Abstract
Since no approved therapies to restore mobility and sensation following spinal cord injury (SCI) currently exist, a better understanding of the cellular and molecular mechanisms following SCI that compromise regeneration or neuroplasticity is needed to develop new strategies to promote axonal regrowth and restore function. Physical trauma to the spinal cord results in vascular disruption that, in turn, causes blood-spinal cord barrier rupture leading to hemorrhage and ischemia, followed by rampant local cell death. As subsequent edema and inflammation occur, neuronal and glial necrosis and apoptosis spread well beyond the initial site of impact, ultimately resolving into a cavity surrounded by glial/fibrotic scarring. The glial scar, which stabilizes the spread of secondary injury, also acts as a chronic, physical, and chemo-entrapping barrier that prevents axonal regeneration. Understanding the formative events in glial scarring helps guide strategies towards the development of potential therapies to enhance axon regeneration and functional recovery at both acute and chronic stages following SCI. This review will also discuss the perineuronal net and how chondroitin sulfate proteoglycans (CSPGs) deposited in both the glial scar and net impede axonal outgrowth at the level of the growth cone. We will end the review with a summary of current CSPG-targeting strategies that help to foster axonal regeneration, neuroplasticity/sprouting, and functional recovery following SCI.
I. INTRODUCTION
When asked in an anonymous questionnaire whether emergency care professionals would desire resuscitative measures following a severe spinal cord injury (SCI), only 37% of physicians and 14% of nurses indicated that they themselves would want measures taken to ensure survival (138). This bleak perception among health care providers of life following SCI derives not only from the devastating permanence of such an injury, but also underscores the dearth of available treatments. In the time that has elapsed since this survey was conducted in the 1990s, our understanding of the molecular mechanisms underlying regenerative failure after SCI has greatly improved, providing hope for novel treatments. Indeed, SCI research has come a long way from Ramon y Cajal’s descriptions of largely abortive regeneration of the “sterile clubs” of severed axonal tips transiently stalled in the glial scar which he believed were destined to die away (56). David and Aguayo’s (81) peripheral nerve graft experiments provided an important appreciation that certain types of central nervous system (CNS) axons do possess some level of an intrinsic ability to regenerate, at least acutely after injury, when presented with a hospitable environment. This galvanized new hope that additional SCI research could lead to functional improvements.
There are ~17,000 new cases of SCI recorded each year in the United States with motor vehicle collisions still contributing to the most common etiology of traumatic cord injury (279a). Alarmingly, since 2012 as the population ages, the number of recorded cases of traumatic SCI due to falls has risen from 16 to 30.5% (92). Clinical presentation is dependent on the severity and location of injury since the long tracts within the cord are arranged somatotopically as they interconnect the brain with autonomic and peripheral nervous systems. Most current acute treatments aim to stabilize the cord and restore homeostasis immediately following injury while long-term treatments mainly seek to manage symptoms arising from maladaptive plasticity and other secondary complications (65, 101, 199).
Our understanding of the extrinsic and intrinsic factors that block axonal regeneration or neuroplasticity has vastly improved with greater elucidation of the course of molecular events that occur following SCI in animal models. However, we need to keep in mind that variation of inflammatory and subsequent glial responses exists between mice and rats (365), different genetic mouse backgrounds (202, 243), and rat strains (312). There is variability in the mechanics of the different injury models as well as deviations in severities of the same injury type (369). In this review, we describe how primary and subsequent secondary injuries contribute to the formation of the glial scar (FIGURE 1). Major cellular components of the glial scar including microglia and peripherally derived leukocytes, astrocytes, oligodendrocyte progenitors, ependymal cells, and pericytes/fibroblasts will be further described in detail. We then discuss how the matrix contents of the glial scar, notably chondroitin sulfate proteoglycans (CSPGs), inhibit axon outgrowth and functional recovery. We also review the inhibitory role of CSPGs in the perineuronal net (PNN). Given that the molecular and cellular cascade of events and possible repair strategies are so expansive, we focus our review from our unique perspective based on modulation of the glial scar by mitigating the inhibitory potential of CSPGs, as well as combinatorial strategies that target the glial scar.
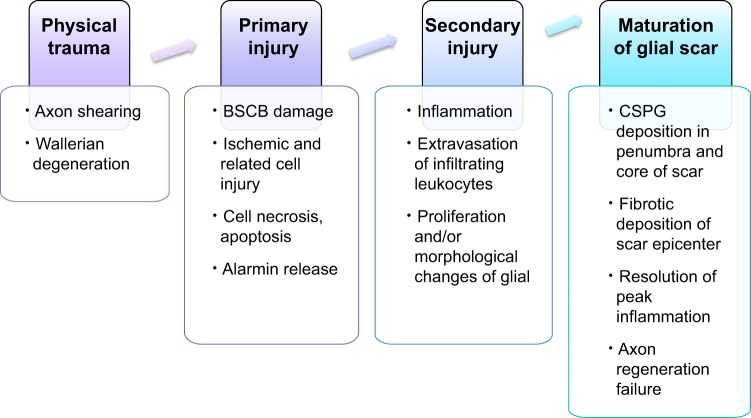
FIGURE 1.
Overview of spinal cord injury pathophysiology. Spinal cord injury can be divided among four progressive stages: physical trauma, primary injury, secondary injury, which ultimately creates a chronically axon-inhibitory structure called the glial scar.
II. DESCRIPTION OF SCI-INDUCED PRIMARY AND SECONDARY INJURY
In rodent models of contusive SCI, the vertebrae and surrounding laminae are excised to expose the cord. The desired amount of force is translated through a clip, piston, or a set amount of weight that is impelled or dropped onto the protracted cord, which causes immediate damage that expands in an elliptical shape rostrally and caudally from the center of impact. While there is some variability in the extent of spared tissue, this model has been useful in recapitulating major morphological and cellular changes present in traumatic human SCI (369). In the contusive model of injury, the force of impact rapidly displaces the cord (FIGURE 2, A–C). While physical trauma is usually brief, tissue remodeling starts immediately, peaks weeks after injury, and persists for the remainder of life.
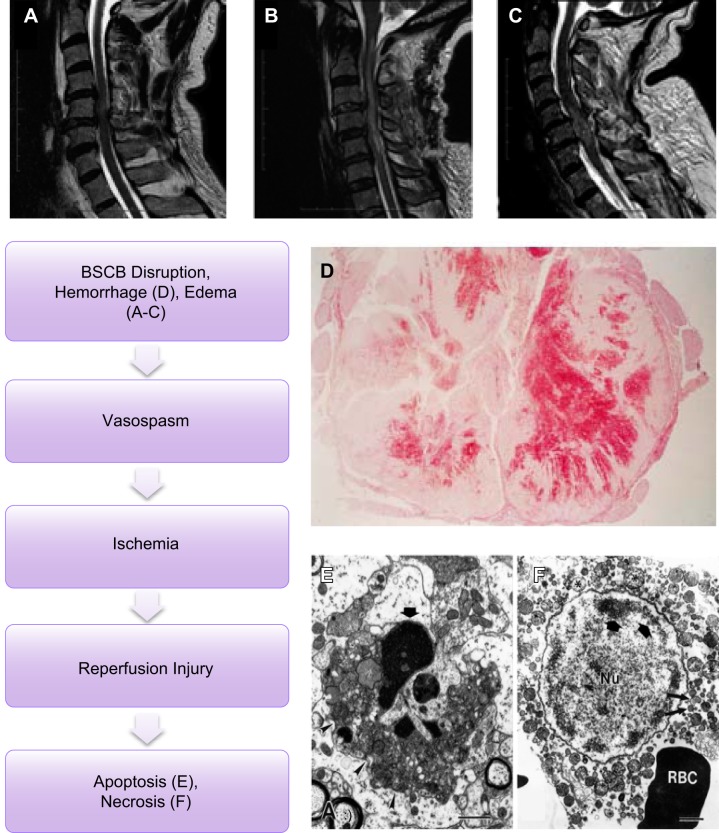
FIGURE 2.
Primary injury. Primary injury caused by direct trauma induces blood spinal cord barrier (BSCB) disruption among other disruptions including ischemia and reperfusion injury. A–C: single (A), multi-level edema (B), and hemorrhage and surrounding edema (C) visualized in human spinal cord injury seen through sagittal T2 MRI with C1–C6 injuries. (From Bozzo et al. J Neurotrauma 28: 1401–1411, 2011. Copyright Mary Ann Liebert, Inc.) D: traumatic injury induces hemorrhaging following BSCB disruption as seen in a human case of C4–5 at 3 days following SCI. (From Tator and Koyanagi. J Neurosurgery 86: 483–492, 1997. TheJNS.org) E and F: ischemia and reperfusion injury result in neuronal and glial apoptosis marked by dense cellular condensation (E) and necrosis marked by cytoplasmic blebbing (F) as seen through EM imaging of the spinal cord. [From Liu et al. (237), with permission from Society for Neuroscience conveyed through Copyright Clearance Center, Inc.]
A. Physical Trauma and Primary Injury
Physical impact to the spinal cord causes symmetrically expanding foci of glial and neuronal necrosis within the lesion epicenter (FIGURE 2F) (146). Necrosis is a disordered phenomenon of cell death characterized by somal swelling, loss of cytoplasmic definition, chromatin aggregation that may leak into the cytoplasm, and spewed organelles resulting in ejection of proteins that contribute to the inflammatory milieu (18). These include the release of alarmins that initiate a reactive state in resident glia and the downstream infiltration of immune cells from the periphery (FIGURE 3) (33).
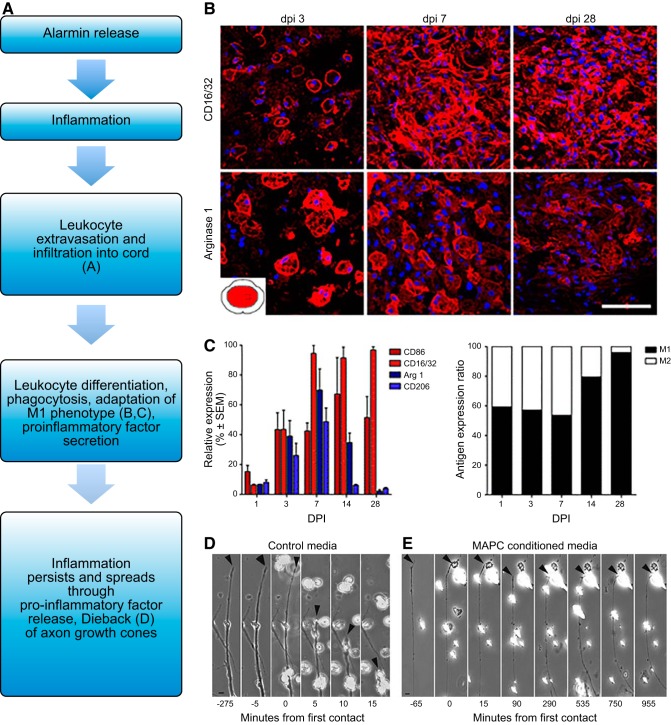
FIGURE 3.
Secondary injury. Secondary injury is marked by inflammation initiated by physical injury and release of alarmins. A–C: leukocyte infiltration through a compromised BSCB and subsequent differentiation can be seen in the lesion of the mouse spinal cord after injury. While both M1 (CD16/32+) and M2 (arginase 1) macrophages are present, the M1 type dominates and persists. [From Kigerl et al. (201), with permission from Society for Neuroscience conveyed through Copyright Clearance Center, Inc.] D and E: M1-like macrophages may induce further damage to axons through dieback, or contact-based retraction of the axon to the soma as seen with peripheral rat axons in vitro. However, immune modulatory treatments such as MAPC stem cells may push macrophages towards an M2-like phenotype which does not cause dieback in vitro even upon axon contact. [From DePaul et al. (89), with permission from Nature Publishing Group.]
In contrast, apoptosis is an ordered, ATP-driven process of cell death marked by tightly packed condensation of the cell body that elicits a far less potent inflammatory signal than necrosis (FIGURE 2E) (209). Neuronal apoptosis seems confined to the early stages of injury (147). However, beyond 24 h post trauma, glial apoptosis persists while neuronal apoptosis diminishes (74, 237). Among glia, astrocytes seem most refractory to apoptosis (146) while oligodendrocyte apoptosis persists in distant white matter several days or longer after impact (29, 237, 374).
Physical impact additionally causes descending and ascending axons along the lesion to shear and then degenerate (277). These severed axons, separated from their somata, undergo stereotypical Wallerian degeneration of the distal aspect of the axon as well as their associated myelin sheaths. Successive axonal dieback (49, 171) due to aggressive macrophage activity (70, 201) and demyelination (41) also occur in the proximal segment which, in turn, may contribute to the list of factors that limit collateral sprouting (75).
Understanding the homeostatic and protective functions of the blood-spinal cord barrier (BSCB) in keeping peripheral immune cells, toxic metabolic products, and other inflammatory substances excluded from the CNS emphasizes the extent of disruption that SCI incurs. The BSCB comprises nonfenestrated endothelial cells which form tight junctions with each other to wrap around blood vessels. The next layer includes endothelial cell-supporting pericytes embedded in the basal lamina which are all enveloped by the endfeet of astrocyte processes (182) that play a major role in tightening the endothelial barrier (24). Upon injury, endothelial cells shed their glycocalyx, and tight junctions are lost leading to breakdown of the BSCB and vascular permeability (255). Further disruption of this coordinated structure leads to edema as a result of the unfettered flow of ions and water. Of course, a major complication of BSCB and blood vessel rupture is hemorrhaging (FIGURE 2D), notably in the grey matter (41, 211). Bleeding occurs within minutes after SCI (18), but can last for days (265) contributing to the cavitation of the lesion site which can further potentiate expansion of the injury area by causing compression further along the cord (393). Neuronal excitotoxicity from the release of ions such as Ca2+ and glutamate also occurs (3, 4). Adding to the homeostatic imbalance following edema includes the unchecked release of neurotransmitters, K+, and Na+ (229) that can continue to drive neuronal oxidative stress, protein aggregation, and lipid peroxidation (132).
An immediate response to staunch the invading flow of blood into the cord is the release of platelet-derived factors that cause vasospasm as well as endothelin-induced constriction of blood vessels, leading to ischemia in the cell-dense area of the grey matter (333, 334). Oxidative stress due to ischemia is another cause of glial apoptosis occurring as soon as 3 h at the lesion epicenter (277). Hypoxia also facilitates expansion of the lesion (211, 382) and contributes to patchy areas of necrosis near the lesion site (18, 41, 414). There is recent evidence that a low-grade ischemia develops slowly but persists chronically in the caudal aspects of the lesioned cord due to the action of trace amines on pericyte contraction (235). Early after injury, the reintroduction of oxygen to ischemic areas instigates reperfusion injury adding to oxidative damage and the release of reactive oxygen species (ROS) that may further contribute to glial apoptosis and inflammation (160). Reperfusion injury also introduces leukocytes to the cord which may inundate the area with other proinflammatory factors such as cytokines and interleukins. As injury resolves, multiple cystic cavities may form in the lesion site with greater incidence if the dura has opened (328). At this point, the lesion epicenter is replete with red blood cells, cellular debris including myelin and subcellular components, necrotic and apoptosing neurons and glia, all of which contribute to the initiation of secondary injury.
Adding to injury is the unregulated influx of peripherally circulating proteins and factors including ROS, inflammatory factors such as tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β, nitric oxide, and fibrinogen (319) [see Garcia et al. (132) for a full review]. While fibrinogen is integral to the formation of blood clots to help resolve hemorrhaging, it also helps initiate astrogliosis (327) and activates microglia (183). Infiltrating ROS and other factors such as inducible nitric oxide synthase (iNOS) contribute to neuronal and glial apoptosis in the first 24 h (424). Even as homeostasis and subsequent BSCB repair occurs, the basal lamina of the BSCB remains damaged, and in the absence of astrocytes in the lesion core, the junctions between endothelial cells fail to reform to their pre-injury state due to decreased expression of tight junction proteins such as claudins and occludens (73). Thus the profuse vasculature within the lesion remains highly abnormal, although much of it is likely resorbed eventually leading to formation of the lesion cavity. Activated endothelial cells also potentiate secondary injury through the release of potent chemoattractants such as interleukin (IL)-16 (275). As a result, the BSCB remains chronically leaky after SCI (285). Another contributor to leakiness is the continued expression of matrix metalloproteases (MMPs), especially MMP-9 from chronically activated endothelial cells (225), oligodendrocyte progenitor cells (343), reactive astrocytes (438), and possibly pericytes (399). Persistent permeability of the BSCB allows for the chronic infiltration of monocytes into the cord (30, 202), which further potentiates the inhibitory effects of the lesion (49).
B. Inflammation Induces Secondary Injury
Primary injury leads directly to a prolonged secondary injury cascade which lasts for weeks until the wound seals and the glial scar matures. Secondary injury is marked by the expansion of tissue damage from the lesion epicenter including inflammation-induced apoptosis of adjacent cells and axotomy of neurons that may have survived the initial impact (115). The magnitude of inflammation and subsequent secondary injury correlates with the extent of immune cell reactivity observed in response to danger-associated molecular patterns or DAMPs (323). DAMPs in the context of SCI include endogenous alarmins, which are normally cell-bound proteins such as ATP, chromatin-associated protein HMGB1, S100, histones, or interleukins such as IL-1α released from cells undergoing necrosis and apoptosis. DAMPs also include cellular debris such as material shed from sheared axons [see Gadani et al. (126) or Kigerl et al. (200) for a full review of DAMPs following CNS injury].
As the name suggests, alarmins serve as a siren to awaken the immune system, initiating and perpetuating a sterile inflammatory response in the CNS (FIGURE 3) (33). ATP is one example of an alarmin released by injury to the CNS that initiates a rapid polarization of proximate microglia (80), which in turn further potentiates inflammatory signals by producing chemokines such as CCL3 (195) and inducing CCL2 release by reactive astrocytes (297). HMGB1, a protein normally sequestered in the nucleus with chromatin, is another classic alarmin that is released quickly following SCI due to necrosing neurons and has been reported to persist chronically after the resolution of the initial inflammatory response (301). HMGB1 activates and further potentiates inflammation by increasing the release of chemokines such as CXCL1/2 from astrocytes (306). Immune cells of the spinal cord become classically activated to assume an M1 phenotype in this environment, which in its simplest terms is marked by iNOS and increased ROS and reactive nitrogen species (FIGURE 3, A–C). This is a change from homeostatic alternatively activated, or M2 phenotypes which describes a heterogeneous population of immune cells that are marked by Arg1 [see Cherry et al. (70) for a detailed discussion]. For example, microglia become activated by the release of alarmins as well as hemorrhaged material, such as iron disgorged from lysed red blood cells. Phagocytosis of iron by monocytes (332, 338) could contribute to the inflammatory milieu through classical activation of microglia (212). Recently, Gadani et al. (127) reported the release of the alarmin IL-33 by oligodendrocytes and grey matter astrocytes in response to SCI. Genetic knockout of IL-33 in mice resulted in the decreased recruitment of monocytes and a reduction in the inflammatory response (127), further demonstrating that alarmins contribute to recruitment, activation, proliferation, and differentiation of peripheral monocytes in the lesion area (87, 126). An understanding of the cascade of molecular events that occur soon after injury can help lead to improved neuroprotective strategies in an attempt to save as much nervous tissue as possible.
C. Microglia
Under normal physiological conditions, microglia are constantly patrolling the CNS by employing an incessant retraction and extension of processes (14, 80, 283). As such, microglia serve a housekeeping function by removing debris, and also act as a first barrier of defense against invading pathogens and injury (287). Microglia are biased toward an M2-like phenotype during normal brain function (70). Following injury, microglia rapidly change their morphology by initially extending their processes toward the damaged site (80). Soon thereafter, they adopt an amoeboid shape (415) before migrating to the lesion area in response to alarmins such as IL-33, IL-1B, and TNF-α released as soon as 15 min after the lesion (127, 323). The acute inflammatory environment post-SCI, therefore, pushes microglia towards a M1-like bias (151, 201) that contributes to a further loss of neurons and increased astrogliosis in discrete regions (405). Histological studies have found a concentration of microglia very early on in the lesion core (143, 326, 344) followed by their migration to the lesion’s margins later on (105). Activated microglia are characterized by deramified and shortened processes that extend rostral and caudal to the lesion (156). At the lesion, microglia clear debris (143), which is all the more important as apoptosis of oligodendrocytes and neurons continues (63, 347, 364). In response to the inflammatory signals released within the lesion, microglia proliferate profusely (143). Proliferation peaks between 3 and 7 days in the lesion epicenter (365) and plateaus 2–4 wk post injury (312). During this time, although microglia may play a beneficial role by clearing cellular debris from the injury site and helping to seal and block the spread of the lesion (167), they have also been reported to contact damaged axons and phagocytose dendrites (143, 415), which may exacerbate synaptic damage. The resolution of microglial activation as the scar forms requires feedback from astrocytes which includes increases of TGF-β and IL-4 and a simultaneous decrease in proinflammatory cytokines such as IL-1β, TNF, and IL-6 (287). However, well after maturation of the glial scar, low-level microglial activation chronically persists in the brain to affect cognitive function (417) as well as in deafferented areas rostral and caudal to the lesion where it may be involved with upregulation of extracellular matrix (ECM) components and circuit remodeling as well as the production of neuropathic pain (91, 151, 156, 415).
D. Leukocytes
Following BSCB damage and subsequent neutrophil infiltration, monocytes influx into the cord where they differentiate into macrophages in the proinflammatory milieu (281). Through a series of stereotyped processes, collectively called extravasation, leukocytes perform rolling adhesion and then transmigrate through the BSCB where they travel towards the source of the chemoattractant (370, 396). Neutrophils are the first reported peripheral immune cell to infiltrate the spinal cord by 3–6 h post injury (66, 381), although their presence in the cord is short lived (117). The amount of neutrophils present in the cord peaks around 1 day post injury where they are associated with necrotic regions (60). At the lesion site, neutrophils help potentiate the inflammatory cascade (66, 145) by activating other immune cells and glia through the release of proinflammatory cytokines and chemokines (60, 371, 372, 396), ROS, and proteases (159).
Extravasated monocytes differentiate into activated macrophages in the injured cord (201) during two peaks: the first at 1 wk post injury, then at 60 days post injury where they have been reported to persist up to 40 days after differentiation (312). M1-activated macrophages dominate the early lesion site (201) and initiate secondary damage through the secretion of enzymes and proinflammatory factors and by potentiating apoptosis (197). Activated immune cells additionally contribute to neuronal and glial cell death by generating ROS through increased NADPH oxidase activity (132, 288). The complex interactions between different cell types of the lesion epicenter further potentiate inflammation. For example, activated oligodendrocyte progenitor cells may further activate macrophages (325) and vice versa (215, 322, 338, 416). The release of TNF-α from activated microglia also activates astrocytes (219), which discharge glutamate to cause neuronal excitotoxicity and further inflammation (132).
Recruited to the core and margins of the lesion (344), macrophages prolifically phagocytose cellular debris, and degenerating tissue remains (143). Macrophages proliferate in greater numbers than microglia and are characterized by their rounder, more amoeboid shape (105). M1 macrophages have been reported to be the dominant macrophage type found at the lesion (201), although other macrophage subtypes based on their chemokine cell-surface receptors are also present (105). M1-promoting genes have been reported to increase up to a month post injury while M2-promoting genes are depressed in the early injury environment (201). Far more than microglia (105), macrophages make intimate contact with the dystrophic ends of axons which promotes axonal retraction or dieback (FIGURE 3D). The dying back phenomenon appears to be mediated, at least in part, by MMP-9 (49, 105, 171). Dieback is terminated once the axonal retraction bulb makes sufficient contacts with NG2+ oligodendrocyte progenitor cells and possibly pericytes where the dystrophic tip makes synaptic-like contacts and stabilizes upon these glial progenitor cells (see below). Thus activated macrophages in the early stages of the development of the lesion core play a major role in regeneration failure.
