Abstract
Understanding how climate change will shape species distributions in the future requires a functional understanding of the demographic responses of animals to their environment. For birds, most of our knowledge of how climate influences population vital rates stems from research in temperate environments, even though most of Earth's avian diversity is concentrated in the tropics. We evaluated effects of Southern Oscillation Index (SOI) and local temperature and rainfall at multiple temporal scales on sex-specific survival of a resident tropical bird, the rufous-and-white wren Thryophilus rufalbus, studied over 15 years in the dry forests of northwestern Costa Rica. We found that annual apparent survival of males was 8% higher than females, more variable over time, and responded more strongly to environmental variation than female survival, which did not vary strongly with SOI or local weather. For males, mean and maximum local temperatures were better predictors of survival than either rainfall or SOI, with high temperatures during the dry season and early wet season negatively influencing survival. These results suggest that, even for species adapted to hot environments, further temperature increases may threaten the persistence of local populations in the absence of distributional shifts.
Keywords: climate change, population dynamics, Southern Oscillation Index, Thryophilus rufalbus, Thryothorus rufalbus, seasonality
1. Introduction
Knowledge of how climate influences survival and fecundity is necessary for understanding the factors that limit species abundance and shape their distributions. The ubiquity, diversity, wide-ranging life histories and movement strategies of birds have made them model organisms for studies of the consequences of climate change for migration [1], population dynamics [2–4] and species distributions [5,6]. However, the overwhelming majority of these studies have focused on migratory species or residents of temperate environments. Considering that avian diversity is higher in the tropics than anywhere else on Earth and that many tropical species are range-restricted [7,8], establishing a better understanding of how climate influences demography of tropical bird populations is essential for predicting consequences of future climate change for the persistence of local populations and species as a whole.
To date, the few long-term, longitudinal studies aimed at elucidating the effects of climate on demography of tropical bird species have revealed widely varying population-level responses to environmental variation both within and among species depending on habitat and species traits, such as diet and geography [9–14]. For example, adult white-collared manakins Manacus candei in Costa Rica exhibited higher annual survival during cooler and wetter (positive) phases of the Southern Oscillation Index (SOI), but this effect was only apparent in young forests and not mature forest [10]. By contrast, adult male wire-tailed manakins Pipra filicauda in Ecuador exhibited higher annual survival during warmer and drier (negative) phases of SOI [12]. Two bird species in the Northern Mariana Islands showed a positive relationship between survival and relative dry-season greenness and a negative relationship between survival and relative wet-season greenness, suggesting that consistent moderate conditions favour survival and that extreme rainfall events negatively affect survival [13]. Whereas these results revealed relationships between climate and survival, albeit in sometimes opposite directions, only one of 20 bird species analysed in Panama revealed a relationship between climate (length of the dry season) and adult survival [11]. Coupled with growing evidence of declines in tropical bird populations [15,16], the observed variation in results of these studies highlights the need for a clearer understanding of how the demography of tropical bird populations responds to climatic variation at different spatio-temporal scales.
Here, we evaluated the effects of climate (SOI) and local weather (temperature and rainfall) at annual, seasonal and monthly time scales on sex-specific annual apparent adult survival of a resident tropical bird, the rufous-and-white wren Thryophilus rufalbus. We collected 15 years of mark–recapture/re-sighting data from a population living in tropical dry forests in the Area de Conservación Guanacaste in northwestern Costa Rica. Tropical dry forests rank among the most imperilled terrestrial habitats globally, and climate change threatens the persistence these forests [17] as well as some of the species that inhabit them [18]. Whereas most studies that have examined survival of tropical birds have generally only considered broad-scale climate indices, we also evaluated effects of local rainfall and temperature on survival and how rainfall influences vegetation greenness, a proxy for resource availability, to provide greater insight into the mechanisms mediating effects of climatic variation on survival.
2. Material and methods
(a). Study species
Rufous-and-white wrens are resident, insectivorous Neotropical songbirds that inhabit the understorey of tropical forests along the Pacific coast of southern Mexico to Panama (figure 1a), as well as parts of northern Colombia and Venezuela [19,20]. Annually, rufous-and-white wrens build nests several weeks in advance of the rainy season and begin laying and incubating eggs following the first large rainfall, typically in early May in our study area. Owing to high levels of nest predation (greater than or equal to 50% of nests are depredated each year [21,22]), pairs will often attempt several nests per year, often continuing into August [23]. Rufous-and-white wrens, like other understorey insectivores, exhibit low levels of breeding dispersal [24]. Low mobility makes this species well suited to demographic studies because measured (apparent) survival should closely approximate actual survival and because, with low levels of movement among populations (immigration and emigration) [24], survival and recruitment will be primary contributors to populations dynamics and persistence. Low mobility also makes insectivorous birds highly vulnerable to habitat disturbance and fragmentation [25,26], further emphasizing the need for expanded knowledge of environment–demography relationships for this and other tropical species.
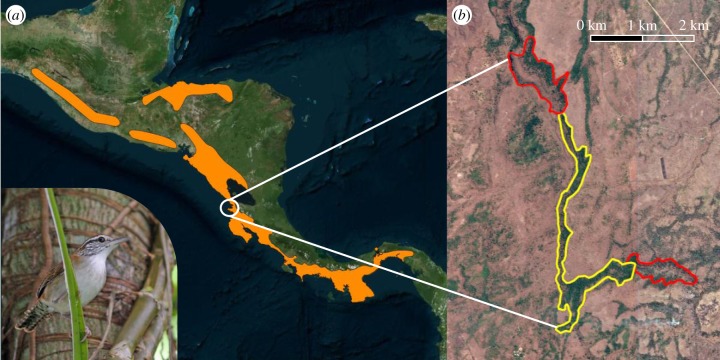
Figure 1.
Distribution of rufous-and-white wrens in Central America and location of the study site. (a) Appearance and year-round distribution (orange) of rufous-and-white wrens in Central America, which also includes parts of Colombia and Venezuela not shown here (species distribution data from [19]). (b) The study site in Sector Santa Rosa of the Area de Conservación Guanacaste in northwestern Costa Rica (10°52′N, 85°36′W). The study site encompassed an area of 125 ha from 2003 to 2005 (yellow), which was expanded by approximately 115 ha (red) in 2006. The background satellite image in (b) was taken at the end of the dry season in May 2013, highlighting the limited availability and patchiness of evergreen forest in comparison to seasonal scrub forest. Photo in (a) courtesy of David Bradley.
