Abstract
Purpose
To evaluate the feasibility and initial functional and anatomical outcomes of transplanting a full-thickness free graft of choroid, retinal pigment epithelium (RPE) along with neurosensory-retina in advanced fibrosis and atrophy associated with end-stage exudative age-related macular degeneration (nvAMD) with and without a concurrent refractory macular hole (MH).
Methods
During vitrectomy, a RPE-choroidal and neurosensory-retinal free graft was harvested in 9 eyes of 9 patients. The RPE-choroidal and neurosensory-retinal free graft was either placed subretinal (n=5), intraretinal to cover the foveal area inside an iatrogenically induced macular hole over the RPE-choroidal graft (n=3) or preretinal (n=1) without a retinotomy wherein both free grafts were placed over the concurrent MH. Silicone oil endotampoande was used in all cases.
Results
Mean follow up was 7±5.5 months (range 3–19). The mean preoperative visual-acuity was ~count fingers (logMAR=2.11, range 2–3), which improved to ~20/800 (logMAR 1.62±0.48, range 0.7–2, p=0.04). Vision was stable in 5 eyes (55.6%) and improved in 4 eyes (44.4%). Reading ability improved in five eyes (55.6%). Post-operative complications were graft atrophy (n=1), epiretinal-membrane (n=1), and dislocation of neurosensory-retina-choroid-RPE free graft (n=1).
Conclusion
Combined autologous RPE-choroid and neurosensory-retinal free graft is a potential surgical alternative in end-stage nvAMD eyes, including concurrent refractory MH.
Keywords: Neurosensory retinal free graft, retinal pigment epithelium choroidal free graft, choroid free graft, age related macular degeneration, disciform scar, fibrotic scar, retinal transplant, choroidal transplant, choroid-RPE transplant, exudative age related macular degeneration
INTRODUCTION
Autologous transplantation of retinal pigment epithelium (RPE) and choroid, first proposed by Peyman et al. is a surgical technique employed when the original RPE is damaged by atrophic changes or by choroidal subfoveal neovascularization that has the rationale to transport healthy RPE and choroid, from the periphery to under the macula. 1 Autologous RPE and choroid transplantation has been shown to be feasible and may induce a significant improvement in visual acuity and reading ability. 2 Stabilization or improvement in visual acuity has now been shown up to several years after surgery, and was achieved in patients with exudative age related macular degeneration (AMD) treated with an autologous free RPE–choroid graft. 2 Our group subsequently reported the feasibility of the RPE and choroidal graft through a peripheral retinotomy. 3,4
There have been several new surgical techniques described recently for large and refractory macular holes. An inverted internal limiting flap (ILM) technique for large macular holes was initially described by Michalewska et al with the rationale of the ILM flap acting as a scaffold to help with hole closure. 5,6 Recently, Grewal and Mahmoud reported the feasibility and efficacy of autologous transplantation of the neurosensory retina to treat large and chronic macular holes. 7,8 They also showed that the neurosensory retinal free graft may integrate with the surrounding retina and lead to functional improvement. 7,8 We hypothesized that an autologous neurosensory retinal free graft overlaid on the autologous RPE-choroid free graft may provide potential for integration with the surrounding retina, possibly leading to improved functional outcomes in cases with atrophy associated with choroidal neovascularization in advanced exudative AMD.
The aims of the present study are to describe our surgical technique of an autologous RPE-choroid free graft combined with a separate neurosensory retinal free graft – subretinal, intraretinal and preretinal; and to report the initial anatomical and functional results, and the complications, in a series of eyes with end-stage exudative AMD including the presence of a concurrent recalcitrant macular hole. Conventionally the term “graft” refers to the procedure of moving tissue from one site to another without bringing its own blood supply with it while the term “patch” is typically used in reference to repair a defect in tissue. Since not all the cases in our series had a defect (macular hole) we have used the term “free graft” throughout the manuscript for the autologous choroid – RPE – neurosensory retinal tissue.
METHODS
SURGICAL TECHNIQUE
A standard 20g (n=8) or 23g (n=1) three-port pars plana vitrectomy was performed with triamcinolone assisted induction of posterior vitreous detachment in eyes not previously vitrectomized. A thorough shave of the vitreous base was performed. Three different techniques were employed for the harvest and placement of the autologous choroid-RPE and neurosensory free graft which are described below:
Subretinal Free Graft Placement (n=5 eyes)
To harvest the neurosensory retinal graft, under chandelier illumination (Alcon Laboratories, Fort Worth, TX, USA), a retinal detachment was induced by injecting balanced salt solution (Balanced Salt Solution; Alcon Laboratories, Fort Worth, TX, USA) into the subretinal space through a 41g needle connected to an active pump of the vitrectomy machine. Then under fluid, a roughly 200° peripheral temporal retinotomy was performed at the ora serrata using curved scissors (DORC International, Netherlands). The temporal retina was folded on the nasal retina to access the subretinal space. The choroidal neovascular membrane (CNV) which was intraoperatively identified as a CNV - fibrosis complex, was removed. Bleeding from the choroidal feeder vessels of the CNV was stopped with gentle pressure or diathermy. A harvest site was selected temporally, with the location chosen by visually selecting an area of normal appearing RPE and taking care to avoid the vortex veins. The area was chosen in the temporal quadrant, superiorly or inferiorly, and the outline was traced with confluent endolaser spots, directed on the RPE. From this site, a free graft comprising of a full thickness free graft of choroid, choriocapillaris, Bruch’s membrane and RPE was removed from the sclera using scissors. After cutting half the perimeter of the free graft, perfluorocarbon liquid (Alcon Laboratories, Fort Worth, TX, USA) was injected into the subretinal space and onto the isolated edges of the free graft, in order to avoid rolling and elevation of the free graft edges. The borders of the choroid in the harvesting site were treated with endodiathermy and bleeding was controlled by intraocular pressure elevation. Additional PFCL was then injected into the sub-retinal space. Once the first half of the free graft was stabilized, the second half was cut with forceps and curved scissors and the choroid-RPE free graft was gently pulled to the sub-foveal area. During this maneuver, the free graft was held with forceps at its anterior edge, in order to prevent it from migrating free into the vitreous cavity and to also avoid damaging it with extensive manipulation. Next, a free graft of peripheral neurosensory retina, the same size as the choroid-RPE free graft, was harvested at the edge of the temporal retinotomy. This autologous free graft of neurosensory retina was then slid under the PFCL to overlay the free graft of choroid. Subretinal PFCL was then slowly aspirated and reinjected in the epiretinal space to flatten the retina on top of the neurosensory retina and choroid-RPE free grafts and to center the fovea on the visually healthy RPE.
