Abstract
The present research measured social reinforcement in rats, using a social-release procedure in which lever presses permitted 10-s access to a familiar social partner. The work requirements for reinforcement increased systematically according to progressive-ratio (PR) schedules. Social and food reinforcement value were compared across blocks of sessions (Experiment 1) and concurrently within the same sessions (Experiment 2). To assess motivational effects, response and reinforcer rates for both reinforcer types were studied under food restriction, social restriction, and combined food and social restriction. Responding was maintained by both reinforcers, albeit at substantially higher levels for food than for social access. Responding for social access decreased to low levels under extinction conditions, demonstrating functional control by the social-reinforcement contingency. Sensitivity to social restriction was seen in some conditions in Experiment 2, in which social reinforcers were earned earlier in the session (at lower food prices) under social restriction than under the other deprivation conditions. Altogether, results are consistent with a social reinforcement conceptualization, and demonstrate an important role for social contact in social release behavior. The study demonstrates a promising set of methods for analyzing and quantifying social reinforcement.
Keywords: empathy, food reinforcement, progressive ratio schedules, rats, social behavior, social reinforcement
1. Introduction
Prosocial behavior has been defined as behavior that produces benefits for another (Cronin, 2012; West, Griffin, & Gardner, 2007), but the mechanisms are not well understood. To the extent that behaving for the good of another incurs costs to the individual, instrumental (cost-benefit) models must assume additional benefits for the individual that outweigh the costs. In some cases, the benefits to the individual are readily apparent, as in some forms of cooperation, with mutual benefits for both organisms (Drea & Carter, 2009; Łopuch, S., & Popik, 2011; Plotnik, Lair, Suphachoksahakun, & de Waal, 2011; Tan & Hackenberg, 2016).
In other cases, the benefits to the individual are less apparent, as in some forms of what might be termed helping or rescue behavior, in which one organism releases another from a trap or restraint (Ben-Ami Bartal, Decety & Mason, 2011; Nowbahari, Scohier, Durand, & Hollis, 2009). In the Ben-Ami Bartal et al. study, for example, two familiar (cagemate) rats were placed in an arena, one of which began each session in a transparent tube-like restrainer. A second rat was unrestrained, and could move freely around the rest of the arena. A latch on one side of the restrainer could be lifted, releasing the restrained rat, for the remainder of the 60-min session. No explicit training was provided, though the response was prompted by lifting the door halfway up at the 30-min mark. After an average of 7 sessions, 23 of the 30 rats acquired the door-opening response; and once the response was acquired, it generally continued to occur in subsequent sessions and with shorter latencies (i.e., earlier in the session). The door-opening response was also selective, in that it occurred only under conditions with a live rat in the restraint; it did not occur when the restraint was empty or occupied by a toy rat.
Door opening under these conditions thus appears to be a learned prosocial response, but how best to explain it? Ben-Ami Bartal et al. (2011) favored an empathy-based explanation, according to which distress is socially transmitted, from the restrained to the free rat via social contagion; this, in turn, motivates pro-social behavior (see also Ben-Ami Bartal et al., 2014; Sato, Tan, Tate, & Okada, 2015). By this view, the prosocial behavior incurs costs that exceed any obvious benefits to the individual, and must therefore be due to altruism.
An alternative, and far simpler, explanation is that door opening for the unrestrained rat is an operant response, established and maintained by social reinforcement: access to the other rat (Schwartz, Silberberg, Casey, Kearns, & Slotnick, 2017; Silberberg, Allouch, Sandfort, Kearns, Karpel, & Slotnick, 2014). This possibility was considered but rejected by Ben-Ami Bartal et al. (2011), largely on the basis of a control condition, in which door opening permitted release but precluded direct social contact with the other rat (which was released into an adjacent chamber). Rats in these no-contact conditions continued to respond, despite the absence of direct social contact. Although this result is seemingly inconsistent with the social reinforcement hypothesis, the rats in these no-contact conditions all had prior social reinforcement histories (i.e., histories in which responding produced direct social contact with the other rat). Silberberg et al. (2014) showed that, without such a history of social contact, social release is not acquired. Once social release is established, however, under conditions with direct social contact, it can be maintained at moderate levels even without direct social contact.
The maintenance of such behavior may be due to conditioned reinforcement effects (i.e., the presence of stimuli correlated with prior social release). It may also be due to uncontrolled sources of social contact. In the Silberberg et al. (2014) study, for example, restrained rats frequently remained in or returned to the restrainer. This is inconsistent with a view of the restraint as aversive and stress-inducing, but can be understood in terms of social proximity. Because the restraint tube was enclosed within the chamber containing the unrestrained rat (with the door opening into an adjacent chamber), leaving the tube increased social distance whereas remaining in the tube reduced it. Social contact may thus serve important functions even in conditions designed to minimize its impact. At the very least, these findings cannot rule out a potentially crucial role for social contact in prior results in the social release paradigm with rats.
Conceptualizing social release in terms of social reinforcement has several advantages. First, it is consistent with a longstanding body of literature showing that contingent access to social stimuli can function as a reinforcer across a variety of procedures, including T-maze and operant tasks, and in a range of species, including chimpanzees (Mason, Hollis, & Sharpe, 1962), capuchin monkeys (Dettmer & Fragaszy, 2000), horses (Sondergaard, Jensen & Nicol, 2011), foxes (Hovland, Akre, FlF, Bakken, Koistinen & Mason, 2011), calves (Holm, Jensen, & Jeppesen, 2002), sows (Kirkden & Pajor, 2006), mice (Martin, Sample, Gregg, & Wood, 2014), and rats (Evans, Duvel, Funk, Lehman, Sparrow, Watson & Neuringer, 1994; Humphreys & Einon, 1981; Wilsoncroft, 1968).
Second, a social reinforcement view opens questions to sharper methods for assessing reinforcing value, more generally. For example, Evans et al. (1994) used operant methods to assess the value of food and social reinforcement in rats. Lever pressing by two groups of female rats produced either 45-s access to another rat (a castrated male cagemate) or 45-s access to food, according to a fixed-ratio (FR) 3 schedule, in which each 3rd press produced food or social access. Lever pressing was acquired, maintained, and extinguished in both groups of rats, consistent with operant functions. Moreover, social and food access appeared to be about equally effective as reinforcers, as there were no significant differences between groups in the number of responses under reinforcement or extinction conditions.
The present study further explores the reinforcing value of social contact in rats. The procedures were designed partly after the Ben-Ami Bartal et al. (2011, 2014) studies, but with free-operant methods like those of Evans et al., (1994) to provide a more detailed analysis of reinforcer value. As in the Ben-Ami Bartal et al. (2011) study, the rats were cagemates of the same sex. The restrained rat was in a transparent tube restraint identical to that used by Ben-Ami Bartal et al. (2011), but the door on one end could be opened by lever presses by the unrestrained rat, as in Evans et al. (1994). Also similar to the Evans et al. study, following a specified period of social interaction, the rats were separated and a new trial started. This permitted repeated opportunities to respond within a session, and a more refined and graded measure of reinforcer value. This contrasts with the binary (all-or-none) measure permitted by Ben-Ami Bartal et al., in which only a single response was permitted in each session (i.e., the door was either opened or it was not).
As a result, the duration of social contact in the Ben-Ami Bartal et al. study was not controlled; once the door had been opened, the two rats remained together for the remainder of the 60-min session. The amount of social contact thus varied depending on the time in the session when the response occurred. From a social reinforcement perspective, social contact is the relevant consequence. Therefore, a procedure in which the duration of this variable (akin to reinforcement magnitude) varies so widely between sessions and subjects is not optimal. Thus, in line with some prior research (e.g., Evans et al., 1994; Holm et al., 2006), we carefully controlled the duration of social contact. Because social reinforcers have been studied far less extensively than other reinforcers, we compared them to more common food reinforcers. Some studies have reported higher levels of responding for food than for social reinforcers. Gilbertson (1975), for example, found that pigeons responded at a higher rate for access to food than to visual access to their mate. Similarly, foxes (Hovland et al., 2011) and mice (Martin et al, 2014) paid higher prices for food than for social access to a same-sex conspecific. Other studies, however, with capuchin monkeys (Dettmer & Fragaszy, 2000) and rats (Ben-Ami Bartal et al., 2011; Evans et al., 1994; Sato et al., 2015), have reported comparable value of social and food reinforcers.
