Abstract
Since the phase-out of pentaBDE in the early 2000s, replacement flame-retardant mixtures including Firemaster 550 (FM 550), Firemaster 600 (FM 600), and organophosphate aryl ester technical mixtures have been increasingly used to treat polyurethane foam in residential upholstered furniture. These mixtures contain isomers of isopropylated and tert-butylated triarylphosphate esters (ITPs and TBPPs), which have similar or greater neuro- and developmental toxicity compared to BDE 47 in high-throughput assays. Additionally, human exposure to ITPs and TBPPs has been demonstrated to be widespread in several recent studies; however, the relative composition of these mixtures has remained largely uncharacterized. Using available authentic standards, the present study quantified the contribution of individual ITP and TBPP isomers in four commercial flame retardant mixtures: FM 550, FM 600, an ITP mixture, and a TBPP mixture. Findings suggest similarities between FM 550 and the ITP mixture, with 2-isopropylphenyl diphenyl phosphate (2IPPDPP), 2,4-diisopropylphenyl diphenyl phosphate (24DIPPDPP), and bis(2-isopropylphenyl) phenyl phosphate (B2IPPPP) being the most prevalent ITP isomers in both mixtures. FM 600 differed from FM 550 in that it contained TBPP isomers instead of ITP isomers. These analytes were also detected and quantified in a house dust standard reference material, SRM 2585, demonstrating their environmental relevance.
Graphical abstract
INTRODUCTION
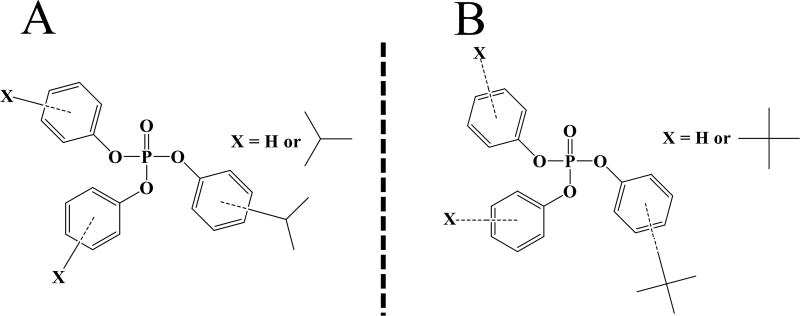
In the early to mid-2000s, the use of polybrominated diphenyl ethers (PBDEs) was globally phased out due to concerns about their persistence, bioaccumulation, and potential toxicity (PBT). One PBDE commercial mixture, PentaBDE, was primarily used in furniture as a flame retardant to meet residential flammability standards; however, new flame retardant chemicals and mixtures have since entered the market. Recent studies by our group demonstrate that many of these new flame retardant mixtures contain alkylated organophosphate aryl esters and, in particular, several types of isopropyl- and tert-butyl-triphenyl phosphates (ITPs and TBPPs, respectively; Figure 1).1,2 In addition to being used as flame retardants, both ITPs and TBPPs are used in hydraulic fluids and as plasticizers in a variety of materials.3,4 In our previous research, we identified ITPs in a common commercial flame retardant mixture (Firemaster 550, FM 550), a mixture of organophosphate and brominated flame retardants, and an ITP mixture.5 Similarly, we identified TBPPs in Firemaster 600 (FM 600) and in a mixture with TPHP that we refer to as the TBPP mixture.2 Despite their documented neuro- and developmental toxicity, there is currently very little information regarding human exposure to ITPs and TBPPs and limited documentation of the presence of these isomers in environmental samples.6,7 This pressing lack of data has been recognized by the U.S. Environmental Protection Agency (EPA), which recently prioritized ITPs as one of five fast-tracked PBT chemicals for further assessment under the 2016 amendment to the Toxic Substances Control Act (TSCA).8 Previous research conducted by our laboratory has demonstrated that exposure to ITP chemicals is very common in the U.S. population and that exposure ranges considerably among individuals, likely based on differential exposure to flame retardant and plasticizer-treated consumer products. For instance, Hammel et al. detected ITPs on silicone wristbands worn by human participants with 100% frequency (n = 40).9 Furthermore, out of five organophosphate flame retardant (PFR) metabolites measured in urine in this study, mono-isopropylphenyl phenyl phosphate (mono-ipPPP), a confirmed metabolite of ITPs, had the highest geometric mean concentration (2.6 ng/mL). Another recent study detected mono-ipPPP in 98% of all tested urine samples (n = 48) and found that, on average, levels of urinary mono-ipPPP were 1.2 times higher in children than in their mothers.10 Other recent studies have used commercial flame-retardant mixtures containing ITPs and TBPPs to assess toxicological endpoints, and yet the composition of these mixtures has not been well-described. For example, FM 550 has been shown to be endocrine disrupting and potentially obesogenic in rats, and exposure in fathead minnows resulted in significant DNA damage.11,12 The ITP and TBPP commercial mixtures have been found to disrupt C. elegans larval development, zebrafish embryonic development, and zebrafish behavior at concentrations in the low-µM range.6,13 The TBPP mixture has also been shown to elevate estradiol serum levels, alter reproductive cycles, and elicit cholesteryl lipidosis in adrenocortical and ovarian interstitial cells of exposed female rats.14 However, because there have been no studies that identify the composition and ITP &TBPP isomer profile of these commercial mixtures, it is difficult to attribute toxicological findings to a specific component(s) of the mixture. Such information will give context to toxicological studies using these mixtures, and give direction to future exposure studies. Moreover, there is considerable confusion in the flame retardant literature regarding the naming and acronyms associated with these compounds, and these mixtures also have a myriad of technical/brand names associated with them from multiple manufacturers (Table 1). For the purposes of this study and to avoid future confusion, individual ITP and TBPP isomer acronyms are used according to their practical abbreviations (PRABs).15 Individual standards for these isomers have become available in the past year, and the present study attempts to alleviate confusion regarding these compounds. Here, we characterize the ITP and TBPP isomer profiles present in FM 550 and FM 600 and two other types of organophosphate commercial mixtures that are currently available on the market.
