Abstract
Ultraviolet (UV)-B radiation-induced root bending has been reported; however, the underlying mechanisms largely remain unclear. Here, we investigate whether and how auxin and flavonoids are involved in UV-B radiation-induced root bending in Arabidopsis using physiological, pharmacological, and genetic approaches. UV-B radiation modulated the direction of root growth by decreasing IAA biosynthesis and affecting auxin distribution in the root tips, where reduced auxin accumulation and asymmetric auxin distribution were observed. UV-B radiation increased the distribution of auxin on the nonradiated side of the root tips, promoting growth and causing root bending. Further analysis indicated that UV-B induced an asymmetric accumulation of flavonoids; this pathway is involved in modulating the accumulation and asymmetric distribution of auxin in root tips and the subsequent redirection of root growth by altering the distribution of auxin carriers in response to UV-B radiation. Taken together, our results indicate that UV-B radiation-induced root bending occurred through a flavonoid-mediated phototropic response to UV-B radiation.
Keywords: ultraviolet (UV)-B, root bending, tropism, auxin, flavonoids
Introduction
Ultraviolet (UV) radiation is classified according to wavelength as UV-A radiation (320–400 nm), UV-B radiation (280–320 nm), and UV-C radiation (200–280 nm). Among these types, UV-B radiation is of prime importance because it has severely damaging effects on plant growth and development despite its small proportion (1.5% of solar radiation reaching the surface of the earth) (Nawkar et al., 2013). It is therefore essential to investigate in detail the effects of UV-B radiation on various aspects of plant growth (Solomon, 2008).
Low doses of UV-B radiation stimulate signaling through the photoreceptor UV RESISTANCE LOCUS 8 (UVR8) (Rizzini et al., 2011). UVR8 interaction with CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) results in HY5-dependent transcriptional responses that induce the accumulation of secondary metabolites involved in protecting against UV, such as flavonoids and other phenolic compounds (Rice-Evans et al., 1997). Vandenbussche et al. (2014) found that UV-B-mediated phototropism in etiolated seedlings is regulated by both phototropin and UV-B photoreceptor UVR8, and UV-B mediates the down-regulation of the expression of auxin-responsive genes by UVR8 pathway.
In general, roots grow in the soil to fix the plants to the ground and absorb water and nutrients (Yokawa et al., 2016). However, in nature, besides during seed germination, the roots are often exposed to sunlight because of strong wind, earthquakes, artificial factors, or animal behaviors (Yokawa et al., 2016). Red light induces a positive phototropic response, whereas blue light induces a negative phototropic response in roots. Both phototropic responses in roots, especially the red-light-induced phototropic response, are weaker than the gravitropic response and thereby frequently masked by the gravitropic response (Ruppel et al., 2001). UV-B radiation induces positive root phototropic bending (Krasylenko et al., 2012); however, the underlying physiological and molecular mechanisms remain unclear.
The asymmetrical redistribution of auxin is considered a principle regulator of the directional growth response in plants (Ruppel et al., 2001; Gilroy, 2008). The auxin influx carrier AUXIN1/LIKE AUX1 (AUX1/LAX) and the auxin efflux carrier PIN-FORMED (PIN) proteins, involved in polar auxin transport (PAT), regulate auxin distribution in the root tip, thereby determining the orientation and extent of cell division in the root meristem as well as root pattern formation (Sabatini et al., 1999). A previous study indicated that auxin plays a fundamental role in UV-disturbed morphology (Ge et al., 2010).
In addition to auxin transport, auxin perception and response also play a role in modulating the root system architecture (RSA) response to environmental cues. The auxin signal transduction pathway is activated by the binding of auxin to its receptor TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX (TIR1/AFB), promoting the degradation of Aux/IAA transcriptional repressors, releasing auxin response factors (ARFs) and activating the expression of auxin-responsive genes (Gray et al., 2001; Liu et al., 2015). Dominant mutations in several auxin/indole-3-acetic acid (Aux/IAA) genes, such as axr2-1, axr3-1, and axr3-3 mutants, result in the inhibition of auxin signaling and disrupt root development (Gray et al., 2001; Nakamura et al., 2006). Yokawa et al. (2016) found that unilateral UV-B radiation (0.3 mW/cm2) induced auxin redistribution to the nonradiated side of roots. UV-B radiation affects different hormonal pathways in various ways, including biosynthesis, transport, and signaling (Vanhaelewyn et al., 2016). However, how the changes in auxin level in roots play a role in the response to UV-B stress remains to be determined.
It has been widely reported that UV-B radiation induces flavonoid production, and one of the proposed functions of flavonoids is to protect plants from potentially harmful UV irradiation (Kootstra, 1994). In fact, the UV-absorbing characteristics of flavonoids have long been considered evidence for the role of these molecules in UV protection. The purified flavonoids naringenin and rutin, as well as flavonoid extracts from apple skin, have been shown to prevent the accumulation of DNA damage (Kootstra, 1994), and plants with decreased levels of flavonoids are more sensitive to UV irradiation (Karabourniotis et al., 1992; Li et al., 1993; Winkel-Shirley, 2002).
Several studies on flavonoid mutants have also suggested a role for flavonoids in PAT (Stenlid, 1976; Jacobs and Rubery, 1988; Muday and DeLong, 2001; Biever et al., 2014). Jacobs and Rubery (1988) found that flavonoids can compete with the synthetic auxin transport inhibitor naphthylphthalamic acid (NPA) to perturb auxin transport. Yin et al. (2014) found that the flavonoid 3-O-rhamnoside-7-O-rhamnoside acts as an endogenous PAT inhibitor in Arabidopsis shoots. In addition, chalcone synthase (CHS)-deficient transparent testa (tt) mutants exhibit elevated auxin transport and altered growth phenotypes (Murphy et al., 2000). Santelia et al. (2008) also found that flavonoids promote asymmetric PIN shifts during gravity stimulation and thereby induce redirection of the basipetal auxin streams necessary for root bending. Silva-Navas et al. (2016) found that unilateral light induces the accumulation of flavonols to promote cell elongation and asymmetric growth in the root transition zone, suggesting that flavonols serve as positional signals. Kuhn et al. (2016) reported that F7RhaT (UGT89C1), a gene encoding a flavonol 7-O-rhamnosyltransferase, affects flavonol rhamnosylation and auxin metabolism, but not auxin transport. This suggests that flavonoids affect auxin distribution not only through the flavonoid-mediated auxin transport pathway but also through F7RhaT (UGT89C1)-modulated flavonol rhamnosylation and the auxin metabolism pathway. Recently, Kuhn et al. (2017) found that flavonols affect auxin transport by regulating PIN2 polarity downregulating PINOID activity.
The UV-B photoreceptor UVR8 can be expressed in roots, thereby conferring roots the ability to sense UV-B radiation (Mo et al., 2015). UV-B radiation-induced root bending toward the source of radiation has been reported in Arabidopsis and barley (Ktitorova et al., 2006; Krasylenko et al., 2012). However, the molecular mechanisms underlying this phenomenon remain largely unclear. The main aim of this work was to investigate the physiological and molecular responses of Arabidopsis roots to UV-B radiation. We found that UV-B radiation reduced auxin levels and led to an asymmetric distribution of auxin in root tips, which induced root bending. Further study indicated the involvement of flavonoids in the IAA-mediated root bending response to UV-B radiation.
Materials and Methods
Plant Growth and Chemical Treatments
The transgenic and mutant Arabidopsis thaliana lines used in this study include the following: DR5:GFP (Ulmasov et al., 1997), DII-VENUS (Brunoud et al., 2012), HS:AXR3NT-GUS (Gray et al., 2001), PIN1:PIN1-GFP (Benkova et al., 2003), PIN2:PIN2-GFP (Blilou et al., 2005), PIN3:PIN3-GFP (Žádníková et al., 2010), PIN7:PIN7-GFP (Blilou et al., 2005), AUX1:AUX1-YFP (Swarup et al., 2004), axr3-3 (CS57505), pin2 (CS8058), aux1-7 (CS9583), yucca (Zhao et al., 2001), uvr8-6 (Rizzini et al., 2011), and tt4-1 (Murphy et al., 2000). The transgenic and mutant lines were confirmed using polymerase chain reaction (PCR).
Arabidopsis seeds were surface sterilized with 50% (v/v) bleach (containing 5% hypochlorite) for 5 min and then rinsed five times with sterile deionized water. The surface-sterilized seeds were sown onto 1/2 Murashige and Skoog (MS) agar medium [Sigma-Aldrich; supplemented with 1% (w/v) agar and 1.5% (w/v) sucrose, pH 5.75] and incubated for 3 days (d) at 4°C in the dark to synchronize germination. The seedlings were grown vertically for 5 d under standard aseptic growth conditions at 22°C with a 16 h light/8 h dark photoperiod.
Ultraviolet-B irradiation was provided by a narrowband UV-B lamps (Philips, TL20W/01-RS, 311 nm) and placed before a vertical plate with no lid and that was covered with cellulose diacetate (0.13 mm, exclude wavelengths lower than 290 nm) to completely block potential UV-C radiation (Casati and Walbot, 2008; He et al., 2011). The desired radiation was obtained by altering the distance from the plate to the lamp. The irradiance was measured by a radiometer (BNU, UV-B, China).
Five-day-old Arabidopsis seedlings were irradiated with 1.6 W m-2 UV-B for 1 h (5.76 KJ m-2) in the presence of simultaneous white light (photon flux density of 100 μmol m-2 s-1) with the Philips daylight lamps and then transferred to normal growth conditions for phenotypic observations. All chemicals were obtained from Sigma-Aldrich.
GUS Staining
The beta-glucuronidase (GUS) histochemical staining was performed according to a previously described method (Hu et al., 2010). Seedlings harboring the GUS reporter gene were incubated at 37°C for 2 h in GUS staining solution with the substrate 1 mM X-Gluc (5-bromo-4-chloro-3-indolyl-β-D-GlcA cyclohexyl-ammonium). Before microscopic examination using a Zeiss Axioskop, the seedlings were incubated in 95% (v/v) ethanol to remove the chlorophyll. At least 20 seedlings were analyzed for each treatment. The experiments were repeated at least three times.
