Abstract
Myocarditis is an inflammatory disease of the myocardium. Viruses, such as enterovirus, adenovirus, parvovirus B19, HHV6 or cytomegalovirus (CMV) and autoimmune diseases are recognized causes of myocarditis. We describe the clinical case of a young Indian woman with SLE and a concomitant acute CMV related myocarditis with favourable outcome after ganciclovir therapy.
CMV myocarditis may range from being a subclinical infection with incidental findings on ECG to a life threating presentation. There are no trials demonstrating the efficacy of antiviral therapy in myocarditis. Case series of patients with CMV myocarditis have reported an excellent clinical outcome after antiviral agents. Lupus Myocarditis (LM) is more prevalent in young females. There are no specific ECG or echocardiographic signs. Treatment strategies of LM are based on corticosteroids, immunosuppressive agents and cardiovascular support, usually with a favorable prognosis, but LM often lead to a severe clinical picture, with mortality of 10.3%. Endomyocardial biopsy (EBM) is recommended as the gold standard but it is very underused in clinical practice, It should be performed in a specialized center but there are concerns on lack of specificity, low negative predictive value, risk of complication, and sampling errors due to the focal nature of myocarditis. Both SLE and CMV are potentially responsible of acute myocarditis. In our knowledge, CMV myocarditis with SLE was described in only one other patient. The initiation of antiviral therapy improved the clinical picture and, in our opinion, it is mandatory when CMV related life threating conditions develop.
Keywords: Myocarditis, Cytomegalovirus, Systemic lupus erythematosus
Introduction
Myocarditis is an inflammatory disease of the myocardium. Viruses, such as enterovirus, adenovirus, parvovirus B19, HHV6 or cytomegalovirus (CMV) and autoimmune diseases are recognized causes of myocarditis. CMV infection has a high prevalence in the general population, ranging between 30 and 100% according to age, socioeconomic status and geographical region. The primary infection in immunocompetent individuals is usually asymptomatic or an influenza-like or mononucleosis-like syndrome with fever, pharyngitis and lymphadenopathy; however the spectrum and intensity of clinical manifestations may be variable and are influenced by the immune status. Immunocompromised hosts such as patients with autoimmune disease, lack of pre-existing CMV-specific immunity and immunosuppressive drugs, are more likely to develop an organ specific clinical disease. The most frequent sites involved are the gastrointestinal tract including the liver, the central nervous system, followed by the blood (hemolytic anaemia, myelosuppression, thrombocytopenia), the eyes and the lungs. In contrast, cardiovascular complications like acute myocarditis are very uncommon, being responsible of 3% of active myocarditis and 0.8% of related dilated cardiomyopathy [[1], [2]]. Systemic lupus erythematosus (SLE) is a chronic auto-immune disorder principally affecting young women and characterized by skin and hematologic manifestations, polyarthritis, renal involvement, and serositis. Myocardial involvement is common, with a current prevalence of 9% [3]. Myocarditis may also be the first manifestation of the disease. Here we describe the clinical case of a young woman with SLE and a concomitant CMV related myocarditis with favorable outcome after ganciclovir therapy. To the best of our knowledge, this is the second case report of CMV myocarditis in SLE in literature [4].
Case presentation
In June 2017, a 37-year-old Indian woman with history of previously diagnosed SLE and Sjogren Syndrome, previously treated with Rituximab and currently on therapy with prednisone 25 mg daily, was referred to our Internal Medicine Department for intermittent fever with shivering, started in the month before hospitalization. She reported no recent contact with sick people or animals, nor recent story of travel. At the time of hospitalization, there was no lymphadenopathy, no hepatosplenomegaly nor cutaneous signs. Chest, cardiological, neurological and abdominal examination were normal. CAT total body, blood cultures and urine cultures were normal. Laboratory exam showed normal blood count, cholestasis and hepatic indices, but ESR, C reactive protein (CRP) and procalcitonin were elevated: 82, 18.72 mg/dl and 2.28 respectively. She had positive dsDNA ab 12.3 U/ml, SSA-RO ab 82 U/ml and SSB-LA 84 U/ml, negative for ribonuclear protein antibody and a reduction of C3 and C4 complement factors were observed. Because of the persistent, high and intermittent fever, resistant to broad-spectrum antimicrobial therapy, the patient was referred to Infectious Diseases department where new blood cultures, serological tests, molecular investigations for viruses (TORCH, HIV serology, HBV and HCV serology) and interferon gamma quantification were taken. All the tests were negative, except for CMV IgM titer that was positive at 49.20 IU/mL (range 18–20) with a negative CMV IgG titer, which suggested an acute CMV infection.
