Abstract
The lower urinary tract (LUT) may be activated by spinal cord stimulation, but the physiological mapping characteristics of LUT activation with noninvasive transcutaneous spinal cord stimulation (TSCS) are not known. The effects of aging on the contractile properties of the detrusor are also not well understood. Therefore, TSCS was applied over the T10/T11 to L6/L7 spinous processes in adult (n = 6) and aged (n = 9) female rhesus macaques. A combination of urodynamic studies and electromyography recordings of the external urethral sphincter (EUS), external anal sphincter (EAS), and pelvic floor muscles was performed. Distinct functional maps were demonstrated for TSCS-evoked detrusor and urethral pressures and for the activation of the EUS, EAS, and pelvic floor muscles. The magnitude of responses for each peripheral target organ was dependent on TSCS location and strength. The strongest detrusor contraction was observed with TSCS at the L1/L2 site in adults and the L3/L4 site in aged subjects. TSCS-evoked bladder pressure at the L1/L2 site was significantly higher for the adults compared with the aged subjects (P < 0.05). Cumulative normalized TSCS-evoked pressures, calculated for five consecutive sites between the T11/T12 and L3/L4 levels, were significantly lower for aged compared with adult subjects (P < 0.05). The aged animals also showed a caudal shift for the TSCS site that generated the strongest detrusor contraction. We conclude that natural aging in rhesus macaques is associated with decreased detrusor contractility, a finding of significant translational research relevance as detrusor underactivity is a common occurrence with aging in humans.
NEW & NOTEWORTHY Transcutaneous spinal cord stimulation (TSCS) was used to map lower urinary tract function in adult and aged rhesus macaques. Aging was associated with decreased peak pressure responses to TSCS, reduced cumulative normalized evoked bladder pressure responses, and a caudal shift for the site generating the strongest TSCS-induced detrusor contraction. We demonstrate the utility of TSCS as a new diagnostic tool for detrusor contractility assessments and conclude that aging is associated with decreased detrusor contractility in primates.
Keywords: cystometrogram, electromyography, transcutaneous spinal cord stimulation, urodynamic studies
INTRODUCTION
The lower urinary tract (LUT) is innervated by a combination of autonomic and somatic motor fibers. Preganglionic sympathetic neurons associated with LUT function reside in the thoracolumbar spinal cord, whereas the somata of preganglionic parasympathetic neurons are located in the conus medullaris (Fowler et al. 2008). Postganglionic sympathetic neurons innervate the urethra and bladder base, whereas postganglionic parasympathetic fibers provide excitatory input to the detrusor and the somatic motor neurons of the Onuf’s nucleus homolog innervate the external urethral sphincter (EUS) (de Groat and Yoshimura 2015). The spinal cord also includes sets of interneurons, which help coordinate micturition reflex functions (Shefchyk 2001). The integrated function of the spinal cord networks promotes detrusor relaxation and EUS tonic activity during the storage phase and efficient detrusor contractions and EUS relaxation during the voiding phase.
Spinal cord stimulation has been introduced as a new approach to activation of neural networks. For instance, epidural stimulation has been demonstrated to enable locomotor function in animal models and humans (Courtine et al. 2009; Harkema et al. 2011). Epidural stimulation has also shown potential utility for activating neural networks associated with LUT function in rodents (Abud et al. 2015; Gad et al. 2014). However, epidural stimulation has a potential limitation as a component of surgical invasiveness, which may limit its utility and translation for select populations, including aged subjects with coexisting conditions that limit surgical candidacy for elective procedures. Recently, transcutaneous spinal cord stimulation (TSCS) has been developed as a noninvasive method to activate motor circuits in the human spinal cord (Gerasimenko et al. 2015; Sayenko et al. 2015). A uniqueness of this approach is that it can recruit dorsal afferents, interneurons, and motor neurons based on the pattern, site, and amplitude of TSCS. Here we performed TSCS in combination with urodynamic studies and electromyography (EMG) recordings of EUS, external anal sphincter (EAS), and pelvic floor muscles to map LUT function in adult and aging rhesus macaques (Macaca mulatta). The objective of the study was to identify and characterize age- and TSCS-dependent functional maps for the LUT with spinally evoked responses and to determine the effect of aging on detrusor contractility in aging rhesus macaques.
METHODS
All animal procedures were performed in facilities that are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International at the California National Primate Research Center, University of California, Davis. The studies were approved by the University of California, Davis Institutional Animal Care and Use Committee and completed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2011). Before studies, all subjects underwent a health check, which included a physical examination and body composition scoring (BCS) (Summers et al. 2012) (Table 1). All subjects were housed indoors in stainless steel cages and exposed to a 12:12-h light-dark cycle. Tap water was freely available, and the animals were fed commercial chow (high-protein diet, Ralston Purina, St. Louis, MO).
