Abstract
Autophagy is a cellular digestion process that contributes to cellular homeostasis and adaptation by the elimination of proteins and damaged organelles. Evidence suggests that dysregulation of autophagy plays a role in neurodegenerative diseases, including motor neuron disorders. Herein, we review emerging evidence indicating the roles of autophagy in physiological motor neuron processes and its function in specific compartments. Moreover, we discuss the involvement of autophagy in the pathogenesis of motor neuron diseases, including spinal cord injury and aging, and recent developments that offer promising therapeutic approaches to mitigate effects of dysregulated autophagy in health and disease.
Introduction
Homeostasis and adaptation (plasticity) in cells requires tight control of cellular compartments and organelles with biogenesis and removal of damaged or excess components via coordinated cell-wide processes. Substantial new information is now available on a range of cellular processes involved in the maintenance of cell structure and function, and although many of these processes reflect common cellular pathways, distinct regulation is present in specialized cells. Autophagy is an evolutionary conserved process in eukaryotes that removes misfolded proteins, protein aggregates, and damaged organelles at baseline levels under normal conditions, and is of crucial importance for proper cell function (8, 78). Many of the molecular discoveries and current understanding of the regulation of autophagy came from analyses in the genetically tractable yeast system (9, 148). Indeed, the process of autophagy and the molecular components are highly conserved across the evolutionary scale. For example, the core autophagy proteins (19 proteins) encoded by autophagy-related genes (ATG) are conserved in eukariotes (30). Physiologically, autophagy plays an important role in homeostasis and ongoing cell turnover (9), and thus has been extensively studied in tissues and organs with rapid turnover, such as the liver and in cancer. However, the role of autophagy in postmitotic cells such as motor neurons is only incompletely understood.
Recent progress in understanding the contribution of dysregulated cellular processes to disease has revealed important roles for global cellular functions across various conditions. In particular, neurological disorders may reflect common mechanisms of cellular dysfunction resulting from various different triggering events. Emerging evidence now places autophagy, as a key regulator of cellular homeostasis and plasticity, on the central stage of disorders associated with motor neuron dysfunction.
Autophagy: A Multi-Step Pathway Important for Homeostasis
Autophagy comprises a series of catabolic processes that involves degradation of cytoplasmic components through lysosomal pathways facilitating the removal of damaged organelles and preventing accumulation of toxic proteins. Autophagy was initially described as a cell response to conditions of nutrient deprivation (starvation), but it is now recognized as a critical process that maintains homeostasis with links to cell metabolism, growth control, balance between cell survival and cell death, immune surveillance, degeneration, plasticity, and aging, among others (30, 104, 148).
There are three distinct classes of autophagy: 1) microautophagy, which refers to the direct invagination of lysosome membranes to engulf cytoplasmic components; 2) chaperone-mediated autophagy, where association of the lysosome membrane protein LAMP-2A with the Hsc70 chaperone mediates lysosomal degradation of intracellular proteins; and 3) macroautophagy (or autophagy, as will be referred to in this review), where the target cellular components are sequestered by a phagophore (an isolated membrane) that eventually forms into an autophagosome by sealing the cargo in a double-membrane structure (44, 134). Hydrolytic degradation of the target cellular components occurs when the autophagosome fuses with the lysosome (9, 102). Indeed, cellular components degraded in autophagosomes include mitochondria, peroxisomes, endoplasmic reticulum, endosomes, lysosomes, lipid droplets, secretory granules, cytoplasmic aggregates, ribosomes, and invading pathogens.
Autophagy can be divided into several stages: initiation (induction), autophagosome expansion and maturation, and degradation and recycling (FIGURE 1). The discovery of autophagy-related genes (ATG), stemming from the pioneering work by Ohsumi and his group (95), has led to the molecular characterization of the steps in the autophagy pathway. This section provides a brief overview of the molecular aspects of autophagy; more detailed reviews on the molecular regulation of autophagy are found elsewhere (75, 118, 133).
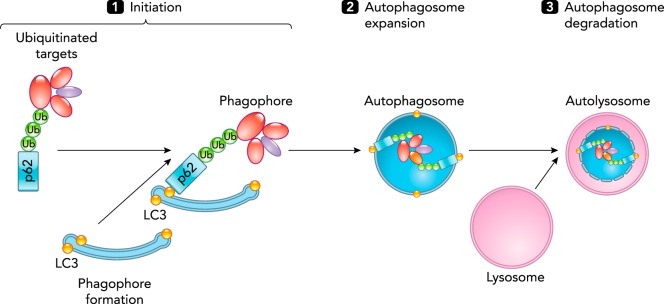
FIGURE 1.
Selective autophagy proceeds in several steps
1: initiation. When recognition of organelles and macromolecular components targeted for degradation occurs, and the phagophore formation begins with ULK1 activation. Ubiquitin-labeled proteins (Ub) and damaged organelles bind to p62 that then binds to LC3 present in the isolated membrane. 2: autophagosome membrane expansion. When phagophores fuse and become elongated. Elongation also involves the recruitment of vesicular membranes and budding from endoplasmic reticulum or mitochondria. As a result of autophagosome expansion, cargo destined for degradation via autophagy is surrounded and engulfed into an autophagosome (double membrane vesicle). 3: autophagosome degradation and recycling. When autophagosomes dock and fuse with lysosomes into an autolysosome. The autophagic body is released into the lysosomal lumen where hydrolases degrade the macromolecular components.
Initiation
In most cells, autophagy occurs at basal levels, but starvation and environmental stressors induce autophagy in various cell types. Autophagy is activated when the un-coordinated 51 (UNC-51)-like kinase1 (ULK1) is phosphorylated and forms a complex with ATG13 and FIP200 (focal adhesion kinase family interacting protein of 200 kD), initiating the formation of the phagophore. Two different kinases, the mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK), differentially regulate ULK1 activation (127). ULK is phosphorylated on two different sites by mTOR and AMPK (67). Cellular stress signals such as starvation suppress mTOR and activate AMPK, both of which result in activation of ULK1 via phosphorylation, albeit at different sites. In addition, AMPK may induce autophagy by inhibiting mTOR further (FIGURE 2). The activated ULK complex (ULK1-ATG13-FIP200) targets a class III PI3K complex, consisting of beclin 1 (ATG6 in yeast), vacuolar protein sorting 15 (VPS15), VPS34, and ATG14 to promote the local production of a pool of phosphatidylinositol 3-phosphate that is specific to autophagosomes (64). The sources to form the phagophore can come from the endoplasmic reticulum (ER), Golgi complex, mitochondria, or plasma membrane via clathrin-mediated endocytosis (3, 40, 50, 116).
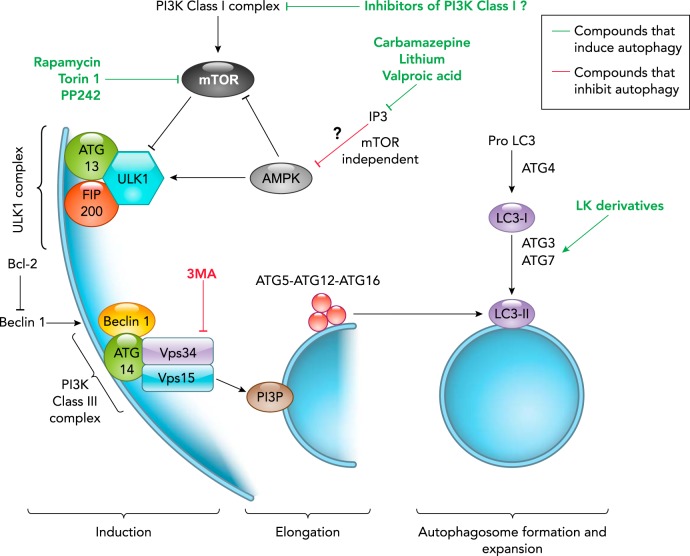
FIGURE 2.
Autophagy induction and possible mechanisms of action of chemical modulators
Autophagosome formation can be initiated via mTOR inhibition or AMPK activation that results in the activation of the ULK complex (ULK1-ATG13-FIP200). mTOR can be inhibited by specific inhibitors including rapamycin, Torin1, and PP242, which therefore induce autophagy. There are some autophagy-inducing agents such as Trehalose and lithium that act through a mTOR-independent pathway that has not been elucidated. After the ULK complex is activated, it targets a class III PI3K complex, consisting of beclin 1, VPS15, VPS34, and ATG14 to promote the local production of PI3P that is specific to autophagosomes. Class III PI3K activity can be inhibited by 3-methyladenine (3-MA), inhibiting autophagy. For autophagosome elongation and formation, the ATG12-ATG5-ATG16 localizes to the outer membrane, where it facilitates the lipidation of LC3 with phosphatidylethanolamine (PE). By cleavage of pro-LC3 by ATG4, LC3-I is formed. Then, LC3-I is processed by ATG7 and ATG3 to be conjugated to PE and form LC3-II. Lanthionine ketimine (LK) compounds induce conversion from LC3-I to LC3-II through a mechanism that is not completely known.