(i). Study site and field methods
Our study took place in Sector Santa Rosa of the Area de Conservación Guanacaste in northwestern Costa Rica (10°52′ N, 85°36′ W; figure 1b). Habitat in Santa Rosa is characterized by a lowland tropical dry forest (elevation range = 225–290 m.a.s.l.) with a relatively open understorey, especially during the dry season [27,28]. Within the dry forest, rufous-and-white wrens inhabit the more mature humid, evergreen sections (figure 1b). The dry season in Santa Rosa typically extends from December to April and the wet season from May to November, although the exact timing and duration of the seasons varies from year to year. Over the course of our study, the first 100 mm of rainfall in the calendar year was reached on 20 May (mean ± s.d. ordinal date = 141 ± 12 days) and all but the final 100 mm of rain had fallen by 6 November (mean ± s.d. ordinal date = 310 ± 11 days), resulting in an average (±s.d.) wet season length of 170 ± 18 days. Annual rainfall ranged from 660 to 3090 mm over the course of our study, with 5 years exceeding 2500 mm of rain (figure 2a).
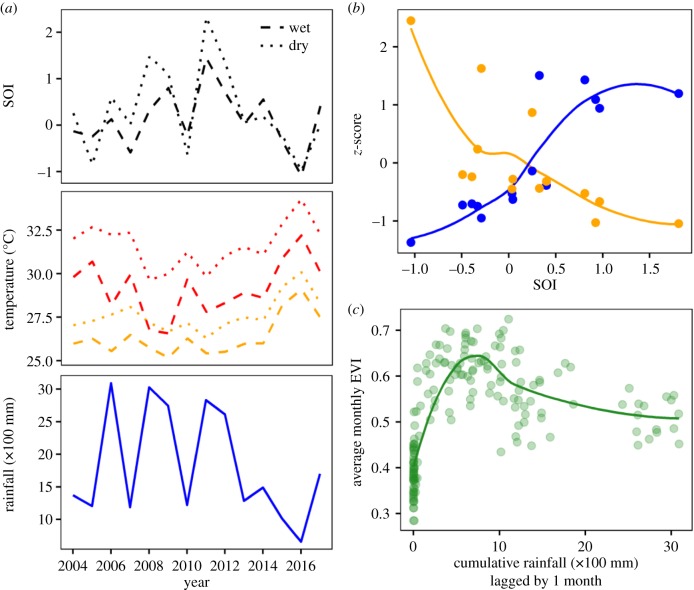
Figure 2.
SOI, local temperature and rainfall, and Enhanced Vegetation Index (EVI) measurements for Santa Rosa National Park, Costa Rica between 2003 and 2017. (a) Time-series of seasonally averaged monthly SOI (black), seasonally averaged daily maximum (red) and mean (orange) temperatures and total annual rainfall (blue). Monthly SOI and daily maximum and mean temperatures were averaged for the dry (April, December–March) and wet (May–November) seasons. Rainfall was summed from April to the following March. (b) z-scores of average annual mean daily temperature (orange) and total annual precipitation (blue) in relation to annual average SOI. (c) Average monthly EVI in relation to cumulative rainfall from the previous month in the Santa Rosa study area. EVI plateaued between 500 and 1000 mm of cumulative rainfall, which was achieved in all but one of the study years. Curves in (b) and (c) were estimated from a local polynomial regression.
We conducted mark–recapture/re-sighting surveys of rufous-and-white wrens from 2003 to 2017. From 2003 to 2005, our study area was 125 ha in size (figure 1b). In 2006, we expanded the study area to 240 ha and continued to monitor this area through to 2017 (figure 1b). Beginning in April of each year, individuals were identified and captured using mist-nets. Each captured individual was marked with a unique combination of three plastic colour leg-bands and one aluminium leg-band and standard morphometric measurements were taken. Males and females were differentiated based on morphometric measurements, the presence or the absence of a brood patch, as well as sex-specific features of their songs [29–31]. Individuals that could not be captured, at least initially, were uniquely identified based on their consistent occupation of the same area or territory and their individually distinctive vocalizations [29,30,32]. All individuals in the population were identified by approximately mid-April of each year. Daily observations of the population continued until the end of June and included recording the territory centres of each bird using a hand-held Global Positioning System. Non-territorial floaters are rare in this population [33].
(b). Environmental data
We used standardized monthly values of the SOI (downloaded from http://www.cpc.ncep.noaa.gov/data/indices/soi) to describe the climatic conditions at our study site. As with many locations in the northern neotropics, Santa Rosa experiences cooler and wetter conditions during positive phases of the Southern Oscillation (La Niña) and warmer and drier conditions (El Niño) during negative phases of the Southern Oscillation [34]. From the standardized monthly values, we calculated average annual (April–March), wet season (May–November) and dry season (April, December–March) SOI for each year of the study. Climate and weather data were summarized beginning in April to coincide with the beginning of the mark–recapture/re-sighting period.
Daily maximum and minimum temperatures (°C) and rainfall (mm) measurements were obtained from weather stations within our study area operated by the Área de Conservación Guanacaste (2003–2015) and by L. Fedigan, A. Melin and K. Jack (2015–2017). Comparison of daily temperature and rainfall measurements from the two stations in 2015 revealed similar measurements (electronic supplementary material, figure S1), justifying the use of weather data from the latter station for the final 3 years of our study (2015–2017). Daily mean temperature was calculated as the mean of the daily minimum and maximum temperatures. Daily mean and maximum temperatures were then averaged at monthly, seasonal (wet/dry) and annual intervals (electronic supplementary material, table S1). Rainfall was summed over the same time intervals and we also calculated cumulative rainfall at monthly intervals, beginning in April and extending through to the following March (e.g. total rainfall for June was calculated as the sum of daily rainfall in June, whereas cumulative rainfall for June was calculated as the sum of daily rainfall in April, May and June; electronic supplementary material, table S1).
We also used the local rainfall data to measure the timing and duration of the wet season. Duration of the wet season was calculated as the number of days between when the first and last 50, 100 and 150 mm of rainfall occurred in each year. Timing of the onset (or end) of the wet season was measured as the ordinal date on which the first (or last) 50, 100, 150, 200, 250 and 300 mm of rain had fallen. A full list of the eight climate and weather variables included in our analyses and the time scales over which they were summarized are presented in electronic supplementary material, table S1.
