Abstract
A common assumption in sexual selection studies is that receivers decode signal information similarly. However, receivers may vary in how they rank signallers if signal perception varies with an individual's sensory configuration. Furthermore, receivers may vary in their weighting of different elements of multimodal signals based on their sensory configuration. This could lead to complex levels of selection on signalling traits. We tested whether multimodal sensory configuration could affect preferences for multimodal signals. We used brown-headed cowbird (Molothrus ater) females to examine how auditory sensitivity and auditory filters, which influence auditory spectral and temporal resolution, affect song preferences, and how visual spatial resolution and visual temporal resolution, which influence resolution of a moving visual signal, affect visual display preferences. Our results show that multimodal sensory configuration significantly affects preferences for male displays: females with better auditory temporal resolution preferred songs that were shorter, with lower Wiener entropy, and higher frequency; and females with better visual temporal resolution preferred males with less intense visual displays. Our findings provide new insights into mate-choice decisions and receiver signal processing. Furthermore, our results challenge a long-standing assumption in animal communication which can affect how we address honest signalling, assortative mating and sensory drive.
Keywords: multimodal sensory processing, preference functions, sexual selection
1. Introduction
Females often choose mating partners based on the quality of the male's signal, which is expected to honestly reflect his body condition, genetic makeup or other relevant traits [1,2]. The behaviours involved in this mate-choice process (male signalling and female decisions) have been widely studied since Darwin [3]. We have assumed for decades that there is a definable relationship that relates male signal quality to a female's estimate of male quality (e.g. [4,5]). This relationship implicitly assumes that all individual females at least qualitatively decode the information in a mating signal in a similar way [4,6,7]. Until recently, we have tended to ignore the possibility that the perception of a given male signal may vary depending on a female's sensory configuration (i.e. her physical, multisensory processing centres which underlie her ability to process sensory information). This is important because differential female sensory configurations may in turn lead to a differential rankings of males between females [8,9].
In short, the relationship between male signal quality and its correlation with male quality viewed from the female's perspective can unravel if individual differences in sensory processing are an important component of female choice. One can imagine that if females vary in their sensory configurations they may subsequently vary in their perception of male quality. Additionally, the information encoded in male signals is generally multimodal (i.e. information is carried across multiple sensory modalities; [10]). The complexity of multimodal signals may exacerbate the likelihood that individual females vary in their ability to decode (and even interpret) signals based on differences in sensory capacity [8,9,11]. Such a scenario would fundamentally change a basic tenet in animal communication (i.e. all receivers perceive variations in the sender's signal to a similar degree) by adding the multiple perceptual dimensions of the receiver to the selective pressures that may affect signal evolution. However, we know relatively little about the relationship between the processing of multimodal signals and subsequent behavioural mating decisions.
Our goal was to examine whether a female's multimodal sensory configuration affected her ability to resolve differences in male songs and visual signals, and ultimately her preference for males. We tested this in female brown-headed cowbirds (Molothrus ater), (hereafter, cowbirds) that use male acoustic and visual displays to make mate-choice decisions [12]. Recent evidence suggests that female cowbirds do vary in their auditory and visual sensory configurations [9,13], and that auditory and visual temporal processing are positively associated [9]. In the present study, we assessed whether this sensory variation played a role in female behavioural mating preferences. Our findings provide novel insights into the mechanistic basis of variation in mate-choice decisions [8,14,15].
The male cowbird courtship display is often multimodal and assumed to be under strong sexual selection [16,17]. Males pair their perched song with a dynamic wingspread display [12] and can display with varying visual intensity (e.g. depth of the bow, width of the wingspread) [18]. Males can also adjust the fine structure of the relatively low frequency, introductory notes of their songs called ‘glugs’ as well as the higher frequency, frequency modulated secondary phrase called ‘P2’ [19,20]. Female cowbirds prefer the multimodal display more than the song or the visual display presented alone [12,21]. We presented female cowbirds, under controlled conditions, with a range of different songs that varied in spectral and temporal acoustic properties (i.e. frequency, Wiener entropy, (i.e. a unitless index measured on a log scale from ‘0,’ or white noise, to minus infinity, a pure tone, [22]) and duration of different song elements; see electronic supplemental material) and with visual displays that varied in intensity (i.e. degree of wingspread, amount of body puffing and depth of the bow; details in Material and methods). As discussed in [9], a female's multimodal sensory filtering capacity (i.e. auditory sensitivity, auditory filter width, visual spatial resolution and visual temporal resolution; details in Material and methods) may alter the female's perception of each potential mate's multimodal signal and subsequently alter her preference for male displays. If so, we would expect to find a significant statistical interaction between our measures of female sensory filtering capacity and properties of the male song or visual display.
We hypothesized that females with higher sensory filtering capacity would have a greater ability to distinguish variation in male signals compared to those with lower sensory filtering capacity [23–25]. Differences in sensory filtering, in turn, should affect a female's ability to choose a mate (i.e. preference would be in part a function of the ability to distinguish between two signals) [8,9,25,26]. Preference functions measure a female's ranking of mates based on specific properties of a male's mating signal [27]; see details of our predicted preference function shapes in the electronic supplementary material. We predicted that (1) females with relatively higher auditory temporal resolution would have steeper preference function slopes relative to the temporal variation in song duration and song Wiener entropy compared to females with lower auditory temporal resolution. We also predicted that (2) females with relatively high auditory sensitivity (i.e. lower auditory thresholds) would have steeper preference function slopes relative to the frequency of the songs compared to females with relatively low auditory sensitivity. Finally, we predicted that (3) females with relatively higher visual temporal resolution and (4) females with higher visual spatial resolution (i.e. higher flicker fusion frequencies and higher density of cones, respectively) would have steeper preference function slopes relative to the intensity of the visual displays (i.e. more puffing, larger wingspreads and deeper bows) compared to females with relatively lower visual temporal resolution and visual spatial resolution.
2. Material and methods
(a). Animal capture and housing
Female cowbirds (N = 20) were wild-caught in decoy traps by the USDA APHIS (Sandusky, OH) in May 2013. Females were transported to Purdue University and individually housed in enclosures (64 × 40 × 64 cm). Birds were provided ad libitum access to mixed seed, grit and water. The lighting schedule followed the natural lighting conditions of West Lafayette, IN (schedule was adjusted weekly and ranged from 14 : 10 light : dark in the summer to 10 : 14 during the winter). All animal care and experimental procedures were approved by Purdue University's Animal Care and Use Committee (PACUC) Protocol # 1111000151.