Table 1.
Demographic information about subjects
| Sex | n | Age, yr | Weight, kg | BCS | Menopause | |
|---|---|---|---|---|---|---|
| Adult | Female | 6 | 9.6 ± 1.8 | 7.8 ± 0.7 | 2.6 ± 0.2 | 0/6 |
| Aged | Female | 9 | 22.7 ± 1.2 | 9.1 ± 0.7 | 3.2 ± 0.3 | 1/9 |
| P < 0.001 | ns | ns | ns |
ns, Not significant.
Animal preparations.
All subjects were initially sedated by an intramuscular dose of ketamine (10 mg/kg). An intravenous catheter was placed, and an endotracheal tube was placed for airway protection. Ketamine was next administered at 10–12 mg·kg−1·h−1 by constant-rate infusion, and the dose was titrated to provide light sedation and immobilization. A triple-lumen 7-Fr transurethral bladder catheter (Life-Tech, Stafford, TX) was placed, and the cystometry and urethral pressure ports were individually connected to a TSD 104A pressure transducer and a MP 150 Data Acquisition System (Biopac Systems, Goleta, CA). Pairs of needle and wire electrodes, consisting of 27-gauge 1-in. needles and 38-gauge insulated wires (1215A-F; Life-Tech), were placed into the bilateral sides of the EUS. For this, the already placed catheter served as a guide, and the needle and wire electrodes were inserted into the EUS on the bilateral sides of the catheter at an approximate depth of 1.5 cm. When each needle and wire electrode entered the EUS muscle, there was a slight increase in tissue resistance and the needle was withdrawn, leaving the hooked wire electrode in place. Paired 22-gauge needles (Hamilton, Reno, NV) were inserted into the EAS, and a pair of patch electrodes were attached to the skin immediately lateral to the rectum for positioning over the levator ani muscle group of the pelvic floor. The EUS, EAS, and pelvic floor electrodes were connected to the MP 150 Data Acquisition System (Biopac Systems) for EMG recordings. Before the subsequent start of stimulation studies, the bladder was emptied, with a syringe attached to the fill port of the bladder catheter. All TSCS and recordings were performed in the setting of an empty bladder to avoid any potential confounding factors associated with partially filled bladders at different age groups.
TSCS and end-organ recordings.
TSCS was applied at the T10/T11 (referred to as T10), T11/T12, T12/L1, L1/L2, L2/L3, L3/L4, L4/L5, L5/L6, and L6/L7 interspinous processes as a monophasic rectangular pulse at 100 V at a frequency of 1 Hz with a pulse width of 1–5 ms in steps of 1 ms with a circular adhesive electrode (1.25-in. diameter) attached to the skin as cathode and a 5 × 10-cm rectangular electrode placed over the abdomen as anode (ValuTrode Cloth Electrodes; Axelgaard, Fallbrook, CA). A total of five stimulation cycles were performed for each pulse width. Concurrent recordings of evoked bladder and urethral pressures and EUS, EAS, and pelvic floor EMG activity were collected. In addition, paired 22-gauge needles (Hamilton) were used for subcutaneous stimulation at L1 to evoke detrusor contractions in all subjects, with a stimulation duration of 5 ms, frequency of 1 Hz, and amplitude of 100 V.
Data analysis.
TSCS-triggered evoked pressure responses in the detrusor and urethra and EMG responses were averaged (n = 5) across each TSCS site and intensity. Peak-to-peak amplitudes were calculated for each subject and reported as means ± SE. Detrusor pressures were normalized to the maximum TSCS-evoked pressure for that subject across all sites. Latency was determined as the time between the leading edge of the stimulation pulse and the peak of the response.
Statistical analysis.
All data are presented as means ± SE. The Shapiro-Wilk test was performed to test for normality of data. Unpaired t-tests were used to compare samples at each TSCS site. One-way repeated-measures ANOVA was used to determine differences between TSCS sites within the adult and aged groups independently. Additionally, a two-way mixed (split) ANOVA was performed with site as repeated independent factor and age as nonrepeated independent factor. To compensate for multiple testing in the experimental design, the post hoc Tukey test was performed. The post hoc Tukey test was also used to identify pairwise differences between groups. A value of P < 0.05 was considered to reflect a statistically significant difference between groups. Exponential regression analysis was performed for the adult and aged groups. All statistical analyses were performed with Origin Pro 2018 (OriginLab, Northampton, MA).
