Abstract
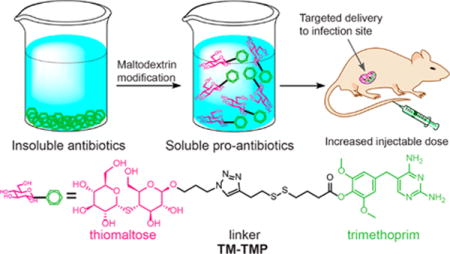
Trimethoprim is one of the most widely used antibiotics in the world. However, its efficacy is frequently limited by its poor water solubility and dose limiting toxicity. Prodrug strategies based on conjugation of oligosaccharides to trimethoprim have great potential for increasing the solubility of trimethoprim and lowering its toxicity, but they have been challenging to develop due to the sensitivity of trimethoprim to chemical modifications, and the rapid degradation of oligosaccharides in serum. In this report, we present a trimethoprim conjugate of maltodextrin termed TM-TMP, which increased the water solubility of trimethoprim by over 100 times, was stable to serum enzymes, and was active against urinary tract infections in mice. TM-TMP is composed of thiomaltose conjugated to trimethoprim, via a self-immolative disulfide linkage, and releases 4′-OH-trimethoprim (TMP-OH) after disulfide cleavage, which is a known metabolic product of trimethoprim and is as potent as trimethoprim. TM-TMP also contains a new maltodextrin targeting ligand composed of thiomaltose, which is stable to hydrolysis by serum amylases and therefore has the metabolic stability needed for in vivo use. TM-TMP has the potential to significantly improve the treatment of a wide number of infections given its high water solubility and the widespread use of trimethoprim.
Graphical abstract
INTRODUCTION
Trimethoprim (TMP) is one of the most widely used antibiotics in the world,1–4 and is used by millions of patients each year.5 For example, TMP is used for the treatment of urinary tract infections (UTIs), Shigellosis, and Pneumocystis pneumonia, either alone or in combination with a sulfonamide.6–10 However, despite its widespread use, TMP’s efficacy is limited by its poor water solubility,11 and drug delivery strategies that can improve the solubility of trimethoprim have the potential to impact multiple areas of medicine.12–18 The poor water solubility of trimethoprim is a significant challenge for patients requiring intravenous dosing of trimethoprim, which is currently used for treating patients with Pneumocystis pneumonia, HIV patients, or patients with complicated UTIs. The current I.V. formulation for trimethoprim requires the use of organic solvents, which minimizes the dose of trimethoprim that can be given, and consequently, moderate levels of trimethoprim resistance cause significant medical problems.8,10,19–21
The conjugation of TMP to maltodextrins has great potential for improving the solubility and efficacy of TMP, due to the high water solubility of the maltodextrins and also their ability to target bacteria in vivo.22,23 However, developing maltodextrin TMP conjugates has been challenging because TMP, like most antibiotics, is very sensitive to structural modifications and conjugation to an oligosaccharide will most likely destroy the efficacy of TMP.24–26 In addition, maltodextrins are rapidly hydrolyzed in serum,27–29 and this poses another challenge with using maltodextrins to increase the water solubility of TMP.
In this report we present a prodrug of trimethoprim termed TM-TMP, which is composed of thiomaltose conjugated to trimethoprim, via a self-immolative disulfide linkage. TM-TMP releases 4′-OH-trimethoprim (TMP-OH) after disulfide cleavage, which is a known metabolic product of trimethoprim and is as potent as trimethoprim (Figure 1).30,31 TM-TMP contains a new maltodextrin targeting ligand composed of thiomaltose, which is stable to hydrolysis by serum amylases and therefore has the metabolic stability needed for in vivo use. A patent has been filed by our laboratory for the use of thiomaltose for the diagnosis and treatment of bacteria infections.32 A novel disulfide self-immolative linkage was also used to connect the thiomaltose to the trimethoprim, which is cleaved by thiols to release TMP-OH, via cyclization of the thiolate with the ester carbonyl (Figure 1B). TM-TMP should improve the efficacy of trimethoprim because it will enhance the water solubility of trimethoprim, and also concentrate trimethoprim to bacterially infected tissues, due to the exclusive expression of maltodextrin transporters on bacteria compared to mammalian cells.33,34
Figure 1.
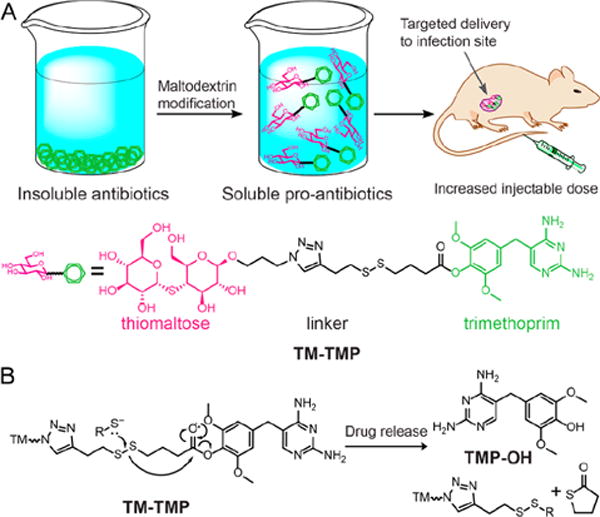
(A) Thiomaltose trimethoprim (TM-TMP): A new trimethoprim prodrug with enhanced water solubility that targets bacteria. (B) Proposed cleavage of TM-TMP in the presence of exogenous thiols.