(b). Experimental stimuli
We developed experimental stimuli by video recording the visual displays and songs of males (details in electronic supplementary material) following Ronald et al. [20,28]. We then quantified different aspects of these visual displays and songs, and classified them in terms of visual display intensity (i.e. degree to which the male extends his wings, puffs his feathers and bows to the receiver) and song potency (i.e. degree to which the song elicits a female copulatory solicitation display, or CSD) [29]. Finally, we paired the visual displays with the songs to have a balanced design with three exemplar videos representing each possible combination of song potency and visual display (i.e. high potency song and high intensity visual display, high potency song and low intensity visual display, low potency song and high intensity visual display, low potency song and low intensity visual display). Videos used in the playback experiment are available in the electronic supplementary material.
(c). Behavioural experiment
Mate-preference trials were conducted from 07.00–13.00 between June and September 2013. Female cowbirds were randomly divided into seven experimental blocks. Females within the same block underwent the experiment together simultaneously. For all trials, females were first exposed to the behavioural experiment and then their auditory and visual filtering capacity was assessed. This procedure, rather than testing filtering capacity both before and after experimental trials, allowed us to limit the total amount of time birds were held in captivity and ensured that the birds were tested within their natural breeding season. Furthermore, some of the visual assessments require the animal to be euthanized and as such did not allow for a before versus after comparison. The details that follow are the experimental procedures for a single block of females.
On day 1 birds were sedated with a combination of ketamine (40–60 mg kg−1) and midazolam (6–8 mg kg−1), and implanted with an oestradiol implant (10 mm crystalline oestrogen, Sigma Chemical Co., St Louis, MO, into Silastic tubing, outer diameter 1.96 mm). Oestrogen implants are commonly used to induce breeding behaviour (CSDs) in cowbirds and other avian species [12,21,30]. On day 13, birds began habituation trials to reduce the probability the females would be startled by the audiovisual playback [12,28]. Birds were randomly selected and then placed in the experimental arena which consisted of an experimental enclosure next to a high-flicker rate LCD television (Sanyo LCD HD-TV, Model # DP26649) and speaker (Saul Mineroff Field Speaker, Model # SME-AFS). After 25 min she was played a short video (4 s) of a related species, the red-winged blackbird (Agelaius phoeniceus), sitting on a perch. She was then returned to her home enclosure and another female was selected. Habituation trials were conducted from 14.00–17.00 and run for three consecutive days so that each bird was exposed to three different videos [28].
Mate-preference trials were run from day 16–28 from 07.00–13.00. Females completed two trials per day for 12 consecutive days. Similar to the habituation trials, females were placed in the experimental arena and after 25 min one of the 12 experimental videos was played [28]. Videos were chosen at random from the 12 videos with one replacement until all 12 were played twice. After the video playback the female was returned to her enclosure for 3 h before her last trial of the day. The day after trials ended (i.e. day 29) females had a blood sample taken via puncture of the left alar wing vein for later oestrogen analysis (see electronic supplementary material). This was done to control statistically for any differences in oestrogen concentration affecting CSD latency or duration (see below). Blood was collected within 2 min of capture with a heparinized collection tube (RAM Scientific Safe-T-Fill) and centrifuged for 5 min at 54 RCF in an Eppendorf centrifuge model 5415D. The plasma was then separated from the red blood cells and stored in a −80°C freezer until subsequent analysis.
We measured two behaviours to quantify female mate preferences: (1) CSD duration, where longer duration indicates greater preference for a male [12,21] and (2) the latency for each female to begin a CSD, where shorter latency is a measure of greater preference for a male [31–33]. Our measures of CSD latency and CSD duration were significantly and negatively correlated (r2 = −0.45, p < 0.001), indicating that displays that elicited long duration CSDs also tended to elicit those CSDs sooner. Two video cameras (HD Everio GZ-E10 and Samsung SMX-F40BN) recorded the behavioural trials so we could quantify these after the trial using Adobe Premiere Pro software [28]. Females that did not give a CSD to a display were coded as having a CSD duration of 0 and no data were entered for CSD latency. All estimates of duration and latency were coded by an unbiased observer (R.Z.) who was blind to the experimental treatment of the videos [28]. K.L.R. also blindly recoded 20% (170 videos) of the total number of videos analysed to ensure repeatability between observers in CSD duration and latency values. K.L.R. and R.Z. were 0.94 repeatable across their calculated values of CSD duration and 0.93 repeatable across their calculated values of CSD latency. Additionally, we found no significant differences when we ran a general liner model between observers for CSD duration (F1,388 = 0.07, p = 0.78) and CSD Latency (F1,274 = 0.44, p = 0.55).
(d). Auditory filtering capacity
Auditory filtering capacity was characterised for each individual female with two traits: (1) auditory sensitivity (i.e. the ability to resolve low intensity sounds such as the low amplitude glugs in the beginning of the cowbird song; [34,35]), and (2) the width of the auditory filter, which gives an index of a fundamental trade-off between auditory frequency resolution (i.e. the ability to discriminate frequencies as different from one another) and auditory temporal resolution (i.e. the ability to resolve rapid temporal changes in a sound like those occurring in a song trill) [36,37]. Narrow auditory filters have longer integration times and enhance frequency resolution. Wide auditory filters have shorter integration times and provide higher auditory temporal resolution [33,34]. The auditory brainstem response was used to provide an index of these two traits [38]. All auditory tests were performed within 2 days after the behavioural experiment ended. Further details of these procedures are described in the electronic supplementary material.
(e). Visual filtering capacity
Visual filtering capacity was characterized for each individual female with two traits: (1) visual temporal resolution, the ability to detect changes in visual signals over time, such as moving stimuli such the movement of the cowbird wingspread [39,40], and (2) visual spatial resolution, the ability to resolve two points as different in visual space [41,42]. Females with higher visual temporal resolution may increase their ability to distinguish different rapid components of male visual displays (i.e. wingspread elements versus the bow). Enhanced spatial visual resolution should allow for better discrimination of male visual signals such as fine differences in the degree of feather puffing (e.g. [23]).
We determined the visual temporal resolution of each female by calculating her flicker fusion frequency (FFF): the frequency of light at which a pulsing stimulus is no longer perceived as pulsing but as a continuous, steady beam [43]. Higher FFF values indicate that the individual can resolve the temporal differences between high-frequency visual stimuli. We determined the spatial visual resolution of each female by estimating the density of cone photoreceptors in the retina, which are involved in the processing of chromatic and achromatic signals under daylight conditions [41,44]. Cone densities have been implicated in visual spatial resolution: higher cone densities indicate higher visual acuity, or the ability to better resolve spatial details in visual stimuli [41,42]. Further details of these procedures are described in the electronic supplementary material.