In the next few days, she developed dyspnea with bilateral basilar pulmonary rales and chest pain accentuated by inspiration and coughing without pericardial rubs and was referred for evaluation to our Cardiology Department. Her ECG showed a diffuse ST segment elevation compatible with pericarditis, subsequently evolved in symmetrical and diffuse T waves inversion. Echocardiography showed normal volume (VTD 65 mL) and wall thickness of the left ventricle (LV), a moderate reduction in LV ejection fraction (LVEF) (40%) due to akinesia of the lateral wall and mid-apical sector of the anterior wall with hypokinesia of the remaining segments, impaired relaxation and a mild circumferential pericardial effusion. Remaining findings were within the normal values. In the same time, laboratory tests revealed a reduction in leukocytes (2690/uL) with spontaneous normalization over a few days, an abrupt and severe normocytic normochromic anemia (7.8 g/dL) with reticulocytes suppression and thrombocytopenia (19000/uL) that were treated with blood and platelet transfusions; proteinuria (30 mg/dL), urobilinogen in urine (12 mg/dL) with normal bilirubin, reduction in pseudocholinesterase (2754 U/L), a slight elevation in aminotransferases, gamma-GT and alkaline phosphatase, an increase in d-dimer with normal fibrinogen and coagulation times, elevation of LDH (1435 U/l) and troponin (1.102 ng/mL), ESR and CRP persistently elevated. A CMV DNA quantitative PCR assay on blood revealed 2130 copies/mL and DNA of other viruses (HSV1, HSV2, HHV6, parvovirus B19, enterovirus and adenovirus) were negative.
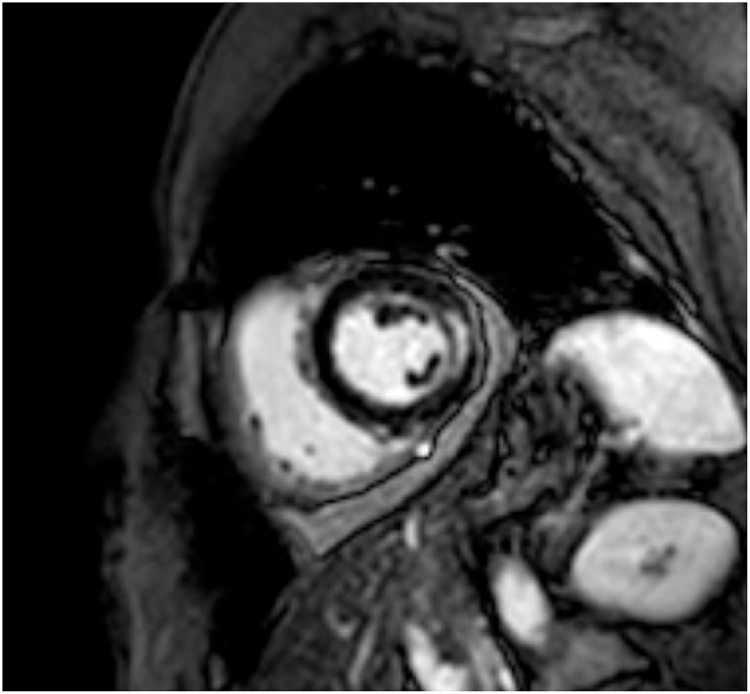
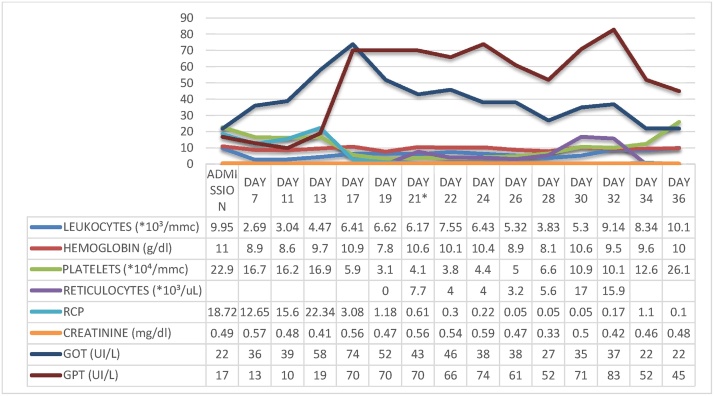
Cardiac Magnetic Resonance (CRM) was suggestive for myocarditis both on T1 and T2 studies and showed a mesocardiac late gadolinium enhancement in the lateral wall (Fig. 1). CMV myocarditis was suspected and she was started on Ganciclovir IV 5 mg/kg (250 mg) twice a day, furosemide and a betablocker (up to tolerated dose), maintaining the same corticosteroid therapy. She then developed a further reduction of systolic function (LVEF 25%), increase in ventricular size compared to the initial evaluation (VTD 105 ml), a restrictive filling pattern, moderate mitral regurgitation, reduction in right ventricular systolic function (TAPSE 14 mm), and increased pericardial effusion (12 mm), clinically manifested as two episodes of pulmonary edema, S3 gallop and hypotension. On the sixth day of ganciclovir therapy added to standard heart failure (HF) therapy, the patient stabilized clinically; platelets, hemoglobin and reticulocytes began to raise up to normal values; ESR, CRP and transaminases normalized (Fig. 2). On the tenth day of antiviral therapy, there were no more signs of heart failure (HF). Echocardiography showed a reduction of LV dimension and a raise in LVEF up to 50% with residual hypokinesia of the lateral wall, mild mitral regurgitation and a reduction until disappearance of the pericardial effusion. The patient had improved hemodynamic without the need of a mechanical circulatory support or inotropic therapy. Treatment with IV ganciclovir was continued for 16 days and then patient was discharged with oral therapy with valganciclovir 900 mg bid for other 7 days. The CMV viremia on blood, taken at the time of discharge, was negative. After one month, routine echocardiography revealed a normal size of the LV with a LVEF of 55%, with neither mitral regurgitation nor pericardial effusion.
