Abstract
Traditional Chinese medicine (TCM)-based herbal therapies have gained increasing popularity worldwide, raising concerns of its efficacy, safety profile and potential interactions with Western medications. Antithrombotic agents are among the most common prescription drugs involved in herb-drug interactions, and this article focused on aspirin, one of the most widely used antiplatelet agents worldwide. We discussed herbs that have potential interactions by exploring Western and TCM approaches to thrombotic events. Common TCM indications for these herbs were also highlighted, including possible scenarios of their concurrent usage with aspirin. With greater awareness and understanding of potential herb-drug interactions, TCM and Western physicians may collaborate more closely to identify, treat and, most importantly, prevent adverse drug events.
Keywords: antiplatelet, aspirin, herb-drug interactions, traditional Chinese medicine
INTRODUCTION
The use of traditional Chinese medicine (TCM)-based herbal medicine has gained increasing acceptance worldwide.(1) It is estimated that one-third of adults in developed countries and more than 80% of people in many developing countries use herbal medicines for illnesses ranging from the common cold to chronic diseases such as heart disease and diabetes.(2) In Singapore, a recent single-centre study revealed that 84% of patients suffering from chronic pain use complementary and alternative medicine, of which the most common is TCM.(3) However, the use of herbal medicines is not without potential hazards. In a study on aged persons seen at a memory disorders clinic, 17% were current users of herbal medication and almost a third of them were at risk of herb-drug interactions.(4) This high prevalence of concurrent usage is further complicated by poor reporting; about 80%–90% of patients do not disclose their use of herbal medicines to healthcare professionals.(5,6) The popularity of TCM use among patients in the local context prompts the need for greater physician awareness of potential risks that may arise from the simultaneous use of prescription drugs.
One of the most common classes of prescription drugs with a risk of herb-drug interactions is antithrombotic agents (including anticoagulants and antiplatelets),(7,8) while other medications include antidepressants, cardiovascular drugs, immunosuppressants, anti-HIV drugs and anticancer drugs.(2) In this article, we reviewed Western and TCM approaches to thrombotic events, identifying potential circumstances for TCM herb-drug interactions with the antiplatelet agent aspirin.
WESTERN AND TCM TREATMENT APPROACHES TO THROMBOTIC EVENTS
Thrombosis refers to haemostasis occurring in the ‘wrong place’, and can be categorised into arterial and venous thrombosis, although overlaps may be present. Arterial thrombosis is the main cause of acute myocardial infarctions, ischaemic stroke and limb gangrene, and is mainly a result of platelet aggregation and abnormalities in the vessel wall. On the other hand, deep vein thrombosis can potentially lead to pulmonary embolism and post-phlebitic syndrome, and is initiated by the activation of the coagulation cascade in the setting of stasis or a hypercoagulable state. Prevention and treatment of thrombotic events in Western medicine is hence targeted at various components of thrombus formation using antiplatelet drugs, anticoagulants and/or fibrinolytic agents.
Of the antiplatelet agents, acetylsalicylic acid, or aspirin, is the most widely used worldwide.(9) Belonging to the heterogeneous class of nonsteroidal anti-inflammatory drugs, its mechanism of action lies in the permanent acetylation and therefore inhibition of cyclooxygenase-1 and -2 (or COX-1 and COX-2). These isoenzymes catalyse the conversion of arachidonic acid to prostaglandin H2, the first step to prostanoid biosynthesis. Hence, aspirin effectively reduces prostanoid production. The constitutive COX-1 isoenzyme is inhibited at low doses, whereas inhibition of the inductive COX-2 requires a higher dose. In human platelets, low-dose aspirin reduces thromboxane A2 (TXA2; primarily COX-1 derived) synthesis, which results in inhibition of platelet aggregation.(10) Clinically, aspirin is widely used for secondary prevention of cardiovascular events in patients with coronary artery, cerebrovascular or peripheral vascular diseases, as well as for primary prevention in patients with high cardiovascular risk.(9) Other antiplatelet drugs, including thienopyridines (ticlopidine, clopidogrel and prasugrel), dipyridamole and glycoprotein (GP) IIb/IIIa receptor antagonists (abciximab, eptifibatide and tirofiban), along with anticoagulants and fibrinolytic agents will not be discussed in this review.
Unlike Western treatment approaches to specific pathologies, treatment in TCM is based on the general constitution of an individual. Hence, diagnosis is based upon identification of disease patterns and translating the pattern of symptoms manifested into specific TCM syndromes that reflect the pathogenesis of the disease. Although other constitutive symptoms accompanying a thrombotic disease may alter the final TCM diagnosis of a patient, one of the most common TCM theories for its pathogenesis is that of Qi or blood stagnation (气滞/血瘀). Consequently, herbs with properties of promoting blood circulation and eliminating stasis (活血化瘀药) are often used to treat these syndromes. For the purpose of this review, a list of TCM herbs with such properties was narrowed down to eight herbs that are commonly used in Singapore. Widely-used herbs of the Qi-supplementing class (补气药), as well as several significant herbs, will also be briefly mentioned.
Taking these approaches into consideration, a search was conducted on the English journal database, PubMed, for articles relating to herb-drug interactions. The search terms included the following key words: ‘herb drug interactions’ OR Latin names of the Chinese herbs AND ‘antiplatelet’ OR ‘warfarin’. Articles published before 31 December 2013 were included in this review. Primary articles cited by reviews found in this manner were also included. Additionally, a TCM pharmacology reference text used in teaching institutions in both China and Singapore was consulted regarding the pharmacology of TCM herbs.(11) TCM herbal formulas, dietary products (e.g. garlic and ginger) and other health supplements not used in TCM (e.g. coenzyme Q and fish oil) were not included in the scope of this review.
HERB-DRUG INTERACTIONS
Herb-drug interactions, as with all drug interactions, can be approached on two main levels, namely: pharmacokinetic and pharmacodynamic interactions. Pharmacokinetic interactions refer to processes that interfere with how the body deals with the drug, including absorption, distribution, metabolism and elimination. Meanwhile, pharmacodynamic interactions affect the action of the drug on the body, resulting in a change in the relationship between drug concentration and resultant drug action.
