Abstract
Mutations of the surfactant protein (SP)-C gene (SFTPC) have been associated with neonatal respiratory distress syndrome (RDS) and childhood interstitial lung disease (ILD). If accurate diagnosis and proper management are delayed, irreversible respiratory failure demanding lung transplantation may ensue. A girl was born at term but was intubated and given exogenous surfactant due to RDS. Cough and tachypnea persisted, and symptoms rapidly progressed at 16 months of age despite treatment with antibiotics, oral prednisolone, methylprednisolone pulse therapy, and intravenous immunoglobulin. At 20 months, she visited our hospital for a second opinion. A computed tomography scan showed a diffuse mosaic pattern with ground-glass opacity and subpleural cysts compatible with ILD. A video-assisted thoracoscopic lung biopsy revealed ILD with eosinophilic proteinaceous material and macrophages in the alveolar space. Bilateral lung transplant from a 30-month-old child was done, and she was discharged in room air without acute complications. Genetic analysis revealed a novel c.203T>A, p.Val68Asp mutation of SP-C, based on the same exon as a known pathogenic mutation, p.Glu66Lys.
Keywords: SFTPC, Surfactant Protein C, Interstitial Lung Disease, Lung Transplantation
Graphical Abstract
INTRODUCTION
Pulmonary surfactant is a complex mixture of phospholipids and proteins that reduces the surface tension of the alveolus by forming a surface-active film at the air-liquid interface.1 Of the four surfactant-associated proteins, surfactant protein (SP)-A, SP-B, SP-C, and SP-D, the hydrophobic SP-B and SP-C are especially essential in the dynamics of phospholipids by promoting the transition between the storage form and the functional surface film.2
SP-C is synthesized from a 197-amino acid precursor (proSP-C), which is encoded by SFTPC on human chromosome 8p21.3. ProSP-C is an integral membrane protein and it undergoes post-translational processing involving palmitoylation, and cleavage of C- and N-terminal propeptides to yield the 3.7 kDa SP-C. Produced exclusively by alveolar type 2 cells, mature SP-C is transferred to the lumen of lamellar bodies and is secreted into the alveolar space with surfactant phospholipids.3 First described by Nogee et al.4 in 2001, mutations of SFTPC have been reported to be associated with a broad spectrum of lung diseases including neonatal respiratory distress syndrome (RDS), childhood interstitial lung disease (ILD), and adult chronic ILD.5,6
In this report, we present a clinical description of a child who suffered neonatal RDS and developed childhood ILD due to a novel heterozygous SFTPC mutation in the non-BRICHOS C-terminal domain (c.203T>A, p.Val68Asp). The child was eventually treated with lung transplantation (LT).