Flavonoid Fluorescence Staining
To measure flavonoid accumulation, we incubated 5-d-old Arabidopsis seedlings in 2-aminoethyl diphenylborinate (DPBA) staining solution containing 0.25% (w/v) DPBA and 0.005% (v/v) Triton X-100 for 5 min as described by Murphy et al. (2000). The seedlings were then washed for 5 min with 50 mM sodium phosphate buffer [plus 0.005% (v/v) Triton X-100, pH 7.0]. After excitation with 488 nm (argon) laser, the DPBA emission was collected at 570–650 nm ranges using LSM710 (Carl Zeiss confocal fluorescence microscope) (Silva-Navas et al., 2016).
Phenotypic Analysis
Ultraviolet-B radiation induced root bending upward from the surface of the medium, and the bending was relatively rigid. After treatment, the seedlings were carefully transferred to glass slides, and then the root tips were observed and photographed. The angle of root bending was quantified using Image J software. At least 60 replicates were measured for each treatment.
qRT-PCR Analysis
RNA was isolated from 5-d-old frozen Arabidopsis seedlings using RNAiso Plus (TaKaRa) according to the manufacturer’s instructions. The concentration of RNA was quantified spectrophotometrically using a NanoQuant spectrophotometer. Reverse transcription was then performed using the PrimeScriptTM RT Reagent Kit with gDNA Eraser (TaKaRa). SYBR-green quantitative reverse transcription (RT)-PCR was performed with Platinum® SYBR® Green qPCR SuperMix-UDG (Invitrogen). ACTIN2 (AT3G18780) and EF1a (AT5G60390) were used as internal controls for quantitative reverse transcription (qRT)-PCR normalization with GeNorm (Czechowski et al., 2005). The gene-specific primers are presented in Supplementary Table S1. Three independent biological replicates and three technical repetitions were performed for each gene. All primer pairs produced only one peak in the DNA melting curves, indicating high primer specificity.
Quantification of IAA
The IAA content was quantified according to Gao et al. (2014) and Liu et al. (2015). Root tips of approximately 0.1 g fresh weight were collected and immediately frozen in liquid nitrogen. After extraction, endogenous IAAs were purified, methylated in a stream of diazomethane gas, and resuspended in 100 μL of ethyl acetate. The endogenous IAA content was analyzed using GC/MS.
Statistical Analysis
For the fluorescence intensity analysis in the full root tips, we selected the full root tip to perform the intensity analysis. For the fluorescence intensity ratio on both sides of the root tips, we selected the left side and right side of the root tip to perform the intensity analysis, respectively, and obtained the intensity value of each side by confocal microscope. The laser power and gain values were kept the same all the time and the signals were below saturation. At least 15 roots were imaged per line for each of three repeats.
All experiments were repeated at least three times, and the results are presented as the means ± SE. The data were analyzed using Image-Pro Plus software (version 4.5.1.29; Media Cybernetics, Carlsbad, CA, United States) and SPSS (Statistic Package for Social Science) software, and the significance of differences was determined by Tukey’s test.
Results
UV-B Radiation Induces Positive Root Phototropic Bending
Several studies have demonstrated that UV-B-induced root bending could occur under natural situation (Ktitorova et al., 2006; Krasylenko et al., 2012). Ktitorova et al. (2006) found that unilateral UV-B radiation (1 W m-2 for 15 min) in barley significantly induced root bending toward the source of radiation. To determine the effects of UV-B radiation on root bending in Arabidopsis, 5-d-old seedlings grown vertically in 1/2 MS medium were subjected to 1–12 W m-2 of UV-B radiation in the presence of simultaneous white light for 15 min (0.9–10.8 KJ m-2). The samples were then transferred to a growth chamber maintained at 22°C under white light (photon flux density of 0.1 mmol m-2 s-1) for 4 h. We found that UV-B radiation caused the roots to bend upward from the surface of the medium and in the direction of the irradiation (Supplementary Figures S1a,b). The roots could not bend when the plate was covered with lid which excludes UV-B radiation, confirmed that root bending phenotype is resulted from UV-B radiation (data not shown).
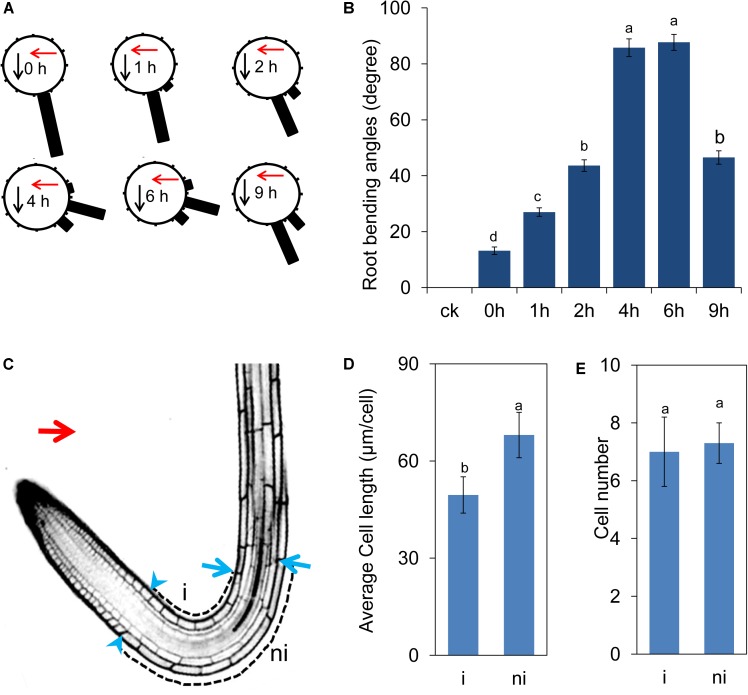
Ultraviolet-B radiation induced root bending in a dosage-dependent manner (Supplementary Figure S1a). We then tested the root bending angles using 1.6 W m-2 UV-B radiation for 15 min–2 h (Supplementary Figure S1c). The angles of root bending peaked when we used 1.6 W m-2 UV-B radiation for 1 h and then transferred to a growth chamber for 4 h (the average angle was 75.56 ± 2.36) (Supplementary Figure S1c). Therefore, we selected the radiation dosage (1.6 W m-2 UV-B radiation for 1 h) for subsequent experiments. After the 1.6 W m-2 UV-B radiation for 1 h, the roots have begun to slightly bending toward the direction of UV-B radiation (0 h after 1 h-treatment) and the angles of root bending peaked at approximately 4–6 h after UV-B radiation and then gradually decreased with root elongation (Figures 1A,B).
FIGURE 1.
UV-B radiation induces root bending. Five-d-old Arabidopsis seedlings grew on vertical plates, irradiated with UV-B for 1 h and then transferred to normal growth conditions for another 9 h. (A,B) The angle of root bending was measured after 0–9 h of treatment, n = 60. The red arrow indicates the direction of UV-B-radiation. (A) Each gravistimulated root was assigned to 1 of the 12 30° sectors on a gravitropism diagram. The length of each bar represents the mean percentage of seedlings assigned to the respective sector. The red and black arrows indicate the direction of UV-B radiation and gravity, respectively. (B) The average root bending angles; ck, before UV-B radiation control. (C–E) UV-B represses cell elongation during UV-B-mediated phototropic response of Col-0 roots (C). The blue arrowheads and arrows represent the last meristematic cells and the cells where the turn initiated in the illuminated (i) and nonilluminated (ni) sides, respectively. The red arrow indicates the direction of UV-B radiation. The average cell length (D) and number of cells (E) in illuminated (i) and nonilluminated (ni) sides. The error bars represent the ±SE, and different letters indicate significantly different values (P < 0.01 by Tukey’s test).
To better understand the nature of root bending in response to UV-B radiation at the cellular level, we measured the length and number of cells on either side of the location of root bending. The cells on the nonradiated side were longer than those on the radiated side (Figures 1C,D), indicating that UV-B radiation induced a rapid response in cell elongation, thereby resulting in differential root growth on either side of the roots. However, the cell number on either side of the location of root bending was not significantly different, indicating that UV-B radiation-induced root bending did not affect cell proliferation between the two sides (Figure 1E).
