Abstract
It has long been thought that respiratory infections are the direct result of acquisition of pathogenic viruses or bacteria, followed by their overgrowth, dissemination, and in some instances tissue invasion. In the last decades, it has become apparent that in contrast to this classical view, the majority of microorganisms associated with respiratory infections and inflammation are actually common members of the respiratory ecosystem and only in rare circumstances do they cause disease. This suggests that a complex interplay between host, environment, and properties of colonizing microorganisms together determines disease development and its severity. To understand the pathophysiological processes that underlie respiratory infectious diseases, it is therefore necessary to understand the host-bacterial interactions occurring at mucosal surfaces, along with the microbes inhabiting them, during symbiosis. Current knowledge regarding host-bacterial interactions during asymptomatic colonization will be discussed, including a plausible role for the human microbiome in maintaining a healthy state. With this as a starting point, we will discuss possible disruptive factors contributing to dysbiosis, which is likely to be a key trigger for pathobionts in the development and pathophysiology of respiratory diseases. Finally, from this renewed perspective, we will reflect on current and potential new approaches for treatment in the future.
I. PREFACE
Respiratory tract infections (RTI) are a major global health concern; worldwide, RTIs are the leading cause of death in childhood and the fourth most common cause of death in adults. At greatest risk for RTI are young children, the elderly, and those who are immunocompromised (136, 228). Traditionally, it has been thought that RTIs are a direct consequence of acquisition, overgrowth and dissemination, and invasion of a limited number of viral and bacterial pathogens (41). However, several decades of research now indicate that most human bacterial pathogens are also capable of asymptomatic colonization, and find their primary ecological niche within the respiratory tract of healthy individuals. Since RTIs are a rare event in comparison to colonization with potential pathogenic organisms, this implies that asymptomatic colonization constitutes the preferred or at least most frequent growth form for the majority of these microorganisms. Thus bacterial pathogenesis is a complex multifaceted process, simultaneously influenced by environmental, host, as well as microbial factors (204).
With the advent of increasingly powerful molecular technologies such as high-throughput sequencing, it has become evident that the human body is itself a complex ecosystem of microorganisms that includes bacteria, fungi, parasites, and viruses. Together these compose the human microbiome (76, 99, 273, 373). Until recently, most effort and interest have been focused on the function of the gut ecosystem in health, which has revealed that the human microbiome is crucial for proper development of mucosal barrier function, healthy immune maturation, nutritional status, and providing direct resistance to infectious disease (399). A state of dysbiosis, defined as microbial imbalance or maladaptation, has on the other hand been associated with disruption of homeostasis at mucosal sites, leading to a disequilibrium between host and microbiome and consequently allowing for a breadth of infectious and inflammatory illnesses.
In contrast to what has long been assumed, considerable experimental evidence now shows that the lower respiratory tract of healthy individuals is not sterile. Similar to the gut, the resident microbiota might even help maintain mucosal homeostasis and prevent airway infections (99). In this review, we would like to postulate that evolutionary processes have led to what is most often a harmonious interaction between host, environment, and the microbial ecosystems within the airways, defined as an equilibrial state that in most instances is capable of self-correction and self-repair when disturbed. In contrast, a disequilibrium, or lack of a stable state between host, environment, and microbiome, may lead to microbial dysbiosis, acute or chronic inflammation, mucosal dysfunction, and consequently the opportunity for infectious diseases. We also describe how this shift in thinking about respiratory infection and inflammation from a health perspective could redefine our view on (future) treatment and prevention of respiratory diseases, using a disturbed equilibrium, rather than the ‟simpleˮ presence of pathogens as a basis for interventions.
II. PHYSIOLOGICAL PROCESSES INVOLVED IN THE EQUILIBRIUM BETWEEN THE HOST AND THE MICROBIAL WORLD
A. Development of Stable Microbial Ecosystems in the Airway
The respiratory tract is the organ system in our body responsible for O2 and CO2 exchange (68). For this purpose, the airway must filter, warm, and humidify inhaled gas and, while doing so, prevent the establishment of noxious infectious agents that may gain access to the system and threaten function and life. Air enters the upper respiratory tract (URT) via the anterior nares and the nasal cavity, moves through the naso- and oropharynx, passes the larynx and vocal cords, and ultimately reaches the lower respiratory tract (LRT). The LRT is composed of the trachea, branching bronchi, bronchioli, and then alveoli, where the critical process of gas exchange occurs. In the URT, multiple sinuses as well as the Eustachian tube drain their mucus and captured debris into the nasal and nasopharyngeal cavity, respectively (68). In the LRT the mucociliary escalator moves mucus-entrapped particles, including aspirated microorganisms from the URT, into the oropharyngeal cavity (131). Once in the oropharynx, mucus and debris are eliminated via swallowing or expulsion.
The oropharynx acts as a crossroad where food and air are directed towards the esophagus and trachea, respectively. In line with the known functionality of the gut microbiome, the microbial ecosystem that occupies the oropharynx most likely also plays a role in host defense and resistance against disruptive events resulting from inhalation, ingestion, or hygienic measures (105, 394). For example, the oropharyngeal microbiome has been suggested to promote barrier function against bacterial pathogens by intrinsic competitive colonization resistance, by stimulating epithelial barrier function via induction of mucus and antimicrobial peptide production, by inducing the downregulation of bacterial ligands, and by increasing tight junction integrity (273). The microbiome inhabiting the respiratory tract might also be involved in ‟instructingˮ the (local) immune system to differentiate between commensal and invasive signals, simultaneously inducing immune regulation and preventing overwhelming inflammatory responses against external insults, and guiding immune maturation over time (99, 204, 314).
The human microbiome in general, and the respiratory microbiome specifically, starts to develop rapidly at the moment a child is born. The bacteria that can be found in the respiratory tract (RT) directly after birth are mostly indistinguishable from other body habitats, such as the skin and gut. In fact, they reflect the infant’s environmental exposures more than the specific niche characteristics. For example, in vaginally delivered children, Lactobacillus, Prevotella, Atopobium, and Sneathia are predominant genera in the URT, whereas in infants born by caesarean section, Staphylococcus spp. and other typical skin inhabitants are initially observed (41, 42). Following this ‟universalˮ exposure, those microorganisms best able to persist and replicate within the RT soon outnumber their competitors. This process is dynamic and continues throughout infancy, resulting from exposure to new environmental factors including those that occur as a result of respiration and feeding. Ultimately, increasingly complex and niche-specific communities emerge (42, 344). Natural selection for the microorganisms best fitted to these niches continues throughout life as the host encounters new environments and changes in diet, becomes a member of larger and different communities (e.g., day care, schools, military institutes, nursing homes), and experiences life events that alter the host’s response to the environment (27–29, 305). FIGURE 1 illustrates how these diverse factors influence the balance between host and microbiome at each anatomical niche and that this is subject to continuous change over time.
FIGURE 1.
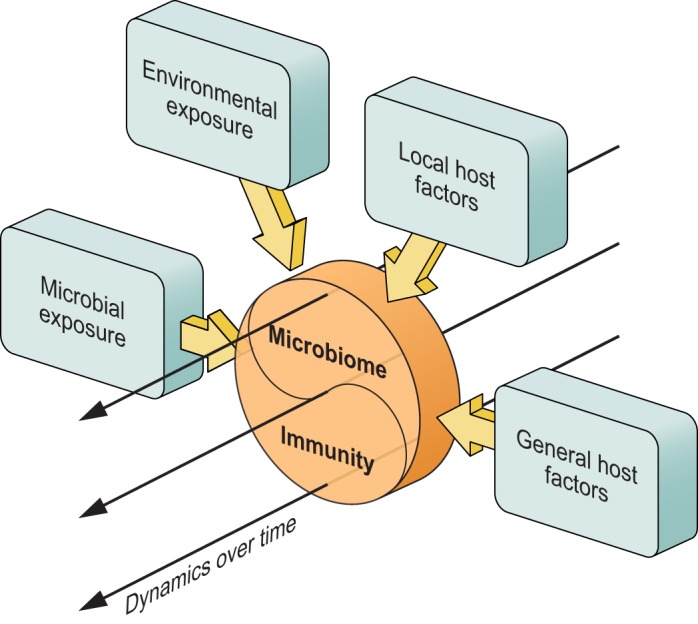
The human body is exposed to a multitude of environmental factors, including interpersonal contact (varying level of crowding), climate, urban or rural environment, air pollution, and food. The body is also exposed to a wide range of microbes from diverse environments, inhaled through air, ingested with food, or encountered via skin contact. These interact with our mucosal and skin surfaces, shaping the microbiome and host immune system, which in turn affect one another over the course of a lifetime as well. In addition, local (anatomy, organ-specific diseases or defects) and systemic host factors, including genetic makeup, age, adaptive immunity, as well as interniche-dependent effects (including effects mediated through the gut-lung axis) contribute to these interactions. At the interface of lung epithelium and outside air, the so-called host-microbiome interface represents the outcome of host factors and environmental and microbial exposures in a temporal (dynamic) manner.
Although a common (core) respiratory microbiome can be defined, including a wide range of mostly aerobic and facultative anaerobic bacteria, relatively large differences in composition between individuals have been observed (28, 261, 344). Moreover, from a microbial perspective, the respiratory tract should be considered a concatenation of overlapping ecosystems. For example, in the nasal cavity, Staphylococci, Corynebacteria, and Streptococci seem to predominate. This is most likely influenced by its proximity to the skin flora and the homology between the epithelial lining of the nasal vestibulum and the facial skin that both originate from the ectoderm during fetal development (261, 334). This skin and nasal epithelium introduces vast amounts of Corynebacteria, which in turn releases products such as short-chain fatty acids that inhibit Streptococcal and support Staphylococcal species (40, 97). The paranasal sinuses, i.e., frontal, ethmoid, sphenoid, and maxillary sinuses, all drain via ostea onto the nasal cavity; also these sinuses are lined with pseudostratified ciliated epithelium and are colonized by highly diverse microbial communities including Lactobacilli, and other lactic acid-producing bacteria such as Carnobacterium, Enterococcus, and Pediococcus spp (4). In the nasopharynx, which is also lined with pseudostratified ciliated epithelium originating from the fetal endoderm, the community composition becomes more complex, although aerobic and facultative anaerobic genera like Moraxella, Staphylococcus, Corynebacterium, Dolosigranulum, Hemophilus, and/or Streptococcus spp. still predominate (28, 344, 352). Although generally found in low abundance, oral bacteria are common in this niche as well. In the oral cavity, the community composition seems to be most complex, with a high diversity of bacterial species including (facultative) anaerobic bacteria such as Prevotella, Gemella, Streptococcus, and Veillonella spp (28, 98). Once entering the larynx, trachea, and lungs, the microbial community changes again, and suddenly contains a mixture of nasopharyngeal and oropharyngeal microbes (20, 87, 104). As indicated, the healthy lungs are not sterile. Instead, they are colonized by diverse groups of microbes, which seem to reach greater diversity more rapidly following birth than even the intestinal microbiota (113, 395). The most prevalent phyla in healthy airways are Bacteroidetes and Firmicutes, and to a lesser extent Proteobacteria and Actinobacteria, with predominance of genera like Streptococcus, Fusobacteria, Pseudomonas, Veillonella, and Prevotella species (20, 21, 104, 250). More details on differences in niche-specific microbial communities were previously reviewed in Reference 99.
Given this temporal and spatial complexity, it is important to first consider what function one likes to study, and then what subniche and submicrobiome ecosystem is relevant for the study of that function when studying the RT microbiome in general. Along those lines, and because colonization of the human nasopharynx most often precedes dissemination of bacteria to other sites where disease occurs, such as the middle ear, sinuses, lungs, as well as meninges and blood, most investigators consider the nasopharynx as the ecological reservoir for potential viral and bacterial respiratory pathogens (12, 162). Within this niche, distinct community profiles have been identified that are highly comparable across the globe (98, 204), implying universal functions. However, so-called beta diversity, i.e., differences in community composition between individuals, is high, implying potentially varying resilience against acquisition of new community members.
Like in any other ecosystem, microbiome resilience is dependent on community diversity as well as the presence of keystone species that may confer direct or indirect protection against new and in some instances pathogenic members (99). Moreover, evenness, defined as the relative abundance of the different bacterial members within a community network, seems important, as it might have a more profound effect on respiratory microbiome stability over time, a phenomenon that has so far only been suggested by molecular epidemiological studies rather than by mechanistic evidence. However, although the nasopharynx seems to constitute the ecological reservoir that samples potential viral and bacterial pathogens from the environment, and the niche from where dissemination to other sites such as the middle ear, sinuses, as well as meninges may occur (12, 162), the eventual niche from where potential pathogens descend towards the lungs by aspiration is most likely the oropharynx (106, 290). The nasopharynx and the oropharynx form an anatomical continuum; therefore, potential pathogens entering the human body through the nose and nasopharynx also enter the oropharyngeal ecosystem (240). Yet, repeated and direct aspiration of saliva containing bacteria from the orpharyngeal cavity is most likely the cause of dissemination to the lungs. In the oropharynx, individual bacteria interact with more complex communities including gastrointestinal, respiratory, and skin bacteria, which could potentially contribute to containment of those potential pathogens (199). This might explain the fact that LRT infections, although caused by similar potential pathogens, are much less common than URT infections.
B. Asymptomatic Colonization of Potential Pathogens
As mentioned previously, the vast majority of bacterial species that cause RTI are common asymptomatic colonizers of the nasal cavities, the nasopharynx, and oropharynx (337). Viruses, on their own, are important and often disruptive occupants of the URT, and important triggers for bacterial pathobionts to transition from asymptomatic colonizers to pathological agents (41). Yet, they too can persist in the RT asymptomatically. The role of viruses in health and disease is further discussed in greater detail in section III.
For the main bacterial pathogens Streptococcus pneumoniae, Hemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus, the colonization rate far exceeds the incidence of infection. For example, it is estimated that the colonization rate with S. pneumoniae at any given time averages ~3 billion individuals worldwide (336), yet “only” 15 million serious infections occur per year (2). This accentuates that these microorganisms have adapted evolutionarily to the ecological niche, where they establish homeostasis with the host and residing microbiota. One way this is accomplished is by rapidly organizing into biofilms, bacterial communities encased by a self-produced matrix (30, 153). Biofilms provide physical advantages such as increased resistance to desiccation and host defense mechanisms (107, 161, 248, 383) and are often polymicrobial in nature in vivo. Colonization with opportunistic pathogens, such as S. pneumoniae, starts during the first weeks after birth and appears to be modulated by the ecological niche these organisms live in and the microbiome they interact with (36, 144, 176). The environmental factors relevant for symbiotic colonization are of great relevance, because colonization also provides a means for transmission of bacteria between individuals (190), and as such promoting evolutionary survival.
1. Epidemiology
The fact that asymptomatic colonization with potential pathogens is very common and the majority of pathogens are human specific suggest that these microorganisms have evolved to colonize the human host and that infection is most likely an accident in response to changes of the occupied environmental niche. Between 10 and 90% of children below the age of 5 are colonized with S. pneumoniae at any given time, with colonization rates being higher in recourse poor settings (65–90%) than in industrialized countries (10–50%) (3, 37, 81, 132, 144, 163, 172, 213, 274, 312). For adults, the colonization rates by conventional cultures are lower, with ~50% colonized individuals in resource-poor settings and 1–15% colonized individuals in industrialized countries (98, 210, 387). The colonization rates for H. influenzae are 30–88% in children and 3–20% in adults (132, 215, 309), and M. catarrhalis can be isolated from 28–81% of children and 2–10% of adults, respectively (309).
Based on their commonality, infants and young children are often colonized with more than one organism (300, 362, 388), with co-colonization patterns depending on the presence of viruses (see sect. III), and potentially driven by changes in the microbiome composition and diversity.
2. Mechanisms involved in establishing asymptomatic colonization
To establish asymptomatic colonization, an incoming organism needs to adapt to a state of microbial symbiosis, and homeostasis with the host mucosal surface and immune system. The mechanisms involved in establishing asymptomatic colonization are not well understood, although an increased interest in this topic is emerging. It is well established that an innate immune response is induced following an encounter with bacterial organisms entering the ecological niche and binding to the nasopharyngeal mucosa and that host factors such as carbon availability (31), oxygen tension (160), and antibodies trigger the bacteria to aggregate and grow as biofilms. Yet the role of the microbiome in this process is not well understood, although it is becoming more plausible that the microbiome modulates how bacteria and co-colonizing bacteria interact with the host (174).
Most information regarding immune evasion of newly acquired potential pathogens has been gathered using S. pneumoniae as a model system as it effectively colonizes mice for long periods of time, which facilitates mechanistic studies. Observational studies suggest that the immune response that is mounted against colonizing bacteria is not always sufficient for clearance (188, 274). Although asymptomatic pneumococcal colonization results in a type I interferon response (142) and this together with production of antimicrobial factors reduces carriage levels (295), this response is most likely not alone responsible for eradication. Instead, cell proliferation and wound healing are activated over time, suggesting that the host focuses on efforts to maintain the integrity of the epithelium in favor of eliminating the bacteria (188). This response likely comes from the early expression of pneumolysin, a cholesterol-dependent toxin produced by pneumococci, that causes initial and local tissue invasion with concomitant cell damage, that is necessary establishment of a more stable colonization (171). Subsequently, a cell-mediated response driven by interleukin (IL)-23-dependent IL-17 response and further enhancement of antimicrobial peptide production induced by IL-22 leads to clearance (200, 226).
Recently, more mechanistic studies have shown that pneumococci actually induce a tolerogenic immune response. Neill et al. (282) found that pneumococci induce transforming growth factor (TGF)-beta1 production from nasopharyngeal cells in vivo, which in turn induces an immune tolerance profile, characterized by high nasopharyngeal T-regulatory cell numbers that were crucial for prolonged carriage of bacteria. It has also been shown that bacterial clearance by immune cells only occurs after recognition of S. pneumoniae by TLR2 on resident macrophages in combination with activation of CD4+ Th17 cells, which in turn drives a cell-mediated response via IL-17 production (71, 397). Bacterial products, including the capsule, have an important role in regulating the immune response and facilitating colonization. Capsule not only protects against phagocytosis, but also suppresses the release of the pro-inflammatory cytokines CXCL8 (IL-8) and IL-6 (211) required for neutrophil-mediated killing. Recently, a subset of unencapsuled strains expressing the pneumococcal surface protein K (PspK) instead of the capsular locus have emerged to effectively colonize the nasopharynx (197). PspK is known to bind to epithelial cells and also to immunoglobulins. However, how PspK interacts with the immune system to avoid eradication and induce stable colonization is not yet understood. These results indicate that pneumococci can modulate the immune system to avoid eradication.
Another way bacteria evade the host immune system is through the establishment of biofilms during colonization, which has been well studied in all organisms mentioned above; biofilms further shield the bacteria from the host response. Pneumococci form biofilms during colonization in vivo, with biofilm bacteria being less invasive and less inflammatory (32, 67, 135, 244, 246). Biofilm growth makes the bacteria not only less recognizable for the immune system, but the solid encapsulation of the biofilms also protects the bacteria from host factors and antibiotics in general (89, 91). In a very similar way, H. influenzae, M. catarrhalis, and S. aureus form (polymicrobial) biofilms to colonize the nasopharynx or oropharynx, plausibly contributing to persistence in these ecological niches (57, 108, 236, 245, 275, 353, 359). For example, in some instances, one species confers passive resistance to antimicrobials to another within biofilms by producing beta-lactamase (297, 376). Alternatively mixed species within a biofilm can ensure optimal usage of limited carbohydrates by cross-feeding on metabolic end products or even cross-respiration (343).
3. Bacterial colonization factors
Bacteria require specific factors to evade the immune system and generate biofilms. Generally, these factors can be grouped into adhesins, proteases, metabolic enzymes and transporters, and regulatory proteins that all variably promote binding to the epithelial mucosa, protect against antibodies and other host components, help to retrieve nutrients, or help to evade the immune system to establish a biofilm. For S. pneumoniae this is exemplified by the requirement of the adhesins CbpA (PspC) and PsrP (246, 272, 323, 329), the presence of the Clp protease (214), and the toxin pneumolysin for optimal colonization (171, 198, 289). Furthermore, regulatory systems such as CiaRH (206, 272), LuxRS (366, 367), and competence induction during colonization create the ideal environment for intra- and interspecies communication and the exchange of genetic material (247, 320, 366, 375). Of note, serine-rich repeat proteins such as pneumococcal PsrP can bind to extracellular DNA (329) alternatively bind to themselves or their ortholog in other Gram-positive species promoting aggregation and biofilm formation (323). Similar to S. pneumoniae, biofilms, adhesins, transporters, and proteases are required for optimal biofilm formation and colonization of both M. catarrhalis (169) and H. influenzae (17, 18, 66, 122, 253, 374, 379). Additionally, for both S. pneumoniae and H. influenzae, optimal biofilm formation requires growth at the nasopharyngeal temperature of 32–34°C (246, 265).
C. Healthy Function of Host-Mucosal Surfaces
At the interface of the host, microbes, and the external world, the mucosa of the respiratory tract has an active role in maintaining homeostasis (FIGURE 2). Whereas the nasopharynx, larynx, and trachea are mostly lined with pseudostratified ciliated columnar epithelial cells including basal cells and goblet cells for mucous production, towards the bronchioles the epithelium gradually changes towards cuboidal epithelium with some cilia, and with club cells instead of goblet cells, producing glycosaminoglycans and secretory proteins involved in both lung maintenance and host defense (346, 350). One such example is hyaluronan, which in its uncleaved form suppresses the inflammatory response, yet when cleaved by bacterial and host proteases, interacts with TLR2 to initiate a lung inflammatory response (184). Another example is secretory IgA, which has the capacity to adhere to pathogens both in its native state and via its Fab portion, thereby neutralizing and aggregating infectious particles (120). The Fc portion of IgA also binds tightly to mucus and entraps bacteria and viruses for subsequent removal via the mucociliary escalator. IgA does so without the activation of complement, a major distinction from IgG (237, 306). However, immune complexes formed by both IgA and IgG induce complement activation in the respiratory tract, although only IgG immune complexes recruit neutrophils (371, 372). The vast majority of the alveolar surface is lined with lipid-rich surfactant with high bacteriostatic capacities, produced by type II alveolar cells (type II pneumocytes) (11, 369).
FIGURE 2.