CASE DESCRIPTION
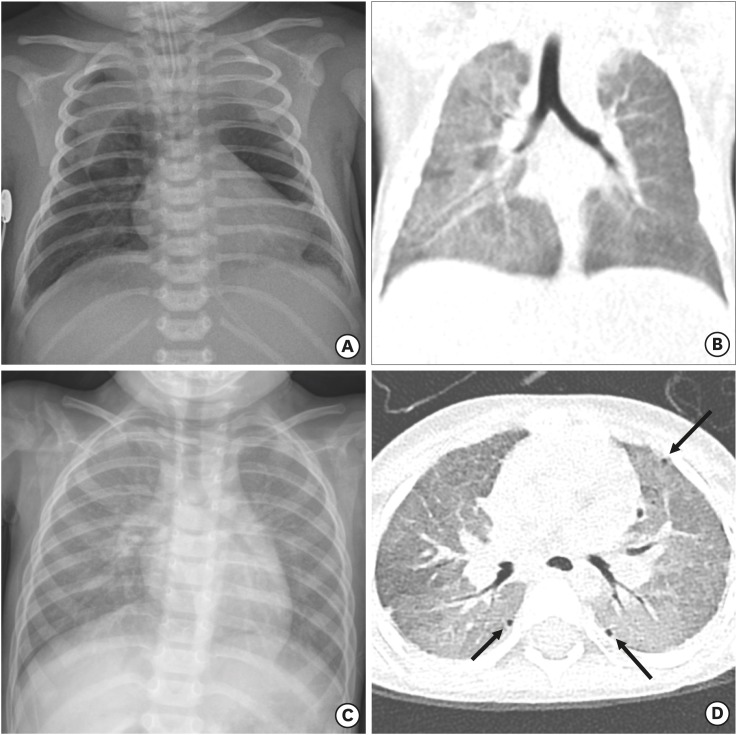
A 20-month-old female patient presented at our emergency room on March 23rd, 2017 with chronic tachypnea, cough, and failure to thrive. She was born at term after an uneventful pregnancy, but developed respiratory distress within hours. She was intubated and given two doses of exogenous surfactant. While on mechanical ventilation, she briefly developed bilateral pneumothorax (Fig. 1A) requiring bilateral chest tubes. After two extubation failures and a one-week course of dexamethasone support, she was successfully weaned off mechanical ventilation. Chest computed tomography (CT) scans showed a diffuse mosaic pattern with ground-glass opacity (GGO) and atelectasis, and nodular or fibrotic lesions on the dependent lung portion (Fig. 1B). She was discharged from the neonatal intensive care unit after 30 days, but subsequently had gradually worsening symptoms of cough, tachypnea, and failure to thrive.
Fig. 1. Sequential chest radiographs and CT. (A) Chest radiograph on the second postnatal day reveals a pneumomediastinum during the treatment with mechanical ventilation. (B) Coronal reconstructed chest CT on postnatal day 18 shows a resolved pneumomediastinum. However, there is residual diffuse GGO in both lungs with the right lung predominance. (C) Chest radiograph at 20 months of age demonstrates recurrence of diffuse haziness in bilateral lungs. (D) Axial chest CT image at 20 months of age shows diffuse GGO with mosaic attenuation and multiple tiny subpleural air cysts (arrows) in both lungs.
CT = computed tomography, GGO = ground-glass opacity.
After 16 months of age, her symptoms were aggravated with concurrent respiratory tract infections. Despite inpatient treatment with antibiotics and several courses of oral prednisolone, her cough and dyspnea worsened, finally a hypoxemia developed on her in room air. Two cycles of methylprednisolone pulse therapy and two cycles of intravenous immunoglobulin were tried, but her symptoms were not alleviated.
At 20 months of age, she was referred to our hospital for a second opinion. The infant showed poor nutritional status, with height of 78.5 cm (third to fifth percentile) and weight 8.2 kg (less than third percentile). Clinically, she had tachypnea with a respiratory rate of 45–60/min, and showed hypoxemia with SpO2 < 90% in room air. Initial venous blood gas analysis was pH 7.39, pCO2 48 mmHg, pO2 47 mmHg, and HCO3 29.1 mmol/L. A chest X-ray showed diffuse haziness on bilateral lung fields (Fig. 1C). She was continued on oral prednisolone with a dosage of 2 mg/kg/day.