In the Ben-Ami Bartal et al. (2011) and Sato et al. (2015) studies, rats were given choices between two doors—one permitting access to a partner rat and one permitting access to a preferred food item (pieces of chocolate). The average latencies were similar between social and food, prompting the authors of both studies to conclude equivalent reinforcer value, although only Sato showed preference data (which door was opened first). Preference depended on the rats’ training histories: when trained with social reinforcement, approximately 70% of the initial choices favored social over food, but when trained with food reinforcement, initial choices were equally divided between social and food. These results suggest that social reinforcers may, under some circumstances, compete successfully with food reinforcers (although it is worth noting that in neither the Ben-Ami Bartal et al. or Sato et al. studies were rats socially or food restricted). Similar results were reported by Evans et al. (1994) with more robust procedures that provided repeated choices, extended exposure to outcomes, and deprivation from the relevant reinforcers. In that study, response rates for a group of rats responding for social reinforcers were comparable to those for a second group responding for food reinforcers.
These results suggest that social reinforcers may, under some circumstances, rival or even surpass food reinforcers in their efficacy. A study by Ikemoto and Panksepp (1992) showed that rats housed in social isolation chose social over food reinforcers on a higher number of trials than rats housed in social groups, suggesting a deprivation-related enhancement of social reinforcement value. In most studies of social reinforcement, however, deprivation is held constant. In neither the Ben-Ami Bartal et al. or Sato et al. studies were relevant deprivation procedures in place: rats had free access to food and social contact outside the experiment. And in the Evans et al. (1994) study, deprivation was arranged by restricting home-cage access to the reinforcers (social or food) between sessions, but these motivational conditions were not an explicit focus of study (i.e., they were not directly manipulated).
In the present study, we systematically explored the impact of motivational variables on the value of social and food reinforcers by restricting post-session access outside the session. In Food-restriction conditions, rats received 60 min post-session access to food. Similarly, in Social-restriction conditions, rats received 60-min post-session access to their cagemate but was otherwise housed alone between sessions. In Combined-restriction conditions, food and social access were limited to 60 min following sessions.
To assess reinforcer value in relation to these different motivational conditions, we used progressive ratio (PR) schedules, in which the work requirement (price) for a reinforcer increased systematically across the session. Such PR schedules have been used widely to assess the incentive value of a range of reinforcers and motivational conditions (Hodos & Kalman, 1963), including food and drink (Pickering, Alsiö, Hulting, & Schiöth, 2009; Stafford & Branch, 1998), sucrose (Sclafani & Ackroff, 2006; Weatherly, King, & Uran, 2003), alcohol (Maccioni et al., 2009; Rodd et al., 2003), nicotine (Donny et al., 1999), and others drugs of abuse (Carroll, Batulis, Landry, & Morgan, 2005; Grasing, Li, He, Parrish, Delich, & Glowa, 2003; Spear & Katz, 1991). The PR schedules were arranged in the present study, either separately (Experiment 1) or concurrently (Experiment 2), under the various restriction conditions described earlier. Together, the experiments were directed to an experimental analysis of social contact as a reinforcer, measured in multiple ways, relative to food reinforcers, and as a function of price and motivational variables. To the extent that social release is understandable in social reinforcement terms, more complex (empathy-based) explanations are unnecessary.
2. Experiment 1
2.1 Methods
2.1.1 Subjects
Six male Long-Evans rats (Rattus norvegicus), approximately 13 weeks of age, were used in this experiment. All rats were previously trained to lever-press in operant chambers during an introductory psychology class before assignment to this study. The 6 rats were divided into three pairs (designated Green, Purple, and Orange) with one rat from each pair designated the unrestrained rat, and the other the restrained rat. When not in the experiment, rats lived in a temperature and light-controlled (12:12 light/dark cycle) colony room, with unrestricted access to water. Depending on the condition of the experiment, rats were housed either individually or together, and with either unrestrained or restricted access to food, as described below.
2.1.2 Apparatus
A schematic drawing of the apparatus is shown in Figure 1. It consisted of two adjoining operant conditioning chambers, both containing a grid floor. The leftmost chamber measured 31 cm × 25 cm × 22 cm, and the rightmost chamber measured 62 cm × 25 cm × 22 cm. The right chamber contained two levers (5 cm × 1.5 cm × 1.5 cm) a pellet receptacle (2 cm diameter), and small light (2 cm diameter) mounted above each lever. The leftmost chamber contained a Plexiglas rodent restrainer (25 by 8.75 by 7.5 cm, Harvard Apparatus, Holliston, MA), separated by a mechanical metal door that opened into the center chamber. The apparatus was controlled VB.net program run on a Macintosh computer located to the right of the chambers.
Fig. 1.
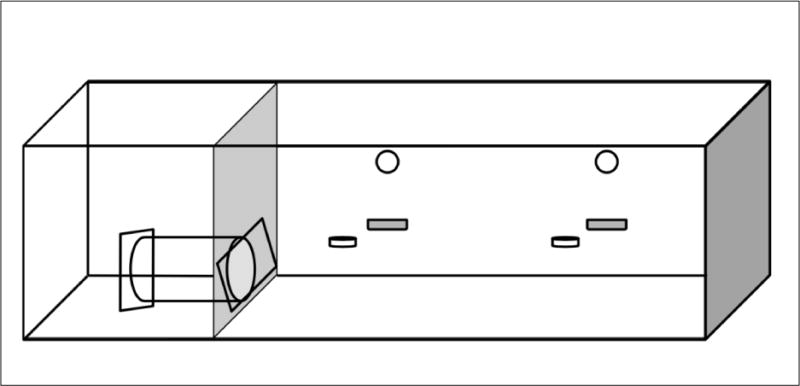
The experimental apparatus: the restrainer on the left of the apparatus held the restrained rat, while the open space in the operant chambers held the unrestrained rat.
2.1.3 Preliminary Training
2.1.3.1 Escape Training
To minimize the delay between lever pressing and social contact, restrained rats were trained to escape (i.e., leave the restraint) soon after the door was opened. Following one 30-min adaptation session with free access to all compartments and the restrainer, the rats underwent a series of conditions in which the restraint door was lifted response-independently (accompanied by a 1 kHz tone of 1-s duration). Escape was defined as the entire body of the rat (except the tail) outside the restrainer. Once in the chamber, rats were allowed to explore for 10 s before being returned to the restrainer for the next trial. Sessions lasted for 10 trials. When escape occurred consistently and with short latencies, the time spent in the restrainer prior to door opening was incrementally increased across sessions according to a variable-time (VT) schedule, which varied the inter-reinforcement interval but had average delays of 10-s (3 sessions), 20-s (3 sessions), and 30-s (14 sessions).
2.1.3.2 Food Reinforcement Training
Unrestrained rats were trained under food restriction (see 2.1.4 below) in four 15-min sessions to press the left lever for 45 mg sucrose banana pellets under a fixed ratio (FR) 1 schedule, in which each lever press produced food accompanied by a 0.5-s 1 kHz tone. During these sessions, only the left lever was active and only the left light was illuminated. The schedule was then changed to a PR 1 schedule, in which the response requirement increased by one response after each food reinforcer. Sessions lasted 30 min in this and all subsequent conditions. Because session duration was held constant, the breakpoint measures are less meaningful here than on traditional PR schedules with a session-termination criterion (e.g., 5 min without a response). We therefore used response rate rather than breakpoint measures in the results.
