Abstract
This review compares two states that lower energy expenditure: non-rapid eye movement (NREM) sleep and torpor. Knowledge on mechanisms common to these states, and particularly on the role of adenosine in NREM sleep, may ultimately open the possibility of inducing a synthetic torpor-like state in humans for medical applications and long-term space travel. To achieve this goal, it will be important, in perspective, to extend the study to other hypometabolic states, which, unlike torpor, can also be experienced by humans.
Introduction
Comparative physiology is the discipline that specifically considers diversity in the modes of solving life’s challenges by using the animal type as the experimental variable. The most critical challenges, such as maintaining energy balance, may be solved with a repertoire of different behavioral states that can vary among animals, and within the same animal, according to the conditions of the internal and external environment. This review will take a comparative approach of examining two behavioral states that share the common outcome of reducing energy expenditure: non-rapid eye movement (NREM) sleep and torpor. We then seek to apply our understanding of these two states to address other physiological conditions that share fully, or in part, the hypometabolic state and autonomic changes seen in torpor and NREM sleep. Understanding these other conditions, including the diving reflex, hypoxia, inflammation-induced hypothermia, motion sickness, and meditation, may allow us to develop models of “synthetic torpor” as the medical science community addresses problems of targeted temperature management and long-term space flight.
NREM sleep, experienced by all mammals, is characterized by a suspension of active contact of the organism with the environment, and by a decrease in energy expenditure with respect to wakefulness, which is obtained through both the assumption of an adequate resting posture and a resetting of homeostatic controls. These characteristics do not belong to sleep in its entirety, because rest and stability of NREM sleep deeply contrast with the irregularity of REM sleep, which is characterized by phasic changes in a variety of autonomic functions and an elevation in metabolic rate, at least as far as the brain is concerned (143).
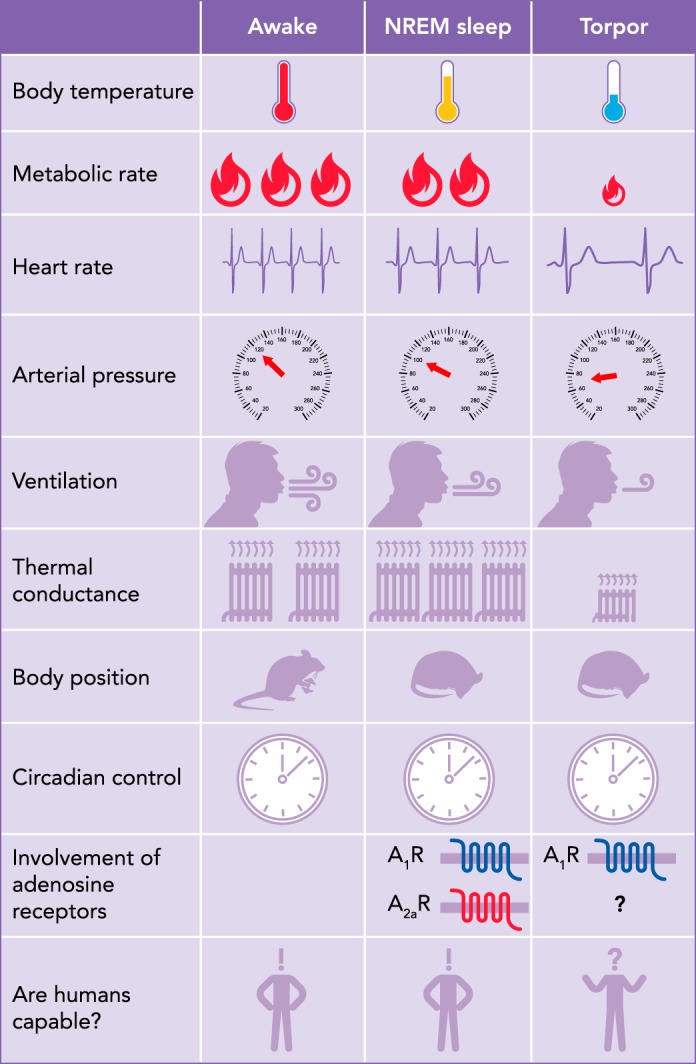
Torpor is a controlled reduction in metabolic rate to levels well below basal metabolic rate. Although many animals utilize torpor, the depth to which they lower their metabolic rate varies widely. This variable hypometabolic state, combined with differences in animal size (~10 g to 200 kg) and in thermal insulation, results in core body temperatures that range from −2.7°C in arctic ground squirrels to 20°C in mice to 30°C in bears. Furthermore, the environmental cues that can induce a torpid state vary among species and include shortened day length, arid conditions, cold ambient temperature, and acute caloric deficits. Nevertheless, it seems plausible that the brain output, i.e., those mechanisms invoked to induce a torpid state, is shared among animals that utilize torpor. That is, the central pathways involved in hypometabolism may be common, albeit that their extent of activation during torpor may vary. Hence, regardless of the cues that send an animal into torpor, we are treating the neural circuity and ensuing physiological conditions under the large umbrella of “torpor.” Most evidence points to a lack of torpor in humans, although there are case studies and anecdotes where thermoregulation can be abandoned in humans (92).
Sleep and Torpor: A Strong Relationship
There are remarkable similarities between sleep and torpor (FIGURE 1). These highly regulated, adaptive, and reversible behaviors require an adequate posture in a safe place, are accompanied by a reduction in muscle tone, and are characterized by a decreased reactivity to the external environment. Moreover, they both represent energy-conserving processes, lying on a continuum of decreasing metabolism and body temperature compared with wakefulness (7). In particular, the resetting of the central regulation of body temperature during the transition from wakefulness to NREM sleep appears to be homologous with that during torpor (57). Accordingly, torpor was hypothesized to represent an evolutionary extension of sleep; such a hypothesis is also strengthened by the remarkable similarities in the electroencephalogram (EEG) between these conditions (56). Indeed, torpor is usually entered through sleep. Walker and colleagues (174) recorded the EEG during entrance in torpor in ground squirrels and showed that the brain temperature started to decrease during either REM or NREM sleep but never during wakefulness. These authors were able to record the characteristic EEG of NREM and REM sleep as long as the brain temperature was maintained above 25°C. In this first period of torpor, the time spent in NREM sleep progressively rose, whereas REM sleep time tended to decrease and disappeared when the brain temperature fell below 25°C. Similar results have been reported during torpor in round-tailed ground squirrels (173), pocket mice (54), and Djungarian hamsters (36). In hamsters, a systematic downward shift of EEG frequency bands was reported as cortical temperature decreased so that EEG slow waves during torpor occurred at frequencies lower than those during euthermia (36). This is of interest because the physiological EEG slow-wave activity (<4.5 Hz) indexes a progressively declining “homeostatic” process, whose initial value increases with the duration of prior wakefulness and which is dissipated in the course of NREM sleep (11). The lack of physiological EEG slow waves during torpor might therefore represent a functional equivalent of sleep deprivation. This is in accordance with the observation that torpor episodes in Djungarian hamsters are followed by an increase in EEG slow-wave activity, which is positively correlated with the length of the episodes and which subsequently declines with time spent asleep (33, 117). Increases in EEG slow-wave activity have also been reported in ground squirrels recorded during the euthermic periods between bouts of torpor (29, 168). In golden-mantled ground squirrels, the slow-wave activity during NREM sleep following arousal from torpor was related to the minimum brain temperature reached during torpor but not to the duration of the torpor bout. This suggests that the sleep debt accumulated during torpor may be due to a brain temperature-dependent effect (81). Data consistent with this suggestion were obtained in European ground squirrels (151). Conversely, the build-up of the homeostatic sleep process during torpor appeared to be slowed down by the lower brain temperature in Djungarian hamsters (34). In an animal that does not enter natural torpor such as the rat, a massive increase in EEG slow-wave activity was recorded in the euthermic period after the pharmacological induction of deep hypothermia (22°C) (22), but its magnitude apparently depended on the extent of the metabolic effort made by the animal to recover euthermia. This would suggest that a relevant part of the sleep debt was accumulated during the rewarming phase. Species differences may also exist in the homeostatic regulation of sleep after exit from torpor. Djungarian hamsters exposed to 1.5 h of sleep deprivation immediately after exit from a torpor episode showed an increase in EEG slow-wave activity during recovery above the level achieved after sleep deprivation (35). In ground squirrels, conversely, the anticipated high level of EEG slow-wave activity typical of recovery NREM sleep did not occur when recovery sleep was delayed by sleep deprivation performed during the first few hours after exit from torpor (80, 152). These differences between findings on Djungarian hamsters vs. ground squirrels may reflect, at least in part, the much lower temperature reached by squirrels than by hamsters during the respective torpor bouts.
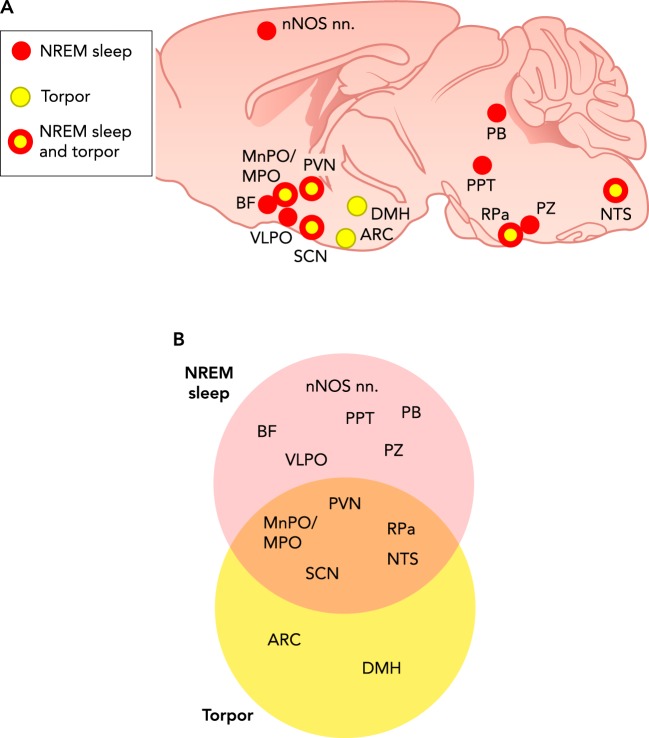
FIGURE 1.
Qualitative comparison of physiological variables and behavior between NREM sleep and natural torpor
NREM, non-rapid eye movement; Tb, body temperature; A1R and A2aR, adenosine receptors type 1 and 2a, respectively.
If NREM sleep and torpor share the common outcome of reducing energy expenditure, it is reasonable to suppose that these behaviors are generated and sustained by at least partly overlapping neural circuits. Our review is not intended as a comprehensive review of the states of NREM sleep and torpor. Rather, we first want to highlight the physiological, behavioral, and neuroanatomical similarities between these states. Then, we apply our understanding of the physiological mechanisms and effectors of NREM sleep and torpor, and in particular of the role of adenosine in NREM sleep (8), to address important questions concerning the mechanisms of physiological adjustments in other hypometabolic states, with an eye toward human applications of “synthetic torpor.”