An examination into the pharmacokinetic properties of aspirin suggests that pharmacokinetic interactions with aspirin are unlikely to occur and can easily be prevented. Upon ingestion, aspirin reaches peak plasma levels after 30–40 minutes and inhibition of platelet function occurs by one hour.(10) Notably, the plasma half-life of aspirin in human circulation is only 15–20 minutes due to hydrolysis in plasma, the liver and erythrocytes.(10) This rapid clearance reduces the window for possible interaction, while the current local practice of advising patients to space out ingestion of TCM and Western medication by two hours may minimise incidences of herb-drug interaction. Furthermore, tamarind is the only herb reported in the literature to solely affect aspirin pharmacokinetics by increasing its bioavailability,(12) and it is not used in TCM practice.
The bulk of potential herb-drug interactions with aspirin, hence, results from pharmacodynamic interactions. Due to the permanent acetylation of COX-1 in platelets, the inhibitory effects of aspirin last for the entire lifespan of the platelet (approximately 8–10 days). Acetylation of megakaryocyte COX enzymes further contributes to the sustained antiplatelet effects.(10) These factors widen the window of opportunity for pharmacodynamic interactions, which increases the chance of clinically significant bleeding episodes. In addition to factors that affect primary haemostasis, it is also vital to consider interactions along the coagulation cascade, as disturbances in secondary haemostasis may further potentiate bleeding in aspirin users.
The following section addresses specific TCM herbs that may potentially interact with aspirin, common TCM indications (based on symptoms recognised by Western physicians) and their proposed mechanism of action.
Significant herbs with potential for herb-drug interactions
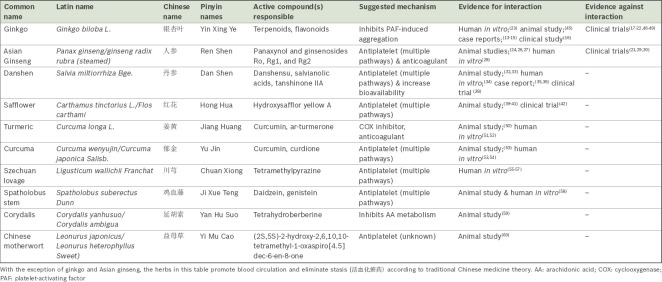
Of the herbs reviewed, four have shown evidence of platelet inhibition and/or interactions with aspirin in vivo/in clinical trials, and are discussed below. The mode of mechanism of these herbs may be found in the Appendix.
Ginkgo
Scientific name: Ginkgo biloba; Chinese name: 银杏叶 (leaves), 白果 (seeds)
Common TCM indications: seeds – chronic cough, asthma, leucorrhoea, urinary incontinence or frequency; leaves – cardiovascular disease, angina, hyperlipidaemia, hypertension, cerebral vasospasm.(11)
Ginkgo is one of the most widely available herbal products in Singapore. It is believed that the medicinal properties of ginkgo leaves are partially due to their effect of reducing blood viscosity. Consequently, there has been much concern and research into potential herb-drug interactions with antithrombotic agents. Numerous case reports of clinically significant bleeding have also been associated with ginkgo, including: a 70-year-old man on aspirin therapy who developed spontaneous hyphaema one week following the consumption of ginkgo tablets;(13) a 33-year-old woman who developed bilateral subdural haematomas and had prolonged bleeding time, which normalised 35 days after stopping ginkgo;(14) and a 71-year-old man who suffered a fatal intracranial bleed associated with Ginkgo biloba and ibuprofen.(15) A retrospective population-based study on adults taking ginkgo alone or in combination with antiplatelet/anticoagulant drugs has shown an increased risk of haemorrhage in patients aged 65 and above and in male patients.(16)
However, various randomised controlled trials involving both healthy adults as well as patients with dementia have not demonstrated any systematic changes in bleeding time and blood coagulation tests with ginkgo extract treatment, nor any clinically significant bleeding episodes that were attributable to the drug.(17-21) A clinical trial involving older adults with cardiovascular risk factors also showed no significant increase in bleeding, both clinically and in investigations of co-ingestion of aspirin and EGb 761 (a widely used and well-standardised ginkgo extract) as compared to aspirin alone.(22) Notably, in a recent study by Koch, it was found that the levels of ginkgolides A, B, C and J that were required for half-maximum inhibition of platelet aggregation were generally more than 100 times higher than the peak plasma values attained after oral ingestion of EGb 761 at recommended doses of 120–240 mg.(23)
As results from controlled studies have consistently demonstrated, ginkgo does not significantly impact haemostasis, nor adversely affect the safety of concurrent ingestion of aspirin or warfarin. Therefore, high-level safety concerns about ginkgo use seem to be unsupported by the currently available evidence.
Asian Ginseng/Red Ginseng (steamed)
Scientific name: Panax ginseng/ginseng radix rubra (steamed); Chinese name: 人参
Common TCM indications: fatigue, cognitive impairment, diabetes, gastrointestinal diseases such as gastritis and ulcers, impotence.(11)
Ginseng is one of the most well-known and valued herbs worldwide. As a testament to its popularity, its use extends well beyond TCM prescriptions to health supplements and even daily diets. Panax ginseng (P. ginseng), however, should not be confused with other species within the same genus, including P. quinquefolius (American ginseng) and P. notoginseng (notoginseng), as they differ both in chemical profiles and haematological effects.(24,25) Various in vitro studies have demonstrated that ginseng inhibits several steps along the platelet aggregation pathway, as well as the coagulation cascade,(24,26-28) suggesting that pharmacodynamic interactions with aspirin may be possible.