2.1.3.3 Social Reinforcement Training
Once food training was complete, all rats received social reinforcement training. In these conditions, both rats were placed in the apparatus, the restrained rat in the restrainer and the unrestrained rat in the center of the open part of the chamber. When the light above the right lever was on, lever presses produced the 1-s tone and opened the door to the restrainer. The left lever light was off and left lever presses produced no scheduled consequences during this training condition. When the restrained rat left the restraint and entered the chamber, the door was closed and this started the 10-s social interaction period, after which the restrained rat was removed and returned to the restrainer for the next trial. During the social interaction period, both levers were temporarily inactivated and the lights extinguished. All pairs received 7 sessions of training under Social-restriction (see 2.1.4 below) on a FR 1 schedule, then were moved onto a PR 1 schedule of reinforcement, like that described above for food reinforcement training.
2.1.4 Experimental procedures
By the end of training, restrained rats were leaving the restraint quickly and reliably, and unrestrained rats had experience with PR 1 schedules of food and social reinforcement (10-s period of social interaction). The rats were then studied under food and social reinforcement across different restriction conditions. The restriction conditions limited access to the relevant reinforcer outside the sessions, and were designed to be as equivalent as possible. In Food-restriction conditions, rats received 60 min access to food after each session, but were otherwise restricted; they had continuous access to their cagemate. In Social-restriction conditions, rats had 60-min post-session access to their cagemate, but were otherwise separated in different cages; they had continuous access to food. In Combined-restriction conditions, rats had 60-min post session access to both food and their cagemate, but had otherwise restricted access to these reinforcers outside the sessions.
Table 1 shows the sequence of experimental conditions and the number of sessions conducted at each. Phase 1 (the first 6 conditions listed in Table 1) constitutes a 2 × 3 within-subject experimental design, with reinforcer type (Food, Social) and deprivation type (Food restriction, Social restriction, and Combined restriction) as variables. The sequence of conditions was selected so as to change only one variable at a time, and each pair of rats was exposed to all six combinations of reinforcer type and deprivation in the same order.
Table 1.
The sequence of conditions in Experiment 1 and the number of sessions conducted at each.
| Number of Sessions
|
|||||
|---|---|---|---|---|---|
| Order | Reinforcer | Restriction | Green | Purple | Orange |
| 1 | Food | Food | 14 | 14 | 14 |
| 2 | Social | Food | 14 | 14 | 14 |
| 3 | Social | Combined | 11 | 11 | 11 |
| 4 | Food | Combined | 16 | 14 | 14 |
| 5 | Food | Social | 11 | 13 | 13 |
| 6 | Social | Social | 10 | 21 | 10 |
| 7 | Extinction | Social | 5 | 3 | 3 |
| 8 | Conditioned Social | Social | 14 | 12 | 9 |
| 9 | Food | Food | 24 | 15 | 16 |
| 10 | Food | Combined | 14 | 19 | 19 |
Conditions 7–8 were control procedures, designed to assess the contribution of additional variables to ongoing performance. The first was an Extinction procedure, in which right lever presses were no longer effective in releasing the restrained rat; the restrained rat was present, but responses neither opened the door nor produced the tone. The second was a Conditioned reinforcement assessment, in which lever presses opened the door and produced the tone, but the restraint was empty and entry was precluded by a second transparent door; the only consequences of responding were the stimuli previously correlated with social release (door opening, tone). Between the two control conditions, lever pressing on the social lever was reinstated for one baseline session on an FR 1 schedule with 10-s social interaction as the reinforcer. Both control conditions (and the baseline sessions that preceded them) were conducted under social restriction. Conditions 9–10 were replications of the Food reinforcer conditions under Food-restriction and Combined-restriction conditions.
When a change in condition included switching of the reinforcer type (i.e., from social to food, or vice versa), unrestrained rats were exposed to a single session under an FR 1 schedule to extinguish responses on the now inactive lever and promote responding on the alternative lever for the newly available reinforcer. Following this transitional session, sessions were conducted in each condition until stability was achieved, defined as (a) minimum of 3 sessions, and (b) absence of monotonic trends in session-to-session response rates. A mechanical error during the Social reinforcement condition under Social-restriction required Rat Purple to undergo additional sessions to achieve stability. These additional sessions included a 1-min socialization period in the operant chamber prior to the beginning of the sessions to help instigate responding on the social reinforcement lever.
2.1.5 Statistical Analysis
All analyses were conducted in R v.3.2.1 (R Core Team, 2016). Linear mixed models were used (R package lme4; Bates et al., 2015) to assess group data with rank-transformed values, appropriate to the small sample size and non-parametric data. For both Social reinforcement and Food reinforcement models, restriction condition was included in the mixed-effects model as a fixed factor, while subject was included as a random factor. ANOVAs were run on the models, with the familywise alpha threshold for significance considered at 0.05. Significant omnibus effects were subsequently analyzed using pairwise contrasts with a false discovery rate correction for multiple comparisons (R package lsmeans; Lenth, 2016).
2.2 Results and Discussion
Figure 2 and Table 2 show the mean number of social reinforcers earned and response rates, respectively, for each rat across the restriction conditions. Lever pressing was established and maintained in all four rats, though at somewhat lower rates (Table 2), producing consistently fewer social reinforcers, under Social restriction than under Food or Combined restriction. There was a main effect of restriction condition on the mean number of social reinforcers earned (F(2,22) = 9.8, p < 0.001). Post-hoc comparisons revealed that significantly more social reinforcers were earned under Food restriction (t=2.56, p = 0.03) and Combined restriction (t = 4.41, p < 0.001) compared to Social restriction alone. There was no significant difference between the mean number of reinforcers earned under Food-restriction and Combined-restriction conditions (t = 1.84, p > 0.05). Because response and reinforcement rates are so highly correlated on ratio schedules like those used here, the same general conclusions would follow from an analysis of response rates.
Fig. 2.
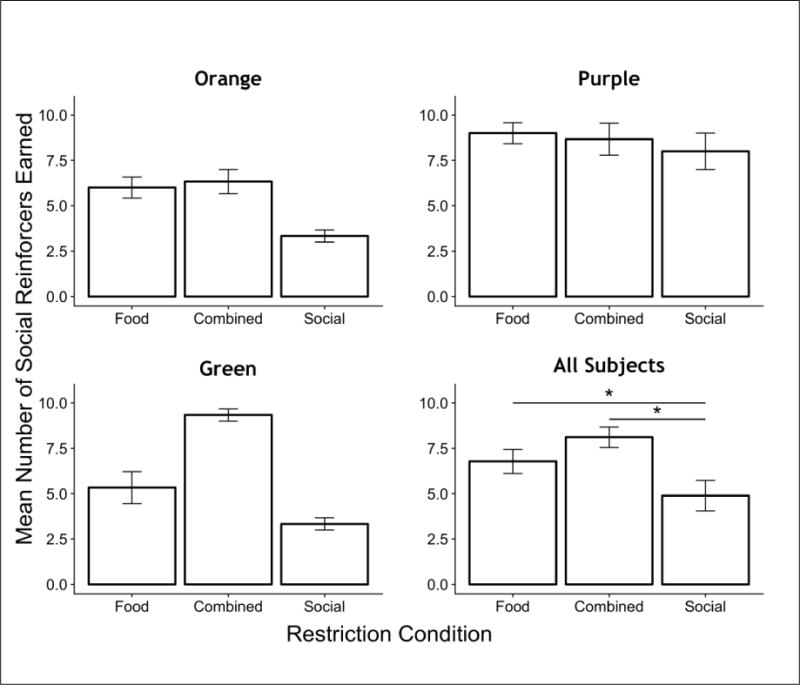
The mean number of social reinforcers earned in Experiment 1, across restriction conditions, both for individual subjects and the group mean. Error bars are standard errors. * p < 0.05
Table 2.
