Abstract
Our improving knowledge of the animal tree of life consistently demonstrates that some taxa diversify more rapidly than others, but what contributes to this variation remains poorly understood. An influential hypothesis proposes that selection arising from competition for mating partners plays a key role in promoting speciation. However, empirical evidence showing a link between proxies of this sexual selection and species richness is equivocal. Here, we collected standardized metrics of sexual selection for a broad range of animal taxa, and found that taxonomic families characterized by stronger sexual selection on males show relatively higher species richness. Thus, our data support the hypothesis that sexual selection elevates species richness. This could occur either by promoting speciation and/or by protecting species against extinction.
Keywords: Bateman gradient, Bateman principles, diversification, macroevolution, species diversity, reproductive isolation
1. Introduction
Surprisingly little is understood about the processes governing the highly uneven distribution of species richness across the animal kingdom [1]. Sexual selection is often invoked to influence species richness by modulating speciation processes, but the theory and empirical data are inconclusive and contentious [2,3].
Several influential theoretical arguments suggest that sexual selection promotes speciation, which could occur through two main routes. First, sexual selection can promote the evolution of divergent phenotypic traits associated with mating success among allopatric populations, which gradually leads to speciation by increasing sexual isolation [4–6]. Second, sexual selection can mediate niche divergence within populations and thus assist ecological speciation by promoting assortative mating [7–9]. However, these intuitive arguments have been disputed by other theories suggesting that increased sexual selection can in fact impede speciation as some forms of sexual selection may promote matings between individuals of different populations (i.e. disassortative mating) and thus elevate gene flow, reducing population divergence [10,11].
Empirical studies testing the role of sexual selection in speciation usually investigate associations between the inferred strength of sexual selection and species richness across phylogenies, while accounting for phylogenetic relatedness [2,3,12]. If sexual selection was to promote speciation, then taxa with more intense sexual selection should experience more speciation events and have higher species richness. Making such a comparison thus requires the use of a uniform measure that meaningfully captures the strength of sexual selection and that is directly comparable among diverse animal taxa.
However, so far, the strength of sexual selection has been approximated through indirect measures relying on traits assumed to have evolved as a result of sexual selection [2,3], such as sexual dichromatism, sexual size dimorphism, mating system or genital size. This body of work has been subject to a meta-analysis [3] which, despite showing a significant overall relationship between sexual selection and species richness, also revealed large inconsistencies across the taxa studied and across the proxies used to measure sexual selection. For instance, when using mating system as a proxy for sexual selection, polyandrous clades have been found to contain more species than monandrous clades across insects [13], but not within butterflies [14]. Likewise, the presence of sexually selected traits predicts taxonomic diversification across ray-finned fishes [15] but not within the Goodeinae family [16]. Moreover, in the most intensely studied and supposedly best understood taxa, the birds, sexual dichromatism has repeatedly been found to be associated with high species richness [17,18], but more recent, robust and powerful studies have surprisingly failed to confirm this pattern [19,20].
The use of such proxies of sexual selection raises several caveats. First, the phenotypic traits used to measure strength of sexual selection are certainly not only driven by sexual selection. For instance, male colouration is often used as a measure of sexual selection but the evolutionary trajectory of this trait is influenced by genetic constraints and other evolutionary forces (e.g. natural selection or random drift) [21,22] obscuring any signal of sexual selection. Second, traits used to measure sexual selection—including dichromatism and size dimorphism—are arguably often used because they are apparent to us human observers, and relatively easy to measure. Obviously, intense sexual selection may not necessarily result in dichromatic and dimorphic species, but may instead manifest itself through more subtle traits such as elaborate behaviours, songs, sexual pheromones, accessory gland secretions or other cryptic post-copulatory processes [23]. Consequently, any approach relying on morphological traits is doomed to provide, at best, only a partial measure of total sexual selection. Third, the use of morphological features often restricts comparisons within certain taxa. For instance, lineages with bioluminescent courtship have a higher species richness than their non-luminous sister lineages [24], but such a comparison is obviously restricted to taxa including species with bioluminescent courtship.
Here, we aim to counter such challenges by using standardized metrics for the expected strength and direction of sexual selection that are derived from Bateman's principles [25,26]. Notably, the Bateman gradient is the regression slope of reproductive success (e.g. number of offspring produced) on mating success (e.g. number of mating partners) and so—unlike other sexual selection proxies used—aims to quantify the fitness benefits gained per additional mating (figure 1). Importantly, the Bateman gradient relies exclusively on individual variation in mating and reproductive success, which allows comparisons across the whole animal kingdom [27,28]. Alongside with the Bateman gradient, we also included the variance in reproductive success (i.e. the opportunity for selection) and the variance in mating success (i.e. the opportunity for sexual selection) as additional measures of sexual selection (figure 1) in our analyses. Despite their limitations (outlined in Material and methods), all three Bateman metrics are well supported measures of the strength and direction of sexual selection [27,29] that are widely used in intra- and interspecific comparisons (e.g. [28,30–33]). We used up to 92 published Bateman metrics—spanning 70 species and 42 families widely distributed across the animal kingdom—to test the hypothesis that sexual selection predicts species richness.
Figure 1.