E. Pleiotropic Effects of Inflammation
Inflammation is pleiotropic in that while both resident and infiltrating immune cells help potentiate their destructive phenotype soon after injury, modulating this early response in a proper way may have beneficial effects (311). Thus selectively blocking proinflammatory factors such as NFκB (40) or curtailing, but not totally eliminating, monocyte infiltration into the cord following injury does help to preserve more myelinated axons, reduce cavitation, and improve functional locomotor activity (145, 310). Specific inhibition of neutrophil entry into the lesion through antibodies targeting rolling adhesion or by other means has also been found to be neuroprotective and beneficial following SCI (19, 137, 381), possibly due to the decrease in proinflammatory factor secretion (372, 396). However, completely abolishing the CNS immune response following injury seems to worsen functional outcome as well as expand the lesion area. For example, genetic ablation of the alarmin IL-33 resulted in the long-lasting failure of monocyte infiltration following SCI but, surprisingly, an eventual decrease in neuronal survival and functional recovery compared with wild-type controls (127). Globally inhibiting inflammation using high doses of glucocorticoids has yielded conflicting results (72, 108, 155) and in some cases increased animal death following SCI (249). To be clear, the medical use of glucocorticoids such as methylprednisolone is no longer recommended in human cases of SCI due to significant complications (339). The wound-healing properties of the late inflammatory response may be one explanation of how complete ablation of inflammation following SCI worsens functional outcome (104, 127, 249).
Inflammation may be modulated in other ways to be beneficial in hastening wound healing and even promoting neurite outgrowth in certain models of CNS injury (37, 288). The inflammatory post-SCI environment, for example, can be modulated by systemic, trophic factor producing stem cells (13) such as multipotential adult progenitor cell treatment (FIGURE 3E) to confer neuroprotection (89, 309, 385, 404) [possibly via their effects in the spleen (88)] and to promote neurite outgrowth despite a growth inhibitory scar barrier (47, 136, 201). Intraspinal injections of anti-inflammatory cytokines such as IL-4 have also been reported to increase M2 or “resolution-phase” macrophages and hasten wound healing (122).
The M1 and M2 paradigm has evolved (69, 318) as more recent studies have begun to stress that these two phenotypes are a continuum instead of a binary (69). Further complicating this story is the innate heterogeneity of both normal microglia and macrophages as well as the heterogeneity of their responses to injury (82). Generally, much of the detrimental effects seen from macrophage interactions following injury have been attributed to their M1-like state. It is M1 macrophages that have been reported to orchestrate glutamate and nitric oxide-induced neuronal death (292, 430). However, through modulation of the processes that convert classically reactive M1 to M2-like macrophages, axonal dieback decreases (47, 89, 426) perhaps through reduction of gliosis and subsequent diminished expression of proinflammatory cytokines such as IL-1β (426). Recently, Kroner et al. (212) have presented a possible explanation as to how this M2 phenotype may be normally repressed. Interestingly, macrophage phenotype (whether they are closer to M1 or M2) seems to be influenced by the type of debris they internalize. Phagocytosis of myelin debris, for example, promoted an M2 bias, whereas phagocytosis of iron, such as that found in red blood cells hemorrhaged into the lesion, pushed classical activation of macrophages towards an M1 bias. This occurred in a TNF-dependent manner which ultimately created more ROS and increased apoptosis in the injury environment.
Taken together, we now appreciate a more nuanced view of inflammation following SCI where although full-forced ablation of the inflammatory response may reduce wound healing and worsen outcome, modulation of the inflammatory milieu towards a “regeneration-associated” phenotype may encourage more favorable outcomes (217).
III. MAJOR CELL TYPES IN THE GLIAL SCAR
With the influx of inflammatory cells, resident glia including astrocytes and oligodendrocyte progenitor cells of the spinal cord become activated and undergo a set of shared characteristics (FIGURES 4 and 6). These include retraction of normally multi-branched processes, enhanced local proliferation in the lesion penumbra (FIGURE 4) (272, 324, 423), and in most cases migration to the lesion margin where they secrete proinflammatory factors. While, over time, the scarring process helps to resolve inflammation, persistent low-grade inflammation by lesion core macrophages and subsequent gliosis remodels the ECM with an increase of fibronectin, collagen, and laminin in the lesion center (109). This substructure is often referred to as the fibrotic component of the scar which becomes replete with pericytes/fibroblasts and macrophages but minimal in microglia (105). The glial component of the scar consists of reactive astrocytes, NG2+ oligodendrocyte precursors, and microglia in the penumbra. Additionally, a gradient of lectican-family CSPGs radiates from the lesion center that creates an inhibitory environment for axons chronically (FIGURE 7) (85). While each cell type is discussed discretely, they interact with each other culminating in a permanently remodeled tissue. We will characterize their reactivity following inflammation, how they contribute to the formation of the glial scar, and how this may influence the scar’s axon growth prohibitive effects.
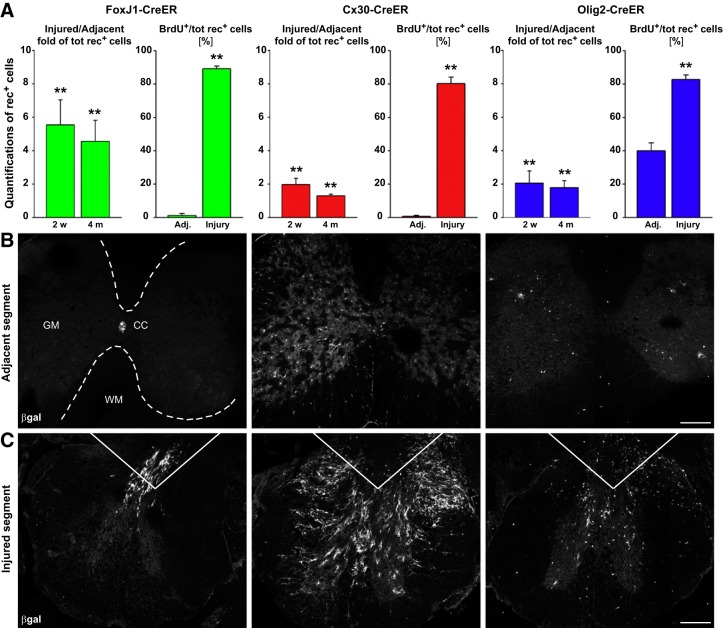
FIGURE 4.
Increased cell proliferation following spinal cord injury. Inflammation following spinal cord injury drives proliferation of many cell types. A–C: quantification of fate-mapped cells in hemisected mouse spinal cords including ependymal (FoxJ1-CreER), astrocyte (Cx30-CreER), and oligodendrocytes progenitor cells (Olig2-CreER) shows proliferation 2 wk and 4 mo following injury. In addition to proliferating, some cell types differentiate including ependymal cells, which differentiate into astrocytes and mature oligodendrocytes. [From Barnabé-Heider et al. (21), with permission from Elsevier.]
FIGURE 6.
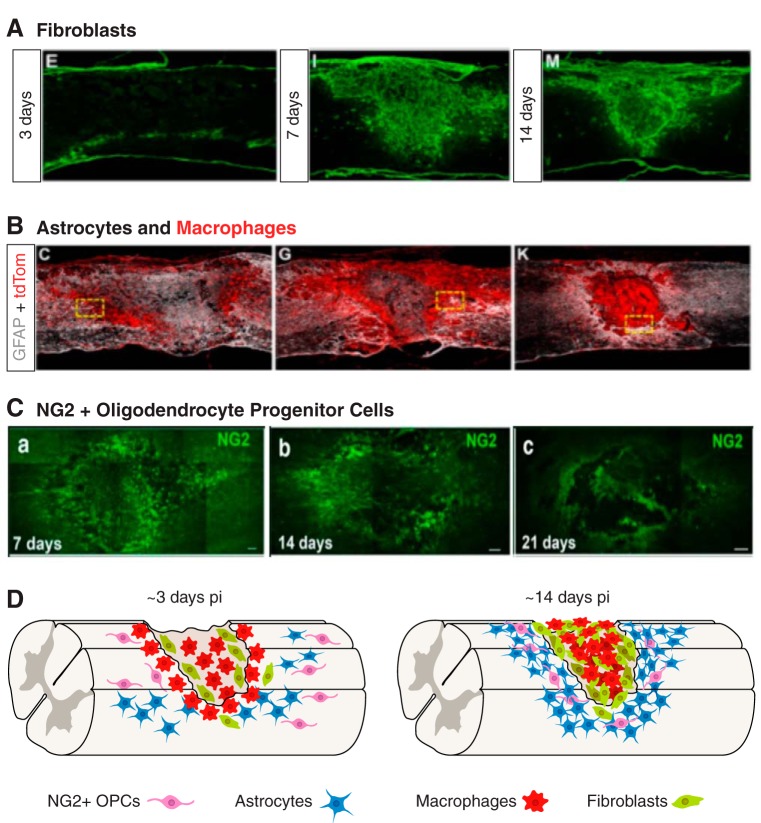
The glial scar is composed of multiple cell types. Cells become activated, proliferate, and together form the glial scar as seen in the following sagittal mouse sections. A: fibroblasts visualized using Col1alpha1 promoter at 3, 7, and 14 days following mouse SCI originate from blood vessels and proliferate to form fibrotic component of glial scar. B: astrocytes (GFAP, white) and hematogenous macrophages (tdTomato driven by lysM promoter, red) at 5, 7, and 14 days following mouse contusive SCI. [A and B from Zhu et al. (444), with permission from Elsevier.] C: NG2 staining of oligodendrocyte progenitor cells in mouse dorsal column crush at 7, 14, and 21 days. [From Filous et al. (113), with permission from Society for Neuroscience conveyed through Copyright Clearance Center, Inc.] D: cartoon depicted progressive glial scar formation. Activated and proliferating fibroblasts and macrophages occupy the lesion core of the glial scar by around 14 days post SCI. Activated astrocytes and NG2+ oligodendrocytes occupy the lesion penumbra.
FIGURE 7.
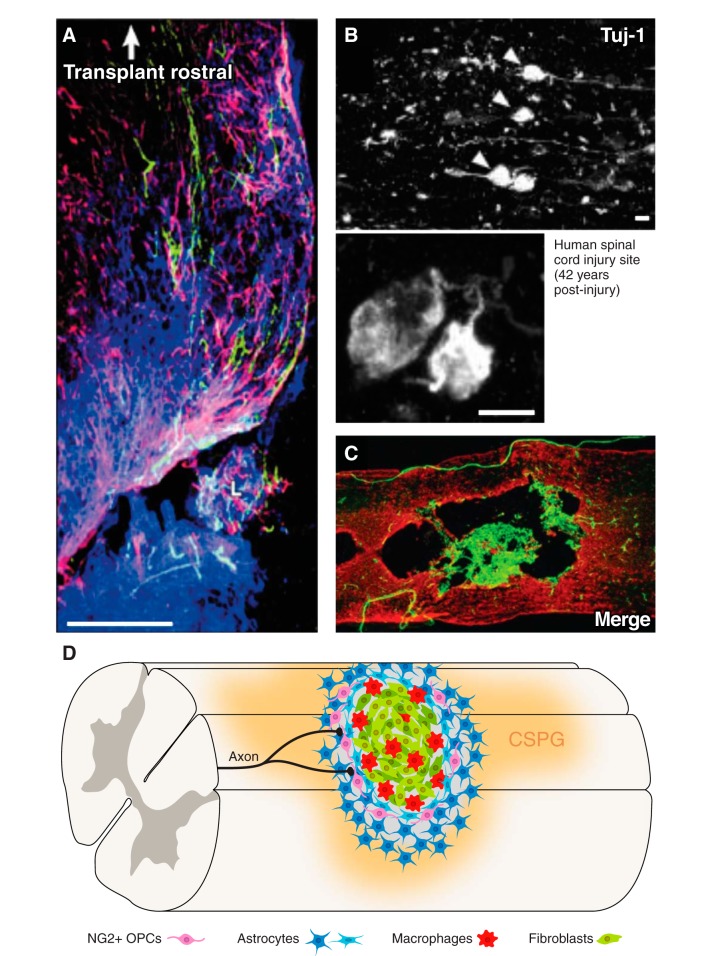
The axon-inhibitory mature glial scar. The mature glial scar becomes a chronically axon-inhibitory structure. A: transplanted dorsal root ganglion neurons (green) are stalled by the glial scar as visualized by GFAP (red) with a gradient of CSPG (CS-56, blue) in rat. [From Davies et al. (85), with permission from Society for Neuroscience conveyed through Copyright Clearance Center, Inc.] B: dystrophic growth cones (arrows and inset, TuJ1, white) are stalled by the glial scar as long as 42 yr in a human case of spinal cord injury. [From Ruschel et al. (330), with permission from AAAS.] C: sagittal section of rat spinal cord 56 days after contusive injury with GFAP (red) and PDGFR-β (green) depicting fibroblasts within the lesion core. [From Zhu et al. (445). Copyright Mary Ann Liebert, Inc.] D: cartoon of mature glial scar depicts reactive astrocytes including palisading astrocytes and NG2+ oligodendrocytes at the lesion penumbra. The lesion core is occupied by macrophages and fibroblasts. Axons become dystrophic as they approach the gradient of CSPGs.
A. Astrocytes and the Glial Scar
Astrocytes are now considered an active player in CNS function contributing to blood-brain barrier maintenance, synaptic physiology, trophic and metabolic support, and a myriad of other homeostatic mechanisms (204). In this section, we briefly review the hallmarks of astrocyte reactivity to the inflammatory environment following SCI and how this response may differ based on a growing appreciation of astrocyte heterogeneity and plasticity. We then describe the astrocytes’ necessary role in acute wound healing and how this tissue remodeling eventually produces a chronic inhibitory structure to axon outgrowth. It is important to emphasize that like the pleiotropic nature of inflammation, astrocytes and the glial scar in general pose a double-edged sword: while they are vital for the acute containment of inflammation, and thus prevention of the expansion of inflammatory processes that can cause greater necrotic damage to cells, reactive scar-forming astrocytes ultimately contribute to the chronic failure of axon regeneration.
1. Astrocytes: a heterogeneous population defined by environmental niches
An area of intense interest is whether phenotypic variances between astrocyte subpopulations could contribute to differences in their ability to hinder or potentiate axon regeneration. Different regions of the CNS can be defined by the heterogeneity of their resident astrocytes (102). Recently, Farmer et al. (106) demonstrated that neurons themselves are capable of diversifying astrocytes through Shh signaling to better contour astrocyte function to different subpopulations of neurons in the cerebellar cortex. Astrocytes are bound to specific regions set after development and while they proliferate at the inner lesion margin after injury, long-distance migration of these newly proliferated astrocytes well beyond their specified regions does not occur (398). The site of injury to the spinal cord, therefore, plays a role in specifying differential astrocytic responses to injury as well as axon regeneration. For instance, protoplasmic astrocytes have been observed to proliferate less than fibrous astrocytes following SCI (103), while juxtavascular astrocytes have been noted to be the source of the majority of newly proliferated astrocytes (20). In another example, Aldh1l1-positive astrocytes prevalent in the spinal cord have been shown to be less conducive to supporting synaptogenesis compared with their brain-derived analogs (186). Additionally, β-amyloid has been reported to activate astrocytes derived from the cortex and hippocampus into a scarlike state, but not those harvested from areas that tend to lack plaque formation such as the spinal cord or cerebellum (174). In fact, astrocytes cocultured with neurons displayed differential effects on neurite branching and length depending on whether they were derived from the medial or lateral sectors of the midbrain (133). Radial astrocytes (tanycytes) located within the median eminence of the hypothalamus do not scar and allow for regeneration of neurohypophysial oxytocinergic and vasopressinergic axons even after penetrating lesions (68). How these differences in environmental glial niches translate to functional benefits following injury in other regions will require further investigation.
2. Reactive astrocytes: heterogeneous response to inflammation
Following exposure to the inflammatory environment post injury, astrocytes become reactive by a process often described incorrectly as astrogliosis (i.e, more astrocytes). Indeed, much of the astroglial response to injury is due to cellular hypertrophy and morphological rearrangements (i.e., reactive astrocytosis) rather than proliferation. Generally, astrocytosis has most commonly been marked by varying upregulation of the intermediate filament decorating proteins glial fibrillary acidic protein (GFAP) and vimentin (422), hypertrophy of the primary branches (373, 412), expansion of normally defined astrocytic domains at the site of injury (408), and very restricted proliferation adjacent to the lesion margin (20, 408). The increase in GFAP, in particular, helps to allow scar astrocytes to form a rigid, densely bundled structure around the lesion (FIGURE 5A) (421).
FIGURE 5.
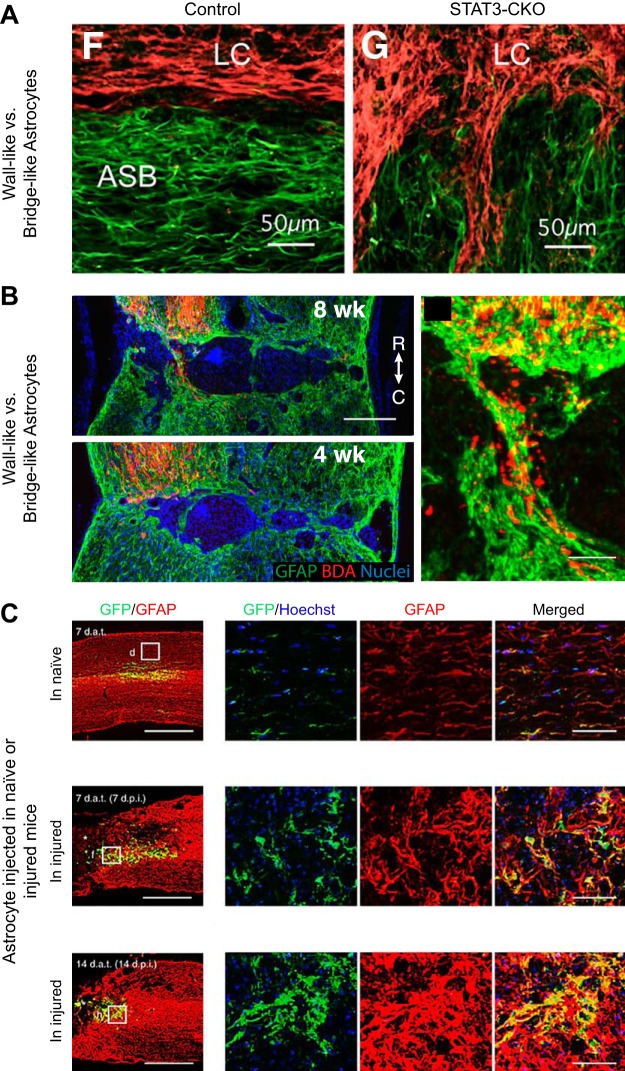
Astrocyte plasticity following spinal cord injury. Astrocytes are a heterogeneous population of glia that are highly plastic and whose phenotype also depends on environmental factors. A: astrocytes (GFAP, green) activated by the post-injury inflamed environment (14 days after mouse crush SCI) become “wall-like” to wall-off fibroblasts (fibronectin, red) of the lesion core (LC) at the astrocyte scar border (ASB). Astrocytes prevented from becoming activated through STAT3-KO, however, become more “bridge-like” instead at the scar border. [From Wanner et al. (408), with permission from Society for Neuroscience conveyed through Copyright Clearance Center, Inc.] B: astrocytes (GFAP, green) in AAV-shPTEN mice create bridges for BDA-labeled cortical spinal tract axons (red) to cross the lesion by 8 wk following crush injury. [From Zukor et al. (447), with permission from Society for Neuroscience conveyed through Copyright Clearance Center, Inc.] C: astrocytes grown in vitro (GFP, green) and transplanted into naive, 7, or 14 days post spinal cord contused mice adopt inflamed “wall-like” or axon-conducive “bridge-like” phenotypes based on their environments as visualized through surrounding astrocyte (GFAP, red) staining. [From Hara et al. (157), with permission from Macmillan Publishers Ltd.]
Astrocytosis occurs as a direct response to physical trauma, although a septic inflammation even without direct physical injury has been shown to induce strong astroglial reactivity (115). For example, a tiny microinjection of zymosan (a potent yeast cell wall inflammogen) into the white matter, while minimally physically destructive, nonetheless was sufficient to locally activate macrophages and cause robust GFAP and CSPG upregulation and rapid migration of astrocytes outwards from the injection site to densely encircle the zone of inflammation (115). Reactive astrocytes in the vicinity of the SCI-induced inflammatory storm respond through intramolecular signaling changes to the myriad of secreted cytokines, alarmins, and other cellular signals. Enhanced calcium signaling in astrocytes as a response to the inflammatory storm, for instance, activates them (191) to promote proreactive transcriptional changes (130). Proinflammatory cytokines, notably TGF-β and fibrinogen infiltrating from the periphery through a compromised BSCB, have been shown to activate SMAD signaling through p75 cleavage and subsequent nuclear pore complex remodeling (327) to enhance GFAP expression (407). The STAT signaling pathway has also been implicated in astrocytosis to initiate hypertrophy (FIGURE 5A) (165, 291, 408). Many of these cellular signaling pathways converge to enhance transcriptional activation and subsequent expression of activation-type proteins such as GFAP (262).
The extent of reactivity and strength of the cellular response and subsequent transcriptional changes are dependent on the type of lesion, regional heterogeneity of the astrocytes themselves, and distance from the injury. Different types of injuries elicit different transcriptional changes in astrocytes (436). Zamanian et al. (436) have found that 50% of the genes upregulated in reactive astrocytes were different between lipopolysaccharide (LPS)-evoked and ischemic injuries. Recently, Liddelow et al. (236) have pinpointed the necessity of microglia to activate a subset of astrocytes through TNF, IL-1, and complement component 1 subcomponent q signaling following LPS administration. In fact, Csf1r knockout mice, which lack microglia, fail to activate this subtype of astrocytes (236). This subset of reactive astrocytes has been reported to upregulate a specific group of genes in response to such proinflammatory signaling and have been found to be potently neurotoxic and detrimental to oligodendrocytes (236). Other microarray or single-cell gene expression studies have confirmed the transcriptional heterogeneity among reactive astrocytes (7, 15, 157, 186, 331). Even in primary astrocyte cultures, single-cell gene expression analyses have revealed transcripts of vimentin and GFAP ranging from 50,000 to <100 which stresses that astrocyte heterogeneity is more complex than a binary expression pattern of proreactive markers (366). Most of these transcriptional changes were found to be transient following injury and resolved around 1 wk as inflammation subsided (79, 161, 320). Additionally, the type of injury, severity of trauma, and the distance of astrocytes away from the lesion core are all critical in eliciting a range of responses (431, 439). Thus the farther away the astrocyte is from the lesion epicenter, even as axons and oligodendrocytes are dying in their midst, the less severe the reactivity (289, 408). Importantly, astrocytes close to the lesion penumbra change their orientation to form an overlapping wall of densely packed and adhered cells that lack fine ramifications (408). This is in contrast to astrocytes farther away from the lesion which display a lessening gradation of hypertrophy including decreased thickening of processes and minimal overlapping of domains. Ultimately, cell signaling and transcriptional changes following the inflammatory storm enable potentiation of astrocytosis including cyotoskeletal protein expression (262), proliferation (21, 22, 408), and secretion of proinflammatory factors (175) to allow for an adequate wall building response to insult.
Reactive astrogliosis (i.e., astrocyte proliferation) is typically confined to the inner margin of the penumbral territory directly adjacent to the inflammatory core (FIGURE 6B). Following injury, reactive astrocytes have been known to derive from ependymal cells (261), NG2+ oligodendrocyte progenitors (443), but mostly from other reactive astrocytes (142). Thus an important question in considering astrocyte heterogeneity is the parental source of newly proliferated astrocytes and where these proliferating niches occur. Following a dorsal spinal cord cut injury, Barnabé-Heider et al. (21) observed that ependymal cells near the center of the lesion gave rise to astrocytes located closest to the inflammatory core, while astrocytes slightly farther into the penumbra of the lesion self-proliferated. However, the critical role of ependymal cells in scar formation has recently been called into question since their contribution appears to be meager at best and seems to be dependent on the need for a direct hit on the cells determined by the precise location and severity of the SCI lesion (321).
Another hallmark of reactive astrocyte wall building is the increased deposition of ECM proteins, notably axon growth inhibitory CSPGs seen in a gradient pattern around the lesion in vivo (84). This aspect of reactive astrocytes will be detailed in another section.