RESULTS AND DISCUSSION
Design of Thiomaltose-Trimethoprim (TM-TMP)
The synthesis of TM-TMP is shown in Figure 2. The key step in the synthesis of TM-TMP was the coupling of azidothiomaltose (TM-N3) with the alkyne modified trimethoprim derivative (2). Briefly, trimethoprim was first selectively demethylated at its 4′ position and then coupled with a disulfide self-immolative linker through DCC/DMAP esterification. The synthesis of azido-thiomaltose is shown in Figure 3. The final product (TM-TMP) was synthesized via Cu-click chemistry and purified by reverse phase HPLC.
Figure 2.
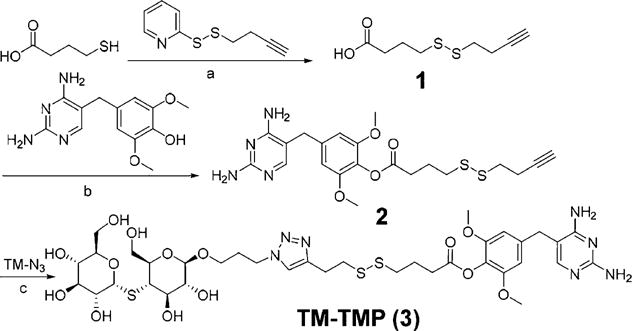
Synthesis of thiomaltose-trimethoprim (TM-TMP). (a) DCM, TEA, rt, 55%. (b) DCC, DMAP, TEA, DMF, rt, 29%. (c) CuI, DIPEA, DMSO, rt, 65%.
Figure 3.
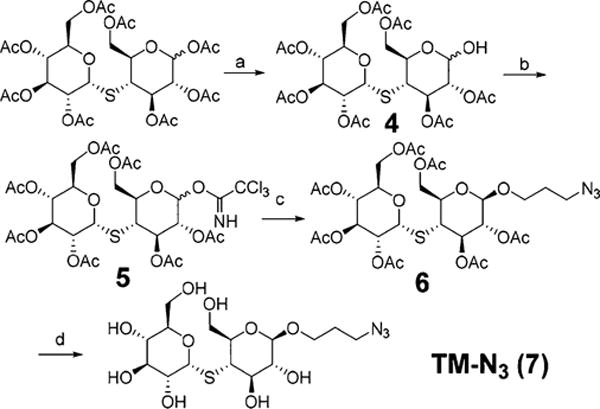
Synthesis of azido-thiomaltose (TM-N3). (a) NH2NH2·HOAc, DMF, rt, 81%. (b) Trichloroacetonitrile, DBU, DCM, rt, 97%. (c) Azidopropanol, TMSOTf, DCM, −20 °C, 45%. (d) MeONa, MeOH, quantitative.
The solubility of trimethoprim in water is less than 1 mg/mL and therefore cannot be delivered to patients at high doses. We hypothesized that the thiomaltose motif in TM-TMP would greatly enhance the water solubility of trimethoprim and also prevent its unfavorable and nonspecific diffusion into mammalian cells. Therefore, we measured the octanol/water partition coefficient (LogP) and water solubility of TM-TMP and compared it to trimethoprim. The LogP of TM-TMP was determined to be −1.42, whereas the logP of TMP was 0.89. In addition, the solubility of TM-TMP in water was determined to be greater than 200 mg/mL; in contrast, the solubility of TMP in water was only 0.4 mg/mL. Therefore, TM-TMP increased the water solubility of TMP by over 250-fold and will enable much higher doses of trimethoprim to be given in vivo. In addition, the low LogP of TM-TMP and its large size should prevent TM-TMP from diffusing into mammalian cells, and this will further increase the dose that TM-TMP can be administered to patients.
Stability of Thiomaltose
A key challenge with using maltodextrins for bacterial targeting is their premature degradation in vivo, as there are numerous enzymes in human tissues, such as amylases and maltases, which are designed to cleave oligosaccharides. Therefore, we investigated the stability of thiomaltose against maltase, the enzyme in the kidney that is responsible for cleaving maltose in humans, and compared its stability to maltose. Thiomaltose or maltose were incubated with 10 units of maltase and the hydrolysis kinetics was determined by quantifying the glucose released. Figure 4 demonstrates that maltose is rapidly hydrolyzed under these conditions, and has a half-life of less than 30 min, whereas thiomaltose had under 1% hydrolysis after 3 h treatment. The thioacetal linkage of thiomaltose therefore significantly extends the stability of maltose to enzymatic hydrolysis and TM-TMP should have the enzymatic stability needed for in vivo use.
Figure 4.
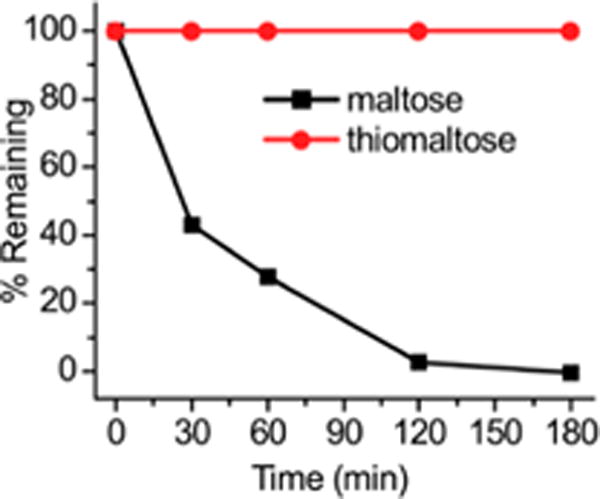
Thiomaltose is stable to maltase hydrolysis. When incubated with maltase, thiomaltose (red) was less than 1% hydrolyzed, whereas maltose (black) was hydrolyzed completely in 3 h. Experiments were done in triplicate and error bars were too small to be visible.
