Abstract
Nonalcoholic fatty liver disease (NAFLD) is a common disease, closely associated with obesity and insulin resistance. We investigated the presence of a subset of myeloid cells associated with metabolic disturbance in the liver of patients with NAFLD and a murine model of obesity-induced liver disease. Gene and protein expression in liver and serum was investigated with RT-PCR or ELISA and correlated to clinical disease. Liver-infiltrating immune cells were isolated from normal or diseased human liver for flow cytometric analysis. In animal experiments, mice were fed a high-fat diet (60% of calories from fat) for 16 wk, or high-fat diet with 30% fructose for 32 wk to induce steatohepatitis and fibrosis. A small molecule inhibitor of CC chemokine receptor 2 (CCR2), CCX872, was administered to some mice. A subset of CD11c+CD206+ immune cells was enriched in human liver tissue, and greater infiltration was observed in NAFLD. The presence of CD11c+CD206+ myeloid cells correlated with systemic insulin resistance. CD11c+CD206+ cells expressed high levels of CCR2, and liver CC chemokine ligand 2 (CCL2) expression was increased in nonalcoholic steatohepatitis and correlated with disease activity. In mice, CCR2 inhibition reduced infiltration of liver CD11b+CD11c+F4/80+ monocytes, which are functional homologs of human CD11c+CD206+ cells, and improved liver injury and glycemic control. A role for CCR2/CCL2 in human NAFLD has long been postulated. These data confirm a role for this chemokine/receptor axis, through mediating adipose and hepatic infiltration of myeloid cells. Inhibition of CCR2 improved hepatic inflammation and fibrosis in murine models of NAFLD. These data confirm the rationale for targeting CCR2 to treat NAFLD.
NEW & NOTEWORTHY These data show for the first time that CD11c+CD206+ myeloid cells, previously associated with human adipose tissue inflammation, infiltrate into liver tissue in nonalcoholic fatty liver disease. These cells express CCR2. Inhibition of CCR2 in mice inhibits hepatic inflammation caused by a murine homolog of these myeloid cells and improves experimental liver disease.
Keywords: immunology, insulin resistance, nonalcoholic fatty liver disease, obesity
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) covers a spectrum of liver pathology from hepatic steatosis (nonalcoholic fatty liver) through the more severe nonalcoholic steatohepatitis (NASH) to cirrhosis (31). NAFLD is present in up to one-third of individuals (6) and is associated with the metabolic syndrome, particularly obesity (31) and insulin resistance (3). NAFLD is becoming the commonest indication for liver transplantation in the US (32), reflecting both the prevalence of the disease and the present lack of effective therapies for advanced disease (25).
There is increasing interest in the role of the innate immune system in obesity and the metabolic syndrome. Myeloid cells infiltrate adipose and liver tissue in patients with NAFLD and contribute to insulin resistance and inflammation. In particular, CD11c+CD206+ monocytes in human adipose tissue are associated with adipocyte necrosis, inflammation, and insulin resistance (30). In mice, a functionally similar subset defined by CD11b+CD11c+F4/80+ contribute to adipose inflammation and systemic insulin resistance (22, 26). CC chemokine receptor 2 (CCR2) mediates obesity-associated macrophage infiltration of adipose and hepatic tissue (19, 29). Mouse experiments have demonstrated that obesity increases hepatic expression of CC chemokine ligand 2 (CCL2) (13, 14, 23, 29), and CD11b+CD11c+F4/80+ express CCR2 (26). Inhibition of the CCR2/CCL2 axis reduces disease activity in mice (4, 8, 21, 27, 33). The CCR2/CCL2 axis in human NAFLD is less well defined, although increased circulating levels of CCL2 are observed (12, 14). We investigated the inflammatory infiltrate in human NAFLD and murine models of obesity-induced liver disease to determine whether functionally important subsets of CCR2+ inflammatory cells are involved in the metabolic dysfunction that characterizes NAFLD.
MATERIALS AND METHODS
Human Tissue
Human tissue and blood were collected from patients with liver disease or healthy controls at University Hospitals Birmingham National Health Service Foundation Trust with full informed consent and research ethics committee approval (REC reference 06/Q2708/11). Liver tissue was obtained from patients undergoing hepatic resection for benign or malignant disease, or liver transplantation for chronic liver disease. In the case of hepatic resection, liver tissue distal to resected lesions was used for analysis. No patient had undergone chemotherapy in the 2 wk before surgery. Liver tissue was placed in formalin or snap frozen before subsequent analysis. Characteristics of these groups and of patients undergoing resection or transplantation as a source of liver tissue are detailed in Table 1. Serum from patients with NAFLD was obtained from two cohorts of patients taking part in the LEAN (liraglutide efficacy and action in nonalcoholic steatohepatitis) and NOBLES (Non Invasive Biomarkers of Liver Fibrosis) studies. LEAN is a randomized controlled trial of liraglutide in patients with NAFLD (2). The serum samples used were taken before randomization. NOBLES is an observational study of biomarkers in patients with liver disease. Finally, a group of healthy volunteers without liver disease donated blood for analysis and served as a control group in enzyme linked immunosorbent assays (ELISA) experiments. This group was drawn from laboratory colleagues and gave consent for their samples to be used for research.
Table 1.
Characteristics of cohorts used for human studies
| Age, yr | BMI, kg/m2 | ALT, IU/l | Diabetes, %Prevalence | |
|---|---|---|---|---|
| Serum | ||||
| NOBLES | 55.1 | 33.9 | 56.1 | 58 |
| Lean | 51.0 | 36.0 | 71.5 | 33 |
| Liver tissue | ||||
| NASH cirrhosis | 56.9 | 32.7 | 37.5 | 88 |
| Non-NASH cirrhosis | 55.3 | 29.3 | 34.1 | 14 |
| Normal | 57.5 | 27.3 | 20.0 | 0 |
BMI, body mass index.
ELISA
Analysis of human serum was performed using commercially available ELISA kits. Serum CCL2 concentration was measured using R&D Systems Quantikine kits (Minneapolis, MN; catalog no. PDCP00), performed according to the manufacturer’s instructions. Recombinant human chemokines were used as a positive control (Peprotech). Samples were diluted in sample buffer 1:4 and run in duplicate. A standard curve was generated from known concentrations of recombinant chemokine and experimental values interpolated from this curve.
