Abstract
The maximum work rate of animals has recently been suggested to be determined by the rate at which excess metabolic heat generated during work can be dissipated (heat dissipation limitation (HDL) theory). As a first step towards testing this theory in wild animals, we experimentally manipulated brood size in breeding marsh tits (Poecile palustris) to change their work rate. Parents feeding nestlings generally operated at above-normal body temperatures. Body temperature in both males and females increased with maximum ambient temperature and with manipulated work rate, sometimes even exceeding 45°C, which is close to suggested lethal levels for birds. Such high body temperatures have previously only been described for birds living in hot and arid regions. Thus, reproductive effort in marsh tits may potentially be limited by the rate of heat dissipation. Females had lower body temperatures, a possible consequence of their brood patch serving as a thermal window facilitating heat dissipation. Because increasing body temperatures are connected to somatic costs, we suggest that the HDL theory may constitute a possible mediator of the trade-off between current and future reproduction. It follows that globally increasing, more stochastic, ambient temperatures may restrict the capacity for sustained work of animals in the future.
Keywords: body temperature, heat dissipation, hyperthermia, maximum work rate, parental effort, reproductive cost
1. Introduction
Rates of resource acquisition and assimilation determine the amount of energy that animals can allocate to different activities and physiological processes. This, in turn, will influence major trade-offs such as future versus current reproduction and quality versus quantity of progeny, ultimately affecting life-history strategies [1]. Thus, it is not surprising that limitations to sustained rates of energy expenditure have attracted much research interest during the last four decades (e.g. [2,3]).
This work has resulted in the formulation of several hypotheses explaining limitations to the amount of work an animal can perform. (i) Environmental limitation: as energy expenditure needs to be fuelled by energy intake, limitations of food availability will restrict work rates, resulting in behaviours such as migration and hibernation to avoid seasonal reductions in food availability. However, access to such resources alone cannot always explain limits to sustained energy expenditure, because studies have shown that animals under ad libitum conditions may still be restricted in their work output. (ii) Central limitation: this hypothesis posits that maximum energy expenditure is limited by the capacity of the alimentary tract [2]. In line with this hypothesis, hummingbirds sometimes perch 75% of the day despite their very high energy requirements, probably owing to constraints on the rate of digestion [4]. (iii) Metabolic theory of ecology: according to this hypothesis, energy expenditure is constrained by the rate at which the circulatory system can deliver nutrients to organs and tissues [5]. Thus, even when energy intake is plentiful and the alimentary tract can process the food fast and efficiently; the distribution of energy to tissues sets the limit to maximum working capacity. These three hypotheses view limitations as inadequate provision of energy to the effector organs/tissues. By contrast, (iv) peripheral limitation: posits that the capacity of the effector organs themselves, such as the ability of muscles to produce physical work and mammary glands to produce milk, constrains maximum work rate [3,6].
These four hypotheses stress the importance of intrinsic rate limits on animal tissues, either owing to sub-optimal assimilation and/or nutrient delivery, or the limited working-capacity of effector organs. Recently, an alternative hypothesis was suggested, the (v) heat dissipation limitation (HDL) theory, proposing that the sustained rate of energy expenditure is limited by the capacity to dissipate excess heat produced as a by-product of all metabolic processes [6]. In a series of classical experiments in laboratory mice during lactation (the most energy demanding activity for female mammals), Hammond & Diamond, and Speakman and co-workers found that females that were manipulated to work harder (via experimentally increased litter size) did not increase food intake and did not produce more milk, despite the fact that the need of the pups increased [7,8]. As a consequence, pups were malnourished and grew slowly and were sometimes even killed by their mothers. These obvious limitations to sustained work rate can be explained by all but the environmental limitation hypothesis (as the mothers were fed ad libitum). However, when lactating females were exposed to cold temperatures (8°C), they responded by increasing food intake and milk production and so raised larger pups than females in warmer (21°C) environments [9]. Lactating females exposed to even warmer (30°C) ambient temperatures decreased food intake and milk production and consequently produced smaller pups than females in other environments [10,11]. Because these results were not compatible with any of the previous (i–iv) hypotheses, Król & Speakman [6,10] formulated the HDL theory to explain the beneficial effect of cold temperature on reproductive performance. This theory subsequently received experimental support, as shaving the back of lactating females to facilitate heat dissipation resulted in increased food intake and milk production ([12]; but see [13]). Thus, to avoid detrimentally high body temperatures [14–16], control females could not employ heat-generating processes to the same extent as shaved females, and, therefore, could not work as hard.
No studies to our knowledge have directly investigated the HDL theory in wild animals in their natural environment. However, male weasels (Mustela nivalis) decreased activity and energy expenditure during days with high ambient temperatures, a result that is consistent with the HDL theory [17]. Furthermore, Guillemette et al. [18] found that flight bouts in migrating eider ducks (Somateria mollissima) are terminated when body temperature approaches a critical level of hyperthermia, and that birds extend both the total time spent flying and maximum flight duration when their rate of heat storage is lower.