3. Reactive astrocytes: differences in immature and mature astrocytic responses to injury
The maturation state of the astrocyte is another major contributing factor to the heterogeneity of its response following injury (27, 168, 278). As an example, immature astrocytes mount a weak reactive response to proteins such as β-amyloid (58), whereas mature astrocytes become highly reactive to the same substrate (329). This difference in reactivity may be one explanation of how immature astrocytes promote axon regeneration better than mature astrocytes (111). Thus astrocytes cultured to maturity for 35 days or longer in vitro do not themselves readily extend past a high concentration of CSPGs, whereas immature astrocytes cultured for less than 2 wk can secrete matrix-degrading enzymes that allow them to cross this inhibitory terrain (111). Differences between immature and mature astrocytes are also seen in vivo in their ability to wound heal. Indeed, transplantation studies have shown that immature astrocytes are far more reparative than mature astrocytes (83, 153, 418). Mature astrocytes transplanted into the brain appear to stimulate increased macrophage and fibroblast entry and cavitation (111). In contrast, immature astrocytes derived from neonatal cortices carefully expelled along the track of a retracting needle within the cingulum bundle (111) or precoated onto nitrocellulose scaffolds and impelled into the adult forebrain have been shown to “knit” together the tissue bordering the lesion as well as reduce scar formation associated with the implant (153, 189, 355). Importantly, axon growth is enhanced when immature astrocytes are present (28, 111, 152) with minimal astroglial scarring (329). Tanycytes of the median eminence may represent a more primitive form of astrocyte which allows for regeneration after injury (67). Intriguingly, in the naked mole rat, reactive astrocytes allow for regeneration in the optic nerve well beyond a crush lesion (302). One may speculate that naked mole rat astrocytes remain in an immature state to allow for axon regeneration much like the primitive radial glia of highly regenerative zebrafish (244). Thus the overall agedness of the CNS environment and the maturational state of astrocytes play a role in differential astrocyte functions.
4. Reactive astrocytes: bridge building or wall building?
In the adult, reactive astrocytes play a multifaceted, complicated role in SCI. Usually, the level of astrocytosis following typical trauma to the spinal cord is severe enough to elicit permanent tissue remodeling and wall building. Histological observations of the glial scar show that the general morphology of astrocytes after typical severe lesions may not be conducive for axon outgrowth. Namely, border-forming astrocytes at the innermost edge of the lesion penumbra create palisading-like patterns with thick hypertrophied processes that densely overlap and pack around the lesion (408). Border-forming astrocytes additionally produce potently inhibitory ECMs in response to a variety of different cell and molecular triggers (57, 157, 336). This three-dimensional, wall-like structure may pose a physical and chemical barrier to axon outgrowth. Interestingly however, the upper surfaces of intensely reactive astrocytes themselves, grown in vitro as a monolayer upon β-amyloid, a potent inducer of reactive astrocytosis, can be conducive to neuronal outgrowth because they position their inhibitory CSPG-laden ECM only on their undersurface abutting the amyloid substrate (57, 342). Astrocytes are, therefore, highly plastic and dynamic depending on their polarity, age, lineage, and extent of the inflammatory environment that they encircle.
To further probe the role of astrocytosis following injury, several groups have utilized transgenic mice to prevent astrocytes from becoming reactive. When STAT3 signaling was specifically perturbed in astrocytes following injury, astrocytosis was decreased with a lack of hypertrophy and reduced GFAP expression compared with wild-type astrocytes (FIGURE 5A) (165, 291). The perturbation of post-injury astrocytosis signaling also prevented the formation of palisading and densely packed wall-building astrocytes at the penumbra (FIGURE 5A) (408). What followed after early disruption of the formation of the glial scar wall included an expansion of the lesion site with increased inflammatory cell infiltration resulting in further loss of function. In studies where GFAP-expressing astrocytes were specifically targeted for cell death, leaky BSCB persisted (107) and inflammatory cells from the periphery increased up to 25-fold (50). As a result, neuronal degeneration increased, as did edema. Moreover, wound repair following stab or crush injury was incomplete as the astrocytic scar border failed to close (50, 107). Ultimately, the interruption of post-injury glial scar formation from the beginning, leading to BSCB related complications, worsened functional recovery through an unchecked inflammatory response. Clearly, completely removing astrocytes in an immediate post-injury environment worsens functional outcomes as seen recently in Anderson et al. (6) (and further discussed below). However, aside from glial scar formation, certain subtypes of reactive astrocytes have a myriad of net positive neuroprotective effects following injury such as expression of key proteins to facilitate stabilization of the chaotic milieu. These include limiting edema through an increase in aquaporin channel 4 expression, limiting neuroexitotoxicity through glutamate transporter upregulation, reducing oxidative stress through glutathione production, and increasing trophic and metabolic support for compromised neurons (112, 362).
But what happens to astrocytes and how do they respond when far less disastrous perturbations occur? Zukor et al. (447) have demonstrated how differences in lesion environments affect reactive mature astrocytes and result in either positive or negative consequences to regeneration. For example, when corticospinal tract axons are strongly growth enhanced through PTEN knockdown after a very thin (<0.5 mm) lesion is created in the spinal cord with limited fibroblast and macrophage infiltration, reactive adult astrocytes could form narrow bridges upon which growth-enhanced axons were able to cross and bypass the lesion (FIGURE 5B). This is in contrast to more expansive lesions that were associated with large numbers of fibroblasts/pericytes and macrophages. The increase in inflammatory and mesenchymally derived cells promoted the construction of a wall around the lesion resulting in regeneration failure. In other situations, when GFAP and vimentin are conditionally knocked out before injury, astrocyte reactivity including stereotyped hypertrophy and proliferation is reduced (262). In paradigms like this where astrocytes are not killed but, rather, prevented from becoming severely hypertrophied and rigid (422), some axon outgrowth and functional improvements can occur (157, 262).
Other strategies involving mild astrocyte manipulations have resulted in increased axonal growth (177, 179, 246). For instance, application of fibroblast growth factor (FGF), which has been previously shown to increase astrocyte plasticity towards a more bipolar, immature phenotype (192), aided axon growth into a peripheral nerve graft (88). Axons have been observed to enter the region of the peripheral nerve graft where reactive astrocytes are aligned and inhibitory proteoglycan matrices are degraded with chondroitinase ABC (ChABC) (216, 254) as opposed to regions containing perpendicularly oriented, wall-like reactive astrocytes (408). In another example, auditory neuroblasts laid superficially along reactive astrocytes of the crushed CNS portion of the eighth nerve, but only with the addition of ChABC, were able to invade the reactive glial environment which acted as a bridge that supported neurite elongation and functional reinnervation (342). Importantly, recent work from the Okada group (157) has elegantly illustrated the plastic nature of astrocytes that better contextualizes the results in Anderson et al. (6), which we believe erroneously interprets that the astrocytic scar always aids axonal regeneration (FIGURE 5C). In Hara et al. (157), reactive astrocytes genetically labeled with GFP under the Nes promoter were FACs sorted from 7-day post-injured spinal cords and grafted into a new cohort of mice that were either intact or injured. Further sequencing 7 days after the graft showed that these astrocytes conformed to the environment of the host, forming scar astrocytes in injured animals or reverting to an unreactive, quiescent state in the naive cord (157). Importantly, astrocyte reprogramming in an injured environment seems to be instigated, at least in part, by fibrotic extracellular matrix material in the lesion core. In particular, type I collagen that is highly secreted following SCI (95) acts upon astrocytes through integrin receptors leading to subsequent N-cadherin signaling to form a tight scar (157). Interfering with specific integrin signaling only during the later scarring phase of astrocytosis after SCI with the use of antibodies reduced the scar (but did not kill the astrocytes) and allowed for significant axonal regeneration around the edges and beyond the lesion. This is in contrast to the overly simplistic interpretation by Anderson et al. (6) that astrocytic scars are wholly beneficial in regeneration because their techniques to kill or misalign scar-forming astrocytes immediately after injury did not lead to spontaneous regeneration. Anderson et al. (6) used transgenic STAT3 knockout mice or mice with ganciclovir-targeted astrocytes under the GFAP promoter. Conditions like this which globally affect astrocytes acutely after injury, in turn, unleash the core of inflammatory macrophages that are highly toxic to regenerating axons (see discussion above and Refs. 50, 231). We take a more nuanced view that astrocytes are highly plastic cells and their ultimate phenotype, either wall building or bridge building, depends on the intensity of the inflammatory environment they occupy and the growth capacity of axons in their vicinity (351).
The view of astrocyte biology following SCI and the appreciation of their polarity and plasticity especially in the presence of very small lesions or where core inflammation and pericyte/fibroblast proliferation are at a minimum have evolved dramatically (77, 350, 351). Astrocytes are a heterogeneous population that conform to different environmental niches and interact extensively with other cell types to potentiate the density of the glial scar. In the midst of very minimal lesions where collagen-producing fibroblasts/pericytes and inflammatory cells are lacking, but also in the presence of robustly growing axons (162, 184), astrocytes will abandon their wall-building role to favor bridge building and can be supportive to regeneration especially when their inhibitory matrices are reduced. However, their reactivity in the vicinity of typical large lesions filled with fibroblastic and immune cells favors wall building, which hinders axon regeneration. Nonetheless, astroglial scar barriers are not absolute and will allow the passage of some regenerating axons if they are maximally growth stimulated (240–242). Like macrophages, we may be able to take advantage of this astrocytic pleiotropy and modulate their reactivity to improve functional outcomes (411).
B. Oligodendrocytes and Their Progenitors
Oligodendrocytes proceed through sequential stages as they differentiate, including a pre-progenitor state and an immature state where the cells transiently express the NG2 CSPG before possessing the ability to myelinate in their mature form (26). In the adult cord, satellite oligodendrocyte progenitors of the grey matter and myelinating oligodendrocyte progenitors of the white matter constantly proliferate to sustain a coterie of progenitor cells as well as to generate increasing numbers of mature oligodendrocytes (21). Oligodendrocyte progenitor cells (OPCs) expressing the purportedly inhibitory NG2 proteoglycan, collectively called polydendrocytes, are one subpopulation that has been the most widely studied in models of SCI since their discovery in the 1980s (233, 367). They are characterized by their branched processes and ability to normally self-renew in adulthood (172). However, other cell types also express the NG2 CSPG. These include pericytes (346), reactive Schwann cells (259), and macrophages (188, 271); thus immature NG2+ oligodendrocyte precursors may be more precisely identified in conjunction with other oligodendrocyte markers (26).
Physical impact to the spinal cord causes mature and immature (i.e., precursor cell) oligodendrocyte apoptosis at the lesion site (146, 237). Oligodendrocyte depletion due to secondary injury may continue for weeks rostral and caudal to the lesion along degenerating axon tracts (234) perhaps as a result of the loss of axon-derived trophic factors. NG2+ OPCs proliferate following injury peaking at 5 days (FIGURE 6C) (245, 435) to accumulate in the lesion epicenter and penumbra (245, 260). This was shown in a model of incomplete spinal cord transection by Barnabé-Heider et al. (21) who used genetic fate mapping in combination with BrdU colabeling to reveal that the rate of oligodendrocyte progenitor proliferation had doubled (FIGURE 4). NG2+ OPCs may also derive from other glial sources. Genetic fate mapping further revealed that a small subset of ependymal cells differentiate into NG2+ oligodendrocytes (21). This phenomenon has been confirmed by other studies (170, 261).
Secondary injury, in part due to increased cytokine levels such as TNF-α from other activated glia, causes OPCs to become reactive resulting in increased expression of the purportedly inhibitory NG2 CSPG. Their increased rates of proliferation (352) owe in part to noncanonical STAT3/SOCS3 signaling (154) and decreased differentiation into mature oligodendrocytes (322). While a few NG2+ OPCs have been shown to differentiate into reactive astrocytes following CNS injury, they are not the main source of de novo astrocytes (208). Some Schwann cells occupying the lesion site (41, 45) have also been shown to differentiate from NG2+ OPCs (437). Activated NG2+ oligodendrocytes also show a change in morphology including withdrawal of long, complex branches into shortened hypertrophied processes concomitant with swelling of the soma and accumulation into dense plaques surrounding the lesion site as a result of enhanced β-catenin signaling following injury (325). NG2+ glia additionally contribute to the inflammatory milieu (215) by activating macrophages and astrocytes (325) and secreting proteases such as MMP-9 (343) that help increase the permeability of the BSCB (232, 285).
What is the impact of these newly proliferated, CSPG-producing NG2+ oligodendrocyte progenitors on axon regeneration in the lesion penumbra? Importantly, these newly proliferated, and supposedly axon inhibitory, NG2+ cells have been shown to highly colocalize with dystrophic axonal growth cones (187, 259, 284). Whether NG2+ OPCs themselves inhibit axon regeneration has been a matter of considerable contention (232, 429). Earlier studies had described how the NG2 CSPG itself inhibits axon regeneration in vitro (98, 359) and in vivo and that this effect could be reversed using an NG2-blocking antibody (245, 378). However, recent studies have revealed that the NG2+ OPC itself can first entrap then stabilize retracting growth cones after a dorsal column lesion despite the extensive presence of macrophages which cause the dying back phenomenon (48). In addition to the proteoglycan NG2, these cells express an abundance of fibronectin and laminin that is likely critical for balancing the ECM and promoting the entrapping rather than repulsive effect on axons. It is also possible that pericytes (which also express NG2, laminin, and fibronectin) may play a role in entrapping dystrophic axons at least transiently within the lesion core.
Closer inspection of the NG2+ OPC and dystrophic growth cone interface has revealed synaptic-like structures by which axon tips may become further stabilized along the lesion penumbra (113, 363). NG2+ cells normally receive excitatory axonal synaptic-like contacts in the developing and adult brain that may likely play a signaling role in allowing the OPC to ‟sense” if a demyelinating event has occurred in its vicinity (32, 128, 446). Following SCI, the abundance of NG2+ OPCs in the lesion penumbra may be providing a safe haven in the midst of fulminant inflammation that serves to stabilize the severed axonal ending through this unusual glia/neuron bond and, unfortunately, helping to prevent them from regenerating further (48, 112, 125). Synaptic-like contacts between regenerating sensory axons that abruptly halt their forward progress upon NG2+ glia has also been documented at the dorsal root entry zone after root crush (363). How long this entrapment lasts may depend on whether the oligodendrocyte progenitor eventually changes its phenotype to become less hospitable. Whether axons eventually dieback to their sustaining collaterals as Cajal had predicted if and when the growth cone escapes this entrapment remains an unanswered question. However, dystrophic growth cones have been observed to persist in the spinal cord scar for 40 yr in a human case of SCI, suggesting that some severed axonal tips can persevere in a doomed, synaptic-like state with glial cells indefinitely (FIGURE 7B) (330). Finding a means to free the dystrophic growth cone from its synaptic entrapment may prove to be therapeutic (384).
Oligodendrocyte progenitors themselves are affected by the CSPG-content of the glial scar which can spread rostral and caudal to the lesion following SCI (FIGURE 6C) (8). While NG2+ OPCs express the NG2 CSPG, other CSPGs of the astroglial scar such as versican and neurocan have been shown to potently inhibit oligodendrocyte myelination (307, 317) which may be contributing to chronic remyelination failure following SCI (34, 46, 100, 394) as well as multiple sclerosis (198). OPCs express the receptor protein tyrosine phosphatase sigma (RPTPσ and see below for further discussion of CSPG receptors), which in the presence of CSPGs inhibit vital oligodendrocyte functions such as proliferation, differentiation, migration, and myelination through ROCK and Rho downstream signaling (307). Oligodendrocyte progenitors in the presence of CSPGs show decreased outgrowth of processes and differentiation into mature, myelinating oligodendrocytes (48, 194, 348). Oligodendrocyte migration is additionally compromised by the sugar moieties of CSPGs, which can be reversed by the sugar-cleaving ability of chondroitinase ABC (194, 349). Changes in Akt and ERK signaling through RPTPσ and CSPG binding also decrease oligodendrocyte survival and maturation (100). NG2+ cells surrounding the lesion penumbra, despite possessing the ability to robustly proliferate following injury, display reduced ability to differentiate to ultimately resume their necessary function of remyelinating denuded axons chronically (377, 394), although disperse remyelination has been shown to occur (166). The proximity of OPCs to the CSPG-rich scar and subsequent CSPG signaling may be one explanation of how oligodendrocyte function is compromised following injury. Thus the CSPG-rich content of the lesion contributes to an environment that serves to curtail remyelination as well as regeneration.
C. Pericytes, Fibroblasts, and the Fibrotic Component of the Glial Scar
Fibroblasts are the major connective tissue cells found throughout the body. These cells provide a structural framework partly through deposition of ECM components, and only invade or are produced in the CNS after injury (1, 109). Following a stab injury, for example, meningeal or perivascular fibroblasts migrate to the lesion where they proliferate and contribute to the development of the fibrotic scar in the lesion epicenter (FIGURE 7C) (59, 109). Fibroblasts aid in the contraction of the lesion and subsequent wound closure, and although the fibrotic scar is essential to the acute healing process after injury, it also contributes a portion of the chronic impediment of the glial scar to axon regeneration (401). Fibroblasts have been shown to decrease neurite length in vitro (286, 329) as well as in vivo (401, 444). Even robustly regenerating PTEN knockdown axons have been reported to avoid the fibroblast-filled lesion epicenter (447). This phenomenon may be due in part to the secretion and deposition of axon-repulsive cues by fibroblasts (303) such as tenascin, versican, and collagen. Recall that type I collagen is a major trigger of astrocytic scar formation (157).
Pericytes have recently been found to contribute to the fibrotic scar following CNS injury by chronically differentiating into fibroblast-like cells (142, 361). Normally, pericytes envelope the blood vessels of the CNS to aid in the control of blood flow, support angiogenesis, help maintain the blood-brain barrier, and perform other homeostatic functions (413). However, following injury and disruption of the BSCB, a particular subset of pericytes delaminate from the basal laminae of the blood vessels they envelope and migrate to the lesion core (142). In a model of spinal cord dorsal hemisection, Görtiz et al. (142) found that the lesion site becomes devoid of blood vessels ~1 day following injury, but by 5 days after injury, blood vessels had regenerated and pericytes had continuously proliferated. This perivascular source of proliferating fibroblasts has also been observed in traumatic brain injuries (97) as well as contusive SCI where the dura mater remains intact (361). However, recent work by Guimaraes-Camboa et al. (149) (through the use of fate mapping with the help of a very specific pericyte marker, tbx18) has posited that pericytes may not be the most prevalent fibrogenic progenitors. However, in support of the fibrogenic pericyte hypothesis, Soderblom et al. (361) genetically labeled collagen1α1-producing cells and showed that this population greatly proliferated to contribute to the fibrotic scar. Like the cell types described in Görtiz et al. (142), Soderblom et al. (361) found that they are also PDGFR-β and CD13 positive. According to these two studies, pericytes at the lesion core lose expression of pericyte-markers, including CD13 and PDGFRα, and upregulate fibrogenic markers such as fibronectin (142). Whether these two mouse studies have independently identified the same source of fibrotic scar formation will need to be clarified along with elucidation of the exact cellular identity of these CD13/PDGFRα+ cells. It is also possible that pericytes (which also express NG2, laminin, and fibronectin) may play a role in entrapping dystrophic axons at least transiently within the lesion core.
Recent studies in mouse models of contusive SCI have additionally shown that the increase of fibroblasts at the lesion site correlates with the infiltration of leukocytes at 7 days post injury where they migrate and concentrate in the lesion core by 14 days (FIGURE 6A) (444, 445). Zhu et al. (444) have further suggested that the delaminated fibroblasts are called to the lesion site through infiltrating macrophages instead of resident microglia as clondronate-induced depletion of macrophage entry greatly decreased formation of the fibrotic component of the scar. This migration of fibroblasts into the cord as well as their activation may be due to TNF and BMP secretion by macrophages (444). TGF-β1 is another potent proinflammatory cytokine expressed in the lesion that has been shown to induce reactive fibrosis in culture (205) which, in turn, has been shown to induce reactivity in other glia such as astrocytes (409). Maturation of the fibrotic scar includes deposition of a basal lamina, formation of the glial limitans structure to sequester fibroblasts from the rest of the CNS (44), and BSCB reformation (255). Basal lamina formation involves cell-cell contact of EphB2 receptors found on fibroblasts and Ephrin-B2 receptors on reactive astrocytes and subsequent secretion of ECM molecules (205). Recent studies involving specific deletion of fibronectin in macrophages have shown that fibroblasts are, indeed, the cell type responsible for the majority of fibronectin secreted in the ECM (445). Soluble fibronectin is possibly integrated directly into the ECM via activated macrophages through integrin receptor activity (445). Overall, this process of fibrotic scar formation is essential to the wound healing process of the lesion after injury. Indeed, genetic, large-scale ablation of pericytes resulted in the enlargement of the lesion and failure of the lesion to contract and close, but discussion was lacking as to whether there was enhanced regeneration (142). Thus it will be important in the future to learn whether specific ablation of pericytes can reduce the fibrotic component of scarring and also stimulate regeneration.
Given the increasing breadth of knowledge regarding fibroblasts or fibroblast-like cells and their interactions with various glial cell types in the forming scar, it is imperative that we consider that no single cell type exists in isolation following SCI. Indeed, glial and fibrotic scar formation is an intricately synchronized and interactive cell process with many potential therapeutic targets for functional recovery.
IV. CHONDROITIN SULFATE PROTEOGLYCANS AND THEIR EFFECTS ON LIMITING REGENERATION/PLASTICITY AFTER SCI
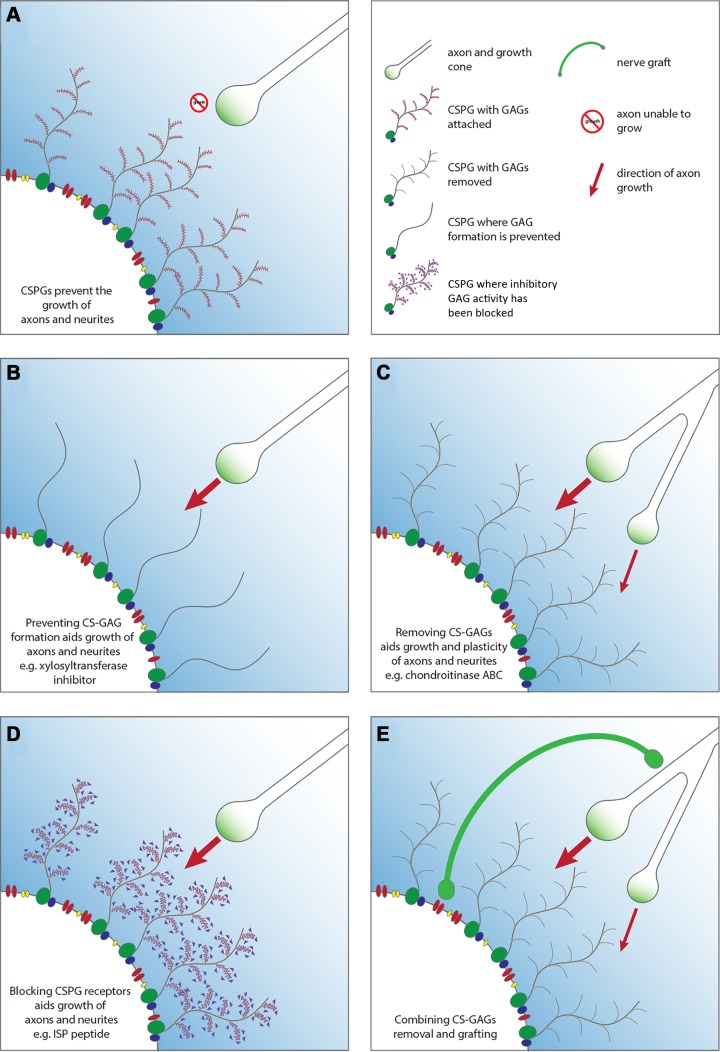
The lesion penumbra surrounding the fibrotic core is marked by reactive glial cells and an abundance of CSPGs (FIGURE 7). While different cells are known to express discrete types of CSPG after SCI, in general, neurocan, versican, brevican, and NG2 predominate at the site of trauma, with expression reaching a plateau 2 wk after the lesion is formed and remaining, albeit at decreased levels, throughout life (11, 12, 51). Interestingly, the axon-inhibitory sugar moieties of CSPGs, glycosaminoglycans or CS-GAGs, are typically sulfated to generate greater amounts of CS-A and CS-C but also CS-E following trauma. The upregulation of CSPGs following SCI is partly caused by the trauma-induced local infusion of blood and fibrinogen which mediates activation of TGF-β (181, 375).
