Abstract
We have investigated the impact of transmitter release site (active zone; AZ) structure on synaptic function by physically rearranging the individual AZ elements in a previously published frog neuromuscular junction (NMJ) AZ model into the organization observed in a mouse NMJ AZ. We have used this strategy, purposefully without changing the properties of AZ elements between frog and mouse models (even though there are undoubtedly differences between frog and mouse AZ elements in vivo), to directly test how structure influences function at the level of an AZ. Despite a similarly ordered ion channel array substructure within both frog and mouse AZs, frog AZs are much longer and position docked vesicles in a different location relative to AZ ion channels. Physiologically, frog AZs have a lower probability of transmitter release compared with mouse AZs, and frog NMJs facilitate strongly during short stimulus trains in contrast with mouse NMJs that depress slightly. Using our computer modeling approach, we found that a simple rearrangement of the AZ building blocks of the frog model into a mouse AZ organization could recapitulate the physiological differences between these two synapses. These results highlight the importance of simple AZ protein organization to synaptic function.
NEW & NOTEWORTHY A simple rearrangement of the basic building blocks in the frog neuromuscular junction model into a mouse transmitter release site configuration predicted the major physiological differences between these two synapses, suggesting that transmitter release site structure and organization is a strong predictor of function.
Keywords: active zone, neuromuscular junction, transmitter release
INTRODUCTION
Chemical transmitter release from presynaptic nerve terminals is triggered by the influx of calcium ions (Ca2+) through voltage-gated Ca2+ channels (VGCCs), which bind to Ca2+ sensor proteins on docked synaptic vesicles, triggering vesicle fusion with the plasma membrane. These events occur within specialized active zone (AZ) regions of the nerve terminal where presynaptic VGCCs colocalize with proteins that regulate synaptic vesicle docking and fusion. Historically, several preparations have been used to investigate the significance of AZ structure for synaptic function (Zhai and Bellen 2004), but the results from these studies have not been in agreement. For example, studies of phasic and tonic synapses in the crayfish neuromuscular junction (NMJ) concluded that AZ structure was not a critical determinant of synaptic function (Millar and Atwood 2004; Walrond and Reese 1985). However, more recent studies have pointed to the importance of AZ organization for synaptic function. For example, studies of hippocampal synapses in the rodent central nervous system (CNS) characterized the structure and organization of AZs (Rollenhagen et al. 2007) and showed that release probability scales with AZ size (Holderith et al. 2012). Scimemi and Diamond (2012) also predicted that sub-AZ organization and the number of presynaptic VGCCs at hippocampal synapses could critically influence synaptic function. Similarly, recent investigations at the large calyx of Held synapse in the rodent brain stem have shown that VGCC number and position relative to synaptic vesicle release sites regulate synaptic strength and plasticity (Han et al. 2011; Sheng et al. 2012). At neuromuscular synapses, AZ structure has long been a focus of investigation (Harlow et al. 2001; Heuser and Reese 1974, 1981; Nagwaney et al. 2009; Pawson et al. 1998; Propst et al. 1986), and previous studies have sought to link VGCC number and position within AZs to synaptic function (Bennett et al. 1997; Chen et al. 2011; Luo et al. 2015; Shahrezaei et al. 2006). Furthermore, several groups have used computer modeling to explore the spatiotemporal profile of Ca2+ influx and the importance of this dynamic process on synaptic vesicle fusion within AZs (Bennett et al. 2000a, 2000b, 2004; Dittrich et al. 2013; Fogelson and Zucker 1985; Matveev et al. 2011).
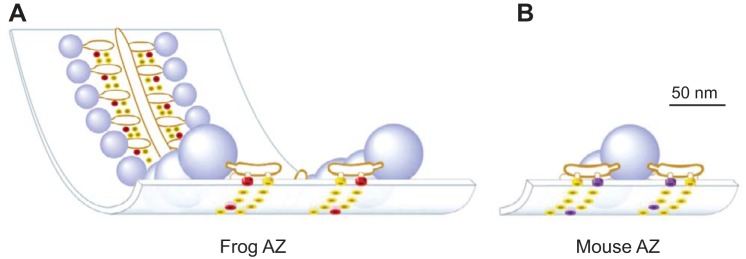
Here we further investigated the relationship between AZ structure and synaptic function using a previously published MCell (Monte Carlo Cell) computer model of the well-defined and highly ordered frog NMJ AZ (validated by the ability of this model to predict a large number of physiological phenomena at this synapse; Dittrich et al. 2013; Ma et al. 2015) and a simple rearrangement of the VGCCs and docked synaptic vesicles in this model into the organization predicted at the mouse NMJ AZ. Recent electron microscopy tomography studies have highlighted significant differences in the highly ordered fine structure of frog and mouse NMJ AZs (Harlow et al. 2001; Nagwaney et al. 2009). Frog AZs are tightly organized into long linear structures consisting of two double rows of presynaptic proteins (thought to include VGCCs) sandwiched between a double row of ~30 docked synaptic vesicles (Fig. 1A). In contrast, AZs at the mouse NMJ are characterized by relatively short AZ segments consisting of two synaptic vesicles and a double row of synaptic proteins on either side (Fig. 1B). We have taken advantage of the well-known and highly ordered structures of neuromuscular AZs to investigate if the characteristic structural differences between frog and mouse NMJ AZs are important determinants of synaptic function. We demonstrate, using a combination of physiological recordings, anatomical investigations, and MCell computer simulations, that differential organization of neuromuscular AZs between frog and mouse could indeed explain the observed differences in probability of release and short-term plasticity characteristics at these synapses. We conclude that our MCell computer model predicts that the differential assembly of the basic building blocks of synapses (presynaptic VGCCs and docked synaptic vesicles within presynaptic AZs) can result in synapses with significantly different functional properties.
Fig. 1.
Schematic diagrams of single active zone (AZ) organization within the frog (A) and mouse (B) neuromuscular junction (NMJ) based on electron microscopy data. Blue spheres = docked synaptic vesicles. Yellow disks = AZ membrane proteins of unknown identity. Red disks = Cav 2.2 type VGCCs. Purple disks = Cav 2.1 type VGCCs. [Adapted from Urbano et al. 2003 with permission from National Academy of Sciences. Copyright (2003) National Academy of Sciences, U.S.A.]
MATERIALS AND METHODS
Ethical Approval
All procedures were performed with strict adherence to the National Institutes of Health guidelines and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Intracellular Recordings at Frog NMJs
Adult male and female northern frogs (Rana pipiens) were obtained in the winter and anesthetized in 0.6% tricaine methane sulfonate (Sigma-Aldrich, St. Louis, MO) in normal frog saline (NFR; 116 mM NaCl, 2 mM KCl, 5mM dextrose, 10 mM HEPES, 1 mM MgCl2, 1.8 mM CaCl2, pH 7.3–7.4) and double pithed. The cutaneous pectoris nerve-muscle preparation was dissected, the nerve was stimulated with a suction electrode, and muscle contractions were blocked by exposure to 1 μM μ-conotoxin PIIIA (Alomone Laboratories, Jerusalem, Israel), a peptide toxin that specifically blocks sodium channels on frog muscle fibers (Shon et al. 1998). All recordings were made at room temperature unless otherwise noted.
Since the time course of multiquantal release during action potential stimuli is distributed slightly in time, quantal content is most accurately calculated as the ratio of the integrals of end-plate currents (EPCs) to the integrals of miniature end-plate currents (mEPCs). To make these accurate measurements of quantal content, two-electrode voltage clamp was used. Voltage-clamp recordings were performed as described previously (supplement of Dittrich et al. 2013). Briefly, microelectrodes were pulled to a resistance of 5–10 MΩ and filled with 3 M potassium chloride. Spontaneous miniature synaptic currents (mEPCs) were collected for 1–2 min in each muscle fiber, and 10–20 single nerve-evoked synaptic currents (EPCs) were collected with an interstimulus interval of 5 s. All voltage-clamp recordings at frog NMJs were made using a holding potential of −85 mV. To calculate quantal content, the integral of the average EPC waveform was divided by the integral of the average mEPC waveform recorded from each muscle fiber. This ratio calculates the average number of quanta that are released following each nerve stimulation.
For measurements of short-term plasticity where relative changes in evoked end-plate potential (EPP) amplitude were measured, current-clamp recordings were performed as described previously (Douthitt et al. 2011; Meriney and Grinnell 1991). Briefly, postsynaptic receptors were partially blocked (using a 10-min exposure to 10 μg/ml alpha-bungarotoxin) such that the amplitude of evoked responses (EPPs) was less than 15 mV. Intracellular recordings of EPPs were made using ~40–60 MΩ borosilicate electrodes filled with 3 M potassium acetate. For these experiments, a pair of EPPs or a train of 5–10 EPPs with varying interstimulus intervals (10–100 Hz) was collected, and relative changes in EPP amplitude were normalized by dividing the amplitude of the second EPP in a pair, or each EPP in a train, by the amplitude of the first EPP in the pair or train.
Intracellular Recordings at Mouse NMJs
Since the size of the NMJ, and therefore the number of AZs, increases with age (Chen et al. 2012), we restricted the age window of mice used in our study to 4–8 wk of age for both estimations of quantal content and AZ counting (bassoon immunohistochemistry). Female mice (outbred Swiss Webster) were euthanized by CO2 inhalation followed by thoracotomy, and intracellular recordings of transmitter release were made from the epitrochleoanconeus (ETA) ex vivo nerve-muscle preparation as previously described (Tarr et al. 2013a). Briefly, the extracellular saline (normal mammalian Ringer; NMR) contained 150 mM NaCl, 5 mM KCl, 11 mM dextrose, 10 mM HEPES, 1 mM MgCl2, and 2 mM CaCl2 (pH 7.3–7.4). The nerve was stimulated with a suction electrode, and muscle contractions were blocked by exposure to 1 μM μ-conotoxin GIIIB (Alomone Laboratories), a peptide toxin that specifically blocks sodium channels on mouse muscle fibers (Hong and Chang 1989). All measurements were made at room temperature (except as noted). For all experiments using mouse neuromuscular synapses, the tissue was oxygenated throughout the dissection and recording period.
For measurements of quantal content, two-electrode voltage-clamp was used (as described above). Spontaneous mEPCs were collected for 1–2 min in each muscle fiber, and then 10–20 EPCs were collected with an interstimulus interval of 5 s. All voltage-clamp recordings at mouse NMJs were made using a holding potential of −90 mV. Quantal content was calculated as described above.
For measurements of short-term plasticity, current-clamp microelectrode recordings were performed using ~40–60 MΩ borosilicate electrodes filled with 3 M potassium acetate. To evaluate effects on short-term synaptic plasticity, either a pair of EPPs or a train of 10 EPPs with varying interstimulus intervals (10–100 Hz) was collected, and relative changes in EPP amplitude were calculated as described above.
Analysis of Electrophysiological Data
All electrophysiology data from frog and mouse preparations were collected using an Axoclamp 900A and digitized at 10 kHz for subsequent analysis using pClamp 10 software. All recordings (voltage and current clamp) were corrected for nonlinear summation (McLachlan and Martin 1981). For voltage-clamp recordings, EPCs were also corrected by considering the driving force [DF = membrane potential – equilibrium potential for acetylcholine receptor channels (EqAChR was set to 0 mV)] and voltage error [VE; where EPCcorrected = EPCmeasured × (DF/(DF − VE)].
Quantification of Active Zones at Frog NMJs
To quantify the number of presynaptic AZs in frog NMJs, the postsynaptic acetylcholine receptor bands were labeled because this has been established as a highly predictive method for identifying the location of presynaptic AZs at the frog NMJ (Cohen et al. 1991; Robitaille et al. 1990). For these experiments, the cutaneous pectoris ex vivo nerve-muscle preparation was dissected and placed in an extracellular saline (NFR as described above) containing 3 μg/ml Alexa 594-conjugated α-bungarotoxin (BTX; Thermo Fisher Scientific, Pittsburgh, PA) for 30 min to label the postsynaptic acetylcholine receptors. This was followed by a 30-min incubation in 5 µg/0.1 ml Alexa 488-peanut lectin (PNA; Thermo Fisher Scientific) to label the extracellular matrix around the nerve terminals and identify the spatial extent of each synapse. The tissue was then washed and fixed in 2% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature and mounted on glass slides under no. 1.5 cover glass using Prolong Gold mounting media (Thermo Fisher Scientific).