Mean response rates (responses per min) plus or minus standard errors in the final 3 sessions of social reinforcement conditions for each rat across restriction conditions in Experiment 1.
| Response Rate
|
|||
|---|---|---|---|
| Restriction | Green | Purple | Orange |
| Food | 0.72 ± 0.22 | 2.08 ± 0.21 | 0.96 ± 0.1 |
| Combined | 2.12 ± 0.19 | 1.22 ± 0.28 | 0.97 ± 0.21 |
| Social | 0.29 ± 0.05 | 1.68 ± 0.58 | 0.26 ± 0.05 |
Figure 3 shows response rates across sessions in the Extinction condition (Condition 7, Table 2), when lever presses were ineffective in producing the social reinforcer. The dashed horizontal reference line corresponds to the response rate in the single baseline session (PR 1 for 10-s social access) prior to the extinction condition. Relative to baseline, responding declined substantially for all three rats, requiring 4–6 sessions to reach low (< 20% of baseline) levels.
Fig. 3.
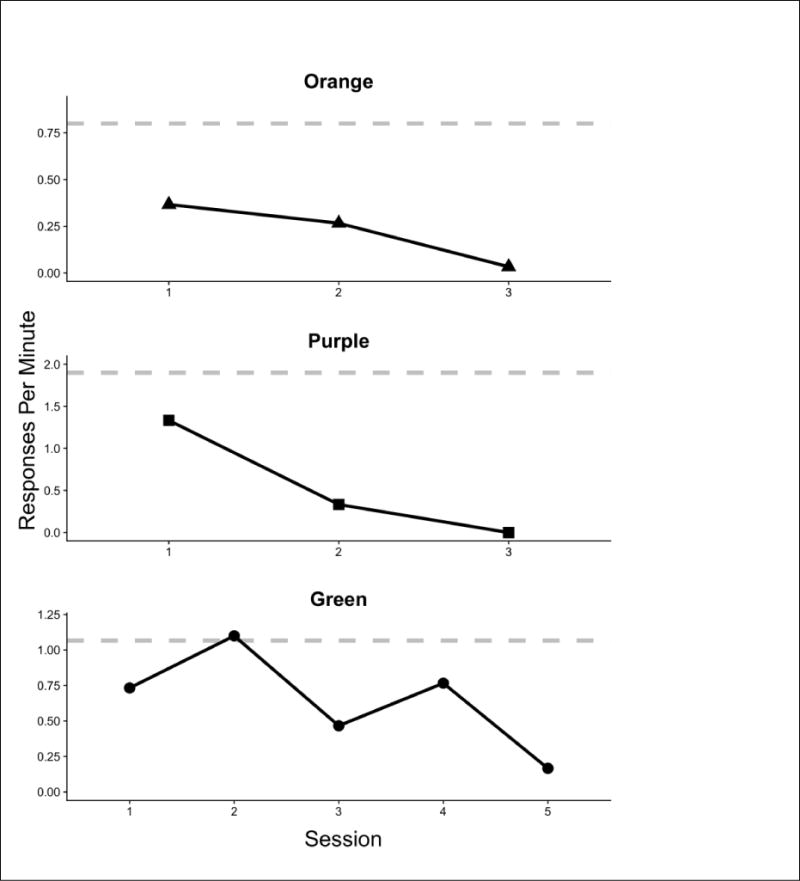
Reponses per minute for each subject in the baseline session (dotted horizontal line) and extinction sessions (solid black line) in Experiment 1.
Figure 4 shows response rates on the social lever in the second control condition, the Conditioned reinforcement probe, designed to assess the contributions of stimuli correlated with social release (Condition 8, Table 2). In this condition, social release was not only ineffective, as in the Extinction condition, but was not even possible (no rat in the restraint). As in Figure 3, the dashed horizontal reference line corresponds to the response rate in the single baseline session between the Extinction and the Conditioned Reinforcement probe condition. For one rat (Orange), responding declined relatively immediately but was highly variable for several sessions before decreasing to low levels (<10% of baseline). For the other two rats, response rates exceeded baseline levels for several sessions, before eventually declining. Although responding ultimately stabilized at low levels (0.25 responses per min), these were within the baseline range. These baseline response rates were considerably lower than those in the session prior to extinction for all three rats (Figure 3).
Fig. 4.
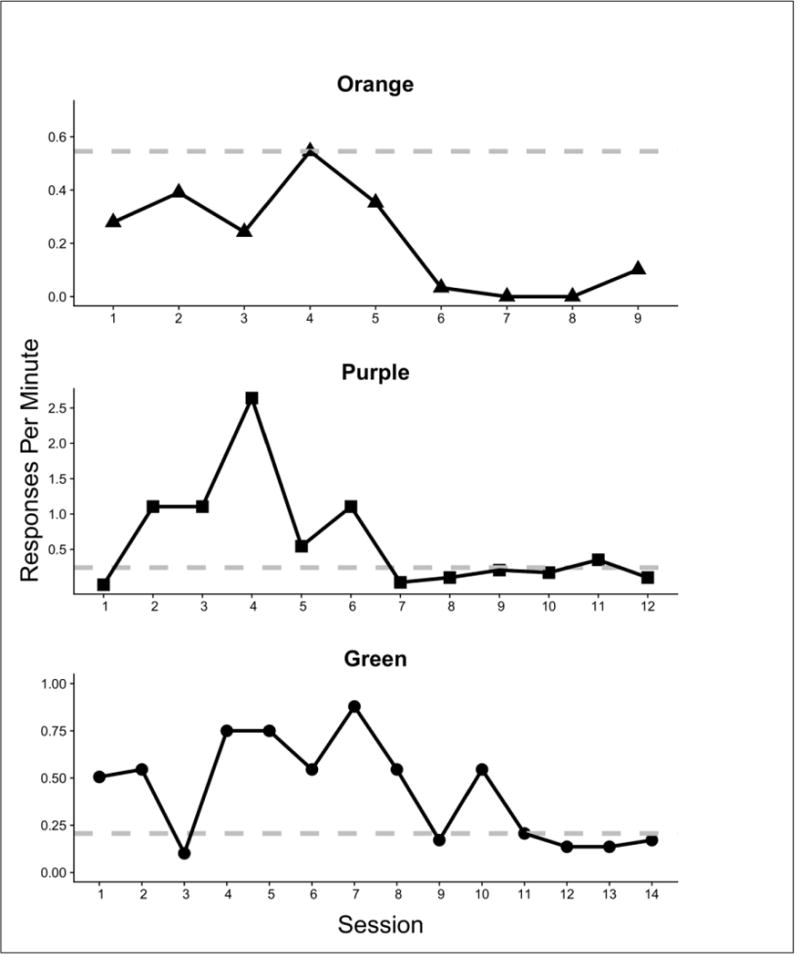
Responses per minute across sessions for each subject in the Social reinforcement probe condition in Experiment 1. Response rate is shown for the baseline session (dotted horizontal line) and extinction sessions (solid black line).
To gain a better understanding of how social contact may serve as reinforcer, we compared responses on the lever for social reinforcement to that of food reinforcement. Figure 5 and Table 3 show the mean number of reinforcers earned and mean response rates, respectively, for food reinforcement for each rat across the restriction conditions. Rats earned consistently more food reinforcers when food was restricted (Food and Combined restriction) than when it was continuously available (Social restriction) outside the sessions. There was a main effect of restriction condition on the mean number of food reinforcers earned (F(2,22) = 96.9, p < 0.001). Post-hoc comparisons revealed that significantly more food reinforcers were earned under Food restriction (t=7.53, p < 0.001) and Combined restriction (t = 13.9, p < 0.001) compared to Social restriction alone. In addition, significantly more food reinforcers were earned under Combined restriction compared to Food restriction alone (t=6.38, p < 0.001). Observation of the results from the replication conditions (Conditions 9 and 10, Table 1), showed the same patterns of responding for food reinforcement compared to the earlier conditions, in which the mean number of food reinforcers earned was higher under Combined restriction compared to Food restriction alone (see Table 3).
