support of this notion, several studies have confirmed elevated plasma levels of catecholamines, including epinephrine and norepinephrine, in urethane-anesthetized rats.1,6
Urethane anesthesia (urethane–droperidol mixture) has been reported to affect cochlear efferent neurons of guinea pigs.3 The maximal discharge rates and sensitivity of cochlear efferent fibers in animals anesthetized with urethane–droperidol appeared to be lower than those anesthetized with pentobarbital–droperidol. Although relatively minor, these issues should be considered before using urethane anesthesia.
One important question we addressed in this study was whether hypoxemia induced by general anesthesia affected the response activity of vestibular neurons in mice. We hypothesized that the countermeasures would prevent changes to vestibular neuronal excitability. Under both ketamine–xylazine and urethane–xylazine anesthesia, mice showed no significant differences in VsEP response parameters over time, regardless of O2-supplementation status. Despite the anticipated risk to function during hypoxemia, compound action potentials of the vestibular nerve were unchanged for 2 h or longer under both anesthetics. These findings indicate that hypoxemia did not reach levels sufficient to compromise vestibular function in the absence of O2 administration. Presumably, the level of hypoxemia required to alter the auditory brainstem response and the VsEP is very low, that is, an SaO2 on the order of 50% or less.13,32 The present findings clearly indicate that standard VsEP protocols, which do not use O2 supplementation, do not normally risk SaO2 levels of hypoxia sufficiently low to compromise VsEP responses.
A second important finding in the present study was that VsEP responses (latencies, amplitudes, and thresholds) did not differ in mice anesthetized with ketamine–xylazine compared with urethane–xylazine when the brain temperature was held at the same set point in the 2 groups. Therefore, urethane–xylazine might be considered for use in standard VsEP testing, especially for long-term experiments lasting more than 2 to 3 h.
The noninvasive standard VsEP protocol includes strict control of rectal temperatures (for example, see reference 21).Rectal temperature is well known to often poorly reflect temperatures in the temporal bone and brain. Under normal conditions, brain temperature may vary during the noninvasive standard preparation despite stability of the rectal core temperature. We addressed this question of varying temperature in the present study by comparing VsEP parameters under standard noninvasive conditions (no control of brain temperature) with those obtained under conditions where brain temperature was critically controlled (Table 3). The results clearly showed that controlling brain temperature prevents random temperature fluctuations and substantially reduced latencies during VsEP testing. The findings also confirm that the longer mean latencies that can be seen in noninvasively prepared mice (for example, Tables 2 and 3) can be attributed to brain temperatures that, on average, are somewhat lower than 36.0 °C during standard VsEP testing. These findings are consistent with previous reports in birds22,29 and mice10 in which the relationship between brain temperature and VsEP parameters was evaluated. The findings suggest that controlling brain temperature may be an appropriate option where experimental treatments are known to or might modify brain temperatures substantially and systematically. This control is clearly important during pharmacologic testing, wherein many drugs can alter brain temperature (for example, diazepam).23 In such cases, direct control of brain temperature is essential for reliable and appropriate interpretation of electrophysiologic results.
The standard noninvasive preparation used commonly in VsEP testing21 is extremely useful in providing a direct evaluation of the peripheral vestibular system under a variety of treatment conditions. The findings of the current study have an important bearing on the physiologic status of animals undergoing standard VsEP testing. The results demonstrate that the anesthetized animals in the short term (that is, less than 2 h) do not normally risk hypoxia or physiologic degradation sufficient to compromise VsEP responses. The results also suggest that the slightly longer latencies commonly seen in VsEP data arise from the natural random variation of brain temperature despite control of rectal temperatures. This variation in brain temperature is typically not a limiting condition for standard VsEP testing. However, as we noted earlier, should it become necessary to prevent systematic changes in temperature, direct control of brain temperature provides a means to do so.
In summary, O2 supplementation readily reduced or eliminated hypoxemia in mice under either ketamine–xylazine or urethane–xylazine anesthesia. Urethane–xylazine anesthesia was longer-lasting, prolonged survival times, and showed less physiologic decline, compared with ketamine–xylazine anesthesia. Given otherwise identical physiologic conditions, VsEP recorded under the 2 different anesthetic regimens did not differ. The findings indicate that brain temperature may be an important determinant of variability in VsEP response metrics in noninvasively prepared animals.