Auxin Is Involved in UV-B-Mediated Root Bending
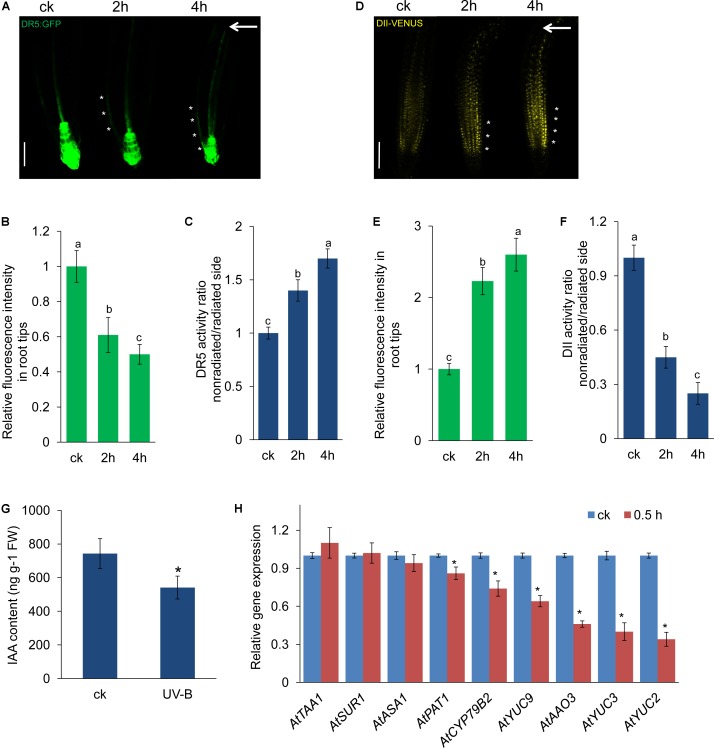
Auxin plays a key role in modulating root growth (Petrášek and Friml, 2009; Baldwin et al., 2013), and root bending in response to UV-B radiation raised the question of whether auxin is involved in this process. Therefore, we examined auxin response using auxin-responsive DR5:GFP marker lines. We found a significant reduction in the expression of DR5:GFP in UV-B-treated roots (Figures 2A,B). Interestingly, we found that UV-B radiation also led to an asymmetric distribution of DR5:GFP fluorescence in the root tips. As shown in Figures 2A,C, UV-B radiation significantly increased the DR5:GFP fluorescence on the nonradiated side of roots. To confirm this finding, we also analyzed IAA perception in root tips using a transgenic line expressing the VENUS protein fused to Aux/IAA–auxin interaction domain II (DII-VENUS) (Brunoud et al., 2012). In the transgenic line, the VENUS signal showed a dose-dependent response to auxin (Brunoud et al., 2012), and UV-B radiation resulted in a dramatic increase in nuclear DII-VENUS fluorescence, suggesting that UV-B radiation reduced IAA perception in root tips (Figures 2D,E). Similarly, we found lower DII-VENUS fluorescence on the nonradiated side of the root–apex transition and elongation zones compared with the radiated side (Figures 2D,F). These results are consistent with Yokawa et al. (2016) and indicate that UV-B radiation led to an asymmetric auxin distribution in the root tips.
FIGURE 2.
UV-B radiation affects auxin distribution in root tips. (A–F) GFP/YFP fluorescence in the roots of 5-d-old DR5:GFP (A) or DII-VENUS (D) seedlings exposed to UV-B radiation for 1 h and then transferred to normal growth conditions for 2–4 h, quantification of DR5:GFP (B) or DII-VENUS (E) fluorescence intensities in root tips, and ratios of DR5:GFP (C) or DII-VENUS (F) signal in the root tips on the nonradiated side versus the radiated side are shown. Bars, 60 μm. White arrows indicate the direction of UV-B radiation. White asterisks indicate more pronounced DR5:GFP signals (A) on the nonradiated side of roots or more pronounced DII-VENUS signals (D) on the radiated side; ck, before UV-B radiation control. The error bars represent the ±SE. Different letters indicate significantly different values (P < 0.05 by Tukey’s test). (G) IAA contents in the roots of wild-type seedlings exposed to UV-B radiation for 1 h and then transferred to normal growth conditions for 2 h. (H) Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) of the expression of auxin biosynthesis-related genes in the wild-type seedlings exposed to UV-B radiation for 1 h and then transferred to normal growth conditions for 0.5 h. The expression levels of the indicated genes in untreated roots (ck) were set to 1. The error bars represent the ±SE. Asterisks (∗) indicate significant differences with respect to the corresponding control (P < 0.01 by Tukey’s test).
We then investigated whether UV-B radiation affects the IAA concentration in roots. As shown in Figure 2G, the IAA level of treated seedlings decreased by 27.2% compared with that of untreated seedlings. To investigate whether these UV-B radiation-reduced IAA concentrations were due to decreased IAA biosynthesis, we performed qRT-PCR to estimate the transcript levels of genes encoding key enzymes in the auxin biosynthesis pathway. The qRT-PCR results demonstrated that UV-B radiation decreased the transcript levels of several IAA biosynthesis genes, including YUCCA2 (YUC2), YUC3, YUC9, ABSCISIC ALDEHYDE OXIDASE3 (AAO3), CYTOCHROME P450 (CYP79B2), and ARABIDOPSIS HOMOLOG OF YEAST PAT1 (PAT1), whereas the gene expression of TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1), SUPERROOT 1 (SUR1), and ATP SULFURYLASE ARABIDOPSIS1 (ASA1) was unaffected (Figure 2H). These data suggest that UV-B radiation resulted in down-regulation of IAA biosynthesis, which affected the auxin concentration in the roots.
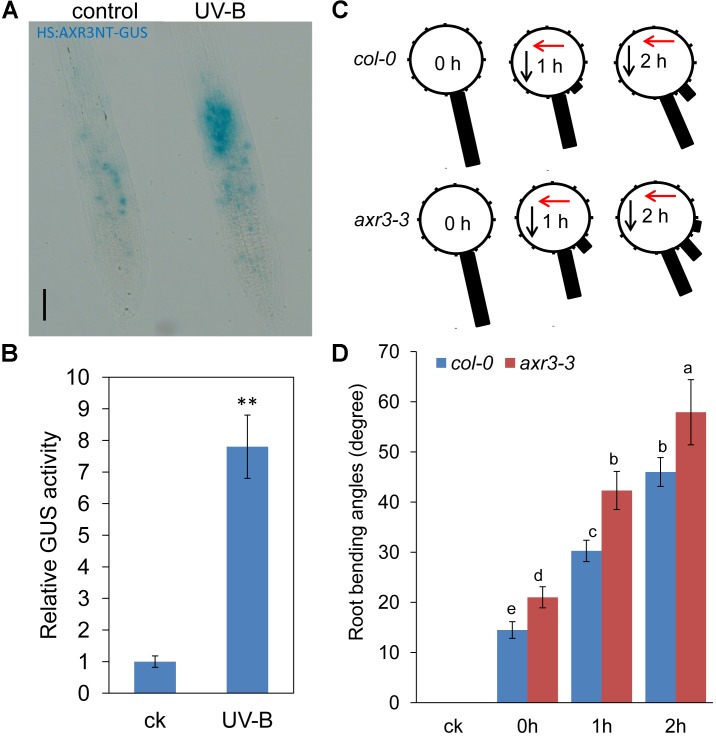
We next employed the HS:AXR3-GUS reporter line (Gray et al., 2001) to examine the effects of UV-B radiation on Aux/IAA stabilization. After heat shock, the AXR3-GUS signal was significantly increased in UV-B-treated roots (Figures 3A,B). These data suggest that UV-B radiation impeded auxin signaling by stabilizing Aux/IAA proteins. To further verify whether Aux/IAA proteins are involved in UV-B-induced root bending, we examined root bending in the gain-of-function axr3-3 mutant after exposure to UV-B radiation. The axr3-3 seedlings exhibited less suppression of PR growth after UV-B radiation than did Col-0 seedlings, indicating that AXR3 is involved in UV-B-induced PR growth inhibition (Supplementary Figure S2). The angles of root bending in axr3-3 seedlings were 39.8% higher after 1 h and 25.9% higher after 2 h compared with those in the Col-0 control seedlings subjected to UV-B radiation (Figures 3C,D), suggesting that the reduced auxin signaling through the increased stability of AXR3 protein increases UV-B-induced root bending.
FIGURE 3.
UV-B radiation increases the stabilization of Aux/IAA. (A) Image of GUS-staining of the roots of 5-d-old HS:AXR3NT-GUS seedlings. Seedlings were heat shocked at 37°C for 2 h, treated with or without UV-B radiation for 1 h, and then transferred to normal growth conditions for 45 min at 23°C, followed by GUS staining. Bars, 60 μm. (B) Relative GUS activity of HS:AXR3NT-GUS, as treated in (A). The error bars represent the SE. Asterisks (∗∗) indicate significant differences with respect to the corresponding control (P < 0.01 by Tukey’s test). (C,D) The angle of root bending of Col-0 and axr3-3 seedlings irradiated with UV-B for 1 h and then transferred to normal growth conditions for 0–2 h; ck, before UV-B radiation control. Col-0, n = 60; axr3-3, n = 60. (C) Each gravistimulated root was assigned to 1 of the 12 30° sectors on a gravitropism diagram. The length of each bar represents the mean percentage of seedlings assigned to the respective sector. The red and black arrows indicate the direction of UV-B radiation and gravity, respectively. (D) The average root bending angles of Col-0 and axr3-3 seedlings. The error bars represent the ±SE, and different letters indicate significantly different values (P < 0.01 by Tukey’s test).
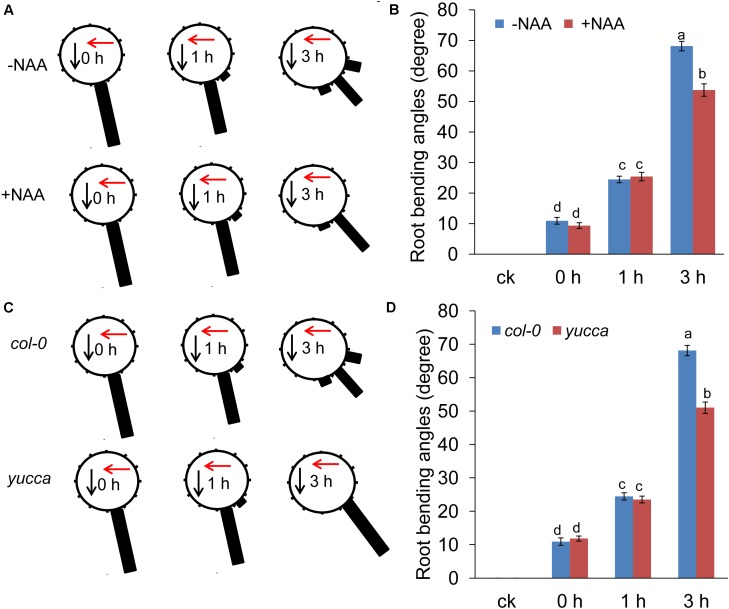
The results presented above suggest that UV-B radiation reduced IAA accumulation in the root tips; this decreased level of auxin may be responsible for the root bending observed in the UV-B-treated seedlings. We tested this hypothesis by applying exogenous auxin. Supplementation with naphthaleneacetic acid (NAA) alleviated UV-B-induced PR growth inhibition (Supplementary Figure S3a) and alleviated UV-B-induced root bending (the average angle was 21% lower in NAA-supplemented roots compared with unsupplemented seedlings after 3 h of treatment) (Figures 4A,B). To confirm this finding, we also analyzed the root bending of yucca, an auxin over-producing mutant (Zhao et al., 2001), upon UV-B radiation. Consistent with the NAA treatment, the yucca mutant showed less suppression of PR growth after UV-B radiation (Supplementary Figures S3b,c) and significantly reduced root bending after exposure to UV-B radiation compared with the wild-type control (the average angles of root bending in yucca seedlings were 25% lower after 3 h of treatment compared with the Col-0 control seedlings subjected to UV-B radiation) (Figures 4C,D).