Fig. 5.
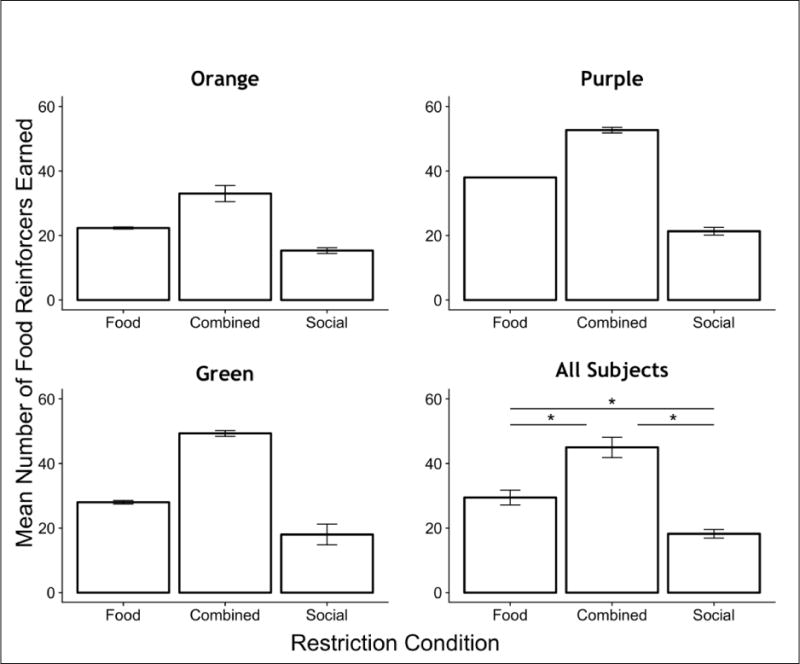
The mean number of food reinforcers earned, across restriction conditions in Experiment 2, both for individual subjects and the group mean. Error bars are standard errors. * p < 0.05
Table 3.
Mean response rates (responses per min) plus or minus standard errors in the final 3 sessions of food reinforcement conditions for each rat across restriction conditions in Experiment 1.
| Response Rate
|
|||
|---|---|---|---|
| Restriction | Green | Purple | Orange |
| Food | 13.6 ± 0.55 | 24.0 ± 1.1 | 9.01 ± 0.2 |
| Combined | 41.5 ± 1.41 | 47.2 ± 1.54 | 19.2 ± 2.9 |
| Social | 6.04 ± 2.07 | 8.03 ± 0.81 | 4.2 ± 0.47 |
| Food | 64.6 ± 4.16 | 41.2 ± 2.4 | 26.5 ± 0.88 |
| Combined | 94.3 ± 4.46 | 100.2 ± 6.36 | 49.7 ± 1.12 |
3. Experiment 2
In Experiment 1, relative reinforcing value of food and social reinforcers was compared across conditions. Another way to assess the relative value is to arrange reinforcers concurrently, as explicit choices. In Experiment 2, rats were given repeated choices between food and social reinforcers in a concurrent PR-PR schedule. The PR schedules operated independently for the two reinforcers, such that the PR increment occurred only for whichever reinforcer had been selected on the previous trial. Thus, while the response cost, or price, of the two options was initially identical, the more times a reinforcer was chosen, the higher its resulting price. We also used a larger PR increment than in Experiment 1 (5 responses, rather than 1, with each reinforcer) to generate higher prices within each session.
3.1 Methods
3.1.1 Subjects and apparatus
The same three pairs of rats (Purple, Orange, and Green) and the same experimental apparatus (Figure 1) were used for Experiment 2.
3.1.2 Procedure
The rats in each pair retained their roles (i.e., restrained rats and unrestrained rats) from the first experiment. Concurrent PR-PR schedules of food and social reinforcement were used; presses on the left lever were reinforced with food whereas presses on the right lever were reinforced with 10-s social interaction. The schedules were initially FR 1 for both options, but increased by 5 responses with each reinforcer earned on a schedule (PR 5); the schedules were independent, in that the PR increment only occurred for the chosen schedule. An intertrial interval (ITI) occurred just following reinforcement of either type, during which the lights above each lever were off and responses had no programmed consequences. The duration of the ITI varied for the two outcomes: 17 s following food reinforcement and 7 s following social reinforcement (accounting for the time needed for 10-s social interaction and 7 s for replacing the partner rat in the restrainer). This was designed to equalize the rate of trial onset, such that choices in trial n would not affect trial onset in trial n+1. The first four trials in each session were forced-choice trials, in which only one option was available (signaled by light above the active lever), designed to bring behavior into contact with the outcomes associated with each lever. The initial position of the active lever in these forced-choice trials was determined randomly, but then strictly alternated, with two trials of each reinforcer type. The remaining trials in the session were choice trials, with both alternatives concurrently available, as signaled by lights above each lever. Sessions lasted 30 min, including the forced-choice trials, and were conducted 5 days per week.
Preferences were assessed across the same three motivational conditions as in Experiment 1: Food restriction, Social restriction, and Combined-restriction. Given that the highest levels of responding were obtained in the Combined-restriction conditions in Experiment 1, this was used as the baseline (A) phase in an A-B-A-C-A within-subject experimental design, with Social- and Food-restriction serving as (B) and (C) phases, respectively. Due to time constraints, Rat Green did not undergo the final return to the Combined-restriction condition. Conditions were run until stability was achieved, defined as the absence of monotonic trends across at least three sessions, as in Experiment 1.
3.1.3 Statistical Analyses
Statistical models were created as described in Experiment 1 above (section 1.1.5).
3.2 Results and Discussion
Table 4 depicts the mean response rates for each subject under food and social reinforcement across the restriction conditions. As in Experiment 1, there were substantial differences in absolute response rates, with food-reinforced responding consistently higher than socially-reinforced responding. Like Experiment 1, we therefore compared each reinforcer to itself, across the three restriction conditions. Figure 6 shows the mean number of social reinforcers and food reinforcers earned across the final 3 sessions per condition.
Table 4.
The sequence of conditions in Experiment 2 and the number of sessions conducted at each.
| Number of Sessions
|
||||
|---|---|---|---|---|
| Order | Restriction Condition | Green | Purple | Orange |
| 1 | Combined | 12 | 17 | 14 |
| 2 | Social | 10 | 11 | 8 |
| 3 | Combined | 26 | 16 | 9 |
| 4 | Food | 13 | 11 | 18 |
| 5 | Combined | N/A | 8 | 11 |
Fig. 6.
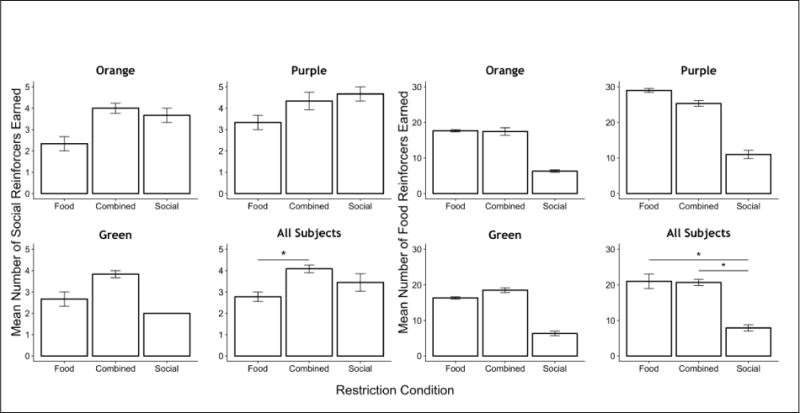
The mean number of social reinforcers earned (left panels) and food reinforcers earned (right panels), across restriction conditions in Experiment 2, both for individual subjects and the group mean. Error bars are standard errors. * p < 0.05
Considering first the social reinforcement conditions (left panels), rats earned the most social reinforcers in the Combined-restriction conditions, when access to both social contact and food was restricted between sessions. Two of three rats (Orange, Purple) also earned more social reinforcers under Social-restriction than Food-restriction conditions. Across rats, there was a main effect of restriction condition on the mean number of social reinforcers earned (F(2,37) = 8.4, p < 0.001). Post-hoc comparisons revealed that significantly more social reinforcers were earned under Combined restriction compared to Food restriction alone (t(37)=4.1, p < 0.001). The number of social reinforcers earned under Social restriction did not differ significantly from that of Combined restriction (t(37)=1.57, p = 0.12) nor Food restriction (t(37)=2.08, p = 0.07).