On the other hand, in an open-label, crossover randomised trial involving 12 healthy male subjects, the international normalised ratio (INR) and platelet aggregation were not affected by treatment with ginseng.(29) Another randomised study conducted on ischaemic stroke patients revealed no impact on the prothrombin time (PT) and INR of patients who received Asian ginseng and warfarin as compared to warfarin alone, suggesting that Asian ginseng does not interact with warfarin to affect the coagulation cascade.(30) Further to this, a randomised crossover trial on ten healthy subjects using Asian ginseng at the manufacturer’s recommended dose showed no effect on in vivo platelet function.(21)
On the whole, despite laboratory evidence of inhibited platelet aggregation by Asian ginseng, clinical evidence indicates that there is little cause for concern regarding increased bleeding risk, and hence pharmacodynamic interactions, when Asian ginseng is taken with aspirin. Pharmacokinetic interactions have also not been reported.
Danshen
Scientific name: Salvia miltiorrhiza; Chinese name: 丹参
Common TCM indications: irregular menstrual bleeding, amenorrhoea, dysmenorrhoea, chest pain, epigastric pain, palpitations, insomnia.(11)
Danshen, the dried roots of Salvia miltiorrhiza, is a common TCM herb known to improve blood circulation and prevent stasis of blood.(11) As such, it has been used for the treatment of various cardiovascular diseases, including acute ischaemic stroke and coronary artery disease.(31) In laboratory studies, danshen has been shown to interact both pharmacodynamically, through various antiplatelet mechanisms,(32,33) and pharmacokinetically(34) with aspirin.
Clinically, there have been case reports of herb-drug interactions between danshen and warfarin leading to significant derangements in clotting profile and bleeding episodes.(35,36) However, it should be noted that these interactions with warfarin may be primarily pharmacokinetic in nature,(37) and therefore do not imply similar risks with aspirin. Nonetheless, a trial conducted in China revealed that sulfotanshinone sodium injection could significantly attenuate angina symptoms in patients with unstable angina on aspirin and baseline therapy, and may be associated with a decreased level of fibrinogen.(38) This suggests that extracts of danshen may have an additive or synergistic effect with aspirin, raising the possibility of herb-drug interactions.
Although current evidence points towards the potential for herb-drug interaction between danshen and aspirin, most of the research consists of laboratory studies that may not necessarily have significant clinical correlation. Clinicians should err on the side of caution and avoid co-administration of danshen and aspirin until more evidence regarding its safety is available.
Safflower
Scientific name: Carthamus tinctorius; Chinese name: 红花
Common TCM indications: amenorrhoea, dysmenorrhoea, chest pain, traumatic injuries.(11)
The safflower plant Carthamus tinctorius has several uses. While it is commercially cultivated for vegetable oil that is extracted from its seeds, the dried flowers are used in TCM, most often for the treatment of gynaecological conditions such as amenorrhoea and dysmenorrhoea. Topical medicated oils and creams containing safflower are also available for bruises. In rat models, safflower has been found to display antiplatelet properties(39,40) and potentiate clopidogrel in preventing thrombosis,(41) suggesting that pharmacodynamic interactions with aspirin may be possible.
In a clinical trial involving patients with acute coronary syndrome, treatment with safflower injection in addition to routine Western therapy (aspirin, clopidogrel, statins, percutaneous coronary intervention, etc) resulted in improvement in clinical symptoms of angina and electrocardiogram changes. Ex vivo analysis later revealed reduced expression of GPIIb/IIIa,(42) suggesting that this may also be a mechanism of action by which platelet aggregation is suppressed by safflower.
While current evidence cannot yet provide definitive proof of herb-drug interactions between safflower and aspirin, caution should be taken, as studies have shown a potential for platelet inhibition.
Other herbs that promote blood circulation and eliminate stasis (活血化瘀药)
In addition to the abovementioned herbs, it is crucial to examine the antiplatelet properties of other herbs that promote blood circulation and eliminate stasis under TCM theory, although there is only laboratory evidence suggesting a potential interaction with aspirin. We elaborate on their proposed mechanisms of interactions in the Appendix. A summary of these herbs and their effects on platelet aggregation and coagulation may be found in Table I.
Table I.
Summary of herbs and their effects on platelet aggregation and coagulation.
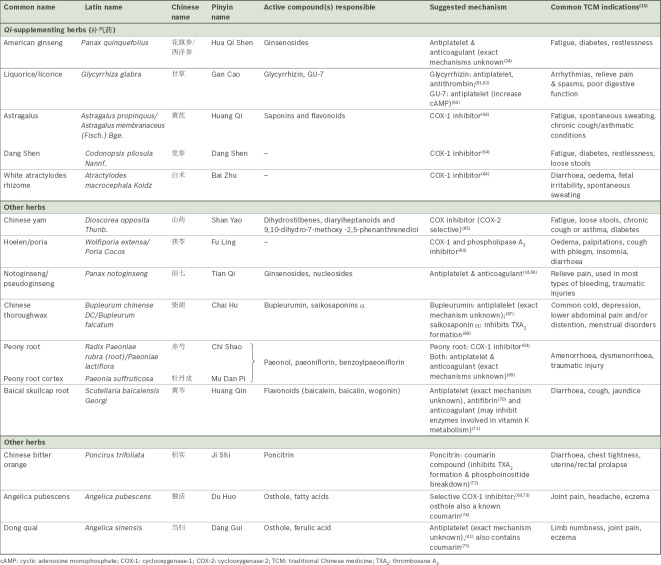
Herbs of the Qi-supplementing class (补气药) and other herbs
As herbs of the Qi-supplementing class are often used to supplement stasis-eliminating herbs in treating syndromes of Qi or blood stagnation, they may also possess inhibitory action on platelets that are fundamental to their pharmacology and are therefore worth a mention. Several other herbs were also reported in the Western literature to contain antithrombotic effects and have thus been included in this review. Their effects are as shown in Table II. However, in comparison to the herbs that promote blood circulation and eliminate stasis (活血化瘀药) discussed earlier, there is significantly less evidence to support a herb-drug interaction with aspirin, and much of this evidence remains experimental in nature. More research is required to clearly elucidate the mechanism of antiplatelet action for these herbs.
Table II.
Summary of Qi-supplementing (补气药) herbs and other herbs, and their effects on platelet aggregation and coagulation.