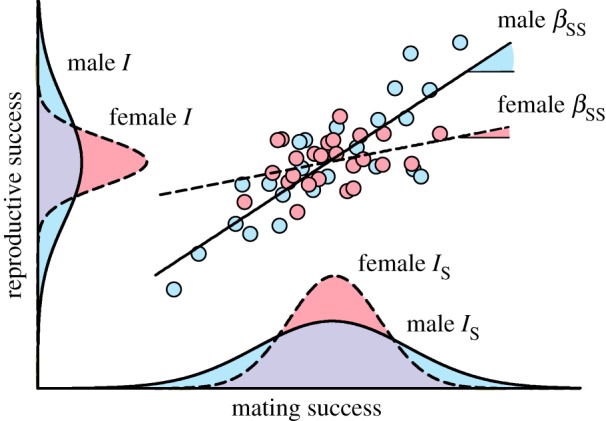
Bateman's three metrics. The variance in reproductive success (e.g. number of offspring produced) and the variance in mating success (e.g. number of mating partners) capture the opportunity for selection (I) and for sexual selection (Is), respectively. High variances indicate high opportunity for (sexual) selection. The regression slope is the Bateman gradient, which thus corresponds to the fitness benefits gained per additional mating. Steep Bateman gradients indicate intense sexual selection. The data points depicted here are fictional and only for illustrative purpose. (Online version in colour.)
2. Material and methods
(a). General approach
We tested for an association between sexual selection and species richness across the animal kingdom using a comparative approach. Specifically, we (i) compiled published estimates of the strength and direction of sexual selection, (ii) reconstructed the phylogeny of the sampled families, and (iii) ran phylogenetic generalized least-squares (PGLS) regressions.
The quantification of sexual selection comprises three interrelated metrics that are all derived from Bateman's principles [25,26]: the Bateman gradient (βss, the slope of an ordinary least-squares regression of reproductive success on mating success), the opportunity for selection (I, the variance in reproductive success) and the opportunity for sexual selection (Is, the variance in mating success) (figure 1). All metrics used are considered to be powerful for quantifying sexual selection and allows comparisons of the opportunity (I, Is) and the actual strength (βss) of (sexual) selection between sexes and among species [27,28,30,34]. Especially, βss represents a particularly informative proxy for the strength of pre-copulatory sexual selection as it aims to measure the fitness return of an additional mating and its sex difference provides an estimate for the direction of sexual selection. On the contrary, variance-based metrics I and Is reflect the maximum strength of selection on offspring production and on mating success, respectively. Despite their great advantages for intra- and interspecific comparisons of sexual selection, all three metrics have limitations. Notably, βss is sensitive to the way in which mating success is assessed [29,35], as it is typically steeper when it relies on the number of genetic partners (i.e. partners with whom a focal individual produced offspring) compared with copulatory mating success (i.e. the actual number of mating partners) [35,36]. Similarly, βss may also depend on how reproductive success is estimated, including the number of fertilized eggs, viable offspring, offspring that reach maturity and recruiting offspring, in which the later stages inevitably include information on offspring quality. In particular, βss has been found to be steeper when measured at later stages [37,38], but there is also evidence for the opposite [39], suggesting that there seems to be no general pattern [29]. Furthermore, it is important to bear in mind that βss only provides the slope of a linear regression, meaning that it does not necessarily imply a causal link between mating success and reproductive success, which can be especially problematic when measuring sexual selection in females (e.g. [38,40]). In addition, variance in reproductive and mating success may not only arise from selection but also from random processes (reviewed in [28]). Moreover, I and Is may depend on the population's mean reproductive success and mating success, respectively [41,42]. Finally, Bateman parameters have been shown to be environment-dependent in terms of being affected by demographic factors (e.g. group size, operational sex ratio [31,33]) and ecological conditions [43–45]. These limitations should be kept in mind when applying Bateman metrics in comparative studies as they can introduce noise into the analysis, which may be the case for the study presented here. However, we do not expect that any of these drawbacks will introduce a systematic bias in our test of how sexual selection predicts species richness, and the use of metrics is superior to that of proxies.
(b). Estimating sexual selection, species richness and phylogenetic affinities
We conducted a systematic literature search to obtain estimates of male and female I, Is and βss. A detailed description of the search protocol including a PRISMA diagram has been published elsewhere [28]. In brief, we screened for relevant studies using ISI Web of Knowledge (Web of Science Core Collection, from 1900 to 2015) with the ‘topic’ search terms defined as ‘Bateman*’ OR ‘opportunit* for selection’ OR ‘opportunit* for sexual selection’ OR ‘selection gradient*’. We only included studies reporting estimates of I, Is and/or βss of both sexes to overcome potential biases arising from non-random sampling of species with particularity strong sexual selection (as advocated by [46]). Specifically, researchers studying sexual selection in only one sex often have some a priori circumstantial evidence that sexual selection operates in that sex leading to an non-representative sampling of effect sizes. We repeated the previous literature search on 1 March 2017 and screened 340 additional studies of which three contained estimates of Is and/or βss [36,47,48]. In total, we extracted 85, 92 and 80 estimates of I, Is and βss, respectively (for both males and females) encompassing 42 families in total. In addition to sex specific-estimates of I, Is and βss, we quantified the sex difference in all these sexual selection metrics. Specifically, we defined ΔI, ΔIs and Δβss as the sex difference in I, Is and βss, respectively, with positive values indicating a male bias. Variance-based metrics ΔI and ΔIs were computed as the coefficient of variation ratio ‘lnCVR’, defined as the natural logarithm of the ratio between the coefficients of variation from two groups [49]. The sex difference in the Bateman gradient Δβss was computed as Hedges's g [50] (see [28] for details). Therefore, overall, the analysis focuses on nine measures of sexual selection: male and female I, Is and βss (n = 6), and the sex difference of these (n = 3).