Figure 1.
(A) General structure of an ITP isomer. (B) General structure of a TBPP isomer. Isopropylation and tert-butylation can occur at the ortho, meta, or para position and the degree of substitution varies among isomers.
Table 1.
Acronyms and Brand Names Associated with ITP and TBPP Isomers
| mixture | acronym or brand name | reference or manufacturer |
|---|---|---|
| ITP | ITP | 30–32 |
| ITP | IPP | 13, 33, 34 |
| ITP | IPTP | 35 |
| ITP | IPDP | 36 |
| ITP | PIP | 37, 38 |
| ITP | IPTPP | 28 |
| ITP | IPPP | 15, 39 |
| ITP | Durad 110, 150, 220, and 300 | Lanxessa |
| ITP | Reofos 35, 50, 65, and 95 | Great Lakes Solutionsb |
| ITP | Phosflex 31L | ICL-IPc |
| ITP | CELLTECH IPPP | Cellular Technologies International, Inc.d |
| ITP | TBPP | 1, 2 |
| TBPP | BPDP | 40–42 |
| TBPP | BTP | 3, 14 |
| TBPP | Durad 110B, 150B, and 220B | Lanxessa |
| TBPP | Phosflex 71B | ICL-IPc |
EXPERIMENTAL SECTION
Materials
Individual, authentic standards of 2-isopropylphenyl diphenyl phosphate (2IPPDPP), 3-isopropylphenyl diphenyl phosphate (3IPPDPP), 4-isopropylphenyl diphenyl phosphate (4IPPDPP), 2,4-diisopropylphenyl diphenyl phosphate (24DIPPDPP), bis(2-isopropylphenyl) phenyl phosphate (B2IPPPP), bis(3-isopropylphenyl) phenyl phosphate (B3IPPPP), bis(4-isopropylphenyl) phenyl phosphate (B4IPPPP), bis(2,4-diisopropylphenyl) phenyl phosphate (B24DIPPPP), tris(3-isopropylphenyl) phosphate (T3IPPP), tris(4-isopropylphenyl) phosphate (T4IPPP), 4-tert-butylphenyl diphenyl phosphate (4tBPDPP), bis(2-tert-butylphenyl) phenyl phosphate (B2tBPPP), bis(4-tert-butylphenyl) phenyl phosphate (B4tBPPP), 13C-TPHP, d15TPHP, 13C-EH-TBB, and 13C-BEH-TEBP were purchased from or provided by Wellington Laboratories (Guelph, Ontario, Canada). TPHP (99% pure) and Tris(4-tert-butylphenyl) phosphate (T4tBPP) were purchased from Sigma-Aldrich (Saint Louis, Missouri). High-performance liquid chromatography grade isooctane was purchased from Honeywell Burdick and Jackson (Muskegon, Michigan). FM 550 was provided by Chemtura (lot no. 77000DI8P), the commercial mixture containing only ITPs was purchased from Jinan Great Chemical Industry Co. (no lot information provided by manufacturer), and the TBPP mixture was produced by Ubichem and obtained from the National Toxicology Program (lot no. M062011NS) for research purposes as part of a materials transfer agreement. The commercial FM 600 mixture itself was not available for analysis; for this reason, a block of FM 600 treated polyurethane foam was obtained from a North Carolina foam manufacturer for analysis.
Commercial Mixture Preparation
Each flame retardant mixture preparation was weighed using a Mettler Toledo A21 Comparator microbalance and dissolved in isooctane to make three low-concentration (~1 µg/mL) and three high-concentration (~5 µg/mL) solutions. FM 600-treated foam was weighed (100 mg) and extracted in triplicate using dichloromethane according to previously published methods.16 For the analysis here, the concentration of FM 600 in the foam was assumed to be 4% by weight, similar to measurements made previously in polyurethane foam.16 Prior to analysis, each solution was spiked with the appropriate internal standards.