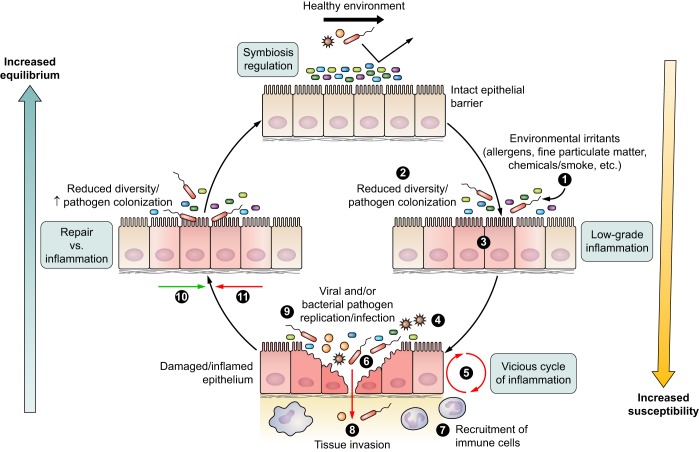
Equilibrium between host mucosal surface and microbial environment is reached when the microbial community (differently colored small rectangle-shaped symbols) is at balance, and the host mucosa is free of damage and inflammation (regulation). Environmental triggers (1) may lead to either reduced microbial diversity (2) or epithelial inflammation or damage (3), resulting in a dysbiotic state. As a consequence, a disequilibrium occurs between microbiome and host, that once triggered by a (mild) disrupting factor (4) like a viral infection (starlike symbols) or other inflammatory trigger, may lead to a series of events including a cascade of epithelial changes and host innate signaling (5), increased colonization with potential pathogenic bacteria (larger red rectangle-shaped symbols) (6), immune cell recruitment (7), and a diminished barrier function allowing pathogen invasion (8). In an attempt to protect the host from pathogen invasion and restore the barrier function, a cycle of inflammatory events is triggered (9) until danger signals disappear (10) and restoration of damage can start to occur (11). At times of restoration and repair, a disequilibrium might still exist, rendering the host vulnerable for new infections induced by external triggers.
Accordingly, type II pneumocytes serve an important role in the production of pulmonary surfactant which helps to lower surface tension along the cell surface (138). Unlike type I cells, which act as the primary cover of the alveolar surface (90–95%) and are responsible for gas exchange and barrier function, type II pneumocytes cover only a fraction of the alveolar surface. Nonetheless, surfactant produced by type II cells prevents alveoli from collapsing. These mechanical defense mechanisms are complemented with antimicrobial peptides, produced by both types of pneumocytes, whose production is increased during inflammation, and with the presence of resident lung leukocytes, in particular activated macrophages and dendritic cells (104). Alveolar epithelial cells also produce chemokines and cytokines that recruit and activate immune cells in damaged or infected areas (386).
All these functions are made possible through an optimal lung fluid homeostasis through regulated ion exchange in the respiratory epithelial cell (254, 286). Aquaporin-5 is essential for water homeostasis in the lung, and knockout mice lacking aquaporin-5 develop cystic fibrosis-like disease (286). Additionally, a coordinated function of chloride secretion and sodium uptake is necessary to maintain fluid volume in the lung and on the respiratory mucosa, which provides for an optimal gas exchange in the lung as well as an optimal mucus composition and mucociliary clearance (254). Similarly, dysregulation of ion transport in the respiratory tract leads to a disequilibrium involved in development of lung disease, which will be discussed below. Altogether, under normal circumstances, these secretions and entrapped components are moved out of the lungs helping to reduce bacterial densities in the airways.
D. Lung Macrophages
Despite their long-established role in pathogen phagocytosis and killing, it is now appreciated that the major roles of airway macrophages are to maintain optimal function and an immune quiescent state in the lungs. Tissue-resident macrophages have multiple roles that impact almost every aspect of homeostasis. In the lungs these include a scavenger function and clearance of physical and cellular debris, such as that mediated by the class A scavenger receptors (14, 15), maintenance of homeostasis despite insult (380), and phagocytosis and killing of aspirated microbes (14, 15, 148, 390), thereby preventing progression to a disease and inflammatory state.
One manner in which inflammation is suppressed in the airways occurs through a subset of alveolar macrophages (AM) that form gap junction channels with the epithelium allowing for communication between these cells and other immune cells located distally. These cell-bound AMs were shown to intercommunicate via synchronized Ca2+ waves, that after LPS challenge of mice, showed immunosuppressive activity of both AM and epithelial inflammation (380). Macrophages are also known to switch between or demonstrate a hybrid state of either pro-inflammatory (termed classical or M1) and anti-inflammatory (termed alternative or M2) activation states. This is dependent on the cytokine milieu that is present in their microenvironment (94). M1 type macrophages metabolize arginine to nitric oxide (NO), a molecule necessary for killing of bacteria, and are found under more pro-inflammatory circumstances. M2 macrophages instead metabolize arginine to ornithine, which limits NO synthesis (271). In general, the role of M2 macrophages is to clear apoptotic cells, mitigate inflammatory responses, and promote wound healing after injury. They do so by having a high phagocytic capacity, producing extracellular matrix components, and secreting IL-10 and angiogenic factors. The M2 activation state is induced by a broad variety of agents including immune complexes, complement, apoptotic cells, cytokines, such as IL-4, IL-13, IL-10, TGF-β, and macrophage colony stimulating factor (38, 121, 127, 278). As would be expected, the majority of macrophages in a resting lung are of the M2 phenotype.
In response to an infection that cannot be constrained, macrophages shift rapidly to an M1 phenotype. This is due to the presence of pro-inflammatory cytokines, such as interferon (IFN)-γ that can be produced by epithelial cells (332). In response to pathogen-associated molecular patterns (PAMPs) and/or alarmins, macrophages secrete the pro-inflammatory cytokines tumor necrosis factor (TNF)-α, IL-6, 1L-1α/β, and chemokines, such as CXCL8/IL-8, CXCL1/KC (26). Thus they work to orchestrate inflammation. Upon disease resolution, M2 cells again appear to help to drive the repair phases (185). Alveolar macrophages thereby prevent an excessive inflammatory response to daily environmental exposures (380), orchestrate the response to less frequent but severe infections, and induce tissue repair (242). Given that the lungs are now known to be colonized with microbes during health, it can be inferred that the presence of bacterial commensals alone is not sufficient to drive resident macrophages to the M1 phenotype.
1. Dendritic cells
Aspirated components are continuously sampled by resident dendritic cells (DCs) located within the airway epithelia, parenchyma, and visceral pleura (331); these cells connect the innate and adaptive arms of the immune system. In response to microbial challenge, and in a pattern recognition receptor-dependent manner, DCs produce the type I and III interferons necessary for an immediate host defense against both bacteria and viruses (151, 191, 222). DCs also process and present antigens to T cells and promote T-cell homing to the lung through their induction of the chemokine receptor CCR4 (262). DCs play a central role in both prevention and promotion of allergic airway inflammation. DCs prevent asthmatic reactions to harmless inhaled antigens by their presentation in a tolerogenic phenotype to T cells, which results in the development of IL-10 producing T regulatory cells and requires the absence of or a weak Toll-like receptor engagement by the DCs (7). A role for plasmacytoid DCs in this process is evidenced by the observation that their deletion resulted in immunoglobulin E sensitization, airway eosinophilia, goblet cell hyperplasia, and Th2 cell cytokine production (96).
Yet DCs are also capable of promoting an allergic Th2-mediated immune response depending on the antigen. When an allergen contains moieties that activate pathogen recognition receptors, such as TLRs, this results in the production of cytokines by DCs that instead promote a Th2 response (19, 302, 360). Thus a homeostatic versus pro-inflammatory response by DCs is dependent on the context during which the DC encounters the antigen and its physical properties. Of note, considerable evidence suggests that AMs are capable of suppressing DC antigen presentation and thus block development of an allergic response (168). This further highlights an important role for AMs in lung homeostasis.
This also suggests that persistent bacterial infections may promote an allergic response. Evidence that this occurs has accumulated from the airway pathogen Mycoplasma pneumoniae, whose CARDS toxin is now known to sensitize individuals for asthma (259). On the contrary, antibodies against certain bacterial surface components such as α-1,3 glucans, which are found on fungi and diverse bacteria including Streptococcus mutans (46), can, when generated in neonatal mice, suppress the subsequent development of an allergic response to common allergens such as from the cockroach (296). The effect of age on inflammatory responses associated with inflammatory disease or infection and its intersection with the microbiota will be discussed in detail in section IIIB.
2. Regulatory T cells
Regulatory T cells (T-regs) are specialized CD4+ T lymphocytes (CD25+Foxp3+) that protect against chronic inflammation, asthma, and development of autoimmunity (54, 201). They are resident within the lungs and play a key role in maintaining homeostasis (229). They function by suppressing CD4+ helper T-cell function. Investigators have shown that pediatric asthma patients have fewer T-regs and that these are more likely to be dysfunctional than those isolated from healthy subjects (158). Likewise, decreased expression of Foxp3 was observed in T-regs from individuals with chronic obstructive pulmonary disease (COPD) (180), suggesting that the activity of these cells is important for immune regulation. Accordingly, their transfer was sufficient to resolve airway inflammation in an OVA sensitized mouse model of airway reactivity; this was found to be dependent on IL-10 production (195). The role of T-regs in mediating interactions between the microbiota and the host has been mostly studied in the intestinal tract. Here, it is clear that T-regs play a pivotal role in intestinal homeostasis with potential pathogens (181). For example, transplantation of normal fecal human flora to germ-free mice resulted in an accumulation of colonic T-regs that was bacterial strain-specific and induced an anti-inflammatory environment (16, 118). In line with this, evidence suggests that T-regs are present from birth, and that their exposure to the local microbiota at this time drives tolerance to antigens (218). As such, and in combination with the generation of appropriate neutralizing antibody, the context and timing of human exposure to symbiotic microorgansims can result in a dysbiotic or protective response against environmental factors. At this point, as far as we are aware, there are very limited studies available that have investigated the role of T-regs in respiratory tract homeostasis, although we foresee this to be of major importance in future studies. Nonetheless, T-reg-mediated protection against for example asthma seems to occur before 6 yr of age (327), suggesting the same may occur for T-reg-mediated homeostasis induced by commensal bacteria that are part of the airway microbiome. The critical role T-regs play during pneumonia and in lung repair during the resolution phase will be discussed in section III.
III. PROCESSES INVOLVED IN A DISEQUILIBRIUM BETWEEN THE HOST AND THE MICROBIAL WORLD
Processes that are involved in disequilibrium between the host and the microbes inhabiting the respiratory tract are complex and can ultimately lead to dysregulation of the immune response resulting in allergic or autoimmune reactions or to dissemination of potential pathogens with resulting infections. Disequilibrium can be caused by microbial factors or external factors, such as antibiotic treatment or environmental irritants that induce changes of the host environment and/or microbial dysbiosis (TABLE 1). Disequilibrium can also be caused by pro-inflammatory host responses resulting from such changes or from external stimuli, such as smoking and air pollution, that result in hyperactivity and dysregulation of the immune response leading to allergic responses or tissue destruction that enables potential bacterial and viral pathogens to more readily induce acute or maintain or aggravate chronic infections (TABLE 1).
Table 1.
Summary of host and environmental events that have been shown to be associated with respiratory microbiota composition and lead to or are involved in acute or chronic respiratory infections and inflammation
| Factor | Impact on Microbiome | Type of Study | Reference Nos. |
|---|---|---|---|
| Microbial factors | |||
| Colonizing environment | Antimicrobial peptide IL-37 induces enhanced resistance of group A Streptococcus to ingestion and killing by human cells. | Human | 235 |
| Influenza A virus infection led to growth of colonizing pneumococci with increased ability to disseminate and cause infection in otherwise sterile sites. | Mouse | 244 | |
| Mice colonized with S. pneumoniae and then infected with influenza A virus showed increased pneumococcal colonization and disease. | Mouse | 103 | |
| Respiratory virus coinfection associated with high pneumococcal colonization density and invasive pneumococcal pneumonia. | Human | 384 | |
| After influenza A virus infection, dispersing bacteria had increased virulence, metabolism, and upregulated genes linked to immune avoidance. | Mouse | 301 | |
| Colonization with S. aureus followed by either influenza A virus coinfection or host physiological changes led to pronounced pneumonia development. | Mouse | 310 | |
| Increased likelihood of colonization with 3 species H. influenzae, S. pneumoniae, and M. catarrhalis and negative associations between S. aureus and S. pneumoniae and S. aureus and H. influenzae. | Human | 300 | |
| S. pneumoniae and M. catarrhalis negatively associated and S. pneumoniae and S.aureus positively asssociated. Colonization rate of H. influenzae high in presence of S. pneumoniae or M. catarrhalis. | Human | 388 | |
| Infection | During acute otitis media (AOM), the microbiome is less diverse and showed a reduced prevalence of commensal families. | Human | 165 |
| Patients with viral pneumonia show high abundance of M. lacunata, but those infected with nonviral pneumonia have high abundance of S. pneumoniae, H. influenzae, and M. catarrhalis. | Human | 321 | |
| Patients with progressive lung disease have reduced bacterial diversity, but total bacterial density remains relatively stable. Patients with a mild form of the disease maintain stable communities. | Human | 398 | |
| Virus-associated upper respiratory infections in the presence of increased abundance of S. pneumoniae, M. catarrhalis, and H. influenzae are associated with progress to more severe (lower) respiratory infections in infants. Individuals with high abundance of Dolosigranulum and Corynebacterium had reduced numbers of future infections. | Human | 352 | |
| Individuals with an acute URTI without otitis media had a microbiota profile with either Dolosogranulum and Corynebacterium or Propionibacteria, Lactococcus and Staphylococcus spp. Actinomyces, Rothia, Neisseria, and Veillonella are associated with AOM. | Human | 219 | |
| Antimicrobial treatment | With prior exposure to antimicrobials, infants with AOM show reduced prevalence of distinct commensal families and increased prevalence of Streptococcaceae and Corynebacteriaceae. | Human | 165 |
| Antibiotic treatment results in pronounced shifts in microbiome community structure. | Human | 398 | |
| Antibiotic treatment results in decreases in Firmicutes and Bacteroidales and increases in taxa including Betaproteobacteria, Gammaproteobacteria, and Bifidobacteriaceae in the gut. | Human | 13 | |
| The reduction in fitness associated with one resistance-conferring mutation in S. aureus is offset in coculture by interactions with microorganisms harboring alternative mutations. | Mouse | 154 | |
| Exposure to antibiotics in the first 2 yr of life delays microbiota maturations, particularly when exposure is between months 6 and 12. | Human | 39 | |
| Gut-lung axis | The commensal bacteria of the gut microbiome directly alter alveolar macrophage function through intestinal bacterial antigen signaling. | Mouse | 79 |
| Gut microbiota protect during pneumococcal pneumonia infection, enhancing alveolar macrophage function. Fecal transplant in microbiota-depleted mice normalizes pulmonary bacteria count. | Mouse | 328 | |
| During bacterial lung infections, the absence of segmented filamentous bacteria (sfb) in the gut can result in more severe S. aureus infection, which reduces when sfb were restored. | Mouse | 133 | |
| In the gut of infants with cystic fibrosis, fluctuations in the abundance of Parabacteroides precedes the chronic P. aeruginosa colonization of the respiratory tract. | Human | 167 | |
| A lack of intestinal microbiota leads to IL-10-mediated inflammatory hyporesponsiveness to bacterial pathogens exacerbating infection. | Mouse | 117 | |
| Host factors | |||
| Host immune response | Complement deficiency results in higher severity of acute lung injury or respiratory distress syndrome, and excessive complement leads to increased alveolar inflammation. | Mouse | 319 |
| During infection with H5N1 virus, acute lung injury is a result of excessive complement activation. Inhibition of complement or treatment of H5N1 significantly reduces lung inflammation. | Mouse | 347 | |
| Bacterial pathogens that produce pore-forming toxins target macrophages for necroptosis, but the early depletion of macrophages during pneumonia is detrimental to the host. | Mouse | 141 | |
| Neutrophil extracellular traps (NETs), produced by neutrophils, have bactericidal properties as well as involvement in disorders of inflammation and autoimmune disease. | Review | 368 | |
| Recruitment of polymorphonuclear leukocytes (PMN) within the first 3 h of intratracheal challenge with pneumococci leads to decreased pulmonary burden. | Mouse | 43 | |
| Neutrophil recruitment increases with S. pneumoniae infection, which if extended in duration is associated with loss of alveolar-capillary barrier function and excessive neutrophilic inflammation. | Mouse/human | 187 | |
| Host age | Age-related decline in immunity or immunosenescence could be a contributor to increased disease susceptibility in the eldery to S. pneumoniae infection in the respiratory tract. | Human | 209 |
| Naive elderly mice had increased proinflammatory expression and prolonged colonization of pneumococci compared with young adults, and delay in clearance was linked to immune dysfunction. | Mouse | 207 | |
| Compared with young mice, elderly mice had lower abundance of Bacteroidetes and upon S. pneumoniae challenge had reduced clearance of the pathogen and lower Bacteroidetes expansion post-infection. | Mouse | 208 | |
| In the oral cavity of elderly healthy individuals, there was reduced microbial diversity, but presence of a diverse group of anaerobic commensals negatively related to overgrowth of potential pathogens. | Human | 98 | |
| Environmental factors | |||
| Mode of birth | Respiratory microbiota development was delayed in caesarean-born infants and was also associated with reduced colonization of commensals like Corynebacterium and Dolosigranulum. | Human | 42 |
| Early in life infants born by caesarean have reduced populations of Bacteroidetes in the gut. During the first year caesarean infants have higher abundance of Clostridiales and Enterobacteriaceae. | Human | 39 | |
| Diet | The first 12–24 mo of life Lactobacillus, Staphylococcus, Megasphaera, and Actinobacteria are high abundant in feces of breast-fed children and Clostridiales and Proteobacteria in formula-fed children. | Human | 39 |
| Air quality | It is possible that the respiratory microbiome metabolizes inhaled pollutants/modulates host inflammatory responses to environmental exposure, so should be considered in exposure effects to inhaled irritants. | Review | 5 |
| Exposure to farms early in life provided a protective effect against asthma and allergy, which could be due to high microbial diversity and modulation of the immune response by the microbiota. | Review | 382 | |
| Proximity to poultry farms is associated with dysbiosis of the respiratory microbiome during and increased incidence of pneumonia. | Human | 341 | |
| Airborne dust and high temperatures are risk factors for bacterial meningitis, and each promoted progression from pneumococcal carriage to pneumonia and disease. | Human | 189 | |
| Home environment | An outbreak of Streptococcus pneumoniae which occurred in a closed community is associated with poverty and crowded living conditions. | Human | 90 |
| Nasopharyngeal bacterial carriage in young children in Greenland is globally high, and domestic crowding increased the likelihood of carriage. | Human | 281 | |
| Pneumoncoccal carriage is significantly associated with floor type, with those living in houses with earth floors fives times more likely to be carriers than those living with cement floors. | Human | 287 | |
| Urbanization is associated with isolation from the outdoor environment and increased space segregation by walls, which could lead to changes in the human microbiome. | Human | 318 | |
| Dog-associated house dust protects mice against allergen- or RSV-mediated airway pathology, which is associated with enrichment of Lactobacilli in the human microbiome. | Mouse | 128 | |
| Following a house move, the microbial community in the new house rapidly converged on the microbial community of the occupants’ former house. | Human | 220 | |
| Travel | Within an aircraft, the air microbiota seems enriched with human (pathogenic) bacteria when compared with outdoor air, which could be an important route of transmission of microorganisms. | Human | 205 |
A. Microbial Factors Leading to a Disequilibrium
1. Transition from colonization to infection
The specific molecular factors and signals that cause changes in the equilibrium between host and microbe in the nasopharynx resulting in the transition from a commensal colonization of the nasopharynx to dissemination and infection of other respiratory sites are not completely understood. The attack rate for each of the common respiratory tract pathogens is astoundingly low with around 10 invasive disease cases per 100,000 colonization events, although variations between strains and serotypes have been detected (50, 55, 157, 324, 340, 361). The overall low attack rate suggests that the major driving force for disequilibrium is related to activation of host systems due to changes of the colonizing environment (67, 235). However, it is important to acknowledge that certain strain backgrounds and serotype characteristics seem to be associated with different colonization efficiencies as well as invasiveness (177, 225, 378), or are differentially sensitive to minor changes in the environment, which could explain within-species differences in attack rates.
In a study by Marks et al. (244), changes in the colonizing environment in the host by influenza A virus infection resulted in release of colonizing pneumococci from biofilm growth and subsequent dissemination of the bacteria into the lungs and into the middle ears of mice, where these organisms caused severe inflammatory disease. This process was likely associated with changes in the microbiota from virus infection, resulting in overgrowth of the pathobiont (103, 384), but also with host immune activation. The effect could be reproduced by treating colonized animals with host stimuli associated with virus infection, such as increased temperature (mimicking fever), high levels of glucose and ATP (associated with virus-induced inflammation and tissue damage), as well as with norepinephrine (associated with the influenza-induced sympato-mimetic response) (146). Of further interest was that biofilms treated with influenza virus, heat, or ATP released bacterial populations had transcriptional profiles that were dramatically different not only from biofilm bacteria, but also from planktonic, broth-grown bacteria (301). These organisms showed an increased virulence and were far more inflammatory than both biofilm and broth-grown, planktonic organisms, which was associated with upregulation of genes linked to immune avoidance (capsule, proteases, choline binding proteins involved in complement evasion, and metabolism genes). This response suggests this to be an evolutionary response to a hostile environment driving attempts of horizontal spread to other hosts, rather than to induce infection (190), since infection will ultimately result in elimination either by death of the host or by clearance of the infection. Similar mechanisms have recently been shown also for the transition from colonization to infection by Staphylococcus aureus (310) and have been suggested for H. influenzae (49). Thus once a dysbiotic environment develops, pathobionts behave differently when compared with a healthy situation; this is certainly associated with changes in host environment but may well be associated also with changed interspecies interactions, and modulated by the resident microflora (300, 388). A wide range of microbial factors, or virulence factors, have been determined to be important in causing or maintaining colonization and causing disease. Excellent reviews specific to virulence factors involved in pathogenesis of disease are available for individuals pathogens (70, 109, 159, 182, 190, 276, 298, 365).
2. Microbiome changes causing disequilibrium and disease
Several studies have shown that the microbiome is less diverse during infections, such as during acute otitis media (AOM) and pneumonia, and that the microbiome is mainly associated with high abundance of S. pneumoniae, H. influenzae, and M. catarrhalis (98, 165, 321, 398). Following the microbiome composition of children over the first year of life, Teo et al. (352) showed that virus-associated infections were accompanied by transient increased abundance of S. pneumoniae, M. catarrhalis, and H. influenzae, which underlines the previously discussed inflammation-mediated enhancement of bacterial colonization. After an infection, it was more likely that the same individual developed a Moraxella abundant healthy microbiome, and was more prone to future infections, suggesting that the state of inflammation accompanying the infectious event modulated or determined the resident microbiota long-term. Individuals with higher abundance of Dolosigranulum and Corynebacterium, however, had fewer infections, suggesting that these bacteria are associated with higher resilience against viral and/or bacterial acquisition or infection or in other ways modulate the inflammatory signals associated with disease transition (352). Similarly, Laufer et al. (219) showed that individuals with an acute URT infection that did not develop otitis media had an increased protective microflora consisting of either Dolosogranulum and Corynebacterium or Propionibacteria, Lactococcus, and Staphylococcus spp. In contrast, individuals with AOM had higher abundance of the Gram-negative lipopolysaccharide (LPS)-expressing Haemophilus or more of the oropharyngeal bacteria Actinomyces, Rothia, Neisseria, and Veillonella in their noses compared with children with uncomplicated respiratory infections, again suggesting that the microbiome composition during health may influence host and pathogen interaction and behavior and determine the balance of asymptomatic colonization versus infection for the accidental pathogens in this niche (219). The question that remains is what is cause and what is consequence, i.e., whether mucosal inflammation leads to an altered microbiota with a parallel and selective overgrowth of pathogenic bacteria thriving in a more inflammatory milieu (207, 209), or whether the microbiota and the pathogens are the actual drivers of inflammation and consequently induction of infectious disease.