Peripheral laser endophotocoagulation was then performed at the edge of the retinotomy being careful to avoid the bare RPE. A direct PFCL-silicone oil (1000 centistokes) exchange completed the surgery. All eyes received silicone oil endotamponade. Silicone oil has not yet been removed. In phakic patients, a standard phacoemulsification and in the bag intraocular lens implantation was performed concurrently.
Intraretinal Free Graft Placement (n=3 eyes)
The same technique was applied to obtain an RPE-choroidal free graft. However, these were cases with an atrophic fovea. An iatrogenic macular hole was induced accidently while injection of subretinal fluid under the macula to induce a retinal detachment. The retina was then attached with PFCL over the RPE-choroidal free graft. A full thickness neurosensory retina free graft was harvested from the temporal equatorial area. The margins of the free graft were not touched with laser, while only the edges of the harvesting site of retina were surrounded with endolaser and with endodiathermy if any bleeding vessels was visible. The free graft was then gently maneuvered to cover the foveal area inside the macular hole over the RPE-choroidal free graft. Then PFCL was placed to flatten the free graft and ensure it was centered to cover the macular hole (Video 1). A direct PFCL-silicone oil exchange was then performed, paying attention not to dislocate the retinal free graft.
Preretinal Free Graft Placement (n=1 eye)
Without inducing a retinal detachment, using a bimanual technique with forceps and pneumatic scissors (Alcon Labs, Fort Worth TX) under chandelier illumination, a full thickness choroid-RPE-neurosensory retina free graft was harvested superonasal to the disc. The margins of the free graft were surrounded with endolaser and any bleeding vessels were treated with endodiathermy. The free graft was then gently maneuvered to cover the foveal area (in this case there was a concurrent macular hole refractory to prior vitrectomy and internal limiting membrane peel) and the area of retinal atrophy. Then PFCL was placed to flatten the free graft and ensure it was centered to cover the macular hole. A direct PFCL-silicone oil exchange was then performed (Video 2).
All patients were instructed to position head down postoperatively or on the nasal side of the operated eye.
Anatomical success was investigated by optical coherence tomography (OCT, Carl Zeiss Zeiss, Dublin, CA, Topcon, Tokyo, Japan or, Heidelberg Engineering, Heidelberg, Germany), fluorescein and indocyanine green (ICG) angiography, OCT angiography (Topcon DRI OCT-1 Atlantis, Topcon, Japan) and fundus autofluorescence (488 nm excitation filter and 500 nm barrier filter) testing when available. Functional success was measured by pre-and post-operative best corrected visual acuity (BCVA) and microperimetry (tested with the scanning laser microscope, MAIA, Centervue, Padova, Italy) when available. Snellen BCVA was converted to its LogMAR equivalent for statistical analysis.
Results
Nine eyes of nine patients (mean age 74 ± 3.4 years, range 68– 79 years) affected by end stage exudative AMD with a fibrotic subretinal scar and atrophy of the external retinal layers underwent surgery. Mean follow up was 7 ± 5.5 (range 4–19 months). All patients were assessed before surgery, and the minimal follow up was 3 months post-operatively. Reading ability was assessed subjectively. We observed a subjective improvement in reading ability in 5/8 eyes. The mean visual acuity preoperatively was ~count fingers (logMAR 2.11, range 2–3). Post-operatively it improved to ~20/800 (logMAR 1.62 ± 0.48, range 0.7–2, p=0.04; paired samples t test, two tailed). Vision was stable in 5 eyes (55.6%) and improved in 4 eyes (44.4%). Preoperative fixation based on reading was absent in all eyes. Reading ability improved postoperatively in 5 eyes (55.6%). Intraoperative complications included a limited subretinal hemorrhage in one eye. Post-operative complications were free graft atrophy (n=1) and epiretinal membrane (n=1) and dislocation of the neurosensory retina from the choroid-RPE free graft when placed in the pre-retinal space (n=1) (Figure 7). None of the eyes developed PVR, retinal detachment or fibrosis of the free graft. None of the eyes reported worse vision following the procedure. Table 1 lists the characteristics of the nine eyes undergoing the free graft.
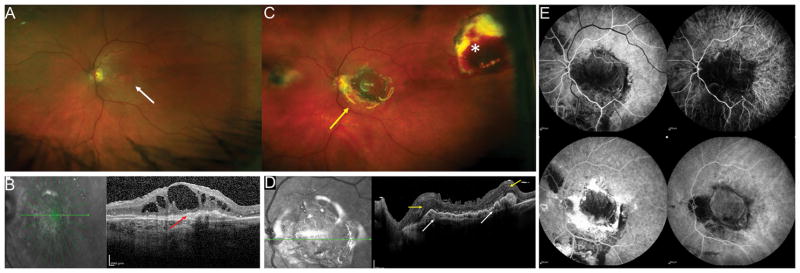
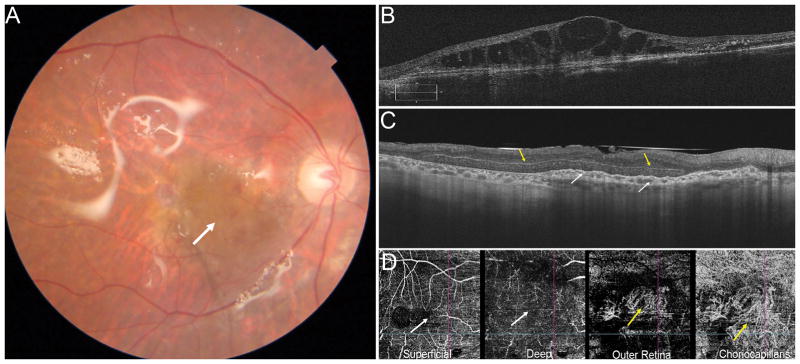
Figure 7.
