Abstract
Dysregulation of the mechanistic target of rapamycin complex 1 (mTORC1) signaling leads to memory deficits and abnormal social behaviors in adults. However, whether mTORC1 is involved in critical periods of early learning remains largely unexplored. Our study addressed this question by investigating imprinting, a form of learning constrained to a sensitive period that supports filial attachment, in newborn chickens. Imprinting to virtual objects and sounds was assessed after acute manipulations of mTORC1. To further understand the role of mTORC1 during the critical period, structural plasticity was analyzed using DiOlistic labeling of dendritic spines. We found that mTORC1 is required for the emergence of experience-dependent preferences and structural plasticity within brain regions controlling behavior. Furthermore, upon critical period closure, pharmacological activation of the AKT/mTORC1 pathway was sufficient to rescue imprinting across sensory modalities. Thus, our results uncover a novel role of mTORC1 in the formation of imprinted memories and experience-dependent reorganization of neural circuits during a critical period.
Introduction
Imprinting, the experience-dependent acquisition of early preferences within a sensitive period1–3, shapes vital behaviors. In worms, imprinting to different odors underlies aversive and appetitive responses2,4. Adult salmons also rely on imprinted odors experienced in early life for navigating towards breeding sites in adulthood1,5. In precocial birds, imprinted memories support the rapid development of filial attachment3,6. Hence, across evolution, imprinting sculpts reproductive and survival behavioral traits. Understanding the mechanisms involved in this form of time-constrained learning can shed light on the principles controlling behavior and its developmental organization.
The mechanistic target of rapamycin complex 1 (mTORC1) plays a pivotal role in long-term memory formation7–12 and different forms of long-term synaptic plasticity7,13,14 in the adult brain. Potentiation of hippocampal synapses and memory consolidation in mice requires activation of mTORC17. Similarly, mTORC1-mediated local translation is required for synaptic plasticity in Aplysia15. The role of mTORC1 in early life is less understood. Yet, recently, it has been reported that mTORC1 signaling supports developmental learning in songbirds16. It is thus possible that mTORC1 mediates experience-dependent reorganization of neural circuits within critical periods. Here we asked if mTORC1-mediated plasticity is relevant for imprinting. To address this question, we investigated the role of mTORC1 in the formation of imprinted memories of newborn chickens where mTORC1-mediated plasticity can be tracked to specific brain regions controlling behavior.
In chickens, protein synthesis is required for the consolidation of imprinted memories17,18. Auditory imprinting, the acquisition of a preference for a given sound during the critical period19, is controlled by dephosphorylation of the eukaryotic translation initiation factor subunit 2 alpha (eIF2α)18. In contrast, the mechanisms regulating experience-dependent regulation of protein synthesis in visual imprinting remain unknown. The mTORC1 complex appears a good candidate to carry out this function given that it regulates translation through three of its downstream targets: the ribosomal protein S6 kinase (S6K)20, the eukaryotic translation initiation factor 4E-binding proteins (4E-BPs)21 and the eukaryotic elongation factor 2 kinase (eEF2K)22,23. Because of its relevance in memory formation in adulthood22,24 and its role in experience-dependent translation22,25, mTORC1 activation might underlie imprinting across sensory modalities.
We found that mTORC1 is recruited within the mediorostral nidopallium/mesopallium (MNM)26,27 and the intermediate medial mesopallium (IMM)28–30, forebrain regions involved in auditory and visual imprinting, respectively. This endogenous activation of mTORC1 was required for the acquisition of imprinted preferences and dendritic spine plasticity. On the other hand, acute pharmacological enhancement of mTORC1 signaling was able to recover imprinting beyond the critical period. These results provide evidence for a previously unknown role of mTORC1 in filial imprinting and reveal that targeting mTORC1 may rejuvenate plasticity upon critical period closure.
Results
mTORC1 controls long-term synaptic plasticity and memory consolidation24. Yet a major outstanding question is whether mTORC1 regulates critical periods in early life. Involvement of mTORC1 in early learning has been shown in juvenile birds, where mTORC1 is required for song learning16. Here, taking advantage of the precocial behavior of newborn chickens and rapid onset of imprinting, we addressed the role of mTORC1 during the critical period for imprinting by combining biochemical assays to assess endogenous activation of mTORC1, behavioral pharmacology and analysis of structural changes correlated to the learning process. In addition, we recovered the imprinting upon closure of the critical period.
Experience-dependent activation of mTORC1
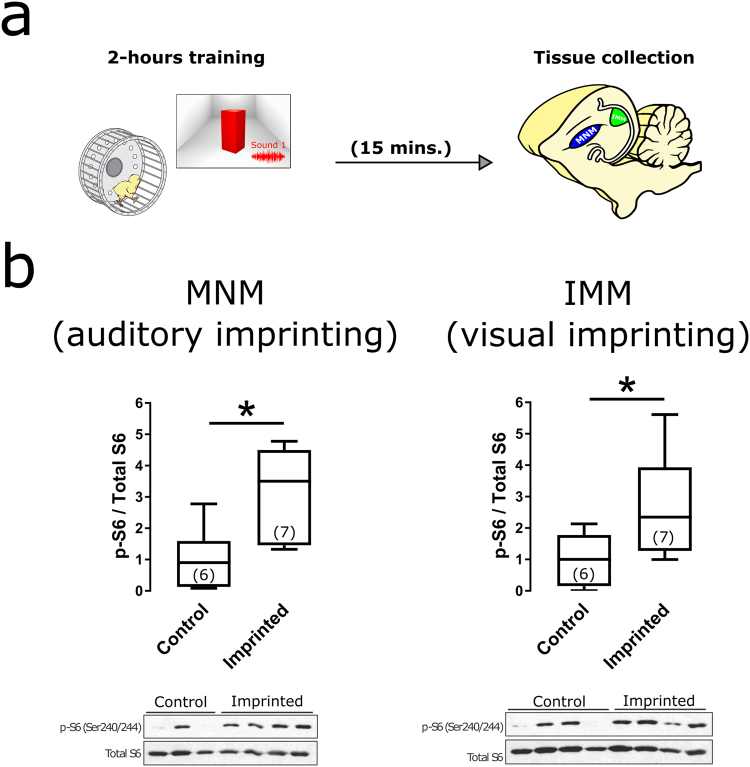
We first verified if mTORC1 was activated by training in MNM and IMM, forebrain areas crucial for auditory and visual imprinting, respectively3,26,30. While there are different read-outs of mTORC1 activation (e.g. 4E-BP phosphorylation), phosphorylation of the ribosomal protein S6 (p-S6) was chosen because it is highly conserved and has been previously used to assess learning-dependent activation of mTORC1 in mice7 and birds16. Endogenous activation of mTORC1 after training was investigated in both MNM and IMM (Fig. 1a), forebrain structures involved in auditory and visual imprinting, respectively31,32.