3. Antimicrobial treatment affects microbiome composition
Evolutionary processes form the basis for a normal equilibrium between host and the environment. Antibiotic treatment during infection is associated with major changes in the microbiome composition, although the microbiome has some levels of resilience and will reform after infection (165, 398). These results combined suggest that there are microbiome species that provide a healthy environment either by modulating the host response and/or regulating pathobiont behavior and that other species are less able to modulate disruptive events. This is also clear from experiments where various bacterial species were introduced, alone or in combination, in germ-free mice, showing that some species were associated with the establishment of a healthy microbiota, whereas others were involved in development of disease phenotypes (16, 118).
However, an alternative explanation for differences in community resilience against antimicrobial treatment is differences in susceptibility to antimicrobials of the commensal flora: selective elimination of those keystone commensals responsible for a strong equilibrium may in fact lead to an imbalanced microbiota and consequently provide a selective advantage for pathogens to overgrow and cause inflammation, whereas intrinsically resistant commensals will show less dysbiosis. This way, especially the commonly resistant group of Proteobacteria may persist and thrive under those circumstances (13). Since Proteobacteria show pro-inflammatory properties, their predominance has been related to induction of Th17-mediated inflammation, leading to a vicious cycle with increased disease incidence (174).
Additionally, genetic adaptation of potentially pathogenic bacteria, in particular, to antimicrobials reduces the ecological effects of treatment. Although antimicrobial resistance usually comes with a cost for the pathogen itself, in practice, they survive and even thrive in the host and population. This might be again a consequence of ecological forces as underlined by Hammer et al. (154); they observed that the reduced fitness associated with one resistance-conferring mutation in S. aureus can be offset by community interactions with microorganisms harboring alternative mutations or via interactions with the human microbiota. Ultimately, this may lead to enhanced growth, virulence factor production, and as a consequence, increased pathogenicity of potential pathogens, especially during or following antimicrobial pressure.
4. The gut-lung axis influences respiratory health
The main reservoir of bacteria and their gene pool, however, are present as part of the gut microbiome. The gut microbiome has been shown to influence host metabolism, and directly regulate systemic immunity. Evidence for the importance of the gut microbiome in respiratory health, also called the gut-lung axis, is accumulating rapidly. As an example, the gut microbiome also influences respiratory health by mediating innate immune functions; for example, several studies have shown that the commensal microbiota directly alters alveolar macrophage function through intestinal bacterial antigen signaling (79, 328). Also, neutrophil recruitment during bacterial lung infections appears to be affected by the absence of segmented filamentous bacteria in the gut (133). In the gut of infants with cystic fibrosis, one has observed that fluctuations in the abundance of specific bacterial taxa preceded the onset of chronic Pseudomonas aeruginosa colonization of the respiratory tract and were related to clinical respiratory outcome (167).
It is therefore no surprise that exposure to antimicrobials early in life has been associated both with major shifts in gut microbiota (133, 328) and expression of genes involved in mucosal defenses (84). In contrast, natural ‟prebioticˮ phenomena have been observed to affect lung health through gut function as well; for example, dog-associated house dust protects against RSV-induced inflammation by mediating Lactobacillus johnsonii enrichment in the gut microbial flora (128). On the other hand, absence of microbial stimuli from the gut microbiome leads to IL-10-mediated inflammatory hyporesponsiveness to bacterial pathogens exacerbating infection (117). An important missing link for most of these studies, however, is whether the observations are primarily the result of microbe-host signaling in the gut with long distance effects (via the gut-lung axis) like what was observed regarding transport of gut metabolites, such as short-chain fatty acids, towards the lung. Alternatively, the result of microbial selection and host-signaling processes taking place in both the gut and the lung may occur in parallel. A third option may be that these microbial processes are indirectly a consequence of host inflammatory processes in the patient and merely a bystander effect. For an extensive overview of the evidence of the gut-lung axis, see References 140, 230, 288, 358.
B. Host Factors
Host factors that contribute to a disequilibrium between the host, microbes, and their environment are diverse and encompass every aspect of host-microbe interactions (TABLE 1). The central consequences involve activation of, and cross talk between, signaling pathways and result in paracrine pro-inflammatory signaling, and as a consequence immune cell infiltration. These pro-inflammatory mechanisms have positive feedbacks at multiple levels, and their prolonged activation state in the lungs can result in tissue dysregulation and over longer periods of time dysfunctional tissue remodeling, which is suggested to provide a basis for chronic respiratory diseases, such as interstitial lung diseases (ILD) and COPD (88, 89).
1. Epithelial factors
As mentioned in section IIB, epithelial cells throughout the respiratory tract contribute to the equilibrium between the host, the microbiome, and colonizing potential pathogens primarily by limiting bacterial overgrowth, actively interacting with other cell constituents in the various respiratory niches, and retaining a physical barrier to the inside of the body. The epithelium does so by producing antimicrobial factors, such as antimicrobial proteins and peptides, surfactant, and other enzymes as well as transporting antibodies to the respiratory lumen that together with the components in mucus and the ciliated cells will kill bacteria, entrap them, and clear them from the airways under certain circumstances. In that sense, the epithelial cell works as a first-responder against bacterial and viral infection (72), yet in allowing asymptomatic pathobionts biofilm colonization.
Critically, and as evident in individuals who have cystic fibrosis, ion homeostasis is crucial for the epithelium to perform these functions and at the same time provide optimal gas exchange. Dysregulation of ion transport of chloride, sodium, and potassium as well as expression of water channels (aquaporins) can lead to disequilibrium and lung disease (254). Mutations in the cystic fibrosis conductance regulator (CFTR) gene results in the inability to transport chloride and thiocyanate ions across epithelial cells. Due to this loss of osmotic potential there is a thickening of mucus leading to its inability to be effectively cleared by the mucociliary escalator. Such individuals are therefore at risk for bacterial overgrowth leading to chronic bacterial infections, in particular those caused by Pseudomonas aeruginosa (303). Similarly, air pollution and industrial exposure to irritants that alone or in combination with reactive oxygen species from oxidative stress cause an aberrant ion transport in the epithelial membrane and cytokine production that promote inflammation (56, 389). Aquaporin-5 is important for fluid homeostasis in the lung, and downregulation of this aquaporin in epithelial cells in combination with an upregulation of the mucin producing gene muc5AC plays an important role in the development of viscous secretions during COPD (391).
2. Secreted factors that activate complement
Complement is a key innate defense protein cascade, whose effector molecules are present in the airway and are involved both in its defense against harmful microbes and in some instances in causing injury (292, 354). Three biochemical pathways activate the complement system: the classical complement pathway, the alternative complement pathway, and the lectin pathway. These are activated by IgG and IgM recognition of antigen, stochastic turnover of serum protein C3, and recognition of bacterial surface components by diverse lectins (see below), respectively. Complement activation initiates a proteolytic cascade that ultimately deposits opsonizing proteins on the surface of the bacterium and leads to the formation of the membrane attack complex (MAC). The MAC complex effectively kills Gram-negative bacteria but is considered irrelevant in the defense against Gram-positive bacteria, although surface deposition occurs (24, 34, 186). Of major importance, especially in the presence of Gram-positive organisms, the chemotactic properties of C3a and C5a serve to recruit neutrophils to the site of activation to kill the invading microorganisms (292). C-reactive protein (CRP), produced by the liver, is also found in mucosal secretions of the airway. CRP binds to phosphorylcholine residues that are present on respiratory tract pathogens, such as S. pneumoniae (266) and H. influenzae (238), and activates complement via the C1q complex in the classical pathway (256). Collectins, including surfactant A and surfactant D, are soluble pattern recognition receptors (PRRs) found in the lower airways (152). Along with proteins such as ficolin and mannose-binding protein, these innate immune effectors recognize conserved oligosaccharide structures on the surface of microorganisms leading not only to their opsonization but also to activation of the complement cascade by various means, including intermediates such as CRP (202, 317). While complement deficiency results in exquisite susceptibility to bacterial infection (92, 292), unregulated excessive complement activation is capable of considerable tissue damage (319); this is particularly evident following viral infection (347). It is of note that both C3a and C5a are capable of triggering degranulation of mast cells and increase vascular permeability, further promoting lung inflammation (292). Thus one must consider that during bacterial dysregulation, activation of complement by various means is occurring with consequences on immune cell recruitment and infiltration.
3. Phagocytic cell activation and cytokine production
Macrophages and dendritic cells are most likely the first immune cells to encounter an aspirated and now replicating pathogen in the airway. It can be speculated that it is for this reason that bacterial pathogens target macrophages for necroptosis with pore-forming toxins, and why their early depletion during pneumonia is detrimental to the host (141). As indicated, above, in response to engagement of Toll-like receptors by pathogen-associated molecular patterns (e.g., LPS) on their surface, M1 macrophages can become activated and secrete pro-inflammatory cytokines such as TNF-α, IFN-γ, and IL-6; chemokines such as CXCL8 (IL-8); T cell stimulating factors such as IL-12; and upon receipt of a second signal such as the aforementioned pore-formation are capable of activating the inflammasome and produce IL-1β and IL-18 (119, 145, 330). Some of these factors, such as IFN-γ, act in an autocrine manner, augmenting the ability of macrophages to kill phagocytosed bacteria. Others serve to activate epithelial and endothelial cells and result in their production of antimicrobial factors and the expression of integrins and selectins that are used by neutrophils and other immune cells for diapedesis and consecutive tissue infiltration (125, 315, 316, 355).
Neutrophils are the primary immune cell responsible for clearance of extracellular bacteria and fungi. These myeloid-derived cells rapidly infiltrate the airway during infectious episodes and can be seen as early as 8–12 h after experimental challenge of animal models with pathogens such as S. pneumoniae (25, 348). Neutrophils kill microorganisms by various means including phagocytosis, degranulation that releases bactericidal molecules from secretory vesicles, the production of reactive oxygen species, protease activation from phagosome acidification, and NETosis (83, 192, 279, 311, 368). Neutrophils contain distinct forms of secretory granules, where secretory vesicles are released first, followed by gelatinase granules, specific granules, and then azurophilic granules (299, 333). These granules contain distinct but also overlapping pro-inflammatory and potentially self-damaging factors such as phospholipases, elastases, and collegenases. Activated neutrophils produce reactive oxygen species (ROS) via the action of NADPH oxidase that is assembled at the plasma membrane following activation (302), and ROS are highly bactericidal but also damage neighboring cells. In turn, neutrophils themselves have been reported to produce IL-1α, IL-6, TNF-α, and other pro-inflammatory factors, supporting the cascade of inflammatory events (299).
Importantly, the prolonged presence of neutrophils in the airway worsens tissue damage. Ideally, once neutrophils are recruited to the airway, pathogen clearance is efficient, and neutrophils are removed via immunoquiescent apoptosis. Extended duration of neutrophils has been linked to loss of alveolar-capillary barrier function and development of disseminated disease; in certain instances, neutrophil depletion during infection has being shown to reduce disease severity (43, 187). Lack of neutrophil apoptosis has been implicated in numerous inflammatory diseases not just within the airway (170).
4. Host-microbiome-environment interactions at the extremes of life
As described above, neonatal host interactions with the microbiome can have consequences related to the development of allergic disease later in life (see also sect. IIIC). Advanced age itself also has an impact on the microbiome composition and its level of resilience. This is thought to occur as a consequence of deterioration of the immune system, called immunosenesence, and the accompanying process of low-grade chronic systemic inflammation, named inflammaging (209). In support of this notion, mouse studies have shown an association between low-grade mucosal inflammation in elderly and respiratory microbiota composition during health, although in this regard cause and consequence are hard to decipher. Furthermore, they showed greater disruption of the preexisting microbiome upon challenge with S. pneumoniae in elderly mice than young adult mice, and reduced clearance of the pathogen, accompanied by a lack of activation of the necessary immune pathways (207, 208), underscoring that in elderly patients a balanced and diverse microbiome might even be more important than in young adults to keep these individuals healthy (277). Also in human adults, reduced diversity and presence of a diverse group of anaerobic commensals in the oral cavity of elderly healthy individuals was observed, commensals that were also negatively related to overgrowth of potential pathogens during pneumonia as well (98). This might not only suggest a role for these bacteria in level of resilience against pathogen overgrowth and disease, but also underline these changes in microbiota composition are a consequence of the inflammaging process, which potentially makes it more difficult to intervene with. Despite this, it is, like with other chronic inflammatory conditions, important to realize that elderly individuals, even those appearing healthy, might respond differently to infectious and inflammatory triggers. Besides increased risk of severe disease, recovery might be slower and less complete, thereby increasing the likelihood of recurrent or exaggerating problems over time (35).
C. External Factors
It has been known that environmental factors are the main drivers of microbial colonization of the respiratory tract from birth onwards (TABLE 1). Environmental effects that not only impact pathogen acquisition and colonization but also the entire microbial population process directly from birth on are the mode of delivery at time of birth, infant feeding, crowding factors, smoking, as well as antibiotic exposure (42, 140, 205). The mode of delivery directly affects the microbial population process of infants, as it is the first encounter with the external microbial world and these microorganisms freely occupy this empty niche (42). Feeding is a second important driver, and thought to drive microbiome development early in life in multiple manners. First, breast milk contains immune-modulatory and protective proteins that may help to prevent pathogen colonization and infection (234). Second, breastmilk contains a wealth of oligosaccharides that may promote beneficial microbes, not only in the gut, but also in the respiratory tract (73, 283, 284). Lastly, breastmilk contains microbes itself, and this may contribute to a healthy development and diversification of the infant’s microbiome (140). Consequently, antibiotic treatment, especially early in life, will alter these microbial population processes dramatically. Antimicrobials have been suggested to have major consequences for immune development and future health (33, 39). Crowding factors are also important for the timing and intensity of microbial exposures, in particular to those that may become pathogenic. As discussed above, the lack of certain microbial exposures or colonization with others may affect susceptibility to infections as well as development of atopic diseases and asthma.
Accordingly, the macroenvironment affecting air quality, including dust, fine particulate matter, allergen, microbe, fungi, and virus exposure, will also influence host-microbe interactions in the respiratory tract over time (5, 124, 203, 230, 249, 341, 382). For example, the interaction between inhaled particles and the host may affect inflammatory processes in two ways, by either preventing or inducing airway inflammation. This is suggested by observations by for example Fujimura et al. (128), who observed that dog-associated house dust protected mice against allergen- or RSV-mediated airway pathology, which was associated with enrichment of Lactobacilli in the human microbiome. However, individuals are also variably exposed to outdoor and indoor environmental pro-inflammatory triggers, a consequence of varying degrees of urbanization. As an example, evidence is accumulating that exposure to fine particulate matter from fuel emissions, working environments, and large-scale farming affect respiratory health by inducing a disequilibrium at the mucosal surfaces (341, 357). These usually chronic long-term exposures might have a dramatic effect on human microbiome development as was underscored by Ruiz-Calderon et al. (318).
Differences in exposure to environmental triggers might also explain the differences in pathogen colonization rates as well as RTI incidence between developed and underdeveloped countries. Environmental factors such as pollutants from indoor wood burning stoves and car exhaust are highly different and may explain some differences. Likewise, family dynamics are different; for example, in the developing world, numerous individuals of different ages belonging to an extended family might be living in close quarters. This occurs less frequently in the industrialized world where families are more nuclear (90, 189, 281, 287, 313). Unfortunately, for none of the above, mechanistic evidence regarding epidemiological associations between environmental factors and microbiome composition is yet available.
Along such lines, the human microbiome seems to influence the local microbial environment, which in turn might affect the microbial community composition of other occupants or family members. In a recent study by Lax et al. (220), it was found that the home microbiome was largely sourced from humans and that following a house move, the microbial community in the new house rapidly converged on the microbial community of the occupants’ former house. Also, analysis of aircraft air microbiome showed enrichment of air microbiota with human (pathogenic) bacteria when compared with outdoor air, supporting that aircraft air is an important route of transmission of microorganisms including infectious agents (205). These data also underscore the complex interactions between the environment and the human host allowing mutual adaptation and coevolution. For an additional overview of environmental drivers of respiratory microbiota, see References 140, 230, 250, 358.
IV. HOW A DISEQUILIBRIUM MAY LEAD TO CLINICAL DISEASES
A. Acute Respiratory Infections
Given the above, development of infection, i.e., pathogenic state, may be the result of a disequilibrium at the mucosal surfaces of the respiratory tract, resulting in microbial dysbiosis, inflammation, and overgrowth of potential pathogens (99, 249). For example, middle ear infections are thought to find their origin in dysbiosis of the upper respiratory tract or nasopharyngeal microbiome. Since that is also the preferred niche for pathobionts like S. pneumoniae, Streptococcus pyogenes, H. influenzae, P. aeruginosa, and S. aureus during asymptomatic colonization, signals arising from dysbiosis (e.g., ATP, raise in temperature) might be the very reason why these pathogens are now able to cause a disease phenotype (243, 363). Alternatively, it may be due to reduced numbers of niche-specific commensals and competitors such as Corynebacterium and Dolosigranulum (219). Obviously, to get from a disequilibrium towards an acute clinical infection, more is needed than low-grade inflammation and microbial dysbiosis. Environmental triggers such as a viral infection, irritants, and allergic responses seem to be needed to tip the balance. For example, inflammation associated with such triggers results in tissue swelling, bacteria ascending up the Eustachian tube, together with dysfunction of the latter, resulting in an acute middle ear infection (326).
Lung infections in otherwise healthy individuals are associated with different pathobionts, although S. pneumoniae and S. aureus are commonly found in lung infections as well. Other bacterial pathogens include Gram-positive microorganisms such as Enterococcus faecium and Gram-negative organisms such as Klebsiella pneumoniae and Enterobacter spp (10, 294). These bacteria all tend to be part of the oropharyngeal more so than the nasopharynx flora. The fact that the oropharynx has closer proximity to the lung, and lung infections are strongly associated with micro-aspiration, especially in older individuals, might explain this coinciding observation. Supportive evidence can be found in recent findings of de Steenhuijsen Piters et al. (98), who observed in individuals with pneumonia that bacterial outgrowth in general, combined with relative overgrowth of individual pathobionts within the oropharyngeal microbiota was strongly correlated with lung disease. Additionally, bacterial overgrowth and disease coincided with the absence of a specific group of facultative anaerobic oral commensals, which suggests their role in containment of pathobiont overgrowth in this niche. These potentially protective anaerobes were of different phylogenetic origin, including Prevotella (Bacteroidetes), Veillonella (Firmicutes), and Leptotrichia (Fusobacteria) spp., which all had previously been associated with absence of other respiratory disease phenotypes such as hospital-acquired pneumonia, periodontitis, asthma, and COPD (44, 69, 164, 325). Whether the key to protection is species specific or lies in the diversity or evenness of the microbial community is not fully understood, although evidence is accumulating that both diversity (45, 241, 263, 304) as well as evenness (63) seem to contribute to mucosal homeostasis. However, here as well, a state of dysbiosis is not directly associated with the actual occurrence of infection: a trigger, such as increased aspiration, viral infection, poor oral hygiene, or a supine position (as seen in institutionalized or hospitalized patients) is again needed to tip the balance towards an actual acute infection (FIGURE 2) (178, 209, 232, 233, 290). For example, studies have shown that influenza A virus infection, through type I and III interferon signaling, inhibits Th17 activation and consequently suppresses IL-23 production and antimicrobial peptide production. In turn, this inhibits immune cell recruitment and bacterial clearance, making the host more susceptible to acute S. pneumoniae and S. aureus lung infection (221, 293). Modulation of the interferon response in the lung by IFN-receptor knockouts or exposure to the anesthetic halothane could reverse this increased susceptibility to bacterial superinfection (239). Interestingly, exposure to influenza virus not only modulates the inflammatory response but has recently been shown to also affect the microbiome composition to bacterial species that promote the growth of Bacteriodetes and Staphylococcus species, including S. aureus (102, 351). This provides an additional dimension to the mechanisms of virally induced trigger of bacterial infection.
It is noteworthy, however, that a bacterial infection is not merely triggered by a concomitant viral infection, but that the respiratory microbiome may in turn also modulate susceptibility to and severity of viral infections. For example, de Steenhuijsen Piters et al. found a strong association between the respiratory microbiota composition during early RSV infection and severity of disease (100). This was strongly linked to increased expression of pro-inflammatory pathways including Th17-mediated immunity and neutrophil and monocyte activation and recruitment (100). Wang et al. (370) published a plausible explanation for this phenomenon; they showed how bacterial communities could increase or dampen influenza-mediated inflammation and lung injury through induction of M1 or M2 alveolar macrophages, respectively.
With the current body of evidence, it is therefore plausible to think that parallel to the level of equilibrium between the host and microbial environment, an additional (environmental) trigger such as viral infections, but potentially also noninfectious triggers such as smoke, air pollution, and allergens are guiding susceptibility to disease, disease phenotype, as well as disease severity. An overview of the events needed to generate disease, based on a series of potential pathways, such as microbial disequilibrium, host-responses affecting susceptibility to and severity of acute infections, is summarized in FIGURE 1.
B. Infections and Inflammation During Chronic Respiratory Disease
There is accumulating evidence that the respiratory microbiome plays a catalyzing role in chronic inflammatory respiratory diseases as well. In upper respiratory infections such as chronic sinusitis, no consistent patterns have emerged to implicate a specific organism as causative. However, data suggest that a less diverse and imbalanced microbiota with reduced immunomodulatory capacities, rather than the specific pathobionts occupying the niche, might drive chronic inflammation in these patients (77, 308, 345). Even more importantly, recent data suggest that microbial diversity combined with the presence of certain Actinobacteria such as Corynebacteria was associated with a better outcome of surgical intervention of chronic sinusitis (308). Since severity scores were not different between patients with a good outcome versus poorer outcome, one might speculate that bacterial diversity and/or the presence of specific commensals limit postoperative outgrowth and infections by pathogens directly, or indirectly modulate wound healing or immune responses (216).