As recently suggested [19], testing the HDL theory in bird models could be especially interesting in view of the higher and more variable body temperatures in this homeothermic lineage compared to mammals [20]. Small birds might be particularly interesting study objects in this context, on account of their higher mass-specific metabolic rates and higher body temperatures [21]. Thus, we manipulated brood size during the nestling feeding stage (which is one of the most energetically demanding times of the year) under a range of ambient temperatures in wild marsh tits (Poecile palustris). Parents taking care of experimentally enlarged broods commonly increase their feeding rate and work load both in marsh tits [22] and in other passerines (e.g. [23–25]). However, nestlings in enlarged broods are typically significantly lighter than those from control and reduced broods [26], indicating that parents are not fully able to compensate for the extra young. Thus, parental energy expenditure is probably either reaching a ceiling, or is traded-off against costs of a further increase in effort. Small passerines have a high daytime body temperature that increases rapidly during flight. We, therefore, predicted that increasing parental effort would result in higher body temperatures and that work rate would be constrained at very high body temperatures, and that any such treatment-related effects would be more pronounced at higher ambient temperatures.
2. Material and methods
(a). General methods
This study was performed during the breeding seasons of 2010 and 2011 in a nest-box breeding population of marsh tits at Revingehed, 20 km east of Lund in southern Sweden (55°42′ N, 13°28′ E). Nest-boxes were wooden and of standardized size (outer dimensions: 220 × 125 × 95 mm (height × width × depth); material thickness 22 mm), with a circular entrance hole of 26 mm that prevented entry by dominant great tits. The nest-box area covers 64 km2 of small deciduous woodlands surrounded by permanent pastures. During the breeding season, we visited the nest-boxes once a week to determine the day of the first egg (under the assumption that one egg is produced per day) and clutch size. To determine the exact date of hatching, boxes were visited daily from one day before estimated hatching to hatching of at least half the clutch. Marsh tits, which are sedentary in the area, are small (10–12 g), cavity nesting passerines that readily uses nest-boxes for breeding. Females at the study site produce a clutch of 5–11 eggs with yearly averages usually between seven and nine eggs [27]. Females incubate alone and both parents feed the nestlings for 19–21 days [28].
(b). Experiment
We created enlarged and reduced broods by moving three or four, six-day old nestlings from reduced to enlarged broods. This resulted in reduced broods being 44.3% (s.d. = 5.5) smaller and enlarged broods 44.8% (s.d. = 7.4) larger compared to their original brood size [26]. We matched the manipulated broods pairwise with respect to hatching date and choose the pairs to minimize travel distance. Unmanipulated broods served as controls and were evenly distributed over the hatching date period of manipulated broods. We manipulated 39 pairs of broods (mean brood size (±s.d.): 4.28 ± 1.25 and 12.41 ± 1.24 in reduced and enlarged broods, respectively) and assigned 46 broods to be controls (7.84 ± 1.48). A total of 66 broods were included in the experiment in 2010 and 58 in 2011. Laying date and original clutch size did not differ between the three brood categories in any of the years (ANOVA, p > 0.15 in all cases). For a detailed description of the manipulation procedure, see [26]. Sample size was reduced owing to predation of one of the parents or the brood. The difference between sample sizes reported here and those in Nilsson & Nord [26] is owing to additional predation events between the nestling days 10 and 14. Thus, the final sample size in this study included 28 enlarged broods (2010: 12; 2011: 16), 37 control broods (2010: 19; 2011: 18) and 26 reduced broods (2010: 15; 2011: 11).
To investigate the effect of parental effort on nocturnal body temperatures, we captured females while they roosted among their brood 9 or 10 nights after hatching. This showed that hard-working females became more hypothermic at night than control females [see 26]. When nestlings were between 13 and 19 days old, we captured the male and the female when they were feeding their nestlings. Upon entry into the nest-box by one of the parents, we ran from a hidden position 20–30 m from the nest-box and swiftly captured the parents within 10–15 s of them entering the nest-box, which ensured standardization of flight/feeding behaviours prior to measurements of body temperature. The majority (80%) of birds were captured on day 14. We managed to capture all males, but two females (tending a control and an enlarged brood, respectively) eluded our capturing efforts. Of the parents captured at nestling day 14, eight females and 11 males were also captured during the next day, which enabled us to calculate body temperature repeatability between days. It turned out that body temperature between days were significantly repeatable for both females (r = 0.62; n = 8; p = 0.029) and males (r = 0.72; n = 11; p = 0.0028).
Within 10 s (usually within 5 s) of capture, we measured core body temperature with a Testo 925 digital thermometer (Testo AG, Lenzkirch, Germany) equipped with a type K thermocouple (Ø = 0.9 mm; ELFA AB, Järfälla, Sweden) calibrated by an accredited calibration laboratory (Nordtec Instrument AB, Göteborg, Sweden). The thermocouple was inserted 12 mm into the cloaca (deeper insertion did not alter the temperature readings as tested during pilot measurements) and we obtained three body temperature readings with the thermocouple in place (inter-reading repeatability; females: r = 0.97; n = 89; p < 0.001; males: r = 0.95; n = 91; p < 0.001 [29]; average used in analyses). Body temperature was stable during the 10 s measurement period i.e. between 10 and 20 s after capture. In males, body temperature decreased by, on average, 0.018°C (s.e. = 0.011) between the first and the third reading. In females, the corresponding body temperature decrease was 0.009°C (s.e. = 0.013). At this time, we also weighed (to the closest 0.1 g) the parents and measured their wing (to the closest 0.5 mm) and tarsus lengths (to the closest 0.1 mm). Sex was determined according to the presence of a brood patch and age according to Svensson [30] or, if already ringed, from our previous records of birds. Unringed birds entering our population when in their third calendar year or later were assigned the age of 3 years. Maximum ambient temperature (highest hourly mean between 18.00 and 18.00 on consecutive days) was collected from a weather station in Lund, 20 km from the study site.