Quantification of Active Zones at Mouse NMJs
To count the number of AZs in mouse NMJs, labeling of the presynaptic protein bassoon has been shown to be an effective and highly localized marker (Nishimune et al. 2004; Nishimune 2012). For these experiments, the ETA ex vivo nerve-muscle preparation was dissected and placed in an extracellular saline (NMR as described above) containing ~3 μg/ml Alexa 594-conjugated α-BTX for 30 min to label the postsynaptic acetylcholine receptors. The tissue was then washed and fixed in 2% (wt/vol) paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature. The fixed tissue was then permeabilized and blocked in a PBS solution containing 2% (wt/vol) bovine serum albumin (BSA), 2% (vol/vol) normal goat serum, and 0.5% Triton X-100. After block, the tissue was incubated in the primary antibody (1:600 dilution of SAP7F407 clone of mouse anti-bassoon IgG subtype 2a; Assay Designs no. VAM-PS003) for 12–16 h on a rocker platform at room temperature. Following washout of the primary antibody, the tissue was incubated in the secondary antibody (1:1,000 dilution of Alexa-488 goat anti-mouse IgG 2a; Molecular Probes no. A21131) for 4–6 h on a rocker platform at room temperature. Finally, after washing in PBS, the tissue was mounted on glass slides under no. 1.5 cover glass using Prolong Gold mounting media.
Confocal Imaging
Mounted tissue was imaged on a Leica TCS SP5 spectral confocal microscope. Alexa-488 labeling was imaged using 488-nm laser excitation and photon collection between 498 and 560 nm. Alexa 594 labeling was imaged using 564-nm laser excitation and photon collection between 571 and 750 nm. Confocal scanning was performed using a resonance scanner at 8,000 Hz, and images were collected using line-scan averaging after 96 sweeps. Regions of interest were scanned using image stacks at 0.25- to 0.5-µm intervals sufficient to include the entire NMJ (typically 10–40 optical slices). Brightest projection images were made from these stacks, and photon collections from both wavelengths were superimposed in pseudocolor to generate composite images. The number of bassoon puncta (representing AZs) within each mouse NMJ was counted by hand within α-bungarotoxin-stained regions of the image. The number of bungarotoxin bands (representing AZs) within each frog NMJ was counted by hand within PNA stained regions of the image. For all AZ counting, each NMJ was counted by at least three individuals and their counts averaged. For purposes of display, some images were processed for deconvolution using the blind deconvolution method within the Slidebook software (Intelligent Imaging Innovations, Ringsby, CT).
MCell Simulations
For our computational investigation, we used MCell version 3.1 (www.mcell.org) to study action potential-triggered vesicle release at the frog and mouse NMJs. MCell is a particle-based, stochastic diffusion-reaction simulator which excels at modeling biological systems with complex 3D geometries (Kerr et al. 2008). Our MCell simulations comprised a spatially realistic model of an AZ region of the frog or mouse NMJ including VGCCs, Ca2+ buffer, and synaptic vesicles, with synaptotagmin and second sensor sites responsible for triggering vesicle release (Dittrich et al. 2013; Ma et al. 2015). During a MCell run of a single or train of APs, VGCCs open stochastically and admit Ca2+ ions into the terminal at a rate determined by the instantaneous driving force. Diffusing Ca2+ ions then bind to Ca2+ buffer molecules distributed throughout the terminal or to Ca2+ sensor sites on synaptic vesicles (synaptotagmin or second sensor sites). Individual synaptic vesicles were then determined to be “released” once they bound sufficient Ca2+ ions in accordance with our excess Ca2+ binding site and energy-based second sensor mechanism (Dittrich et al. 2013; Ma et al. 2015).
Frog NMJ model.
In this study, we employed the same MCell model of the frog NMJ AZ as described in Dittrich et al. (2013) and Ma et al. (2015), including key geometric parameters. Briefly, the model consists of 26 synaptic vesicles of 50-nm diameter arranged in a double row (Fig. 1). Located between the double row of vesicles is a trough containing 26 VGCCs at a 1:1 channel to vesicle stoichiometry (Luo et al. 2011) and at locations suggested by published estimates (Heuser et al. 1979; Pawson et al. 1998; Stanley et al. 2003). The bottom surface of each synaptic vesicle contains 8 synaptotagmin molecules, each with 5 Ca2+ binding sites, as well as 16 second Ca2+ sensor sites (“Y sites”). Vesicle fusion occurs according to the 16-Y-site energy model as previously described (Ma et al. 2015). VGCCs open stochastically with voltage-dependent channel kinetics and have a ~20% open probability (Luo et al. 2011) such that on average ~5 VGCCs open per action potential (AP) stimulation event within the AZ. The Ca2+ binding kinetics to sensor sites on synaptic vesicles, and to the 2 mM of static Ca2+ buffer located throughout the terminal, were identical to the values used previously (Ma et al. 2015).
Mouse NMJ model.
Since we wanted to investigate the impact of AZ structure on NMJ function, our mouse NMJ AZ comprised the same molecular elements used in the frog model (synaptic vesicles with synaptotagmin and second sensor sites, VGCCs, Ca2+ buffers) with identical kinetic parameters. Thus we built the mouse NMJ model by rearranging the frog NMJ molecular elements into a mouse NMJ AZ organization based on published structural estimates for the mouse (Nagwaney et al. 2009; Fig. 1). It is estimated that each mouse NMJ AZ contains two docked synaptic vesicles and a double row of transmembrane particles, some of which are thought to be VGCCs, on either side of docked synaptic vesicles (Nagwaney et al. 2009). Our MCell model contains six AZs spaced ~500 nm apart on the presynaptic membrane to be consistent with previously reported AZ distributions (Chen et al. 2012; Fukunaga et al. 1983; Fukuoka et al. 1987; Ruiz et al. 2011). We included six AZs in the mouse model compared with the single AZ in the frog model since this enabled us to study possible Ca2+ mediated cross-talk between these small AZs, and also enhanced our sampling ability of release events during a single simulation run. Each mouse AZ in our model contains four VGCCs, two each on either side of the two docked synaptic vesicles. The distance between the center of each channel and the bottom of its closely-associated vesicle is ~32 nm (Nagwaney et al. 2009). The details of each synaptic vesicle in the mouse model, including their number, arrangement, and the binding kinetics of synaptotagmin and second sensor sites, are identical to the frog NMJ model. Similarly, the opening kinetics of VGCCs, as well as the number and binding kinetics of Ca2+ buffer molecules, are identical to the frog model. Finally, vesicle release was determined according to the 16-Y-site energy model as described in Ma et al. (2015).
Runtime logistics.
For our simulations, we used MCell version 3.1 with a custom-compressed binary reaction data output format to enable efficient storage and analysis of the large simulation output files. The simulation algorithms used in MCell have been described previously (Kerr et al. 2008). For each simulation condition, we typically conducted a large number of statistically independent runs (5,000–10,000) and used these to compute statistical averages and errors. Our simulations used a small time step of 10 ns to ensure that diffusing ions could properly sample the confined spaces in the AZs models, especially in the vicinity of synaptic vesicles. With our simulations, we are able to track Ca2+ ions entering the presynaptic space from individual VGCCs and could thus determine the origin of Ca2+ ions that contributed to the release of specific synaptic vesicles. The simulations were analyzed using custom scripts written in Go and Python. All simulations were conducted on the Axon cluster at the Pittsburgh Supercomputing Center (64 quad-core, 2.5-GHz Intel Xeon E5420 CPUs) and analyzed on several large-memory workstations at the Pittsburgh Supercomputing Center.
Statistical Analysis
Statistical analysis was performed using either GraphPad Prism Version 5.0 (GraphPad Software, La Jolla, CA) or Origin 7 (OriginLab, Northampton, MA). Data are presented as means ± SE unless otherwise noted; α = 0.05 for all statistical tests. For the MCell simulation outputs, data analysis was performed using Python and Go scripts.
RESULTS
Average Probability of Release per Active Zone and Synaptic Vesicle
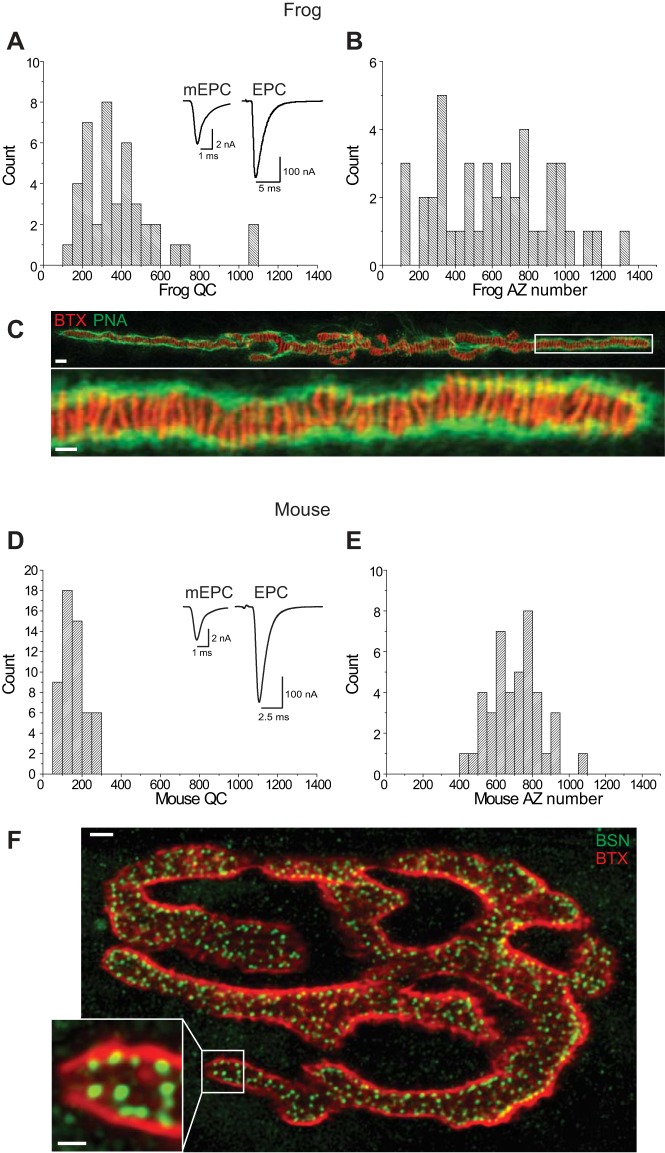
We started our exploration of AZ structure and function relationships by quantifying several key functional differences between the mouse and frog NMJ. In particular, we measured the average probability of vesicle release per AZ (Pr-AZ) and per synaptic vesicle within each AZ (Pr-SV), and the characteristics of short-term plasticity. To determine Pr-AZ and Pr-SV at the frog and mouse NMJ, we measured both the quantal content and the number of AZs at synapses in both preparations (Fig. 2).
Fig. 2.