Reduced microbial diversity as well as the presence and absence of specific bacteria are also related to increased inflammation in lung diseases such as COPD, cystic fibrosis (CF), idiopathic pulmonary fibrosis (IPF), and asthma (80, 175, 267). For example, in COPD, a strong correlation between disease and presence of more pro-inflammatory Proteobacteria (especially H. influenzae) and absence of Firmicutes (Streptococcus spp.) and low inflammatory Bacteroidetes (mostly Prevotella spp.) were observed (349). Also, in acute exacerbations of diseases in patients with chronic lung problems such as COPD and CF, overgrowth of primarily oropharyngeal pathogens such as nontypeable H. influenzae and Pseudomonas spp. has been observed (105). Again, decreased diversity in general, as well as the absence of specific commensal bacteria such as Prevotella spp., is associated with exacerbations of disease in CF and COPD patients (155). This central, possibly even antagonistic, role for anaerobic species like Prevotella might be a consequence of its intrinsic immunomodulatory capacities: in contrast to Gram-negative bacteria of the Proteobacteria (like H. influenzae), the tetra- and penta-acylated LPS of Bacteroidetes spp. has little TLR4 stimulatory activity (217). Bacteroidetes-associated LPS even antagonizes hexa-acylated LPS-mediated TLR4 signaling, dampening downstream signaling, thereby potentially contributing to mucosal homeostasis and preventing pathogen overgrowth (129).
For long it has been known that the presence and abundance of Pseudomonas is directly associated with exacerbations of CF disease and not affected by treatment, the latter emphasizing a high resilience of this bacterial spp. against antimicrobial agents (88, 114). In contrast, recent evidence showed that pathogenicity of P. aeruginosa seems to be at least in part mediated by cosignaling (cooperation) of other bacteria, which is underlined by studies of antimicrobial efficacy against Pseudomonas infections, where antimicrobial regimens for which Pseudomonas is intrinsically resistant still reduce symptoms, suggesting secondary microbes to be involved in exacerbation of Pseudomonas-related disease (314). The mechanisms behind these observations are still unknown, although studies on coinfection with S. aureus and P. aeruginosa have shown to increase Pseudomonas persistence, expansion, and virulence by direct metabolic interactions (126, 260). It is unfortunately still unclear which commensal species might likely contain Pseudomonas from overgrowth or which ones seem to support its pro-inflammatory role. In the light of severity of this disease and the limited treatment options available, expanded investigations on pathogenesis of Pseudomonas infections towards a microbial community perspective should be prioritized.
In recent years, a paradigm shift has also occurred with regard to our understanding of asthma pathogenesis; where previously sterile inflammation, autoimmune phenomena, and allergies were blamed as the cause for this clinical disease, there is now a plausible role for the microbial respiratory ecosystem in maintaining and inducing exacerbation of asthma (111, 115, 147, 217). Again, potential pathogens like Hemophilus, Pseudomonas, Lactobacillus, and Rickettsia species were enriched in lungs of asthmatic patients, whereas Prevotella, Streptococcus, and Veillonella species had a reduced presence, especially in the neutrophilic asthma phenotype.
In addition, reduced bacterial diversity was correlated with decreased lung function and increased inflammation in asthmatic patients, again suggesting a role for the microbial ecosystem in mucosal homeostasis (101, 338) and chronic immune dysregulation (194, 392). Aside from the development of appropriate B-cell responses and T-regs during the neonatal period (as discussed above), experts in the field have suggested that many of these processes occur as part of a vicious cycle of inflammation and epithelial damage. Even if the initial trigger is long gone, the underlying inflammation together with the recent mucosal damage may have resulted in a cyclic process of mucosal surface changes, and selection and outgrowth of pro-inflammatory bacteria, which in turn may maintain a dysbiotic state (230, 269). One example of this is the CARDS toxin produced by Mycoplasma pneumoniae (258, 259).
Although asthma is generally treated with immunosuppressive and/or modulating medication like inhalation corticosteroids or long-acting beta-agonists (75), in case microbes are triggering or maintaining an infectious and/or inflammatory situation, a true equilibrium might not be restored with these medicines alone. That is the reason why lately exacerbations or prevention of exacerbations in especially severe neutrophilic asthma in particular is treated with macrolides, which has fairly good coverage for potential pathogenic bacteria, but also have immunomodulatory properties helping to prevent virus-induced illness as well (385). Although macrolides might reduce the load of pro-inflammatory bacteria, it is unlikely that they help to restore the healthy commensals theoretically needed to keep the potential pathogens at bay and induce a regulatory state allowing repair and restoration of healthy mucosal barrier. Much work needs to be done to elucidate when antimicrobials might be useful, what antimicrobials might be most optimal, and how to counterbalance their potential side effects on the microbiome.
Finally, a new area of chronic respiratory disease has been identified where environment-host-microbiome interactions may be crucial for development, maintenance, and/or deterioration of ILD (268, 270), since pilot data not only showed microbial overgrowth during exacerbations of disease, but also a reduction in Gram-positive bacteria and an increase in Gram-negative bacteria of the Proteobacteria family during exacerbations. Others have found a specific association with the potential pathogens Staphylococcus and Streptococcus and deterioration of lung function over time, strengthening the idea that bacterial changes are not only a consequence of exacerbations but potentially also of their presence during basal nonclinical periods of disease (156). Much needs to be done, however, to unravel whether respiratory infections, severe or recurrent, are also the foundation in childhood or young adulthood for development of syndromes like COPD and ILD later in life.
V. HOW TREATMENT STRATEGIES AFFECT THE MICROBIOME
A. Current Interventions That Potentially Impact the Microbiome
The current treatments and preventions of symptomatic respiratory tract infection rely primarily on vaccines and antibiotics. Although these approaches are life-saving, they both have unanticipated consequences and limitations that fail to take the complete ecological niche into account. These problems and alternatives will be discussed here.
1. Vaccines
In most countries, recommended immunizations for children under 6 yr includes vaccines against the bacterial airway pathogens H. influenzae type b, the pneumococcus Bordetella pertussis, as well as seasonal influenza and other viruses capable of airway replication including measles, mumps, and rubella (1, 65, 82). These interventions have reduced the morbidity and mortality by these pathogens dramatically, especially in the highest risk group for such infections, children under 5 yr of age. However, it is fair to presume that blocking colonization of the microorganism or reducing their infectious burden following an exposure has consequences on the composition of the host microbiome and potentially its stability when challenged. Herein, we discuss the pneumococcal vaccine as a prototype with vaccines for Hemophilus having similar concerns.
The original pneumococcal conjugate vaccine, PCV7 (Prevnar), containing 7 out of the 97 identified serotypes (134) was designed to protect against invasive pneumococcal disease (IPD), and immediately on its introduction in child vaccination in the year 2000, it showed a significant effect (1, 52, 112, 179). The protection was over 95% against infection caused by vaccine serotypes and ~90% against IPD caused by all serotypes in the United States. Associated with this, a marked reduction and almost complete eradication of meningitis cases was also observed (183, 307). Additionally, a significant herd immunity in elderly and other nonvaccinated individuals was observed due to the fact that the vaccine effectively reduced colonization with the vaccine serotypes, thus minimizing spread (1).
However, the main problem with the vaccines have been that eradicated serotypes have since been replaced by non-vaccine serotypes that initially had a higher level of antibiotic resistance, and in turn this kept the overall level of pneumococcal colonization unaffected (60, 173, 356, 377). Additionally and recently, novel unencapsulated strains have emerged in colonization and disease (196). This has been addressed by developing conjugate vaccines with additional serotypes. PCV10 (Synflorix), where eight capsular polysaccharides are conjugated to H. influenzae protein D, and Prevnar 13, a similar vaccine to the original Prevnar (PCV7) but including the serotypes that appeared post-PCV7, are two examples, and Prevnar-15 is currently undergoing clinical studies (255). However, despite the success of these vaccines in reducing mortality and morbidity in target populations, they fail to protect to any major degree against common local pneumococcal infections, such as all-serotype pneumococcal AOM (123) and nondisseminating pneumonia (1). Also, there is an ongoing evolution in the respiratory tract with expansion of new and minor serotypes and clones both due to the expanded opportunity in the absence of more successful serotypes from the past and to successful serotypes/clones in the current vaccine receiving novel genetic material, including genes for switching capsule (51, 85, 86, 130). It is now known that the vaccine has changed the selective forces present within the nasopharynx, resulting in an increase in the relative abundance of non-pneumococcal streptococci and anaerobic bacteria, as well as Hemophilus and Staphylococci (29, 95, 342). Along these lines, the current colonization rates with S. aureus are increased from those observed in the late 1990s (37, 95, 335, 342, 396), with colonization rates between 10 and 50%, dependent on age. Although the two potential pathogens are hard to compare with respect to health risk, these findings at least indicate a possibly unintended negative impact of eradication of specific serotypes on child health (29, 165). Although direct protection against IPD has increased with release of an expanded 13-valent PCV13 vaccine (23), the long-term protection, due to serotype replacement, is unclear, and it has been proposed that other means of protection through protein candidates may provide a better solution (143). Of note, no studies have investigated the effect on the microbiome after immunization with a protein antigen. Importantly, all these vaccines eradicate colonization, which on the one hand provides elimination of the pathobiont but may provide other problems as it is uncertain what organisms will fill the niche instead and what health effects the change in the microbiome-pathobiont balance will provide in the future.
An alternative approach has recently been suggested, based on the differences in surface expression of proteins in biofilm bacteria that colonize the nasopharynx and biofilm released bacteria that cause infection (301). By using a systemic immunization protocol, proteins were chosen that were highly expressed in virulent organisms and had a low expression in colonizing biofilms. With the use of this approach, animals immunized with 10 conserved surface proteins, of which PspA was one, and where colonized animals were challenged with influenza A virus to trigger AOM, pneumonia, and sepsis, showed that immunization protected against and eradicated bacteria causing disease, but had no effect on colonization (149, 223, 224). Similar results were obtained by Glover et al. (137) when immunizing with PcpA that protected against pneumococcal pneumonia and sepsis, but did not affect colonization. Therefore, this approach may be more beneficial compared with total eradication as it would not affect the potentially beneficial colonization of Streptococci and simultaneously have less of an effect on the remaining microbial community members in the nasopharynx.
2. Antibiotics
Immunodeficient, immunosuppressed, or individuals with chronic morbidities that place them at risk for infection may take continuous prophylactic antimicrobials to prevent acquisition of potential pathogenic bacteria or fungi (62, 212, 291). For example, children with sickle cell disease are often on prophylactic penicillin treatment for the first 5 years of life to prevent invasive bacterial infections, such as that caused by the pneumococcus (53, 166). Of note, pneumococcal disease is promoted by host inflammation, which upregulates the bacterial ligand platelet-activating factor receptor. This occurs during sickle cell disease (264) and is one example of a dysbiotic host environment enabling disease. In turn, prophylactic antimicrobial therapy results in individuals being colonized with a subset of pneumococci that are distinct from those found in the general community, but maintain their virulence (59). Along similar lines, individuals with COPD are often placed on prophylactic macrolide therapy, which is not only antimicrobial but also anti-inflammatory, inhibiting activities of both the innate and adaptive immune system (285). While the benefits of these treatments against invasive infection are clear-cut and in some instances lifesaving, this treatment obviously results in changes in the normal airway microbiome. This in turn can lead to greater susceptibility to infection by dysbiosis in combination with selection of resistant strains of pathogenic bacteria (64, 352).
Antibiotic resistance in respiratory tract organisms has increased to the degree where several organisms are monitored closely in the CDC’s active bacterial core surveillance program. Antibiotic resistance in general has become so extensive that the World Health Organization recently reported the risk of a post-antibiotic era if alternative approaches were not taken (381). While prudent use of antibiotics is in many instances life saving, antibiotics also alter the polymicrobial interactions in the respiratory tract and have deleterious effects on the human microbiome in general (93, 312, 364). Early antibiotic treatment is now also thought to be one reason for the development of allergies (251); thus the development of novel antimicrobial agents needs to be more targeted and have less effects on the microbiome. At minimum, broad-acting antibiotics should be used judiciously.
B. Possible Interventions That Do Not Disrupt the Microbiome
A more targeted way to interfere with infection or colonization is to use molecules that are not in themselves antibiotics, but interfere in other ways with the disequilibrium processes discussed earlier (9). One such way would be to resensitize antibiotic-resistant bacteria to the antibiotic they are resistant to either by interfering with bacterial physiology (74, 110, 244) or by inhibiting drug efflux pumps (116, 252), although these still may alter microbial community compositions. Other ways that are more targeted are to use bacteriophages specific for a certain host species (58, 393) or to specifically interfere with biofilm formation of individual bacterial species using antibodies or peptides interfering with quorum sensing (6, 47, 48). Finally, a fair amount of effort is put into activating or modulating the immune system to better eliminate pathogens. Examples are statins that induce NET formation by neutrophils (78) to facilitate autophagosome formation against enteric pathogens (231) or to boost innate immunity or block host factors required for bacteria to replicate (280).
1. Probiotics
Probiotics have been used successfully to reduce disease with S. pneumoniae and induce higher protective immunity against colonization, so far mostly in animal studies (227). One form of a probiotic for infants and young children is human milk. Breastfeeding is known to protect infants against respiratory and other infections. Studies by Biesbroek et al. (27) showed a strong association between breastfeeding and a protective microbiome in infants. Recent information suggests that one important component that directs the development of the microflora, at least in the gut but likely also in the respiratory tract, is the numerous carbohydrate species (around 200) present in human milk, that constitute specific growth substrates for “healthy” microbiome species and cannot be used by the infant host for energy (193, 283). Human milk also contains immunomodulatory factors that together with milk oligosaccharides play a role in shaping a healthy immune system and preventing dysbiosis in the form of inflammatory diseases such as allergies and autoimmune disorders (61).
2. Microbiome as a target
Considering that there is ample evidence for a role of the more complex microbial ecosystem in pathogenesis of especially chronic respiratory infections and inflammation, this information can also be used to reevaluate diagnostic (139) and therapeutic strategies (322). Infections that are (partially) driven by their biogeographical context can also be targeted from their polymicrobial and environmental perspective. For example, Pseudomonas infections are commonly enhanced by commensal interactions; depleting the commensals instead of the antimicrobial resistant pathogen by antimicrobial components, including synthetic antimicrobial peptides, might likely result in improvement of clinical symptoms, even if the pathogen itself will not disappear.
In line with the accumulating evidence that reconstitution of the gut microbiota is highly effective in fighting enteric infectious diseases (257, 339), restoring or ‟transplantingˮ a healthy respiratory ecosystem for example by combining a limited number of community members that behave as keystone species for a balanced microbial ecosystem might be within reach (228). Alternatively, strategies that support host barriers, for example (re-)introduction of commensal species like Bifidobacteria that enhance mucosal barrier functions and reproduce themselves by itself or in conjunction with the host anti-inflammatory molecules that fight potential pathogenic microorganisms are likely to become candidates for new therapies as well (228). Finally, during chronic infections, biofilm formation is currently significantly complicating the treatment and recovery, especially limiting the effects of antimicrobial therapy (22, 67). However, certain commensal species might actually be capable of destroying biofilm matrix, thereby reexposing the pathogen to effective eradication strategies; an example of this is the use of Staphylococcus epidermidis which not only destroys S. aureus biofilms, but also enhances S. aureus susceptibility to host antimicrobial peptides (150).
VI. CONCLUSIONS
In conclusion, a new era has arrived where the rapid development of technology has allowed us to characterize respiratory health and disease in much more detail and from multiple angles including microbiome-derived and host response data. This detailed information allows us to obtain a much better picture of host-microbe-environmental interactions leading to potential ways to sustain health and avoid acute or chronic infectious diseases. Moreover, once we will be capable of integrating these data with developmental information and other patient characteristics in a standardized manner, personalized or precision medicine becomes within reach, allowing us to immediate clinical decision making and improve abilities to monitor therapeutic responses for the individual patient (22, 67, 76).
GRANTS
This work was supported by Division of Intramural Research, National Institute of Allergy and Infectious Diseases Grant AI114800, National Institute of General Medical Sciences Grant AI117309-01, National Institute on Deafness and Other Communication Disorders Grant AI121614-01, National Institute on Aging (U.S. National Institute on Aging) Grant AG055144, Nederlandse Organisatie voor Wetenschappelijk Onderzoek (Netherlands Organisation for Scientific Research) Grant vidi-91715359, and the Chief Scientist Office Grant SCAD/16/03.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Rebecca Watson for her support.
Address for reprint requests and other correspondence: D. Bogaert, Center for Inflammation Research, Queens Medical Research Institute, University of Edinburgh, Edinburgh, UK (e-mail: d.bogaert@ed.ac.uk).
