Abstract
The nano-cellulose derived nano-biofilm keeps a magnificent role in medical, biomedical, bioengineering and pharmaceutical industries. Plant biomaterial is naturally organic and biodegradable. This study has been highlighted as one of the strategy introducing biomass based nano-bioplastic (nanobiofilm) to solve dependency on petroleum and environment pollution because of non-degradable plastic. The data study was carried out to investigate the nano-biopolymer (nanocellulose) based nano-biofilm data from corn leaf biomass coming after bioprocess technology without chemicals. Corn leaf biomass was used to produce biodegradable nano-bioplastic for medical and biomedical and other industrial uses. Data on water absorption, odor, pH, cellulose content, shape and firmness, color coating and tensile strength test have been exhibited under standardization of ASTM (American standard for testing and materials). Moreover, the chemical elements of nanobiofilm like K+, CO3−−, Cl−, Na+ showed standard data using the EN (166).
Keywords: Nanocellulose, Nanobiofilm, Nanobioplastic, Biodegradable, Corn leaf
Specification table
| Subject area | Biological chemistry, Biochemistry |
| More specific subject area | Nanocellulose based nanobiofilm bioplastic from plant biomass |
| Type of data | Physicochemical, mechanical (Table and Figure) |
| How data were acquired | SEM, pH meter, spectrophotometer, Tensile test, absorption test, burning test, bore, shape and size test, chemical test by ASTM and EN standard. |
| Data format | Row data were collected and analyzed |
| Experimental factors | Single factor |
| Experimental features | Three replicates were used in the experiment as Randomized Complete Design (CRD). The sample was selected randomly from the different lots. |
| Data source location | Kuala Lumpur, Malaysia and Hail, KSA |
| Data accessibility | This is an innovative data, not yet published elsewhere. |
Value of the data
-
1.
Data have been highlighted bearing innovative information on nano-biofilm or other definite biomaterials for medical, biomedical and bioengineering industries from corn leaf biomass.
-
2.
Data exhibited a outstanding and an innovative research. Data would be a valuable to the related researcher and academician, on nano-biofilm production using plant biomass as plant biomaterial.
-
3.
Data investigated the appropriate quality of nano-cellulose based nano-biofilm plant biomaterial production using agro-biomass according to the ASTM (American standard for testing and materials) and EN (European Norms) standardization.
-
4.
Data can be explored for the future studies in the related research community all over the world.
1. Data
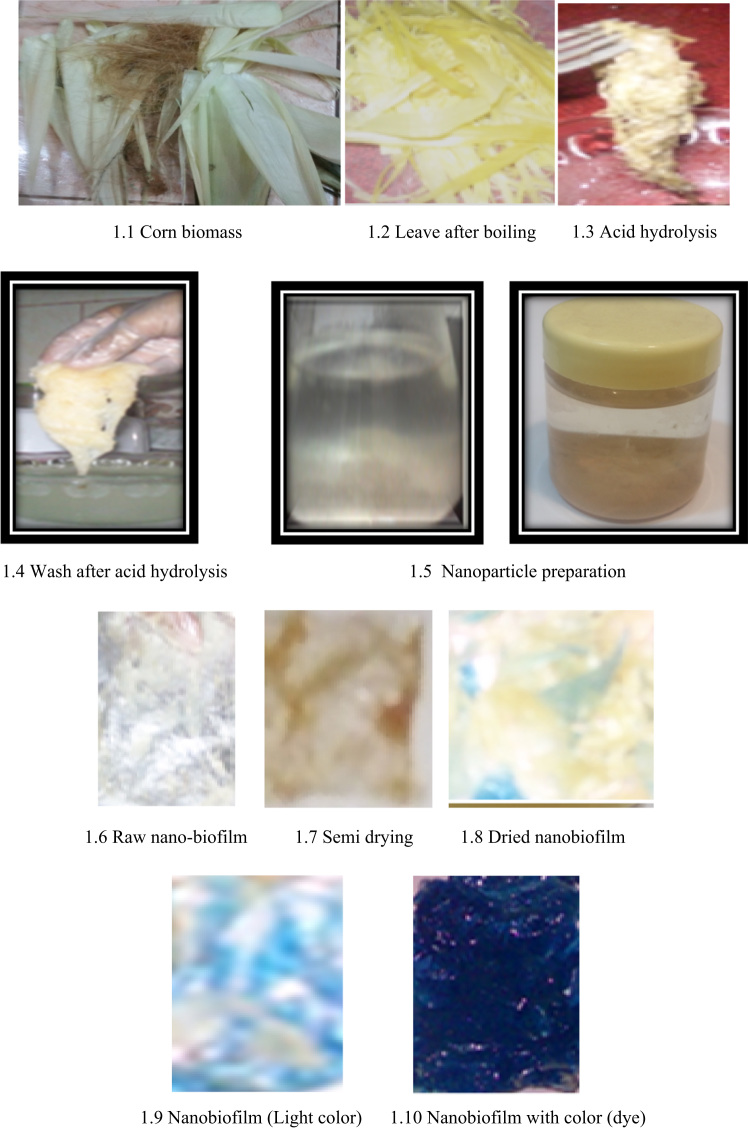