Common Physiological Changes in NREM Sleep and Torpor
The daily rest period is characterized by a circadian drop in body temperature, which results from a decrease in heat production and from an increase in heat dissipation due to cutaneous vasodilation (78). In humans, NREM sleep, which covers most of the subjective rest period, reduces energy expenditure by approximately one-third (13, 73), with body and brain temperatures regulated at a lower level (175). NREM sleep per se also decreases energy expenditure in small model organisms such as mice, particularly when long, consolidated wake-sleep episodes are taken into account (181). The fall in metabolic rate during NREM sleep is accompanied by a decrease in heart rate and arterial pressure, both in human subjects and in small model animals (145). This decrease in arterial pressure may result from the decrease in heart rate, itself due to a balance of cardiac sympathetic withdrawal and parasympathetic activation (87), in the absence of a compensatory increase in stroke volume and/or peripheral resistance (74). Indeed, recent evidence indicates that, at least in mice, the decrease in arterial pressure during NREM sleep depends critically from a reduction in sympathetic vasoconstriction (87), possibly due to a reduction in sympathetic activity to the vasculature of skeletal muscles (147), kidneys (177), and skin (163). However, since both arterial pressure and regional vascular resistance may decrease during NREM sleep, peripheral blood flow to non-skin organs may not change greatly during NREM sleep (26, 82, 93, 115).
Torpor is a behavior that results in energy savings through a reduction in metabolic rate to a much greater extent compared with NREM sleep. Similarly to what occurs during NREM sleep, the autonomic nervous system is intimately involved in all stages of a bout of torpor. During entrance into torpor and during the bout of torpor, parasympathetic activity to the heart is markedly increased. During this time, heart rate slows, whereas its variability increases, with periodic increases in heart rate coupled to ventilation (60, 101, 102, 183, 184). The parasympathetic dominance during entry into torpor occurs as sympathetic activity withdraws (6). However, although sympathetic outflow to the heart and brown fat decreases during entrance to torpor, other organs experience an elevation in sympathetic activity. For example, total peripheral resistance appears to be elevated fourfold with reduced thermal conductance during a bout of torpor in the mouse, suggesting a net elevation of vasoconstriction (62, 155). Despite the vasoconstriction, low arterial pressure is a hallmark of torpor (60, 88, 155). In addition, sympathetic activity to white fat increases during caloric-restriction-induced torpor, with the result of lowering leptin release and elevating lipolysis and free fatty acid release to the blood (128, 156, 159). The sympathetic nervous system also appears important during arousal from torpor, when heart rate is maximal and brown adipose tissue is sympathetically activated for heat production (62, 112, 159).
In humans, the decrease in energy expenditure during NREM sleep is associated with an even greater relative decrease in minute ventilation, leading to a small but significant rise in the end-tidal partial pressure of carbon dioxide and a fall in the partial pressure of oxygen (38). The minute-to-minute variability of respiratory rate and tidal volume is also lower during NREM sleep than during wakefulness (161). These effects of NREM sleep on respiratory control in human subjects are also seen in small model organisms. In particular, NREM sleep in mice blunts the chemoreflex responses to hypoxia, decreases respiratory rate and/or tidal volume compared with wakefulness (106), and shows the lowest values of breath-to-breath respiratory variability (144). When animals near the onset of torpor breathe, ventilation occurs in concert with increased electromyogram activity (either a result of shivering or postural changes) and tachycardia (100, 157). Interestingly, ventilation during torpor decreases to a greater extent than does metabolic rate, leading to a slight respiratory acidosis (100, 101), similar to what occurs during NREM sleep.
Central Neural Pathways of NREM Sleep and Torpor
A great deal is known about the control of NREM sleep, including brain areas that trigger NREM sleep, effector areas of the brain that induce physiological changes, and some of the molecular mechanisms involved, such as adenosine signaling. On the other hand, information on the neural structures involved in torpor is still scant, despite its potentially dramatic clinical and practical applications (19). Little is known also about the basic molecular mechanisms of torpor. In particular, although several lines of evidence suggest a role for adenosine signaling in torpor, much critical evidence concerning the nature of this role and the sources of adenosine during torpor is still missing.
The NREM sleep-promoting pathways are thought to include sleep-active neurons releasing gamma-amino-butyric-acid (GABA) and are located in the cortex (these neurons also express neuronal nitric oxide synthase), basal forebrain, hypothalamus (ventrolateral and median preoptic nuclei), and medulla (parafacial zone) (137) (FIGURE 2). It is still unclear which portion of the central autonomic network is specifically responsible for mediating the autonomic changes during NREM sleep. However, a set of data-driven testable hypotheses has been proposed (145), positing a central role for projections of the ventrolateral preoptic nucleus of the hypothalamus, a key structure that promotes NREM sleep, to at least four different pathways (25, 123, 141) (FIGURE 3). The first pathway was posited to target the paraventricular nucleus of the hypothalamus (171), which is an autonomic master switch controlling parasympathetic and sympathetic outflow. The second pathway may involve “command neurons” of the pedunculopontine nucleus (42), which themselves project to pre-sympathetic neurons of the rostral ventrolateral medulla. The third pathway may involve the parabrachial nucleus (133), which modulates the baroreflex through the nucleus of the solitary tract (40). The last pathway may involve the median preoptic nucleus of the hypothalamus, which modulates thermoregulatory pathways including the medial preoptic area of the hypothalamus and the rostral medullary raphe (103).
FIGURE 2.
Common and disparate neural structures potentially involved in NREM sleep and torpor
The figure shows neural structures that, based on the available evidence, are potentially involved in NREM sleep and torpor. Cf. the text for details and references. A: the brain structures are shown on a sagittal section of the mouse brain and marked in color code: red and yellow circles, structures thought to be involved only in NREM sleep and torpor, respectively; yellow circles with red rim, structures potentially involved both in NREM sleep and in torpor. This grouping is emphasized in B, where the same brain structures are inscribed in Venn diagrams corresponding to the NREM sleep and torpor structure sets. nNOS nn, sleep-active cortical neurons containing both gamma-amino-butyric acid (GABA) and neuronal nitric oxide synthase (nNOS); BF, basal forebrain; MPO, hypothalamic medial preoptic area; MnPO, VLPO, SCN, ARC, PVN, and DMH, hypothalamic median preoptic, ventrolateral preoptic, suprachiasmatic, arcuate, paraventricular, and dorsomedial nuclei, respectively; PB, parabrachial nucleus; PPT, pedunculopontine nucleus; RPa, raphe pallidus; PZ, parafacial zone; NTS, nucleus of the solitary tract.
FIGURE 3.
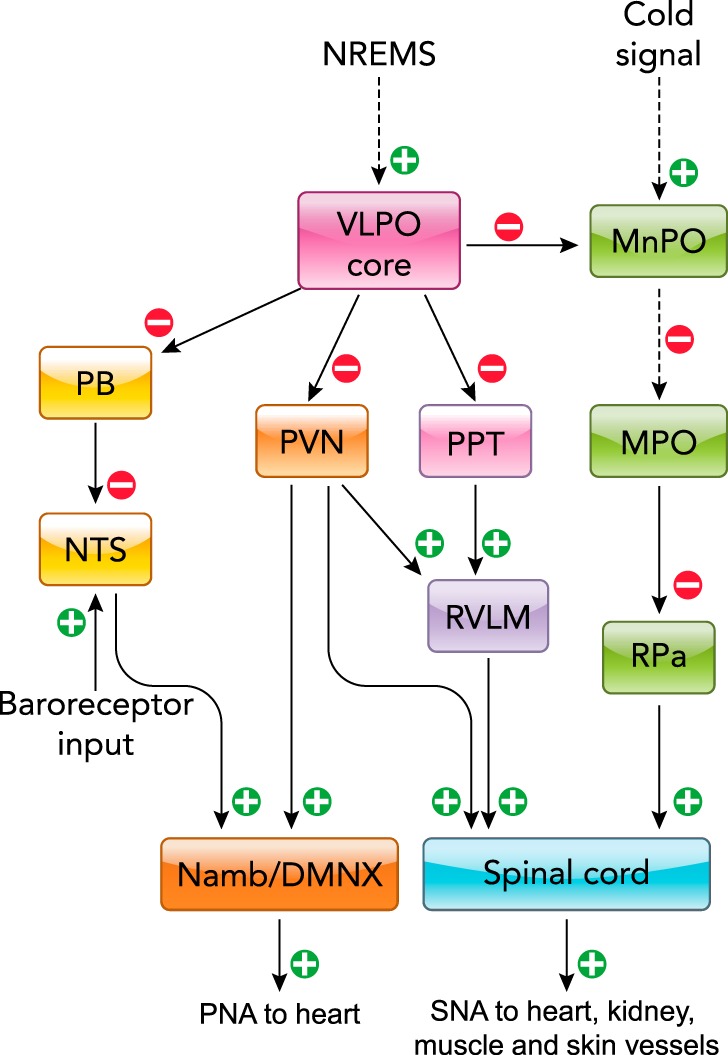
Diagram indicating postulated central pathways mediating autonomic changes during NREM sleep
The broken lines indicate indirect connections, including one or more relay nuclei. Namb/DMNX, nucleus ambiguous/dorsal motor nucleus of the vagus nerve. Figure was modified from Ref 145, with permission from the American Physiological Society.
Although little is known about the neural structures mediating torpor (FIGURE 2), some hypotheses can be proposed by examining the available behavioral, physiological, and genetic data. We can categorize the regions of the brain that are 1) sensing the environment for torpor induction and 2) evoking the behavioral and physiological responses of torpor. In facultative heterotherms, the environmental cues that trigger daily torpor are at least two: negative energy balance and cold exposure. Cold exposure activates cold-sensing areas such as the parabrachial nucleus (107), which normally would stimulate cold defense by multi-synaptically activating the pre-sympathetic neurons of the raphe pallidus that control sympathetic innervation of the brown adipose tissue (104). The activation of brown adipose thermogenesis would be incompatible with the metabolic reduction characteristic of torpor. This suggests that, during torpor entrance, neurons that would activate brown fat are actually inhibited. A parsimonious site for that inhibition may be the pre-sympathetic neurons of the raphe pallidus themselves. On the other hand, the fact that the drop in body temperature during torpor is associated with a decrease in cutaneous blood flow suggests that pre-sympathetic neurons controlling cutaneous blood vessels, also located within the raphe pallidus, remain active. There should therefore be at least two distinct populations of intermingled neurons within the raphe pallidus relevant to torpor induction: 1) those that control sympathetic activity to cutaneous blood vessels, which would remain active during torpor; 2) those that control sympathetic activity to the brown adipose tissue, which would be inhibited during torpor. The specific source of such inhibition is currently unknown, but some direct and indirect inhibitory afferents to the raphe pallidus have been identified in studies conducted mostly on rats. However, since rats do not enter natural torpor, it is possible that these findings do not directly translate to torpor-prone species such as mice. These inhibitory afferents originate from forebrain areas and include 1) direct GABAergic projections from the preoptic area of the hypothalamus (105); 2) projections from the paraventricular nucleus of the hypothalamus (90); 3) a direct cholinergic inhibitory pathway from the dorsomedial hypothalamus (66), which is tonically active in anaesthetized rats (28) and inactive in awake mice (66); 4) projections from the commissural nucleus of the solitary tract (89); and 5) noradrenergic projections from the ventrolateral medulla (91). It is also interesting to note that, during torpor, a specific heat-saving posture is maintained. The neural pathways controlling thermoregulatory posture are largely unknown but may include the hypothalamic preoptic area, whose lesions induce an array of thermoregulatory responses that includes changes in posture (160). On the other hand, it has been shown that the activity of the solitary tract nucleus neurons show distinct adaptations during torpor in Syrian hamsters, potentially sustaining signal processing, including of baroceptive inputs, while conserving energy at the same time (139, 140).