76-year-old M with exudative age related macular degeneration with a concurrent large macular hole refractory to prior internal limiting membrane removal and chorioretinal scarring from prior retinal detachment repair. Preoperative OCT (A, middle) shows a large refractory macular hole with 2400-micron largest basal diameter and 1600-micron inner opening diameter. Following a combined autologous neurosensory retinal and choroidal free graft with silicone oil tamponade (B), post-operative day 1 OCT (C) shows the choroidal graft plugging the macular hole, the neurosensory retinal graft had dislocated. Postoperative photograph at 1 month shows the choroidal free graft covering the macular hole, free graft harvest site and a silicone oil tamponade. OCT at 2 months (C) and shows improved integrated of the choroidal free graft tissue in the macular hole with surrounding retinal tissue. Fluorescein angiogram (C, right) shows blockage from the subretinal heme, staining around the choroidal graft site and no leakage. The subretinal hemorrhage resolved. OCT at 12 months shows resolution of subretinal fluid and further improved integration of the choroidal free graft with the retinal tissue and closure of the macular hole and ICG shows blockage in the area of the choroidal graft with no abnormal vascular complex or leakage. The vision was improved from HM preoperative to 20/200E@1 meter 12 months postoperatively with subjective improvement as well.
Table 1.
Characteristics of the nine eyes undergoing the combined choroid and neurosensory retinal graft
Yr = years; M = months; FU = follow up; BCVA = best corrected visual acuity; CNV = choroidal neovascular membrane
| Age (Yr) |
FU (M) |
Diagnosis | Location of Choroid and Neurosensory Retinal Flap Harvest |
Location of Free Flaps |
Preop Snellen BCVA |
PostOp Snellen BCVA |
Preop Fixation (based on reading) |
Post op Fixation |
Intraop complications |
Postop Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 78 | 12 | CNV with fibrotic scar | Periphery | Subretinal | Count Fingers | Count Fingers | Absent | Absent | None | |
| 79 | 6 | CNV with fibrotic scar | Periphery | Subretinal | Count Fingers | Count Fingers | Absent | Absent | None | Graft Atrophy |
| 70 | 8 | CNV with fibrotic scar | Periphery | Subretinal | Count Fingers | Count Fingers | Absent | Absent | None | |
| 74 | 5 | CNV with fibrotic scar | Mid periphery | Subretinal | Count Fingers | 20/400 | Absent | Closer to Center | None | |
| 72 | 4 | CNV with fibrotic scar | Mid periphery | Subretinal | Count Fingers | 20/400 | Absent | Absent | None | |
| 70 | 5 | CNV with fibrotic scar | Mid periphery | Intraretinal | Count Fingers | 20/400 | Absent | Closer to Center | None | |
| 68 | 5 | CNV with fibrotic scar | Mid periphery | Intraretinal | Count Fingers | Count Fingers | Absent | Absent | None | Epiretinal Membrane |
| 74 | 4 | CNV with fibrotic scar | Mid periphery | Intraretinal | Count Fingers | 20/100 | Absent | Closer to Center | None | |
| 76 | 19 | CNV with fibrotic scar + FTMH | Mid periphery | Preretinal | Hand Motion | Count Fingers | Absent | Absent | Limited Subretinal Hemorrhage | Migration of neurosensory retinal flap |
In most eyes after free graft translocation, by OCT, the retinal free graft appeared, in the subretinal transplant group, well integrated between the original retina and the RPE-choroidal free graft, separated by a hyper-reflective line (Figures 1–6), and, in the intraretinal transplant group, well integrated with the surrounding retina. There was quick resolution of intraretinal fluid in the native retina in most patients within one week after surgery. In most cases (Figures 1,3), ICGA revealed choroidal vessels starting at the margin of the free graft with healthy and original underlying choroidal vasculature (Figure 1). There was some component of intraretinal fluid in all eyes preoperatively, which resolved following the graft in all eyes. Microperimetry revealed areas of significant sensitivity along margins of the retinal free graft, and over the choroidal free graft in several cases (Figure 5). In the cases of improvement in vision, microperimetry revealed fixation and retinal sensitivity over the choroidal free graft.
Figure 1.
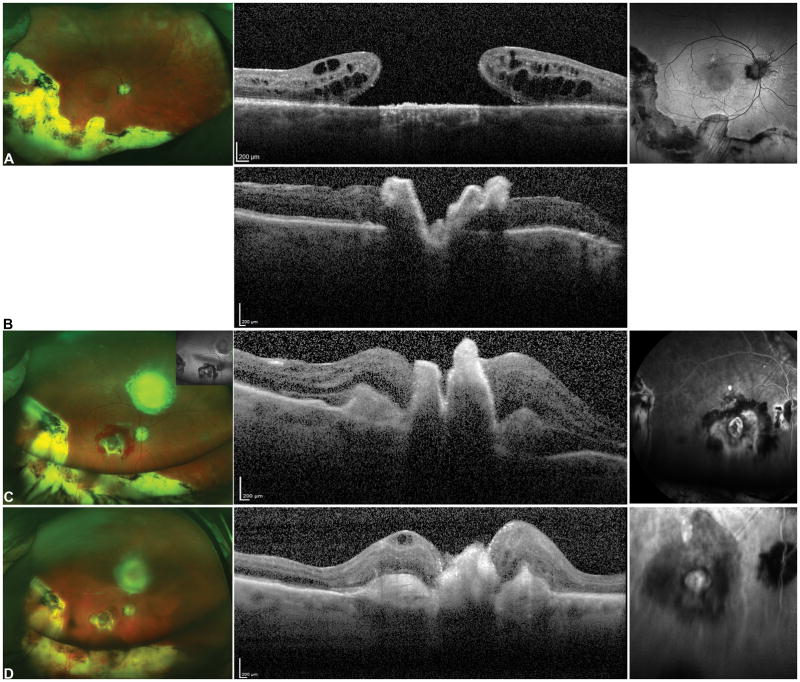
Exudative age related macular degeneration (AMD) with a disciform scar covering the macular region (A). OCT shows cystic intraretinal fluid overlying the subretinal hyperreflective material with loss of the outer retinal layers (B red arrow).