REFERENCES
- 1.Pneumococcal conjugate vaccine for childhood immunization–WHO position paper. Wkly Epidemiol Rec 82: 93–104, 2007. [PubMed] [Google Scholar]
- 2.Pneumococcal vaccines WHO position paper–2012. Wkly Epidemiol Rec 87: 129–144, 2012. [PubMed] [Google Scholar]
- 3.Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, Wanjiru E, Jomo J, Musyimi R, Lipsitch M, Scott JA. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One 7: e30787, 2012. doi: 10.1371/journal.pone.0030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abreu NA, Nagalingam NA, Song Y, Roediger FC, Pletcher SD, Goldberg AN, Lynch SV. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med 4: 151ra124, 2012. doi: 10.1126/scitranslmed.3003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adar SD, Huffnagle GB, Curtis JL. The respiratory microbiome: an underappreciated player in the human response to inhaled pollutants? Ann Epidemiol 26: 355–359, 2016. doi: 10.1016/j.annepidem.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal C, Jimenez JC, Lee H, Chlipala GE, Ratia K, Federle MJ. Identification of Quorum-Sensing Inhibitors Disrupting Signaling between Rgg and Short Hydrophobic Peptides in Streptococci. MBio 6: e00393-15, 2015. doi: 10.1128/mBio.00393-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med 8: 1024–1032, 2002. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 9.Allen HK, Trachsel J, Looft T, Casey TA. Finding alternatives to antibiotics. Ann N Y Acad Sci 1323: 91–100, 2014. doi: 10.1111/nyas.12468. [DOI] [PubMed] [Google Scholar]
- 10.Anand N, Kollef MH. The alphabet soup of pneumonia: CAP, HAP, HCAP, NHAP, and VAP. Semin Respir Crit Care Med 30: 3–9, 2009. doi: 10.1055/s-0028-1119803. [DOI] [PubMed] [Google Scholar]
- 11.Andreeva AV, Kutuzov MA, Voyno-Yasenetskaya TA. Regulation of surfactant secretion in alveolar type II cells. Am J Physiol Lung Cell Mol Physiol 293: L259–L271, 2007. doi: 10.1152/ajplung.00112.2007. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous; Advisory Committee on Immunization Practices . Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 49, RR-9: 1–35, 2000. [PubMed] [Google Scholar]
- 13.Arat S, Spivak A, Van Horn S, Thomas E, Traini C, Sathe G, Livi GP, Ingraham K, Jones L, Aubart K, Holmes DJ, Naderer O, Brown JR. Microbiome changes in healthy volunteers treated with GSK1322322, a novel antibiotic targeting bacterial peptide deformylase. Antimicrob Agents Chemother 59: 1182–1192, 2015. doi: 10.1128/AAC.04506-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, Kobzik L. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am J Respir Cell Mol Biol 35: 474–478, 2006. doi: 10.1165/rcmb.2006-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp Med 200: 267–272, 2004. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500: 232–236, 2013. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 17.Bakaletz LO, Baker BD, Jurcisek JA, Harrison A, Novotny LA, Bookwalter JE, Mungur R, Munson RS Jr. Demonstration of Type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect Immun 73: 1635–1643, 2005. doi: 10.1128/IAI.73.3.1635-1643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barenkamp SJ, St Geme JW III. Genes encoding high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae are part of gene clusters. Infect Immun 62: 3320–3328, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry J, Loh Z, Collison A, Mazzone S, Lalwani A, Zhang V, Davidson S, Wybacz E, Garlanda C, Mantovani A, Mattes J, Foster PS, Phipps S. Absence of Toll-IL-1 receptor 8/single immunoglobulin IL-1 receptor-related molecule reduces house dust mite-induced allergic airway inflammation in mice. Am J Respir Cell Mol Biol 49: 481–490, 2013. doi: 10.1165/rcmb.2012-0425OC. [DOI] [PubMed] [Google Scholar]
- 20.Beck JM, Young VB, Huffnagle GB. The microbiome of the lung. Transl Res 160: 258–266, 2012. doi: 10.1016/j.trsl.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck JM, Schloss PD, Venkataraman A, Twigg H III, Jablonski KA, Bushman FD, Campbell TB, Charlson ES, Collman RG, Crothers K, Curtis JL, Drews KL, Flores SC, Fontenot AP, Foulkes MA, Frank I, Ghedin E, Huang L, Lynch SV, Morris A, Palmer BE, Schmidt TM, Sodergren E, Weinstock GM, Young VB; Lung HIV Microbiome Project . Multicenter Comparison of Lung and Oral Microbiomes of HIV-infected and HIV-uninfected Individuals. Am J Respir Crit Care Med 192: 1335–1344, 2015. doi: 10.1164/rccm.201501-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beloin C, Renard S, Ghigo JM, Lebeaux D. Novel approaches to combat bacterial biofilms. Curr Opin Pharmacol 18: 61–68, 2014. doi: 10.1016/j.coph.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Shimol S, Givon-Lavi N, Leibovitz E, Raiz S, Greenberg D, Dagan R. Near-elimination of otitis media caused by 13-valent pneumococcal conjugate vaccine (PCV) serotypes in southern Israel shortly after sequential introduction of 7-valent/13-valent PCV. Clin Infect Dis 59: 1724–1732, 2014. doi: 10.1093/cid/ciu683. [DOI] [PubMed] [Google Scholar]
- 24.Berends ET, Dekkers JF, Nijland R, Kuipers A, Soppe JA, van Strijp JA, Rooijakkers SH. Distinct localization of the complement C5b-9 complex on Gram-positive bacteria. Cell Microbiol 15: 1955–1968, 2013. doi: 10.1111/cmi.12170. [DOI] [PubMed] [Google Scholar]
- 25.Bhowmick R, Tin Maung N, Hurley BP, Ghanem EB, Gronert K, McCormick BA, Leong JM. Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. J Immunol 191: 5115–5123, 2013. doi: 10.4049/jimmunol.1300522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5, 2007. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 27.Biesbroek G, Bosch AA, Wang X, Keijser BJ, Veenhoven RH, Sanders EA, Bogaert D. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am J Respir Crit Care Med 190: 298–308, 2014. doi: 10.1164/rccm.201401-0073OC. [DOI] [PubMed] [Google Scholar]
- 28.Biesbroek G, Tsivtsivadze E, Sanders EA, Montijn R, Veenhoven RH, Keijser BJ, Bogaert D. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med 190: 1283–1292, 2014. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 29.Biesbroek G, Wang X, Keijser BJ, Eijkemans RM, Trzciński K, Rots NY, Veenhoven RH, Sanders EA, Bogaert D. Seven-valent pneumococcal conjugate vaccine and nasopharyngeal microbiota in healthy children. Emerg Infect Dis 20: 201–210, 2014. doi: 10.3201/eid2002.131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanchette KA, Orihuela CJ. Future perspective on host-pathogen interactions during bacterial biofilm formation within the nasopharynx. Future Microbiol 7: 227–239, 2012. doi: 10.2217/fmb.11.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanchette KA, Shenoy AT, Milner J II, Gilley RP, McClure E, Hinojosa CA, Kumar N, Daugherty SC, Tallon LJ, Ott S, King SJ, Ferreira DM, Gordon SB, Tettelin H, Orihuela CJ. Neuraminidase A-Exposed Galactose Promotes Streptococcus pneumoniae Biofilm Formation during Colonization. Infect Immun 84: 2922–2932, 2016. doi: 10.1128/IAI.00277-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanchette-Cain K, Hinojosa CA, Akula Suresh Babu R, Lizcano A, Gonzalez-Juarbe N, Munoz-Almagro C, Sanchez CJ, Bergman MA, Orihuela CJ. Streptococcus pneumoniae biofilm formation is strain dependent, multifactorial, and associated with reduced invasiveness and immunoreactivity during colonization. MBio 4: e00745-13, 2013. doi: 10.1128/mBio.00745-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaser MJ. Antibiotic use and its consequences for the normal microbiome. Science 352: 544–545, 2016. doi: 10.1126/science.aad9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blom AM, Bergmann S, Fulde M, Riesbeck K, Agarwal V. Streptococcus pneumoniae phosphoglycerate kinase is a novel complement inhibitor affecting the membrane attack complex formation. J Biol Chem 289: 32499–32511, 2014. doi: 10.1074/jbc.M114.610212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boe DM, Boule LA, Kovacs EJ. Innate immune responses in the ageing lung. Clin Exp Immunol 187: 16–25, 2017. doi: 10.1111/cei.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 4: 144–154, 2004. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 37.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rümke HC, Verbrugh HA, Hermans PW. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363: 1871–1872, 2004. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 38.Bohlson SS, O’Conner SD, Hulsebus HJ, Ho MM, Fraser DA. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front Immunol 5: 402, 2014. doi: 10.3389/fimmu.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, D Lieber A, Wu F, Perez-Perez GI, Chen Y, Schweizer W, Zheng X, Contreras M, Dominguez-Bello MG, Blaser MJ. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 8: 343ra82, 2016. doi: 10.1126/scitranslmed.aad7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bomar L, Brugger SD, Yost BH, Davies SS, Lemon KP. Corynebacterium accolens Releases Antipneumococcal Free Fatty Acids from Human Nostril and Skin Surface Triacylglycerols. MBio 7: e01725-15, 2016. doi: 10.1128/mBio.01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog 9: e1003057, 2013. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosch AATM, Levin E, van Houten MA, Hasrat R, Kalkman G, Biesbroek G, de Steenhuijsen Piters WAA, de Groot PCM, Pernet P, Keijser BJF, Sanders EAM, Bogaert D. Development of Upper Respiratory Tract Microbiota in Infancy is Affected by Mode of Delivery. EBioMedicine 9: 336–345, 2016. doi: 10.1016/j.ebiom.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bou Ghanem EN, Clark S, Roggensack SE, McIver SR, Alcaide P, Haydon PG, Leong JM. Extracellular Adenosine Protects Against Streptococcus pneumoniae Lung Infection by Regulating Pulmonary Neutrophil Recruitment. PLoS Pathog 11: e1005126, 2015. doi: 10.1371/journal.ppat.1005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bousbia S, Papazian L, Saux P, Forel JM, Auffray JP, Martin C, Raoult D, La Scola B. Repertoire of intensive care unit pneumonia microbiota. PLoS One 7: e32486, 2012. doi: 10.1371/journal.pone.0032486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boutin S, Graeber SY, Weitnauer M, Panitz J, Stahl M, Clausznitzer D, Kaderali L, Einarsson G, Tunney MM, Elborn JS, Mall MA, Dalpke AH. Comparison of microbiomes from different niches of upper and lower airways in children and adolescents with cystic fibrosis. PLoS One 10: e0116029, 2015. doi: 10.1371/journal.pone.0116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res 45: 69–86, 2011. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brackman G, Coenye T. Quorum sensing inhibitors as anti-biofilm agents. Curr Pharm Des 21: 5–11, 2015. doi: 10.2174/1381612820666140905114627. [DOI] [PubMed] [Google Scholar]
- 48.Brandstetter KA, Jurcisek JA, Goodman SD, Bakaletz LO, Das S. Antibodies directed against integration host factor mediate biofilm clearance from Nasopore. Laryngoscope 123: 2626–2632, 2013. doi: 10.1002/lary.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brockson ME, Novotny LA, Mokrzan EM, Malhotra S, Jurcisek JA, Akbar R, Devaraj A, Goodman SD, Bakaletz LO. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Mol Microbiol 93: 1246–1258, 2014. doi: 10.1111/mmi.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis 187: 1424–1432, 2003. doi: 10.1086/374624. [DOI] [PubMed] [Google Scholar]
- 51.Brueggemann AB, Pai R, Crook DW, Beall B. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog 3: e168, 2007. doi: 10.1371/journal.ppat.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brugger SD, Frey P, Aebi S, Hinds J, Mühlemann K. Multiple colonization with S. pneumoniae before and after introduction of the seven-valent conjugated pneumococcal polysaccharide vaccine. PLoS One 5: e11638, 2010. doi: 10.1371/journal.pone.0011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bundy DG, Muschelli J, Clemens GD, Strouse JJ, Thompson RE, Casella JF, Miller MR. Preventive Care Delivery to Young Children With Sickle Cell Disease. J Pediatr Hematol Oncol 38: 294–300, 2016. doi: 10.1097/MPH.0000000000000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burchell JT, Wikstrom ME, Stumbles PA, Sly PD, Turner DJ. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells. Am J Physiol Lung Cell Mol Physiol 296: L307–L319, 2009. doi: 10.1152/ajplung.00521.2007. [DOI] [PubMed] [Google Scholar]
- 55.Calix JJ, Dagan R, Pelton SI, Porat N, Nahm MH. Differential occurrence of Streptococcus pneumoniae serotype 11E between asymptomatic carriage and invasive pneumococcal disease isolates reflects a unique model of pathogen microevolution. Clin Infect Dis 54: 794–799, 2012. doi: 10.1093/cid/cir953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Canella R, Martini M, Borriello R, Cavicchio C, Muresan XM, Benedusi M, Cervellati F, Valacchi G. Modulation of Chloride Currents in Human Lung Epithelial Cells Exposed to Exogenous Oxidative Stress. J Cell Physiol 232: 1817–1825, 2017. doi: 10.1002/jcp.25705. [DOI] [PubMed] [Google Scholar]
- 57.Carruthers MD, Tracy EN, Dickson AC, Ganser KB, Munson RS Jr, Bakaletz LO. Biological roles of nontypeable Haemophilus influenzae type IV pilus proteins encoded by the pil and com operons. J Bacteriol 194: 1927–1933, 2012. doi: 10.1128/JB.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carson CF, Riley TV. Non-antibiotic therapies for infectious diseases. Commun Dis Intell Q Rep 27, Suppl: S143–S146, 2003. [PubMed] [Google Scholar]
- 59.Carter R, Wolf J, van Opijnen T, Muller M, Obert C, Burnham C, Mann B, Li Y, Hayden RT, Pestina T, Persons D, Camilli A, Flynn PM, Tuomanen EI, Rosch JW. Genomic analyses of pneumococci from children with sickle cell disease expose host-specific bacterial adaptations and deficits in current interventions. Cell Host Microbe 15: 587–599, 2014. doi: 10.1016/j.chom.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 29: 304–309, 2010. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castanys-Muñoz E, Martin MJ, Vazquez E. Building a Beneficial Microbiome from Birth. Adv Nutr 7: 323–330, 2016. doi: 10.3945/an.115.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castón JJ, Linares MJ, Gallego C, Rivero A, Font P, Solís F, Casal M, Torre-Cisneros J. Risk factors for pulmonary Aspergillus terreus infection in patients with positive culture for filamentous fungi. Chest 131: 230–236, 2007. doi: 10.1378/chest.06-0767. [DOI] [PubMed] [Google Scholar]
- 63.Castro-Nallar E, Shen Y, Freishtat RJ, Pérez-Losada M, Manimaran S, Liu G, Johnson WE, Crandall KA. Integrating microbial and host transcriptomics to characterize asthma-associated microbial communities. BMC Med Genomics 8: 50, 2015. doi: 10.1186/s12920-015-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Catry B, Dewulf J, Maes D, Pardon B, Callens B, Vanrobaeys M, Opsomer G, de Kruif A, Haesebrouck F. Effect of Antimicrobial Consumption and Production Type on Antibacterial Resistance in the Bovine Respiratory and Digestive Tract. PLoS One 11: e0146488, 2016. doi: 10.1371/journal.pone.0146488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention (CDC) Progress toward control of rubella and prevention of congenital rubella syndrome—worldwide, 2009. MMWR Morb Mortal Wkly Rep 59: 1307–1310, 2010. [PubMed] [Google Scholar]
- 66.Chang A, Kaur R, Michel LV, Casey JR, Pichichero M. Haemophilus influenzae vaccine candidate outer membrane protein P6 is not conserved in all strains. Hum Vaccin 7: 102–105, 2011. doi: 10.4161/hv.7.1.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chao Y, Marks LR, Pettigrew MM, Hakansson AP. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front Cell Infect Microbiol 4: 194, 2015. doi: 10.3389/fcimb.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, Bushman FD, Collman RG. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 184: 957–963, 2011. doi: 10.1164/rccm.201104-0655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Charlson ES, Chen J, Custers-Allen R, Bittinger K, Li H, Sinha R, Hwang J, Bushman FD, Collman RG. Disordered microbial communities in the upper respiratory tract of cigarette smokers. PLoS One 5: e15216, 2010. doi: 10.1371/journal.pone.0015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of Mycoplasma pneumoniae: an update. Indian J Med Microbiol 34: 7–16, 2016. doi: 10.4103/0255-0857.174112. [DOI] [PubMed] [Google Scholar]
- 71.Chen L, Guo S, Wu L, Hao C, Xu W, Zhang J. Effects of recombinant IL-17F intranasal inoculation against Streptococcus pneumoniae infection in a murine model. Biotechnol Appl Biochem 62: 393–400, 2015. doi: 10.1002/bab.1286. [DOI] [PubMed] [Google Scholar]
- 72.Cheng G, Wang LC, Fridlender ZG, Cheng GS, Chen B, Mangalmurti NS, Saloura V, Yu Z, Kapoor V, Mozdzanowska K, Moon E, Sun J, Kreindler JL, Cohen NA, Caton AJ, Erikson J, Albelda SM. Pharmacologic activation of the innate immune system to prevent respiratory viral infections. Am J Respir Cell Mol Biol 45: 480–488, 2011. doi: 10.1165/rcmb.2010-0288OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chichlowski M, German JB, Lebrilla CB, Mills DA. The influence of milk oligosaccharides on microbiota of infants: opportunities for formulas. Annu Rev Food Sci Technol 2: 331–351, 2011. doi: 10.1146/annurev-food-022510-133743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiosis G, Boneca IG. Selective cleavage of d-Ala-d-Lac by small molecules: re-sensitizing resistant bacteria to vancomycin. Science 293: 1484–1487, 2001. doi: 10.1126/science.1060324. [DOI] [PubMed] [Google Scholar]
- 75.Chipps BE, Corren J, Israel E, Katial R, Lang DM, Panettieri RA Jr, Peters SP, Farrar JR. Asthma Yardstick: practical recommendations for a sustained step-up in asthma therapy for poorly controlled asthma. Ann Allergy Asthma Immunol 118: 133–142.e3, 2017. doi: 10.1016/j.anai.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 76.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 13: 260–270, 2012. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi EB, Hong SW, Kim DK, Jeon SG, Kim KR, Cho SH, Gho YS, Jee YK, Kim YK. Decreased diversity of nasal microbiota and their secreted extracellular vesicles in patients with chronic rhinosinusitis based on a metagenomic analysis. Allergy 69: 517–526, 2014. doi: 10.1111/all.12374. [DOI] [PubMed] [Google Scholar]
- 78.Chow OA, von Köckritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell Host Microbe 8: 445–454, 2010. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun 82: 4596–4606, 2014. doi: 10.1128/IAI.02212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, Zhang Y, Surendra A, Gong Y, Elizabeth Tullis D, Yau YC, Waters VJ, Hwang DM, Guttman DS. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 5: 10241, 2015. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coles CL, Labrique A, Saha SK, Ali H, Al-Emran H, Rashid M, Christian P, West KP Jr, Klemm R. Newborn vitamin A supplementation does not affect nasopharyngeal carriage of Streptococcus pneumoniae in Bangladeshi infants at age 3 months. J Nutr 141: 1907–1911, 2011. doi: 10.3945/jn.111.141622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Committee on Infectious Diseases, American Academy of Pediatrics Recommended Childhood and Adolescent Immunization Schedule–United States, 2016. Pediatrics 137: e20154531, 2016. doi: 10.1542/peds.2015-4531. [DOI] [PubMed] [Google Scholar]
- 83.Cowland JB, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev 273: 11–28, 2016. doi: 10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 84.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, Kim SG, Li H, Gao Z, Mahana D, Zárate Rodriguez JG, Rogers AB, Robine N, Loke P, Blaser MJ. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158: 705–721, 2014. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, McGee L, von Gottberg A, Song JH, Ko KS, Pichon B, Baker S, Parry CM, Lambertsen LM, Shahinas D, Pillai DR, Mitchell TJ, Dougan G, Tomasz A, Klugman KP, Parkhill J, Hanage WP, Bentley SD. Rapid pneumococcal evolution in response to clinical interventions. Science 331: 430–434, 2011. doi: 10.1126/science.1198545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Croucher NJ, Kagedan L, Thompson CM, Parkhill J, Bentley SD, Finkelstein JA, Lipsitch M, Hanage WP. Selective and genetic constraints on pneumococcal serotype switching. PLoS Genet 11: e1005095, 2015. doi: 10.1371/journal.pgen.1005095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cui L, Morris A, Huang L, Beck JM, Twigg HL III, von Mutius E, Ghedin E. The microbiome and the lung. Ann Am Thorac Soc 11, Suppl 4: S227–S232, 2014. doi: 10.1513/AnnalsATS.201402-052PL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cuthbertson L, Rogers GB, Walker AW, Oliver A, Green LE, Daniels TW, Carroll MP, Parkhill J, Bruce KD, van der Gast CJ. Respiratory microbiota resistance and resilience to pulmonary exacerbation and subsequent antimicrobial intervention. ISME J 10: 1081–1091, 2016. doi: 10.1038/ismej.2015.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dabernat H, Geslin P, Megraud F, Bégué P, Boulesteix J, Dubreuil C, de La Roque F, Trinh A, Scheimberg A. Effects of cefixime or co-amoxiclav treatment on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children with acute otitis media. J Antimicrob Chemother 41: 253–258, 1998. doi: 10.1093/jac/41.2.253. [DOI] [PubMed] [Google Scholar]
- 90.Dagan R, Gradstein S, Belmaker I, Porat N, Siton Y, Weber G, Janco J, Yagupsky P. An outbreak of Streptococcus pneumoniae serotype 1 in a closed community in southern Israel. Clin Infect Dis 30: 319–321, 2000. doi: 10.1086/313645. [DOI] [PubMed] [Google Scholar]
- 91.Dagan R, Leibovitz E, Greenberg D, Yagupsky P, Fliss DM, Leiberman A. Dynamics of pneumococcal nasopharyngeal colonization during the first days of antibiotic treatment in pediatric patients. Pediatr Infect Dis J 17: 880–885, 1998. doi: 10.1097/00006454-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Dalia AB, Weiser JN. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell Host Microbe 10: 486–496, 2011. doi: 10.1016/j.chom.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74: 417–433, 2010. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio 4: e00264-13, 2013. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dawood FS, Chaves SS, Pérez A, Reingold A, Meek J, Farley MM, Ryan P, Lynfield R, Morin C, Baumbach J, Bennett NM, Zansky S, Thomas A, Lindegren ML, Schaffner W, Finelli L; Emerging Infections Program Network . Complications and associated bacterial coinfections among children hospitalized with seasonal or pandemic influenza, United States, 2003-2010. J Infect Dis 209: 686–694, 2014. doi: 10.1093/infdis/jit473. [DOI] [PubMed] [Google Scholar]
- 96.De Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 200: 89–98, 2004. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Steenhuijsen Piters WA, Bogaert D. Unraveling the Molecular Mechanisms Underlying the Nasopharyngeal Bacterial Community Structure. MBio 7: e00009-16, 2016. doi: 10.1128/mBio.00009-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Steenhuijsen Piters WA, Huijskens EG, Wyllie AL, Biesbroek G, van den Bergh MR, Veenhoven RH, Wang X, Trzciński K, Bonten MJ, Rossen JW, Sanders EA, Bogaert D. Dysbiosis of upper respiratory tract microbiota in elderly pneumonia patients. ISME J 10: 97–108, 2016. doi: 10.1038/ismej.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Steenhuijsen Piters WA, Sanders EA, Bogaert D. The role of the local microbial ecosystem in respiratory health and disease. Philos Trans R Soc Lond B Biol Sci 370: 20140294, 2015. doi: 10.1098/rstb.2014.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Steenhuijsen Piters WA, Heinonen S, Hasrat R, Bunsow E, Smith B, Suarez-Arrabal MC, Chaussabel D, Cohen DM, Sanders EA, Ramilo O, Bogaert D, Mejias A. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am J Respir Crit Care Med 194: 1104–1115, 2016. doi: 10.1164/rccm.201602-0220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Denner DR, Sangwan N, Becker JB, Hogarth DK, Oldham J, Castillo J, Sperling AI, Solway J, Naureckas ET, Gilbert JA, White SR. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol 137: 1398–1405.e3, 2016. doi: 10.1016/j.jaci.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deriu E, Boxx GM, He X, Pan C, Benavidez SD, Cen L, Rozengurt N, Shi W, Cheng G. Influenza Virus Affects Intestinal Microbiota and Secondary Salmonella Infection in the Gut through Type I Interferons. PLoS Pathog 12: e1005572, 2016. doi: 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Diavatopoulos DA, Short KR, Price JT, Wilksch JJ, Brown LE, Briles DE, Strugnell RA, Wijburg OL. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J 24: 1789–1798, 2010. doi: 10.1096/fj.09-146779. [DOI] [PubMed] [Google Scholar]