As previously discussed, the nucleus of the solitary tract not only may contribute to inhibit the raphe pallidus during torpor but also may represent a key component of the central autonomic network of NREM sleep. The nucleus of the solitary tract is a major gateway through which visceral afferent signals reach the brain (31). It is thus intriguing that this nucleus may represent the neuroanatomical “common ground” between NREM sleep and torpor, two states that share an energy-conserving function.
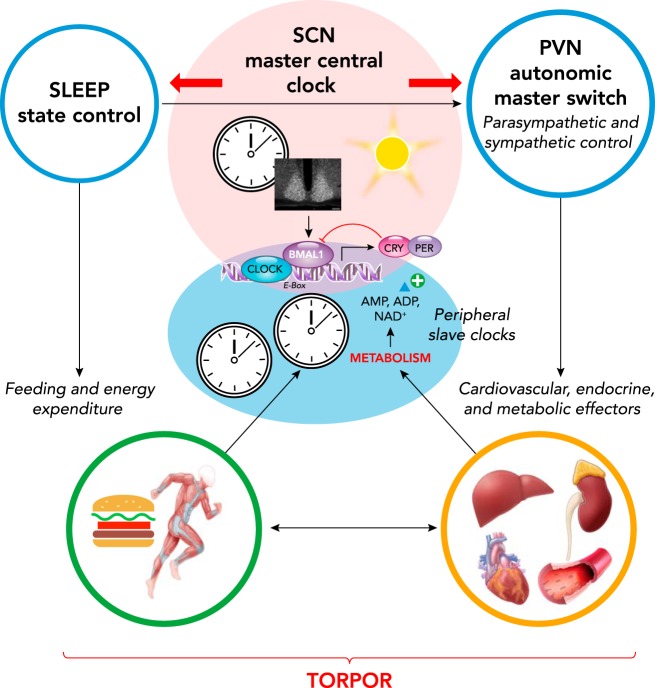
Another region within the hypothalamus that appears important for the control of torpor is the suprachiasmatic nucleus, which is the master circadian clock responsible for the circadian rhythms of sleep (39) and cardiovascular variables (65). The master circadian clock has a strong influence over whether an animal engages in a bout of torpor, as shown by several groups on a variety of organisms (59, 63, 112). The suprachiasmatic nucleus also controls separate populations of pre-sympathetic and pre-parasympathetic neurons by projecting to the hypothalamic paraventricular nucleus (15) and may thereby contribute to shape the autonomic correlates of the torpor bouts. The specific role of the suprachiasmatic nucleus in torpor induction could be viewed as a modality for the brain to align the metabolic needs satisfied by torpor with the circadian rhythm, given that specific changes in the suprachiasmatic nucleus molecular clock have a very limited effect on body temperature (129) (FIGURE 4).
FIGURE 4.
Circadian and sleep-related control of autonomic and metabolic factors that may modulate torpor
CLOCK, BMAL1, CRY, and PER, key proteins coded by homonymous core clock genes; AMP and ADP, adenosine mono- and diphosphate, respectively; NAD+, oxidized nicotinamide adenine dinucleotide.
Negative energy balance is also a necessary prerequisite for caloric-restriction-induced torpor, suggesting a role of central areas controlling food intake. Many areas within the hypothalamus have been identified with a role in the control of food intake (138), and their discussion is beyond the scope of this review. However, one nucleus seems of particular importance: the arcuate nucleus. Several lines of evidence suggest this nucleus is a main candidate for the regulation of entry into daily torpor, including 1) neuropeptide Y (NPY) neurons in the arcuate nucleus tonically express c-Fos during fasting in mice (176); 2) mice deficient in NPY or mice with lesions in the arcuate nucleus do not enter extended torpor bouts (50); and 3) intracerebroventricular injection of NPY induces a torpor-like state in hamsters with a NPY-Y1 receptor-dependent mechanism (32).
Although there is at least a partial knowledge of the brain areas, such as the arcuate nucleus, that may be involved in torpor entrance, much less is known about brain areas involved in the maintenance of torpor and even less about the areas involved in the arousal from torpor. Important information in this respect is provided by a systematic study of c-fos mRNA expression, which was measured as an index of cell activation, in the brain of 13-lined ground squirrels during different phases of the torpor bouts (14). During entrance into torpor, this study detected activation of the hypothalamic medial preoptic area, which, as previously mentioned, plays a key role in thermoregulatory pathways, and of the reticular thalamic nucleus, which may contribute to gate afferent information to the cortex. During the maintenance of torpor, ground squirrels showed increased activity of the hypothalamic suprachiasmatic nucleus, the master circadian clock, whereas the medial preoptic area was active during arousal from torpor. Intriguingly, torpor maintenance also entailed increased activity of the epithelial cells of the choroid plexus and of tanycytes, whereas arousal from torpor entailed increased activity of ependymal cells, suggesting a hitherto unrecognized involvement of cerebrospinal fluid dynamics in torpor maintenance and termination (14). Accordingly, tanycytes have been suggested as regulators of seasonal cycles in neuroendocrine functions (84). It has also been suggested that the increased activity of the choroid plexus during torpor maintenance is due, at least in part, to increased activity of histaminergic fibers (14). Accordingly, the density of histaminergic fibers and brain histamine levels were found to be increased during torpor in golden-mantled ground squirrels, particularly in the hypothalamus and hippocampus (134). During torpor in this species, the hippocampus showed an increase in excitatory H1 and H2 histamine receptors, and a decrease in inhibitory H3 histamine receptors (135), whereas inhibitory H3 histamine receptor binding was increased in the cerebral cortex, globus pallidus, and substantia nigra (136). Djungarian hamsters also showed an upregulation of histamine H3 receptors during torpor in multiple hypothalamic nuclei, including the arcuate, suprachiasmatic, and dorsomedial nuclei (58). Therefore, although much critical evidence is still missing, the available data are at least consistent with a role for the histamine system in torpor maintenance.
Pharmacological evidence on neurotransmitter antagonists that induce arousal from torpor may also provide valuable insights on the brain areas that are active during torpor. As far as we know, only antagonists of opioid (164), adenosine (64, 71), and NMDA-type glutamate receptors (70) have been shown to induce arousal from torpor. Interestingly, orexin is involved in cold defense (21, 162), but its mRNA levels do not change during torpor (58). By contrast, recent data on orexin-neuron-deficient mice indicate that orexin neurons play a protective/compensatory role in fasting-induced natural torpor (48). However, the same study showed that orexin neurons instead act as enhancers in the induction phase and compensators during the recovery phase of adenosine-induced synthetic torpor (48), which will be discussed in the following sections of this review. The roles of the brain orexin transmission in torpor therefore still await clarification.
Adenosine Plays a Major Role in NREM Sleep. Does It Play a Role in Torpor?
Another intriguing commonality between NREM sleep and torpor is the potential role of adenosine: this molecule plays an important role in controlling NREM sleep (10) and may mediate a pathway for torpor induction. Adenosine binds four different receptors named A1R, A2aR, A2bR, and A3R (45). Adenosine monophosphate (AMP), the singly phosphorylated form of adenosine, is also an A1R agonist (130). All of these adenosine receptors are expressed in the brain (12, 45, 153), and there is evidence that at least brain A1R and A2aR play a role in sleep control. Mice lacking A1R selectively in neurons that express the CaMKII promoter show reductions in EEG slow-wave activity during NREM sleep at baseline and during recovery after sleep deprivation (9), and a loss of the normal decay of EEG slow-wave activity during NREM sleep across the light (rest) phase (8). The NREM sleep homeostasis is also impaired in rats by knock-down (167) or antagonism (49) of A1R in the cholinergic basal forebrain, which is the only brain region in which adenosine levels in cats increase progressively during sleep deprivation and remain elevated in the first hours of sleep recovery (126). However, A1R knockout mice show normal levels of NREM sleep time and EEG slow-wave activity at baseline and during recovery after sleep deprivation (150). This indicates that the role of the A1R expressed in CaMKII-expressing neurons and in the basal forebrain in driving EEG slow-wave activity and NREM sleep homeostasis is prominent but redundant. The A2aR are also involved in sleep control, since A2aR knockout mice are insensitive to the wake-promoting effects of caffeine (61) and do not show the NREM sleep time rebound after sleep deprivation (55). Recent chemo- and optogenetic experiments on mice suggest that A2aR in the striatum (179) and nucleus accumbens core (113) also play important roles in inducing NREM sleep during the active period of the day but not in enhancing EEG slow-wave activity during NREM sleep. Blockade of A2aR in the hypothalamic lateral preoptic area of rats also decreases NREM sleep time without affecting EEG slow-wave activity (98), whereas A2aR blockade in the basal forebrain has no detectable effect on NREM sleep homeostasis (49). These data indicate that A2aR in structures including the striatum, nucleus accumbens, and lateral hypothalamus plays an important role in inducing NREM sleep, particularly during the active period, but not in enhancing EEG slow-wave activity.
Compared with sleep, much less is known about the role of adenosine in torpor. Infusion of adenosine or AMP peripherally or centrally induces a fall in metabolic rate and body temperature similar to that found in natural torpor, even in rats, which do not naturally enter torpid states (18, 68, 71, 158, 170). Pharmacological experiments on mice suggest that A2aR and A2bR do not play any major role in this effect (3). The low body temperature that is induced by peripheral infusion of A1R and A3R agonists can be both cardiac-mediated (154) and mast-cell-dependent (18), whereas neurons of the medullary nucleus of the solitary tract may be key for the temperature-lowering effect of central A1R agonists, at least in rats (170). Caloric restriction increases the central sensitivity to adenosine receptor agonists in rats and winter-adapted arctic ground squirrels, with longer and deeper drops in body temperature (68, 71). As detailed in a later section of this review, the hypothermia that is induced by adenosine receptor agonists has some physiological features in common with natural torpor, but other features are quite different (172). This is not to say that adenosine signaling is not important for torpor. Rather, a few studies have shown the importance of adenosine tone in the induction and maintenance of torpor. For example, central administration of A1R antagonists into Syrian hamsters, arctic ground squirrels, or mice prevents torpor, or, if given during a torpor bout, causes arousal from torpor (64, 71, 164). Importantly, however, neither A1R nor A3R are required for fasting-induced torpor (18). One critical piece of evidence missing to link adenosine accumulation to torpor induction and maintenance is the demonstration of adenosine build-up (e.g., through microdialysis experiments) before torpor onset, possibly in association with an increase in NREM sleep time and EEG slow-wave activity. Along these lines, it would be critical to test whether sleep rebound after torpor (116) also reflects adenosine build-up, and whether enhancing adenosine build-up with sleep deprivation increases the probability of torpor. The current state of our understanding of torpor and adenosine leads to the conclusion that adenosine tone likely is permissive for torpor entry, but much more work is needed to show definitively that adenosine action is a trigger for naturally occurring torpor.
Where Does Adenosine Come from to Permit Torpor Entry?