Quantal content and AZ number at frog and mouse NMJs. A: histogram plot of the distribution of quantal content (QC) measurements determined using 2-electrode voltage clamp at frog NMJs. Inset shows sample mEPP (left; average of 300 events) and EPP (right; average of 15 events) recordings. B: histogram plot of the distribution of AZ numbers measured across frog NMJs. C: representative image of a frog NMJ stained with Alexa-488 peanut lectin (PNA, green) to outline synapses, and Alexa-594 α-bungarotoxin (BTX, red) to stain postsynaptic acetylcholine receptors and predict the location of AZs (red stripes). White box in the top image identifies the region that is enlarged in the bottom image. Scale bars = 5 µm (top) and 2 µm (bottom). D: histogram plot of the distribution of quantal content measurements determined using 2-electrode voltage clamp at mouse NMJs. Inset shows sample mEPP (left; average of 300 events) and EPP (right; average of 15 events) recordings. E: histogram plot of the distribution of AZ numbers measured across mouse NMJs. F: representative image of a mouse NMJ stained with Alexa-488 conjugated secondary antibody that recognized an anti-bassoon primary antibody to identify the location of AZs (BSN, green), and Alexa-594 α-bungarotoxin (BTX, red) to stain postsynaptic acetylcholine receptors and identify the extent of each NMJ. White box in the larger image identifies the region that is enlarged in the inset image. Scale bars = 5 µm (large image) and 2 µm (inset).
At the frog NMJ, average quantal content was 414.9 ± 40.4 (n = 43 synapses from 5 frogs; Fig. 2A). We determined an average AZ number per terminal of 616.4 ± 45.6 (n = 44 synapses from 10 frogs; Fig. 2, B and C). The large range in values (AZ number and quantal content) observed between synapses is likely due to the large range of postsynaptic muscle fiber diameters across this preparation as presynaptic nerve terminals scale in length (and AZ number) to compensate. Previous reports have shown that quantal content is linearly correlated with AZ number (Dorlöchter et al. 1993). Assuming functional homogeneity across AZs, we calculated an average Pr-AZ of 0.67 (the ratio of average quantal content to average AZ count). Since the number of vesicles that are docked at a frog NMJ averages 30 (Heuser et al. 1979; Pawson et al. 1998; Rizzoli and Betz 2005), dividing the Pr-AZ by 30 synaptic vesicles yields an average Pr-SV of 0.022. Therefore, these data lead to the conclusion that the average Pr-SV is very low at the frog NMJ.
At the mouse NMJ, we measured a quantal content of 157.8 ± 7.9 (n = 54 synapses from 6 mice; Fig. 2D). We calculated an average number of AZs per terminal of 703.5 ± 22 (n = 42 synapses from 6 mice; Fig. 2, E and F). Assuming functional homogeneity across AZs, we could then calculate the average Pr-AZ to be 0.22 (the ratio of average quantal content to average number of AZs). Since the number of vesicles that are docked at a mouse NMJ AZ averages 2 (Nagwaney at al. 2009), the average Pr-SV at the mouse NMJ equals 0.11, and thus is five times higher than at the frog NMJ.
Short-Term Plasticity Characteristics at the Frog and Mouse NMJ
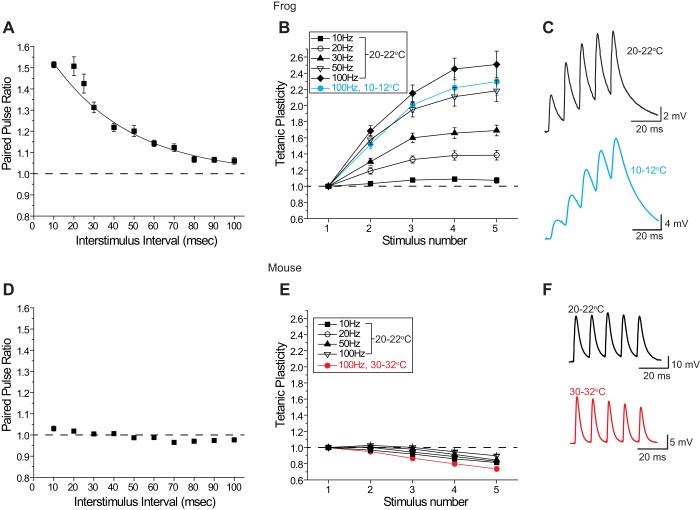
Using both paired stimuli and short trains of stimuli at the frog NMJ we observed relatively strong facilitation across synapses. The paired pulse ratio (PPR) averaged 1.51 ± 0.015 (n = 325 synapses from 31 frogs) when measured with a 10-ms interstimulus interval and gradually declined as the interstimulus interval was lengthened, with a time constant of ~40 ms (Fig. 3A). At a 100-ms interstimulus interval there was little change in the second synaptic response as compared with the first (PPR = 1.06 ± 0.015 n = 47 synapses from 6 frogs; Fig. 3A). When the magnitude of transmitter release was measured during a short stimulus train from 10 to 100 Hz, facilitation was mild at 10 Hz (maximum 1.09 ± 0.03 at the 4th pulse in a train), and increased in magnitude to a maximum of 2.51 ± 0.16 at the 5th pulse in a 100-Hz train (n = 23 synapses from 3 frogs; Fig. 3, B and C). These observations are consistent with previous observations of strong facilitation at the frog NMJ when measured under physiological conditions (Meriney and Grinnell 1991; Sosa and Zengel 1993). As northern frogs are cold-blooded animals whose NMJs function in ambient environmental temperatures, we also examined tetanic potentiation during 100-Hz trains at 10–12°C and found a similar facilitatory response (n = 31 synapses from 2 frogs; Fig. 3, B and C), indicating that facilitation at frog AZs is present at room temperature (20–22°C) and at colder temperatures (10–12°C).
Fig. 3.
Short-term plasticity measured at frog (A–C) and mouse (D–F) NMJs. A: plot of the changes in paired-pulse ratio (EPP2/EPP1) at the frog NMJ when 2 stimuli were delivered at varying interstimulus intervals (10–100 ms). Paired-pulse facilitation is very sensitive to interstimulus interval at the frog NMJ. B: plot of tetanic potentiation, normalized to the amplitude of the first EPP (EPPx/EPP1), when 5 stimuli were delivered to the frog motor nerve at varying frequencies (10–100 Hz). Data plotted in black were collected at room temperature (20–22°C); data plotted in blue were recorded at 10–12°C. The magnitude of tetanic potentiation is very sensitive to stimulus frequency at the frog NMJ. C: sample EPPs recorded from a frog NMJ during a 100-Hz stimulus train at 20–22°C (black) and 10–12°C (blue). D: plot of the changes in paired-pulse ratio (EPP2/EPP1) at the mouse NMJ when 2 stimuli were delivered at varying interstimulus intervals (10–100 ms). Paired-pulse facilitation is minimal and relatively insensitive to interstimulus interval at the mouse NMJ. E: plot of tetanic potentiation, normalized to the amplitude of the first EPP (EPPx/EPP1), when 5 stimuli were delivered to the mouse motor nerve at varying frequencies (10–100 Hz). Data plotted in black were collected at room temperature (20–22°C); data plotted in red were recorded at 30–32°C. At the mouse NMJ, mild tetanic potentiation gives way to mild depression at room temperature, and this form of short-term plasticity is relatively insensitive to stimulus frequency. Mild depression persists even at warmer temperatures (30–32°C). F: sample EPPs recorded from a mouse neuromuscular junction during a 100-Hz stimulus train at 20–22°C (black) and 30–32°C (red).
Next, we sought to determine the short-term plasticity characteristics at the mouse NMJ using both paired-pulse and high-frequency train protocols with varying interstimulus intervals. Figure 3D shows a plot of the paired-pulse ratio (PPR) for each of the 10 interstimulus intervals tested. We observed little variability in PPR regardless of the interstimulus interval (maximum 1.03 ± 0.01 at a 10-ms interstimulus interval; n = 37 synapses from 5 mice; Fig. 3D). Similarly, there was little variability in short-term plasticity during trains of 5 stimuli at varying frequencies (Fig. 3E). Trains at 10, 20, 50, and 100 Hz were remarkably similar to one another, showing very little to no facilitation during the first few pulses (maximum 1.03 ± 0.01 at the second pulse in a 100-Hz train) and mild depression to 80–90% of the first EPP by the 5th stimulus in the train (n = 27 synapses from 3 mice). Therefore, unlike the frog NMJ, which shows strong frequency-dependent short-term facilitation (see above; Ma et al. 2015), the mouse NMJ consistently lacks significant facilitation, followed by mild depression, irrespective of stimulus frequency. These observations are consistent with previous reports at the mouse NMJ when short-term synaptic plasticity is studied in healthy synapses with physiological levels of extracellular Ca2+ (David and Barrett 2003; Flink and Atchison 2002), unlike the nonphysiological, low-extracellular-Ca2+ conditions that are often used to strongly reduce the probability of release, inducing facilitation (e.g., Hirata et al. 1999; Nanou et al. 2016b; Urbano et al. 2003). Furthermore, because mice are warm-blooded animals whose NMJs function in a temperature-controlled environment, we also examined tetanic potentiation during 100-Hz stimulus trains at 30–32°C and found a similar mild depression (n = 29 synapses from 3 mice; Fig. 3, E and F). Therefore, mild depression during short stimulus trains at the mouse NMJ is not restricted to measurements at room temperature, and also occurs at warmer temperatures.
Using MCell Modeling to Study AZ Structure-Function Relationships
Since Pr-SV and the short-term synaptic plasticity described above are thought to emerge from characteristics within AZs, and the fine structure of frog and mouse AZs are well known, we wondered to what extent AZ organization alone could contribute to these physiological properties. To explore these structure-function relationships we used 3D diffusion-reaction simulations via MCell to examine the physiological impact of AZ geometry, in particular the number and position of presynaptic VGCCs relative to docked synaptic vesicles.
We had previously developed an MCell-based spatially realistic model of AZ function at the frog NMJ (Dittrich et al. 2013; Luo at al. 2015; Ma et al. 2015). Our simulations provided insight into many experimentally inaccessible aspects of synaptic function and the microscopic mechanisms underlying Ca2+-triggered synaptic vesicle release. In particular, we were able to propose an excess Ca2+ binding site model of vesicle release (Dittrich et al. 2013), which integrated well-known synaptic physiology with recent biochemical and structural insights into the nature of the vesicle release machinery. In addition, we found that short-term synaptic facilitation could be faithfully modeled by a second Ca2+ sensor protein with lower affinity and slower kinetics (Ma et al. 2015; similar to what has recently been reported in the CNS; Jackman et al. 2016).
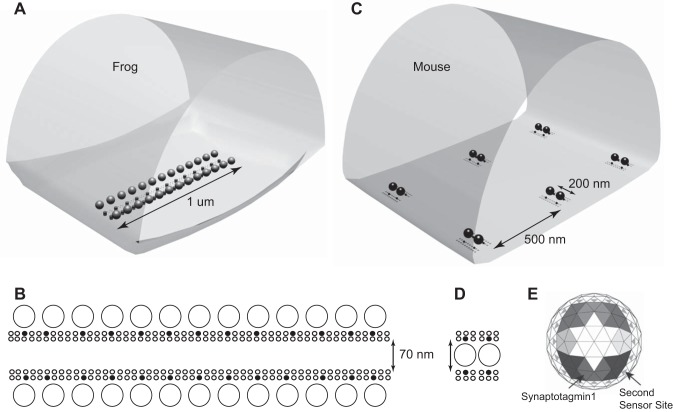
The structure of our previous frog AZ model (Fig. 4, A and B) is based on extensive experimental data, and consists of two rows of 13 synaptic vesicles on each lateral edge of the AZ. Freeze-fracture and EM tomography provide evidence for the presence of ~200 transmembrane particles within each AZ arranged into two double rows between these vesicles (Harlow et al. 2001; Heuser and Reese 1981; Pawson et al. 1998). We previously showed that only a small fraction of these transmembrane proteins are likely to be VGCCs and that each synaptic vesicle is on average associated with only a single VGCC (Luo et al. 2011, 2015). Therefore, in this study in which we sought to evaluate in isolation the impact of AZ organization on synaptic function, we did not change any of the inherent properties of the elements in the frog model (including VGCC biophysical properties, release sensor sensitivity, etc.) when making rearrangements that represented mouse AZ organization. By not changing the intrinsic properties of the AZ elements in our model, we could investigate, in isolation, the role that structure could play on function. The typical AZ at the mouse NMJ is structurally quite distinct (Nagwaney et al. 2009). Each mouse AZ is relatively short and consists of approximately two synaptic vesicles, with ~20–25 transmembrane particles arranged in two double rows on either side of the two vesicles (Nagwaney et al. 2009). Because we wanted to conserve the conclusions regarding VGCC distribution within AZs previously established in our frog NMJ studies (Dittrich et al. 2013; Luo et al. 2011, 2015), we assumed in our mouse MCell model that only a small fraction of AZ particles observed in EM studies would correspond to functional VGCCs. The organizational differences between frog and mouse NMJ AZs are highlighted in Figs. 1 and 4. Again, to maintain consistency between frog and mouse model properties, synaptic vesicles in each model contained 8 synaptotagmin molecules (each with 5 Ca2+ ion binding sites), and 16 second Ca2+ sensor sites as described in our previously frog MCell model that most closely matched physiological properties of the frog NMJ (Ma et al. 2015; Fig. 4E).