CURRENT EVIDENCE AND ITS LIMITATIONS
The growing popularity of TCM and ‘natural’ herbal supplements worldwide, along with a rising interest in the pharmacology of these herbs, has led to increased research in this field of study. More importantly, it has also raised questions regarding the safety of these herbs as well as their suitability for simultaneous use with Western medications. Reports of herb-drug interactions following concurrent use of TCM and Western medication are not uncommon; consumers and physicians should not assume that because herbs are natural, they are comparatively milder and do not possess significant pharmacological activity. On the contrary, this review has compiled evidence that various herbs belonging to the main classes of stasis-eliminating herbs and Qi-supplementing herbs do indeed possess antiplatelet properties, which may contribute in part to their medicinal effect. Hence, concern for potential adverse effects from herb-drug interactions between antiplatelet agents, such as aspirin, and TCM herbs may be warranted.
A closer inspection of the evidence presented, however, revealed that most herbs have only demonstrated antiplatelet activity in laboratory studies, while the clinical significance of such findings remains limited. For instance, drug levels may not reach effective concentrations in vivo following oral ingestion to be able to achieve significant inhibition of platelet function. This was suggested by Beckert et al, who investigated the effects of several herbs at the manufacturer’s recommended doses and found no significant interaction,(21) and was clearly demonstrated by Koch et al(23) in the case of ginkgo. There is a need for investigations to move beyond animal models and in vitro laboratory experiments to in vivo randomised controlled trials and retrospective studies, which are better representations of the effects of the herbs clinically.
Case reports of bleeding episodes or herb-drug interactions were also complicated by poor reporting of herb details, as well as lack of proof of medication compliance and physiologic disturbance. For instance, both case reports regarding herb-drug interactions between warfarin and danshen did not specify any detail regarding danshen use by the patient – vital information such as dosage and method of preparation were unavailable.(35,36) In the case of ginkgo, it has been noted that documenting exactly what the patient was taking and confirming this by a visual inspection of the actual product was rarely carried out.(43) A standard scoring system has been proposed for the assessment of causality of adverse drug reactions in a case report, which includes the following factors: previous reports of adverse drug reaction, dechallenge and rechallenge, appropriate timing, ruling out of other possible causes, trial with placebo and proof of a toxic concentration.(44) These aspects should be presented in future case reports to strengthen current evidence of herb-drug interactions.
Further to this, several fundamental considerations limit the usefulness of current evidence in clinical practice. Firstly, the reductionistic approach of isolating single components of a herb may not reflect the final antiplatelet property of the herb, as the various components found within may interact with one another to alter its overall pharmacodynamic profile. Methods of preparation may also differ between experimental and commercial/prescribed products, resulting in differences in the active components derived as well. It is, hence, more appropriate to use extracts of herbs that are available in commercial products or TCM preparations for the investigation of antiplatelet properties. Most importantly, the effects of herbs were considered in isolation in the literature reviewed. In reality, when prescribed by TCM physicians, herbs are often used in combination as herbal formulas, with their properties being used complementarily for a synergistic effect. This is an established concept behind TCM prescriptions, allowing physicians to maximise therapeutic benefits while minimising potential side effects within each formula. Such a practice would inevitably result in changes to the herb-drug interaction profile, making adverse effects more unpredictable.
These numerous confounding factors indicate that the clinical significance of the above evidence has to be interpreted critically. Nevertheless, physician awareness and patient education are crucial in identifying potential herb-drug interactions. Hence, clinicians should continue to practise with caution, actively seeking TCM use for a comprehensive drug history. This is especially important in light of the trend of patients not disclosing their use of herbal products to attending physicians. An example of a list of questions physicians can ask is shown in Box 1. In the event of a suspected herb-drug interaction, information on the specific dosage and ingredients of the TCM preparation or herbal product should be elicited. Clinicians may also encourage patients to obtain the ingredients of TCM formulas from their TCM practitioner or provide product labels for more details.
Box 1.

Questions to ask when screening for traditional Chinese medicine (TCM)/herbal supplement use.
Although this review has only addressed the potential herb-drug interactions with aspirin, other antithrombotic agents such as clopidogrel may also be implicated due to possible pharmacodynamic interactions. Clinicians should counsel patients on antithrombotic agents accordingly regarding concurrent use of TCM products, applying the information provided by this review. All patients, especially high-risk groups such as the elderly and patients with pre-existing bleeding diathesis, must also be actively reviewed for side effects of clinically significant bleeding.
CONCLUSION
In the face of rising TCM use in Singapore, it is imperative that both TCM and Western physicians be more aware of the possible benefits and risks associated with concurrent use of TCM and Western therapies. Many herbs have demonstrated potential for antiplatelet activity, possibly enhancing the drug effect of aspirin and thereby causing clinically significant bleeding episodes. Clinicians must therefore educate themselves regarding these herbs to be able to adequately diagnose and treat patients, while avoiding potential side effects that may not only hinder therapy but also cause serious morbidity and mortality. However, more concrete evidence is required to accurately determine the level of risks associated with these herbs, as well as the mechanisms involved. With more research dedicated to understanding herb-drug interactions, it may even be possible to combine Western and TCM herbs in the future for greater therapeutic outcome with minimal adverse effects. At the present juncture, however, close monitoring and avoidance may be the only solution to negative herb-drug interactions.
ACKNOWLEDGEMENTS
We would like to thank Dr Khoo Kei Siong, Senior Consultant, Parkway Cancer Centre, Singapore, and Physician Zhong Xi Ming, Senior Physician, Eu Yan Sang Integrative Health Pte Ltd, Singapore, for their guidance and feedback. We would also like to acknowledge Ms Eva Shen and Ms Alicia Lim from Eu Yan Sang Integrative Health Pte Ltd, Singapore, for providing administrative assistance during the process.