Figure 1.
Experience-dependent activation of mTORC1. (a) Tissue samples from IMM (green) and MNM (blue) were collected for western blotting, 15 minutes after training. (b) Phosphorylation of the S6 ribosomal protein in MNM (left) and IMM (right) was increased in trained (N = 7) chickens compared to controls (N = 6). Representative western blots are shown below each panel. Box plots show mean and interquartile. Error bars show 10–90 percentile, *indicates p < 0.05 from two-sample t-test. The number of animals used in each group is listed inside parenthesis. (Illustrations by Michael Beckert).
Western blots from MNM samples showed increased activation of mTORC1 compared to untrained animals, as indicated by the increased p-s6/total-S6 ratio (Fig. 1b) (Two-sample t-test, p = 0.0176). Thus, training activated mTORC1 signaling in the auditory imprinting-relevant area MNM. This effect could not be explained only by lack of movement in control animals since only chickens that significantly moved the wheel within the first half hour of training were included in the sample.
In addition, we examined mTORC1 activation in IMM, an area involved in visual imprinting3. Consistently, IMM samples from trained chickens also showed higher p-S6/total-S6 ratios than control animals (Fig. 1b) (Two-sample t-test, p = 0.0348). Therefore, the training method used to induce imprinting led to mTORC1 activation in both auditory and visual imprinting areas.
mTORC1 signaling is required for imprinting
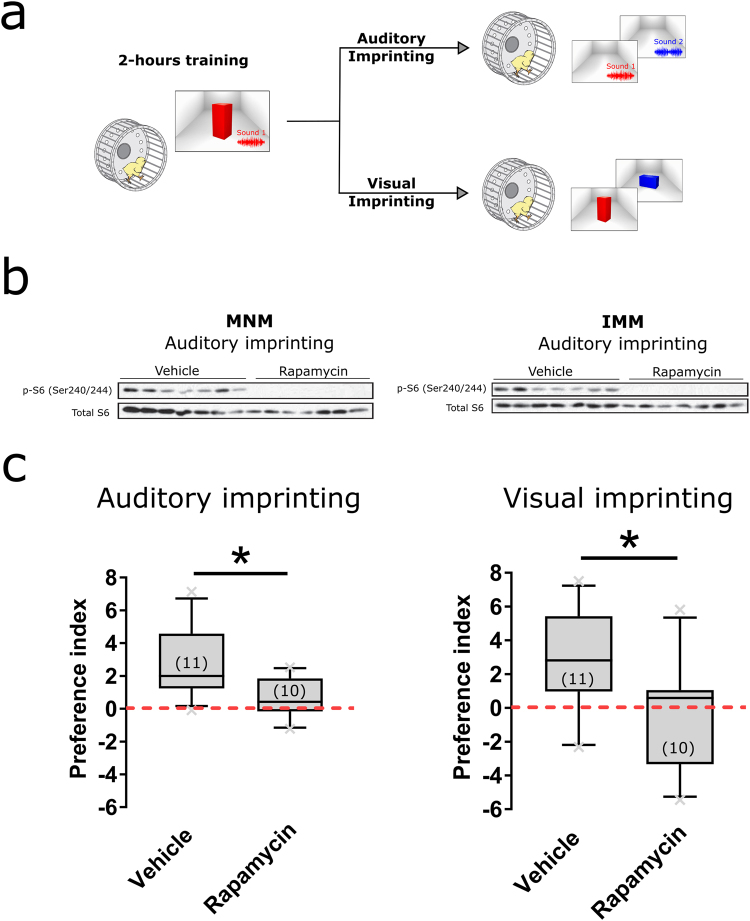
We tested whether inactivation of mTORC1 affects memory formation specifically. Imprinting develops rapidly (2-hour training), which allows acute pharmacological manipulations, reducing the potential confound of compensatory mechanisms induced by prolonged treatments. Taking advantage of these features we investigated whether activation of mTORC1 supports behavioral and structural plasticity in imprinting. To investigate whether mTORC1 activation is required for the formation of imprinted memories, we injected the mTORC1 inhibitor rapamycin to chickens, prior to training (Fig. 2a,b).
Figure 2.
mTORC1 blockade impairs auditory and visual imprinting. (a) Chickens were trained for two hours and injected either with rapamycin (N = 11) or vehicle (N = 10) prior to training. Visual and auditory imprinting were separately tested the day after training. (b) Rapamycin (N = 7) strongly reduced phosphorylation of S6 within MNM (left) and IMM (right) compared to vehicle-injected chickens (N = 7). (c) Vehicle- (N = 11), but not rapamycin-injected (N = 10) chickens showed auditory (left) and visual (right) imprinting the day after training. Box plots show mean and interquartile. Error bars show 10–90 percentile, *indicates p < 0.05 from two-sample t-test. Number of animals used for each group is inside parenthesis.(Illustrations by Michael Beckert).
Consistently with our western blot analysis, mTORC1 blockade abolished both auditory and visual imprinting (Fig. 2c) (Two sample t-test, pAuditory-imprinting = 0.0067, pVisual-imprinting = 0.0363). Chicks treated with rapamycin did not show a preference for the sound experienced during training (Fig. 2c). In contrast, vehicle-treated animals showed auditory imprinting 24 hours after training (Fig. 2c). Rapamycin also blocked visual imprinting (Fig. 2c). On the other hand, chickens injected with vehicle developed a normal preference for the object presented during training. Importantly, no change in basal locomotion was observed between vehicle- and rapamycin-injected chickens (Supplementary Fig. S1a). Together our results demonstrate that long-term auditory and visual imprinting requires mTORC1 activation during the critical period.