Cleavage Kinetics of TM-TMP
TM-TMP is designed to circulate in tissue, target bacteria, and then release free trimethoprim due to exogenous thiol mediated cleavage. We investigated the cleavage kinetics of TM-TMP in the presence of glutathione. TM-TMP was mixed with a 10 mM concentration of glutathione (GSH), in PBS, at 37 °C, and then analyzed via HPLC at 10 and 30 min. Figure 5A demonstrates that glutathione can cleave TM-TMP and catalyze the release of free TMP-OH. For example, TM-TMP was completely hydrolyzed after 10 min of incubation with glutathione, and under these conditions, it generated an equimolar concentration of free TMP-OH. In contrast, in the absence of GSH, TM-TMP was stable in PBS for at least 2 days without any observable degradation.
Figure 5.
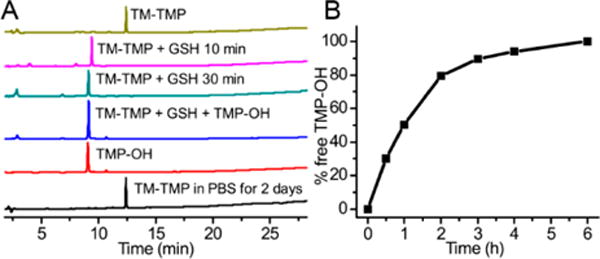
Release kinetics of TM-TMP. (A) Chromatograms monitored at 254 nm of TM-TMP in water (yellow), TM-TMP (100 μM) incubated with 10 mM GSH for 10 min (purple) and 30 min (green), TM-TMP incubated with GSH mixed with TMP-OH (blue), TMP-OH in water (red), TM-TMP in PBS at 37 after 2 days (black). (B) Cleavage kinetics of TM-TMP (100 μM) in FBS.
In addition, we also tested the stability of TM-TMP in serum. TM-TMP was mixed with fetal bovine serum (FBS), and the released TM-TMP was assayed via HPLC. Figure 5B demonstrates that TM-TMP has a half-life of approximately 1 h in serum, presumably due to the presence of thiols in the serum (around 500 μM).35,36 TM-TMP is a low-molecular-weight hydrophilic compound that should rapidly diffuse through tissue, and therefore its 1 h serum stability half-life should be sufficient for it to target bacteria in vivo.
Uptake of Thiomaltose Conjugates
Thiomaltose contains a thioacetal linkage and the tolerance of oligosaccharide transporters to the thioacetal linkage has never been investigated. We therefore synthesized a fluorophore labeled thiomaltose conjugate, thiomaltose-perylene (TM-P, Figure 6A), to examine the uptake of TM-P and its specificity for bacteria. A 500 μL suspension of E. coli (O.D. = 0.5) was incubated with 20 μM TM-P for 2 h. The bacterial cells were washed with PBS and lysed. The fluorescence intensity of the sample was measured and normalized to the protein content. Similarly, 105 macrophage cells were incubated with 20 μM TM-P for 2 h and the cells were lysed. The specificity of TM-P for bacteria was determined by comparing the fluorescence intensity in bacteria versus macrophages, normalized to intracellular protein content. Figures 6B demonstrates that TM-P has high specificity for bacteria, as the uptake of TM-P was 98-fold higher in bacteria when compared to mammalian cells. Thus, the thioacetal linkage is well tolerated by oligosaccharide transporters, and thiomaltose has similar specificity for bacteria over mammalian cells as endogenous oligosaccharide targeting ligands, such as maltose and maltohexaose. We further tested the specificity of TM-P with lamB mutant E. coli, which is a mutant strain that lacks the outer membrane maltodextrin transporter lamB and cannot internalize maltodextrins.37,38 As shown in Figure 6C, the mutant strain had a 2.5-fold decrease in uptake compared to wild-type E. coli. A fluorescent image of TM-P treated E. coli was also obtained to further validate the ability of E. coli to internalize TM-P (see Supporting Information, Figure S11).
Figure 6.
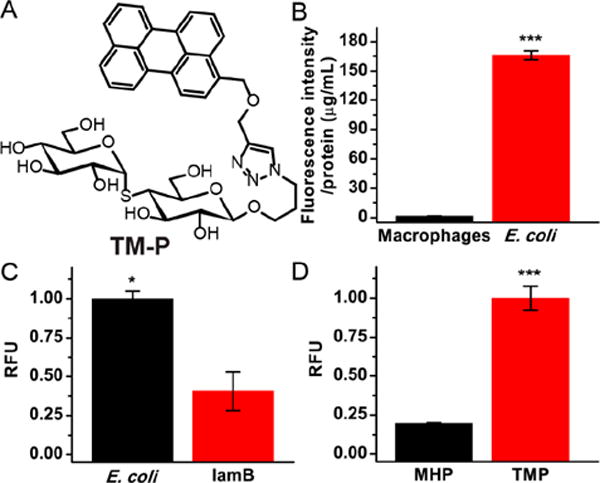
Uptake studies using TM-P. (A) Structure of TM-P. (B) Uptake of thiomaltose-perylene in bacteria was 98-fold higher than in macrophages. (C) Uptake of thiomaltose-perylene in wild-type E. coli was 2.5-fold higher than that in lamB mutant. (D) Uptake of thiomaltose-perylene in bacteria was 6-fold higher than that of maltohexaose-perylene. The statistical significances were determined using a two-sample Student’s t-test (*p ≤ 0.05, ***p ≤ 0.001).