In total, we extracted 85, 92 and 80 estimates of I, IS and βss, respectively (for both males and females), encompassing 70 species and 42 families. Taxonomic sampling was inevitably biased by the availability of studies, and birds and arthropods were most common. However, studies have been carried out in a wide range of families from across the animal kingdom (electronic supplementary material, figure S1) and the comparative methods used will counter any statistical problems arising from phylogenetic non-independence (see below).
We assessed the number of species for each of the 42 sampled families from the Catalogue of Life database (http://www.catalogueoflife.org/) on the 8 March 2017, excluding extinct taxa. Note that we did not test for relationships between the number of extinct species and sexual selection metrics as we consider our knowledge of extinct species highly heterogeneous due to varying research efforts among taxa. Like all taxonomic levels, family is arguably an arbitrary unit as families may vary in the elapsed time period during which species could diversify. In order to account for this potentially confounding effect, we obtained estimates of the crown age (i.e. the age of the most recent common ancestor of the extant members of the clade) for the sampled families from the TimeTree database [51] and corrected for it statistically (see below). Finally, we also retrieved divergence times from the TimeTree database to reconstruct the phylogeny of all sampled families.
(c). Statistical analysis
We used PGLS regressions to test whether sexual selection predicts species number at the family level. First, we obtained family-mean estimates for sexual selection metrics by either using the arithmetic mean (i.e. for male and female I, Is) or, if possible, by computing family-mean effect sizes (i.e. for male and female βss; and Δβss, ΔI, ΔIs) from random-effects models using the R package metafor version 1.9.2 [52]. We excluded the female estimate of Is of the family Iguanidae from the statistical analysis as it turned out to be a clear outlier (χ2 = 35.01, p < 0.001), but this exclusion did not qualitatively affect the results. Family-mean estimates of sexual selection were then used as predictor variables in PGLS regressions with the log-transformed number of species defined as the response variable. We also tested for nonlinear relationships between sexual selection metrics and species richness by adding a quadratic term to PGLS regressions. In an additional run of PGLS regressions we included family crown age as a covariate to account for among-family variation in the time period that species diversified (see above). However, we could only obtain published estimates of family crown age for a subset of all sampled families (i.e. 33 out of 42 families), meaning that we had less statistical power in these additional tests. All PGLS regressions were carried out using the gls function of the R package nlme version 3.1-131 assuming a Brownian motion model of evolution [53]. The number of sexual selection metrics extracted for each family varied between 1 and 11 (mean ± s.e.: I, 2.02 ± 0.27; Is, 2.19 ± 0.31; βss, 1.90 ± 0.27). To account for these differences in precision of the estimated family-specific strength of sexual selection, we weighted all PGLS regressions by the number of estimates used to compute family-mean effect sizes.
3. Results
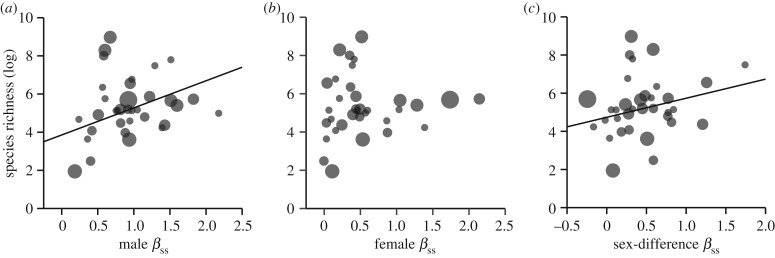
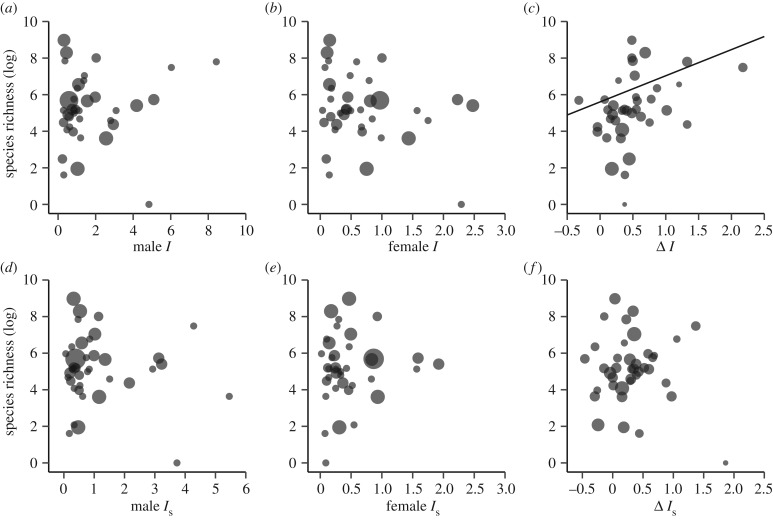
Animal families differ significantly in all Bateman metrics used to quantify sexual selection (table 1) and, importantly, three of these measures significantly predicted species richness (table 2). Specifically, we found that the strength of selection on mating success (βss) in males but not in females was positively correlated with species richness (figures 2 and 3a,b). As a consequence, the sex difference in βss also predicted species richness with families characterized by a steeper βss in males relative to females encompassed more species (figure 3c). Likewise, ΔI predicted species richness (table 2). Families with a more male-biased opportunity for selection contained more species (figure 4c). By contrast, none of the other variance-based estimates of selection were associated with species richness (table 2; figure 4). Quadratic models provided support for a nonlinear relationship between species richness and male βss, but not for any other tested sexual selection metric (electronic supplementary material, table S1).