Fig. 4.
Diagrams of MCell models used for frog and mouse active zones. A: visualization output of the geometry used for the frog AZ MCell model. A single AZ is modeled along with the surrounding intraterminal space. The walls of this space are reflective to simulate the potential contribution from neighboring AZs. B: organization of elements within the modeled frog AZ. A total of 26 docked synaptic vesicles (large circles) are positioned laterally and adjacent to two double rows of membrane proteins (small circles), some of which (filled circles) are VGCCs. C: visualization output of the geometry used for the mouse AZ MCell model. A total of six AZs are modeled along with the surrounding intraterminal space. The walls of this space are reflective to simulate the potential contribution from neighboring AZs. D: organization of elements within the modeled mouse AZ. A total of 2 docked synaptic vesicles (large circles) are positioned between two double rows of membrane proteins (small circles), some of which (filled circles) are VGCCs. E: the undersurface of each docked synaptic vesicle is populated with the Ca2+ binding sites of eight synaptotagmin 1 molecules (shaded large triangles; differences in shading depict the 5 binding sites for each of the 8 synaptotagmin 1 molecules), and 16 second Ca2+ sensor sites (small gray triangles). The kinetic values for Ca2+ ion binding to these sites are reported in Ma et al. (2015).
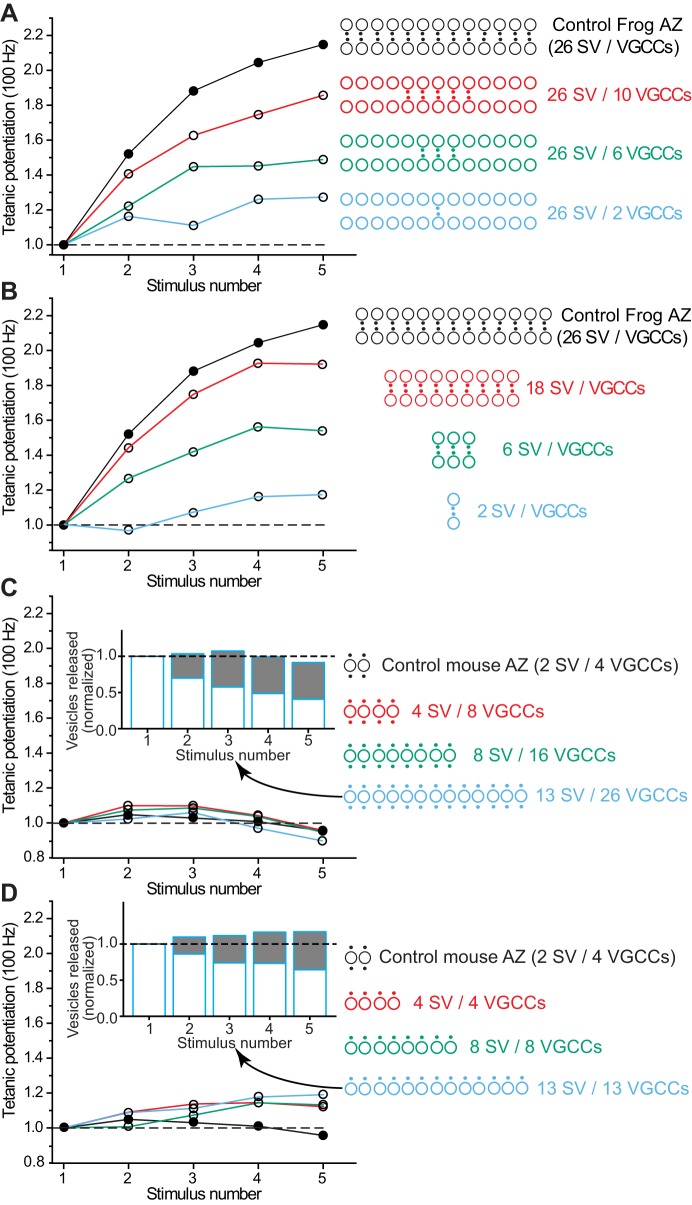
Given that our MCell model of the frog NMJ AZ could accurately predict frog NMJ function (Dittrich et al. 2013; Luo et al. 2015; Ma et al. 2015), we hypothesized that our modeling approach could shed light on the impact of AZ structure on synaptic function. In particular, we wanted to investigate the degree to which the structural differences in the frog and mouse AZs, as described above and shown in Fig. 4, could explain their functional differences. We started our investigation by assembling an initial model of a mouse NMJ AZ based on the basic synaptic building blocks taken from the frog AZ. Specifically, we assembled a mouse AZ from two single vesicle release sites taken from our frog model, each consisting of a synaptic vesicle and two closely associated VGCCs, one on either side at positions constrained by the experimentally observed locations of transmembrane particles identified in electron microscopy images (Fig. 4, C and D). At the frog NMJ, freeze-fracture electron microscopic data leads to the conclusion that AZs have ~200 transmembrane particles (Pawson et al. 1998), ~30 of which are predicted to be functional VGCCs (Luo et al. 2011). In contrast, the mouse AZ has ~20–25 transmembrane particles (Nagwaney et al. 2009) and if the same fraction of these particles represents VGCCs, we would thus expect about four functional VGCCs, which we arranged bilaterally next to vesicles. The distance between the closest VGCC and synaptic vesicle was set at 32 nm, which agrees with experimental estimates for the distance of transmembrane particles (Nagwaney et al. 2009). Figure 4D shows a schematic view of our final mouse AZ model.
Apart from the structural rearrangement of single vesicle release sites from the frog into a mouse AZ configuration, we made no additional changes to any property of the model components. In particular, we left the properties of the key molecular machinery involved in vesicle release unchanged, including the gating properties of the VGCCs (which are P/Q-type in the mouse vs. N-type in the frog), synaptotagmin Ca2+ sensors, facilitation sensors, Ca2+ buffer, and the vesicle release model. In summary, all properties of the elements within our mouse AZ model were identical to what has been reported previously for our frog model (Ma et al. 2015).
MCell Simulations of Mouse NMJ AZ Model During Single Action Potential Stimuli
We then ran MCell simulations of single AP-triggered vesicle release within our mouse AZ model and found good qualitative agreement with all our experimental constraints. Our simulations resulted in a Pr-AZ of 0.13. Dividing this value by the two synaptic vesicles in each mouse AZ resulted in a Pr-SV = 0.065. In our mouse AZ model, the presence of two VGCCs close to each synaptic vesicle increased the release probability per vesicle (Pr-SV) following each action potential in our MCell model from 0.017 in the frog to 0.065 in the mouse, a roughly fourfold increase, which is a similar relationship to what we observed in our ex vivo recordings, where we calculated that the mouse AZ has a fivefold higher average Pr-SV than estimated in frog. This difference in Pr-SV between frog and mouse likely results from a difference in the number of VGCC per docked synaptic vesicle (twice as many in the mouse), coupled with the known nonlinear relationship between Ca2+ ion concentration and transmitter release magnitude (Dodge and Rahamimoff 1967). The precise difference between our measured Pr-AZ in ex vivo recordings (Pr-AZ = 0.22) and our MCell model of mouse AZ organization that was created as a simple rearrangement of the elements in the frog MCell model (Pr-AZ = 0.13) may reflect specific differences in the properties or numbers of some of the AZ elements between frog and mouse NMJs. However, for the purposes of this study that focused on the defined impact of a simple rearrangement of AZ organization on synaptic function, those specific differences are beyond the scope of this report.
In addition, our mouse AZ model also predicted two key known properties of this synapse. Figure 5A shows the simulated distribution of transmitter release events following an AP, which is in good agreement with previously published experimental data regarding the distribution of synaptic latencies (Wang et al. 2010) and synaptic delay (Bukharaeva et al. 2007; Katz and Miledi 1965). Last, the known steep dependence of transmitter release on the external Ca2+ concentration (Ca2+ release relationship, CRR) is also predicted by this mouse model (Fig. 5B). Our simulated value for CRR was 3.8, which is in good agreement with previously published experimental data (Smith 1988). Thus, under a single action potential stimulus paradigm, a simple rearrangement of frog AZ elements into a mouse AZ structure closely recapitulates the experimentally observed probability of vesicle release, CRR, and distribution of release events at the mouse NMJ.
Fig. 5.
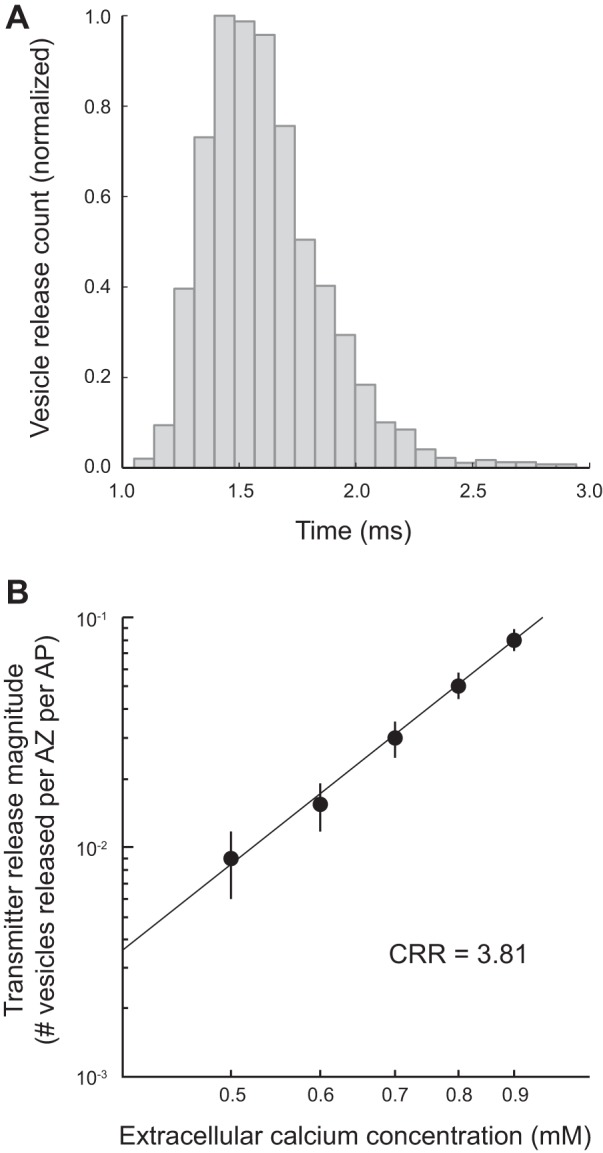
Distribution of transmitter release latencies and the Ca2+-release relationship (CRR) predicted by the mouse MCell model. A: histogram plot of the time course of single vesicle release events in the mouse MCell model following a presynaptic action potential closely matching previously published physiology data (Wang et al. 2010). B: plot of the changes in the magnitude of transmitter release (number of vesicles released per AZ per AP) as the extracellular Ca2+ concentration was varied in the mouse MCell model. When plotted on a log scale, these data are fit by line with a slope of 3.81, closely matching the known 4th-order relationship between extracellular Ca2+ and transmitter release.