- 104.Dickson RP, Huffnagle GB. The Lung Microbiome: New Principles for Respiratory Bacteriology in Health and Disease. PLoS Pathog 11: e1004923, 2015. doi: 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 384: 691–702, 2014. doi: 10.1016/S0140-6736(14)61136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, Curtis JL. Bacterial Topography of the Healthy Human Lower Respiratory Tract. MBio 8: e02287-16, 2017. doi: 10.1128/mBio.02287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Domenech M, García E, Prieto A, Moscoso M. Insight into the composition of the intercellular matrix of Streptococcus pneumoniae biofilms. Environ Microbiol 15: 502–516, 2013. doi: 10.1111/j.1462-2920.2012.02853.x. [DOI] [PubMed] [Google Scholar]
- 108.Drago L, De Vecchi E, Torretta S, Mattina R, Marchisio P, Pignataro L. Biofilm formation by bacteria isolated from upper respiratory tract before and after adenotonsillectomy. APMIS 120: 410–416, 2012. doi: 10.1111/j.1600-0463.2011.02846.x. [DOI] [PubMed] [Google Scholar]
- 109.Duell BL, Su YC, Riesbeck K. Host-pathogen interactions of nontypeable Haemophilus influenzae: from commensal to pathogen. FEBS Lett 590: 3840–3853, 2016. doi: 10.1002/1873-3468.12351. [DOI] [PubMed] [Google Scholar]
- 110.Dwivedi GR, Maurya A, Yadav DK, Khan F, Darokar MP, Srivastava SK. Drug Resistance Reversal Potential of Ursolic Acid Derivatives against Nalidixic Acid- and Multidrug-resistant Escherichia coli. Chem Biol Drug Des 86: 272–283, 2015. doi: 10.1111/cbdd.12491. [DOI] [PubMed] [Google Scholar]
- 111.Earl CS, An SQ, Ryan RP. The changing face of asthma and its relation with microbes. Trends Microbiol 23: 408–418, 2015. doi: 10.1016/j.tim.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Egere U, Townend J, Roca A, Akinsanya A, Bojang A, Nsekpong D, Greenwood B, Adegbola RA, Hill PC. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal carriage in newborns in rural Gambia: a randomised controlled trial. PLoS One 7: e49143, 2012. doi: 10.1371/journal.pone.0049143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Einarsson GG, Comer DM, McIlreavey L, Parkhill J, Ennis M, Tunney MM, Elborn JS. Community dynamics and the lower airway microbiota in stable chronic obstructive pulmonary disease, smokers and healthy non-smokers. Thorax 71: 795–803, 2016. doi: 10.1136/thoraxjnl-2015-207235. [DOI] [PubMed] [Google Scholar]
- 114.Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B, Martinez FJ, Huffnagle GB. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 6: e16384, 2011. doi: 10.1371/journal.pone.0016384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Essilfie AT, Simpson JL, Dunkley ML, Morgan LC, Oliver BG, Gibson PG, Foster PS, Hansbro PM. Combined Haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax 67: 588–599, 2012. doi: 10.1136/thoraxjnl-2011-200160. [DOI] [PubMed] [Google Scholar]
- 116.Fadli M, Chevalier J, Saad A, Mezrioui NE, Hassani L, Pages JM. Essential oils from Moroccan plants as potential chemosensitisers restoring antibiotic activity in resistant Gram-negative bacteria. Int J Antimicrob Agents 38: 325–330, 2011. doi: 10.1016/j.ijantimicag.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 117.Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM, Souza DG. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol 188: 1411–1420, 2012. doi: 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- 118.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med 6: 220ra11, 2014. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fang R, Hara H, Sakai S, Hernandez-Cuellar E, Mitsuyama M, Kawamura I, Tsuchiya K. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect Immun 82: 2310–2317, 2014. doi: 10.1128/IAI.01572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fasching CE, Grossman T, Corthésy B, Plaut AG, Weiser JN, Janoff EN. Impact of the molecular form of immunoglobulin A on functional activity in defense against Streptococcus pneumoniae. Infect Immun 75: 1801–1810, 2007. doi: 10.1128/IAI.01758-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ferrante CJ, Leibovich SJ. Regulation of Macrophage Polarization and Wound Healing. Adv Wound Care (New Rochelle) 1: 10–16, 2012. doi: 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fink DL, St Geme JW III. Chromosomal expression of the Haemophilus influenzae Hap autotransporter allows fine-tuned regulation of adhesive potential via inhibition of intermolecular autoproteolysis. J Bacteriol 185: 1608–1615, 2003. doi: 10.1128/JB.185.5.1608-1615.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fletcher MA, Fritzell B. Pneumococcal conjugate vaccines and otitis media: an appraisal of the clinical trials. Int J Otolaryngol 2012: 312935, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Freidl GS, Spruijt IT, Borlée F, Smit LA, van Gageldonk-Lafeber AB, Heederik DJ, Yzermans J, van Dijk CE, Maassen CB, van der Hoek W. Livestock-associated risk factors for pneumonia in an area of intensive animal farming in the Netherlands. PLoS One 12: e0174796, 2017. doi: 10.1371/journal.pone.0174796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Froy O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol 7: 1387–1397, 2005. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 126.Frydenlund Michelsen C, Hossein Khademi SM, Krogh Johansen H, Ingmer H, Dorrestein PC, Jelsbak L. Evolution of metabolic divergence in Pseudomonas aeruginosa during long-term infection facilitates a proto-cooperative interspecies interaction. ISME J 10: 1323–1336, 2016. doi: 10.1038/ismej.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fuentes L, Roszer T, Ricote M. Inflammatory mediators and insulin resistance in obesity: role of nuclear receptor signaling in macrophages. Mediators Inflamm 2010: 219583, 2010. doi: 10.1155/2010/219583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, Boushey HA, Zoratti E, Ownby D, Lukacs NW, Lynch SV. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci USA 111: 805–810, 2014. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fukata M, Arditi M. The role of pattern recognition receptors in intestinal inflammation. Mucosal Immunol 6: 451–463, 2013. doi: 10.1038/mi.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galanis I, Lindstrand A, Darenberg J, Browall S, Nannapaneni P, Sjöström K, Morfeldt E, Naucler P, Blennow M, Örtqvist Å, Henriques-Normark B. Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden. Eur Respir J 47: 1208–1218, 2016. doi: 10.1183/13993003.01451-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers 1: e24997, 2013. doi: 10.4161/tisb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.García-Rodríguez JA, Fresnadillo Martínez MJ. Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother 50, Suppl S2: 59–74, 2002. doi: 10.1093/jac/dkf506. [DOI] [PubMed] [Google Scholar]
- 133.Gauguet S, D’Ortona S, Ahnger-Pier K, Duan B, Surana NK, Lu R, Cywes-Bentley C, Gadjeva M, Shan Q, Priebe GP, Pier GB. Intestinal Microbiota of Mice Influences Resistance to Staphylococcus aureus Pneumonia. Infect Immun 83: 4003–4014, 2015. doi: 10.1128/IAI.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin Microbiol Rev 28: 871–899, 2015. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gilley RP, Orihuela CJ. Pneumococci in biofilms are non-invasive: implications on nasopharyngeal colonization. Front Cell Infect Microbiol 4: 163, 2014. doi: 10.3389/fcimb.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386: 743–800, 2015. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Glover DT, Hollingshead SK, Briles DE. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect Immun 76: 2767–2776, 2008. doi: 10.1128/IAI.01126-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta 1408: 79–89, 1998. doi: 10.1016/S0925-4439(98)00060-X. [DOI] [PubMed] [Google Scholar]
- 139.Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the Leap from Research Laboratory to Clinic: Challenges and Opportunities for Next-Generation Sequencing in Infectious Disease Diagnostics. MBio 6: e01888-15, 2015. doi: 10.1128/mBio.01888-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gollwitzer ES, Marsland BJ. Impact of Early-Life Exposures on Immune Maturation and Susceptibility to Disease. Trends Immunol 36: 684–696, 2015. doi: 10.1016/j.it.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 141.González-Juarbe N, Gilley RP, Hinojosa CA, Bradley KM, Kamei A, Gao G, Dube PH, Bergman MA, Orihuela CJ. Pore-Forming Toxins Induce Macrophage Necroptosis during Acute Bacterial Pneumonia. PLoS Pathog 11: e1005337, 2015. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity 36: 166–174, 2012. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grabenstein JD, Klugman KP. A century of pneumococcal vaccination research in humans. Clin Microbiol Infect 18, Suppl 5: 15–24, 2012. doi: 10.1111/j.1469-0691.2012.03943.x. [DOI] [PubMed] [Google Scholar]
- 144.Gray BM, Converse GM III, Dillon HC Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis 142: 923–933, 1980. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 145.Greaney AJ, Leppla SH, Moayeri M. Bacterial Exotoxins and the Inflammasome. Front Immunol 6: 570, 2015. doi: 10.3389/fimmu.2015.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Grebe KM, Takeda K, Hickman HD, Bailey AL, Embry AC, Bennink JR, Yewdell JW. Cutting edge: sympathetic nervous system increases proinflammatory cytokines and exacerbates influenza A virus pathogenesis. J Immunol 184: 540–544, 2010. doi: 10.4049/jimmunol.0903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, Carroll MP, Bruce KD, Howarth PH. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One 9: e100645, 2014. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Green GM, Kass EH. The Role of the Alveolar Macrophage in the Clearance of Bacteria from the Lung. J Exp Med 119: 167–176, 1964. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Greene CJ, Marks LR, Hu JC, Reddinger R, Mandell L, Roche-Hakansson H, King-Lyons ND, Connell TD, Hakansson AP. Novel Strategy To Protect against Influenza Virus-Induced Pneumococcal Disease without Interfering with Commensal Colonization. Infect Immun 84: 1693–1703, 2016. doi: 10.1128/IAI.01478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature 430: 1024–1027, 2004. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 151.Guilliams M, Lambrecht BN, Hammad H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol 6: 464–473, 2013. doi: 10.1038/mi.2013.14. [DOI] [PubMed] [Google Scholar]
- 152.Haczku A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J Allergy Clin Immunol 122: 861–879, 2008. doi: 10.1016/j.jaci.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2: 95–108, 2004. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 154.Hammer ND, Cassat JE, Noto MJ, Lojek LJ, Chadha AD, Schmitz JE, Creech CB, Skaar EP. Inter- and intraspecies metabolite exchange promotes virulence of antibiotic-resistant Staphylococcus aureus. Cell Host Microbe 16: 531–537, 2014. doi: 10.1016/j.chom.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Han MK, Huang YJ, Lipuma JJ, Boushey HA, Boucher RC, Cookson WO, Curtis JL, Erb-Downward J, Lynch SV, Sethi S, Toews GB, Young VB, Wolfgang MC, Huffnagle GB, Martinez FJ. Significance of the microbiome in obstructive lung disease. Thorax 67: 456–463, 2012. doi: 10.1136/thoraxjnl-2011-201183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, Moore BB, White ES, Flaherty KR, Huffnagle GB, Martinez FJ; COMET Investigators . Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med 2: 548–556, 2014. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hanage WP, Kaijalainen TH, Syrjänen RK, Auranen K, Leinonen M, Mäkelä PH, Spratt BG. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect Immun 73: 431–435, 2005. doi: 10.1128/IAI.73.1.431-435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol 119: 1258–1266, 2007. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 159.Hassan F. Molecular mechanisms of moraxella catarrhalis-induced otitis media. Curr Allergy Asthma Rep 13: 512–517, 2013. doi: 10.1007/s11882-013-0374-8. [DOI] [PubMed] [Google Scholar]
- 160.Hathaway LJ, Bättig P, Mühlemann K. In vitro expression of the first capsule gene of Streptococcus pneumoniae, cpsA, is associated with serotype-specific colonization prevalence and invasiveness. Microbiology 153: 2465–2471, 2007. doi: 10.1099/mic.0.2006/005066-0. [DOI] [PubMed] [Google Scholar]
- 161.Hernández-Jiménez E, Del Campo R, Toledano V, Vallejo-Cremades MT, Muñoz A, Largo C, Arnalich F, García-Rio F, Cubillos-Zapata C, López-Collazo E. Biofilm vs. planktonic bacterial mode of growth: which do human macrophages prefer? Biochem Biophys Res Commun 441: 947–952, 2013. doi: 10.1016/j.bbrc.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 162.Heron MP, Smith BL. Deaths: leading causes for 2003. Natl Vital Stat Rep 55: 1–92, 2007. [PubMed] [Google Scholar]
- 163.Hill PC, Townend J, Antonio M, Akisanya B, Ebruke C, Lahai G, Greenwood BM, Adegbola RA. Transmission of Streptococcus pneumoniae in rural Gambian villages: a longitudinal study. Clin Infect Dis 50: 1468–1476, 2010. doi: 10.1086/652443. [DOI] [PubMed] [Google Scholar]
- 164.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WO. Disordered microbial communities in asthmatic airways. PLoS One 5: e8578, 2010. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hilty M, Qi W, Brugger SD, Frei L, Agyeman P, Frey PM, Aebi S, Mühlemann K. Nasopharyngeal microbiota in infants with acute otitis media. J Infect Dis 205: 1048–1055, 2012. doi: 10.1093/infdis/jis024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hirst C, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database Syst Rev 11: CD003427, 2014. [DOI] [PubMed] [Google Scholar]
- 167.Hoen AG, Li J, Moulton LA, O’Toole GA, Housman ML, Koestler DC, Guill MF, Moore JH, Hibberd PL, Morrison HG, Sogin ML, Karagas MR, Madan JC. Associations between Gut Microbial Colonization in Early Life and Respiratory Outcomes in Cystic Fibrosis. J Pediatr 167: 138–147.e1, 2015. doi: 10.1016/j.jpeds.2015.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med 177: 397–407, 1993. doi: 10.1084/jem.177.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hoopman TC, Liu W, Joslin SN, Pybus C, Sedillo JL, Labandeira-Rey M, Laurence CA, Wang W, Richardson JA, Bakaletz LO, Hansen EJ. Use of the chinchilla model for nasopharyngeal colonization to study gene expression by Moraxella catarrhalis. Infect Immun 80: 982–995, 2012. doi: 10.1128/IAI.05918-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hornstein T, Lehmann S, Philipp D, Detmer S, Hoffmann M, Peter C, Wesselborg S, Unfried K, Windolf J, Flohé S, Paunel-Görgülü A. Staurosporine resistance in inflammatory neutrophils is associated with the inhibition of caspase- and proteasome-mediated Mcl-1 degradation. J Leukoc Biol 99: 163–174, 2016. doi: 10.1189/jlb.3A1114-537RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hotomi M, Yuasa J, Briles DE, Yamanaka N. Pneumolysin plays a key role at the initial step of establishing pneumococcal nasal colonization. Folia Microbiol (Praha) 61: 375–383, 2016. doi: 10.1007/s12223-016-0445-z. [DOI] [PubMed] [Google Scholar]
- 172.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 124: e1–e11, 2009. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116: e408–e413, 2005. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 174.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol 136: 874–884, 2015. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 52: 2813–2823, 2014. doi: 10.1128/JCM.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huebner RE, Dagan R, Porath N, Wasas AD, Klugman KP. Lack of utility of serotyping multiple colonies for detection of simultaneous nasopharyngeal carriage of different pneumococcal serotypes. Pediatr Infect Dis J 19: 1017–1020, 2000. doi: 10.1097/00006454-200010000-00019. [DOI] [PubMed] [Google Scholar]
- 177.Hyams C, Trzcinski K, Camberlein E, Weinberger DM, Chimalapati S, Noursadeghi M, Lipsitch M, Brown JS. Streptococcus pneumoniae capsular serotype invasiveness correlates with the degree of factor H binding and opsonization with C3b/iC3b. Infect Immun 81: 354–363, 2013. doi: 10.1128/IAI.00862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Iki H, Sawa S, Teranishi T, Tomita M, Nishii K, Yamada K. Effect of exercise therapy on cytokine secretion in the saliva of bedridden patients. J Phys Ther Sci 28: 2871–2876, 2016. doi: 10.1589/jpts.28.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis 14: e197–e209, 2010. doi: 10.1016/j.ijid.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 180.Isajevs S, Taivans I, Strazda G, Kopeika U, Bukovskis M, Gordjusina V, Kratovska A. Decreased FOXP3 expression in small airways of smokers with COPD. Eur Respir J 33: 61–67, 2009. doi: 10.1183/09031936.00145307. [DOI] [PubMed] [Google Scholar]
- 181.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 27: 313–338, 2009. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 182.Jalalvand F, Riesbeck K. Haemophilus influenzae: recent advances in the understanding of molecular pathogenesis and polymicrobial infections. Curr Opin Infect Dis 27: 268–274, 2014. doi: 10.1097/QCO.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 183.Jayasinghe S, Menzies R, Chiu C, Toms C, Blyth CC, Krause V, McIntyre P. Long-term Impact of a “3 + 0” Schedule for 7- and 13-Valent Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Australia, 2002-2014. Clin Infect Dis 64: 175–183, 2017. doi: 10.1093/cid/ciw720. [DOI] [PubMed] [Google Scholar]
- 184.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 185.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol 47: 417–426, 2012. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Joiner KA, Brown EJ, Frank MM. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol 2: 461–491, 1984. doi: 10.1146/annurev.iy.02.040184.002333. [DOI] [PubMed] [Google Scholar]
- 187.José R, Williams A, Sulikowski M, Brealey D, Brown J, Chambers R. Regulation of neutrophilic inflammation in lung injury induced by community-acquired pneumonia. Lancet 385, Suppl 1: S52, 2015. doi: 10.1016/S0140-6736(15)60367-1. [DOI] [PubMed] [Google Scholar]
- 188.Joyce EA, Popper SJ, Falkow S. Streptococcus pneumoniae nasopharyngeal colonization induces type I interferons and interferon-induced gene expression. BMC Genomics 10: 404, 2009. doi: 10.1186/1471-2164-10-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jusot JF, Neill DR, Waters EM, Bangert M, Collins M, Bricio Moreno L, Lawan KG, Moussa MM, Dearing E, Everett DB, Collard JM, Kadioglu A. Airborne dust and high temperatures are risk factors for invasive bacterial disease. J Allergy Clin Immunol 139: 977–986.e2, 2017. doi: 10.1016/j.jaci.2016.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6: 288–301, 2008. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 191.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194: 863–869, 2001. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 189: 2689–2695, 2012. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Karav S, Le Parc A, Leite Nobrega de Moura Bell JM, Frese SA, Kirmiz N, Block DE, Barile D, Mills DA. Oligosaccharides Released From Milk Glycoproteins Are Selective Growth Substrates for Infant-Associated Bifidobacteria. Appl Environ Microbiol 82: 3622–3630, 2016. doi: 10.1128/AEM.00547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kawano H, Kayama H, Nakama T, Hashimoto T, Umemoto E, Takeda K. IL-10-producing lung interstitial macrophages prevent neutrophilic asthma. Int Immunol 28: 489–501, 2016. doi: 10.1093/intimm/dxw012. [DOI] [PubMed] [Google Scholar]
- 195.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med 202: 1539–1547, 2005. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Keller LE, Robinson DA, McDaniel LS. Nonencapsulated Streptococcus pneumoniae: Emergence and Pathogenesis. MBio 7: e01792-15, 2016. doi: 10.1128/mBio.01792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Keller LE, Jones CV, Thornton JA, Sanders ME, Swiatlo E, Nahm MH, Park IH, McDaniel LS. PspK of Streptococcus pneumoniae increases adherence to epithelial cells and enhances nasopharyngeal colonization. Infect Immun 81: 173–181, 2013. doi: 10.1128/IAI.00755-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Khan MN, Coleman JR, Vernatter J, Varshney AK, Dufaud C, Pirofski LA. An ahemolytic pneumolysin of Streptococcus pneumoniae manipulates human innate and CD4+ T-cell responses and reduces resistance to colonization in mice in a serotype-independent manner. J Infect Dis 210: 1658–1669, 2014. doi: 10.1093/infdis/jiu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, Tonetti MS, Wade WG, Zaura E. The oral microbiome: an update for oral healthcare professionals. Br Dent J 221: 657–666, 2016. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 200.Kim BJ, Lee S, Berg RE, Simecka JW, Jones HP. Interleukin-23 (IL-23) deficiency disrupts Th17 and Th1-related defenses against Streptococcus pneumoniae infection. Cytokine 64: 375–381, 2013. doi: 10.1016/j.cyto.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 201.Kim J, Lahl K, Hori S, Loddenkemper C, Chaudhry A, deRoos P, Rudensky A, Sparwasser T. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol 183: 7631–7634, 2009. doi: 10.4049/jimmunol.0804308. [DOI] [PubMed] [Google Scholar]
- 202.Kjaer TR, Thiel S, Andersen GR. Toward a structure-based comprehension of the lectin pathway of complement. Mol Immunol 56: 413–422, 2013. doi: 10.1016/j.molimm.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 203.Ko FW, Chan KP, Hui DS, Goddard JR, Shaw JG, Reid DW, Yang IA. Acute exacerbation of COPD. Respirology 21: 1152–1165, 2016. doi: 10.1111/resp.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Koppen I, Bosch A, Sanders E, Van Houten M, Bogaert D. The respiratory microbiota during health and disease: a paediatric perspective. PNEU 6: 90–100, 2015. doi: 10.15172/pneu.2015.6/656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Korves TM, Piceno YM, Tom LM, Desantis TZ, Jones BW, Andersen GL, Hwang GM. Bacterial communities in commercial aircraft high-efficiency particulate air (HEPA) filters assessed by PhyloChip analysis. Indoor Air 23: 50–61, 2013. doi: 10.1111/j.1600-0668.2012.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kowalko JE, Sebert ME. The Streptococcus pneumoniae competence regulatory system influences respiratory tract colonization. Infect Immun 76: 3131–3140, 2008. doi: 10.1128/IAI.01696-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Krone CL, Trzciński K, Zborowski T, Sanders EA, Bogaert D. Impaired innate mucosal immunity in aged mice permits prolonged Streptococcus pneumoniae colonization. Infect Immun 81: 4615–4625, 2013. doi: 10.1128/IAI.00618-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Krone CL, Biesbroek G, Trzciński K, Sanders EA, Bogaert D. Respiratory microbiota dynamics following Streptococcus pneumoniae acquisition in young and elderly mice. Infect Immun 82: 1725–1731, 2014. doi: 10.1128/IAI.01290-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Krone CL, van de Groep K, Trzciński K, Sanders EA, Bogaert D. Immunosenescence and pneumococcal disease: an imbalance in host-pathogen interactions. Lancet Respir Med 2: 141–153, 2014. doi: 10.1016/S2213-2600(13)70165-6. [DOI] [PubMed] [Google Scholar]