Table 1.
Among-family variation in sexual selection metrics. Results from random-effects models with family as a moderator variable are shown.
| response | K | R2 (%) | QM | d.f. | p-value |
|---|---|---|---|---|---|
| ΔI (lnCVR) | 85 | 69.23 | 110.85 | 37 | <0.001 |
| ΔIs (lnCVR) | 92 | 63.59 | 111.23 | 39 | <0.001 |
| male βss (Fisher's z) | 80 | 66.01 | 142.86 | 33 | <0.001 |
| female βss (Fisher's z) | 80 | 48.30 | 96.93 | 33 | <0.001 |
| Δβss (Hedges's g) | 80 | 52.14 | 84.87 | 33 | <0.001 |
Table 2.
Relationship between sexual selection and species richness inferred from PGLS regressions.
| predictor | estimate | s.e. | d.f. | F-value | p-value |
|---|---|---|---|---|---|
| male I | 0.08 | 0.17 | 36 | 0.25 | 0.622 |
| female I | −0.33 | 0.31 | 36 | 1.16 | 0.288 |
| ΔI (lnCVR) | 1.43 | 0.44 | 36 | 10.44 | 0.003 |
| male Is | 0.08 | 0.25 | 38 | 0.11 | 0.744 |
| female Is | −0.13 | 0.52 | 37 | 0.06 | 0.805 |
| ΔIs (lnCVR) | 0.50 | 0.65 | 38 | 0.60 | 0.444 |
| male βss (Fisher's z) | 1.42 | 0.47 | 32 | 9.10 | 0.005 |
| female βss (Fisher's z) | 0.46 | 0.42 | 32 | 1.20 | 0.282 |
| Δβss (Hedges's g) | 1.00 | 0.46 | 32 | 4.79 | 0.036 |
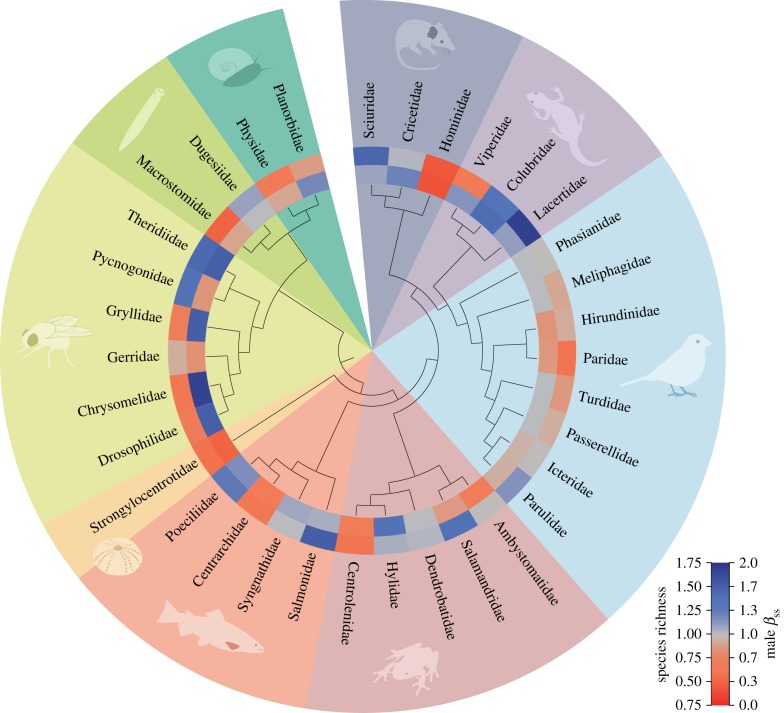
Figure 2.
Male Bateman gradient predicts species richness across 34 animal families. Phylogenetic tree of the studied families with their levels of species richness (inner ring) and the strength of sexual selection in males as estimated by the Bateman gradient (outer ring). Colour cells represent family averages. (Online version in colour.)
Figure 3.
Bubble plots and PGLS regressions showing the effect of family species richness (log transformed) on (a) male, (b) female and (c) sex difference (male−female) values of the Bateman gradient (βss). Bubbles represent family means, and their sizes are proportional to the number of estimates. Regression slopes that differ significantly from zero are shown. See main text for full statistics. Note that the PGLS regressions account for phylogenetic relatedness, unlike bubble plots, which should thus only be considered for visual aid.
Figure 4.
Bubble plots and PGLS regressions showing the effect of family species richness (log-transformed) on male, female and sex difference (male−female) of (a–c) the opportunity for selection (Is) and (d–f) the opportunity for sexual election (Is). Regression slopes that differ significantly from zero are shown.
As expected, species richness depended on the family crown age (linear regression: F1,31 = 20.61, p < 0.001, R2 = 0.38). We accounted for this potentially confounding effect by adding crown age as a covariate in PGLS regressions testing the effect of sexual selection metrics on species richness. In these additional analyses the above-mentioned effects remained statistically significant except for Δβss, which only tended to be positively correlated with species richness (electronic supplementary material, table S2).