Mouse AZ Model Exhibits No Short-Term Facilitation
Since our mouse AZ model provided good agreement with experimental observations following a single action potential stimulus, we next wondered if we could also model the dramatic difference in short-term plasticity observed experimentally when comparing frog and mouse NMJs. Indeed, when simulating paired-pulse plasticity at different interstimulus intervals, our MCell-based frog and mouse models recapitulated the striking experimental difference between these two synapses (Fig. 6, A and B). Furthermore, when using high-frequency trains of stimuli (5 pulses at 100 Hz), our MCell simulations closely predicted the strong potentiation at the frog NMJ in contrast to mild depression at the mouse NMJ (Fig. 6C). Thus a simple reorganization of single vesicle release sites from a frog AZ configuration into a mouse AZ configuration resulted in significantly different short-term plasticity properties that matched what we observed experimentally.
Fig. 6.
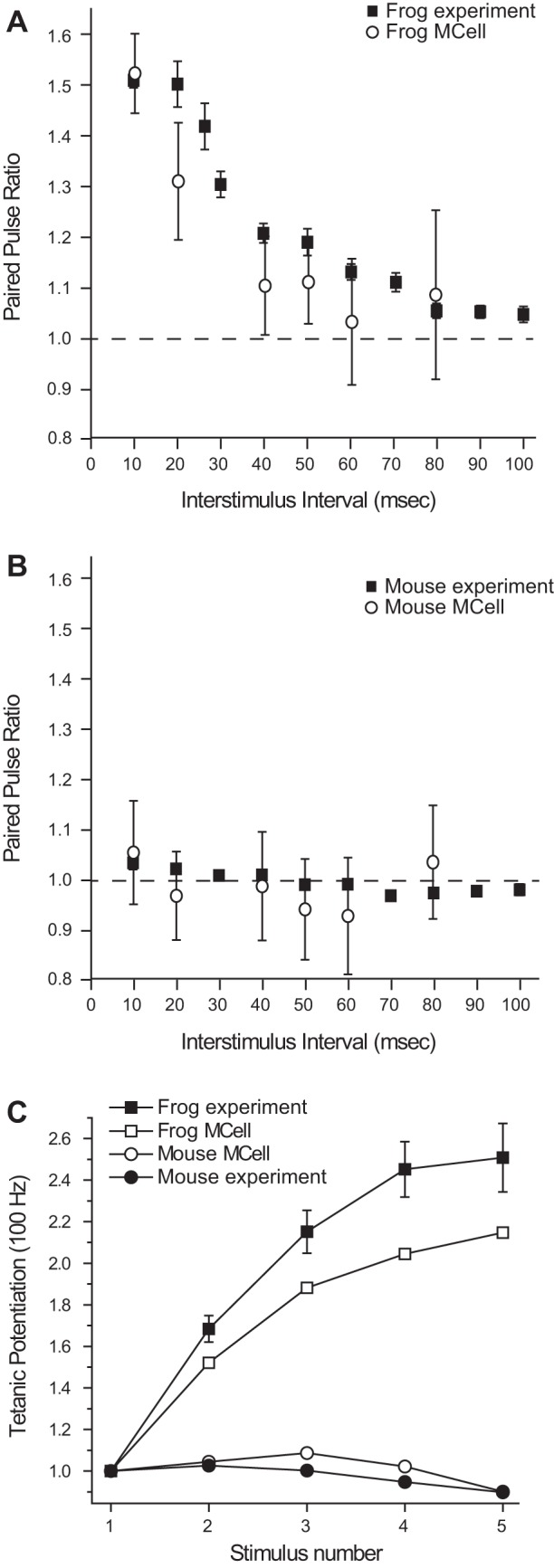
Differences in short-term plasticity observed between frog and mouse NMJs are reproduced by a simple rearrangement in the organization of elements in the frog MCell model into the organization observed in the mouse AZ. A and B: plot of changes in the paired-pulse ratio at the frog (A) and mouse (B) NMJ when the interstimulus interval is changed. The experimental data collected (filled squares) are closely matched by the MCell simulation data (open circles) in both cases. C: plot of tetanic potentiation at 100 Hz in the frog (filled squares) and mouse (filled circles) is closely matched by MCell simulation of the frog AZ (open squares) and the mouse AZ (open circles) models.
These data raise the question of the sub-AZ mechanisms responsible for this dramatic change in synaptic function. Using MCell, we tracked each Ca2+ ion within our simulations and determined when and where they bound during vesicle fusion events. During a short train of APs at 100 Hz within the frog model, we plotted the number of vesicle fusion events that were triggered completely by de novo Ca2+ ion binding (binding that occurred only during the particular stimulus in which the vesicle fused) compared with vesicle fusion events that were triggered in part by Ca2+ ions that remained bound from a previous stimulus event, so-called residual bound Ca2+. These later events thus represented the fusion of synaptic vesicles that had partially bound Ca2+ from a previous stimulus event (Fig. 7). This persistent Ca2+ binding occurred via binding of Ca2+ ions to second-sensor sites on vesicles which had a slower off rate than our synaptotagmin 1 sites (see Ma et al. 2015) and were thus more likely to remain bound during a subsequent stimulation event. At the frog AZ, a large amount of persistent Ca2+ binding to vesicles played an important role in the observed short-term plasticity (Fig. 7A). In contrast, in our mouse AZ model, the relatively small amount of persistent bound Ca2+ resulted in little to no facilitation, and slight depression later in the pulse sequence (Fig. 7B).
Fig. 7.
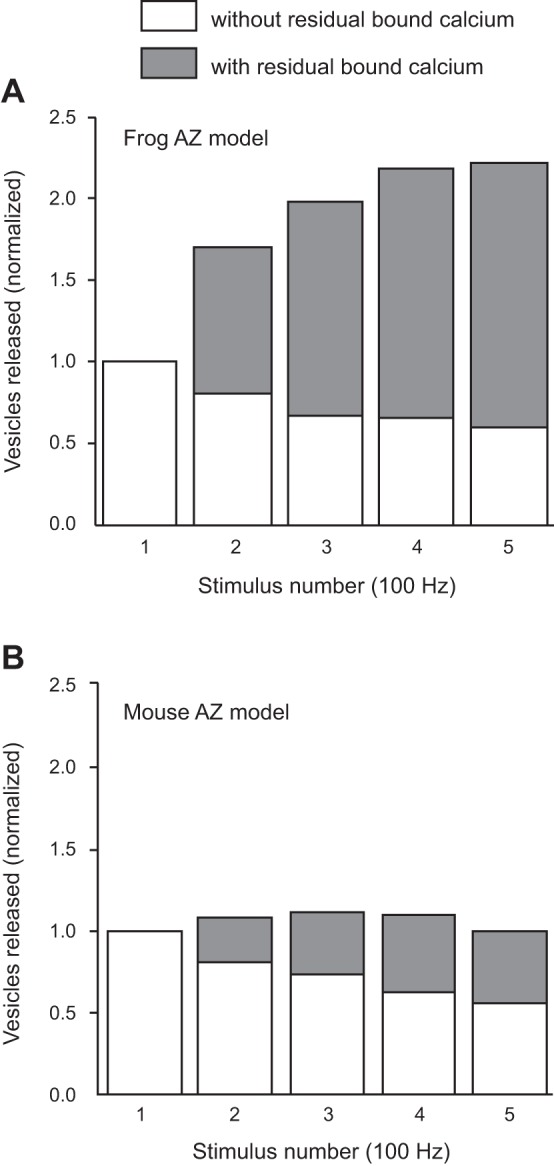
Differences between frog and mouse MCell AZ models in the fraction of vesicle fusion events that included residual bound Ca2+ ions. A: in the frog AZ model, the vesicles that fuse during a train of action potentials at 100 Hz have a strongly increasing fraction of Ca2+ ions that remain bound from a prior action potential stimulus. B: in the mouse AZ model, there are fewer vesicle fusion events that are partially triggered by residual bound Ca2+ ions than observed in the frog model (A).
In assessing the underlying reason for these differences between frog and mouse AZs, we made several additional observations. We determined the number of VGCCs that opened during each action potential in the frog and mouse models. Even though both models employed the same VGCC gating scheme, and thus each channel in these AZs opened with a probability of ~0.2, the large difference in the total number of VGCCs in frog and mouse AZs led to a corresponding difference in the number of Ca2+ channels that opened with each action potential (Fig. 8). Importantly, whereas frog AZs almost always experienced multiple VGCC openings per action potential (mean ± SD = 4.8 ± 1.0) with only rare failures (Fig. 8A), at the mouse AZ, more than 40% of action potential stimuli failed to open any VGCC in a single AZ, and most of the time only a single VGCC opened in each AZ (Fig. 8B). Because the frog AZ experienced multiple VGCC openings per stimulation event, and contained tens of vesicles, this resulted in significant persistent Ca2+ bound vesicles, whereas vesicle fusion remained relatively rare (Pr-AZ = 0.67; Luo et al. 2015). In effect, during a stimulation event, each synaptic vesicle in the frog AZ bound Ca2+ ions from several VGCCs at varying distances from its location. In contrast, the few VGCC openings in the mouse AZ per stimulation event, combined with only two docked vesicles, resulted in little docked vesicle persistent Ca2+ binding. Furthermore, in the mouse we found very little diffusion of Ca2+ ions between neighboring AZs separated by 500 nm from each other. Thus individual AZs in the mouse NMJ acted essentially as isolated units. The above data provide an explanation of why there was significantly more release of persistently Ca2+ bound synaptic vesicles in frog AZs resulting in strong facilitation compared with mouse AZs (Fig. 7).
Fig. 8.
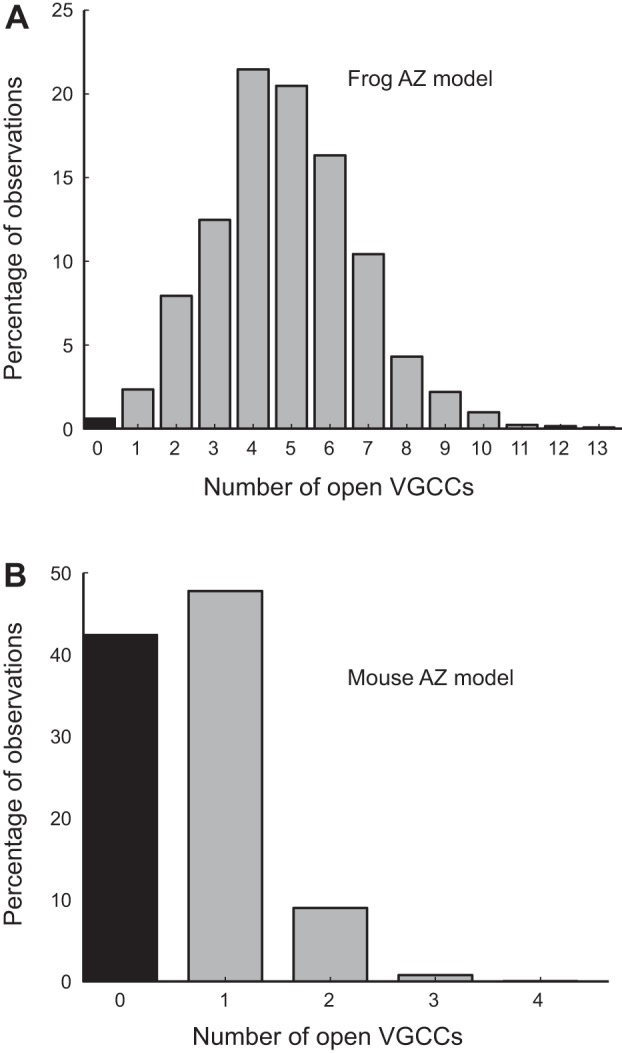
The number of VGCCs that open in frog and mouse AZ MCell models. Although both frog and mouse AZ models have VGCCs with a Po during an action potential of ~0.2, since the frog AZ has 26 VGCCs, the average number of VGCCs that open during an action potential in the frog AZ model (A) is much greater than in the mouse AZ model (B). Within one AZ in the mouse model, more than 40% of action potentials do not open any VGCCs (black bar; B), and most action potentials open only one VGCC. Within the frog AZ model, most action potential stimuli open 3–7 VGCCs, and failures (black bar; A) are rare.