Autophagy can target degradation of specific cargoes such as mitochondria, peroxisomes, ribosomes, and protein aggregates through cargo-specific autophagy receptors that facilitate cargo sequestration into autophagosomes (30). These receptors commonly mediate targeted substrate binding to the microtubule-associated protein light chain 3 (LC3) isoforms present on newly developing autophagosomes (FIGURE 1). As a result, autophagy cargo receptors act as molecular bridges that capture ubiquitinated proteins targeted for degradation by the autophagy pathway and complement the UPS. There are several autophagic receptors that promote clearance of ubiquitinated proteins, peroxisomes, and even internalized pathogens (24, 30, 60). One autophagy cargo receptor that recognizes ubiquitin protein and has been associated with neurodegenerative diseases is the p62 protein, also called sequestosome 1 (SQSTM1). p62/SQSTM1 is commonly found in inclusion bodies containing polyubiquitinated protein aggregates and is degraded by autophagy (63, 106).
Autophagosome Expansion
Two ubiquitin-like conjugating systems are involved in the elongation of phagophores: the ATG12-ATG5-ATG16L system and the phosphatidylethanolamine (PE)-LC3 system. ATG12-ATG5-ATG16 localizes to the outer membrane, where it facilitates the lipidation of LC3 with PE (64). By cleavage of pro-LC3 by ATG4B, the cytosolic form of LC3 (LC3-I) is formed. Then, LC3-I is processed by ATG7 and ATG3 to be conjugated to PE and form LC3-II (FIGURE 2). Therefore, LC3-II puncta visualized by immunofluorescence reflect the number of autophagosomes. In selective autophagy, the specific interaction between p62/SQSTM1 and LC3 is instrumental in mediating autophagic degradation of the p62-positive bodies (106).
Autophagosome Degradation and Recycling
Autophagosomes are then transported in proximity with endosomes or lysosomes along microtubules by dynein-dynactin complexes (115). Autophagosomes fuse with lysosomes or late endosomes (that later fuse with lysosomes) during its retrograde transport. Therefore, in neurons, autophagosome retrograde transport is particularly important to complete the digestion process (59, 76). Through some non-ATG components and proteins that are also required for the formation of late endosomes (endosomal sorting complex, soluble N-ethylmaleimide-sensitive factor attachment proteins receptors, RAB proteins, and ATPases), the fusion with endosomes or lysosomes occurs (33, 77, 120). In lysosomes, degradation occurs with hydrolytic enzymes (proteases, lipases, nucleases), which are activated when the lysosomal lumen becomes acidified by the vacuolar ATPase (vATPase), a large protein complex on the lysosomal membrane that imports hydrogen ions (121). Lysosomes can also influence in the initial stages of autophagy through a signaling complex in the vATPase that includes the transcription factor EB (TFEB). During starvation (low amino acid concentration), mTOR activity and phosphorylation of TFEB are inhibited, which releases TFEB to translocate to the nucleus and induce transcription of genes that support increased autophagy (109, 149).
Autophagosome Trafficking and Transport in Neurons
Neurons are postmitotic cells, composed of different compartments: a soma or cell body, a dendritic arborization, and an axon. Neurons need to have a robust housekeeping process such as autophagy to ensure the quality and integrity of proteins and organelles in the cytoplasm. The detailed process of autophagy in these compartmentalized cells and the physiological relevance has only begun to be elucidated. Some recent work has investigated the compartmentalization of autophagy and autophagosome transport in neurons, but not specifically in motor neurons.
There is now evidence that autophagy pathways may occur preferentially across the various compartments in neurons. Using dual-color live-cell imaging, a recent study investigated the constitutive autophagosome initiation in cultured primary dorsal root ganglion and hippocampal neurons. Under basal conditions without starvation or environmental stressors, autophagosomes are generated predominantly in the presynaptic terminal and distal axon, with membranes and proteins coming from the ER rather than from plasma or mitochondrial-derived membranes (86).
Presynaptic terminals are particularly susceptible to accumulation of synaptic components as a result of repeated cycles of synaptic vesicle release and retrieval. Presynaptic terminals are thought to contain ~200 synaptic vesicles, ~300,000 proteins within a volume of ~0.4 μm3 (144). After an action potential, neurotransmitters are released at the synapse involving fusion of synaptic vesicles with the plasma membrane, followed by retrieval of fused vesicle membrane by endocytosis. The synaptic vesicle cycle can thus lead to accumulation of proteins and other membrane components that need to be degraded and recycled via autophagy to maintain the efficiency of neurotransmission and facilitate plasticity (e.g., strengthening) of synapses with sprouting and remodeling pre- and postsynaptically (FIGURE 3).
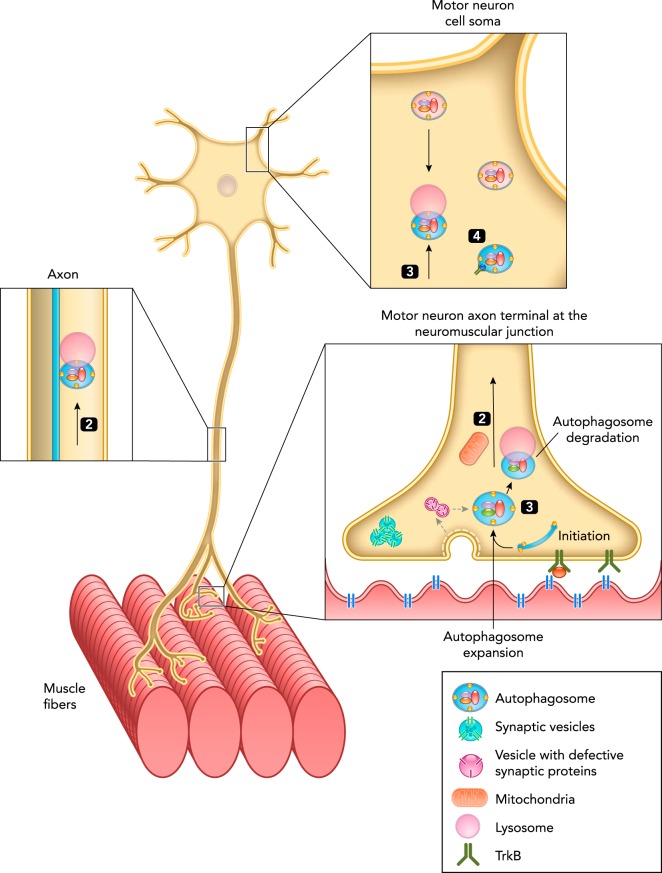
FIGURE 3.
Model for autophagy compartmentalization: autophagy initiation and formation in the axon terminal and autophagosome transport in motor neurons
At the axon terminal, defective synaptic proteins, synaptic components, and organelles, such as mitochondria, are recycled by autophagy. Synaptic vesicles are retrieved after neurotransmitter exocytosis by clathrin-mediated endocytosis and can be restored to the vesicle pool or recycle or end up in autophagosomes by a mechanism that remains elusive. Autophagosomes can fuse with lysosomes (1) locally at the axon terminal, or be transported retrogradely in the axon and fuse with lysosomes (2) during axon transport or at the cell soma (3). Autophagosomes can also serve as signaling structures from axon terminals to the cell soma, e.g., by containing BDNF bound to the TrkB receptor from axon terminals, dendrites, and the cell soma (4). This signaling role for autophagosomes links BDNF/TrkB signaling, defective autophagy, and neurodegeneration.
Autophagosomes are transported within axons to the soma in a dynein-dependent manner (17, 59), and enter the soma and remain confined within the somatodendritic domain (87), enabling fusion with lysosomes enriched in the soma. This retrograde trafficking of autophagosomes highlights the importance of evaluating autophagy across cell compartments in conditions of environmental stress or disease.