APPENDIX
Ginkgo
The parts of the plant used for medicinal effect include the seeds and leaves, which differ in clinical effects. While ginkgo seeds are more often used in traditional Chinese medicine (TCM) as compared to ginkgo leaves, extracts of ginkgo leaves are sold as commercial products locally. These extracts were initially studied in the form of EGb 761, a standardised extract containing approximately 24% flavone glycosides (primarily quercetin, kaempferol and isorhamnetin) and 6% terpene lactones (2.8%–3.4% ginkgolides A, B and C, and 2.6%–3.2% bilobalide).(76) Although EGb 761 has not been approved by the United States Food and Drug Administration, commercial products of ginkgo supplements are standardised in a similar manner. These products are primarily recommended for age-related cognitive decline and for slowing the progress of neurodegenerative disorders, such as Alzheimer’s disease and other forms of dementia.(77) Other reported uses of ginkgo include dizziness, tinnitus, depression, anxiety, peripheral vascular disease and age-related macular degeneration. Experimental evidence (both in vitro and ex vivo) has shown that the main mechanism for its herb-drug interaction with aspirin is pharmacodynamic in nature, via the inhibition of platelet-activating factor-mediated aggregation of platelets.(23,46)
Ginseng
The major bioactive components found in Asian ginseng are saponins, including ginsenosides Ro, Rg1, and Rg2, but panaxynol has also been found to play a role in its antiplatelet activity.(26) Commercially, ginseng products may take the form of capsules, extracts, tonic drinks and wines, and are commonly recommended to boost immunity. In vitro experiments conducted using extracts of Asian ginseng have demonstrated that panaxynol and ginsenosides inhibit adenosine triphosphate release from platelets,(26) one of the pathways involved in the activation of platelet aggregation. Panaxynol further inhibits thromboxane formation, a crucial step downstream.(26) In addition, a lipophilic fraction of Panax ginseng was found to increase intracellular cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate levels, and thereby inhibit thrombin- or collagen-induced platelet aggregation in rat platelets in vitro. Coagulation time of thrombin (TT) and activated partial thromboplastin was also prolonged in the experiment, suggesting inhibition of the coagulation cascade.(27) The anticoagulation activity of Asian ginseng was confirmed in a recent in vitro study, with its extract as well as its active component ginsenoside Rg2 showing the strongest anticoagulation activity among extracts from the same genus.(28) While steaming has been known to increase the antiplatelet and anticoagulant properties of notoginseng, this form of processing has been shown to have no effect on the antiplatelet effect of Asian ginseng.(24)
Danshen
While intravenous injections of danshen and its bioactive compound have been used in China,(31,38) only oral forms – powders, pills and liquids – are available in Singapore. Of its various chemical constituents, pharmacological research has focused on three active components: danshensu, salvianolic acid B and tanshinone IIA. Their combined effects include coronary artery dilation, improved microcirculation and protection against ischaemia-reperfusion injury in the heart and brain.(37) Research investigating the effects of salvianolic acids has demonstrated in vitro and in vivo inhibition of platelet aggregation in rats.(33) A study by Fan et al suggested that the mechanism of action for salvianolic acid A (SAA) involves increasing the cAMP level, possibly through activating adenylyl cyclase, leading to platelet inhibition. Fan et al also showed that SAA reduced blood viscosity in vivo, although there was no effect on the coagulation profile of rats treated with intravenous SAA.(32) Danshen also interacts pharmacokinetically with salicylate, an active metabolite of aspirin, displacing salicylate from protein binding to increase its free concentration.(34)
Safflower
Research has primarily focused on a group of flavonoids isolated from safflower, known as carthamins yellow, with hydroxysafflor yellow A (HSYA) as one of its main components. HSYA has been found to reduce platelet aggregation in rat models in two separate studies,(39,40) although conflicting evidence has been presented regarding its effect on coagulation profile. When its effect on thrombosis in rats was investigated, treatment with aqueous extracts of safflower potentiated the effect of clopidogrel on bleeding time, although it did not prolong bleeding time when given alone.(41) Prothrombin time and TT were also prolonged with safflower treatment alone in the same study.
Other herbs that promote blood circulation and eliminate stasis (活血化瘀药)
Turmeric (scientific name: Curcuma longa L.; Chinese name: 姜黄) is often used in the TCM context for the treatment of rheumatism as well as other symptoms belonging to the syndrome of Qi/blood stagnation, including chest pain, dysmenorrhoea and amenorrhoea.(11) Studies have suggested that curcumin and ar-turmerone, both major constituents of turmeric, are potent inhibitors of cyclooxygenase enzymes (although less so than aspirin), thereby inhibiting platelet aggregation.(50-52) Curcuma (scientific name: Curcuma wenyujin/Curcuma aromatica Salisb.; Chinese name: 郁金), another species within the same genus, is commonly used for the treatment of chest and flank pain, dysmenorrhoea, epilepsy and jaundice.(11) It also contains curcumin and thus inhibits platelet aggregation in a similar fashion.(54) Curdione, another compound isolated from the essential oil of curcuma, also increases cAMP levels leading to platelet inhibition.(53)