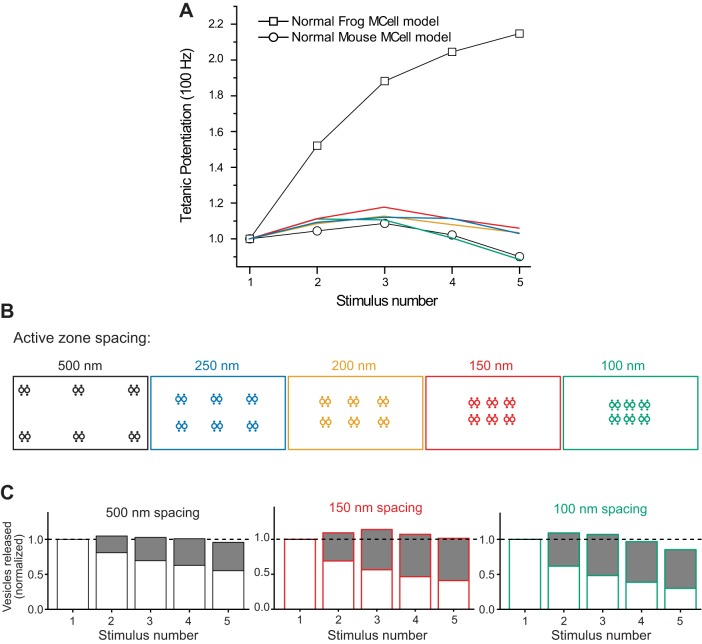
To further explore the impact of the mouse AZ organization on short-term synaptic plasticity, we tested the role of AZ spacing. To examine this issue, we varied the distance between each of the small AZs in the mouse model from 500 nm in our control model to a 250-, 200-, 150-, and 100-nm spacing (Fig. 9). We found that as the mouse AZs were positioned closer to one another, tetanic stimulation resulted in little change, with only mild facilitation (less than 20%) observed at 250-, 200-, and 150-nm AZ spacing, and mild depression that was similar to control (500-nm AZ spacing) when AZs were only 100 nm apart from one another (Fig. 9, A and B). We next examined the proportion of release events derived from docked vesicles that either had residual bound Ca2+ from prior action potentials in the train, or did not have any residual bound Ca2+ (Fig. 9C). We found that as AZ spacing was decreased in the mouse model, there was an increase in release of residual bound vesicles, but there was also a decrease in release of vesicles that did not have any residual bound Ca2+. These data lead to the conclusion that close AZ spacing in the mouse model nerve terminal resulted in both an increase in vesicle depletion (depression), and vesicles with residual bound Ca2+ (facilitation). Therefore, closely positioning mouse AZs together does not create the strong facilitation observed in the frog AZ.
Fig. 9.
The impact on tetanic potentiation of moving modeled mouse AZs closer together. A: plot of tetanic potentiation during a 100-Hz action potential train. Open squares and circles recapitulate the control MCell model data for frog and mouse AZs (see Fig. 6C). Plots in blue, yellow, red, and green represent short-term plasticity for the model in which the 6 modeled AZs are 250, 200, 150, and 100 nm apart from one another as color coded in the diagrams in B. C: plots of the fraction of vesicle fusion events that included residual bound Ca2+ ions (as described in Fig. 7) for 3 AZ spacing arrangements that resulted in the extremes in short-term synaptic plasticity at the modeled mouse AZ (500, 150, and 100 nm AZ spacing, and color coded as in A and B).
The difference in short-term plasticity observed when AZs were organized in a frog or mouse arrangement might also be due to the number of VGCC-docked synaptic vesicle complexes (single vesicle release sites) present in the linear array, and the stoichiometry between VGCCs and docked synaptic vesicles. To explore these issues, we either varied the length of the frog AZ by removing VGCC-vesicle complexes, or varied the number of VGCCs available near the long linear array of docked synaptic vesicles by removing only VGCCs from the AZ (Fig. 10, A and B). Both manipulations resulted in less facilitation during a 100-Hz train. However, even shortening the frog AZ to only 2 vesicles (the same as in a mouse AZ) resulted in more facilitation than was observed in the mouse NMJ (Fig. 10B). The difference between this shortened frog AZ (with only 2 vesicles) and the control mouse AZ (that also contains 2 vesicles) is that there are fewer VGCCs per docked synaptic vesicle in the frog AZ (only 1) compared with the mouse (where there are 2 VGCCs per docked vesicle). The importance of the stoichiometric relationship between VGCCs and docked synaptic vesicles was also evident when only VGCCs were removed from the frog AZ (Fig. 10A). Under these conditions, two VGCCs surrounded by excess docked synaptic vesicles resulted in more facilitation during a 100-Hz stimulus train than when both VGCCs and docked synaptic vesicles were reduced to only two (compare light blue data plotted in Fig. 10, A and B).
Fig. 10.
The impact on tetanic potentiation of altering the length of modeled frog and mouse AZs. A: the control frog model (filled circles) generates strong tetanic potentiation during a 100-Hz stimulus train, but this potentiation significantly weakens as the number of VGCCs is reduced (open circles). The diagrams at the right depict the organization of VGCCs (black dots) relative to synaptic vesicles (large open circles) as VGCCs are removed (plots and diagrams are color coded for clarity). B: the control frog model (filled circles) generates strong tetanic potentiation during a 100-Hz stimulus train, but this potentiation significantly weakens as the number of both VGCCs and docked synaptic vesicles are reduced. The diagrams at the right depict the organization of VGCCs (black dots) and synaptic vesicles (large open circles) as both are removed (plots and diagrams are color coded for clarity). C: the control mouse model (filled circles) generates mild tetanic depression during a 100-Hz stimulus train, and this mild depression does not change significantly as the mouse AZ arrangement is lengthened (adding two VGCCs for each additional docked synaptic vesicle). The diagrams at the right depict the organization of VGCCs (black dots) and docked synaptic vesicles (large open circles) as both are added (plots and diagrams are color coded for clarity). Inset: plot of the fraction of vesicle fusion events that included residual bound Ca2+ ions for the longest AZ plotted in blue. D: the control mouse model (filled circles) generates mild tetanic depression during a 100-Hz stimulus train, but this mild depression reverses to mild tetanic potentiation when the mouse AZ arrangement is changed and lengthened by only adding one VGCC for each docked synaptic vesicle. The diagrams at the right depict the organization of VGCCs (black dots) and docked synaptic vesicles (large open circles) as both are added (plots and diagrams are color coded for clarity). Inset: plot of the fraction of vesicle fusion events that included residual bound Ca2+ ions for the longest AZ plotted in blue.
We also took the approach of lengthening the control mouse AZ to examine effects on tetanic potentiation during a 100-Hz train of action potentials (Fig. 10, C and D). Interestingly, we found that lengthening the mouse AZ did not increase tetanic potentiation during a 100-Hz action potential train (Fig. 10C), and this was reminiscent of the effects when AZ spacing was reduced (Fig. 9). We speculated that this might be due to the presence of twice as many VGCCs per synaptic vesicle (increasing the probability of release) compared with the VGCC-synaptic vesicle arrangement in the frog AZ. To reduce the number of VGCCs in this lengthened mouse AZ, we removed half of the VGCCs such that there was only one VGCC per docked synaptic vesicle (Fig. 10D). This arrangement resulted in an increase in tetanic potentiation as this AZ arrangement was lengthened; however, the tetanic potentiation measured did not reach the level observed in the control frog AZ. Therefore, the organization of VGCCs and docked synaptic vesicles in a frog AZ appears to be optimized for strong tetanic potentiation. These experiments demonstrate that the structural organization of AZs, in particular the number and arrangement of VGCCs with respect to docked synaptic vesicles, can have a major impact on AZ function.
DISCUSSION
The transmitter release site at synapses displays a remarkable diversity in structural forms (Zhai and Bellen 2004). Over the last decade, a number of common themes relating synaptic structure to function have emerged.
The first theme relates to how the number of AZs within individual synapses correlates with their strength. Small mammalian CNS synapses often contain a single or a small number of AZs (Clarke et al. 2012), each of which is organized into a disk-shaped array that is often characterized by a “particle web” (Dresbach et al. 2001; Phillips et al. 2001). Functionally, these small synapses are often also characterized by a low probability of transmitter release (Goda and Südhof 1997; Tarr et al. 2013b). On the other hand, large synapses like the NMJ or the calyx of Held in the auditory brain stem contain hundreds of AZs, which result in highly reliable transmitter release and synaptic strength (Dondzillo et al. 2010; Sätzler et al. 2002; Tarr et al. 2013b; Taschenberger et al. 2002). Studies of frog NMJs have shown that AZ size and number are better indicators of transmitter release magnitude than total synapse size (Herrera et al. 1985; Propst and Ko 1987). Thus synaptic strength appears to be positively correlated with an increase in the number and size of AZs within terminals.
A second theme in synaptic structure and function pertains to the internal organization of individual AZs. Recent freeze-fracture immunoelectron microscopy has revealed that presynaptic VGCCs within AZs at both small CNS boutons and at large calyx of Held synapses are arranged into small clusters (Althof et al. 2015; Nakamura et al. 2015). In addition to this conservation of AZ nanoarchitecture across synapse types, there appears to be heterogeneity in the size of individual AZs at large synapses like the calyx of Held. This, in turn, results in significant variability in the number of VGCCs within each AZ and corresponding variations in vesicular release probability (Nakamura et al. 2015; Sheng et al. 2012) and short-term synaptic plasticity (Sheng et al. 2012). Therefore, variations in sub-AZ organization within the calyx of Held appear to significantly impact their functional output. Similar structure-function relationships have been described across small CNS boutons where VGCC number appears to scale with AZ area and significantly influences transmitter release probability (Holderith et al. 2012). Furthermore, it was recently shown that the coupling of VGCCs and docked synaptic vesicles tightens during the development of small cortical synapses, and that these changes correlate with predicted alterations in probability of transmitter release and short-term synaptic plasticity (Baur et al. 2015). Overall, it appears that heterogeneity in the organization within AZs, especially with respect to the number and spatial localization of VGCCs and docked synaptic vesicles, can be a strong determinant of synaptic function.
Recent studies have used a combination of electrophysiology, imaging, and computer modeling to suggest that AZs of synapses may be constructed by differential assembly of unitary building blocks, so-called single vesicle release sites (Tarr et al. 2013b) akin to the “secretosome” described by Bennett (1996). These single vesicle release sites consist of a synaptic vesicle and a small number of associated VGCCs (Luo et al. 2015; Tarr et al. 2013b), and their arrangement into AZs directly affects synaptic release and facilitation. It is possible that AZs are arranged in different organizational structures that might be fine-tuned to construct individual synapses with properties that are appropriate for each synapse physiologically.
In considering the species-specific benefits of arranging VGCCs and docked synaptic vesicles within AZs at the NMJ in different organizational structures, it is useful to consider the environmental conditions of both frogs and mice. In all species, the function of the NMJ is to release sufficient transmitter to reliably bring the postsynaptic muscle cell to threshold for triggering an action potential and eliciting a muscle twitch. The “safety factor” at neuromuscular synapses (releasing more transmitter than is required to bring the postsynaptic muscle cell to threshold) ensures that most synapses reliably trigger an action potential in the postsynaptic muscle cell (Wood and Slater 2001). When comparing frogs and mice in the context of the data presented in this report, one issue that might be relevant is the range of temperatures within which the NMJ needs to function reliably. The mouse is a warm-blooded animal, and as such, the mouse NMJ does not experience variation in temperature. Under these conditions, a relatively stable amount of transmitter release during a brief burst of action potential activity maintains release above the safety factor for eliciting a postsynaptic action potential. In contrast, the cold-blooded frog NMJ is required to maintain effective function over a wide range of environmental temperatures. The magnitude of transmitter released from the frog NMJ has been reported to be sensitive to temperature, especially at very cold temperatures where transmitter release decreases (Adams 1989; Barrett et al. 1978). In fact, at the frog sartorius NMJ there are very few NMJs that do not release enough transmitter to bring the postsynaptic muscle to threshold at room temperature (only ~6%), but this number increases to 42% at 5°C and 59% at 2.5°C (Adams 1989). Therefore, at cold temperatures, tetanic potentiation of transmitter release during short trains of activity might be required at the frog NMJ to recover reliable communication between nerve and muscle. It is possible that the frog AZ has evolved an organizational structure that creates strong facilitation during short trains of action potential activity (especially at cold temperatures; see Fig. 3, B and C) that serves to aid in bringing the postsynaptic membrane to threshold even at colder temperatures when quantal content is low.