Statistical analyses were performed using SAS Enterprise Guide 6.1 (SAS Institute Inc., Cary, NC, USA). The difference between male and female core body temperature was analysed using a paired-sample t-test between parents provisioning at the same nest. To test which variables could explain significant variation in female or male core body temperature, we used mixed-effect models fitted with restricted maximum-likelihood with female or male identity as the random variable. In the original model, we included maximum ambient temperature, female age (2–7 years), day of measurement and brood size as covariates, and experimental category (reduced, control or enlarged brood) and year as fixed factors. We also included the interaction between year and experimental category and year and brood size, and between maximum ambient temperature and experimental category and maximum ambient temperature and brood size. All initial models were reduced by backward elimination of non-significant variables until only significant terms (p < 0.05) remained. Owing to multicollinearity between brood size and experimental category, we exchanged these variables for each other when they remained in final models and re-fitted models with maximum-likelihood parameter estimation. We then calculated the Akaike information criterion (AIC) for these models, and selected the model with the lowest AIC as final [31].
3. Results
We found no significant difference between experimental categories in female mass at nestling day 14 [26]. However, mean nestling mass at the same time differed between categories with nestlings from enlarged broods (mean = 11.3 g; s.e. = 0.15) being significantly smaller than nestlings from both control (mean = 11.8 g; s.e. = 0.13) and reduced (mean = 12.0 g; s.d. = 0.16) broods, whereas nestlings mass between control and reduced broods did not differ [26].
Males and females had significantly different core body temperature during nestling feeding (t88 = 3.34; p = 0.0012), with males (mean = 43.46°C; s.d. = 0.77) operating at higher body temperatures than females (mean = 43.17°C; s.d. = 0.70). Overall, body temperatures during nestling feeding were well above those measured at other times of the year with the highest individual body temperatures greater than 45°C (male: 45.40°C and female: 45.50°C).
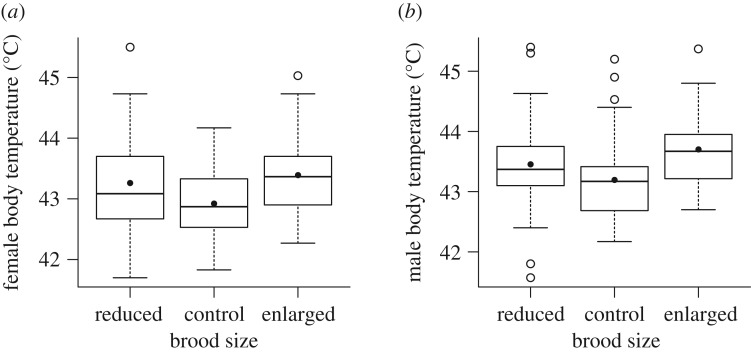
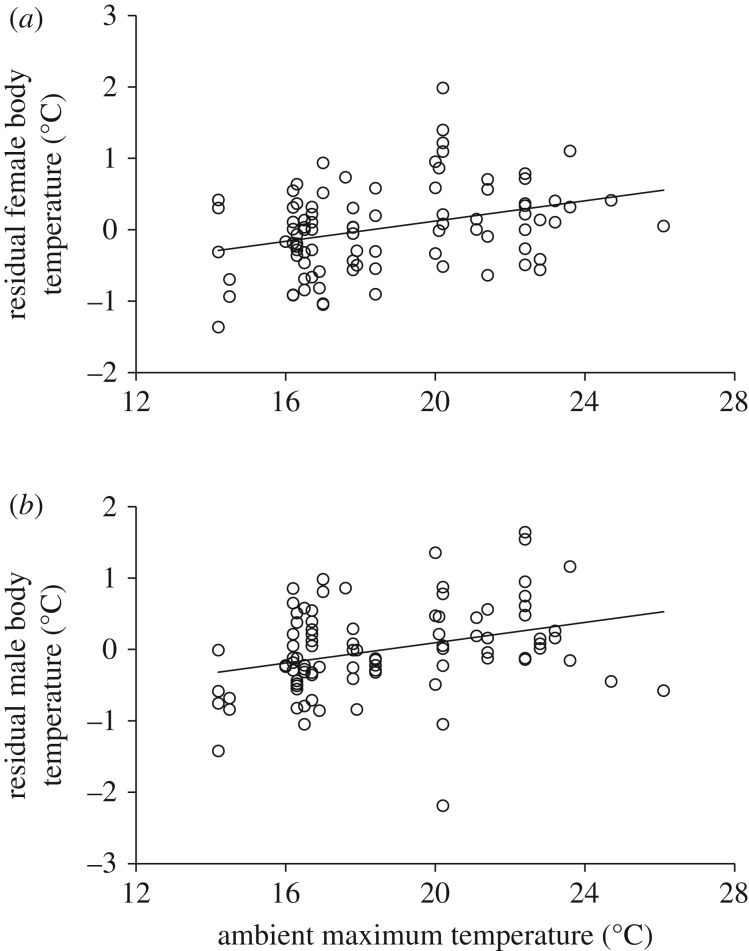
The final model for explaining variation in core body temperature within each sex included experimental category (figure 1) in both females (F2,79.6 = 7.01; p = 0.0016) and males (F2,92.8 = 5.10; p = 0.0079). Model fit was reduced when experimental category was exchanged for brood size (females: ΔAIC = 10.1; males: ΔAIC = 3.6). Females tending both enlarged and reduced broods had significantly higher body temperatures than those tending control broods (figure 1a). In males, individuals feeding an enlarged brood had higher body temperatures than those feeding control broods (figure 1b). Both the female and male models also included maximum ambient temperature and year. The maximum ambient temperature also affected parental body temperature (figure 2) as body temperature increased with maximum ambient temperature in both females (F1,90.5 = 10.5; p = 0.0017; b = 0.069) and males (F1,68.4 = 7.94; p = 0.0063; b = 0.067). During 2011, both females (F1,56.3 = 14.5; p = 0.0003) and males (F1,82.6 = 15.2; p = 0.0002) had significantly higher body temperatures when feeding their nestlings than during 2010, the average difference being 0.40°C and 0.51°C for females and males, respectively. Mean maximum ambient temperature did not differ significantly between the years (2010: mean = 18.8°C; s.d. = 3.19; 2011: mean = 19.5; s.d. = 3.53; t-test28 = 0.53; p = 0.6).
Figure 1.