(f). Statistical analyses
We used two behavioural measures to explore how sensory filtering capacity interacts with song and visual display to affect female mate preferences: (1) the CSD duration and (2) the latency between signal onset to the beginning of the CSD. We reduced the dimensionality of the male display properties using two-factor analyses with varimax rotation in SAS (Proc FACTOR in v. 9.3). The details of the factor analyses are published elsewhere [26]; we provide only an overview here and a table summarizing these results in the electronic supplementary material. Song and bow properties were treated separately. The song factor analysis yielded three significant factors when using the broken stick model [45] to determine eigenvalues thresholds, and accounted for 77% of the variation in song characteristics. Song factor 1 (λ = 3.11) was positively correlated with glug 1 (the first introductory element) frequency, and positively correlated with glug 2 frequency, Wiener entropy and duration. Song factor 2 (λ = 2.57) was positively correlated with both the duration of glug 1 and the inter-glug interval (IGI), and negatively correlated with glug 1 Wiener entropy. Song factor 3 (λ = 2.03) was negatively correlated with P2 frequency, and positively correlated with P2 duration and Wiener entropy.
The visual factor analysis yielded two factors that were significantly above the broken stick model thresholds [45] and accounted for 82% of the variation. Visual display factor 1 (λ = 3.87) was positively correlated with the amount of body feather puffing, wingspread width, the time interval between the start of the display and the first wing pump, and the time interval between the start of the display and when the song began. Visual display factor 2 (λ = 1.88) was positively correlated with the total duration of the display and the depth of the bow.
We then modelled mate preferences (i.e. CSD duration or latency) using repeated measures mixed models (Proc MIXED in SAS) with bird ID as the subject variable. Independent factors included our factor scores for song and visual display properties (e.g. song factor 1–3 and visual display factor 1 and 2, see Results), all sensory traits and four interactions of interest based on the predictions articulated in the Introduction: (1) song × auditory filter width, (2) song × auditory sensitivity, (3) visual display × FFF and (4) visual display × cone density. We specified an autoregressive covariance structure and the Kenward–Roger method was used to calculate the denominator degrees of freedom. Besides the main effects above, we also included the following independent factors: trial order (i.e. the order in which bird was placed in the experiment on a given day), trial day and eye axial size (as individuals with larger eyes may have greater numbers of cones and thus higher spatial resolving power) [46]. The dependent variables and eye axial size were log10 transformed to normalize residuals. We found that the statistical model with the best fit (i.e. lowest AIC value) did not include experimental block or oestrogen values as covariates. Because the results were otherwise qualitatively similar (i.e. no change in significance levels), we only present this best-fitting model. For all models, non-significant interactions were removed based on descending F-values. We also examined the possibility of three-way interactions between each of our song and visual display factor scores and sensory traits. However, none of these three-way interactions were statistically significant and were, therefore, removed from all the models.
3. Results
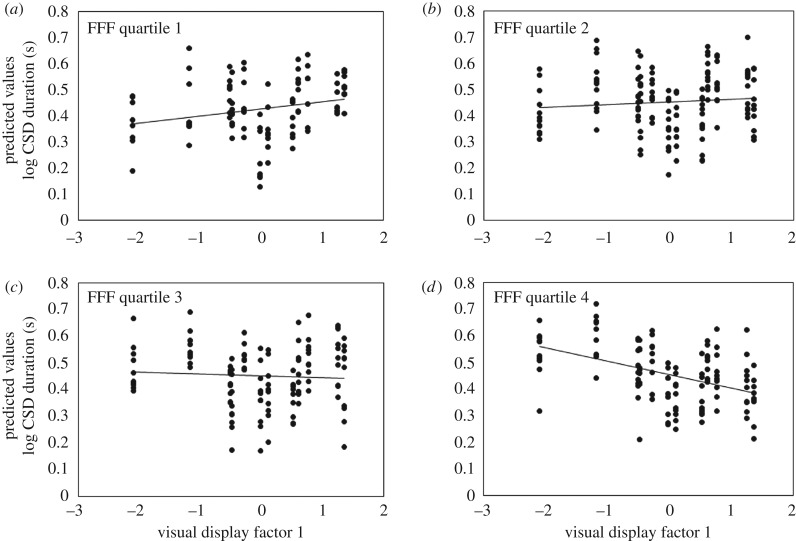
Female preference functions were significantly affected by two interaction effects related to male display properties and female sensory configuration. (1) Female auditory temporal resolution interacted with male song factor 3 to affect female CSD duration (table 1 and figure 1a–d), and (2) female visual temporal resolution interacted with male visual display intensity factor 1 to affect CSD duration (table 1, figure 2a–d). We plotted preference functions for four categories of females representing sensory quartiles ranging from the females with the lowest resolution (figures 1a and 2a), mid-low resolution (figures 1b and 2b), mid-high resolution (figures 1c and 2c), to the highest resolution (figures 1d and 2d) to illustrate how preference function slope changes with sensory properties of the birds. Regression lines were calculated from the best-fit lines of the predictive values of CSD duration (figures 1a–d and 2a–d).
Table 1.
Statistical models of female mate preferences as indexed by CSD duration and CSD latency. The factors result from a factor analysis; refer to text for more information. Italicized values indicate statistical significance while β indicates the slope of the function between a continuous independent variable and the dependent variables.