SRM 2585 Analysis
Approximately 300 mg of SRM 2585 (National Institute of Standards and Technology, Gaithersburg, MD) was spiked with 13C-TPHP and extracted three times in dichloromethane with sonication. SRM extracts (n = 4) were cleaned using a Supelclean ENVI-Florisil SPE cartridges (6 mL; 1.0 g; Supelco, Bellefonte, Pennsylvania) using previously published methods.17 ITP and TBPP isomers were eluted using 10 mL of ethyl acetate. Eluents were concentrated under nitrogen and solvent-exchanged to hexane prior to analysis.
Component Quantification
EH-TBB and BEH-TEBP were quantified with previously described GC/ENCI–MS methods using 13C-EH-TBB and 13C-BEH-TEBP as internal standards.5 TPHP and individual ITP and TBPP isomers were quantified with the previously described GC/EI-MS methods using 13C-TPHP as an internal standard.16 Briefly, the quantification of TPHP, ITP, and TBPP isomers was performed using an Agilent (Wilmington, DE) gas chromatograph (model 7890A) mass spectrometer (model 5975C) operating in electron-impact (EI) mode. Pressurized temperature vaporization (PTV) injection was employed in the inlet, and a 0.25 mm (I.D.) × 30 m fused silica capillary column coated with 5% phenyl methylpolysiloxane (J&W Scientific, 0.25 µm film thickness) was used in the GC to resolve the analytes. Helium was used as the carrier gas in the GC with a constant flow rate of 1.3 mL/min. The inlet was set to a temperature of 80 °C for 0.3 min and then ramped to 300 °C at a rate of 600 °C/min to efficiently transfer samples to the head of the GC column. The GC oven was held at 80 °C for 2 min, ramped to 250 °C at 20 °C/min, ramped to 260 °C at 1.5 °C/min, then ramped to 300 °C at 25 °C/min and held at 300 °C for 20 min. The transfer line temperature was held at 300 °C, and the ion source was maintained at 200 °C. Table 2 outlines the m/z ions and retention times used for the quantification of ITP and TBPP isomers.
Table 2.
m/z Ions and Retention Times Used for Quantification of ITP and TBPP Isomers
| ITP or TBPP isomer | m/zquantifier | m/zqualifier | retention time (min)a |
|---|---|---|---|
| TPHP | 325 | 326 | 14.49 |
| 2IPPDPP | 251 | 368 | 15.82 |
| 3IPPDPP | 353 | 368 | 17.09 |
| 4IPPDPP | 353 | 368 | 17.71 |
| 24DIPPDPP | 145 | 160 | 18.21 |
| B2IPPPP | 251 | 410 | 17.46 |
| B3IPPPP | 395 | 410 | 19.04 |
| B4IPPPP | 395 | 410 | 20.18 |
| B24DIPPPP | 145 | 160 | 21.78 |
| T3IPPP | 452 | 438 | 21.10 |
| T4IPPP | 452 | 438 | 23.56 |
| 2tBPDPP | 367 | 382 | 17.03 |
| 4tBPDPP | 367 | 382 | 18.64 |
| B2tBPPP | 423 | 438 | 19.38 |
| B4tBPPP | 423 | 438 | 21.52 |
| T4tBPP | 479 | 494 | 26.14 |
As observed on a 30 m DB5-MS Agilent J&W column.
Quality Assurance and Quality Control
Laboratory blanks were included in both the commercial mixture preparation (n = 3) and the SRM 2585 analysis (n = 6). All of the samples were blank-corrected using the average blank level. Method detection limits (MDLs) were calculated using 3 times the standard deviation of the average lab blanks and were normalized to the amount of dust extracted for the SRM 2585 analysis. MDLs are reported in Table S1 for each isomer. Linear calibration curves were constructed using a five-point calibration for the quantification of each isomer, with r2 values ranging from 0.9985 to 0.9999. To evaluate the recoveries of ITP and TBPP isomers in house dust, a matrix spike experiment was performed using a low and a high dose of isomers. Samples of SRM 2585 were spiked with either a low dose (50 ng) or a high dose (500 ng) of all ITP and TBPP isomers and extracted, in triplicate, according to the methods detailed above. 13C TPHP was added prior to GC–MS analysis to quantify recovery of each analyte. Recoveries ranged from 72.4 ± 1.0% to 109.9 ± 10.7% (see Table S2) for the various isomers.