RESULTS
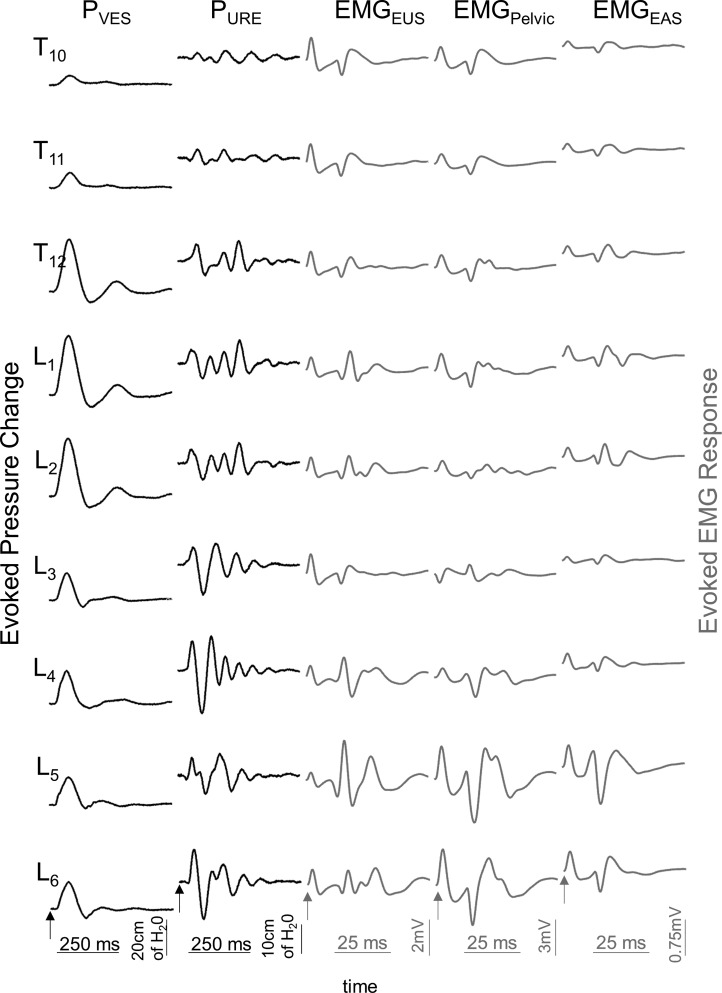
A total of 15 female rhesus macaques were included in the studies (Table 1). The age range was 3.9–14.9 yr for adults (n = 6) and 19.2–30.2 yr for the aged subjects (n = 9). TSCS was sequentially applied over the T10–L6 interspinous process spaces in an attempt to evoke a pressure change in the detrusor (PVES) and urethra (PURE) and to activate the EUS (EMGEUS), EAS (EMGEAS), and pelvic floor (EMGPelvic) muscles (Fig. 1).
Fig. 1.
Representative example of evoked pressure changes from the bladder (PVES) and urethra (PURE) and evoked EMG responses from the external urethral sphincter (EMGEUS), pelvic floor (EMGPelvic), and external anal sphincter (EMGEAS) after TSCS applied sequentially over the T10/T11 through L6/L7 vertebral levels in an adult rhesus macaque. TSCS was applied as a monophasic rectangular pulse at 100 V at a frequency of 1 Hz and with a pulse width of 5 ms. Note that the evoked pressure changes and EMG responses vary with TSCS applied to the different vertebral levels and that the site for the maximum response also varies between different outcome measures. Arrows at bottom of the recordings indicate the stimulation artifact.
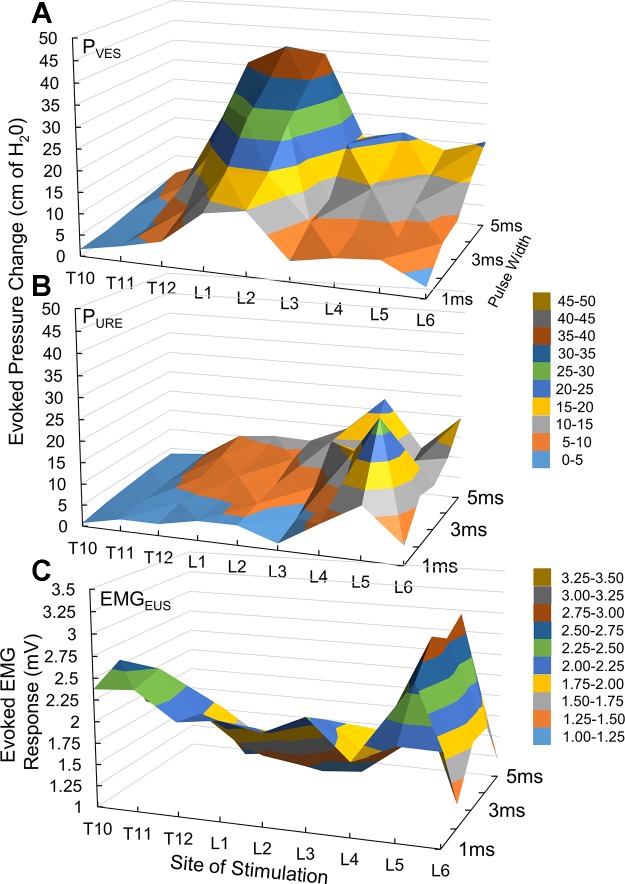
In adult subjects, the maximum PVES was detected in response to TSCS at L1 (n = 5/6) and L2 (n = 1/6) and the maximum PURE was detected at L4 (n = 3). The pattern of evoked responses showed end-organ specificity and a dependence on segmental site of stimulation and stimulus strength, allowing for the generation of separate functional maps of activation for the different pelvic organ and floor functions in individual subjects (Fig. 2).