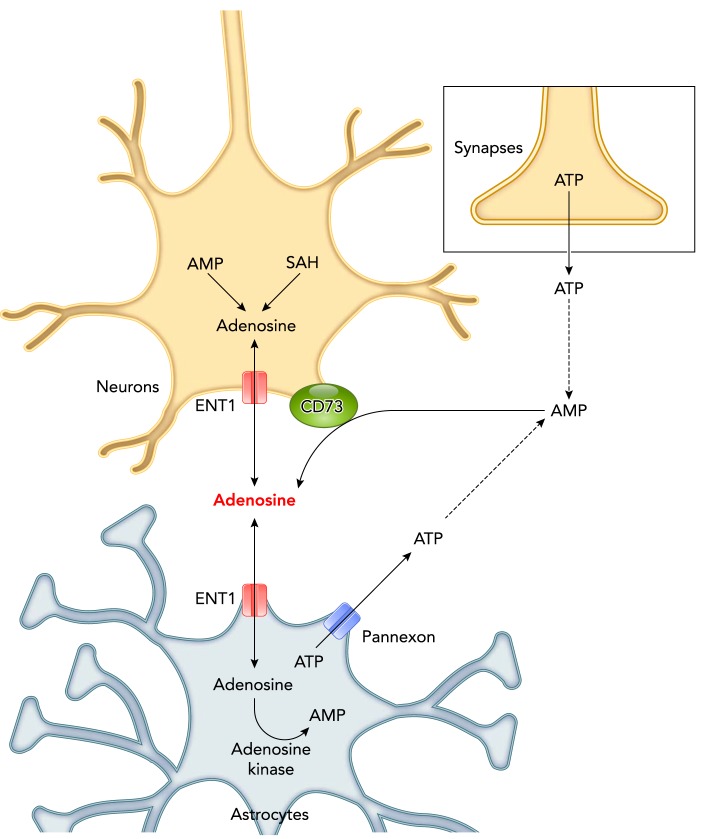
Adenosine is generated intracellularly by hydrolysis of AMP or S-adenosylhomocysteine at a rate increasing with the imbalances between ATP synthesis and utilization rates (FIGURE 5). Intracellular adenosine may diffuse to the interstitial fluid through equilibrative membrane nucleoside transporters (44). Adenosine may thus act as a “retaliatory metabolite” that is released by cells to retaliate against stimuli that would cause excessive ATP breakdown (109). Brain extracellular adenosine may also result from the phosphohydrolysis of AMP catalyzed by CD73, an ecto-5′-nucleotidase on brain cell surface (27). Extracellular AMP mainly results from the degradation of extracellular ATP (44), which may be released as a co-transmitter by neurons (16).
FIGURE 5.
Sources of adenosine potentially involved in the control of NREM sleep and torpor
SAH, S-adenosylhomocysteine; ENT1, equilibrative nucleoside transporter 1. Cf. the text for details and references.
It is still unknown to what extent the adenosine or AMP molecules that may permit entry into spontaneous torpor result from extracellular ATP degradation or are transported from the cytoplasm of neurons or glia (FIGURE 5). However, there is evidence that both intracellularly and extracellularly produced adenosine molecules play a role in the control of sleep, potentially representing a source of experimental approaches translatable to torpor research. In particular, mice knockout for ENT1 (equilibrative nucleoside transporter 1) shows a decrease in NREM sleep time during the light (rest) period in the face of a preserved build-up of brain extracellular adenosine levels during sleep deprivation, and of a normal increase in EEG slow-wave activity during recovery of NREM sleep (75). This apparent lack of effect of ENT1 deficiency on NREM sleep homeostasis may be, at least in part, because ENT1 transporters also mediate adenosine transport from the extracellular space into astrocytes (122), where adenosine kinase degrades adenosine. Accordingly, targeted deficiency of adenosine kinase in astrocytes enhances the increase in EEG slow-wave activity during NREM sleep after sleep deprivation (8). Conversely, constitutive overexpression of adenosine kinase in mice increases wake time during the active period and markedly decreases EEG slow-wave activity during NREM sleep at baseline and after sleep deprivation (118). Concerning the role of extracellularly produced adenosine, CD73 knockout mice lack the rebound of NREM sleep time and show a limited increase in EEG slow-wave activity during sleep recovery after deprivation (182). These mice also show a mild increase in NREM sleep time at baseline, which may occur, at least in part, because the impairment of CD73-dependent clearance increases extracellular ATP levels (182). Although ATP may not act on adenosine receptors without being first degraded to nucleosides (45), extracellular ATP may stimulate microglial release of interleukin 1 and tumor necrosis factor alpha, which then act back on neurons to increase NREM sleep time and EEG slow-wave activity (79). Data have been produced in support of a role of ATP exocytosis by astrocytes (gliotransmission) in the control of sleep homeostasis (52). However, these data have been questioned based on imperfect astrocyte specificity of the glial fibrillary acidic protein (GFAP) promoter (46). Nonetheless, astrocytes also release ATP with other mechanisms, including the recently discovered pannexon channels (30). Accordingly, pannexin 1 knockout mice show reduced NREM sleep time during the active period (76).
The potential for translation of these experimental approaches to torpor research is highlighted by the recent findings that AMP-induced hypometabolism duration is increased in CD73 knockout mice (111) and that pharmacological inhibition of adenosine kinase causes profound hypothermia in mice (41). The study of spontaneous torpor in mice knockout for ENT1, adenosine kinase, CD73, or pannexin1 has, to our knowledge, not yet been undertaken, and represents a promising avenue for future research.
Adenosine Receptor Agonists Depress Metabolism. Does the Hypometabolic State Elicited by Adenosine Receptor Agonists Induce Physiological Changes That Mimic Natural Torpor?
Because central administration of A1R agonists into small mammals induces metabolic depression with a concomitant drop in body temperature, the question arises whether this “synthetic torpor” has the same physiological features of natural torpor. Synthetic torpor shares many characteristics with natural torpor, but some differences remain (FIGURE 6). In rats, the activation of A1R by central pharmacological agonist administration induces a steep reduction in heart rate that is accompanied by a significant increase in arterial pressure (170). The EEG waves seen in synthetic torpor induced by central A1R agonist delivery also seem to show an increase in spectral power at the slower frequencies (170) that differs from the EEG changes in natural torpor (77). It is of course possible that the diffusion of the A1R agonist to other areas besides the ones mediating torpor causes such differences. Even when used in mice, the A1R agonist N6-cyclohexyl-adenosine (CHA) induces a state that differs from natural torpor in terms of cardiovascular and metabolic regulation (172). No data are currently available concerning the existence of a sleep rebound after CHA-induced synthetic torpor. This was instead shown in synthetic torpor induced with a different modality: the inhibition of neurons within the raphe pallidus (22), suggesting that the physiology of synthetic torpor may change according to the procedure used for its induction (see FIGURE 6).
FIGURE 6.
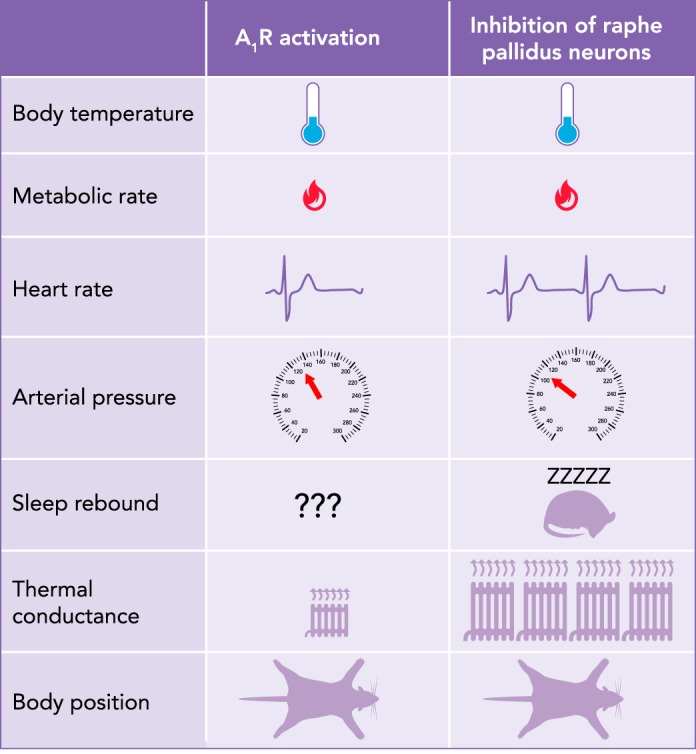
Qualitative comparison of physiological variables and behavior between synthetic torpor induced by A1R activation vs. raphe pallidus inhibition
Tb, body temperature; A1R, adenosine receptors.
Humans Do Not Hibernate. Do Other Achievable Hypometabolic States in Humans Resemble Torpor?
In addition to NREM sleep and torpor, animals can undergo a hypometabolic state as an adaptive response to particular environmental conditions. One such condition is submersion under water. The ensuing diving reflex comprises a suite of physiological changes that include marked bradycardia, peripheral vasoconstriction, and apnea. The bradycardia commonly found in diving mammals has often been taken as a proxy for an assumed fall in metabolic rate (17). Moreover, studies with direct measurement of oxygen consumption in humans and some species of seals have shown that metabolic rate can be depressed during the dive (4, 17, 148), although this hypometabolic state may not become manifest in other species of seals and aquatic birds (17). Diving birds and mammals can experience acute hypoxia (127), and the reduction in oxygen availability itself might contribute to the hypometabolic state (89).
As with both NREM sleep and torpor, the physiological effects of the diving response are mediated through action of the autonomic nervous system. The time scale of the physiological effects are wildly different, of course, ranging from hours/days in torpor to seconds/minutes in diving. Although the extent of the physiological changes of diving can vary quantitatively between animals that primarily/exclusively live in water (whales and dolphins) vs. semi-aquatic mammals (beavers) vs. land animals that submerge periodically (humans), it is conceivable that they share the same qualitative features, so we treat those physiological responses as a group. Here, we draw on examples that range from diving birds, to muskrats, to seals, to rats. The physiological changes in torpor and the diving reflex are striking in their similarities. The sympathetic nervous system mediates the peripheral vasoconstriction that occurs during the diving response (85). During the diving response, blood is redistributed to those tissues with a greater oxygen need during the dive, namely the central nervous system and heart (17, 114). The parasympathetic nervous system, via vagal activation of the muscarinic receptors on the sino-atrial node, mediates the bradycardia of diving (17, 124, 142). Although cardiac sympathetic fibers are co-activated with vagal fibers during the dive (108, 121, 125), the activation of the vagus nerve outcompetes the cardiac accelerator fibers (47, 124, 125, 142), possibly by inhibiting release of norepinephrine from nerve endings and/or lowering the sensitivity of the target cells to catecholamines (83). The third arm of the diving reflex is apnea (119). Similar to some hibernating animals, diving animals, in particular small semi-aquatic mammals and birds, can go several minutes without an attempt to take a breath, despite the hypercapnia and low oxygen content of the blood (96, 99).
The regions of the brain that are activated during diving are only known in small part and are studied mainly in the rat, a species that does not enter natural torpor (94, 120). Not surprisingly, the sensing arm of the diving response involves afferent nerves and brain regions that are quite different from those in NREM sleep and torpor, although the autonomic outflow areas are similar. Initiation of the diving reflex can be evoked simply by immersion of the snout, which activates the anterior ethmoidal nerve (119). Nasopharyngeal stimulation of this nerve leads to profound bradycardia produced by parasympathetic preganglionic cardiac motoneurons of the nucleus ambiguous (166), with a concomitant activation of the trigeminal sensory complex (132). The medullary dorsal horn of the spinal trigeminal nucleus seems to be critical for the coordination of cardiac, vascular, and respiratory consequences of diving (119). There is also evidence that signals from trigeminal afferent fibers reach the nucleus of the solitary tract (31, 97). Intense bradycardia would produce a collapse of the peripheral blood flow. However, this is contrasted during diving by an increase in resistances of cutaneous, muscular, and splanchnic circulations driven by the rostral ventrolateral medulla, which is sufficient to result in an increase in arterial pressure (95). Whether adenosine plays any role in the diving reflex is simply not known at this time.