Due to the high CSPG content within the glial scar, it is not surprising that axon regeneration and plasticity are hindered following SCI (FIGURE 8A) (5, 23, 35, 39, 98, 116, 123, 359, 380). The classic demonstration of this inhibitory effect came from Davies et al. (84) who used microtransplantation of adult dorsal root ganglion neurons into degenerating myelin rostral to a lesion of the dorsal columns where, surprisingly, they could regenerate axons robustly among myelin debris as well as reactive intratract astrocytes, demonstrating that reactive astrocytes farther from the lesion can be axon growth permissive. However, once they reached scarred areas just outside of the lesion core that contained wall-building astrocytes and abundant CSPGs, they halted their progress and formed dystrophic endings. Considering the importance of CSPGs to recent research in the SCI field, we will detail the contribution of CSPGs to growth cone dystrophy and discuss their newly discovered cognate receptors (RPTPσ and LAR) as well as putative downstream signaling cascades. Finally, we will end this section with the role of CSPGs in limiting axonal plasticity via the PNN.
FIGURE 8.
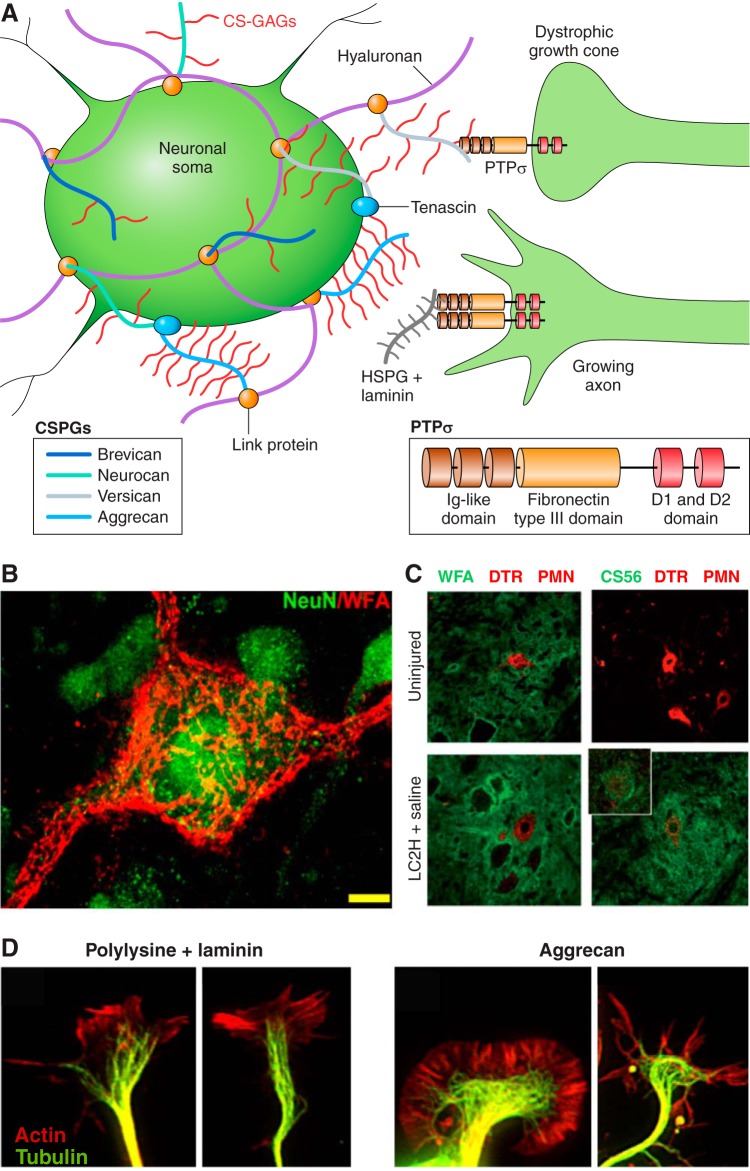
Chondroitin sulfate proteoglycans and the growth cone. Chondroitin sulfate proteoglycans (CSPGs) of the glial scar inhibit axon outgrowth through the protein tyrosine phosphatase sigma receptor (PTPσ). A: CSPGs consist of a protein core with varying glycosaminoglycans. CSPGs attach to hyaluronan through linker proteins to form the perineuronal net (PNN) surrounding the soma of select neurons. The glycosaminoglycan segment of CSPGs binds to transmembrane receptor PTPσ to contribute to receptor monomerization causing growth cone dystrophy. Heparan sulfate proteoglycans binding to PTPσ promote their oligomerization to allow for axon growth. B: the PNN is visualized through WFA staining of glycosaminoglycans. [From Massey et al. (251), with permission from Society for Neuroscience conveyed through Copyright Clearance Center, Inc.] C: CSPGs visualized through CS56 antibody staining (green) are upregulated following C2 hemisecton and surround dextran amine Texas Red (DTR)-labeled phrenic motor neurons (PMN). [From Alilain et al. (5), with permission from Macmillan Publishers Ltd.] D: axonal growth cones on polylysine and laminin are visualized through actin (red) and tubulin (green) staining. Dystrophic growth cones are visualized through tubulin and actin staining of adult mouse dorsal root ganglion neurons on aggrecan. (From Hur et al. Proc Natl Acad Sci USA 108: 5057–5062, 2011, with permission from PNAS.)
A. Chondroitin Sulfate Proteoglycans and the Growth Cone
Depending on the geometry of the assay, in response to substrate-bound CSPGs in vitro, growth cones typically either turn or enter a dystrophic state (FIGURE 8D). The repulsive turning effect was initially demonstrated with in vitro stripe assays where neurons would deviate abruptly to avoid growing on CSPG-rich regions (98, 359). The stripe assay promotes outgrowth upon alternating patterns of permissive versus inhibitory substrates to mimic the environment that a neurite might experience at the point of a sharp cellular boundary (359, 360). Through this assay, it has been determined that neurite growth and rate of extension, calcium signaling, growth cone morphology, and filipodial behavior are all significantly affected by the GAG portion of CSPGs (31, 213, 358, 359, 395).
The strength of the stripe assay is its ability to model special in vivo situations such as that which occurs at the glial roof plate barrier that acts in the fashion of a guard-rail to the ascending dorsal columns and descending CST axons during development of the spinal cord (196, 359). Here, an immediate change in substrate, or substrate concentration, dramatically affects neurite growth and guidance to induce turning. However, most often in vivo, changes in substrate concentration occur more gradually in space or time. For example, in the vicinity of the glial scar, neurites attempting to regrow will come into contact with gradually increasing concentrations of CSPGs as they struggle within the lesion epicenter (350). This geometry has been reflected with the development of the spot assay (392). This assay generates a smooth gradient of proteoglycan within a defined area of the spot rim, similar to that which occurs in the glial scar penumbra. Initially this technique was used to show that dorsal root ganglion axons will enter but then stall upon an increasing gradient of aggrecan, eventually resulting in the formation of dystrophic end bulbs (FIGURE 8D) (392). More recently, it has been used to identify CSPG receptor upregulation as well as the critical demonstration that the inhibitory effect of CSPG gradients upon growth cone advance is not due to repulsion but, rather, to an over adhesion of the growth cone to the inhibitory substrate at a particular point within the gradient (221). Such super-adhesion-mediated growth cone inhibition also occurs in relation to NG2+ oligodendrocyte precursor cells which can entrap axonal growth cones via synaptic-like connections (48, 113). It is likely that in vivo growth cone dystrophy is also caused, at least in part, by such overly adhesive interactions with the CSPG GAG chains presented by OPCs (98, 110, 123, 178, 220, 266, 279, 293, 357).
B. Chondroitin Sulfate Proteoglycans Mediate Signaling in the Growth Cone
Another way to overcome the effects of proteoglycans on growth cones would be to interrupt the downstream signaling pathway through which CSPGs mediate their dystrophy producing effect. A number of studies have identified the RhoA/ROCK signaling pathway as being instrumental in causing growth cone collapse, which can precede dystrophy (76, 99), through the prevention of microtubule formation (247). Indeed, use of the specific inhibitors C3 coenzyme or Y-27632 in vivo facilitates axonal growth following SCI (90, 120, 268, 316). Nonetheless, CSPG-mediated growth cone collapse may be further instigated by a number of alternative signaling receptors and pathways. For example, epidermal growth factor receptor (EGFR) is thought to control the calcium signaling within neurites and is inhibited in the presence of CSPGs (78, 210). Furthermore, CSPGs interfere with integrin binding and signaling (2, 296, 379, 448).
The failure of axon growth on CSPGs has recently been attributed to the activation of protein kinase A (PKA) (214). These data are interesting as cAMP, which activates PKA, is known to promote regeneration of optic nerves (216, 269). Indeed, this key intracellular signaling molecule has been shown to increase neuronal regeneration in a number of models (55, 71, 314, 315) through its effect on PKA (131, 239, 335), and by increasing growth factor receptor translocation from the cytoplasm to the cell membrane (263). This may suggest that the effect of PKA activation on neuronal growth is dependent on the specific range of extracellular matrix molecules that the growth cone contacts.
C. Chondroitin Sulfate Proteoglycan Receptor Discovery and Mediation of Growth Inhibition by Excess Adhesion
Less than a decade ago, our best evidence suggested that CSPGs mediate their effect on axon guidance and growth cone dystrophy solely through their negative charge (273) or via interactions that impede growth-promoting factors. Recently, however, a few CSPG receptors have been identified that may directly initiate these inhibitory effects. These are the adhesion-promoting, synaptogenesis-related LAR family receptor phosphatases, RPTPσ and LAR. The third family member, RPTPδ, has not yet been studied in relation to CSPG inhibitory function.
RPTPσ acts as a functional receptor for specific CSPGs (FIGURE 8A) (124, 345). RPTPσ−/− mice demonstrated increased outgrowth of granule and dorsal root ganglion neurons when grown on substrates containing CSPGs (124, 345), while no such effect was shown following growth on inhibitory myelin substrates (345). As such, these receptors have been shown to be of primary importance in the control of neurite outgrowth after CNS trauma where CSPGs are increased. For example, RPTPσ−/− mice demonstrate enhanced regeneration of the injured CST and growth of sensory axons into an SCI lesion site (124, 345). Similar regenerative capacity through knockout of RPTPσ has been shown in peripheral and optic nerve crush models (124, 258, 345, 387, 442). Interestingly, McLean et al. (258) further demonstrated that RPTPσ−/− mice showed faster rates of peripheral nerve regeneration following sciatic nerve crush and bidirectional growth of fibers when nerve transection was combined with immediate repair or allografting. Gardner and Habecker (135) have recently shown that the effect of CSPG-RPTPσ interactions can also effect sympathetic reinnervation following denervation within the forming scar of the heart following ischemia-reperfusion injury. RPTPσ−/− mice showed hyper-reinnervation of proteoglycan-rich, cardiac scar tissue following recovery unlike RPTPσ+/− animals, which displayed no regeneration into the scar as well as marked susceptibility to generate arrhythmias. As such, CS-RPTPσ interactions can be shown to directly affect capacity and rate of regeneration following trauma and scar formation in both the CNS and peripheral nervous system (PNS).
It is believed that four positively charged lysine residues in the first immunoglobulin domain of the RPTPσ receptor mediate the specific interaction with GAGs (345, 432) on both CS and HS molecules (FIGURE 8A) (10, 432). Sulfation patterning additionally affects binding of specific CSPGs to RPTPσ. Due to the nature of electrostatic interactions, the bifunctional RPTPσ is likely to bind most strongly with CS-D and CS-E. Given that the latter of these molecules is highly upregulated after CNS injury (42, 93, 432), this makes the receptor a strong candidate to target in a treatment strategy (42, 221). Importantly, the downstream effectors of the RPTPσ signaling pathway have yet to be identified, meaning that the mechanism through which CSPGs mediate inhibitory signaling is not yet fully elucidated. It is possible that it converges on the Rho/ROCK pathway and aids inhibition of Erk signaling by dephosphorlyation (100, 221).
The second receptor that mediates the cell growth inhibitory function of CSPGs is LAR. Among other things, LAR−/− mice demonstrate developmental deficits within the septohippocampal cholinergic fiber system (207, 428), suggesting an effect in the organization of neural networks, especially through the formation and regulation of synapses (169). LAR binds to itself (428), to HSPGs (121), and to CSPGs (114) (in a dose-dependent manner). Further downstream, the CSPG-LAR interaction has been shown to increase RhoA signaling and inactivate Akt, indicating a convergence of all CSPG mechanisms of action (100, 114).
Following traumatic CNS injury, LAR−/− mice have improved regeneration within the corticospinal tract and serotonergic sprouting leading to some functional recovery of the locomotor system (114, 421). This effect may be a result of the homophilic binding exhibited by the receptor which has been identified as growth promoting (428). However, these results are not consistent as LAR−/− mice have also exhibited reduced recovery following entorhinal cortex and sciatic nerve injury (419). Nonetheless, this complication means that the receptor may be growth promoting or inhibiting depending on a complex series of interactions. This, combined with the fact that LAR is not known to bind to CS-A or CS-C (267), may limit its use as a treatment for SCI and means more research is required into the mechanism of LAR action.
Recently, the Nogo receptors NgR1 and NgR3 (93) have also been recognized as CSPG receptors. Nogo−/− mice demonstrate only modest functional regeneration following injury (203, 353, 441), perhaps indicative that targeting this molecule alone is not sufficient to enable axon regeneration following SCI. Dickendesher et al. (93) recently demonstrated high-affinity binding between the receptors NgR1 and NgR3 and CSPGs, specifically CS-B, CS-D, CS-E, and HS (42, 267). Indeed, the binding patterns of RPTPσ, NgR1, and NgR3 fundamentally overlap. However, double knockout NgR1−/− and NgR3−/− mice showed significant axonal growth following injury not exhibited by either knockout alone (93). Regeneration was further enhanced in the triple NgR1−/−, NgR3−/−, and RPTPσ−/− knockout. This is highly suggestive of the important role that CSPG signaling has on regenerative capacity after traumatic injury.
D. Perineuronal Net Characterization
A key function of CSPGs within the adult CNS is their role in formation of PNNs (FIGURE 8, A and B). These extracellular reticular structures were originally identified by Camillo Golgi [reviewed by Celio et al. (64)]. The most widely addressed role of PNNs is their capacity to limit plasticity within the CNS. The PNN is activity dependent and forms around the soma and proximal neurites (FIGURE 8, B and C) appearing in the CNS at the time of synapse maturation and the closure of critical periods during development (148, 253). However, PNNs have additionally been implicated in a multitude of other homeostatic functions such as neuroprotection (43) and memory consolidation (141, 250, 308, 327). In addition, they are thought to play a role in the etiology of various neurological disorders such as addiction (354, 425), schizophrenia (254, 299, 300), Alzheimer’s disease (17, 305, 427), depression (150, 290), and epilepsy (298). While biochemical analysis suggests that only 1.3% of total CS-GAGs in the adult rodent brain are associated with PNNs (86), their critical role in the proper functioning of the CNS cannot be understated. While PNNs surround many neuronal subtypes, they are secreted in great abundance by parvalbumin-expressing GABAergic interneurons, many of which express the voltage-dependent potassium channel Kv3.1b (43, 164). They also surround fast-spiking interneurons (96, 274) and occur about many of the neurons in the spinal cord including motor neurons (253).
1. Perineuronal nets impede functional plasticity following SCI
The importance of PNNs to plasticity within the CNS is indicated by the rapid and robust degree of PNN remodeling that occurs in deafferented neuropil in centers distal to a lesion. For instance, Massey and co-workers (250, 251) showed that by 2–3 wk post injury, the PNNs were dramatically upregulated around rat dorsal column nuclei neurons following a mid-cervical dorsal column tract lesion that spared light touch sensory information from the thumb and forefinger (FIGURE 8B). Furthermore, evidence suggested that these upregulated nets were curtailing plasticity as their removal in the cuneate nucleus via ChABC allowed significant sprouting into its denervated portions from the intact sensory axons coming from the first two digits. Whether the spread of fibers throughout the nucleus improves or impedes function has not yet been determined. It has been demonstrated that thoracic SCI in a rodent model mediates decreases in parvalbumin expression and atrophy of GABAergic interneurons in hindlimb, sensory, and motor cortices (139, 140, 295). Furthermore, changes were noted in PNN CSPG-GAGs at the boundary between the motor forelimb and sensorimotor hindlimb cortex. Changes in the PNN may help tightly regulate the remodeling and plasticity that occurs in the cortex after SCI for the animal to acquire some degree of endogenous locomotor functional recovery.
PNNs also increase in areas surrounding motor neurons within the spinal cord well below the level of injury (295). An example of this occurs in the respiratory system. Alilain et al. (5) demonstrated that paralysis of the hemidiaphragm ipsilateral to a full C2 hemisection was persistent over time and leads to an increase of the PNN around phrenic motor neurons at level C4 (FIGURE 8C). However, acute removal of CS-GAGs at C4 by injection of ChABC enabled some, albeit minimal, return of function to the previously paralyzed side of the diaphragm muscle. We have recently assessed this treatment in animals up to 1.5 yr after injury where we hypothesized that a slow sprouting of re-crossed projections [the so-called latent crossed phrenic pathway; Alilain et al. (5)] over time, even in the presence of the PNN, might be unmasked by digesting the matrix via chondroitinase (410). We were surprised to see a rapid (as early as 1 wk) and robust recovery in diaphragm EMG activity in these long chronically paralyzed animals that was virtually indistinguishable from uninjured controls. Remarkably, the recovery after chronic stages was far greater than that which occurred at acute stages. In both studies, we show substantial reductions in both PNNs and CSPGs following ChABC application and sprouting of serotonergic axons, which are also critical in the control of breathing.
V. EXTRACELLULAR MATRIX-TARGETING REPAIR STRATEGIES
There are broadly three ways to remove the inhibitory influence of CSPGs (FIGURE 9). The first is to target the CSPG itself. The most commonly used method is via the enzymatic degradation of the CS-GAG chains which increases regeneration/plasticity at, or distant from, the site of injury. Other successful methods include preventing CSPG formation and inhibiting the proteoglycan signaling mechanism. These will each be described in turn.
FIGURE 9.
SCI repair strategies involving CSPG modification. A: CSPGs within the ECM and PNNs are upregulated following spinal cord injury. This molecule creates a physical and chemical barrier to the growth of axons. B: preventing the formation of CSPGs through processes such as xylosyltransferase inhibition, the use of xyloside, or N-acetylgalactosaminyltransferase-1 deletion removes the inhibitory component from the ECM enabling axonal growth. C: the catabolism of CS-GAGs through use of the bacterial enzyme ChABC removes the major inhibitory component of the glial scar and ECM facilitating axonal growth. The enzyme additionally facilitates plasticity through axonal and neuronal sprouting and synaptogenesis while encouraging neuronal protection. D: the inhibition of the CSPG receptor through the use of drugs such as ISP and ILP masks the inhibitory signals from the proteoglycans facilitating axonal and neuronal growth. E: combination treatment strategies may further facilitate repair following SCI, here demonstrating the use of ChABC with a peripheral nerve graft to facilitate axonal growth, sprouting, and plasticity as well as the formation of functional axonal connections.
A. Removing Chondroitin Sulfate-Glycosaminoglycans
The catabolism of CS-GAG chains to remove the inhibitory influence of CSPGs following SCI has the possible advantage of being a delayed strategy. As such, it can be applied at both acute and chronic time points after trauma, meaning that the positive effects of astrogliosis can occur before the treatment is applied. This would have the potential to maximize repair and recovery after SCI. Sulfatases, which target the catabolism of specific moieties of sulfated GAG chains such as CS-A, have been shown to enhance axonal regeneration in vitro (403) and in vivo (434). However, the most highly studied and successful of these methods is via the use of the GAG degrading enzyme ChABC. The bacterial enzyme acts to cleave GAG polymers from CS-A through E, into tetrasaccharides and disaccharides via an eliminative mechanism, preventing CSPG-matrix glycoprotein interactions (176, 313, 388). It was first applied in vitro and ex vivo to enable axonal outgrowth on CSPG substrates and glial scar explants (36, 256, 257, 329, 450). Indeed, ChABC treatment increases outgrowth over inhibitory astrocytic cell lines (356), through oligodendrocyte lineage cells (12), and facilitates neurite and axonal growth on spinal cord cryosections. The potential use of ChABC for in vivo SCI treatment was initially exemplified by Lemons et al. (230) who used the enzyme to degrade the CS-GAG chains of the glial scar following contusion. Subsequently, ChABC application was convincingly shown to mediate anatomical and functional regeneration with polysynaptic activity observed caudal to the injury site following a dorsal column crush (39).
The effects of ChABC upon axon regeneration following SCI have subsequently been demonstrated in a large number of studies using a variety of injury models and species including rats, hamsters, rabbits, cats, pigs, and squirrel monkeys. Indeed, ChABC enzyme-mediated axon regeneration has been shown in nigrostriatal (270), serotonergic (389), rubrospinal (433), and corticospinal tracts (39). Data suggest that ChABC can mediate recovery in the most severe of spinal injuries. Caggiano et al. (54) showed that intrathecal application of ChABC improved locomotor and autonomic bladder function following a clip compression injury. Furthermore, following contusion injury, ChABC has been shown to mediate some functional improvements in locomotor and urinary systems, albeit with modest anatomical regeneration (54, 193). However, the more dramatic effects of the use of ChABC have been in hemisected animals (180). Interestingly, Cafferty et al. (53) used a transgenic approach to express ChABC under a GFAP promotor. This enabled enzyme delivery to be targeted to areas of astrocytosis and demonstrated increased regeneration of CST axons into the site of a hemisection injury. Similarly, sensory and motor projections have shown functional growth into previously denervated areas (52, 368). These data may suggest that treatment with ChABC needs to occur at the optimal time and location after injury to evoke the maximal functional effect. Interestingly, recent studies have demonstrated that ChABC treatment leads to an increase of OPCs at the site of injury, meaning that treatment with the enzyme could facilitate remyelination following SCI (223, 307).
A critical effect of the application of ChABC is to promote plasticity from projections that survive after CNS trauma leading to functionally beneficial rewiring of undamaged systems in both rodent and non-human primate models (38, 134, 250, 251, 397). For example, numerous studies have shown that administration of the enzyme promoted serotonergic, corticospinal, and sensory fiber sprouting in areas where CS-GAG degradation was prominent (5, 23, 52, 193, 251, 402). Indeed, through anatomical tracing and electrophysiological techniques, collateral sprouting of forelimb sensory afferents into the partially denervated brain stem nuclei were shown to be functionally active following cervical dorsal column injury and ChABC injection (251). Similarly, Galtrey et al. (129) have shown that inaccurate repair following PNS injury can be overcome through ChABC injection into the spinal cord, causing increased neuronal sprouting. However, ChABC-induced sprouting does not always make functional connections (389). This highlights the need to drive the induced plasticity via appropriate activity and, thus, following Hebb’s postulate, stronger functional connections will be made (see below).
A number of studies have shown that ChABC can be neuroprotective and decrease CSPG-mediated axonal atrophy (62). Carter and co-workers (61, 62) demonstrated that CS-GAG removal by ChABC acts retrogradely to reduce degenerative changes of injured corticospinal and rubrospinal neurons, shown by a restoration in cell body soma size in acute and chronic injury models. This is an effect indicative of neuroprotection. Furthermore, ChABC has been shown to reduce macrophage-mediated axonal dieback following SCI (49). The precise mechanism through which ChABC mediates neuroprotective effects is not clear, although it may involve immune cell modulation (25, 94). However, these data clearly show that, if applied in the subacute stages following spinal trauma, ChABC activity can remove several of the negative effects of increased CSPG upregulation.
Despite ample evidence for the success of a ChABC-mediated treatment strategy for SCI, there are potential problems with its application. Primary among these are that the half-life of the enzyme is relatively short in vivo at ~3–10 days (342, 386) or 2–3 wk with glycerol, albumin, or trehalose stabilizers (67, 224, 280). This limits the time frame in which a single treatment application could evoke change in the CNS after injury. As such, either more invasive delivery procedures may need to be clinically employed, or the enzyme will need further modification. In a recent study, Lee et al. (224) produced a thermostabilized ChABC which remained active, albeit at low levels, up to 6 wk after SCI. However, this treatment has not demonstrated a functional recovery following SCI in vivo. Alternatively, a number of recent studies have utilized gene therapy to modify the enzyme to enable its secretion from mammalian cells (185, 206). Perhaps the most successful of these has shown that the modified enzyme can cause extensive in vivo CS-GAG removal resulting in CST sprouting following injury to the dorsal columns (276, 440). Furthermore, it has been shown that a lenti-viral form of ChABC can increase neuroprotection and enhance sensory-motor function from acute to chronic stages following spinal contusion injury (25). Despite more data being required, these studies demonstrate how innovations in ChABC can make it more applicable for clinical use.