Polymerase Chain Reaction
RNA was isolated by homogenizing liver tissue in Trizol (Life Technologies). Chloroform was added, and samples were centrifuged at top speed in a microfuge for 15 min. The upper aqueous layer was removed, isopropanol was added, and samples were centrifuged at 12,000 rpm for 15 min. The resulting RNA pellet was washed in 70% ethanol and resuspended in nuclease-free water. Purity and concentration of total RNA was determined spectrophotometrically. cDNA was prepared from RNA using Taqman reagents (Life Technologies), according to the manufacturer’s instructions. Briefly, 2 μl of RNA were combined with random hexamers, reverse transcriptase, RNase inhibitor, magnesium chloride, and a buffer solution. This mixture was heated to 25°C for 10 min, 37°C for 30 min, 95°C for 5 min, and then cooled to 4°C. Probe/primer mixes for genes of interest and appropriate controls were obtained from Taqman (Life Technologies) and made up with Taqman reagents. A 96-well plate was used for reactions, with wells containing cDNA, primer/probe mix (CCL2 primer/probe mix catalog no. Hs00234140_m1; 18S mix catalog no. Hs03003631_g1) and Taqman Master Mix. Three replicates were used for both the gene of interest and housekeeping gene. 18S has been shown to have the lowest level of variability across stages of alcoholic liver disease, suggesting it is reliable as a housekeeping gene in steatohepatitis (5). PCR experiments were performed using a Roche Lightcycler 480 machine. A single quantification measurement was taken during each cycle.
Isolation of Leukocytes
Isolation of leukocytes from human liver or blood.
Mononuclear cells were isolated from blood or liver. Liver was washed with phosphate-buffered saline (PBS) to remove blood and digested nonenzymatically using GentleMACS (Miltenyi). The resulting homogenate was passed through a sterile 70-μm mesh. The homogenate was then washed in PBS until a clear supernatant was achieved. Liver homogenate or whole blood was layered over a density gradient (Lypmhoprep, CedarLane) to isolate mononuclear cells, which were aspirated from the interface and washed in PBS three times before further analysis.
Isolation of leukocytes from murine liver tissue.
Mice were killed by CO2 inhalation and cervical dislocation. Blood samples were taken by left ventricular puncture. PBS was gently infused into the left ventricle to flush end organs of blood before harvesting. The liver were removed, immediately divided, and placed into RPMI, formalin, or snap frozen in liquid nitrogen. To isolate leukocytes, a segment of liver was coarsely chopped with scissors before mechanical dissociated by gently passing homogenate through a 75-μm sieve. The resulting homogenate was washed in PBS until a clear supernatant was achieved. For analysis of mouse liver, whole homogenate was incubated with fluorescently tagged antibodies as described below, and CD45 was used to identify leukocytes.
Flow Cytometry Analysis of Leukocytes
Isolated cells were suspended in 100 μl at 1 × 106 cells/ml in MACS buffer (PBS containing 2% fetal calf serum and 1 mM EDTA) and incubated with antibodies. After incubation for 20 min at room temperature, cells were washed, resuspended in PBS, and analyzed by flow cytometry using a Beckman Coulter Cyan. Cells stained with single colors were analyzed for compensation, and appropriate isotype controls were used to define the negative populations.
Animal Experiments
Mouse experiments were performed at ChemoCentryx Inc., Mountain View, CA. C57/Bl6 mice were purchased from Charles River Laboratories and were housed in the research locations for at least 3 days before investigations were started. Animals were housed according to local and national standards. Animal housing was maintained at 23°C with 12:12-h light-dark cycles. Male C57/Black 6 (C57/Bl6) mice bred in controlled clean conditions were used for all experiments and were aged 6–8 wk at the start of experiments.
Two animal models were used: high-fat diet (HFD) to induce steatohepatitis, or HFD in combination with 30% fructose in drinking water to induce steatohepatitis with fibrosis. The HFD provided 60% of calories from fat (by overall weight, this is provided 31% by lard and 3% by soybean oil). HFD and control diet were obtained from Teklad. HFD was administered for 16 wk; HFD plus fructose was administered for 32 wk. In each case, a control group of littermates was fed a control diet (10% of calories from fat) with normal drinking water for the duration of the experiment. At the end of experiments, mice were killed by CO2 inhalation.
Chemokine receptor antagonism.
A small molecule inhibitor of CCR2 (CCX872) was manufactured by ChemoCentryx (Mountain View, CA). CCX872 was dissolved in 1% hydroxypropyl methylcellulose (HPMC) and administered to mice by subcutaneous injection at a dose of 30 mg/kg daily. An equivalent volume of 1% HPMC was given in control experiments. A maximum volume of 350 μl was used.
Triglyceride content of murine liver tissue.
Triglyceride content of murine liver tissue was assessed using a commercially available colorimetric assay kit (Cayman Chemical, Ann Arbor, MI), according to the manufacturer’s instructions. In short, 400 mg of liver tissue were suspended in 2 ml of assay diluent and homogenized. Ten microliters of homogenate were added to wells of a 96-well plate, and each sample was assayed in triplicate. Triglycerides were enzymatically hydrolyzed to free fatty acids and glycerol using the supplied enzyme mixture. After 15-min incubation, color change was measured with a plate reader (Synergy HT, BioTek) by measuring absorbance at 540 nm. A standard curve was generated by assaying known concentrations of triglyceride, and the triglyceride concentration of samples was interpolated from this curve and expressed as milligrams per gram of liver tissue.
Fibrosis content of liver tissue.
Entire lobes of mouse livers were immersed in formalin immediately after harvesting and subsequently embedded in paraffin. Sections of 10-μm thickness were stained with Sirius red (Sigma Aldrich) to detect collagen deposition. Briefly, sections were dewaxed and stained with hematoxylin before being stained with Sirius red for 1 h. Sections were then dehydrated and mounted. Fibrosis was quantified by calculating percent area of collagen deposition using ImageJ software (National Institutes of Health; version 1.48). Two Sirius red-stained slides per animal were taken at different depth, with 18 images taken randomly per slide for a total of 36 images per animal for collagen quantification. All pathological evaluations were made by a pathologist on a random and blinded basis.
Glycemic Control
Glucose metabolism in mice was assessed with insulin and glucose challenge experiments. Insulin challenge was performed by administering 0.75 U/kg of insulin (Sigma Aldrich) to nonfasted mice via intraperitoneal injection. Plasma glucose was measured with an AccuCheck glucometer (Roche, Basel, Switzerland) using a drop of blood from a tail vein. Plasma glucose was measured at baseline and 15, 30, 60, 90, and 120 min following administration of insulin. Mice were fasted overnight before glucose tolerance tests. Glucose (Sigma Aldrich) was administered at 2 g/kg of glucose (as 45% glucose solution), given by gastric lavage. Plasma glucose was measured at baseline and 15, 30, 60, 90, and 120 min after administration of glucose using an AccuCheck glucometer and drops of blood from tail vein.
