TO THE EDITOR
Chronic urticaria (CU) is defined as the appearance of pruritic migratory wheals, angioedema, or both on most days of the week for more than 6 weeks. First line therapies for CU are high dose H1 and H2 anti-histamines along with leukotriene receptor antagonists. Second line therapies traditionally consist of immunomodulatory agents. In 2013, omalizumab (humanized monoclonal antibody against IgE) was shown in a phase 3 multicenter randomized controlled trial to be a highly-effective second line therapy for patients with CU refractory to antihistamines and received FDA approval in 2014.1 After 12 weeks of treatment, ~40% of patients with CU demonstrated a complete response and 50–70% showed a partial response to therapy with omalizumab.2 In addition to there being many non-responders, there is often a delayed response of up to 6 months to observe therapeutic benefit and treatment can be costly.2 Therefore, biomarkers which would predict response are desirable.
Unlike asthma, where omalizumab is dosed based on a nomogram of serum IgE and weight, in CU omalizumab is dosed at a fixed 150 or 300 mg dose every month based on results of the ASTERIA I, ASTERIA II, and GLACIAL trials.3 While Zheng et al reported that baseline serum IgE was not predictive of omalizumab clinical response,4 further analysis of the data does suggest reduced efficacy amongst those with the lowest IgE concentrations.4 Two retrospective analyses by Metz et. al. (n = 51)5 and Viswanathan et. al. (n = 16)6 did not show significant differences in serum IgE concentrations between omalizumab responders and non-responders. However, none of the aforementioned work stratified low versus normal/high IgE levels and then analyzed for differences in clinical response to omalizumab.
Therefore, we performed a multicenter retrospective chart review of 137 patients, greater than 12 years of age, with antihistamine-refractory CU seen at the University of Virginia (UVA) and Johns Hopkins University (JHU) outpatient allergy clinics between 2011 and 2016. This study was approved by the UVA Health System Institutional Review Board (UVA IRB-HSR #18100) and de-identified data was provided by JHU. Serum IgE was measured prior to initiation of omalizumab and known cases of galactose-alpha-1, 3-galactose allergy or other causes of increased IgE were excluded from the analyses. All subjects in the study were treatment-naïve to omalizumab and 130 of 136 (95.6%) were not on other immunomodulatory medications for their CIU at study enrollment. Clinical response to omalizumab was subjectively defined by documented clinical response to omalizumab by the prescribing provider and/or continuation of omalizumab beyond 4 months of therapy. Partial responders were considered responders. χ2 test was used to compare categorical response data. Serum IgE concentration was modeled as a restricted cubic spline function of the serum IgE concentration with linear and nonlinear components and concomitant variables: age, sex and weight (kg) were the logistic regression predictor variables. Unique associations were tested based on the log-adjusted odds ratio. A p <0.05 was considered significant.
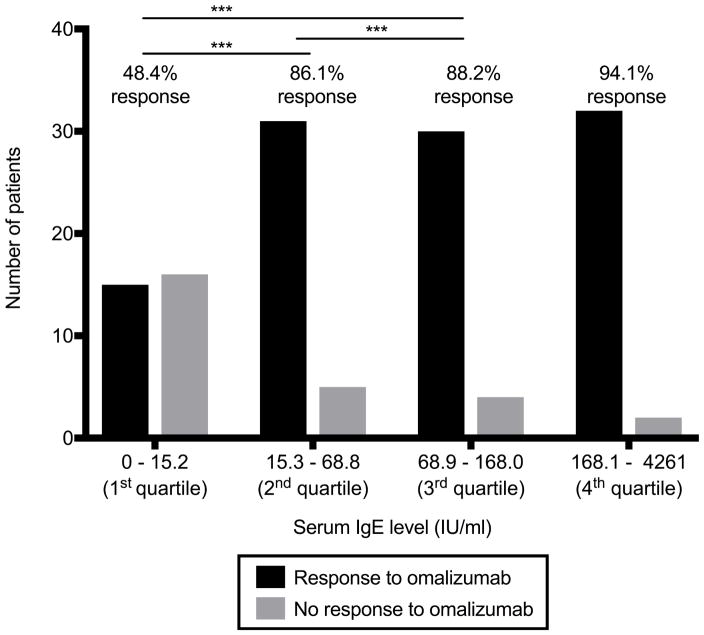
Patients’ characteristics are summarized in Table E1. The average age was 45 (±15) years, majority were women (73.7%), and nearly half had detectable thyroid autoantibodies, all epidemiologically consistent with CU. When patients were subdivided into serum IgE quartiles (1st 0–15.2 IU/mL, 2nd 15.3–68.8 IU/mL, 3rd 68.9–168.0 IU/mL, 4th 168.1–4261 IU/mL), their response to omalizumab differed significantly. Patients with a serum IgE in the 1st quartile had a 48.4% response rate to omalizumab compared to 86.1%, 88.2%, and 94.1% response rates for the 2nd, 3rd, and 4th quartiles respectively (Figure 1, p<0.001, multivariate logistic regression model). The adjusted odds ratio for response to omalizumab was 13.81 (95%CI: [3.58, 48.45]) for individuals whose serum IgE concentration was at the 75th percentile (168.0 IU/mL) than for those at the 25th percentile (15.2 IU/mL) (p<0.001). Thus, having a low baseline serum IgE ≤15.2 IU/mL would predict a lower likelihood of response to omalizumab, suggesting serum IgE could be utilized as a biomarker.