While the CS-GAGs targeted through the use of ChABC remove the major inhibitors, the core protein and the leftover ChABC-resistant sugar stubs remain (337). Another way to remove CSPGs, and specifically target the core protein following SCI, is to take advantage of the endogeonous CNS CSPG regulators. There are a number of identified proteases that are able to mediate this function and act to degrade the proteoglycan core proteins. Perhaps the most widely studied is the one known to act all over the body, the disintegrin-like and metalloproteinase with thrombospondin type 1 motif 4 (ADAMTS4) (9, 227, 383). It is thought that experience-dependent plasticity within the CNS is mediated through a tPA-ADAMTS4 signaling pathway (252, 294). Functional regeneration may be increased following SCI through the upregulation of ADAMTS4 expression by the introduction of IL-1α (228). Alternatively, microarray studies have identified a host of MMPs, which act in the CNS to breakdown CSPGs (190). These molecules are known to be upregulated in response to neuronal stimulation (163, 248) and can aid recovery following SCI (222, 228).
The problem with increasing MMPs or ADAMTS4 activity and expression after SCI is the method through which they operate. Following SCI, CSPG production changes the ECM which expresses a higher quantity of CS-GAGs. These chains provide a significant physical barrier to the action of both MMPs or ADAMTS4 meaning that their functional activity following SCI may be limited. However, recent studies have shown that combining ADAMTS4 activity with ChABC application can have an additive effect on neurite outgrowth in vitro (78). These data illustrate how full degradation of the entire CSPG in a spatially and temporally specific manor may mediate neuronal growth following injury and highlight this combination as a potential SCI treatment strategy.
B. Preventing Chondroitin Sulfate-Glycosaminoglycan Formation
An alternative method to prevent the inhibitory effect of CSPGs following SCI is not to degrade them once in place, but to prevent their formation from the beginning. However, this has the disadvantage of needing to be applied within the acute phase of injury, limiting clinical applicability. The classic method of preventing CSPG formation involves the use of a DNA enzyme to inhibit xylosyltransferase (XT)-1 mRNA. This enzyme glycosylates the core protein of CSPGs, and without its functioning, proteoglycan deposition is predominantly inhibited and axon regeneration can be stimulated (144). However, some CSPG synthesis was evident in this experiment about the lesion penumbra, largely attributed to the function of XT-2.
To have the benefit of reduced CSPG allowed plasticity and axonal growth after trauma, many groups have focused on inhibiting the formation of CS-GAGs while enabling the core protein to be expressed. For example, Takeuchi et al. (376) have recently described the use of a transgenic animal which prevented CS synthesis through deletion of N-acetylgalactosaminyltransferase-1. These mice showed superior recovery from SCI than both control and ChABC-treated animals, possibly due to the endogenous upregulation of HS. Similarly, the use of the molecule xyloside to compete with the attachment of the CS-GAGs to the CSPG protein core reduces the inhibitory effects of the proteoglycan on neuronal growth by decreasing their deposition about the site of injury in SCI and multiple sclerosis models (223, 449). A number of molecules that have a similar function on CS formation have likewise been used with comparable effects including 4-fluoro-glucosamine (282) and 4-methyl-umbelliferyl-β-d-xylopyranoside (340, 341). Similarly, prevention of GAG polymerization using siRNA against chondroitin polymerizing factor has been shown to successfully decrease the CS-GAGs expressed and secreted by astrocytes, facilitating the growth of cerebellar granule cell neurites (218). Keough et al. (198) have recently described the generation of a new CSPG synthesis inhibitor, fluorosamine. This molecule likely interferes with the enzymatic conversion of UDP-N-acetylglucosamine to UDP-N-acetyl-galactosamine by 4-epimerase (282). CSPGs have long been known to inhibit OPC differentiation, process outgrowth, and remyelination (198, 223, 348). Fluorosamine acted in vivo to reduce CSPGs, in turn facilitating OPC process outgrowth and remyelination in models of multiple sclerosis.
C. Manipulating Chondroitin Sulfate-Glycosaminoglycan Receptors and Signaling
The final method employed to evoke recovery from SCI by negating the inhibitory influence of CSPGs is to target the proteoglycan signaling mechanism. Again, this treatment strategy has the advantage of being able to be applied at both acute and chronic stages after injury. Lang et al. (221) demonstrated that RPTPσ activation in adult sensory neurons by a gradient of CSPGs directly caused growth cones to enter an entrapped dystrophic state. Furthermore, it was shown that the CSPG receptors RPTPσ and LAR occurred at high concentrations within these dystrophic growth cones. The researchers designed an innovative peptide-mimetic for the PTPσ wedge termed the intracellular sigma peptide (ISP) which enabled a change in the growth cone even after it had entered an endball state, allowing for advancement across the CSPG-laden territory. Use of the peptide delivered systemically following severe thoracic contusion resulted in remarkable functional recovery of the locomotor and urinary systems, possibly mediated, in part, through serotonergic sprouting and reinnervation caudal to the site of injury (221). Recently, Paveliev et al. (304) have used the CS-binding protein pleiotrophin (a heparin-binding growth-associated molecule) to facilitate neural regeneration in vitro and in vivo. In preventing CS binding to PTPσ, they demonstrated increased sprouting following mild cortical and spinal cord injury, although this may additionally be linked to rapid reestablishment of the blood supply following trauma. Inhibition of the LAR receptor through the peptide ILP (114, 420) has been shown in vitro to promote axonal growth on CSPG substrates by activating Src, FAK, and TrkB as well as a number of downstream molecules (114, 221, 428). In vivo, cord injured mice were shown to have superior locomotor function than controls, again possibly caused by increased axonal sprouting of serotonergic fibers caudal to the site of injury (114). These data hold great promise for the treatment of SCI and, potentially, a number of neurological conditions in which CSPGs play a definitive role. Furthermore, these exciting results provide great incentive for the identification of receptors for CS-A and CS-C which, if inhibited, may provide further functional benefit after CNS trauma.
D. Combinatorial Strategies
Because SCI is a multifaceted problem, it is unlikely that any one treatment alone can affect the large number of obstacles that occur following trauma. Indeed, this may be the reason why many disparate treatments produce similar modest levels of recovery in SCI animal models. Thus combining treatment strategies that target contrasting mechanisms is being investigated by many laboratories in an attempt to maximize functional benefit. To this end, ChABC has been used widely in combination treatment strategies to aid functional recovery. For example, Bai et al. (16) utilized ChABC with the β2-adrenoceptor agonist clenbutarol following complete T10 transection to increase cAMP levels, facilitating neuroprotection. Only the combined treatment group showed enhanced anatomical and functional recovery 2–3 mo following injury. The use of ChABC to remove the inhibitory CSPG barrier preventing neuronal growth has been used most effectively in combination with tissue grafts and peripheral nerve bridges. The enzymatic modulation of the glial scar at the entrances to the bridge, in particular with the further addition of FGF, facilitates integration of the transplanted tissue with the host and facilitates the regrowth of axons through and beyond the graft tissue (88). Tom et al. (391) demonstrated this through the application of ChABC with a peripheral nerve graft (PNG) after chronic contusion injury, demonstrating the formation of functional synapses and some behavioral recovery. This strategy has been further developed through the addition of virally mediated exogenous BDNF to the combination (390). While the number of regenerating axons did not increase, the combination did enhance the number of neurons trans-synaptically activated following stimulation of the graft. Similarly, the combination of ChABC with a PNG and acidic fibroblast growth factor has been used in rodent models following complete T8 transection to mediate remarkable axonal regeneration from brain stem centers leading to recovery of urination (88). Furthermore, the use of ChABC combined with a PNG has been used to facilitate functional recovery of the respiratory motor system following acute cervical hemisection (5). Three months following the injury, the combined treatment group showed the maximal return of diaphragm function and experiments suggested that the treatment strategy further led to a beneficial rewiring of respiratory motor circuitry. The positive effects of combining ChABC treatment with grafting techniques have been shown in numerous other publications and animal models (118, 119, 173, 193, 226, 400).
In addition to causing the catabolism of the glial scar, ChABC can act to increase plasticity within the injured spinal cord below the level of injury especially when combined with targeted exercise strategies. It has been shown that combining ChABC with task-specific physical rehabilitation following acute and subchronic injury can augment this plasticity, mediating specific functional recovery (134, 402). Combining ChABC treatment with other strategies which reduce inhibition in the extrinsic environment of the spinal cord has also yielded some success. Zhao et al. (440) showed that the combination of ChABC, rehabilitation, and anti-Nogo-A treatment increased the degree of axon sprouting and regeneration better than either treatment acting alone. Furthermore, Wang et al. (406) demonstrated that ChABC added to preconditioning with a sciatic nerve lesion and use of a soluble Nogo receptor decoy (NgR310-Fc) enabled axonal growth to extend not only through, but beyond the injury site. It will be exciting to learn whether the various systemically delivered treatment strategies for overcoming CSPG-mediated inhibition when combined with appropriate rehabilitation will produce enhanced recovery sufficiently robust to provide hope for clinical translation especially at chronic stages after injury.
VI. CONCLUDING REMARKS
The etiology of regeneration failure following SCI has evolved from a simplistic view that the astrocyte scar is the solely culpable mechanical obstacle to axonal regrowth to a richer, multicellular and molecular perspective that involves astrocytes, immune cells, perictyes and fibroblasts as well as oligodendrocyte progenitors, and the intricate ways they all interact to form an axon entrapping environment. While this review concentrated on the potently inhibitory effects of upregulated CSPGs in the scar as well as the PNN and the emerging ways this family of proteoglycans could be perturbed to promote regeneration or sprouting, understanding the complexity of the glial/fibrotic scar and net has underscored the necessity for combinatorial treatments. In particular, the fibrotic component of scar, produced by pericyte and/or meningeal fibroblast associations with astrocytes and each other, and manipulations to specifically alter these interactions have been relatively less well studied than the glial cells themselves. From our perspective, we would like to see the field further pursue combinatorial treatment paradigms especially targeted to the chronic stage since the millions of people worldwide who suffer from SCI are well past their moment of injury. The rather daunting problems that the long-injured cord introduces to hinder the regrowth of damaged axon pathways has frustrated attempts to devise promising regenerative approaches. The exciting discovery of the first known receptors on neurons that mediate the regrowth inhibitory effects of the entrapping matrix molecules has opened the door to the production of specific blocking peptides as well as long-acting enzymes that can be used to manipulate these extracellular culprits and their receptors. When combined with additional strategies to enhance intrinsic neuronal growth potential (238) as well as targeted rehabilitative therapy (158, 264), we may be able to elicit recovery even after a near lifetime of paralysis.
GRANTS
P. M. Warren was funded by the Welcome Trust ISSF Fellowship with the University of Leeds. P. M. Warren and J. Silver were funded by the International Spinal Research Trust, Wings for Life, and the Neilsen Foundation. A. P. Tran and J. Silver were funded by National Institute of Neurological Disorders and Stroke Grant NS025713, The Hong Kong Spinal Cord Injury Fund, The Brumagin/Nelsen Fund, and The Kaneko Family Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Jared Cregg, Marc DePaul, and Bradley Lang for their review and comments.
A. P. Tran and P. M. Warren contributed equally to the work.
Address for reprint requests and other correspondence: J. Silver, Dept. of Neurosciences, School of Medicine, Robbins E653, Case Western Reserve University, 10900 Euclid Ave., Cleveland OH, 44106 (e-mail: jxs10@case.edu).
REFERENCES
- 1.Abnet K, Fawcett JW, Dunnett SB. Interactions between meningeal cells and astrocytes in vivo and in vitro. Brain Res Dev Brain Res 59: 187–196, 1991. doi: 10.1016/0165-3806(91)90099-5. [DOI] [PubMed] [Google Scholar]
- 2.Afshari FT, Kwok JC, Fawcett JW. Astrocyte-produced ephrins inhibit Schwann cell migration via VAV2 signaling. J Neurosci 30: 4246–4255, 2010. doi: 10.1523/JNEUROSCI.3351-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na+-K+-ATPase, the Na+-H+ exchanger, and the Na+-Ca2+ exchanger. J Neurosci 16: 545–552, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal SK, Nashmi R, Fehlings MG. Role of L- and N-type calcium channels in the pathophysiology of traumatic spinal cord white matter injury. Neuroscience 99: 179–188, 2000. doi: 10.1016/S0306-4522(00)00165-2. [DOI] [PubMed] [Google Scholar]
- 5.Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 475: 196–200, 2011. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532: 195–200, 2016. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson D, Wilhelmsson U, Nilsson M, Kubista M, Ståhlberg A, Pekna M, Pekny M. Plasticity response in the contralesional hemisphere after subtle neurotrauma: gene expression profiling after partial deafferentation of the hippocampus. PLoS One 8: e70699, 2013. doi: 10.1371/journal.pone.0070699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews EM, Richards RJ, Yin FQ, Viapiano MS, Jakeman LB. Alterations in chondroitin sulfate proteoglycan expression occur both at and far from the site of spinal contusion injury. Exp Neurol 235: 174–187, 2012. doi: 10.1016/j.expneurol.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem 284: 31493–31497, 2009. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aricescu AR, McKinnell IW, Halfter W, Stoker AW. Heparan sulfate proteoglycans are ligands for receptor protein tyrosine phosphatase sigma. Mol Cell Biol 22: 1881–1892, 2002. doi: 10.1128/MCB.22.6.1881-1892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci 20: 2427–2438, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci 22: 2225–2236, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci 20: 637–647, 2017. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 14.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670, 2007. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 15.Bachoo RM, Kim RS, Ligon KL, Maher EA, Brennan C, Billings N, Chan S, Li C, Rowitch DH, Wong WH, DePinho RA. Molecular diversity of astrocytes with implications for neurological disorders. Proc Natl Acad Sci USA 101: 8384–8389, 2004. doi: 10.1073/pnas.0402140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai F, Peng H, Etlinger JD, Zeman RJ. Partial functional recovery after complete spinal cord transection by combined chondroitinase and clenbuterol treatment. Pflugers Arch 460: 657–666, 2010. doi: 10.1007/s00424-010-0852-y. [DOI] [PubMed] [Google Scholar]
- 17.Baig S, Wilcock GK, Love S. Loss of perineuronal net N-acetylgalactosamine in Alzheimer’s disease. Acta Neuropathol 110: 393–401, 2005. doi: 10.1007/s00401-005-1060-2. [DOI] [PubMed] [Google Scholar]
- 18.Balentine JD. Pathology of experimental spinal cord trauma. I. The necrotic lesion as a function of vascular injury. Lab Invest 39: 236–253, 1978. [PubMed] [Google Scholar]
- 19.Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem 88: 1335–1344, 2004. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- 20.Bardehle S, Krüger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, Dimou L, Götz M. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16: 580–586, 2013. doi: 10.1038/nn.3371. [DOI] [PubMed] [Google Scholar]
- 21.Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7: 470–482, 2010. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Barrett CP, Guth L, Donati EJ, Krikorian JG. Astroglial reaction in the gray matter lumbar segments after midthoracic transection of the adult rat spinal cord. Exp Neurol 73: 365–377, 1981. doi: 10.1016/0014-4886(81)90272-7. [DOI] [PubMed] [Google Scholar]
- 23.Barritt AW, Davies M, Marchand F, Hartley R, Grist J, Yip P, McMahon SB, Bradbury EJ. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci 26: 10856–10867, 2006. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol 70: 194–206, 2011. doi: 10.1002/ana.22421. [DOI] [PubMed] [Google Scholar]
- 25.Bartus K, James ND, Didangelos A, Bosch KD, Verhaagen J, Yáñez-Muñoz RJ, Rogers JH, Schneider BL, Muir EM, Bradbury EJ. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. J Neurosci 34: 4822–4836, 2014. doi: 10.1523/JNEUROSCI.4369-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81: 871–927, 2001. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 27.Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH. Astrocyte development and heterogeneity. Cold Spring Harb Perspect Biol 7: a020362, 2015. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bähr M, Przyrembel C, Bastmeyer M. Astrocytes from adult rat optic nerves are nonpermissive for regenerating retinal ganglion cell axons. Exp Neurol 131: 211–220, 1995. doi: 10.1016/0014-4886(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 29.Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron 36: 375–386, 2002. doi: 10.1016/S0896-6273(02)01005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133: 433–447, 2010. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beller JA, Kulengowski B, Kobraei EM, Curinga G, Calulot CM, Bahrami A, Hering TM, Snow DM. Comparison of sensory neuron growth cone and filopodial responses to structurally diverse aggrecan variants, in vitro. Exp Neurol 247: 143–157, 2013. doi: 10.1016/j.expneurol.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergles DE, Roberts JDB, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405: 187–191, 2000. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 33.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5, 2007. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 34.Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma 2: 299–315, 1985. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- 35.Borisoff JF, Chan CCM, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci 22: 405–416, 2003. doi: 10.1016/S1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 36.Bovolenta P, Wandosell F, Nieto-Sampedro M. Neurite outgrowth inhibitors associated with glial cells and glial cell lines. Neuroreport 5: 345–348, 1993. doi: 10.1097/00001756-199312000-00042. [DOI] [PubMed] [Google Scholar]
- 37.Bowes AL, Yip PK. Modulating inflammatory cell responses to spinal cord injury: all in good time. J Neurotrauma 31: 1753–1766, 2014. doi: 10.1089/neu.2014.3429. [DOI] [PubMed] [Google Scholar]
- 38.Bowes C, Massey JM, Burish M, Cerkevich CM, Kaas JH. Chondroitinase ABC promotes selective reactivation of somatosensory cortex in squirrel monkeys after a cervical dorsal column lesion. Proc Natl Acad Sci USA 109: 2595–2600, 2012. doi: 10.1073/pnas.1121604109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bradbury EJ, Moon LDF, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416: 636–640, 2002. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 40.Brambilla R, Bracchi-Ricard V, Hu W-H, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202: 145–156, 2005. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bresnahan JC. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J Neurol Sci 37: 59–82, 1978. doi: 10.1016/0022-510X(78)90228-9. [DOI] [PubMed] [Google Scholar]
- 42.Brown JM, Xia J, Zhuang B, Cho K-S, Rogers CJ, Gama CI, Rawat M, Tully SE, Uetani N, Mason DE, Tremblay ML, Peters EC, Habuchi O, Chen DF, Hsieh-Wilson LC. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci USA 109: 4768–4773, 2012. doi: 10.1073/pnas.1121318109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brückner G, Brauer K, Härtig W, Wolff JR, Rickmann MJ, Derouiche A, Delpech B, Girard N, Oertel WH, Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia 8: 183–200, 1993. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- 44.Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci 23: 7789–7800, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunge MB, Holets VR, Bates ML, Clarke TS, Watson BD. Characterization of photochemically induced spinal cord injury in the rat by light and electron microscopy. Exp Neurol 127: 76–93, 1994. doi: 10.1006/exnr.1994.1082. [DOI] [PubMed] [Google Scholar]
- 46.Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol 59: 75–89, 1993. [PubMed] [Google Scholar]
- 47.Busch SA, Hamilton JA, Horn KP, Cuascut FX, Cutrone R, Lehman N, Deans RJ, Ting AE, Mays RW, Silver J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J Neurosci 31: 944–953, 2011. doi: 10.1523/JNEUROSCI.3566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J. Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage-induced axonal dieback after spinal cord injury. J Neurosci 30: 255–265, 2010. doi: 10.1523/JNEUROSCI.3705-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busch SA, Horn KP, Silver DJ, Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. J Neurosci 29: 9967–9976, 2009. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23: 297–308, 1999. doi: 10.1016/S0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 51.Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, Brook GA. NG2 and phosphacan are present in the astroglial scar after human traumatic spinal cord injury. BMC Neurol 9: 32, 2009. doi: 10.1186/1471-2377-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cafferty WBJ, Bradbury EJ, Lidierth M, Jones M, Duffy PJ, Pezet S, McMahon SB. Chondroitinase ABC-mediated plasticity of spinal sensory function. J Neurosci 28: 11998–12009, 2008. doi: 10.1523/JNEUROSCI.3877-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cafferty WBJ, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci 27: 2176–2185, 2007. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma 22: 226–239, 2005. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- 55.Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron 22: 89–101, 1999. doi: 10.1016/S0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 56.Cajal SRY. Degeneration & Regeneration of the Nervous System. New York: Hafner, 1959. [Google Scholar]
- 57.Canning DR, Höke A, Malemud CJ, Silver J. A potent inhibitor of neurite outgrowth that predominates in the extracellular matrix of reactive astrocytes. Int J Dev Neurosci 14: 153–175, 1996. doi: 10.1016/0736-5748(96)00004-4. [DOI] [PubMed] [Google Scholar]
- 58.Canning DR, McKeon RJ, DeWitt DA, Perry G, Wujek JR, Frederickson RCA, Silver J. β-Amyloid of Alzheimer’s disease induces reactive gliosis that inhibits axonal outgrowth. Exp Neurol 124: 289–298, 1993. doi: 10.1006/exnr.1993.1199. [DOI] [PubMed] [Google Scholar]
- 59.Carbonell AL, Boya J. Ultrastructural study on meningeal regeneration and meningo-glial relationships after cerebral stab wound in the adult rat. Brain Res 439: 337–344, 1988. doi: 10.1016/0006-8993(88)91491-6. [DOI] [PubMed] [Google Scholar]
- 60.Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol 151: 77–88, 1998. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- 61.Carter LM, McMahon SB, Bradbury EJ. Delayed treatment with chondroitinase ABC reverses chronic atrophy of rubrospinal neurons following spinal cord injury. Exp Neurol 228: 149–156, 2011. doi: 10.1016/j.expneurol.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 62.Carter LM, Starkey ML, Akrimi SF, Davies M, McMahon SB, Bradbury EJ. The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury. J Neurosci 28: 14107–14120, 2008. doi: 10.1523/JNEUROSCI.2217-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 103: 203–218, 2001. doi: 10.1016/S0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 64.Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci 21: 510–515, 1998. doi: 10.1016/S0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- 65.Chang E, Ghosh N, Yanni D, Lee S, Alexandru D, Mozaffar T. A Review of Spasticity Treatments: Pharmacological and Interventional Approaches. Crit Rev Phys Rehabil Med 25: 11–22, 2013. doi: 10.1615/CritRevPhysRehabilMed.2013007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chatzipanteli K, Yanagawa Y, Marcillo AE, Kraydieh S, Yezierski RP, Dietrich WD. Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. J Neurotrauma 17: 321–332, 2000. doi: 10.1089/neu.2000.17.321. [DOI] [PubMed] [Google Scholar]
- 67.Chau CH, Shum DKY, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. FASEB J 18: 194–196, 2004. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- 68.Chauvet N, Prieto M, Alonso G. Tanycytes present in the adult rat mediobasal hypothalamus support the regeneration of monoaminergic axons. Exp Neurol 151: 1–13, 1998. doi: 10.1006/exnr.1998.6784. [DOI] [PubMed] [Google Scholar]