Thiomaltose should be internalized by a wide variety of oligosaccharide transporters, such as the maltodextrin transporters (lamB and others) and other general pores (OmpF, OmpC, and others).37 We compared the uptake of TM-P against maltohexaose conjugated to perylene (MH-P),22 as it was previously reported that longer oligosaccharides were less effectively internalized by E. coli.39 As shown in Figure 6D, the uptake of TM-P is about 4–5-fold higher than MH-P, which is consistent with previous studies demonstrating that maltose is internalized more efficiently than maltohexaose.39,40 In addition, TM-P was stable in serum, and showed no detectable degradation in serum after 24 h incubation with fetal bovine serum (FBS), whereas MH-P was rapidly degraded in FBS (see Supporting Information, Figure S8). Finally, HPLC analysis of the bacteria lysate from E. coli (BL21(DE3)) treated with TM-P demonstrated that it was stable in the intracellular environment of the bacteria, and remained as a single peak HPLC, even after internalization by bacteria (see Supporting Information, Figure S9). A similar thiomaltose conjugate, TM-IR780, was also synthesized to further prove the stability of thiomaltose conjugates (see Supporting Information, Figure S10).
In Vitro Activity of TMP-OH and TM-TMP
TM-TMP releases TMP-OH after reduction by thiols, which binds E. coli dihydrofolate reductase (DHFR) as tightly as TMP, and had a similar minimum inhibitory concentration (MIC) value against bacteria as TMP.41 We observed that TMP-OH had a similar activity to TMP on a lab strain of E. coli (BL21(DE3)), and both TMP and TMP-OH had an MIC around 1 μM against BL21(DE3) (see Supporting Information, Figure S12). We therefore further tested the activity of TMP and TM-TMP on a clinical E. coli strain (E. coli 209) As expected, addition of 500 μM of GSH into the incubation broth significantly increased the efficacy of the TM-TMP, and the MIC of TM-TMP with GSH approached the MIC of free TMP, which was about 0.86 μM (Figure 7).
Figure 7.
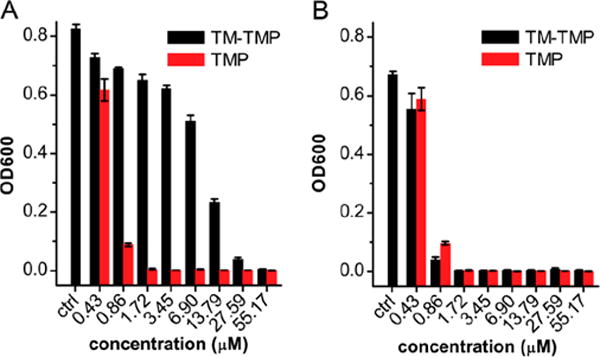
TM-TMP is active against E. coli and has similar efficacy to TMP in the presence of GSH. In vitro activity of TM-TMP against E. coli 209. (A) OD600 of E. coli after 24 h incubation with various concentrations of TM-TMP (black) and TMP (red), in the absence of GSH. (B) OD600 of E. coli after 24 h incubation with various concentration of TM-TMP (black) and TMP (red), in the presence of 500 μM of GSH.
In Vivo Activity of TM-TMP in a UTI Mouse Model
We selected urinary tract infection (UTI) caused by uropathogenic E. coli as an initial testbed for TM-TMP. There is an urgent need for new UTI treatment strategies because of the increasing frequency of UTIs caused by drug-resistant E. coli. Furthermore, UTIs are the most common community-acquired bacterial infection in the world, and affect more than 250 million people globally.42 Trimethoprim is one of the drugs used for treating UTIs, but it is frequently ineffective because of dose limiting toxicity and low water solubility, and therefore, strategies that can lower TMP’s toxicity and increase its water solubility have great potential.
Here, mice were transurethrally infected with a 50 μL suspension containing (1–2) × 107 of a clinical E. coli strain (E. coli 209), and the infection was allowed to develop for 2 days. The mice were then treated with either TMP or TM-TMP, injected via tail vein, at a dose of 10 mg/kg equivalent of TMP per mouse per day for 3 days. At day 5 of infection, the mice were sacrificed, and both kidneys and bladders were collected for enumeration of E. coli colony forming units (CFU).
Figure 8 demonstrates that TM-TMP is active in treating UTIs in a mouse model. For example, after 3 days of treatment, most of the bladders (7/9) from TM-TMP treated mice were cured and had no detectable bacteria, while in TMP treated mice, 5 mice had evidence of infection. Statistical analysis with the Mann–Whitney U test showed that the TM-TMP group was significantly different from the control group, while the TMP group was not. However, no statistical difference was observed between TM-TMP group and TMP group, due to the large errors in both groups. In the kidneys, TM-TMP treated mice had a lower number of bacteria (777 CFUs/mL, geometric mean), while the TMP treated group had an average of 925 CFUs/mL and the untreated group’s average was even higher at 5169 CFUs/mL, though no statistical significance was observed. The greater effect of TM-TMP on the bladder than the kidney infection may be due to higher concentrations of TM-TMP in the bladder than in the tissue of the kidneys.
Figure 8.