RESULTS AND DISCUSSION
Firemaster 550 and ITP Mixture
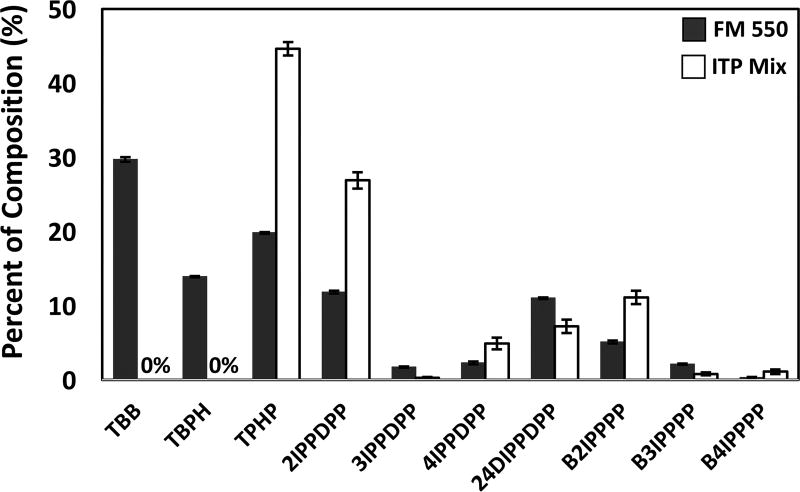
The percent composition of each ITP isomer present in the FM 550 and ITP mixture, respectively, is shown in Table 3 and is presented on a percent w/w basis. The FM 550 mixture consisted of approximately 44% of the brominated components, with the remaining mass composed of TPHP and the ITPs. Like the FM 550 mixture, the ITP mixture consisted of very similar ITP isomers, particularly TPHP, 2IPPDPP, and B2IPPPP, which were the dominant compounds in the mixture. Notably, the contribution of the individual organophosphate components in the ITP mixture is roughly twice that of their contribution in FM 550, suggesting a common ITP formulation and/or synthesis (Figure 2). T4IPPP was not detected in either of the mixtures. Our analyses accounted for 97.8 ± 1.5% of FM 550 and 97.0 ± 5.3% of the ITP mixture.
Table 3.
Percent Composition (w/w) of Individual ITP Isomers in FM 550 and the ITP Mixturea
| Component Name |
Structure | Mass Fraction (w/w) in FM 550 |
Mass Fraction (w/w) in ITP Mix |
|---|---|---|---|
| EH-TBB |
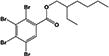
|
29.7 ± 0.3% | none |
| BEH-TEBP |
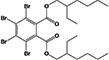
|
13.9 ± 0.1% | none |
| TPHP |
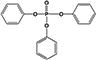
|
19.8 ± 0.1% | 44.6 ± 0.9% |
| 2IPPDPP |
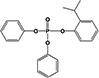
|
11.8 ± 0.2% | 26.9 ± 1.1% |
| 3IPPDPP |
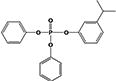
|
1.7 ± 0.1% | 0.3 ± 0.1% |
| 4IPPDPP |
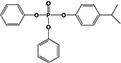
|
2.3 ± 0.2% | 4.9 ± 0.8% |
| 24DIPPDPP |
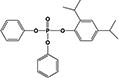
|
11.0 ± 0.1% | 7.2 ± 0.9% |
| B2IPPPP |
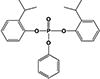
|
5.1 ± 0.2% | 11.1 ± 0.9% |
| B3IPPPP |
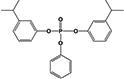
|
2.1 ± 0.1% | 0.8 ± 0.2% |
| B4IPPPP |
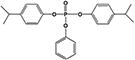
|
0.3 ± 0.1% | 1.1 ± 0.3% |
| T3IPPP |
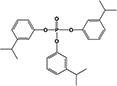
|
0.1 ± 0.04% | 0.1 ± 0.05% |
The total percentage accounted for was 97.8 ± 1.5% for FM 550 and 97.0 ± 5.3% for the ITP mix.
Figure 2.
A comparison of the contributions of individual components in the FM 550 and ITP mixture suggests a common ITP formulation. FM 550 is shown in black, and the ITP mixture is shown in white. The two brominated components of FM 550, EH-TBB and BEH-TEBP, comprise ~43.6% of the mixture and are absent in the ITP mixture.