A recent study suggests another function for autophagosomes in neurons related to their retrograde axonal transport. Autophagosomes may convey neurotrophic signals from the presynaptic terminals to the cell soma (FIGURE 3). For instance, signaling endosomes containing the brain-derived neurotrophic factor (BDNF) receptor tropomyosin receptor kinase subtype B (TrkB) were in fact late-stage LC3/Rab7-positive autophagosomes (72). These findings highlight a link between autophagy and BDNF/TrkB signaling at presynaptic terminals.
Autophagosomes generated at the presynaptic terminal could also fuse with local lysosomes as an alternative pathway (FIGURE 3). Although presynaptic terminals have a low density of lysosomes compared with the soma, local degradation may be important to recycle presynaptic components, e.g., in cases of high-frequency stimulation when bulk endocytosis is evident. For instance, when endosomal acidification was inhibited in cultured neurons using bafilomycin A1 (an inhibitor of the V-type ATPase), the generation of synaptic vesicles from bulk endosomes was arrested, and the generation of small endosomes was retarded (18). Thus evidence suggests that local degradation of endosomes after bulk endocytosis is important to generate new synaptic vesicles.
In the soma, there is also generation of autophagosomes, but these are less mobile and tend to cluster (87). Indeed, autophagosome initiation was infrequent in the midaxon, soma, or dendrites. The higher density of lysosomes at the soma is consistent with autophagy and other degradation pathways completing their final steps at the soma, where recycling of degraded components and energetic demands are most likely met (higher ER, ribosome, and mitochondria density).
Physiological Regulation of Autophagy
Metabolic demands are well-characterized regulators of autophagy across a variety of cells, including neurons. Neurons respond to energy balance and changes in food intake by modulating autophagy (65). Studies initially suggested that the brain is protected from the acute effects of starvation, including autophagy cell responses (99). However, it has been recently shown that this is not the case, and food restriction induces upregulation of autophagy in cortical, Purkinje, and hypothalamic neurons (1, 65). In motor neurons, autophagy is also upregulated in response to starvation (132). A key link between metabolism and autophagy is the kinase complex mTOR (129). Under baseline conditions, mTOR inhibits autophagy by phosphorylation of ULK1/ATG1. Starvation activates AMPK and suppresses mTOR, both of which activate ULK1, triggering initiation of autophagy.
Trophic factors such as neurotrophins are also important modulators of autophagy in neurons. Recent studies indicate that neuron survival during development is regulated by trophic factors, at least in part, by regulating autophagy. Motor neuron survival during development (specifically during the postnatal period of programmed motor neuron cell death) is influenced by a number of trophic factors (6, 10, 35, 105, 126), including BDNF. Indeed, BDNF/TrkB signaling is likely indispensable for maintenance of motor neuron numbers during motor neuron elimination (21, 26, 27). In neurons, it has been demonstrated that BDNF/TrkB signaling contributes to cell survival via activation of the PI3K/Akt/mTOR pathway and modulation of autophagy (14). It is important to mention that the autophagy response to BDNF treatment in neurons differs according to nutritional conditions and brain regions. For example, in in vitro cortical neurons with oxygen deprivation, BDNF promoted cell viability via the upregulation of autophagy by inhibiting the PI3K/Akt/mTOR pathway (14). While in in vitro cultures of hippocampal neurons under no nutritional stress, BDNF treatment acting also through the PI3K/Akt/mTOR pathway reduced autophagosomes and autophagy flux (130). In vivo studies showed that animals with conditional deletion of BDNF in the neural lineage exhibit increased LC3 and decreased p62 levels in the brain. These results are in line with BDNF reducing autophagy flux under no nutritional stress. Also, suppression of autophagy was shown to be a crucial component of the BDNF/TrkB signaling mediating synaptic plasticity in hippocampal neurons (103).
Although most studies have used brain-cultured neurons and examined autophagy at the neuron soma, BDNF/TrkB signaling at the presynaptic terminal also plays an important role in the development and maintenance of adult neuromuscular junctions (83, 85, 90, 91). Thus examining the role of BDNF/TrkB signaling at the neuromuscular junction in regulating autophagy may reveal novel therapeutic targets to halt or reverse effects on motor neurons.
Autophagy in motor neurons can also be induced by physiologically relevant levels of neuronal stimulation. At the presynaptic terminal of neuromuscular junctions in Drosophila, high-frequency stimulation results in a rapid increase in the formation of LC3 puncta (132, 141). In rat hippocampal neurons, neuronal stimulation induces autophagosome formation pre- and postsynaptically (128). These studies indicate that, during neuronal activity and metabolic stress, autophagy may also serve the special needs of the synapse in protein turnover.
Another important regulator of autophagy is oxygen. Hypoxia increases autophagy in neuronal cells and plays a role in neonatal hypoxic/ischemic (H/I) brain injury. However, it is unclear whether autophagy participates as a pro- or anti-death factor in the execution of neuron death after H/I injury (11, 69). The impact of hypoxia in motor neuron autophagy under different circumstances, such as injury, remains to be elucidated.
In summary, a variety of environmental conditions and other factors modulate autophagy in motor neurons. The balance of autophagy is important to neuronal survival and plasticity, with impaired autophagy contributing to cell dysfunction and excessive autophagy leading to cell death likely via apoptosis (102, 136). In neurons, defects in autophagy may also culminate in progressive neurodegeneration (52, 70).
Autophagy in Motor Neuron Health and Disease
In neurons, autophagosomes are continuously generated in the terminal axon under baseline conditions (86). Knockout mice for several ATG genes showed that the continuous clearance of diffuse cytosolic proteins through basal autophagy is important for preventing accumulation of abnormal proteins, which can disrupt neural function and ultimately lead to neurodegeneration (102). Importantly, mice deficient for ATG5 or ATG7 showed deficits in motor function and evidence of fragmentation at neuromuscular junctions in the tibialis anterior muscle (12, 52, 70, 71). Consistent with the role of ATG5 and ATG7 in autophagosome formation, the protein levels of LC3-II and the presence of double membrane vacuoles (autophagosomes) in neurons of mutant mice were abolished. These impairments in autophagosome formation resulted in axonal degeneration and massive neuronal death, indicating the essential role of autophagy in neuronal survival.
Synaptic plasticity may be regulated by autophagy pathways (14, 103), and digestion of axonal remnants during axonal pruning was reported to be mediated by autophagy (131). These effects of autophagy likely contribute to the regulation of neurotransmission, likely at presynaptic sites, but there is minimal information on such effects at this time. Whether trophic signaling at the neuromuscular junction (e.g., via BDNF/TrkB signaling) contributes to the regulation of autophagy in motor neurons also remains to be established.
Impaired autophagy has been implicated in a number of neurodegenerative diseases, with defects in one or more of the autophagy stages: initiation, expansion, or degradation (Table 1). Despite the great interest and recognition of the importance of autophagy to motor neuron health, studies do not consistently report effects on autophagy to elucidate the impairment and stage affected. For instance, the often transient accumulation of autophagosomes due to increased induction may be misinterpreted as accumulation due to inefficient clearance of sequestered cargoes (impaired autophagy flux). Novel techniques permit a more complete evaluation of the impairment in autophagy flux and capitalize on modulation (either positive or negative) at any of the various steps in autophagy (13, 61).
Table 1.
Autophagy dysfunction in motor neuron diseases
| Step | Autophagy Impairment | Motor Neuron Disease | Proposed Autophagy Defect | References |
|---|---|---|---|---|
| Initiation | Impaired substrate recognition, sequestration, or autophagosome formation | Amyotrophic lateral sclerosis (ALS) | Mutations in UBQLIN2 and p62/SQSTM1 (essential in substrate recognition) | 23, 31, 39 |
| Spinal muscular atrophy | Increase Beclin1 (essential protein for membrane isolation) | 38 | ||
| Deregulation of Calpain (calcium-activated protease, which regulates autophagy through mTOR-independent pathway) | 110 | |||
| Autophagosome expansion | Impaired autophagy flux (increase in autophagosome synthesis without affecting turnover) | Spinal cord injury | Mutation in Atg7 (impairment in autophagosome formation in astrocytes) | 100 |
| Autophagosome degradation and recycling | Impaired retrograde axonal transport (decrease fusion with lysosomes or late endosomes), maturation of early endosomes or lysosomal digestion | Amyotrophic lateral sclerosis (ALS) | Mutation in dynactin (essential for retrograde transport) | 74, 107, 115, 138 |
| Rab5 suppression (protein involved in maturation of early endosomes) | ||||
| Spinal muscular atrophy | Mutations in dynein motor complex (essential for retrograde transport) | 19, 32 | ||
| Spinal cord injury | Decreased levels of lysosomal protease cathepsin D | 82 |
Deficits in autophagy flux may result from impairments in substrate recognition. Autophagy deficiency leads to several neurodegenerative diseases causing synaptic dysfunction and axonal degeneration. The upregulation of autophagy can be considered a potential therapeutic strategy. However, it is important to study whether the loss of autophagy in a particular context is caused by block in autophagy flux or lower levels of autophagy induction.