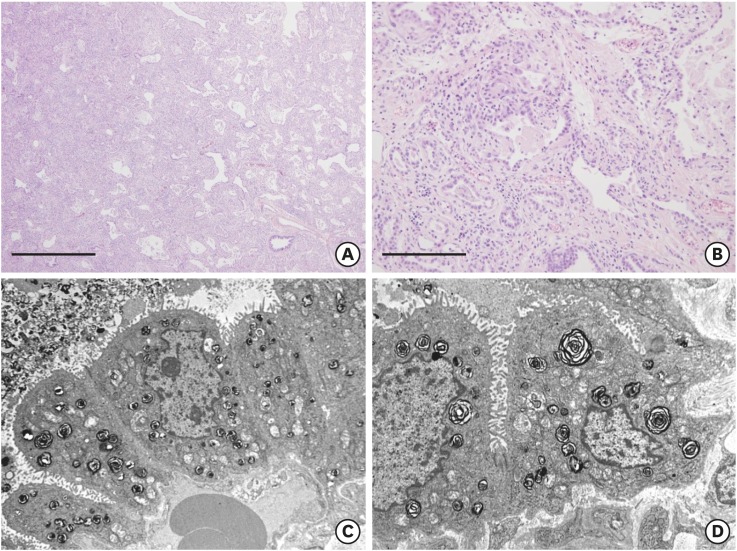
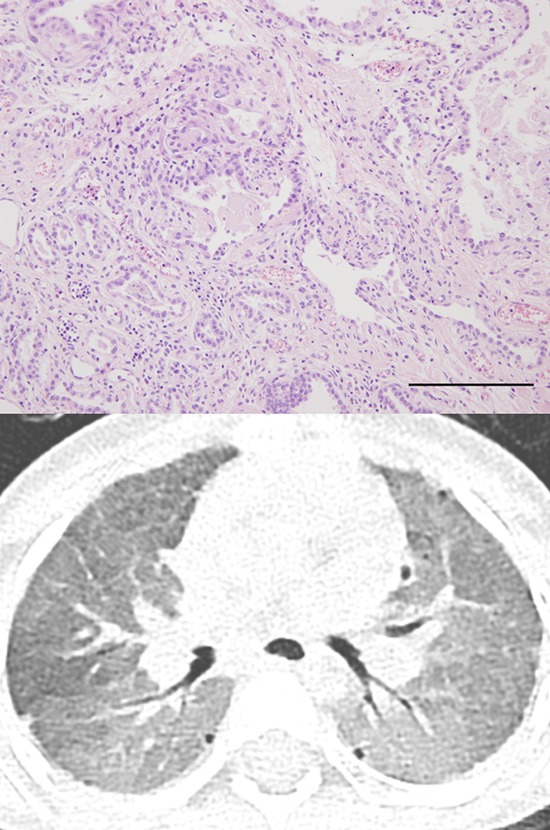
A CT scan on hospital day four showed a diffuse mosaic pattern with GGO and subpleural cysts that was compatible with ILD (Fig. 1D). Lung biopsy via video-assisted thoracoscopic surgery (VATS) revealed ILD with interstitial lymphocytic infiltration, type II pneumocyte hyperplasia, eosinophilic proteinaceous material, and alveolar macrophage accumulation in the alveoli on light microscopy (Fig. 2A and B), and the relatively normal appearing lamellar bodies on electron microscopy (Fig. 2C and D). Genetic analysis was performed for SFTPB, SFTPC, and ATP-binding cassette subfamily A, member 3 (ABCA3). However, the results were only available three months later.
Fig. 2. Histological (hematoxylin and eosin stain) and ultrastructural findings of the lung. (A) Uniformly diffuse interstitial fibrosis and inflammatory cell infiltration are observed (× 40, scale bar = 1 mm). (B) Eosinophilic proteinaceous material and alveolar macrophages are identified in alveolar spaces (× 200, scale bar = 200 μm). (C, D) Ultrastructurally, the alveoli are lined by type II pneumocytes, which have a relatively normal appearing lamellar form of surfactant in shape and number.
Because a surfactant deficiency was strongly suspected, she was listed at the Korean Network for Organ Sharing (KONOS) as a LT candidate. Within one week, she underwent successful bilateral LT from a 30-month-old child. The patient was weaned off mechanical ventilation seven days after LT. Oxygen was completely tapered off 18 days post-LT. A chest CT at one-month post-LT revealed resolution of GGO with minimal segmental and sub-segmental atelectasis due to size discrepancy of the thoracic cage. She was discharged 39 days post-LT without acute complications.
At four months post-LT, she had minimal problems. She had no respiratory symptoms and could run without difficulty. The genetic analysis revealed a novel heterozygous c.203T>A, p.Val68Asp mutation of SFTPC, based on the same exon as a known pathogenic mutation, p.Glu66Lys.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Seoul National University College of Medicine (Reg. No. 1710-002-889). Informed consent was submitted by the caregiver of the patient.
