Abstract
Birds and mammals have developed numerous strategies for replacing worn feathers and hair. Moulting usually occurs on an annual basis; however, moults that take place twice per year (biannual moults) also occur. Here, we review the forces driving the evolution of various moult strategies, focusing on the special case of the complete biannual moult as a convergence of selection pressures across birds and mammals. Current evidence suggests that harsh environmental conditions or seasonality (e.g. larger variation in temperatures) drive evolution of a biannual moult. In turn, the biannual moult can respond to secondary selection that results in phenotypic alteration such as colour changes for mate choice dynamics (sexual selection) or camouflage requirements (natural selection). We discuss the contributions of natural and sexual selection to the evolution of biannual moulting strategies in the contexts of energetics, niche selection, functionality and physiological mechanisms. Finally, we suggest that moult strategies are directly related to species niche because environmental attributes drive the utility (e.g. thermoregulation, camouflage, social dynamics) of the hair or feathers. Functional efficiency of moult may be undermined if the pace of evolution fails to match that of the changing climate. Thus, future research should seek to understand the plasticity of moult duration and phenology, especially in the context of annual cycles.
Keywords: feathers, fur, moult, life history, annual cycle, camouflage
1. Introduction
Hair and feathers are non-living keratinous structures that degrade through wear and breakage as they age. This reduced functionality can reduce individual fitness by compromising flight [1,2], thermoregulation [3] and mating abilities [4]. Because the structures are non-living, the only mechanism for damage repair is complete replacement through shedding (a protracted, year-round replacement) or moult (a contracted, punctuated replacement) [5]. Though some species forgo migration and feeding events during the period when fur/feathers are replaced [6], no species has been documented to skip an entire moult cycle, suggesting its key importance to endotherm life cycles [7,8]. Despite this importance, moulting is one of the most poorly studied life-history events, particularly in mammals, but also in birds [9].
Birds and mammals exhibit a wide variety of moulting strategies [10,11]. Most can be simplified and divided into two categories: replacement of fur or feathers after 12 months (hereafter, annual moult) and replacement of some or all fur or feathers twice per year (hereafter, incomplete or complete biannual moult). By definition, the first moult occurs after breeding and produces basic, non-breeding plumages (in birds, body and flight feathers replaced) or winter pelages (in mammals). The second moult of the year is almost always incomplete [8,12], producing the alternate breeding plumage (in birds, body feathers replaced) or summer pelage (mammals). In some species, however, all feathers or fur are replaced during a complete second moult. In addition, some species can slow or halt a moult [13] due to nutritional deficiency or migration timing constraints and continue later (hereafter, facultative split moult) or, in extreme cases, break the moult cycle (hereafter, partial moult) [14]. Still other species may replace fur during a protracted, year-round process (hereafter, continuous moult) [15] or may take more than one year to perform a complete moult (hereafter, biennial moult) [16,17]. Finally, some species exhibit a catastrophic or simultaneous moulting strategy where plumage or pelage function is temporarily compromised as feathers or fur are shed rapidly. The range of moulting strategies are subject to a wide range of selective forces (table 1); understanding the factors driving the variation in moult strategies is important for predicting future impacts of global change.
Table 1.
Descriptions and examples of main moulting strategies in birds and mammals. Strategies are colour coded to match figure 1.
| hair/feather replacement strategy | description | environmental conditions | example mammal species | example bird species |
|---|---|---|---|---|
continuous shedding 
|
Individuals replace fur or feathers during a protracted, year-round process. | Typical in animals that experience limited seasonality in resource availability or ambient conditions. | domestic dogs [15], sea otters [18] | mousebirds [19] |
annual moult 
|
Individuals replace pelage/plumage once per year. | Typical in seasonally homogeneous areas. | harbour seals [20], bent-winged bats [21] | bullfinches [22], lesser redpolls [23] |
| SUBSET: Catastrophic moult. Individuals rapidly shed all pelage and plumage, such that pelage or plumage function is compromised, and feeding does not occur. |
Typical of species that reside in aquatic environments such that insulative, waterproof, and hydrodynamic functions of pelage and plumage are crucial. | northern elephant seals [24], southern elephant seals [25], Hawaiian monk seals [26] | Adelie and emperor penguins [27] | |
| SUBSET: Simultaneous moult. Individuals rapidly shed flight feathers, such that plumage function is compromised. Feeding does occur during this time. |
The same as above. | To our knowledge, does not occur in mammals. | common eiders [28], lesser snow geese [29] | |
complete biannual moult 
|
Individuals replace pelage/plumage twice per year, usually to meet camouflage and insulation requirements. | Typical of polar latitudes where conditions can be snowy and cold during the winter. | Arctic, mountain, and snowshoe hares [30], least, long-tailed, and short-tailed weasels [31], Peary caribou [32], collared lemmings [33], Siberian hamsters [34], ground squirrels [35], arctic foxes [36] | rock, willow, and white-tailed ptarmigan [37], willow warblers [38], black-chested prinias [39] |
incomplete biannual moult 
|
Individuals grow thicker winter pelage or plumage and then shed into their thinner summer pelage or plumage during spring to allow heat exchange. Thus, the covering is a composite of retained and new fur/feathers. | Typical of temperate latitudes where it can be wet and cold in the winter but not snowy, and hot in the summer. Alternatively, species in high-latitude environments that do not rely on snow camouflage for survival. | ferrets [40], elk [41], mink [42], snow leopards [43], deer [44], moose [45], squirrels [46], white-footed mice [47], shrews [48] | grey-headed albatrosses [49], barred warblers [50], painted buntings [51] |
| split moult | Animals can stop the moult and continue the moult later. | Typical in areas where food supplies or weather conditions are unpredictable or periodic. | To our knowledge, does not occur in mammals. | barred warblers [52], common whitethroats [53], spectacled warblers [54] (see appendix 1 in Norman [55]) |
Here, we review contributions of natural and sexual selection to the frequency and timing of bird and mammal moults in the context of energetics, ecological niches, functions and physiological mechanisms. For simplicity, we limit the scope of our review to sexually mature adults (i.e. no juvenile plumages).
2. Functional roles and forms of pelage and plumage
The evolution of feathers and fur has allowed endothermic vertebrates to inhabit both land and sea [56,57]. Plumages and pelages serve a variety of functions, such as providing thermal insulation by creating an air barrier between bare skin and surrounding ambient conditions [53], enhancing camouflage and/or mate attraction through coloration, providing mechanical protection and altering fluid flow to minimize drag in flying and swimming species [13,53,58]. In mammals, fur generally includes long, coarse guard hairs, and numerous fine, short underhairs [59]. Birds have a more diverse set of above-skin coverings including several types of feathers (flight, down, tail, contour, semiplume, bristle, filoplume) that vary widely in their function and form. For example, flight feathers that provide thrust (primaries) and lift (secondaries) are characterized by windproof surfaces of interlocking microstructures that allow birds to manoeuvre in the air. By contrast, down feathers have exceptional insulative properties that out-perform nearly all man-made materials.
Plumage and pelage morphologies of temperate/polar birds and mammals differ from those of tropical birds and mammals [13]. For example, tropical mammals rarely have fur longer than 20 mm [60], while arctic and high temperate mammals can have fur up to 70 mm, with relatively fine, abundant underhairs. Similarly, temperate and tropical birds have fewer down feathers and shorter contour feathers than those residing in polar areas [61]. While fur and feathers primarily provide insulation for animals in cool climates, they can also reflect solar radiation to reduce heat gain in hot climates. For instance, plumage reflectance is 65–69% higher for white plumage relative to black plumage and is thus beneficial for tropical birds nesting in open habitat [62]; however, white plumage may be less advantageous as wind speed increases, because white plumage limits convective cooling and thus retains a higher heat load [63]. Alternatively, white feathers and fur camouflage polar species such as snow petrels Pagodroma nivea and arctic foxes Vulpes lagopus in their snow-covered habitats.