mTORC1-mediated structural plasticity
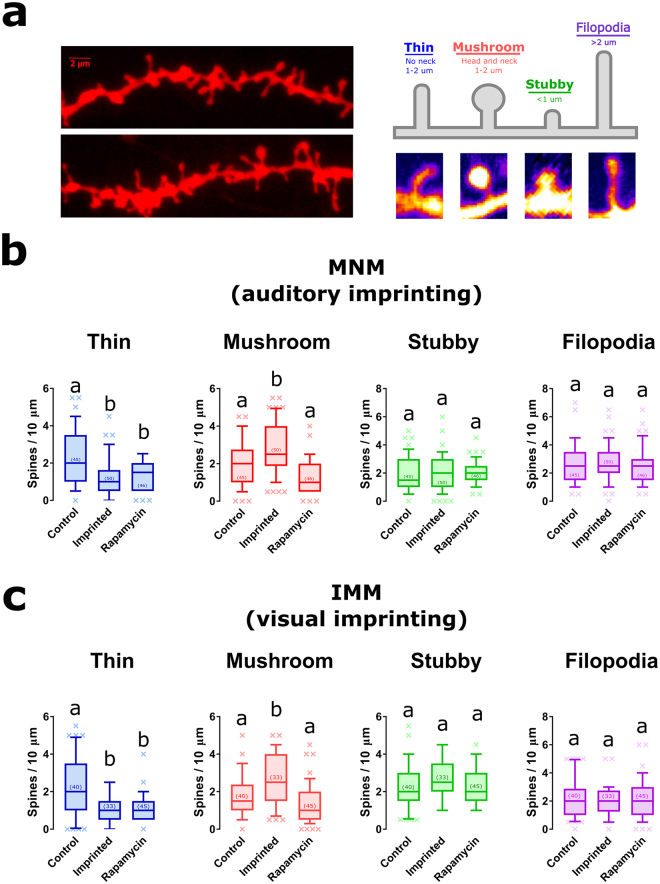
Long-term changes in the organization of neural circuits are hypothesized to support memory storage33. Hence, to shed light on how mTORC1 could underlie imprinting, we analyzed experience-dependent alterations in spine morphology, the cellular compartment receiving most excitatory inputs34. To achieve this, we combined DiOlistic labeling and confocal imaging of dendrites within MNM and IMM (Fig. 3a).
Figure 3.
Structural plasticity in MNM and IMM is disrupted by mTORC1 inactivation. (a) Spines were imaged using DiOlistic labeling and confocal imaging (left) to image dendrites in IMM (top, left) and MNM (bottom, left). Dendritic spines were classified in thin, mushroom, stubby and filopodia based on length and presence or absence of a neck (top, right). Different groups can be distinguished under confocal imaging (bottom, right). (b,c) Imprinted chickens show a decrease in thin spines (blue) and an increase in mushroom spines (red) compared to control animals. Structural plasticity of mushroom spines was blocked in chickens treated with rapamycin. No changes in stubby (purple) or filopodia (green) were detected. Box plots show mean and interquartile. Error bars show 10–90 percentile; different letters indicate statistically significant differences (p < 0.05) between groups from Kruskal-Wallis test, Dunn’s multiple comparisons test. Number of dendrites analyzed for each group is inside parenthesis.
Because spine morphology is informative about the functional state of the embedded synapses34, we classified them in stubby, filopodia, thin and mushroom spine types (Fig. 3). To test whether structural plasticity of spines required mTORC1 signaling, we injected chickens with either vehicle or rapamycin prior to training and tissue processing.
As previously shown18, imprinting triggered an increase in the number of mushroom spines in MNM and IMM that was paralleled with a reduction in thin spines. In MNM, rapamycin blocked the increase in mushroom spines (Fig. 3b) (Kruskal-Wallis test, Dunn’s multiple comparisons test, p < 0.001). However, the reduction in the number of thin spines after training was not blocked by rapamycin (Fig. 3b) (Kruskal-Wallis test, Dunn’s multiple comparisons test, p = 0.037). On the other hand, no changes in filopodia or stubby spines were detected in rapamycin-treated animals or controls.
Rapamycin injection also blocked structural plasticity in IMM. In chickens where mTORC1 was inactivated, IMM neurons did not exhibit an increase in the number of mushroom spines (Fig. 3c) (Kruskal-Wallis test, Dunn’s multiple comparisons test, p < 0.001) compared to the control group. Yet, as in MNM, rapamycin-treated chickens showed fewer thin spines after training, compared to untrained ones (Fig. 3c) (Kruskal-Wallis test, Dunn’s multiple comparisons test, p < 0.01). No significant changes in the number of stubby or filopodia spines were observed after training in IMM (Fig. 3b,c). These results indicate that mTORC1 activation is required for structural plasticity in both MNM and IMM.
mTORC1 activation is recovers imprinting after closure of the critical period
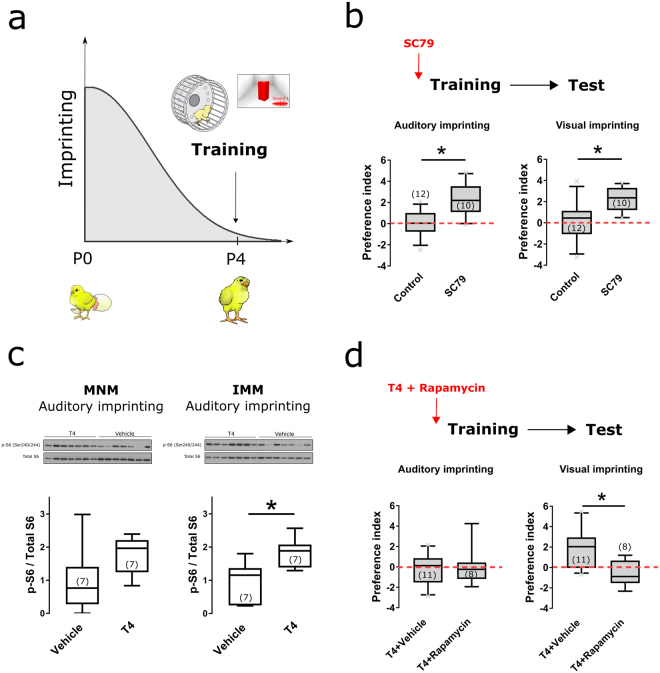
Because blockade of mTORC1 impaired both behavioral and structural plasticity, we aimed to test whether enhancing mTORC1 activity could restore plasticity upon closure of the critical period (Fig. 4a). Four-day old chickens, which are unable to become imprinted18,29, were injected with the AKT activator SC7916,35. We found that this manipulation recovered imprinting in both sensory modalities (Fig. 4b) (Two sample t-test, pAuditory-imprinting = 0.0015, pVisual-imprinting = 0.0085). These results indicate that mTORC1 activation is sufficient to restore plasticity upon critical period closure. No significant difference in basal locomotion between vehicle and SC79-injected groups was detected (Supplementary Fig. S1b).