FIGURE 4.
Auxin is involved in UV-B-induced root bending. (A,B) The angle of root bending of the wild-type Col-0 seedlings irradiated with UV-B for 1 h and then transferred to normal growth conditions plus 0 nM NAA or 10 nM NAA for 0–3 h, n = 60. (A) Each gravistimulated root was assigned to 1 of the 12 30° sectors on a gravitropism diagram. The length of each bar represents the mean percentage of seedlings assigned to the respective sector. (B) The average root bending angles. (C,D) The angle of root bending of Col-0 and yucca seedlings irradiated with UV-B for 1 h and then transferred to normal growth conditions for 0–3 h. Col-0, n = 60; yucca, n = 60. (C) Each gravistimulated root was assigned to 1 of the 12 30° sectors on a gravitropism diagram. The length of each bar represents the mean percentage of seedlings assigned to the respective sector. (D) The average root bending angles of Col-0 and yucca seedlings. The red and black arrows indicate the direction of UV-B radiation and gravity, respectively; ck, before UV-B radiation control. The error bars represent the ±SE, and different letters indicate significantly different values (P < 0.01 by Tukey’s test).
AUX1 and PIN2 Are Involved in Decreased Auxin Accumulation and Asymmetric Auxin Distribution in Root Tips
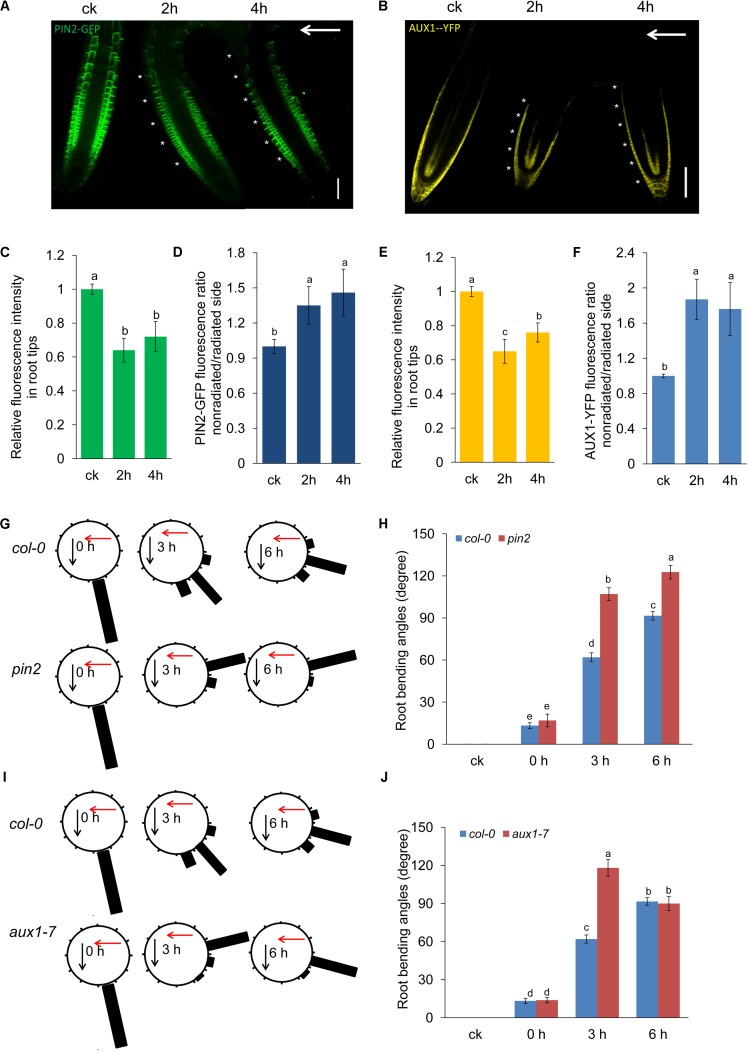
Mutants related to auxin transport, such as aux1 and pin2, exhibit a defect in phototropic or gravitropic responses (Petrášek and Friml, 2009; Baldwin et al., 2013; Cui et al., 2013). Therefore, UV-B-induced root bending could be modulated by auxin carriers. To investigate this possibility, we examined auxin carrier levels using transgenic lines expressing AUX1:YFP, PIN1:GFP, PIN2:GFP, PIN3:GFP, and PIN7:GFP. We found that UV-B radiation markedly repressed the abundance of PIN2 and AUX1 (Figures 5A,B,D,E), whereas the abundance of PIN1, PIN3, and PIN7 was largely unaltered (Supplementary Figure S4). Furthermore, we found that signals for both PIN2:GFP and AUX1:YFP were stronger on the nonradiated side of the roots (Figures 5A,C,D,F).
FIGURE 5.
PIN2 and AUX1 are involved in UV-B-induced root bending. GFP/YFP fluorescence in the roots of 5-d-old PIN2:GFP (A) or AUX1:YFP (D) seedlings exposed to UV-B radiation for 1 h and then transferred to normal growth conditions for 2 and 4 h, quantification of PIN2:GFP (B) or AUX1:YFP (E) fluorescence intensities in root tips and ratios of PIN2:GFP (C) or AUX1:YFP (F) signals in the root tip on the nonradiated side versus the radiated side are presented. Bars, 50 μm. White arrows indicate the direction of UV-B radiation. White asterisks indicate more pronounced PIN2:GFP (A) or AUX1:YFP (D) signals on the nonradiated side of roots. (G–I) The angle of root bending of Col-0, pin2 (G,H), and aux1-7 (I,J) seedlings irradiated with UV-B for 1 h and then transferred to normal growth conditions for 0–6 h. Col-0, n = 60; pin2, n = 60; aux1-7, n = 60. (G,I) Each gravistimulated root was assigned to 1 of the 12 30° sectors on a gravitropism diagram. The length of each bar represents the mean percentage of seedlings assigned to the respective sector. The red and black arrows indicate the direction of UV-B radiation and gravity, respectively. (H,J) The average root bending angles of Col-0, pin2 (H), and aux1-7 (J) seedlings; ck, before UV-B radiation control. The error bars represent the ±SE, and different letters indicate significantly different values (P < 0.01 by Tukey’s test).
We next investigated the roles of PIN2 and AUX1 in UV-B-induced root bending using pin2 and aux1 mutants. The loss of gravitropism in the aux1 and pin2 mutants led to root bending. However, different from the agravitropic root bending that cling to agar medium, UV-B radiation induced root bending upward from the surface of the medium and toward the source of radiation. Therefore, the direction of agravitropic root bending of aux1 and pin2 was distinct from the UV-B radiation-induced root bending. The degree of nonirradiated control (ck) root bending of aux1 and pin2 was 0 (it did not show any degree toward the direction of UV-B radiation). Both the pin2 and aux1 mutants showed less suppression of PR growth than did the Col-0 seedlings after UV-B radiation (Supplementary Figure S5). The average angles of root bending were 72.7% higher after 3 h and 34% higher after 6 h in pin2 seedlings (Figures 5G,H), and 90.5% higher after 3 h in aux1-7 seedlings (Figures 5I,J) compared with the Col-0 control seedlings subjected to UV-B radiation. These results indicate that PIN2 and AUX1 are involved in generating the asymmetric auxin distribution underlying the root bending response to UV-B radiation.
To further confirm the effect of auxin transport in root bending, we also used NPA, an auxin transport inhibitor. The average angles of root bending of NPA-treated Col-0 seedlings were 16.8% higher after 4 h and 28.6% higher after 6 h compared with the control (NPA-untreated) seedlings subjected to UV-B radiation (Supplementary Figure S6).
Involvement of Flavonoids in UV-B-Disturbed Auxin Distribution in Root Tips
To further investigate the molecular mechanisms underlying UV-B-induced root bending, we analyzed the transcript profiles in roots via high-throughput RNA-seq (Supplementary Materials and Methods) followed by qRT-PCR. We compared the transcripts obtained at 0.5 and 2 h after UV-B treatment. Relative to the gene expression levels under control conditions, 1436 genes were down-regulated and 557 genes were up-regulated in roots after 0.5 h of UV-B treatment; 1710 genes were down-regulated, and 1040 genes were up-regulated in roots after 2 h of UV-B treatment (Supplementary Figure S7 and Supplementary Table S2). The differentially expressed genes showed enrichment in the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of photosynthesis, carbon fixation, and flavonoid biosynthesis, among others, due to either 0.5 or 2 h of UV-B treatment (Supplementary Figure S8 and Supplementary Table S3). The qRT-PCR results strongly agreed with the RNA-seq results (R2 = 0.6339); this finding verified the accuracy of the RNA-seq results (Supplementary Figure S9).