When analyzing the number of food reinforcers earned (right panels), we found a significant effect of restriction condition (F(2,37) = 47.4, p < 0.001). Post-hoc comparisons demonstrated that the number of food reinforcers earned was significantly higher under Combined restriction (t(37)=9.31, p <0.001) and Food restriction (t(37)=7.89, p <0.001) when either were compared to behavior under Social restriction alone. The mean number of food reinforcers earned did not differ significantly, however, between Combined restriction and Food restriction alone (t(37)=0.18, p =0.86).
In addition to these global (session-wide) measures, we analyzed within-session patterns of responding. As would be expected from the global measures, more food than social reinforcers were selected overall, but the likelihood of selecting a social reinforcer increased as a function of the price of food. Figure 7 shows the PR price of food when the first social reinforcer each session was earned across the three restriction conditions. Each point is taken from each of the last three stable sessions of every subject. The points in the combined restriction condition are mean PR prices across all three of the combined restriction condition sessions (A). Social reinforcers were selected much earlier in the session (at lower PR food prices) in Social-restriction conditions than in the other restriction conditions, showing sensitivity to social motivational variables not seen with the more global (session-wide) measures.
Fig. 7.
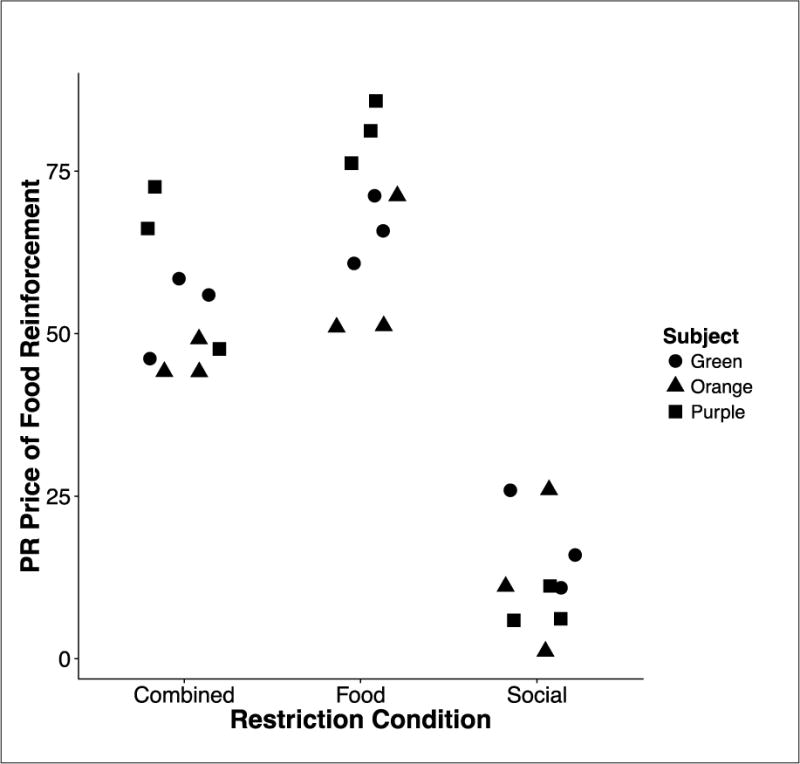
The PR price on the food lever at which the first response on the social lever was made, compared across restriction conditions for individual rats during each of the final three sessions of the three main conditions in Experiment 2.
4. General Discussion
The overall pattern of results show that rats’ lever pressing was under functional control of social reinforcement contingencies, in which responses produced access to a partner rat: responding occurred at modest but consistent levels when it produced social release, and quickly decreased to low levels when the social release contingency was discontinued. These results are in line with those of Evans et al. (1994), who found that lever pressing was maintained by social access but decreased substantially when it no longer produced access to the social partner. In their study, this pattern of extinction held both under conditions in which the reinforcement compartment was empty, and when it contained a social partner. The latter conditions more closely resemble those used in the present study, in which the social partner was present and the unrestrained rat was socially deprived. Such conditions include the critical requirements for an extinction procedure, in which responses are ineffective but motivational variables are in place (i.e., the response is ineffective in producing the reinforcer but the motivation to produce it remains high).
Social contact was not the only consequence of door-opening, however; responses also produced correlated stimuli (e.g., tone, door opening). Results from the Conditioned reinforcement probe condition in Experiment 1 (Figure 4) show the important contribution of these correlated stimuli. It is perhaps worth noting that baseline response rates were quite low in the session prior to the probe condition, perhaps reflecting the immediately prior extinction condition. As a result, there was a narrow range of responding within which to observe an effect. Even so, however, the continued response-dependent presentation of these stimuli sustained a good deal of behavior in the absence of social release, similar to prior research (Ben-Ami Bartal et al., 2011; Silberberg et al., 2014). The rats all had extensive histories of social release: 40 sessions per subject, on average (all with correlated stimuli), by the time of the conditioned reinforcement probe. These stimuli were presumably functioning as conditioned reinforcers, via long-term correlation with social release, but additional analysis is needed to isolate specific controlling variables.
Conceptualizing social release in social reinforcement terms is consistent with a growing body of research across species showing that access to social stimuli reinforces behavior that produces it (see Trezza, Campolongo, & Vanderschuren, 2011, for a review). It has proven useful in studies of this sort to compare social reinforcers against more common reinforcers, such as food. Prior studies with rats have found social and food reinforcers to be roughly equivalent (Ben-Ami Bartal et al., 2011; Sato et al., 2015), including the Evans et al. (1994) study with operant methods and deprivation schedules similar to the present study. In contrast, we found that food reinforcers generated consistently higher levels of absolute responding than social reinforcers.
Such differences in absolute level are likely due to methodological factors that dampen the relative efficacy of social reinforcement. To begin with, obtained reinforcer delays were longer for social contact than for food, owing both to procedural features (the food-producing lever was closer to the food cup than the door-producing lever was to the restraint) and to the nature of the social reinforcement contingency (in which direct social access depended on the behavior of both rats). To equate such differences in obtained reinforcer delays, future research might consider yoking food delays to the obtained delays to social contact.
The reinforcer duration we used (10 s) was also relatively brief, and there are good reasons to think that longer social access times would increase social reinforcer value. Evans et al. (1994), who found comparable levels of responding for social and food reinforcers, used 45-s access time; in some studies, social access times are even longer (Hovland et al., 2011). Research has also shown an important role for reinforcer quality (Feuerbacher & Wynne, 2015; Holm et al., 2002). An important priority for future research therefore is to systematically explore the functions relating the value of a social reinforcer value to its magnitude, both quantity (e.g., duration of social access) and quality (e.g., type of social interaction).
Despite such differences in absolute levels of responding, there are interesting parallels in socially reinforced and food reinforced behavior in the present study. Both reinforcers were sensitive to motivational variables, defined in terms of restricted access outside the session. Food reinforced responding in both experiments was higher when food was otherwise restricted, showing a clear deprivation effect. Socially reinforced behavior showed a similar type of sensitivity to motivational variables in Experiment 2; rats produced more social reinforcers when social contact outside the sessions was restricted than when it was continuously available (comparing responding under Food restriction to Combined restriction, which included the addition of social restriction). Moreover, choices of social reinforcers occurred earlier (at lower FR food prices) in the session when social interaction was restricted outside the session. Both results indicate clear sensitivity of socially reinforced behavior to deprivation variables.