Like turmeric, Szechuan lovage (scientific name: Ligusticum wallichii Franchat; Chinese name: 川芎) has also been used for rheumatism, headaches and manifestations of Qi/blood stagnation such as chest, flank or abdominal pain.(11) Tetramethylpyrazine, the key component responsible for its antiplatelet properties, has been shown to inhibit phosphoinositide breakdown and thromboxane A2 (TXA2) formation,(57) increase nitric oxide production in human platelets,(56) increase intracellular cAMP and reduce glycoprotein (GP) IIb/IIIa expression.(55)
Spatholobus stem is another herb with stasis-eliminating properties. It is primarily used in the treatment of menstrual irregularities, amenorrhoea and dysmenorrhoea, rheumatism, numbness or paralysis.(11) Components of Spatholobus stem (scientific name: Spatholobus suberectus Dunn; Chinese name: 鸡血藤) have demonstrated antiplatelet properties by blocking the binding of fibrinogen to GPIIb/IIIa and suppressing TXA2 formation.(58)
Corydalis (scientific name: Corydalis yanhusuo/Corydalis ambigua; Chinese name: 延胡索), a herb used for menstrual irregularities, dysmenorrhoea, traumatic injuries and all types of pain, including chest and gastric pain,(11) may inhibit platelet aggregation via its constituent, tetrahydroberberine. Tetrahydroberberine inhibits arachidonic acid metabolism and thereby inhibits platelet aggregation.(59) Chinese motherwort (scientific name: Leonurus japonicus; Chinese name: 益母草), a herb used in the treatment of amenorrhoea, dysmenorrhoea, traumatic injuries and rashes, has also been shown to inhibit adenosine diphosphate-induced platelet aggregation, but the exact mechanism has not been reported.(60)
REFERENCES
- 1.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy--from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–31. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 2.Zhou SF, Zhou ZW, Li CG, et al. Identification of drugs that interact with herbs in drug development. Drug Discov Today. 2007;12:664–73. doi: 10.1016/j.drudis.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Tan MG, Win MT, Khan SA. The use of complementary and alternative medicine in chronic pain patients in Singapore: a single-centre study. Ann Acad Med Singapore. 2013;42:133–7. [PubMed] [Google Scholar]
- 4.Dergal JM, Gold JL, Laxer DA, et al. Potential interactions between herbal medicines and conventional drug therapies used by older adults attending a memory clinic. Drugs Aging. 2002;19:879–86. doi: 10.2165/00002512-200219110-00005. [DOI] [PubMed] [Google Scholar]
- 5.Saw JT, Bahari MB, Ang HH, Lim YH. Potential drug-herb interaction with antiplatelet/anticoagulant drugs. Complement Ther Clin Pract. 2006;12:236–41. doi: 10.1016/j.ctcp.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Canter PH, Ernst E. Herbal supplement use by persons aged over 50 years in Britain: frequently used herbs, concomitant use of herbs, nutritional supplements and prescription drugs, rate of informing doctors and potential for negative interactions. Drugs Aging. 2004;21:597–605. doi: 10.2165/00002512-200421090-00004. [DOI] [PubMed] [Google Scholar]
- 7.Sood A, Sood R, Brinker FJ, et al. Potential for interactions between dietary supplements and prescription medications. Am J Med. 2008;121:207–11. doi: 10.1016/j.amjmed.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Posadzki P, Watson L, Ernst E. Herb-drug interactions: an overview of systematic reviews. Br J Clin Pharmacol. 2013;75:603–18. doi: 10.1111/j.1365-2125.2012.04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ongo DL. Harrison's principles of internal medicine. 18th ed. New York: McGraw-Hill; 2012. [Google Scholar]
- 10.Patrono C, Rocca B. Aspirin and other COX-1 inhibitors. Handb Exp Pharmacol. 2012;210:137–64. doi: 10.1007/978-3-642-29423-5_6. [DOI] [PubMed] [Google Scholar]
- 11.学敏 高, editor. 中药学 (新世纪(第二版)) 2nd ed. 中国中医药出版社; 2007. Chinese. [Google Scholar]
- 12.Mustapha A, Yakasai IA, Aguye IA. Effect of Tamarindus indica L. on the bioavailability of aspirin in healthy human volunteers. Eur J Drug Metab Pharmacokinet. 1996;21:223–6. doi: 10.1007/BF03189717. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblatt M, Mindel J. Spontaneous hyphema associated with ingestion of Ginkgo biloba extract. N Engl J Med. 1997;336:1108. doi: 10.1056/NEJM199704103361518. [DOI] [PubMed] [Google Scholar]
- 14.Rowin J, Lewis SL. Spontaneous bilateral subdural hematomas associated with chronic Ginkgo biloba ingestion. Neurology. 1996;46:1775–6. doi: 10.1212/wnl.46.6.1775. [DOI] [PubMed] [Google Scholar]
- 15.Meisel C, Johne A, Roots I. Fatal intracerebral mass bleeding associated with Ginkgo biloba and ibuprofen. Atherosclerosis. 2003;167:367. doi: 10.1016/s0021-9150(03)00015-7. [DOI] [PubMed] [Google Scholar]
- 16.Chan AL, Leung HW, Wu JW, Chien TW. Risk of hemorrhage associated with co-prescriptions for Ginkgo biloba and antiplatelet or anticoagulant drugs. J Altern Complement Med. 2011;17:513–7. doi: 10.1089/acm.2010.0295. [DOI] [PubMed] [Google Scholar]
- 17.Schneider LS, DeKosky ST, Farlow MR, et al. A randomized, double-blind, placebo-controlled trial of two doses of Ginkgo biloba extract in dementia of the Alzheimer's type. Curr Alzheimer Res. 2005;2:541–51. doi: 10.2174/156720505774932287. [DOI] [PubMed] [Google Scholar]
- 18.Köhler S, Funk P, Kieser M. Influence of a 7-day treatment with Ginkgo biloba special extract EGb 761 on bleeding time and coagulation: a randomized, placebo-controlled, double-blind study in healthy volunteers. Blood Coagul Fibrinolysis. 2004;15:303–9. doi: 10.1097/00001721-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327–32. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- 20.Bal Dit Sollier C, Caplain H, Drouet L. No alteration in platelet function or coagulation induced by EGb761 in a controlled study. Clin Lab Haematol. 2003;25:251–3. doi: 10.1046/j.1365-2257.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 21.Beckert BW, Concannon MJ, Henry SL, Smith DS, Puckett CL. The effect of herbal medicines on platelet function: an in vivo experiment and review of the literature. Plast Reconstr Surg. 2007;120:2044–50. doi: 10.1097/01.prs.0000295972.18570.0b. [DOI] [PubMed] [Google Scholar]