Fig. 2.
Representative examples of evoked pressure changes in the bladder (A) and urethra (B) and of evoked EMG responses from the EUS (C). Recordings are presented in a heat map representation to indicate the influence of the site of stimulation and pulse width duration on the evoked responses. Note that the site of stimulation for the maximum evoked response differs between the 3 outcome measures.
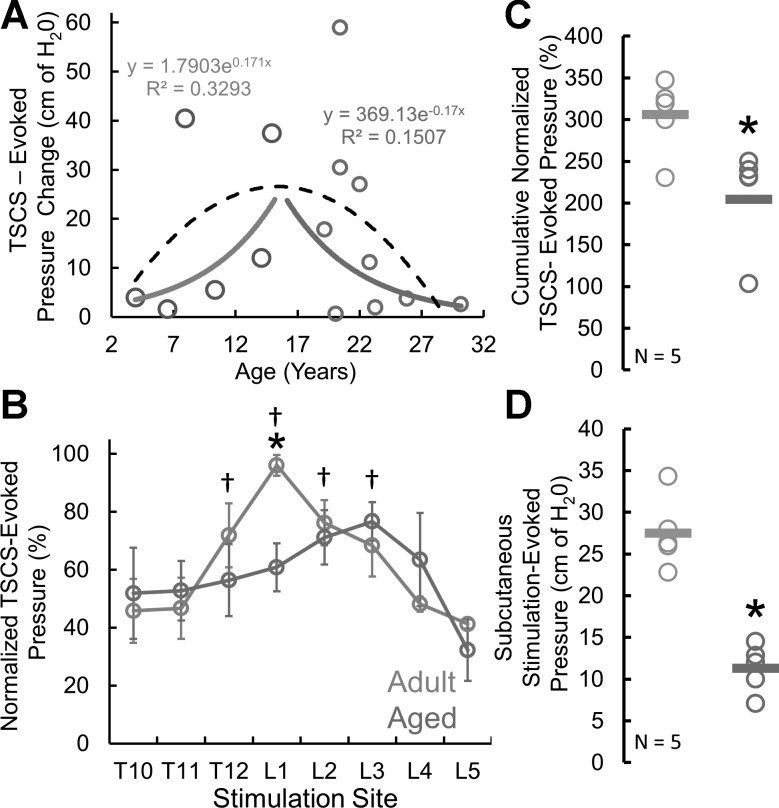
To determine the effect of age on detrusor contractility, we calculated the amplitude of the TSCS-evoked detrusor pressure change by TSCS site and duration for all subjects (n = 15). The TSCS-evoked pressure changes were compared against subject age. The adult and aged groups were studied both as a combined group and separately. When regarded as a single group of subjects, the TSCS-evoked pressure change increased with age until the midteens, followed by a gradual decline in the response (Fig. 3A). When the animals were divided into adult (<18 yr) and aged (>18 yr) groups, both linear and exponential regression analysis showed a positive regression for the adult animals and a negative regression for the aged subjects (Fig. 3A). The detrusor contractility of adult and aged rhesus macaques was also studied across the T10–L5 stimulation sites (Fig. 3B). The bladder pressure response to TSCS at the L1 site was significantly higher for adult compared with aged subjects (df = 13, t = 3.2876, P = 0.0059). The site for maximal response was at the L1 site for the adult subjects and more caudally located at L3 for the aged subjects. Two-way mixed (split) ANOVA analysis identified multiple differences across TSCS sites in the adult group but no differences between sites in the aged animals (df = 7, F = 2.22, P = 0.0405). In addition, the cumulative normalized TSCS-evoked pressures were calculated for five consecutive stimulation sites between the T11 and L3 levels and showed a significantly lower pressure response for aged compared with adult subjects (df = 8, t = 2.5626, P = 0.0335) (Fig. 3C). To identify the potential impact of skin resistance, subcutaneous needles were used as stimulation electrodes at L1 to evoke detrusor contractions. Subcutaneous stimulation-evoked pressures at L1 showed significantly lower responses in the aged group compared with adults (df = 8, t = 5.6318, P = 0.0005) (Fig. 3D).
