Abstract
Epigenetics is the study of heritable mechanisms that can modify gene activity and phenotype without modifying the genetic code. The basis for the concept of epigenetics originated more than 2,000 yr ago as a theory to explain organismal development. However, the definition of epigenetics continues to evolve as we identify more of the components that make up the epigenome and dissect the complex manner by which they regulate and are regulated by cellular functions. A substantial and growing body of research shows that nutrition plays a significant role in regulating the epigenome. Here, we critically assess this diverse body of evidence elucidating the role of nutrition in modulating the epigenome and summarize the impact such changes have on molecular and physiological outcomes with regards to human health.
I. INTRODUCTION
Diet/nutrition has long been known to alter phenotype and play a significant role in health and disease of all living organisms (136). However, before the discovery of epigenetic mechanisms, there was an incomplete understanding of how dietary components modulate cell function, and why people vary in their responses and requirements for nutrients. Most importantly, while transient effects could potentially be explained by nongenomic mechanisms, it was unclear how dietary differences could exert seemingly stable long-term phenotypic changes. The discovery of epigenetic modifications opened a conceptual door, whereby diet and other exogenous factors could induce stable and mitotically heritable genomic changes that act in conjunction with transcriptional machinery to modify gene expression without modifying the genetic code. Environmentally induced epigenetic changes are now proposed to be responsible for a significant portion of normal and disease-related phenotypic differences that cannot be explained by differences in DNA sequence (9, 151).
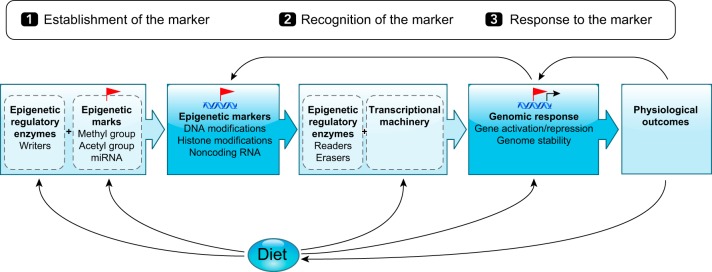
As shown in FIGURE 1, epigenetic regulation of the genome is a complex phenomenon that consists of three main steps: 1) establishment of an epigenetic marker(s) that acts as a flag to signal specific regulatory activity, 2) recognition of the epigenetic marker(s) and interpretation of what it encodes by epigenetic regulators, and 3) genomic and cellular response to the epigenetic marker based on the interpretation of the message encoded. To date, there are three recognized categories of epigenetic markers in mammals: DNA modifications and posttranslational histone modifications, which are covalent modifications to DNA and histone proteins, respectively, and noncoding RNA which act by binding directly to RNA, DNA, and proteins. Epigenetic regulatory enzymes, known as writers, readers, and erasers, are responsible for establishment, recognition, and removal of the epigenetic marker, respectively. Readers are particularly important for interpretation of the epigenetic marker, which usually involves changes to the underlying secondary DNA structure, known as chromatin, and recruitment or inhibition of transcriptional machinery at the locus. Epigenetic changes can induce genomic and cellular responses that subsequently define physiological outcomes in an organism. However, as shown in FIGURE 1, epigenetic changes can also be an outcome of cellular changes due to gross physiological changes. Therefore, it is often difficult to map out the cause-response pathway between phenotype and epigenotype.
FIGURE 1.
Epigenetic regulation of the genome. Components required and pathway of events leading to epigenetic regulation of the genome. Arrows below show components of this pathway that are altered by diet. Arrows above indicate potential for epigenetic changes to be a response to genomic or physiological outcomes rather than a causal factor.
A growing number of studies demonstrate epigenetic changes associated with dietary depletion or supplementation. We summarize these findings in TABLE 1 and discuss them throughout the manuscript. However, the exact relationship between diet and the epigenome remains unclear for most nutrients. In the simplest proposed models, nutrients contribute the molecules that comprise the epigenetic mark (e.g., methyl groups for DNA methylation). However, in more complex models, nutrients seemingly interact directly with and either induce or repress activity of epigenetic regulatory enzymes or they act as intermediate signaling molecules for dietary regulation of cell metabolism. All of these models are still under development in terms of fully understanding the interactions between diet and the epigenome, the relevant dose-response effects, the key molecules involved, and the tangible impact on human health. Below we discuss the research to date that addresses this role of diet.
Table 1.
Summary of literature findings on nutrition and epigenetics
| Nutrient Classification | Nutrient(s) | Dietary Change(s) | Developmental Window of Exposure | Model/Experimental System | Epigenetic Mechanism Perturbed | Epigenetic Outcome | Affected Cell/Tissue Type | Physiological Outcome Associated With Epigenetic Change | Reference Nos. |
|---|---|---|---|---|---|---|---|---|---|
| Fatty acids | Acetate, butyrate, and propionate | Supplementation | NA | Mouse (germ free) | Histone modifications | Changes in various posttranslational histone modifications | Liver and colon | ND | 112 |
| Fatty acids | Butyrate | Supplementation | NA | Pig cell line | Histone modifications | Reduced HDAC activity; histone hyperacetylation | Alveolar macrophage cell line | ND | 217 |
| Fatty acids | Butyrate | Supplementation | NA | Human cell line | Histone modifications | Reduced HDAC activity; histone hyperacetylation | Breast cancer cell line | ND | 43 |
| Fatty acids | Docosahexaenoic acid (DHA) | Supplementation | Gestation | Humans | DNA methylation | Hypermethylation at IGF2; Hypermethylation at H19 | Cord blood | Positive correlation with maternal body mass index (BMI) | 117 |
| Fatty acids | Fat and sucrose | Supplementation | NA | Mouse | Histone modifications | Changes in various posttranslational histone modifications | Liver and colon | ND | 112 |
| Macronutrients | Fat | Supplementation | Postweaning | Mouse | DNA methylation | 232 differentially methylated regions | Adipose tissue | Obesity and impaired glucose metabolism | 144 |
| Macronutrients | Fat | Supplementation | Gestation | Mouse | DNA methylation | Hypomethylation at leptin | Visceral fat, muscle, liver | Increased body weight, decreased glucose tolerance and insulin sensitivity | 104 |
| Macronutrients | Fat | Supplementation | Adult | Mouse | Histone modifications | Persistent changes in H3K4me2 levels and chromatic accessibility | Liver | ND | 121 |
| Macronutrients | Fat | Supplementation | Gestation | Mouse | Histone modifications | Depletion of acetylation and enrichment of H3K9 methylation at adiponectin, increased methylation of H3K20 at leptin | Adipose tissue | Metabolic syndrome-like phenotype | 135 |
| Macronutrients | Fat | Supplementation | Gestation | Rat | Histone modifications | Enrichment H3K27me3 and depletion H3K9me3 at Pepck | Liver | Fatty liver and impaired glucose metabolism | 227 |
| Macronutrients | Fat | Supplementation | Adult | Mouse | Histone modifications | Depletion of H3 acetylation | Brain | Learning and memory deficits | 178 |
| Macronutrients | Fat | Supplementation | Adult | Mouse | Noncoding RNA | Downregulation of miR-26a | Liver | Obesity and impaired glucose metabolism | 65 |
| Macronutrients | Fat | Supplementation | Gestation | Mouse | Noncoding RNA | Downregulation of miR-615-5p, miR-3079-5p, miR-124*, and miR-101b*; upregulation of miR-143* | Liver | Obesity and impaired glucose metabolism | 227 |
| Macronutrients | Fat and cholesterol | Supplementation | Adult | Mouse | Noncoding RNA | Upregulation of LeXis | Liver | Obesity and impaired glucose metabolism | 171 |
| Macronutrients | Fat and starch | Supplementation and depletion | Adult | Rat | Histone modifications | Enrichment of mono-, di-, and trimethylation of histone H3K4 at Si and Sglt1 | Gut | ND | 92 |
| Methyl donors | Betaine | Supplementation | Gestation | Pig | Noncoding RNA | Upregulation of miR-130b, miR-181a, and mir-181d | Brain | ND | 186 |
| Methyl donors | Choline | Depletion | Gestation | Rat | DNA methylation | Hypermethylation at Igf2DMR2; Hypomethylation at Dnmt1 | Liver | ND | 111 |
| Methyl donors | Choline | Supplementation | Gestation | Humans | DNA methylation | Positive correlation at CRH and NR3C1; increased methyltransferase expression | Placenta | ND | 95 |
| Methyl donors | Choline | Supplementation | Gestation | Rat | Histone modifications | Enrichment of H3K9Me2 and H3K27Me3; hypermethylation at G9a and Suv39 h1 | Liver and brain | ND | 44 |
| Methyl donors | Choline | Supplementation | Gestation | Humans | Histone modifications | Enrichment of H3K9me2 | Placenta | ND | 95 |
| Methyl donors | Choline and betaine | Depletion | Gestation | Mouse | Histone modifications | Depletion of H3K9 methylation at calbindin 1 promoter | Brain | Decreased neural progenitor cell (NPC) proliferation; increased apoptosis in cultured NPCs | 137 |
| Methyl donors | Folate | Depletion | Postweaning | Mouse | DNA methylation | Hypermethylation at Mthfr | Brain | ND | 122 |
| Methyl donors | Folate | NA | Gestation | Humans | DNA methylation | Negative and positive correlation with methylation at 433 CpGs in 320 genes | Cord blood | ND | 99 |
| Methyl donors | Folic acid | Supplementation | Gestation | Humans | DNA methylation | Hypermethylation at ZFP57 | CD4(+) cells | ND | 2 |
| Methyl donors | Folic acid | Supplementation | Pre- and during gestation | Humans | DNA methylation | Hypomethylation at H19/IGF2 | Cord blood | ND | 89 |
| Methyl donors | Folic acid | Supplementation | Periconception | Humans | DNA methylation | Hypermethylation at IGF2 | Whole blood | ND | 184 |
| Methyl donors | Folic acid | Supplementation | Gestation | Humans | DNA methylation | Hypermethylation at IGF2, hypomethylation at PEG3 and LINE-1 elements | Whole blood | ND | 74 |
| Methyl donors | Folic acid | Supplementation | Gestation | Humans | DNA methylation | Negative correlation with methylation at LINE-1 elements | Cord blood | ND | 64 |
| Methyl donors | Folic acid | Supplementation | Gestation | Humans | Histone modifications | Enrichment of H3/H4 acetylation at the ZFP57 promoter | Cord blood | ND | 2 |
| Methyl donors | Folic acid | Supplementation | NA | Human cell line | Noncoding RNA | Hypermethylation and increased expression of miR-203 and miR-375 | Cervical cells | ND | 76 |
| Methyl donors | Methionine | Depletion | Gestation | Mouse | Histone modifications | Depletion of H3K4me3, H3K27me3, and H3K4me2 | Liver | ND | 138 |
| Methyl donors | Methionine, choline | Depletion | Postweaning | Rat | DNA methylation | Global hypomethylation | Liver | Hepatocellular carcinoma | 213 |
| Methyl donors | Methionine, choline, betaine | Depletion | Gestation | Mouse | DNA methylation | Hypomethylation at Cdkn3 | Brain | Decreased proliferation of neural progenitor cells in fetal brain | 150 |
| Methyl donors | Methionine, choline, betaine, and vitamin B12 | Supplementation | Preconception to lactation | Mouse | DNA methylation | Hypermethylation at AxinFu | Tail | Kinky tail | 207 |
| Methyl donors | Methionine, choline, betaine, vitamin B12, folic acid, zinc | Supplementation | Gestation | Mouse | DNA methylation | Hypermethylation at Avy | Hair folicle | Coat color change, fat mass increase | 36, 208, 215 |
| Methyl donors | Methionine, choline, folic acid, vitamin B12 | Depletion | Postweaning | Mouse | DNA methylation | Hypermethylation at Igf2DMR2 | Kidney | ND | 210 |
| Micronutrients | Ascorbic acid (vitamin C) | Supplementation | NA | Mouse embryonic stem cells | DNA hydroxymethylation | Enhanced activity of Tet1 and Tet2, increased oxidation of 5mC (5-formylcytosine and 5-carboxylcytosine), global hypomethylation | ES cells | ND | 220 |
| Micronutrients | Biotin | Supplementation | NA | Human cell line | Histone modifications | Enrichment of H4K12 and H2AK9 biotinylation | Cell lines | ND | 30 |
| Micronutrients | Folate, zinc, and vitamins A, B12, C, and D | Supplementation | Pre- and periconception | Humans | DNA methylation | Hypomethylation at IGF2R and GTL2–2 | Cord blood | ND | 37 |
| Nutrient and caloric restriction | Various | Depletion | Periconception | Humans | DNA methylation | Hypomethylation at IGF2 | Whole blood | ND | 82 |
| Nutrient and caloric restriction | Various | Depletion | Gestation | Humans | DNA methylation | Differential methylation at INSIGF, GNASAS, MEG3, IL10, LEP, and ABCA1 | Whole blood | ND | 196 |
| Nutrient and caloric restriction | Various | Depletion | Early childhood | Humans | DNA methylation | Differential methylation at IFNG, VIPR2, ZBTB9, SYNGAP1, ABCF1, COMT, and DCTN1 | Whole blood | Increased risk of ADHD | 156 |
| Nutrient and caloric restriction; methyl donor | Various | Depletion | Pre- and periconception | Humans | DNA methylation | Hypo- and hypermethylation at several genes (BOLA3, LOC654433, EXD3, ZFYVE28, PARD6G, RBM46, and ZNF678) | Infant lymphocytes and hair follicles | ND | 50 |
| Phytochemicals | Genistein | Supplementation | Gestation | Mouse | DNA methylation | Hypermethylation at Avy | Brain, tail, kidney, liver | Coat color change, body weight decrease | 49 |
| Phytochemicals | Polyphenols (catechin, epicatechin, epigallocatechin-3-O-gallate) and bioflavonoids (quercetin, fisetin, and myricetin) | NA | NA | In vitro | DNA methylation | Inhibition of DNMT1 activity, hypomethylation | NA | ND | 120 |
| Phytochemicals | Sulforaphane | Supplementation | NA | Human cell line | DNA methylation | Hypomethylation at Cyclin D2, global hypomethylation | Benign prostate hyperplasia (BPH-1) cells | ND | 90 |
| Protein-energy restriction | Gross calories | Depletion | Gestation | Mouse | DNA methylation | Genome-wide hypo- and hypermethylation | Sperm | Low birth weight, early adiposity, reduced stem cells, impaired pancreatic function, glucose intolerance | 161 |
| Protein-energy restriction | Gross calories | Depletion | Pre- and periconception | Sheep | DNA methylation | Hypomethylation at H19/Igf2 ICR | Adrenal gland | Decreased adrenal mass and increased cortisol response to stress | 225 |
| Protein-energy restriction | Gross calories | Depletion | Adult | Nonhuman primates | Noncoding RNA | Reversal of age-associated miRNA profiles | Skeletal muscle | ND | 139 |
| Protein-energy restriction | Gross calories | Depletion | Adult | Mouse | Noncoding RNA | Downregulation of miR-181a-1*, miR-30e, and miR-34a | Brain | ND | 105 |
| Protein-energy restriction | Gross calories | Depletion | Adult | Fly | Noncoding RNA | Upregulation of miR-310 family | Whole body | ND | 33 |
| Protein-energy restriction | Protein | Depletion | Gestation | Rat | DNA methylation | Global Hypermethylation | Liver | Reduced growth | 164 |
| Protein-energy restriction | Protein | Depletion | Gestation | Rat | DNA methylation | Hypomethylation of glucocorticoid receptor and PPARa | Liver | ND | 126 |
| Protein-energy restriction | Protein | Depletion | Gestation | Rat | DNA methylation | Hypermethylation at Hnf4a | Pancreatic islets | T2D like phenotype | 173 |
| Protein-energy restriction | Protein to high fat | Depletion to supplementation | Gestation to postweaning | Rat | DNA methylation | Hypermethylation at H19 | Adipocytes | Increased adiposity, decreased insulin sensitivity | 34 |
NA, not applicable; ND, not determined.
II. DEFINING THE MAMMALIAN EPIGENOME
The genome encodes the basic instructions for cellular function in all living things. The genome of a single fertilized mammalian egg (zygote) contains one copy of DNA from each parent that must be accurately replicated and propagated to all daughter cells during mitotic cell division. Therefore, all of an individual’s somatic cells throughout the body share the same DNA throughout life (with the exception of cells with naturally occurring copy number variation and de novo mutations). This is despite the fact that cells in the body have highly diverse functions in the different tissues and organs and at different stages of development. Functional differences in genetically identical cell types is achieved through cell type specific differences in interpretation of the DNA code. The epigenome can be defined broadly as a complex network of signals that are recognized by cellular machinery and used to interpret the DNA. However, unlike the DNA code, the epigenome can be altered between cell types and developmental stages to allow for spatial and temporal specific interpretation of the DNA. The plasticity and multiplicity of these epigenetic signals allow for a diverse array of stable cellular changes without altering the integrity of the primary DNA code.
A primitive concept of epigenetics was conceived well before DNA and genes were recognized as an essential part of life. This was first recorded by Aristotle (384–322 B.C.) in his theory of development proposing that complex organisms developed in a progressive manner from a simple parentally contributed germplasm (6). In the mid 17th century, William Harvey coined the term epigenesis to describe the progressive developmental stages of chicken embryos (47). At the time, epigenesis contradicted previous development hypotheses that proposed enlargement of a preformed complex individual. To date, the role of prepatterned events versus stochastic events during development remains under debate (181).