Statistical Analysis
Data are expressed as means ± SE for normally expressed data, and median and interquartile range (IQR) for skewed data. Normality was assessed with the Kolmogorov-Smirnov test. Normally distributed data were compared between groups with Student’s t-test, and the Mann-Whitney test was used for skewed data. Variance across multiple groups, for example, over a range of concentrations, was analyzed with one-way ANOVA. Survival analysis was analyzed by Kaplan-Meier curves with P values assessed with log-rank test. Median time to death in animals that died was also calculated. All authors had access to the study data and reviewed and approved the final manuscript. Data were analyzed using Prism version 5.
RESULTS
CD14+CD11c+CD206+ Monocytes Are Enriched in NAFLD Liver Tissue
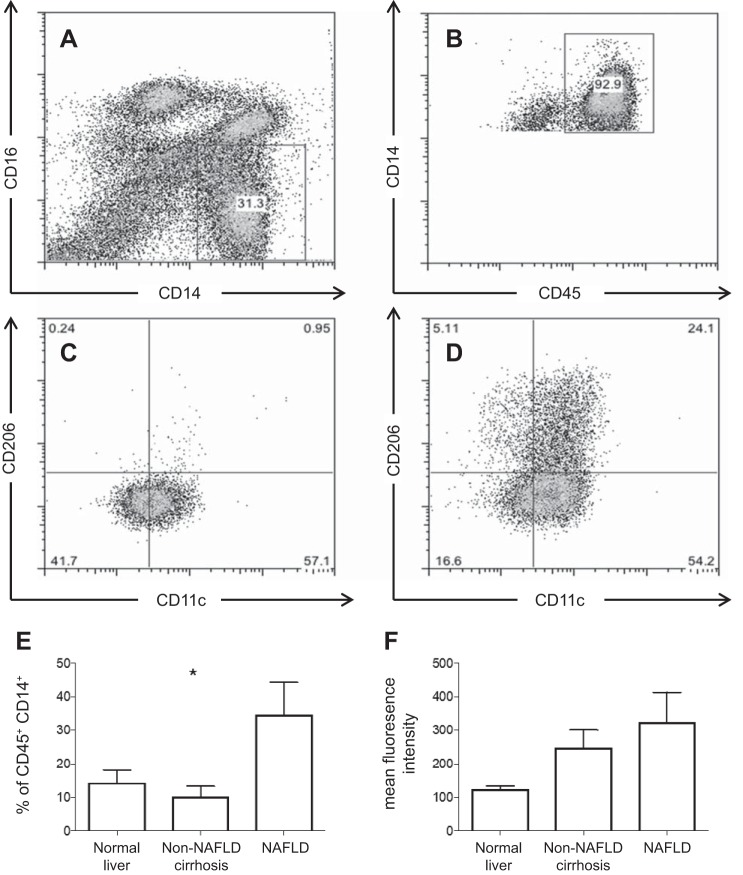
Immune cells that express CD14+CD11c+CD206+ have been detected in human adipose tissue and associated with insulin resistance (30). We examined the presence of these cells in human blood and liver. Liver tissue from patients with NAFLD (n = 8), other liver disease (alcoholic liver disease, n = 4; primary sclerosing cholangitis, n = 3; primary biliary cholangitis, n = 2; cryptogenic cirrhosis, n = 1; hemochromatosis, n = 1) or without liver disease (n = 5) was analyzed. Very few CD14+CD11c+CD206+ were observed in peripheral blood, whereas these cells were enriched in liver tissue (Fig. 1). The frequency of intrahepatic CD11c+CD206+ monocytes, as a percentage of CD45+CD14+ cells, differed significantly between types of liver disease (Kruskal-Wallis, P = 0.023), with highest frequency of cells seen in NAFLD (Fig. 1E). Mean fluorescence intensity of CD11c and CD206 showed a tendency to be greater in NAFLD, although this did not reach statistical significant (Kruskal-Wallis, P = 0.056) (Fig. 1F). No differences in expression of CD11c and CD206 were seen between noncirrhotic and cirrhotic liver tissue (data not shown).
Fig. 1.
CD11c+CD206+ monocytes are enriched in liver tissue. A and B: gating strategy to identify CD45+CD14+ monocytes. Representative samples of peripheral blood (C) and liver infiltrating monocytes (D) from the same individual (E) are shown. Liver tissue from patients with NAFLD (n = 8) showed a greater proportion of CD11c+CD206+ monocytes as a proportion of CD45+CD14+ monocytes, compared with other chronic liver disease (alcoholic liver disease, n = 4; primary sclerosing cholangitis, n = 3; primary biliary cholangitis, n = 2; hemochromatosis, n = 1; cryptogenic cirrhosis, n = 1) or normal liver (n = 5). *P < 0.05 by Kruskal-Wallis. F: mean fluorescence intensity of CD11c+CD206+ cells by liver disease (Kruskal-Wallis, P = 0.056).
CD14+CD11c+CD206+ Monocytes Are Associated with Insulin Resistance and Express CCR2 in NAFLD
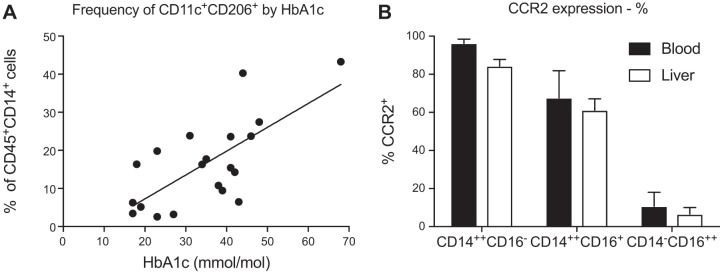
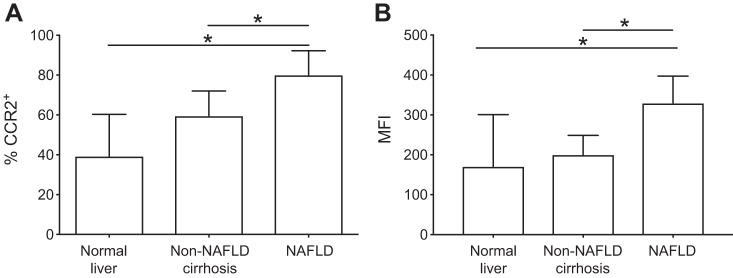
A correlation between the proportion of intrahepatic CD14+CD11c+CD206+ monocytes and glycosylated hemoglobin (HbA1c) was observed in liver infiltrating monocytes isolated from patients with chronic liver disease (r2 = 0.499, P = 0.0005) (Fig. 2A). No significant correlation was observed with age, body mass index or alanine aminotransferase (ALT) (Table 2). In both blood and liver, CCR2 expression was largely restricted to CD14+ monocytes, particularly the classical CD14++CD16− subset (Fig. 2B). The overall frequency of CCR2+ cells in blood or liver tissue did not vary significantly by etiology of liver disease (one-way ANOVA, P = 0.236). However, CCR2+ expression on CD14+CD11c+CD206+ monocytes was higher in NAFLD compared with normal liver tissue or non-NAFLD liver disease in terms of the percentage of CD14+CD11c+CD206+ cells that expressed CCR2 (Fig. 3A) and the mean fluorescence intensity of CCR2 (Fig. 3B).