- 22.Gardner CD, Zehnder JL, Rigby AJ, Nicholus JR, Farquhar JW. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787–93. doi: 10.1097/MBC.0b013e3282f102b1. [DOI] [PubMed] [Google Scholar]
- 23.Koch E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine. 2005;12:10–6. doi: 10.1016/j.phymed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Lau AJ, Toh DF, Chua TK, et al. Antiplatelet and anticoagulant effects of Panax notoginseng: comparison of raw and steamed Panax notoginseng with Panax ginseng and Panax quinquefolium. J Ethnopharmacol. 2009;125:380–6. doi: 10.1016/j.jep.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 25.Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry. 2011;72:689–99. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo SC, Teng CM, Lee JC, et al. Antiplatelet components in Panax ginseng. Planta Med. 1990;56:164–7. doi: 10.1055/s-2006-960916. [DOI] [PubMed] [Google Scholar]
- 27.Park HJ, Lee JH, Song YB, Park KH. Effects of dietary supplementation of lipophilic fraction from Panax ginseng on cGMP and cAMP in rat platelets and on blood coagulation. Biol Pharm Bull. 1996;19:1434–9. doi: 10.1248/bpb.19.1434. [DOI] [PubMed] [Google Scholar]
- 28.Li CT, Wang HB, Xu BJ. A comparative study on anticoagulant activities of three Chinese herbal medicines from the genus Panax and anticoagulant activities of ginsenosides Rg1 and Rg2. Pharm Biol. 2013;51:1077–80. doi: 10.3109/13880209.2013.775164. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Williams KM, Liauw WS, et al. Effect of St John's wort and ginseng on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;57:592–9. doi: 10.1111/j.1365-2125.2003.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SH, Ahn YM, Ahn SY, Doo HK, Lee BC. Interaction between warfarin and Panax ginseng in ischemic stroke patients. J Altern Complement Med. 2008;14:715–21. doi: 10.1089/acm.2007.0799. [DOI] [PubMed] [Google Scholar]
- 31.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Fan HY, Fu FH, Yang MY, et al. Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb Res. 2010;126:e17–22. doi: 10.1016/j.thromres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Tang MK, Ren DC, Zhang JT, Du GH. Effect of salvianolic acids from Radix Salviae miltiorrhizae on regional cerebral blood flow and platelet aggregation in rats. Phytomedicine. 2002;9:405–9. doi: 10.1078/09447110260571634. [DOI] [PubMed] [Google Scholar]
- 34.Gupta D, Jalali M, Wells A, Dasgupta A. Drug-herb interactions: unexpected suppression of free Danshen concentrations by salicylate. J Clin Lab Anal. 2002;16:290–4. doi: 10.1002/jcla.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu CM, Chan JC, Sanderson JE. Chinese herbs and warfarin potentiation by ‘danshen’. J Intern Med. 1997;241:337–9. doi: 10.1046/j.1365-2796.1997.134137000.x. [DOI] [PubMed] [Google Scholar]
- 36.Izzat MB, Yim AP, El-Zufari MH. A taste of Chinese medicine! Ann Thorac Surg. 1998;66:941–2. doi: 10.1016/s0003-4975(98)00624-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Zuo Z, Chow MS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45:1345–59. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 38.Yan FF, Liu YF, Liu Y, Zhao YX. Sulfotanshinone Sodium Injection could decrease fibrinogen level and improve clinical outcomes in patients with unstable angina pectoris. Int J Cardiol. 2009;135:254–5. doi: 10.1016/j.ijcard.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Li HX, Han SY, Wang XW, et al. Effect of the carthamins yellow from Carthamus tinctorius L. on hemorheological disorders of blood stasis in rats. Food Chem Toxicol. 2009;47:1797–802. doi: 10.1016/j.fct.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Duan JA, Tang Y, et al. Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation. J Ethnopharmacol. 2012;139:381–7. doi: 10.1016/j.jep.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Wang N. Antithrombotic effects of Danggui, Honghua and potential drug interaction with clopidogrel. J Ethnopharmacol. 2010;128:623–8. doi: 10.1016/j.jep.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Zhu YF, Luo HM, Deng ZL, et al. [Effects of the Chinese patent medicine, Honghua Injection, on platelet glycoprotein IIb/III a receptors in patients with acute coronary syndrome: a randomized controlled trial] Zhong Xi Yi Jie He Xue Bao. 2012;10:318–23. doi: 10.3736/jcim20120311. Chinese. [DOI] [PubMed] [Google Scholar]
- 43.Bone KM. Potential interaction of Ginkgo biloba leaf with antiplatelet or anticoagulant drugs: what is the evidence? Mol Nutr Food Res. 2008;52:764–71. doi: 10.1002/mnfr.200700098. [DOI] [PubMed] [Google Scholar]
- 44.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 45.Ryu KH, Han HY, Lee SY, et al. Ginkgo biloba extract enhances antiplatelet and antithrombotic effects of cilostazol without prolongation of bleeding time. Thromb Res. 2009;124:328–34. doi: 10.1016/j.thromres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Chung KF, Dent G, McCusker M, et al. Effect of a ginkgolide mixture (BN 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet. 1987;1:248–51. doi: 10.1016/s0140-6736(87)90066-3. [DOI] [PubMed] [Google Scholar]
- 47.Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425–32. doi: 10.1111/j.1365-2125.2005.02322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aruna D, Naidu MU. Pharmacodynamic interaction studies of Ginkgo biloba with cilostazol and clopidogrel in healthy human subjects. Br J Clin Pharmacol. 2007;63:333–8. doi: 10.1111/j.1365-2125.2006.02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guinot P, Caffrey E, Lambe R, Darragh A. Tanakan inhibits platelet-activating-factor-induced platelet aggregation in healthy male volunteers. Haemostasis. 1989;19:219–23. doi: 10.1159/000215920. [DOI] [PubMed] [Google Scholar]