Autophagy in Motor Neuron Diseases
Autophagosome formation is impaired in chronic neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS). Like many other neurodegenerative disorders, a key pathological hallmark of ALS is the mislocalization of protein aggregates and the presence of cytoplasmic aggregates in motor neurons and surrounding cells (114), both of which suggest defects in the machinery that regulates protein homeostasis. Evidence from ALS animal models and postmortem samples suggests defects in autophagosome formation (80, 124). For instance, aggregates of transactive response (TAR) DNA-binding protein 43 (TDP-43) present in ALS neurons can be abated by inducing autophagy via mTOR inhibition, an effect that promotes autophagosome formation. Indeed, TDP-43 is localized to its proper nuclear compartment following mTOR inhibition, resulting in a reduction of TDP-43 accumulation (142). Of note, TDP-43 aggregate formation was also inhibited by enhancing autophagy flux through an mTOR-independent pathway (42). Aggregate formations were significantly decreased in the presence of trehalose through the AMPK pathway (92). In the superoxide dismutase 1 (SOD1) ALS model, upregulation of autophagy by AMPK activators decreases aggregation and promotes clearance of mutant SOD1 (22, 58). Autophagosome formation is also impaired when there is incorrect identification of ubiquitinated misfolded proteins. Mutations in UBQLIN2, a protein that promotes correct identification of ubiquitinated misfolded protein by p62/SQTM1, altered the autophagy process leading to neurodegeneration (23, 39). Alterations in beclin1, an important protein in initiation of autophagosome formation, contribute to neurodegeneration in an SOD1 model of disease (101).
Impairments in other stages of autophagy have also been related to the pathogenesis of ALS (20). For example, the rescue of impaired dynein-driven retrograde transport led to motor neuron survival and amelioration of the ALS disease phenotype in SOD1G93A mice (146), consistent with the importance of retrograde transport for the elimination of aggregate vacuoles. These results indicate that inducing autophagy countered the formation and accumulation of ALS-associated protein aggregates.
It is also possible that aberrant or excessive autophagy contributes to neurodegeneration in ALS. A recent study showed that autophagy plays distinct roles early and late in the ALS disease progression, with inhibition of autophagy accelerating neuromuscular denervation but reducing glial inflammation and extending the animal’s lifespan (119). These important, seemingly confounding, findings require further study.
In spinal muscle atrophy (SMA), a genetic neuromuscular disorder characterized by degeneration of motor neurons in the anterior horn of the spinal cord, changes in autophagy, specifically an increase in autophagosomes, were reported. An increase could be due to a rise in autophagosome production or a decrease in autophagic flux, and yet previous reports have yielded conflicting results. Using both in vitro model of SMA as well as motor neurons obtained from a survival motor neuron protein (SMN) knockdown model, Garcera et al., showed that reductions in SMN protein resulted in increased autophagosome production but not alterations in the autophagic flux (38). Furthermore, this group showed that the LC3-II increase could be counteracted by Bcl-xL overexpression, which inhibits autophagy by binding to Beclin1 required for the initiation of autophagosome formation (108). These results together suggest that an increase of Beclin1-dependent autophagy could be one of the mechanisms responsible for motor neuron degeneration in the SMN knockdown model. However, Periyakaruppiah et al. reported an increase of p62 protein level in motor neuron cultures from SMA mice, suggesting a decreased autophagic flux. In addition, the proteasome pathway may play a role in the degradation of SMN protein when the autophagic flux is reduced (110). These seemingly conflicting results might be the consequence of the nonspecific nature of autophagy manipulations, as well as the complex and distinct roles that autophagy could play in disease progression. Future studies could specifically determine the effects of changes in autophagy pathways at the various stages of disease using targeted interventions in relevant in vivo and in vitro models.
Autophagy in Spinal Cord Injury
The molecular mechanisms responsible for the susceptibility to motor neuron death following spinal cord injury (SCI) and the progressive loss of motor neurons are not completely known. Several studies indicate an important role for autophagy, including upregulation of the autophagy-related protein LC3 in the spinal cord of animals following SCI (62, 82). Motor neuron loss appears to depend on the severity of injury and duration of exposure to the contusion. In the clinical setting, early decompression is a well-documented and perhaps the only known therapeutic option that exerts short- and long-term benefits (36, 151). Accordingly, cellular mechanisms mediating the response to injury, including removal of damaged organelles, i.e., autophagy, are likely to play an essential role in mediating neuronal death. For instance, motor neurons showing impaired autophagy flux also displayed increased caspase 12 and cleaved caspase 3 following contusion injury (82), supporting a role for impaired autophagy in mediating neuronal apoptosis. Dysfunction in autophagy pathways has been associated with cell death in other organ systems, and thus cell-targeting therapies that modulate autophagy pathways in the central nervous system may constitute a novel and important therapeutic target.
Autophagy pathways may also contribute to the effects of trophic factor signaling to the extent and recovery from SCI. A recent study showed an association between motor neuron survival, increased BDNF expression, and autophagy (measured by LC3 protein expression) following simvastatin administration in a contusion model of injury (37). BDNF/TrkB signaling also plays important roles in neuroplasticity and in promoting functional recovery after SCI (41, 45, 46, 57, 88, 89, 94), and it is possible that the neuroprotective effects of BDNF may be mediated by regulation of autophagy flux (14). Future studies could explicitly explore these complex interactions between trophic factors and autophagy in the context of injury, cell survival, and degeneration.
Another role of autophagy after SCI involves regulation of the inflammation response after injury. Recent evidence suggests that, following acute cortical injury, there is a differential region-specific remodeling of the astrocytic mitochondrial network in response to inflammation (100). Whereas astrocytes invaded by pro-inflammatory cells undergo mitochondrial fragmentation, astrocytes within the penumbra of the lesion undergo mitochondrial elongation. Most importantly, the induction of autophagy is what appears to maintain mitochondrial architecture. Deletion of ATG7, required for autophagosome formation, prevented the reestablishment of tubular mitochondria leading to reactive oxygen species accumulation and cell death.
Autophagy in Motor Neuron Aging
There is now substantial interest in autophagy as a contributor to the aging process and neurodegeneration (98, 104). In general, autophagy reportedly is impaired with age (12, 145). The efficacy of autophagic clearance is impaired as a result of aging in many tissues and organisms (48, 84, 111, 147, 150). For instance, autophagy has been shown to be critical for suppression of memory impairments with aging (48) and for the delayed aging related to caloric restriction (147). In the human brain, it was demonstrated that autophagic core machinery (including ATG5 and ATG7) is downregulated during normal aging (81).
One possible result of the age-related impaired autophagy in motor neurons is muscle fiber denervation, possibly resulting from disrupted axonal trafficking and reduced organelle clearance (2), whereas loss of motor neurons in old age may represent a terminal, late effect (98, 139). As discussed before, neurotrophins, including BDNF, are important for motor neuron survival (6, 10, 35, 105, 126). Therefore, age-related neuromuscular dysfunction may reflect loss of trophic support and disrupted protective mechanisms within motor neurons, including autophagy. Multiple studies, have focused on morphological changes at the neuromuscular junction in old age (25, 28, 29, 43, 47, 112, 140). Indeed, retraction of presynaptic terminals, disaggregation, and fragmentation of the motor end-plate has been reported across muscles. The role of autophagy in age-related motor neuron dysfunction or death is presently not known.
Therapies Targeting the Autophagy Pathway
Modulating the autophagy pathway has potential therapeutic application. It is worth noting that intermittent fasting and/or caloric restriction may have therapeutic interest since caloric restriction is feasible in humans, does not have a high risk, and is often performed for a variety of reasons. The degree of caloric restriction necessary to consistently elicit the starvation response and induce autophagy is not presently known in humans, particularly as it relates to the possible upregulation of autophagy in neurons. Much work has focused on identifying small molecules that inhibit or enhance autophagy, and most of the progress in developing autophagy inhibitors and testing their therapeutic potential has been made in cancer cells since induction of autophagy is associated with resistance of cancer cells to chemotherapeutic agents (51, 93, 113, 135, 143). Some pharmacological treatments have also been tested, targeting autophagy in the central nervous system. In this section, we review promising compounds that modulate autophagy based on their mechanism of action and divide them into mTOR-dependent and -independent pathways (FIGURE 2).