Fig. 2.
Monocytes were isolated from liver tissue from patients with or without NAFLD and analyzed by flow cytometry. A: the frequency of CD11c+CD206+ monocytes in liver tissue correlated with insulin resistance, measured by HbA1c. n = 24; r2 = 0.499. B: CCR2 percent expression was greater on CD14++CD16− monocytes with a nonsignificant reduction of CCR2 expression on all intrahepatic monocytes.
Table 2.
Correlation of frequency of intrahepatic CD11c+CD206+ monocytes with clinical parameters
| Correlation with Intrahepatic CD11c+CD206+ Monocytes (as %CD14+), r2 | P Value | |
|---|---|---|
| HbA1c | 0.50 | <0.001 |
| ALT | 0.02 | 0.551 |
| BMI | 0.04 | 0.388 |
| Age | 0.16 | 0.084 |
Fig. 3.
Monocytes were isolated from liver tissue and analyzed by flow cytometry. CCR2 expression was higher on CD11c+CD206+ monocytes isolated from NAFLD liver tissue (n = 8) compared with non-NAFLD cirrhosis (n = 11) or normal liver tissue (n = 5) with regard to percentage of CCR2+ cells (normal, median 39.4%, IQR 40.1; non-NAFLD cirrhosis 59.7%, IQR 24.9; NAFLD 80.1%, IQR 24.7; A) and mean fluorescent intensity (normal 171, IQR 163.7; non-NAFLD cirrhosis 200.6, IQR 80.1; NAFLD 3,299, IQR 144.4; B). Data are shown as median and IQR; n = 23 in each case. *P < 0.05 by Mann-Whitney test.
CCL2 Is Upregulated in NAFLD
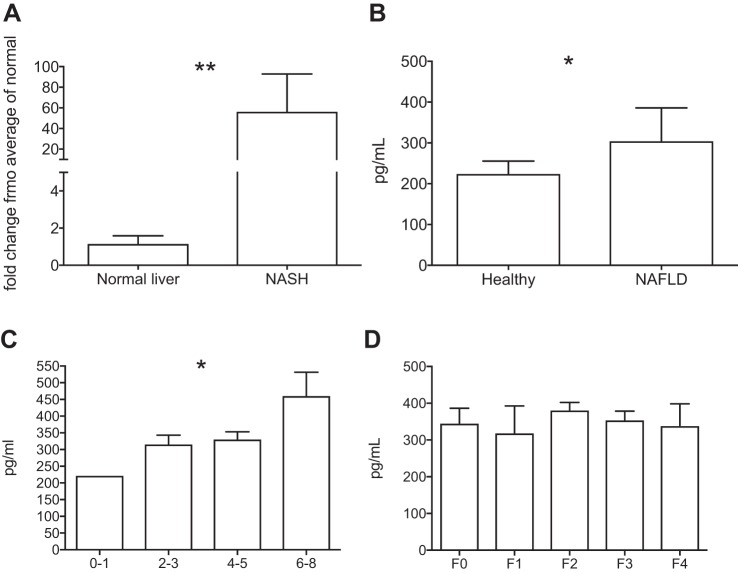
CCL2 gene expression in liver tissue was analyzed by quantitative real-time PCR using 18S as a housekeeping gene. CCL2 gene expression was significantly upregulated in liver tissue from patients with NAFLD undergoing transplantation (Mann-Whitney test, P = 0.009) (Fig. 4A). The concentration of CCL2 was measured by ELISA in serum of individuals with biopsy-proven NAFLD (n = 20) or healthy volunteers (n = 10). Serum CCL2 concentration was significantly higher in patients with NAFLD compared with healthy volunteers [median 305.1 pg/ml (IQR 211.8–385.7) vs. 224.7 (105.2–255.4), Mann-Whitney test, P = 0.021] (Fig. 4B). NAFLD was assessed histologically by independent pathologists using the NAFLD activity score (NAS) proposed by Kleiner et al. (15). Serum CCL2 concentration was higher in individuals with more severe histological inflammation [assessed with the NAS (15)] (one-way ANOVA, P = 0.025), but levels did not correlate with fibrosis stage (one-way ANOVA, P = 0.347) (Fig. 4, C and D). When individual components of the NAS were considered, serum CCL2 concentration was associated with higher lobular inflammation score (one-way ANOVA, P = 0.043), but not with steatosis or hepatocyte ballooning, consistent with the known role of CCL2 as a monocyte chemoattractant.
Fig. 4.
A: RNA was isolated from liver tissue, and CCL2 gene expression was analyzed by semiquantitative PCR. CCL2 gene expression was significantly increased in liver tissue from patients with NASH (n = 6) compared with normal liver tissue (n = 6) (Mann-Whitney test, **P < 0.01). B: serum concentration of CCL2 measured by ELISA was higher in NAFLD (n = 20) compared with healthy volunteers (n = 10) (Mann-Whitney test, *P < 0.05). C: serum concentration of CCL2 increased with increasing disease activity as measured by the NAS score (one-way ANOVA, *P < 0.05). D: no relation was seen with fibrosis stage (one-way ANOVA, P > 0.05).
Inhibition of CCR2 Reduces Accumulation of F4/80+CD11c+ Monocytes in Murine Steatohepatitis
CD11b+CD11c+F4/80+ monocytes are found in adipose tissue in experimentally induced obesity in mice and are functionally similar to CD11c+CD206+ monocytes in humans. To investigate the effect of CCR2 inhibition in obesity-induced steatohepatitis, 26 male C57/Bl6 mice were given HFD with 60% of calories from fat for 16 wk. After 8 wk of HFD, the mice were divided into two groups: 13 were treated daily with CCX872 (30 mg·kg−1·day−1, administered by subcutaneous injection), and 13 received an equivalent volume of vehicle (1% HPMC). A further eight littermates were given the control diet for the duration of the experiment.