3. Metabolic costs of moult
A biannual moult is expected when the energetic or fitness cost of producing a new pelage/plumage is less than the cost incurred by having suboptimal pelage/plumage coloration or insulation during different seasons. Although the sedentary nature of moulting animals minimizes transport costs [28], the moulting process (in combination, energy content of new tissue, production efficiency of new tissue, and compromised thermoregulation) incurs considerable costs above those required for basal maintenance. In small terrestrial mammals, pelage accounts for between 4% [24] and 15% [64] of total body mass. These pelage proportions exceed those of large mammals (1.7% fur in Weddell seals [65]; 3.4% in fur and skin of northern elephant seals [66]; 4–4.5% in muskoxen [67]), probably because the smaller mammals have larger surface area (i.e. fur) to body mass ratios [68]. The energetics of moulting mammals have been studied almost exclusively in phocid seals (family Phocidae) with most studies reporting minimal [24] or no [64] added metabolic cost aside from the reduced activity. To our knowledge, no estimates exist for the energetic efficiency of fur production in mammals.
Moult energetics have been more extensively investigated in avian species. Plumages account for 4% [65] to 20% [66] of total body dry mass of birds. Less than 30% of energy used by moulting birds is thought to be incorporated into feathers [67]; the remaining energy is expended on the increases in thermoregulatory costs from the associated skin perfusion [68], increases in flight costs from reduced wing area [69] and production of tissues needed for feather synthesis [70]. It is difficult to disentangle the contributions of thermoregulation, protein deposition and efficiency to the cost of the moult; as a result, most researchers report the overall metabolic increase during the moult. Moulting costs vary by species and can be large [71,72], with metabolic rate increasing by 10% in red knots Calidris canutus [73], 12% in common eiders Somateria mollissima [28], 15–16% in blue jays Cyanocitta cristata and scrub jays Aphelocoma californica [74], 58% in white-crowned sparrows Zonotrichia leucophrys, and 82% in white-plumed honeyeaters Lichenostomus penicillatus [75] relative to non-moulting individuals [76]. The energy cost of feather synthesis increases proportionally with basal metabolic rate [76], such that small birds have higher mass-specific moult costs relative to large birds.
The highly variable moulting costs can be explained by interactions between moulting strategies, life histories and environmental conditions. Rapid moults tend to occur in animals that experience greater mortality or energetic costs due to reduced functionality of fur or feathers [8,10]. For instance, follicular growth requires perfusion to maintain skin temperature above a certain threshold [77], which could exacerbate heat loss during the moulting period in cold climates [78,79]. Because the duration of favourable seasons decreases at high latitudes (e.g. ‘seasons of stress, seasons of opportunity’ [80]), moults in polar resident and breeding birds tend to be shorter than in tropical birds [81,82]. By contrast, under less seasonal conditions (e.g. tropical regions), a more prolonged moult maximizes energetic efficiency because it avoids high daily costs of thermoregulation and fur growth [81]; as a result, tropical avian moults are usually slow [83]. We propose that the necessity of optimizing energetic expenditures coupled with the apparently high cost of moult provides a strong selection pressure for convergence of moulting durations within environmental niches.
4. Selection pressures and moulting strategies
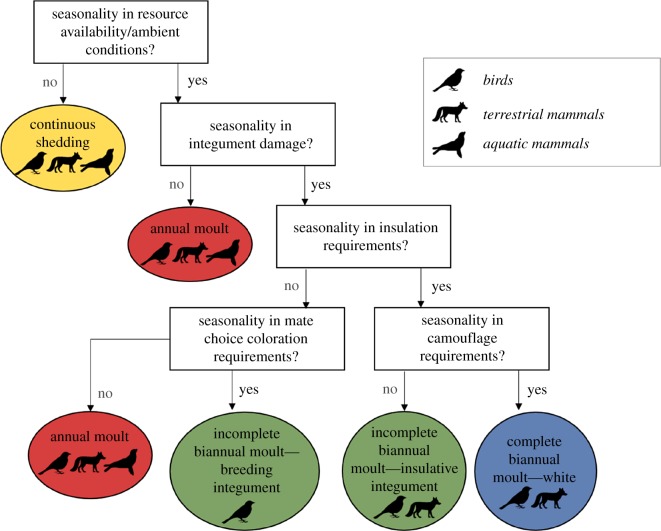
Birds and mammals that inhabit comparable environmental niches must solve similar social, thermal and energetic problems to survive and reproduce [84]. Because these selective pressures constrain moulting strategies, similar moulting strategies have evolved across avian and mammalian species where niches overlap [10]. For example, while an annual moult is usually sufficient to offset normal fur or feather degradation rates, biannual moults are particularly common in species of birds and mammals that occupy harsh habitats or use seasonal plumages for territory defence or mate attraction [11,13,85]. It is important to consider differing moulting strategies that may arise under ecological or social selection forces (figure 1) [86].
Figure 1.
Selective pressures (boxes) on moulting strategies (ovals), including the group of endotherms that typically exhibits each strategy. Note that catastrophic moult is an extreme case of the annual moult.
The highly ornamented breeding plumages of many avian species are well-known examples of sexual selection [87]. Many species (e.g. mandarin ducks Aix galericulata, Indian peacocks Pavo cristatus) have evolved colourful plumages because of female preference for more ornamented males [87]. The strong sexual selection for male birds to grow brightly coloured body feathers (i.e. alternate plumage) prior to the breeding season is usually facilitated by an incomplete second moult (i.e. biannual moult), which allows animals to return to a more cryptic plumage during the rest of the year [86]. Birds have tetrachromatic colour vision [88], which creates opportunities for heritable variations in plumage colour. Conversely, mammals generally have dichromatic vision with relatively poor colour sensitivity. Limited colour vision restricts the utility of colour in mating displays and thus minimizes sexual selective pressures for evolution of ornamental fur pigmentation in mammals [89]; here, the natural selective forces for crypsis dominate. As a result, coloration of most mammals is duller than many avian species and sexual dichromatism is nearly absent in mammals. Notable exceptions are primates and marsupials, which have retained trichromatic vision [90] and use bright colours (e.g. faces of mandrills Mandrillus sphinx, rumps of hamadryas baboons Papio hamadryas and chests of geladas Theropithecus gelada) for intraspecific communication. However, these colours result from structural components in the skin rather than replaceable fur [90] and thus are independent from the pelage moult [91].
At least in mammals, some species with no sexual selection on pelage colour still undergo two complete moults per year. Strong seasonality in temperatures, such as occur in arctic, alpine and temperate climates, require animals to either avoid temperature extremes through migration or to adapt to seasonal camouflage and insulation requirements. Thus, the selective forces of seasonal habitat transformations affect both migrants and residents in different ways, requiring increased insulation, increased camouflage or increased replacement due to degradation. We discuss each of these components below.