| log CSD duration |
log CSD latency |
|||||||
|---|---|---|---|---|---|---|---|---|
| effect | d.f. | F value | p value | slope (β) | d.f. | F value | p value | slope (β) |
| song factor 1 | ||||||||
| G1 frequency | 1, 365 | 1.91 | 0.17 | 0.02 ± 0.01 | 1, 294 | 5.95 | 0.02 | −0.01 ± 0.004 |
| G2 frequency, entropy, duration | ||||||||
| song factor 2 | ||||||||
| G1 entropy (-) and duration | 1, 365 | 29.77 | <0.001 | −0.06 ± 0.01 | 1, 310 | 5.95 | 0.02 | −0.01 ± 0.004 |
| IGI duration | ||||||||
| song factor 3 | ||||||||
| P2 frequency (-), entropy, duration | 1, 368 | 9.2 | 0.003 | 0.22 ± 0.07 | 1, 333 | 5.78 | 0.02 | 0.009 ± 0.004 |
| visual display factor 1 | ||||||||
| body ‘puffing,’ wing-extension, song and wing pump beginning | 1, 390 | 8.46 | 0.004 | 0.22 ± 0.08 | 1, 319 | 0.92 | 0.34 | 0.004 ± 0.004 |
| visual display factor 2 | ||||||||
| display duration and bow depth | 1, 371 | 2.58 | 0.11 | 0.02 ± 0.01 | 1, 304 | 40.67 | <0.001 | −0.03 ± 0.004 |
| photoreceptor density | 1, 72.6 | 0.65 | 0.42 | −0.000002 ± 0.000003 | 1, 97 | 5.17 | 0.03 | −0.0000002 ± 0.000001 |
| FFF | 1, 72.7 | 2.88 | 0.09 | 0.003 ± 0.002 | 1, 90.6 | 0.04 | 0.84 | −0.00008 ± 0.0004 |
| FFF × visual display factor 1 | 1, 394 | 7.37 | 0.007 | |||||
| minimum auditory threshold | 1, 72.7 | 3.38 | 0.07 | −0.006 ± 0.003 | 1, 111 | 0.2 | 0.65 | 0.0004 ± 0.0009 |
| ERB | 1, 72.7 | 4.73 | 0.03 | −0.0008 ± 0.0004 | 1, 98.7 | 4.44 | 0.04 | 0.0002 ± 0.0001 |
| ERB × song factor 3 | 1, 365 | 10.66 | 0.001 | |||||
| trial day | 1, 102 | 15.45 | <0.001 | −0.02 ± 0.005 | 1, 108 | 1.69 | 0.2 | 0.002 ± 0.001 |
| experimental order | 1, 332 | 23.72 | <0.001 | −0.01 ± 0.003 | 1, 266 | 6.86 | 0.01 | 0.003 ± 0.001 |
| log(eye axial length) | 1, 72.7 | 2.63 | 0.11 | 2.1 ± 1.27 | 1, 89 | 6.86 | 0.01 | −0.84 ± 0.32 |
Figure 1.
Significant interaction between female (N = 20) auditory temporal resolution (i.e. auditory filter width measured via equivalent rectangular bandwidth, ERB) and the ending portion of the male cowbird song (i.e. the P2, song factor 3, see text for more details) on female CSD duration. As factor 3 increases, the P2 decreases in frequency but increases in Wiener entropy and duration. Each point illustrates a single female response to a stimulus. To illustrate the interaction, we divided females into quartiles based on their ERB values: (a) lowest 25% of ERB values, (b) mid-low ERB values (26–50%), (c) mid-high ERB values (51–75%), and (d) highest ERB values (greater than 76%). However, ERB value was treated as a continuous variable in the statistical model. Note the change in slope from panel (a) to panel (d) females with narrow auditory filters preferred songs with longer duration, higher Wiener entropy and lower average frequency while females with wider auditory filters preferred songs with shorter duration, lower Wiener entropy and higher average frequencies.
Figure 2.
Significant interaction between female (N = 20) visual temporal resolution (i.e. FFF) and male visual display intensity (e.g. visual display factor 1, see text for more details) on female CSD duration. Each point illustrates a single female response to a stimulus. To illustrate the interaction, we divided females into quartiles based on their FFF values: (a) lowest 25% of FFF values, (b) mid-low FFF values (26–50%), (c) mid-high FFF values (51–75%) and (d) highest FFF values (greater than 76%). However, FFF value was treated as a continuous variable in the statistical model. Note the change in slope from panel (a) to panel (d) females with the lowest visual temporal resolution preferred the highest intensity displays and females with the highest visual temporal resolution preferred the lowest intensity displays.
Females with relatively poor auditory temporal resolution but better frequency selectively (i.e. narrow auditory filters) preferred the P2 portion of the song with longer duration, higher Wiener entropy and lower average frequency (figure 1a). By contrast, females with relatively good auditory temporal resolution and poor frequency selectivity (i.e. wider auditory filters) preferred the P2 portion of the song with shorter duration, lower Wiener entropy and higher average frequencies (figure 1d). Females with relatively intermediate auditory temporal resolution showed intermediate levels of preference irrespective of the properties of the P2 portion of the song (figure 1b,c). This supports our first prediction in which we predicted that females with relatively higher auditory temporal resolution would have steeper preference function slopes relative to the temporal variation in song duration and Wiener entropy.
In comparison, female visual temporal resolution affected a female's perception of male visual display (table 1 and figure 2a–d). Specifically, females with relatively low visual temporal resolution preferred higher-intensity visual displays with more body puffing and wing-extension (figure 2a), while females with relatively higher visual temporal resolution preferred less intense visual displays with less body puffing and wing-extension (figure 2d). Females with relatively intermediate temporal visual resolution showed intermediate levels of preference irrespective of the properties of the intensity of the visual display (figure 2b,c). This finding shows mixed support for our third prediction; female visual temporal resolution did indeed affect her preferences for male visual display but in the opposite direction we had originally predicted.
We did not find significant interactions between female auditory sensitivity (prediction 2) or visual spatial resolution (prediction 4) and properties of the male song and visual displays, respectively, in relation to female preference functions. Nevertheless, female photoreceptor density was significantly and negatively associated with CSD latency. Thus, females with higher visual spatial resolution (i.e. higher density of cones) tended to make their mating decisions faster.
There were no significant effects on CSD latency of interactions between sensory configuration and male song or visual display properties (table 1). Nevertheless, we did find some significant main effects (table 1). Song factors 1 and 2 were significant, suggesting that females tended to start their CSDs sooner if song glugs had relatively higher frequency and longer durations, and had higher Wiener entropy in glug 2 (table 1). Females also tended to begin their CSDs sooner when P2 frequency was higher, had lower Wiener entropy and was shorter (i.e. song factor 3) and when the visual display was longer and had deeper bows (i.e. visual display factor 2; table 1). Additionally, females tended to begin their CSDs sooner when auditory filters were relatively narrow (table 1).
We also found some significant covariates across our measures of CSD duration and latency (table 1): CSD duration was negatively associated with trial day and trial order such that as the experiment progressed on a given day (i.e. trial order) and across different days (i.e. trial day) female CSDs became shorter. Additionally, as the trial order increased females also took longer to begin a CSD (table 1). Finally, eye axial length was found to be negatively associated with CSD latency but not CSD duration: females with higher spatial resolution (i.e. larger eyes) were quicker to make their mate-choice decisions.
4. Discussion
We found that female sensory filtering of male multimodal signals affects female mating preferences. This effect was apparent for the temporal processing of the auditory and visual components of male signals. We demonstrated that individual variation in female temporal auditory and visual resolutions can affect decision-making when females are exposed to male signals that vary in auditory and visual properties. This finding, along with our recent work detailing how receivers can vary in their sensory configurations [9], provides new insights into how receivers decode multimodal signals and contradicts a long-standing tenet in animal communication that females are qualitatively similar in how they decode male signals [4,6,7].