Six months after surgery, the retinal pigment epithelium (RPE)-choroid graft is visualized in the center of the macular region (C, yellow arrow) and the RPE-choroid graft temporal harvest site is flat (white asterisk). The peripheral superotemporal harvesting site of the retinal graft is not visible in the picture.
Postoperative OCT (D) shows the RPE-choroid graft, with rounded hyporeflective spaces due to dilatation of choroidal vessels inside the RPE-choroid graft (white arrows). The neurosensory retinal graft appears well integrated between the native macula, and the RPE-choroid graft separated by a hyper-reflective line (yellow arrows). The retinal layers are not well recognizable. There is resolution of the intraretinal fluid. The silicone oil reflex is visible. Postoperative fluorescein angiography (FA) and Indocyanine Green (ICG) Angiography images (E) showing the complete revascularization of the choroidal graft. Dynamic ICG angiography revealed choroidal vessels starting at the margin of the graft. Feeder vessels of the choroidal patch seemed to grow in sites of contacts of the choroidal graft with healthy and original underlying choroidal vasculature (yellow arrow).
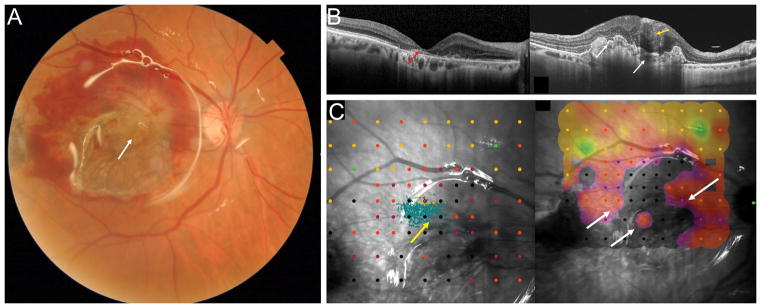
Figure 6.
74-year-old female with end-stage CNV that progressed to a fibrovascular scar with OCT showing lamellar macular hole like configuration with very thin residual fovea and loss of retinal layers, outer retinal tabulation (white asterisk) and subretinal hyperreflective subretinal material (red arrow). Postoperative ultra-wide-field fundus photograph showing the RPE-choroid graft in the center of the macular region and the neurosensory retinal graft covering the macular hole (yellow arrow). The harvest site of the retinal graft (white arrow) and area of RPE-choroid graft (white asterisk) are seen temporally. Postoperative OCT 5 months after combined RPE-choroid free graft and intraretinal retinal graft (C). The RPE-choroid graft is vascularized as shown by the ovoidal dark hyporeflective spaces. The intraretinal retina graft is integrated in the surrounding retina. Postoperative microperimetry (D) showing fixation if on the area of the choroid graft and close to the nasal margin of the retinal graft. Fixation is stable with P2= 75%. The sensitivity map shows a spot of sensitivity over the choroid and retina grafts. Vision improved from 2 to 0.7 logMAR.
Figure 3.
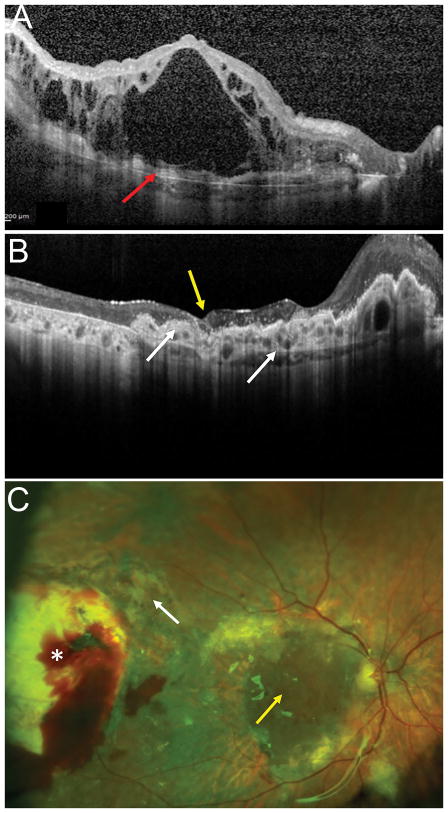
OCT (A) showing advanced exudative AMD with subretinal hyperreflective material, extensive loss of outer retinal layers and overlying cystic intraretinal fluid. Postoperative OCT (B) showing the RPE-choroid graft in the macula area, with a dome-shaped elevation nasally due to dilatation of choroidal vessels and an irregular surface secondary to contraction (white arrows). The neurosensory retinal free graft appears well integrated with the original retina (yellow arrow), and there is resolution of the intraretinal fluid. Postoperative fundus photograph (C) showing the RPE-choroidal free graft and the neurosensory retinal graft in the center of the macular region (yellow arrow). The harvest sites of the neurosensory retinal free graft (white arrow) and the RPE-choroid free graft (white asterisk) are seen temporally.
Figure 5.
Fundus photograph after surgery (A) showing the RPE-choroid free graft in the center of the macular region with the overlying neurosensory retinal graft (white arrow). The harvest sites of the RPE-choroid free graft temporally and the neurosensory retinal free graft superotemporally are not visible in the picture. Preoperative OCT (B, left) showing an atrophic macular area with loss of the outer retinal layers and highly reflective subretinal material (red arrow). Postoperative OCT (B, right) showing the RPE-choroid graft with dilatation of choroidal vessels and an irregular surface (white arrows). The choroidal patch is vascularized as shown by the ovoidal dark hyporeflective spaces. The intraretinal neurosensory retinal free graft appears well integrated (yellow arrow) with some inner retinal boundaries visible overlying the RPE-choroidal free graft. Postoperative microperimety (C) and fixation maps (4-2 strategy). The area of fixation was at superotemporal border of the retinal free graft (yellow arrow) overlying the area of the choroidal graft. There was a dense scotoma with complete loss of sensitivity, however there are areas of low sensitivity along on the retinal free graft, in the superotemporal, nasal and temporal sides and over the choroidal graft (white arrows).