There are other much less understood hypothermic/hypometabolic conditions such as meditation (2), motion sickness (110), inflammation-induced hypothermia (131, 149), and hypoxia per se, i.e., independent of the dive (89). The meditative state can obviously only be characterized in humans and not in other animals. Limited work in humans shows that some meditative states may result in an increased parasympathetic outflow with a resultant fall in metabolic rate, heart rate, and ventilation rate, albeit not to the levels achieved during torpor bouts (43, 67, 165, 178). Due to the lack of animal studies on meditation, the mechanistic changes, including central pathways and potential role of adenosine, during meditation have not been examined. Motion sickness is caused by conflicting information coming from the vestibular and the visual inputs. Motion sickness may represent a hypometabolic state, since it is characterized by a reduction in core temperature (110) in the face of reduced NREM sleep time (37), but the central pathways mediating such effects are unknown. Severe inflammation can also cause hypothermia, which may help reduce tissue damage (86). Whereas several hypotheses have been suggested on the mechanism of hypothermia in the course of severe inflammation, such as a role of peripheral release of interleukin 10, many aspects of this response are still unknown (53). In the case of hypoxia per se, the mechanism that leads to a reduction in body temperature is clearly related to the diminished oxygen availability. Among areas involved in thermoregulation, the NTS was shown to be one of the key relays in mediating such effects (89). Second-messenger (adenosine 3′:5′ cyclic monophosphate and inositol 1,4,5-trisphosphate) accumulation in the hypothalamic preoptic area may also be affected (72, 180).
Conclusions
The knowledge of the mechanisms that regulate the condition of torpor is the first step toward the induction of such condition in human subjects. Humans engage in NREM sleep and other potentially hypometabolic states such as the diving reflex, meditation, motion sickness, inflammation-induced hypothermia, and hypoxia. So, is it possible to use these pathways to induce a synthetic torpor-like state? This would be of great interest for medical purposes. Hypothermia induced by physical means (external cooling or infusion of cold fluids) is currently used to reduce neuronal damage resulting from cardiac arrest, since it slows down the activity of enzymes responsible for cellular damage, limits the formation of free radicals, stabilizes cell membranes, and reduces the oxygen demand of ischemic areas (5). However, forced hypothermia induces homeostatic responses for cold defense such as shivering (146). Hypothermia can also induce blood coagulopathies as well as changes in electrolyte balance, reductions in cardiac function, especially in terms of rhythm generation, and immune suppression (1). Such an array of problematic outcomes effectively limits the safe use of forced hypothermia. Synthetic torpor may represent a safe, alternative procedure to induce a controlled hypothermia without the adverse side-effects of forced hypothermia (19). The induction of synthetic torpor could also facilitate long-duration space exploration for humans (24, 51) and animal experimentation (51), by reducing the amount of food required, the level of psychological stress for the crew, and the disuse atrophy of skeletal muscle and bones (20). The investigation of the neural networks and molecular mechanisms shared among NREM sleep and natural torpor is therefore relevant to identify and test more suitable targets to induce synthetic torpor in human subjects. So far, only two effective procedures were shown to effectively induce synthetic torpor in animals that do not enter natural torpor: the inhibition of the neurons within the raphe pallidus (22) and the central activation of A1R (69, 170). In this latter case, the inhibition of thermogenesis appears to be mediated by the activation of neurons in the nucleus of the solitary tract (170), possibly triggering a general reassembling of the entire thermoregulatory network (169). In perspective, the search for the way to obtain effective and safe synthetic torpor could also be expanded to other much less understood hypothermic/hypometabolic conditions such as the diving reflex, meditation, motion sickness, inflammation-induced hypothermia, and hypoxia.
Acknowledgments
Supported by National Heart, Lung, and Blood Institute Grant R15 HL-120072-01 (to S.J.S.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: A.S., M.C., G.Z., and S.J.S. conceived and designed research; A.S. prepared figures; A.S., M.C., G.Z., and S.J.S. drafted manuscript; A.S., M.C., G.Z., and S.J.S. edited and revised manuscript; A.S., M.C., G.Z., and S.J.S. approved final version of manuscript.
References
- 1.Alshimemeri A. Therapeutic hypothermia after cardiac arrest. Ann Card Anaesth 17: 285–291, 2014. doi: 10.4103/0971-9784.142065. [DOI] [PubMed] [Google Scholar]
- 2.Amihai I, Kozhevnikov M. The influence of Buddhist meditation traditions on the autonomic system and attention. BioMed Res Int 2015: 731579, 2015. doi: 10.1155/2015/731579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R, Sheehan MJ, Strong P. Characterization of the adenosine receptors mediating hypothermia in the conscious mouse. Br J Pharmacol 113: 1386–1390, 1994. doi: 10.1111/j.1476-5381.1994.tb17151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson S, Chamberlain MR, Musgrove S, Partusch A, Tice KRJ, Thorp DB. Is V̇O2supressed during nonapnoeic facial submersion? Appl Physiol Nutr Metab 41: 1171–1176, 2016. doi: 10.1139/apnm-2016-0268. [DOI] [PubMed] [Google Scholar]
- 5.Arrich J, Holzer M, Havel C, Müllner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2: CD004128, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atgie C, Nibbelink M, Ambid L. Sympathoadrenal activity and hypoglycemia in the hibernating garden dormouse. Physiol Behav 48: 783–787, 1990. doi: 10.1016/0031-9384(90)90227-U. [DOI] [PubMed] [Google Scholar]
- 7.Berger RJ. Slow wave sleep, shallow torpor and hibernation: homologous states of diminished metabolism and body temperature. Biol Psychol 19: 305–326, 1984. doi: 10.1016/0301-0511(84)90045-0. [DOI] [PubMed] [Google Scholar]
- 8.Bjorness TE, Dale N, Mettlach G, Sonneborn A, Sahin B, Fienberg AA, Yanagisawa M, Bibb JA, Greene RW. An adenosine-mediated glial-neuronal circuit for homeostatic sleep. J Neurosci 36: 3709–3721, 2016. doi: 10.1523/JNEUROSCI.3906-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorness TE, Greene RW. Adenosine and sleep. Curr Neuropharmacol 7: 238–245, 2009. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci 29: 1267–1276, 2009. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borbély AA, Baumann F, Brandeis D, Strauch I, Lehmann D. Sleep deprivation: effect on sleep stages and EEG power density in man. Electroencephalogr Clin Neurophysiol 51: 483–495, 1981. doi: 10.1016/0013-4694(81)90225-X. [DOI] [PubMed] [Google Scholar]
- 12.Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, Gessi S. The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67: 74–102, 2015. doi: 10.1124/pr.113.008540. [DOI] [PubMed] [Google Scholar]
- 13.Bouma HR, Kroese FGM, Kok JW, Talaei F, Boerema AS, Herwig A, Draghiciu O, van Buiten A, Epema AH, van Dam A, Strijkstra AM, Henning RH. Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine-1-phosphate. Proc Natl Acad Sci USA 108: 2052–2057, 2011. doi: 10.1073/pnas.1008823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bratincsák A, McMullen D, Miyake S, Tóth ZE, Hallenbeck JM, Palkovits M. Spatial and temporal activation of brain regions in hibernation: c-fos expression during the hibernation bout in thirteen-lined ground squirrel. J Comp Neurol 505: 443–458, 2007. doi: 10.1002/cne.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 464: 36–48, 2003. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- 16.Burnstock G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology 104: 4–17, 2016. doi: 10.1016/j.neuropharm.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Butler PJ, Jones DR. Physiology of diving of birds and mammals. Physiol Rev 77: 837–899, 1997. doi: 10.1152/physrev.1997.77.3.837. [DOI] [PubMed] [Google Scholar]
- 18.Carlin JL, Jain S, Gizewski E, Wan TC, Tosh DK, Xiao C, Auchampach JA, Jacobson KA, Gavrilova O, Reitman ML. Hypothermia in mouse is caused by adenosine A1and A3receptor agonists and AMP via three distinct mechanisms. Neuropharmacology 114: 101–113, 2017. doi: 10.1016/j.neuropharm.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cerri M. The central control of energy expenditure: exploiting torpor for medical applications. Annu Rev Physiol 79: 167–186, 2017. doi: 10.1146/annurev-physiol-022516-034133. [DOI] [PubMed] [Google Scholar]
- 20.Cerri M. Consciousness in hibernation and synthetic torpor. J Integr Neurosci 16, s1: S19–S26, 2017. doi: 10.3233/JIN-170063. [DOI] [PubMed] [Google Scholar]
- 21.Cerri M, Del Vecchio F, Mastrotto M, Luppi M, Martelli D, Perez E, Tupone D, Zamboni G, Amici R. Enhanced slow-wave EEG activity and thermoregulatory impairment following the inhibition of the lateral hypothalamus in the rat. PLoS One 9: e112849, 2014. doi: 10.1371/journal.pone.0112849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerri M, Mastrotto M, Tupone D, Martelli D, Luppi M, Perez E, Zamboni G, Amici R. The inhibition of neurons in the central nervous pathways for thermoregulatory cold defense induces a suspended animation state in the rat. J Neurosci 33: 2984–2993, 2013. doi: 10.1523/JNEUROSCI.3596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerri M, Tinganelli W, Negrini M, Helm A, Scifoni E, Tommasino F, Sioli M, Zoccoli A, Durante M. Hibernation for space travel: impact on radioprotection. Life Sci Space Res (Amst) 11: 1–9, 2016. doi: 10.1016/j.lssr.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci 22: 977–990, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cianci T, Zoccoli G, Lenzi P, Franzini C. Regional splanchnic blood flow during sleep in the rabbit. Pflugers Arch 415: 594–597, 1990. doi: 10.1007/BF02583511. [DOI] [PubMed] [Google Scholar]
- 27.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73). Purinergic Signal 2: 351–360, 2006. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conceição EPS, Madden CJ, Morrison SF. Tonic inhibition of brown adipose tissue sympathetic nerve activity via muscarinic acetylcholine receptors in the rostral raphe pallidus. J Physiol 595: 7495–7508, 2017. doi: 10.1113/JP275299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daan S, Barnes BM, Strijkstra AM. Warming up for sleep? Ground squirrels sleep during arousals from hibernation. Neurosci Lett 128: 265–268, 1991. doi: 10.1016/0304-3940(91)90276-Y. [DOI] [PubMed] [Google Scholar]