The higher a female's auditory frequency resolution (i.e. the narrower her auditory filters), the more she preferred songs with an ending P2 that had a lower frequency and longer duration. This part of the song is produced at a high frequency (5–10 kHz) which is potentially in the range of frequencies where neural phaselocking becomes too weak to allow for accurate processing of the frequency properties of a sound [47]. The intriguing pattern here is that females with high-frequency resolution preferred lower-frequency songs whose frequency properties are likely to be processed more reliably, particularly if the song element has a long duration. Alternatively, temporal information would still be resolvable in the higher frequencies in this range, and females that process temporal information with higher resolution (i.e. those with broad auditory filters) appear to prefer P2 elements that are higher frequency and shorter in duration. Parenthetically, previous literature [19] suggested that the P2 element is not an important component of the cowbird song with regard to female mate preferences, but our findings suggest otherwise.
Contrary to our predictions, females with higher visual temporal resolution actually preferred less intense visual displays (i.e. low amounts of puffing, shorter wingspreads and less time between beginning the song and the first wing pump). This is relevant given that previous studies have found that female golden-collared manakins have larger brain areas (i.e. ventrolateral mesopallium) associated with the processing of male rapid acrobatic courtship displays compared to males [48], and female guppies with larger brains (and hence, potentially greater cognitive abilities) prefer more colourful males [49]. One possibility is that female cowbirds with relatively higher temporal visual resolution may be able to gather the visual information from the male signal with lower intensity displays, and that females with lower temporal visual resolution may need a greater intensity male visual display to glean a similar signal content. This finding may shed some light on previous results showing that female cowbirds prefer lower intensity, female-directed displays compared to higher-intensity, male-directed displays [21].
This is not the first example of females preferring lower intensity visual displays. Female satin bowerbirds (Ptilonorhynchus violaceus) show age-dependent preferences with younger females preferring to evaluate male bowers and decorations and older females preferring to evaluate the intense behavioural displays [50]. This difference may be driven by the fact that younger females tend to be more threatened by the male displays [51]. Successful male bowerbirds, in turn, appear to adjust their courtship intensity depending on female receptivity to visually intense displays [52]. In our study, we were unable to differentiate between our wild-caught adult females based on age, but future work should examine whether age-related differences in preference are underscored by differences in age-related differences in visual physiology. It is possible that younger females are more visually sensitive to intense displays and, therefore, prefer low-intensity displays or, like in the bowerbirds, evaluate other components of the display. Furthermore, similar to bowerbirds, male cowbirds have also been shown to rely on adult female cues (e.g. fast wing-flick) during song development [53], which can influence their mating success [54]. In both bowerbirds and cowbirds, therefore, females with variable preferences (perhaps driven by variation in perception) can provide feedback to males so that they modify their courtship efforts, ultimately affecting their mating success and the pattern of selection on the male displays.
Our results suggest that both the temporal resolutions of auditory and visual signals are critical in female cowbird mating decisions. This is especially important given the fine temporal synchronization of the male cowbird multimodal display itself; indeed, the most intense portions of the visual display occur during the silent portions of the song [55]. The timing between visual and acoustic signals has been shown to influence female preferences for multimodal signals in several species [56–58]. Thus, female cowbirds may be paying close attention to the timing between male auditory and visual signals. Interestingly, female cowbirds that are particularly good at resolving auditory temporal information are also particularly good at resolving visual temporal information [9]. This is further evidence of individual differences between females in the population and may provide a mechanism for variation in mate choice for multimodal signals.
Some of the interactions between sensory filtering capacity and male signal characteristics were not significantly related to our indices of choice. For example, a female's auditory sensitivity did not affect her song preference nor did a female's visual spatial resolution affect her preference for visual display intensity. Nevertheless, we did find a main effect indicating that females with greater visual spatial resolution make their mating decisions faster, regardless of the type of male visual display. It is possible that females with higher visual spatial resolution are better able to resolve details in the visual displays overall and thus began their CSDs sooner.
Individual variation in sensory traits has been documented in a wide range of species from birds [13,23,59], fish [60,61], and human [62] and non-human primates [63,64]. This variation may result from differences in a variety of factors including genetics [61,65], development [66–70] and current condition [71–73]. Few studies have previously addressed how current condition influences unimodal sensory configuration and ultimately mate-choice decisions (but see [25,26]); but none has investigated this in a multimodal animal communication context [10]. Our experiments relied on natural variation in the sensory configuration of a sample of females. Future work should focus on how the source of the variation (e.g. developmental or condition) affects mate choice and the strength of sexual selection for different male signals [8].
Overall, variation in female sensory filtering capacity as well as perception (see also [49]) can now be considered as factors underlying differences in mating preferences. These results can affect how we address classic problems in animal communication, such as honest signalling, assortative mating and sensory drive (see [8]). For instance, directional aspects of sexual selection on male signals can qualitatively switch based on the female's sensory traits: if females in a population have low visual temporal resolution, males could increase the chances of mating by displaying low intensity visual signals, as seems to be the case from previous studies in cowbirds [21]. The implication is that selection may favour males that tune their signals to the frequency distribution of sensory traits in the female population, which could potentially be a driving factor in the geographical variation in male signals (e.g. dialects, [74]). Additionally, if variation in female multimodal sensory configuration is driven by factors that also affect her body condition (e.g. females in better condition may have greater ability to resolve signals), females that are in good condition may be able to resolve differences between males based on their multimodal signals and preferentially pair with high-quality males. This could lead to assortative mating between condition-matched mating partners because females in good condition may pair with males of similar condition, leaving females in relatively poorer condition, and, therefore, unable to discriminate between males, to pair with the remaining males in the population. However, the details of this assortative mating will depend on the relative scaling across sensory modalities between sensory capacity and condition. This pattern of assortative mating suggests that male signals would not follow the predictions of typical directional sexual selection [3] and, therefore, selection on mating signals may be difficult to measure in systems where the distribution of sensory capabilities of the receiver population is an important component of signal evolution.
5. Conclusion
Differences between females in their perception of male multimodal courtship signals have the potential to change the strength and direction of sexual selection on male signals. We found evidence to suggest that females vary in the auditory and visual sensory processing, and that those differences can affect a female's ranking of potential male mating partners. These data suggest that, contrary to a long-standing tenet in animal communication and sexual selection, female's sensory configuration affects her preferences for male mating signals. Male multimodal signals, therefore, may not be designed to reach a single female model but rather to advertise to a population of females that will differ in how they process and respond to male signals. The implication is that we might expect rapid and random geographical divergence in signal design.