Lastly, we extracted monthly, 1 km2 resolution Enhanced Vegetation Index (EVI) measurements from the Terra MODIS dataset MOD13A3 [35] for the full 15-year study period to explore the relationship between local rainfall and vegetation greenness in our study area. MOD13A3 data were retrieved from the online DAAC2Disk download manager, courtesy of the NASA EOSDIS Land Processes Distributed Active Archive Center and USGS/Earth Resources Observation and Science Center (https://lpdaac.usgs.gov/data_access/daac2disk). Similar to Saracco et al. [13], we removed cloud-contaminated pixels (pixel reliability code = 3), extracted monthly EVI values for the six 1 km2 pixels overlapping our study area using R [36] and package ‘raster’ [37], and then averaged values for the six pixels to acquire a monthly average EVI. We related monthly EVI to cumulative rainfall for the previous month using local polynomial regression implemented using the ‘loess’ function in R with a smoothing parameter (or span) equal to 0.9 (figure 2c).
(c). Statistical analyses
We estimated sex-specific annual apparent survival and recapture/re-sighting probabilities using a Cormack–Jolly–Seber (CJS) model [38]. Hereafter, we use the word ‘survival’ in place of ‘annual apparent survival’, and ‘re-encounter probability’ in place of ‘recapture/re-sighting probability’. We analysed the CJS model in a Bayesian framework using Markov chain Monte Carlo (MCMC) simulations, which we implemented in JAGS [39] from R [36] using the package ‘jagsUI’ [40]. The CJS model was formulated using a multinomial array [41,42].
To quantify temporal variability, survival φ and re-encounter probability p of each sex s was modelled on a logit scale as a function of its mean μ and temporal residual ɛt:
| 2.1 |
and
| 2.2 |
We evaluated effects of climate, local weather and seasonal timing and duration on survival by fitting a series of univariable models that included the climate and weather variables described above (see also electronic supplementary material, table S1). Relative variable importance was evaluated based on the magnitudes of the standardized slope estimates (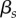 in equation (2.3)) and their uncertainty (95% credible intervals). We also evaluated the relative importance of variables using the indicator variable method [43], which can be an effective means of identifying influential predictors under high estimation uncertainty [44]. By this method, each covariate β is multiplied by an indicator variable γ that is drawn from a Bernoulli distribution with a prior probability of 0.5. At each MCMC iteration, γ takes a value of 1 or 0, causing the variable to be included or excluded from the model, respectively. If a variable is important for model fit, then γ will be included more times than not, causing the posterior mean of γ to approach 1. By contrast, a variable that does little to improve model fit will often be excluded from the model, such that the posterior mean of γ will tend to 0. We only fitted models containing a single predictor variable at a time due to SOI, temperature and rainfall being highly correlated (figure 2). The structure of the models was as follows:
in equation (2.3)) and their uncertainty (95% credible intervals). We also evaluated the relative importance of variables using the indicator variable method [43], which can be an effective means of identifying influential predictors under high estimation uncertainty [44]. By this method, each covariate β is multiplied by an indicator variable γ that is drawn from a Bernoulli distribution with a prior probability of 0.5. At each MCMC iteration, γ takes a value of 1 or 0, causing the variable to be included or excluded from the model, respectively. If a variable is important for model fit, then γ will be included more times than not, causing the posterior mean of γ to approach 1. By contrast, a variable that does little to improve model fit will often be excluded from the model, such that the posterior mean of γ will tend to 0. We only fitted models containing a single predictor variable at a time due to SOI, temperature and rainfall being highly correlated (figure 2). The structure of the models was as follows:
| 2.3 |
Vague prior distributions were specified for all parameters: 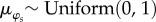 ;
; 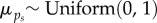 ;
; 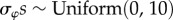 ;
; 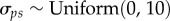 ;
; 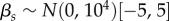 . We ran three independent chains with different starting values for 250 000 iterations. We used a burn-in of 50 000 iterations and kept every 50th sample, resulting in 12 000 posterior samples for each model parameter. For modelling, climatic and weather variables were converted to z-scores by subtracting the mean of the variable across all years from the value for each year and dividing by the standard deviation of the variable across years, such that each variable had a mean of 0 and standard deviation of 1. Convergence of model chains was assessed using the Gelman-Rubin
. We ran three independent chains with different starting values for 250 000 iterations. We used a burn-in of 50 000 iterations and kept every 50th sample, resulting in 12 000 posterior samples for each model parameter. For modelling, climatic and weather variables were converted to z-scores by subtracting the mean of the variable across all years from the value for each year and dividing by the standard deviation of the variable across years, such that each variable had a mean of 0 and standard deviation of 1. Convergence of model chains was assessed using the Gelman-Rubin 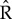 diagnostic statistic [45]. In the results, we present estimated survival and re-encounter probabilities on the real scale, whereas effect size and variance estimates are on the logit scale.
diagnostic statistic [45]. In the results, we present estimated survival and re-encounter probabilities on the real scale, whereas effect size and variance estimates are on the logit scale.
Goodness-of-fit of the CJS models was evaluated using the Freeman–Tukey statistic [46]. With this method, expected data simulated from the CJS model are compared to the observed data. Bayesian p-values greater than 0.5 indicate that the expected data are more variable than the observed data, whereas p-values less than 0.5 indicate that expected data are less variable than the observed data. A model that fits the data perfectly will result in a Bayesian p-value of 0.5. We found no evidence of lack of fit for any models. For the null model (equation (2.1)), Bayesian p-values were 0.58 for females and 0.41 for males (electronic supplementary material, figure S2). Across all models that contained environmental covariates, Bayesian p-values averaged 0.41 for males (range = 0.32–0.60) and 0.58 for females (range = 0.51–0.62).