DISCUSSION
In this report, we describe a novel heterozygous mutation of SFTPC associated with full-term neonatal RDS, progressive respiratory difficulty consistent with childhood ILD, and eventual LT. There are many reported cases of SFTPC mutations associated with RDS, childhood ILD, or adult ILD. This case is unique in that the patient had neonatal RDS requiring exogenous surfactant and mechanical ventilation, continued to have respiratory symptoms throughout infancy, and showed acute aggravation with respiratory infection. The neonatal presentation of our case originally raised suspicion for a surfactant mutation abnormality of ABCA3. 7 The SFTPB mutation also results in neonatal acute RDS in full-term infants, but usually does not respond to supportive therapies including surfactant replacement, and patients usually dies within 6 months.8 However, polymerase chain reaction and full-length sequencing of the coding regions of SFTPB and ABCA3 revealed no known or unknown variants.
We detected a c.203T>A in exon 2 of SFTPC, which is p.Val68Asp in the non-BRICHOS C-terminal of proSP-C. This is a novel mutation that has not been listed in the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk/ac/index.php), but it is very closely located on the same exon as a known pathogenic mutation, p.Glu66Lys. In vitro studies have shown that mutations in the non-BRICHOS C-terminal region (i.e., I73T, E66K) disrupt proSP-C trafficking and processing by accumulation in the endosomal pathway directly, or after deposition in the plasma membrane.5,9 While this causes abnormal delivery of the mutant proSP-C, the wild-type isoforms undergo normal trafficking to yield detectable 3.7 kDa mature SP-C in bronchoalveolar lavage fluid.10 Thus, the pathogenesis of these SP-C mutants is a toxic gain of function, not a lack of normal SP-C. The natural course of SP-C mutation varies widely from childhood-onset mild chronic lung disease to infantile acute respiratory failure resulting in death, even within the same genotype.11
After LT, our patient, for the first time in her entire 24 months of life, was not tachypneic nor coughing all day. She was running freely without any respiratory symptoms and gained weight to reach the third percentile of her age. However, as this was the first case of ILD due to surfactant deficiency in Korea, there are some limitations in the diagnosis and treatment. Most importantly, SFTPB, SFTPC, and ABCA3 sequencing was not a readily available test in any lab in Korea. After initiating a new gene sequencing test, over three months are required to obtain the results. Our patient was rapidly deteriorating when she was referred to our hospital, and owing to her progressive respiratory failure we could not wait the three months required for the genetic diagnosis before deciding on a treatment. Thus, when the CT scan revealed a diffuse mosaic pattern with GGO and subpleural cysts that was compatible with ILD, a VATS lung biopsy was done according to the American Thoracic Society Children's ILD (chILD) protocol. This was done to rule out other causes of ILD, such as diffuse lung developmental disorders, neuroendocrine cell hyperplasia of infancy, and pulmonary interstitial glycogenosis.12
While waiting for the genetic diagnosis, we had to determine a treatment plan. Until then anti-inflammatory treatment with two cycles of intravenous immunoglobulin, two cycles of methylprednisolone, and long-term oral prednisolone had already failed to prevent progression. Recently, some cases of successful treatment without LT are being reported. They used hydroxychloroquine (HCQ) to wean off the mechanical ventilation and supplementary oxygen11,13,14 because HCQ has anti-inflammatory properties and inhibits the intracellular proSP-C processing.15 However, despite the use of HCQ, several other cases reported the disease progression resulting in chronic ventilator care with tracheostomy, LT, or death.11,16,17 Moreover, the pathologic finding in this subject was not exactly the same as those ILDs that may have benefit from HCQ administration. Furthermore, in such age of < 2 years size-matched donors are extremely hard to encounter. Therefore, we voted for LT, an aggressive but definitive treatment option for all differential diagnoses: ABCA3, SFTPB, and SFTPC, rather than losing the donor for supportive management with HCQ.