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank Megan Gall, Kenneth Henry, Megan Saenger, Ellis Loew, Patrice Helm and Amanda Ensminger for their help troubleshooting the collection of physiological data. Thank you to Ruiyu Zeng (RZ) for her help coding the behavioural videos for analysis and to David White for providing the brown-headed cowbird songs we used as playback stimuli. Finally, thanks to the Lucas, Fernández-Juricic and Bernal laboratories, two anonymous reviewers, Associate Editor, Dr Hannah Rowland and reviewer, Dr James Higham, for their insightful comments on earlier drafts of this manuscript.
Ethics
All research was conducted under appropriate permits for collection and use of brown-headed cowbirds
Data accessibility
Data are deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.21jf0t1 [75].
Authors' contributions
K.L.R. ran behavioural trials and collected all physiological data. K.L.R., J.R.L. and E.F.J. designed the study, ran statistical analyses and drafted the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by the National Science Foundation (NSF) (IOS 1146986) to E.F.J. and (IOS 1121728) to J.R.L. This investigation was also supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32HD049336. K.L.R. was supported by the NSF Graduate Research Fellowship Program, Sigma Xi Graduate Student Research Grant, and an Animal Behavior Society graduate student research grant.
References
- 1.Searcy WA, Nowicki S. 2005. Evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Weaver RJ, Koch RE, Hill GE.. 2017. What maintains signal honesty in animal colour displays used in mate choice? Phil. Trans. R. Soc. Lond. B. 372, 20160343 ( 10.1098/rstb.2016.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546. ( 10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 5.Johnstone RA. 1994. Honest signaling, perceptual error and the evolution of all-or-nothing displays. Proc. R. Soc. Lond. B 256, 169–175. ( 10.1098/rspb.1994.0066) [DOI] [Google Scholar]
- 6.Godfray HCJ. 1991. Signaling of need by offspring to their parents. Nature 352, 328–330. ( 10.1038/352328a0) [DOI] [Google Scholar]
- 7.Smith JM. 1991. Honest signaling-the philip sidney game. Anim. Behav. 42, 1034–1035. ( 10.1016/S0003-3472(05)80161-7) [DOI] [Google Scholar]
- 8.Ronald KL, Fernández-Juricic E, Lucas JR. 2012. Taking the sensory approach: how individual differences in sensory perception can influence mate choice. Anim. Behav. 84, 1283–1294. ( 10.1016/j.anbehav.2012.09.015) [DOI] [Google Scholar]
- 9.Ronald KL, Sesterhenn TM, Fernández-Juricic E, Lucas JR. 2017. The sensory substrate of multimodal communication in brown-headed cowbirds: are females sensory ‘specialists’ or generalists? J. Comp. Physiol. A. 203, 935–943. ( 10.1007/s00359-017-1203-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higham JP, Hebets EA. 2013. An introduction to multimodal communication. Behav. Ecol. Sociobiol. 67, 1381–1388. ( 10.1007/s00265-013-1590-x) [DOI] [Google Scholar]
- 11.Hebets EA, Papaj DR. 2005. Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197–214. ( 10.1007/s00265-004-0865-7) [DOI] [Google Scholar]
- 12.O'Loghlen AL, Rothstein SI. 2010. It's not just the song: male visual displays enhance female sexual responses to song in brown-headed cowbirds. Condor 112, 615–621. ( 10.1525/cond.2010.090216) [DOI] [Google Scholar]
- 13.Ronald KL, Ensminger AL, Shawkey MD, Lucas JR, Fernandez-Juricic E. 2017. Testing a key assumption in animal communication: between-individual variation in female visual systems alters perception of male signals. Biol. Open. 6, 1771–1783. ( 10.1242/bio.028282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennions MD, Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 72, 283–327. ( 10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 15.Ah-King M, Gowaty PA. 2016. A conceptual review of mate choice: stochastic demography, within-sex phenotypic plasticity, and individual flexibility. Ecol. Evol. 6, 4607–4642. ( 10.1002/ece3.2197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokel DA, Rothstein S. 1991. The basis for female choice in an avian brood parasite. Behav. Ecol. Sociobiol. 29, 39–45. ( 10.1007/BF00164293) [DOI] [Google Scholar]
- 17.Woolfenden BE, Gibbs HL, Sealy SG. 2002. High opportunity for sexual selection in both sexes of an obligate brood parasitic bird, the brown-headed cowbird (Molothrus ater). Behav. Ecol. Sociobiol. 52, 417–425. ( 10.1007/s00265-002-0529-4) [DOI] [Google Scholar]
- 18.O'Loghlen AL, Rothstein SI. 2010. Multimodal signalling in a songbird: male audiovisual displays vary significantly by social context in brown-headed cowbirds. Anim. Behav. 79, 1285–1292. ( 10.1016/j.anbehav.2010.03.001) [DOI] [Google Scholar]
- 19.West MJ, King AP, Eastzer DH, Staddon JER. 1979. Bioassay of isolate cowbird song. J. Comp. Physiol. Psychol. 93, 124–133. ( 10.1037/h0077577) [DOI] [Google Scholar]
- 20.Ronald KL, Skillman T, Lin A, Li Q, Fernández-Juricic E, Lucas JR. 2015. Watch your tone: social conditions modulate singing strategies. Ethology 121, 1104–1115. ( 10.1111/eth.12425) [DOI] [Google Scholar]
- 21.O'Loghlen AL, Rothstein SI. 2012. When less is best: female brown-headed cowbirds prefer less intense male displays. PLoS ONE 7, 8 ( 10.1371/journal.pone.0036130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. 2000. A procedure for an automated measurement of song similarity. Anim. Behav. 59, 1167–1176. ( 10.1006/anbe.1999.1416) [DOI] [PubMed] [Google Scholar]
- 23.Ensminger AL, Fernández-Juricic E. 2014. Individual variation in cone photoreceptor density in house sparrows: implications for between-individual differences in visual resolution and chromatic contrast. PLoS ONE 9, e111854 ( 10.1371/journal.pone.0111854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry KS, Gall MD, Bidelman GM, Lucas JR. 2011. Songbirds tradeoff auditory frequency resolution and temporal resolution. J. Comp. Physiol. A 197, 351–359. ( 10.1007/s00359-010-0619-0) [DOI] [PubMed] [Google Scholar]