Box plots of female (a) and male (b) core body temperature in relation to experimental brood size category (reduced, control, enlarged). Box plots show medians, quartiles, ranges, outliers as well as means as a black dot. Tukey post hoc tests: reduced versus control; females: p = 0.025; males: p = 0.16; control versus enlarged; females: p = 0.0006; males: p = 0.0019; reduced versus enlarged; females: p = 0.3; males: p = 0.13.
Figure 2.
The relationship between residual female (a) and male (b) core body temperature (°C) and maximum ambient temperature (°C). Residuals were calculated from mixed-effect models (where bird identity was used as a random variable) with male and female body temperature, respectively, as dependent variables and year and experimental brood size category (reduced, control, enlarged) as explanatory variables. Equation of the lines; female: Y = 0.069X − 1.29; male: Y = 0.067X − 1.26.
4. Discussion
All of the hypotheses outlined in the introduction could potentially explain the inability of parents to adequately feed nestlings in enlarged broods. However, as both male and female marsh tits tending such broods were significantly more hyperthermic than those tending control broods, we suggest that the HDL theory is also compatible with our results. We, therefore, propose that hard-working parents seemingly had problems to dissipate excess heat produced during foraging and nestling-feeding flights. In line with this, when estimating energy demands of parents feeding nestlings in the different experimental categories (see the electronic supplementary material, data), we found that parents of control broods had 14.8% lower energy expenditure during the active day than parents of enlarged broods (electronic supplementary material, table S1). The highest recorded body temperature (45.40°C and 45.50°C; see Results) were almost 2°C higher than the assumed hyperthermia threshold (above which all physical activity ceased) in migrating eider ducks (42.6°C; [18]) and comparable with the highest records for passerines in tropical and arid regions [32–34].
The most important avenue for dry heat loss in a flying bird is from forced convection, particularly from the sparsely insulated areas under the wing, the head and the legs [35]. Thus, when parents enter a cavity to feed nestlings, their ability to dissipate heat generated during flight is drastically reduced, both as a result of folded wings and of much reduced air flow over the body, compared with flying. By capturing and handling parents in this state, we may have prolonged the period of reduced heat dissipation capacity. However, because our repeated body temperature measures over a 10 s period did not indicate any buildup of a heat load, we do not believe that the handling affected our estimates of core body temperature. Moreover, even if heat dissipation rate is reduced when the bird is not flying, heat production rate should also be considerably lower when the bird is resting inside the nest-box compared to when in flight.
Also parents tending reduced broods had higher body temperatures than those in the control treatment, indicating that there was an increase in heat-generating processes also in this group. This is supported by the fact that females with reduced broods engaged in energy-saving behaviours during the night [26], by roosting in nest-boxes in higher proportions despite increased perceived predation risk and by entering deeper nocturnal hypothermia than control females (NB: males never roost in nest-boxes in our population). This indicates that these females had spent more energy than control females during the day, precluding them from accumulating enough fat reserves to maintain nocturnal body temperature at normal levels and necessitating behavioural mitigation to further conserve energy [26].