As mTOR dependent, the most common molecule used to induce autophagy is rapamycin (and its analogs CCI-779, RAD001, AP23573), a lipophilic macrolide antibiotic first isolated from Streptomyces Hygroscopicus (34). Autophagy induction by rapamycin occurs through inhibition of the kinase activity of mTOR (66). Similar to rapamycin, four compounds (perhexilene, niclosamide, amiodarone, and rottlerin) were identified in a cell-based screen for compounds that increase autophagosome number (5). The exact targets of these compounds remain unknown, however. In addition, two ATP-competitive small-molecule mTOR inhibitors, PP242 and Torin1, were found to directly inhibit both mTORC1 and mTORC2 (137). Details on the exact mechanisms responsible for autophagy modulation with these compounds need to be addressed. Another alternative to regulate mTOR is through PI3 kinases, but highly selective pharmacological agents may be required since PI3 kinases have opposing roles at points upstream and downstream of mTOR with activation of class Ia PI3K at the plasma membrane activating mTOR (and inhibiting) autophagy (68), and downstream activation of the class III PI3 kinase Vsp34 increasing the local concentration of PI3P and inducing autophagy (4). There is minimal information on pharmacological treatments that specifically target class I PI3K signaling. Class III PI3K activity can be inhibited by 3-methyladenine (3-MA), wortmannin and 2-(4-morpholinyl)-8-phenylchromone (LY294002) (7, 125). Furthermore, mTOR-dependent signals can also be regulated by AMPK, but the pathways and molecules responsible remain unclear, and thus interpretation of molecular mechanisms is challenging. For instance, treatment with an AMPK inhibitor (compound C) and treatment with an AMPK activator (AICA riboside) both inhibit autophagy (96, 97). All of these compounds are widely used as tools to test whether protein-degradation processes are autophagy dependent. However, most have additional off-target effects (e.g., protein, lipid and nucleotide synthesis, energy metabolism) that may confound interpretation in certain contexts and limit therapeutic application.
In an mTOR-independent pathway, inositol 1,4,5-trisphosphate (IP3) negatively regulates autophagy. Mood-stabilizing drugs that induce autophagy, e.g., carbamazepine, lithium, and valproic acid, reduce intracellular inositol levels (123). Another potent mTOR-independent autophagy enhancer is the disaccharide trehalose that has been shown to induce autophagy and enhance clearance of aggregate-prone proteins in vitro and in vivo (117, 122). Trehalose is thought to function as a chemical chaperone, owing to its ability to influence protein folding through direct protein-trehalose interactions. Interestingly, trehalose protects Drosophila and mammalian cells from hypoxic and anoxic injury, and the mechanism of this protection is related to a decrease in protein denaturation (15, 16, 49). It is possible that, under a variety of stresses, different types of mechanisms are activated for cell recovery, some that enhance protein integrity and limit protein degradation, and others (like autophagy) that help to remove proteins that are damaged. In this sense, trehalose can enhance protein integrity and limit protein degradation under different types of stresses (heat stress, oxidant injury, anoxia) while also inducing autophagy.
Specifically in the central nervous system, lanthionine ketimine (LK) is an amino acid metabolite found in mammalian brain tissue at low concentrations that possesses potent neuroprotective, neurotrophic, and anti-inflammatory properties (54, 73). Both LK, and its cell-permeable derivative LKE, activate autophagy flux in rat glioma and human neuroblastoma cells (53, 55). A recent study using systemic LKE in the SOD1G93A mouse model of ALS showed promising, albeit modest, effects on survival (54). Lanthionine ketimine ester treatment also showed substantially diminished cognitive decline, brain amyloid-A peptide deposition, and phospho-Tau accumulation in a mouse model of Alzheimer’s disease (56).
Several caveats to the above-mentioned studies obfuscate interpretation of the effects of regulating autophagy in disease. First, there is no good method for accurately quantifying autophagy in vivo, and especially in human patients. This makes it difficult to verify that drugs that increase autophagic flux in cultured cells are also doing so in vivo. Second, agents that activate autophagy also have other effects, for example mTOR inhibitors also affect protein, nucleotide, and lipid synthesis as well as glutamine metabolism and glycolysis, all via autophagy-independent mechanisms (79). This makes it difficult to know whether the beneficial effects are due to increased autophagy or other effects. A major hurdle for the field is to develop better ways to stimulate autophagy in a more specific manner. In summary, although more work is needed to fully elucidate the mechanisms of these compounds and their therapeutic potential, upregulation of autophagy remains a promising therapeutic approach for neurodegenerative and motor neuron diseases.
Conclusions
Growing evidence supports the important roles that autophagy plays in different tissues, in particular its unique role in the nervous system. Very little is known about the role of autophagy during normal motor neuron function, the specific role of autophagy in neuromuscular dysfunction, and the trophic factor-dependent mechanisms responsible for changes in motoneuron autophagy. Autophagy can be modulated positively and negatively at several steps. Future studies should aim to investigate the cellular background explaining such differences in the physiology and pathophysiology of motor neurons, as well as the differences in the regulation of autophagy in the different motor neuron compartments. Also, the roles of trophic interactions at the neuromuscular junction possibly contributing to impaired autophagy in motor neurons are important to determine, particularly as it relates to trophic signaling. This collective knowledge constitutes a foundation for the development of targeted therapies to mitigate motor neuron diseases or aging effects on neuromuscular performance.
Acknowledgments
Supported by National Institutes of Health Grants AG-057052, AG-044615, and HL-096750.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: M.G.P. and C.B.M. prepared figures; M.G.P. drafted manuscript; M.G.P., G.C.S., and C.B.M. edited and revised manuscript; M.G.P., G.C.S., and C.B.M. approved final version of manuscript.
References
- 1.Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy 6: 702–710, 2010. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol 206: 655–670, 2014. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701, 2008. doi: 10.1083/jcb.200803137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J 410: 1–17, 2008. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 5.Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, Nabi IR, Roberge M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One 4: e7124, 2009. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baloh RH, Enomoto H, Johnson EM Jr, Milbrandt J. The GDNF family ligands and receptors - implications for neural development. Curr Opin Neurobiol 10: 103–110, 2000. doi: 10.1016/S0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 7.Blommaart EF, Krause U, Schellens JP, Vreeling-Sindelárová H, Meijer AJ. The phosphatidylinositol 3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in isolated rat hepatocytes. Eur J Biochem 243: 240–246, 1997. doi: 10.1111/j.1432-1033.1997.0240a.x. [DOI] [PubMed] [Google Scholar]
- 8.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci 28: 6926–6937, 2008. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boya P, Reggiori F, Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol 15: 713–720, 2013. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol 11: 287–296, 2001. doi: 10.1016/S0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 11.Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis 32: 329–339, 2008. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, Blaauw B, Friguet B, Bottinelli R, Rudolf R, Sandri M. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Reports 8: 1509–1521, 2014. doi: 10.1016/j.celrep.2014.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castillo K, Valenzuela V, Matus S, Nassif M, Oñate M, Fuentealba Y, Encina G, Irrazabal T, Parsons G, Court FA, Schneider BL, Armentano D, Hetz C. Measurement of autophagy flux in the nervous system in vivo. Cell Death Dis 4: e917, 2013. doi: 10.1038/cddis.2013.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen A, Xiong LJ, Tong Y, Mao M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep 8: 1011–1016, 2013. doi: 10.3892/mmr.2013.1628. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Haddad GG. Role of trehalose phosphate synthase and trehalose during hypoxia: from flies to mammals. J Exp Biol 207: 3125–3129, 2004. doi: 10.1242/jeb.01133. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Ma E, Behar KL, Xu T, Haddad GG. Role of trehalose phosphate synthase in anoxia tolerance and development in Drosophila melanogaster. J Biol Chem 277: 3274–3279, 2002. doi: 10.1074/jbc.M109479200. [DOI] [PubMed] [Google Scholar]