Data show the nano-biofilm production procedure derived from nanocellulose based corn leaf biomass (Fig. 1). Data observe the nanosized biofilm as nanocellulose detected by Transmission Electron Microscopy (TEM) (Fig. 2) (Table 1). In Table 2, data exhibit the negligible water percent absorbed by nanobiomaterial based nano-biofilm. Moreover, data of the odor, color of flame and speed of burning represented by burning test were no odor, yellow–orange flame and slow speed of burning respectively which were under the standardization of burning test (Table 3). In addition, data describe on the color dying time for drying at different hours (Table 4). Table 5 shows the tensile strength and tensile modulus for the nano-bioplastic derived nanobiofilm. Data mentioned in Table 6, show the positive shape and size test for the nano-biofilm. In Table 7, data observe the value on pH and cellulose. In addition, chemical elements test data from nanobiofilm samples like K+, CO3−−, Cl−, Na+ were measured and represented data using the EN (166)) standardization (Table 8).
Fig. 1.
Nanobiofilm production procedure from corn leaf samples.
Fig. 2.
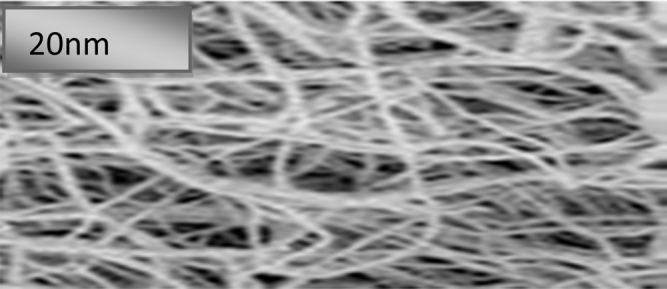
Nanocellulose size measurement.
Table 1.
Measurement of nanocellulose by Transmission Electron Microscopy (TEM).
| Materials | Nanocellulose size |
|---|---|
| Corn leaves sample as raw material of Nano-biofilm | 20 nm |
| Nanoparticle size | 1–100 nm |
Table 2.
Determination of water absorption by ASTM D570.
| Materials | Water absorption | ASTM D570 |
|---|---|---|
| Water absorption | ||
| Nano-biofilm | 0.03% | Water absorption by ASTM is 0–0.16%. |
| Synthetic film | 0–0.16% |
Table 3.
Odor test according to the ASTM D3801.
| Materials | Odor | Color of flame | Speed of burning | Spark or not |
|---|---|---|---|---|
| Nano-biofilm | No odor | Yellow–orange | Slow | Spark |
| Synthetic film | ||||
| By ASTM D3801 | Low odor | Yellow–orange | Slow | Spark |
Table 4.
Color coating dye was used as the mode of application by ASTM B 117.
| Materials | Coating dye test | ASTM B117 |
|---|---|---|
| (Drying time) | Maximum 2 h | |
| Nano-biofilm | 1.3 h | Max. 2 h |
| Synthetic film | 2 h (max). | |
| By ASTM B 117 |
Table 5.
Determination of tensile test by using ASTM by ASTM D5083.
| Materials | Tensile strength (MPa/kg.m3) | Tensile Modulus (GPa) |
|---|---|---|
| Nano-biofilm | 74.0 | 1.1 |
| Synthetic film | ||
| By ASTM D5083 | 70–230 (ASTM) | 1.0–3.0 (ASTM) |
Table 6.
Firmness test represented by bore and crack test.
| Shape, size and Bore and test |
||
|---|---|---|
| Materials | Firmness test | Firmness test Shape and size |
| Bore test ASTM D2925 | by ASTM D500 (Resistant Character) | |
| Nanobiofilm | No bore symptom | No swell or shrink |
| Synthetic film | No bore symptom | No swell or shrink |
Table 7.
pH and cellulose determination from corn waste biomass sample.
| Test | pH | Cellulose |
|---|---|---|
| Corn samples | 7.3 ± 0.01 | 65.5% ± 0.05 |
| Plastic film sample | Alkaline ≥ 7 | (It is zero if from gas or oil sample, if from cellulose sample it is up to 70%) |
Mean±SE (standard Error, n = 3).