In the current study, we have explored the functional significance for the differential assembly of single vesicle release sites and demonstrate that the arrangement of these presynaptic elements can regulate specific aspects of synaptic function. Taking advantage of the well-known differences in function and structural organization between the highly ordered AZs at frog and mouse neuromuscular synapses, we demonstrated that the arrangement of presynaptic VGCCs and docked synaptic vesicles within neuromuscular AZs significantly determines the probability of transmitter release and short-term synaptic plasticity at these synapses. Here, we have looked at AZ organization alone, with no changes to the functional properties of any of the AZ elements. For example, we did not alter the gating properties of the Ca2+ channels in frog (N-type channels) and mouse MCell models (P/Q-type channels), even though it is possible that they are different (future studies can examine the impact of these specific differences). The approach of keeping the properties of AZ elements unchanged was purposeful so that we could examine in isolation the impact of AZ organization. Because we could predict the differences between frog and mouse physiology with this approach, our results add to the growing evidence that presynaptic AZ structure has a major impact on synaptic function. In particular, we show that differential assembly of single vesicle release sites can in fact be used to tune key synaptic properties like short-term facilitation. This raises the interesting prospect that such a mechanism could be used to tune synaptic transmission by adding or removing single vesicle release sites or by changing the intrinsic spatial relationship between VGCCs and synaptic vesicles.
The critical dependence of short-term synaptic plasticity on AZ organization has not previously been appreciated, and frog and mouse neuromuscular synapses provide an opportunity to explore these relationships carefully. Our study highlights the importance of single vesicle release site organization on the spatiotemporal profile of presynaptic Ca2+ ions during action potential activity, a critical determinant of short-term synaptic plasticity. Furthermore, we show that a high docked synaptic vesicle-to-VGCC ratio appears to favor facilitation during short tetanic trains of stimulation (see Fig. 10). In this report, we have exclusively focused on these AZ structure-function relationships and have not explored the other mechanisms that have been shown to shape short-term synaptic plasticity. It is clear from a variety of reports that short-term synaptic plasticity can also be regulated by changes in action potential shape that alters the probability of release (reviewed in von Gersdorff and Borst 2002), neuronal Ca2+-sensor proteins (Nanou et al. 2016a, 2016b; Sippy et al. 2003), the expression of various synaptotagmin types (Jackman et al. 2016; Tejero et al. 2016), intraterminal Ca2+ buffer concentration and distribution (Blatow et al. 2003; Jackson and Redman 2003; Matveev et al. 2004), the phosphorylation state of AZ proteins (Srinivasan et al. 2008), and a variety of metaplasticity and neuromodulatory events (Becker et al. 2013; Goussakov et al. 2000; Li et al. 1998; Lozovaya et al. 2011). Here, we do not intend to suggest that these factors are less important than AZ organization, but rather study in isolation the impact of the number and position of presynaptic Ca2+ channels and docked synaptic vesicles, and the resultant differences in the spatiotemporal profile of presynaptic Ca2+ during action potential activity, with the knowledge that short-term synaptic plasticity can be further affected by the other mechanisms listed above.
Finally, in this report, we have restricted our modeling of the mouse NMJ AZ to the simple rearrangement of elements from our frog NMJ AZ model, with no modifications to the properties of elements. We used this approach to make the point that organization of AZ elements was the primary determinant of function, but we recognize that the mouse AZ might have additional refinements that are likely required to more closely match finer details of synaptic function, and to recapitulate all aspects of mouse NMJ function (particularly after pharmacological or disease manipulation). These refinements remain as the subject of future investigations.
GRANTS
This work was supported by National Institutes of Health Grant R01-NS-090644 (S. D. Meriney and M. Dittrich). This work used the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation (NSF) Grant Number OCI-1053575, specifically, Comet, Stampede and Bridges. The Bridges system is supported by NSF Award Number ACI-1445606, at the Pittsburgh Supercomputing Center (PSC).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.L., J.M., T.B.T., A.E.H., L.K., M.S.T., B.S.V., H.P.R., S.D.M., and M.D. performed experiments; R.L., J.M., T.B.T., A.E.H., L.K., M.S.T., B.S.V., H.P.R., S.D.M., and M.D. analyzed data; R.L., A.E.H., and S.D.M. prepared figures; R.L., T.B.T., A.E.H., S.D.M., and M.D. edited and revised manuscript; R.L., J.M., T.B.T., A.E.H., L.K., M.S.T., B.S.V., H.P.R., S.D.M., and M.D. approved final version of manuscript; T.B.T., A.E.H., S.D.M., and M.D. conceived and designed research; T.B.T., A.E.H., S.D.M., and M.D. interpreted results of experiments; S.D.M. drafted manuscript.
ACKNOWLEDGMENTS
We thank K. Ojala and S. Aldrich for critical reading of the manuscript.
REFERENCES
- Adams BA. Temperature and synaptic efficacy in frog skeletal muscle. J Physiol 408: 443–455, 1989. doi: 10.1113/jphysiol.1989.sp017469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althof D, Baehrens D, Watanabe M, Suzuki N, Fakler B, Kulik Á. Inhibitory and excitatory axon terminals share a common nano-architecture of their Cav2.1 (P/Q-type) Ca2+ channels. Front Cell Neurosci 9: 315, 2015. doi: 10.3389/fncel.2015.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN, Botz D, Chang DB, Mahaffey D. Temperature-sensitive aspects of evoked and spontaneous transmitter release at the frog neuromuscular junction. J Physiol 279: 253–273, 1978. doi: 10.1113/jphysiol.1978.sp012343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur D, Bornschein G, Althof D, Watanabe M, Kulik A, Eilers J, Schmidt H. Developmental tightening of cerebellar cortical synaptic influx-release coupling. J Neurosci 35: 1858–1871, 2015. doi: 10.1523/JNEUROSCI.2900-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Klein EM, Striepens N, Mihov Y, Schlaepfer TE, Reul J, Goossens L, Schruers K, Kendrick KM, Hurlemann R. Nicotinic acetylcholine receptors contribute to learning-induced metaplasticity in the hippocampus. J Cogn Neurosci 25: 986–997, 2013. doi: 10.1162/jocn_a_00383. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Neuromuscular transmission at an active zone: the secretosome hypothesis. J Neurocytol 25: 869–891, 1996. doi: 10.1007/BF02284848. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Farnell L, Gibson WG. The probability of quantal secretion near a single calcium channel of an active zone. Biophys J 78: 2201–2221, 2000a. doi: 10.1016/S0006-3495(00)76769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Farnell L, Gibson WG. The probability of quantal secretion within an array of calcium channels of an active zone. Biophys J 78: 2222–2240, 2000b. doi: 10.1016/S0006-3495(00)76770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Farnell L, Gibson WG. The facilitated probability of quantal secretion within an array of calcium channels of an active zone at the amphibian neuromuscular junction. Biophys J 86: 2674–2690, 2004. doi: 10.1016/S0006-3495(04)74323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Gibson WG, Robinson J. Probabilistic secretion of quanta and the synaptosecretosome hypothesis: evoked release at active zones of varicosities, boutons, and endplates. Biophys J 73: 1815–1829, 1997. doi: 10.1016/S0006-3495(97)78212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron 38: 79–88, 2003. doi: 10.1016/S0896-6273(03)00196-X. [DOI] [PubMed] [Google Scholar]
- Bukharaeva EA, Samigullin D, Nikolsky EE, Magazanik LG. Modulation of the kinetics of evoked quantal release at mouse neuromuscular junctions by calcium and strontium. J Neurochem 100: 939–949, 2007. doi: 10.1111/j.1471-4159.2006.04282.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin β2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. J Neurosci 31: 512–525, 2011. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol 520: 434–452, 2012. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke GL, Chen J, Nishimune H. Presynaptic active zone density during development and synaptic plasticity. Front Mol Neurosci 5: 12, 2012. doi: 10.3389/fnmol.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MW, Jones OT, Angelides KJ. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J Neurosci 11: 1032–1039, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett EF. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J Physiol 548: 425–438, 2003. doi: 10.1113/jphysiol.2002.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich M, Pattillo JM, King JD, Cho S, Stiles JR, Meriney SD. An excess-calcium-binding-site model predicts neurotransmitter release at the neuromuscular junction. Biophys J 104: 2751–2763, 2013. doi: 10.1016/j.bpj.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol 193: 419–432, 1967. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondzillo A, Sätzler K, Horstmann H, Altrock WD, Gundelfinger ED, Kuner T. Targeted three-dimensional immunohistochemistry reveals localization of presynaptic proteins Bassoon and Piccolo in the rat calyx of Held before and after the onset of hearing. J Comp Neurol 518: 1008–1029, 2010. doi: 10.1002/cne.22260. [DOI] [PubMed] [Google Scholar]
- Dorlöchter M, Meurer S, Wernig A. Acetylcholine receptor bars and transmitter release in frog neuromuscular junctions. Neuroscience 52: 987–999, 1993. doi: 10.1016/0306-4522(93)90545-Q. [DOI] [PubMed] [Google Scholar]
- Douthitt HL, Luo F, McCann SD, Meriney SD. Dynasore, an inhibitor of dynamin, increases the probability of transmitter release. Neuroscience 172: 187–195, 2011. doi: 10.1016/j.neuroscience.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Qualmann B, Kessels MM, Garner CC, Gundelfinger ED. The presynaptic cytomatrix of brain synapses. Cell Mol Life Sci 58: 94–116, 2001. doi: 10.1007/PL00000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flink MT, Atchison WD. Passive transfer of Lambert-Eaton syndrome to mice induces dihydropyridine sensitivity of neuromuscular transmission. J Physiol 543: 567–576, 2002. doi: 10.1113/jphysiol.2002.021048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson AL, Zucker RS. Presynaptic calcium diffusion from various arrays of single channels. Implications for transmitter release and synaptic facilitation. Biophys J 48: 1003–1017, 1985. doi: 10.1016/S0006-3495(85)83863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci USA 80: 7636–7640, 1983. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T, Engel AG, Lang B, Newsom-Davis J, Vincent A. Lambert-Eaton myasthenic syndrome: II. Immunoelectron microscopy localization of IgG at the mouse motor end-plate. Ann Neurol 22: 200–211, 1987. doi: 10.1002/ana.410220204. [DOI] [PubMed] [Google Scholar]
- Goda Y, Südhof TC. Calcium regulation of neurotransmitter release: reliably unreliable? Curr Opin Cell Biol 9: 513–518, 1997. doi: 10.1016/S0955-0674(97)80027-0. [DOI] [PubMed] [Google Scholar]
- Goussakov IV, Fink K, Elger CE, Beck H. Metaplasticity of mossy fiber synaptic transmission involves altered release probability. J Neurosci 20: 3434–3441, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron 69: 304–316, 2011. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog’s neuromuscular junction. Nature 409: 479–484, 2001. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- Herrera AA, Grinnell AD, Wolowske B. Ultrastructural correlates of naturally occurring differences in transmitter release efficacy in frog motor nerve terminals. J Neurocytol 14: 193–202, 1985. doi: 10.1007/BF01258447. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Morphology of synaptic vesicle discharge and reformation at the frog neuromuscular junction. In: Synaptic Transmission and Neuronal Interaction, edited by Bennett MVL. New York: Raven, 1974, vol. 28, chapt. 4, p. 59–78. Soc. Gen. Physiol. Series [Google Scholar]