- 210.Krone CL, Wyllie AL, van Beek J, Rots NY, Oja AE, Chu ML, Bruin JP, Bogaert D, Sanders EA, Trzciński K. Carriage of Streptococcus pneumoniae in aged adults with influenza-like-illness. PLoS One 10: e0119875, 2015. doi: 10.1371/journal.pone.0119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Küng E, Coward WR, Neill DR, Malak HA, Mühlemann K, Kadioglu A, Hilty M, Hathaway LJ. The pneumococcal polysaccharide capsule and pneumolysin differentially affect CXCL8 and IL-6 release from cells of the upper and lower respiratory tract. PLoS One 9: e92355, 2014. doi: 10.1371/journal.pone.0092355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Küpeli E, Ulubay G, Akkurt ES, Öner Eyüboğlu F, Sezgin A. Long-term pulmonary infections in heart transplant recipients. Exp Clin Transplant 13, Suppl 1: 356–360, 2015. doi: 10.6002/ect.mesot2014.P205. [DOI] [PubMed] [Google Scholar]
- 213.Kwambana BA, Barer MR, Bottomley C, Adegbola RA, Antonio M. Early acquisition and high nasopharyngeal co-colonisation by Streptococcus pneumoniae and three respiratory pathogens amongst Gambian new-borns and infants. BMC Infect Dis 11: 175, 2011. doi: 10.1186/1471-2334-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kwon HY, Ogunniyi AD, Choi MH, Pyo SN, Rhee DK, Paton JC. The ClpP protease of Streptococcus pneumoniae modulates virulence gene expression and protects against fatal pneumococcal challenge. Infect Immun 72: 5646–5653, 2004. doi: 10.1128/IAI.72.10.5646-5653.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.LaCross NC, Marrs CF, Gilsdorf JR. Population structure in nontypeable Haemophilus influenzae. Infect Genet Evol 14: 125–136, 2013. doi: 10.1016/j.meegid.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lam K, Schleimer R, Kern RC. The Etiology and Pathogenesis of Chronic Rhinosinusitis: a Review of Current Hypotheses. Curr Allergy Asthma Rep 15: 41, 2015. doi: 10.1007/s11882-015-0540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Larsen JM, Musavian HS, Butt TM, Ingvorsen C, Thysen AH, Brix S. Chronic obstructive pulmonary disease and asthma-associated Proteobacteria, but not commensal Prevotella spp., promote Toll-like receptor 2-independent lung inflammation and pathology. Immunology 144: 333–342, 2015. doi: 10.1111/imm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature 478: 250–254, 2011. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. Microbial communities of the upper respiratory tract and otitis media in children. MBio 2: e00245-10, 2011. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, Gibbons SM, Larsen P, Shogan BD, Weiss S, Metcalf JL, Ursell LK, Vázquez-Baeza Y, Van Treuren W, Hasan NA, Gibson MK, Colwell R, Dantas G, Knight R, Gilbert JA. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345: 1048–1052, 2014. doi: 10.1126/science.1254529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Lee B, Robinson KM, McHugh KJ, Scheller EV, Mandalapu S, Chen C, Di YP, Clay ME, Enelow RI, Dubin PJ, Alcorn JF. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 309: L158–L167, 2015. doi: 10.1152/ajplung.00338.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.LeMessurier KS, Häcker H, Chi L, Tuomanen E, Redecke V. Type I interferon protects against pneumococcal invasive disease by inhibiting bacterial transmigration across the lung. PLoS Pathog 9: e1003727, 2013. doi: 10.1371/journal.ppat.1003727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li Y, Beitelshees M, Fang L, Hill A, Ahmadi MK, Chen M, Davidson BA, Knight P III, Smith RJ Jr, Andreadis ST, Hakansson AP, Jones CH, Pfeifer BA. In situ pneumococcal vaccine production and delivery through a hybrid biological-biomaterial vector. Sci Adv 2: e1600264, 2016. doi: 10.1126/sciadv.1600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li Y, Hill A, Beitelshees M, Shao S, Lovell JF, Davidson BA, Knight PR III, Hakansson AP, Pfeifer BA, Jones CH. Directed vaccination against pneumococcal disease. Proc Natl Acad Sci USA 113: 6898–6903, 2016. doi: 10.1073/pnas.1603007113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li Y, Weinberger DM, Thompson CM, Trzciński K, Lipsitch M. Surface charge of Streptococcus pneumoniae predicts serotype distribution. Infect Immun 81: 4519–4524, 2013. doi: 10.1128/IAI.00724-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203: 2271–2279, 2006. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Licciardi PV, Toh ZQ, Dunne E, Wong SS, Mulholland EK, Tang M, Robins-Browne RM, Satzke C. Protecting against pneumococcal disease: critical interactions between probiotics and the airway microbiome. PLoS Pathog 8: e1002652, 2012. doi: 10.1371/journal.ppat.1002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, Rudan I, Campbell H, Cibulskis R, Li M, Mathers C, Black RE; Child Health Epidemiology Reference Group of WHO and UNICEF . Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379: 2151–2161, 2012. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 229.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity 31: 438–449, 2009. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lloyd CM, Marsland BJ. Lung Homeostasis: Influence of Age, Microbes, and the Immune System. Immunity 46: 549–561, 2017. doi: 10.1016/j.immuni.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 231.Lo JH, Kulp SK, Chen CS, Chiu HC. Sensitization of intracellular Salmonella enterica serovar Typhimurium to aminoglycosides in vitro and in vivo by a host-targeted antimicrobial agent. Antimicrob Agents Chemother 58: 7375–7382, 2014. doi: 10.1128/AAC.03778-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Loeb M, McGeer A, McArthur M, Walter S, Simor AE. Risk factors for pneumonia and other lower respiratory tract infections in elderly residents of long-term care facilities. Arch Intern Med 159: 2058–2064, 1999. doi: 10.1001/archinte.159.17.2058. [DOI] [PubMed] [Google Scholar]
- 233.Loeb M, Neupane B, Walter SD, Hanning R, Carusone SC, Lewis D, Krueger P, Simor AE, Nicolle L, Marrie TJ. Environmental risk factors for community-acquired pneumonia hospitalization in older adults. J Am Geriatr Soc 57: 1036–1040, 2009. doi: 10.1111/j.1532-5415.2009.02259.x. [DOI] [PubMed] [Google Scholar]
- 234.Lönnerdal B. Bioactive proteins in breast milk. J Paediatr Child Health 49, Suppl 1: 1–7, 2013. doi: 10.1111/jpc.12104. [DOI] [PubMed] [Google Scholar]
- 235.Love JF, Tran-Winkler HJ, Wessels MR. Vitamin D and the human antimicrobial peptide LL-37 enhance group a streptococcus resistance to killing by human cells. MBio 3: e00394-12, 2012. doi: 10.1128/mBio.00394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Luke NR, Jurcisek JA, Bakaletz LO, Campagnari AA. Contribution of Moraxella catarrhalis type IV pili to nasopharyngeal colonization and biofilm formation. Infect Immun 75: 5559–5564, 2007. doi: 10.1128/IAI.00946-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lund FE, Schuer K, Hollifield M, Randall TD, Garvy BA. Clearance of Pneumocystis carinii in mice is dependent on B cells but not on P. carinii-specific antibody. J Immunol 171: 1423–1430, 2003. doi: 10.4049/jimmunol.171.3.1423. [DOI] [PubMed] [Google Scholar]
- 238.Lysenko E, Richards JC, Cox AD, Stewart A, Martin A, Kapoor M, Weiser JN. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein-mediated killing. Mol Microbiol 35: 234–245, 2000. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 239.MacDonald BA, Chakravarthy KV, Davidson BA, Mullan BA, Alluri R, Hakansson AP, Knight PR. Halothane modulates the type i interferon response to influenza and minimizes the risk of secondary bacterial pneumonia through maintenance of neutrophil recruitment in an animal model. Anesthesiology 123: 590–602, 2015. doi: 10.1097/ALN.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol 15: 259–270, 2017. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mans JJ, von Lackum K, Dorsey C, Willis S, Wallet SM, Baker HV, Lamont RJ, Handfield M. The degree of microbiome complexity influences the epithelial response to infection. BMC Genomics 10: 380, 2009. doi: 10.1186/1471-2164-10-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol 229: 176–185, 2013. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 243.Marchisio P, Bianchini S, Baggi E, Fattizzo M, Galeone C, Torretta S, Principi N, Esposito S. A retrospective evaluation of microbiology of acute otitis media complicated by spontaneous otorrhea in children living in Milan, Italy. Infection 41: 629–635, 2013. doi: 10.1007/s15010-012-0371-1. [DOI] [PubMed] [Google Scholar]
- 244.Marks LR, Davidson BA, Knight PR, Hakansson AP. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. MBio 4: e00438-13, 2013. doi: 10.1128/mBio.00438-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Marks LR, Mashburn-Warren L, Federle MJ, Hakansson AP. Streptococcus pyogenes biofilm growth in vitro and in vivo and its role in colonization, virulence, and genetic exchange. J Infect Dis 210: 25–34, 2014. doi: 10.1093/infdis/jiu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Marks LR, Parameswaran GI, Hakansson AP. Pneumococcal interactions with epithelial cells are crucial for optimal biofilm formation and colonization in vitro and in vivo. Infect Immun 80: 2744–2760, 2012. doi: 10.1128/IAI.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Marks LR, Reddinger RM, Hakansson AP. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. MBio 3: e00200-12, 2012. doi: 10.1128/mBio.00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Marks LR, Reddinger RM, Hakansson AP. Biofilm formation enhances fomite survival of Streptococcus pneumoniae and Streptococcus pyogenes. Infect Immun 82: 1141–1146, 2014. doi: 10.1128/IAI.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Marsland BJ, Gollwitzer ES. Host-microorganism interactions in lung diseases. Nat Rev Immunol 14: 827–835, 2014. doi: 10.1038/nri3769. [DOI] [PubMed] [Google Scholar]
- 250.Marsland BJ, Trompette A, Gollwitzer ES. The Gut-Lung Axis in Respiratory Disease. Ann Am Thorac Soc 12, Suppl 2: S150–S156, 2015. [DOI] [PubMed] [Google Scholar]
- 251.Martel MJ, Rey E, Malo JL, Perreault S, Beauchesne MF, Forget A, Blais L. Determinants of the incidence of childhood asthma: a two-stage case-control study. Am J Epidemiol 169: 195–205, 2009. doi: 10.1093/aje/kwn309. [DOI] [PubMed] [Google Scholar]
- 252.Martins M, Dastidar SG, Fanning S, Kristiansen JE, Molnar J, Pagès JM, Schelz Z, Spengler G, Viveiros M, Amaral L. Potential role of non-antibiotics (helper compounds) in the treatment of multidrug-resistant Gram-negative infections: mechanisms for their direct and indirect activities. Int J Antimicrob Agents 31: 198–208, 2008. doi: 10.1016/j.ijantimicag.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 253.Mason KM, Raffel FK, Ray WC, Bakaletz LO. Heme utilization by nontypeable Haemophilus influenzae is essential and dependent on Sap transporter function. J Bacteriol 193: 2527–2535, 2011. doi: 10.1128/JB.01313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Matalon S, Bartoszewski R, Collawn JF. Role of epithelial sodium channels in the regulation of lung fluid homeostasis. Am J Physiol Lung Cell Mol Physiol 309: L1229–L1238, 2015. doi: 10.1152/ajplung.00319.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.McFetridge R, Meulen AS, Folkerth SD, Hoekstra JA, Dallas M, Hoover PA, Marchese RD, Zacholski DM, Watson WJ, Stek JE, Hartzel JS, Musey LK. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 33: 2793–2799, 2015. doi: 10.1016/j.vaccine.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 256.McGrath FD, Brouwer MC, Arlaud GJ, Daha MR, Hack CE, Roos A. Evidence that complement protein C1q interacts with C-reactive protein through its globular head region. J Immunol 176: 2950–2957, 2006. doi: 10.4049/jimmunol.176.5.2950. [DOI] [PubMed] [Google Scholar]
- 257.McKenney PT, Pamer EG. From Hype to Hope: The Gut Microbiota in Enteric Infectious Disease. Cell 163: 1326–1332, 2015. doi: 10.1016/j.cell.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Medina JL, Brooks EG, Chaparro A, Dube PH. Mycoplasma pneumoniae CARDS toxin elicits a functional IgE response in Balb/c mice. PLoS One 12: e0172447, 2017. doi: 10.1371/journal.pone.0172447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Medina JL, Coalson JJ, Brooks EG, Le Saux CJ, Winter VT, Chaparro A, Principe MF, Solis L, Kannan TR, Baseman JB, Dube PH. Mycoplasma pneumoniae CARDS toxin exacerbates ovalbumin-induced asthma-like inflammation in BALB/c mice. PLoS One 9: e102613, 2014. doi: 10.1371/journal.pone.0102613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Michelsen CF, Christensen AM, Bojer MS, Høiby N, Ingmer H, Jelsbak L. Staphylococcus aureus alters growth activity, autolysis, and antibiotic tolerance in a human host-adapted Pseudomonas aeruginosa lineage. J Bacteriol 196: 3903–3911, 2014. doi: 10.1128/JB.02006-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mika M, Mack I, Korten I, Qi W, Aebi S, Frey U, Latzin P, Hilty M. Dynamics of the nasal microbiota in infancy: a prospective cohort study. J Allergy Clin Immunol 135: 905–12.e11, 2015. doi: 10.1016/j.jaci.2014.12.1909. [DOI] [PubMed] [Google Scholar]
- 262.Mikhak Z, Strassner JP, Luster AD. Lung dendritic cells imprint T cell lung homing and promote lung immunity through the chemokine receptor CCR4. J Exp Med 210: 1855–1869, 2013. doi: 10.1084/jem.20130091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Millares L, Ferrari R, Gallego M, Garcia-Nuñez M, Pérez-Brocal V, Espasa M, Pomares X, Monton C, Moya A, Monsó E. Bronchial microbiome of severe COPD patients colonised by Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 33: 1101–1111, 2014. doi: 10.1007/s10096-013-2044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Miller ML, Gao G, Pestina T, Persons D, Tuomanen E. Hypersusceptibility to invasive pneumococcal infection in experimental sickle cell disease involves platelet-activating factor receptor. J Infect Dis 195: 581–584, 2007. doi: 10.1086/510626. [DOI] [PubMed] [Google Scholar]
- 265.Mokrzan EM, Ward MO, Bakaletz LO. Type IV pilus expression is upregulated in nontypeable Haemophilus influenzae biofilms formed at the temperature of the human nasopharynx. J Bacteriol 198: 2619–2630, 2016. doi: 10.1128/JB.01022-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mold C, Nakayama S, Holzer TJ, Gewurz H, Du Clos TW. C-reactive protein is protective against Streptococcus pneumoniae infection in mice. J Exp Med 154: 1703–1708, 1981. doi: 10.1084/jem.154.5.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, Cookson WO, Maher TM, Moffatt MF. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 190: 906–913, 2014. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Molyneaux PL, Cox MJ, Wells AU, Kim HC, Ji W, Cookson WO, Moffatt MF, Kim DS, Maher TM. Changes in the respiratory microbiome during acute exacerbations of idiopathic pulmonary fibrosis. Respir Res 18: 29, 2017. doi: 10.1186/s12931-017-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Molyneaux PL, Mallia P, Cox MJ, Footitt J, Willis-Owen SA, Homola D, Trujillo-Torralbo MB, Elkin S, Kon OM, Cookson WO, Moffatt MF, Johnston SL. Outgrowth of the bacterial airway microbiome after rhinovirus exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 188: 1224–1231, 2013. doi: 10.1164/rccm.201302-0341OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Molyneaux PL, Willis-Owen SAG, Cox MJ, James P, Cowman S, Loebinger M, Blanchard A, Edwards LM, Stock C, Daccord C, Renzoni EA, Wells AU, Moffatt MF, Cookson WOC, Maher TM. Host-Microbial Interactions in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 195: 1640–1650, 2017. doi: 10.1164/rccm.201607-1408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Muñoz-Elías EJ, Marcano J, Camilli A. Isolation of Streptococcus pneumoniae biofilm mutants and their characterization during nasopharyngeal colonization. Infect Immun 76: 5049–5061, 2008. doi: 10.1128/IAI.00425-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Murdoch DR. How recent advances in molecular tests could impact the diagnosis of pneumonia. Expert Rev Mol Diagn 16: 533–540, 2016. doi: 10.1586/14737159.2016.1156536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mureithi MW, Finn A, Ota MO, Zhang Q, Davenport V, Mitchell TJ, Williams NA, Adegbola RA, Heyderman RS. T cell memory response to pneumococcal protein antigens in an area of high pneumococcal carriage and disease. J Infect Dis 200: 783–793, 2009. doi: 10.1086/605023. [DOI] [PubMed] [Google Scholar]
- 275.Murphy TF, Bakaletz LO, Smeesters PR. Microbial interactions in the respiratory tract. Pediatr Infect Dis J 28, Suppl: S121–S126, 2009. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- 276.Murphy TF, Faden H, Bakaletz LO, Kyd JM, Forsgren A, Campos J, Virji M, Pelton SI. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J 28: 43–48, 2009. doi: 10.1097/INF.0b013e318184dba2. [DOI] [PubMed] [Google Scholar]
- 277.Murray MA, Chotirmall SH. The Impact of Immunosenescence on Pulmonary Disease. Mediators Inflamm 2015: 692546, 2015. doi: 10.1155/2015/692546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20, 2014. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Narayana Moorthy A, Narasaraju T, Rai P, Perumalsamy R, Tan KB, Wang S, Engelward B, Chow VT. In vivo and in vitro studies on the roles of neutrophil extracellular traps during secondary pneumococcal pneumonia after primary pulmonary influenza infection. Front Immunol 4: 56, 2013. doi: 10.3389/fimmu.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Nathan C. Fresh approaches to anti-infective therapies. Sci Transl Med 4: 140sr2, 2012. doi: 10.1126/scitranslmed.3003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Navne JE, Børresen ML, Slotved HC, Andersson M, Melbye M, Ladefoged K, Koch A. Nasopharyngeal bacterial carriage in young children in Greenland: a population at high risk of respiratory infections. Epidemiol Infect 144: 3226–3236, 2016. doi: 10.1017/S0950268816001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Neill DR, Coward WR, Gritzfeld JF, Richards L, Garcia-Garcia FJ, Dotor J, Gordon SB, Kadioglu A. Density and duration of pneumococcal carriage is maintained by transforming growth factor β1 and T regulatory cells. Am J Respir Crit Care Med 189: 1250–1259, 2014. doi: 10.1164/rccm.201401-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Newburg DS, Morelli L. Human milk and infant intestinal mucosal glycans guide succession of the neonatal intestinal microbiota. Pediatr Res 77: 115–120, 2015. doi: 10.1038/pr.2014.178. [DOI] [PubMed] [Google Scholar]
- 284.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res 61: 2–8, 2007. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 285.Ni W, Shao X, Cai X, Wei C, Cui J, Wang R, Liu Y. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One 10: e0121257, 2015. doi: 10.1371/journal.pone.0121257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nomura J, Horie I, Seto M, Nagai K, Hisatsune A, Miyata T, Isohama Y. All-trans retinoic acid increases expression of aquaporin-5 and plasma membrane water permeability via transactivation of Sp1 in mouse lung epithelial cells. Biochem Biophys Res Commun 351: 1048–1053, 2006. doi: 10.1016/j.bbrc.2006.10.159. [DOI] [PubMed] [Google Scholar]
- 287.Nyandiko WM, Greenberg D, Shany E, Yiannoutsos CT, Musick B, Mwangi AW. Nasopharyngeal Streptococcus pneumoniae among under-five year old children at the Moi Teaching and Referral Hospital, Eldoret, Kenya. East Afr Med J 84: 156–162, 2007. [PubMed] [Google Scholar]
- 288.O’Dwyer DN, Dickson RP, Moore BB. The Lung Microbiome, Immunity, and the Pathogenesis of Chronic Lung Disease. J Immunol 196: 4839–4847, 2016. doi: 10.4049/jimmunol.1600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ogunniyi AD, LeMessurier KS, Graham RM, Watt JM, Briles DE, Stroeher UH, Paton JC. Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect Immun 75: 1843–1851, 2007. doi: 10.1128/IAI.01384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Pace CC, McCullough GH. The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia 25: 307–322, 2010. doi: 10.1007/s00455-010-9298-9. [DOI] [PubMed] [Google Scholar]
- 291.Pandit C, Hsu P, van Asperen P, Mehr S. Respiratory manifestations and management in children with Common Variable Immunodeficiency. Paediatr Respir Rev 19: 56–61, 2016. [DOI] [PubMed] [Google Scholar]
- 292.Pandya PH, Wilkes DS. Complement system in lung disease. Am J Respir Cell Mol Biol 51: 467–473, 2014. doi: 10.1165/rcmb.2013-0485TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Parker D. Impact of Type I and III Interferons on Respiratory Superinfections Due to Multidrug-Resistant Pathogens. J Infect Dis 215, suppl_1: S58–S63, 2017. doi: 10.1093/infdis/jiw466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Parker D, Ahn D, Cohen T, Prince A. Innate Immune Signaling Activated by MDR Bacteria in the Airway. Physiol Rev 96: 19–53, 2016. doi: 10.1152/physrev.00009.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, Ratner AJ, Fitzgerald KA, Schindler C, Prince A. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. MBio 2: e00016-11, 2011. doi: 10.1128/mBio.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Patel P, Kearney JF. Immunological Outcomes of Antibody Binding to Glycans Shared between Microorganisms and Mammals. J Immunol 197: 4201–4209, 2016. doi: 10.4049/jimmunol.1600872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Perez AC, Pang B, King LB, Tan L, Murrah KA, Reimche JL, Wren JT, Richardson SH, Ghandi U, Swords WE. Residence of Streptococcus pneumoniae and Moraxella catarrhalis within polymicrobial biofilm promotes antibiotic resistance and bacterial persistence in vivo. Pathog Dis 70: 280–288, 2014. doi: 10.1111/2049-632X.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pérez-Dorado I, Galan-Bartual S, Hermoso JA. Pneumococcal surface proteins: when the whole is greater than the sum of its parts. Mol Oral Microbiol 27: 221–245, 2012. doi: 10.1111/j.2041-1014.2012.00655.x. [DOI] [PubMed] [Google Scholar]
- 299.Perobelli SM, Galvani RG, Gonçalves-Silva T, Xavier CR, Nóbrega A, Bonomo A. Plasticity of neutrophils reveals modulatory capacity. Braz J Med Biol Res 48: 665–675, 2015. doi: 10.1590/1414-431X20154524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis 14: 1584–1591, 2008. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Pettigrew MM, Marks LR, Kong Y, Gent JF, Roche-Hakansson H, Hakansson AP. Dynamic changes in the Streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza A virus infection. Infect Immun 82: 4607–4619, 2014. doi: 10.1128/IAI.02225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Phipps S, Lam CE, Kaiko GE, Foo SY, Collison A, Mattes J, Barry J, Davidson S, Oreo K, Smith L, Mansell A, Matthaei KI, Foster PS. Toll/IL-1 signaling is critical for house dust mite-specific helper T cell type 2 and type 17 [corrected] responses. Am J Respir Crit Care Med 179: 883–893, 2009. doi: 10.1164/rccm.200806-974OC. [DOI] [PubMed] [Google Scholar]
- 303.Pier GB. Role of the cystic fibrosis transmembrane conductance regulator in innate immunity to Pseudomonas aeruginosa infections. Proc Natl Acad Sci USA 97: 8822–8828, 2000. doi: 10.1073/pnas.97.16.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Poroyko V, Meng F, Meliton A, Afonyushkin T, Ulanov A, Semenyuk E, Latif O, Tesic V, Birukova AA, Birukov KG. Alterations of lung microbiota in a mouse model of LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol 309: L76–L83, 2015. doi: 10.1152/ajplung.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Prevaes SM, de Winter-de Groot KM, Janssens HM, de Steenhuijsen Piters WA, Tramper-Stranders GA, Wyllie AL, Hasrat R, Tiddens HA, van Westreenen M, van der Ent CK, Sanders EA, Bogaert D. Development of the Nasopharyngeal Microbiota in Infants with Cystic Fibrosis. Am J Respir Crit Care Med 193: 504–515, 2016. doi: 10.1164/rccm.201509-1759OC. [DOI] [PubMed] [Google Scholar]