Figure 4.
mTORC1 underlies recovering of imprinting upon closure of the critical period. (a) Chickens were trained on P4, when the critical period is closed and mTORC1 activation was manipulated to assess its role in the critical period. (b) Chickens were injected with the AKT activator SC79 before training. Activation of mTORC1 restored both auditory (left) and visual (right) imprinting (NControl = 12, NSC79 = 10). (c) Injections of the thyroid hormone thyroxine (T4) enhanced mTORC1 signaling in IMM (right) but not MNM (left) (NVehicle = 7, NT4 = 7). (d) Injections of T4 on P4 had no effect on auditory imprinting (left). Re-opening the critical period for visual imprinting by thyroid hormone (T4) also requires mTORC1 activation (right) (NT4 = 11, NT4+Rapamycin = 8). Box plots show mean and interquartile. Error bars show 10–90 percentile, *indicates p < 0.05 from two-sample t-test. Number of animals used for each group is inside parenthesis (Illustrations by Michael Beckert).
To further examine the ability to recover imprinting after the critical period by manipulation of the mTORC1 pathway, we explored other potential ways for mTORC1 activation. Thyroid hormones, in particular thyroxine (T4), have been shown to restore visual imprinting in P429. As shown in Fig. 4c, systemic injections of T4 strongly activated mTORC1 in IMM but not significantly in MNM (Two sample t-test, pIMM = 0.0092, pMNM = 0.10). Thus, we asked whether mTORC1 was required for the T4-dependent reopening of the critical period for visual imprinting. In fact, T4 rescued visual imprinting in P4 chickens but did not affect auditory imprinting (Fig. 4d). Remarkably, T4-dependent recovery of visual imprinting was abolished if mTORC1 was inactivated with rapamycin (Fig. 4d) (Two sample t-test, p = 0.0037). Basal locomotion was not different across experimental groups (Supplementary Fig. S1c).
Together these findings demonstrate the requirement of mTORC1 in auditory and visual imprinting. Furthermore, we show that pharmacological enhancement of mTORC1 could be a novel strategy to reopen critical periods.
Discussion
The mechanistic target of rapamycin complex 1 (mTORC1) regulates a variety of cellular functions, ranging from autophagy to proliferation21. Activation of this protein complex in the brain mediates neuronal plasticity7,14,36,37, long-term memory formation7,8,38, biological rhythms39,40 and social behaviors41–43. While most studies aimed to uncover mTORC1 function in the adult brain, here we demonstrated that mTORC1 activation is required for imprinting and circuit re-organization during a critical period in newborn chicks. Furthermore, we showed that imprinting can be recovered, once the critical period is closed, through acute enhancement of mTORC1 signaling.
mTORC1 is recruited within imprinted circuits
Western blot analysis showed that exposure to virtual objects and artificial sounds activated mTORC1 in forebrain areas relevant for visual and auditory imprinting. This activation was required for visual and auditory, imprinting. However, our approach is not able to identify the contribution of specific neuronal types to the observed increase in the p-S6/Total-S6 ratio, which could contribute to variability across samples. Future studies should target mTORC1 activation within specific cell-types (e.g. inhibitory interneurons). This might be particularly important given that, as shown in the visual cortex of mice44, maturation of parvalbumin positive (PV) cells sets the onset of sensitive periods. Similarly, these neurons seem to be also important for imprinting. An experience-dependent increase in the number of PV-cells after filial imprinting has been reported45. It is possible that mTORC1 is heavily involved in the maturation of PV-cells. This hypothesis is likely since genetic ablation of PTEN, an inhibitor of mTORC1 activation, increases the proportion of PV-neurons in the mouse neocortex46.
mTORC1 mediates structural plasticity of dendritic spines, the main site receiving excitatory inputs in neurons34. Changes in spine morphology are tightly associated with potentiation and depression of synaptic function47 and learning experiences48. These changes are indicative of circuit reorganization48. In particular, spine enlargement and acquisition of mushroom shape, is associated with increased AMPA currents49 and longer spine stability50,51. We found that rapamycin injections blocked the increase in mushroom spines detected 24 hours after imprinting training in both MNM and IMM. Similarly, mTORC1 appears to mediate spine plasticity in cultured neurons52. While spine plasticity might suggest that mTORC1-mediated post-synaptic changes are important for imprinting, mTORC1 could also be a key player in regulating pre-synaptic processes, as shown previously in the hippocampus of mice14. Notably, training triggered a reduction in the number of thin spines. This process resonates with a form of homeostatic plasticity described in the hippocampus53, where potentiation of a subset of spines is counterbalanced by synapse elimination within the same dendrites. In our case, the experience-dependent reduction of thin spines was not blocked by mTORC1 inactivation, suggesting that this process is mTORC1 independent. It would be interesting to assess if in the hippocampus, rapamycin blocks spine potentiation but not synapse elimination. This might be the case since manipulation of mTORC1 in cultured neurons particularly affects structural plasticity of mushroom spines but not of other subtypes52.
Protein synthesis regulation by mTROC1: a potential downstream mechanism underlying imprinting
The downstream mechanisms controlled by mTORC1 to support imprinting remain unclear. However, it is likely that mTORC1-mediated protein synthesis, through S6K, 4E-BPs and eEF2K21,24, underlies critical period plasticity. mTORC1 phosphorylates S6K and 4E-BPs to regulate translation initiation21,54, a mechanism known to mediate memory consolidation in adults7,22. On the other hand, mTORC1 can control the elongation of nascent polypeptide chains through phosphorylation of eEFK215. In Aplysia, the elongation factor 2 (eEF2) supports long-term facilitation of synaptic function even when translation initiation is blocked25. Because of its potential for controlling translation at different levels, mTORC1 might be a ‘master’ regulator of experience-dependent protein synthesis necessary for imprinting. In contrast, other translational control mechanisms involved in imprinting, such as dephosphorylation of the translation initiation factor eIF2α18, may have more subtle and specific roles. For instance, eIF2α-mediated translation regulates auditory, but not visual, imprinting18. To better understand how experience recruits eIF2α and mTORC1 within imprinted circuits, species tractable by genetic approaches might be required, such as C. elegans, where imprinting has also been shown2,4.