3. Results
From 2003 to 2017, we marked and monitored the survival of a total of 314 rufous-and-white wrens (175 males and 139 females). The number of wrens monitored each year ranged from 22 to 70, with an average of 44. Mean annual survival probability of rufous-and-white wrens over the 15-year period was 0.60 [95% credible interval (CI) = 0.52, 0.68] for males (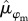 ) and 0.52 [95% CI = 0.44, 0.59] for females (
) and 0.52 [95% CI = 0.44, 0.59] for females (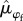 ), a difference of 8% between the two sexes (Pr[
), a difference of 8% between the two sexes (Pr[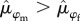 ] = 0.94; figure 3). Survival of males was more variable over time compared with females (
] = 0.94; figure 3). Survival of males was more variable over time compared with females (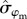 = 0.42 versus
= 0.42 versus 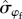 = 0.22; Pr[
= 0.22; Pr[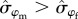 ] = 0.79). Of the 160 wrens encountered in more than 1 year of the study, 55% remained within 100 m of where they were initially encountered, 26% had a net dispersal of 100–400 m, and 14% had a net dispersal of 400–1000 m. Only 6% (n = 9) dispersed greater than 1 km. Re-encounter probabilities were high for both sexes (
] = 0.79). Of the 160 wrens encountered in more than 1 year of the study, 55% remained within 100 m of where they were initially encountered, 26% had a net dispersal of 100–400 m, and 14% had a net dispersal of 400–1000 m. Only 6% (n = 9) dispersed greater than 1 km. Re-encounter probabilities were high for both sexes (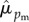 = 0.96 [95% CI = 0.87, 0.99] versus
= 0.96 [95% CI = 0.87, 0.99] versus 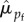 = 0.93 [95% CI = 0.80, 0.99]).
= 0.93 [95% CI = 0.80, 0.99]).
Figure 3.
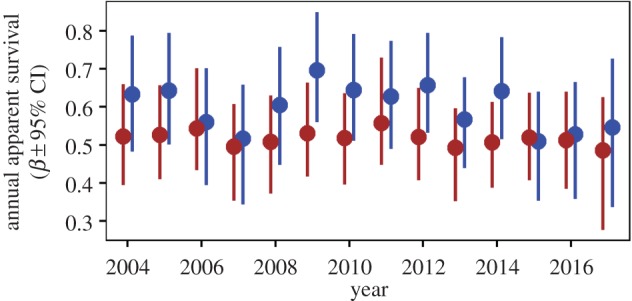
Sex-specific annual apparent survival probabilities of rufous-and-white wrens in Santa Rosa National Park, Costa Rica between 2003 and 2017. Male (blue) and female (maroon) survival were estimated using a Cormack–Jolly–Seber model.
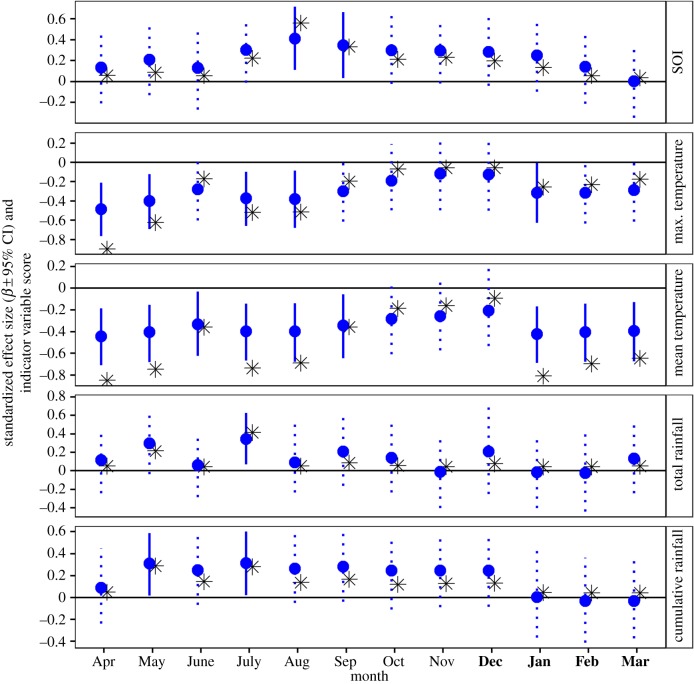
Annual survival of male rufous-and-white wrens was related to SOI during the wet season, whereas female survival was unrelated to annual or seasonal SOI (figure 4). Male survival was positively related to wet-season SOI, primarily in August and September (figure 5). Consistent with positive phases of the SOI coinciding with cooler and wetter weather in Santa Rosa (figure 2b), male survival was negatively related to temperatures during the wet season (figure 4). Effects of rainfall on male survival were strongest for May and July (figure 5), but were overall weaker than effects of temperature. Female survival was unrelated to annual and seasonal temperatures and rainfall (figure 4). The only wet season weather variable for which there was some (albeit weak) evidence of a relationship to female survival was total September rainfall (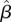 = 0.33, 95% CI = 0.02, 0.66; electronic supplementary material, figure S3). In 2006, 2009 and 2011, over 500 mm of rain fell in September compared with less than 400 mm in all other years. This coincided with an increase in female survival of 2.6%, 1.4% and 4.0% above the long-term average.
= 0.33, 95% CI = 0.02, 0.66; electronic supplementary material, figure S3). In 2006, 2009 and 2011, over 500 mm of rain fell in September compared with less than 400 mm in all other years. This coincided with an increase in female survival of 2.6%, 1.4% and 4.0% above the long-term average.
Figure 4.
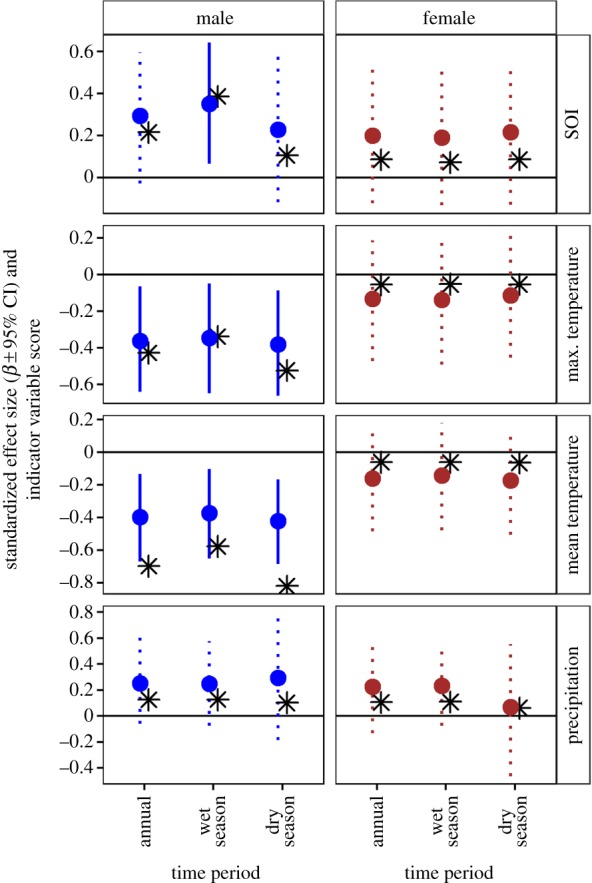
Effects of climate and local weather on annual survival of rufous-and-white wrens. Points show slope estimates (±95% CI) from Cormack–Jolly–Seber models relating annual survival to SOI or local weather (daily mean and maximum temperature and total precipitation) averaged over annual and seasonal (wet/dry) time periods. Asterisks show indicator variable scores, which range from 0 (unimportant) to 1 (important). For visualization, indicator variable scores were multiplied by −1 if the corresponding effect size was negative. Credible intervals that overlap zero are represented with dotted lines. (Online version in colour.)