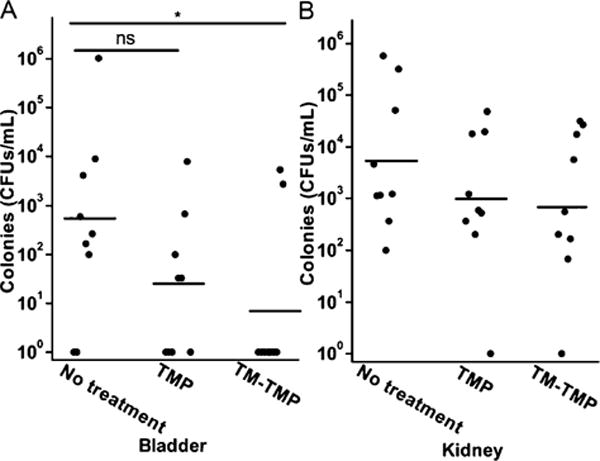
TM-TMP is active at treating UTIs in a mouse model. In vivo activity of TM-TMP against E. coli 209 in the UTI mouse model. (A) CFU counts of bladder samples. (B) CFU counts of kidney. Solid circles represent experimental results, and horizontal lines indicate the geometric mean. *P < 0.05; ns, not significant, one-tailed Mann–Whitney U test.
CONCLUSIONS
In summary, we have developed a new trimethoprim prodrug composed of trimethoprim conjugated to thiomaltose. A disulfide self-immolative linker was developed to connect the thiomaltose with the trimethoprim. TM-TMP is stable under normal buffered conditions, has a half-life of about 1 h in complete serum, but is rapidly cleaved under high concentrations of glutathione, to release the free trimethoprim–OH. Thiomaltose is also fully resistant to enzymatic hydrolysis and is metabolically stable. TM-TMP has over 200 times better water solubility than unmodified trimethoprim, and has an MIC of around 1 μM on E. coli in the presence of 500 μM of glutathione. TM-TMP was shown to be at least as effective as TMP alone in the treatment of UTI in a mouse model, which validates the design of the prodrug. Thiomaltose conjugation via a self-immolative linkage provides a convenient method for increasing the solubility of antibiotics and improving their efficacy, and has the potential to be adapted to a wide variety of hydrophobic antibiotics.
EXPERIMENTAL PROCEDURES
General Methods
1H NMR spectra were recorded in CDCl3, CD3OD, or D2O on a Bruker AVB-400 spectrometer at 298 K. TMS (δ (ppm)H = 0.00) was used as the internal reference. 13C NMR spectra were recorded in either CDCl3, CD3OD, and D2O at a 100 MHz on a Bruker AVB-400 spectrometer, using the central resonances of CDCl3 (δ (ppm)C = 77.0) and methanol (δ (ppm)C = 50.4) as the internal references. Chemical shifts are reported in ppm and multiplicities are indicated by s (singlet), d (doublet), t (triplet), q (quartet), dd (doublet of doublets), and m (multiplet). Coupling constants, J, are reported in hertz (Hz). High-resolution mass spectra (HRMS) were obtained on a AB SCIEX TOF/TOF 5800 system and are reported as m/z (relative intensity). Chemicals and solvents were purchased from Aldrich or VWR and used without further purification. All reactions were performed under anhydrous conditions under N2 or argon and monitored by TLC on MD Millipore Silica Gel 60 F254 Glass Plates. Detection was accomplished by examination under UV light (254 nm) and by charring with 20% sulfuric acid in methanol.
Synthetic compounds were purified with either flash chromatography using silica gel (230–400 mesh) or reverse phase HPLC. The HPLC was performed on a Shimadzu system with a CBM-20A Prominence communication bus module, a SPD-M20A Prominence Diode Array detector, a DGU-20A5 Prominence degasser, and two LC-6AD pumps. The column used for the HPLC purification was an XBridge BEH C8 OBD prep column (5 μm, 19 × 150 mm).
Compound Synthesis
The synthesis and the characterization of new compounds are shown in detail in the Supporting Information.
LogP Analysis
To a solution of TM-TMP (200 μL of 100 μM in DI water) was added 200 μL of 1-octanol. The mixture was vortexed for 2 min and then left to stand until the two phases separated clearly. Both the aqueous phase and the organic phase were then analyzed with an analytical HPLC, monitoring at 254 nm. The column used for the HPLC analysis was an XBridge BEH C8 column (5 μm, 4.6 × 150 mm), and the elution was performed with 0.1% TFA in H2O (A) and 0.1% TFA in acetonitrile (B). The gradient used was: 0–4 min, 5% B; 4–23 min, 5% to 100% B; 23–25 min, 100% B. The peak representing TM-TMP was integrated and the LogP was calculated as Log10(areaoctanol/areawater).
Maltase Hydrolysis
To 100 μL of 20 μM of thiomaltose or maltose solution was added 100 μL of 20 unit/mL maltase (Sigma, α-Glucosidase from Saccharomyces cerevisiae) in PBS. The solution was incubated at room temperature, and the glucose concentration was determined using a glucose assay kit (Sigma) at 30, 60, 120, and 180 min, following the method described in the kit manual. This was done in triplicate and the average was reported.
Solubility of TM-TMP
To a 100 mg of lyophilized TM-TMP powder, 500 μL of water was added, which yielded a clear viscous solution.