FIGURE 1.
Response to omalizumab therapy categorized by serum IgE quartile (IU/L) with the number of subjects representing the y-axis. Analysis conducted with nonlinear regression modeling.
Interestingly, there was a trend towards greater likelihood of response to omalizumab treatment by men compared to women (p= 0.052, adjusted odds ratio for response 4.79 (95% CI [0.99, 23.18])) (Table 1). No correlation of efficacy was found with age or weight (Table 1). In subjects in whom the basophil activation test (BAT)7 was performed (n=74), there was a trend towards lower likelihood of omalizumab response in those with a positive test, consistent with previous work7, although this did not reach statistical significance (p = 0.074) (Table E2). No correlation of efficacy was found with thyroid autoantibody status (Table E2).
TABLE 1.
Responder analysis, multivariate logistic regression model
| Variable | Ratio | Adjusted Odds Ratio [95% CI] | P-value |
|---|---|---|---|
| IgE | 2nd Quartile (68.8 IU/ml) : 1st Quartile (15.2 IU/ml) | 3.81 [1.94, 7.46] | <0.001 |
| 3rd Quartile (168.0 IU/ml) : 1st Quartile (15.2 IU/ml) | 13.81 [3.58, 48.45] | <0.001 | |
| 3rd Quartile (168.0 IU/ml): 2nd Quartile (68.8 IU/ml) | 3.46 [1.84, 6.50] | <0.001 | |
| Age | 2nd Quartile (44.0 yrs) : 1st Quartile (34.0 yrs) | 1.19 [0.85, 1.68] | 0.308 |
| 3rd Quartile (53.0 yrs) : 1st Quartile (34.0 yrs) | 1.40 [0.73, 2.67] | 0.308 | |
| 3rd Quartile (53.0 yrs): 2nd Quartile (44 0 yrs) | 1.17 [0.86, 1.59] | 0.308 | |
| Sex | Male : Female | 4.79 [0.99, 23.18] | 0.052 |
| Weight | 2nd Quartile (83.0 kg) : 1st Quartile (73 kg) | 1.04 [0.85, 1.28] | 0.685 |
| 3rd Quartile (102 kg) : 1st Quartile (73 kg) | 1.13 [0.62, 2.06] | 0.685 | |
| 3rd Quartile (102 kg): 2nd Quartile (83.0 kg) | 1.08 [0.73, 1.61] | 0.685 |
Although the exact etiology of CU is unknown, autoimmunity is one current prevailing hypothesis. Indirect evidence of putative basophil and mast cell activating autoantibodies is provided by a positive BAT or autologous serum/plasma skin test. Some evidence suggests that these autoantibodies are directed against either IgE or the IgE receptor. Further evidence for an autoimmune etiology is the strong association of CU with thyroid autoantibodies,8 including in this cohort. Omalizumab binds to the Fc region of free serum IgE and prevents binding to the high-affinity IgE receptor (FcεRI), which subsequently leads to the down-regulation of its expression on the surface of basophils and mast cells.9 Thus, one hypothesis is that omalizumab could function by preventing IgE from binding to FcεRI on mast cells and basophils, thereby removing the target of the IgG autoantibodies. However, several lines of evidence argue against this being the mechanism of omalizumab in CU. Particularly, a positive BAT negatively predicts omalizumab response,7 clinical efficacy does not correlate with autoimmune phenotypes, and the time frame for clinical improvement with omalizumab (sometimes less than one week) is not associated with an observed difference in expression of FcεRI on cutaneous mast cells.8
Our current study finds an association between the lack of serum IgE and lower likelihood of omalizumab response. To explain these observations, we propose that CU comprises two disease processes. One is driven by auto-reactive IgG and is characterized by female predominance, lower serum IgE, a positive BAT, and less responsiveness to omalizumab. The other is IgE mediated (perhaps to self-antigens) and is characterized by equal sex prevalence, higher serum IgE and greater responsiveness to omalizumab. Further prospective studies are needed to test this hypothesis and identify other potential biomarkers of response to omalizumab.