By 1942, Conrad Waddington proposed the term epigenetics (a combination of “epigenesis” and “genetics”) to describe the study of developmental processes that connect genotype to phenotype, which he called the “epigenotype” (200). Although he incorrectly believed that, similar to yeast, the presence or absence of particular genes determined the epigenotype (47, 78, 119), he was far ahead of his time in proposing that perturbation of epigenotypes early in life could cause “far-reaching abnormalities in many different organs and tissues” (200). Waddington (201) also popularized the idea that epigenotype is responsible for cell fate during development proposing that totipotent embryonic cells differentiate by means of factors that “steer” the developing systems into “particular channels” (i.e., landscapes).
It was another 40 yrs before an experimental model definitively demonstrated that changes in epigenetic markings in the genome were capable of changing cell fate in a stable manner (98). The currently accepted model of how epigenotype regulates phenotype is that it acts by regulating accessibility of DNA, RNA, and protein targets to the cellular machinery required to properly replicate, translate, and transcribe the genome. In this way, epigenetic mechanisms play a critical role in determining development, health, and disease outcomes.
A. Epigenetic Markers
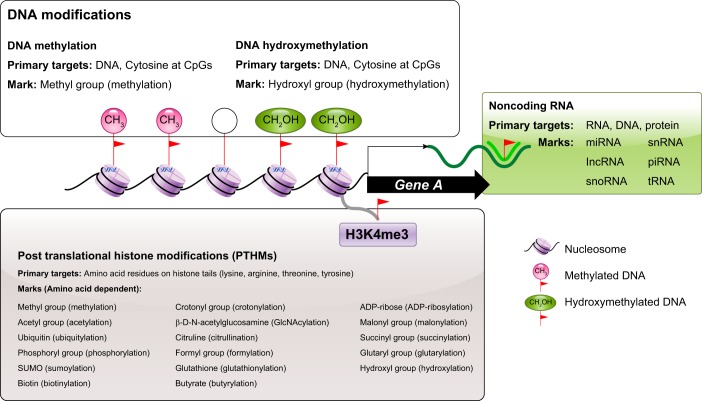
As illustrated in FIGURE 2, what we know of the epigenome today is that it is composed of three major types of epigenetic markers and their regulatory proteins. Known mammalian epigenetic markers include 1) covalent DNA modifications: methylation and hydroxymethylation of the cytosine at CpG dinucleotides; 2) numerous covalent posttranslational modifications made to the tails of histone proteins that make up the core of nucleosomes around which the DNA is wrapped; and 3) numerous sizes and types of noncoding RNA that bind to DNA, RNA, and protein targets. These different epigenetic markers usually act in a cooperative manner to determine chromatin conditions either favorable or inhibitory of gene expression.
FIGURE 2.
Epigenetic markers and targets. Three categories of epigenetic markers, their primary targets, and most common marks found in mammals. Closed circles represent methylated CpGs, shaded circles represent hydroxmethylated CpGs, and open circles represent unmodified CpGs.
1. DNA modifications
DNA methylation is the most widely studied epigenetic mark since it was the first epigenetic marker identified that could regulate gene expression and thereby mediate the relationship between genotype and phenotype. Because of its stability and prevalence in higher organisms, DNA methylation was proposed early on in the 1970s to be the mechanism by which cells stably maintained their differentiated state during division and also for switching genes on/off during development (84, 86, 167). Experimental evidence later supported this model by showing that inhibition of methylation by 5-azacytidine could change cell differentiation status (98) and that allelic methylation differences in the absence of DNA sequence differences can differentially regulate transcription of alleles at genes nearby in a phenomenon known as genomic imprinting (10, 11).
DNA becomes methylated by the addition of a methyl group (CH3) to carbon five in cytosine to generate 5-methylcytosine (5mC). In mammals, 5mC occurs primarily at palindromic CG dinucleotides such that both strands of DNA can be methylated (188). Methylated CA, CC, and CT dinucleotides can also be detected at lower frequency, but their role in the mammalian genome remains unclear (80). 5mC is generated by enzymatic activity of DNA methyltransferases (DNMTs). These writers of 5mC function by transferring a methyl group from S-adenosylmethionine (SAM) to cytosine (152). DNMT3a and -3b are responsible for de novo methylation of unmethylated CpGs while DNMT1 recognizes hemimethylated CpGs (usually at newly replicated DNA) and methylates the other strand. 5mC is recognized (read) by methyl-CpG binding domain proteins, such as MeCP2, which bind to the methylated locus and recruit additional factors necessary for transcriptional regulation (202).
The presence of 5mC at a locus is associated with either gene repression or activation depending on proximity to DNA regulatory elements such as promoters, transcript splice sites, enhancers, or insulators (97). For example, while 5mC at promoter regions is often associated with gene repression, 5mC in the gene body (introns and exons) is often associated with gene activation (97). Accumulating evidence suggests that 5mC does not initiate gene silencing but rather acts as a mechanism of maintaining the silenced state of a gene (97). Therefore, the function of 5mC is likely preceded by other repressive epigenetic marks. Data suggest that with the exception of cases where 5mC is directly within a transcription binding site, the impact of CpG methylation is often determined by mean methylation across a cluster/domain of CpGs versus differences at individual CpGs (75, 93).
5-Hydroxymethylcytosine (5hmC) is a relatively new epigenetic marker, and its role in genome regulation is less well understood. Until recently, most conventional detection methods (e.g., bisulfite conversion) could not differentiate between 5mC and 5hmC. However, new methods that allow for distinction of 5mC and 5hmC show that it is highly prevalent in specific cell types (e.g., stem cells and brain cells) and linked to gene activation (182). Initially, conversion of 5mC to 5hmC was proposed to function as either by causing loci to become unrecognizable to 5mC writers or readers or as an intermediate stage before full demethylation (passive mechanism–replication without remethylation) since 5hmC cannot be directly remethylated (189, 190). However, recent studies show that 5hmC has its own readers, such as UHRF2, and thus likely functions in a manner distinct from methylated and unmethylated cytosine (27, 129).
Unlike the DNA sequence, 5mC marks are changeable and can be removed passively by failure to remethylate the new daughter strand during DNA replication (216). 5mC can also be removed actively through either deamination of 5mC by the apolipoprotein B mRNA editing enzyme catalytic polypeptide-like (APOBEC) (108) or by conversion of the methyl group to a hydroxyl group by ten-eleven-translocation (TET) enzymes (158, 189). Deamination converts the cytosine base to thymine causing a genetic mutation that must be fixed by base excision repair to maintain the integrity of the genome (108). Hydroxymethylation mediated by TET enzymes generates 5hmC, which can be further modified to 5-formylcytosine (5fC) and 5-carboxylcytosine (5aC), the latter which can be recognized and converted back to cytosine by base excision repair (81).
2. Posttranslational histone modifications
The development of chromatin immunoprecipitation (ChIP) methods in 1984 (70) made possible the identification of histone proteins bound to DNA and enabled characterization of their role in epigenetic regulation of the genome. DNA is organized in the nucleus by sections that are wrapped around protein complexes called nucleosomes (115). The nucleosome is the key component of chromatin, and nucleosomal occupancy (arrangement and density at a locus) determines chromatin compaction and accessibility of the DNA to transcriptional machinery (115). Each nucleosome consists of eight histone proteins, two copies each of four core histones: H2A, H2B, H3, and H4, around which 147 bp of DNA is wrapped (115). Variants of these core histone proteins alternate to make up the nucleosome, but these occur at lower frequency. Histones function primarily by determining nucleosome occupancy to generate “active” versus closed “inactive” chromatin configuration. This function is regulated by the presence of covalent posttranslational histone modifications (PTHMs) made to the amino-terminal “tails” of histones (FIGURE 2) (185).
The role of PTHMs as epigenetic regulators of gene expression was demonstrated for the first time in 1996 when acetyl group attachment to the histone tails of nucleosomes altered expression of genes at the site of modification (21, 193). To date, numerous PTHMs have been identified including, but not limited to, methylation (addition of 1, 2, or 3 methyl groups), acetylation, propionylation, 2-hydroxiosbutyrylation, succinylation, phosphorylation, ubiquitylation, biotinylation, GlcNAcylation, citrullination, crotonylation, formylation, glutathionylation, butyrylation, ADP-ribosylation, malonylation, hydroxylation, oxidation, and proline isomerization (FIGURE 2) (226). These modifications are written, read, and erased by epigenetic regulatory enzymes that are specific to the modification (and in the case of methylation, the number of methyl groups) and amino acid residue being modified. In fact, a recent assessment reported that humans have at least 50 different histone methyltransferases (HMTs, methylation writers), 28 different histone methylation demethylases (HDMs, methylation erasers), and 147 different histone methylation readers (218).
The functions of PTHMs are histone and tail-location dependent and are described to act in a “combinatorial or sequential fashion . . . [to] specify unique downstream functions” in what is known as the “histone code” (185). This code has not yet been fully deciphered; however, functional characterization for some common PTHMs are well studied. Some well-characterized examples include the classification of H3K4, H3K36, and H3K79 methylation as “active” marks and H3K9, H3K27, and H4K20 methylation as “silencing” marks (17). An exception to this rule is found when both active and repressive marks are present, which was recently shown to indicate regions that are silenced but poised for quick activation when required by the cell (16). The histone code is recognized by chromatin remodeling complexes that act on the chromatin by rearranging nucleosome spacing to create a transcriptionally accessible state (heterochromatin) or a transcriptionally repressed state (euchromatin) (128).
Active histone modifications have been shown to coincide with active DNA methylation marks, and many examples of “crosstalk” between the two modifications have been described. In one example, hyperacetylation of lysines on histones H3 and H4 leads to release of DNMT1 from the DNMT1/proliferating cell nuclear antigen (PCNA) complex, and this results in hypomethylation of DNA (103). The interdependence of these epigenetic mechanisms has been extensively reviewed (26, 54, 109, 168).
3. Noncoding RNAs
Noncoding RNAs represent a more recently discovered mechanism of epigenetic regulation (123). The first evidence that the transcriptome contained many non-protein coding RNAs was reported in 2002 (102). We now realize that these RNAs contribute an important mechanism of epigenetic regulation of the genome (48). Unlike protein coding genes, noncoding RNA do not need to be translated into proteins to function. Furthermore, this class of epigenetic markers can interact with DNA, RNA, or protein to regulate gene expression, transcription, and posttranscriptional activity, respectively (85).
There are numerous types of noncoding RNA, classified by size, localization, function, and targets (25). However, the most well-characterized in terms of epigenetic function and modulation by diet are microRNA (miRNA). MiRNA are short, ~22-bp nucleotide long, noncoding RNA that are generated from longer transcripts by two RNase III proteins, Drosha and Dicer (72). MiRNA bind complementary mRNA targets via the RISC complex and mark targeted mRNA for cleavage, degradation, or translational repression depending on the degree of base pair matching (170).
Although miRNA activity can be modified by changes in transcriptional levels of the full-length parent mRNA, it is more likely to be modified by changes in miRNA biogenesis, which are temporally and spatially regulated (72). MiRNA activity is also dependent on cellular localization, timing, and interactions with multiple targets. Therefore, these remain the most challenging epigenetic mechanism to accurately assess. Interestingly, many miRNAs are regulated at the transcriptional level by DNA methylation. For example, DNA methylation of miRNA 1451–5p inhibits its expression (53). DNA methylation, histone modifications, and noncoding RNA are known to work together through separate but collaborative functions (185).
B. Plasticity of the Epigenome: Reprogramming, Epimutations, Stability, and Heritability
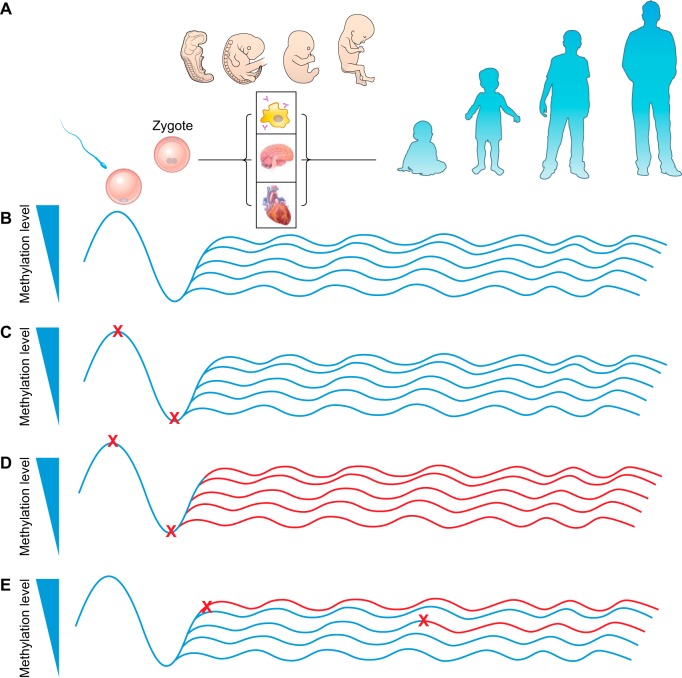
Changes in of the level of 5mC across the genome occur naturally in a temporally and spatially dependent manner. The greatest extent of normal change occurs during embryonic development when DNA methylation levels cycle from high to low in a process known as epigenetic reprogramming (165, 172). During reprogramming, the genome undergoes widespread erasure of 5mC marks followed by reestablishment in a cell lineage specific manner (FIGURE 3A). This event occurs at separate times in germ cells and somatic cells. Interestingly, as 5mC marks are depleted, 5hmC marks are enriched temporarily during this erasure process before also being depleted (140). This resetting of the epigenome is an intrinsic part of mammalian development and is required to transition from a single cell zygote into a multicellular, multiorgan organism. While resetting of the epigenome also helps in part to prevent transmission of epimutations between generations, it may also be a window of vulnerability of the epigenome to stress-induced epimutations since it requires a great deal of precision for the machinery responsible in reestablishing (from “memory”) the epigenetic marks specific to each cell type in the body.
FIGURE 3.
Proposed model of timing of epimutation event and stability over lifespan. Model shows how the timing of environmentally induced epimutations may differentially affect epigenetic state across the lifespan. A: human developmental stages across lifespan. B: unperturbed DNA methylation levels at different stages of human development depicted in A. Methylation levels are shown increasing on the y-axis; each horizontal blue line indicates unperturbed methylation levels for different cell lineages in body, and waves in line indicate naturally occurring minor fluctuations in methylation levels over time. C–E: epimutation initiated during different stages of development (red “X” indicates timing of event) and mitotic inheritance/stability over the developmental timeline (red line indicates perturbed epigenetic state). C: epimutation initiated in germ cells but reset during somatic cell epigenetic reprogramming. D: epimutation initiated in germ cells or zygote and not reset during somatic cell epigenetic reprogramming affects multiple cell lineages and may persist throughout lifespan. E: epimutation initiated after somatic cell lineage determination/epigenetic reprogramming is usually cell lineage specific.
Establishment of the epigenetic patterns required for cell lineage specific development is inherited from the previous generation, but to date the mechanism responsible for this memory is unclear. Furthermore, it is unclear how it is determined that cell lineages have fully established the appropriate epigenetic marks to switch to maintenance mechanisms such that the epigenome is mitotically inherited every time the cell replicates (28). This involves remethylation of the newly methylated copy of DNA, reassembly of the nucleosome structure with the appropriate histone modifications, retargeting of noncoding RNA to their RNA, DNA, and protein targets in the new cell and reassembly of all the complexes required to carry out these events (28).
Epimutations, or heritable/stable changes to the epigenome that are not part of the normal cyclic developmentally regulated pattern of epigenetic programming, can either occur due to stochastic errors in establishment or maintenance mechanisms or due to exogenous stressors such as changes in age, environment, health, and diet. These are not necessarily deleterious changes but may also be adaptive changes that do not have a detrimental effect on health. The rate of epimutations is estimated to be one to two orders of magnitude higher than the rate of genetic mutation in the soma (15). Since there are no known dedicated repair mechanisms for damage/changes to the epigenome, it is thought that epimutations once generated are likely maintained until they can be reprogrammed during embryonic development. Therefore, timing of insult plays a major role in the cells or organs to be affected. Epimutations occurring in the germ cell (germline epimutation) will most likely be reset/“repaired” during somatic cell development and those that are not repaired will likely persist into all of the soma in a non-cell type-dependent manner (FIGURE 3, C and D). Likewise, epimutations occurring in the zygote before the first cell division are also more likely to propagate into all of the soma in a non-cell type-dependent manner (FIGURE 3D). However, epimutations occurring after reestablishment of the epigenome during reprogramming will only be present in the individual cell/tissue types initially affected and not throughout the organism resulting in an epigenetic mosaic phenotype in that tissue (FIGURE 3E). Therefore, unless the timing of insult is known, one of the main challenges to understanding the relationship between epigenotype and phenotype is an inability to pinpoint the primary causal epimutation responsible for phenotypic outcomes.
III. ROLE OF DIET/NUTRITION IN REGULATING THE EPIGENOME
As introduced above, dietary changes can induce epigenetic changes (49, 137, 207, 211, 215). It would be logical to propose that dietary modulation of the epigenome is an intrinsic feature that allows cells to adapt their metabolic state to best match food availability. Nutrition and energy metabolism are among the most important functions needed for organismal fitness and survival and therefore are potent drivers of evolution (57). Growing evidence suggests that some epigenetic mechanisms may have evolved in part to more rapidly sense and respond to changes in nutrient availability (66, 79, 180). Such “nutrient responsive” epigenetic mechanisms could act directly on nutrient metabolism genes to up- or downregulate pathways that regulate bioavailability of the altered nutrient, or could act on metabolic pathways downstream of the altered nutrient. To understand the full impact of diet-induced epigenetic changes on human health, ongoing studies need to identify causal nutrients, direct mechanisms by which nutrients induce epigenetic changes, the timing of exposure where individuals are susceptible to change (windows of susceptibility), the extent of change, the stability of epimutations, and mechanisms by which epigenetic changes lead to change in physiological function or disease. To date, science has identified a number of nutrients that modify epigenetics in animal models, with some modest data from human studies. For some of these causal nutrients, viable mechanisms have been identified by which these nutrients induce epigenetic changes, but few such mechanisms are proven. For a few of these nutrients, periods of exposure have been identified where individuals are susceptible to epigenetic change, and there is some evidence as to stability of these epimutations. Little is understood about the mechanisms where nutrient-caused changes in epigenetics results in health or disease. Below we discuss the nutrients for which we can reasonably address the above criteria. The collection of studies discussed showing dietary modulation of the epigenome have been summarized in TABLE 1.