Which were then the activities or processes that increased heat production in brood-reduced males and females up to the level of parents tending enlarged broods (figure 1)? The high and repeatable diurnal body temperatures were probably not a result of increased feeding effort in reduced broods, because nestlings in these broods (despite being fewer) were not heavier than control nestlings. Parents of reduced broods may perceive the change in brood size as an act of predation, which could potentially result in increased levels of stress hormones, e.g. corticosterone [36]. Increased levels of stress hormones have been found to be associated with increased metabolic rate in mammals [37] and in some birds [38]; but see [39]. However, parents relieved of feeding duties have also been found to advance their onset of moult to overlap the nestling feeding period [40]. As moult results in a substantial increase in energy expenditure [41,42], moulting birds will produce more heat than non-moulting ones (electronic supplementary material, table S1). In fact, our estimates of metabolic rate for parents feeding different brood categories (see the electronic supplementary material, data), revealed that a moulting parent of a reduced brood would expend considerably more energy than a parent tending a control brood (+9.4%), and only somewhat less than a parent of an enlarged brood (−4.9%) (electronic supplementary material, table S1). The effect of an experimental reduction in brood size on parental physiology, as well as its generality, clearly requires further investigation.
This study is, to our knowledge, the first verification of a relationship between ambient temperature and high body temperatures in a passerine at temperate latitudes and under natural conditions. Although such relationships are common in arid and tropical environments (e.g. [32–34]), even modest increases in ambient temperatures resulted in an increase in body temperature in hard-working parents also at a temperate latitude. Parents with the highest body temperatures in our study (greater than 45°C) were close to predicted lethal levels for passerines (approx. 46°C) [43,44]. Furthermore, birds from arid and hot areas, subjected to very hot environments (greater than 46°C) in captive conditions, seem to avoid body temperatures greater than 45°C [33,34]. For example, when the body temperature of birds in a hot and dry environment in Nigeria were measured directly after catching them in mist-nets (i.e. shortly after a terminated flight bout), very few individuals had body temperatures greater than 45°C [32]. Thus, hardworking marsh tit parents in temperate areas, attain a heat load comparable of birds living in extremely hot environments.
Even though we can only provide correlational support for the HDL theory, it is intriguing that even unmanipulated nestling-feeding marsh tit parents operate at high body temperatures. As shown in our study, a further increase in work rate may increase this already high body temperature to close-to-lethal levels. This indicates that any further increases in work rate could be constrained by an inability to dissipate heat generated by physical work. Further support for the HDL theory comes from the fact that males, on average, had higher body temperatures than females. This is probably not a result of higher feeding effort in males, as we have previously found that female marsh and blue tits (Cyanistes caeruleus, which are closely related, and ecologically similar, species) at the same study site feed slightly more intensively than males [45,46]. Instead, we propose that females are able to operate at lower body temperatures on account of their unfeathered and richly vascularized brood patch. It is, thus, conceivable that this structure serves as a thermal window that greatly facilitates heat dissipation [19].
If the HDL theory should function as a mediator of reproductive costs, i.e. underpin the trade-off between current and future reproduction, one would predict both decreased work rate and gradually increased somatic costs as body temperature increases. When animals approach very high body temperatures, work rate may be traded against maintained somatic integrity in both mammals [47], including humans [48] and birds (eider ducks, [18]). However, before approaching lethal levels, there is good evidence that increased body temperatures are connected to costs. Even though hyperthermia may confer significant energy- and water-conservation benefits [49], metabolic rate and evaporation still increases gradually with increasing body temperatures [33,34]. Thus, hyperthermia is likely to generate metabolic and water costs. Furthermore, biochemical and physiological processes are suggested to be temperature-dependent, and that the rate of various cellular processes has been optimized to normothermic body temperatures [50]. In ectotherms, this is exemplified by marked co-adaptation between body temperature and optimal physiological performance that differs depending on the thermal environment to which animals are adapted [51]. Additionally, levels of stress hormones increase fast with heat stress in vertebrates [52]. Maybe the most commonly described cost of sub-lethal high body temperatures is an increase in the production of reactive oxygen subjects [14–16], with downstream consequences such as increased lipid peroxidation [53]. This might explain why high body temperatures have been found to reduce current reproductive success in mammals [54]. Thus, at sub-lethal hyperthermic body temperatures, organisms may have to pay a somatic cost to sustained work rate. The HDL theory may, therefore, constitute one possible mediator of the trade-off between current and future reproduction.
As body temperature is affected by ambient temperature, it is evident that an increase in the latter will force birds to reduce work rate to retain water and to keep body temperature below lethal levels. Failure to do so might explain catastrophic avian mortality events during heat waves (reviewed in [55]). The incidence of such heat waves is predicted to increase under climate change [56]. However, it is also possible that the present gradual increase in ambient temperatures could affect avian life-history strategies. In our study, we found a consistent increase in body temperature as ambient temperature increased, even in our relatively cold habitat and in already hyperthermic birds. Thus, breeding birds might ultimately be required to decrease work rate when ambient temperature increases to escape increased, hyperthermia-related, somatic cost. This could result in reduced brood sizes or increased nestling mortality, or activity shifts of nestling feeding away from the hottest parts of the day [57]. Any such effects are probably even more pronounced at already warmer latitudes. Ultimately, global warming may, thus, directly select for changes in life-history strategies. In relation to this, it is intriguing that brood size [58], basal metabolic rate [59] and (especially) peak metabolic rate [60] decrease towards the equator. Yet, decreased feeding effort predicted by our own study may well be compensated for by reduced nocturnal thermogenic costs [26] with increasing ambient temperature at high latitudes.