- 25.Toomey MB, McGraw KJ. 2012. Mate choice for a male carotenoid-based ornament is linked to female dietary carotenoid intake and accumulation. Bmc Evol. Biol. 12, 3 ( 10.1186/1471-2148-12-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruska KP, Ung US, Fernald RD. 2012. The African cichlid fish Astatotilapia burtoni uses acoustic communication for reproduction: sound production, hearing, and behavioral significance. PLoS ONE 7, 13 ( 10.1371/journal.pone.0037612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner WE. 1998. Measuring female mating preferences. Anim. Behav. 55, 1029–1042. ( 10.1006/anbe.1997.0635) [DOI] [PubMed] [Google Scholar]
- 28.Ronald KL, Zeng RY, White DJ, Fernández-Juricic E, Lucas JR. 2017. What makes a multimodal signal attractive? A preference function approach. Behav. Ecol. 28, 677–687. ( 10.1093/beheco/arx015) [DOI] [Google Scholar]
- 29.West MJ, King AP, Eastzer DH. 1981. Validating the female bioassay of cowbird song-relating differences in song potency to mating success. Anim. Behav. 29, 490–501. ( 10.1016/S0003-3472(81)80110-8) [DOI] [Google Scholar]
- 30.Hunt KE, Wingfield JC. 2004. Effect of estradiol implants on reproductive behavior of female Lapland longspurs (Calcarius lapponicus). Gen. Comp. Endocrinol. 137, 248–262. ( 10.1016/j.ygcen.2004.03.015) [DOI] [PubMed] [Google Scholar]
- 31.Wells KD, Schwartz JJ. 1984. Vocal communication in a neotropical treefrog, Hyla ebraccata: advertisement calls. Anim. Behav. 32, 405–420. ( 10.1016/S0003-3472(84)80277-8) [DOI] [Google Scholar]
- 32.Simmons LW. 1989. Kin recognition and its influence on mating preferences of the field cricket, Gryllus bimaculatus (Degeer). Anim. Behav. 38, 68–77. ( 10.1016/S0003-3472(89)80066-1) [DOI] [Google Scholar]
- 33.Wignall AE, Kemp DJ, Herberstein ME. 2014. Extreme short-term repeatability of male courtship performance in a tropical orb-web spider. Behav. Ecol. 25, 1083–1088. ( 10.1093/beheco/aru083) [DOI] [Google Scholar]
- 34.Konishi M. 1970. Comparative neurophysiological studies of hearing and vocalizations in songbirds. Z. Vergl. Physiol. 66, 257 ( 10.1007/BF00297829) [DOI] [Google Scholar]
- 35.Dooling RJ, Zoloth SR, Baylis JR. 1978. Auditory sensitvity, equal loudness, temporal resolving power, and vocalizations in house finch (Carpodacus-mexicanus). J. Comp. Physiol. Psychol. 92, 867–876. ( 10.1037/h0077529) [DOI] [PubMed] [Google Scholar]
- 36.Moore BCJ. 1993. Frequeny analysis and pitch perception. In Human psychophysics (eds Yost WA, Popper AN, Fay RR), pp. 56–115. New York, NY: Springer. [Google Scholar]
- 37.Viemeister NF, Plack CJ. 1993. Time analysis. In Human psychophysics (eds Yost WA, Popper AN, Fay RR), pp. 116–154. New York, NY: Springer. [Google Scholar]
- 38.Hall JW. 2007. New handbook of auditory evoked responses. Boston, MA: Pearson. [Google Scholar]
- 39.Healy K, McNally L, Ruxton GD, Cooper N, Jackson AL. 2013. Metabolic rate and body size are linked with perception of temporal information. Anim. Behav. 86, 685–696. ( 10.1016/j.anbehav.2013.06.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagura N, Kanai R, Orgs G, Haggard P. 2012. Ready steady slow: action preparation slows the subjective passage of time. Proc. R. Soc. Lond. B 279, 4399–4406. ( 10.1098/rspb.2012.1339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams DR, Coletta NJ. 1987. Cone spacing and the visual resolution limit. J. Opt. Soc. Am. A 4, 1514–1523. ( 10.1364/JOSAA.4.001514) [DOI] [PubMed] [Google Scholar]
- 42.Pettigrew JD, Dreher B, Hopkins CS, McCall MJ, Brown M. P. 1988. Peak density and distribution of ganglion cells in the retinae of microchiropteran Bats: implications for visual acquity. Brain Behav. Evolut. 32, 39–56. ( 10.1159/000116531) [DOI] [PubMed] [Google Scholar]
- 43.Lisney TJ, Ekesten B, Tauson R, Hastad O, Odeen A. 2012. Using electroretinograms to assess flicker fusion frequency in domestic hens Gallus gallus domesticus. Vis. Res. 62, 125–133. ( 10.1016/j.visres.2012.04.002) [DOI] [PubMed] [Google Scholar]
- 44.Hart NS. 2001. Variations in cone photoreceptor abundance and the visual ecology of birds. J. Comp. Physiol. A 187, 685–697. ( 10.1007/s00359-001-0240-3) [DOI] [PubMed] [Google Scholar]
- 45.Jackson DA. 1993. Stopping rules in principal components analysis: A comparison of heuristic and statistical approaches. Ecol. 74, 2204–2214. ( 10.2307/1939574) [DOI] [Google Scholar]
- 46.Tamura T, Wisby WJ. 1963. The visual sense of pelagic fishes, especially the visual axis and accommodation. Bull. Mar. Sci. Gulf and Caribbean 13, 433–448. [Google Scholar]
- 47.Koppl C. 1997. Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J. Neurosci. 17, 3312–3321. ( 10.1523/JNEUROSCI.17-09-03312.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Day LB, Fusani L, Kim C, Schlinger BA. 2011. Sexually dimorphic neural phenotypes in golden-collared manakins (Manacus vitellinus). Brain Behav. Evolut. 77, 206–218. ( 10.1159/000327046) [DOI] [PubMed] [Google Scholar]
- 49.Corral-Lopez A, et al. 2017. Female brain size affects the assessment of male attractiveness during mate choice. Sci. Adv. 3, e1601990 ( 10.1126/sciadv.1601990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coleman SW, Patricelli GL, Borgia G. 2004. Variable female preferences drive complex male displays. Nature 428, 742–745. ( 10.1038/nature02419) [DOI] [PubMed] [Google Scholar]
- 51.Uy JAC, Patricelli GL, Borgia G. 2001. Complex mate searching in the satin bowerbird Ptilonorhynchus violaceus. Am. Nat. 158, 530–542. ( 10.1086/323118) [DOI] [PubMed] [Google Scholar]