Re-opening critical periods through translational control mechanisms
Mechanisms controlling experience-dependent protein synthesis during critical periods can offer novel routes to rejuvenate plasticity. Enhancing eIF2α-mediated translation, for example, can recover auditory imprinting in chickens after the critical period18. Since mTORC1 can control protein synthesis, its manipulation could hold promise for restoring critical period plasticity across species. Consolidation of ocular dominant plasticity (ODP), the experience-dependent reorganization of visual binocular responses in the primary visual cortex (V1) of mammals, requires mTORC1 activation during sleep55. However, no attempt to recover ODP through mTORC1 activation after critical period closure has been pursued55. Our results demonstrate that direct enhancement of AKT/mTORC1 signaling is sufficient to restore visual and auditory imprinting upon critical period closure. Yet, these data cannot rule out that AKT activation by SC79 restores imprinting through downstream targets other than mTORC1. In addition, we showed that mTORC1 is recruited in the reopening of the critical period by thyroxine, one of the thyroid hormones capable of restoring visual imprinting29. Interestingly, during the critical period for birdsong acquisition in zebra finches, thyroid hormones levels increase56, as well as mTORC1 activation in response to the tutor’s song16. Our results raise the intriguing question of whether thyroid hormones also engage mTORC1 in songbirds to support behavioral plasticity. It remains untested whether activation of mTORC1 can rescue song learning outside of the critical period.
mTORC1 as key regulator of complex behaviors during development
In addition to its role in imprinting, mTORC1 may be important in time-constrained learning supporting the development of other brain functions, such as social behaviors. Yet the lack of early behavioral readouts in most vertebrates has stalled progress in understanding the early roles of mTORC1. Recently, in one of the few studies investigating mTORC1 in juveniles, it was shown that repeated inactivation of mTORC1 in songbirds impairs acquisition of the tutor’s song16. This result is consistent with our findings showing that rapamycin injections block imprinting. However, while acute rapamycin administration inactivates mTORC157, repeated exposure to rapamycin also reduces the activity of the mTOR complex 2 (mTORC2)58, involved in actin remodeling regulation59,60. Thus, in the case of songbird learning16 it has no yet been ruled out that both mTOR complexes have a role in the behavioral plasticity during the critical period. The single-trial nature of imprinting in chickens allowed us to acutely perform gain- and loss-of-function manipulations on mTORC1, which is less likely to affect mTORC2 signaling. Yet, mTORC2 might still play a role in imprinting. To address this important question, specific genetic manipulations and pharmacological tools will be required.
Conclusions
Together, our results demonstrate that mTORC1 mediates behavioral and structural plasticity in newborn chickens, supporting the idea that mTORC1 controls the storage of long-term memories since birth. We also showed that pharmacological activation of mTORC1 could help to reinstruct brain function after critical windows are closed, opening a potential avenue for therapeutic interventions to recover brain plasticity.
Methods
Animals
White Leghorn chicken eggs (E14-17) were received from a vendor (Charles River supplier) every week and incubated (Grumbach incubator, compact S84) at 37–38 °C in darkness until hatching. Humidity and temperature were constantly monitored to ensure proper embryonic development and normal hatching. On hatching day, chicks were transferred to individual compartments inside a brooder (Brinsea TLC-5). Temperature was kept at 37–38 C, and chickens were reared in the dark until the time of the experiments. Because chickens are capable of eating and drinking in darkness, water and food was provided ad libitum. This method of rearing does not affect health, locomotion and sensory acuity of chicks29. All the experiments were approved by the institutional animal care committee (IACUC) at Albert Einstein College of Medicine (protocol 20140910). All experiments were performed in accordance with relevant guidelines and regulations.
Imprinting training and preference test
Experiments and analysis described in this section were performed blind to treatment, between 8 AM to 6 PM. Both female and male chicks were used. In this study these data were pooled. To isolate chickens from environmental noise, training and tests were carried inside a sound proof chamber (IAC acoustics) at 37 °C. The day of the experiment, subjects were first placed under a white light for 30 minutes. This step has been proven to enhance imprinting training efficiency61. After priming with light, chickens were moved inside a running wheel equipped with a ring of magnets to assess locomotion (Med Associates). The wheel (internal diameter = 18 cm) was connected to a counter and a motherboard (Med Associates, DIG-700G, DIG-726). Data was stored in a computer for offline analysis.
In front of the running wheel, a monitor (ACER LCD, 17′′) and a speaker were placed. Videos of animated figures, blue or red, were generated using the open source program Blender (http://www.blender.org/). Animated objects were designed to move inside a virtual environment. Both animated objects covered the same area and followed identical trajectories on the screen. Expansion and contraction animation effects were added, matching the timing of sounds employed to test auditory imprinting. Two distinct sounds (labeled ‘sound 1’ and ‘sound 2’) were generated using Audacity software (2.1.0). The frequency range (0–3 KHz) was the same for all sounds, within the chickens’ audible range. Sound 1 contained sharp frequency steps and sound 2 frequency sweeps (ramps). A command script written in Matlab controlled the Med Associates equipment through a USB DAQ card (National instruments USB-6008). Each sound was played 12 times per minute, with an inter-stimulus interval of 3 seconds.
Training consisted in the presentation of audiovisual stimuli in bouts of 4 minutes with an inter-bout interval of 1 minute. During intervals, the speaker remained silent and the room was in complete darkness. If chickens were inactive within the first half-hour of the training, they were not considered for this study (N = 3). Training length was 120 minutes for all experiments and imprinting was tested one day after training.
Independent assessment of auditory and visual imprinting was achieved with a sequential test, where novel and imprinted stimuli were presented in alternation, in contrast to other studies that have used a simultaneous choice test29. The presentation of stimuli in sequence during tests allowed us to randomize stimulus presentation, measure baseline locomotion and evaluate the response to novel and imprinted stimuli independently. Each test included 5 presentations of the imprinted stimulus and 5 presentations of the novel stimulus. The duration of each presentation was 1 minute. The amount of locomotion inside the running wheel when no stimulus was presented, was measured during 30-second time windows immediately preceding each trial. This value, referred here to as baseline locomotion, was included in the preference score metric as a normalization factor. Imprinted and novel stimuli were alternated over 5 consecutive blocks. The first stimulus that started the sequence was picked randomly. Randomization of stimulus presentations prevents the emergence of biases and motivation changes over time. Importantly, no innate biases for the stimuli used was detected (Supplementary Fig. S2).