Acknowledgments
This work was supported by the American Academy of Audiology Foundation, the Nebraska Tobacco Settlement Biomedical Research Foundation, and the Department of Special Education and Communication Disorders of the University of Nebraska–Lincoln.
References
- 1.Armstrong JM, Lefèvre-Borg F, Scatton B, Cavero I. 1982. Urethane inhibits cardiovascular responses mediated by the stimulation of α2 adrenoceptors in the rat. J Pharmacol Exp Ther 223:524–535. [PubMed] [Google Scholar]
- 2.Bejanian M, Jones BL, Syapin PJ, Finn DA, Alkana RL. 1991. Brain temperature and ethanol sensitivity in C57 mice: a radiotelemetric study. Pharmacol Biochem Behav 39:457–463. https://doi.org/10.1016/0091-3057(91)90208-J. [DOI] [PubMed] [Google Scholar]
- 3.Brown MC. 1989. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 40:93–109. https://doi.org/10.1016/0378-5955(89)90103-2. [DOI] [PubMed] [Google Scholar]
- 4.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 5.Cloutier MM, Thrall RS. 2008. The respiratory system, p 415–478. In: Koeppen BM, Stanton BA, editors. Berne and Levy physiology, 6th ed. St Louis (MO): Mosby Elsevier. [Google Scholar]
- 6.De Jonge A, Santing PN, Timmermans PBMWM, Van Zwieten PA. 1982. Functional role of cardiac presynaptic α2 adrenoceptors in the bradycardia of α2 adrenoceptors agonists in pentobarbitone and urethane-anesthetized normotensive rats. J Auton Pharmacol 2:87–96. https://doi.org/10.1111/j.1474-8673.1982.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 7.Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blümel G. 1984. A comparative study with various anesthetics in mice (pentobarbitone, ketamine–xylazine, carfentanyl–etomidate). Res Exp Med (Berl) 184:159–169. https://doi.org/10.1007/BF01852390. [DOI] [PubMed] [Google Scholar]
- 8.Flecknell PA. 1993. Anesthesia and perioperative care. Methods Enzymol 225:16–33. https://doi.org/10.1016/0076-6879(93)25005-M. [DOI] [PubMed] [Google Scholar]
- 9.Franks NP. 2008. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9:370–386. https://doi.org/10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 10.Gaines GC. [Internet]. 2012. Generators of mammalian vestibular surface responses to head motion (Doctoral dissertation, East Carolina University) [Cited 19 October 2017]. Available at: http://hdl.handle.net/10342/4067. [Google Scholar]
- 11.Giles TD, Quiroz AC, Burch GE. 1969. Hemodynamic alterations produced by prolonged urethane anesthesia in the intact dog. Am Heart J 78:281–282. https://doi.org/10.1016/0002-8703(69)90023-4. [DOI] [PubMed] [Google Scholar]
- 12.Hara K, Harris RA. 2002. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg 94:313–318. [DOI] [PubMed] [Google Scholar]
- 13.Hildesheimer M, Muchnik C, Rubinstein M. 1987. Cochlear action potentials threshold and systemic arterial pO2. Laryngoscope 97:204–207. [PubMed] [Google Scholar]
- 14.Jones SM, Erway LC, Bergstrom RA, Schimenti JC, Jones TA. 1999. Vestibular responses to linear acceleration are absent in otoconia-deficient C57BL/6JEi-het mice. Hear Res 135:56–60. https://doi.org/10.1016/S0378-5955(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 15.Jones SM, Erway LC, Johnson KR, Yu H, Jones TA. 2004. Gravity receptor function in mice with graded otoconial deficiencies. Hear Res 191:34–40. https://doi.org/10.1016/j.heares.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Jones SM, Jones TA. 2016. Vestibular sensory evoked potentials, p 501–532. In: Jacobson GP, Shepard NT, editors. Balance function assessment and management, 2nd ed. San Diego (CA): Plural Publishing. [Google Scholar]
- 17.Jones TA. 1992. Vestibular short latency responses to pulsed linear acceleration in unanesthetized animals. Electroencephalogr Clin Neurophysiol 82:377–386. https://doi.org/10.1016/0013-4694(92)90007-5. [DOI] [PubMed] [Google Scholar]
- 18.Jones TA, Jones SM. 1999. Short latency compound action potentials from mammalian gravity receptor organs. Hear Res 136:75–85. https://doi.org/10.1016/S0378-5955(99)00110-0. [DOI] [PubMed] [Google Scholar]