4. Discussion
Studying the role of sexual selection on speciation is challenging, and previous empirical data arising from comparative studies are equivocal [2,3]. Here, we used an alternative way to estimate the strength and direction of sexual selection, through the Bateman gradient, which allowed us to avoid many of the caveats of previous sexual selection proxies used. The results showed elevated species richness in families with steeper male βss, which clearly supports the hypothesis that sexual selection promotes speciation.
Our results showed that species richness was predicted by the steepness of the Bateman gradient in males but not in females. Such a result may suggest that speciation rate is more affected by sexual selection operating on males compared to females. However, we think that the components of sexual selection captured by the Bateman gradients may better suit how sexual selection operates in males than in females. In particular, the Bateman gradients focus on the fitness benefits of additional matings and so may neglect other fitness components (e.g. post-copulatory selection, offspring quality) that may be key for female sexual selection. Although it is appreciated that strong sexual selection on males could either accelerate speciation by increasing divergence of traits which are targets of mate choice or inhibit speciation due to increased male–male competition [54], the fact that our results specifically highlight sexual selection on males implies that the first effect is much more prevalent across the animal kingdom.
Importantly, Bateman gradients do not capture every component of sexual selection equally well, and should be interpreted accordingly. For instance, post-copulatory sexual selection may represent an important component of total sexual selection (e.g. [36,55–57]), which is poorly quantified by the Bateman gradients. Specifically, when pre- and post-copulatory sexual selection interact (e.g. high-quality males mate more and produce high-quality sperm or, alternatively, males mating more experience sperm depletion), Bateman gradients can over- or underestimate total sexual selection. Moreover, offspring quality is usually not considered in Bateman studies, meaning that fitness benefits of mate choice are poorly reflected in Bateman gradients. Therefore, our study may miss additional components of sexual selection that are involved in speciation but not captured by Bateman metrics (e.g. [13]).
Unlike our findings on βss, we did not detect any relationship between species richness and Is in males, females or the sex difference therein. Given our findings on the Bateman gradient and the fact that Is and βss are typically positively correlated [28], we suspect that the absence of an effect has methodological rather than biological grounds. In fact, Is represents presumably the most controversial metric for quantifying sexual selection (e.g. [41,58]). This is not only because Is also captures random variation in mating success [59] but also because Is has been demonstrated to depend on mean mating success observed in the studied population as a consequence of (i) an nonlinear relationship between mating success and its variance [60–62], and/or (ii) the fact that mating success is usually measured as an integer [42]. It is very likely that random variation in (and the mean of) mating success can differ substantially across contexts within a species and among species. Such concerns clearly impose limitations on the applicability of Is as a proxy for sexual selection in interspecific comparisons such as our meta-analysis. Although we do not believe that these issues induce a systematic bias in the relationship between sexual selection and species richness found here, they are likely to increase noise in our predictor variable, which may render moderate and small effects undetected.
These potential shortcomings of using variance-based metrics as proxies for an upper limit of selection also apply for the opportunity of selection I. But despite potentially being a noisy metric, we found a positive relationship between the sex bias in I and species richness. This finding is especially interesting in the context of the role of sexual selection for species extinction, which also affects species richness. As such, the positive association we found between metrics of (sexual) selection and species richness may be mediated by speciation, by species extinction, or by both. There is controversy on whether sexual selection promotes or prevents species from extinction [63,64]. On the one hand, it has been argued that sexual selection can increase extinction rates by promoting sexual conflict, which may reduce the total reproductive output of a population [65] or by causing a runaway processes [66] that may lead to extreme male traits which come at a cost of lower viability (reviewed in [67]). On the other hand, stronger net selection on males relative to females has been proposed to purge deleterious alleles at a low demographic cost, which may allow populations to adapt more efficiently to novel environments [6,68–70]. Given that I sums up all variance in reproductive success arising from viability, fecundity and sexual selection, it can be considered a proxy for net selection, where a male bias indicates that the sexual selection on males overrides fecundity and viability selection in females [28]. Hence, though speculative, our findings on the sex difference in I are in accordance with the idea that stronger net selection on males protects species from extinction.
Although there are numerous ways in which sexual selection might act on traits involved in mating and fertilization success, and how these may in turn influence evolutionary dynamics, our results support that sexual selection on males is associated with an increase in species richness across the broad range of animal families sampled here, even after controlling for family age. More detailed taxon-specific studies are required to disentangle the myriad ways in which sexual selection might act to increase species richness, and these may still differ between animal groups.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to N. Anthes and I. K. Häderer for contributing in the extraction of the Bateman gradients, and to K. Bòth for drawing the animal specimens of figure 2. We also thank S. Dall, B. Caspers and four anonymous referees for useful comments on the manuscript, and N. W. Bailey, J. Rayner and W. Schneider for helpful discussion.
Data accessibility
The dataset has been uploaded in the electronic supplementary material.
Authors' contributions
T.J. conceived the study. T.J. and L.M.-O. collected the data from the literature. T.J. statistically analysed the data. T.J., E.H.M., M.G.R. and L.M.-O. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by grants from the Swiss National Science Foundation to T. J. (PA00P3-145375/1) and to L.M.-O. (P2BSP3_158842 and P300PA_171516).