Many high-latitude species have evolved behavioural strategies to cope with the extreme cold, including hibernation in brown bears Ursus arctos [92], under-snow lairs in ruffed grouse Bonasa umbellus [93], ‘behavioural wintering’ in European badgers Meles meles [94] and under-snow social aggregations in red-backed voles Myodes gapperi [95]. In contrast, species that are active above the snow rely heavily on insulation of the pelage or plumage during winter [59]. These species often have a biannual moult wherein a more insulative winter pelage or plumage replaces that of summer. In mammals, underfur from the winter pelage can vary in density, length, diameter, colour and texture, and guard hairs can be finer and longer to increase their insulation [59]. These anatomical changes have been observed in many species such as ferrets Mustela putorius furo [40], elk Cervus canadensis [41], mink Mustela vison [42], snow leopards Panthera uncia [43], white-tailed deer Odocoileus virginianus [44], moose Alces alces [45], grey squirrels Sciurus carolinensis [46], white-footed mice Peromyscus leucopus [47] and lesser white-toothed shrews Crocidura suaveolens [48]. Winter pelages can decrease the lower critical temperatures of red foxes Vulpes vulpes and porcupines Erethizon dorsatum by approximately 20°C [59]. For these high-latitude mammals, meeting insulation requirements does not require a colour change, so rather than a full second moult per year, these species typically grow a thicker pelage before the winter and then shed into their thinner summer pelage during spring to allow heat exchange. We consider this an incomplete moult because the summer shedding process is a partial loss of previous pelage (and occasional replacement of some fur) rather than growth of an entirely new pelage. Polar resident birds show a similar pattern of enhanced insulation in the basic (winter) plumage. During winter, non-migratory house sparrows Passer domesticus increase plumage weight by 70% [80], and goldfinches Carduelis carduelis increase plumage weight by up to 50% [96]. The purpose of the added winter pelage or plumage in these species is probably for thermoregulatory advantage rather than cryptic or breeding coloration.
When habitats are snow-covered, a combination of camouflage and thermoregulatory selection pressures has driven a biannual moult that facilitates an entirely white, thick winter pelage/plumage. Because summer pelage is usually brown, black or grey, these species typically facilitate their autumn and spring pelage changes by complete shedding of the previous pelage (i.e. complete biannual moult) rather than adding to the fur already grown. For example, to camouflage with seasonal snowfall in high latitude environments, rock, willow and white-tail ptarmigan Lagopus spp. alternate between pigmented, summer plumage and white, winter plumage [97] with longer winter feathers (42% longer contour feathers, 29% longer down feathers) than in summer [61]. Some terrestrial mammals such as Arctic, mountain and snowshoe hares Lepus spp. [30], least, long-tailed and short-tailed weasels Mustela spp. [31], Peary caribou Rangifer tarandus pearyi [98], collared lemmings Dicrostonyx groenlandicus [33], Siberian hamsters Phodopus sungorus [34], and arctic foxes V. lagopus [36] complete an analogous biannual moult to grow a more insulative white pelage (figure 2).
Figure 2.
(a) Rock ptarmigan Lagopus muta (photographs by Jared Hughey) and (b) snowshoe hares Lepus americanus (research photographs by Mills lab) both undergo complete biannual moults, shedding into a thicker, white plumage/pelage before winter and a thinner, dark plumage/pelage before summer. (Online version in colour.)
In addition to seasonal coloration and thermoregulation requirements, moulting strategies can also reflect the rate of degradation of features or fur. In temperate and tropical species, pelage or plumage degradation can result from abrasive vegetation, wind and sand [59]. Likewise, the plumages of birds in humid climates are subject to feather-degrading bacteria [99]. The melanin associated with darker feathers increases feather keratin thickness (abrasion resistance) and solar absorption (above optimal temperature for microbe growth); thus, darker feathers tend to be found in more humid environments, termed Gloger's rule. In high-latitude species, exposure to UV radiation during summer and to extreme cold during winter degrades pelage/plumage [85] by denaturing keratin and other structural proteins [100]. The ambient conditions and food availability of high-latitude environments are inherently seasonal and thus provide strong selection pressures relative to tropical habitats that are relatively benign and homogeneous [101]. Thus, it is no surprise that the presence of the biannual moult can be explained more by environmental conditions than by phylogenetic relationships among birds and mammals.
5. Special considerations for aquatic species
Semi-aquatic animals have additional selection pressures from the increased thermal conductivity of water. When submerged, water replaces the insulating air layer between fur and reduces the thermal resistance of fur by 84–92% [102]. For diving animals like phocid seals (family Phocidae), water pressure at depth diminishes the utility of fur insulation; instead, phocid seals rely almost exclusively on blubber for insulation. These blubber stores enable phocid seals to exploit seasonally available prey and withstand lower ambient temperatures than would be possible if they relied on fur alone; consequently, phocids have a wide niche and inhabit both polar and non-polar environments (10 polar species, 8 non-polar species). In contrast, sea lions and fur seals (family Otariidae) rely heavily on pelage for insulation and inhabit almost exclusively temperate and tropical environments (1 polar species, 13 non-polar species), with the Antarctic fur seals Arctocephalus gazella having denser fur than other species. These aquatic mammals are not required to coordinate pelages with seasonal changes due to the seasonally homogeneous colour and temperature of their marine environments and thus only exhibit a single moult per year [10], with phocid seals moulting more rapidly than otariids. Sea otters Enhydra lutris, by contrast, replace fur continuously, probably due to their reliance on extremely thick pelage (up to 140 000 hairs cm−2 [18]) for aquatic thermoregulation.
Some pinniped and avian species undergo an extreme annual moult that involves a rapid, nearly simultaneous shedding of all pelage or plumage [24,27]. This is generally termed the ‘catastrophic moult’ although a consistent definition has not yet been established. Northern elephant seals Mirounga angustirostris, southern elephant seals Mirounga leonina, Hawaiian monk seals Neomonachus schauinslandi and penguins (order Sphenisciformes) are the only species described in the literature to moult this way [24,26,27]. In the pinniped literature, catastrophic moult refers to moulting of a thick epidermal layer in conjunction with hair loss (i.e. peeling skin sheets attached to hair roots, in contrast to small flakes of skin as in some Weddell seals Leptonychotes weddellii [103]) [24–26], and all catastrophic moulting species are known to fast during hair replacement. In the avian literature, the distinction between catastrophic and non-catastrophic moult seems to be the duration of moult, with penguins moulting all feathers in 13–34 days (relative to a couple months [104] or more [105] in ordinary moult) while fasting [27]. The regeneration of skin and fur requires elevated skin temperature and surface blood flow [77] so concurrent moulting and feeding would result in drastic thermoregulatory losses in the highly thermally conductive marine environment. Similarly, moulting impedes the insulative, waterproof and hydrodynamic functions of penguin plumage that are crucial for underwater foraging; as a result, these animals fast for the entire duration of the moult. Thus, across taxa, animals with catastrophic moults appear to meet two criteria: (1) they lose function of their pelage or plumage during the moult, and (2) they do not feed during the moult. To our knowledge, no terrestrial mammals undergo catastrophic moults.
Some birds, including common eiders S. mollissima (36 day moult [28]), lesser snow geese Chen caerulescens caerulescens (less than one month moult [29]), Hawaiian gallinules Gallinula galeata sandvicensis (21–54 day moult [106]) and grebes (order Podicipedidae, approximately 20 day moult [107]) undergo a quick simultaneous wing moult that renders them flightless; however, they do not fast during this moult, and the moult is not referred to as ‘catastrophic’ in the literature. The high energetic cost of the catastrophic and simultaneous moults [24] precludes a twice-per-year moult in these species; these strategies serve as interesting contrasts to the longer moults of many species in less thermally challenging environments.