- 17.Cheng XT, Zhou B, Lin MY, Cai Q, Sheng ZH. Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes. J Cell Biol 209: 377–386, 2015. doi: 10.1083/jcb.201412046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung G, Cousin MA. Synaptic vesicle generation from activity-dependent bulk endosomes requires calcium and calcineurin. J Neurosci 33: 3370–3379, 2013. doi: 10.1523/JNEUROSCI.4697-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta 1762: 1094–1108, 2006. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Cipolat Mis MS, Brajkovic S, Frattini E, Di Fonzo A, Corti S. Autophagy in motor neuron disease: Key pathogenetic mechanisms and therapeutic targets. Mol Cell Neurosci 72: 84–90, 2016. doi: 10.1016/j.mcn.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, Friedman B, Wiegand S, Vejsada R, Kato AC, Dechiara TM, Yancopoulos GD. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature 375: 235–238, 1995. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- 22.Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E, Galbiati M, Fontana E, Marino M, Carra S, Bendotti C, De Biasi S, Poletti A. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet 19: 3440–3456, 2010. doi: 10.1093/hmg/ddq257. [DOI] [PubMed] [Google Scholar]
- 23.Deng HX, Chen W, Hong ST, Boycott KM, Gorrie GH, Siddique N, Yang Y, Fecto F, Shi Y, Zhai H, Jiang H, Hirano M, Rampersaud E, Jansen GH, Donkervoort S, Bigio EH, Brooks BR, Ajroud K, Sufit RL, Haines JL, Mugnaini E, Pericak-Vance MA, Siddique T. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477: 211–215, 2011. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deretic V. Autophagy in infection. Curr Opin Cell Biol 22: 252–262, 2010. doi: 10.1016/j.ceb.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol 45: 389–393, 2010. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci 16: 5361–5371, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 368: 147–150, 1994. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 28.Fahim MA, Holley JA, Robbins N. Scanning and light microscopic study of age changes at a neuromuscular junction in the mouse. J Neurocytol 12: 13–25, 1983. doi: 10.1007/BF01148085. [DOI] [PubMed] [Google Scholar]
- 29.Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol 11: 641–656, 1982. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- 30.Farré JC, Subramani S. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat Rev Mol Cell Biol 17: 537–552, 2016. doi: 10.1038/nrm.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fecto F, Yan J, Vemula SP, Liu E, Yang Y, Chen W, Zheng JG, Shi Y, Siddique N, Arrat H, Donkervoort S, Ajroud-Driss S, Sufit RL, Heller SL, Deng HX, Siddique T. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol 68: 1440–1446, 2011. doi: 10.1001/archneurol.2011.250. [DOI] [PubMed] [Google Scholar]
- 32.Ferrucci M, Fulceri F, Toti L, Soldani P, Siciliano G, Paparelli A, Fornai F. Protein clearing pathways in ALS. Arch Ital Biol 149: 121–149, 2011. doi: 10.4449/aib.v149i1.1258. [DOI] [PubMed] [Google Scholar]
- 33.Filimonenko M, Isakson P, Finley KD, Anderson M, Jeong H, Melia TJ, Bartlett BJ, Myers KM, Birkeland HC, Lamark T, Krainc D, Brech A, Stenmark H, Simonsen A, Yamamoto A. The selective macroautophagic degradation of aggregated proteins requires the PI3P-binding protein Alfy. Mol Cell 38: 265–279, 2010. doi: 10.1016/j.molcel.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fleming A, Noda T, Yoshimori T, Rubinsztein DC. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol 7: 9–17, 2011. doi: 10.1038/nchembio.500. [DOI] [PubMed] [Google Scholar]
- 35.Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibáñez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science 268: 1495–1499, 1995. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 36.Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma 28: 1371–1399, 2011. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao K, Wang G, Wang Y, Han D, Bi J, Yuan Y, Yao T, Wan Z, Li H, Mei X. Neuroprotective effect of simvastatin via inducing the autophagy on spinal cord injury in the rat model. BioMed Res Int 2015: 260161, 2015. doi: 10.1155/2015/260161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcera A, Bahi N, Periyakaruppiah A, Arumugam S, Soler RM. Survival motor neuron protein reduction deregulates autophagy in spinal cord motoneurons in vitro. Cell Death Dis 4: e686, 2013. doi: 10.1038/cddis.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gellera C, Tiloca C, Del Bo R, Corrado L, Pensato V, Agostini J, Cereda C, Ratti A, Castellotti B, Corti S, Bagarotti A, Cagnin A, Milani P, Gabelli C, Riboldi G, Mazzini L, Sorarù G, D’Alfonso S, Taroni F, Comi GP, Ticozzi N, Silani V; SLAGEN Consortium . Ubiquilin 2 mutations in Italian patients with amyotrophic lateral sclerosis and frontotemporal dementia. J Neurol Neurosurg Psychiatry 84: 183–187, 2013. doi: 10.1136/jnnp-2012-303433. [DOI] [PubMed] [Google Scholar]
- 40.Geng J, Nair U, Yasumura-Yorimitsu K, Klionsky DJ. Post-Golgi Sec proteins are required for autophagy in Saccharomyces cerevisiae. Mol Biol Cell 21: 2257–2269, 2010. doi: 10.1091/mbc.E09-11-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gill LC, Gransee HM, Sieck GC, Mantilla CB. Functional recovery after cervical spinal cord injury: Role of neurotrophin and glutamatergic signaling in phrenic motoneurons. Respir Physiol Neurobiol 226: 128–136, 2016. doi: 10.1016/j.resp.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomes C, Escrevente C, Costa J. Mutant superoxide dismutase 1 overexpression in NSC-34 cells: effect of trehalose on aggregation, TDP-43 localization and levels of co-expressed glycoproteins. Neurosci Lett 475: 145–149, 2010. doi: 10.1016/j.neulet.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci 6: 208, 2014. doi: 10.3389/fnagi.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon PB, Seglen PO. Prelysosomal convergence of autophagic and endocytic pathways. Biochem Biophys Res Commun 151: 40–47, 1988. doi: 10.1016/0006-291X(88)90556-6. [DOI] [PubMed] [Google Scholar]
- 45.Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. J Neurotrauma 32: 185–193, 2015. doi: 10.1089/neu.2014.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One 8: e64755, 2013. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greising SM, Vasdev AK, Zhan WZ, Sieck GC, Mantilla CB. Chronic TrkB agonist treatment in old age does not mitigate diaphragm neuromuscular dysfunction. Physiol Rep 5: e13103, 2017. doi: 10.14814/phy2.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KS, Schroeder S, Stunnenberg HG, Sinner F, Magnes C, Pieber TR, Dipt S, Fiala A, Schenck A, Schwaerzel M, Madeo F, Sigrist SJ. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci 16: 1453–1460, 2013. doi: 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- 49.Haddad GG. Tolerance to low O2: lessons from invertebrate genetic models. Exp Physiol 91: 277–282, 2006. doi: 10.1113/expphysiol.2005.030767. [DOI] [PubMed] [Google Scholar]
- 50.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141: 656–667, 2010. doi: 10.1016/j.cell.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han J, Hou W, Goldstein LA, Lu C, Stolz DB, Yin XM, Rabinowich H. Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem 283: 19665–19677, 2008. doi: 10.1074/jbc.M710169200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441: 885–889, 2006. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 53.Harris-White ME, Ferbas KG, Johnson MF, Eslami P, Poteshkina A, Venkova K, Christov A, Hensley K. A cell-penetrating ester of the neural metabolite lanthionine ketimine stimulates autophagy through the mTORC1 pathway: evidence for a mechanism of action with pharmacological implications for neurodegenerative pathologies. Neurobiol Dis 84: 60–68, 2015. doi: 10.1016/j.nbd.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hensley K, Christov A, Kamat S, Zhang XC, Jackson KW, Snow S, Post J. Proteomic identification of binding partners for the brain metabolite lanthionine ketimine (LK) and documentation of LK effects on microglia and motoneuron cell cultures. J Neurosci 30: 2979–2988, 2010. doi: 10.1523/JNEUROSCI.5247-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hensley K, Denton TT. Alternative functions of the brain transsulfuration pathway represent an underappreciated aspect of brain redox biochemistry with significant potential for therapeutic engagement. Free Radic Biol Med 78: 123–134, 2015. doi: 10.1016/j.freeradbiomed.2014.10.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hensley K, Gabbita SP, Venkova K, Hristov A, Johnson MF, Eslami P, Harris-White ME. A derivative of the brain metabolite lanthionine ketimine improves cognition and diminishes pathology in the 3 × Tg-AD mouse model of Alzheimer disease. J Neuropathol Exp Neurol 72: 955–969, 2013. doi: 10.1097/NEN.0b013e3182a74372. [DOI] [PubMed] [Google Scholar]