A. Dietary Modulation of DNA Modifications
1. Diet and epigenetics in the honeybee
One of the best examples of a profound and seemingly direct diet-mediated epigenetic effect occurs in honeybee colonies where the behavior, function, lifespan, and morphology of genetically identical bees can be changed in response to a specific food. All larvae are initially fed with “royal jelly,” but worker larvae are soon switched to a diet of pollen and nectar, whereas queen larvae are fed royal jelly throughout their larval and adult development (31). The chemical composition of this jelly is only partially understood, and it contains proteins, amino acids, fatty acids, and vitamins including histone deacetylase inhibitors [phenyl butyrate (31)], microRNAs, and other factors. MRJP1, the most abundant protein in royal jelly (134), is involved in queen differentiation via activation of p70 S6 kinase and the epidermal growth factor receptor (EGFR)-mediated signaling pathway (101, 134). It is hypothesized that these mediate epigenetic effects in the bee. Although all of the epigenetically active components of royal jelly are yet to be described, we know that silencing DNMT3 expression in honeybee larvae mimics the effect of royal jelly in that the larvae normally destined to become workers develop into queens with fully developed ovaries (113); there are over 550 differentially methylated genes between queens and workers (31, 59, 91). Thus diet and epigenetics allow different phenotypes to be generated from identical genomes. Epigenetically mediated effects of a similar, but smaller proportion have been identified in mammals (see discussion below on methyl-indicator mice).
2. Nutrients as methyl group donors
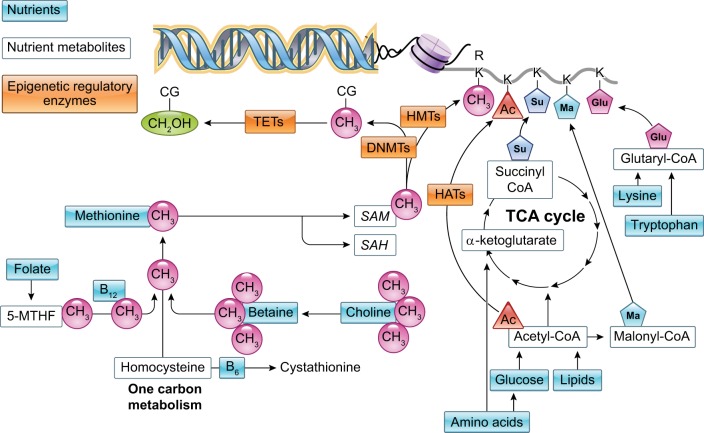
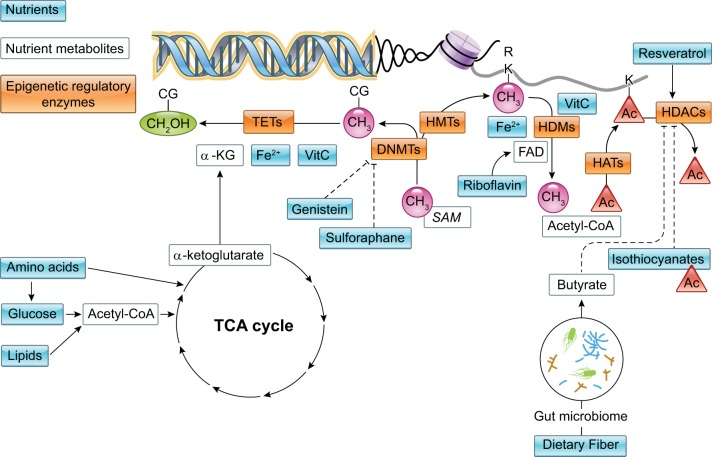
DNA and histone methylation are directly dependent on the availability of methyl groups derived from diet (methyl-folate, choline, betaine, or methionine). During one carbon metabolism, these nutrients are metabolized in pathways dependent on other nutrients such as vitamins B12 or B6 (224) to form S-adenosylmethionine (AdoMet), the metabolite source of methyl groups for DNA methylation (FIGURE 4). 5mC is generated when a methyl group is transferred from AdoMet to cytosine by DNMTs (FIGURE 4). Methylation of the histone tail residues lysine and arginine also utilizes a methyl group from AdoMet via HMT activity (FIGURE 4). When AdoMet donates a methyl group, S-adenosylhomocysteine (AdoHcy) is formed, which competes with AdoMet for the substrate binding site on all methyltransferases; thus methylation potential is proportional to the ratio of concentrations of AdoHcy to AdoMet in the tissue (132).
FIGURE 4.
Nutrients as donors of epigenetic marks. Components of the one carbon metabolism pathway and TCA cycle that directly contribute the epigenetic marks for DNA and histone modifications. C, cytosine; G, guanine; CH3, methyl group; CH2OH, hydroxylmethyl group; Ac, acetyl group; Su, succinyl group; Ma, malonyl group; Glu, glutaryl group; TETs, ten-eleven-translocation enzymes; DNMTs, DNA methyltransferase enzymes; HMTs, histone methyltransferase enzymes; HATs, histone acetyltransferase enzymes; K, lysine; R, arginine; 5-MTHF, 5-methyltetrahydrofolate; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine.
The first suggestion that diet could modify DNA methylation was reported in 1984, when Poirer and colleagues (213) observed that rats fed a diet very low in methyl donors had decreased methylation of cytosines in hepatic nuclear DNA. These changes were associated with the development of liver cancers on this diet (68, 148, 213). Subsequently, these diet-induced changes in hepatic DNA methylation were associated with changes in hepatic gene expression (204). Normally, epigenetic marks are established very early in life and then are copied and maintained during cell replications (49). However, it is common for epigenetic marks to be erased and changed in cancer cells. Thus it is difficult to be sure whether diet changes or the carcinogenic transformation in methyl-deficient liver was the cause of changes in epigenetic marks in these studies.
The relationships between dietary methyl donors and epigenetic modifications became more apparent in studies using methylation indicator-mouse models in which changes in DNA methylation resulted in easily visible and long-lasting phenotypes in offspring. Mice bearing the dominant mutation ‟viable yellowˮ (Avy) allele of the agouti gene have an insertion of an intracisternal A particle (IAP) sequence preceding the first coding exon, placing the gene under the control of the IAP promoter/enhancer. IAP expression is epigenetically regulated (215); the proximal IAP long terminal repeat is normally hypomethylated when Avy is expressed. This results in obesity and hyperinsulinemia, as well as in yellow hair (215). When the IAP repeat is hypermethylated, Avy is not transcribed while the normal A allele is, and the mouse is lean and brown. Wolff et al. (215) observed that feeding pregnant Avy/A mice a diet high in methionine, choline, betaine, vitamin B12, and folic acid resulted in offspring born with hypermethylated IAP locus and less Avy expression, and they were more likely to be lean and brown rather than the normal fat and yellow phenotype (215). This effect of dietary methyl donors on Avy only occurred when the male parental allele was expressed (40). There was a critical period during which the epigenetic marks were established, and after this time window had passed, the marks were stable; for IAP, DNA methylation status was determined in utero and was maintained thereafter, thus returning mice to normal diets after birth did not change IAP DNA methylation or restore mouse phenotype. There is some conflicting evidence as to how these diet-induced epigenetic changes can be inherited transgenerationally (40, 211).
Waterland confirmed and expanded these observations about perinatal diet and Avy expression (208). Also, he used another methylation indicator mouse to show that maternal dietary intake modulated other fetal epigenetic marks. The Axin fused [Axin(Fu)] gene, when expressed, causes mice to develop kinks in their tails during development. When pregnant mice were fed diets high in methionine, choline, betaine, and vitamin B12, Axin(Fu) was hypermethylated and its expression was suppressed, and progeny had tails with fewer kinks (207). Again, the sensitive period for maternal diet changes was the in utero period during which the epigenetic marks were normally established; returning mice to normal diets after birth did not change these epigenetic marks or the tail phenotype (207).
These studies in methyl-indicator mouse strains show that the dietary intake of the pregnant mother modulates epigenetic marking in the fetus. However, both indicator models study methylation of a gene that contains a retrotransposon, likely inserted by a retrovirus into the human genome. It is possible that retrotransposons behave differently, in terms of susceptibility to epigenetic regulation, than do other regions of DNA (58). For this reason, it is important to determine whether DNA methylation in other genes is altered by dietary methyl donors.
Imprinted genes, for which the paternal or the maternal gene is preferentially expressed, also can be modulated by diet. H19 and Igf2 are two coregulated imprinted genes that share enhancers and a differentially methylated imprinting control region, ICR (11, 13, 46). An important growth-stimulating factor, insulin-like growth factor II (IGF2), is expressed from the paternal allele. H19 is maternally expressed and encodes a long noncoding RNA that negatively regulates Igf2 expression. Decreased expression of H19 is associated with increased expression of Igf2 (34). Methylation at a differentially methylated region within the Igf2 coding region (Igf2DMR2) is sensitive to diet during gestation. Igf2DMR2 in fetal liver was hypermethylated when pregnant rats were fed choline-deficient diets (111). This occurred in parallel with hypomethylation of the regulatory CpGs within the maintenance DNA methyltransferase 1 (Dnmt1) gene (69, 111), leading to its overexpression and increased gene-specific DNA methylation (111).
The effects of maternal methyl donor availability on epigenetic marks are not limited to Igf2 and H19. Feeding pregnant mice a diet low in choline, betaine, and methionine decreased gene-specific DNA methylation of Cdkn3, which encodes for kinase-associated phosphatase (Kap) in fetal brain. These methylation changes correlated with increased expression of Cdkn3, which inhibits cell cycling (150), and decreased proliferation of neural progenitor cells in fetal brain (39, 150). In people, the effect of maternal choline intake (930 vs. 480 mg/day) on the epigenetic state and expression of cortisol-regulating genes in women was studied in placenta and cord venous blood. Women eating more choline had hypermethylated promoter region of the corticotrophin releasing hormone (CRH) and glucocorticoid receptor (NR3C1) genes in the placenta. This was associated with lower placental CRH transcript abundance. In addition, there was increased expression of several placental methyltransferases (95). Thus there is a reasonable body of evidence in animal models, and a small amount of data from human studies, showing that the availability of methyl donors in the diet can modify DNA methylation. The most compelling evidence comes from studies that manipulate diet during pregnancy and examine fetal DNA methylation; we previously discussed how early development is a period when cells are susceptible to changing epigenetic marks. There are limited data that these effects of early-life exposure to supplemented dietary methyl donors are heritable, as such exposure was shown to increase epigenetic variation in isogenic mice over six generations (124).
We do not yet fully understand the exact mechanisms whereby dietary methyl group intake alters the epigenome. The obvious mechanism has been previously discussed: that the ratio of AdoMet to AdoHcy concentrations in tissues is changed by the diet, and that this directly alters the activity of DNA methyltransferases. However, in many of the above studies, investigators note regions of DNA that are hypermethylated, while others are hypomethylated after the dietary exposure. This suggests more complex mechanisms underlie the effects of dietary methyl donors on the epigenome.
3. Folic acid availability and DNA methylation
To reduce the number of babies born with neural tube defects, fortification of the food supply with folic acid in the United States and in number of other countries is mandatory (7). In addition, 0.4 mg folic acid per day (or higher) is recommended worldwide before and in the very early stages of pregnancy by obstetricians. Based on the animal studies discussed earlier, it is reasonable that maternal intake of folic acid could alter epigenetic marks. Meta-analysis of two European pregnancy cohorts of Caucasian ancestry in which epigenome-wide DNA methylation was assessed in 1,988 samples of newborn cord blood, observed that methylation of 443 CpGs (in 320 genes; 416 hypomethylated, 27 hypermethylated) was significantly associated with increased maternal plasma folate concentrations. Of the 365 CpGs associated with folate that were able to be matched to a gene transcript, 43 CpGs were significantly associated with altered expression of nearby genes (99). A smaller study investigated DNA methylation in CD4(+) and antigen-presenting cells isolated from neonatal cord blood in women who were folic acid supplemented during pregnancy and observed differential methylation upstream of the gene ZFP57, a regulator of DNA methylation during development (2). ZFP57 mRNA expression was higher in the high folate group relative to the low folate group (2). Maternal supplementation with folic acid before and during pregnancy modified DNA methylation at two differentially methylated regions (DMRs) at the H19/IGF2 region that regulate IGF2 expression in umbilical cord blood leukocytes of their infants (89). Compared with infants born to women reporting no folic acid intake before or during pregnancy, methylation levels at an H19 DMR decreased with increasing folic acid intake. This methylation decrease was most pronounced in male infants (89). In a separate study, IGF2 DMR methylation was assessed; children of mothers who used folic acid had a 4.5% higher methylation of the IGF2 DMR than did children who were not exposed to folic acid. IGF2 DMR methylation of the children also was associated with AdoMet blood concentrations of the mother but not of the child (184). In a prospective cohort study of pregnancies in the United Kingdom between 2000 and 2006, folic acid supplement use after 12 wk of gestation was associated with a higher level of CpG methylation in IGF2 and reduced methylation in both paternally expressed gene 3 (PEG3) and long interspersed nuclear element 1 (LINE-1) in DNA from blood (74). Other studies report that concentrations of folate-associated intermediates in cord blood during late pregnancy were negatively correlated with the level of methylation of LINE-1 repetitive elements in cord blood lymphocyte samples in offspring of mothers taking daily folic acid supplements during pregnancy (64).
Folate metabolism is regulated by the enzyme 5,10-methylenetetrahydrofolate reductase (MTHFR) which catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is required for homocysteine remethylation to methionine. When Balb/c mice were fed a folate-deficient diet, methylation of CpGs in a region immediately upstream of the Mthfr translational start site was decreased in liver, and expression of the gene was increased. This observation is consistent with a role for folate-dependent gene methylation in regulation of Mthfr, since several transcription start sites were identified in this region (122).
Is the above data just an extension of methyl-donor effects on the epigenome? 5-Methyl-tetrahydrofolate has an important role in 1-carbon metabolism pathways leading to AdoMet synthesis, but other forms of folate (e.g., 10-formyl-tetrahydrofolate) are important for purine synthesis. Food contains multiple forms of folates, but not folic acid, which is a synthetic form used in supplements that must be converted to tetrahydrofolate before it can be used in mammalian cells. Pregnant women supplemented with folic acid accumulate unmetabolized folic acid in maternal and umbilical cord samples (157), which can compete with tetrahydrofolates for enzyme binding sites. Accumulation of unmetabolized folic acid can perturb fetal development (141). Thus it may be important to consider the source of folates when interpreting studies on folate effects on the epigenome. As noted earlier, these human data are from observational studies and can only be used to identify associations that support hypotheses identified in cell and animal models.
4. Protein-energy restriction and DNA methylation
Dietary methyl donor status of the pregnant female is not the only diet modulator of DNA methylation. Protein restriction is used as a model for maternal malnutrition. Fetuses born of dams fed a protein-restricted diet during pregnancy have global DNA hypermethylation in liver (164) as well as locus-specific changes in DNA methylation. In juvenile and adult offspring of rat dams fed a protein-restricted diet during pregnancy, the glucocorticoid receptor and PPARα promoters are hypomethylated in liver (22, 126). Similar changes in fetal liver glucocorticoid receptor DNA methylation were seen when pig sows were fed a low-protein diet (94). Pregnant mice who were protein restricted had offspring in which hepatic DNA had more than 200 differentially methylated genes (199). Offspring of rat dams fed a low-protein diet during pregnancy and lactation exhibit hypermethylated and repressed transcription factor hepatocyte nuclear factor 4 (Hnf4α) in pancreatic islets (173). Feeding pregnant rats a low-protein diet and then feeding their pups a high-fat, normal-protein diet resulted in increased expression of Igf2 in adipose tissue due to increased methylation of cytosines in H19 (34). Low-protein diet fed to rats at the time of conception also resulted in a decrease in H19 and Igf2 expression (114). We do not know the exact mechanisms whereby protein restriction causes changes in DNA methylation, and some investigators hypothesize that protein restriction limits methionine intake that causes alterations in the AdoMet pathways discussed earlier (127).
Energy restriction during prenatal life in mice alters male germline DNA methylation and results in differentially methylated regions that are associated with altered gene expression in the offspring of these males (161). Thus paternal diet in utero, even when the nutritional status is normalized later in life, causes epigenetic reprogramming in their offspring. Energy restriction during pregnancy also modifies DNA methylation in the fetus. Offspring of sheep fed a modestly energy-restricted diet during pregnancy had decreased methylation in Igf2/H19 in the adrenal gland with decreased Igf2 mRNA expression (225). Conversely, overfeeding also modifies DNA methylation. Offspring of mouse dams fed a high-fat diet during late gestation had decreased methylation of the leptin gene and increased expression of leptin, but had increased methylation of genes encoding the adiponectin and leptin receptors resulting in reduced mRNA expression of these receptors (104).