- 69.Cherry JD, Olschowka JA, O’Banion MK. Are “resting” microglia more “m2”? Front Immunol 5: 594, 2014. doi: 10.3389/fimmu.2014.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cherry JD, Olschowka JA, O’Banion MK. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11: 98, 2014. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chierzi S, Ratto GM, Verma P, Fawcett JW. The ability of axons to regenerate their growth cones depends on axonal type and age, and is regulated by calcium, cAMP and ERK. Eur J Neurosci 21: 2051–2062, 2005. doi: 10.1111/j.1460-9568.2005.04066.x. [DOI] [PubMed] [Google Scholar]
- 72.Chikuda H, Yasunaga H, Takeshita K, Horiguchi H, Kawaguchi H, Ohe K, Fushimi K, Tanaka S. Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emerg Med J 31: 201–206, 2014. doi: 10.1136/emermed-2012-202058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res 2: 492–516, 2011. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Citron BA, Arnold PM, Sebastian C, Qin F, Malladi S, Ameenuddin S, Landis ME, Festoff BW. Rapid upregulation of caspase-3 in rat spinal cord after injury: mRNA, protein, and cellular localization correlates with apoptotic cell death. Exp Neurol 166: 213–226, 2000. doi: 10.1006/exnr.2000.7523. [DOI] [PubMed] [Google Scholar]
- 75.Collyer E, Catenaccio A, Lemaitre D, Diaz P, Valenzuela V, Bronfman F, Court FA. Sprouting of axonal collaterals after spinal cord injury is prevented by delayed axonal degeneration. Exp Neurol 261: 451–461, 2014. doi: 10.1016/j.expneurol.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 76.Conrad S, Schluesener HJ, Trautmann K, Joannin N, Meyermann R, Schwab JM. Prolonged lesional expression of RhoA and RhoB following spinal cord injury. J Comp Neurol 487: 166–175, 2005. doi: 10.1002/cne.20561. [DOI] [PubMed] [Google Scholar]
- 77.Cregg JM, DePaul MA, Filous AR, Lang BT, Tran A, Silver J. Functional regeneration beyond the glial scar. Exp Neurol 253: 197–207, 2014. doi: 10.1016/j.expneurol.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cua RC, Lau LW, Keough MB, Midha R, Apte SS, Yong VW. Overcoming neurite-inhibitory chondroitin sulfate proteoglycans in the astrocyte matrix. Glia 61: 972–984, 2013. doi: 10.1002/glia.22489. [DOI] [PubMed] [Google Scholar]
- 79.Darmanis S, Sloan SA, Zhang Y, Enge M, Caneda C, Shuer LM, Hayden Gephart MG, Barres BA, Quake SR. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci USA 112: 7285–7290, 2015. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8: 752–758, 2005. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 81.David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214: 931–933, 1981. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 82.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12: 388–399, 2011. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 83.Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJ. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J Biol 5: 7, 2006. doi: 10.1186/jbiol35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390: 680–683, 1997. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 85.Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci 19: 5810–5822, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem 281: 17789–17800, 2006. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 87.Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol 152: 1197–1206, 2001. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DePaul MA, Lin C-Y, Silver J, Lee Y-S. Peripheral Nerve Transplantation Combined with Acidic Fibroblast Growth Factor and Chondroitinase Induces Regeneration and Improves Urinary Function in Complete Spinal Cord Transected Adult Mice. PLoS One 10: e0139335, 2015. doi: 10.1371/journal.pone.0139335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DePaul MA, Palmer M, Lang BT, Cutrone R, Tran AP, Madalena KM, Bogaerts A, Hamilton JA, Deans RJ, Mays RW, Busch SA, Silver J. Intravenous multipotent adult progenitor cell treatment decreases inflammation leading to functional recovery following spinal cord injury. Sci Rep 5: 16795, 2015. doi: 10.1038/srep16795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 22: 6570–6577, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 212: 337–347, 2008. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Vivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50: 365–372, 2012. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- 93.Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat Neurosci 15: 703–712, 2012. doi: 10.1038/nn.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ. Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J Neurosci 34: 16424–16432, 2014. doi: 10.1523/JNEUROSCI.2927-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Didangelos A, Puglia M, Iberl M, Sanchez-Bellot C, Roschitzki B, Bradbury EJ. High-throughput proteomics reveal alarmins as amplifiers of tissue pathology and inflammation after spinal cord injury. Sci Rep 6: 21607, 2016. doi: 10.1038/srep21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dityatev A, Brückner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol 67: 570–588, 2007. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- 97.Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res 60: 55–69, 2000. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- 98.Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci 14: 7616–7628, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dubreuil CI, Winton MJ, McKerracher L. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol 162: 233–243, 2003. doi: 10.1083/jcb.200301080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dyck SM, Alizadeh A, Santhosh KT, Proulx EH, Wu C-L, Karimi-Abdolrezaee S. Chondroitin Sulfate Proteoglycans Negatively Modulate Spinal Cord Neural Precursor Cells by Signaling Through LAR and RPTPσ and Modulation of the Rho/ROCK Pathway. Stem Cells 33: 2550–2563, 2015. doi: 10.1002/stem.1979. [DOI] [PubMed] [Google Scholar]
- 101.Edgerton VR, Harkema S. Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother 11: 1351–1353, 2011. doi: 10.1586/ern.11.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol 2: 175–186, 2006. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ertürk A, Becker K, Jährling N, Mauch CP, Hojer CD, Egen JG, Hellal F, Bradke F, Sheng M, Dodt HU. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc 7: 1983–1995, 2012. doi: 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- 104.Evaniew N, Belley-Côté EP, Fallah N, Noonan VK, Rivers CS, Dvorak MF. Methylprednisolone for the Treatment of Patients with Acute Spinal Cord Injuries: A Systematic Review and Meta-Analysis. J Neurotrauma 33: 468–481, 2016. doi: 10.1089/neu.2015.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Evans TA, Barkauskas DS, Myers JT, Hare EG, You JQ, Ransohoff RM, Huang AY, Silver J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol 254: 109–120, 2014. doi: 10.1016/j.expneurol.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Bally BP, Chen GG, Théroux J-F, Peng J, Bourque CW, Charron F, Ernst C, Sjöström PJ, Murai KK. Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351: 849–854, 2016. doi: 10.1126/science.aab3103. [DOI] [PubMed] [Google Scholar]
- 107.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24: 2143–2155, 2004. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fehlings MG, Wilson JR, Cho N. Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery 61, Suppl 1: 36–42, 2014. doi: 10.1227/NEU.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 109.Fernández-Klett F, Priller J. The fibrotic scar in neurological disorders. Brain Pathol 24: 404–413, 2014. doi: 10.1111/bpa.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, Muir E, Levine JM, Geller HM, Rogers JH, Faissner A, Fawcett JW. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci 19: 8778–8788, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Filous AR, Miller JH, Coulson-Thomas YM, Horn KP, Alilain WJ, Silver J. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev Neurobiol 70: 826–841, 2010. doi: 10.1002/dneu.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Filous AR, Silver J. “Targeting astrocytes in CNS injury and disease: a translational research approach”. Prog Neurobiol 144: 173–187, 2016. doi: 10.1016/j.pneurobio.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Filous AR, Tran A, Howell CJ, Busch SA, Evans TA, Stallcup WB, Kang SH, Bergles DE, Lee S-I, Levine JM, Silver J. Entrapment via synaptic-like connections between NG2 proteoglycan+ cells and dystrophic axons in the lesion plays a role in regeneration failure after spinal cord injury. J Neurosci 34: 16369–16384, 2014. doi: 10.1523/JNEUROSCI.1309-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fisher D, Xing B, Dill J, Li H, Hoang HH, Zhao Z, Yang X-L, Bachoo R, Cannon S, Longo FM, Sheng M, Silver J, Li S. Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci 31: 14051–14066, 2011. doi: 10.1523/JNEUROSCI.1737-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci 19: 8182–8198, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol 209: 294–301, 2008. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain 129: 3249–3269, 2006. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 118.Fouad K, Pearse DD, Tetzlaff W, Vavrek R. Transplantation and repair: combined cell implantation and chondroitinase delivery prevents deterioration of bladder function in rats with complete spinal cord injury. Spinal Cord 47: 727–732, 2009. doi: 10.1038/sc.2009.10. [DOI] [PubMed] [Google Scholar]
- 119.Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci 25: 1169–1178, 2005. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fournier AE, Takizawa BT, Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 23: 1416–1423, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fox AN, Zinn K. The heparan sulfate proteoglycan syndecan is an in vivo ligand for the Drosophila LAR receptor tyrosine phosphatase. Curr Biol 15: 1701–1711, 2005. doi: 10.1016/j.cub.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 122.Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, López-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia 64: 2079–2092, 2016. doi: 10.1002/glia.23041. [DOI] [PubMed] [Google Scholar]
- 123.Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol 125: 669–680, 1994. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fry EJ, Chagnon MJ, López-Vales R, Tremblay ML, David S. Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia 58: 423–433, 2010. doi: 10.1002/glia.20934. [DOI] [PubMed] [Google Scholar]
- 125.Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave K-A. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485: 517–521, 2012. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gadani SP, Walsh JT, Lukens JR, Kipnis J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron 87: 47–62, 2015. doi: 10.1016/j.neuron.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85: 703–709, 2015. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gallo V, Mangin J-M, Kukley M, Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol 586: 3767–3781, 2008. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galtrey CM, Asher RA, Nothias F, Fawcett JW. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain 130: 926–939, 2007. doi: 10.1093/brain/awl372. [DOI] [PubMed] [Google Scholar]
- 130.Gao K, Wang CR, Jiang F, Wong AYK, Su N, Jiang JH, Chai RC, Vatcher G, Teng J, Chen J, Jiang YW, Yu ACH. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia 61: 2063–2077, 2013. doi: 10.1002/glia.22577. [DOI] [PubMed] [Google Scholar]
- 131.Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, Spencer T, Mellado W, Kandel ER, Filbin MT. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 44: 609–621, 2004. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 132.Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediators Inflamm 2016: 9476020, 2016. doi: 10.1155/2016/9476020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garcia-Abreu J, Silva LC, Tovar FF, Onofr- GR, Cavalcante LA, Moura Neto V. Compartmental distribution of sulfated glycosaminoglycans in lateral and medial midbrain astroglial cultures. Glia 17: 339–344, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 134.García-Alías G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci 12: 1145–1151, 2009. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- 135.Gardner RT, Habecker BA. Infarct-derived chondroitin sulfate proteoglycans prevent sympathetic reinnervation after cardiac ischemia-reperfusion injury. J Neurosci 33: 7175–7183, 2013. doi: 10.1523/JNEUROSCI.5866-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci 29: 3956–3968, 2009. doi: 10.1523/JNEUROSCI.3992-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Geremia NM, Bao F, Rosenzweig TE, Hryciw T, Weaver L, Dekaban GA, Brown A. CD11d Antibody Treatment Improves Recovery in Spinal Cord-Injured Mice. J Neurotrauma 29: 539–550, 2012. doi: 10.1089/neu.2011.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gerhart KA, Koziol-McLain J, Lowenstein SR, Whiteneck GG. Quality of life following spinal cord injury: knowledge and attitudes of emergency care providers. Ann Emerg Med 23: 807–812, 1994. doi: 10.1016/S0196-0644(94)70318-3. [DOI] [PubMed] [Google Scholar]
- 139.Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT, Mueggler T, Baltes C, Rudin M, Weber B, Schwab ME. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci 13: 97–104, 2010. doi: 10.1038/nn.2448. [DOI] [PubMed] [Google Scholar]
- 140.Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zörner B, Schneider R, Baltes C, Rudin M, Weber B, Schwab ME. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci 29: 12210–12219, 2009. doi: 10.1523/JNEUROSCI.1828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gogolla N, Caroni P, Lüthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science 325: 1258–1261, 2009. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- 142.Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science 333: 238–242, 2011. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 143.Greenhalgh AD, David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci 34: 6316–6322, 2014. doi: 10.1523/JNEUROSCI.4912-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grimpe B, Silver J. A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond lesions in the spinal cord. J Neurosci 24: 1393–1397, 2004. doi: 10.1523/JNEUROSCI.4986-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci 24: 4043–4051, 2004. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol 168: 273–282, 2001. doi: 10.1006/exnr.2001.7628. [DOI] [PubMed] [Google Scholar]
- 147.Grossman SD, Wolfe BB, Yasuda RP, Wrathall JR. Changes in NMDA receptor subunit expression in response to contusive spinal cord injury. J Neurochem 75: 174–184, 2000. doi: 10.1046/j.1471-4159.2000.0750174.x. [DOI] [PubMed] [Google Scholar]
- 148.Guimarães A, Zaremba S, Hockfield S. Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J Neurosci 10: 3014–3024, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guimarães-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell 20: 345–359.e5, 2017. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guirado R, Perez-Rando M, Sanchez-Matarredona D, Castrén E, Nacher J. Chronic fluoxetine treatment alters the structure, connectivity and plasticity of cortical interneurons. Int J Neuropsychopharmacol 17: 1635–1646, 2014. doi: 10.1017/S1461145714000406. [DOI] [PubMed] [Google Scholar]
- 151.Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience 161: 895–903, 2009. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haas C, Fischer I. Human astrocytes derived from glial restricted progenitors support regeneration of the injured spinal cord. J Neurotrauma 30: 1035–1052, 2013. doi: 10.1089/neu.2013.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Haas C, Neuhuber B, Yamagami T, Rao M, Fischer I. Phenotypic analysis of astrocytes derived from glial restricted precursors and their impact on axon regeneration. Exp Neurol 233: 717–732, 2012. doi: 10.1016/j.expneurol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hackett AR, Lee D-H, Dawood A, Rodriguez M, Funk L, Tsoulfas P, Lee JK. STAT3 and SOCS3 regulate NG2 cell proliferation and differentiation after contusive spinal cord injury. Neurobiol Dis 89: 10–22, 2016. doi: 10.1016/j.nbd.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Haghighi SS, Agrawal SK, Surdell D Jr, Plambeck R, Agrawal S, Johnson GC, Walker A. Effects of methylprednisolone and MK-801 on functional recovery after experimental chronic spinal cord injury. Spinal Cord 38: 733–740, 2000. doi: 10.1038/sj.sc.3101074. [DOI] [PubMed] [Google Scholar]
- 156.Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci 26: 4308–4317, 2006. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y, Okada S. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med 23: 818–828, 2017. doi: 10.1038/nm.4354. [DOI] [PubMed] [Google Scholar]
- 158.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil 93: 1508–1517, 2012. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 159.Harlan JM. Consequences of leukocyte-vessel wall interactions in inflammatory and immune reactions. Semin Thromb Hemost 13: 434–444, 1987. doi: 10.1055/s-2007-1003520. [DOI] [PubMed] [Google Scholar]
- 160.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord 41: 369–378, 2003. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 161.Hawrylycz MJ, Lein ES, Guillozet-Bongaarts AL, Shen EH, Ng L, Miller JA, van de Lagemaat LN, Smith KA, Ebbert A, Riley ZL, Abajian C, Beckmann CF, Bernard A, Bertagnolli D, Boe AF, Cartagena PM, Chakravarty MM, Chapin M, Chong J, Dalley RA, David Daly B, Dang C, Datta S, Dee N, Dolbeare TA, Faber V, Feng D, Fowler DR, Goldy J, Gregor BW, Haradon Z, Haynor DR, Hohmann JG, Horvath S, Howard RE, Jeromin A, Jochim JM, Kinnunen M, Lau C, Lazarz ET, Lee C, Lemon TA, Li L, Li Y, Morris JA, Overly CC, Parker PD, Parry SE, Reding M, Royall JJ, Schulkin J, Sequeira PA, Slaughterbeck CR, Smith SC, Sodt AJ, Sunkin SM, Swanson BE, Vawter MP, Williams D, Wohnoutka P, Zielke HR, Geschwind DH, Hof PR, Smith SM, Koch C, Grant SGN, Jones AR. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature 489: 391–399, 2012. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hawthorne AL, Hu H, Kundu B, Steinmetz MP, Wylie CJ, Deneris ES, Silver J. The unusual response of serotonergic neurons after CNS injury: lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. J Neurosci 31: 5605–5616, 2011. doi: 10.1523/JNEUROSCI.6663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hayashi N, Miyata S, Yamada M, Kamei K, Oohira A. Neuronal expression of the chondroitin sulfate proteoglycans receptor-type protein-tyrosine phosphatase beta and phosphacan. Neuroscience 131: 331–348, 2005. doi: 10.1016/j.neuroscience.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 164.Härtig W, Brauer K, Bigl V, Brückner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res 635: 307–311, 1994. doi: 10.1016/0006-8993(94)91452-4. [DOI] [PubMed] [Google Scholar]
- 165.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28: 7231–7243, 2008. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hesp ZC, Goldstein EZ, Miranda CJ, Kaspar BK, McTigue DM. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J Neurosci 35: 1274–1290, 2015. doi: 10.1523/JNEUROSCI.2568-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hines DJ, Hines RM, Mulligan SJ, Macvicar BA. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia 57: 1610–1618, 2009. doi: 10.1002/glia.20874. [DOI] [PubMed] [Google Scholar]
- 168.Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. Identification of Positionally Distinct Astrocyte Subtypes whose Identities Are Specified by a Homeodomain Code. Cell 133: 510–522, 2008. doi: 10.1016/j.cell.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hofmeyer K, Treisman JE. The receptor protein tyrosine phosphatase LAR promotes R7 photoreceptor axon targeting by a phosphatase-independent signaling mechanism. Proc Natl Acad Sci USA 106: 19399–19404, 2009. doi: 10.1073/pnas.0903961106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol 498: 525–538, 2006. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci 28: 9330–9341, 2008. doi: 10.1523/JNEUROSCI.2488-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci 20: 2218–2228, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci 26: 7405–7415, 2006. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Höke A, Canning DR, Malemud CJ, Silver J. Regional differences in reactive gliosis induced by substrate-bound beta-amyloid. Exp Neurol 130: 56–66, 1994. doi: 10.1006/exnr.1994.1185. [DOI] [PubMed] [Google Scholar]
- 175.Hu X, Yuan Y, Wang D, Su Z. Heterogeneous astrocytes: active players in CNS. Brain Res Bull 125: 1–18, 2016. doi: 10.1016/j.brainresbull.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 176.Huang W, Matte A, Suzuki S, Sugiura N, Miyazono H, Cygler M. Crystallization and preliminary X-ray analysis of chondroitin sulfate ABC lyases I and II from Proteus vulgaris. Acta Crystallogr D Biol Crystallogr 56: 904–906, 2000. doi: 10.1107/S0907444900005102. [DOI] [PubMed] [Google Scholar]
- 177.Hurtado A, Cregg JM, Wang HB, Wendell DF, Oudega M, Gilbert RJ, McDonald JW. Robust CNS regeneration after complete spinal cord transection using aligned poly-l-lactic acid microfibers. Biomaterials 32: 6068–6079, 2011. doi: 10.1016/j.biomaterials.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Iijima N, Oohira A, Mori T, Kitabatake K, Kohsaka S. Core protein of chondroitin sulfate proteoglycan promotes neurite outgrowth from cultured neocortical neurons. J Neurochem 56: 706–708, 1991. doi: 10.1111/j.1471-4159.1991.tb08207.x. [DOI] [PubMed] [Google Scholar]
- 179.Iseda T, Nishio T, Kawaguchi S, Yamanoto M, Kawasaki T, Wakisaka S. Spontaneous regeneration of the corticospinal tract after transection in young rats: a key role of reactive astrocytes in making favorable and unfavorable conditions for regeneration. Neuroscience 126: 365–374, 2004. doi: 10.1016/j.neuroscience.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 180.Iseda T, Okuda T, Kane-Goldsmith N, Mathew M, Ahmed S, Chang Y-W, Young W, Grumet M. Single, high-dose intraspinal injection of chondroitinase reduces glycosaminoglycans in injured spinal cord and promotes corticospinal axonal regrowth after hemisection but not contusion. J Neurotrauma 25: 334–349, 2008. doi: 10.1089/neu.2007.0289. [DOI] [PubMed] [Google Scholar]
- 181.Jahan N, Hannila SS. Transforming growth factor β-induced expression of chondroitin sulfate proteoglycans is mediated through non-Smad signaling pathways. Exp Neurol 263: 372–384, 2015. doi: 10.1016/j.expneurol.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 182.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325: 253–257, 1987. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 183.Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med 17: 568–573, 2011. doi: 10.2119/molmed.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jin Y, Dougherty SE, Wood K, Sun L, Cudmore RH, Abdalla A, Kannan G, Pletnikov M, Hashemi P, Linden DJ. Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury. Neuron 91: 748–762, 2016. doi: 10.1016/j.neuron.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jin Y, Ketschek A, Jiang Z, Smith G, Fischer I. Chondroitinase activity can be transduced by a lentiviral vector in vitro and in vivo. J Neurosci Methods 199: 208–213, 2011. doi: 10.1016/j.jneumeth.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.John Lin C-C, Yu K, Hatcher A, Huang T-W, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, Mohila CA, Ahmed N, Patel AJ, Arenkiel BR, Noebels JL, Creighton CJ, Deneen B. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20: 396–405, 2017. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol 182: 399–411, 2003. doi: 10.1016/S0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 188.Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci 22: 2792–2803, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Joosten EAJ, Veldhuis WB, Hamers FPT. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J Neurosci Res 77: 127–142, 2004. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- 190.Kaczmarek L, Lapinska-Dzwonek J, Szymczak S. Matrix metalloproteinases in the adult brain physiology: a link between c-Fos, AP-1 and remodeling of neuronal connections? EMBO J 21: 6643–6648, 2002. doi: 10.1093/emboj/cdf676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M. Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc Natl Acad Sci USA 110: 11612–11617, 2013. doi: 10.1073/pnas.1300378110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kang W, Balordi F, Su N, Chen L, Fishell G, Hébert JM. Astrocyte activation is suppressed in both normal and injured brain by FGF signaling. Proc Natl Acad Sci USA 111: E2987–E2995, 2014. doi: 10.1073/pnas.1320401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci 30: 1657–1676, 2010. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Karus M, Ulc A, Ehrlich M, Czopka T, Hennen E, Fischer J, Mizhorova M, Qamar N, Brüstle O, Faissner A. Regulation of oligodendrocyte precursor maintenance by chondroitin sulphate glycosaminoglycans. Glia 64: 270–286, 2016. doi: 10.1002/glia.22928. [DOI] [PubMed] [Google Scholar]
- 195.Kataoka A, Tozaki-Saitoh H, Koga Y, Tsuda M, Inoue K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J Neurochem 108: 115–125, 2009. doi: 10.1111/j.1471-4159.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- 196.Katori S, Noguchi-Katori Y, Itohara S, Iwasato T. Spinal RacGAP α-Chimaerin Is Required to Establish the Midline Barrier for Proper Corticospinal Axon Guidance. J Neurosci 37: 7682–7699, 2017. doi: 10.1523/JNEUROSCI.3123-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kawabata H, Setoguchi T, Yone K, Souda M, Yoshida H, Kawahara K, Maruyama I, Komiya S. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine 35: 1109–1115, 2010. doi: 10.1097/BRS.0b013e3181bd14b6. [DOI] [PubMed] [Google Scholar]
- 198.Keough MB, Rogers JA, Zhang P, Jensen SK, Stephenson EL, Chen T, Hurlbert MG, Lau LW, Rawji KS, Plemel JR, Koch M, Ling C-C, Yong VW. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat Commun 7: 11312, 2016. doi: 10.1038/ncomms11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Khurana SR, Garg DS. Spasticity and the use of intrathecal baclofen in patients with spinal cord injury. Phys Med Rehabil Clin N Am 25: 655–669, 2014. doi: 10.1016/j.pmr.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 200.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol 258: 5–16, 2014. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29: 13435–13444, 2009. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol 494: 578–594, 2006. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim JE, Li S, GrandPré T, Qiu D, Strittmatter SM. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron 38: 187–199, 2003. doi: 10.1016/S0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 204.Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics 7: 338–353, 2010. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kimura-Kuroda J, Teng X, Komuta Y, Yoshioka N, Sango K, Kawamura K, Raisman G, Kawano H. An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol Cell Neurosci 43: 177–187, 2010. doi: 10.1016/j.mcn.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 206.Klüppel M. Efficient secretion of biologically active chondroitinase ABC from mammalian cells in the absence of an N-terminal signal peptide. Mol Cell Biochem 351: 1–11, 2011. doi: 10.1007/s11010-010-0705-1. [DOI] [PubMed] [Google Scholar]