Fig. 1.
T2 weighted sequence after gadolinium infusion demonstrated mesocardiac late enhancement in the mid lateral wall (short axis view).
Fig. 2.
Laboratory tests shows evolution of liver enzymes levels, ESV, RCP and haemachrome before and during ganciclovir therapy (*ganciclovir therapy started; ESV = erythrocyte sedimentation velocity; RCP = reactive c-protein; AST = serum glutamic oxaloacetic transaminase; ALT = serum glutamate-pyruvate transaminase; ALKP = alkaline phosphatase; GGT = gamma-glutamyltransferase).
Discussion
CMV myocarditis may be subclinical, with incidental finding on ECG (non-specific repolarization abnormalities, widespread ST segment elevations, T wave inversion) and as LV regional wall motion alterations or diffuse hypokinesia [4], symptoms develop as such acute HF, intractable arrhythmias and cardiogenic shock [5]. Our patient had the most common presentation of CMV myocarditis, as described in case series [[6], [7]] which reports an high prevalence in young patients (range 20–40 years), usually presenting with fever, chest pain and signs and symptoms of HF. Features such as mononucleosis-like signs, petechiae or hematological alterations are rare, while the association with elevation of transaminases is almost ubiquitously described. Even though there are no trials demonstrating the efficacy of antiviral therapy in myocarditis, case series of patients with CMV myocarditis suspected on the basis of viral serology, clinical picture and imaging, have reported an excellent clinical outcome after antiviral agents use versus a fatal outcome both in immunocompetent, mainly with fulminant presentation, and in the immunosuppressed population, including SLE [4].
Three major case series on Lupus Myocarditis (LM) [[8], [9], [10]] show that this condition is more prevalent in young females, with a high SLEDAI score, independently of concomitant corticosteroid treatment and duration of the disease. Like other forms of myocarditis, there are no specific ECG or echocardiographic signs, although LM is frequently associated with pleural and pericardial effusions during SLE flares and with a LVEF impairment trough global or mid-apical hypokinesis with a Takotsubo-like pattern [11]. Current treatment strategies of LM are based on clinical experience rather than randomized trials and usual therapy consists of corticosteroids, immunosuppressive agents and cardiovascular support, usually with a favorable prognosis [11]. Despite LM often leading to a severe clinical picture, with high prevalence of fulminant form and an overall mortality of 10.3%, it has often a good outcome after the acute phase.
Despite endomyocardial biopsy (EBM) recommended as the gold standard for diagnosis [12], it is very underused in clinical practice (as shown in the clinical case and series reported), considering that it should be performed in a specialized center and the concerns on lack of specificity, low predictive negative value, risk of complication, and sampling errors due to the focal nature of myocarditis [[13], [14]]. Giving such limitations, the “clinically suspected myocarditis” diagnostic criteria were elaborated, which rely on the sum of clinical presentation, ECG features, myocardiocytolysis markers, functional and structural abnormalities on cardiac imaging and tissue characterization by CMR (Lake Louise criteria) [14]. The diagnosis of CMV myocarditis was made, as in almost of the previously reported case series, fulfilling such criteria and on the basis of high CMV IgM titers and a positive CMV DNA quantitative PCR assay. CRM provides non-invasive tissue characterization of the myocardium and can support the diagnosis of myocarditis. It is not an alternative, even though it has demonstrated good correlation compared to EMB, with a sensitivity of 67%, specificity of 91% and positive predictive value of 91% [5]. On the other hand, EMB with immunohistochemistry and viral genome analysis are capable to identify the underlying etiology and to differentiate between autoimmune and viral causes of myocarditis, which have different treatment options. Thus, EBM appears to be mandatory in myocarditis with fulminant presentation and when an autoimmune form is suspected for a safe immunosuppression (PCR virus negative myocarditis). In our scenario, the coexistence of an autoimmune disease (SLE) and the detection of a positive serology and PCR DNA amplification together with clinical features suggestive of an acute CMV infection required the use of both immunosuppressive and antiviral therapy. Based on these premises and the clinical response to ganciclovir therapy, it was decided not to proceed with EMB, as such evaluation would not have provided further diagnostic and therapeutic elements.