Table 8.
Chemical element determination nanocellulose sample.
| Chemical Element | Corn sample based Biofilm (PPM) | Synthetic Plastic film By EN (European Standard EN166.) |
|---|---|---|
| K+ | 9.7 ± 0.5 | 10 |
| Na+ | 4.2 ± 0.2 | 5 |
| Cl− | 0.55 ± 0.01 | 2 |
| CO3−− | 139.1 ± 1.1 | 5–440 |
Mean ± SE (standard Error, n = 3).
2. Experimental design, materials and methods
2.1. Sample collection and preparation
Five kg corn stalk new leaves were collected from the farmers field, Kuala Lumpur Malaysia and Hail regional area, KSA. Leaves were randomly chosen from both area and removed from corn stalk and washed to clean. Washed leaves were sliced by scissors and boiled (Fig. 1). Then it was blended by blender. After blending it was again ground for fine mixing by motor and pestle and put it to the beaker.
2.2. pH determination
The pH was determined by using Horiba Scientific pH meter, Japan.
2.3. Cellulose determination
The quantitative determination of cellulose content from the corn sample was made by anthrone reagent [1]. A standard curve was drawn by measuring the absorbance of known concentration of cellulose solutions at 620 nm. Anthrone reagent consisted of acetic nitric reagent, 67% sulfuric acid, 10 ml anthrone solution. The tubes were then kept the boiling water bath for 16 min. The mixture (centrifuged sample 0.2 g/10 ml and chemical mixture) was then cooled in ice bath for 2–3 min and made it normal at room temperature. To measure cellulose content, 3 ml of unknown cellulose solution was filled into a test tube, followed by addition of 3 ml of anthrone reagent and its absorbance at 620 nm was measured.
2.4. Samples pyrolysis
Blended and ground sample was heated at 150 °C in pressure cooker for 2 hours at 30 psi until the sample become liquid paste. After heating the liquid fiber samples were cooled down. A 0.8% (w/v) sodium chlorite (NaClO2) solution and acetic acid were added to acidify the NaClO2 solution until the pH reached 4.5. The fibers were boiled in NaClO2 solution for 3 h at 70–80 °C whereby the ratio of fiber to NaClO2 solution was set to 1: 30. The bleaching process was repeated for five times until fiber became white and then filtered. After being filtered, the residue was washed for several times with distilled water and dried in air. The bleached cellulose obtained was heated to 70–80 °C in 5% (w/v) sodium sulfite solution for 2 h. The fibers were filtered, washed, and dried in the air. After being dried, the fibers were treated in 17.5% (w/v) sodium hydroxide (NaOH) solution for 2 h. The residue was washed for several times with distilled water.
2.5. Nano-particle preparation by acid hydrolysis
Fiber sample was hydrolyzed (100 ml/50 g sample) by hydrochloric acid (HCl 99% pure) to make it micro to nano size particle for 12 h. The water bath (60 °C) was used during the process of hydrolysis occurred. After 12 h the samples were separated by separation funnel and washed by distilled water five times (Fig. 1).
2.6. Nanoparticle measurement
Nano particle size was measured by Transmission electron microscopy (TEM) (Fig. 2). TEM images were obtained using a JEM-2100 transmission electron microscope operated at 120 kV. For TEM sample preparation, the nanocellulose particles were deposited on a carbon-coated grid by placing a drop of a very dilute cellulose nanofiber suspension on the grid and then allowed to dry in order to evaporate the liquid.
2.7. Plasticizer mixture
Acetic acid 5% (5 ml/100 g cellulose sample), 5 ml/100 g (polyvinyl chloride), and starch powder 20%, and 20% water were added with the 500 g of cellulose (65.5%) samples. Later 10 ml/100 g PVC (polyvinyl chloride) and glycerin (5 ml/100 g) were added with the mixture of cellulose samples and waited for 10 min to mix up well. Then the mixture was heated at 150 °C in the oven for 30 min at 30 psi pressure followed by pyrolysis method as ASTM standard until visual plasticity occurred in the oven for nanobioplastic film material. The raw nanobiofilm were taken it out from the oven and kept it in room temperature at 28 °C for cooling down for 10 min. Then raw nanobiofilm was put in the aluminum foils to make it dry for 30 min. Al last, semidried nano-bioplastic film was oven dried at 80 °C for 2 h. The completely dried nanobioplastic film was used for different tests to investigate the fitness.
2.8. Testing fitness
2.8.1. Absorption test (as ASTM D570) [2]
For the water absorption test, the specimens were dried in an oven for a specified time and temperature and then placed into the desiccator to cool. Immediately upon cooling the specimens were weighed. The material was then emerged in water at agreed upon conditions, often 23 °C for 48 h. Specimens were removed, patted dry with a lint free cloth and weighed (Fig. 3). The diameter of disk was 5 cm and 2 mm thick. Water absorption was calculated.