- 207.Kolkman MJM, Streijger F, Linkels M, Bloemen M, Heeren DJ, Hendriks WJAJ, Van der Zee CEEM. Mice lacking leukocyte common antigen-related (LAR) protein tyrosine phosphatase domains demonstrate spatial learning impairment in the two-trial water maze and hyperactivity in multiple behavioural tests. Behav Brain Res 154: 171–182, 2004. doi: 10.1016/j.bbr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 208.Komitova M, Serwanski DR, Lu QR, Nishiyama A. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia 59: 800–809, 2011. doi: 10.1002/glia.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279–289, 2008. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Koprivica V, Cho K-S, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310: 106–110, 2005. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- 211.Koyanagi I, Tator CH. Effect of a single huge dose of methylprednisolone on blood flow, evoked potentials, and histology after acute spinal cord injury in the rat. Neurol Res 19: 289–299, 1997. doi: 10.1080/01616412.1997.11740815. [DOI] [PubMed] [Google Scholar]
- 212.Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83: 1098–1116, 2014. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 213.Krull CE, Oland LA, Faissner A, Schachner M, Tolbert LP. In vitro analyses of neurite outgrowth indicate a potential role for tenascin-like molecules in the development of insect olfactory glomeruli. J Neurobiol 25: 989–1004, 1994. doi: 10.1002/neu.480250808. [DOI] [PubMed] [Google Scholar]
- 214.Kuboyama T, Luo X, Park K, Blackmore MG, Tojima T, Tohda C, Bixby JL, Lemmon VP, Kamiguchi H. Paxillin phosphorylation counteracts proteoglycan-mediated inhibition of axon regeneration. Exp Neurol 248: 157–169, 2013. doi: 10.1016/j.expneurol.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kucharova K, Stallcup WB. NG2-proteoglycan-dependent contributions of oligodendrocyte progenitors and myeloid cells to myelin damage and repair. J Neuroinflammation 12: 161, 2015. doi: 10.1186/s12974-015-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kurimoto T, Yin Y, Omura K, Gilbert H-Y, Kim D, Cen L-P, Moko L, Kügler S, Benowitz LI. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci 30: 15654–15663, 2010. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kwon MJ, Yoon HJ, Kim BG. Regeneration-associated macrophages: a novel approach to boost intrinsic regenerative capacity for axon regeneration. Neural Regen Res 11: 1368–1371, 2016. doi: 10.4103/1673-5374.191194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci 27: 14494–14501, 2007. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lafortune L, Nalbantoglu J, Antel JP. Expression of tumor necrosis factor alpha (TNF alpha) and interleukin 6 (IL-6) mRNA in adult human astrocytes: comparison with adult microglia and fetal astrocytes. J Neuropathol Exp Neurol 55: 515–521, 1996. doi: 10.1097/00005072-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 220.Lander AD, Fujii DK, Gospodarowicz D, Reichardt LF. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol 94: 574–585, 1982. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng Y-L, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPσ promotes recovery after spinal cord injury. Nature 518: 404–408, 2015. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J Neurosci 23: 11127–11135, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lau LW, Keough MB, Haylock-Jacobs S, Cua R, Döring A, Sloka S, Stirling DP, Rivest S, Yong VW. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann Neurol 72: 419–432, 2012. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- 224.Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci USA 107: 3340–3345, 2010. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee JY, Na WH, Choi HY, Lee KH, Ju BG, Yune TY. Jmjd3 mediates blood-spinal cord barrier disruption after spinal cord injury by regulating MMP-3 and MMP-9 expressions. Neurobiol Dis 95: 66–81, 2016. doi: 10.1016/j.nbd.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 226.Lee SH, Kim Y, Rhew D, Kuk M, Kim M, Kim WH, Kweon O-K. Effect of the combination of mesenchymal stromal cells and chondroitinase ABC on chronic spinal cord injury. Cytotherapy 17: 1374–1383, 2015. doi: 10.1016/j.jcyt.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 227.Lemarchant S, Pruvost M, Hébert M, Gauberti M, Hommet Y, Briens A, Maubert E, Gueye Y, Féron F, Petite D, Mersel M, do Rego J-C, Vaudry H, Koistinaho J, Ali C, Agin V, Emery E, Vivien D. tPA promotes ADAMTS-4-induced CSPG degradation, thereby enhancing neuroplasticity following spinal cord injury. Neurobiol Dis 66: 28–42, 2014. doi: 10.1016/j.nbd.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 228.Lemke AK, Sandy JD, Voigt H, Dreier R, Lee JH, Grodzinsky AJ, Mentlein R, Fay J, Schünke M, Kurz B. Interleukin-1alpha treatment of meniscal explants stimulates the production and release of aggrecanase-generated, GAG-substituted aggrecan products and also the release of pre-formed, aggrecanase-generated G1 and m-calpain-generated G1-G2. Cell Tissue Res 340: 179–188, 2010. doi: 10.1007/s00441-010-0941-4. [DOI] [PubMed] [Google Scholar]
- 229.Lemke M, Demediuk P, McIntosh TK, Vink R, Faden AI. Alterations in tissue Mg2+, Na+ and spinal cord edema following impact trauma in rats. Biochem Biophys Res Commun 147: 1170–1175, 1987. doi: 10.1016/S0006-291X(87)80192-4. [DOI] [PubMed] [Google Scholar]
- 230.Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp Neurol 160: 51–65, 1999. doi: 10.1006/exnr.1999.7184. [DOI] [PubMed] [Google Scholar]
- 231.Levine J, Kwon E, Paez P, Yan W, Czerwieniec G, Loo JA, Sofroniew MV, Wanner IB. Traumatically injured astrocytes release a proteomic signature modulated by STAT3-dependent cell survival. Glia 64: 668–694, 2016. doi: 10.1002/glia.22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Levine J. The reactions and role of NG2 glia in spinal cord injury. Brain Res 1638, Pt B: 199–208, 2016. doi: 10.1016/j.brainres.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci 7: 2721–2731, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Li Y, Field PM, Raisman G. Death of oligodendrocytes and microglial phagocytosis of myelin precede immigration of Schwann cells into the spinal cord. J Neurocytol 28: 417–427, 1999. doi: 10.1023/A:1007026001189. [DOI] [PubMed] [Google Scholar]
- 235.Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, Sanelli L, Fenrich KK, Di Narzo AF, Dracheva S, Winship IR, Fouad K, Bennett DJ. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 23: 733–741, 2017. doi: 10.1038/nm.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487, 2017. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 17: 5395–5406, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu Y, Wang X, Li W, Zhang Q, Li Y, Zhang Z, Zhu J, Chen B, Williams PR, Zhang Y, Yu B, Gu X, He Z. A Sensitized IGF1 Treatment Restores Corticospinal Axon-Dependent Functions. Neuron 95: 817–833.e4, 2017. doi: 10.1016/j.neuron.2017.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron 35: 605–623, 2002. doi: 10.1016/S0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 240.Lu P, Jones LL, Tuszynski MH. Axon regeneration through scars and into sites of chronic spinal cord injury. Exp Neurol 203: 8–21, 2007. doi: 10.1016/j.expneurol.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 241.Lu P, Tuszynski MH. Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol 209: 313–320, 2008. doi: 10.1016/j.expneurol.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci 24: 6402–6409, 2004. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Luchetti S, Beck KD, Galvan MD, Silva R, Cummings BJ, Anderson AJ. Comparison of immunopathology and locomotor recovery in C57BL/6, BUB/BnJ, and NOD-SCID mice after contusion spinal cord injury. J Neurotrauma 27: 411–421, 2010. doi: 10.1089/neu.2009.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lyons DA, Talbot WS. Glial cell development and function in zebrafish. Cold Spring Harb Perspect Biol 7: a020586, 2015. doi: 10.1101/cshperspect.a020586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lytle JM, Chittajallu R, Wrathall JR, Gallo V. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia 57: 270–285, 2009. doi: 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ma M, Wei P, Wei T, Ransohoff RM, Jakeman LB. Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response. J Comp Neurol 474: 469–486, 2004. doi: 10.1002/cne.20149. [DOI] [PubMed] [Google Scholar]
- 247.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 285: 895–898, 1999. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 248.Maolood N, Hardin-Pouzet H, Grange-Messent V. Matrix metalloproteinases MMP2 and MMP9 are upregulated by noradrenaline in the mouse neuroendocrine hypothalamus. Eur J Neurosci 27: 1143–1152, 2008. doi: 10.1111/j.1460-9568.2008.06099.x. [DOI] [PubMed] [Google Scholar]
- 249.Marcon RM, de Barros Filho TEP, Oliveira RP, Cristante AF, Taricco MA, Colares G, Barbarini AF, Teixeira WGJ, de Souza FI. Experimental study on the action of methylprednisolone on Wistar rats before spinal cord injury. Acta Ortop Bras 18: 26–30, 2010. doi: 10.1590/S1413-78522010000100005. [DOI] [Google Scholar]
- 250.Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NG, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol 209: 426–445, 2008. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J, Silver J, Onifer SM. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci 26: 4406–4414, 2006. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Mataga N, Mizuguchi Y, Hensch TK. Experience-dependent pruning of dendritic spines in visual cortex by tissue plasminogen activator. Neuron 44: 1031–1041, 2004. doi: 10.1016/j.neuron.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 253.Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci 22: 7536–7547, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, Woo T-UW. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol Psychiatry 74: 427–435, 2013. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Mautes AE, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther 80: 673–687, 2000. [PubMed] [Google Scholar]
- 256.Mckeon RJ, Höke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol 136: 32–43, 1995. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- 257.Mckeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci 11: 3398–3411, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.McLean J, Batt J, Doering LC, Rotin D, Bain JR. Enhanced rate of nerve regeneration and directional errors after sciatic nerve injury in receptor protein tyrosine phosphatase sigma knock-out mice. J Neurosci 22: 5481–5491, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol 65: 406–420, 2006. doi: 10.1097/01.jnen.0000218447.32320.52. [DOI] [PubMed] [Google Scholar]
- 260.McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci 21: 3392–3400, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Meletis K, Barnabé-Heider F, Carlén M, Evergren E, Tomilin N, Shupliakov O, Frisén J. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 6: e182, 2008. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Menet V, Prieto M, Privat A, Ribotta MG. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci USA 100: 8999–9004, 2003. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MGJ Jr, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron 21: 681–693, 1998. doi: 10.1016/S0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mignardot J-B, Le Goff CG, van den Brand R, Capogrosso M, Fumeaux N, Vallery H, Anil S, Lanini J, Fodor I, Eberle G, Ijspeert A, Schurch B, Curt A, Carda S, Bloch J, von Zitzewitz J, Courtine G. A multidirectional gravity-assist algorithm that enhances locomotor control in patients with stroke or spinal cord injury. Sci Transl Med 9: 3621, 2017. doi: 10.1126/scitranslmed.aah3621. [DOI] [PubMed] [Google Scholar]
- 265.Mihai G, Nout YS, Tovar CA, Miller BA, Schmalbrock P, Bresnahan JC, Beattie MS. Longitudinal comparison of two severities of unilateral cervical spinal cord injury using magnetic resonance imaging in rats. J Neurotrauma 25: 1–18, 2008. doi: 10.1089/neu.2007.0338. [DOI] [PubMed] [Google Scholar]
- 266.Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol 127: 1703–1715, 1994. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mironova YA, Giger RJ. Where no synapses go: gatekeepers of circuit remodeling and synaptic strength. Trends Neurosci 36: 363–373, 2013. doi: 10.1016/j.tins.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 22: 319–330, 2003. doi: 10.1016/S1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 269.Monsul NT, Geisendorfer AR, Han PJ, Banik R, Pease ME, Skolasky RLJ Jr, Hoffman PN. Intraocular injection of dibutyryl cyclic AMP promotes axon regeneration in rat optic nerve. Exp Neurol 186: 124–133, 2004. doi: 10.1016/S0014-4886(03)00311-X. [DOI] [PubMed] [Google Scholar]
- 270.Moon LDF, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci 4: 465–466, 2001. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- 271.Moransard M, Dann A, Staszewski O, Fontana A, Prinz M, Suter T. NG2 expressed by macrophages and oligodendrocyte precursor cells is dispensable in experimental autoimmune encephalomyelitis. Brain 134: 1315–1330, 2011. doi: 10.1093/brain/awr070. [DOI] [PubMed] [Google Scholar]
- 272.Moreno-Manzano V, Rodríguez-Jiménez FJ, García-Roselló M, Laínez S, Erceg S, Calvo MT, Ronaghi M, Lloret M, Planells-Cases R, Sánchez-Puelles JM, Stojkovic M. Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells 27: 733–743, 2009. doi: 10.1002/stem.24. [DOI] [PubMed] [Google Scholar]
- 273.Mörgelin M, Paulsson M, Malmström A, Heinegård D. Shared and distinct structural features of interstitial proteoglycans from different bovine tissues revealed by electron microscopy. J Biol Chem 264: 12080–12090, 1989. [PubMed] [Google Scholar]
- 274.Morris NP, Henderson Z. Perineuronal nets ensheath fast spiking, parvalbumin-immunoreactive neurons in the medial septum/diagonal band complex. Eur J Neurosci 12: 828–838, 2000. doi: 10.1046/j.1460-9568.2000.00970.x. [DOI] [PubMed] [Google Scholar]
- 275.Mueller CA, Schluesener HJ, Conrad S, Pietsch T, Schwab JM. Spinal cord injury-induced expression of the immune-regulatory chemokine interleukin-16 caused by activated microglia/macrophages and CD8+ cells. J Neurosurg Spine 4: 233–240, 2006. doi: 10.3171/spi.2006.4.3.233. [DOI] [PubMed] [Google Scholar]
- 276.Muir EM, Fyfe I, Gardiner S, Li L, Warren P, Fawcett JW, Keynes RJ, Rogers JH. Modification of N-glycosylation sites allows secretion of bacterial chondroitinase ABC from mammalian cells. J Biotechnol 145: 103–110, 2010. doi: 10.1016/j.jbiotec.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Muradov JM, Ewan EE, Hagg T. Dorsal column sensory axons degenerate due to impaired microvascular perfusion after spinal cord injury in rats. Exp Neurol 249: 59–73, 2013. doi: 10.1016/j.expneurol.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature 438: 360–363, 2005. doi: 10.1038/nature04139. [DOI] [PubMed] [Google Scholar]
- 279.Nakanishi K, Aono S, Hirano K, Kuroda Y, Ida M, Tokita Y, Matsui F, Oohira A. Identification of neurite outgrowth-promoting domains of neuroglycan C, a brain-specific chondroitin sulfate proteoglycan, and involvement of phosphatidylinositol 3-kinase and protein kinase C signaling pathways in neuritogenesis. J Biol Chem 281: 24970–24978, 2006. doi: 10.1074/jbc.M601498200. [DOI] [PubMed] [Google Scholar]
- 279a.National Spinal Cord Injury Statistical Center Facts and Figures at a Glance. Birmingham, AL: Univ. of Alabama at Birmingham, 2017. [Google Scholar]
- 280.Nazari-Robati M, Khajeh K, Aminian M, Fathi-Roudsari M, Golestani A. Co-solvent mediated thermal stabilization of chondroitinase ABC I form Proteus vulgaris. Int J Biol Macromol 50: 487–492, 2012. doi: 10.1016/j.ijbiomac.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 281.Neirinckx V, Cantinieaux D, Coste C, Rogister B, Franzen R, Wislet-Gendebien S. Concise review: spinal cord injuries: how could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells 32: 829–843, 2014. doi: 10.1002/stem.1579. [DOI] [PubMed] [Google Scholar]
- 282.Nigro J, Wang A, Mukhopadhyay D, Lauer M, Midura RJ, Sackstein R, Hascall VC. Regulation of heparan sulfate and chondroitin sulfate glycosaminoglycan biosynthesis by 4-fluoro-glucosamine in murine airway smooth muscle cells. J Biol Chem 284: 16832–16839, 2009. doi: 10.1074/jbc.M109.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308: 1314–1318, 2005. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 284.Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol 31: 437–455, 2002. doi: 10.1023/A:1025783412651. [DOI] [PubMed] [Google Scholar]
- 285.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci 22: 7526–7535, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Noble M, Fok-Seang J, Cohen J. Glia are a unique substrate for the in vitro growth of central nervous system neurons. J Neurosci 4: 1892–1903, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96, Pt A: 29–41, 2015. doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol 93: 875–881, 2013. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Oberheim NA, Takano T, Han X, He W, Lin JHC, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci 29: 3276–3287, 2009. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ohira K, Takeuchi R, Iwanaga T, Miyakawa T. Chronic fluoxetine treatment reduces parvalbumin expression and perineuronal nets in gamma-aminobutyric acidergic interneurons of the frontal cortex in adult mice. Mol Brain 6: 43, 2013. doi: 10.1186/1756-6606-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12: 829–834, 2006. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 292.Olmos G, Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm 2014: 861231, 2014. doi: 10.1155/2014/861231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Oohira A, Matsui F, Katoh-Semba R. Inhibitory effects of brain chondroitin sulfate proteoglycans on neurite outgrowth from PC12D cells. J Neurosci 11: 822–827, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Oray S, Majewska A, Sur M. Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron 44: 1021–1030, 2004. doi: 10.1016/j.neuron.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 295.Orlando C, Raineteau O. Integrity of cortical perineuronal nets influences corticospinal tract plasticity after spinal cord injury. Brain Struct Funct 220: 1077–1091, 2015. doi: 10.1007/s00429-013-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Orlando C, Ster J, Gerber U, Fawcett JW, Raineteau O. Perisynaptic chondroitin sulfate proteoglycans restrict structural plasticity in an integrin-dependent manner. J Neurosci 32: 18009–18017, 2012. doi: 10.1523/JNEUROSCI.2406-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Panenka W, Jijon H, Herx LM, Armstrong JN, Feighan D, Wei T, Yong VW, Ransohoff RM, MacVicar BA. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci 21: 7135–7142, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pantazopoulos H, Berretta S. In Sickness and in Health: Perineuronal Nets and Synaptic Plasticity in Psychiatric Disorders. Neural Plast 2016: 9847696, 2016. doi: 10.1155/2016/9847696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pantazopoulos H, Markota M, Jaquet F, Ghosh D, Wallin A, Santos A, Caterson B, Berretta S. Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: a postmortem study on the amygdala. Transl Psychiatry 5: e496, 2015. doi: 10.1038/tp.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Pantazopoulos H, Woo T-UW, Lim MP, Lange N, Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 67: 155–166, 2010. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Papatheodorou A, Stein A, Bank M, Sison CP, Gibbs K, Davies P, Bloom O. High-Mobility Group Box 1 (HMGB1) Is Elevated Systemically in Persons with Acute or Chronic Traumatic Spinal Cord Injury. J Neurotrauma 34: 746–754, 2017. doi: 10.1089/neu.2016.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Park KK, Luo X, Mooney SJ, Yungher BJ, Belin S, Wang C, Holmes MM, He Z. Retinal ganglion cell survival and axon regeneration after optic nerve injury in naked mole-rats. J Comp Neurol 525: 380–388, 2017. doi: 10.1002/cne.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Pasterkamp RJ, Giger RJ, Ruitenberg M-J, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J. Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci 13: 143–166, 1999. doi: 10.1006/mcne.1999.0738. [DOI] [PubMed] [Google Scholar]
- 304.Paveliev M, Fenrich KK, Kislin M, Kuja-Panula J, Kulesskiy E, Varjosalo M, Kajander T, Mugantseva E, Ahonen-Bishopp A, Khiroug L, Kulesskaya N, Rougon G, Rauvala H. HB-GAM (pleiotrophin) reverses inhibition of neural regeneration by the CNS extracellular matrix. Sci Rep 6: 33916, 2016. doi: 10.1038/srep33916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pearson BL, Corley MJ, Vasconcellos A, Blanchard DC, Blanchard RJ. Heparan sulfate deficiency in autistic postmortem brain tissue from the subventricular zone of the lateral ventricles. Behav Brain Res 243: 138–145, 2013. doi: 10.1016/j.bbr.2012.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pedrazzi M, Patrone M, Passalacqua M, Ranzato E, Colamassaro D, Sparatore B, Pontremoli S, Melloni E. Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J Immunol 179: 8525–8532, 2007. doi: 10.4049/jimmunol.179.12.8525. [DOI] [PubMed] [Google Scholar]
- 307.Pendleton JC, Shamblott MJ, Gary DS, Belegu V, Hurtado A, Malone ML, McDonald JW. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp Neurol 247: 113–121, 2013. doi: 10.1016/j.expneurol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 308.Pizzorusso T. Neuroscience. Erasing fear memories. Science 325: 1214–1215, 2009. doi: 10.1126/science.1179697. [DOI] [PubMed] [Google Scholar]
- 309.Pluchino S, Zanotti L, Deleidi M, Martino G. Neural stem cells and their use as therapeutic tool in neurological disorders. Brain Res Brain Res Rev 48: 211–219, 2005. doi: 10.1016/j.brainresrev.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 310.Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol 158: 351–365, 1999. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 311.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci 9: 481–493, 2008. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 312.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol 377: 443–464, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 313.Prabhakar V, Raman R, Capila I, Bosques CJ, Pojasek K, Sasisekharan R. Biochemical characterization of the chondroitinase ABC I active site. Biochem J 390: 395–405, 2005. doi: 10.1042/BJ20050532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34: 895–903, 2002. doi: 10.1016/S0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 315.Qiu J, Cai D, Filbin MT. A role for cAMP in regeneration during development and after injury. Prog Brain Res 137: 381–387, 2002. doi: 10.1016/S0079-6123(02)37029-8. [DOI] [PubMed] [Google Scholar]
- 316.Ramer LM, Borisoff JF, Ramer MS. Rho-kinase inhibition enhances axonal plasticity and attenuates cold hyperalgesia after dorsal rhizotomy. J Neurosci 24: 10796–10805, 2004. doi: 10.1523/JNEUROSCI.3337-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ranjan M, Hudson LD. Regulation of tyrosine phosphorylation and protein tyrosine phosphatases during oligodendrocyte differentiation. Mol Cell Neurosci 7: 404–418, 1996. doi: 10.1006/mcne.1996.0029. [DOI] [PubMed] [Google Scholar]
- 318.Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19: 987–991, 2016. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 319.Rebhun J, Madorsky JG, Glovsky MM. Proteins of the complement system and acute phase reactants in sera of patients with spinal cord injury. Ann Allergy 66: 335–338, 1991. [PubMed] [Google Scholar]
- 320.Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci 27: 6607–6619, 2007. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Ren Y, Ao Y, O’Shea TM, Burda JE, Bernstein AM, Brumm AJ, Muthusamy N, Ghashghaei HT, Carmichael ST, Cheng L, Sofroniew MV. Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury. Sci Rep 7: 41122, 2017. doi: 10.1038/srep41122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Rhodes KE, Raivich G, Fawcett JW. The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience 140: 87–100, 2006. doi: 10.1016/j.neuroscience.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 323.Rice T, Larsen J, Rivest S, Yong VW. Characterization of the early neuroinflammation after spinal cord injury in mice. J Neuropathol Exp Neurol 66: 184–195, 2007. doi: 10.1097/01.jnen.0000248552.07338.7f. [DOI] [PubMed] [Google Scholar]