- 306.Puchelle E, Zahm JM, Girard F, Bertrand A, Polu JM, Aug F, Sadoul P. Mucociliary transport in vivo and in vitro. Relations to sputum properties in chronic bronchitis. Eur J Respir Dis 61: 254–264, 1980. [PubMed] [Google Scholar]
- 307.Rajasingham CR, Bonsu BK, Chapman JI, Cohen DM, Barson WJ. Serious neurologic sequelae in cases of meningitis arising from infection by conjugate vaccine-related and nonvaccine-related serogroups of Streptococcus pneumoniae. Pediatr Infect Dis J 27: 771–775, 2008. doi: 10.1097/INF.0b013e3181710976. [DOI] [PubMed] [Google Scholar]
- 308.Ramakrishnan VR, Hauser LJ, Feazel LM, Ir D, Robertson CE, Frank DN. Sinus microbiota varies among chronic rhinosinusitis phenotypes and predicts surgical outcome. J Allergy Clin Immunol 136: 334–42.e1, 2015. doi: 10.1016/j.jaci.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 309.Rawlings BA, Higgins TS, Han JK. Bacterial pathogens in the nasopharynx, nasal cavity, and osteomeatal complex during wellness and viral infection. Am J Rhinol Allergy 27: 39–42, 2013. doi: 10.2500/ajra.2013.27.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Reddinger RM, Luke-Marshall NR, Hakansson AP, Campagnari AA. Host Physiologic Changes Induced by Influenza A Virus Lead to Staphylococcus aureus Biofilm Dispersion and Transition from Asymptomatic Colonization to Invasive Disease. MBio 7: e01235-16, 2016. doi: 10.1128/mBio.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416: 291–297, 2002. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 312.Revai K, McCormick DP, Patel J, Grady JJ, Saeed K, Chonmaitree T. Effect of pneumococcal conjugate vaccine on nasopharyngeal bacterial colonization during acute otitis media. Pediatrics 117: 1823–1829, 2006. doi: 10.1542/peds.2005-1983. [DOI] [PubMed] [Google Scholar]
- 313.Riley ID. Pneumonia research in Papua New Guinea: 1967-1986. P N G Med J 53: 106–118, 2010. [PubMed] [Google Scholar]
- 314.Rogers GB, Wesselingh S. Precision respiratory medicine and the microbiome. Lancet Respir Med 4: 73–82, 2016. doi: 10.1016/S2213-2600(15)00476-2. [DOI] [PubMed] [Google Scholar]
- 315.Rosseau S, Selhorst J, Wiechmann K, Leissner K, Maus U, Mayer K, Grimminger F, Seeger W, Lohmeyer J. Monocyte migration through the alveolar epithelial barrier: adhesion molecule mechanisms and impact of chemokines. J Immunol 164: 427–435, 2000. doi: 10.4049/jimmunol.164.1.427. [DOI] [PubMed] [Google Scholar]
- 316.Rowlands DJ, Islam MN, Das SR, Huertas A, Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS, Bhattacharya S, Bhattacharya J. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J Clin Invest 121: 1986–1999, 2011. doi: 10.1172/JCI43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Roy N, Ohtani K, Matsuda Y, Mori K, Hwang I, Suzuki Y, Inoue N, Wakamiya N. Collectin CL-P1 utilizes C-reactive protein for complement activation. Biochim Biophys Acta 1860: 1118–1128, 2016. doi: 10.1016/j.bbagen.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 318.Ruiz-Calderon JF, Cavallin H, Song SJ, Novoselac A, Pericchi LR, Hernandez JN, Rios R, Branch OH, Pereira H, Paulino LC, Blaser MJ, Knight R, Dominguez-Bello MG. Walls talk: microbial biogeography of homes spanning urbanization. Sci Adv 2: e1501061, 2016. doi: 10.1126/sciadv.1501061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Russkamp NF, Ruemmler R, Roewe J, Moore BB, Ward PA, Bosmann M. Experimental design of complement component 5a-induced acute lung injury (C5a-ALI): a role of CC-chemokine receptor type 5 during immune activation by anaphylatoxin. FASEB J 29: 3762–3772, 2015. doi: 10.1096/fj.15-271635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sakai F, Talekar S, Klugman K, Vidal J. Expression of Virulence-Related Genes in the Nasopharynx of Healthy Children. PLoS One 8: e67147, 2013. doi: 10.1371/journal.pone.0067147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sakwinska O, Bastic Schmid V, Berger B, Bruttin A, Keitel K, Lepage M, Moine D, Ngom Bru C, Brüssow H, Gervaix A. Nasopharyngeal microbiota in healthy children and pneumonia patients. J Clin Microbiol 52: 1590–1594, 2014. doi: 10.1128/JCM.03280-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Salami O, Marsland BJ. Has the airway microbiome been overlooked in respiratory disease? Genome Med 7: 62, 2015. doi: 10.1186/s13073-015-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Sanchez CJ, Shivshankar P, Stol K, Trakhtenbroit S, Sullam PM, Sauer K, Hermans PW, Orihuela CJ. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog 6: e1001044, 2010. doi: 10.1371/journal.ppat.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Sandgren A, Sjostrom K, Olsson-Liljequist B, Christensson B, Samuelsson A, Kronvall G, Henriques Normark B. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J Infect Dis 189: 785–796, 2004. doi: 10.1086/381686. [DOI] [PubMed] [Google Scholar]
- 325.Sands KM, Twigg JA, Lewis MA, Wise MP, Marchesi JR, Smith A, Wilson MJ, Williams DW. Microbial profiling of dental plaque from mechanically ventilated patients. J Med Microbiol 65: 147–159, 2016. doi: 10.1099/jmm.0.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Schilder AG, Chonmaitree T, Cripps AW, Rosenfeld RM, Casselbrant ML, Haggard MP, Venekamp RP. Otitis media. Nat Rev Dis Primers 2: 16063, 2016. doi: 10.1038/nrdp.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Schröder PC, Illi S, Casaca VI, Lluis A, Böck A, Roduit C, Depner M, Frei R, Genuneit J, Pfefferle PI, Roponen M, Weber J, Braun-Fahrländer C, Riedler J, Dalphin JC, Pekkanen J, Lauener R, von Mutius E, Schaub B; PASTURE study group . A switch in regulatory T cells through farm exposure during immune maturation in childhood. Allergy 72: 604–615, 2017. doi: 10.1111/all.13069. [DOI] [PubMed] [Google Scholar]
- 328.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, de Vos WM, van der Poll T, Wiersinga W. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65: 575–583, 2016. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Schulte T, Mikaelsson C, Beaussart A, Kikhney A, Deshmukh M, Wolniak S, Pathak A, Ebel C, Löfling J, Fogolari F, Henriques-Normark B, Dufrêne YF, Svergun D, Nygren PA, Achour A. The BR domain of PsrP interacts with extracellular DNA to promote bacterial aggregation; structural insights into pneumococcal biofilm formation. Sci Rep 6: 32371, 2016. doi: 10.1038/srep32371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Searles S, Gauss K, Wilkison M, Hoyt TR, Dobrinen E, Meissner N. Modulation of inflammasome-mediated pulmonary immune activation by type I IFNs protects bone marrow homeostasis during systemic responses to Pneumocystis lung infection. J Immunol 191: 3884–3895, 2013. doi: 10.4049/jimmunol.1301344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Sertl K, Takemura T, Tschachler E, Ferrans VJ, Kaliner MA, Shevach EM. Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med 163: 436–451, 1986. doi: 10.1084/jem.163.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Sharma M, Sharma S, Roy S, Varma S, Bose M. Pulmonary epithelial cells are a source of interferon-gamma in response to Mycobacterium tuberculosis infection. Immunol Cell Biol 85: 229–237, 2007. doi: 10.1038/sj.icb.7100037. [DOI] [PubMed] [Google Scholar]
- 333.Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. Granule protein processing and regulated secretion in neutrophils. Front Immunol 5: 448, 2014. doi: 10.3389/fimmu.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Shilts MH, Rosas-Salazar C, Tovchigrechko A, Larkin EK, Torralba M, Akopov A, Halpin R, Peebles RS, Moore ML, Anderson LJ, Nelson KE, Hartert TV, Das SR. Minimally Invasive Sampling Method Identifies Differences in Taxonomic Richness of Nasal Microbiomes in Young Infants Associated with Mode of Delivery. Microb Ecol 71: 233–242, 2016. doi: 10.1007/s00248-015-0663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Shopsin B, Mathema B, Martinez J, Ha E, Campo ML, Fierman A, Krasinski K, Kornblum J, Alcabes P, Waddington M, Riehman M, Kreiswirth BN. Prevalence of methicillin-resistant and methicillin-susceptible Staphylococcus aureus in the community. J Infect Dis 182: 359–362, 2000. doi: 10.1086/315695. [DOI] [PubMed] [Google Scholar]
- 336.Short K, Diavatopoulos D. Nasopharyngeal colonization with Streptococcus pneumoniae. In: Streptococcus pneumoniae: Molecular Mechanisms of Host-Pathogen Interactions, edited by Orihuela C, Hammerschmidt S, Brown J. London: Elsevier, Academic Press, 2015, p. 279–292. doi: 10.1016/B978-0-12-410530-0.00015-6 [DOI] [Google Scholar]
- 337.Siegel SJ, Weiser JN. Mechanisms of Bacterial Colonization of the Respiratory Tract. Annu Rev Microbiol 69: 425–444, 2015. doi: 10.1146/annurev-micro-091014-104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Simpson JL, Daly J, Baines KJ, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Hugenholtz P, Willner D, Gibson PG. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J 47: 792–800, 2016. doi: 10.1183/13993003.00405-2015. [DOI] [PubMed] [Google Scholar]
- 339.Singh R, Nieuwdorp M, ten Berge IJ, Bemelman FJ, Geerlings SE. The potential beneficial role of faecal microbiota transplantation in diseases other than Clostridium difficile infection. Clin Microbiol Infect 20: 1119–1125, 2014. doi: 10.1111/1469-0691.12799. [DOI] [PubMed] [Google Scholar]
- 340.Sleeman KL, Griffiths D, Shackley F, Diggle L, Gupta S, Maiden MC, Moxon ER, Crook DW, Peto TE. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis 194: 682–688, 2006. doi: 10.1086/505710. [DOI] [PubMed] [Google Scholar]
- 341.Smit LAM, Boender GJ, de Steenhuijsen Piters WAA, Hagenaars TJ, Huijskens EGW, Rossen JWA, Koopmans M, Nodelijk G, Sanders EAM, Yzermans J, Bogaert D, Heederik D. Increased risk of pneumonia in residents living near poultry farms: does the upper respiratory tract microbiota play a role? Pneumonia (Nathan) 9: 3, 2017. doi: 10.1186/s41479-017-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Spijkerman J, Prevaes SM, van Gils EJ, Veenhoven RH, Bruin JP, Bogaert D, Wijmenga-Monsuur AJ, van den Dobbelsteen GP, Sanders EA. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One 7: e39730, 2012. doi: 10.1371/journal.pone.0039730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Stacy A, Fleming D, Lamont RJ, Rumbaugh KP, Whiteley M. A Commensal Bacterium Promotes Virulence of an Opportunistic Pathogen via Cross-Respiration. MBio 7: e00782-16, 2016. doi: 10.1128/mBio.00782-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Stearns JC, Davidson CJ, McKeon S, Whelan FJ, Fontes ME, Schryvers AB, Bowdish DM, Kellner JD, Surette MG. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J 9: 1268, 2015. doi: 10.1038/ismej.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Stevens WW, Lee RJ, Schleimer RP, Cohen NA. Chronic rhinosinusitis pathogenesis. J Allergy Clin Immunol 136: 1442–1453, 2015. doi: 10.1016/j.jaci.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sun R, Zhou Q, Ye X, Takahata T, Ishiguro A, Kijima H, Nukiwa T, Saijo Y. A change in the number of CCSP(pos)/SPC(pos) cells in mouse lung during development, growth, and repair. Respir Investig 51: 229–240, 2013. doi: 10.1016/j.resinv.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 347.Sun S, Zhao G, Liu C, Wu X, Guo Y, Yu H, Song H, Du L, Jiang S, Guo R, Tomlinson S, Zhou Y. Inhibition of complement activation alleviates acute lung injury induced by highly pathogenic avian influenza H5N1 virus infection. Am J Respir Cell Mol Biol 49: 221–230, 2013. doi: 10.1165/rcmb.2012-0428OC. [DOI] [PubMed] [Google Scholar]
- 348.Surewaard BG, Trzciński K, Jacobino SR, Hansen IS, Vughs MM, Sanders EA, van der Ende A, van Strijp JA, de Haas CJ. Pneumococcal immune evasion: ZmpC inhibits neutrophil influx. Cell Microbiol 15: 1753–1765, 2013. [DOI] [PubMed] [Google Scholar]
- 349.Sze MA, Dimitriu PA, Suzuki M, McDonough JE, Campbell JD, Brothers JF, Erb-Downward JR, Huffnagle GB, Hayashi S, Elliott WM, Cooper J, Sin DD, Lenburg ME, Spira A, Mohn WW, Hogg JC. Host Response to the Lung Microbiome in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 192: 438–445, 2015. doi: 10.1164/rccm.201502-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis 5: 255–273, 2011. doi: 10.1177/1753465810396539. [DOI] [PubMed] [Google Scholar]
- 351.Tarabichi Y, Li K, Hu S, Nguyen C, Wang X, Elashoff D, Saira K, Frank B, Bihan M, Ghedin E, Methé BA, Deng JC. The administration of intranasal live attenuated influenza vaccine induces changes in the nasal microbiota and nasal epithelium gene expression profiles. Microbiome 3: 74, 2015. doi: 10.1186/s40168-015-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Holt BJ, Hales BJ, Walker ML, Hollams E, Bochkov YA, Grindle K, Johnston SL, Gern JE, Sly PD, Holt PG, Holt KE, Inouye M. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17: 704–715, 2015. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Tikhomirova A, Kidd SP. Haemophilus influenzae and Streptococcus pneumoniae: living together in a biofilm. Pathog Dis 69: 114–126, 2013. doi: 10.1111/2049-632X.12073. [DOI] [PubMed] [Google Scholar]
- 354.Till GO, Johnson KJ, Kunkel R, Ward PA. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest 69: 1126–1135, 1982. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sørensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol 171: 6690–6696, 2003. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 356.Tocheva AS, Jefferies JM, Rubery H, Bennett J, Afimeke G, Garland J, Christodoulides M, Faust SN, Clarke SC. Declining serotype coverage of new pneumococcal conjugate vaccines relating to the carriage of Streptococcus pneumoniae in young children. Vaccine 29: 4400–4404, 2011. doi: 10.1016/j.vaccine.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 357.Torén K, Qvarfordt I, Bergdahl IA, Järvholm B. Increased mortality from infectious pneumonia after occupational exposure to inorganic dust, metal fumes and chemicals. Thorax 66: 992–996, 2011. doi: 10.1136/thoraxjnl-2011-200707. [DOI] [PubMed] [Google Scholar]
- 358.Torow N, Marsland BJ, Hornef MW, Gollwitzer ES. Neonatal mucosal immunology. Mucosal Immunol 10: 5–17, 2017. doi: 10.1038/mi.2016.81. [DOI] [PubMed] [Google Scholar]
- 359.Torretta S, Marchisio P, Drago L, Baggi E, De Vecchi E, Garavello W, Nazzari E, Pignataro L, Esposito S. Nasopharyngeal biofilm-producing otopathogens in children with nonsevere recurrent acute otitis media. Otolaryngol Head Neck Surg 146: 991–996, 2012. doi: 10.1177/0194599812438169. [DOI] [PubMed] [Google Scholar]
- 360.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 457: 585–588, 2009. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Trzciński K, Li Y, Weinberger DM, Thompson CM, Cordy D, Bessolo A, Malley R, Lipsitch M. Effect of Serotype on Pneumococcal Competition in a Mouse Colonization Model. MBio 6: e00902-15, 2015. doi: 10.1128/mBio.00902-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Van den Bergh MR, Biesbroek G, Rossen JW, de Steenhuijsen Piters WA, Bosch AA, van Gils EJ, Wang X, Boonacker CW, Veenhoven RH, Bruin JP, Bogaert D, Sanders EA. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS One 7: e47711, 2012. doi: 10.1371/journal.pone.0047711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Van Dongen TM, Venekamp RP, Wensing AM, Bogaert D, Sanders EA, Schilder AG. Acute otorrhea in children with tympanostomy tubes: prevalence of bacteria and viruses in the post-pneumococcal conjugate vaccine era. Pediatr Infect Dis J 34: 355–360, 2015. doi: 10.1097/INF.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 364.Veenhoven R, Bogaert D, Uiterwaal C, Brouwer C, Kiezebrink H, Bruin J, IJzerman E, Hermans P, de Groot R, Zegers B, Kuis W, Rijkers G, Schilder A, Sanders E. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet 361: 2189–2195, 2003. doi: 10.1016/S0140-6736(03)13772-5. [DOI] [PubMed] [Google Scholar]
- 365.Vernatter J, Pirofski LA. Current concepts in host-microbe interaction leading to pneumococcal pneumonia. Curr Opin Infect Dis 26: 277–283, 2013. doi: 10.1097/QCO.0b013e3283608419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Vidal JE, Howery KE, Ludewick HP, Nava P, Klugman KP. Quorum-sensing systems LuxS/autoinducer 2 and Com regulate Streptococcus pneumoniae biofilms in a bioreactor with living cultures of human respiratory cells. Infect Immun 81: 1341–1353, 2013. doi: 10.1128/IAI.01096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Vidal JE, Ludewick HP, Kunkel RM, Zähner D, Klugman KP. The LuxS-dependent quorum-sensing system regulates early biofilm formation by Streptococcus pneumoniae strain D39. Infect Immun 79: 4050–4060, 2011. doi: 10.1128/IAI.05186-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Vorobjeva NV, Pinegin BV. Neutrophil extracellular traps: mechanisms of formation and role in health and disease. Biochemistry (Mosc) 79: 1286–1296, 2014. doi: 10.1134/S0006297914120025. [DOI] [PubMed] [Google Scholar]
- 369.Voyno-Yasenetskaya TA, Dobbs LG, Williams MC. Regulation of ATP-dependent surfactant secretion and activation of second-messenger systems in alveolar type II cells. Am J Physiol 261, Suppl: 105–109, 1991. [DOI] [PubMed] [Google Scholar]
- 370.Wang J, Li F, Sun R, Gao X, Wei H, Li LJ, Tian Z. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun 4: 2106, 2013. doi: 10.1038/ncomms3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ward PA, Johnson KJ, Till GO. Complement and experimental respiratory failure. Intensive Care Med 12: 17–21, 1986. doi: 10.1007/BF00315362. [DOI] [PubMed] [Google Scholar]
- 372.Warren JS, Kunkel SL, Johnson KJ, Ward PA. In vitro activation of rat neutrophils and alveolar macrophages with IgA and IgG immune complexes. Implications for immune complex-induced lung injury. Am J Pathol 129: 578–588, 1987. [PMC free article] [PubMed] [Google Scholar]
- 373.Watanabe Y, Ogino Y, Ubukata E, Sakamoto Y, Matsuzaki O, Shimizu N. A case of a gallbladder cancer with marked hypercalcemia and leukocytosis. Jpn J Med 28: 722–726, 1989. doi: 10.2169/internalmedicine1962.28.722. [DOI] [PubMed] [Google Scholar]
- 374.Webb DC, Cripps AW. Secondary structure and molecular analysis of interstrain variability in the P5 outer-membrane protein of non-typable Haemophilus influenzae isolated from diverse anatomical sites. J Med Microbiol 47: 1059–1067, 1998. doi: 10.1099/00222615-47-12-1059. [DOI] [PubMed] [Google Scholar]
- 375.Wei H, Håvarstein LS. Fratricide is essential for efficient gene transfer between pneumococci in biofilms. Appl Environ Microbiol 78: 5897–5905, 2012. doi: 10.1128/AEM.01343-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Weimer KE, Juneau RA, Murrah KA, Pang B, Armbruster CE, Richardson SH, Swords WE. Divergent mechanisms for passive pneumococcal resistance to β-lactam antibiotics in the presence of Haemophilus influenzae. J Infect Dis 203: 549–555, 2011. doi: 10.1093/infdis/jiq087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet 378: 1962–1973, 2011. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Weinberger DM, Trzciński K, Lu YJ, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog 5: e1000476, 2009. doi: 10.1371/journal.ppat.1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Weiser JN, Shchepetov M, Chong ST. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun 65: 943–950, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 506: 503–506, 2014. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.WHO Antimicrobial Resistance: Global Report of Surveillance. Geneva: WHO, 2014, p. 1–256. [Google Scholar]
- 382.Wlasiuk G, Vercelli D. The farm effect, or: when, what and how a farming environment protects from asthma and allergic disease. Curr Opin Allergy Clin Immunol 12: 461–466, 2012. doi: 10.1097/ACI.0b013e328357a3bc. [DOI] [PubMed] [Google Scholar]
- 383.Wolcott RD, Ehrlich GD. Biofilms and chronic infections. JAMA 299: 2682–2684, 2008. doi: 10.1001/jama.299.22.2682. [DOI] [PubMed] [Google Scholar]
- 384.Wolter N, Tempia S, Cohen C, Madhi SA, Venter M, Moyes J, Walaza S, Malope-Kgokong B, Groome M, du Plessis M, Magomani V, Pretorius M, Hellferscee O, Dawood H, Kahn K, Variava E, Klugman KP, von Gottberg A. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis 210: 1649–1657, 2014. doi: 10.1093/infdis/jiu326. [DOI] [PubMed] [Google Scholar]
- 385.Wong EH, Porter JD, Edwards MR, Johnston SL. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med 2: 657–670, 2014. doi: 10.1016/S2213-2600(14)70107-9. [DOI] [PubMed] [Google Scholar]
- 386.Wong MH, Johnson MD. Differential response of primary alveolar type I and type II cells to LPS stimulation. PLoS One 8: e55545, 2013. doi: 10.1371/journal.pone.0055545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Wyllie AL, Rümke LW, Arp K, Bosch AA, Bruin JP, Rots NY, Wijmenga-Monsuur AJ, Sanders EA, Trzciński K. Molecular surveillance on Streptococcus pneumoniae carriage in non-elderly adults; little evidence for pneumococcal circulation independent from the reservoir in children. Sci Rep 6: 34888, 2016. doi: 10.1038/srep34888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Xu Q, Almudervar A, Casey JR, Pichichero ME. Nasopharyngeal bacterial interactions in children. Emerg Infect Dis 18: 1738–1745, 2012. doi: 10.3201/eid1811.111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Xu YM, Gao YM, Wu DD, Yu FY, Zang ZS, Yang L, Yao Y, Cai NL, Zhou Y, Chiu JF, Ching YP, Lau AT. Aberrant cytokine secretion and zinc uptake in chronic cadmium-exposed lung epithelial cells. Proteomics Clin Appl 11: 1600059, 2017. doi: 10.1002/prca.201600059. [DOI] [PubMed] [Google Scholar]
- 390.Yajjala VK, Thomas VC, Bauer C, Scherr TD, Fischer KJ, Fey PD, Bayles KW, Kielian T, Sun K. Resistance to Acute Macrophage Killing Promotes Airway Fitness of Prevalent Community-Acquired Staphylococcus aureus Strains. J Immunol 196: 4196–4203, 2016. doi: 10.4049/jimmunol.1600081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Yang M, Wang Y, Zhang Y, Zhang F, Zhao Z, Li S, Zhang J, Cao X, Zhang D. S-allylmercapto-l-cysteine modulates MUC5AC and AQP5 secretions in a COPD model via NF-кB signaling pathway. Int Immunopharmacol 39: 307–313, 2016. doi: 10.1016/j.intimp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 392.Yang X, Jiang Y, Wang C. Does IL-17 Respond to the Disordered Lung Microbiome and Contribute to the Neutrophilic Phenotype in Asthma? Mediators Inflamm 2016: 6470364, 2016. doi: 10.1155/2016/6470364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Yosef I, Manor M, Kiro R, Qimron U. Temperate and lytic bacteriophages programmed to sensitize and kill antibiotic-resistant bacteria. Proc Natl Acad Sci USA 112: 7267–7272, 2015. doi: 10.1073/pnas.1500107112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Zaura E, Nicu EA, Krom BP, Keijser BJ. Acquiring and maintaining a normal oral microbiome: current perspective. Front Cell Infect Microbiol 4: 85, 2014. doi: 10.3389/fcimb.2014.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Zemanick ET, Wagner BD, Robertson CE, Stevens MJ, Szefler SJ, Accurso FJ, Sagel SD, Harris JK. Assessment of airway microbiota and inflammation in cystic fibrosis using multiple sampling methods. Ann Am Thorac Soc 12: 221–229, 2015. doi: 10.1513/AnnalsATS.201407-310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis 5: 275–286, 2005. doi: 10.1016/S1473-3099(05)70112-2. [DOI] [PubMed] [Google Scholar]
- 397.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119: 1899–1909, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA 109: 5809–5814, 2012. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zmora N, Zeevi D, Korem T, Segal E, Elinav E. Taking it Personally: Personalized Utilization of the Human Microbiome in Health and Disease. Cell Host Microbe 19: 12–20, 2016. doi: 10.1016/j.chom.2015.12.016. [DOI] [PubMed] [Google Scholar]