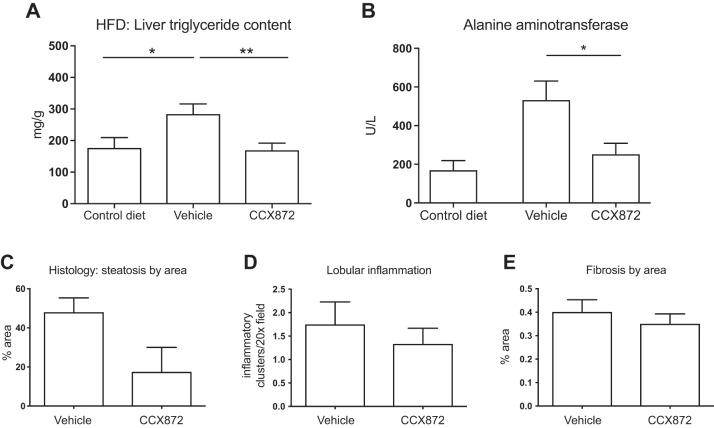
Steatosis, assessed by measuring triglyceride content of liver tissue, was markedly increased after 16 wk of HFD. Mice treated with CCX872 had significantly less triglyceride accumulation in comparison with vehicle-treated mice (169.6 ± 21.20 vs. 284.2 ± 31.9 mg/g, Student’s t-test, P = 0.007), with levels reduced to those seen in animals receiving a control diet (Fig. 5A). Serum ALT was significantly lower in CCX872-treated mice (mean ALT: 252.5 ± 56.02 vs. 532.8 ± 98.07 IU/ml, Student’s t-test, P = 0.028) (Fig. 5B). The reduction in hepatic steatosis was confirmed histologically (Fig. 5C), but histological features of inflammation and fibrosis did not differ between groups (Fig. 5, D and E).
Fig. 5.
Improvements in steatohepatitis with inhibition of CCR2. Thirteen animals in each group were given HFD with daily administration of vehicle or CCX872, and a further eight animals were given a control diet for 16 wk. A: triglyceride content was measured with a colorimetric assay. CCR2 inhibition reduced triglyceride accumulation (*P < 0.05, **P < 0.01 by Student’s t-test). B: CCR2 inhibition reduced serum ALT (*P < 0.05 by Student’s t-test). Histological assessment of liver disease confirmed reduced steatosis, as assessed by area of staining (C), but there were no differences in histological inflammation (D) or histological fibrosis (E).
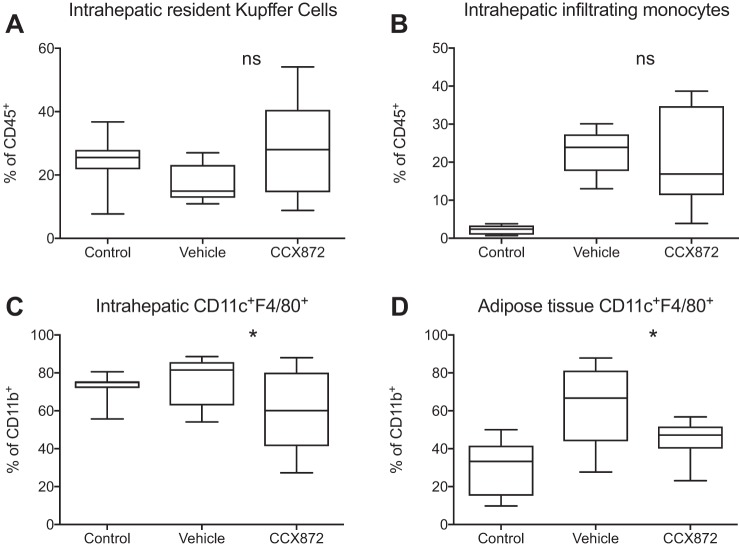
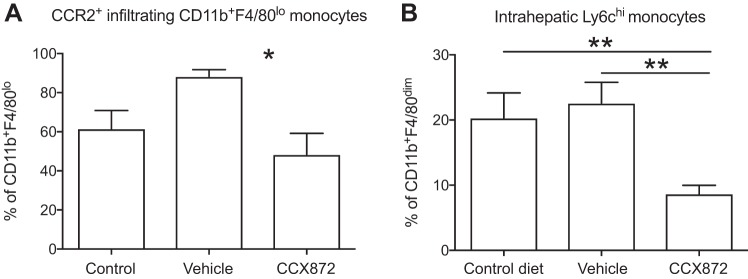
Flow cytometric analysis of isolated liver-infiltrating immune cells revealed an increase in CD11b+F4/80low cells in all HFD-fed mice. No differences were seen between groups with regard to intrahepatic frequencies of CD11b+F4/80hi Kupffer cells or overall CD11b+F4/80low infiltrating monocytes (Fig. 6, A and B). However, fewer CCR2 expressing monocytes were seen in CCX872-treated mice (Fig. 7A), and CCR2 inhibition reduced liver infiltration with Ly6chi monocytes (Fig. 7B). HFD feeding resulted in higher intrahepatic and adipose tissue frequencies of CD11b+CD11c+F4/80+ cells, an immune cell population functionally similar to CD11c+CD206+ cells in humans, which are implicated in the development of obesity-mediated insulin resistance (19). The frequency of CD11b+CD11c+F4/80+ cells in both adipose and liver tissue was significantly reduced after treatment with CCX872 (Fig. 6, C and D).
Fig. 6.
Myeloid cells from liver and adipose tissue from mice given a HFD were analyzed by flow cytometry. Treatment with a small molecule inhibitor of CCR2 did not affect proportions of intrahepatic Cd11b+F4/80hi Kupffer cells (A) or overall infiltrating CD11b+F4/80low monocytes (B) [Mann-Whitney test to compare vehicle and CCX872 groups, P > 0.05, nonsignificant (ns)]. CCR2 antagonism reduced infiltration of CD11c+F4/80+ cells into liver tissue (C) (Mann-Whitney test to compare vehicle and CCX872 groups, *P < 0.05) and adipose tissue (D) (Mann-Whitney test to compare vehicle and CCX872 groups, t-test, *P < 0.05). Data are shown as boxes to denote IQR, with line at median and whiskers showing maximum and minimum values.
Fig. 7.