Although hairless, at least four polar cetacean species undergo a similar catastrophic moult of their epidermis: killer whales Orcinus orca [108], southern right whales Eubalaena australis [109], belugas Delphinapterus leucas [110] and bowhead whales Balaena mysticetus [111]. All cetaceans experience selective pressures to deter ectoparasitic and commensal organisms (e.g. lice, barnacles, diatoms) from attaching to the skin [112] by continuously replacing their vascularized skin. For polar cetaceans, the extremely cold sea temperatures probably make prolonged skin perfusion energetically costly [108]. To avoid large heat loss associated with skin profusion in cold water, these species migrate to warmer waters and replace/exfoliate their skin in a concentrated period [113]. In these cases, migration to moulting habitats can result in considerable metabolic costs.
6. Physiological mechanics of pelage and plumage replacement
Physiological drivers of avian and mammalian moults are generally similar, with age, sex, condition and reproductive status affecting the timing and duration of moult [20,114]. Internal factors (biological clocks, body condition) exert control via nervous and endocrine processes, and rely on external cues (zeitgebers, such as photoperiod and temperature cycles) for synchronization [115]. In combination, these mechanisms coordinate and sequence moult with other life history events, such as migration and reproduction, and align them with optimal environmental conditions [116].
A variety of hormones interact to regulate moult: thyroxine and progesterone promote hair and feather synthesis, whereas oestrogen and cortisol suppress it [20]. Corticosterone is downregulated during moult because it appears to negatively affect feather quality [117]. Thyroxine influences moult onset [73] and duration by increasing metabolic activity of feather forming cells in a permissive rather than causal manner [118]. The timing of peak prolactin is linked to (and slightly precedes) moult start date [66], and prolactin and thyroxine appear mechanistically linked [119,120]. Apart from species that exhibit moult-breeding overlap, moult initiation is inhibited by elevated levels of gonadal hormones such as oestrogen and testosterone. Consequently, sexually immature or reproductively unsuccessful individuals often initiate moult earlier than successful breeders, probably due to the reduction in levels of sex steroids. Moult timing is also influenced by body condition, which is driven by resource availability and reproductive output. Poor body condition, associated with increased cortisol levels, has been found to suppress thyroid hormones [121], causing slower and longer moult [117]. For instance, lower food abundance has been found to delay moult onset in harbour seals Phoca vitulina [20], while food abundance has been found to advance moult onset in swamp sparrows Melospiza georgiana [122]. Indeed, birds in superior body condition often advance moult timing and replace plumage more rapidly [123], possibly due to their lower circulating corticosterone. We note that endocrine control, which we have greatly simplified here, is not the only regulatory mechanism for moult. The roles of intrinsic and extrinsic factors for regulating moult phenology are topics of current research. See [124] and [116] for detailed reviews.
Synthesis and secretion of hormones that regulate moult are coordinated in part by seasonal cues that affect the pituitary gland primarily through melatonin signalling and hypothalamic control. Experimental manipulations of temperature and photoperiod have both been found to induce changes in winter pelage [59]. In snowshoe hares Lepus americanus, the winter moult was entirely suppressed when air temperature warmed by 7°C [30]. Conversely, cold exposure has delayed and shortened the spring moult in short-tailed weasels Mustela erminea [125], and accelerated the autumn moult of the white-footed mouse Peromyscus leucopus [47].
The species-specific reliance on photoperiod or temperature cues has evolved based on environment. For instance, photoperiod appears to be the critical driver of moult in high-latitude birds and mammals, while temperature and nutrition can modulate its timing. On the other hand, tropical residents and species that are subject to consistent annual daylength may rely heavily on non-photoperiodic cues such as temperature and rainfall [124]. Amphibious mammals such as pinnipeds apparently use a combination of cues for moult onset, including endogenous rhythms, changes in photoperiod, sea temperature, air temperature and body condition [126]. In turn, moult onset cues decide how species respond to global change; for example, migratory birds that depend on photoperiod cues for moult onset are expected to respond with less phenotypic plasticity than those cued by temperature [127].
7. Feedbacks between moult and global change
By changing the colour or insulation of pelage and plumage, the biannual moult can increase seasonal functionality; however, a biannual moult may be maladaptive under global change scenarios. If the pace of evolution fails to match that of climate warming [128], the functional efficiency of moult may be undermined. For instance, phenological mismatches between snow presence and snowshoe hare Lepus americanus pelage coloration could compromise crypsis and lead to elevated predation risk [129]. In ambush predators such as snow leopards Panthera uncia, similarly compromised crypsis could lead to diminished foraging success. Evidence for phenotypic plasticity to variable conditions has been found in mountain hares Lepus timidus, which tend to have slower spring moults (white to brown pelage coloration) in colder springs [130] and faster winter moults (brown to white) during colder falls [131]. Other studies have demonstrated that life histories can limit the flexibility of moult duration and phenology, and thus limit adaptive capacities. For example, long-distance migrants have advanced their phenologies less than short-distance migrants [127] because they have no information about phenology on the breeding grounds while in their wintering grounds [115]. Differential rates of phenological flexibility can lead to progressively mismatched seasonal timing between interacting species [115]. As a result, phenological plasticity can have population-level consequences under climate change.
8. Conclusion
In his seminal paper on mammalian moulting strategies in 1970, Ling [13] noted that ‘moult patterns … may be very different in closely related species … and very similar in widely separated taxonomic groups'. Here, we synthesize evidence that environmental conditions are important in determining the frequency of moulting in birds and mammals. Because the functional roles of pelage and plumage are defined by environmental niches, moulting strategies across taxa converge as a function of environmental conditions [6]. In endotherms that inhabit higher latitudes, plumages and pelages play distinct seasonal roles [10] in camouflage (pelage colour polyphenism [129]), insulation and mate attraction. In birds, the biannual moult evolved from the ancestral state of a single summer moult [11,132,133] as a response to energetic and environmental selection factors. We suggest that the same could be true in mammals, giving the moult similar adaptive functions across avian and mammalian taxa. Comparative studies across taxa that share life-history characteristics provide insight into the wide range of functional roles that have caused strategies to emerge. Researchers should take care to document species-typical moult routines and place these routines within the framework of other critical life-history events and their environmental niches.
Acknowledgements
Thank you to Ryan S. Terrill, Claire Nasr, Parker Forman and Amy Kirkham for reviewing previous drafts, and to Diane O'Brien, Brian Barnes and Ward Testa for helpful discussions.
Data accessibility
This article has no additional data.