Discussion
In this pilot investigation, we demonstrate the initial outcomes of a combined neurosensory retina, RPE and choroid autologous free graft in eyes with advanced fibrosis and atrophy from end stage exudative AMD.
Options for end stage exudative AMD with fibrosis and atrophy are limited. This surgical technique may be an alternative for the select group of patients with AMD who have not benefitted from, or cannot undergo, other standard treatments. All patients in our series had received multiple anti-vascular endothelial growth factor (VEGF) injections prior, but none in the one year preceding surgery due to the fibrotic scar as there was felt to be poor potential for visual improvement with further injections. Transplantation of an autologous graft of RPE, Bruch membrane, choriocapillaris, and choroid has been well described previously with varied outcomes. 1,9,10 Long term results with BCVA, retinal sensitivity data, and fixation on the graft have shown that it is the addition of the graft, rather than simply the removal of submacular hemorrhage and CNV, that was responsible for the preservation of macular function. 11 The sub-macular surgery trial also demonstrated that a solitary excision of the subretinal membrane did not result in a markedly better visual outcome than observation alone or laser treatment further suggesting the impact of the graft. 12 The autologous transplantation limited to RPE and choroid provides no improvement in eyes with external retinal damage. 3,4 It is possible that the addition of the neurosensory retinal free graft as in our technique allows restoration of outer retinal layers, better integration of the free graft into the surrounding tissue, potentially resulting in better visual outcomes. There are several potential hypotheses on the possible mechanisms for this. One possibility is that the neurosensory retinal and choroidal free graft firstly serves as a scaffold, and in cases with a MH it forms a plug to seal the hole and secludes communication between the vitreous and subretinal space. 8
Previously, placement of a full-thickness free graft of choroid, choriocapillaris, Bruch’s membrane and RPE from the periphery to the subfoveal area of the same eye, after performing a 180° peripheral retinotomy and removing subfoveal CNV has been described. Macular translocation reports have shown that the retina could recover some function when relocated to a healthy area of RPE and choroid. Stanga et al. in 2002 described subfoveal translocation of an autologous RPE–choroid sheet that was cut from the edge of the RPE defect after CNV extraction. 9 Van Meurs and Van den Biesen first described subfoveal transplantation of an RPE–choroid graft that was isolated from the mid-periphery. 13,14 It was initially thought that patients with long-standing visual loss and central retinal degeneration may not profit from attempts to improve the subretinal environment. One option would be to consider patients eligible for free graft translocation with very recent loss of (reading) vision independent of the type of membrane and independent of RPE atrophy.
Subretinal surgery may not only cause damage to RPE and Bruch membrane, but may also cause damage to photoreceptors and the outer retina, as reflected by the incidence of MH. We dragged the free graft from its excision site to its final location under perfluorocarbon liquid. The advantage of this method in contrast to manipulation of the free graft in the water-filled eye is that the free graft does not curl and can be placed in a controlled and safe fashion. Although this could theoretically result in coating of the free graft and affecting the interaction of RPE and photoreceptors after reattaching the retina, it is unlikely given the high surface tension of PFCL and since it was entirely removed. Short-term PFCL in the sub-retinal space has not been shown to be associated with significant retinal toxicity in experimental eyes. 15 In our technique, we aspirated the subretinal PFCL after the neurosensory retinal graft was placed over the choroid and RPE graft and injected it in the epiretinal space to flatten the grafts for good apposition as well as to confirm centration of the fovea on the neurosensory retina-choroid-RPE graft.
Even a limited postoperative hemorrhage surrounding the free graft, but not covering it, is often enough to induce fibrous encapsulation of the free graft at least at its choroidal side and thus inhibit free graft revascularization and functioning. It is also difficult to determine whether RPE from the periphery maintains identical functional abilities as compared with RPE from the macular area. In postmortem eyes, it has been shown that the expression of selected genes related to phagocytosis as well as genes expressing factors responsible for integrity and maintenance of the choroid are equally distributed in the macula area and periphery. 16
Revascularization of the free graft is often followed by a reorganization of the choroidal vessels indicating the large plasticity of the choroidal vessels. Vascularization pattern of the previously studied grafts have suggested connection of the preexisting choroidal channels of the graft with those of the underlying recipient choroid. 2,13,17–19 Maajiwee et al suggested that vascularised choroidal vessels of the graft run parallel to one another and differ from the radially arranged choroidal vascular system in the macula area. 20 During CNV removal, choroidal structure should be left unharmed as much as possible, and that the dimension of the graft must be large enough to connect to an area of preserved choroid. 2,20
At one week after surgery, in our series the retina showed disappearance of intraretinal fluid. Reading ability improved subjectively in 5/9 eyes postoperatively. FA and ICG images showed revascularization of the choroidal free graft. Dynamic ICG angiography in some eyes showed vessels starting at the margin of the free graft. Feeder vessels seemed to grow in sites of contact of the free graft with the underlying choroidal vasculature. In eyes where postoperative OCT angiography was obtained, the retinal free graft did not show any signs of neovascularization although the scans are limited by segmentation challenges. In most eyes, the subretinal retinal free graft was seen to be well integrated under the host retina, separated by a hyper-reflective line. On postoperative OCT, edges of the free graft could be easily identified in the subfoveal area as a layer of RPE and choroid with open dark spaces corresponding to perfused choroidal vessels. Postoperative OCT scans appeared to illustrate bridging tissue in the area of the retinal and choroidal grafts indicating integration of the retinal graft tissue. The increase in retinal sensitivity with corresponding improvement in visual acuity and subjective reduction in scotoma suggests the possibility of the autologous neurosensory retinal transplant to maintain retinal function although longer follow up and additional cases will help establish the role of retinal free grafts in possible functional recovery. Most of the cases in our series showed a gradual restoration of the external limiting membrane and ellipsoid zone suggesting some potential for outer retinal restoration at the free graft edges.