There is much less data about the relationships between diet pattern and DNA methylation in humans. Available data are derived from observational studies because there are no controlled intervention studies. Undernutrition in pregnancy is associated with both hypo- and hypermethylation of DNA in the children born of these pregnancies (50, 196, 209). Seasonal variations in diet can be extreme in some populations. In The Gambia, West Africa, there is a protein-energy-limited rainy (‟hungryˮ) season and a better nourished dry (‟harvestˮ) season. There are significant seasonal variations in methyl-donor nutrient intake of mothers around the time of conception; maternal periconceptional plasma concentrations of folate, riboflavin, methionine, betaine, and the AdoMet/AdoHcy ratio were higher in the rainy season (50). The concentrations of these maternal biomarkers were associated with increased/decreased DNA methylation postnatally at several genes in infant lymphocytes and hair follicles (BOLA3, LOC654433, EXD3, ZFYVE28, PARD6G, RBM46, and ZNF678). DNA methylation was highest in children conceived in the protein-energy-limited rainy (hungry) season compared with children conceived in the dry (harvest) season (50, 209).
The effects of protein-energy malnutrition at the time of conception were studied in 350 births with periconceptional exposure to the Dutch famine of 1944–45 selected from three birth clinics, as well as in 290 births from these clinics born before or after the famine as unexposed controls and 307 same-sex siblings of either birth group as unexposed family controls. Blood DNA samples were collected at a mean age of 58. In people exposed to periconceptional famine in early development, compared with same-sex siblings conceived before or after the famine, there was a 5% decrease in DNA methylation at the IGF2 gene locus (82). When two repetitive elements, LINE-1 (long interspersed nucleotide element 1) and Sat2 (Satellite 2 DNA sequence) were analyzed, no relation between overall global DNA methylation in adults and periconceptional famine exposure was observed (131).
Because undernutrition is associated with both hyper- and hypomethylation in specific regions of DNA, the underlying mechanisms likely involve more than just variation in methyl-donor status. Many of the specific genes that are differentially methylated in the above studies are important for regulation of growth, suggesting there could be an adaptive change in metabolism in response to diet restriction, but the selection of genes to be studied was likely biased by knowledge of their function.
5. Phytochemical supplementation and DNA methylation
Foods also can contain bioactive phytochemicals that alter DNA methylation. Sulforaphane, a bioactive component of cruciferous vegetables, downregulates DNMT1 and induces demethylation of cyclin D2 (CCND2) in cell culture (FIGURE 5) (90). Genistein (a phytoestrogen in soy beans) also inhibits DNMT1 in cell culture (62). When genistein was fed to pregnant Avy methylation indicator-mice during pregnancy, at levels comparable to humans consuming high-soy diets, they had offspring with shifted coat color (yellow to brown), and this phenotypic change was significantly associated with increased methylation of six CpG sites at the IAP upstream of the transcription start site of the Agouti gene (45, 49). Although the mechanism of epigenetic perturbation is unclear, interestingly, this outcome is similar to that observed when methyl donors were depleted using the same genetic model (discussed above). This clearly reflects the sensitivity of the a Avy allele to dietary changes and the fact that these are likely indirect effects of the two diets.
FIGURE 5.
Nutrients as regulators of enzymatic activity. Nutrients and nutrient metabolites that alter activity of epigenetic regulatory enzymes. Solid arrows indicate increased enzymatic activity in the presence of the nutrient shown, and lines with blunted ends represent decreased/inhibited activity in the presence of nutrient shown. C, cytosine; G, guanine; CH3, methyl group; CH2OH, hydroxymethyl group; Ac, acetyl group; Fe2+, iron; VitC, vitamin C; FAD, flavin adenine dinucleotide; TETs, ten-eleven-translocation enzymes; DNMTs, DNA methyltransferase enzymes; HMTs, histone methyltransferase enzymes; HATs, histone acetyltransferase enzymes.
Polyphenols in tea (catechin, epicatechin, epigallocatechin-3-O-gallate) and bioflavonoids in tea (quercetin, fisetin, and myricetin) inhibit DNMT1-mediated DNA methylation in a concentration-dependent manner in vitro (FIGURE 5) (120), possibly by elevating AdoHcy concentrations (a competitive inhibitor of AdoMet-dependent methylations).
6. Docosahexaenoic acid supplementation and DNA methylation
Docosahexaenoic acid (DHA) is an omega-3 fatty acid used frequently as a nutritional supplement. DNA methylation status was assessed at IGF2 promoter 3 (IGF2 P3), IGF2 differentially methylated region (DMR), and the H19 DMR in cord blood mononuclear cells of pregnant women supplemented with DHA (DHA supplemented, n = 131; control group, n = 130). Women received 400 mg DHA daily or a placebo from gestation week 18–22 to parturition. DNA methylation levels at only one CpG in IGF2 P3 were significantly higher in the DHA group than the control group infants. There were no changes at the nine other CpGs (including the H19 DMR) assessed (117). In the same study population, DNA methylation states in Th1, Th2, Th17, and regulatory T-relevant genes as well as LINE1 repetitive elements of cord blood mononuclear cells were assessed (118). No significant difference in promoter methylation levels was shown between supplemented and control groups for the genes analyzed. This study monitored DNA from cord blood, which might not reflect changes in the epigenome of target tissues that express IGF2. The observed differential methylation of a single CpG may not alter the expression of the gene, and no data were presented that DHA treatment was associated with altered gene expression or altered growth of the children.
7. Micronutrient supplementation and DNA methylation
DNA methylation at 12 DMRs was analyzed in cord blood samples from 58 offspring of women participating in a double-blind randomized-controlled trial of pre- and periconceptional micronutrient supplementation (including folate, zinc, and vitamins A, B12, C, and D). The authors observed sex-specific effects of micronutrient supplementation, reducing methylation levels in DMRs in IGF2R in girls and in GTL2–2 in boys (37). These are observational data that need to be supported by experimental data in model systems before it is concluded that some component of micronutrient supplements is an epigenetic modifier.
8. Ascorbic acid, iron, and DNA hydroxymethylation
As discussed earlier, we do not fully understand the functions of 5hmC; therefore, less has been shown in the way of dietary modulation of 5hmC. We do know that the catalytic activity of 5hmC writers, TET1, TET2, and TET3 dioxygenases, depends on divalent iron (Fe2+) as a cofactor and α-ketoglutarate as a cosubstrate; additionally, some cases require ascorbate as another cofactor for full catalytic activity (FIGURE 5) (220, 222). Iron, α-ketoglutarate, and ascorbate are all derived from diet metabolism.
B. Dietary Modulation of PTHMs
1. Diet and histone methylation
A number of chromatin-modifying enzymes require cofactors derived from the diet. Flavin adenine dinucleotide (FAD) is derived from the vitamin riboflavin (vitamin B2) and is a required cofactor for the histone demethylase LSD1 (FIGURE 5) (100). LSD1 binds to PGC1a, PDK4, FATP1, and ATGL and represses their transcription in a manner that is associated with loss of H3K4 methylation (100). The JmjC domain-containing histone demethylases are α-ketoglutarate -dependent dioxygenases that derive this substrate from nutrient metabolism; activity of these enzymes is also iron dependent (FIGURE 5) (100) (see earlier discussion of the α-ketoglutarate-dependent TET enzymes that modify DNA methylation). This link to the tricarboxylic acid (TCA) cycle of metabolism may explain why manipulating diet fat intake alters histone methylation. Feeding rats a high-starch/low-fat diet induced mono-, di-, and trimethylation of histone H3K4 on the promoter and transcribed regions of the Si and Sglt1 genes (92). Feeding mice a high fat diet resulted in persistent changes (maintained after diet was ceased) in H3K4me2 levels in liver, and these changes were associated with altered accessibility of the chromatin and alter gene expression (121). In offspring of pregnant mice fed a high-fat diet during pregnancy, there was lower acetylation and higher methylation of histone H3 at lysine 9 of the promoter of adiponectin in adipose tissue, and methylation of histone 4 at lysine 20 in the leptin promoter was significantly higher (135).
Similar to DNA methylation (discussed above), histone methylation is dependent on the availability of AdoMet produced from dietary methyl-group donors (FIGURE 4). Feeding mouse dams a diet low in choline and betaine during pregnancy resulted in hypomethylation of H3K9 upstream of the RE1 binding site in the calbindin 1 promoter (137). The levels of H3K9Me2 and H3K27Me3 were increased by in utero choline supplementation of rats, whereas the levels of H3K4Me2, associated with active promoters, were highest in prenatal low-choline rats (44). Feeding mouse dams a diet low in choline and betaine during pregnancy decreased the expression and activity of G9a histone methylase in fetal brain (137), and choline supplementation during gestation increased expression of mRNA and protein of G9a and SUV39H1 methyltransferases (the promoters of G9a and Suv39h1 were hypermethylated in low choline embryos) (44). Methionine restriction in mice also modifies histone methylation in liver, reducing H3K4me3, H3K27me3, and H3K4me2 levels (138). Women with higher intake of choline (930 vs. 480 mg/day) had more dimethylated histone H3 at lysine 9 (H3K9me2) in placental chromatin (95).
2. Diet and histone acylation
Multiple components of the diet and diet metabolism are the primary substrates for histone acetylases. Acetyl-CoA that is generated through the TCA cycle is the universal source of acetyl groups for histone acetylation. In addition, several other important histone modifications, including but not limited to malonyl-CoA, glutaryl-CoA, and succinyl-CoA, are all produced through a variety of metabolic pathways, both catabolic and anabolic (FIGURE 4). Lysine 2-hydroxyisobutyrylation is likely to result from an enzymatic reaction using hydroxyisobutyrylCoA as a cofactor that derives from 2-hydroxyisobutyrate [perhaps derived from environmental exposure to methyl tert-butyl ether (MTBE)] (41).
Multiple components of the diet inhibit histone deacetylases (HDACs) (162). Some of these components are generated by gut microbiota-dependent mechanisms. Plants consumed in the diet (especially crucifera) contain isothiocyanates that are competitive inhibitors of class I/II HDACs (but not class III HDACs), and when consumed they result in activation of genes by increasing the acetylation of H3 and H4 (FIGURE 5) (103). Butyrate, a short-chain fatty acid produced by microbiome-mediated fermentation of undigested carbohydrates, also is a class I/II HDAC inhibitor (FIGURE 5) (43, 52, 112, 217). This has been proposed as a mechanism connecting diet, the epigenome, and cancer prevention by dietary fiber (107). β-Hydroxybutyrate, a ketone body, is produced after prolonged exercise or starvation. β-Hydroxybutyrate inhibits the activities of class I/II HDACs (100).
Class III HDACs (also known as ‟sirtuinsˮ) require nicotinamide adenine dinucleotide (NAD+) as a cofactor and are sensitive to changes in cellular NAD+ status; NAD is derived from nicotinamide in the diet (nicotinamide is the biologically active form of niacin) (71). Elevated nicotinamide concentrations result in feedback inhibition of sirtuin activity (71). This dependence and interaction with NAD may explain why exercise and calorie restriction change sirtuin activity (71). Seven sirtuins have been identified in humans and are distributed with different cellular locations (38). Nuclear sirtuin 1 modifies transcription of certain genes by modifying histone acetylation, while cytosolic sirtuin 1 is thought to sense nutrient status (calorie restriction) and modify metabolism via modifying histone acetylation and thereby changing expression of genes controlling fatty acid oxidation and mobilization, gluconeogenesis, and insulin secretion (174). Sirtuin 2 is located in the cytosol and functions in the regulation of tubulin (38). Sirtuin 3 is located both in the nucleus as a sensor of cellular stress and in the mitochondria as a regulator of mitochondrial function; this sirtuin may regulate adaptive thermogenesis (174). Sirtuin 3 has several metabolically related functions; it can protect the cell from reactive oxygen species (ROS) by activating superoxide dismutase 2 (88), and long-chain acyl-CoA dehydrogenase (LCAD; an enzyme involved in fatty acid oxidation) is a target for SIRT3 deacetylation during prolonged fasting, resulting in the activation of fatty acid breakdown (83). Sirtuin 3 also deacylates the mitochondrial acetyl-CoA synthetase at K642, thereby activating the enzyme and controlling the entry of acetate into the TCA cycle (174). Sirtuin 4 (mitochondrial) is also involved in the regulation of mitochondrial energy metabolism. It catalyzes the ADP-ribosylation of glutamate dehydrogenase (GDH), inhibiting GDH activity (174). GDH controls the entry of glutamate from amino acid metabolism into the TCA cycle. Sirtuin 4 also targets leucine metabolism and insulin secretion (4). Sirtuin 4 regulates leucine metabolism by controlling the acylation status (and thereby activity) of enzymes in the metabolic pathway (4). The major mechanism by which leucine stimulates insulin secretion involves the allosteric activation of GDH, which as discussed above, is a target of sirtuin 4 (4). Sirtuin 5 is located in mitochondria and regulates the uric acid cycle (146). Sirtuin 6 regulates glucose homeostasis by inhibiting the glucose transporter Glut1, and multiple glycolytic genes in a coordinated fashion, in part because sirtuin 6 corepresses the transcription factor Hif1α by deacetylating histone 3 lysine 9 (H3K9) at the promoters of these genes (Hif1α increases glucose uptake and upregulates glycolysis) (60, 175, 228). Sirtuin 6 deacetylates the histone acetyltransferase GCN5, thereby enhancing its activity and this increases the acetyl levels of PPARγ coactivator 1α (PGC-1α; a transcriptional activator of gluconeogenesis), leading to a reduction in PGC-1α activity, which in turn leads to suppressed gluconeogenesis (51). Sirtuin 6 also acts to increase insulin sensitivity in skeletal muscle and liver (3). Sirtuin 6-deficient mice die early in life because most glucose is used to produce lactate by lactate dehydrogenase releasing less ATP than does oxidative phosphorylation (228). As part of this altered metabolism, brown adipose tissue and muscle remove excessive amounts glucose from blood resulting in lethal hypoglycemia (60). Sirtuin 7 is a positive regulator of Pol I transcription and has a role in responding to cellular stress (38).
Resveratrol (from dietary intake of plant products such as blueberries and red wine) increases the affinity of sirtuin 1 to acylated histones, thereby enhancing deacylation (FIGURE 5) (20).
3. Diet and other histone modifications
Histone lysine crotonylation is functionally different from lysine acetylation in that it marks either active promoters or potential enhancers (191). The cytoplasmic/nuclear metabolic enzyme acyl-CoA synthetase 2 forms crotonyl-CoA from the short-chain fatty acid (SCFA) crotonate in mammalian cells (169). This fatty acid is formed by bacteria (18) that are likely part of the human gut microbiome.
Biotin is a B-vitamin coenzyme for carboxylase enzymes needed for the synthesis of fatty acids, isoleucine, and valine and for gluconeogenesis. Specific biotinylation sites have been identified in lysines: K9, K13, K125, K127, and K129 in histone H2A; K4, K9, and K18 in histone H3 and K8 and K12 in histone H4; H4K12bio participates in gene repression (30, 77). H4K12bio colocalizes with the repression marker H3K9me2 (30). H4K12 and H2AK9 were more biotinylated if their growth medium contained more biotin (human cell lines were cultured in biotin-defined media representing concentrations observed in plasma from biotin-deficient individuals, normal individuals, and users of biotin supplements) (30). This suggests that dietary manipulation of biotin status should alter histone biotinylation; ~20% of the United States population reports taking biotin supplements (30).
C. Dietary Modulation of Noncoding RNAs
1. Diet-induced miRNA changes
DNA methylation of miR-203 and miR375 was higher when cells were grown in medium containing more folic acid (76). Expression of miR-615-5p, miR-3079-5p, miR-124*, and miR-101b* were downregulated, whereas miR-143* was upregulated, in livers from offspring from high-choline diet-fed dams (227). Maternal betaine supplementation in piglets upregulated miR-130b, miR-181a, and miR-181d in fetal brain hippocampus (187).
Caloric restriction alters miRNA expression. During aging in skeletal muscle of rhesus monkeys, miRNA expression patterns changed, but caloric restriction reversed these alterations (139). In the brain of calorie-restricted mice, there was an age-dependent decreased expression of microRNAs miR-181a-1*, miR-30e, and miR-34a, with a corresponding gain in Bcl-2 expression, and decreases in pro-apoptosis genes such as Bax contributing to the gain in neuronal survival in caloric restricted fed mice (105). miR-310 family miRs are expressed when Drosophila are starved and are suppressed when they are fed (33).
2. miRNA regulation of metabolism
Only a small number of studies examined the interactions between metabolism, diet, and the expression of noncoding RNAs, and most of these have studied microRNAs (miRs).
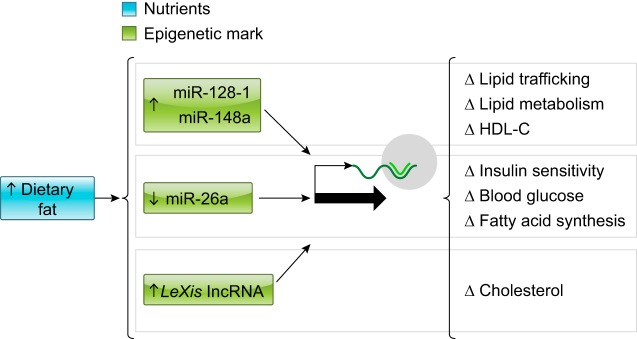
Data show that diet-induced changes in miRNAs can significantly modulate glucose and lipid metabolism (FIGURE 6). miR-93 regulates adiposity via inhibition of Sirt7 and Tbx3, miR-378a-3p enhances adipogenesis by targeting MAPK1, and miR-140–5p promotes adipocyte differentiation into mature adipocytes (53). miR-145 inhibits porcine preadipocyte differentiation by targeting IRS1 (53). MiR-145a-5p promotes adipocyte proliferation and is negatively correlated with obesity (53). Overexpression and antisense targeting of miR-128-1 or miR-148a in high-fat diet-fed C57BL/6J and Apoe-null mice resulted in altered hepatic expression of proteins involved in lipid trafficking and metabolism (FIGURE 6) (203). miR-26a regulates insulin signaling and metabolism of glucose and lipids; miR-26a was downregulated in two obese mouse models compared with control animals (65). Compared with lean individuals, overweight humans had decreased expression of miR-26a in the liver (65). miR-223 induces the glucose transporter 4 (GLUT4) protein (130). Inhibition of miR-29a in primary mouse islets caused β-cell silencing of the MCT1 transporter which is involved in insulin secretion (159). Knockdown of miR-194 in L6 skeletal muscle cells induced an increase in basal and insulin-stimulated glucose uptake and glycogen synthesis. This occurred in conjunction with an increased glycolysis, indicated by elevated lactate production (116).