Authors' contributions
R.S.B., J.M.B. and G.A.B. conceived of the study; R.S.B. carried out the literature review; R.S.B., J.M.B. and G.A.B. wrote and revised the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation Graduate Research Fellowship Program under grant no. DGE-1242789 to R.S.B. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
References
- 1.Jovani R, Blas J. 2004. Adaptive allocation of stress-induced deformities on bird feathers. J. Evol. Biol. 17, 294–301. ( 10.1111/j.1420-9101.2003.00680.x) [DOI] [PubMed] [Google Scholar]
- 2.Swaddle JP, Lockwood R. 2003. Wingtip shape and flight performance in the European Starling Sturnus vulgaris. Ibis 145, 457–464. ( 10.1046/j.1474-919X.2003.00189.x) [DOI] [Google Scholar]
- 3.Dawson TJ, Blaney CE, Munn AJ, Krockenberger A, Maloney S.K. 2000. Thermoregulation by kangaroos from mesic and arid habitats: influence of temperature on routes of heat loss in eastern grey kangaroos (Macropus giganteus) and red kangaroos (Macropus rufus). Physiol. Biochem. Zool. 73, 374–381. ( 10.1086/316751) [DOI] [PubMed] [Google Scholar]
- 4.Smith HG, Montgomerie R. 1991. Sexual selection and the tail ornaments of North American barn swallows. Behav. Ecol. Sociobiol. 28, 195–201. ( 10.1007/BF00172171) [DOI] [Google Scholar]
- 5.Boily P. 1995. Theoretical heat flux in water and habitat selection of phocid seals and beluga whales during the annual molt. J. Theor. Biol. 172, 235–244. ( 10.1006/jtbi.1995.0020) [DOI] [Google Scholar]
- 6.Ryder TB, Parker PG, Blake JG, Loiselle BA. 2009. It takes two to tango: reproductive skew and social correlates of male mating success in a lek-breeding bird. Proc. R. Soc. Lond. B 276, 2377–2384. ( 10.1098/rspb.2009.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennycuick C. 1975. Mechanics of flight. Avian Biol. 5, 1–75. [Google Scholar]
- 8.Humphrey PS, Parkes KC. 1959. An approach to the study of molts and plumages. The Auk 76, 1–31. ( 10.2307/4081839) [DOI] [Google Scholar]
- 9.Bridge ES. 2011. Mind the gaps: what's missing in our understanding of feather molt. The Condor 113, 1–4. ( 10.1525/cond.2011.100228) [DOI] [Google Scholar]
- 10.Ling J. 1972. Adaptive functions of vertebrate molting cycles. Am. Zool. 12, 77–93. ( 10.1093/icb/12.1.77) [DOI] [Google Scholar]
- 11.Svensson E, Hedenstrom A. 1999. A phylogenetic analysis of the evolution of moult strategies in Western Palearctic warblers (Aves: Sylviidae). Biol. J. Linn. Soc. 67, 263–276. ( 10.1111/j.1095-8312.1999.tb01864.x) [DOI] [Google Scholar]
- 12.Pyle P. 1997. Molt limits in North American passerines. North Am. Bird Bander 22, 49–89. [Google Scholar]
- 13.Ling JK. 1970. Pelage and molting in wild mammals with special reference to aquatic forms. Quart. Rev. Biol. 45, 16–54. ( 10.1086/406361) [DOI] [PubMed] [Google Scholar]
- 14.Scheiman DM, Dunning JB Jr. 2004. A case of arrested molt in the bobolink. North Am. Bird Bander 29, 105–108. [Google Scholar]
- 15.Gunaratnam P, Wilkinson G. 1983. A study of normal hair growth in the dog. J. Small Anim. Pract. 24, 445–453. ( 10.1111/j.1748-5827.1983.tb00384.x) [DOI] [Google Scholar]
- 16.Snyder NF, Johnson EV, Clendenen DA. 1987. Primary molt of California condors. Condor 89, 468–485. ( 10.2307/1368637) [DOI] [Google Scholar]
- 17.Edwards AE. 2008. Large-scale variation in flight feather molt as a mechanism enabling biennial breeding in albatrosses. J. Avian Biol. 39, 144–151. ( 10.1111/j.2007.0908-8857.04139.x) [DOI] [Google Scholar]
- 18.Kuhn RA, Ansorge H, Godynicki S, Meyer W. 2010. Hair density in the Eurasian otter Lutra lutra and the sea otter Enhydra lutris. Acta Theriol. 55, 211–222. ( 10.4098/j.at.0001-7051.014.2009) [DOI] [Google Scholar]
- 19.Moreau R, Wilk A. 1947. The moult and gonad cycles of three species of birds at five degrees south of the equator. Proc. Zool. Soc. Lond. 117, 345–364. ( 10.1111/j.1096-3642.1947.tb00523.x) [DOI] [Google Scholar]
- 20.Daniel RG, Jemison LA, Pendleton GW, Crowley S.M. 2003. Molting phenology of harbor seals on Tugidak Island, Alaska. Mar. Mamm. Sci. 19, 128–140. ( 10.1111/j.1748-7692.2003.tb01097.x) [DOI] [Google Scholar]
- 21.Dwyer P. 1963. Seasonal changes in pelage of Miniopterus schreibersi blepotis (Chiroptera) in north-eastern NSW. Austr. J. Zool. 11, 290–300. ( 10.1071/ZO9630290) [DOI] [Google Scholar]
- 22.Newton I. 1966. The moult of the bullfinch Pyrrhula pyrrhula. Ibis 108, 41–67. ( 10.1111/j.1474-919X.1966.tb07251.x) [DOI] [Google Scholar]
- 23.Evans P. 1966. Autumn movements, moult and measurements of the lesser redpoll Carduelis flammea cabaret. Ibis 108, 183–216. ( 10.1111/j.1474-919X.1966.tb07267.x) [DOI] [Google Scholar]
- 24.Worthy G, Morris P, Costa D, Le Boeuf B. 1992. Moult energetics of the northern elephant seal (Mirounga angustirostris). J. Zool. 227, 257–265. ( 10.1111/j.1469-7998.1992.tb04821.x) [DOI] [Google Scholar]
- 25.Ling J, Thomas C. 1967. The skin and hair of the southern elephant seal, Mirounga leonina (L.). II. pre-natal and early post-natal development and moulting. Aust. J. Zool. 15, 349–365. ( 10.1071/ZO9670349) [DOI] [Google Scholar]
- 26.Kenyon KW, Rice D.W. 1959. Life history of the Hawaiian monk seal. Pacific Sci. 13, 215–252. [Google Scholar]
- 27.Davis LS, Darby J.T. 2012. Penguin biology. Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- 28.Guillemette M, Pelletier D, Grandbois J-M, Butler PJ. 2007. Flightlessness and the energetic cost of wing molt in a large sea duck. Ecology 88, 2936–2945. ( 10.1890/06-1751.1) [DOI] [PubMed] [Google Scholar]
- 29.Ankney CD. 1979. Does the wing molt cause nutritional stress in lesser snow geese? The Auk 96, 68–72. [Google Scholar]
- 30.Hewson R. 1958. Moults and winter whitening in the mountain hare Lepus timidus scoticus, Hilzheimer. Proc. Zool. Soc. Lond. 131, 99–108. ( 10.1111/j.1096-3642.1958.tb00635.x) [DOI] [Google Scholar]
- 31.Bissonnette TH, Bailey E.E. 1944. Experimental modification and control of molts and changes of coat-color in weasels by controlled lighting. Ann. N Y Acad. Sci. 45, 221–260. ( 10.1111/j.1749-6632.1944.tb47953.x) [DOI] [Google Scholar]
- 32.Gunn A, Miller F, Thomas D. 1981. The current status and future of Peary caribou Rangifer tarandus pearyi on the Arctic Islands of Canada. Biol. Conserv. 19, 283–296. ( 10.1016/0006-3207(81)90004-5) [DOI] [Google Scholar]
- 33.Reynolds P, Lavigne D. 1989. Photoperiodic effects on post-weaning growth and food consumption in the collared lemming Dicrostonyx groenlandicus. J. Zool. 218, 109–121. ( 10.1111/j.1469-7998.1989.tb02529.x) [DOI] [Google Scholar]