Our computation of the preference indexes normalized differences between locomotion to novel and imprinted stimuli by the average baseline locomotion in the wheel when no stimulus was presented. Therefore, to measure imprinting strength, we calculated a preference index (PI), PI = ∑(ImprintedSTL − NovelSTL)/BaselineA where STL indicates stimulus-triggered locomotion either during presentation of the imprinted stimulus (ImprintedSTL) or presentation of the novel stimulus (NovelSTL), and baselineA refers to the average baseline locomotion across the experiment. An advantage of this quantification over previous methods is that: (1) it considers basal locomotion before each stimulus presentation, and (2) it normalizes locomotion during stimulus presentation trials by average locomotion.
Pharmacological manipulation of mTORC1 activation
Rapamycin specifically blocks mTORC17,8. We used a rapamycin dosage shown to impair LTM when injected systemically before a 2-hour training (20 mg/Kg62, N = 10) diluted in 4% ethanol, 5% Tween-80 and 5% PEG-400. We confirmed mTORC1 blockade by rapamycin with Western blotting (Fig. 2b). Behavioral measures of imprinting were performed 24 hours after training. Vehicle-injected chicks were used for comparison (N = 11).
To enhance mTORC1 signaling outside of the critical period (P4) we employed two pharmacological agents: (1) we used the AKT activatorSC7916,35,63 (0.04 mg/g, N = 10) diluted in diluted in 5% Tween-80 and 5% PEG-400 to enhance mTORC1 activity; and (2) Thyroxine (30 µg/100 gr, N = 11), which is known to extend the sensitive period for visual imprinting29, after verification that such manipulation strongly activates mTORC1 within IMM (Fig. 4c). SC79-treated group was compared with vehicle-injected chickens (N = 12). To evaluate if the previously reported29 T4-mediated re-opening of the critical period requires mTORC1 activation, we administered both T4 and rapamycin (N = 8) to P4 chicks before training. All drugs used in our study were injected systemically (i.p.) 30 minutes prior to training.
Western blotting
Lysates of IMM and MNM (anatomical boundaries described below) were obtained from brain tissue, punched out from 0.75- to 1 mm-thick sagittal brain slices collected from imprinted (N = 7) and control (N = 6) animals. We used antibodies against the phosphorylated ribosomal protein p-s6 (Cell Signaling Cat #2215) and total s6 protein (Cell Signaling Cat #2217), following standard protocols described before64. Control tissue samples were obtained from chickens that ran on the wheel towards a screen displaying only a static image of an empty room.
Dendritic spine analysis
Chicks were euthanized, and brains were rapidly removed and sank in PFA (4%) for 1 hour before being transferred to PBS. Fixation period was set to 1 hour because over-fixation damages the cellular membrane, affecting DiI spread. Sagittal slices (200 µM thick) were made with a vibratome (Leica VT 1000S). The DiOlistic technique was used to obtain sparse labeling of neurons. In brief, tungsten beads coated with a lipophilic dye (DiI) were delivered with a modified gene gun. After allowing the DiI to spread for 24 hours at room temperature, slices were mounted using ProLong Gold mounting media. Confocal imaging (Zeiss LSM 510 Meta Duo V2) was used to collect Z-stacks (63×, zoom 3) from dendritic arbors of MNM and IMM neurons. The criteria to define regions of interest within a dendrite were based on distance from the soma (50–75 µm), always after the first dendritic bifurcation.
All images were taken blind to experimental groups and treatment. IMM is located 2.5 mm from the dorsal surface of the forebrain and 0.5–1 mm from the caudal edge, limited below and laterally by the lateral ventricle. MNM stereotaxic coordinates are: 0.5–1 mm lateral from the midline, 3 mm ventral from the dorsal surface of the brain and 5 mm rostral from the caudal edge of the forebrain, below the lateral pallial lamina that separates the hyperpallium and mesopallium65. All compared samples were processed the same day, using the same protocol, and images were taken with equal microscope settings. As in other comparative testing, control animals were housed in the same conditions as trained animals but presented with a screen displaying the static image of a virtual empty room.
Image J software (Version 1.50a) was used to count spines in each group by a subject blind to treatments. To enhance spine contrast, a multicolored lookup table (Fire) was used. Two 10 μm segments were marked randomly along each secondary dendritic branch. Spines along each of the two segments were counted by a blind experimenter. The spines‘ head width, presence of neck and overall length were used for classifying them in stubby (length <1 μm), thin (1 <length <2 μm, no clear head or neck), mushroom (1< length <2 μm, head and neck) and filopodia (length >2 μm)34,66,67.
Statistical analyses
SigmaPlot (Systat Software) was used for statistical analysis. Data distribution normality was assessed using the Shapiro-Wilk and F-test to evaluate differences in variance. When variance was significantly different across datasets, the Welch’s correction was used. Statistics were based on the two-sided Student’s t test, or the two-way ANOVA and Bonferroni post-hoc test for multiple comparisons of normally distributed samples. Otherwise the Mann-Whitney or the Kruskal-Wallis and Dunn’s multiple comparisons tests were used. Within-group variation is indicated by box plots showing the mean, interquartile and 10–90 percentiles (error bars) for each distribution. p < 0.05 was the criterion for significance.
Electronic supplementary material
Acknowledgements
We thank Dan Sanes, Suzanne Zukin, Saleem Nicola, Bryen Jordan and Tiago Goncalves for their critical discussion and comments on the study. We also thank Michael Beckert for helping with illustrations. This work was supported by the Konishi Neuroethology Research Award to GB, by NIH grant number DC007690 and a pilot grant from the Rose F Kennedy Intellectual and Developmental Disabilities Research Center (RFK-IDDRC) to JLP and grants from the National Institutes of Health to MCM (NIMH 096816, NINDS 076708).