Cleavage Kinetics of TM-TMP
To 90 μL of PBS or 10 mM GSH or FBS was added 10 μL of 1 mM TM-TMP in PBS. The solution was incubated at 37 °C and analyzed with HPLC using the same conditions used for the LogP analysis. For the TM-TMP cleavage in FBS, peaks representing TM-TMP and TMP-OH were integrated, and % TMP-OH released was calculated as the areaTMP-OH/(areaTM-TMP + areaTMP-OH). Over the analytical time frame, the sum of two peak areas was constant, indicating 1:1 conversion of TM-TMP into TMP-OH.
Uptake of TM-P
To a 500 μL suspension of E. coli (BL21(DE3), O.D600 = 0.5) was added 10 μL of 1 mM TM-P or MH-P. The bacterial cells were incubated at 37 °C for 2 h and then centrifuged at 10 000 rpm for 5 min. The supernatant was discarded and the cell pellet was washed with 0.5 mL of PBS for 3 times, via centrifugation. The cell pellet was then lysed with 0.5 mL of bacterial lysis buffer (Thermo Scientific B-PER Bacterial Protein Extraction Reagents). The fluorescence intensity of the sample was measured and the protein concentration was determined using a Pierce BCA protein assay kit.
Similarly, 105 macrophage cells were incubated with 20 μM TM-P for 2 h and the cells were lysed with 0.5 mL of mammalian cell lysis buffer.
For the lamB mutant study, to a 1 mL suspension of E. coli (108 CFUs, control strain (ATCC 33456), or lamB mutant strain (JW3992-1, Yale CGSC)) was added 5 μL of 1 mM TM-P. The bacterial cells were incubated at 37 °C for 1 h and then centrifuged at 10 000 rpm for 5 min. The supernatant was discarded and the cell pellet was washed with 1 mL of PBS for 3 times, via centrifugation. The cell pellet was then suspended into 100 μL of PBS, and the fluorescent intensity was evaluated by using the plate readers.
In Vitro Antibacterial Activity
To a 96-well plate well containing 200 μL of LB medium (with or without 0.5 mM GSH) was added 1 μL of E. coli (OD600 = 0.5) and 2 μL of stock drug solution (TM-TMP in PBS and TMP-OH in DMSO). The plate was incubated at 37 °C, shaken at 190 rpm for 24 h, and the OD600 was measured using a Tecan Infinite 200 plate reader.
UTI Infection Model
Seven-week-old female C3H/HeOuJ (Jackson laboratories) were transurethrally infected with a 50 μL suspension containing (1–2) × 107 CFU of E. coli under 3% isoflurane. Mice were deprived of water for 1 h and had urine expelled from their bladders immediately before inoculation. The animals were allowed to recover from anesthesia and were given water 1 h later. The experiment was divided into control and experimental groups, each group containing 9 mice. Control groups included mice untreated with any drug, and mice treated with trimethoprim (Chem-Impex International Inc. Wood Dale, IL), while the experimental group was treated with thiomaltose-trimethoprim (TM-TMP). The drugs were injected every 12 h for 3 days at concentration of 10 mg/kg, intravenously. Extraintestinal pathogenic E. coli 209 were used in this experiment and were isolated from a patient with a bloodstream infection. To prepare the inoculum, bacteria were grown under static conditions in Luria broth at 37 °C to induce the expression of type 1 pili as previously described.43 E. coli 209 is trimethoprim sensitive.
Mice were euthanized at day 5 (after the end of treatment). The bladder and both kidneys of each animal were removed and homogenized in sterile PBS after which the homogenates were serially diluted and plated onto LB agar (Difco Laboratories) in triplicate. The number of E. coli colonies on each plate was counted after overnight incubation at 37 °C and was used to calculate the total number of bacteria in each bladder or pair of kidneys.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health RO1 EB2000801A1 (N.M. and W.R.T) and R33AI119115 (N.M. and L.R.). We thank Santanu Maity for chemistry advice.
ABBREVIATIONS
- TM
thiomaltose
- TMP
trimethoprim
- TM-TMP
thiomaltose trimethoprim conjugate
- TM-P
thiomaltose perylene conjugate
- TMP-OH
4′-OH-trimethoprim
- MH-P
maltohexaose perylene conjugate
- LogP
octanol/water partition coefficient
- UTI
urinary tract infection
- TM-N3
azidothiomaltose
- FBS
fetal bovine serum
- GSH
glutathione
- CFU
colony forming unit
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bioconjchem.8b00177.
Experimental details and synthetic protocols for making TM-TMP (PDF)
ORCID
Xiaojian Wang: 0000-0002-1502-8781
Notes
The authors declare the following competing financial interest(s): N.M. and W.R.T are equity holders in Microbial Medical, a company focused on using maltodextrins to target bacterial infections.