FIGURE 6.
Epigenetic markers as mediators of nutrient signaling. Example of pathways of mediation of nutrient signaling via noncoding RNA molecules.
The mir-310 family (mir-310/mir-311/mir-312/mir-313) are important regulators of Drosophila metabolic status; they target three regulatory factors (Rab23, DHR96, and Ttk) of the Hedgehog (Hh) pathway and thereby modify dietary response. miR310 family knockouts in Drosophila accumulate lipid in liver (33).
MiRNAs also regulate some genes of methyl metabolism; miR-125b targets DNMT3b in vascular smooth muscle (23), while miR-22 and miR-29b directly target rat Mthfr and Mat1 genes, respectively (110) A number of miRNAs are epigenetically regulated by DNA methylation, these include miRs 375, 149, 27b (125), 196b (87), 203, 375 (76), 129-2/-137/-935/-3663/-3665 and -428 (214), 211 (223), 153 (67), 145a-5p (53) and many more. DNA methylation of miR 1451–5p inhibits its expression (53). There were greater than twofold expression alterations of the miR-379/miR-410 miRNAs in the livers of mice fed a low-choline and low-folate diet (198).
Long noncoding RNAs (lncRNA) also influence metabolism. Liver X receptors (LXRs) are transcriptional regulators of cellular and systemic cholesterol homeostasis. The noncoding RNA LeXis is a modulator of LXR signaling on hepatic lipid metabolism, contributing to the ability of LXRs to inhibit cholesterol synthesis (171). Hepatic LeXis expression is induced in response to a high in fat and cholesterol diet, and it affects the DNA interactions of RALY, a ribonucleoprotein that acts as a transcriptional cofactor for cholesterol biosynthetic genes in the mouse liver (FIGURE 6) (171). Manipulation of LeXis concentrations in the liver alters expression of genes involved in cholesterol biosynthesis and subsequently alters cholesterol levels in the liver and plasma (171).
D. Heritability of Diet-Induced Epigenetic Changes
Although it is well accepted that diet-induced epigenetic changes can be mitotically heritable, the question of how changes induced in one generation can persist into the next generation remains a topic of debate. Despite the fact that the epigenome is proposed to be reset between every generation to avoid transmission of epimutations, recent studies show that diet-induced epigenetic changes may be transmitted to the following unexposed generations through the germline. Such multigenerational effects can persist for as many as three generations after dietary insult, in which case they are deemed “transgenerationally inherited” (5, 32, 40, 163). Evidence in mice for diet-induced multigenerational epigenetic changes was first shown at IAP regulated metastable epialleles Avy and axin-fused (discussed in sect. IIIA2). Since then a growing number of studies report epigenetic changes in the unexposed offspring of parental dietary challenges, sometimes with subsequent transmission to future generations. For example, preconception paternal diet (low protein and high fat) in rodents was shown to alter energy metabolism pathways in the unexposed offspring (24, 149). This demonstrates transmission of the insult of dietary modulation of either the male germline genome/epigenome or semen composition in a way that perturbed offspring metabolic programming.
We and others also show in different ways that intergenerational epigenetic inheritance after maternal treatment of various diets (high fat, caloric restriction, vitamin D depletion) during gestation (56, 161, 219). Interestingly, we found that consistent diet-dependent epigenetic changes may be present in somatic cells of two generations, but not in the intermediate germline (219). This suggests an indirect mechanism of inheritance that remains to be defined. Unfortunately, it is difficult to rule out the role of DNA sequence mutations, and one recent report suggests that stoichastic epigenetic variation and changes in DNA sequence explain most of the epigenetic changes reported in dietary studies (179). Furthermore, the direct path of inheritance is difficult to determine in these cases since many studies use intercrosses to generate offspring. It also remains challenging to distinguish between germline effects and maternal uterine effects.
Evidence of epigenetic inheritance in humans remains limited in part due to the challenge of time, cost, and participant retention rate required for longitudinal studies. More importantly, even if these challenges could be overcome, humans live in an uncontrolled environment and therefore throughout the lifespan are exposed to a variety of environmental factors, which makes forming direct links specifically between diet and epigenetic outcomes challenging.
IV. DIET-RELATED HEALTH/DISEASES MEDIATED BY EPIGENETIC CHANGES
Nutrition and other environmental factors during prenatal and early postnatal development influence developmental plasticity and alter susceptibility to adult cardiovascular disease, type 2 diabetes, and obesity (9); neuropsychiatric diseases (61); immune/inflammatory diseases (29); and cancer (205) [the Developmental Origins of Health and Disease (DOHaD) hypothesis (142)]. Maternal nutrition is a key contributor to diseases that originate during development since offspring are fully dependent on maternal nutrient availability during gestation and lactation. However, paternal nutrition also can affect children’s epigenetic marks; newborns from obese fathers showed altered methylation overall and significant hypomethylation at the IGF2 gene (55).
Epigenetic changes are proposed as a major mechanism of DOHaD since early development is a critical time for epigenetic programming (153). It is hypothesized that diet-mediated dysregulation of epigenetic programming during early development leads to downstream effects on health that may last throughout the lifetime. It is unclear whether these developmentally attained epimutations are reversible later in life. However, it is possible that early detection of these epimutations might allow for preventative measures to decrease or eliminate some physiological outcomes.
While there are many diet-related epigenetic changes associated with health outcomes, paths of causality often remain unclear since most human studies are carried out using blood or buccal samples as proxies for the target organ of interest because the disease tissue/cell type is usually inaccessible. In cases where direct evidence of causality is absent, epigenetic changes still represent important biomarkers of disease prediction, diagnosis, or progression. While the number of distinct populations in which an association is detected does not necessarily determine the importance of the finding, consistent findings among multiple populations of different ethnicities (e.g., European and non-European ancestry) do provide support of broader application. Below we discuss epigenetic changes linked to diet and associated with some of the most commonly occurring physiological outcomes.
A. Metabolic Syndrome and Related Diseases
Metabolic syndrome (MetS) is characterized by the presence of three or more risk factors including but not limited to increased waist circumference as well as high triglyceride levels, cholesterol levels, blood pressure, or fasting blood sugar (indicative of insulin resistance) (73). The greater the number of risk factors a person has, the greater their risk for heart disease, diabetes, and stroke. Although the exact cause of MetS is unclear, diet-induced obesity is strongly correlated with MetS. Epigenome-wide association studies (EWAS) have identified MetS-related changes in DNA methylation at genes that may have causal functions by regulating food intake, energy expenditure, and lipid metabolism (e.g., SOCS3, CPT1A, ABCG1) (1, 42, 133). Gene methylation has also been proposed for use as predictive biomarkers of MetS. For example, Yoo et al. (221) demonstrate that methylation levels at POMC (proopiomelanocortin, regulates appetite and energy expenditure) in cord blood at birth have positively correlated with later childhood triglycerides and insulin levels. MicroRNAs with roles in lipid homeostasis have also been strongly associated with developing MetS. For example, circulating miR-122, which regulates hepatic lipid metabolism and is linked to insulin resistance, was recently associated with the risk of developing MetS (212).
Nonalcoholic fatty liver disease (NAFLD) is the abnormal accumulation of lipids in the liver which is associated with subsequent inflammation and fibrosis known as nonalcoholic steatohepatitis (NASH). NAFLD is usually associated with MetS and has been linked to diets enriched in fructose, trans fats, and saturated fats (8, 35, 63, 145, 154). Animal studies have primarily identified NAFLD-related epigenetic changes in pathways related to glucose and fatty acid metabolism. Zhou et al. (229) show that high-fat diet (HFD) during gestation and lactation in a Sprague-Dawley rat model results in fatty livers in adult female offspring that express significantly higher levels of Pepck mRNA and protein. Pepck plays an important role in energy metabolism, acting as a substrate for gluconeogenesis and glyceroneogenesis. In these samples, the Pepck promoter was enriched for RNA polymerase II (PolII) but showed inverse correlations between repressive histone marks H3K27me3 (enriched) and H3K9me3 (depleted). In support of this finding, analyses of NAFLD liver biopsies showed upregulation of PEPCK mRNA and repression of MiR-10b in patients with inflammation (12). The authors proposed that PEPCK expression is induced by PPARα, a downstream target of miR-10b, which is inhibited by KLF6. Epigenetic regulation of proliferator-activated receptor γ coactivator 1 α (PPARGC1A), an upstream regulator of insulin signaling genes including PEPCK, is also associated with NAFLD (166). PPARGC1A methylation in liver biopsies of NAFLD patients is positively correlated with PPARGC1A expression and insulin resistance (183).
Type 2 diabetes mellitus (T2D) is a growing global epidemic characterized by insulin resistance. Causal factors include both genetic and environmental contributors including diet and environmental pollutants. The disease is characterized by insulin resistance and is closely linked to obesity although not all T2D patients are obese. A recent genome-wide methylation study comparing adipose tissue from HFD and low-fat diet (LFD) mice identified 232 differentially methylated regions (DMRs) associated with diet, 183 associated with weight, 235 associated with fasting glucose, and 294 associated with insulin sensitivity (144). Interestingly, Pepck promoter was hypermethylated in the HFD group compared with the LFD group (144). The authors also show that among diet-related DMRs, hypermethylated regions were in or near genes involved in lipid metabolism while hypomethylated regions were in or near genes involved in inflammation. Genome-wide methylation analyses of human adipose tissue from lean and obese insulin-resistant participants determined that more than 50% of the mouse adipose tissue DMRs overlapped with this human data set, most of which (68%) had a consistent direction of methylation change.
Cardiovascular disease (CVD) is strongly associated with MetS and in particular with obesity, diabetes, and hyperlipidemia. Studies on the Dutch famine birth cohort demonstrate that exposure to the famine specifically during early gestation is associated with increased risk of coronary artery disease (155). Later studies determined that several genes linked to metabolic and cardiovascular disease were perturbed epigenetically in this cohort. In particular, DNA methylation at INSR was positively correlated with birth weight, and methylation at CPT1A was positively correlated with LDL cholesterol levels (194, 195). A separate study by the same group demonstrated that several genes linked to CVD were differentially methylated in association with prenatal exposure to famine including INSIGF, GNASAS, MEG3, IL10, LEP, and ABCA1 (196).
B. Brain Development and Function
The important role of epigenetic mechanisms in brain development and function is well known. However, progress in this field towards understanding mechanisms of diet-induced changes is limited due primarily to inaccessibility to brain from live human subjects combined with the complexity of epigenetic signatures of different cell types in the brain.
Cognition as defined by information processing, conception, language learning, memory, problem solving, and decision making is determined in part by brain development during early childhood. A study on the effect of early childhood nutrition determined that malnutrition within the first year of life is associated with differential methylation in blood of several key neurodevelopmental and neuropsychiatric genes including IFNG, VIPR2, ZBTB9, SYNGAP1, ABCF1, COMT, and DCTN1 (156). In addition, methylation at IFNG, VIPR2, COMT, DCTN1 antisense RNA 1, and ABCF1 was associated with the ADHD index (a measure predicting attention-deficit/hyperactivity disorder), while methylation at XBTB9 and SYNGAP1 was associated with IQ scores within this cohort. This initial genome-wide study was performed in human blood; therefore, mechanistic relevance to brain development and function is possible, but remains speculative. In support of mechanistic roles, rats exposed to a low protein diet in utero exhibited attention deficits in adulthood and reduced gene expression at COMT and IFNG (156). Epigenetic regulation of these gene expression changes was not investigated in this study. In mice, diets low in choline during pregnancy resulted in fetal brains that did not form the layers of frontal cortex properly (206); such changes in brain development could underlie some of the effects of malnutrition in children on brain function. Interestingly, a recent review of data in this field observed that maternal high-fat-induced obesity, metabolic syndrome, and insulin resistance may also be linked to mental health dysfunction in offspring (19).
Alzheimer’s disease (AD) is a devastating neurodegenerative disease associated with aging. The direct cause of AD remains unclear, but epidemiological evidence is emerging that links AD to circulating concentrations of homocysteine and B vitamins (143, 197). Epigenetic analyses demonstrate that Alzheimer’s and Alzheimer’s-like disease brain tissues have increased levels of histone acetylation, compared with neurologically normal brain (14, 147, 176). In a recent study, Alzheimer’s-like symptoms (defects in metabolic phenotypes, locomoter activity, learning activity, and memory) induced by HFD were almost fully alleviated by combined treatment with a histone deacetylase inhibitor, suberoylanilie hydroxamic acid (SAHA) (178). Specifically, histone H3 acetylation (epigenetically regulated by SAHA) was downregulated in the HFD group and partially rescued by combined treatment with SAHA.
C. Cancer
Cancer is a complex multifaceted disease primarily defined by abnormal cellular proliferation and migration. Links between diet, cancer, and epigenetic changes are not well elucidated. However, recent studies emphasize a link between folate, vitamin B12, or vitamin C levels and epigenetic changes associated with lung and breast cancer (96). Cancer has long been linked to abnormal methylation and histone modification at important cell cycle regulators and DNA repair enzymes and more recently linked to abnormal nucleosome positioning, noncoding RNA, and genetic mutations in epigenetic regulators (177). In these cases, epigenetic disruption of gene expression mirrors changes caused by genetic mutations. For example, both genetic mutations and epigenetic changes at Breast cancer 1 (BRCA1), a DNA repair enzyme, are linked to breast and ovarian cancer (192). Although the role of diet in these changes is still speculative, a recent study showed that high plasma folate levels (>24.4 ng/ml) in women with the BRCA1 or BRCA2 genetic mutation increases risk of cancer up to 3.2-fold compared with women with low folate concentrations (106).
V. CONCLUSIONS
Dietary changes play a significant role in modulation of the epigenome, including DNA methylation, posttranslational histone modifications, and noncoding RNAs. Such diet-induced epigenetic changes may provide the key mechanistic link between diet-induced disease and thus may serve as targets of intervention. Importantly, research to date shows that nutrients play an essential and direct role in normal epigenetic mechanisms, often providing the substrate or signal for establishment or maintenance of epigenetic states. Thus, through epigenetic mechanisms in part, nutrition helps define phenotype and explain why cells and organs that share a common genetic code can be functionally and structurally different.
It is suggested that diet-induced epimutations early in life increase risk for a variety of chronic diseases in later life and may even be transmitted to future generations similar to DNA sequence mutations. However, proving causality of epigenetic changes in humans is challenging. In fact, most of these findings remain limited to animal model studies, and translation to humans is restricted due to important physiological differences between the species, and limited accessibility in obtaining the appropriate cell types/tissues for epigenetic diagnostic tests in medicine. In humans, we often measure epigenetic marks in proxy tissues/cell types such that can be acquired by noninvasive methods, such as blood and buccal cells. However, this method is potentially flawed in that we often cannot distinguish between differences in cell composition and true epigenetic changes, and furthermore, we often cannot investigate the cell-type specific changes for inaccessible tissues. Therefore, most human studies remain restricted to identifying biomarkers or targets for measuring susceptibility by association. Development of relevant ex vivo methods for human cell types and use of reprogrammed cells such as induced pluripotent stem (iPS) cells will greatly aid in this effort.
Effects of diet on the epigenome are timing specific such that there is seemingly increased sensitivity during early development. Maternal diet changes during early development can result in epigenetic changes in the fetus that are stable even after the dietary changes have resumed normality. These changes in epigenetic marks can be associated with significant modification of developmental programs in brain and other tissues (206). There is also growing evidence that seasonal variation in food supply during early development acts to modify human epigenetic marks (209). Thus diet-induced changes in epigenetic regulation may be an intrinsic adaptive mechanism whereby the diet can modify expression of genes regulating metabolism to adjust for long-term changes in the food supply. Inheritance of such changes could be a logical adaptation in a population, and future exploration of this topic would support a paradigm in which familial inheritance of disease risk is conveyed not only by genes but also by epigenetic information accumulated over previous generations. However, much more needs to be understood about where, how, and why such epigenetic changes are stably transmitted through multiple generations and whether they can be reversed.
GRANTS
This work was supported by National Institutes of Health Grants DK 115380, DK 056350, and ES 023849A.
DISCLOSURES
S. H. Zeisel is founder of Nutrigene Sciences, LLC, a company in which he owns stock equity. S. H. Zeisel serves on the Scientific Advisory Boards of Metabolon and of SNPitty. None of these companies has a financial interest in the outcomes of this paper. S. H. Zeisel has research support from Nestle Nutrition and Balchem that is not related to this paper.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: S. H. Zeisel, Univ. of North Carolina at Chapel Hill, 500 Laureate Way, Rm. 2218, Kannapolis, NC 28081 (e-mail: steven_zeisel@unc.edu).