Firemaster 600 and TBPP Mixture
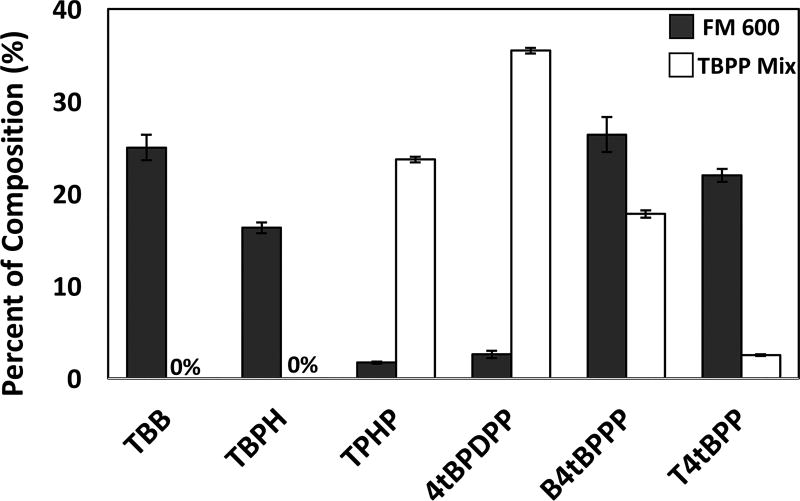
The percent composition of each TBPP isomer present in the FM 600 and TBPP mixture is shown in Table 4 and is presented on a percent w/w basis. In the case of the FM 600 analysis, we used foam as a standard, which could be a limitation affecting the accuracy of our characterization. We assumed that the foam was treated with 4% FM 600 by weight.16 Using that assumption, the foam treated with FM 600 contained approximately 40% of the brominated compounds, with the remaining mass being composed of TPHP and TBPP isomers. The organophosphate formulation of FM 600 was dominated by B4tBPPP and T4tBPP isomers, with relatively little TPHP or 4tBPDPP present in the mixture. In contrast, the TBPP mixture was composed of higher percentages of TPHP and 4tBPDPP (Figure 3). In total, we could account for 95.3 ± 5.2% of the FM 660 mixture and 79.5 ± 1.1% of the TBPP mixture. 2tBPDPP and B2tBPPP were not detected in either FM 600 or the TBPP mixture, but chromatographic peaks for 3tBPDPP and B3tBPPP were observed for both mixtures (Figure S1). These peaks were presumed to be the meta-isomers based on their mass spectra (m/z = 382 for 3tBPDPP and m/z = 438 for B3tBPPP), retention times between ortho- and para-substituted isomers, and lack of matching with the ortho- and para-substituted isomers. Standards of meta-TBPP isomers are not commercially available, so quantification of 3tBPDPP and B3tBPPP was not possible.
Table 4.
Percent Composition (w/w) of Individual TBPP Isomers in FM 600-Treated Foam and the TBPP Mixturea
| Component Name |
Structure | Mass Fraction (w/w) in FM 600- treated foam |
Mass Fraction (w/w) in TBPP Mix |
|---|---|---|---|
| EH-TBB |
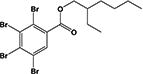
|
25.0 ± 1.4% | none |
| BEH-TEBP |
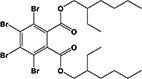
|
16.3 ± 0.6% | none |
| TPHP |
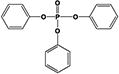
|
1.7 ± 0.1% | 23.7 ± 0.3% |
| 4tBPDPP |
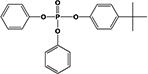
|
2.6 ± 0.4% | 35.5 ± 0.3% |
| B4tBPPP |
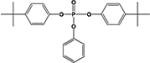
|
26.4 ± 1.9% | 17.8 ± 0.4% |
| T4tBPP |
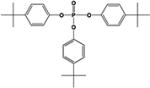
|
22.0 ± 0.7% | 2.5 ± 0.1% |
The total percentage accounted was 95.3 ± 5.2% for FM 600-treated foam and 79.5 ± 1.1% for the TBPP mixture. The concentration of FM 600 in the foam was assumed to be 4% by weight16
Figure 3.
A comparison of the contributions of individual components in the FM 600 and TBPP mixture suggests a disparate TBPP formulation. FM 600 is shown in gray, and the TBPP mixture is shown in white. Strikingly, FM 600 contained relatively little TPHP and mono-substituted TBPP isomer compared to the TBPP mixture. The concentration of FM 660 in the foam was assumed to be 4% by weight.16
Concentrations in SRM 2585
Levels of ITP and TBPP isomers in house dust standard reference material 2585 are shown in Table 5. Deuterated TPHP was used as a recovery standard, and the percent recovery of 13C-TPHP was 95.7 ± 6.2%. Measured concentrations of TPHP were 1002.13 ± 52.88 ng/g, similar to those reported previously.17,18 2IPPDPP, 4IPPDPP, 4tBPDPP, and B4BPDPP were the most prevalent ITP and TBPP isomers in SRM 2585, all of which had concentrations of >200 ng/g. In contrast, T3IPPP, T4IPPP, 2tBPDPP, and B2tBPPP were not detected in SRM 2585 above MDL levels (0.67, 1.07, 0.61, and 0.98 ng/g, respectively.) While individual isomer levels are lower than that of other organophosphate flame retardants, ΣITP and ΣTBPP levels (1098.28 and 762.09 ng/g, respectively) approach and, in some cases, exceed levels that have been reported for TPHP, TCEP, and TCPP previously.18,19 These concentrations suggest the potential for chronic human exposure to ITP and TBPP isomers via inadvertent dust ingestion and hand-to-mouth contact. In addition, the fact that SRM 2585 was prepared from dust collected in 1993 and 1994 suggests that ITP and TBPP use had been ongoing before PentaBDE was phased out. Interestingly, it has been reported that the ITP isomers were originally developed as tricresyl phosphate replacements due to the erratic supply and increasing cost of cresols in the 1970s. Similarly, TBPP isomers were introduced in the 1970s for use in hydraulic applications.4 However, to the authors’ knowledge, this is the first demonstration of their presence in SRM 2585.