References
- 1.Forsch RA, Queener SF, Rosowsky A. Preliminary in vitro studies on two potent, water-soluble trimethoprim analogues with exceptional species selectivity against dihydrofolate reductase from Pneumocystis carinii and Mycobacterium avium. Bioorg Med Chem Lett. 2004;14:1811–1815. doi: 10.1016/j.bmcl.2003.12.103. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Model Lists of Essential Medicines (20th list) http://www.who.int/medicines/publications/essentialmedicines/en/ (Accessed Nov. 18, 2017)
- 3.Lawrenson RA, Logie JW. Antibiotic failure in the treatment of urinary tract infections in young women. J Antimicrob Chemother. 2001;48:895–901. doi: 10.1093/jac/48.6.895. [DOI] [PubMed] [Google Scholar]
- 4.Ho JMW, Juurlink DN. Considerations when prescribing trimethoprim–sulfamethoxazole. Can Med Assoc J. 2011;183:1851–1858. doi: 10.1503/cmaj.111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks LA, Taylor THJ, Hunkler RJ. U.S. Outpatient Antibiotic Prescribing, 2010. N Engl J Med. 2013;368:1461–1462. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- 6.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mabeck CE, Vejlsgaard R. Treatment of urinary tract infections in general practice with sulfamethizole, trimethoprim or co-trimazine (sulphadiazine-trimethoprim) J Antimicrob Chemother. 1980;6:701–708. doi: 10.1093/jac/6.6.701. [DOI] [PubMed] [Google Scholar]
- 8.Teva Parenteral Medicines, I. SULFAMETHOXAZOLE, AND TRIMETHOPRIM - sulfamethoxazole and trimethoprim injection. http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=16388 (Accessed Jan. 10, 2016)
- 9.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents: recommendations from the Centers for Disease Control and Prevention the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. https://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf (Accessed Jul. 30, 2016)
- 10.Hughes WT. Pneumocystis carinii pneumonia: new approaches to diagnosis, treatment and prevention. Pediatr Infect Dis J. 1991;10:391–399. [PubMed] [Google Scholar]
- 11.Yin D-p, Liu M-x, Fu H-l, Shu G, Zhou J-y, Qing X-y, Wu W-b. Solubility of Trimethoprim in Selected Pure Solvents and (Water + Ethanol/2-Propanol) Mixed-Solvent Systems. J Chem Eng Data. 2016;61:404–411. [Google Scholar]
- 12.Li N, Zhang YH, Xiong XL, Li ZG, Jin XH, Wu YN. Study of the physicochemical properties of trimethoprim with β-cyclodextrin in solution. J Pharm Biomed Anal. 2005;38:370–374. doi: 10.1016/j.jpba.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Li N, Zhang YH, Wu YN, Xiong XL, Zhang YH. Inclusion complex of trimethoprim with β-cyclodextrin. J Pharm Biomed Anal. 2005;39:824–829. doi: 10.1016/j.jpba.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Garnero C, Zoppi A, Genovese D, Longhi M. Studies on trimethoprim:hydroxypropyl-β-cyclodextrin: aggregate and complex formation. Carbohydr Res. 2010;345:2550–2556. doi: 10.1016/j.carres.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Kubota D, Macedo OFL, Andrade GRS, Conegero LS, Almeida LE, Costa NB, Jr, Gimenez IF. Structural and theoretical-experimental physicochemical study of trimethoprim/randomly methylated-β-cyclodextrin binary system. Carbohydr Res. 2011;346:2746–2751. doi: 10.1016/j.carres.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Macedo OFL, Andrade GRS, Conegero LS, Barreto LS, Costa NB, Jr, Gimenez IF, Almeida LE, Kubota D. Physicochemical study and characterization of the trimethoprim/2-hydroxypropyl-γ-cyclodextrin inclusion complex. Spectrochim Acta, Part A. 2012;86:101–106. doi: 10.1016/j.saa.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Sun H, Seshadri M, Lingard S, Monaghan W, Faoagali J, Chan E, McDonald HW, Houston TA, King MA, Peak I. Antibacterial activity of β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin trimethoprim complexes. American Journal of Microbiology. 2011;2:1–8. [Google Scholar]
- 18.Figueiras A, Cardoso O, Veiga F, de Carvalho RB, Ballaro G. Preparation and characterization of Trimethoprim inclusion complex with Methyl-β-Cyclodextrin and determination of its antimicrobial activity. Pharm Anal Acta. 2015;6:405–409. [Google Scholar]
- 19.Sattler FR, Remington JS. Intravenous trimethoprim-sulfamethoxazole therapy for pneumocystis carinii pneumonia. Am J Med. 1981;70:1215–1221. doi: 10.1016/0002-9343(81)90830-5. [DOI] [PubMed] [Google Scholar]
- 20.Shehab N, Lewis CL, Streetman DD, Donn SM. Exposure to the pharmaceutical excipients benzyl alcohol and propylene glycol among critically ill neonates. Pediatr Crit Care Med. 2009;10:256–259. doi: 10.1097/PCC.0b013e31819a383c. [DOI] [PubMed] [Google Scholar]