Fig. 3.
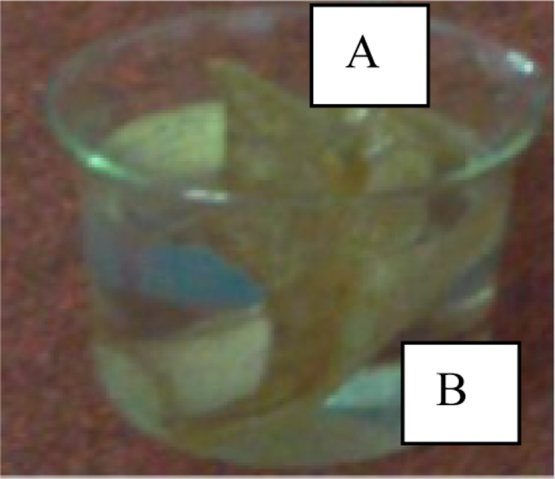
Absorption test. Nanobiofilm (A) in the water cylinder (B).
2.8.2. Odor test
It was burnt by using gas burner. Odor, color of flame, speed of burning and spark were observed by visual observation and compared with the synthetic bumper by ASTM D3801 (Fig. 4).
Fig. 4.

Burning test.
2.8.3. Tensile/tension test
Tensile test was done by Universal Test Machine for bioPlastics as ASTM D5083 [3].
2.8.3.1. Test procedure
Specimens were placed in the grips of a Universal Test Machine at a specified grip separation and pulled until failure. For ASTM D5083 the test speed was measured by the material specification. The default test speed was 5 mm/min (0.2 in/min), but modulus determination was made at 1.5 mm/min (0.057 in/min). A strain gauge was used to determine the elongation and tensile modulus. Max Load Capacity was 50 kN/m2 depending upon the reinforcement and type.
2.8.3.2. Sample size
The standard specimen for ASTM [3] has a constant rectangular cross section, 25 mm (1 in) wide and at least 250 mm (10 mm) long. Thickness can be between 1.5 mm (0.057 in) and 14 mm (0.55 in). Optional tabs can be bonded to the ends of the specimen to prevent gripping damage. Intertek PTL can machine the specimens from larger samples and bond tabs if requested. Tensile Strength (MPa or PSI) was displayed from tensile test.
2.8.4. Color test
Spray coating dye was used as the mode of application. It was attached properly with plastic and dried after 1 h (Fig. 1).
2.8.5. Shape and size test
By the hammer it was continuously beaten for 2 min and pulled on for 5 min. There was no change of its shape and size.
2.8.6. Firmness test: (Bore test)
Bioplastic film was hit by the hammer of 1 kg on the screw set on the biofilm. Hit was completed for 5 min.
2.8.7. Chemical element test
Chemical element like K+, CO3−−, Cl−, Na+ were tested using different meters. K+ and Na+ were tested by LAQUA twin K+ meter and LAQUA twin Na+ meter (Horiba, Japan). CO3−, and Cl− were tested by Photometer PF-3,version A (Macherey-Nagel, Germany). In the case of all chemical elements positive results exhibited and compared to the synthetic plastic in the laboratory using the EN (166) standardization (Fig. 1) [4].
2.8.8. Statistical analysis
Randomized Complete Design (CRD). The sample was selected randomly from the different lots in the experiment. Standard deviation was calculated from the mean of the replicates and Standard error was analyzed from standard deviation using 3 replicates of the samples where necessary (n = 3) (n = replicate).
Acknowledgement
Authors are thankful to the Department of Biology, Faculty of Science, University of Hail for financial support for this project (UOH/S-12A). He is also thankful to his PhD and MS students, Biotechnology Program, Institute of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur, Malaysia for the assistance of this project.
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.dib.2018.02.053.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.David M.U. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969;2(9):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- 2.ASTM International Plastics. American Standards for Testing and Materials American National Standards Institute, 2013. 〈http://webstore.ansi.org/FindStandards.aspx?Action=displaydept&DeptID=1590&Acro=ASTM&DpName=ASTM〉 International: Plastics.
- 3.ASTM D5083. Tensile Testing of Reinforced Thermosetting Plastics. Intertek Plastics Technology Laboratories, 2012. 〈http://www.ptli.com/testlopedia/tests/Thermosetting-Tensile-ASTM.asp〉.
- 4.EC, European Commission. Nanomaterials. 18 October, 2011. 〈http://ec.europa.eu/atwork/programmes/index_en.htm〉.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material