Table 5.
ITP and TBPP Levels in House Dust SRM 2585 (n = 4)
| ITP or TBPP isomer | Avg. (ng/g dust) | Std. Dev. (ng/g dust) |
|---|---|---|
|
| ||
| TPHP | 1002.13 | 52.88 |
| 2IPPDPP | 475.48 | 12.20 |
| 3IPPDPP | 32.28 | 3.79 |
| 4IPPDPP | 224.75 | 22.56 |
| 24DIPPDPP | 120.29 | 15.15 |
| B2IPPPP | 110.25 | 9.55 |
| B3IPPPP | 88.76 | 14.04 |
| B4IPPPP | 30.21 | 14.06 |
| B24DIPPPP | 16.26 | 3.38 |
| T3IPPP | N.D. | -- |
| T4IPPP | N.D. | -- |
|
| ||
| ΣITPs | 1098.28 | 56.40 |
|
| ||
| 2tBPDPP | N.D. | -- |
| 4tBPDPP | 405.27 | 92.28 |
| B2tBPPP | N.D. | -- |
| B4tBPPP | 332.79 | 32.59 |
| T4tBPP | 24.03 | 2.24 |
|
| ||
| ΣTBPPs | 762.09 | 122.33 |
Not detected.
Implications
There is growing evidence that the organophosphate components in these mixtures may elicit adverse health effects, and human exposure to them has been demonstrated to be widespread in recent years.9,20–22 A recent epidemiological study found a significant association between the urinary metabolite of ITPs and decreases in successful pregnancy outcomes.23 Identification of the predominant ITP and TBPP isomers in commonly used commercial flame retardant mixtures will aid in hazard characterization and the ongoing, prioritized risk assessment of these compounds by the U.S. EPA. With the exception of FM 600, all of these mixtures contain ≥20% TPHP, a compound with known toxicological activity.24–26 Interestingly, FM 600 has very low TPHP content, potentially due to distillation or other treatment following isomerization. The four mixtures in this study are complex, containing many different components, each with potentially unique health hazards and physicochemical properties. These findings should be taken into account when designing and interpreting toxicological studies. Furthermore, it should be recognized that there are many different manufacturers of these mixtures, and mixture formulations may differ among manufacturers and across lots. It is possible that the minor components of the ITP mixture (<1% w/w) are synthesis byproducts and might also vary from lot to lot. In addition, the flame retardant mixtures analyzed here are not the only sources of ITP and TBPP isomers to the environment; ITP and TBPP isomers also have uses as plasticizers and in hydraulic fluids.27–29 Future work should focus on identifying sources of these isomers, the degree of human exposure to these isomers, and the toxicological assessment of individual isomers.
Supplementary Material
Acknowledgments
The authors are grateful to Wellington Laboratories, Inc. for graciously providing individual ITP and TBPP standards. We also thank the National Toxicology Program for providing the TBPP mixture for research purposes as part of a materials transfer agreement. We thank J. Doering for her contribution to the TOC art.
Funding
This work was funded by the National Institute of Environmental Health Sciences (R01 ES016099 to H.M.S.).
Footnotes
ASSOCIATED CONTENT
- A figure showing a total ion chromatogram of the TBPP mixture. Two tables showing method detection limits and matrix spike recoveries. (PDF)
The authors declare no competing financial interest.