Supplementary Material
Acknowledgements
We thank Sieglinde Kundisch for help in the field.
Ethics
All procedures performed involving animals were in accordance with the ethical standards of the institution and comply with national legislation and were approved by the Malmö/Lund Animal Care committee prior to the start of experiments (permit nos. M 237-07, M 235-10). Catching and ringing of adults and young was performed under licence from the Swedish Ringing Centre (licence no. 475).
Data accessibility
The datasets supporting this article are available at Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.2q2h5) [61].
Authors' contributions
J.-Å.N. and A.N. conceived and designed the study. J.-Å.N. and A.N. performed the field work. J.-Å.N. analysed the data and wrote the first draft of the manuscript with continuous input on both processes from A.N.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by grants from the Swedish Research Council (grant no. 621-2009-5194) to J.-Å.N. and the Lund Animal Protection Foundation, the Helge Ax:son Johnson Foundation and the Swedish Research Council (grant no. 637-2013-7442) to A.N.
References
- 1.van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 2.Drent RH, Daan S. 1980. The prudent parent: energetic adjustments in avian breeding. Ardea 86, 225–252. [Google Scholar]
- 3.Hammond KA, Diamond J. 1997. Maximal sustained energy budgets in humans and animals. Nature 386, 457–462. ( 10.1038/386457a0) [DOI] [PubMed] [Google Scholar]
- 4.Diamond JM, Karasov WH, Phan D, Carpenter FL. 1986. Digestive physiology is a determinant of foraging bout frequency in hummingbirds. Nature 320, 62–63. ( 10.1038/320062a0) [DOI] [PubMed] [Google Scholar]
- 5.West GB, Brown JH, Enquist BJ. 1999. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284, 1677–1679. ( 10.1126/science.284.5420.1677) [DOI] [PubMed] [Google Scholar]
- 6.Speakman JR, Król E. 2010. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746. [DOI] [PubMed] [Google Scholar]
- 7.Hammond KA, Diamond J. 1992. An experimental test for a ceiling on sustained metabolic rate in lactating mice. Physiol. Zool. 65, 952–977. ( 10.1086/physzool.65.5.30158552) [DOI] [Google Scholar]
- 8.Johnson MS, Thomson SC, Speakman JR. 2001. Limits to sustained energy intake. I. Lactation in the laboratory mouse Mus musculus. J. Exp. Biol. 204, 1925–1935. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MS, Speakman JR. 2001. Limits to sustained energy intake. V. Effect of cold-exposure during lactation in Mus musculus. J. Exp. Biol. 204, 1967–1977. [DOI] [PubMed] [Google Scholar]
- 10.Król E, Speakman JR. 2003. Limits to sustained energy intake. VI. Energetics of lactation in laboratory mice at thermoneutrality. J. Exp. Biol. 206, 4255–4266. ( 10.1242/jeb.00674) [DOI] [PubMed] [Google Scholar]
- 11.Król E, Speakman JR. 2003. Limits to sustained energy intake. VII. Milk energy output in laboratory mice at thermoneutrality. J. Exp. Biol. 206, 4267–4281. ( 10.1242/jeb.00675) [DOI] [PubMed] [Google Scholar]
- 12.Król E, Murphy M, Speakman JR. 2007. Limits to sustained energy intake. X. Effects of fur removal on reproductive performance in laboratory mice. J. Exp. Biol. 210, 4233–4243. ( 10.1242/jeb.009779) [DOI] [PubMed] [Google Scholar]
- 13.Zhao ZJ, Chi QS, Cao J. 2010. Milk energy output during peak lactation in shaved Swiss mice. Physiol. Behav. 101, 59–66. ( 10.1016/j.physbeh.2010.04.017) [DOI] [PubMed] [Google Scholar]
- 14.Lin H, Decuypere E, Buyse J. 2006. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A 144, 11–17. ( 10.1016/j.cbpa.2006.01.032) [DOI] [PubMed] [Google Scholar]
- 15.Jimenez AG, Williams JB. 2014. Rapid changes in cell physiology as a result of acute thermal stress in house sparrows, Passer domesticus. J. Thermal Biol. 46, 31–39. ( 10.1016/j.jtherbio.2014.10.001) [DOI] [PubMed] [Google Scholar]
- 16.Vesco APD, Gasparino E, Grieser DO, Zancanela V, Voltolini DM, Khatlab AS, Guimarães SEF, Soares MAM, Neto ARO. 2015. Effects of methionine supplementation on the expression of protein deposition-related genes in acute heat stress-exposed broilers. PLoS ONE 10, e0115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zub K, Fletcher QE, Szafrańska PA, Konarzewski M. 2013. Male weasels decrease activity and energy expenditure in response to high ambient temperatures. PLoS ONE 8, e72646 ( 10.1371/journal.pone.0072646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillemette M, Woakes AJ, Larochelle J, Polymeropoulos ET, Granbois J-M, Butler PJ, Pelletier D, Frappell PB, Portugal SJ. 2016. Does hyperthermia constrain flight duration in a short-distance migrant? Phil. Trans. R. Soc. B 371, 20150386 ( 10.1098/rstb.2015.0386) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grémillet D, Meslin L, Lescroël A. 2012. Heat dissipation limit theory and the evolution of avian functional traits in a warming world. Funct. Ecol. 25, 1001–1006. ( 10.1111/j.1365-2435.2012.02048.x) [DOI] [Google Scholar]