Fig. 3.
Effects of aging on detrusor contractility. A: plot of TSCS-evoked pressure change at the L1 site of stimulation in each subject across all ages: adults (light gray) and aged (dark gray). Note an initial increase in TSCS-evoked pressure change with age and a peak effect in the midteens followed by a gradual decline in TSCS-evoked pressure change with aging. Separate exponential regression curves for the adult and aged cohorts show a positive regression for the adult and a negative regression for the aged subjects. However, a combined regression across all ages show a binomial distribution (black trace). B: normalized TSCS-evoked pressures at the T10–L5 site. *Significantly larger response at L1 for adult vs. aged animals (P < 0.05), as well as a caudal change for the site of maximum response in the aged cohort. †Significant differences across sites for the adults [2-way mixed (split) ANOVA]. Note that no differences were identified between sites of the aged subjects. C: cumulative normalized TSCS-evoked pressure was calculated for 5 consecutive stimulation sites, T11–L3. *Significantly lower evoked response for aged vs. adult subjects (P < 0.05). Each circle represents a subject, with the bold bar representing the mean response (n = 5). D: subcutaneous stimulation-evoked pressures at L1. *Significantly lower response in aged vs. adults (P < 0.05). Each circle represents a subject, with the bold bar representing the mean response (n = 5).
DISCUSSION
TSCS was applied over the thoracolumbar spine to record pressure and EMG responses from various components of the LUT in female rhesus macaques. Distinct functional maps were demonstrated for TSCS-evoked detrusor and urethral pressures as well as for the activation of the external sphincters and pelvic floor muscles. The magnitude of the responses for each peripheral target organ was dependent on TSCS location and strength. Comparisons of TSCS-induced bladder contractions between adult and aged subjects showed decreased detrusor contractility in the aged cohort and a caudal shift of the stimulation site that generated the strongest detrusor contraction in aged rhesus macaques. In humans, impaired bladder contractility is common during aging, can be diagnosed by urodynamic studies, and includes cystometrogram demonstration of bladder contractions with reduced strength and/or duration, impaired flow during voiding, and incomplete bladder emptying (Aldamanhori and Chapple 2017; Miyazato et al. 2013).
Anatomical considerations.
TSCS applied at different thoracolumbar interspinous processes provides electrical stimulation of the lumbar spinal cord and conus medullaris. However, there is a normal anatomical variability between subjects for the caudal extent of the conus medullaris within the spinal canal of primates. In humans, magnetic resonance imaging (MRI) studies have shown that the location for the tip of the conus may range from the T11/T12 to L2/L3 vertebrae levels (Morimoto et al. 2013; Wilson and Prince 1989). In rhesus macaques, recent MRI studies have shown that the conus medullaris tapers over a length of 1.5 ± 0.3 vertebral units, with the top and tip of the conus medullaris at 2.0 ± 0.3 and 3.6 ± 0.4 vertebral units from the thoracolumbar junction, respectively (Ohlsson et al. 2017). This anatomical feature of the lumbosacral spinal cord provides a rationale for some of the observed intersubject variability related to the most optimal TSCS location for the activation of peripheral target responses. Because of this anatomical relationship between the lumbar spine and the caudal portion of the spinal cord in rhesus macaques, any TSCS rostral to the L2/L3 vertebral level is expected to have activated both spinal circuits and lumbosacral dorsal roots, whereas TSCS below the L3 vertebra activated spinal circuits by stimulation of the lumbosacral dorsal roots of the cauda equina.
Knowledge of the rostrocaudal distribution of autonomic and motor neurons in the lumbosacral spinal cord is a critical factor for the effectiveness of mapping and modulation of spinal cord networks controlling the LUT. Detrusor contractions are caused by parasympathetic projections from the conus medullaris, and the preganglionic parasympathetic neurons are located in the S1/S2 spinal cord segments in rhesus macaques (Nadelhaft et al. 1983). Somatic motor neurons of the Onuf’s nucleus homolog innervate the EUS and EAS and are located in the ventral horn of the L7 and S1 segments, and the levator ani muscle group of the pelvic floor is innervated by motor neurons in S1/S2 segments in rhesus macaques (Gross et al. 2017; Vanderhorst et al. 2000). The limited rostrocaudal distribution and extensive anatomical overlap for these neuronal populations facilitate the selective activation of the LUT and pelvic floor by TSCS.
Aging in rhesus macaques.