References
- 1.Butlin RK, Bridle JR, Schluter D. 2009. Speciation and patterns of biodiversity. In Speciation and patterns of diversity (eds RK Butlin, JR Bridle, D Schluter), pp. 1–14. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Ritchie MG. 2007. Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 38, 79–102. ( 10.1146/annurev.ecolsys.38.091206.095733) [DOI] [Google Scholar]
- 3.Kraaijeveld K, Kraaijeveld-Smit FJL, Maan ME. 2011. Sexual selection and speciation: the comparative evidence revisited. Biol. Rev. 86, 367–377. ( 10.1111/j.1469-185X.2010.00150.x) [DOI] [PubMed] [Google Scholar]
- 4.Lande R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725. ( 10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lande R. 1982. Rapid origin of sexual isolation and character divergence in a cline. Evolution 36, 213–223. ( 10.1111/j.1558-5646.1982.tb05034.x) [DOI] [PubMed] [Google Scholar]
- 6.Lorch PD, Proulx S, Rowe L, Day T. 2003. Condition-dependent sexual selection can accelerate adaptation. Evol. Ecol. Res. 5, 867–881. [Google Scholar]
- 7.Turner GF, Burrows MT. 1995. A model of sympatric speciation by sexual selection. Proc. R. Soc. Lond. B 260, 287–292. ( 10.1098/rspb.1995.0093) [DOI] [Google Scholar]
- 8.Dieckmann U, Doebeli M. 1999. On the origin of species by sympatric speciation. Nature 400, 354–357. ( 10.1038/22521) [DOI] [PubMed] [Google Scholar]
- 9.Gavrilets S, Waxman D. 2002. Sympatric speciation by sexual conflict. Proc. Natl Acad. Sci. USA 99, 10 533–10 538. ( 10.1073/pnas.152011499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Servedio MR, Bürger R. 2014. The counterintuitive role of sexual selection in species maintenance and speciation. Proc. Natl Acad. Sci. USA 111, 8113–8118. ( 10.1073/pnas.1316484111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker GA, Partridge L. 1998. Sexual conflict and speciation. Phil. Trans. R. Soc. Lond. B 353, 261–274. ( 10.1098/rstb.1998.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panhuis TM, Butlin R, Zuk M, Tregenza T. 2001. Sexual selection and speciation. Trends Ecol. Evol. 16, 364–371. ( 10.1016/S0169-5347(01)02160-7) [DOI] [PubMed] [Google Scholar]
- 13.Arnqvist G, Edvardsson M, Friberg U, Nilsson T. 2000. Sexual conflict promotes speciation in insects. Proc. Natl Acad. Sci. USA 97, 10 460–10 464. ( 10.1073/pnas.97.19.10460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage MJG, Parker GA, Nylin S, Wiklund C. 2002. Sexual selection and speciation in mammals, butterflies and spiders. Proc. R. Soc. Lond. B 269, 2309–2316. ( 10.1098/rspb.2002.2154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mank JE. 2007. Mating preferences, sexual selection and patterns of cladogenesis in ray-finned fishes. J. Evol. Biol. 20, 597–602. ( 10.1111/j.1420-9101.2006.01251.x) [DOI] [PubMed] [Google Scholar]
- 16.Ritchie MG, Webb SA, Graves JA, Magurran AE, Macias Garcia C. 2005. Patterns of speciation in endemic Mexican Goodeid fish: sexual conflict or early radiation? J. Evol. Biol. 18, 922–929. ( 10.1111/j.1420-9101.2005.00919.x) [DOI] [PubMed] [Google Scholar]
- 17.Møller AP, Cuervo JJ. 1998. Speciation and feather ornamentation in birds. Evolution 52, 859–869. ( 10.1111/j.1558-5646.1998.tb03710.x) [DOI] [PubMed] [Google Scholar]
- 18.Owens IPF, Bennett PM, Harvey PH. 1999. Species richness among birds: body size, life history, sexual selection or ecology? Proc. R. Soc. Lond. B 266, 933–939. ( 10.1098/rspb.1999.0726) [DOI] [Google Scholar]
- 19.Morrow EH, Pitcher TE, Arnqvist G. 2003. No evidence that sexual selection is an ‘engine of speciation’ in birds. Ecol. Lett. 6, 228–234. ( 10.1046/j.1461-0248.2003.00418.x) [DOI] [Google Scholar]
- 20.Huang H, Rabosky DL. 2014. Sexual selection and diversification: reexamining the correlation between dichromatism and speciation rate in birds. Am. Nat. 184, E101–E114. ( 10.1086/678054) [DOI] [PubMed] [Google Scholar]
- 21.Bossu CM, Near TJ. 2015. Ecological constraint and the evolution of sexual dichromatism in darters. Evolution 69, 1219–1231. ( 10.1111/evo.12655) [DOI] [PubMed] [Google Scholar]
- 22.Maia R, Rubenstein DR, Shawkey MD. 2016. Selection, constraint, and the evolution of coloration in African starlings. Evolution 70, 1064–1079. ( 10.1111/evo.12912) [DOI] [PubMed] [Google Scholar]
- 23.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 24.Ellis EA, Oakley TH. 2016. High rates of species accumulation in animals with bioluminescent courtship displays. Curr. Biol. 26, 1–6. ( 10.1016/j.cub.2016.05.043) [DOI] [PubMed] [Google Scholar]