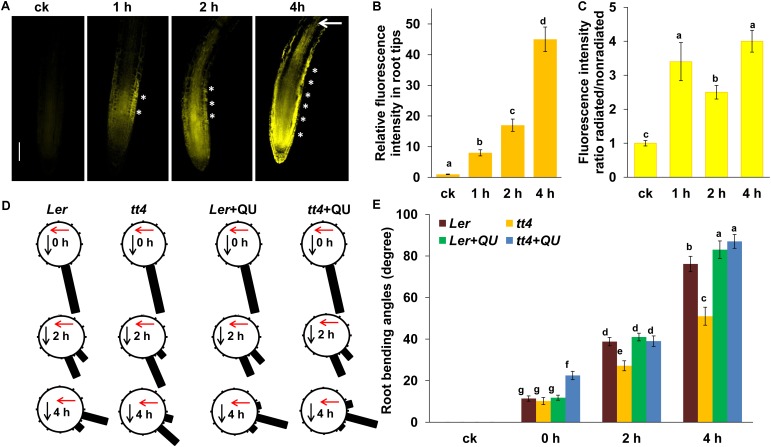
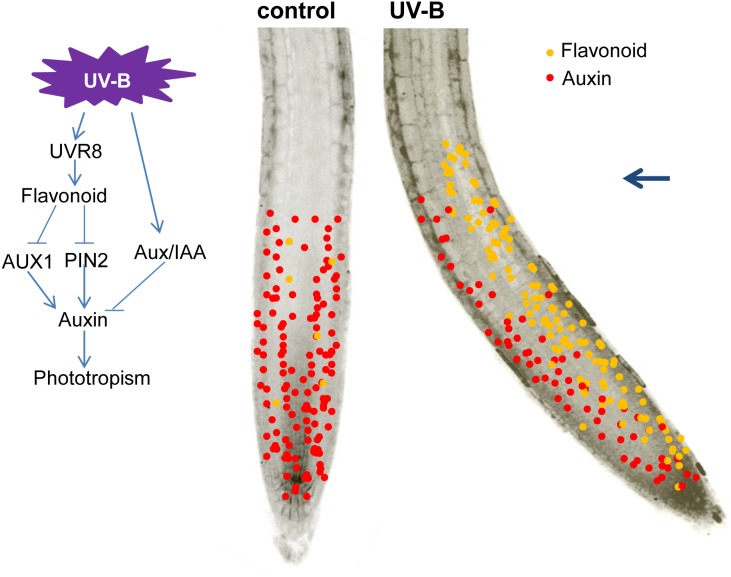
The RNA-seq analysis showed that UV-B radiation significantly induced the expression of flavonoid biosynthesis-related genes, and the results were consistent with previous reports that UV-B activates the expression of flavonoid biosynthesis-related genes (Rice-Evans et al., 1997; Nawkar et al., 2013). As it has been documented that flavonoids can affect auxin distribution (Jacobs and Rubery, 1988; Muday and DeLong, 2001) and root phototropism (Silva-Navas et al., 2016), the possible role of flavonoids in UV-B-induced root bending was also investigated in our study. First, flavonoids were stained with diphenylborinic acid 2-aminoethyl ester (DPBA), which predominantly detects quercetin (QU) and kaempferol, two natural flavonoids in plants (Murphy et al., 2000; Lewis et al., 2011). DPBA fluorescence was dramatically increased in the roots of Col-0 seedlings after UV-B radiation compared with the untreated controls (Figure 6). Interestingly, we found that UV-B radiation led to an asymmetric distribution of flavonoid in the root tips. As shown in Figure 6, UV-B radiation significantly increased DPBA fluorescence on the radiated side of roots.
FIGURE 6.
Flavonoids are involved in UV-B-induced root bending. (A) DPBA fluorescence in the roots of 5-d-old wild-type Col-0 seedlings exposed to UV-B radiation for 1 h and then transferred to normal growth conditions for 1, 2, and 4 h. Bars, 100 μm. (B,C) Quantification of DPBA fluorescence intensities in root tips (B), and ratios of DPBA fluorescence intensities in the root tips on the radiated side versus the nonradiated side (C) are shown. (D,E) The angle of root bending of Ler and tt4-1 seedlings irradiated with UV-B for 1 h and then transferred to normal growth conditions plus 0 nM QU or 100 nM QU for 1–4 h, n = 60. (D) Each gravistimulated root was assigned to 1 of the 12 30° sectors on a gravitropism diagram. The length of each bar represents the mean percentage of seedlings assigned to the respective sector. The red and black arrows indicate the direction of UV-B radiation and gravity, respectively. (E) The average root bending angles of Ler and tt4-1 seedlings; ck, before UV-B radiation control. White asterisks indicate more pronounced DPBA fluorescence on the radiated side of roots. The error bars represent the ±SE, and different letters indicate significantly different values (P < 0.01 by Tukey’s test).
To investigate whether flavonoids are involved in UV-B-induced root bending, we analyzed root bending in the flavonoid biosynthesis-defective mutant transparent testa 4-1(tt4-1) after UV-B radiation. The tt4-1 mutant exhibited significantly lower flavonoid levels in the roots compared with wild-type seedlings following exposure to UV-B radiation, as indicated by DPBA fluorescence (Supplementary Figure S10). The tt4-1 mutant showed markedly greater suppression of PR growth than did the wild-type seedlings after UV-B radiation (Supplementary Figure S11). The tt4-1 mutant also showed significantly reduced root bending after UV-B radiation compared with the wild-type control. The average angles of root bending were 30% lower after 2 h, and 33% lower after 4 h in tt4-1 seedlings compared with the wild-type control seedlings subjected to UV-B radiation (Figures 6B,C).
To further confirm this observation, we also analyzed the effect of exogenous QU, a natural flavonoid, on UV-B radiation-induced root bending. Exogenous application of QU resulted in a greater degree of root bending compared with UV-B radiation alone (the average angles of root bending were 6 and 43.6% higher after 2 h, and 9 and 71% higher after 4 h in QU-supplemented Ler and tt4 seedlings, respectively, compared with unsupplemented seedlings subjected to UV-B radiation), and the QU-supplemented tt4 mutant showed a similar root bending degree compared with the QU-supplemented Ler seedlings after 2 h of UV-B radiation (Figures 6B,C). These data indicate that UV-B-induced root bending occurs, at least partially, through the UV-B-mediated rapid accumulation of flavonoids in roots.
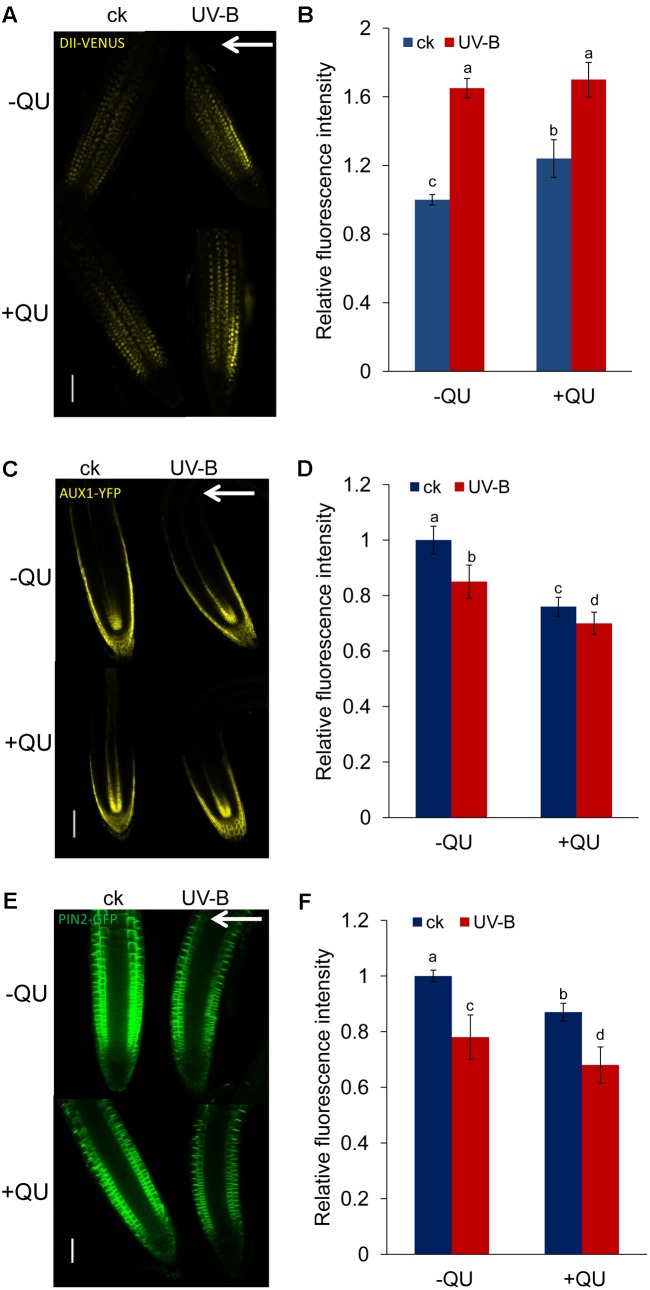
We next examined whether and how flavonoids modulate auxin distribution in root tips subjected to UV-B radiation. We first used an auxin-perceptive DII-VENUS marker line to monitor possible changes in auxin distribution in UV-B-treated roots in the presence or absence of exogenous QU. UV-B radiation reduced auxin distribution in root tips, and treatment with QU alone increased the DII-VENUS expression in root tips, indicating that exogenous QU reduces the distribution of auxin in root tips (Figures 7A,B). Nonetheless, supplementation with QU did not significantly increase DII-VENUS expression in UV-B-treated roots. It might be that UV-B induces dramatic accumulation of flavonoids in root tips; thus, exogenous flavonoid cannot further impact the phenotype.
FIGURE 7.
UV-B radiation influences auxin accumulation and transport via flavonoids. GFP/YFP fluorescence in the roots of 5-d-old DII-VENUS (A), AUX1:YFP (C), and PIN2:GFP (E) seedlings exposed to 1.6 W m-2 UV-B radiation for 1 h and then transferred to normal growth conditions plus 0 nM QU or 100 nM QU for 4 h. Quantification of DII-VENUS (B), AUX1:YFP (D), and PIN2:GFP (F) fluorescence intensities in the root tips is presented. Bars, 50 μm. White arrows indicate the direction of UV-B-radiation; ck, before UV-B radiation control. The error bars represent the SE, and different letters indicate significantly different values (P < 0.05 by Tukey’s test).