- 34.Logan A. 1978. Pelage color cycles and hair follicle tyrosinase activity in the Siberian hamster. J. Investigative Dermatol. 71, 295–298. [DOI] [PubMed] [Google Scholar]
- 35.Butterworth B.B. 1958. Molt patterns in the barrow ground squirrel. J. Mamm. 39, 92–97. ( 10.2307/1376614) [DOI] [Google Scholar]
- 36.Fuglei E, Ims RA. 2008. Global warming and effects on the arctic fox. Sci. Prog. 91, 175–191. ( 10.3184/003685008X327468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cramp S. 1980. Handbook of the birds of Europe, the Middle East and north Africa. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Underhill L, Prys-Jones R, Dowsett R, Herroelen P, Johnson D, Lawn M, Norman S, Pearson D, Tree A. 1992. The biannual primary moult of willow warblers Phylloscopus trochilus in Europe and Africa. Ibis 134, 286–297. ( 10.1111/j.1474-919X.1992.tb03811.x) [DOI] [Google Scholar]
- 39.Herremans M. 1999. Biannual complete moult in the Black-chested Prinia Prinia flavicans. Ibis 141, 115–124. ( 10.1111/j.1474-919X.1999.tb04270.x) [DOI] [Google Scholar]
- 40.Harvey NE, Macfarlane W. 1958. The effects of day length on the coat-shedding cycles, body weight, and reproduction of the ferret. Aust. J. Biol. Sci. 11, 187–199. ( 10.1071/BI9580187) [DOI] [Google Scholar]
- 41.Parker KL, Robbins CT. 1984. Thermoregulation in mule deer and elk. Can. J. Zool. 62, 1409–1422. ( 10.1139/z84-202) [DOI] [Google Scholar]
- 42.Martinet L, Mondain-Monval M, Monnerie R. 1992. Endogenous circannual rhythms and photorefractoriness of testis activity, moult and prolactin concentrations in mink (Mustela vison). J. Reproduct. Fert. 95, 325–338. ( 10.1530/jrf.0.0950325) [DOI] [PubMed] [Google Scholar]
- 43.Hemmer H. 1972. Uncia uncia. Mamm. Spec. 20, 1–5. ( 10.2307/3503882) [DOI] [Google Scholar]
- 44.Silver H, Colovos N, Holter J, Hayes H. 1969. Fasting metabolism of white-tailed deer. J. Wildl. Manage. 33, 490–498. ( 10.2307/3799370) [DOI] [Google Scholar]
- 45.Renecker LA, Hudson RJ. 1986. Seasonal energy expenditures and thermoregulatory responses of moose. Can. J. Zool. 64, 322–327. ( 10.1139/z86-052) [DOI] [Google Scholar]
- 46.Elton C. 1951. Some aspects of the biology of the grey squirrel (Sciurus carolinensis) in Great Britain. Proc. Zool. Soc. Lond. 121, 427–459. [Google Scholar]
- 47.Lynch G.R. 1973. Seasonal changes in thermogenesis, organ weights, and body composition in the white-footed mouse, Peromyscus leucopus. Oecologia 13, 363–376. ( 10.1007/BF01825526) [DOI] [PubMed] [Google Scholar]
- 48.Rood J. 1965. Observations on population structure, reproduction and molt of the Scilly shrew. J. Mamm. 46, 426–433. ( 10.2307/1377629) [DOI] [Google Scholar]
- 49.Prince P, Rodwell S, Jones M, Rothery P. 1993. Moult in Black-browed and Grey-headed Albatrosses Diomedea melanophris and D. chrysostoma. Ibis 135, 121–131. ( 10.1111/j.1474-919X.1993.tb02823.x) [DOI] [Google Scholar]
- 50.Hasselquist D, Hedenström A, Lindström Å, Bensch S. 1988. The seasonally divided flight feather moult in the barred warbler Sylvia nisoria: a new moult pattern for European passerines. Ornis Scandinavica 19, 280–286. ( 10.2307/3676722) [DOI] [Google Scholar]
- 51.Thompson C.W. 1991. The sequence of molts and plumages in painted buntings and implications for theories of delayed plumage maturation. Condor 93, 209–235. ( 10.2307/1368938) [DOI] [Google Scholar]
- 52.Lindström Å, Pearson DJ, Hasselquist D, Hedenström A, Bensch S, Åkesson S. 1993. The moult of Barred Warblers Sylvia nisoria in Kenya—evidence for a split wing-moult pattern initiated during the birds' first winter. Ibis 135, 403–409. ( 10.1111/j.1474-919X.1993.tb02112.x) [DOI] [Google Scholar]
- 53.Jenni L, Winkler R. 1994. Moult and ageing of European passerines. London, UK: A&C Black. [Google Scholar]
- 54.Talabante C. 2014. Atypical moult of the secondary feathers in Spectacled Warbler Sylvia conspicillata. Ring. Migr. 29, 44–46. ( 10.1080/03078698.2014.932627) [DOI] [Google Scholar]
- 55.Norman S. 1991. Suspended split-moult systems: an alternative explanation for some species of Palearctic migrants. Ringing and Migration 12, 135–138. [Google Scholar]
- 56.Smith HM. 1960. Evolution of chordate structure: an introduction to comparative anatomy. New York, NY: Holt, Rinehart and Winston. [Google Scholar]
- 57.Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz RB, Jiang T-X, Chuong C.-M. 2004. Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 48, 249 ( 10.1387/ijdb.15272390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokolov W. 1962. Adaptations of the mammalian skin to the aquatic mode of life. Nature 195, 464–466. ( 10.1038/195464a0) [DOI] [Google Scholar]
- 59.Marchand P.J. 2014. Life in the cold: an introduction to winter ecology. Lebanon, NH: University Press of New England. [Google Scholar]
- 60.Davenport J. 2012. Animal life at low temperature. Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- 61.Nuttall M. 2012. Encyclopedia of the Arctic. Abingdon, UK: Routledge. [Google Scholar]
- 62.Ellis H.I. 1980. Metabolism and solar radiation in dark and white herons in hot climates. Physiol. Zool. 53, 358–372. ( 10.1086/physzool.53.4.30157874) [DOI] [Google Scholar]
- 63.Walsberg G, Campbell G, King J. 1978. Animal coat color and radiative heat gain: a re-evaluation. J. Comp. Physiol. B 126, 211–222. ( 10.1007/BF00688930) [DOI] [Google Scholar]
- 64.Ashwell-Erickson SM, Elsner R. 1981. The energy cost of free existence for Bering Sea harbor and spotted seals. Fairbanks, AK: University of Alaska. [Google Scholar]
- 65.Turček FJ. 1966. On plumage quantity in birds. Ekologia Polska Series A 14, 617–634. [Google Scholar]
- 66.Dawson A. 2006. Control of molt in birds: association with prolactin and gonadal regression in starlings. Gen. Comp. Endocrinol. 147, 314–322. ( 10.1016/j.ygcen.2006.02.001) [DOI] [PubMed] [Google Scholar]
- 67.Murphy ME. 1996. Energetics and nutrition of molt. In Avian energetics and nutritional ecology (ed. Carey C.), pp. 158–198. Berlin, Germany: Springer. [Google Scholar]
- 68.Beltran RS, Testa JW, Burns JM. 2017. An agent-based bioenergetics model for predicting impacts of environmental change on a top marine predator, the Weddell seal. Ecol. Modelling 351, 36–50. ( 10.1016/j.ecolmodel.2017.02.002) [DOI] [Google Scholar]
- 69.Kiat Y, Izhaki I, Sapir N. 2016. Determinants of wing-feather moult speed in songbirds. Evol. Ecol. 30, 783–795. ( 10.1007/s10682-016-9838-3) [DOI] [Google Scholar]
- 70.Dietz MW, Daan S, Masman D. 1992. Energy requirements for molt in the kestrel Falco tinnunculus. Physiol. Zool. 65, 1217–1235. ( 10.1086/physzool.65.6.30158276) [DOI] [Google Scholar]