Author Contributions
G.B.- performed all behavioral experiments, extraction of tissue samples and diolistic labeling, designed the mTORC1 loss- and gain- of function experiments, helped with the interpretation of the behavioral and western blotting results, wrote the manuscript with input from all other authors; J.L.J.- performed all the western blots, helped with experimental design; E.D.- counted, measured and classified dendritic spines; M.C.M.- designed the mTORC1 loss- and gain- of function experiments, helped with the interpretation of the behavioral and western blotting results, wrote the manuscript with input from all other authors; J.L.P.- writing original draft, project administration, writing review and editing, conceived and designed the study, directed and supervised the experiments, wrote the manuscript with input from all other authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-26479-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gervasio Batista, Email: gervasio.batista@phd.einstein.yu.edu.
Jose L. Pena, Email: jose.pena@einstein.yu.edu
References
- 1.Nevitt GA, Dittman AH, Quinn TP, Moody WJ. Evidence for a peripheral olfactory memory in imprinted salmon. Proc. Natl. Acad. Sci. USA. 1994;91:4288–92. doi: 10.1073/pnas.91.10.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remy J-J, Hobert O. An interneuronal chemoreceptor required for olfactory imprinting in C. elegans. Science. 2005;309:787–90. doi: 10.1126/science.1114209. [DOI] [PubMed] [Google Scholar]
- 3.Horn G. Pathways of the past: the imprint of memory. Nat. Rev. Neurosci. 2004;5:108–20. doi: 10.1038/nrn1324. [DOI] [PubMed] [Google Scholar]
- 4.Jin X, Pokala N, Bargmann CI. Distinct Circuits for the Formation and Retrieval of an Imprinted Olfactory Memory. Cell. 2016;164:632–43. doi: 10.1016/j.cell.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bett NN, Hinch SG, Dittman AH, Yun S-S. Evidence of Olfactory Imprinting at an Early Life Stage in Pink Salmon (Oncorhynchus gorbuscha) Sci. Rep. 2016;6:36393. doi: 10.1038/srep36393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insel TR, Young LJ. The neurobiology of attachment. Nat. Rev. Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- 7.Stoica L, et al. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc. Natl. Acad. Sci. USA. 2011;108:3791–6. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jobim PFC, et al. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol. Learn. Mem. 2012;97:105–12. doi: 10.1016/j.nlm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Tischmeyer W, et al. Rapamycin-sensitive signalling in long-term consolidation of auditory cortex-dependent memory. Eur. J. Neurosci. 2003;18:942–50. doi: 10.1046/j.1460-9568.2003.02820.x. [DOI] [PubMed] [Google Scholar]
- 10.Bekinschtein P, et al. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol. Learn. Mem. 2007;87:303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Dash PK, Orsi SA, Moore AN. Spatial Memory Formation and Memory-Enhancing Effect of Glucose Involves Activation of the Tuberous Sclerosis Complex-Mammalian Target of Rapamycin Pathway. J. Neurosci. 2006;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puighermanal E, et al. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat. Neurosci. 2009;12:1152–8. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- 13.Tang SJ, et al. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. USA. 2002;99:467–72. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Younts TJ, et al. Presynaptic Protein Synthesis Is Required for Long-Term Plasticity of GABA Release. Neuron. 2016;92:479–492. doi: 10.1016/j.neuron.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCamphill, P. K., Ferguson, L. & Sossin, W. S. A decrease in eukaryotic elongation factor 2 phosphorylation is required for local translation of sensorin and long-term facilitation in Aplysia. J. Neurochem. 10.1111/jnc.14030 (2017). [DOI] [PubMed]
- 16.Ahmadiantehrani S, London SE. Bidirectional manipulation of mTOR signaling disrupts socially mediated vocal learning in juvenile songbirds. Proc. Natl. Acad. Sci. 2017;114:9463–9468. doi: 10.1073/pnas.1701829114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibbs M, Lecanuet J. Disruption of imprinting by memory inhibitors. Anim. Behav. 1981;29:572–580. doi: 10.1016/S0003-3472(81)80120-0. [DOI] [Google Scholar]
- 18.Batista, G., Johnson, J. L., Dominguez, E., Costa-Mattioli, M. & Pena, J. L. Translational control of auditory imprinting and structural plasticity by eIF2α. Elife5 (2016). [DOI] [PMC free article] [PubMed]
- 19.Scheich H. Neural correlates of auditory filial imprinting. J. Comp. Physiol. A. 1987;161:605–19. doi: 10.1007/BF00603664. [DOI] [PubMed] [Google Scholar]
- 20.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 21.Laplante M, Sabatini DMM. mTOR signaling in growth control and disease. Cell. 2012;149:274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buffington SA, Huang W, Costa-Mattioli M. Translational Control in Synaptic Plasticity and Cognitive Dysfunction. Annu. Rev. Neurosci. 2014;37:17–38. doi: 10.1146/annurev-neuro-071013-014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thoreen CC, et al. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–13. doi: 10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graber TE, McCamphill PK, Sossin WS. A recollection of mTOR signaling in learning and memory. Learn. Mem. 2013;20:518–530. doi: 10.1101/lm.027664.112. [DOI] [PubMed] [Google Scholar]
- 25.McCamphill PK, Farah CA, Anadolu MN, Hoque S, Sossin WS. Bidirectional regulation of eEF2 phosphorylation controls synaptic plasticity by decoding neuronal activity patterns. J. Neurosci. 2015;35:4403–17. doi: 10.1523/JNEUROSCI.2376-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bock J, Braun K. Blockade of N-methyl-D-aspartate receptor activation suppresses learning-induced synaptic elimination. Proc. Natl. Acad. Sci. USA. 1999;96:2485–90. doi: 10.1073/pnas.96.5.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bock J, Thode C, Hannemann O, Braun K, Darlison MGG. Early socio-emotional experience induces expression of the immediate-early gene Arc/arg3.1 (activity-regulated cytoskeleton-associated protein/activity-regulated gene) in learning-relevant brain regions of the newborn chick. Neuroscience. 2005;133:625–33. doi: 10.1016/j.neuroscience.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 28.Nakamori T, et al. Demonstration of a neural circuit critical for imprinting behavior in chicks. J. Neurosci. 2010;30:4467–80. doi: 10.1523/JNEUROSCI.3532-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi S, et al. Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat. Commun. 2012;3:1081. doi: 10.1038/ncomms2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCabe BJ, Horn G, Bateson PP. Effects of restricted lesions of the chick forebrain on the acquisition of filial preferences during imprinting. Brain Res. 1981;205:29–37. doi: 10.1016/0006-8993(81)90717-4. [DOI] [PubMed] [Google Scholar]