- 30.Dahl G. ATP release through pannexon channels. Philos Trans R Soc Lond B Biol Sci 370: 20140191, 2015. doi: 10.1098/rstb.2014.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 32.Dark J, Pelz KM. NPY Y1 receptor antagonist prevents NPY-induced torpor-like hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 294: R236–R245, 2008. doi: 10.1152/ajpregu.00587.2007. [DOI] [PubMed] [Google Scholar]
- 33.Deboer T, Tobler I. Natural hypothermia and sleep deprivation: common effects on recovery sleep in the Djungarian hamster. Am J Physiol 271: R1364–R1371, 1996. [DOI] [PubMed] [Google Scholar]
- 34.Deboer T, Tobler I. Sleep regulation in the Djungarian hamster: comparison of the dynamics leading to the slow-wave activity increase after sleep deprivation and daily torpor. Sleep 26: 567–572, 2003. doi: 10.1093/sleep/26.5.567. [DOI] [PubMed] [Google Scholar]
- 35.Deboer T, Tobler I. Slow waves in the sleep electroencephalogram after daily torpor are homeostatically regulated. Neuroreport 11: 881–885, 2000. doi: 10.1097/00001756-200003200-00044. [DOI] [PubMed] [Google Scholar]
- 36.Deboer T, Tobler I. Temperature dependence of EEG frequencies during natural hypothermia. Brain Res 670: 153–156, 1995. doi: 10.1016/0006-8993(94)01299-W. [DOI] [PubMed] [Google Scholar]
- 37.Del Vecchio F, Nalivaiko E, Cerri M, Luppi M, Amici R. Provocative motion causes fall in brain temperature and affects sleep in rats. Exp Brain Res 232: 2591–2599, 2014. doi: 10.1007/s00221-014-3899-8. [DOI] [PubMed] [Google Scholar]
- 38.Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax 37: 840–844, 1982. doi: 10.1136/thx.37.11.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Easton A, Meerlo P, Bergmann B, Turek FW. The suprachiasmatic nucleus regulates sleep timing and amount in mice. Sleep 27: 1307–1318, 2004. doi: 10.1093/sleep/27.7.1307. [DOI] [PubMed] [Google Scholar]
- 40.Eguchi K, Satoh T. Convergence of sleep-wakefulness subsystems onto single neurons in the region of cat’s solitary tract nucleus. Arch Ital Biol 118: 331–345, 1980. [PubMed] [Google Scholar]
- 41.Eisner C, Kim S, Grill A, Qin Y, Hoerl M, Briggs J, Castrop H, Thiel M, Schnermann J. Profound hypothermia after adenosine kinase inhibition in A1AR-deficient mice suggests a receptor-independent effect of intracellular adenosine. Pflugers Arch 469: 339–347, 2017. doi: 10.1007/s00424-016-1925-3. [DOI] [PubMed] [Google Scholar]
- 42.el Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res 76: 519–529, 1989. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- 43.Farrow JT, Hebert JR. Breath suspension during the transcendental meditation technique. Psychosom Med 44: 133–153, 1982. doi: 10.1097/00006842-198205000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Fredholm BB. Adenosine–a physiological or pathophysiological agent? J Mol Med (Berl) 92: 201–206, 2014. doi: 10.1007/s00109-013-1101-6. [DOI] [PubMed] [Google Scholar]
- 45.Fredholm BB, Ijzerman AP, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol Rev 63: 1–34, 2011. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita T, Chen MJ, Li B, Smith NA, Peng W, Sun W, Toner MJ, Kress BT, Wang L, Benraiss A, Takano T, Wang S, Nedergaard M. Neuronal transgene expression in dominant-negative SNARE mice. J Neurosci 34: 16594–16604, 2014. doi: 10.1523/JNEUROSCI.2585-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furilla RA, Jones DR. The relationship between dive and pre-dive heart rates in restrained and free dives by diving ducks. J Exp Biol 127: 333–348, 1987. [Google Scholar]
- 48.Futatsuki T, Yamashita A, Ikbar KN, Yamanaka A, Arita K, Kakihana Y, Kuwaki T. Involvement of orexin neurons in fasting- and central adenosine-induced hypothermia. Sci Rep 8: 2717, 2018. doi: 10.1038/s41598-018-21252-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gass N, Porkka-Heiskanen T, Kalinchuk AV. The role of the basal forebrain adenosine receptors in sleep homeostasis. Neuroreport 20: 1013–1018, 2009. doi: 10.1097/WNR.0b013e32832d5859. [DOI] [PubMed] [Google Scholar]
- 50.Gluck EF, Stephens N, Swoap SJ. Peripheral ghrelin deepens torpor bouts in mice through the arcuate nucleus neuropeptide Y signaling pathway. Am J Physiol Regul Integr Comp Physiol 291: R1303–R1309, 2006. doi: 10.1152/ajpregu.00232.2006. [DOI] [PubMed] [Google Scholar]
- 51.Griko Y, Regan MD. Synthetic torpor: a method for safely and practically transporting experimental animals aboard spaceflight missions to deep space. Life Sci Space Res (Amst) 16: 101–107, 2018. doi: 10.1016/j.lssr.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61: 213–219, 2009. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harden LM, Rummel C, Laburn HP, Damm J, Wiegand F, Poole S, Gerstberger R, Roth J. Critical role for peripherally-derived interleukin-10 in mediating the thermoregulatory manifestations of fever and hypothermia in severe forms of lipopolysaccharide-induced inflammation. Pflugers Arch 466: 1451–1466, 2014. doi: 10.1007/s00424-013-1371-4. [DOI] [PubMed] [Google Scholar]
- 54.Harris DV, Walker JM, Berger RJ. A continuum of slow-wave sleep and shallow torpor in the pocket mouse Perognathus longimembris. Physiol Zool 57: 428–434, 1984. doi: 10.1086/physzool.57.4.30163344. [DOI] [Google Scholar]
- 55.Hayaishi O, Urade Y, Eguchi N, Huang ZL. Genes for prostaglandin d synthase and receptor as well as adenosine A2A receptor are involved in the homeostatic regulation of nrem sleep. Arch Ital Biol 142: 533–539, 2004. [PubMed] [Google Scholar]
- 56.Heller HC, Ruby NF. Sleep and circadian rhythms in mammalian torpor. Annu Rev Physiol 66: 275–289, 2004. doi: 10.1146/annurev.physiol.66.032102.115313. [DOI] [PubMed] [Google Scholar]
- 57.Heller HC, Walker JM, Florant GL, Glotzbach SF, Berger RJ. Sleep and hibernation: electrophysiological and thermoregulatory homologies. In: Strategies in Cold, edited by Hudson JW. Cambridge, MA: Academic Press, 1978, p. 225–265. doi: 10.1016/B978-0-12-734550-5.50012-0. [DOI] [Google Scholar]
- 58.Herwig A, Ivanova EA, Lydon H, Barrett P, Steinlechner S, Loudon AS. Histamine H3 receptor and orexin A expression during daily torpor in the Djungarian hamster (Phodopus sungorus). J Neuroendocrinol 19: 1001–1007, 2007. doi: 10.1111/j.1365-2826.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 59.Herwig A, Revel F, Saboureau M, Pévet P, Steinlechner S. Daily torpor alters multiple gene expression in the suprachiasmatic nucleus and pineal gland of the Djungarian hamster (Phodopus sungorus). Chronobiol Int 23: 269–276, 2006. doi: 10.1080/07420520500522424. [DOI] [PubMed] [Google Scholar]
- 60.Horwitz BA, Chau SM, Hamilton JS, Song C, Gorgone J, Saenz M, Horowitz JM, Chen (陳昭吟) C-Y. Temporal relationships of blood pressure, heart rate, baroreflex function, and body temperature change over a hibernation bout in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 305: R759–R768, 2013. doi: 10.1152/ajpregu.00450.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci 8: 858–859, 2005. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 62.Hudson JW, Scott IM. Daily torpor in the laboratory mouse, Mus musculus Var Albino. Physiol Zool 52: 205–218, 1979. doi: 10.1086/physzool.52.2.30152564. [DOI] [Google Scholar]
- 63.Hut RA, Pilorz V, Boerema AS, Strijkstra AM, Daan S. Working for food shifts nocturnal mouse activity into the day. PLoS One 6: e17527, 2011. doi: 10.1371/journal.pone.0017527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iliff BW, Swoap SJ. Central adenosine receptor signaling is necessary for daily torpor in mice. Am J Physiol Regul Integr Comp Physiol 303: R477–R484, 2012. doi: 10.1152/ajpregu.00081.2012. [DOI] [PubMed] [Google Scholar]
- 65.Janssen BJA, Tyssen CM, Duindam H, Rietveld WJ. Suprachiasmatic lesions eliminate 24-h blood pressure variability in rats. Physiol Behav 55: 307–311, 1994. doi: 10.1016/0031-9384(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 66.Jeong JH, Lee DK, Blouet C, Ruiz HH, Buettner C, Chua S Jr, Schwartz GJ, Jo YH. Cholinergic neurons in the dorsomedial hypothalamus regulate mouse brown adipose tissue metabolism. Mol Metab 4: 483–492, 2015. doi: 10.1016/j.molmet.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jevning R, Wallace RK, Beidebach M. The physiology of meditation: a review. A wakeful hypometabolic integrated response. Neurosci Biobehav Rev 16: 415–424, 1992. doi: 10.1016/S0149-7634(05)80210-6. [DOI] [PubMed] [Google Scholar]
- 68.Jinka TR, Carlson ZA, Moore JT, Drew KL. Altered thermoregulation via sensitization of A1 adenosine receptors in dietary-restricted rats. Psychopharmacology (Berl) 209: 217–224, 2010. doi: 10.1007/s00213-010-1778-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jinka TR, Combs VM, Drew KL. Translating drug-induced hibernation to therapeutic hypothermia. ACS Chem Neurosci 6: 899–904, 2015. doi: 10.1021/acschemneuro.5b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jinka TR, Rasley BT, Drew KL. Inhibition of NMDA-type glutamate receptors induces arousal from torpor in hibernating arctic ground squirrels (Urocitellus parryii). J Neurochem 122: 934–940, 2012. doi: 10.1111/j.1471-4159.2012.07832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jinka TR, Tøien Ø, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. J Neurosci 31: 10752–10758, 2011. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones CA, Perez E, Amici R, Luppi M, Baracchi F, Cerri M, Dentico D, Zamboni G. Lithium affects REM sleep occurrence, autonomic activity and brain second messengers in the rat. Behav Brain Res 187: 254–261, 2008. doi: 10.1016/j.bbr.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 73.Kayaba M, Park I, Iwayama K, Seya Y, Ogata H, Yajima K, Satoh M, Tokuyama K. Energy metabolism differs between sleep stages and begins to increase prior to awakening. Metabolism 69: 14–23, 2017. doi: 10.1016/j.metabol.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 74.Khatri IM, Freis ED. Hemodynamic changes during sleep. J Appl Physiol 22: 867–873, 1967. doi: 10.1152/jappl.1967.22.5.867. [DOI] [PubMed] [Google Scholar]
- 75.Kim T, Ramesh V, Dworak M, Choi DS, McCarley RW, Kalinchuk AV, Basheer R. Disrupted sleep-wake regulation in type 1 equilibrative nucleoside transporter knockout mice. Neuroscience 303: 211–219, 2015. doi: 10.1016/j.neuroscience.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kovalzon VM, Moiseenko LS, Ambaryan AV, Kurtenbach S, Shestopalov VI, Panchin YV. Sleep-wakefulness cycle and behavior in pannexin1 knockout mice. Behav Brain Res 318: 24–27, 2017. doi: 10.1016/j.bbr.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 77.Krauchi K, Deboer T. The interrelationship between sleep regulation and thermoregulation. Front Biosci (Landmark Ed) 15: 604–625, 2010. doi: 10.2741/3636. [DOI] [PubMed] [Google Scholar]
- 78.Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol 267: R819–R829, 1994. [DOI] [PubMed] [Google Scholar]