Figure 5.
Effects of monthly SOI and local weather on annual apparent survival of male rufous-and-white wrens. Points show slope estimates (± 95% CI) from Cormack–Jolly–Seber models relating annual apparent survival to monthly SOI and local weather. Months that occur during the dry season are indicated with bold text on the x-axis. Asterisks show indicator variable scores, which range from 0 (unimportant) to 1 (important). For visualization, indicator variable scores were multiplied by −1 if the corresponding effect size was negative. Credible intervals that overlap zero are represented with dotted lines. (Online version in colour.)
Although we did not find evidence for an effect of dry-season SOI on male survival, temperatures during this season had a strong negative effect on male survival (figure 4). In particular, mean and maximum temperatures in April, coinciding with the end of the dry season, had the strongest effects of any climatic or local weather variable on male survival (figure 5). The weakest temperature effects were in October, November and December, coinciding with peak rainfall, the end of the wet season and beginning of the dry season, respectively (figure 5). We found no evidence for effects of dry season temperature on female survival (figure 4). There was some evidence of a positive relationship between February precipitation and female survival (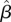 = 0.41, 95% CI = 0.04, 0.91; electronic supplementary material, figure S3), but this pattern was primarily due to 2 years (2006 and 2011) in which 10–15 mm of rain fell in February (compared with less than 2 mm in all other years). In those same years, female survival was 2.6 and 4.0% higher than the long-term average.
= 0.41, 95% CI = 0.04, 0.91; electronic supplementary material, figure S3), but this pattern was primarily due to 2 years (2006 and 2011) in which 10–15 mm of rain fell in February (compared with less than 2 mm in all other years). In those same years, female survival was 2.6 and 4.0% higher than the long-term average.
We found some evidence for timing of the onset of the wet season influencing annual survival of males. Male survival was higher when the first 150–250 mm of rain fell earlier in the year (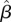 150 mm = −0.28, 95% CI = −0.56, −0.01;
150 mm = −0.28, 95% CI = −0.56, −0.01; 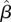 200 mm = −0.27, 95% CI = −0.56, 0.0;
200 mm = −0.27, 95% CI = −0.56, 0.0; 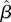 250 mm = −0.29, 95% CI = −0.60, 0.00; electronic supplementary material, figure S4), but, as with cumulative and total rainfall, timing of the onset of the wet season was a weaker predictor of male survival than temperature. Timing of the end of the wet season and total duration of the wet season were not strong predictors of rufous-and-white wren survival for either sex, nor was the start of the wet season for female survival (electronic supplementary material, figure S4).
250 mm = −0.29, 95% CI = −0.60, 0.00; electronic supplementary material, figure S4), but, as with cumulative and total rainfall, timing of the onset of the wet season was a weaker predictor of male survival than temperature. Timing of the end of the wet season and total duration of the wet season were not strong predictors of rufous-and-white wren survival for either sex, nor was the start of the wet season for female survival (electronic supplementary material, figure S4).
4. Discussion
Our results show that local temperatures, particularly during the dry season and early wet season, are important predictors of annual apparent survival of adult male rufous-and-white wrens, with higher temperatures leading to lower survival. There was also evidence that average wet season SOI, rainfall during the early wet season and timing of the onset of the wet season influenced male survival, but generally these effects were weaker when compared with those of temperature. Similarly, length of the wet season did not strongly influence adult survival of either sex [11]. Although broad-scale climate indices are often better at predicting population processes than local weather [47], and have been shown to predict survival of several tropical birds [10,12], our results show the value of including both local and broad-scale climate and weather measurements when available.
In contrast to male survival, female survival was relatively insensitive to both local weather and broad-scale climatic variation. On average, female survival was 8% lower than male survival, a similar difference to that observed between male and female buff-breasted wrens Cantorchilus leucotis [48]. This, coupled with the result that survival of females was also more constant over time relative to males, suggests a limiting factor that was relatively constant among years, which may have overridden local- and broad-scale climate effects. One such possibility is that females suffer higher costs of reproduction compared with males. Tropical birds often exhibit high nest predation rates and high re-nesting rates, both generally [49–51] and in our study population specifically [21]. In species where females perform all of the incubation and the majority of nestling provisioning, such as rufous-and-white wrens [31,52], this may result in females incurring high reproductive costs which could lead to higher mortality after breeding compared with males. However, identifying how differential costs of reproduction between males and females could contribute to sex differences in survival is complicated by the fact that costs of reproduction may also vary with climate and other environmental factors. Future studies examining drivers of the costs of reproduction in tropical species and impacts of costs of reproduction on other demographic processes are needed.
The positive relationship between SOI and annual survival that we observed contributes to a growing understanding of how climate influences demography of tropical bird species. The pattern of higher survival during cooler and wetter (positive) phases of the SOI is consistent with that observed for white-collared manakins in young forest in northeastern Costa Rica [10], but opposite to that observed for wire-tailed manakins in Ecuador, which had higher survival during warmer and drier (negative) phases of the SOI [12]. For insectivores in dry forests, such as rufous-and-white wrens, survival may be higher when conditions are cooler and wetter due to reduced heat-induced thermoregulatory costs [53]. Indeed, resident tropical species and insectivores have lower basal metabolic rates compared with migrants and frugivorous/granivorous species, respectively, suggesting their ability to tolerate thermal fluctuations may be more limited [8]. That temperature had a stronger influence on survival compared with rainfall lends further support to thermoregulatory costs as a contributor to the observed climate and weather effects on survival. An important implication of this result is that, even for species accustomed to living in hot environments, temperature increases may threaten the persistence of local populations in the absence of distributional shifts.