The results above suggest that the UV-B-regulated auxin distribution involved in root bending is modulated by AUX1 and PIN2. To further explore the role of QU in the auxin distribution response to UV-B radiation, we analyzed the levels of AUX1 and PIN2 expression in UV-B-treated roots in the presence or absence of exogenous QU using transgenic lines expressing AUX1:YFP and PIN2:GFP. Treatment with QU alone markedly decreased AUX1:YFP (Figures 7C,D) and PIN2:GFP (Figures 7E,F) fluorescence, and QU supplementation further reduced AUX1 and PIN2 level in UV-B-treated roots (Figures 7C–F), suggesting that QU reduced auxin transport by affecting the levels of these auxin carriers.
UVR8 Is Involved in UV-B-Mediated Root Bending
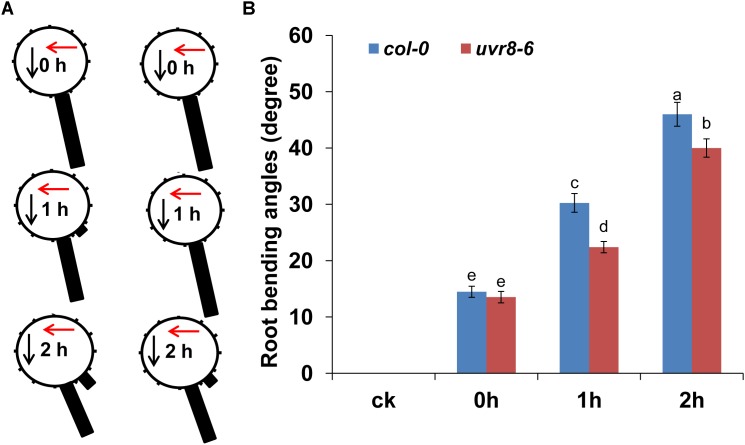
Ultraviolet-B radiation activates MPK3 and MPK6 via the MKP1 signaling pathway (González Besteiro et al., 2011; Nawkar et al., 2013). Exposure to UV-B also initiates signaling through the UVR8 pathway (Rizzini et al., 2011). The stress-induced MAPK pathway and the UVR8-mediated photomorphogenesis pathway are independent of each other and coordinately determine plant UV-B tolerance. Thus, we used mkp1, mpk3, mpk6, and uvr8-6 mutants to ascertain whether UV-B-mediated root bending is MKP1 dependent or UVR8 dependent. Although the root bending response to UV-B radiation was similar in mkp1, mpk3, and mpk6 mutants compared with the wild-type plants (Supplementary Figure S12), a lower response was observed in uvr8-6 (Figure 8). The average angles of root bending in uvr8-6 seedlings were 30% lower after 1 h and 13% lower after 2 h compared with the col-0 control seedlings subjected to UV-B radiation. Because the uvr8 mutant is hypersensitive to UV-B radiation, we analyzed whether UV-B radiation induced root growth cessation. After UV-B radiation, both uvr8-6 and col-0 seedlings showed a reduced primary root (PR) growth, and the PR growth of uvr8-6 seedlings gradually recovered to a similar level compared with col-0 seedling after 4 d of treatment when the UV-B-radiated seedlings were transferred to normal condition (Supplementary Figure S13), indicating that UV-B radiation did not result in root growth cessation in the uvr8 mutant. Taken together, these data indicate that UV-B-mediated root bending is at least partially dependent on the UVR8 signaling pathway.
FIGURE 8.
UVR8 is involved in UV-B-induced root bending. The angle of root bending of col-0 and uvr8-6 mutant seedlings irradiated with UV-B for 1 h and then transferred to normal growth conditions for 0–2 h. col-0, n = 60; uvr8-6, n = 60. (A) Each gravistimulated root was assigned to 1 of the 12 30° sectors on a gravitropism diagram. The length of each bar represents the mean percentage of seedlings assigned to the respective sector. The red and black arrows indicate the direction of UV-B-radiation and gravity, respectively. (B) The average root bending angles of col-0 and uvr8-6 seedlings; ck, before UV-B radiation control. The error bars represent the ±SE, and different letters indicate significantly different values (P < 0.01 by Tukey’s test).
Previous studies have reported that UV-B activates the expression of flavonoid biosynthesis-related genes through the UVR8 signaling pathway (Rice-Evans et al., 1997; Nawkar et al., 2013). We thus also analyzed flavonoid accumulation in uvr8 roots. As shown in Supplementary Figure S10, the DPBA fluorescence was significantly lower in the roots of the uvr8-6 mutant compared with the wild-type seedlings after UV-B radiation, indicating that UV-B-induced flavonoid production depends on UVR8.
Discussion
Stress-induced root bending is a common phenomenon in plants (Li and Zhang, 2008). Salt modulates root growth direction by inducing root bending as a salt-avoidance tropism (Li and Zhang, 2008). Light locally induces a root light avoidance mechanism, allowing roots to bend and escape from the light (Zhang et al., 2013; Silva-Navas et al., 2016). Different from the avoidance tropism of root bending, UV-B induces root bending toward the irradiated direction. It is well known that the penetration capacity of UV radiation is limited. Brumfield (1953) found that the cell division of only the outer layer of the root tip meristem was suppressed when roots were irradiated with UV-C because these cells were not protected by the root cap. Ktitorova et al. (2006) also found that UV-B radiation-induced cell division cessation and cell vacuolation did not occur in the distal meristem zone, which is protected by the root cap. Therefore, in UV-B radiation-induced root bending toward the irradiated direction, the root cap would increase the protection of root meristem cells from UV-B radiation.
UV-B Radiation Induces Root Bending by Modulating Auxin Perception and Distribution in Roots
Auxin perception and signaling both play roles in tropistic responses (Band et al., 2012). Disrupting the auxin responsiveness of expanding epidermal cells by expressing a mutant form of the Aux/IAA17 protein, the axr3-1 mutant lacks root gravitropism (Swarup et al., 2005). Vandenbussche et al. (2014) found that UV-B radiation results in the down-regulation of the expression of auxin-responsive genes. Our results indicate that UV-B perturbs auxin signaling by stabilizing Aux/IAA proteins, as indicated by HS:AXR3-GUS expression, and the gain-of-function axr3-3 mutant showed a greater extent of root bending than did the wild-type control. These results support the hypothesis that UV-B radiation induces root bending by reducing auxin signaling through increase of Aux/IAA stabilization in roots. Further study will investigate whether other Aux/IAA proteins are also UV-B target and involved in UV-B-mediated auxin signaling.
In addition to auxin perception, the auxin content in roots and the asymmetric distribution of auxin are known to contribute to root bending during tropistic responses (Sun et al., 2008). These effects are potentially regulated by the expression of auxin biosynthesis-related genes and auxin carriers (Abas et al., 2006). Indeed, UV-B radiation decreased the transcript levels of several IAA biosynthesis genes, such as YUC2, YUC3, YUC9, AAO3, CYP79B2, and PAT1, suggesting that UV-B radiation decreases auxin content in roots by down-regulating IAA biosynthesis-related gene expression. Mutants related to auxin transport, such as aux1 and pin2, exhibit defects in gravitropic responses (Petrášek and Friml, 2009; Haga and Sakai, 2012). Previous studies showed that an asymmetric distribution of auxin carriers is needed to generate asymmetric auxin distribution during root gravitropism and the root phototropic response (Zhang et al., 2013). In this study, we observed reduced auxin accumulation in root tips and asymmetric auxin distribution during the root bending response to UV-B radiation. We found that UV-B radiation increased auxin distribution on the nonradiated side of the root–apex transition and elongation zones; at this site, auxin promoted growth and caused root bending by asymmetric cell elongation. Further examination showed that UV-B radiation induced the asymmetric distribution of AUX1 and PIN2 on both sides of the root tips. The nonradiated side of the roots showed stronger PIN2:GFP and AUX1:YFP signals than did the radiated side, suggesting a role for these increased PIN2 and AUX1 levels in the higher auxin distribution observed on the nonradiated side. Our findings demonstrate that a reduction in the abundance and asymmetric distribution of AUX1 and PIN2 in roots exposed to UV-B radiation may interfere with the distribution of auxin within root meristem cells as well as auxin transport during the root bending response to UV-B radiation. Such changes may promote root bending to modify the direction of root growth. Mutation of AUX1 and PIN2 would disrupt auxin transport and reduce the asymmetric auxin distribution in UV-B-radiated roots. However, we found that aux1-7 and pin2 mutants are more sensitive to UV-B-induced root bending than Col-0 plants. These results indicated that, in addition to asymmetric distribution of auxin induced by UV-B, reduction of auxin accumulation in UV-B-radiated root tips also led to root agravitropic response, and thereby aggravating root bending. However, the detailed molecular mechanisms involved in how the modulation of the root agravitropic response are involved in UV-B-mediated root bending and the possible interaction between root agravitropic response and phototropic bending in response to UV-B radiation remain to be further explored.
Despite the asymmetric auxin distribution during root gravitropism and the root phototropic response-induced root bending, auxin accumulated on the concave side of gravistimulated roots, but it accumulated on the convex side of the roots in response to unilateral blue light stimulation (Zhang et al., 2013) and UV-B radiation (in this study). The difference between the gravitropism assays and UV-B radiation may be due to the following reasons: (1) UV-B radiation significantly reduced auxin accumulation in roots by repressing auxin biosynthesis (evidence from qRT-PCR), transport (evidence from AUX1:YFP and PIN2-GFP), and signaling (evidence from HS:AXR3-GUS reporter), and thereby inhibited root growth. (2) Unilateral UV-B radiation significantly increased auxin distribution on the nonradiated side (convex side) of the roots. Greater auxin accumulation on the nonradiated side (convex side) of roots resulted in higher H+ efflux, thereby promoting cell wall acidification on the side (Rubery and Sheldrake, 1974; Yan et al., 2016) and ultimately leading to asymmetric growth and subsequent root bending. Taken together, these data indicate that although UV-B radiation reduces total auxin accumulation in roots, it increases auxin distribution on the nonradiated side (convex side) of roots, thereby promoting growth on the nonradiated side (convex side) and ultimately resulting in root growth toward the radiation.