- 71.King JR, Murphy ME. 1985. Periods of nutritional stress in the annual cycles of endotherms: fact or fiction? Am. Zool. 25, 955–964. ( 10.1093/icb/25.4.955) [DOI] [Google Scholar]
- 72.Murphy M, King J. 1991. Nutritional aspects of avian molt. In Acta XX Congressus Internationalis Ornithologici (ed. Bell BD.), pp. 2186–2193. Wellington, New Zealand: New Zealand Ornithological Congress Trust Board. [Google Scholar]
- 73.Vézina F, Gustowska A, Jalvingh KM, Chastel O, Piersma T. 2009. Hormonal correlates and thermoregulatory consequences of molting on metabolic rate in a northerly wintering shorebird. Physiol. Biochem. Zool. 82, 129–142. ( 10.1086/596512) [DOI] [PubMed] [Google Scholar]
- 74.Bancroft GT, Woolfenden G.E. 1982. The molt of scrub jays and blue jays in Florida. Ornithol. Monogr. 29, 1–51. ( 10.2307/40166767) [DOI] [Google Scholar]
- 75.Hoye BJ, Buttemer WA. 2011. Inexplicable inefficiency of avian molt? Insights from an opportunistically breeding arid-zone species, Lichenostomus penicillatus. PLoS ONE 6, e16230 ( 10.1371/journal.pone.0016230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindström Å, Visser GH, Daan S. 1993. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 66, 490–510. ( 10.1086/physzool.66.4.30163805) [DOI] [Google Scholar]
- 77.Feltz ET, Fay FH. 1966. Thermal requirements in vitro of epidermal cells from seals. Cryobiology 3, 261–264. ( 10.1016/S0011-2240(66)80020-2) [DOI] [PubMed] [Google Scholar]
- 78.Paterson W, Sparling CE, Thompson D, Pomeroy PP, Currie JI, McCafferty DJ. 2012. Seals like it hot: changes in surface temperature of harbour seals (Phoca vitulina) from late pregnancy to moult. J. Therm. Biol 37, 454–461. ( 10.1016/j.jtherbio.2012.03.004) [DOI] [Google Scholar]
- 79.Lustick S. 1970. Energy requirements of molt in cowbirds. The Auk 87, 742–746. ( 10.2307/4083708) [DOI] [Google Scholar]
- 80.Gill FB. 1995. Ornithology. Basingstoke, UK: Macmillan. [Google Scholar]
- 81.Helm B, Gwinner E. 1999. Timing of postjuvenal molt in African (Saxicola torquata axillaris) and European (Saxicola torquata rubicola) stonechats: effects of genetic and environmental factors. The Auk 116, 589–603. ( 10.2307/4089321) [DOI] [Google Scholar]
- 82.Holberton RL, Dufty AM Jr, Greenberg R, Marra P. 2005. Hormones and variation in life history strategies of migratory and non-migratory birds. In Birds of Two worlds: the ecology and evolution of migration (eds Marra P, Greenburg E), pp. 290–302. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 83.Fogden M. 1972. The seasonality and population dynamics of equatorial forest birds in Sarawak. Ibis 114, 307–343. ( 10.1111/j.1474-919X.1972.tb00831.x) [DOI] [Google Scholar]
- 84.Irving L. 1972. Arctic life of birds and mammals, including man New York, NY: Springer. [Google Scholar]
- 85.Terrill RS.2018. Evolutionary interactions of feather molt in birds. Lectured delivered 27 February, Occidental College, Los Angeles, CA.
- 86.Ralph CL. 1969. The control of color in birds. Am. Zool. 9, 521–530. ( 10.1093/icb/9.2.521) [DOI] [PubMed] [Google Scholar]
- 87.Zahavi A. 1975. Mate selection—a selection for a handicap. J. Theor. Biol. 53, 205–214. ( 10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 88.Vorobyev M, Osorio D, Bennett AT, Marshall N, Cuthill I. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633. ( 10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 89.Caro T. 2005. The adaptive significance of coloration in mammals. BioScience 55, 125–136. ( 10.1641/0006-3568(2005)055%5B0125:TASOCI%5D2.0.CO;2) [DOI] [Google Scholar]
- 90.Prum RO, Torres R.H. 2004. Structural colouration of mammalian skin: convergent evolution of coherently scattering dermal collagen arrays. J. Exp. Biol. 207, 2157–2172. ( 10.1242/jeb.00989) [DOI] [PubMed] [Google Scholar]
- 91.Vessey SH, Morrison J.A. 1970. Molt in free-ranging rhesus monkeys, Macaca mulatta. J. Mamm. 51, 89–93. ( 10.2307/1378535) [DOI] [Google Scholar]
- 92.Jacoby ME, Hilderbrand GV, Servheen C, Schwartz CC, Arthur SM, Hanley TA, Robbins CT, Michener R. 1999. Trophic relations of brown and black bears in several western North American ecosystems. J. Wildl. Manage. 63, 921–929. ( 10.2307/3802806) [DOI] [Google Scholar]
- 93.Garbutt A, Leatherland J, Middleton A. 1979. Seasonal changes in serum thyroid hormone levels in ruffed grouse maintained under natural conditions of temperature and photoperiod. Can. J. Zool. 57, 2022–2027. ( 10.1139/z79-266) [DOI] [Google Scholar]
- 94.Maurel D, Coutant C, Boissin-Agasse L, Boissin J. 1986. Seasonal moulting patterns in three fur bearing mammals: the European badger (Meles meles L.), the red fox (Vulpes vulpes L.), and the mink (Mustela vison). A morphological and histological study. Can. J. Zool. 64, 1757–1764. ( 10.1139/z86-265) [DOI] [Google Scholar]
- 95.West SD. 1977. Midwinter aggregation in the northern red-backed vole, Clethrionomys rutilus. Can. J. Zool. 55, 1404–1409. ( 10.1139/z77-183) [DOI] [Google Scholar]
- 96.Dawson WR, Carey C. 1976. Seasonal acclimatization to temperature in cardueline finches. J. Comp. Physiol. B 112, 317–333. ( 10.1007/BF00692302) [DOI] [Google Scholar]
- 97.Tickell WLN. 2003. White plumage. Waterbirds 26, 1–12. ( 10.1675/1524-4695(2003)026%5B0001:WP%5D2.0.CO;2) [DOI] [Google Scholar]
- 98.Choy CA, Popp BN, Kaneko JJ, Drazen JC. 2009. The influence of depth on mercury levels in pelagic fishes and their prey. Proc. Natl Acad. Sci. USA 106, 13 865–13 869. ( 10.1073/pnas.0900711106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burtt EH Jr, Ichida JM. 2004. Gloger's rule, feather-degrading bacteria, and color variation among song sparrows. The Condor 106, 681–686. ( 10.1650/7383) [DOI] [Google Scholar]
- 100.Blanco G, Frías O, Garrido-Fernández J, Hornero-Méndez D. 2005. Environmental-induced acquisition of nuptial plumage expression: a role of denaturation of feather carotenoproteins? Proc. R. Soc. Lond. B 272, 1893–1900. ( 10.1098/rspb.2005.3157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barta Z, McNamara JM, Houston AI, Weber TP, Hedenström A, Fero O. 2008. Optimal moult strategies in migratory birds. Phil. Trans. R. Soc. B 363, 211–229. ( 10.1098/rstb.2007.2136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kvadsheim P, Aarseth J. 2002. Thermal function of phocid seal fur. Mar. Mamm. Sci. 18, 952–962. ( 10.1111/j.1748-7692.2002.tb01084.x) [DOI] [Google Scholar]
- 103.Green K, Burton HR, Watts DJ. 1995. Studies of the weddell seal in the vestfold hills, east Antarctica. Kingston, Australia: Australian Antarctic Division. [Google Scholar]