Rhesus macaques represent a useful model for studies of aging, as they show a high degree of genetic homology and physiology of, for instance, cardiovascular, immunological, and reproductive functions similar to humans (Didier et al. 2016; Rogers et al. 2006). Rhesus macaques and humans also demonstrate similar risk factors for many pathological processes and diseases (Simmons 2016).
Aging occurs approximately three times faster in rhesus macaques compared with humans, and the animals are commonly considered to be aged when ~20 yr old (Simmons 2016). In female macaques, onset of menarche varies but commonly occurs when the animals are 3–5 yr old and menopause typically occurs at 25–26 yr of age (Gilardi et al. 1997; Walker and Herndon 2008). In the present study, all subjects except for the oldest aged animal, which was postmenopausal, showed cyclical genital bleeding.
We showed that aging was associated with decreased detrusor contractility in female rhesus macaques. The findings were supported by both a decreased TSCS-evoked bladder contraction pressure change and cumulative TSCS-evoked pressure responses across multiple stimulation sites in aged compared with adult rhesus macaques. In addition, the stimulation site that generated maximal pressure change in the bladder for aged subjects was two vertebral levels more caudal compared with the corresponding site in adults. Decreased detrusor contractility is also an important feature in underactive bladder, a human condition associated with aging (Aldamanhori and Chapple 2017; Miyazato et al. 2013). However, the present studies do not differentiate between potential mechanisms for the observed differences. For instance, it is possible that both neurogenic mechanisms and intrinsic changes in the bladder wall may have contributed to the decreased detrusor contractility in the aged subjects. Future studies will determine the relative contribution of neurogenic vs. myogenic mechanisms to age-associated decreased detrusor contractility in primates.
TSCS as a diagnostic tool to identify neurophysiological biomarkers of aging.
A variety of electrical stimulation techniques have been proposed to induce or modulate a detrusor contraction, including direct electrical stimulation of the pelvic nerve (Holmquist and Olin 1968), pelvic plexus (Walter et al. 2005), and the sacral nerve (Granger et al. 2013). Percutaneous stimulation of the tibial nerve was effective in modulating voiding function in clinical studies of human subjects with an overactive bladder (Peters et al. 2010). In recent studies, epidural stimulation in both acute and chronic preparations has shown activation of spinal cord networks and modulation of reflex micturition after spinal cord injury in rats (Abud et al. 2015; Gad et al. 2014). However, all of the above approaches require a degree of surgical invasiveness to access peripheral nerves or the use of surgically implanted devices. In contrast, TSCS represents a noninvasive strategy and can access spinal cord networks involved with leg control and locomotion in humans (Gerasimenko et al. 2015; Sayenko et al. 2015). We also investigated the potential impact on evoked detrusor pressures by an age-related change in skin resistance. For this purpose, subcutaneous needle electrodes were used to evoke detrusor contractions in all subjects. Interestingly, similar patterns and significant differences in evoked detrusor pressures between adult and aged subjects were identified, suggesting that the observed differences were not due to age-related skin changes. Here, TSCS was shown to be able to activate the LUT and serve as a diagnostic tool for the identification of a decreased detrusor contractility in aged rhesus macaques.
For TSCS, the reference electrode is commonly placed over the abdominal wall in cats (Musienko et al. 2012) and humans (Sayenko et al. 2015), and the anode was similarly placed over the abdomen for the present investigation. It is not possible to exclude the presence of abdominal contractions as a result of TSCS, as stimulation of afferent fibers and spinal cord networks will activate both autonomic outflow from the spinal cord as well as efferent motor fibers. However, the applied TSCS resulted in a single-peak detrusor contraction and pressure change. There was an absence of any complex or delayed pressure peaks after TSCS. If conduction of electrical current had extended through soft tissues, outside of the spinal networks, complex or delayed pressure peaks would have been expected. In addition, there was an ~12-ms latency between TSCS at L1 and EUS EMG responses in rhesus macaques, a latency that is consistent with a spinal cord-induced response. Any abdominal contraction that would have resulted from the conduction of current through soft tissues would have been expected to show a longer latency. At stronger stimulus strengths, the latency remained the same but the EUS EMG amplitude increased without any widening of waveform. Because the cathode was placed over the spine and the anode over the belly, the stimulation was expected to primarily cause activation of the dorsal roots and spinal cord. This assumption was also validated by reversing the polarity of the electrodes in early studies, placing the cathode over the belly and the anode over the spine, and the reverse electrode arrangement resulted in markedly lower evoked EMG responses and pressure changes from detrusor activation. However, future studies will need to determine the most optimal electrode placement, including the best location for the reference electrode, for the collection of evoked responses without the potential confounding influence of accessory muscle activation.