- 25.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368. ( 10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 26.Arnold SJ. 1994. Bateman principles and the measurement of sexual selection in plants and animals. Am. Nat. 144, S126–S149. ( 10.1086/285656) [DOI] [Google Scholar]
- 27.Mobley KB. 2014. Mating systems and the measurement of sexual selection. In Animal behaviour: how and why animals do things they do (ed. Yasukawa K.), pp. 99–144. Santa Barbara, CA: Praeger. [Google Scholar]
- 28.Janicke T, Häderer IK, Lajeunesse MJ, Anthes N. 2016. Darwinian sex roles confirmed across the animal kingdom. Sci. Adv. 2, e1500983 ( 10.1126/sciadv.1500983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anthes N, Häderer IK, Michiels NK, Janicke T. 2017. Measuring and interpreting sexual selection metrics: evaluation and guidelines. Methods Ecol. Evol. 8, 918–931. ( 10.1111/2041-210X.12707) [DOI] [Google Scholar]
- 30.Fritzsche K, Arnqvist G. 2013. Homage to Bateman: sex roles predict sex differences in sexual selection. Evolution 67, 1926–1936. ( 10.1111/evo.12086) [DOI] [PubMed] [Google Scholar]
- 31.Mills SC, Grapputo A, Koskela E, Mappes T. 2007. Quantitative measure of sexual selection with respect to the operational sex ratio: a comparison of selection indices. Proc. R. Soc. B 274, 143–150. ( 10.1098/rspb.2006.3639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones AG, Rosenqvist G, Berglund A, Arnold SJ, Avise JC. 2000. The Bateman gradient and the cause of sexual selection in a sex-role-reversed pipefish. Proc. R. Soc. Lond. B 267, 677–680. ( 10.1098/rspb.2000.1055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janicke T, Morrow EH. 2018. Operational sex ratio predicts the opportunity and direction of sexual selection across animals. Ecol. Lett. 21, 384–391. ( 10.1111/ele.12907) [DOI] [PubMed] [Google Scholar]
- 34.Henshaw JM, Kahn AT, Fritzsche K. 2016. A rigorous comparison of sexual selection indexes via simulations of diverse mating systems. Proc. Natl Acad. Sci. USA 113, E300–E308. ( 10.1073/pnas.1518067113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collet JM, Dean RF, Worley K, Richardson DS, Pizzari T. 2014. The measure and significance of Bateman's principles. Proc. R. Soc. B 281, 20132973 ( 10.1098/rspb.2013.2973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marie-Orleach L, Janicke T, Vizoso DB, David P, Schärer L. 2016. Quantifying episodes of sexual selection: insights from a transparent worm with fluorescent sperm. Evolution 70, 314–328. ( 10.1111/evo.12861) [DOI] [PubMed] [Google Scholar]
- 37.Walker LK, Ewen JG, Brekke P, Kilner RM. 2014. Sexually selected dichromatism in the hihi Notiomystis cincta: multiple colours for multiple receivers. J. Evol. Biol. 27, 1522–1535. ( 10.1111/jeb.12417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerlach NM, McGlothin JW, Parker PG, Ketterson ED. 2012. Reinterpreting Bateman gradients: multiple mating and selection in both sexes of a songbird species. Behav. Ecol. 23, 1078–1088. ( 10.1093/beheco/ars077) [DOI] [Google Scholar]
- 39.Fitze PS, Le Galliard J-F. 2011. Inconsistency between differet measures of sexual selection. Am. Nat. 178, 256–268. ( 10.1086/660826) [DOI] [PubMed] [Google Scholar]
- 40.Ketterson ED, Parker PG, Raouf SA, Nolan V Jr, Ziegenfus C, Chandler CR. 1998. The relative impact of extra-pair fertilizations on variation in male and female reproductive success in dark-eyed juncos (Junco hyemalisi). Ornithol. Monogr. 49, 81–101. ( 10.2307/40166719) [DOI] [Google Scholar]
- 41.Klug H, Heuschele J, Jennions MD, Kokko H. 2010. The mismeasurement of sexual selection. J. Evol. Biol. 23, 447–462. ( 10.1111/j.1420-9101.2009.01921.x) [DOI] [PubMed] [Google Scholar]
- 42.Jennions MD, Kokko H, Klug H. 2012. The opportunity to be misled in studies of sexual selection. J. Evol. Biol. 25, 591–598. ( 10.1111/j.1420-9101.2011.02451.x) [DOI] [PubMed] [Google Scholar]
- 43.Mobley KB, Jones AG. 2009. Environmental, demographic, and genetic mating system variation among five geographically distinct dusky pipefish (Syngnathus floridae) populations. Mol. Ecol. 18, 1476–1490. ( 10.1111/j.1365-294X.2009.04104.x) [DOI] [PubMed] [Google Scholar]
- 44.Janicke T, David P, Chapuis E. 2015. Environment-dependent sexual selection: Bateman's parameters under varying levels of food availability. Am. Nat. 185, 756–768. ( 10.1086/681128) [DOI] [PubMed] [Google Scholar]
- 45.Morimoto J, Pizzari T, Wigby S. 2016. Developmental environment effects on sexual selection in male and female Drosophila melanogaster. PLoS ONE 11, 1–27. ( 10.1371/journal.pone.0154468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kokko H, Jennions MD. 2015. Describing mate choice in a biased world: comments on Edward and Dougherty & Shuker. Behav. Ecol. 26, 320–321. ( 10.1093/beheco/arv005) [DOI] [Google Scholar]