There are different hypotheses to suggest the role of the neurosensory retina in visual improvement in such cases. It has been demonstrated that rod photoreceptors and their postsynaptic partners show structural and functional remodeling ability suggesting long-lasting plastic changes. 21 Although the mechanisms that mediate the genesis of normal rod and cone synapses are unknown, an inherent molecular flexibility in forming synaptic partnerships may provide an adaptive advantage for the survival of the retina. 22 Neovascularization of the transplanted choroid-RPE graft has been demonstrated in porcine studies. 10 Porcine studies have also shown survival of autologous porcine RPE-choroid free grafts, with preservation of polarity and some remaining pigment distribution, in both donor and recipient RPE layers, up to 30 days of follow-up. 23 Ectopic synaptogenesis may be a common step in the disease progression of different forms of retinal degeneration that include photoreceptor death as a feature, both in rat and mammalian retina. 24,25 Mammalian and rat studies have also demonstrated that the rod bipolar cell dendrites have the capability to make alternative connections, when the preferred contacts are apparently not available. 24,25 Morphological plasticity involving rod bipolar cells and horizontal cells after injury to the retina has been demonstrated. 26 Demonstration of ectopic synapses in a rat model suggested that ectopic synaptogenesis could occur as a result of photoreceptor degeneration, even when the rods and cones are developmentally normal. The adult human eye may harbor two different populations of neuroepithelial stem/progenitor cells; a non-glial population located in the proximal pars plana around peripheral cysts in addition to a population with Müller glia characteristics. 27
Based on our initial experience, we believe the size of the choroidal free graft should be 3–4 disc diameters large and the retinal free graft should be no more than 0.5 disc diameters larger than the size of MH (when present) to allow for appropriate handling and positioning of the graft, increase the chances of retaining the graft in the MH following direct PFC-silicone oil exchange, and, permit MH closure despite some decentration of the graft postoperatively. However, despite the larger and thicker retinal graft we experienced graft displacement in the single case where a preretinal graft was performed. We recommend positioning an edge of the retinal free graft within the MH prior to instilling perfluorcarbon, performing a relatively high pressure perfluorocarbon-silicone oil exchange followed by strict face down positioning. Alternatively, the choroid-RPE and retinal grafts should be harvested in a single layer in such cases so that the retinal graft can stay in position attached to the heavier choroidal graft. Our case had a large recalcitrant macular hole with outer retinal atrophy and although there was closure of the macular hole with the choroid bridging the retinal defect and resulting in improved vision, it is important to note that the mechanism of the choroid and/or neurosensory retina graft closing the macular hole is still in the process of being fully elucidated. It is arguable that potentially any scaffold such as internal limiting membrane or posterior lens capsule could also serve as a scaffold to close the macular hole but neither of these were available in our case. 5 Longer term follow-up is imperative to further evaluate the role of combined choroid-neurosensory retina grafts in such advanced cases with macular atrophy or scarring.
Our study has several limitations including its retrospective nature, limited follow-up to date on a limited number of cases, unknown potential long-term complications, and lack of controls. Specifically, our eyes have short term follow up and silicone oil removal has not yet been performed. It is important to acknowledge that long term outcomes and complications such as retinal detachment and proliferative vitreoretinopathy need to evaluated with further follow up. Patients would want to know whether it is worthwhile to undergo the surgery, the chances of closing the MH (when applicable) and the level of vision expected. In our series, visual improvement was noted in 57.1% of eyes. Overall the procedure was safe and well tolerated in the short term. It is however important to recognize that this is a complex surgical maneuver which needs familiarity with working with the tissues that are not normally handled during vitreoretinal surgery such as the choroid and also accessing the subretinal space. The intricacy of the various maneuvers involved in this surgery is something that all surgeons may not be comfortable with. While further studies with longer follow up and larger sample sizes are certainly needed to answer these questions, large studies of this technique may be difficult to achieve.
In conclusion, while acknowledging these limitations we present encouraging pilot surgical outcomes of autologous neurosensory retinal transplant with choroidal-RPE transplant for late exudative macular degeneration with and without chronic refractory macular hole. Such patients did not previously have surgical options available and this technique may provide the basis of a surgical technique upon which other improvements can be built and serve an important tool in the surgical armamentarium for management of such challenging cases.
Supplementary Material
Intraoperative video showing the technique for harvest of retinal pigment epithelium choroid free graft along with a neurosensory retinal free graft with subretinal placement of retinal pigment epithelium choroid free graft and intraretinal placement of neurosensory retinal free graft.
Intraoperative video showing the technique for harvest of a combined retinal pigment epithelium choroid free graft along with a neurosensory retinal free graft with preretinal placement of the combined graft.
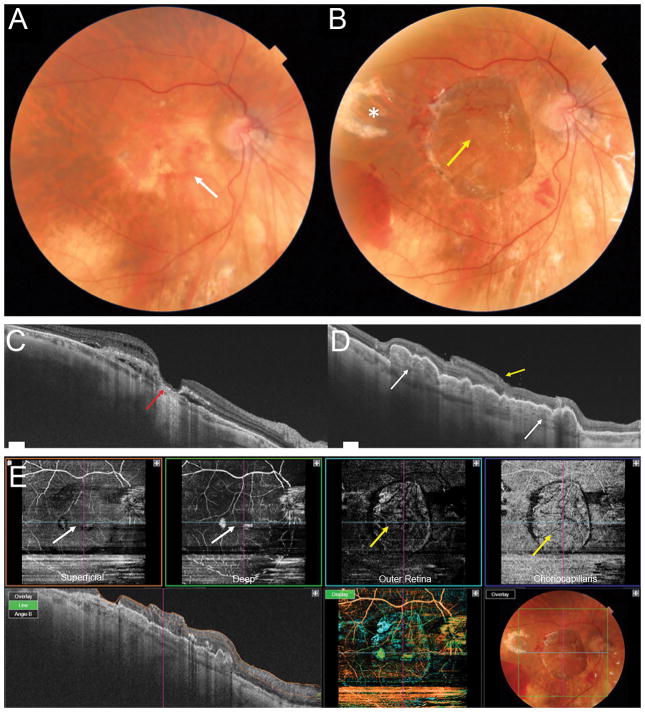
Figure 2.