A potential limitation for all forms of neuromodulation using electrical stimulation to activate the LUT is detrusor-sphincter dyssynergia (Gaunt and Prochazka 2006). The present study, using a single stimulation approach for diagnostic purposes, also shows coactivation of both the detrusor and EUS. However, when a single-pulse stimulation is changed to a tonic stimulation pattern, the spinal cord circuitry may respond by generating a physiological pattern of activation and inhibition of appropriate end organs, as has been suggested in prior studies on LUT function, including reflex micturition, after spinal cord injury in rats (Abud et al. 2015; Gad et al. 2014). In the present study of nonhuman primates the signature of neurophysiological mapping was investigated as a possible diagnostic tool, but functional studies of coordinated reflex micturition were not performed. Future studies will determine whether TSCS, using a tonic pattern of stimulation, may be used to overcome detrusor-sphincter dyssynergia and augment LUT function also in primates.
Conclusions.
TSCS demonstrated decreased detrusor contractility in the aged cohort compared with the adult control group. We show that decreased detrusor contractility in aged rhesus macaques shares features of detrusor underactivity in humans during aging. TSCS may be used as a noninvasive tool to identify changes in detrusor contractility as a neurophysiological biomarker of aging. Future studies will be needed to determine whether TSCS may also have utility as a therapeutic strategy to augment detrusor function in the setting of LUT impairments.
GRANTS
The studies were supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the National Institutes of Health (NIH) Office of the Director (P51 OD-011107), and an NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant (UL1 TR-001881).
DISCLOSURES
P. N. Gad and V. R. Edgerton hold shareholder interest in NeuroRecovery Technologies and hold certain inventorship rights on intellectual property licensed by The Regents of the University of California to NeuroRecovery Technologies and its subsidiaries.
AUTHOR CONTRIBUTIONS
P.N.G., V.R.E., and L.A.H. conceived and designed research; P.N.G., K.L.C., and L.A.H. performed experiments; P.N.G., N.K., and L.A.H. analyzed data; P.N.G., V.R.E., and L.A.H. interpreted results of experiments; P.N.G. and L.A.H. prepared figures; P.N.G. and L.A.H. drafted manuscript; P.N.G., N.K., K.L.C., V.R.E., and L.A.H. edited and revised manuscript; P.N.G., N.K., K.L.C., V.R.E., and L.A.H. approved final version of manuscript.
REFERENCES
- Abud EM, Ichiyama RM, Havton LA, Chang HH. Spinal stimulation of the upper lumbar spinal cord modulates urethral sphincter activity in rats after spinal cord injury. Am J Physiol Renal Physiol 308: F1032–F1040, 2015. doi: 10.1152/ajprenal.00573.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldamanhori R, Chapple CR. Underactive bladder, detrusor underactivity, definition, symptoms, epidemiology, etiopathogenesis, and risk factors. Curr Opin Urol 27: 293–299, 2017. doi: 10.1097/MOU.0000000000000381. [DOI] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12: 1333–1342, 2009. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Anatomy and physiology of the lower urinary tract. Handb Clin Neurol 130: 61–108, 2015. doi: 10.1016/B978-0-444-63247-0.00005-5. [DOI] [PubMed] [Google Scholar]
- Didier ES, MacLean AG, Mohan M, Didier PJ, Lackner AA, Kuroda MJ. Contributions of nonhuman primates to research on aging. Vet Pathol 53: 277–290, 2016. doi: 10.1177/0300985815622974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad PN, Roy RR, Zhong H, Lu DC, Gerasimenko YP, Edgerton VR. Initiation of bladder voiding with epidural stimulation in paralyzed, step trained rats. PLoS One 9: e108184, 2014. doi: 10.1371/journal.pone.0108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt RA, Prochazka A. Control of urinary bladder function with devices: successes and failures. Prog Brain Res 152: 163–194, 2006. doi: 10.1016/S0079-6123(05)52011-9. [DOI] [PubMed] [Google Scholar]
- Gerasimenko Y, Gorodnichev R, Puhov A, Moshonkina T, Savochin A, Selionov V, Roy RR, Lu DC, Edgerton VR. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J Neurophysiol 113: 834–842, 2015. doi: 10.1152/jn.00609.2014. [DOI] [PubMed] [Google Scholar]
- Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod 57: 335–340, 1997. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Granger N, Chew D, Fairhurst P, Fawcett JW, Lacour SP, Craggs M, Mosse CA, Donaldson N, Jeffery ND. Use of an implanted sacral nerve stimulator to restore urine voiding in chronically paraplegic dogs. J Vet Intern Med 27: 99–105, 2013. doi: 10.1111/jvim.12011. [DOI] [PubMed] [Google Scholar]