Asymmetric Flavonoid Accumulation in Roots Is Associated With UV-B-Mediated Root Bending Through Decreased Auxin Accumulation and Induction of Asymmetric Auxin Distribution in Root Tips
It is believed that one of the important aspects by which flavonoids protect plants from UV-B irradiation is their UV-absorbing characteristics (Kootstra, 1994). Flavonoids can be synthesized in the root elongation zone and accumulate in the root tips of plants subjected to UV-B radiation (Karabourniotis et al., 1992; Li et al., 1993; Winkel-Shirley, 2002). Santelia et al. (2008) found that flavonoids could induce an asymmetric distribution of auxin in root tips, thus resulting in root bending. UV-B radiation induces positive root phototropic bending. A possible explain is that UV-B causes a destruction of auxin on the illuminated side of the roots, and UV-B would likely not penetrate to the shaded side of the tissue and thus an asymmetric IAA distribution would result. UV-B radiation markedly induces flavonoid production and the increased flavonoids would protect roots from UV-B irradiation. Therefore, we wondered whether the flavonoids play a protective role in UV-B-irradiated roots, or flavonoid itself also plays a role in modulating the root system development response to UV-B radiation. Indeed, we found that UV-B-induced flavonoids affected auxin distribution by altering the abundance of auxin carriers in the root tips, thereby modulating the direction of root growth. Several lines of evidence support this conclusion. First, we demonstrated that flavonoids are needed for UV-B-induced root bending. The flavonoid biosynthesis-defective mutant tt4 showed significantly reduced root bending in response to UV-B radiation compared with the wild-type controls. A similar result was also reported by Silva-Navas et al. (2016), i.e., that the tt4 mutant showed a reduced root phototropic response to light. Second, physiological analysis showed that exogenous application of QU reduced auxin accumulation in the root tips and resulted in a greater extent of root bending compared with UV-B radiation alone. Third, exogenous QU supplementation reduced the abundance of AUX1 and PIN2 in root tips. Fourth, we confirmed that UV-B-induced flavonoid production in the root tips depends on UVR8, and loss of function uvr8 mutant shows a reduced response to UV-B radiation. Silva-Navas et al. (2016) found that light induced an asymmetric accumulation of flavonoids, thereby resulting in asymmetric growth in the root transition zone. Consistent with their results, we also observed an asymmetric accumulation of flavonoids in the UV-B-radiated root tips. UV-B radiation significantly increased flavonoid production on the radiated side of roots, as indicated by DPBA fluorescence. The increased accumulation of flavonoids on the radiated side of roots resulted in reduced auxin transport and subsequently reduced auxin distribution on that side, and ultimately asymmetric root growth.
Yokawa et al. (2016) found that UV-B radiation induced ROS accumulation in the root tips. PR growth could be regulated by the auxin pathway and ROS pathway independently (Tsukagoshi et al., 2010). In addition to acting as auxin transport inhibitors, flavonoids could also act as ROS scavengers. ROS could also induce flavonoid production (Silva-Navas et al., 2016). Further study will elucidate the possible interaction between ROS and flavonoid in mediating UV-B-induced root bending.
We found that the uvr8 mutant displayed less root bending compared with the wild-type control under UV-B radiation. Consistent with these results, the uvr8 mutant accumulated a lower level of flavonoids in the roots compared with the wild-type control in response to UV-B radiation. A previous study reported that the single mutants mpk3 and mpk6 exhibited enhanced UV-B tolerance, suggesting a genetically defined in vivo role for these kinases in UV-B stress signaling (González Besteiro et al., 2011). We found that the mkp1, mpk3, and mpk6 single mutants exhibited a root bending phenotype that was similar to that of the wild-type control. These results suggest that UV-B-induced root bending depends on the UVR8 signaling pathway, but not on the MKP1–MPK3/6 signaling pathway.
In this study, we have showed that UV-B radiation inhibits PR growth and induces root bending. We found that most of mutants that showed a higher root bending also had a less inhibition of PR growth exposed to UV-B radiation. These results support the hypothesis that the root cap would increase the protection of root meristem cells from UV-B radiation when the root bending toward the irradiated direction, and thereby alleviating UV-B-induced PR growth inhibition. Another possible reason is that the more rapid growth rate would result in higher root bending. However, we found that the auxin over-producing yucca mutant that had a lower root bending but it showed a decrease in PR growth inhibition. These results support the view that the root growth and bending in response to gravity, light, or abiotic stresses occurs through multiple overlapping mechanisms and that UV-B radiation may act as an input for one of multiple responses (Wolverton et al., 2002; Bai et al., 2013).
In summary, our data indicate that UVR8-dependent flavonoid production and its asymmetric accumulation in root tips are associated with UV-B-mediated root phototropic bending through decreased auxin accumulation and induction of asymmetric auxin distribution in root tips by modulating the distribution of AUX1 and PIN2 (Figure 9). These findings provide new insight into how UV-B radiation regulates root growth through a flavonoid-mediated phototropic response to UV-B radiation.
FIGURE 9.
A proposed model of UV-B-mediated root bending. Blue arrow indicates the direction of UV-B radiation.
Author Contributions
JX conceived the study and designed the experiments. JW, PZ, RW, and LS carried out the experiments. PZ, JW, RW, HZ, WW, and JX analyzed the data. JX wrote the manuscript. PZ, JW, HZ, WW, and JX revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Yingtang Lu and Prof. Shaojian Zheng for providing AUX1:YFP and yucca seeds. We gratefully acknowledge the Central Laboratory of the Xishuangbanna Tropical Botanical Garden for providing research facilities.
Footnotes
Funding. This work was supported by the National Key Research and Development Program of China (2016YFC0501901), China National Natural Sciences Foundation (31772383 and 31272239), Qinghai innovation platform construction project (2017-ZJ-Y20), and Yunnan Province Foundation for academic leader (2014HB043).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.00618/full#supplementary-material
References
- Abas L., Benjamins R., Malenica N., Paciorek T., Wirniewska J., Moulinier-Anzola J. C., et al. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. 8 249–256. 10.1038/ncb1369 [DOI] [PubMed] [Google Scholar]
- Bai H., Murali B., Barber K., Wolverton C. (2013). Low phosphate alters lateral root setpoint angle and gravitropism. 100 175–182. 10.3732/ajb.1200285 [DOI] [PubMed] [Google Scholar]
- Baldwin K. L., Strohm A. K., Masson P. H. (2013). Gravity sensing and signal transduction in vascular plant primary roots. 100 126–142. 10.3732/ajb.1200318 [DOI] [PubMed] [Google Scholar]
- Band L. R., Wells D. M., Larrieu A., Sun J., Middleton A. M., French A. P., et al. (2012). Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. 109 4668–4673. 10.1073/pnas.1201498109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkova E., Michniewicz M., Sauer M., Teichmann T., Seifertova D., Jurgens G., et al. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. 115 591–602. 10.1016/S0092-8674(03)00924-3 [DOI] [PubMed] [Google Scholar]
- Biever J. J., Brinkman D., Gardner G. (2014). UV-B inhibition of hypocotyl growth in etiolated Arabidopsis thaliana seedlings is a consequence of cell cycle arrest initiated by photodimer accumulation. 65 2949–2961. 10.1093/jxb/eru035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I., Xu J., Wildwater M., Willemsen V., Paponov I., Friml J., et al. (2005). The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. 433 39–44. 10.1038/nature03184 [DOI] [PubMed] [Google Scholar]
- Brumfield R. T. (1953). The effect of ultraviolet irradiation on cell division and elongation in timothy roots. 39 366–370. 10.1073/pnas.39.5.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoud G., Wells D. M., Oliva M., Larrieu A., Mirabet V., Burrow A. H., et al. (2012). A novel sensor to map auxin response and distribution at high spatio-temporal resolution. 482 103–106. 10.1038/nature10791 [DOI] [PubMed] [Google Scholar]
- Casati P., Walbot V. (2008). Maize lines expressing RNAi to chromatin remodeling factors are similarly hypersensitive to UV-B radiation but exhibit distinct transcriptome responses. 3 216–229. 10.4161/epi.3.4.6631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D., Zhao J., Jing Y., Fan M., Liu J., Wang Z., et al. (2013). The Arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. 9:e1003759. 10.1371/journal.pgen.1003759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T., Stitt M., Altmann T., Udvardi M. K., Scheible W. R. (2005). Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. 139 5–17. 10.1104/pp.105.063743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Yuan H. M., Hu Y. Q., Li J., Lu Y. T. (2014). Mutation of Arabidopsis CATALASE2 results in hyponastic leaves by changes of auxin levels. 37 175–188. 10.1111/pce.12144 [DOI] [PubMed] [Google Scholar]
- Ge L., Peer W., Robert S., Swarup R., Ye S., Prigge M., et al. (2010). Arabidopsis ROOT UVB SENSITIVE2/WEAK AUXIN RESPONSE1 is required for polar auxin transport. 22 1749–1761. 10.1105/tpc.110.074195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S. (2008). Plant tropisms. 18 R275–R277. 10.1016/j.cub.2008.02.033 [DOI] [PubMed] [Google Scholar]