Food availability is another mechanism that could explain increased survival during cooler and wetter conditions. Insect abundance in the understorey has been shown to increase with moisture in the Guanacaste region [54], suggesting that conditions that are wetter, but not so wet as to limit access to food by inhibiting foraging [55], could improve survival through increased food availability [56]. However, evidence from a comparative study of rufous-and-white wren and buff-breasted wren reproductive biology in Columbia found that insect abundance in the leaf litter, where rufous-and-white wrens primarily forage, did not vary between wet and dry seasons [22]. Moreover, we found that vegetation greenness, a proxy for resource availability, in Santa Rosa plateaued between 600 and 1000 mm of cumulative rainfall (figure 2c). This amount of rainfall was surpassed in all but one of the study years (figure 2a), implying that, even in the driest of years, conditions were sufficiently wet to provide the resources necessary for survival. These two pieces of evidence, coupled with the result that both the amount of rainfall and the timing and duration of the wet seasons had weaker effects on survival compared with temperature, provide further evidence that higher thermoregulatory costs associated with hot temperatures was likely the main mechanism by which climate and weather limited survival.
The opposite effects of SOI on survival of two frugivorous manakin species observed by Wolfe et al. [10] and Ryder & Sillett [12] points to the importance of considering how variation in SOI manifests locally. Indeed, Wolfe et al. [10] found little effect of SOI on manakin survival in mature forests despite strong effects on survival in young forests, showing that responses to climate can vary within species and over small spatial scales. Vast differences in average annual rainfall, which exceeds 5000 mm in northeast Costa Rica compared with under 3000 mm in Ecuador, might explain the opposite effects of SOI on manakin survival if manakins in Ecuador are less adapted to cooler and wetter conditions compared with those in northeast Costa Rica which experience almost double the annual rainfall. Whatever the cause of the discrepancy, variation in responses to climate among and within species seen by comparing our results with those of previous studies [10–13] highlights the need to consider the local environment, such as forest type (open versus closed and dry versus wet) and elevation [55], and species traits, such as their diet and foraging behaviour [11], when predicting and interpreting effects of climate on the dynamics of tropical bird populations. The observed diversity of responses to climatic variation similarly highlights the need to study a broader range of species that represent the range of life histories and habitats in the tropics. Such studies are needed in order to detect emergent patterns of how climatic variation shapes population dynamics across tropical taxa.
In summary, our results provide much-needed information on the demography of a tropical bird, including sex differences in survival and the effects of climatic variables at different spatio-temporal scales thereon. Although declines of tropical bird species have primarily been attributed to direct habitat loss and degradation caused by humans, the observed negative effects of increased temperature on survival in an undisturbed habitat reveals a mechanism by which climate change could drive future population declines in the tropics independent of habitat loss. That the distribution and composition of tropical dry forest itself will probably shift with climate change [17] is likely to only exacerbate the problem in the absence of concurrent distributional shifts by the species that inhabit these forests. Future studies that consider climate and local weather effects on all vital rates simultaneously, and how those vital rates contribute to population growth rate, will further clarify the population dynamical consequences of short-term variation and long-term trends in climate for tropical species.
Supplementary Material
Acknowledgements
We thank members of the Mennill Laboratory who aided in collecting mark–recapture/re-sighting data, particularly Nicole Barker, Sarah Tremain-Douglas, Kristina Hick, Kristin Kovach, Anneka Osmun, Lincoln Savi and Stephanie Topp, as well as many research assistants over the 15 years of this study. We thank the Area de Conservación Guanacaste (ACG), especially Roger Blanco and María Marta Chavarria for ongoing logistical support for this long-term study. We thank the ACG for providing access to 2003–2015 weather data, and we thank Linda Fedigan, Kathy Jack, and Amanda Melin for providing access to 2015–2017 weather data.
Ethics
The long-term field study on rufous-and-white wrens was conducted with permission of the Area de Conservación Guanacaste, the government of Costa Rica (MINAE), and the University of Windsor Animal Care Committee.
Data accessibility
Data and model code supporting this article are available on Dryad: http://dx.doi.org/10.5061/dryad.k785p43 [57].
Authors' contributions
D.J.M. initiated the long-term study in 2003 and, together with his students, collected and collated the mark–recapture data; D.J.M. led the field investigation from 2003 to 2010, B.A.G. from 2011 to 2015 and Z.A.K. from 2015 to 2017. B.K.W., D.J.M. and D.R.N. designed the survival analysis. B.K.W. analysed the data. The authors shared in writing the manuscript.
Competing interests
We declare no competing interests.
Funding
Funding for this field study came from three Discovery Grants and one Discovery Accelerator Supplement from the Natural Sciences and Engineering Research Council of Canada (NSERC) and from the University of Windsor.