References
- 1.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol. 2012;46(24):13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper EM, Kroeger G, Davis K, Clark CR, Ferguson PL, Stapleton HM. Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ. Sci. Technol. 2016;50(19):10653–10660. doi: 10.1021/acs.est.6b01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latendresse JR, Brooks CL, Capen CC. Pathologic Effects of Butylated Triphenyl Phosphate-Based Hydraulic Fluid and Tricresyl Phosphate on the Adrenal Gland, Ovary, and Testis in the Fischer-344 Rat. Toxicol. Pathol. 1994;22(4):341–352. doi: 10.1177/019262339402200401. [DOI] [PubMed] [Google Scholar]
- 4.Weil ED, Levchik S, Moy P. Flame and Smoke Retardants in Vinyl Chloride Polymers – Commercial Usage and Current Developments. J. Fire Sci. 2006;24:211–236. [Google Scholar]
- 5.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, Mcclean MD, Webster TF. Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 2008;42:6910–6916. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 6.Behl M, Hsieh J-H, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES, Jarema KA, Padilla S, et al. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 2015;52(B):181–193. doi: 10.1016/j.ntt.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 7.McGee SP, Konstantinov A, Stapleton HM, Volz DC. Aryl phosphate esters within a major PentaBDE replacement product induce cardiotoxicity in developing zebrafish embryos: potential role of the aryl hydrocarbon receptor. Toxicol. Sci. 2013;133(1):144–156. doi: 10.1093/toxsci/kft020. [DOI] [PubMed] [Google Scholar]
- 8.United States Environmental Protection Agency News Release from Headquarters. U.S. EPA; Washington, DC: 2016. U.S. EPA EPA Acts on New Chemical Law to Fast-Track Five Chemicals. [Google Scholar]
- 9.Hammel S, Hoffman K, Webster TF, Anderson KA, Stapleton HM. Measuring Personal Exposure to Organophosphate Flame Retardants using Silicone Wristbands and Hand Wipes. Environ. Sci. Technol. 2016;50(8):4483–4491. doi: 10.1021/acs.est.6b00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 2014;48(17):10432–10438. doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- 11.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and endocrine disrupting effects of the flame retardant mixture Firemaster® 550 in rats: an exploratory assessment. J. Biochem. Mol. Toxicol. 2013;27(2):124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bearr JS, Stapleton HM, Mitchelmore CL. Accumulation and DNA damage in fathead minnows (Pimephales promelas) exposed to 2 brominated flame-retardant mixtures, Firemaster 550 and Firemaster BZ-54. Environ. Toxicol. Chem. 2010;29(3):722–729. doi: 10.1002/etc.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL. Advanced Morphological — Behavioral Test Platform Reveals Neurodevelopmental Defects in Embryonic Zebrafish Exposed to Comprehensive Suite of Halogenated and Organophosphate Flame Retardants. Toxicol. Sci. 2015;145(1):177–195. doi: 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latendresse JR, Brooks CL, Capen CC. Toxic Effects of Butylated Triphenyl Phosphate-based Hydraulic Fluid and Tricresyl Phosphate in Female F344 Rats. Vet. Pathol. 1995;32(4):394–402. doi: 10.1177/030098589503200408. [DOI] [PubMed] [Google Scholar]
- 15.Bergman Å, Rydén A, Law RJ, de Boer J, Covaci A, Alaee M, Birnbaum L, Petreas M, Rose M, Sakai S, et al. A novel abbreviation standard for organobromine, organochlorine and organophosphorus flame retardants and some characteristics of the chemicals. Environ. Int. 2012;49:57–82. doi: 10.1016/j.envint.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, Webster TF. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ. Sci. Technol. 2009;43:7490–7495. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Eede N, Dirtu AC, Ali N, Neels H, Covaci A. Multi-residue method for the determination of brominated and organophosphate flame retardants in indoor dust. Talanta. 2012;89:292–300. doi: 10.1016/j.talanta.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Van den Eede N, Dirtu AC, Neels H, Covaci A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 2011;37:454–461. doi: 10.1016/j.envint.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Bergh C, Luongo G, Wise S, Östman C. Organophosphate and phthalate esters in standard reference material 2585 organic contaminants in house dust. Anal. Bioanal. Chem. 2012;402(1):51–59. doi: 10.1007/s00216-011-5440-2. [DOI] [PubMed] [Google Scholar]
- 20.Belcher SM, Cookman CJ, Patisaul HB, Stapleton HM. In vitro assessment of human nuclear hormone receptor activity and cytotoxicity of the flame retardant mixture FM 550 and its triarylphosphate and brominated components. Toxicol. Lett. 2014;228(2):93–102. doi: 10.1016/j.toxlet.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang M, Webster TF, Ferguson PL, Stapleton HM. Characterizing the peroxisome proliferator-activated receptor (PPARγ) ligand binding potential of several major flame retardants, their metabolites, and chemical mixtures in house dust. Environ. Health Perspect. 2014;123(2):166–172. doi: 10.1289/ehp.1408522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman K, Butt CM, Chen A, Limkakeng AT, Stapleton HM. High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products. Environ. Sci. Technol. 2015;49(24):14554–14559. doi: 10.1021/acs.est.5b03577. [DOI] [PubMed] [Google Scholar]