Conclusion
Both SLE and CMV are potentially responsible for acute myocarditis. In most cases, a CMV infection in SLE patients predominantly carries gastrointestinal tract complications, while a new CMV infection as a cause of myocarditis has been described, in our knowledge, only in one other patient with SLE. Our patient did not develop the clinical picture during a SLE flare and had CMV related clinical features like elevation of aminotransferases and development of medullary suppression with severe anemia and thrombocytopenia. The initiation of antiviral therapy improved the clinical picture and, in our opinion, it is mandatory when CMV related life threatening conditions develop.
References
- 1.Roubille C., Brunel A.S., Gahide G., Kovacsik H.V., Quellec A. Cytomegalovirus (CMV) and acute myocarditis in an immunocompetent patient. Intern Med. 2010;49(2):131–133. doi: 10.2169/internalmedicine.49.2313. [DOI] [PubMed] [Google Scholar]
- 2.Andréoletti L., Lévêque N., Boulagnon C., Brasselet C., Fornes P. Viral causes of human myocarditis. Arch Cardiovasc Dis. 2009;102(June–July (6–7)):559–568. doi: 10.1016/j.acvd.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Thomas G., Cohen Aubart F., Chiche L., Haroche J., Hié M., Hervier B. Lupus myocarditis: initial presentation and longterm outcomes in a multicentric series of 29 patients. J Rheumatol. 2017;44(January (1)):24–32. doi: 10.3899/jrheum.160493. [DOI] [PubMed] [Google Scholar]
- 4.Padala S.K., Kumar A., Padala S. Fulminant cytomegalovirus myocarditis in an immunocompetent host: resolution with oral valganciclovir. Tex Heart Inst J. 2014;41(October (5)):523–529. doi: 10.14503/THIJ-13-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollack A., Kontorovich A.R., Fuster V., Dec G.W. Viral myocarditis – diagnosis, treatment options, and current controversies. Nat Rev Cardiol. 2015;12(November (11)):670–680. doi: 10.1038/nrcardio.2015.108. [DOI] [PubMed] [Google Scholar]
- 6.Magno Palmeira M., Umemura Ribeiro H.Y., Garcia Lira Y., Machado Jucá Neto F.O., da Silva Rodrigues I.A., Fernandes da Paz L.N. Heart failure due to cytomegalovirus myocarditis in immunocompetent young adults: a case report. BMC Res Notes. 2016;9(August (391)) doi: 10.1186/s13104-016-2181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Zhu Y.L., Li M.T., Gao N., You X., Wu Q.J. Lupus myocarditis: a case-control study from China. Chin Med J (Engl) 2015;128(October (19)):2588–2594. doi: 10.4103/0366-6999.166029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zawadowski G.M., Klarich K.W., Moder K.G., Edwards W.D., Cooper L.T., Jr. A contemporary case series of lupus myocarditis. Lupus. 2012;21(November (13)):1378–1384. doi: 10.1177/0961203312456752. [DOI] [PubMed] [Google Scholar]
- 9.Ishimori M.L., Agarwal M., Beigel R., Ng R.K., Firooz N., Weisman M.H. Systemic lupus erythematosus cardiomyopathy a case series demonstrating a reversible form of left venticular dysfunction. Echocardiography. 2014;31(May (5)):563–568. doi: 10.1111/echo.12425. [DOI] [PubMed] [Google Scholar]
- 10.Appenzeller S., Pineau C.A., Clarke A.E. Acute lupus myocarditis: clinical features and outcome. Lupus. 2011;20:981–988. doi: 10.1177/0961203310395800. [DOI] [PubMed] [Google Scholar]
- 11.Caforio A.L., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B. European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(September (33)):2636–2648. doi: 10.1093/eurheartj/eht210. 2648-2648d. [DOI] [PubMed] [Google Scholar]
- 12.Cooper L.T., Baughman K.L., Feldman A.M., Frustaci A., Jessup M., Kuhl U. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 13.From A.M., Maleszewski J.J., Rihal C.S. Current status of endomyocardial biopsy. Mayo Clinic Proc. 2011;86:1095–1102. doi: 10.4065/mcp.2011.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedrich M.G., Sechtem U., Schulz-Menger J., Holmvang G., Alakija P., Cooper L.T. International consensus group on cardiovascular magnetic resonance in myocarditis. cardiovascular magnetic resonance in myocarditis: a JACCWhite paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]