- 19.Jones TA, Jones SM, Colbert S. 1998. The adequate stimulus for avian short latency vestibular responses to linear translation. J Vestib Res 8:253–272. https://doi.org/10.1016/S0957-4271(97)00072-4. [PubMed] [Google Scholar]
- 20.Jones TA, Jones SM, Vijayakumar S, Brugeaud A, Bothwell M, Chabbert C. 2011. The adequate stimulus for mammalian linear vestibular evoked potentials (VsEPs). Hear Res 280:133–140. https://doi.org/10.1016/j.heares.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones TA, Lee C, Gaines GC, Grant JW. 2015. On the high-frequency transfer of mechanical stimuli from the surface of the head to the macular neuroepithelium of the mouse. J Assoc Res Otolaryngol 16:189–204. https://doi.org/10.1007/s10162-014-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones TA, Pedersen TL. 1989. Short-latency vestibular responses to pulsed linear acceleration. Am J Otolaryngol 10:327–335. https://doi.org/10.1016/0196-0709(89)90108-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee C, Jones TA. 2016. Effects of meclizine and diazepam on mammalian vestibular function. Poster presented at Annual Meeting American Academy of Audiology, AudiologyNOW! 2016, Phoenix, Arizona, 13–15 April 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maggi CA, Manzini S, Parlani M, Meli A. 1984. An analysis of the effects of urethane on cardiovascular responsiveness to catecholamines in terms of its interference with Ca2+ mobilization from both intra- and extracellular pools. Experientia 40:52–59. https://doi.org/10.1007/BF01959102. [DOI] [PubMed] [Google Scholar]
- 25.Maggi CA, Meli A. 1986a. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 1: general considerations. Experientia 42:109–114. https://doi.org/10.1007/BF01952426. [DOI] [PubMed] [Google Scholar]
- 26.Maggi CA, Meli A. 1986b. Suitability of urethane anesthesia for physiopharmacological investigations in various systems. Part 2: cardiovascular system. Experientia 42:292–297. https://doi.org/10.1007/BF01942510. [DOI] [PubMed] [Google Scholar]
- 27.Maggi CA, Meli A. 1986c. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: other systems and conclusions. Experientia 42:531–537. https://doi.org/10.1007/BF01946692. [DOI] [PubMed] [Google Scholar]
- 28.Moroni F, Corradetti R, Casamenti F, Moneti G, Pepeu G. 1981. The release of endogenous GABA and glutamate from the cerebral cortex in the rat. Naunyn Schmiedebergs Arch Pharmacol 316:235–239. https://doi.org/10.1007/BF00505655. [DOI] [PubMed] [Google Scholar]
- 29.Nazareth AM, Jones TA. 1998. Central and peripheral components of short latency vestibular responses in the chicken. J Vestib Res 8:233–252. https://doi.org/10.1016/S0957-4271(97)00076-1. [PubMed] [Google Scholar]
- 30.Otto K. 2004. Anesthesia, analgesia, and euthanasia, p 555–569. Chapter 34. In: Hedrich H, Bullock G, editors. The laboratory mouse. London (UK): Elsevier Academic Press. [Google Scholar]
- 31.Patel PM, Patel HH, Roth DM. 2011. General anesthetics and therapeutic gases, p 553–554. In: Brunton LL, Chabner BA, Knollman B, editors. Goodman and Gilman's the pharmacological basis of therapeutics, 12th ed. New York (NY): McGraw–Hill Companies. [Google Scholar]
- 32.Sohmer H, Freeman S, Schmuel M. 1989. ABR threshold is a function of blood oxygen level. Hear Res 40:87–91. https://doi.org/10.1016/0378-5955(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 33.Tsukamoto A, Serizawa K, Sato R, Yamazaki J, Inomata T. 2015. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp Anim 64:57–64. https://doi.org/10.1538/expanim.14-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X, Jones SM, Yamoah EN, Lundberg YW. 2008. Otoconin-90 deletion leads to imbalance but normal hearing: a comparison with other otoconia mutants. Neurosci 153:289–299. https://doi.org/10.1016/j.neuroscience.2008.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]