- Heuser JE, Reese TS. Structural changes after transmitter release at the frog neuromuscular junction. J Cell Biol 88: 564–580, 1981. doi: 10.1083/jcb.88.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol 81: 275–300, 1979. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Nakagawa M, Urbano FJ, Rosato-Siri MD, Moreira JE, Uchitel OD, Sugimori M, Llinás R. Reduced facilitation and vesicular uptake in crustacean and mammalian neuromuscular junction by T-588, a neuroprotective compound. Proc Natl Acad Sci USA 96: 14588–14593, 1999. doi: 10.1073/pnas.96.25.14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderith N, Lorincz A, Katona G, Rózsa B, Kulik A, Watanabe M, Nusser Z. Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat Neurosci 15: 988–997, 2012. doi: 10.1038/nn.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Chang CC. Use of geographutoxin II (mu-conotoxin) for the study of neuromuscular transmission in mouse. Br J Pharmacol 97: 934–940, 1989. doi: 10.1111/j.1476-5381.1989.tb12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Turecek J, Belinsky JE, Regehr WG. The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 529: 88–91, 2016. doi: 10.1038/nature16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Redman SJ. Calcium dynamics, buffering, and buffer saturation in the boutons of dentate granule-cell axons in the hilus. J Neurosci 23: 1612–1621, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. The measurement of synaptic delay, and the time course of acetylcholine release at the neuromuscular junction. Proc R Soc Lond B Biol Sci 161: 483–495, 1965. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Kerr RA, Bartol TM, Kaminsky B, Dittrich M, Chang JC, Baden SB, Sejnowski TJ, Stiles JR. Fast Monte Carlo simulation methods for biological reaction-diffusion systems in solution and on surfaces. SIAM J Sci Comput 30: 3126–3149, 2008. doi: 10.1137/070692017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Weiss SR, Chuang DM, Post RM, Rogawski MA. Bidirectional synaptic plasticity in the rat basolateral amygdala: characterization of an activity-dependent switch sensitive to the presynaptic metabotropic glutamate receptor antagonist 2S-alpha-ethylglutamic acid. J Neurosci 18: 1662–1670, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya N, Mukhtarov M, Tsintsadze T, Ledent C, Burnashev N, Bregestovski P. Frequency-dependent cannabinoid receptor-independent modulation of glycine receptors by endocannabinoid 2-AG. Front Mol Neurosci 4: 13, 2011. doi: 10.3389/fnmol.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Dittrich M, Cho S, Stiles JR, Meriney SD. Transmitter release is evoked with low probability predominately by calcium flux through single channel openings at the frog neuromuscular junction. J Neurophysiol 113: 2480–2489, 2015. doi: 10.1152/jn.00879.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Dittrich M, Stiles JR, Meriney SD. Single-pixel optical fluctuation analysis of calcium channel function in active zones of motor nerve terminals. J Neurosci 31: 11268–11281, 2011. doi: 10.1523/JNEUROSCI.1394-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Kelly L, Ingram J, Price TJ, Meriney SD, Dittrich M. New insights into short-term synaptic facilitation at the frog neuromuscular junction. J Neurophysiol 113: 71–87, 2015. doi: 10.1152/jn.00198.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev V, Bertram R, Sherman A. Calcium cooperativity of exocytosis as a measure of Ca2+ channel domain overlap. Brain Res 1398: 126–138, 2011. doi: 10.1016/j.brainres.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matveev V, Zucker RS, Sherman A. Facilitation through buffer saturation: constraints on endogenous buffering properties. Biophys J 86: 2691–2709, 2004. doi: 10.1016/S0006-3495(04)74324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan EM, Martin AR. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol 311: 307–324, 1981. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriney SD, Grinnell AD. Endogenous adenosine modulates stimulation-induced depression at the frog neuromuscular junction. J Physiol 443: 441–455, 1991. doi: 10.1113/jphysiol.1991.sp018843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AG, Atwood HL. Crustacean phasic and tonic motor neurons. Integr Comp Biol 44: 4–13, 2004. doi: 10.1093/icb/44.1.4. [DOI] [PubMed] [Google Scholar]
- Nagwaney S, Harlow ML, Jung JH, Szule JA, Ress D, Xu J, Marshall RM, McMahan UJ. Macromolecular connections of active zone material to docked synaptic vesicles and presynaptic membrane at neuromuscular junctions of mouse. J Comp Neurol 513: 457–468, 2009. doi: 10.1002/cne.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Harada H, Kamasawa N, Matsui K, Rothman JS, Shigemoto R, Silver RA, DiGregorio DA, Takahashi T. Nanoscale distribution of presynaptic Ca2+ channels and its impact on vesicular release during development. Neuron 85: 145–158, 2015. doi: 10.1016/j.neuron.2014.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Sullivan JM, Scheuer T, Catterall WA. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to short-term synaptic plasticity in hippocampal neurons. Proc Natl Acad Sci USA 113: 1062–1067, 2016a. doi: 10.1073/pnas.1524636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanou E, Yan J, Whitehead NP, Kim MJ, Froehner SC, Scheuer T, Catterall WA. Altered short-term synaptic plasticity and reduced muscle strength in mice with impaired regulation of presynaptic CaV2.1 Ca2+ channels. Proc Natl Acad Sci USA 113: 1068–1073, 2016b. doi: 10.1073/pnas.1524650113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune H. Active zones of mammalian neuromuscular junctions: formation, density, and aging. Ann N Y Acad Sci 1274: 24–32, 2012. doi: 10.1111/j.1749-6632.2012.06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature 432: 580–587, 2004. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- Pawson PA, Grinnell AD, Wolowske B. Quantitative freeze-fracture analysis of the frog neuromuscular junction synapse. I. Naturally occurring variability in active zone structure. J Neurocytol 27: 361–377, 1998. doi: 10.1023/A:1006942909544. [DOI] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron 32: 63–77, 2001. doi: 10.1016/S0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Propst JW, Herrera AA, Ko CP. A comparison of active zone structure in frog neuromuscular junctions from two fast muscles with different synaptic efficacy. J Neurocytol 15: 525–534, 1986. doi: 10.1007/BF01611734. [DOI] [PubMed] [Google Scholar]
- Propst JW, Ko C-P. Correlations between active zone ultrastructure and synaptic function studied with freeze-fracture of physiologically identified neuromuscular junctions. J Neurosci 7: 3654–3664, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci 6: 57–69, 2005. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron 5: 773–779, 1990. doi: 10.1016/0896-6273(90)90336-E. [DOI] [PubMed] [Google Scholar]
- Rollenhagen A, Sätzler K, Rodríguez EP, Jonas P, Frotscher M, Lübke JHR. Structural determinants of transmission at large hippocampal mossy fiber synapses. J Neurosci 27: 10434–10444, 2007. doi: 10.1523/JNEUROSCI.1946-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz R, Cano R, Casañas JJ, Gaffield MA, Betz WJ, Tabares L. Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J Neurosci 31: 2000–2008, 2011. doi: 10.1523/JNEUROSCI.4663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sätzler K, Söhl LF, Bollmann JH, Borst JG, Frotscher M, Sakmann B, Lübke JH. Three-dimensional reconstruction of a calyx of Held and its postsynaptic principal neuron in the medial nucleus of the trapezoid body. J Neurosci 22: 10567–10579, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Diamond JS. The number and organization of Ca2+ channels in the active zone shapes neurotransmitter release from Schaffer collateral synapses. J Neurosci 32: 18157–18176, 2012. doi: 10.1523/JNEUROSCI.3827-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrezaei V, Cao A, Delaney KR. Ca2+ from one or two channels controls fusion of a single vesicle at the frog neuromuscular junction. J Neurosci 26: 13240–13249, 2006. doi: 10.1523/JNEUROSCI.1418-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng J, He L, Zheng H, Xue L, Luo F, Shin W, Sun T, Kuner T, Yue DT, Wu LG. Calcium-channel number critically influences synaptic strength and plasticity at the active zone. Nat Neurosci 15: 998–1006, 2012. doi: 10.1038/nn.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon KJ, Olivera BM, Watkins M, Jacobsen RB, Gray WR, Floresca CZ, Cruz LJ, Hillyard DR, Brink A, Terlau H, Yoshikami D. mu-Conotoxin PIIIA, a new peptide for discriminating among tetrodotoxin-sensitive Na channel subtypes. J Neurosci 18: 4473–4481, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippy T, Cruz-Martín A, Jeromin A, Schweizer FE. Acute changes in short-term plasticity at synapses with elevated levels of neuronal calcium sensor-1. Nat Neurosci 6: 1031–1038, 2003. doi: 10.1038/nn1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DO. Muscle-specific decrease in presynaptic calcium dependence and clearance during neuromuscular transmission in aged rats. J Neurophysiol 59: 1069–1082, 1988. doi: 10.1152/jn.1988.59.4.1069. [DOI] [PubMed] [Google Scholar]
- Sosa MA, Zengel JE. Use of mu-conotoxin GIIIA for the study of synaptic transmission at the frog neuromuscular junction. Neurosci Lett 157: 235–238, 1993. doi: 10.1016/0304-3940(93)90745-7. [DOI] [PubMed] [Google Scholar]
- Srinivasan G, Kim JH, von Gersdorff H. The pool of fast releasing vesicles is augmented by myosin light chain kinase inhibition at the calyx of Held synapse. J Neurophysiol 99: 1810–1824, 2008. doi: 10.1152/jn.00949.2007. [DOI] [PubMed] [Google Scholar]
- Stanley EF, Reese TS, Wang GZ. Molecular scaffold reorganization at the transmitter release site with vesicle exocytosis or botulinum toxin C1. Eur J Neurosci 18: 2403–2407, 2003. doi: 10.1046/j.1460-9568.2003.02948.x. [DOI] [PubMed] [Google Scholar]
- Tarr TB, Dittrich M, Meriney SD. Are unreliable release mechanisms conserved from NMJ to CNS? Trends Neurosci 36: 14–22, 2013b. doi: 10.1016/j.tins.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr TB, Malick W, Liang M, Valdomir G, Frasso M, Lacomis D, Reddel SW, Garcia-Ocano A, Wipf P, Meriney SD. Evaluation of a novel calcium channel agonist for therapeutic potential in Lambert-Eaton myasthenic syndrome. J Neurosci 33: 10559–10567, 2013a. doi: 10.1523/JNEUROSCI.4629-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H, Leão RM, Rowland KC, Spirou GA, von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron 36: 1127–1143, 2002. doi: 10.1016/S0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Tejero R, Lopez-Manzaneda M, Arumugam S, Tabares L. Synaptotagmin-2, and -1, linked to neurotransmission impairment and vulnerability in spinal muscular atrophy. Hum Mol Genet 25: 4703–4716; 2016. doi: 10.1093/hmg/ddw297. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Piedras-Rentería ES, Jun K, Shin HS, Uchitel OD, Tsien RW. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci USA 100: 3491–3496, 2003. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Borst JG. Short-term plasticity at the calyx of Held. Nat Rev Neurosci 3: 53–64, 2002. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- Walrond JP, Reese TS. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J Neurosci 5: 1118–1131, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Pinter MJ, Rich MM. Ca2+ dependence of the binomial parameters p and n at the mouse neuromuscular junction. J Neurophysiol 103: 659–666, 2010. doi: 10.1152/jn.00708.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol 64: 393–429, 2001. doi: 10.1016/S0301-0082(00)00055-1. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Bellen HJ. The architecture of the active zone in the presynaptic nerve terminal. Physiology (Bethesda) 19: 262–270, 2004. doi: 10.1152/physiol.00014.2004. [DOI] [PubMed] [Google Scholar]