The 20th official International Society for Heart and Lung Transplantation (ISHLT) Registry Report of 2017 showed that during the period of January to June 2016, the indications for LT among the < 1-year age group included surfactant disorder in 30% of all infants, second to pulmonary hypertension accounting for 37%. In children aged 1 to 5 years, pulmonary hypertension accounted for 46.2% of LT, and 8.3% of surfactant disorders.8
According to the 2016 annual report of KONOS, the average waiting period for a lung transplant is 116 days. In a case of humidifier disinfectant-associated ILD, the patient had to wait 100 days on extracorporeal membrane support waiting for a donor lung.18 A longer waiting period results in extended time of hospitalization and ventilator support, increasing the risk of treatment-related morbidities and subsequent worse post-transplant outcomes. In a Japanese case of SFTPC mutation, the 10-year-old patient underwent living-donor right single-lobe lung transplantation from her mother.19 In Korea, live donor LT is illegal, thus elongating the waiting period for LT. Fortunately, our patient had the opportunity to receive a donor lung in such a short time.
The limitation to our case report is that we cannot yet anticipate the long-term outcome of this patient. The overall survival after LT is 5.4 years in children and 5.9 years in adults for patients who underwent transplant between January 1990 and June 2015. Bronchiolitis obliterans, the most common form of chronic lung allograft dysfunction, occurs in more than 50% of patients by 5 years after transplant.18 A multi-disciplinary team of pediatric pulmonologists, thoracic surgeons, organ transplantation team, and infectious disease specialists is closely monitoring this patient for possible future complications.
To our knowledge, this is the first case of a genetically diagnosed SP mutation causative of neonatal RDS or childhood ILD in Korea. Thus, the patient was delayed in diagnosis even though she presented with symptoms within hours after birth. Had the mutation been detected earlier, various treatment options might have been explored before her respiratory failure and subsequent VATS lung biopsy and emergency LT. Recent guidelines suggest that, if a patient presents with full-term neonatal RDS or childhood ILD, SP deficiency should be considered, and genetic testing should precede biopsy if possible.12,20 Thus, there is a need for a national center where gene testing of SPs can be done rapidly and efficiently.
In conclusion, we report a clinical description of a child who suffered neonatal RDS and developed childhood ILD due to a novel heterozygous SFTPC mutation in the non-BRICHOS C-terminal domain. The patient was successfully treated with LT.
ACKNOWLEDGMENTS
The authors acknowledge the kid who donated his precious lung to this desperate girl.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Kim YT, Suh DI. Investigation: Park JS, Choi YJ, Park S, Chae JH, Park JD, Cho YJ, Kim WS, Seong MW, Park SH, Kwon D, Chung DH. Writing - original draft: Park JS, Suh DI. Writing - review & editing: Choi YJ, Suh DI.
References
- 1.Hawgood S. Pulmonary surfactant apoproteins: a review of protein and genomic structure. Am J Physiol. 1989;257(2 Pt 1):L13–L22. doi: 10.1152/ajplung.1989.257.2.L13. [DOI] [PubMed] [Google Scholar]
- 2.Weaver TE, Johnson Conkright J. Functions of surfactant proteins B and C. Annu Rev Physiol. 2001;63(1):555–578. doi: 10.1146/annurev.physiol.63.1.555. [DOI] [PubMed] [Google Scholar]
- 3.Beers MF, Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005;67(1):663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- 4.Nogee LM, Dunbar AE, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344(8):573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 5.Brasch F, Griese M, Tredano M, Johnen G, Ochs M, Rieger C, et al. Interstitial lung disease in a baby with a de novo mutation on the SFTPC gene. Eur Respir J. 2004;24(1):30–39. doi: 10.1183/09031936.04.00000104. [DOI] [PubMed] [Google Scholar]