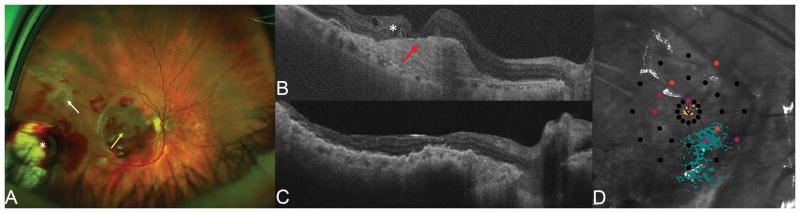
Patient with advanced exudative AMD (A) with RPE atrophy in the macular region (white arrow). OCT (C) showing lamellar macular hole configuration with a very thin residual foveal floor, significant loss of outer retinal layers and RPE atrophy (red arrow), overlying intraretinal fluid and subretinal hyporeflective spaces. Postoperative photograph 1 month after surgery (B) showing the choroidal-RPE graft in the center of the macular region and the neurosensory retinal graft covering the macular hole (yellow arrow). The temporal harvest site of the neurosensory retinal graft is shown with the white asterisk. Postoperative OCT 3 months after surgery (D) showing RPE-choroidal patch in the macula area, with rounded hyporeflective round spaces due to dilatation of choroidal vessels inside the graft (white arrows). The neurosensory retinal graft (yellow arrow) appears well integrated into the macular hole and is partly lying over the original retina, separated by a hyper-reflective line. Postoperative OCT angiography (E, scan area 6 x 6 mm) 3 months after surgery. The choroidal graft (yellow arrows) is visualized on the en face images at the level of the outer retina and choriocapillaris. The superficial and deep retinal vasculature appear grossly preserved in the area of the original overlying neurosensory retina (white arrows), however the scan is limited by artifact and segmentation errors.
Figure 4.
Postoperative fundus photograph showing the RPE-choroidal free graft in the center of the macula (A. white arrow). The neurosensory retinal free graft is under the native retina. The temporal harvest site of RPE-choroidal patch and the superotemporal harvest site of the retinal patch are not visible. Preoperative OCT showing advanced exudative AMD with subretinal hyperreflective material, loss of outer retinal layers and overlying cystic intraretinal fluid (B). Postoperative OCT (C) showing the RPE-choroid graft in the macula area, with a dome-shaped aspect due to dilatation of choroidal vessels and an irregular surface secondary to contraction of the RPE-choroid graft (white arrows). The neurosensory retinal free graft is well integrated between the native retina and the RPE-choroidal graft, separated by a hyper-reflective line, (yellow arrow) and there is relative preservative of the retinal architecture. OCT angiography (D, scan area 6 x 6 mm) shows the area of the RPE-Choroid graft seen on the outer retina and choriocapillaris slabs (yellow arrows). While the superficial capillary plexus is relatively well preserved (left white arrow), the deep capillary plexus which is likely in the area of the neurosensory graft (right white arrow) is not well visualized. The scan is however limited by artifact and segmentation errors.
Summary Statement.
We demonstrate the feasibility of transplanting a full-thickness autologous free graft of choroid, retinal pigment epithelium along with neurosensory-retina in eyes with advanced fibrosis and atrophy in end-stage exudative age-related macular degeneration including the presence of an associated refractory macular hole. Some degree of functional improvement was noted in 55.6% eyes.
Footnotes
Previous Presentation: Presented at the Duke Advanced Vitreous Surgery Course Meeting April 2017
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Additional Contributions: None.
Funding/Support: None
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Tamer Mahmoud reports participation in an advisory board for Dutch Ophthalmic (Exeter, NH), Alimera, and Spark Therapeutics. Research support as investigator for Genentech. Dr Grewal reports participation in an advisory board for Allergan and Regeneron. No other disclosures were reported.