- 52.Patricelli GL, Uy JAC, Walsh G, Borgia G. 2002. Male displays adjusted to female's response: macho courtship by the satin bowerbird is tempered to avoid frightening the female. Nature 415, 279–780. ( 10.1038/415279a) [DOI] [PubMed] [Google Scholar]
- 53.West MJ, King AP. 1988. Female visual displays affect the development of male song in the cowbird. Nature 334, 244–246. ( 10.1038/334244a0) [DOI] [PubMed] [Google Scholar]
- 54.Smith VA, King AP, West MJ. 2002. The context of social learning: association patterns in a captive flock of brown-headed cowbirds. Anim. Behav. 63, 23–35. ( 10.1006/anbe.2001.1886) [DOI] [Google Scholar]
- 55.Cooper BG, Goller F. 2004. Multimodal signals: enhancement and constraint of song motor patterns by visual display. Science 303, 544–546. ( 10.1126/science.1091099) [DOI] [PubMed] [Google Scholar]
- 56.Reichert MS, Hobel G. 2015. Modality interactions alter the shape of acoustic mate preference functions in gray treefrogs. Evolution 69, 2384–2398. ( 10.1111/evo.12750) [DOI] [PubMed] [Google Scholar]
- 57.Reichert MS, Symes LB, Hobel G.. 2016. Lighting up sound preferences: cross-modal influences on the precedence effect in treefrogs. Anim. Behav. 119, 151–159. ( 10.1016/j.anbehav.2016.07.003) [DOI] [Google Scholar]
- 58.Taylor RC, Ryan MJ. 2013. Interactions of multisensory components perceptually rescue tungara frog mating signals. Science 341, 273–274. ( 10.1126/science.1237113) [DOI] [PubMed] [Google Scholar]
- 59.Henry KS, Lucas JR. 2010. Auditory sensitivity and the frequency selectivity of auditory filters in the Carolina chickadee, Poecile carolinensis . Anim. Behav. 80, 497–507. ( 10.1016/j.anbehav.2010.06.012) [DOI] [Google Scholar]
- 60.Fuller RC, Fleishman LJ, Leal M, Travis J, Loew E. 2003. Intraspecific variation in retinal cone distribution in the bluefin killifish, Lucania goodei. J. Comp. Physiol. A. 189, 609–616. ( 10.1007/s00359-003-0435-x) [DOI] [PubMed] [Google Scholar]
- 61.Smith AR, et al. 2011. Intraspecific cone opsin expression variation in the cichlids of Lake Malawi. Mol. Ecol. 20, 299–310. ( 10.1111/j.1365-294X.2010.04935.x) [DOI] [PubMed] [Google Scholar]
- 62.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. 1990. Human photoreceptor topography. J. Comp. Neurol. 292, 497–523. ( 10.1002/cne.902920402) [DOI] [PubMed] [Google Scholar]
- 63.Jacobs GH, Blakeslee B. 1984. Individual variations in color vision among squirrel monkeys (Saimiri sciurues) of different geographical origins. J. Comp. Psychol. 98, 347–357. ( 10.1037/0735-7036.98.4.347) [DOI] [PubMed] [Google Scholar]
- 64.Hiramatsu C, Melin AD, Allen WL, Dubuc C, Higham JP. 2017. Experimental evidence that primate trichromacy is well suited for detecting primate social colour signals. Proc. R. Soc. Lond. B. 284, 9 ( 10.1098/rspb.2016.2458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carroll J, et al. 2012. The effect of cone opsin mutations on retinal structure and the integrity of the photoreceptor mosaic. Invest. Ophth. Vis. Sci. 53, 8006–8015. ( 10.1167/iovs.12-11087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nowicki S, Searcy WA, Peters S. 2002. Brain development, song learning and mate choice in birds: a review and experimental test of the ‘nutritional stress hypothesis'. J. Comp. Physiol. A 188, 1003–1014. ( 10.1007/s00359-002-0361-3) [DOI] [PubMed] [Google Scholar]
- 67.Hart NS, Lisney TJ, Collin SP. 2006. Cone photoreceptor oil droplet pigmentation is affected by ambient light intensity. J. Exp. Biol. 209, 4776–4787. ( 10.1242/jeb.02568) [DOI] [PubMed] [Google Scholar]
- 68.Dangles O, Irschick D, Chittka L, Casas J. 2009. Variability in sensory ecology: expanding the bridge between physiology and evolutionary biology. Q. Rev. Biol. 84, 51–74. ( 10.1086/596463) [DOI] [PubMed] [Google Scholar]
- 69.Farrell TM, Morgan A, MacDougall-Shackleton SA. 2016. Developmental stress impairs performance on an association task in male and female songbirds, but impairs auditory learning in females only. Anim. Cogn. 19, 1–14. ( 10.1007/s10071-015-0908-7) [DOI] [PubMed] [Google Scholar]
- 70.Schmidt KL, McCallum ES, MacDougall-Shackleton EA, MacDougall-Shackleton SA. 2013. Early-life stress affects the behavioural and neural response of female song sparrows to conspecific song. Anim. Behav. 85, 825–837. ( 10.1016/j.anbehav.2013.01.029) [DOI] [Google Scholar]
- 71.Lynch KS, Wilczynski W. 2008. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav. Evol. 71, 143–150. ( 10.1159/000111460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knott B, Berg ML, Morgan ER, Buchanan KL, Bowmaker JK, Bennett ATD. 2010. Avian retinal oil droplets: dietary manipulation of colour vision? Proc. R. Soc. B 277, 953–962. ( 10.1098/rspb.2009.1805) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoder KM, Vicario DS. 2012. To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. Behav. Neurosci. 126, 17–28. ( 10.1037/a0026673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson KE, Rothstein SI, Fleischer RC, O'Loghlen AL. 2005. Large-scale movement patterns between song dialects in brown-headed cowbirds (Molothrus ater). Auk 122, 803–818. ( 10.1642/0004-8038(2005)122%5B0803:LMPBSD%5D2.0.CO;2) [DOI] [Google Scholar]
- 75.Ronald KL, Fernández-Juricic E, Lucas JR. 2018. Data from: Mate choice in the eye and ear of the beholder? Female multimodal sensory configuration influences her preferences Dryad Digital Repository. ( 10.5061/dryad.21jf0t1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ronald KL, Fernández-Juricic E, Lucas JR. 2018. Data from: Mate choice in the eye and ear of the beholder? Female multimodal sensory configuration influences her preferences Dryad Digital Repository. ( 10.5061/dryad.21jf0t1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are deposited in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.21jf0t1 [75].