- 23.Carignan C, Minguez-Alarcon L, Butt C, Williams PL, Meeker JD, Stapleton HM, Toth TL, Ford JB, Hauser R. Urinary Concentrations of Organophosphate Flame Retardant Metabolites and Pregnancy Outcomes among Women Undergoing in Vitro Fertilization. Environ. Health Perspect. 2017;125(8) doi: 10.1289/EHP1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X, Ji K, Jo A, Moon H-B, Choi K. Effects of TDCPP or TPP on gene transcriptions and hormones of HPG axis, and their consequences on reproduction in adult zebrafish (Danio rerio) Aquat. Toxicol. 2013;134:104–111. doi: 10.1016/j.aquatox.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Pillai HK, Fang M, Beglov D, Kozakov D, Vajda S, Stapleton HM, Webster TF, Schlezinger JJ. Ligand Binding and Activation of PPARγ by Firemaster 550: Effects on Adipogenesis and Osteogenesis in Vitro. Environ. Health Perspect. 2014;122:1225–1232. doi: 10.1289/ehp.1408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ. Health Perspect. 2009;118(3):318–323. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David MD, Seiber JN. Analysis of Organophosphate Hydraulic Fluids in U.S. Air Force Base Soils. Arch. Environ. Contam. Toxicol. 1999;36(3):235–241. doi: 10.1007/s002449900466. [DOI] [PubMed] [Google Scholar]
- 28.Sundkvist AM, Olofsson U, Haglund P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 2010;12:943–951. doi: 10.1039/b921910b. [DOI] [PubMed] [Google Scholar]
- 29.Wensing M, Uhde E, Salthammer T. Plastics additives in the indoor environment - Flame retardants and plasticizers. Sci. Total Environ. 2005;339:19–40. doi: 10.1016/j.scitotenv.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Phillips AL, Chen A, Rock KD, Horman B, Patisaul HB, Stapleton HM. Transplacental and Lactational Transfer of Firemaster® 550 Components in Dosed Wistar Rats. Toxicol. Sci. 2016;153(2):246–257. doi: 10.1093/toxsci/kfw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du Z, Wang G, Gao S, Wang Z. Aryl organophosphate flame retardants induced cardiotoxicity during zebrafish embryogenesis: By disturbing expression of the transcriptional regulators. Aquat. Toxicol. 2015;161:25–32. doi: 10.1016/j.aquatox.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 32.Scanlan LD, Loguinov AV, Teng Q, Antczak P, Dailey KP, Nowinski DT, Kornbluh J, Lin XX, Lachenauer E, Arai A, et al. Gene Transcription, Metabolite and Lipid Profiling in Eco-Indicator Daphnia magna Indicate Diverse Mechanisms of Toxicity by Legacy and Emerging Flame-Retardants. Environ. Sci. Technol. 2015;49(12):7400–7410. doi: 10.1021/acs.est.5b00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol. Teratol. 2015;52(B):194–209. doi: 10.1016/j.ntt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Behl M, Rice JR, Smith MV, Co CA, Bridge MF, Hsieh J-H, Freedman JH, Boyd WA. Editor’s Highlight: Comparative Toxicity of Organophosphate Flame Retardants and Polybrominated Diphenyl Ethers to Caenorhabditis elegans. Toxicol. Sci. 2016;154(2):241–252. doi: 10.1093/toxsci/kfw162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tung EWY, Ahmed S, Peshdary V, Atlas E. Firemaster® 550 and its components isopropylated triphenyl phosphate and triphenyl phosphate enhance adipogenesis and transcriptional activity of peroxisome proliferator activated receptor (Pparγ) on the adipocyte protein 2 (aP2) promoter. PLoS One. 2017;12(4):e0175855. doi: 10.1371/journal.pone.0175855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babich MA. CPSC Staff Preliminary Risk Assessment of Flame Retardant (FR) Chemicals in Upholstered Furniture Foam. U.S. Consumer Product Safety Commission Directorate for Health Sciences; Bethesda, MD: 2006. [Google Scholar]
- 37.Bittner PM, Ferrante J, Hatlelid KM, Babich M. An Evaluation of the Toxicity Data for Selected Flame Retardant Chemicals. Toxicologist. 2001;60:2014A. [Google Scholar]
- 38.Ferrante J. Toxicity review of aromatic phosphate plasticizers. Memorandum updated from Jacqueline Ferrante, Pharmacologist, Division of Health Sciences, to Ronald L.Medford, Assistant Executive Director for Hazard Identification and Reduction. U.S. Consumer Product Safety Commission; Washington, DC: 1999. [Google Scholar]
- 39.European Flame Retardants Association (EFRA) Overview of classification and regulatory status for FRs used in transport. EFRA; Brussels, Belgium: 2015. [Google Scholar]
- 40.Heitkamp MA, Freeman JP, Cerniglia CE. Biodegradation of tert-Butylphenyl Diphenyl Phosphate. Appl. Environ. Microbiol. 1986;51(2):316–322. doi: 10.1128/aem.51.2.316-322.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schang G, Robaire B, Hales BF. Organophosphate Flame Retardants Act as Endocrine-Disrupting Chemicals in MA-10 Mouse Tumor Leydig Cells. Toxicol. Sci. 2016;150(2):499–509. doi: 10.1093/toxsci/kfw012. [DOI] [PubMed] [Google Scholar]
- 42.Kosarac I, Kubwabo C, Foster WG. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC–MS/MS) J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016;1014:24–30. doi: 10.1016/j.jchromb.2016.01.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.