- 79.Krueger JM, Taishi P, De A, Davis CJ, Winters BD, Clinton J, Szentirmai E, Zielinski MR. ATP and the purine type 2 X7 receptor affect sleep. J Appl Physiol (1985) 109: 1318–1327, 2010. doi: 10.1152/japplphysiol.00586.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Larkin JE, Heller HC. Sleep after arousal from hibernation is not homeostatically regulated. Am J Physiol 276: R522–R529, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Larkin JE, Heller HC. Temperature sensitivity of sleep homeostasis during hibernation in the golden-mantled ground squirrel. Am J Physiol Regul Integr Comp Physiol 270: R777–R784, 1996. [DOI] [PubMed] [Google Scholar]
- 82.Lenzi P, Cianci T, Leonardi GS, Martinelli A, Franzini C. Muscle blood flow changes during sleep as a function of fibre type composition. Exp Brain Res 74: 549–554, 1989. doi: 10.1007/BF00247356. [DOI] [PubMed] [Google Scholar]
- 83.Levy MN. Sympathetic-parasympathetic interactions in the heart. Circ Res 29: 437–445, 1971. doi: 10.1161/01.RES.29.5.437. [DOI] [PubMed] [Google Scholar]
- 84.Lewis JE, Ebling FJ. Tanycytes as regulators of seasonal cycles in neuroendocrine function. Front Neurol 8: 79, 2017. doi: 10.3389/fneur.2017.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lin YC. Autonomic nervous control of cardiovascular response during diving in the rat. Am J Physiol 227: 601–605, 1974. [DOI] [PubMed] [Google Scholar]
- 86.Liu E, Lewis K, Al-Saffar H, Krall CM, Singh A, Kulchitsky VA, Corrigan JJ, Simons CT, Petersen SR, Musteata FM, Bakshi CS, Romanovsky AA, Sellati TJ, Steiner AA. Naturally occurring hypothermia is more advantageous than fever in severe forms of lipopolysaccharide- and Escherichia coli-induced systemic inflammation. Am J Physiol Regul Integr Comp Physiol 302: R1372–R1383, 2012. doi: 10.1152/ajpregu.00023.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lo Martire V, Silvani A, Alvente S, Bastianini S, Berteotti C, Valli A, Zoccoli G. Modulation of sympathetic vasoconstriction is critical for the effects of sleep on arterial pressure in mice. J Physiol 596: 591–608, 2018. doi: 10.1113/JP275353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lyman CP, O’Brien RC. Circulatory changes in the thirteen-lined ground squirrel during the hibernating cycle. Bull Mus Comp Zool Harv 124: 353–372, 1960. [Google Scholar]
- 89.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 566: 559–573, 2005. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R831–R843, 2009. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Madden CJ, Tupone D, Cano G, Morrison SF. α2 Adrenergic receptor-mediated inhibition of thermogenesis. J Neurosci 33: 2017–2028, 2013. doi: 10.1523/JNEUROSCI.4701-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Magnifico F, Pierangeli G, Barletta G, Candela C, Montagna P, Bonavina G, Cortelli P. Paroxysmal episodic central thermoregulatory failure. Neurology 58: 1300–1302, 2002. doi: 10.1212/WNL.58.8.1300. [DOI] [PubMed] [Google Scholar]
- 93.Mancia G, Baccelli G, Zanchetti A. Regulation of renal circulation during behavioral changes in the cat. Am J Physiol 227: 536–542, 1974. [DOI] [PubMed] [Google Scholar]
- 94.McCulloch PF. Animal models for investigating the central control of the Mammalian diving response. Front Physiol 3: 169, 2012. doi: 10.3389/fphys.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCulloch PF, Dinovo KM, Connolly TM. The cardiovascular and endocrine responses to voluntary and forced diving in trained and untrained rats. Am J Physiol Regul Integr Comp Physiol 298: R224–R234, 2010. doi: 10.1152/ajpregu.00592.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meir JU, Stockard TK, Williams CL, Ponganis KV, Ponganis PJ. Heart rate regulation and extreme bradycardia in diving emperor penguins. J Exp Biol 211: 1169–1179, 2008. doi: 10.1242/jeb.013235. [DOI] [PubMed] [Google Scholar]
- 97.Menétrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J Comp Neurol 255: 439–450, 1987. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- 98.Methippara MM, Kumar S, Alam MN, Szymusiak R, McGinty D. Effects on sleep of microdialysis of adenosine A1 and A2a receptor analogs into the lateral preoptic area of rats. Am J Physiol Regul Integr Comp Physiol 289: R1715–R1723, 2005. doi: 10.1152/ajpregu.00247.2005. [DOI] [PubMed] [Google Scholar]
- 99.Milsom B. Breathless–by choice. Biologist (London) 47: 239–242, 2000. [PubMed] [Google Scholar]
- 100.Milsom WK, Jackson DC. Hibernation and gas exchange. In: Comprehensive Physiology. Hoboken, NJ: John Wiley & Sons, Inc, 2011. doi: 10.1002/cphy.c090018. [DOI] [PubMed] [Google Scholar]
- 101.Milsom WK, Zimmer MB, Harris MB. Vagal control of cardiorespiratory function in hibernation. Exp Physiol 86: 791–796, 2001. doi: 10.1111/j.1469-445X.2001.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 102.Morhardt JE. Heart rates, breathing rates and the effects of atropine and acetylcholine on white-footed mice (Peromyscus sp.) during daily torpor. Comp Biochem Physiol 33: 441–457, 1970. doi: 10.1016/0010-406X(70)90360-9. [DOI] [PubMed] [Google Scholar]
- 103.Morrison SF. Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci 196: 14–24, 2016. doi: 10.1016/j.autneu.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 16: 74–104, 2011. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci 22: 4600–4610, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 292: R127–R136, 2007. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nalivaiko E, De Pasquale CG, Blessing WW. Electrocardiographic changes associated with the nasopharyngeal reflex in conscious rabbits: vago-sympathetic co-activation. Auton Neurosci 105: 101–104, 2003. doi: 10.1016/S1566-0702(03)00048-1. [DOI] [PubMed] [Google Scholar]
- 109.Newby AC. Adenosine and the concept of ‘retaliatory metabolites’. Trends Biochem Sci 9: 42–44, 1984. doi: 10.1016/0968-0004(84)90176-2. [DOI] [Google Scholar]
- 110.Ngampramuan S, Cerri M, Del Vecchio F, Corrigan JJ, Kamphee A, Dragic AS, Rudd JA, Romanovsky AA, Nalivaiko E. Thermoregulatory correlates of nausea in rats and musk shrews. Oncotarget 5: 1565–1575, 2014. doi: 10.18632/oncotarget.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O’Brien WG III, Ling HS, Zhao Z, Lee CC. New insights on the regulation of the adenine nucleotide pool of erythrocytes in mouse models. PLoS One 12: e0180948, 2017. doi: 10.1371/journal.pone.0180948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oelkrug R, Heldmaier G, Meyer CW. Torpor patterns, arousal rates, and temporal organization of torpor entry in wildtype and UCP1-ablated mice. J Comp Physiol B 181: 137–145, 2011. doi: 10.1007/s00360-010-0503-9. [DOI] [PubMed] [Google Scholar]
- 113.Oishi Y, Xu Q, Wang L, Zhang BJ, Takahashi K, Takata Y, Luo YJ, Cherasse Y, Schiffmann SN, de Kerchove d’Exaerde A, Urade Y, Qu WM, Huang ZL, Lazarus M. Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat Commun 8: 734, 2017. doi: 10.1038/s41467-017-00781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ollenberger GP, Matte G, Wilkinson AA, West NH. Relative distribution of blood flow in rats during surface and submerged swimming. Comp Biochem Physiol A Mol Integr Physiol 119: 271–277, 1998. doi: 10.1016/S1095-6433(97)00427-3. [DOI] [PubMed] [Google Scholar]
- 115.Oosterhof H, Lander M, Aarnoudse JG. Behavioural states and Doppler velocimetry of the renal artery in the near term human fetus. Early Hum Dev 33: 183–189, 1993. doi: 10.1016/0378-3782(93)90144-J. [DOI] [PubMed] [Google Scholar]
- 116.Palchykova S, Crestani F, Meerlo P, Tobler I. Sleep deprivation and daily torpor impair object recognition in Djungarian hamsters. Physiol Behav 87: 144–153, 2006. doi: 10.1016/j.physbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Palchykova S, Deboer T, Tobler I. Selective sleep deprivation after daily torpor in the Djungarian hamster. J Sleep Res 11: 313–319, 2002. doi: 10.1046/j.1365-2869.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 118.Palchykova S, Winsky-Sommerer R, Shen HY, Boison D, Gerling A, Tobler I. Manipulation of adenosine kinase affects sleep regulation in mice. J Neurosci 30: 13157–13165, 2010. doi: 10.1523/JNEUROSCI.1359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Panneton WM. The mammalian diving response: an enigmatic reflex to preserve life? Physiology (Bethesda) 28: 284–297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Panneton WM, Gan Q, Juric R. The rat: a laboratory model for studies of the diving response. J Appl Physiol (1985) 108: 811–820, 2010. doi: 10.1152/japplphysiol.00600.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paton JFR, Nalivaiko E, Boscan P, Pickering AE. Reflexly evoked coactivation of cardiac vagal and sympathetic motor outflows: observations and functional implications. Clin Exp Pharmacol Physiol 33: 1245–1250, 2006. doi: 10.1111/j.1440-1681.2006.04518.x. [DOI] [PubMed] [Google Scholar]
- 122.Peng L, Huang R, Yu AC, Fung KY, Rathbone MP, Hertz L. Nucleoside transporter expression and function in cultured mouse astrocytes. Glia 52: 25–35, 2005. doi: 10.1002/glia.20216. [DOI] [PubMed] [Google Scholar]
- 123.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18: 9996–10015, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ponganis PJ. Diving Physiology of Marine Mammals and Seabirds. Cambridge, UK: Cambridge University Press, 2015. doi: 10.1017/CBO9781139045490. [DOI] [Google Scholar]
- 125.Ponganis PJ, McDonald BI, Tift MS, Williams CL. Heart rate regulation in diving sea lions: the vagus nerve rules. J Exp Biol 220: 1372–1381, 2017. doi: 10.1242/jeb.146779. [DOI] [PubMed] [Google Scholar]
- 126.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience 99: 507–517, 2000. doi: 10.1016/S0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 127.Ramirez JM, Folkow LP, Blix AS. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu Rev Physiol 69: 113–143, 2007. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- 128.Rayner DV. The sympathetic nervous system in white adipose tissue regulation. Proc Nutr Soc 60: 357–364, 2001. doi: 10.1079/PNS2001101. [DOI] [PubMed] [Google Scholar]
- 129.Riedel CS, Georg B, Jørgensen HL, Hannibal J, Fahrenkrug J. Mice lacking EGR1 have impaired clock gene (BMAL1) oscillation, locomotor activity, and body temperature. J Mol Neurosci 64: 9–19, 2018. doi: 10.1007/s12031-017-0996-8. [DOI] [PubMed] [Google Scholar]
- 130.Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, Zylka MJ. AMP is an adenosine A1 receptor agonist. J Biol Chem 287: 5301–5309, 2012. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 10: 2193–2216, 2005. doi: 10.2741/1690. [DOI] [PubMed] [Google Scholar]
- 132.Rybka EJ, McCulloch PF. The anterior ethmoidal nerve is necessary for the initiation of the nasopharyngeal response in the rat. Brain Res 1075: 122–132, 2006. doi: 10.1016/j.brainres.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 133.Saito H, Sakai K, Jouvet M. Discharge patterns of the nucleus parabrachialis lateralis neurons of the cat during sleep and waking. Brain Res 134: 59–72, 1977. doi: 10.1016/0006-8993(77)90925-8. [DOI] [PubMed] [Google Scholar]