REFERENCES
- 1.Ali O, Cerjak D, Kent JW Jr, James R, Blangero J, Carless MA, Zhang Y. Methylation of SOCS3 is inversely associated with metabolic syndrome in an epigenome-wide association study of obesity. Epigenetics 11: 699–707, 2016. doi: 10.1080/15592294.2016.1216284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amarasekera M, Martino D, Ashley S, Harb H, Kesper D, Strickland D, Saffery R, Prescott SL. Genome-wide DNA methylation profiling identifies a folate-sensitive region of differential methylation upstream of ZFP57-imprinting regulator in humans. FASEB J 28: 4068–4076, 2014. doi: 10.1096/fj.13-249029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JG, Ramadori G, Ioris RM, Galiè M, Berglund ED, Coate KC, Fujikawa T, Pucciarelli S, Moreschini B, Amici A, Andreani C, Coppari R. Enhanced insulin sensitivity in skeletal muscle and liver by physiological overexpression of SIRT6. Mol Metab 4: 846–856, 2015. doi: 10.1016/j.molmet.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KA, Huynh FK, Fisher-Wellman K, Stuart JD, Peterson BS, Douros JD, Wagner GR, Thompson JW, Madsen AS, Green MF, Sivley RM, Ilkayeva OR, Stevens RD, Backos DS, Capra JA, Olsen CA, Campbell JE, Muoio DM, Grimsrud PA, Hirschey MD. SIRT4 Is a Lysine Deacylase that Controls Leucine Metabolism and Insulin Secretion. Cell Metab 25: 838–855.e15, 2017. doi: 10.1016/j.cmet.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308: 1466–1469, 2005. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aristotle On the Generation of Animals, edited by Platt A. Adelaide, Australia: Electronic Scholarly Publishing, 1942. https://ebooks.adelaide.edu.au/a/aristotle/generation/contents.html. doi: 10.4159/DLCL.aristotle-generation_animals.1942 [DOI] [Google Scholar]
- 7.Arth A, Kancherla V, Pachón H, Zimmerman S, Johnson Q, Oakley GP Jr. A 2015 global update on folic acid-preventable spina bifida and anencephaly. Birth Defects Res A Clin Mol Teratol 106: 520–529, 2016. doi: 10.1002/bdra.23529. [DOI] [PubMed] [Google Scholar]
- 8.Asrih M, Jornayvaz FR. Diets and nonalcoholic fatty liver disease: the good and the bad. Clin Nutr 33: 186–190, 2014. doi: 10.1016/j.clnu.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab 13: 364–368, 2002. doi: 10.1016/S1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 10.Barlow DP, Stöger R, Herrmann BG, Saito K, Schweifer N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature 349: 84–87, 1991. doi: 10.1038/349084a0. [DOI] [PubMed] [Google Scholar]
- 11.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature 351: 153–155, 1991. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 12.Bechmann LP, Vetter D, Ishida J, Hannivoort RA, Lang UE, Kocabayoglu P, Fiel MI, Muñoz U, Patman GL, Ge F, Yakar S, Li X, Agius L, Lee YM, Zhang W, Hui KY, Televantou D, Schwartz GJ, LeRoith D, Berk PD, Nagai R, Suzuki T, Reeves HL, Friedman SL. Post-transcriptional activation of PPAR alpha by KLF6 in hepatic steatosis. J Hepatol 58: 1000–1006, 2013. doi: 10.1016/j.jhep.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature 405: 482–485, 2000. doi: 10.1038/35013100. [DOI] [PubMed] [Google Scholar]
- 14.Benito E, Urbanke H, Ramachandran B, Barth J, Halder R, Awasthi A, Jain G, Capece V, Burkhardt S, Navarro-Sala M, Nagarajan S, Schütz AL, Johnsen SA, Bonn S, Lührmann R, Dean C, Fischer A. HDAC inhibitor-dependent transcriptome and memory reinstatement in cognitive decline models. J Clin Invest 125: 3572–3584, 2015. doi: 10.1172/JCI79942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett-Baker PE, Wilkowski J, Burke DT. Age-associated activation of epigenetically repressed genes in the mouse. Genetics 165: 2055–2062, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, Fry B, Meissner A, Wernig M, Plath K, Jaenisch R, Wagschal A, Feil R, Schreiber SL, Lander ES. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125: 315–326, 2006. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 17.Black JC, Van Rechem C, Whetstine JR. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol Cell 48: 491–507, 2012. doi: 10.1016/j.molcel.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blažič M, Kosec G, Baebler Š, Gruden K, Petković H. Roles of the crotonyl-CoA carboxylase/reductase homologues in acetate assimilation and biosynthesis of immunosuppressant FK506 in Streptomyces tsukubaensis. Microb Cell Fact 14: 164, 2015. doi: 10.1186/s12934-015-0352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolton JL, Bilbo SD. Developmental programming of brain and behavior by perinatal diet: focus on inflammatory mechanisms. Dialogues Clin Neurosci 16: 307–320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280: 17187–17195, 2005. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 21.Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84: 843–851, 1996. doi: 10.1016/S0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 22.Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr 97: 435–439, 2007. doi: 10.1017/S0007114507352392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao C, Zhang H, Zhao L, Zhou L, Zhang M, Xu H, Han X, Li G, Yang X, Jiang Y. miR-125b targets DNMT3b and mediates p53 DNA methylation involving in the vascular smooth muscle cells proliferation induced by homocysteine. Exp Cell Res 347: 95–104, 2016. doi: 10.1016/j.yexcr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, Bock C, Li C, Gu H, Zamore PD, Meissner A, Weng Z, Hofmann HA, Friedman N, Rando OJ. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143: 1084–1096, 2010. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157: 77–94, 2014. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10: 295–304, 2009. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 27.Chen R, Zhang Q, Duan X, York P, Chen GD, Yin P, Zhu H, Xu M, Chen P, Wu Q, Li D, Samarut J, Xu G, Zhang P, Cao X, Li J, Wong J. The 5-Hydroxymethylcytosine (5hmC) Reader UHRF2 Is Required for Normal Levels of 5hmC in Mouse Adult Brain and Spatial Learning and Memory. J Biol Chem 292: 4533–4543, 2017. doi: 10.1074/jbc.M116.754580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet 15: 93–106, 2014. doi: 10.1038/nrg3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen T, Liu HX, Yan HY, Wu DM, Ping J. Developmental origins of inflammatory and immune diseases. Mol Hum Reprod 22: 858–865, 2016. doi: 10.1093/molehr/gaw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chew YC, West JT, Kratzer SJ, Ilvarsonn AM, Eissenberg JC, Dave BJ, Klinkebiel D, Christman JK, Zempleni J. Biotinylation of histones represses transposable elements in human and mouse cells and cell lines and in Drosophila melanogaster. J Nutr 138: 2316–2322, 2008. doi: 10.3945/jn.108.098673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chittka A, Chittka L. Epigenetics of royalty. PLoS Biol 8: e1000532, 2010. doi: 10.1371/journal.pbio.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chong S, Whitelaw E. Epigenetic germline inheritance. Curr Opin Genet Dev 14: 692–696, 2004. doi: 10.1016/j.gde.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Çiçek IO, Karaca S, Brankatschk M, Eaton S, Urlaub H, Shcherbata HR. Hedgehog Signaling Strength Is Orchestrated by the mir-310 Cluster of MicroRNAs in Response to Diet. Genetics 202: 1167–1183, 2016. doi: 10.1534/genetics.115.185371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Claycombe KJ, Uthus EO, Roemmich JN, Johnson LK, Johnson WT. Prenatal low-protein and postnatal high-fat diets induce rapid adipose tissue growth by inducing Igf2 expression in Sprague Dawley rat offspring. J Nutr 143: 1533–1539, 2013. doi: 10.3945/jn.113.178038. [DOI] [PubMed] [Google Scholar]
- 35.Conlon BA, Beasley JM, Aebersold K, Jhangiani SS, Wylie-Rosett J. Nutritional management of insulin resistance in nonalcoholic fatty liver disease (NAFLD). Nutrients 5: 4093–4114, 2013. doi: 10.3390/nu5104093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr 132, Suppl: 2393S–2400S, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Cooper WN, Khulan B, Owens S, Elks CE, Seidel V, Prentice AM, Belteki G, Ong KK, Affara NA, Constância M, Dunger DB. DNA methylation profiling at imprinted loci after periconceptional micronutrient supplementation in humans: results of a pilot randomized controlled trial. FASEB J 26: 1782–1790, 2012. doi: 10.1096/fj.11-192708. [DOI] [PubMed] [Google Scholar]
- 38.Covington JD, Bajpeyi S. The sirtuins: markers of metabolic health. Mol Nutr Food Res 60: 79–91, 2016. doi: 10.1002/mnfr.201500340. [DOI] [PubMed] [Google Scholar]
- 39.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr 133: 3614–3618, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cropley JE, Suter CM, Beckman KB, Martin DI. Germ-line epigenetic modification of the murine A vy allele by nutritional supplementation. Proc Natl Acad Sci USA 103: 17308–17312, 2006. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai L, Peng C, Montellier E, Lu Z, Chen Y, Ishii H, Debernardi A, Buchou T, Rousseaux S, Jin F, Sabari BR, Deng Z, Allis CD, Ren B, Khochbin S, Zhao Y. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat Chem Biol 10: 365–370, 2014. doi: 10.1038/nchembio.1497. [DOI] [PubMed] [Google Scholar]
- 42.Das M, Sha J, Hidalgo B, Aslibekyan S, Do AN, Zhi D, Sun D, Zhang T, Li S, Chen W, Srinivasan SR, Tiwari HK, Absher D, Ordovas JM, Berenson GS, Arnett DK, Irvin MR. Association of DNA Methylation at CPT1A Locus with Metabolic Syndrome in the Genetics of Lipid Lowering Drugs and Diet Network (GOLDN) Study. PLoS One 11: e0145789, 2016. doi: 10.1371/journal.pone.0145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 133, Suppl: 2485S–2493S, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Davison JM, Mellott TJ, Kovacheva VP, Blusztajn JK. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J Biol Chem 284: 1982–1989, 2009. doi: 10.1074/jbc.M807651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Day JK, Bauer AM, DesBordes C, Zhuang Y, Kim BE, Newton LG, Nehra V, Forsee KM, MacDonald RS, Besch-Williford C, Huang TH, Lubahn DB. Genistein alters methylation patterns in mice. J Nutr 132, Suppl: 2419S–2423S, 2002. [DOI] [PubMed] [Google Scholar]
- 46.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell 64: 849–859, 1991. doi: 10.1016/0092-8674(91)90513-X. [DOI] [PubMed] [Google Scholar]
- 47.Deichmann U. Epigenetics: the origins and evolution of a fashionable topic. Dev Biol 416: 249–254, 2016. doi: 10.1016/j.ydbio.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Deveson IW, Hardwick SA, Mercer TR, Mattick JS. The Dimensions, Dynamics, and Relevance of the Mammalian Noncoding Transcriptome. Trends Genet 33: 464–478, 2017. doi: 10.1016/j.tig.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect 114: 567–572, 2006. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE, Dyer RA, Fulford AJ, Guan Y, Laritsky E, Silver MJ, Swan GE, Zeisel SH, Innis SM, Waterland RA, Prentice AM, Hennig BJ. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun 5: 3746, 2014. doi: 10.1038/ncomms4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominy JE Jr, Lee Y, Jedrychowski MP, Chim H, Jurczak MJ, Camporez JP, Ruan HB, Feldman J, Pierce K, Mostoslavsky R, Denu JM, Clish CB, Yang X, Shulman GI, Gygi SP, Puigserver P. The deacetylase Sirt6 activates the acetyltransferase GCN5 and suppresses hepatic gluconeogenesis. Mol Cell 48: 900–913, 2012. doi: 10.1016/j.molcel.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donohoe DR, Holley D, Collins LB, Montgomery SA, Whitmore AC, Hillhouse A, Curry KP, Renner SW, Greenwalt A, Ryan EP, Godfrey V, Heise MT, Threadgill DS, Han A, Swenberg JA, Threadgill DW, Bultman SJ. A gnotobiotic mouse model demonstrates that dietary fiber protects against colorectal tumorigenesis in a microbiota- and butyrate-dependent manner. Cancer Discov 4: 1387–1397, 2014. doi: 10.1158/2159-8290.CD-14-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du J, Cheng X, Shen L, Tan Z, Luo J, Wu X, Liu C, Yang Q, Jiang Y, Tang G, Li X, Zhang S, Zhu L. Methylation of miR-145a-5p promoter mediates adipocytes differentiation. Biochem Biophys Res Commun 475: 140–148, 2016. doi: 10.1016/j.bbrc.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 54.Du J, Johnson LM, Jacobsen SE, Patel DJ. DNA methylation pathways and their crosstalk with histone methylation. Nat Rev Mol Cell Biol 16: 519–532, 2015. doi: 10.1038/nrm4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunford AR, Sangster JM. Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: a systematic review. Diabetes Metab Syndr 11, Suppl 2: S655–S662, 2017. doi: 10.1016/j.dsx.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Dunn GA, Bale TL. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 152: 2228–2236, 2011. doi: 10.1210/en.2010-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature 517: 302–310, 2015. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ekram MB, Kang K, Kim H, Kim J. Retrotransposons as a major source of epigenetic variations in the mammalian genome. Epigenetics 7: 370–382, 2012. doi: 10.4161/epi.19462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elango N, Hunt BG, Goodisman MA, Yi SV. DNA methylation is widespread and associated with differential gene expression in castes of the honeybee, Apis mellifera. Proc Natl Acad Sci USA 106: 11206–11211, 2009. doi: 10.1073/pnas.0900301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Etchegaray JP, Zhong L, Mostoslavsky R. The histone deacetylase SIRT6: at the crossroads between epigenetics, metabolism and disease. Curr Top Med Chem 13: 2991–3000, 2013. doi: 10.2174/15680266113136660213. [DOI] [PubMed] [Google Scholar]
- 61.Faa G, Manchia M, Pintus R, Gerosa C, Marcialis MA, Fanos V. Fetal programming of neuropsychiatric disorders. Birth Defects Res C Embryo Today 108: 207–223, 2016. doi: 10.1002/bdrc.21139. [DOI] [PubMed] [Google Scholar]
- 62.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr 137, Suppl: 223S–228S, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Ferolla SM, Ferrari TC, Lima ML, Reis TO, Tavares-Jr WC, Couto OF, Vidigal PV, Fausto MA, Couto CA. Dietary patterns in Brazilian patients with nonalcoholic fatty liver disease: a cross-sectional study. Clinics (Sao Paulo) 68: 11–17, 2013. doi: 10.6061/clinics/2013(01)OA03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fryer AA, Nafee TM, Ismail KM, Carroll WD, Emes RD, Farrell WE. LINE-1 DNA methylation is inversely correlated with cord plasma homocysteine in man: a preliminary study. Epigenetics 4: 394–398, 2009. doi: 10.4161/epi.4.6.9766. [DOI] [PubMed] [Google Scholar]
- 65.Fu X, Dong B, Tian Y, Lefebvre P, Meng Z, Wang X, Pattou F, Han W, Wang X, Lou F, Jove R, Staels B, Moore DD, Huang W. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J Clin Invest 125: 2497–2509, 2015. doi: 10.1172/JCI75438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Füllgrabe J, Klionsky DJ, Joseph B. The return of the nucleus: transcriptional and epigenetic control of autophagy. Nat Rev Mol Cell Biol 15: 65–74, 2014. doi: 10.1038/nrm3716. [DOI] [PubMed] [Google Scholar]
- 67.Ghasemi A, Fallah S, Ansari M. MiR-153 as a Tumor Suppressor in Glioblastoma Multiforme is Downregulated by DNA Methylation. Clin Lab 62: 573–580, 2016. doi: 10.7754/Clin.Lab.2015.150738. [DOI] [PubMed] [Google Scholar]
- 68.Ghoshal AK, Farber E. The induction of liver cancer by dietary deficiency of choline and methionine without added carcinogens. Carcinogenesis 5: 1367–1370, 1984. doi: 10.1093/carcin/5.10.1367. [DOI] [PubMed] [Google Scholar]
- 69.Ghoshal K, Li X, Datta J, Bai S, Pogribny I, Pogribny M, Huang Y, Young D, Jacob ST. A folate- and methyl-deficient diet alters the expression of DNA methyltransferases and methyl CpG binding proteins involved in epigenetic gene silencing in livers of F344 rats. J Nutr 136: 1522–1527, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilmour DS, Lis JT. Detecting protein-DNA interactions in vivo: distribution of RNA polymerase on specific bacterial genes. Proc Natl Acad Sci USA 81: 4275–4279, 1984. doi: 10.1073/pnas.81.14.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guedes-Dias P, Oliveira JM. Lysine deacetylases and mitochondrial dynamics in neurodegeneration. Biochim Biophys Acta 1832: 1345–1359, 2013. doi: 10.1016/j.bbadis.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 72.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15: 509–524, 2014. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 73.Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation 108: 1541–1545, 2003. doi: 10.1161/01.CIR.0000088845.17586.EC. [DOI] [PubMed] [Google Scholar]
- 74.Haggarty P, Hoad G, Campbell DM, Horgan GW, Piyathilake C, McNeill G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am J Clin Nutr 97: 94–99, 2013. doi: 10.3945/ajcn.112.042572. [DOI] [PubMed] [Google Scholar]
- 75.Hansen KD, Timp W, Bravo HC, Sabunciyan S, Langmead B, McDonald OG, Wen B, Wu H, Liu Y, Diep D, Briem E, Zhang K, Irizarry RA, Feinberg AP. Increased methylation variation in epigenetic domains across cancer types. Nat Genet 43: 768–775, 2011. doi: 10.1038/ng.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hao M, Zhao W, Zhang L, Wang H, Yang X. Low folate levels are associated with methylation-mediated transcriptional repression of miR-203 and miR-375 during cervical carcinogenesis. Oncol Lett 11: 3863–3869, 2016. doi: 10.3892/ol.2016.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hassan YI, Zempleni J. A novel, enigmatic histone modification: biotinylation of histones by holocarboxylase synthetase. Nutr Rev 66: 721–725, 2008. doi: 10.1111/j.1753-4887.2008.00127.x. [DOI] [PubMed] [Google Scholar]