Myeloid cells from liver and adipose tissue from mice given a HFD were analyzed by flow cytometry. Treatment with a small molecule inhibitor of CCR2 reduced hepatic infiltration with CCR2+CD11b+F4/80lo monocytes (A) (Student’s t-test, *P < 0.05) and infiltration of liver tissue by proinflammatory Ly6chi cells (B) (**P < 0.05 by Student’s t-test).
Inhibition of CCR2 Reduces Scarring in Murine Steatohepatitis and Fibrosis
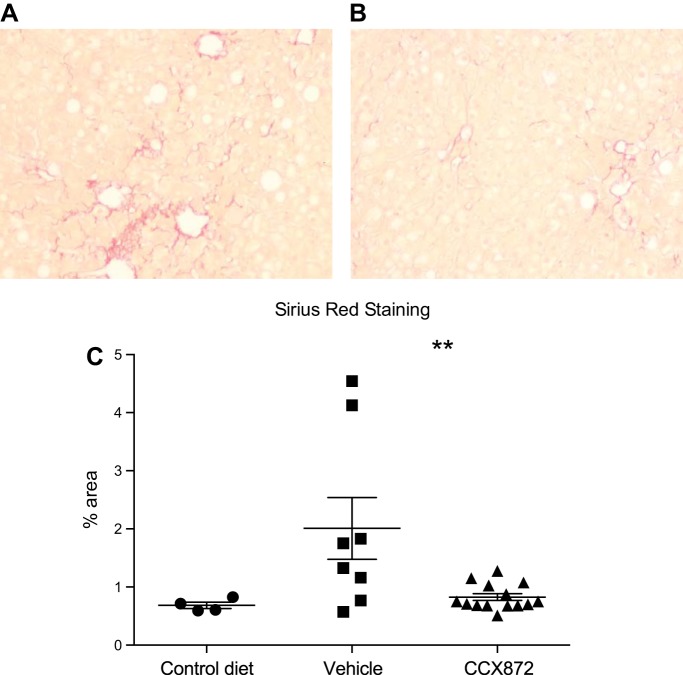
Only mild hepatic fibrosis was seen after 16 wk of HFD (Fig. 5E). As fibrosis is an important prognostic marker in human NAFLD (1, 9), we sought to assess the effects of CCR2 antagonism on the development of fibrosis. Fructose intake is associated with more severe fibrosis in human NAFLD (24) and has been shown to cause fibrosis in animal models of NAFLD (7, 16). We used HFD and fructose to induce fibrosis and assess the effect of CCR2 antagonism. Twenty-two mice were given HFD and 30% fructose for 32 wk. CCX872 or vehicle was administered daily for the final 8 wk of the experiment, each to 11 mice. A further four littermates were given the control diet for the duration of the experiment.
Consistent with initial experiments, after 32 wk, lower ALT concentrations were observed in CCX872-treated animals (median 67.0 vs. 251.5 IU/l, P < 0.006 by Mann-Whitney test), although, interestingly, they were lower than seen after a shorter period of HFD diet alone. Hepatic fibrosis, assessed by area of scarring on histology, was significantly reduced in the livers of mice receiving CCX872 [mean area 0.83 (SD 0.22) vs. 2.01% (1.5), P = 0.01 by Student’s t-test] (Fig. 8).
Fig. 8.
22 C57/Bl6 mice were fed HFD with 30% fructose in drinking water, or control diet without fructose, for 32 wk. CCR2 antagonism with a small molecule inhibitor, CCX872, reduced fibrosis compared with vehicle control. Representative pictures of liver sections from control (A) and CCX872-treated animals (B) are shown. C: fibrosis as assessed by percentage of collagen area by Sirius red staining of liver sections. Values are means and SE. **P < 0.05 by Student’s t-test.
CCR2 Antagonism Improves Glucose Metabolism in Mice Given HFD
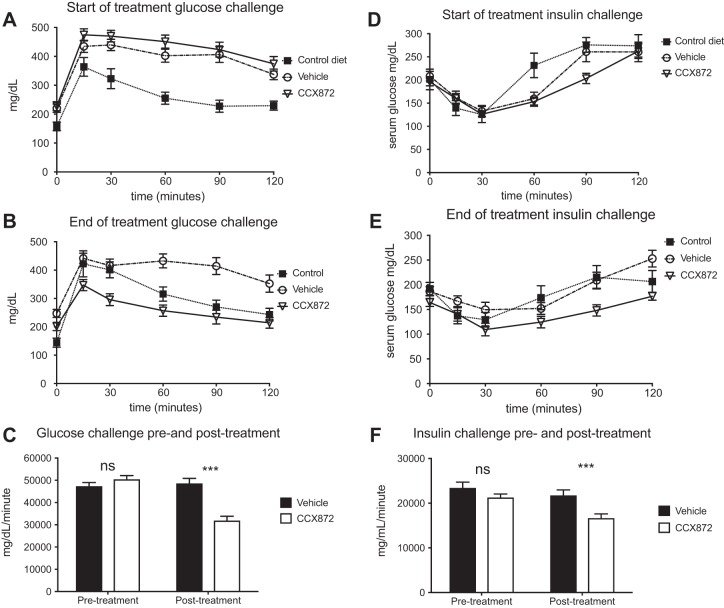
At the start of the treatment period (after 8 wk of HFD), response to a glucose load was similar between CCX872 and vehicle-treated mice (Fig. 9, A and C). However, after 8 wk of treatment, there was a significant improvement in the response of CCX872 mice compared with vehicle-treated mice (area under the curve: 48,545 vs. 31,795 mg·dl−1·min−1, Student’s t-test, P < 0.001) (Fig. 9, B and C). Insulin challenge was performed by administering a standard dose of 0.75 U/g of insulin by intraperitoneal injection to nonfasted mice. At the start of the treatment period, changes in plasma glucose concentration in response to insulin were similar in both groups of mice fed HFD (Fig. 9, D and F). After a further 8 wk of HFD and treatment with CCX872 or vehicle, there was a significant difference between groups (area under the curve: 21,719 vs. 16,553 mg·dl−1·min−1, Student’s t-test, P < 0.001) (Fig. 9, D and F).
Fig. 9.
CCR2 antagonism improved glycemic control in mice on a HFD with CCR2. Glycemic control was assessed at the beginning and end of the treatment period with glucose tolerance tests and insulin challenges. Mice in each group showed similar responses at the start of the treatment period [glucose (A) and insulin (D)]. At the time of death, mice treated with CCX872 showed significantly improved response to glucose and insulin (B and E, respectively). When assessed by measuring area under the curve, statistically significant changes were seen [glucose (C) and insulin (F)]. ***P < 0.001 by Student’s t-test.