- 134.Sallmen T, Beckman AL, Stanton TL, Eriksson KS, Tarhanen J, Tuomisto L, Panula P. Major changes in the brain histamine system of the ground squirrel Citellus lateralis during hibernation. J Neurosci 19: 1824–1835, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sallmen T, Lozada AF, Anichtchik OV, Beckman AL, Leurs R, Panula P. Changes in hippocampal histamine receptors across the hibernation cycle in ground squirrels. Hippocampus 13: 745–754, 2003. doi: 10.1002/hipo.10120. [DOI] [PubMed] [Google Scholar]
- 136.Sallmen T, Lozada AF, Anichtchik OV, Beckman AL, Panula P. Increased brain histamine H3 receptor expression during hibernation in golden-mantled ground squirrels. BMC Neurosci 4: 24, 2003. doi: 10.1186/1471-2202-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron 93: 747–765, 2017. doi: 10.1016/j.neuron.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 139.Sekizawa S, Horowitz JM, Horwitz BA, Chen CY. Realignment of signal processing within a sensory brainstem nucleus as brain temperature declines in the Syrian hamster, a hibernating species. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 198: 267–282, 2012. doi: 10.1007/s00359-011-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sekizawa S, Horwitz BA, Horowitz JM, Chen CY. Protection of signal processing at low temperature in baroreceptive neurons in the nucleus tractus solitarius of Syrian hamsters, a hibernating species. Am J Physiol Regul Integr Comp Physiol 305: R1153–R1162, 2013. doi: 10.1152/ajpregu.00165.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci 18: 4705–4721, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Signore PE, Jones DR. Effect of pharmacological blockade on cardiovascular responses to voluntary and forced diving in muskrats. J Exp Biol 198: 2307–2315, 1995. [DOI] [PubMed] [Google Scholar]
- 143.Silvani A, Asti V, Berteotti C, Ferrari V, Franzini C, Lenzi P, Wild J, Grant DA, Walker AM, Zoccoli G. Sleep-dependent changes in cerebral oxygen consumption in newborn lambs. J Sleep Res 15: 206–211, 2006. doi: 10.1111/j.1365-2869.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 144.Silvani A, Berteotti C, Bastianini S, Cohen G, Lo Martire V, Mazza R, Pagotto U, Quarta C, Zoccoli G. Cardiorespiratory anomalies in mice lacking CB1 cannabinoid receptors. PLoS One 9: e100536, 2014. doi: 10.1371/journal.pone.0100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Silvani A, Dampney RA. Central control of cardiovascular function during sleep. Am J Physiol Heart Circ Physiol 305: H1683–H1692, 2013. doi: 10.1152/ajpheart.00554.2013. [DOI] [PubMed] [Google Scholar]
- 146.So HY. Therapeutic hypothermia. Korean J Anesthesiol 59: 299–304, 2010. doi: 10.4097/kjae.2010.59.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328: 303–307, 1993. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 148.Sparling CE, Fedak MA. Metabolic rates of captive grey seals during voluntary diving. J Exp Biol 207: 1615–1624, 2004. doi: 10.1242/jeb.00952. [DOI] [PubMed] [Google Scholar]
- 149.Steiner AA, Flatow EA, Brito CF, Fonseca MT, Komegae EN. Respiratory gas exchange as a new aid to monitor acidosis in endotoxemic rats: relationship to metabolic fuel substrates and thermometabolic responses. Physiol Rep 5: e13100, 2017. doi: 10.14814/phy2.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res 12: 283–290, 2003. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 151.Strijkstra AM, Daan S. Ambient temperature during torpor affects NREM sleep EEG during arousal episodes in hibernating European ground squirrels. Neurosci Lett 221: 177–180, 1997. doi: 10.1016/S0304-3940(96)13321-8. [DOI] [PubMed] [Google Scholar]
- 152.Strijkstra AM, Daan S. Dissimilarity of slow-wave activity enhancement by torpor and sleep deprivation in a hibernator. Am J Physiol Regul Integr Comp Physiol 275: R1110–R1117, 1998. [DOI] [PubMed] [Google Scholar]
- 153.Sun Y, Huang P. Adenosine A2B receptor: from cell biology to human diseases. Front Chem 4: 37, 2016. doi: 10.3389/fchem.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Swoap SJ. The pharmacology and molecular mechanisms underlying temperature regulation and torpor. Biochem Pharmacol 76: 817–824, 2008. doi: 10.1016/j.bcp.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Swoap SJ, Gutilla MJ. Cardiovascular changes during daily torpor in the laboratory mouse. Am J Physiol Regul Integr Comp Physiol 297: R769–R774, 2009. doi: 10.1152/ajpregu.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The full expression of fasting-induced torpor requires beta 3-adrenergic receptor signaling. J Neurosci 26: 241–245, 2006. doi: 10.1523/JNEUROSCI.3721-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Swoap SJ, Körtner G, Geiser F. Heart rate dynamics in a marsupial hibernator. J Exp Biol 220: 2939–2946, 2017. doi: 10.1242/jeb.155879. [DOI] [PubMed] [Google Scholar]
- 158.Swoap SJ, Rathvon M, Gutilla M. AMP does not induce torpor. Am J Physiol Regul Integr Comp Physiol 293: R468–R473, 2007. doi: 10.1152/ajpregu.00888.2006. [DOI] [PubMed] [Google Scholar]
- 159.Swoap SJ, Weinshenker D. Norepinephrine controls both torpor initiation and emergence via distinct mechanisms in the mouse. PLoS One 3: e4038, 2008. doi: 10.1371/journal.pone.0004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Szymusiak R, Satinoff E. Acute thermoregulatory effects of unilateral electrolytic lesions of the medial and lateral preoptic area in rats. Physiol Behav 28: 161–170, 1982. doi: 10.1016/0031-9384(82)90118-4. [DOI] [PubMed] [Google Scholar]
- 161.Tabachnik E, Muller NL, Bryan AC, Levison H. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol Respir Environ Exerc Physiol 51: 557–564, 1981. [DOI] [PubMed] [Google Scholar]
- 162.Takahashi Y, Zhang W, Sameshima K, Kuroki C, Matsumoto A, Sunanaga J, Kono Y, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for prostaglandin E2-induced fever and defence against environmental cooling in mice. J Physiol 591: 5623–5643, 2013. doi: 10.1113/jphysiol.2013.261271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takeuchi S, Iwase S, Mano T, Okada H, Sugiyama Y, Watanabe T. Sleep-related changes in human muscle and skin sympathetic nerve activities. J Auton Nerv Syst 47: 121–129, 1994. doi: 10.1016/0165-1838(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 164.Tamura Y, Shintani M, Inoue H, Monden M, Shiomi H. Regulatory mechanism of body temperature in the central nervous system during the maintenance phase of hibernation in Syrian hamsters: involvement of β-endorphin. Brain Res 1448: 63–70, 2012. doi: 10.1016/j.brainres.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 165.Tang Y-Y, Ma Y, Fan Y, Feng H, Wang J, Feng S, Lu Q, Hu B, Lin Y, Li J, Zhang Y, Wang Y, Zhou L, Fan M. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci USA 106: 8865–8870, 2009. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taylor EW, Al-Ghamdi MS, Ihmied IH, Wang T, Abe AS. The neuranatomical basis of central control of cardiorespiratory interactions in vertebrates. Exp Physiol 86: 771–776, 2001. doi: 10.1111/j.1469-445X.2001.tb00043.x. [DOI] [PubMed] [Google Scholar]
- 167.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci 23: 4278–4287, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Trachsel L, Edgar DM, Heller HC. Are ground squirrels sleep deprived during hibernation? Am J Physiol Regul Integr Comp Physiol 260: R1123–R1129, 1991. [DOI] [PubMed] [Google Scholar]
- 169.Tupone D, Cano G, Morrison SF. Thermoregulatory inversion: a novel thermoregulatory paradigm. Am J Physiol Regul Integr Comp Physiol 312: R779–R786, 2017. doi: 10.1152/ajpregu.00022.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci 33: 14512–14525, 2013. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Uschakov A, Gong H, McGinty D, Szymusiak R. Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur J Neurosci 23: 3284–3296, 2006. doi: 10.1111/j.1460-9568.2006.04860.x. [DOI] [PubMed] [Google Scholar]
- 172.Vicent MA, Borre ED, Swoap SJ. Central activation of the A1 adenosine receptor in fed mice recapitulates only some of the attributes of daily torpor. J Comp Physiol B 187: 835–845, 2017. doi: 10.1007/s00360-017-1084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Walker JM, Garber A, Berger RJ, Heller HC. Sleep and estivation (shallow torpor): continuous processes of energy conservation. Science 204: 1098–1100, 1979. doi: 10.1126/science.221974. [DOI] [PubMed] [Google Scholar]
- 174.Walker JM, Glotzbach SF, Berger RJ, Heller HC. Sleep and hibernation in ground squirrels (Citellus spp): electrophysiological observations. Am J Physiol Regul Integr Comp Physiol 233: R213–R221, 1977. [DOI] [PubMed] [Google Scholar]
- 175.Walker JM, Haskell EH, Berger RJ, Heller HC. Hibernation at moderate temperatures: a continuation of slow wave sleep. Experientia 37: 726–728, 1981. doi: 10.1007/BF01967947. [DOI] [PubMed] [Google Scholar]
- 176.Wu Q, Lemus MB, Stark R, Bayliss JA, Reichenbach A, Lockie SH, Andrews ZB. The temporal pattern of cfos activation in hypothalamic, cortical, and brainstem nuclei in response to fasting and refeeding in male mice. Endocrinology 155: 840–853, 2014. doi: 10.1210/en.2013-1831. [DOI] [PubMed] [Google Scholar]
- 177.Yoshimoto M, Sakagami T, Nagura S, Miki K. Relationship between renal sympathetic nerve activity and renal blood flow during natural behavior in rats. Am J Physiol Regul Integr Comp Physiol 286: R881–R887, 2004. doi: 10.1152/ajpregu.00105.2002. [DOI] [PubMed] [Google Scholar]
- 178.Young JD, Taylor E. Meditation as a voluntary hypometabolic state of biological estivation. News Physiol Sci 13: 149–153, 1998. [DOI] [PubMed] [Google Scholar]
- 179.Yuan XS, Wang L, Dong H, Qu WM, Yang SR, Cherasse Y, Lazarus M, Schiffmann SN, d’Exaerde AK, Li RX, Huang ZL. Striatal adenosine A2A receptor neurons control active-period sleep via parvalbumin neurons in external globus pallidus. eLife 6: e29055, 2017. doi: 10.7554/eLife.29055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zamboni G, Jones CA, Domeniconi R, Amici R, Perez E, Luppi M, Cerri M, Parmeggiani PL. Specific changes in cerebral second messenger accumulation underline REM sleep inhibition induced by the exposure to low ambient temperature. Brain Res 1022: 62–70, 2004. doi: 10.1016/j.brainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 181.Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol 581: 649–663, 2007. doi: 10.1113/jphysiol.2007.129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zielinski MR, Taishi P, Clinton JM, Krueger JM. 5′-Ectonucleotidase-knockout mice lack non-REM sleep responses to sleep deprivation. Eur J Neurosci 35: 1789–1798, 2012. doi: 10.1111/j.1460-9568.2012.08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zosky GR. The parasympathetic nervous system: its role during torpor in the fat-tailed dunnart (Sminthopsis crassicaudata). J Comp Physiol B 172: 677–684, 2002. doi: 10.1007/s00360-002-0295-7. [DOI] [PubMed] [Google Scholar]
- 184.Zosky GR, Larcombe AN. The parasympathetic nervous system and its influence on heart rate in torpid western pygmy possums, Cercatetus concinnus (Marsupialia: Burramyidae). Zoology (Jena) 106: 143–150, 2003. doi: 10.1078/0944-2006-00108. [DOI] [PubMed] [Google Scholar]