- 20.Prinzinger R, Pressmar A, Schleucher E. 1991. Body temperature in birds. Comp. Biochem. Physiol. A 99, 499–506. ( 10.1016/0300-9629(91)90122-S) [DOI] [Google Scholar]
- 21.McNab BK. 1966. An analysis of the body temperatures of birds. Condor 68, 47–55. ( 10.2307/1365174) [DOI] [Google Scholar]
- 22.Nilsson J-Å. 2002. Metabolic consequences of hard work. Proc. R. Soc. Lond. B 269, 1735–1739. ( 10.1098/rspb.2002.2071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nur N. 1984. Feeding frequencies of nestling blue tits (Parus caeruleus): costs, benefits and a model of optimal feeding frequency. Oecologia 65, 125–137. ( 10.1007/BF00384475) [DOI] [PubMed] [Google Scholar]
- 24.Sanz JJ, Tinbergen JM. 1999. Energy expenditure, nestling age, and brood size: an experimental study of parental behavior in the great tit Parus major. Behav. Ecol. 10, 598–606. ( 10.1093/beheco/10.5.598) [DOI] [Google Scholar]
- 25.García-Navas V, Sanz JJ. 2010. Flexibility in the foraging behavior of blue tits in response to short-term manipulations of brood size. Ethology 116, 744–754. [Google Scholar]
- 26.Nilsson J-Å, Nord A. 2017. The use of the nest for parental roosting and thermal consequences of the nest for nestlings and parents. Behav. Ecol. Sociobiol. 71, 171 ( 10.1007/s00265-017-2400-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nilsson J-Å. 1991. Clutch size determination in the marsh tit (Parus palustris). Ecology 72, 1757–1762. ( 10.2307/1940974) [DOI] [Google Scholar]
- 28.Nilsson J-Å, Svensson M. 1993. Fledging in altricial birds: parental manipulation or sibling competition? Anim. Behav. 46, 379–386. ( 10.1006/anbe.1993.1200) [DOI] [Google Scholar]
- 29.Lessells CM, Boag PT. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121. ( 10.2307/4087240) [DOI] [Google Scholar]
- 30.Svensson L. 1992. Identification guide to European passerines. Norfolk, UK: British Trust for Ornithology. [Google Scholar]
- 31.Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. 2006. SAS for mixed models. Cary, NC: SAS Institute Inc. [Google Scholar]
- 32.Nilsson J-Å, Molokwu MN, Olsson O. 2016. Body temperature regulation in hot environments. PLoS ONE 11, e0161481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKechnie AE, Gerson AR, McWhorter TJ, Smith EK, Talbot WA, Wolf BO. 2017. Avian thermoregulation in the heat: evaporative cooling in five Australian passerines reveals within-order biogeographic variation in heat tolerance. J. Exp. Biol. 220, 2436–2444. ( 10.1242/jeb.155507) [DOI] [PubMed] [Google Scholar]
- 34.Smith EK, O'Neill JJ, Gerson AR, McKechnie AE, Wolf BO. 2017. Avian thermoregulation in the heat: resting metabolism, evaporative cooling and heat tolerance in Sonoran Desert songbirds. J. Exp. Biol. 220, 3290–3300. ( 10.1242/jeb.161141) [DOI] [PubMed] [Google Scholar]
- 35.Ward S, Rayner JMV, Möller U, Jackson DM, Nachtigall W, Speakman JR. 1999. Heat transfer from starlings Sturnus vulgaris during flight. J. Exp. Biol. 202, 1589–1602. [DOI] [PubMed] [Google Scholar]
- 36.Clinchy M, Zanette L, Boonstra R, Wingfield JC, Smith JNM. 2004. Balancing food and predator induced chronic stress in songbirds. Proc. R. Soc. Lond. B 271, 2473–2479. ( 10.1098/rspb.2004.2913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasse CG, Long AK, Gillooly JF. 2016. Energetics of stress: linking plasma cortisol levels to metabolic rate in mammals. Biol. Lett. 12, 20150867 ( 10.1098/rsbl.2015.0867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimeno B, Hau M, Verhulst S. 2017. Strong association between corticosterone levels and temperature-dependent metabolic rate in individual zebra finches. J. Exp. Biol. 220, 4426–4431. ( 10.1242/jeb.166124) [DOI] [PubMed] [Google Scholar]
- 39.Buttemer WA, Astheimer LB, Wingfield JC. 1991. The effect of corticosterone on standard and metabolic rates of small passerine birds. J. Comp. Physiol. B 161, 427–431. [DOI] [PubMed] [Google Scholar]
- 40.Morales J, Moreno J, Merino S, Sanz JJ, Tomás G, Arriero E, Lobato E, la Puente JM. 2007. Early moult improves local survival and reduces reproductive output in female pied flycatchers. Écoscience 14, 31–39. ( 10.2980/1195-6860(2007)14%5B31:EMILSA%5D2.0.CO;2) [DOI] [Google Scholar]