There were also interactive effects of the motivational conditions. The highest levels of socially reinforced responding were seen in the Combined restriction conditions, suggesting that food restriction enhanced the efficacy of social contact as a reinforcer. Similarly, food reinforced responding in Experiment 1 peaked under the combined restriction conditions, suggesting that social restriction enhanced the efficacy of food as a reinforcer. These types of interactions between qualitatively different reinforcers may suggest that the social and food reinforcers were functioning as at least partial economic complements (i.e., shifts in reinforcer value in the same direction). More refined methods utilizing cross-price elasticity analyses will provide a clearer picture of the interactions between social and other reinforcers, including different types of social reinforcement. The present methods are well suited to this type of research.
Although we did not directly measure behavior during the reinforcement periods, informal observations suggested that the social interactions were mutually reinforcing. The restrained rat generally exited the tube shortly after the door opening, and the unrestrained rat generally moved toward the tube, both of which facilitated social contact. Moreover, the interactions themselves were largely positive (e.g., grooming, play). Interestingly, the unrestrained rats would often enter and spend time in the restraint tube. Together with observations reported by Silberberg et al. (2014), this suggests that access to the tube may (perhaps in addition to social release) contribute to the lever pressing by the unrestrained rat. In fact, it appeared that access to the tube was reinforcing for the restrained and unrestrained rats alike, as both entered during reinforcement periods. On its face, the observation that restrained rats would ever return to the restraint runs contrary to the notion that the restraint is aversive (i.e., a source of acute distress), required by empathy-based accounts. Direct measurement of such behavior, however, is needed for a clearer picture of the nature of the social interaction, and the degree to which social release is motivated primarily by aversive or reinforcing contingencies: minimizing distress or enhancing social contact?
It will also prove useful in this context to supplement behavioral measures with ultrasonic vocalization (USV) methods. Prior research has shown that USV in the 50 kHz range are associated with a range of positive outcomes, including food (Yuki & Okanoya, 2014) and social access (Willey & Spear, 2013), whereas USV in the 23 kHz range are associated with distress calls (Borta, Wohr, & Schwarting, 2005). If social release is governed more by access to social contact than by avoiding distress, this should be reflected in the USV profile: relatively higher in the 50 kHz than in the 23 kHz range. On the other hand, the reverse would be true if distress was the main factor: relatively higher in the 23 kHz range. In any case, detailed measurement, using both behavioral and USV methods, is a critical part of a comprehensive understanding of social release.
Ben-Ami Bartal et al. (2011) measured USV in the 23 kHz range, commonly associated with distress calls, in the context of their social release procedure. If social release is motivated by empathic concern for the restrained rat, then one might expect door opening to occur in the presence of distress calls by the restrained rat, as this is when obvious distress is most apparent. Contrary to this expectation, distress calls occurred initially, but decreased over time, several sessions prior to when social release began occurring. There was thus no relationship in the Ben-Ami Bartal et al. study between acute distress (at least as indexed by USV) and social release.
The preponderance of evidence to date, therefore, does not favor empathy-based explanations. Even in those cases reported to involve empathy (Ben-Ami Bartal et al., 2011; Sato et al., 2015), other variables, including most notably access to social reinforcement, provide an equally plausible, yet simpler, explanation (Schwartz et al., 2017; Silberberg et al., 2014). Social reinforcement is not only more parsimonious, it is more scientifically productive, in that it builds on existing scientific knowledge, based on well-established principles.
The present study shows the utility of viewing social release through a social reinforcement lens, grounded in established methods for quantifying the value of reinforcers more generally. To identify an important role for social reinforcement, however, is not to deny the importance of other variables; escaping from acute distress (including that induced by another animal) and accessing social contact may each contribute to social release – perhaps even at different times in the development of the behavior. Determining the relative contributions of these (and possibly other) mechanisms is an important priority for future research, to which present methods are well suited. Such research is crucial to an overall analysis of social behavior, regardless of one’s theoretical predilections.
Table 5.
Mean response rates (responses per min) plus or minus standard errors for each reinforcer type in the final 3 sessions of each conditions or each rat across restriction conditions in Experiment 2.
| Social Response Rate | Food Response Rate | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Restriction | Green | Purple | Orange | Green | Purple | Orange |
| Combined | 1.03 ± 0.1 | 1.69 ± 0.6 | 1.2 ± 0.18 | 14.9 ± 5.1 | 48.0 ± 5.45 | 17.6 ± 2.0 |
| Social | 0.2 ± 0.02 | 1.0 ± 0.29 | 0.89 ± 0.17 | 2.27 ± 0.42 | 8.0 ± 1.8 | 2.1 ± 0.26 |
| Combined | 0.88 ± 0.19 | 0.74 ± 0.24 | 1.13 ± 0.12 | 27.7 ± 1.03 | 41.1 ± 4.58 | 17.8 ± 3.22 |
| Food | 0.48 ± 0.15 | 0.46 ± 0.11 | 0.24 ± 0.08 | 18.5 ± 0.82 | 63.2 ± 2.67 | 22.8 ± 1.59 |
| Combined | N/A | 0.64 ± 0.06 | 0.76 ± 0.06 | N/A | 55.9 ± 1.47 | 33.2 ± 0.99 |
Highlights.
Rats responded for food and social contact across food and/or social-restriction conditions.
Schedules of reinforcement were presented singly (EXP 1) and concurrently (EXP 2).
Rats displayed sensitivity to deprivation conditions in a reinforcer-specific manner.
Responses on the social lever extinguished after decoupling the social reinforcement contingency.
Acknowledgments
The many technical contributions of Greg Wilkinson to the project are gratefully acknowledged.
Funding. This research was supported in part by grant 026127 from the National Institute on Drug Abuse to T.D.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Notes. The data from Experiment 1 served as L.C.H.’s Senior Thesis at Reed College. Some of these data were presented at the 2017 SQAB annual symposia in Denver, CO.
Compliance with ethical standards
Ethics statement. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experimental procedures were in accord with the policies maintained by the Reed College Institutional Animal Care and Use Committee.
Conflicts of interest. The authors declare that they have no conflicts of interest.