- González Besteiro M. A., Bartels S., Albert A., Ulm R. (2011). Arabidopsis MAP kinase phosphatase 1 and its target MAP kinases 3 and 6 antagonistically determine UV-B stress tolerance, independent of the UVR8 photoreceptor pathway. 68 727–737. 10.1111/j.1365-313X.2011.04725.x [DOI] [PubMed] [Google Scholar]
- Gray W. M., Kepinski S., Rouse D., Leyser O., Estelle M. (2001). Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. 414 271–276. 10.1038/35104500 [DOI] [PubMed] [Google Scholar]
- Haga K., Sakai T. (2012). PIN auxin efflux carriers are necessary for pulse-induced but not continuous light-induced phototropism in Arabidopsis. 160 763–776. 10.1104/pp.112.202432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. M., Zhang Z., Wang R. B., Chen Y. P. (2011). UV-B-induced stomatal closure occurs via ethylene-dependent NO generation in Vicia faba. 38 293–302. 10.1071/FP10219 [DOI] [PubMed] [Google Scholar]
- Hu Y. Q., Liu S., Yuan H. M., Li J., Yan D. W., Zhan J. F., et al. (2010). Functional comparison of catalase genes in the elimination of photorespiratory H2O2 using promoter- and 3′- untranslated region exchange experiments in the Arabidopsis cat2 photorespiratory mutant. 33 1656–1670. 10.1111/j.1365-3040.2010.02171.x [DOI] [PubMed] [Google Scholar]
- Jacobs M., Rubery P. H. (1988). Naturally occurring auxin transport regulators. 241 346–349. 10.1126/science.241.4863.346 [DOI] [PubMed] [Google Scholar]
- Karabourniotis G., Papadopoulos K., Papamarkou M., Manetas Y. (1992). Ultraviolet-B radiation absorbing capacity of leaf hairs. 86 414–418. 10.1111/j.1399-3054.1992.tb01337.x [DOI] [Google Scholar]
- Kootstra A. (1994). Protection from UV-B-induced DNA damage by flavonoids. 26 771–774. 10.1007/BF00013762 [DOI] [PubMed] [Google Scholar]
- Krasylenko Y. A., Yemets A. I., Sheremet Y. A., Blume Y. B. (2012). Nitric oxide as a critical factor for perception of UV-B irradiation by microtubules in Arabidopsis. 145 505–515. 10.1111/j.1399-3054.2011.01530.x [DOI] [PubMed] [Google Scholar]
- Ktitorova I. N., Demchenko N. P., Kalimova I. B., Demchenko K. N., Skobeleva O. V. (2006). Cellular analysis of UV-B-induced barley root subapical swelling. 53 824–836. 10.1134/S1021443706060148 [DOI] [Google Scholar]
- Kuhn B. M., Errafi S., Bucher R., Dobrev P., Geisler M., Bigle R. L., et al. (2016). 7-Rhamnosylated Flavonols modulate homeostasis of the plant hormone auxin and affect plant development. 291 5385–5395. 10.1074/jbc.M115.701565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn B. M., Nodzyński T., Errafi S., Bucher R., Gupta S., Ringli C. (2017). Flavonol-induced changes in PIN2 polarity and auxin transport in the Arabidopsis thaliana rol1-2 mutant require phosphatase activity. 7:41906. 10.1038/srep41906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. R., Ramirez M. V., Miller N. D., Vallabhaneni P., Ray W. K., Helm R. F., et al. (2011). Auxin and ethylene induce flavonol accumulation through distinct transcriptional networks. 156 144–164. 10.1104/pp.111.172502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Ou-Lee T. M., Raba R., Amundson R. G., Last R. L. (1993). Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. 5 171–179. 10.1105/tpc.5.2.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang W. S. (2008). Salt-avoidance tropism in Arabidopsis thaliana. 3 351–353. 10.4161/psb.3.5.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li R. J., Han T. T., Cai W., Fu Z. W., Lu Y. T. (2015). Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. 168 343–356. 10.1104/pp.15.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo M., Yokawa K., Wan Y., Baluška F. (2015). How and why do root apices sense light under the soil surface? 6:775. 10.3389/fpls.2015.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muday G. K., DeLong A. (2001). Polar auxin transport: controlling where and how much. 6 535–542. 10.1016/S1360-1385(01)02101-X [DOI] [PubMed] [Google Scholar]
- Murphy A., Peer W. A., Taiz L. (2000). Regulation of auxin transport by aminopeptidases and endogenous flavonoids. 211 315–324. 10.1007/s004250000 [DOI] [PubMed] [Google Scholar]
- Nakamura A., Nakajima N., Goda H., Shimada Y., Hayashi K., Nozaki H., et al. (2006). Arabidopsis Aux/IAA genes are involved in brassinosteroid-mediated growth responses in a manner dependent on organ type. 45 193–205. 10.1111/j.1365-313X.2005.02582.x [DOI] [PubMed] [Google Scholar]
- Nawkar G. M., Maibam P., Park J. H., Sahi V. P., Lee S. Y., Kang C. H. (2013). UV-induced cell death in plants. 14 1608–1628. 10.3390/ijms14011608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek J., Friml J. (2009). Auxin transport routes in plant development. 136 2675–2688. 10.1242/dev.030353 [DOI] [PubMed] [Google Scholar]
- Rice-Evans C. A., Miller N. J., Papaga G. (1997). Antioxidant properties of phenolic compounds. 2 152–159. 10.1016/S1360-1385(97)01018-2 [DOI] [Google Scholar]
- Rizzini L., Favory J. J., Cloix C., Faggionato D., O’Hara A., Kaiserli E., et al. (2011). Perception of UV-B by the Arabidopsis UVR8 protein. 332 103–106. 10.1126/science.1200660 [DOI] [PubMed] [Google Scholar]
- Rubery P. H., Sheldrake A. R. (1974). Carrier-mediated auxin transport. 118 101–121. 10.1007/BF00388387 [DOI] [PubMed] [Google Scholar]
- Ruppel N. J., Hangarter R. P., Kiss J. Z. (2001). Red-light-induced positive phototropism in Arabidopsis roots. 212 424–430. 10.1007/s004250000410 [DOI] [PubMed] [Google Scholar]
- Sabatini S., Beis D., Wolkenfelt H., Murfett J., Guilfoyle T., Malamy J., et al. (1999). An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. 99 463–472. 10.1016/S0092-8674(00)81535-4 [DOI] [PubMed] [Google Scholar]
- Santelia D., Henrichs S., Vincenzetti V., Sauer M., Bigler L., Klein M., et al. (2008). Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. 283 31218–31226. 10.1074/jbc.M710122200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Navas J., Moreno-Risueño M. A., Manzano C., Téllez-Robledo B., Navarro-Neila S., Carrasco V., et al. (2016). Flavonols mediate root phototropism and growth through regulation of proliferation-to-differentiation transition. 28 1372–1387. 10.1105/tpc.15.00857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon K. R. (2008). Effects of ozone depletion and UV-B radiation on humans and the environment. 46 185–202. 10.3137/ao.460109 [DOI] [Google Scholar]
- Sun F., Zhang W., Hu H., Li B., Wang Y., Zhao Y., et al. (2008). Salt modulates gravity signaling pathway to regulate growth direction of primary roots in Arabidopsis. 146 178–188. 10.1104/pp.107.109413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlid G. (1976). Effects of flavonoids on the polar transport of auxins. 38 262–266. 10.1111/j.1399-3054.1976.tb04001.x [DOI] [Google Scholar]
- Swarup R., Kargul J., Marchant A., Zadik D., Rahman A., Mills R., et al. (2004). Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. 16 3069–3083. 10.1105/tpc.104.024737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R., Kramer E. M., Perry P., Knox K., Leyser H. M., Haseloff J., et al. (2005). Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. 7 1057–1065. 10.1038/ncb1316 [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H., Busch W., Benfey P. N. (2010). Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. 143 606–616. 10.1016/j.cell.2010.10.020 [DOI] [PubMed] [Google Scholar]
- Ulmasov T., Murfett J., Hagen G., Guilfoyle T. J. (1997). Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. 9 1963–1971. 10.1105/tpc.9.11.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F., Tilbrook K., Fierro A. C., Marchal K., Poelman D., Van Der Straeten D., et al. (2014). Photoreceptor-mediated bending towards UV-B in Arabidopsis. 7 1041–1052. 10.1093/mp/ssu039 [DOI] [PubMed] [Google Scholar]
- Vanhaelewyn L., Prinsen E., Van Der Straeten D., Vandenbussche F. (2016). Hormone-controlled UV-B responses in plants. 67 4469–4482. 10.1093/jxb/erw261 [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2002). Biosynthesis of flavonoids and effects of stress. 5 218–223. 10.1016/S1369-5266(02)00256-X [DOI] [PubMed] [Google Scholar]
- Wolverton C., Ishikawa H., Evans M. L. (2002). The kinetics of root gravitropism: dual motors and sensors. 21 102–112 10.1007/s003440010053 [DOI] [PubMed] [Google Scholar]
- Yan S., Zhang T., Dong S., McLamore E. S., Wang N., Shan X., et al. (2016). MeJA affects root growth by modulation of transmembrane auxin flux in the transition zone. 35 256–265. 10.1007/s00344-015-9530-9 [DOI] [Google Scholar]
- Yin R., Han K., Heller W., Schaffner A. R. (2014). Kaempferol 3-O-rhamnoside-7-O-rhamnoside is an endogenous flavonol inhibitor of polar auxin transport in Arabidopsis shoots. 201 466–475. 10.1111/nph.12558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokawa K., Kagenishi T., Baluška F. (2016). UV-B induced generation of reactive oxygen species promotes formation of BFA-induced compartments in cells of Arabidopsis root apices. 6:1162. 10.3389/fpls.2015.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žádníková P., Petrášek J., Marhavý P., Raz V., Vandenbussche F., Ding Z., et al. (2010). Role of PIN-mediated auxin efflux in apical hook development of Arabidopsis thaliana. 137 607–617. 10.1242/dev.041277 [DOI] [PubMed] [Google Scholar]
- Zhang K. X., Xu H. H., Yuan T. T., Zhang L., Lu Y. T. (2013). Blue-light-induced PIN3 polarization for root negative phototropic response in Arabidopsis. 76 308–321. 10.1111/tpj.12298 [DOI] [PubMed] [Google Scholar]
- Zhao Y. D., Christensen S. K., Fankhauser C., Cashman J. R., Cohen J. D., Weigel D., et al. (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. 291 306–309. 10.1126/science.291.5502.306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.