- 78.Hawthorne DC. A Deletion in Yeast and Its Bearing on the Structure of the Mating Type Locus. Genetics 48: 1727–1729, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hayakawa K, Hirosawa M, Tabei Y, Arai D, Tanaka S, Murakami N, Yagi S, Shiota K. Epigenetic switching by the metabolism-sensing factors in the generation of orexin neurons from mouse embryonic stem cells. J Biol Chem 288: 17099–17110, 2013. doi: 10.1074/jbc.M113.455899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Y, Ecker JR. Non-CG Methylation in the Human Genome. Annu Rev Genomics Hum Genet 16: 55–77, 2015. doi: 10.1146/annurev-genom-090413-025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333: 1303–1307, 2011. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 105: 17046–17049, 2008. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science 187: 226–232, 1975. doi: 10.1126/science.1111098. [DOI] [PubMed] [Google Scholar]
- 85.Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet 16: 71–84, 2015. doi: 10.1038/nrg3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hotchkiss RD. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem 175: 315–332, 1948. [PubMed] [Google Scholar]
- 87.Hou YY, You JJ, Yang CM, Pan HW, Chen HC, Lee JH, Lin YS, Liou HH, Liu PF, Chi CC, Ger LP, Tsai KW. Aberrant DNA hypomethylation of miR-196b contributes to migration and invasion of oral cancer. Oncol Lett 11: 4013–4021, 2016. doi: 10.3892/ol.2016.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13: 225–238, 2012. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hoyo C, Murtha AP, Schildkraut JM, Jirtle RL, Demark-Wahnefried W, Forman MR, Iversen ES, Kurtzberg J, Overcash F, Huang Z, Murphy SK. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 6: 928–936, 2011. doi: 10.4161/epi.6.7.16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hsu A, Wong CP, Yu Z, Williams DE, Dashwood RH, Ho E. Promoter de-methylation of cyclin D2 by sulforaphane in prostate cancer cells. Clin Epigenetics 3: 3, 2011. doi: 10.1186/1868-7083-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hunt BG, Brisson JA, Yi SV, Goodisman MA. Functional conservation of DNA methylation in the pea aphid and the honeybee. Genome Biol Evol 2: 719–728, 2010. doi: 10.1093/gbe/evq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inoue S, Honma K, Mochizuki K, Goda T. Induction of histone H3K4 methylation at the promoter, enhancer, and transcribed regions of the Si and Sglt1 genes in rat jejunum in response to a high-starch/low-fat diet. Nutrition 31: 366–372, 2015. doi: 10.1016/j.nut.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 93.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33, Suppl: 245–254, 2003. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 94.Jia Y, Li R, Cong R, Yang X, Sun Q, Parvizi N, Zhao R. Maternal low-protein diet affects epigenetic regulation of hepatic mitochondrial DNA transcription in a sex-specific manner in newborn piglets associated with GR binding to its promoter. PLoS One 8: e63855, 2013. doi: 10.1371/journal.pone.0063855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jiang X, Yan J, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Caudill MA. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J 26: 3563–3574, 2012. doi: 10.1096/fj.12-207894. [DOI] [PubMed] [Google Scholar]
- 96.Johanning GL, Heimburger DC, Piyathilake CJ. DNA methylation and diet in cancer. J Nutr 132: 3814S–3818S, 2002. [DOI] [PubMed] [Google Scholar]
- 97.Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 13: 484–492, 2012. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- 98.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell 20: 85–93, 1980. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 99.Joubert BR, den Dekker HT, Felix JF, Bohlin J, Ligthart S, Beckett E, Tiemeier H, van Meurs JB, Uitterlinden AG, Hofman A, Håberg SE, Reese SE, Peters MJ, Andreassen BK, Steegers EA, Nilsen RM, Vollset SE, Midttun Ø, Ueland PM, Franco OH, Dehghan A, de Jongste JC, Wu MC, Wang T, Peddada SD, Jaddoe VW, Nystad W, Duijts L, London SJ. Maternal plasma folate impacts differential DNA methylation in an epigenome-wide meta-analysis of newborns. Nat Commun 7: 10577, 2016. doi: 10.1038/ncomms10577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaelin WG Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell 153: 56–69, 2013. doi: 10.1016/j.cell.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kamakura M. Royalactin induces queen differentiation in honeybees. Nature 473: 478–483, 2011. doi: 10.1038/nature10093. [DOI] [PubMed] [Google Scholar]
- 102.Kapranov P, Cawley SE, Drenkow J, Bekiranov S, Strausberg RL, Fodor SP, Gingeras TR. Large-scale transcriptional activity in chromosomes 21 and 22. Science 296: 916–919, 2002. doi: 10.1126/science.1068597. [DOI] [PubMed] [Google Scholar]
- 103.Kaufman-Szymczyk A, Majewski G, Lubecka-Pietruszewska K, Fabianowska-Majewska K. The Role of Sulforaphane in Epigenetic Mechanisms, Including Interdependence between Histone Modification and DNA Methylation. Int J Mol Sci 16: 29732–29743, 2015. doi: 10.3390/ijms161226195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khalyfa A, Carreras A, Hakim F, Cunningham JM, Wang Y, Gozal D. Effects of late gestational high-fat diet on body weight, metabolic regulation and adipokine expression in offspring. Int J Obes 37: 1481–1489, 2013. doi: 10.1038/ijo.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khanna A, Muthusamy S, Liang R, Sarojini H, Wang E. Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging (Albany NY) 3: 223–236, 2011. doi: 10.18632/aging.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim SJ, Zuchniak A, Sohn KJ, Lubinski J, Demsky R, Eisen A, Akbari MR, Kim YI, Narod SA, Kotsopoulos J. Plasma folate, vitamin B-6, and vitamin B-12 and breast cancer risk in BRCA1- and BRCA2-mutation carriers: a prospective study. Am J Clin Nutr 104: 671–677, 2016. doi: 10.3945/ajcn.116.133470. [DOI] [PubMed] [Google Scholar]
- 107.Kim YS, Milner JA. Dietary modulation of colon cancer risk. J Nutr 137, Suppl: 2576S–2579S, 2007. [DOI] [PubMed] [Google Scholar]
- 108.Knisbacher BA, Gerber D, Levanon EY. DNA Editing by APOBECs: A Genomic Preserver and Transformer. Trends Genet 32: 16–28, 2016. doi: 10.1016/j.tig.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 109.Kondo Y. Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei Med J 50: 455–463, 2009. doi: 10.3349/ymj.2009.50.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Koturbash I, Melnyk S, James SJ, Beland FA, Pogribny IP. Role of epigenetic and miR-22 and miR-29b alterations in the downregulation of Mat1a and Mthfr genes in early preneoplastic livers in rats induced by 2-acetylaminofluorene. Mol Carcinog 52: 318–327, 2013. doi: 10.1002/mc.21861. [DOI] [PubMed] [Google Scholar]
- 111.Kovacheva VP, Mellott TJ, Davison JM, Wagner N, Lopez-Coviella I, Schnitzler AC, Blusztajn JK. Gestational choline deficiency causes global and Igf2 gene DNA hypermethylation by up-regulation of Dnmt1 expression. J Biol Chem 282: 31777–31788, 2007. doi: 10.1074/jbc.M705539200. [DOI] [PubMed] [Google Scholar]
- 112.Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, Keller MP, Attie AD, Rey FE, Denu JM. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol Cell 64: 982–992, 2016. doi: 10.1016/j.molcel.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kucharski R, Maleszka J, Foret S, Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science 319: 1827–1830, 2008. doi: 10.1126/science.1153069. [DOI] [PubMed] [Google Scholar]
- 114.Kwong WY, Miller DJ, Ursell E, Wild AE, Wilkins AP, Osmond C, Anthony FW, Fleming TP. Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction 132: 265–277, 2006. doi: 10.1530/rep.1.01038. [DOI] [PubMed] [Google Scholar]
- 115.Lai WKM, Pugh BF. Understanding nucleosome dynamics and their links to gene expression and DNA replication. Nat Rev Mol Cell Biol 18: 548–562, 2017. doi: 10.1038/nrm.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Latouche C, Natoli A, Reddy-Luthmoodoo M, Heywood SE, Armitage JA, Kingwell BA. MicroRNA-194 Modulates Glucose Metabolism and Its Skeletal Muscle Expression Is Reduced in Diabetes. PLoS One 11: e0155108, 2016. doi: 10.1371/journal.pone.0155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee HS, Barraza-Villarreal A, Biessy C, Duarte-Salles T, Sly PD, Ramakrishnan U, Rivera J, Herceg Z, Romieu I. Dietary supplementation with polyunsaturated fatty acid during pregnancy modulates DNA methylation at IGF2/H19 imprinted genes and growth of infants. Physiol Genomics 46: 851–857, 2014. doi: 10.1152/physiolgenomics.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee HS, Barraza-Villarreal A, Hernandez-Vargas H, Sly PD, Biessy C, Ramakrishnan U, Romieu I, Herceg Z. Modulation of DNA methylation states and infant immune system by dietary supplementation with ω-3 PUFA during pregnancy in an intervention study. Am J Clin Nutr 98: 480–487, 2013. doi: 10.3945/ajcn.112.052241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee JY, Terakawa T, Qi Z, Steinfeld JB, Redding S, Kwon Y, Gaines WA, Zhao W, Sung P, Greene EC. Base triplet stepping by the Rad51/RecA family of recombinases. Science 349: 977–981, 2015. doi: 10.1126/science.aab2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee WJ, Shim JY, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol 68: 1018–1030, 2005. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- 121.Leung A, Trac C, Du J, Natarajan R, Schones DE. Persistent Chromatin Modifications Induced by High Fat Diet. J Biol Chem 291: 10446–10455, 2016. doi: 10.1074/jbc.M115.711028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lévesque N, Leclerc D, Gayden T, Lazaris A, De Jay N, Petrillo S, Metrakos P, Jabado N, Rozen R. Murine diet/tissue and human brain tumorigenesis alter Mthfr/MTHFR 5′-end methylation. Mamm Genome 27: 122–134, 2016. doi: 10.1007/s00335-016-9624-0. [DOI] [PubMed] [Google Scholar]
- 123.Lewis CJ, Pan T, Kalsotra A. RNA modifications and structures cooperate to guide RNA-protein interactions. Nat Rev Mol Cell Biol 18: 202–210, 2017. doi: 10.1038/nrm.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li CC, Cropley JE, Cowley MJ, Preiss T, Martin DI, Suter CM. A sustained dietary change increases epigenetic variation in isogenic mice. PLoS Genet 7: e1001380, 2011. doi: 10.1371/journal.pgen.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li X, Wu Y, Liu A, Tang X. MiR-27b is epigenetically downregulated in tamoxifen resistant breast cancer cells due to promoter methylation and regulates tamoxifen sensitivity by targeting HMGB3. Biochem Biophys Res Commun 477: 768–773, 2016. doi: 10.1016/j.bbrc.2016.06.133. [DOI] [PubMed] [Google Scholar]
- 126.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr 135: 1382–1386, 2005. [DOI] [PubMed] [Google Scholar]
- 127.Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPAR alpha promoter of the offspring. Br J Nutr 100: 278–282, 2008. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lorch Y, Maier-Davis B, Kornberg RD. Mechanism of chromatin remodeling. Proc Natl Acad Sci USA 107: 3458–3462, 2010. doi: 10.1073/pnas.1000398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lu H, Bhoopatiraju S, Wang H, Schmitz NP, Wang X, Freeman MJ, Forster CL, Verneris MR, Linden MA, Hallstrom TC. Loss of UHRF2 expression is associated with human neoplasia, promoter hypermethylation, decreased 5-hydroxymethylcytosine, and high proliferative activity. Oncotarget 7: 76047–76061, 2016. doi: 10.18632/oncotarget.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu H, Buchan RJ, Cook SA. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc Res 86: 410–420, 2010. doi: 10.1093/cvr/cvq010. [DOI] [PubMed] [Google Scholar]
- 131.Lumey LH, Terry MB, Delgado-Cruzata L, Liao Y, Wang Q, Susser E, McKeague I, Santella RM. Adult global DNA methylation in relation to pre-natal nutrition. Int J Epidemiol 41: 116–123, 2012. doi: 10.1093/ije/dyr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lupu DS, Orozco LD, Wang Y, Cullen JM, Pellegrini M, Zeisel SH. Altered methylation of specific DNA loci in the liver of Bhmt-null mice results in repression of Iqgap2 and F2rl2 and is associated with development of preneoplastic foci. FASEB J 31: 2090–2103, 2017. doi: 10.1096/fj.201601169R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mamtani M, Kulkarni H, Dyer TD, Göring HH, Neary JL, Cole SA, Kent JW, Kumar S, Glahn DC, Mahaney MC, Comuzzie AG, Almasy L, Curran JE, Duggirala R, Blangero J, Carless MA. Genome- and epigenome-wide association study of hypertriglyceridemic waist in Mexican American families. Clin Epigenetics 8: 6, 2016. doi: 10.1186/s13148-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mandacaru SC, do Vale LH, Vahidi S, Xiao Y, Skinner OS, Ricart CA, Kelleher NL, de Sousa MV, Konermann L. Characterizing the Structure and Oligomerization of Major Royal Jelly Protein 1 (MRJP1) by Mass Spectrometry and Complementary Biophysical Tools. Biochemistry 56: 1645–1655, 2017. doi: 10.1021/acs.biochem.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 153: 2823–2830, 2012. doi: 10.1210/en.2011-2161. [DOI] [PubMed] [Google Scholar]
- 136.McGinnis JM, Nestle M. The Surgeon General’s Report on Nutrition and Health: policy implications and implementation strategies. Am J Clin Nutr 49: 23–28, 1989. [DOI] [PubMed] [Google Scholar]
- 137.Mehedint MG, Niculescu MD, Craciunescu CN, Zeisel SH. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J 24: 184–195, 2010. doi: 10.1096/fj.09-140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mentch SJ, Mehrmohamadi M, Huang L, Liu X, Gupta D, Mattocks D, Gómez Padilla P, Ables G, Bamman MM, Thalacker-Mercer AE, Nichenametla SN, Locasale JW. Histone Methylation Dynamics and Gene Regulation Occur through the Sensing of One-Carbon Metabolism. Cell Metab 22: 861–873, 2015. doi: 10.1016/j.cmet.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mercken EM, Majounie E, Ding J, Guo R, Kim J, Bernier M, Mattison J, Cookson MR, Gorospe M, de Cabo R, Abdelmohsen K. Age-associated miRNA alterations in skeletal muscle from rhesus monkeys reversed by caloric restriction. Aging (Albany NY) 5: 692–703, 2013. doi: 10.18632/aging.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Messerschmidt DM, Knowles BB, Solter D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev 28: 812–828, 2014. doi: 10.1101/gad.234294.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mikael LG, Deng L, Paul L, Selhub J, Rozen R. Moderately high intake of folic acid has a negative impact on mouse embryonic development. Birth Defects Res A Clin Mol Teratol 97: 47–52, 2013. doi: 10.1002/bdra.23092. [DOI] [PubMed] [Google Scholar]
- 142.Mochizuki K, Hariya N, Honma K, Goda T. Relationship between epigenetic regulation, dietary habits, and the developmental origins of health and disease theory. Congenit Anom (Kyoto) 57: 184–190, 2017. doi: 10.1111/cga.12213. [DOI] [PubMed] [Google Scholar]
- 143.Mohajeri MH, Troesch B, Weber P. Inadequate supply of vitamins and DHA in the elderly: implications for brain aging and Alzheimer-type dementia. Nutrition 31: 261–275, 2015. doi: 10.1016/j.nut.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 144.Multhaup ML, Seldin MM, Jaffe AE, Lei X, Kirchner H, Mondal P, Li Y, Rodriguez V, Drong A, Hussain M, Lindgren C, McCarthy M, Näslund E, Zierath JR, Wong GW, Feinberg AP. Mouse-human experimental epigenetic analysis unmasks dietary targets and genetic liability for diabetic phenotypes. Cell Metab 21: 138–149, 2015. doi: 10.1016/j.cmet.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Fagà E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 37: 909–916, 2003. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 146.Nakagawa T, Guarente L. Urea cycle regulation by mitochondrial sirtuin, SIRT5. Aging (Albany NY) 1: 578–581, 2009. doi: 10.18632/aging.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Narayan PJ, Lill C, Faull R, Curtis MA, Dragunow M. Increased acetyl and total histone levels in post-mortem Alzheimer’s disease brain. Neurobiol Dis 74: 281–294, 2015. doi: 10.1016/j.nbd.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 148.Newberne PM, Rogers AE. Labile methyl groups and the promotion of cancer. Annu Rev Nutr 6: 407–432, 1986. doi: 10.1146/annurev.nu.06.070186.002203. [DOI] [PubMed] [Google Scholar]
- 149.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 467: 963–966, 2010. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- 150.Niculescu MD, Craciunescu CN, Zeisel SH. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J 20: 43–49, 2006. doi: 10.1096/fj.05-4707com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oestreich AK, Moley KH. Developmental and Transmittable Origins of Obesity-Associated Health Disorders. Trends Genet 33: 399–407, 2017. doi: 10.1016/j.tig.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet 19: 219–220, 1998. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 153.Ong ML, Lin X, Holbrook JD. Measuring epigenetics as the mediator of gene/environment interactions in DOHaD. J Dev Orig Health Dis 6: 10–16, 2015. doi: 10.1017/S2040174414000506. [DOI] [PubMed] [Google Scholar]