- 324.Robel S, Berninger B, Götz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci 12: 88–104, 2011. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 325.Rodriguez JP, Coulter M, Miotke J, Meyer RL, Takemaru K, Levine JM. Abrogation of β-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J Neurosci 34: 10285–10297, 2014. doi: 10.1523/JNEUROSCI.4915-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, Rechavi G, Schwartz M. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med 5: e171, 2008. doi: 10.1371/journal.pmed.0050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Romberg C, Yang S, Melani R, Andrews MR, Horner AE, Spillantini MG, Bussey TJ, Fawcett JW, Pizzorusso T, Saksida LM. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci 33: 7057–7065, 2013. doi: 10.1523/JNEUROSCI.6267-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Rooney GE, Endo T, Ameenuddin S, Chen B, Vaishya S, Gross L, Schiefer TK, Currier BL, Spinner RJ, Yaszemski MJ, Windebank AJ. Importance of the vasculature in cyst formation after spinal cord injury. J Neurosurg Spine 11: 432–437, 2009. doi: 10.3171/2009.4.SPINE08784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Rudge JS, Silver J. Inhibition of neurite outgrowth on astroglial scars in vitro. J Neurosci 10: 3594–3603, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Ruschel J, Hellal F, Flynn KC, Dupraz S, Elliott DA, Tedeschi A, Bates M, Sliwinski C, Brook G, Dobrindt K, Peitz M, Brüstle O, Norenberg MD, Blesch A, Weidner N, Bunge MB, Bixby JL, Bradke F. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 348: 347–352, 2015. doi: 10.1126/science.aaa2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rusnakova V, Honsa P, Dzamba D, Ståhlberg A, Kubista M, Anderova M. Heterogeneity of astrocytes: from development to injury - single cell gene expression. PLoS One 8: e69734, 2013. doi: 10.1371/journal.pone.0069734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sahinkaya FR, Milich LM, McTigue DM. Changes in NG2 cells and oligodendrocytes in a new model of intraspinal hemorrhage. Exp Neurol 255: 113–126, 2014. doi: 10.1016/j.expneurol.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sandler AN, Tator CH. Effect of acute spinal cord compression injury on regional spinal cord blood flow in primates. J Neurosurg 45: 660–676, 1976. doi: 10.3171/jns.1976.45.6.0660. [DOI] [PubMed] [Google Scholar]
- 334.Sandler AN, Tator CH. Review of the effect of spinal cord trama on the vessels and blood flow in the spinal cord. J Neurosurg 45: 638–646, 1976. doi: 10.3171/jns.1976.45.6.0638. [DOI] [PubMed] [Google Scholar]
- 335.Sands WA, Palmer TM. Regulating gene transcription in response to cyclic AMP elevation. Cell Signal 20: 460–466, 2008. doi: 10.1016/j.cellsig.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 336.Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci 30: 5843–5854, 2010. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Schmalfeldt M, Bandtlow CE, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Brain derived versican V2 is a potent inhibitor of axonal growth. J Cell Sci 113: 807–816, 2000. [DOI] [PubMed] [Google Scholar]
- 338.Schonberg DL, McTigue DM. Iron is essential for oligodendrocyte genesis following intraspinal macrophage activation. Exp Neurol 218: 64–74, 2009. doi: 10.1016/j.expneurol.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Schroeder GD, Kwon BK, Eck JC, Savage JW, Hsu WK, Patel AA. Survey of Cervical Spine Research Society members on the use of high-dose steroids for acute spinal cord injuries. Spine 39: 971–977, 2014. doi: 10.1097/BRS.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 340.Schwartz NB, Galligani L, Ho PL, Dorfman A. Stimulation of synthesis of free chondroitin sulfate chains by beta-d-xylosides in cultured cells. Proc Natl Acad Sci USA 71: 4047–4051, 1974. doi: 10.1073/pnas.71.10.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Schwartz NB. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem 252: 6316–6321, 1977. [PubMed] [Google Scholar]
- 342.Sekiya T, Holley MC, Hashido K, Ono K, Shimomura K, Horie RT, Hamaguchi K, Yoshida A, Sakamoto T, Ito J. Cells transplanted onto the surface of the glial scar reveal hidden potential for functional neural regeneration. Proc Natl Acad Sci USA 112: E3431–E3440, 2015. doi: 10.1073/pnas.1501835112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Seo JH, Miyamoto N, Hayakawa K, Pham L-DD, Maki T, Ayata C, Kim K-W, Lo EH, Arai K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest 123: 782–786, 2013. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 6: e1000113, 2009. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326: 592–596, 2009. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Shimizu F, Sano Y, Abe M-A, Maeda T, Ohtsuki S, Terasaki T, Kanda T. Peripheral nerve pericytes modify the blood-nerve barrier function and tight junctional molecules through the secretion of various soluble factors. J Cell Physiol 226: 255–266, 2011. doi: 10.1002/jcp.22337. [DOI] [PubMed] [Google Scholar]
- 347.Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res 50: 798–808, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 348.Siebert JR, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem 119: 176–188, 2011. doi: 10.1111/j.1471-4159.2011.07370.x. [DOI] [PubMed] [Google Scholar]
- 349.Siebert JR, Stelzner DJ, Osterhout DJ. Chondroitinase treatment following spinal contusion injury increases migration of oligodendrocyte progenitor cells. Exp Neurol 231: 19–29, 2011. doi: 10.1016/j.expneurol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 350.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci 5: 146–156, 2004. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 351.Silver J. The glial scar is more than just astrocytes. Exp Neurol 286: 147–149, 2016. doi: 10.1016/j.expneurol.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 352.Simon C, Götz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia 59: 869–881, 2011. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- 353.Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron 38: 201–211, 2003. doi: 10.1016/S0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 354.Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, Darling RA, Brown TE, Sorg BA. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci 35: 4190–4202, 2015. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Smith GM, Silver J. Transplantation of immature and mature astrocytes and their effect on scar formation in the lesioned central nervous system. Prog Brain Res 78: 353–361, 1988. doi: 10.1016/S0079-6123(08)60304-0. [DOI] [PubMed] [Google Scholar]
- 356.Smith-Thomas LC, Fok-Seang J, Stevens J, Du JS, Muir E, Faissner A, Geller HM, Rogers JH, Fawcett JW. An inhibitor of neurite outgrowth produced by astrocytes. J Cell Sci 107: 1687–1695, 1994. [DOI] [PubMed] [Google Scholar]
- 357.Smith-Thomas LC, Stevens J, Fok-Seang J, Faissner A, Rogers JH, Fawcett JW. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J Cell Sci 108: 1307–1315, 1995. [DOI] [PubMed] [Google Scholar]
- 358.Snow DM, Brown EM, Letourneau PC. Growth cone behavior in the presence of soluble chondroitin sulfate proteoglycan (CSPG), compared to behavior on CSPG bound to laminin or fibronectin. Int J Dev Neurosci 14: 331–349, 1996. doi: 10.1016/0736-5748(96)00017-2. [DOI] [PubMed] [Google Scholar]
- 359.Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol 109: 111–130, 1990. doi: 10.1016/S0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- 360.Snow DM, Mullins N, Hynds DL. Nervous system-derived chondroitin sulfate proteoglycans regulate growth cone morphology and inhibit neurite outgrowth: a light, epifluorescence, and electron microscopy study. Microsc Res Tech 54: 273–286, 2001. doi: 10.1002/jemt.1140. [DOI] [PubMed] [Google Scholar]
- 361.Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J, Krishnan V, Lai-Hsu C, Park KK, Tsoulfas P, Lee JK. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 33: 13882–13887, 2013. doi: 10.1523/JNEUROSCI.2524-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol 7: a020420, 2015. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Son Y-J. Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration? Neural Regen Res 10: 346–348, 2015. doi: 10.4103/1673-5374.153672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat Med 5: 943–946, 1999. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- 365.Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol 462: 223–240, 2003. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 366.Ståhlberg A, Andersson D, Aurelius J, Faiz M, Pekna M, Kubista M, Pekny M. Defining cell populations with single-cell gene expression profiling: correlations and identification of astrocyte subpopulations. Nucleic Acids Res 39: e24, 2011. doi: 10.1093/nar/gkq1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci 7: 2737–2744, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Starkey ML, Bartus K, Barritt AW, Bradbury EJ. Chondroitinase ABC promotes compensatory sprouting of the intact corticospinal tract and recovery of forelimb function following unilateral pyramidotomy in adult mice. Eur J Neurosci 36: 3665–3678, 2012. doi: 10.1111/ejn.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Steward O, Willenberg R. Rodent spinal cord injury models for studies of axon regeneration. Exp Neurol 287: 374–383, 2017. doi: 10.1016/j.expneurol.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 370.Stirling DP, Liu S, Kubes P, Yong VW. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci 29: 753–764, 2009. doi: 10.1523/JNEUROSCI.4918-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol 31: 318–324, 2010. doi: 10.1016/j.it.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Summers L, Kangwantas K, Nguyen L, Kielty C, Pinteaux E. Adhesion to the extracellular matrix is required for interleukin-1 beta actions leading to reactive phenotype in rat astrocytes. Mol Cell Neurosci 44: 272–281, 2010. doi: 10.1016/j.mcn.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Sun D, Lye-Barthel M, Masland RH, Jakobs TC. Structural remodeling of fibrous astrocytes after axonal injury. J Neurosci 30: 14008–14019, 2010. doi: 10.1523/JNEUROSCI.3605-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Sun F, Lin C-LG, McTigue D, Shan X, Tovar CA, Bresnahan JC, Beattie MS. Effects of axon degeneration on oligodendrocyte lineage cells: dorsal rhizotomy evokes a repair response while axon degeneration rostral to spinal contusion induces both repair and apoptosis. Glia 58: 1304–1319, 2010. doi: 10.1002/glia.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Susarla BTS, Laing ED, Yu P, Katagiri Y, Geller HM, Symes AJ. Smad proteins differentially regulate transforming growth factor-β-mediated induction of chondroitin sulfate proteoglycans. J Neurochem 119: 868–878, 2011. doi: 10.1111/j.1471-4159.2011.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Takeuchi K, Yoshioka N, Higa Onaga S, Watanabe Y, Miyata S, Wada Y, Kudo C, Okada M, Ohko K, Oda K, Sato T, Yokoyama M, Matsushita N, Nakamura M, Okano H, Sakimura K, Kawano H, Kitagawa H, Igarashi M. Chondroitin sulphate N-acetylgalactosaminyl-transferase-1 inhibits recovery from neural injury. Nat Commun 4: 2740, 2013. doi: 10.1038/ncomms3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Talbott JF, Loy DN, Liu Y, Qiu MS, Bunge MB, Rao MS, Whittemore SR. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp Neurol 192: 11–24, 2005. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Tan AM, Colletti M, Rorai AT, Skene JHP, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci 26: 4729–4739, 2006. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Tan CL, Kwok JCF, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci 31: 6289–6295, 2011. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Tang X, Davies JE, Davies SJA. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res 71: 427–444, 2003. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- 381.Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience 79: 1177–1182, 1997. doi: 10.1016/S0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 382.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg 75: 15–26, 1991. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 383.Tauchi R, Imagama S, Natori T, Ohgomori T, Muramoto A, Shinjo R, Matsuyama Y, Ishiguro N, Kadomatsu K. The endogenous proteoglycan-degrading enzyme ADAMTS-4 promotes functional recovery after spinal cord injury. J Neuroinflammation 9: 53, 2012. doi: 10.1186/1742-2094-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Tedeschi A, Dupraz S, Laskowski CJ, Xue J, Ulas T, Beyer M, Schultze JL, Bradke F. The Calcium Channel Subunit Alpha2delta2 Suppresses Axon Regeneration in the Adult CNS. Neuron 92: 419–434, 2016. doi: 10.1016/j.neuron.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 385.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA 99: 3024–3029, 2002. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Tester NJ, Plaas AH, Howland DR. Effect of body temperature on chondroitinase ABC’s ability to cleave chondroitin sulfate glycosaminoglycans. J Neurosci Res 85: 1110–1118, 2007. doi: 10.1002/jnr.21199. [DOI] [PubMed] [Google Scholar]
- 387.Thompson KM, Uetani N, Manitt C, Elchebly M, Tremblay ML, Kennedy TE. Receptor protein tyrosine phosphatase sigma inhibits axonal regeneration and the rate of axon extension. Mol Cell Neurosci 23: 681–692, 2003. doi: 10.1016/S1044-7431(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 388.Tkalec AL, Fink D, Blain F, Zhang-Sun G, Laliberte M, Bennett DC, Gu K, Zimmermann JJ, Su H. Isolation and expression in Escherichia coli of cslA and cslB, genes coding for the chondroitin sulfate-degrading enzymes chondroitinase AC and chondroitinase B, respectively, from Flavobacterium heparinum. Appl Environ Microbiol 66: 29–35, 2000. doi: 10.1128/AEM.66.1.29-35.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Tom VJ, Kadakia R, Santi L, Houlé JD. Administration of chondroitinase ABC rostral or caudal to a spinal cord injury site promotes anatomical but not functional plasticity. J Neurotrauma 26: 2323–2333, 2009. doi: 10.1089/neu.2009.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Tom VJ, Sandrow-Feinberg HR, Miller K, Domitrovich C, Bouyer J, Zhukareva V, Klaw MC, Lemay MA, Houlé JD. Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Exp Neurol 239: 91–100, 2013. doi: 10.1016/j.expneurol.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houlé JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. J Neurosci 29: 14881–14890, 2009. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J Neurosci 24: 6531–6539, 2004. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Tomko P, Farkaš D, Čížková D, Vanický I. Longitudinal enlargement of the lesion after spinal cord injury in the rat: a consequence of malignant edema? Spinal Cord 55: 255–263, 2017. doi: 10.1038/sc.2016.133. [DOI] [PubMed] [Google Scholar]
- 394.Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 486: 373–383, 2005. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- 395.Treloar HB, Ray A, Dinglasan LA, Schachner M, Greer CA. Tenascin-C is an inhibitory boundary molecule in the developing olfactory bulb. J Neurosci 29: 9405–9416, 2009. doi: 10.1523/JNEUROSCI.2356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Trivedi A, Olivas AD, Noble-Haeusslein LJ. Inflammation and spinal cord injury: infiltrating leukocytes as determinants of injury and repair processes. Clin Neurosci Res 6: 283–292, 2006. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Tropea D, Caleo M, Maffei L. Synergistic effects of brain-derived neurotrophic factor and chondroitinase ABC on retinal fiber sprouting after denervation of the superior colliculus in adult rats. J Neurosci 23: 7034–7044, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SPJ, Merkle F, Kessaris N, Alvarez-Buylla A, Richardson WD, Rowitch DH. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337: 358–362, 2012. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Underly RG, Levy M, Hartmann DA, Grant RI, Watson AN, Shih AY. Pericytes as Inducers of Rapid, Matrix Metalloproteinase-9-Dependent Capillary Damage during Ischemia. J Neurosci 37: 129–140, 2017. doi: 10.1523/JNEUROSCI.2891-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Vavrek R, Pearse DD, Fouad K. Neuronal populations capable of regeneration following a combined treatment in rats with spinal cord transection. J Neurotrauma 24: 1667–1673, 2007. doi: 10.1089/neu.2007.0290. [DOI] [PubMed] [Google Scholar]
- 401.Vogelaar CF, König B, Krafft S, Estrada V, Brazda N, Ziegler B, Faissner A, Müller HW. Pharmacological Suppression of CNS Scarring by Deferoxamine Reduces Lesion Volume and Increases Regeneration in an In Vitro Model for Astroglial-Fibrotic Scarring and in Rat Spinal Cord Injury In Vivo. PLoS One 10: e0134371, 2015. doi: 10.1371/journal.pone.0134371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci 31: 9332–9344, 2011. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci 121: 3083–3091, 2008. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Wang L, Shi J, van Ginkel FW, Lan L, Niemeyer G, Martin DR, Snyder EY, Cox NR. Neural stem/progenitor cells modulate immune responses by suppressing T lymphocytes with nitric oxide and prostaglandin E2. Exp Neurol 216: 177–183, 2009. doi: 10.1016/j.expneurol.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 405.Wang X, Cao K, Sun X, Chen Y, Duan Z, Sun L, Guo L, Bai P, Sun D, Fan J, He X, Young W, Ren Y. Macrophages in spinal cord injury: phenotypic and functional change from exposure to myelin debris. Glia 63: 635–651, 2015. doi: 10.1002/glia.22774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wang X, Hasan O, Arzeno A, Benowitz LI, Cafferty WBJ, Strittmatter SM. Axonal regeneration induced by blockade of glial inhibitors coupled with activation of intrinsic neuronal growth pathways. Exp Neurol 237: 55–69, 2012. doi: 10.1016/j.expneurol.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Wang Y, Moges H, Bharucha Y, Symes A. Smad3 null mice display more rapid wound closure and reduced scar formation after a stab wound to the cerebral cortex. Exp Neurol 203: 168–184, 2007. doi: 10.1016/j.expneurol.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 408.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci 33: 12870–12886, 2013. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Wanner IB, Deik A, Torres M, Rosendahl A, Neary JT, Lemmon VP, Bixby JL. A new in vitro model of the glial scar inhibits axon growth. Glia 56: 1691–1709, 2008. doi: 10.1002/glia.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Warren PM, Macfarlane PM, Silver J, Alilain WJ. Extensive recovery of respiratory motor function at chronic and super-chronic time points following cervical spinal cord injury. Program No. 523.10. 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience, 2014. [Google Scholar]
- 411.White RE, Jakeman LB. Don’t fence me in: harnessing the beneficial roles of astrocytes for spinal cord repair. Restor Neurol Neurosci 26: 197–214, 2008. [PMC free article] [PubMed] [Google Scholar]
- 412.Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA 103: 17513–17518, 2006. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci 14: 1398–1405, 2011. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Wozniewicz B, Filipowicz K, Swiderska SK, Deraka K. Pathophysiological mechanism of traumatic cavitation of the spinal cord. Paraplegia 21: 312–317, 1983. [DOI] [PubMed] [Google Scholar]
- 415.Wu D, Miyamoto O, Shibuya S, Okada M, Igawa H, Janjua NA, Norimatsu H, Itano T. Different expression of macrophages and microglia in rat spinal cord contusion injury model at morphological and regional levels. Acta Med Okayama 59: 121–127, 2005. doi: 10.18926/AMO/31950. [DOI] [PubMed] [Google Scholar]
- 416.Wu J, Yoo S, Wilcock D, Lytle JM, Leung PY, Colton CA, Wrathall JR. Interaction of NG2(+) glial progenitors and microglia/macrophages from the injured spinal cord. Glia 58: 410–422, 2010. doi: 10.1002/glia.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci 34: 10989–11006, 2014. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wunderlich G, Stichel CC, Schroeder WO, Müller HW. Transplants of immature astrocytes promote axonal regeneration in the adult rat brain. Glia 10: 49–58, 1994. doi: 10.1002/glia.440100107. [DOI] [PubMed] [Google Scholar]
- 419.Xie Y, Yeo TT, Zhang C, Yang T, Tisi MA, Massa SM, Longo FM. The leukocyte common antigen-related protein tyrosine phosphatase receptor regulates regenerative neurite outgrowth in vivo. J Neurosci 21: 5130–5138, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Xie Y, Massa SMS, Ensslen-Craig SES, Major DLD, Yang T, Tisi MAM, Derevyanny VDV, Runge WOW, Mehta BPB, Moore LAL, Brady-Kalnay SMS, Longo FMF. Protein-tyrosine phosphatase (PTP) wedge domain peptides: a novel approach for inhibition of PTP function and augmentation of protein-tyrosine kinase function. J Biol Chem 281: 16482–16492, 2006. doi: 10.1074/jbc.M603131200. [DOI] [PubMed] [Google Scholar]
- 421.Xu B, Park D, Ohtake Y, Li H, Hayat U, Liu J, Selzer ME, Longo FM, Li S. Role of CSPG receptor LAR phosphatase in restricting axon regeneration after CNS injury. Neurobiol Dis 73: 36–48, 2015. doi: 10.1016/j.nbd.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Xu K, Malouf AT, Messing A, Silver J. Glial fibrillary acidic protein is necessary for mature astrocytes to react to beta-amyloid. Glia 25: 390–403, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 423.Xu Y, Kitada M, Yamaguchi M, Dezawa M, Ide C. Increase in bFGF-responsive neural progenitor population following contusion injury of the adult rodent spinal cord. Neurosci Lett 397: 174–179, 2006. doi: 10.1016/j.neulet.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 424.Xu Z, Wang B-R, Wang X, Kuang F, Duan X-L, Jiao X-Y, Ju G. ERK1/2 and p38 mitogen-activated protein kinase mediate iNOS-induced spinal neuron degeneration after acute traumatic spinal cord injury. Life Sci 79: 1895–1905, 2006. doi: 10.1016/j.lfs.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 425.Xue Y-X, Xue L-F, Liu J-F, He J, Deng J-H, Sun S-C, Han H-B, Luo Y-X, Xu L-Z, Wu P, Lu L. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J Neurosci 34: 6647–6658, 2014. doi: 10.1523/JNEUROSCI.5390-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Yang C, Li X, Sun L, Guo W, Tian W. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J Neural Eng 14: 026005, 2017. doi: 10.1088/1741-2552/aa596b. [DOI] [PubMed] [Google Scholar]
- 427.Yang S, Cacquevel M, Saksida LM, Bussey TJ, Schneider BL, Aebischer P, Melani R, Pizzorusso T, Fawcett JW, Spillantini MG. Perineuronal net digestion with chondroitinase restores memory in mice with tau pathology. Exp Neurol 265: 48–58, 2015. doi: 10.1016/j.expneurol.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Yang T, Yin W, Derevyanny VD, Moore LA, Longo FM. Identification of an ectodomain within the LAR protein tyrosine phosphatase receptor that binds homophilically and activates signalling pathways promoting neurite outgrowth. Eur J Neurosci 22: 2159–2170, 2005. doi: 10.1111/j.1460-9568.2005.04403.x. [DOI] [PubMed] [Google Scholar]
- 429.Yang Z, Suzuki R, Daniels SB, Brunquell CB, Sala CJ, Nishiyama A. NG2 glial cells provide a favorable substrate for growing axons. J Neurosci 26: 3829–3839, 2006. doi: 10.1523/JNEUROSCI.4247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Yawata I, Takeuchi H, Doi Y, Liang J, Mizuno T, Suzumura A. Macrophage-induced neurotoxicity is mediated by glutamate and attenuated by glutaminase inhibitors and gap junction inhibitors. Life Sci 82: 1111–1116, 2008. doi: 10.1016/j.lfs.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 431.Yeh T-H, Lee DY, Gianino SM, Gutmann DH. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia 57: 1239–1249, 2009. doi: 10.1002/glia.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Yi J-H, Katagiri Y, Yu P, Lourie J, Bangayan NJ, Symes AJ, Geller HM. Receptor protein tyrosine phosphatase σ binds to neurons in the adult mouse brain. Exp Neurol 255: 12–18, 2014. doi: 10.1016/j.expneurol.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Yick L-W, So K-F, Cheung P-T, Wu W-T. Lithium chloride reinforces the regeneration-promoting effect of chondroitinase ABC on rubrospinal neurons after spinal cord injury. J Neurotrauma 21: 932–943, 2004. doi: 10.1089/0897715041526221. [DOI] [PubMed] [Google Scholar]
- 434.Yoo M, Khaled M, Gibbs KM, Kim J, Kowalewski B, Dierks T, Schachner M. Arylsulfatase B improves locomotor function after mouse spinal cord injury. PLoS One 8: e57415, 2013. doi: 10.1371/journal.pone.0057415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia 50: 247–257, 2005. doi: 10.1002/glia.20176. [DOI] [PubMed] [Google Scholar]
- 436.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci 32: 6391–6410, 2012. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Zawadzka M, Rivers LE, Fancy SPJ, Zhao C, Tripathi R, Jamen F, Young K, Goncharevich A, Pohl H, Rizzi M, Rowitch DH, Kessaris N, Suter U, Richardson WD, Franklin RJM. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 6: 578–590, 2010. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Zhang H, Adwanikar H, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinases and neurotrauma: evolving roles in injury and reparative processes. Neuroscientist 16: 156–170, 2010. doi: 10.1177/1073858409355830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MSB, Li G, Duncan JA III, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MGH, Barres BA. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89: 37–53, 2016. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Zhao R-R, Muir EM, Alves JN, Rickman H, Allan AY, Kwok JC, Roet KCD, Verhaagen J, Schneider BL, Bensadoun J-C, Ahmed SG, Yáñez-Muñoz RJ, Keynes RJ, Fawcett JW, Rogers JH. Lentiviral vectors express chondroitinase ABC in cortical projections and promote sprouting of injured corticospinal axons. J Neurosci Methods 201: 228–238, 2011. doi: 10.1016/j.jneumeth.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron 38: 213–224, 2003. doi: 10.1016/S0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 442.Zhou H-X, Li X-Y, Li F-Y, Liu C, Liang Z-P, Liu S, Zhang B, Wang T-Y, Chu T-C, Lu L, Ning G-Z, Kong X-H, Feng S-Q. Targeting RPTPσ with lentiviral shRNA promotes neurites outgrowth of cortical neurons and improves functional recovery in a rat spinal cord contusion model. Brain Res 1586: 46–63, 2014. doi: 10.1016/j.brainres.2014.08.048. [DOI] [PubMed] [Google Scholar]
- 443.Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol 4: 19–26, 2008. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- 444.Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR, Lee JK. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol Dis 74: 114–125, 2015. doi: 10.1016/j.nbd.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zhu Y, Soderblom C, Trojanowsky M, Lee D-H, Lee JK. Fibronectin Matrix Assembly after Spinal Cord Injury. J Neurotrauma 32: 1158–1167, 2015. doi: 10.1089/neu.2014.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci 10: 321–330, 2007. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci 33: 15350–15361, 2013. doi: 10.1523/JNEUROSCI.2510-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci 18: 5203–5211, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol 34: 41–54, 1998. doi:10.1002/(SICI)1097-4695(199801)34:1<41::AID-NEU4>3.0.CO;2-C. [PubMed] [Google Scholar]
- 450.Zuo J, Neubauer D, Dyess K, Ferguson TA, Muir D. Degradation of chondroitin sulfate proteoglycan enhances the neurite-promoting potential of spinal cord tissue. Exp Neurol 154: 654–662, 1998. doi: 10.1006/exnr.1998.6951. [DOI] [PubMed] [Google Scholar]