- 21.Koch BCP, Zabirova SR, van der Nagel BCH, Hanff LM. Development and Validation of an HPLC-UV Assay to Quantify Plasma Levels of Sulfametrol: A Preferential Antibiotic in Children. Ther Drug Monit. 2015;37:670–674. doi: 10.1097/FTD.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 22.Ning X, Lee S, Wang Z, Kim D, Stubblefield B, Gilbert E, Murthy N. Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nat Mater. 2011;10:602–607. doi: 10.1038/nmat3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning X, Seo W, Lee S, Takemiya K, Rafi M, Feng X, Weiss D, Wang X, Williams L, Camp VM, et al. PET Imaging of Bacterial Infections with Fluorine-18-Labeled Maltohexaose. Angew Chem, Int Ed. 2014;53:14096–14101. doi: 10.1002/anie.201408533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung ME, Yang EC, Vu BT, Kiankarimi M, Spyrou E, Kaunitz J. Glycosylation of Fluoroquinolones through Direct and Oxygenated Polymethylene Linkages as a Sugar-Mediated Active Transport System for Antimicrobials. J Med Chem. 1999;42:3899–3909. doi: 10.1021/jm990015b. [DOI] [PubMed] [Google Scholar]
- 25.Milner SJ, Carrick CT, Kerr KG, Snelling AM, Thomas GH, Duhme-Klair AK, Routledge A. Probing bacterial uptake of glycosylated ciprofloxacin conjugates. ChemBioChem. 2014;15:466–471. doi: 10.1002/cbic.201300512. [DOI] [PubMed] [Google Scholar]
- 26.Karoli T, Mamidyala SK, Zuegg J, Fry SR, Tee EHL, Bradford TA, Madala PK, Huang JX, Ramu S, Butler MS, et al. Structure aided design of chimeric antibiotics. Bioorg Med Chem Lett. 2012;22:2428–2433. doi: 10.1016/j.bmcl.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Ohneda A, Yamagata S, Tsutsumi K, Fujiwara H. Distribution of Maltose Intravenously Administered to Rabbits and Its Metabolism in the Kidney. Tohoku J Exp Med. 1974;112:141–154. doi: 10.1620/tjem.112.141. [DOI] [PubMed] [Google Scholar]
- 28.Tahara Y, Fukuda M, Yamamoto Y, Noma Y, Yamato E, Cha T, Yoneda H, Ikegami H, Hirota M, Shima K. Metabolism of intravenously administered maltose in renal tubules in humans. Am J Clin Nutr. 1990;52:689–693. doi: 10.1093/ajcn/52.4.689. [DOI] [PubMed] [Google Scholar]
- 29.Young SJM, Weser E. The metabolism of circulating maltose in man. J Clin Invest. 1971;50:986–991. doi: 10.1172/JCI106592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth B, Strelitz JZ, Rauckman BS. 2,4-Diamino-5-benzylpyrimidines and analogs as antibacterial agents. 2 C-Alkylation of pyrimidines with Mannich bases and application to the synthesis of trimethoprim and analogs. J Med Chem. 1980;23:379–384. doi: 10.1021/jm00178a007. [DOI] [PubMed] [Google Scholar]
- 31.Damsten MC, de Vlieger JSB, Niessen WMA, Irth H, Vermeulen NPE, Commandeur JNM. Trimethoprim: Novel Reactive Intermediates and Bioactivation Pathways by Cytochrome P450s. Chem Res Toxicol. 2008;21:2181–2187. doi: 10.1021/tx8002593. [DOI] [PubMed] [Google Scholar]
- 32.Goodman M, Taylor RW, Takemiya K, Murthy N, Mohammed R, Ning X. Saccharide analogs and agents for the diagnosis and therapy of bacterial infections. WO2016044846A1. International Patent. 2016 Mar 24;
- 33.Gopal S, Berg D, Hagen N, Schriefer EM, Stoll R, Goebel W, Kreft J. Maltose and Maltodextrin Utilization by Listeria monocytogenes Depend on an Inducible ABC Transporter which Is Repressed by Glucose. PLoS One. 2010;5:e10349. doi: 10.1371/journal.pone.0010349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gowrishankar G, Namavari M, Jouannot EB, Hoehne A, Reeves R, Hardy J, Gambhir SS. Investigation of 6-[18F]-Fluoromaltose as a Novel PET Tracer for Imaging Bacterial Infection. PLoS One. 2014;9:e107951. doi: 10.1371/journal.pone.0107951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brigham MP, Stein WH, Moore S. The Concentrations Of Cysteine And Cystine In Human Blood Plasma. J Clin Invest. 1960;39:1633–1638. doi: 10.1172/JCI104186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Look MP, Rockstroh JK, Rao GS, Kreuzer KA, Barton S, Lemoch H, Sudhop T, Hoch J, Stockinger K, Spengler U, et al. Serum selenium, plasma glutathione (GSH) and erythrocyte glutathione peroxidase (GSH-Px)-levels in asymptomatic versus symptomatic human immunodeficiency virus-1 (HIV-1)-infection. Eur J Clin Nutr. 1997;51:266–272. doi: 10.1038/sj.ejcn.1600401. [DOI] [PubMed] [Google Scholar]
- 37.Freundlieb S, Ehmann U, Boos W. Facilitated diffusion of p-nitrophenyl-alpha-D-maltohexaoside through the outer membrane of Escherichia coli. Characterization of LamB as a specific and saturable channel for maltooligosaccharides. J Biol Chem. 1988;263:314–320. [PubMed] [Google Scholar]
- 38.Klebba PE. Mechanism of maltodextrin transport through LamB. Res Microbiol. 2002;153:417–424. doi: 10.1016/s0923-2508(02)01340-2. [DOI] [PubMed] [Google Scholar]
- 39.Luckey M, Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980;77:167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dippel R, Boos W. The maltodextrin system of Escherichia coli: metabolism and transport. J Bacteriol. 2005;187:8322–8331. doi: 10.1128/JB.187.24.8322-8331.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hryniewicz K, Szczypa K, Sulikowska A, Jankowski K, Betlejewska K, Hryniewicz W. Antibiotic susceptibility of bacterial strains isolated from urinary tract infections in Poland. J Antimicrob Chemother. 2001;47:773–780. doi: 10.1093/jac/47.6.773. [DOI] [PubMed] [Google Scholar]
- 42.Ronald AR, Nicolle LE, Stamm E, Krieger J, Warren J, Schaeffer A, Naber KG, Hooton TM, Johnson J, Chambers S, et al. Urinary tract infection in adults: research priorities and strategies. Int J Antimicrob Agents. 2001;17:343–348. doi: 10.1016/s0924-8579(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 43.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