- 154.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 48: 993–999, 2008. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Painter RC, de Rooij SR, Bossuyt PM, Simmers TA, Osmond C, Barker DJ, Bleker OP, Roseboom TJ. Early onset of coronary artery disease after prenatal exposure to the Dutch famine. Am J Clin Nutr 84: 322–327, 2006. [DOI] [PubMed] [Google Scholar]
- 156.Peter CJ, Fischer LK, Kundakovic M, Garg P, Jakovcevski M, Dincer A, Amaral AC, Ginns EI, Galdzicka M, Bryce CP, Ratner C, Waber DP, Mokler D, Medford G, Champagne FA, Rosene DL, McGaughy JA, Sharp AJ, Galler JR, Akbarian S. DNA Methylation Signatures of Early Childhood Malnutrition Associated With Impairments in Attention and Cognition. Biol Psychiatry 80: 765–774, 2016. doi: 10.1016/j.biopsych.2016.03.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Plumptre L, Masih SP, Ly A, Aufreiter S, Sohn KJ, Croxford R, Lausman AY, Berger H, O’Connor DL, Kim YI. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr 102: 848–857, 2015. doi: 10.3945/ajcn.115.110783. [DOI] [PubMed] [Google Scholar]
- 158.Ponnaluri VK, Maciejewski JP, Mukherji M. A mechanistic overview of TET-mediated 5-methylcytosine oxidation. Biochem Biophys Res Commun 436: 115–120, 2013. doi: 10.1016/j.bbrc.2013.05.077. [DOI] [PubMed] [Google Scholar]
- 159.Pullen TJ, da Silva Xavier G, Kelsey G, Rutter GA. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol Cell Biol 31: 3182–3194, 2011. doi: 10.1128/MCB.01433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Radford EJ, Ito M, Shi H, Corish JA, Yamazawa K, Isganaitis E, Seisenberger S, Hore TA, Reik W, Erkek S, Peters AHFM, Patti ME, Ferguson-Smith AC. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 345: 1255903, 2014. doi: 10.1126/science.1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rajendran P, Williams DE, Ho E, Dashwood RH. Metabolism as a key to histone deacetylase inhibition. Crit Rev Biochem Mol Biol 46: 181–199, 2011. doi: 10.3109/10409238.2011.557713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rakyan VK, Beck S. Epigenetic variation and inheritance in mammals. Curr Opin Genet Dev 16: 573–577, 2006. doi: 10.1016/j.gde.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 164.Rees WD, Hay SM, Brown DS, Antipatis C, Palmer RM. Maternal protein deficiency causes hypermethylation of DNA in the livers of rat fetuses. J Nutr 130: 1821–1826, 2000. [DOI] [PubMed] [Google Scholar]
- 165.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 293: 1089–1093, 2001. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 166.Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA 100: 4012–4017, 2003. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet 14: 9–25, 1975. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- 168.Rose NR, Klose RJ. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim Biophys Acta 1839: 1362–1372, 2014. doi: 10.1016/j.bbagrm.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sabari BR, Tang Z, Huang H, Yong-Gonzalez V, Molina H, Kong HE, Dai L, Shimada M, Cross JR, Zhao Y, Roeder RG, Allis CD. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol Cell 58: 203–215, 2015. doi: 10.1016/j.molcel.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Saetrom P, Snøve O Jr, Rossi JJ. Epigenetics and microRNAs. Pediatr Res 61: 17R–23R, 2007. doi: 10.1203/pdr.0b013e318045760e. [DOI] [PubMed] [Google Scholar]
- 171.Sallam T, Jones MC, Gilliland T, Zhang L, Wu X, Eskin A, Sandhu J, Casero D, Vallim TQ, Hong C, Katz M, Lee R, Whitelegge J, Tontonoz P. Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding RNA LeXis. Nature 534: 124–128, 2016. doi: 10.1038/nature17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Samson M, Jow MM, Wong CC, Fitzpatrick C, Aslanian A, Saucedo I, Estrada R, Ito T, Park SK, Yates JR III, Chu DS. The specification and global reprogramming of histone epigenetic marks during gamete formation and early embryo development in C. elegans. PLoS Genet 10: e1004588, 2014. doi: 10.1371/journal.pgen.1004588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sandovici I, Smith NH, Nitert MD, Ackers-Johnson M, Uribe-Lewis S, Ito Y, Jones RH, Marquez VE, Cairns W, Tadayyon M, O’Neill LP, Murrell A, Ling C, Constância M, Ozanne SE. Maternal diet and aging alter the epigenetic control of a promoter-enhancer interaction at the Hnf4a gene in rat pancreatic islets. Proc Natl Acad Sci USA 108: 5449–5454, 2011. doi: 10.1073/pnas.1019007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab 7: 104–112, 2008. doi: 10.1016/j.cmet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 175.Sebastián C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, Ram O, Truelove J, Guimaraes AR, Toiber D, Cosentino C, Greenson JK, MacDonald AI, McGlynn L, Maxwell F, Edwards J, Giacosa S, Guccione E, Weissleder R, Bernstein BE, Regev A, Shiels PG, Lombard DB, Mostoslavsky R. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 151: 1185–1199, 2012. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sen N. Epigenetic regulation of memory by acetylation and methylation of chromatin: implications in neurological disorders, aging, and addiction. Neuromolecular Med 17: 97–110, 2015. doi: 10.1007/s12017-014-8306-x. [DOI] [PubMed] [Google Scholar]
- 177.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis 31: 27–36, 2010. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sharma S, Taliyan R. Epigenetic modifications by inhibiting histone deacetylases reverse memory impairment in insulin resistance induced cognitive deficit in mice. Neuropharmacology 105: 285–297, 2016. doi: 10.1016/j.neuropharm.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 179.Shea JM, Serra RW, Carone BR, Shulha HP, Kucukural A, Ziller MJ, Vallaster MP, Gu H, Tapper AR, Gardner PD, Meissner A, Garber M, Rando OJ. Genetic and Epigenetic Variation, but Not Diet, Shape the Sperm Methylome. Dev Cell 35: 750–758, 2015. doi: 10.1016/j.devcel.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shin HJ, Kim H, Oh S, Lee JG, Kee M, Ko HJ, Kweon MN, Won KJ, Baek SH. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature 534: 553–557, 2016. doi: 10.1038/nature18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Solter D. Preformation Versus Epigenesis in Early Mammalian Development. Curr Top Dev Biol 117: 377–391, 2016. doi: 10.1016/bs.ctdb.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 182.Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, Wang J, Zhang L, Looney TJ, Zhang B, Godley LA, Hicks LM, Lahn BT, Jin P, He C. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol 29: 68–72, 2011. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sookoian S, Rosselli MS, Gemma C, Burgueño AL, Fernández Gianotti T, Castaño GO, Pirola CJ. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology 52: 1992–2000, 2010. doi: 10.1002/hep.23927. [DOI] [PubMed] [Google Scholar]
- 184.Steegers-Theunissen RP, Obermann-Borst SA, Kremer D, Lindemans J, Siebel C, Steegers EA, Slagboom PE, Heijmans BT. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS One 4: e7845, 2009. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Strahl BD, Allis CD. The language of covalent histone modifications. Nature 403: 41–45, 2000. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 186.Sun Q, Li X, Jia Y, Pan S, Li R, Yang X, Zhao R. Maternal betaine supplementation during gestation modifies hippocampal expression of GR and its regulatory miRNAs in neonatal piglets. J Vet Med Sci 78: 921–928, 2016. doi: 10.1292/jvms.15-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sun Q, Li X, Jia Y, Pan S, Li R, Yang X, Zhao R. Maternal betaine supplementation during gestation modifies hippocampal expression of GR and its regulatory miRNAs in neonatal piglets. J Vet Med Sci 78: 921–928, 2016. doi: 10.1292/jvms.15-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Szyf M. DNA methylation and demethylation as targets for anticancer therapy. Biochemistry (Mosc) 70: 533–549, 2005. doi: 10.1007/s10541-005-0147-7. [DOI] [PubMed] [Google Scholar]
- 189.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324: 930–935, 2009. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development 139: 1895–1902, 2012. doi: 10.1242/dev.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tan M, Luo H, Lee S, Jin F, Yang JS, Montellier E, Buchou T, Cheng Z, Rousseaux S, Rajagopal N, Lu Z, Ye Z, Zhu Q, Wysocka J, Ye Y, Khochbin S, Ren B, Zhao Y. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell 146: 1016–1028, 2011. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tang Q, Cheng J, Cao X, Surowy H, Burwinkel B. Blood-based DNA methylation as biomarker for breast cancer: a systematic review. Clin Epigenetics 8: 115, 2016. doi: 10.1186/s13148-016-0282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Taunton J, Hassig CA, Schreiber SL. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272: 408–411, 1996. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 194.Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, Slieker RC, Stok AP, Thijssen PE, Müller F, van Zwet EW, Bock C, Meissner A, Lumey LH, Eline Slagboom P, Heijmans BT. Corrigendum: DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun 6: 7740, 2015. doi: 10.1038/ncomms8740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, Slieker RC, Stok AP, Thijssen PE, Müller F, van Zwet EW, Bock C, Meissner A, Lumey LH, Eline Slagboom P, Heijmans BT. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun 5: 5592, 2014. doi: 10.1038/ncomms6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet 18: 4046–4053, 2009. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Troesch B, Weber P, Mohajeri MH. Potential Links between Impaired One-Carbon Metabolism Due to Polymorphisms, Inadequate B-Vitamin Status, and the Development of Alzheimer’s Disease. Nutrients 8: 803, 2016. doi: 10.3390/nu8120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tryndyak VP, Marrone AK, Latendresse JR, Muskhelishvili L, Beland FA, Pogribny IP. MicroRNA changes, activation of progenitor cells and severity of liver injury in mice induced by choline and folate deficiency. J Nutr Biochem 28: 83–90, 2016. doi: 10.1016/j.jnutbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 199.Van Straten EM, Bloks VW, Huijkman NC, Baller JF, van Meer H, Lütjohann D, Kuipers F, Plösch T. The liver X-receptor gene promoter is hypermethylated in a mouse model of prenatal protein restriction. Am J Physiol Regul Integr Comp Physiol 298: R275–R282, 2010. doi: 10.1152/ajpregu.00413.2009. [DOI] [PubMed] [Google Scholar]
- 200.Waddington CH. The epigenotype. Endeavour 1: 18–20, 1942. [Google Scholar]
- 201.Waddington CH. The Strategy of the Genes London: Routledge Library Editions: 20th Century Science, 1957, p. 259. [Google Scholar]
- 202.Wade PA. Methyl CpG binding proteins: coupling chromatin architecture to gene regulation. Oncogene 20: 3166–3173, 2001. doi: 10.1038/sj.onc.1204340. [DOI] [PubMed] [Google Scholar]
- 203.Wagschal A, Najafi-Shoushtari SH, Wang L, Goedeke L, Sinha S, deLemos AS, Black JC, Ramírez CM, Li Y, Tewhey R, Hatoum I, Shah N, Lu Y, Kristo F, Psychogios N, Vrbanac V, Lu YC, Hla T, de Cabo R, Tsang JS, Schadt E, Sabeti PC, Kathiresan S, Cohen DE, Whetstine J, Chung RT, Fernández-Hernando C, Kaplan LM, Bernards A, Gerszten RE, Näär AM. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat Med 21: 1290–1297, 2015. doi: 10.1038/nm.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wainfan E, Poirier LA. Methyl groups in carcinogenesis: effects on DNA methylation and gene expression. Cancer Res 52, Suppl: 2071s–2077s, 1992. [PubMed] [Google Scholar]
- 205.Walker CL, Ho SM. Developmental reprogramming of cancer susceptibility. Nat Rev Cancer 12: 479–486, 2012. doi: 10.1038/nrc3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang Y, Surzenko N, Friday WB, Zeisel SH. Maternal dietary intake of choline in mice regulates development of the cerebral cortex in the offspring. FASEB J 30: 1566–1578, 2016. doi: 10.1096/fj.15-282426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Waterland RA, Dolinoy DC, Lin JR, Smith CA, Shi X, Tahiliani KG. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis 44: 401–406, 2006. doi: 10.1002/dvg.20230. [DOI] [PubMed] [Google Scholar]
- 208.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol 23: 5293–5300, 2003. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Waterland RA, Kellermayer R, Laritsky E, Rayco-Solon P, Harris RA, Travisano M, Zhang W, Torskaya MS, Zhang J, Shen L, Manary MJ, Prentice AM. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet 6: e1001252, 2010. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum Mol Genet 15: 705–716, 2006. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- 211.Waterland RA, Travisano M, Tahiliani KG. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. FASEB J 21: 3380–3385, 2007. doi: 10.1096/fj.07-8229com. [DOI] [PubMed] [Google Scholar]
- 212.Willeit P, Skroblin P, Moschen AR, Yin X, Kaudewitz D, Zampetaki A, Barwari T, Whitehead M, Ramírez CM, Goedeke L, Rotllan N, Bonora E, Hughes AD, Santer P, Fernández-Hernando C, Tilg H, Willeit J, Kiechl S, Mayr M. Circulating MicroRNA-122 Is Associated With the Risk of New-Onset Metabolic Syndrome and Type 2 Diabetes. Diabetes 66: 347–357, 2017. doi: 10.2337/db16-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wilson MJ, Shivapurkar N, Poirier LA. Hypomethylation of hepatic nuclear DNA in rats fed with a carcinogenic methyl-deficient diet. Biochem J 218: 987–990, 1984. doi: 10.1042/bj2180987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wilting SM, Miok V, Jaspers A, Boon D, Sørgård H, Lando M, Snoek BC, van Wieringen WN, Meijer CJ, Lyng H, Snijders PJ, Steenbergen RD. Aberrant methylation-mediated silencing of microRNAs contributes to HPV-induced anchorage independence. Oncotarget 7: 43805–43819, 2016. doi: 10.18632/oncotarget.9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J 12: 949–957, 1998. [PubMed] [Google Scholar]
- 216.Wu SC, Zhang Y. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 11: 607–620, 2010. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Xiong H, Guo B, Gan Z, Song D, Lu Z, Yi H, Wu Y, Wang Y, Du H. Butyrate upregulates endogenous host defense peptides to enhance disease resistance in piglets via histone deacetylase inhibition. Sci Rep 6: 27070, 2016. doi: 10.1038/srep27070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu Y, Zhang S, Lin S, Guo Y, Deng W, Zhang Y, Xue Y. WERAM: a database of writers, erasers and readers of histone acetylation and methylation in eukaryotes. Nucleic Acids Res 45, D1: D264–D270, 2017. doi: 10.1093/nar/gkw1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xue J, Schoenrock SA, Valdar W, Tarantino LM, Ideraabdullah FY. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin Epigenetics 8: 107, 2016. doi: 10.1186/s13148-016-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, Jiao Y, Li C, Liu S, Wu D, Gu W, Yang YG, Xu GL, Wang H. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. J Am Chem Soc 135: 10396–10403, 2013. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 221.Yoo JY, Lee S, Lee HA, Park H, Park YJ, Ha EH, Kim YJ. Can proopiomelanocortin methylation be used as an early predictor of metabolic syndrome? Diabetes Care 37: 734–739, 2014. doi: 10.2337/dc13-1012. [DOI] [PubMed] [Google Scholar]
- 222.Young JI, Züchner S, Wang G. Regulation of the Epigenome by Vitamin C. Annu Rev Nutr 35: 545–564, 2015. doi: 10.1146/annurev-nutr-071714-034228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yu H, Yang W. MiR-211 is epigenetically regulated by DNMT1 mediated methylation and inhibits EMT of melanoma cells by targeting RAB22A. Biochem Biophys Res Commun 476: 400–405, 2016. doi: 10.1016/j.bbrc.2016.05.133. [DOI] [PubMed] [Google Scholar]
- 224.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 26: 229–250, 2006. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang S, Rattanatray L, MacLaughlin SM, Cropley JE, Suter CM, Molloy L, Kleemann D, Walker SK, Muhlhausler BS, Morrison JL, McMillen IC. Periconceptional undernutrition in normal and overweight ewes leads to increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. FASEB J 24: 2772–2782, 2010. doi: 10.1096/fj.09-154294. [DOI] [PubMed] [Google Scholar]
- 226.Zhao Y, Garcia BA. Comprehensive Catalog of Currently Documented Histone Modifications. Cold Spring Harb Perspect Biol 7: a025064, 2015. doi: 10.1101/cshperspect.a025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zheng J, Zhang Q, Mul JD, Yu M, Xu J, Qi C, Wang T, Xiao X. Maternal high-calorie diet is associated with altered hepatic microRNA expression and impaired metabolic health in offspring at weaning age. Endocrine 54: 70–80, 2016. doi: 10.1007/s12020-016-0959-9. [DOI] [PubMed] [Google Scholar]
- 228.Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, Clish CB, Vaitheesvaran B, Iliopoulos O, Kurland I, Dor Y, Weissleder R, Shirihai OS, Ellisen LW, Espinosa JM, Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140: 280–293, 2010. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhou D, Wang H, Cui H, Chen H, Pan YX. Early-life exposure to high-fat diet may predispose rats to gender-specific hepatic fat accumulation by programming Pepck expression. J Nutr Biochem 26: 433–440, 2015. doi: 10.1016/j.jnutbio.2014.10.009. [DOI] [PubMed] [Google Scholar]