References
- 1.Peyman GA, Blinder KJ, Paris CL, Alturki W, Nelson NC, Jr, Desai U. A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg. 1991;22(2):102–108. [PubMed] [Google Scholar]
- 2.Maaijwee K, Heimann H, Missotten T, Mulder P, Joussen A, van Meurs J. Retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: long-term results. Graefes Arch Clin Exp Ophthalmol. 2007;245(11):1681–1689. doi: 10.1007/s00417-007-0607-4. [DOI] [PubMed] [Google Scholar]
- 3.Parolini B, Alkabes M, Baldi A, Pinackatt S. Visual Recovery after Autologous Retinal Pigment Epithelium and Choroidal Patch in a Patient with Choroidal Neovascularization Secondary to Angioid Streaks: Long-Term Results. Retin Cases Brief Rep. 2016;10(4):368–372. doi: 10.1097/ICB.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 4.Cereda MG, Parolini B, Bellesini E, Pertile G. Surgery for CNV and autologous choroidal RPE patch transplantation: exposing the submacular space. Graefes Arch Clin Exp Ophthalmol. 2010;248(1):37–47. doi: 10.1007/s00417-009-1201-8. [DOI] [PubMed] [Google Scholar]
- 5.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117(10):2018–2025. doi: 10.1016/j.ophtha.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Adelman RA, Nawrocki J. TEMPORAL INVERTED INTERNAL LIMITING MEMBRANE FLAP TECHNIQUE VERSUS CLASSIC INVERTED INTERNAL LIMITING MEMBRANE FLAP TECHNIQUE: A Comparative Study. Retina. 2015;35(9):1844–1850. doi: 10.1097/IAE.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 7.Grewal DS, Fine HF, Mahmoud TH. Management of Challenging Macular Holes: Current Concepts and New Surgical Techniques. Ophthalmic Surg Lasers Imaging Retina. 2016;47(6):508–513. doi: 10.3928/23258160-20160601-01. [DOI] [PubMed] [Google Scholar]
- 8.Grewal DS, Mahmoud TH. Autologous Neurosensory Retinal Free Flap for Closure of Refractory Myopic Macular Holes. JAMA Ophthalmol. 2016;134(2):229–230. doi: 10.1001/jamaophthalmol.2015.5237. [DOI] [PubMed] [Google Scholar]
- 9.Stanga PE, Kychenthal A, Fitzke FW, et al. Retinal pigment epithelium translocation after choroidal neovascular membrane removal in age-related macular degeneration. Ophthalmology. 2002;109(8):1492–1498. doi: 10.1016/s0161-6420(02)01099-0. [DOI] [PubMed] [Google Scholar]
- 10.Maaijwee KJ, van Meurs JC, Kirchhof B, et al. Histological evidence for revascularisation of an autologous retinal pigment epithelium--choroid graft in the pig. Br J Ophthalmol. 2007;91(4):546–550. doi: 10.1136/bjo.2006.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zeeburg EJ, Maaijwee KJ, Missotten TO, Heimann H, van Meurs JC. A free retinal pigment epithelium-choroid graft in patients with exudative age-related macular degeneration: results up to 7 years. Am J Ophthalmol. 2012;153(1):120–127. e122. doi: 10.1016/j.ajo.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 12.MacLaren RE, Bird AC, Sathia PJ, Aylward GW. Long-term results of submacular surgery combined with macular translocation of the retinal pigment epithelium in neovascular age-related macular degeneration. Ophthalmology. 2005;112(12):2081–2087. doi: 10.1016/j.ophtha.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 13.van Meurs JC, Van Den Biesen PR. Autologous retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: short-term follow-up. Am J Ophthalmol. 2003;136(4):688–695. doi: 10.1016/s0002-9394(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 14.van Meurs JC, ter Averst E, Hofland LJ, et al. Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. Br J Ophthalmol. 2004;88(1):110–113. doi: 10.1136/bjo.88.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Queiroz JM, Jr, Blanks JC, Ozler SA, Alfaro DV, Liggett PE. Subretinal perfluorocarbon liquids. An experimental study. Retina. 1992;12(3 Suppl):S33–39. [PubMed] [Google Scholar]
- 16.Aisenbrey S, Lafaut BA, Szurman P, et al. Macular translocation with 360 degrees retinotomy for exudative age-related macular degeneration. Arch Ophthalmol. 2002;120(4):451–459. doi: 10.1001/archopht.120.4.451. [DOI] [PubMed] [Google Scholar]
- 17.Joussen AM, Joeres S, Fawzy N, et al. Autologous translocation of the choroid and retinal pigment epithelium in patients with geographic atrophy. Ophthalmology. 2007;114(3):551–560. doi: 10.1016/j.ophtha.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Heussen FM, Fawzy NF, Joeres S, et al. Autologous translocation of the choroid and RPE in age-related macular degeneration: 1-year follow-up in 30 patients and recommendations for patient selection. Eye (Lond) 2008;22(6):799–807. doi: 10.1038/sj.eye.6702823. [DOI] [PubMed] [Google Scholar]
- 19.Degenring RF, Cordes A, Schrage NF. Autologous translocation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Acta Ophthalmol. 2011;89(7):654–659. doi: 10.1111/j.1755-3768.2010.01867.x. [DOI] [PubMed] [Google Scholar]
- 20.Maaijwee K, Van Den Biesen PR, Missotten T, Van Meurs JC. Angiographic evidence for revascularization of an rpe-choroid graft in patients with age-related macular degeneration. Retina. 2008;28(3):498–503. doi: 10.1097/IAE.0b013e318159ec24. [DOI] [PubMed] [Google Scholar]
- 21.Specht D, Tom Dieck S, Ammermuller J, Regus-Leidig H, Gundelfinger ED, Brandstatter JH. Structural and functional remodeling in the retina of a mouse with a photoreceptor synaptopathy: plasticity in the rod and degeneration in the cone system. Eur J Neurosci. 2007;26(9):2506–2515. doi: 10.1111/j.1460-9568.2007.05886.x. [DOI] [PubMed] [Google Scholar]
- 22.Cowan WM, Fawcett JW, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Bueno I, Rodriguez de la Rua E, Hileeto D, et al. Histology and immunochemistry evaluation of autologous translocation of retinal pigment epithelium-choroid graft in porcine eyes. Acta Ophthalmol. 2013;91(2):e125–132. doi: 10.1111/aos.12001. [DOI] [PubMed] [Google Scholar]
- 24.Peng YW, Senda T, Hao Y, Matsuno K, Wong F. Ectopic synaptogenesis during retinal degeneration in the royal college of surgeons rat. Neuroscience. 2003;119(3):813–820. doi: 10.1016/s0306-4522(03)00153-2. [DOI] [PubMed] [Google Scholar]
- 25.Peng YW, Hao Y, Petters RM, Wong F. Ectopic synaptogenesis in the mammalian retina caused by rod photoreceptor-specific mutations. Nat Neurosci. 2000;3(11):1121–1127. doi: 10.1038/80639. [DOI] [PubMed] [Google Scholar]
- 26.Lewis GP, Linberg KA, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998;39(2):424–434. [PubMed] [Google Scholar]
- 27.Johnsen EO, Froen RC, Albert R, et al. Activation of neural progenitor cells in human eyes with proliferative vitreoretinopathy. Exp Eye Res. 2012;98:28–36. doi: 10.1016/j.exer.2012.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperative video showing the technique for harvest of retinal pigment epithelium choroid free graft along with a neurosensory retinal free graft with subretinal placement of retinal pigment epithelium choroid free graft and intraretinal placement of neurosensory retinal free graft.
Intraoperative video showing the technique for harvest of a combined retinal pigment epithelium choroid free graft along with a neurosensory retinal free graft with preretinal placement of the combined graft.