- 6.Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59(11):977–980. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bullard JE, Wert SE, Nogee LM. ABCA3 deficiency: neonatal respiratory failure and interstitial lung disease. Semin Perinatol. 2006;30(6):327–334. doi: 10.1053/j.semperi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Goldfarb SB, Levvey BJ, Cherikh WS, Chambers DC, Khush K, Kucheryavaya AY, et al. Registry of the International Society for Heart and Lung Transplantation: twentieth pediatric lung and heart-lung transplantation report—2017; Focus Theme: allograft ischemic time. J Heart Lung Transplant. 2017;36(10):1070–1079. doi: 10.1016/j.healun.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Stevens PA, Pettenazzo A, Brasch F, Mulugeta S, Baritussio A, Ochs M, et al. Nonspecific interstitial pneumonia, alveolar proteinosis, and abnormal proprotein trafficking resulting from a spontaneous mutation in the surfactant protein C gene. Pediatr Res. 2005;57(1):89–98. doi: 10.1203/01.PDR.0000147567.02473.5A. [DOI] [PubMed] [Google Scholar]
- 10.Thouvenin G, Taam RA, Flamein F, Guillot L, Le Bourgeois M, Reix P, et al. Characteristics of disorders associated with genetic mutations of surfactant protein C. Arch Dis Child. 2010;95(6):449–454. doi: 10.1136/adc.2009.171553. [DOI] [PubMed] [Google Scholar]
- 11.Salerno T, Peca D, Menchini L, Schiavino A, Boldrini R, Esposito F, et al. Surfactant protein C-associated interstitial lung disease; three different phenotypes of the same SFTPC mutation. Ital J Pediatr. 2016;42(1):23. doi: 10.1186/s13052-016-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, et al. An official American Thoracic Society clinical practice guideline: Classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188(3):376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen DM, Waltz DA. Hydroxychloroquine and surfactant protein C deficiency. N Engl J Med. 2005;352(2):207–208. doi: 10.1056/NEJM200501133520223. [DOI] [PubMed] [Google Scholar]
- 14.Arıkan-Ayyıldız Z, Caglayan-Sozmen S, Isık S, Deterding R, Dishop MK, Couderc R, et al. Survival of an infant with homozygous surfactant protein C (SFTPC) mutation. Pediatr Pulmonol. 2014;49(3):E112–E115. doi: 10.1002/ppul.22976. [DOI] [PubMed] [Google Scholar]
- 15.Beers MF, Chem JB. Inhibition of cellular processing of surfactant protein C by drugs affecting intracellular pH gradients. J Biol Chem. 1996;271(24):14361–14370. doi: 10.1074/jbc.271.24.14361. [DOI] [PubMed] [Google Scholar]
- 16.Litao MK, Hayes D, Jr, Chiwane S, Nogee LM, Kurland G, Guglani L. A novel surfactant protein C gene mutation associated with progressive respiratory failure in infancy. Pediatr Pulmonol. 2017;52(1):57–68. doi: 10.1002/ppul.23493. [DOI] [PubMed] [Google Scholar]
- 17.Peca D, Boldrini R, Johannson J, Shieh JT, Citti A, Petrini S, et al. Clinical and ultrastructural spectrum of diffuse lung disease associated with surfactant protein C mutations. Eur J Hum Genet. 2015;23(8):1033–1041. doi: 10.1038/ejhg.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jhang WK, Park SJ, Lee E, Yang SI, Hong SJ, Seo JH, et al. The first successful heart-lung transplant in a Korean child with humidifier disinfectant-associated interstitial lung disease. J Korean Med Sci. 2016;31(5):817–821. doi: 10.3346/jkms.2016.31.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Sano K, Ogiwara N, Kobayashi N. A novel surfactant protein C L55F mutation associated with interstitial lung disease alters subcellular localization of proSP-C in A549 cells. Pediatr Res. 2016;79(1-1):27–33. doi: 10.1038/pr.2015.178. [DOI] [PubMed] [Google Scholar]
- 20.Gupta A, Zheng SL. Genetic disorders of surfactant protein dysfunction: when to consider and how to investigate. Arch Dis Child. 2017;102(1):84–90. doi: 10.1136/archdischild-2012-303143. [DOI] [PubMed] [Google Scholar]