- 57.Hernandez-Torres V, Gransee HM, Mantilla CB, Wang Y, Zhan WZ, Sieck GC. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury. J Neurophysiol 117: 537–544, 2017. doi: 10.1152/jn.00654.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev 23: 2294–2306, 2009. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hollenbeck PJ. Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J Cell Biol 121: 305–315, 1993. doi: 10.1083/jcb.121.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson CW, Melia TJ, Yamamoto A. Modulating macroautophagy: a neuronal perspective. Future Med Chem 4: 1715–1731, 2012. doi: 10.4155/fmc.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaizuka T, Morishita H, Hama Y, Tsukamoto S, Matsui T, Toyota Y, Kodama A, Ishihara T, Mizushima T, Mizushima N. An autophagic flux probe that releases an internal control. Mol Cell 64: 835–849, 2016. doi: 10.1016/j.molcel.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 62.Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine 36: E1427–E1434, 2011. doi: 10.1097/BRS.0b013e3182028c3a. [DOI] [PubMed] [Google Scholar]
- 63.Katsuragi Y, Ichimura Y, Komatsu M. p62/SQSTM1 functions as a signaling hub and an autophagy adaptor. FEBS J 282: 4672–4678, 2015. doi: 10.1111/febs.13540. [DOI] [PubMed] [Google Scholar]
- 64.Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol 16: 461–472, 2015. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]
- 65.Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab 14: 173–183, 2011. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110: 163–175, 2002. doi: 10.1016/S0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 290: 1717–1721, 2000. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 172: 454–469, 2008. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441: 880–884, 2006. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 71.Komatsu M, Wang QJ, Holstein GR, Friedrich VL Jr, Iwata J, Kominami E, Chait BT, Tanaka K, Yue Z. Essential role for autophagy protein Atg7 in the maintenance of axonal homeostasis and the prevention of axonal degeneration. Proc Natl Acad Sci USA 104: 14489–14494, 2007. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kononenko NL, Claßen GA, Kuijpers M, Puchkov D, Maritzen T, Tempes A, Malik AR, Skalecka A, Bera S, Jaworski J, Haucke V. Retrograde transport of TrkB-containing autophagosomes via the adaptor AP-2 mediates neuronal complexity and prevents neurodegeneration. Nat Commun 8: 14819, 2017. doi: 10.1038/ncomms14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kotaka K, Nagai J, Hensley K, Ohshima T. Lanthionine ketimine ester promotes locomotor recovery after spinal cord injury by reducing neuroinflammation and promoting axon growth. Biochem Biophys Res Commun 483: 759–764, 2017. doi: 10.1016/j.bbrc.2016.12.069. [DOI] [PubMed] [Google Scholar]
- 74.Laird FM, Farah MH, Ackerley S, Hoke A, Maragakis N, Rothstein JD, Griffin J, Price DL, Martin LJ, Wong PC. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J Neurosci 28: 1997–2005, 2008. doi: 10.1523/JNEUROSCI.4231-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lamb CA, Yoshimori T, Tooze SA. The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol 14: 759–774, 2013. doi: 10.1038/nrm3696. [DOI] [PubMed] [Google Scholar]
- 76.Larsen KE, Sulzer D. Autophagy in neurons: a review. Histol Histopathol 17: 897–908, 2002. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- 77.Lee JA, Gao FB. Inhibition of autophagy induction delays neuronal cell loss caused by dysfunctional ESCRT-III in frontotemporal dementia. J Neurosci 29: 8506–8511, 2009. doi: 10.1523/JNEUROSCI.0924-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee S, Sato Y, Nixon RA. Primary lysosomal dysfunction causes cargo-specific deficits of axonal transport leading to Alzheimer-like neuritic dystrophy. Autophagy 7: 1562–1563, 2011. doi: 10.4161/auto.7.12.17956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Kim SG, Blenis J. Rapamycin: one drug, many effects. Cell Metab 19: 373–379, 2014. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li L, Zhang X, Le W. Altered macroautophagy in the spinal cord of SOD1 mutant mice. Autophagy 4: 290–293, 2008. doi: 10.4161/auto.5524. [DOI] [PubMed] [Google Scholar]
- 81.Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C, Yankner BA, Scherzer CR, Yuan J. Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci USA 107: 14164–14169, 2010. doi: 10.1073/pnas.1009485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu S, Sarkar C, Dinizo M, Faden AI, Koh EY, Lipinski MM, Wu J. Disrupted autophagy after spinal cord injury is associated with ER stress and neuronal cell death. Cell Death Dis 6: e1582, 2015. doi: 10.1038/cddis.2014.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363: 350–353, 1993. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 84.Loos B, Klionsky DJ, Wong E. Augmenting brain metabolism to increase macro- and chaperone-mediated autophagy for decreasing neuronal proteotoxicity and aging. Prog Neurobiol 156: 90–106, 2017. doi: 10.1016/j.pneurobio.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 85.Lu B. Acute and long-term synaptic modulation by neurotrophins. Prog Brain Res 146: 137–150, 2004. [DOI] [PubMed] [Google Scholar]
- 86.Maday S, Holzbaur EL. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell 30: 71–85, 2014. doi: 10.1016/j.devcel.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maday S, Holzbaur EL. Compartment-specific regulation of autophagy in primary neurons. J Neurosci 36: 5933–5945, 2016. doi: 10.1523/JNEUROSCI.4401-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mantilla CB, Gransee HM, Zhan WZ, Sieck GC. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol 247: 101–109, 2013. doi: 10.1016/j.expneurol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mantilla CB, Greising SM, Stowe JM, Zhan WZ, Sieck GC. TrkB kinase activity is critical for recovery of respiratory function after cervical spinal cord hemisection. Exp Neurol 261: 190–195, 2014. doi: 10.1016/j.expneurol.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mantilla CB, Stowe JM, Sieck DC, Ermilov LG, Greising SM, Zhang C, Shokat KM, Sieck GC. TrkB kinase activity maintains synaptic function and structural integrity at adult neuromuscular junctions. J Appl Physiol (1985) 117: 910–920, 2014. doi: 10.1152/japplphysiol.01386.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve 29: 381–386, 2004. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- 92.Mardones P, Rubinsztein DC, Hetz C. Mystery solved: Trehalose kickstarts autophagy by blocking glucose transport. Sci Signal 9: fs2, 2016. doi: 10.1126/scisignal.aaf1937. [DOI] [PubMed] [Google Scholar]
- 93.Mariño G, Salvador-Montoliu N, Fueyo A, Knecht E, Mizushima N, López-Otín C. Tissue-specific autophagy alterations and increased tumorigenesis in mice deficient in Atg4C/autophagin-3. J Biol Chem 282: 18573–18583, 2007. doi: 10.1074/jbc.M701194200. [DOI] [PubMed] [Google Scholar]
- 94.Martínez-Gálvez G, Zambrano JM, Diaz Soto JC, Zhan WZ, Gransee HM, Sieck GC, Mantilla CB. TrkB gene therapy by adeno-associated virus enhances recovery after cervical spinal cord injury. Exp Neurol 276: 31–40, 2016. doi: 10.1016/j.expneurol.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsuura A, Tsukada M, Wada Y, Ohsumi Y. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192: 245–250, 1997. doi: 10.1016/S0378-1119(97)00084-X. [DOI] [PubMed] [Google Scholar]
- 96.Meijer AJ, Codogno P. AMP-activated protein kinase and autophagy. Autophagy 3: 238–240, 2007. doi: 10.4161/auto.3710. [DOI] [PubMed] [Google Scholar]
- 97.Meley D, Bauvy C, Houben-Weerts JH, Dubbelhuis PF, Helmond MT, Codogno P, Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem 281: 34870–34879, 2006. doi: 10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 98.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature 451: 1069–1075, 2008. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell 15: 1101–1111, 2004. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B, Conzelmann KK, Götz M, Winklhofer KF, Hrelia S, Bergami M. Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab 18: 844–859, 2013. doi: 10.1016/j.cmet.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 101.Nassif M, Valenzuela V, Rojas-Rivera D, Vidal R, Matus S, Castillo K, Fuentealba Y, Kroemer G, Levine B, Hetz C. Pathogenic role of BECN1/Beclin 1 in the development of amyotrophic lateral sclerosis. Autophagy 10: 1256–1271, 2014. doi: 10.4161/auto.28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nikoletopoulou V, Papandreou ME, Tavernarakis N. Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ 22: 398–407, 2015. doi: 10.1038/cdd.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nikoletopoulou V, Sidiropoulou K, Kallergi E, Dalezios Y, Tavernarakis N. Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metab 26: 230–242.e5, 2017. doi: 10.1016/j.cmet.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 104.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med 19: 983–997, 2013. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 105.Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron 17: 195–197, 1996. doi: 10.1016/S0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 106.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282: 24131–24145, 2007. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 107.Pasquali L, Ruffoli R, Fulceri F, Pietracupa S, Siciliano G, Paparelli A, Fornai F. The role of autophagy: what can be learned from the genetic forms of amyotrophic lateral sclerosis. CNS Neurol Disord Drug Targets 9: 268–278, 2010. doi: 10.2174/187152710791292594. [DOI] [PubMed] [Google Scholar]