References
- 1.Both C, Visser ME. 2001. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298. ( 10.1038/35077063) [DOI] [PubMed] [Google Scholar]
- 2.Sæther B-E, Tufto J, Engen S, Jerstad K, Røstad OW, Skåtan JE. 2000. Population dynamical consequences of climate change for a small temperate songbird. Science 287, 854–856. ( 10.1126/science.287.5454.854) [DOI] [PubMed] [Google Scholar]
- 3.Both C, Bouwhuis S, Lessells CM, Visser ME. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83. ( 10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 4.van Gils JA, et al. 2016. Body shrinkage due to Arctic warming reduces red knot fitness in tropical wintering range. Science 352, 819–821. ( 10.1126/science.aad6351) [DOI] [PubMed] [Google Scholar]
- 5.Şekercioğlu CH, Schneider SH, Fay JP, Loarie SR. 2008. Climate change, elevational range shifts, and bird extinctions. Conserv. Biol. 22, 140–150. ( 10.1111/j.1523-1739.2007.00852.x) [DOI] [PubMed] [Google Scholar]
- 6.Wauchope HS, Shaw JD, Varpe Ø, Lappo EG, Boertmann D, Lanctot RB, Fuller RA. 2017. Rapid climate-driven loss of breeding habitat for Arctic migratory birds. Glob. Change Biol. 23, 1085–1094. ( 10.1111/gcb.13404) [DOI] [PubMed] [Google Scholar]
- 7.Harris JBC, Şekercioğlu CH, Sodhi NS, Fordham DA, Paton DC, Brook BW. 2011. The tropical frontier in avian climate impact research. Ibis 153, 877–882. ( 10.1111/j.1474-919X.2011.01166.x) [DOI] [Google Scholar]
- 8.Şekercioğlu ÇH, Primack RB, Wormworth J. 2012. The effects of climate change on tropical birds. Biol. Conserv. 148, 1–18. ( 10.1016/j.biocon.2011.10.019) [DOI] [Google Scholar]
- 9.Gibbs HL, Grant PR. 1987. Adult survivorship in Darwin's ground finch (Geospiza) populations in a variable environment. J. Anim. Ecol. 56, 797–813. ( 10.2307/4949) [DOI] [Google Scholar]
- 10.Wolfe JD, Ralph CJ, Elizondo P. 2015. Changes in the apparent survival of a tropical bird in response to the El Niño Southern Oscillation in mature and young forest in Costa Rica. Oecologia 178, 715–721. ( 10.1007/s00442-015-3256-z) [DOI] [PubMed] [Google Scholar]
- 11.Brawn JD, Benson TJ, Stager M, Sly ND, Tarwater CE. 2017. Impacts of changing rainfall regime on the demography of tropical birds. Nat. Clim. Change 7, 133–136. ( 10.1038/nclimate3183) [DOI] [Google Scholar]
- 12.Ryder TB, Sillett TS. 2016. Climate, demography and lek stability in an Amazonian bird. Proc. R. Soc. B 283, 20152314 ( 10.1098/rspb.2015.2314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saracco JF, Radley P, Pyle P, Rowan E, Taylor R, Helton L, LaDeau SL. 2016. Linking vital rates of landbirds on a tropical island to rainfall and vegetation greenness. PLoS ONE 11, e0148570 ( 10.1371/journal.pone.0148570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox DTC, Cresswell W. 2014. Mass gained during breeding positively correlates with adult survival because both reflect life history adaptation to seasonal food availability. Oecologia 174, 1197–1204. ( 10.1007/s00442-013-2859-5) [DOI] [PubMed] [Google Scholar]
- 15.Latta SC, Tinoco BA, Graham CH. 2011. Patterns and magnitude of temporal change in avian communities in the Ecuadorian Andes. The Condor 113, 24–40. ( 10.1525/cond.2011.090252) [DOI] [Google Scholar]
- 16.Blake JG, Loiselle BA. 2015. Enigmatic declines in bird numbers in lowland forest of eastern Ecuador may be a consequence of climate change. PeerJ 3, e1177 ( 10.7717/peerj.1177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miles L, Newton AC, DeFries RS, Ravilious C, May I, Blyth S, Kapos V, Gordon JE. 2006. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 33, 491–505. ( 10.1111/j.1365-2699.2005.01424.x) [DOI] [Google Scholar]
- 18.Campos FA, Jack KM, Fedigan LM. 2015. Climate oscillations and conservation measures regulate white-faced capuchin population growth and demography in a regenerating tropical dry forest in Costa Rica. Biol. Conserv. 186, 204–213. ( 10.1016/j.biocon.2015.03.017) [DOI] [Google Scholar]
- 19.BirdLife International and Handbook of the Birds of the World. 2016. Bird species distribution maps of the world. See http://datazone.birdlife.org/species/requestdis.
- 20.Stotz DF, Fitzpatrick JW, Parker TA III, Moskovits DK. 1996. Neotropical birds: ecology and conservation. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Douglas SB, Heath DD, Mennill DJ. 2012. Low levels of extra-pair paternity in a Neotropical duetting songbird, the rufous-and-white wren (Thryothorus rufalbus). The Condor 114, 393–400. ( 10.1525/cond.2012.110028) [DOI] [Google Scholar]
- 22.Ahumada JA. 2001. Comparison of the reproductive biology of two Neotropical wrens in an unpredictable environment in northeastern Colombia. The Auk 118, 191–210. ( 10.2307/4089768) [DOI] [Google Scholar]
- 23.Stiles FG, Skutch AF. 1989. A guide to the birds of Costa Rica. Ithaca, NY: Cornell University Press. [Google Scholar]
- 24.Graham BA, Heath DD, Mennill DJ. 2017. Dispersal influences genetic and acoustic spatial structure for both males and females in a tropical songbird. Ecol. Evol. 7, 10 089–10 102. ( 10.1002/ece3.3456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Şekercioğlu ÇH, Ehrlich PR, Daily GC, Aygen D, Goehring D, Sandí RF. 2002. Disappearance of insectivorous birds from tropical forest fragments. Proc. Natl Acad. Sci. USA 99, 263–267. ( 10.1073/pnas.012616199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stratford JA, Stouffer PC. 1999. Local extinctions of terrestrial insectivorous birds in a fragmented landscape near Manaus, Brazil. Conserv. Biol. 13, 1416–1423. ( 10.1046/j.1523-1739.1999.98494.x) [DOI] [Google Scholar]
- 27.Graham BA, Sandoval L, Dabelsteen T, Mennill DJ. 2017. A test of the acoustic adaptation hypothesis in three types of tropical forest: degradation of male and female rufous-and-white wren songs. Bioacoustics 26, 37–61. ( 10.1080/09524622.2016.1181574) [DOI] [Google Scholar]
- 28.Gillespie TW, Grijalva A, Farris CN. 2000. Diversity, composition, and structure of tropical dry forests in Central America. Plant Ecol. 147, 37–47. ( 10.1023/A:1009848525399) [DOI] [Google Scholar]
- 29.Mennill DJ, Vehrencamp SL. 2005. Sex differences in singing and duetting behavior of neotropical rufous-and-white wrens (Thryothorus rufalbus). The Auk 122, 175–186. ( 10.1642/0004-8038(2005)122%5B0175:SDISAD%5D2.0.CO;2) [DOI] [Google Scholar]
- 30.Mennill DJ. 2006. Aggressive responses of male and female rufous-and-white wrens to stereo duet playback. Anim. Behav. 71, 219–226. ( 10.1016/j.anbehav.2005.05.006) [DOI] [Google Scholar]
- 31.Topp SM, Mennill DJ. 2008. Seasonal variation in the duetting behaviour of rufous-and-white wrens (Thryothorus rufalbus). Behav. Ecol. Sociobiol. 62, 1107–1117. ( 10.1007/s00265-007-0538-4) [DOI] [Google Scholar]
- 32.Harris AJ, Wilson DR, Graham BA, Mennill DJ. 2016. Estimating repertoire size in a songbird: a comparison of three techniques. Bioacoustics 25, 211–224. ( 10.1080/09524622.2016.1138416) [DOI] [Google Scholar]
- 33.Battiston MM, Wilson DR, Graham BA, Kovach KA, Mennill DJ. 2015. Rufous-and-white wrens Thryophilus rufalbus do not exhibit a dear enemy effects towards conspecific or heterospecific competitors. Curr. Zool. 61, 23–33. ( 10.1093/czoolo/61.1.23) [DOI] [Google Scholar]
- 34.Malhi Y, Wright J. 2004. Spatial patterns and recent trends in the climate of tropical rainforest regions. Phil. Trans. R. Soc. B 359, 311–329. ( 10.1098/rstb.2003.1433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Didan K.2015. MOD13A3 MODIS/Terra vegetation Indices monthly L3 global 1 km SIN grid V006. NASA EOSDIS Land Processes DAAC. See . . [DOI]
- 36.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 37.Hijmans RJ.2016. raster: geographic data analysis and modeling. See https://CRAN.R-project.org/package=raster .