- 47.Turnell BR, Shaw KL. 2015. High opportunity for postcopulatory sexual selection under field conditions. Evolution 69, 2094–2104. ( 10.1111/evo.12721) [DOI] [PubMed] [Google Scholar]
- 48.Devost E, Turgeon J. 2016. The combined effects of pre- and post-copulatory processes are masking sexual conflict over mating rate in Gerris buenoi. J. Evol. Biol. 29, 167–177. ( 10.1111/jeb.12772) [DOI] [PubMed] [Google Scholar]
- 49.Nakagawa S, Poulin R, Mengersen K, Reinhold K, Engqvist L, Lagisz M, Senior AM. 2015. Meta-analysis of variation: ecological and evolutionary applications and beyond. Methods Ecol. Evol. 6, 143–152. ( 10.1111/2041-210X.12309) [DOI] [Google Scholar]
- 50.Hedges L, Olkin I. 1985. Statistical methods for meta-analysis. New York, NY: Academic Press. [Google Scholar]
- 51.Hedges SB, Dudley J, Kumar S. 2006. TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics 22, 2971–2972. ( 10.1093/bioinformatics/btl505) [DOI] [PubMed] [Google Scholar]
- 52.Viechtbauer W. 2010. Journal of statistical software. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 53.Paradis E. 2012. Analysis of phylogenetics and evolution with R. New York, NY: Springer. [Google Scholar]
- 54.Debelle A, Ritchie MG, Snook RR. 2016. Sexual selection and assortative mating: an experimental test. J. Evol. Biol. 29, 1307–1316. ( 10.1111/jeb.12855) [DOI] [PubMed] [Google Scholar]
- 55.Collet J, Richardson DS, Worley K, Pizzari T. 2012. Sexual selection and the differential effect of polyandry. Proc. Natl Acad. Sci. USA 109, 8641–8645. ( 10.1073/pnas.1200219109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pélissié B, Jarne P, Sarda V, David P. 2014. Disentangling precopulatory and postcopulatory sexual selection in polyandrous species. Evolution 68, 1320–1331. ( 10.1111/evo.12353) [DOI] [PubMed] [Google Scholar]
- 57.Evans JP, Garcia-Gonzalez F. 2016. The total opportunity for sexual selection and the integration of pre- and post-mating episodes of sexual selection in a complex world. J. Evol. Biol. 29, 2338–2361. ( 10.1111/jeb.12960) [DOI] [PubMed] [Google Scholar]
- 58.Krakauer AH, Webster MS, Duval EH, Jones AG, Shuster SM. 2011. The opportunity for sexual selection: not mismeasured, just misunderstood. J. Evol. Biol. 24, 2064–2071. ( 10.1111/j.1420-9101.2011.02317.x) [DOI] [PubMed] [Google Scholar]
- 59.Sutherland WJ. 1985. Chance can produce a sex difference in variance in mating success and explain Batemans data. Anim. Behav. 33, 1349–1352. ( 10.1016/s0003-3472(85)80197-4) [DOI] [Google Scholar]
- 60.Fairbairn DJ, Wilby AE. 2001. Inequality of opportunity: measuring the potential for sexual selection. Evol. Ecol. Res. 3, 667–686. [Google Scholar]
- 61.Downhower JF, Blumer LS, Brown L. 1987. Opportunity for selection: an appropriate measure for evaluating variation in the potential for selection? Evolution 41, 1395–1400. ( 10.1111/j.1558-5646.1987.tb02476.x) [DOI] [PubMed] [Google Scholar]
- 62.Ruzzante DE, Hamilton DC, Kramer DL, Grant JWA. 1996. Scaling of the variance and the quantification of resource monopolization. Behav. Ecol. 7, 199–207. ( 10.1093/beheco/7.2.199) [DOI] [Google Scholar]
- 63.Holman L, Kokko H. 2013. The consequences of polyandry for population viability, extinction risk and conservation. Phil. Trans. R. Soc. B 368, 20120053 ( 10.1098/rstb.2012.0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Candolin U, Heuschele J. 2008. Is sexual selection beneficial during adaptation to environmental change? Trends Ecol. Evol. 23, 446–452. ( 10.1016/j.tree.2008.04.008) [DOI] [PubMed] [Google Scholar]
- 65.Holland B, Rice WR. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA 96, 5083–5088. ( 10.1073/pnas.96.9.5083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: Clarendon Press. [Google Scholar]
- 67.Arnqvist G, Rowe L. 2005. Sexual conflict. Princeton, NJ: Princeton University Press. [Google Scholar]
- 68.Whitlock MC, Agrawal AF. 2009. Purging the genome with sexual selection: reducing mutation load through selection on males. Evolution 63, 569–582. ( 10.1111/j.1558-5646.2008.00558.x) [DOI] [PubMed] [Google Scholar]
- 69.Lumley AJ, et al. 2015. Sexual selection protects against extinction. Nature 522, 470–473. ( 10.1038/nature14419) [DOI] [PubMed] [Google Scholar]
- 70.Almbro M, Simmons LW. 2014. Sexual selection can remove an experimentally induced mutation load. Evolution 68, 295–300. ( 10.1111/evo.12238) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset has been uploaded in the electronic supplementary material.