References
- Belke TW. Running and responding reinforced by the opportunity to run: Effect of reinforcer duration. Journal of the Experimental Analysis of Behavior. 1997;67:337–351. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334:1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Rodgers DA, Sarria MSB, Decety J, Mason P. Pro-social behavior in rats is modulated by social experience. eLife. 2013;3:e01385, 1–16. doi: 10.7554/eLife.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- Borta A, Wohr M, Schwarting RKW. Rat ultrasonic vocalization in aversively motivated situations and the role of individual differences in anxiety-related behavior. Behavioural Brain Research. 2005;166:271–80. doi: 10.1016/j.bbr.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Bradshaw CM, Szabadi E, Bevan P. Relationship between response rate and reinforcement frequency in variable-interval schedules: the effect of the concentration of sucrose reinforcement. Journal of the Experimental Analysis of Behavior. 1978;29:447–452. doi: 10.1901/jeab.1978.29-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Batulis DK, Landry KL, Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology. 2005;180:414–426. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- Cronin KA. Prosocial behaviour in animals: the influence of social relationships, communication, and rewards. Animal Behaviour. 2012;84:1085–1093. [Google Scholar]
- Dettmer E, Fragaszy D. Determining the value of social companionship to captive tufted capuchin monkeys (Cebus apella) Journal of Applied Animal Welfare Science. 2000;3:293–304. [Google Scholar]
- de Villiers P. Choice in concurrent schedules and a quantitative formulation of the law of effect. In: Honig WK, Staddon JER, editors. Handbook of operant behavior. Englewood Cliffs, NJ: Prentice-Hall; 1977. pp. 223–287. [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharid MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology. 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Drea CM, Carter AN. Cooperative problem solving in a social carnivore. Animal Behaviour. 2009;78(4):967–977. [Google Scholar]
- Evans MJ, Duvel A, Funk ML, Lehman B, Sparrow J, Watson NT, Neuringer A. Social reinforcement of operant behavior in rats: A methodological note. Journal of the Experimental Analysis of Behavior. 1994;62:149–156. doi: 10.1901/jeab.1994.62-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerbacher EN, Wynne CD. Shut up and pet me! Domestic dogs (Canis lupus familiaris) prefer petting to vocal praise in concurrent and single-alternative choice procedures. Behavioral Processes. 2015;110:47–59. doi: 10.1016/j.beproc.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Gilbertson DW. Courtship as a reinforcement for key pecking in the pigeon, Columba livia. Animal Behaviour. 1975;23:735–744. [Google Scholar]
- Grasing K, Li N, He S, Parrish C, Delich J, Glowa J. A new progressive ratio schedule for support of morphine self-administration in opiate dependent rats. Psychopharmacology. 2003;168:387–396. doi: 10.1007/s00213-003-1442-x. [DOI] [PubMed] [Google Scholar]
- Harzem P, Lowe CF, Priddle-Higson PJ. Inhibiting function of reinforcement: Magnitude effects on variable-interval schedules. Journal of the Experimental Analysis of Behavior. 1978;30:1–10. doi: 10.1901/jeab.1978.30-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. Journal of the Experimental Analysis of Behavior. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Jensen MB, Jeppesen LL. Calves’ motivation for access to two different types of social contact measured by operant conditioning. Applied Animal Behaviour Science. 2002;79:175–194. [Google Scholar]
- Hovland AL, Akre AK, Flø A, Bakken M, Koistinen T, Mason GJ. Two’s company? Solitary vixens’ motivations for seeking social contact. Applied Animal Behaviour Science. 2011;135:110–120. [Google Scholar]
- Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Animal Behaviour. 1981;29:259–270. [Google Scholar]
- Ikemoto S, Panksepp J. The effects of early social isolation on the motivation for social play in juvenile rats. Developmental Psychobiology. 1992;25:261–274. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- Kirkden RD, Pajor EA. Motivation for group housing in gestating sows. Animal Welfare. 2006;15:119–130. [Google Scholar]
- Lenth RV. Least-Squares Means: The R Package lsmeans. Journal of Statistical Software. 2016;69 1rnal. [Google Scholar]
- Łopuch S, Popik P. Cooperative behavior of laboratory rats (Rattus norvegicus) in an instrumental task. Journal of Comparative Psychology. 2011;125(2):250–253. doi: 10.1037/a0021532. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Carai AM, Kaupmann K, Guery S, Froestl W, Leite-Morris KA, Gessa GL, Colombo G. Reduction of alcohol’s reinforcing and motivational properties by the positive allosteric modulator of the GABAB Receptor, BHF177, in alcohol-preferring rats. Alcoholism: Clinical and Experimental Research. 2009;33:1749–1756. doi: 10.1111/j.1530-0277.2009.01012.x. [DOI] [PubMed] [Google Scholar]
- Martin L, Sample H, Gregg M, Wood C. Validation of operant social motivation paradigms using BTBR T+tf/J and C57BL/6J inbred mouse strains. Brain and Behavior. 2014;4:754–764. doi: 10.1002/brb3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WA, Hollis JH, Sharpe LG. Differential responses of chimpanzees to social stimulation. Journal of Comparative and Physiological Psychology. 1962;55:1105–1110. [Google Scholar]
- Nowbahari E, Scohier A, Durand L, Hollis KL. Ants, Cataglyphis cursor, use precisely directed rescue behavior to free entrapped relatives. PLoS One. 2009;4:e6573. doi: 10.1371/journal.pone.0006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering C, Alsiö J, Hulting AL, Schiöth HB. Withdrawal from free-choice high-fat high-sugar diet induces craving only in obesity-prone animals. Psychopharmacology. 2009;204:431–443. doi: 10.1007/s00213-009-1474-y. [DOI] [PubMed] [Google Scholar]
- Plotnik JM, Lair R, Suphachoksahakun W, de Waal FB. Elephants know when they need a helping trunk in a cooperative task. Proceedings of the National Academy of Sciences. 2011;108(12):5116–5121. doi: 10.1073/pnas.1101765108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2016. [Google Scholar]
- Reed P. Multiple determinants of the effects of reinforcement magnitude on free-operant response rates. Journal of the Experimental Analysis of Behavior. 1991;55:109–123. doi: 10.1901/jeab.1991.55-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Ting-Kai L, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Sato N, Tan L, Tate K, Okada M. Rats demonstrate helping behavior toward a soaked conspecific. Animal Cognition. 2015;18:1039–1047. doi: 10.1007/s10071-015-0872-2. [DOI] [PubMed] [Google Scholar]
- Schwartz LP, Silberberg A, Casey AH, Kearns DN, Slotnick B. Does a rat release a soaked conspecific due to empathy? Animal Cognition. 2017;20(2):299–308. doi: 10.1007/s10071-016-1052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Nutrient-conditioned flavor preference and incentive value measured by progressive ratio licking in rats. Physiology & Behavior. 2006;88:88–94. doi: 10.1016/j.physbeh.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Silberberg A, Allouch C, Sandfort S, Kearns D, Karpel H, Slotnik B. Desire for social contact, not empathy, may explain “rescue” behavior in rats. Animal Cognition. 2014;17:609–617. doi: 10.1007/s10071-013-0692-1. [DOI] [PubMed] [Google Scholar]
- Søndergaard E, Jensen MB, Nicol CJ. Motivation for social contact in horses measured by operant conditioning. Applied Animal Behaviour Science. 2011;132:131–137. [Google Scholar]
- Spear DJ, Katz JL. Cocaine and food as reinforcers: Effects of reinforce magnitude and response requirement under second-order fixed-ratio and progressive-ratio schedules. Journal of the Experimental Analysis of Behavior. 1991;56:261–275. doi: 10.1901/jeab.1991.56-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, Branch MN. Effects of step size and break-point criterion on progressive-ratio performance. Journal of the Experimental Analysis of Behavior. 1998;70:123–138. doi: 10.1901/jeab.1998.70-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Hackenberg TD. Functional analysis of mutual behavior in laboratory rats (Rattus norvegicus) Journal of Comparative Psychology. 2016;130:13–23. doi: 10.1037/com0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJ. Evaluating the rewarding nature of social interactions in laboratory animals. Developmental Cognitive Neuroscience. 2011;1:444–458. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherly JN, King BM, Uran EL. Upcoming food-pellet reinforcement alters rats’ lever pressing for liquid sucrose delivered by a progressive-ratio schedule. Behavioural Processes. 2003;63:73–86. doi: 10.1016/s0376-6357(03)00033-0. [DOI] [PubMed] [Google Scholar]
- West SA, Griffin AS, Gardner A. Social semantics: altruism, cooperation, mutualism, strong reciprocity, and group selection. Journal of Evolutionary Biology. 2007;20:415–432. doi: 10.1111/j.1420-9101.2006.01258.x. [DOI] [PubMed] [Google Scholar]
- Willey AR, Spear LP. The effects of pre-test social deprivation on a natural reward incentive test and concomitant 50kHz ultrasonic vocalization production in adolescent and adult male Sprague-Dawley rats. Behavioural Brain Research. 2013;245:107–112. doi: 10.1016/j.bbr.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsoncroft WE. Babies by bar-press: maternal behavior in the rat. Behavior Research Methods. 1968;1:229–230. [Google Scholar]
- Yuki S, Okanoya K. Behavioral correlates of 50-kHz ultrasonic vocalizations in rats. Animal Behavior and Cognition. 2014;1:452–463. [Google Scholar]