- 50.Lee HS. Antiplatelet property of Curcuma longa L. rhizome-derived ar-turmerone. Bioresour Technol. 2006;97:1372–6. doi: 10.1016/j.biortech.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Srivastava KC, Bordia A, Verma SK. Curcumin, a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prostaglandins Leukot Essent Fatty Acids. 1995;52:223–7. doi: 10.1016/0952-3278(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 52.Shah BH, Nawaz Z, Pertani SA, et al. Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+signaling. Biochem Pharmacol. 1999;58:1167–72. doi: 10.1016/s0006-2952(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 53.Xia Q, Wang X, Xu DJ, Chen XH, Chen FH. Inhibition of platelet aggregation by curdione from Curcuma wenyujin essential Oil. Thromb Res. 2012;130:409–14. doi: 10.1016/j.thromres.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Jantan I, Raweh SM, Sirat HM, et al. Inhibitory effect of compounds from Zingiberaceae species on human platelet aggregation. Phytomedicine. 2008;15:306–9. doi: 10.1016/j.phymed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Liu SY, Sylvester DM. Antiplatelet activity of tetramethylpyrazine. Thromb Res. 1994;75:51–62. doi: 10.1016/0049-3848(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 56.Sheu JR, Kan YC, Hung WC, Lin CH, Yen MH. The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase. Life Sci. 2000;67:937–47. doi: 10.1016/s0024-3205(00)00686-x. [DOI] [PubMed] [Google Scholar]
- 57.Sheu JR, Kan YC, Hung WC, Ko WC, Yen MH. Mechanisms involved in the antiplatelet activity of tetramethylpyrazine in human platelets. Thromb Res. 1997;88:259–70. doi: 10.1016/s0049-3848(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 58.Lee BJ, Jo IY, Bu Y, et al. Antiplatelet effects of Spatholobus suberectus via inhibition of the glycoprotein IIb/IIIa receptor. J Ethnopharmacol. 2011;134:460–7. doi: 10.1016/j.jep.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 59.Xuan B, Wang W, Li DX. Inhibitory effect of tetrahydroberberine on platelet aggregation and thrombosis. Zhongguo Yao Li Xue Bao. 1994;15:133–5. [PubMed] [Google Scholar]
- 60.Xiong L, Zhou QM, Peng C, et al. Sesquiterpenoids from the herb of Leonurus japonicus. Molecules. 2013;18:5051–8. doi: 10.3390/molecules18055051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Francischetti IM, Monteiro RQ, Guimarães JA. Identification of glycyrrhizin as a thrombin inhibitor. Biochem Biophys Res Commun. 1997;235:259–63. doi: 10.1006/bbrc.1997.6735. [DOI] [PubMed] [Google Scholar]
- 62.Mendes-Silva W, Assafim M, Ruta B, et al. Antithrombotic effect of Glycyrrhizin, a plant-derived thrombin inhibitor. Thromb Res. 2003;112:93–8. doi: 10.1016/j.thromres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 63.Tawata M, Yoda Y, Aida K, et al. Anti-platelet action of GU-7, a 3-arylcoumarin derivative, purified from glycyrrhizae radix. Planta Med. 1990;56:259–63. doi: 10.1055/s-2006-960951. [DOI] [PubMed] [Google Scholar]
- 64.Prieto JM, Recio MC, Giner RM, et al. Influence of traditional Chinese anti-inflammatory medicinal plants on leukocyte and platelet functions. J Pharm Pharmacol. 2003;55:1275–82. doi: 10.1211/0022357021620. [DOI] [PubMed] [Google Scholar]
- 65.Yang MH, Yoon KD, Chin YW, Park JH, Kim J. Phenolic compounds with radical scavenging and cyclooxygenase-2 (COX-2) inhibitory activities from Dioscorea opposita. Bioorg Med Chem. 2009;17:2689–94. doi: 10.1016/j.bmc.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 66.Wang J, Huang ZG, Cao H, et al. Screening of anti-platelet aggregation agents from Panax notoginseng using human platelet extraction and HPLC-DAD-ESI-MS/MS. J Sep Sci. 2008;31:1173–80. doi: 10.1002/jssc.200700507. [DOI] [PubMed] [Google Scholar]
- 67.Kim SY, Yun-Choi HS. Platelet anti-aggregating activities of bupleurumin from the aerial parts of Bupleurum falcatum. Arch Pharm Res. 2007;30:561–4. doi: 10.1007/BF02977649. [DOI] [PubMed] [Google Scholar]
- 68.Chang WC, Hsu FL. Inhibition of platelet activation and endothelial cell injury by flavan-3-ol and saikosaponin compounds. Prostaglandins Leukot Essent Fatty Acids. 1991;44:51–6. doi: 10.1016/0952-3278(91)90144-t. [DOI] [PubMed] [Google Scholar]
- 69.Koo YK, Kim JM, Koo JY, et al. Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa. Pharmazie. 2010;65:624–8. [PubMed] [Google Scholar]
- 70.Kubo M, Matsuda H, Tani T, et al. Studies on Scutellariae radix XII. Anti-thrombic actions of various flavonoids from Scutellariae radix. Chem Pharm Bull (Tokyo) 1985;33:2411–5. doi: 10.1248/cpb.33.2411. [DOI] [PubMed] [Google Scholar]
- 71.Liu XF, Liu ML, Iyanagi T, et al. Inhibition of rat liver NAD(P)H: quinone acceptor oxidoreductase (DT-diaphorase) by flavonoids isolated from the Chinese herb scutellariae radix (Huang Qin) Mol Pharmacol. 1990;37:911–5. [PubMed] [Google Scholar]
- 72.Teng CM, Li HL, Wu TS, Huang SC, Huang TF. Antiplatelet actions of some coumarin compounds isolated from plant sources. Thromb Res. 1992;66:549–57. doi: 10.1016/0049-3848(92)90309-x. [DOI] [PubMed] [Google Scholar]
- 73.Liu JH, Zschocke S, Reininger E, Bauer R. Inhibitory effects of Angelica pubescens fbiserrata on 5-lipoxygenase and cyclooxygenase. Planta Med. 1998;64:525–9. doi: 10.1055/s-2006-957507. [DOI] [PubMed] [Google Scholar]
- 74.Hoult JR, Paya M. Pharmacological and biochemical actions of simple coumarins: natural products with therapeutic potential. Gen Pharmacol. 1996;27:713–22. doi: 10.1016/0306-3623(95)02112-4. [DOI] [PubMed] [Google Scholar]
- 75.Page RL, 2nd, Lawrence JD. Potentiation of warfarin by dong quai. Pharmacotherapy. 1999;19:870–6. doi: 10.1592/phco.19.10.870.31558. [DOI] [PubMed] [Google Scholar]
- 76.EGb 761: ginkgo biloba extract, Ginkor. Drugs R D. 2003;4:188–93. doi: 10.2165/00126839-200304030-00009. [DOI] [PubMed] [Google Scholar]
- 77.Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;1:CD003120. doi: 10.1002/14651858.CD003120.pub3. [DOI] [PubMed] [Google Scholar]