- 104.Holmgren N, Hedenström A. 1995. The scheduling of molt in migratory birds. Evol. Ecol. 9, 354–368. ( 10.1007/BF01237759) [DOI] [Google Scholar]
- 105.Bridge E. 2006. Influences of morphology and behavior on wing-molt strategies in seabirds. Mar. Ornithol. 34, 7–19. [Google Scholar]
- 106.DesRochers DW, Butler LK, Silbernagle MD, Reed J.M. 2009. Observations of molt in an endangered rallid, the Hawaiian Moorhen. Wilson J. Ornithol. 121, 148–153. ( 10.1676/08-064.1) [DOI] [Google Scholar]
- 107.Stout BE, Cooke F. 2003. Timing and location of wing molt in horned, red-necked and western grebes in North America. Waterbirds 26, 88–93. ( 10.1675/1524-4695(2003)026%5B0088:TALOWM%5D2.0.CO;2) [DOI] [Google Scholar]
- 108.Durban J, Pitman R. 2012. Antarctic killer whales make rapid, round-trip movements to subtropical waters: evidence for physiological maintenance migrations? Biol. Lett. 8, 274–277. ( 10.1098/rsbl.2011.0875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reeb D, Best PB, Kidson S.H. 2007. Structure of the integument of southern right whales, Eubalaena australis. Anat. Rec. 290, 596–613. ( 10.1002/ar.20535) [DOI] [PubMed] [Google Scholar]
- 110.Aubin DS, Smith TG, Geraci J. 1990. Seasonal epidermal molt in beluga whales, Delphinapterus leucas. Can. J. Zool. 68, 359–367. ( 10.1139/z90-051) [DOI] [Google Scholar]
- 111.Chernova O, Shpak O, Kiladze A, Azarova V, Rozhnov V. 2016. Summer molting of bowhead whales Balaena mysticetus Linnaeus, 1758, of the Okhotsk Sea population. Dokl Biol. Sci. 471, 261–265. ( 10.1134/S0012496616060028) [DOI] [PubMed] [Google Scholar]
- 112.Fish FE, Howle LE, Murray MM. 2008. Hydrodynamic flow control in marine mammals. Integr. Comp. Biol. 48, 788–800. ( 10.1093/icb/icn029) [DOI] [PubMed] [Google Scholar]
- 113.Fortune SM, Koski WR, Higdon JW, Trites AW, Baumgartner MF, Ferguson SH. 2017. Evidence of molting and the function of ‘rock-nosing’ behavior in bowhead whales in the eastern Canadian Arctic. PLoS One 12, e0186156 ( 10.1371/journal.pone.0186156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morales J, Moreno J, Merino S, Tomás G, Arriero E, Lobato E, Martínez-de la Puente J. 2009. Early moult improves local survival and reduces reproductive output in female pied flycatchers. Ecoscience 14, 31–39. [Google Scholar]
- 115.Helm B, Ben-Shlomo R, Sheriff MJ, Hut RA, Foster R, Barnes BM, Dominoni D. 2013. Annual rhythms that underlie phenology: biological time-keeping meets environmental change. Proc. R. Soc. Lond. B 280, 20130016 ( 10.1098/rspb.2013.0016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zimova M, Hackländer K, Good JM, Melo-Ferreira J, Alves PC, Mills LS. In press. Function and underlying mechanisms of seasonal colour moulting in mammals and birds: what keeps them changing in a warming world? Biol. Rev. [DOI] [PubMed] [Google Scholar]
- 117.Romero LM, Strochlic D, Wingfield J.C. 2005. Corticosterone inhibits feather growth: potential mechanism explaining seasonal down regulation of corticosterone during molt. Comp. Biochem. Physiol. A 142, 65–73. ( 10.1016/j.cbpa.2005.07.014) [DOI] [PubMed] [Google Scholar]
- 118.Jenni-Eiermann S, Jenni L, Piersma T. 2002. Temporal uncoupling of thyroid hormones in Red Knots: T3 peaks in cold weather, T4 during moult. J. für Ornithol. 143, 331–340. ( 10.1007/BF02465483) [DOI] [Google Scholar]
- 119.Goldsmith A, Nicholls T. 1984. Changes in plasma prolactin in male starlings during testicular regression under short days compared with those during photorefractoriness. J. Endocrinol. 102, 353–356. ( 10.1677/joe.0.1020353) [DOI] [PubMed] [Google Scholar]
- 120.Goldsmith A, Nicholls T, Plowman G. 1985. Thyroxine treatment facilitates prolactin secretion and induces a state of photorefractoriness in thyroidectomized starlings. J. Endocrinol. 104, 99–103. ( 10.1677/joe.0.1040099) [DOI] [PubMed] [Google Scholar]
- 121.St. Aubin D, Geraci J. 1988. Capture and handling stress suppresses circulating levels of thyroxine (T4) and triiodothyronine (T3) in beluga whales Delphinapterus leucas. Physiol. Zool. 61, 170–175. ( 10.1086/physzool.61.2.30156148) [DOI] [Google Scholar]
- 122.Danner RM, Greenberg RS, Danner JE, Walters J.R. 2015. Winter food limits timing of pre-alternate moult in a short-distance migratory bird. Funct. Ecol. 29, 259–267. ( 10.1111/1365-2435.12322) [DOI] [Google Scholar]
- 123.Saino N, et al. 2015. Light-level geolocators reveal covariation between winter plumage molt and phenology in a trans-Saharan migratory bird. Oecologia 178, 1105–1112. ( 10.1007/s00442-015-3299-1) [DOI] [PubMed] [Google Scholar]
- 124.Payne RB, Farner D, King J. 1972. Mechanisms and control of molt. Avian Biology 2, 103–155. ( 10.1016/B978-0-12-249402-4.50012-7) [DOI] [Google Scholar]
- 125.Rust CC. 1962. Temperature as a modifying factor in the spring pelage change of short-tailed weasels. J. Mamm. 43, 323–328. ( 10.2307/1376938) [DOI] [Google Scholar]
- 126.Schop J, Aarts G, Kirkwood R, Cremer JS, Brasseur SM. 2017. Onset and duration of gray seal (Halichoerus grypus) molt in the Wadden Sea, and the role of environmental conditions. Mar. Mamm. Sci. 33, 830–846. [Google Scholar]
- 127.Rubolini D, Saino N, Møller AP. 2010. Migratory behaviour constrains the phenological response of birds to climate change. Clim. Res. 42, 45–55. ( 10.3354/cr00862) [DOI] [Google Scholar]
- 128.Stocker T. 2014. Climate change 2013: the physical science basis: Working Group I contribution to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 129.Mills LS, Zimova M, Oyler J, Running S, Abatzoglou JT, Lukacs PM. 2013. Camouflage mismatch in seasonal coat color due to decreased snow duration. Proc. Natl Acad. Sci. USA 110, 7360–7365. ( 10.1073/pnas.1222724110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Watson A. 1963. The effect of climate on the colour changes of mountain hares in Scotland. Proc. Zool. Soc. Lond. 141, 823–835. ( 10.1111/j.1469-7998.1963.tb01629.x) [DOI] [Google Scholar]
- 131.Flux JE. 1970. Life history of the Mountain hare (Lepus timidus scoticus) in north-east Scotland. J. Zool. 161, 75–123. ( 10.1111/j.1469-7998.1970.tb02171.x) [DOI] [Google Scholar]
- 132.Guallar S, Figuerola J. 2016. Factors influencing the evolution of moult in the non-breeding season: insights from the family Motacillidae. Biol. J. Linn. Soc. 118, 774–785. [Google Scholar]
- 133.Pyle P, Leitner WA, Lozano-Angulo L, Avilez-Teran F, Swanson H, Limón EG, Chambers MK. 2009. Temporal, spatial, and annual variation in the occurrence of molt-migrant passerines in the Mexican monsoon region. The Condor 111, 583–590. ( 10.1525/cond.2009.090085) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.