DISCUSSION
NAFLD is a common condition closely related to obesity and the metabolic syndrome. Progressive disease is typified by hepatic inflammation in the form of steatohepatitis and fibrosis (11). We report here that a subset of monocytes that express both CD11c and CD206 are enriched in the liver of patients with NAFLD, and their presence is associated with insulin resistance. A similar subset has been reported previously in human adipose tissue, but not in liver tissue (30). We show that intrahepatic CD11c+CD206+ monocytes express CCR2, and its principal ligand, CCL2, is overexpressed in NAFLD liver tissue, suggesting that the CCR2/CCL2 axis may promote trafficking of CD11c+CD206+ monocytes to the liver in NAFLD. This would suggest that tageting CCR2 would be of therapeutic benefit in NAFLD.
To test this hypothesis, we investigated the role of CCR2 in trafficking of proinflammatory myeloid cells in a mouse model of NAFLD, where HFD feeding causes insulin resistance, steatohepatitis, and hepatic fibrosis. When a small molecule inhibitor of CCR2 was administered to mice, the numbers of liver and adipose tissue infiltrating CD11b+CD11c+F4/80+ cells were reduced, accompanied by improvements in liver histology and glycemic control.
The transition from simple steatosis to NASH is associated with hepatic inflammation and the development of insulin resistance even in the absence of overt diabetes mellitus. The present study suggests that a specific subset of liver tissue infiltrating monocytes provides the link between hepatic inflammation and insulin resistance. Wentworth at el. (30) reported that the presence of proinflammatory CD11c+CD206+ monocytes in subcutaneous and omental adipose tissue of obese individuals was associated with insulin resistance. This was mediated in part through the inhibition of the action of insulin on adipocytes. We now report the same subset of monocytes in the livers of patients with NASH. In contrast to Wentworth, we detected high levels of CCR2 on CD11c+CD206+ cells in the liver. A comparable subset of monocytes in mice is defined by F4/80 and CD11c expression. These cells express CCR2 and use it to infiltrate adipose tissue (19). Our data confirm and extend these observations by showing that pharmacological inhibition of CCR2 reduces not only adipose tissue infiltration, but also hepatic infiltration by this subset. A crucial role for these cells in disease pathogenesis was suggested by our finding of a strong correlation between the frequency of CD11c+CD206+ cells in the liver and clinical measurement of insulin resistance. Thus local hepatic insulin resistance may be mediated in part through inflammation caused by this monocyte subset recruited to the liver in response to increased CCL2 expression. Thus the improvement in glucose metabolism observed in mice is likely to be multifactorial. Improved adipose tissue inflammation will improves insulin resistance at this site, whereas reduced hepatic inflammation is also likely to improve hepatic glucose metabolism.
There have been several studies that examine the CCR2/CCL2 axis in the murine models of liver disease. Inhibition of CCR2/CCL2 either through genetic manipulation (23, 29) or pharmaceutical targeting (4, 17, 21, 27, 33) leads to improvements in steatosis, inflammation, or fibrosis, with variation dependent on the model employed. Many of these preclinical pharmaceutical studies have relied on transgenic mice (28, 33), or used nonphysiological methods, such as administration of carbon tetrachloride (4) or streptozotocin (17), or deficient diets (4, 21), and as such are of limited translational value. Our data presented here deliberately used diets that mimic high-fat and/or high-carbohydrate diets, which are a feature of human liver disease. This is a particular contrast to the study by Lefebvre et al. (17), who induced NASH in part by using streptozotocin to kill pancreatic islet cells.
Increased CCL2 expression in human NAFLD has been described previously by Haukeland et al. (12), who reported higher levels of circulating CCL2 in NAFLD and in progressive disease. Our data confirm this finding and, by correlating CCL2 blood levels with histological features seen on liver biopsies taken at the same time, show that CCL2 expression correlates with hepatic inflammation but not fibrosis. We also show increased liver-specific expression of CCL2 in patients with NAFLD, although these data have limitations through the number of samples used for analysis of liver-specific CCL2 expression and the necessary reliance of samples from patients with advanced disease to analyze liver inflammation. CCL2 is the major chemokine ligand for the receptor CCR2, which mediates myeloid cell trafficking into tissues. Intrahepatic monocytes as a group express low levels of CCR2, but this is not the case for CD11c+CD206+ monocytes, which maintain high levels of CCR2, suggesting that CCL2/CCR2 interactions may be more important for the recruitment and positioning of these cells in liver tissue. Based on these findings, we hypothesized that inhibiting CCR2 would reduce transmigration of monocytes into adipose and liver tissue. This was confirmed in mice where inhibition of CCR2 using a small molecule CCR2 inhibitor reduced accumulation of the corresponding murine subset of monocytes associated with reduced steatohepatitis and improved metabolic parameters. Several studies have reported on the use of a variety of pharmaceutical inhibitors of CCR2 in murine fatty liver disease, administered in a variety of routes and in a variety of disease models (21, 27, 33). The human data presented here confirm that CCR2 antagonism may be of benefit in NAFLD, and indeed a phase II trial of a joint CCR2/CCR5 inhibitor, cencriviroc, reported in 2016 shows benefit on hepatic fibrosis (17). The recently published results of the dual CCR2/CCR5 inhibitor, cencriviroc, in clinical NAFLD show some changes in inflammatory activity and encouraging improvements in fibrosis compared with placebo treatment. The most obvious difference between CCX872 and cencriviroc is the additional effect on CCR5, which may bring additional benefits in the setting of liver disease. Interestingly, when cenicriviroc was compared with CCX872 in the methinone-choline-deficient diet model of steatohepatitis, greater improvement in ALT and fibrosis was observed with CCX872 (20), although the methinone-choline-deficient diet is not noted for causing a great deal of fibrosis and also lacks relevance to human NAFLD. CCR2 antagonists have been used in clinical trials in a variety of diseases where their use seems safe. Treatment of NAFLD would likely require administration over at least months and possibly years, and as such long-term safety is important. One important aspect of long-term use is the impact of CCR2 inhibition on carcinogenesis. Any effects are difficult to predict at present, as animal models yield conflicting data (18), and CCR2 inhibition is being trialed for use in, for example, pancreatic cancer.
In conclusion, we suggest that a particular subset of monocytes is associated with progressive disease NAFLD, and that infiltration of liver by this subset is driven, at least in part, by CCL2/CCR2 signaling. Inhibition of this axis in NAFLD may be a rational means of improving hepatic and adipose tissue inflammation to prevent progressive liver disease.