- 108.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122: 927–939, 2005. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Peña-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L, Xie XJ, Corey DR, Brugarolas J. Regulation of TFEB and V-ATPases by mTORC1. EMBO J 30: 3242–3258, 2011. doi: 10.1038/emboj.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Periyakaruppiah A, de la Fuente S, Arumugam S, Bahí N, Garcera A, Soler RM. Autophagy modulators regulate survival motor neuron protein stability in motoneurons. Exp Neurol 283, Pt A: 287–297, 2016. doi: 10.1016/j.expneurol.2016.06.032. [DOI] [PubMed] [Google Scholar]
- 111.Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis 84: 39–49, 2015. doi: 10.1016/j.nbd.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 112.Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve 21: 887–895, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 113.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112: 1809–1820, 2003. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramesh N, Pandey UB. Autophagy dysregulation in ALS: when protein aggregates get out of hand. Front Mol Neurosci 10: 263, 2017. doi: 10.3389/fnmol.2017.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O’Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet 37: 771–776, 2005. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- 116.Ravikumar B, Moreau K, Rubinsztein DC. Plasma membrane helps autophagosomes grow. Autophagy 6: 1184–1186, 2010. doi: 10.4161/auto.6.8.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodríguez-Navarro JA, Rodríguez L, Casarejos MJ, Solano RM, Gómez A, Perucho J, Cuervo AM, García de Yébenes J, Mena MA. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis 39: 423–438, 2010. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 118.Rogov V, Dötsch V, Johansen T, Kirkin V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell 53: 167–178, 2014. doi: 10.1016/j.molcel.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 119.Rudnick ND, Griffey CJ, Guarnieri P, Gerbino V, Wang X, Piersaint JA, Tapia JC, Rich MM, Maniatis T. Distinct roles for motor neuron autophagy early and late in the SOD1G93Amouse model of ALS. Proc Natl Acad Sci USA 114: E8294–E8303, 2017. doi: 10.1073/pnas.1704294114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rusten TE, Stenmark H. How do ESCRT proteins control autophagy? J Cell Sci 122: 2179–2183, 2009. doi: 10.1242/jcs.050021. [DOI] [PubMed] [Google Scholar]
- 121.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10: 623–635, 2009. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 122.Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem 282: 5641–5652, 2007. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 123.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170: 1101–1111, 2005. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sasaki S. Autophagy in spinal cord motor neurons in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 70: 349–359, 2011. doi: 10.1097/NEN.0b013e3182160690. [DOI] [PubMed] [Google Scholar]
- 125.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA 79: 1889–1892, 1982. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sendtner M, Pei G, Beck M, Schweizer U, Wiese S. Developmental motoneuron cell death and neurotrophic factors. Cell Tissue Res 301: 71–84, 2000. doi: 10.1007/s004410000217. [DOI] [PubMed] [Google Scholar]
- 127.Shang L, Wang X. AMPK and mTOR coordinate the regulation of Ulk1 and mammalian autophagy initiation. Autophagy 7: 924–926, 2011. doi: 10.4161/auto.7.8.15860. [DOI] [PubMed] [Google Scholar]
- 128.Shehata M, Matsumura H, Okubo-Suzuki R, Ohkawa N, Inokuchi K. Neuronal stimulation induces autophagy in hippocampal neurons that is involved in AMPA receptor degradation after chemical long-term depression. J Neurosci 32: 10413–10422, 2012. doi: 10.1523/JNEUROSCI.4533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol 15: 155–162, 2014. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 130.Smith ED, Prieto GA, Tong L, Sears-Kraxberger I, Rice JD, Steward O, Cotman CW. Rapamycin and interleukin-1β impair brain-derived neurotrophic factor-dependent neuron survival by modulating autophagy. J Biol Chem 289: 20615–20629, 2014. doi: 10.1074/jbc.M114.568659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Song JW, Misgeld T, Kang H, Knecht S, Lu J, Cao Y, Cotman SL, Bishop DL, Lichtman JW. Lysosomal activity associated with developmental axon pruning. J Neurosci 28: 8993–9001, 2008. doi: 10.1523/JNEUROSCI.0720-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Soukup SF, Kuenen S, Vanhauwaert R, Manetsberger J, Hernández-Díaz S, Swerts J, Schoovaerts N, Vilain S, Gounko NV, Vints K, Geens A, De Strooper B, Verstreken P. A LRRK2-dependent endophilina phosphoswitch is critical for macroautophagy at presynaptic terminals. Neuron 92: 829–844, 2016. doi: 10.1016/j.neuron.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 133.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol 16: 495–501, 2014. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 134.Strømhaug PE, Berg TO, Fengsrud M, Seglen PO. Purification and characterization of autophagosomes from rat hepatocytes. Biochem J 335: 217–224, 1998. doi: 10.1042/bj3350217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mulé JJ, Pledger WJ, Wang HG. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 9: 1142–1151, 2007. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tan CC, Yu JT, Tan MS, Jiang T, Zhu XC, Tan L. Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiol Aging 35: 941–957, 2014. doi: 10.1016/j.neurobiolaging.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 137.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032, 2009. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tresse E, Salomons FA, Vesa J, Bott LC, Kimonis V, Yao TP, Dantuma NP, Taylor JP. VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes and this function is impaired by mutations that cause IBMPFD. Autophagy 6: 217–227, 2010. doi: 10.4161/auto.6.2.11014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21: 4249–4258, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Valdez G, Tapia JC, Kang H, Clemenson GD Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA 107: 14863–14868, 2010. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vanhauwaert R, Kuenen S, Masius R, Bademosi A, Manetsberger J, Schoovaerts N, Bounti L, Gontcharenko S, Swerts J, Vilain S, Picillo M, Barone P, Munshi ST, de Vrij FMS, Kushner SA, Gounko NV, Mandemakers W, Bonifati V, Meunier FA, Soukup SF, Verstreken P. The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. EMBO J 36: 1392–1411, 2017. doi: 10.15252/embj.201695773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang IF, Tsai KJ, Shen CK. Autophagy activation ameliorates neuronal pathogenesis of FTLD-U mice: a new light for treatment of TARDBP/TDP-43 proteinopathies. Autophagy 9: 239–240, 2013. doi: 10.4161/auto.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.White E, Karp C, Strohecker AM, Guo Y, Mathew R. Role of autophagy in suppression of inflammation and cancer. Curr Opin Cell Biol 22: 212–217, 2010. doi: 10.1016/j.ceb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wilhelm BG, Mandad S, Truckenbrodt S, Kröhnert K, Schäfer C, Rammner B, Koo SJ, Claßen GA, Krauss M, Haucke V, Urlaub H, Rizzoli SO. Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344: 1023–1028, 2014. doi: 10.1126/science.1252884. [DOI] [PubMed] [Google Scholar]
- 145.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: effects of calorie restriction and life-long exercise. Exp Gerontol 45: 138–148, 2010. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xie Y, Zhou B, Lin MY, Wang S, Foust KD, Sheng ZH. Endolysosomal deficits augment mitochondria pathology in spinal motor neurons of asymptomatic fALS mice. Neuron 87: 355–370, 2015. doi: 10.1016/j.neuron.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y, Fu L. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res 264: 82–90, 2014. doi: 10.1016/j.bbr.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 148.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol 12: 814–822, 2010. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu L, McPhee CK, Zheng L, Mardones GA, Rong Y, Peng J, Mi N, Zhao Y, Liu Z, Wan F, Hailey DW, Oorschot V, Klumperman J, Baehrecke EH, Lenardo MJ. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 465: 942–946, 2010. doi: 10.1038/nature09076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yu Y, Feng L, Li J, Lan X, A L, Lv X, Zhang M, Chen L. The alteration of autophagy and apoptosis in the hippocampus of rats with natural aging-dependent cognitive deficits. Behav Brain Res 334: 155–162, 2017. doi: 10.1016/j.bbr.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 151.Zhu H, Feng YP, Young W, You SW, Shen XF, Liu YS, Ju G. Early neurosurgical intervention of spinal cord contusion: an analysis of 30 cases. Chin Med J (Engl) 121: 2473–2478, 2008. [PubMed] [Google Scholar]