- Gross C, Ellison B, Buchman AS, Terasawa E, VanderHorst VG. A novel approach for assigning levels to monkey and human lumbosacral spinal cord based on ventral horn morphology. PLoS One 12: e0177243, 2017. doi: 10.1371/journal.pone.0177243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377: 1938–1947, 2011. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist B, Olin T. Electromicturition in female dogs at pelvic nerve stimulation. An urethrocystographic study. Scand J Urol Nephrol 2: 129–135, 1968. doi: 10.3109/00365596809136982. [DOI] [PubMed] [Google Scholar]
- Miyazato M, Yoshimura N, Chancellor MB. The other bladder syndrome: underactive bladder. Rev Urol 15: 11–22, 2013. [PMC free article] [PubMed] [Google Scholar]
- Morimoto T, Sonohata M, Kitajima M, Mawatari M, Konishi H, Otani K, Kikuchi S. The termination level of the conus medullaris and lumbosacral transitional vertebrae. J Orthop Sci 18: 878–884, 2013. doi: 10.1007/s00776-013-0461-7. [DOI] [PubMed] [Google Scholar]
- Musienko P, Courtine G, Tibbs JE, Kilimnik V, Savochin A, Garfinkel A, Roy RR, Edgerton VR, Gerasimenko Y. Somatosensory control of balance during locomotion in decerebrated cat. J Neurophysiol 107: 2072–2082, 2012. doi: 10.1152/jn.00730.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadelhaft I, Roppolo J, Morgan C, de Groat WC. Parasympathetic preganglionic neurons and visceral primary afferents in monkey sacral spinal cord revealed following application of horseradish peroxidase to pelvic nerve. J Comp Neurol 216: 36–52, 1983. doi: 10.1002/cne.902160105. [DOI] [PubMed] [Google Scholar]
- Ohlsson M, Nieto JH, Christe KL, Villablanca JP, Havton LA. Radiographic and magnetic resonance imaging identification of thoracolumbar spine variants with implications for the positioning of the conus medullaris in rhesus macaques. Anat Rec (Hoboken) 300: 300–308, 2017. doi: 10.1002/ar.23495. [DOI] [PubMed] [Google Scholar]
- Peters KM, Carrico DJ, Perez-Marrero RA, Khan AU, Wooldridge LS, Davis GL, Macdiarmid SA. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 183: 1438–1443, 2010. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Rogers J, Garcia R, Shelledy W, Kaplan J, Arya A, Johnson Z, Bergstrom M, Novakowski L, Nair P, Vinson A, Newman D, Heckman G, Cameron J. An initial genetic linkage map of the rhesus macaque (Macaca mulatta) genome using human microsatellite loci. Genomics 87: 30–38, 2006. doi: 10.1016/j.ygeno.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Sayenko DG, Atkinson DA, Dy CJ, Gurley KM, Smith VL, Angeli C, Harkema SJ, Edgerton VR, Gerasimenko YP. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J Appl Physiol (1985) 118: 1364–1374, 2015. doi: 10.1152/japplphysiol.01128.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefchyk SJ. Sacral spinal interneurones and the control of urinary bladder and urethral striated sphincter muscle function. J Physiol 533: 57–63, 2001. doi: 10.1111/j.1469-7793.2001.0057b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons HA. Age-associated pathology in rhesus macaques (Macaca mulatta). Vet Pathol 53: 399–416, 2016. doi: 10.1177/0300985815620628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers L, Clingerman KJ, Yang X. Validation of a body condition scoring system in rhesus macaques (Macaca mulatta): assessment of body composition by using dual-energy X-ray absorptiometry. J Am Assoc Lab Anim Sci 51: 88–93, 2012. [PMC free article] [PubMed] [Google Scholar]
- Vanderhorst VG, Terasawa E, Ralston HJ 3rd, Holstege G. Monosynaptic projections from the nucleus retroambiguus to motoneurons supplying the abdominal wall, axial, hindlimb, and pelvic floor muscles in the female rhesus monkey. J Comp Neurol 424: 233–250, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Walker ML, Herndon JG. Menopause in nonhuman primates? Biol Reprod 79: 398–406, 2008. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JS, Wheeler JS, Fitzgerald MP, McDonnell A, Wurster RD. A spinal cord injured animal model of lower urinary tract function: observations using direct bladder and pelvic plexus stimulation with model microstimulators. J Spinal Cord Med 28: 246–254, 2005. [PubMed] [Google Scholar]
- Wilson DA, Prince JR. John Caffey award. MR imaging determination of the location of the normal conus medullaris throughout childhood. AJR Am J Roentgenol 152: 1029–1032, 1989. doi: 10.2214/ajr.152.5.1029. [DOI] [PubMed] [Google Scholar]