GRANTS
R. Parker received support from a Clinical Research Training Fellowship from the Medical Research Council Grant no. G1100448, and a Sheila Sherlock Traveling Fellowship in Hepatology awarded by the Royal College of Physicians, London. ChemoCentryx Inc. designed and developed the small molecule inhibitor of chemokine C receptor 2, CCX872, which is a proprietary compound.
DISCLOSURES
Z. Miao, L. Ertl, K. Ebsworth, M.J. Walters, T. Baumart, D. Newland, J. McMahon, P. Zhang, R. Singh, J. Campbell, I. Charo, and T.J. Schall are employees of ChemoCentryx Inc. No conflicts of interest, financial or otherwise, are declared by R. Parker, C.J. Weston, C.J. Corbett, M.J. Armstrong, P.N. Newsome, and D.H. Adams.
AUTHOR CONTRIBUTIONS
R.P., M.J.W., J.C., and D.H.A. conceived and designed research; R.P., Z.M., C.C., M.J.A., L.E., K.E., M.J.W., T.B., J.M., and J.C. performed experiments; R.P., C.J.W., Z.M., C.C., M.J.A., and I.C. analyzed data; R.P., C.J.W., Z.M., and J.C. interpreted results of experiments; R.P. and Z.M. prepared figures; R.P. drafted manuscript; R.P., C.J.W., Z.M., M.J.A., P.Z., R.S., P.N.N., I.C., T.J.S., and D.H.A. edited and revised manuscript; R.P., C.J.W., Z.M., C.C., M.J.A., L.E., K.E., M.J.W., T.B., D.N., J.M., P.Z., R.S., J.C., P.N.N., I.C., T.J.S., and D.H.A. approved final version of manuscript.
REFERENCES
- 1.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149: 389–397.e10, 2015. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong MJ, Barton D, Gaunt P, Hull D, Guo K, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN; LEAN trial team . Liraglutide efficacy and action in non-alcoholic steatohepatitis (LEAN): study protocol for a phase II multicentre, double-blinded, randomised, controlled trial. BMJ Open 3: e003995, 2013. doi: 10.1136/bmjopen-2013-003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong MJ, Hazlehurst JM, Hull D, Guo K, Borrows S, Yu J, Gough SC, Newsome PN, Tomlinson JW. Abdominal subcutaneous adipose tissue insulin resistance and lipolysis in patients with non-alcoholic steatohepatitis. Diabetes Obes Metab 16: 651–660, 2014. doi: 10.1111/dom.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeck C, Wehr A, Karlmark KR, Heymann F, Vucur M, Gassler N, Huss S, Klussmann S, Eulberg D, Luedde T, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61: 416–426, 2012. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 5.Boujedidi H, Bouchet-Delbos L, Cassard-Doulcier AM, Njiké-Nakseu M, Maitre S, Prévot S, Dagher I, Agostini H, Voican CS, Emilie D, Perlemuter G, Naveau S. Housekeeping gene variability in the liver of alcoholic patients. Alcohol Clin Exp Res 36: 258–266, 2012. doi: 10.1111/j.1530-0277.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- 6.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395, 2004. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 7.Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, Masuoko H, Gores G. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol 301: G825–G834, 2011. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cynis H, Kehlen A, Haegele M, Hoffmann T, Heiser U, Fujii M, Shibazaki Y, Yoneyama H, Schilling S, Demuth HU. Inhibition of glutaminyl cyclases alleviates CCL2-mediated inflammation of non-alcoholic fatty liver disease in mice. Int J Exp Pathol 94: 217–225, 2013. doi: 10.1111/iep.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 61: 1547–1554, 2015. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 11.Gadd VL, Skoien R, Powell EE, Fagan KJ, Winterford C, Horsfall L, Irvine K, Clouston AD. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 59: 1393–1405, 2014. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 12.Haukeland JW, Damås JK, Konopski Z, Løberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjøro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol 44: 1167–1174, 2006. doi: 10.1016/j.jhep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirovski G, Dorn C, Huber H, Moleda L, Niessen C, Wobser H, Schacherer D, Buechler C, Wiest R, Hellerbrand C. Elevated systemic monocyte chemoattractrant protein-1 in hepatic steatosis without significant hepatic inflammation. Exp Mol Pathol 91: 780–783, 2011. doi: 10.1016/j.yexmp.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313–1321, 2005. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistreri WF, Woods SC, Seeley RJ. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 52: 934–944, 2010. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D, Richards T, Yoneyama H, Jenkins H, Wolfgang G, Friedman SL. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One 11: e0158156, 2016. doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Knight DA, Snyder LA, Smyth MJ, Stewart TJ. A role for CCL2 in both tumor progression and immunosurveillance. OncoImmunology 2: e25474, 2013. doi: 10.4161/onci.25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117: 175–184, 2007. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miao Z, Newland D, Ertl L, Parker R, McMahon J, Zhang P, Adams DH, Schall TJ, Charo I. Reduction of liver fibrosis by CCR2 antagonist CCX872 in murine models of NASH (Abstract). Hepatology 64, Suppl 1: 763A, 2016. [Google Scholar]
- 21.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol 302: G1310–G1321, 2012. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen MTA, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 23.Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW Jr. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59: 916–925, 2010. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 48: 993–999, 2008. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker R, Hodson J, Rowe IAC. Systematic review: current evidence in non-alcoholic fatty liver disease lacks relevance to patients with advanced fibrosis. J Gastroenterol Hepatol 32: 950–956, 2017. doi: 10.1111/jgh.13625. [DOI] [PubMed] [Google Scholar]
- 26.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8: 301–309, 2008. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura Y, Sugimoto M, Murayama T, Minami M, Nishikaze Y, Ariyasu H, Akamizu T, Kita T, Yokode M, Arai H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb 17: 219–228, 2010. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
- 28.Tamura Y, Sugimoto M, Murayama T, Ueda Y, Kanamori H, Ono K, Ariyasu H, Akamizu T, Kita T, Yokode M, Arai H. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol 28: 2195–2201, 2008. doi: 10.1161/ATVBAHA.108.168633. [DOI] [PubMed] [Google Scholar]
- 29.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 59: 1648–1656, 2010. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140: 124–131, 2011. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 32.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the US. Hepatology 59: 2188–2195, 2014. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 33.Yang SJ, IglayReger HB, Kadouh HC, Bodary PF. Inhibition of the chemokine (C-C motif) ligand 2/chemokine (C-C motif) receptor 2 pathway attenuates hyperglycaemia and inflammation in a mouse model of hepatic steatosis and lipoatrophy. Diabetologia 52: 972–981, 2009. doi: 10.1007/s00125-009-1309-8. [DOI] [PubMed] [Google Scholar]