- 31.Wallhäusser E, Scheich H. Auditory imprinting leads to differential 2-deoxyglucose uptake and dendritic spine loss in the chick rostral forebrain. Brain Res. 1987;428:29–44. doi: 10.1016/0165-3806(87)90080-0. [DOI] [PubMed] [Google Scholar]
- 32.McCabe BJ, Davey JE, Horn G. Impairment of learning by localized injection of an N-methyl-D-aspartate receptor antagonist into the hyperstriatum ventrale of the domestic chick. Behav. Neurosci. 1992;106:947–53. doi: 10.1037/0735-7044.106.6.947. [DOI] [PubMed] [Google Scholar]
- 33.Poo M, Stevens C. What is memory? The present state of the engram. BMC Biol. 2016;14:40. doi: 10.1186/s12915-016-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17:381–6. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 35.Jo H, et al. Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc. Natl. Acad. Sci. USA. 2012;109:10581–6. doi: 10.1073/pnas.1202810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raab-Graham KF, Haddick PCG, Jan YN, Jan LY. Activity- and mTOR-dependent suppression of Kv1.1 channel mRNA translation in dendrites. Science. 2006;314:144–8. doi: 10.1126/science.1131693. [DOI] [PubMed] [Google Scholar]
- 37.Slipczuk L, et al. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons, R. G., Gafford, G. M. & Helmstetter, F. J. Translational Control via the Mammalian Target of Rapamycin Pathway Is Critical for the Formation and Stability of Long-Term Fear Memory in Amygdala Neurons. J. Neurosci. 26 (2006). [DOI] [PMC free article] [PubMed]
- 39.Cao R, et al. Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling. Neuron. 2013;79:712–24. doi: 10.1016/j.neuron.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao R, Lee B, Cho H-Y, Saklayen S, Obrietan K. Photic regulation of the mTOR signaling pathway in the suprachiasmatic circadian clock. Mol. Cell. Neurosci. 2008;38:312–24. doi: 10.1016/j.mcn.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang G, et al. Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gkogkas CG, et al. Autism-related deficits via dysregulated eIF4E-dependent translational control. Nature. 2013;493:371–7. doi: 10.1038/nature11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber KM, Klann E, Costa-Mattioli M, Zukin RS. Dysregulation of Mammalian Target of Rapamycin Signaling in Mouse Models of Autism. J. Neurosci. 2015;35:13836–42. doi: 10.1523/JNEUROSCI.2656-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugiyama S, et al. Experience-Dependent Transfer of Otx2 Homeoprotein into the Visual Cortex Activates Postnatal Plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 45.Nakamori T, Kato T, Sakagami H, Tanaka K, Ohki-Hamazaki H. Regulation of visual Wulst cell responsiveness by imprinting causes stimulus-specific activation of rostral cells. Sci. Rep. 2017;7:42927. doi: 10.1038/srep42927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogt D, Cho KKA, Lee AT, Sohal VS, Rubenstein JLR. The Parvalbumin/Somatostatin Ratio Is Increased in Pten Mutant Mice and by Human PTEN ASD Alleles. Cell Rep. 2015;11:944–956. doi: 10.1016/j.celrep.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishiyama J, Yasuda R. Biochemical Computation for Spine Structural Plasticity. Neuron. 2015;87:63–75. doi: 10.1016/j.neuron.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamprecht R, LeDoux J. Structural plasticity and memory. Nat. Rev. Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 49.Matsuzaki M, et al. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 2001;4:1086–92. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parnass Z, Tashiro A, Yuste R. Analysis of spine morphological plasticity in developing hippocampal pyramidal neurons. Hippocampus. 2000;10:561–568. doi: 10.1002/1098-1063(2000)10:5<561::AID-HIPO6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 51.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 52.Henry FE, Hockeimer W, Chen A, Mysore SP, Sutton MA. Mechanistic target of rapamycin is necessary for changes in dendritic spine morphology associated with long-term potentiation. Mol. Brain. 2017;10:50. doi: 10.1186/s13041-017-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourne JN, Harris KM. Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus. 2011;21:354–373. doi: 10.1002/hipo.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–45. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seibt J, et al. Protein Synthesis during Sleep Consolidates Cortical Plasticity In Vivo. Current Biology. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi S, et al. Sex Differences in Brain Thyroid Hormone Levels during Early Post-Hatching Development in Zebra Finch (Taeniopygia guttata) PLoS One. 2017;12:e0169643. doi: 10.1371/journal.pone.0169643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–9. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 58.Sarbassov DD, et al. Prolonged Rapamycin Treatment Inhibits mTORC2 Assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 59.Huang W, et al. mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat. Neurosci. 2013;16:441–8. doi: 10.1038/nn.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou H, Huang S. The complexes of mammalian target of rapamycin. Curr. Protein Pept. Sci. 2010;11:409–24. doi: 10.2174/138920310791824093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolhuis J, Cook S, Horn G. Getting better all the time: improving preference scores reflect increases in the strength of filial imprinting. Anim. Behav. 2000;59:1153–1159. doi: 10.1006/anbe.2000.1413. [DOI] [PubMed] [Google Scholar]
- 62.Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol. Learn. Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bangi E, Murgia C, Teague AGS, Sansom OJ, Cagan RL. Functional exploration of colorectal cancer genomes using Drosophila. Nat. Commun. 2016;7:13615. doi: 10.1038/ncomms13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Costa-Mattioli M, et al. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puelles, L., Martinez-de-la-Torre, M., Paxinos, G., Watson, C. & Martinez, S. The chick brain in stereotaxic coordinates. (Elsevier, 2007).
- 66.Chakravarthy S, et al. Postsynaptic TrkB signaling has distinct roles in spine maintenance in adult visual cortex and hippocampus. Proc. Natl. Acad. Sci. USA. 2006;103:1071–6. doi: 10.1073/pnas.0506305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanders J, Cowansage K, Baumgärtel K, Mayford M. Elimination of dendritic spines with long-term memory is specific to active circuits. J. Neurosci. 2012;32:12570–8. doi: 10.1523/JNEUROSCI.1131-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.