- 41.Lindström Å, Visser GH, Daan S. 1993. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 66, 490–510. ( 10.1086/physzool.66.4.30163805) [DOI] [Google Scholar]
- 42.Cyr NE, Wikelski M, Romero LM. 2008. Increased energy expenditure but decreased stress responsiveness during molt. Physiol. Biochem. Zool. 81, 452–462. ( 10.1086/589547) [DOI] [PubMed] [Google Scholar]
- 43.Calder WA. 1964. Gaseous metabolism and water relations of the zebra finch, Taeniopygia castanotis. Physiol. Zool. 37, 400–413. ( 10.1086/physzool.37.4.30152758) [DOI] [Google Scholar]
- 44.Williams JB, Shobrak M, Wilms TM, Arif IA, Khan HA. 2012. Climate change and animals in Saudi Arabia. Saudi J. Biol. Sci. 19, 121–130. ( 10.1016/j.sjbs.2011.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Råberg L, Nilsson J-Å, Ilmonen P, Stjernman M, Hasselquist D. 2000. The cost of an immune response: vaccination reduces parental effort. Ecol. Lett. 3, 382–386. ( 10.1046/j.1461-0248.2000.00154.x) [DOI] [Google Scholar]
- 46.Nilsson J-Å. 2003. Ectoparasitism in marsh tits: costs and functional explanations. Behav. Ecol. 14, 175–181. ( 10.1093/beheco/14.2.175) [DOI] [Google Scholar]
- 47.Marino FE. 2004. Anticipatory regulation and avoidance of catastrophe during exercise-induced hyperthermia. Comp. Biochem. Physiol. B 139, 561–569. ( 10.1016/j.cbpc.2004.09.010) [DOI] [PubMed] [Google Scholar]
- 48.Tucker R, Marle T, Lambert EV, Noakes TD. 2006. The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J. Physiol. 574, 905–915. ( 10.1113/jphysiol.2005.101733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nord A, Williams JB. 2015. The energetic costs of incubation. In Nests, eggs, and incubation: new ideas about avian reproduction (eds Deeming DC, Reynolds SJ), pp. 152–170. Oxford, UK: Oxford University Press. [Google Scholar]
- 50.Boyles JG, Seebacher F, Smit B, McKechnie AE. 2011. Adaptive thermoregulation in endotherms may alter responses to climate change. Integr. Comp. Biol. 51, 676–690. ( 10.1093/icb/icr053) [DOI] [PubMed] [Google Scholar]
- 51.Hochachka PW, Somero GN. 2002. Biochemical adaptation: mechanism and process in physiological evolution. Oxford, UK: Oxford University Press. [Google Scholar]
- 52.Jessop TS, Lane ML, Teasdale L, Stuart-Fox D, Wilson RS, Careau V, Moore IT. 2016. Multiscale evaluation of thermal dependence in the glucocorticoid response of vertebrates. Am. Nat. 188, 342–356. ( 10.1086/687588) [DOI] [PubMed] [Google Scholar]
- 53.Azad KMA, Kikusato M, Hoque AM, Toyomizu M. 2010. Effect of chronic heat stress on performance and oxidative damage in different strains of chickens. J. Poult. Sci. 47, 333–337. ( 10.2141/jpsa.010025) [DOI] [Google Scholar]
- 54.Hansen PJ. 2017. Effects of heat stress on mammalian reproduction. Phil. Trans. R. Soc. B 364, 3341–3350. ( 10.1098/rstb.2009.0131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKechnie AE, Wolf BO. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253–256. ( 10.1098/rsbl.2009.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.IPCC. 2013. Climate change 2013: the physical science basis. New York, NY: Oxford University Press. [Google Scholar]
- 57.Wiley EM, Ridley AR. 2016. The effects of temperature on offspring provisioning in a cooperative breeder. Anim. Behav. 117, 187–195. ( 10.1016/j.anbehav.2016.05.009) [DOI] [Google Scholar]
- 58.Lack D. 1947. The significance of clutch-size, parts 1 and 2. Ibis 89, 302–352. ( 10.1111/j.1474-919X.1947.tb04155.x) [DOI] [Google Scholar]
- 59.Wiersma P, Muñoz-Garcia A, Walker A, Williams JB. 2007. Tropical birds have a slow pace of life. Proc. Natl Acad. Sci. USA 104, 9340–9345. ( 10.1073/pnas.0702212104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiersma P, Chappell MA, Williams JB. 2007. Cold- and excerise-induced peak metabolic rates in tropical birds. Proc. Natl Acad. Sci. USA 104, 20 866–20 871. ( 10.1073/pnas.0707683104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilsson J-Å, Nord A. 2018. Data from: Testing the heat dissipation limit theory in a breeding passerine Dryad Digital Repository. ( 10.5061/dryad.2q2h5) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nilsson J-Å, Nord A. 2018. Data from: Testing the heat dissipation limit theory in a breeding passerine Dryad Digital Repository. ( 10.5061/dryad.2q2h5) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available at Dryad Digital Repository (http://dx.doi.org/10.5061/dryad.2q2h5) [61].