- 38.Lebreton J-D, Burnham KP, Clobert J, Anderson DR. 1992. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 62, 67–118. ( 10.2307/2937171) [DOI] [Google Scholar]
- 39.Plummer M.2003. JAGS: a program for analysis of Bayesian graphical models using Gibbs sampling. See http://mcmc-jags.sourceforge.net/ .
- 40.Kellner K.2017. jagsUI: a wrapper around ‘rjags’ to streamline ‘JAGS’ analyses. See https://CRAN.R-project.org/package=jagsUI .
- 41.Kéry M, Schaub M. 2012. Bayesian population analysis using WinBUGS: a hierarchical perspective. Waltham, MA: Academic Press. [Google Scholar]
- 42.Schaub M, Jakober H, Stauber W. 2013. Strong contribution of immigration to local population regulation: evidence from a migratory passerine. Ecology 94, 1828–1838. ( 10.1890/12-1395.1) [DOI] [PubMed] [Google Scholar]
- 43.Kuo L, Mallick B. 1998. Variable selection for regression models. Sankhyā Indian J. Stat. Ser. B 60, 65–81. [Google Scholar]
- 44.Mutshinda CM, Finkel ZV, Irwin AJ. 2013. Which environmental factors control phytoplankton populations? A Bayesian variable selection approach. Ecol. Model. 269, 1–8. ( 10.1016/j.ecolmodel.2013.07.025) [DOI] [Google Scholar]
- 45.Brooks SP, Gelman A. 1998. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 7, 434–455. ( 10.1080/10618600.1998.10474787) [DOI] [Google Scholar]
- 46.Brooks SP, Catchpole EA, Morgan BJT, Barry SC. 2000. On the Bayesian analysis of ring-recovery data. Biometrics 56, 951–956. ( 10.1111/j.0006-341X.2000.00951.x) [DOI] [PubMed] [Google Scholar]
- 47.Hallett TB, Coulson T, Pilkington JG, Clutton-Brock TH, Pemberton JM, Grenfell BT. 2004. Why large-scale climate indices seem to predict ecological processes better than local weather. Nature 430, 71–75. ( 10.1038/nature02708) [DOI] [PubMed] [Google Scholar]
- 48.Gill SA, Haggerty TM. 2012. A comparison of life-history and parental care in temperate and tropical wrens. J. Avian Biol. 43, 461–471. ( 10.1111/j.1600-048X.2012.05637.x) [DOI] [Google Scholar]
- 49.Ryder TB, Durães R, Tori WP, Hidalgo JR, Loiselle BA, Blake JG. 2008. Nest survival for two species of manakins (Pipridae) in lowland Ecuador. J. Avian Biol. 39, 355–358. ( 10.1111/j.0908-8857.2008.04290.x) [DOI] [Google Scholar]
- 50.Brawn JD, Angehr G, Davros N, Robinson WD, Styrsky JN, Tarwater CE. 2011. Sources of variation in the nesting success of understory tropical birds. J. Avian Biol. 42, 61–68. ( 10.1111/j.1600-048X.2010.04897.x) [DOI] [Google Scholar]
- 51.Styrsky JN, Brawn JD. 2011. Annual fecundity of a Neotropical bird during years of high and low rainfall. The Condor 113, 194–199. ( 10.1525/cond.2011.100051) [DOI] [Google Scholar]
- 52.Kahn ZA. 2017. Vocal duetting behaviour in neotropical wren: insights into paternity guarding and parental commitment. Windsor, Ontario, Canada: University of Windsor. [Google Scholar]
- 53.du Plessis KL, Martin RO, Hockey PAR, Cunningham SJ, Ridley AR. 2012. The costs of keeping cool in a warming world: implications of high temperatures for foraging, thermoregulation and body condition of an arid-zone bird. Glob. Change Biol. 18, 3063–3070. ( 10.1111/j.1365-2486.2012.02778.x) [DOI] [PubMed] [Google Scholar]
- 54.Janzen DH, Schoener TW. 1968. Differences in insect abundance and diversity between wetter and drier sites during a tropical dry season. Ecology 49, 96–110. ( 10.2307/1933565) [DOI] [Google Scholar]
- 55.Boyle WA, Norris DR, Guglielmo CG. 2010. Storms drive altitudinal migration in a tropical bird. Proc. R. Soc. B 277, 2511–2519. ( 10.1098/rspb.2010.0344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Studds CE, Marra PP. 2007. Linking fluctuations in rainfall to nonbreeding season performance in a long-distance migratory bird, Setophaga ruticilla. Clim. Res. 35, 115–122. ( 10.3354/cr00718) [DOI] [Google Scholar]
- 57.Woodworth BK, Norris DR, Graham BA, Kahn ZA, Mennill DJ. 2018. Data from: Hot temperatures during the dry season reduce survival of a resident tropical bird Dryad Digital Repository. ( 10.5061/dryad.k785p43) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Woodworth BK, Norris DR, Graham BA, Kahn ZA, Mennill DJ. 2018. Data from: Hot temperatures during the dry season reduce survival of a resident tropical bird Dryad Digital Repository. ( 10.5061/dryad.k785p43) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and model code supporting this article are available on Dryad: http://dx.doi.org/10.5061/dryad.k785p43 [57].