Supplementary Material
Clinical Implications.
Low serum IgE (≤15.2 IU/mL) in patients with chronic urticaria correlated with significantly lower omalizumab treatment response. This holds potential as a clinically relevant biomarker to predict response and may help elucidate the immune mechanism of omalizumab in this disorder.
Acknowledgments
This work was supported by NIH grants: R56AI120055 (LB), AI057438 (LB), AI100799 (LB), R01AI116658 (SSS) and AI116658 Supplement (EO) as well as the University of Virginia School of Medicine (MGL).
Footnotes
Conflicts of interest: Sarbjit S. Saini receives research support from Novartis. The rest of the authors declare that they have no relevant conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the Treatment of Chronic Idiopathic or Spontaneous Urticaria. N Engl J Med. 2013;368:924–35. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan A, Ferrer M, Bernstein J, Antonova E, Trzaskoma B, Raimundo K, et al. Timing and duration of omalizumab response in patients with chronic idiopathic/spontaneous urticaria. J Allergy Clin Immunol. 2016;137(2):474–481. doi: 10.1016/j.jaci.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Tonacci A, Billeci L, Pioggia G, Navarra M, Gangemi S. Omalizumab for the Treatment of Chronic Idiopathic Urticaria: Systematic Review of the Literature. Pharmacotherapy. 2017;37(4):464–80. doi: 10.1002/phar.1915. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y, Kha L, Wada R, Jin J, Putnam W, Rosén K. Population PK-PD and Exposure-Response Modeling and Simulation to Support Dose Recommendations of Xolair in Chronic Idiopathic Urticaria/Chronic Spontaneous Urticaria. Poster presented at: American Association of Pharmaceutical Scientists National Biotechnology Conference; May 19–21, 2014; San Diego, CA. (Presentation not publically available) [Google Scholar]
- 5.Metz M, Ohanyan T, Church M, Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: A retrospective clinical analysis. J Dermatological Science. 2014;73(1):57–62. doi: 10.1016/j.jdermsci.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan R, Moss M, Mathur S. Retrospective analysis of the efficacy of omalizumab in chronic refractory urticaria. Allergy Asthma Proc. 2013;34(5):446–52. doi: 10.2500/aap.2013.34.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palacios T, Stillman L, Borish L, Lawrence M. Lack of basophil CD203c-upregulating activity as an immunological marker to predict response to treatment with omalizumab in patients with symptomatic chronic urticaria. J Allergy Clin Immunol Pract. 2016;4(3):529–30. doi: 10.1016/j.jaip.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altrichter S, Peter HJ, Pisareveskaja D, Metz M, Martus P, Maurer M. IgE Mediated Autoallergy against Thyroid Peroxidase – A Novel Pathomechanism of Chronic Spontaneous Urticaria? PLoS One. 2011;6:e14794. doi: 10.1371/journal.pone.0014794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J Allergy Clin Immunol. 2004;111(3):527–30. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.