Abstract
Chronic stress exerts vagally dependent effects to disrupt gastric motility; previous studies have shown that, among other nuclei, A2 neurons are involved in mediating these effects. Several studies have also shown robust in vitro and in vivo effects of α2-adrenoceptor agonists on vagal motoneurons. We have demonstrated previously that brainstem vagal neurocircuits undergo remodeling following acute stress; however, the effects following brief periods of chronic stress have not been investigated. Our aim, therefore, was to test the hypothesis that different types of chronic stress influence gastric tone and motility by inducing plasticity in the response of vagal neurocircuits to α2-adrenoreceptor agonists. In rats that underwent 5 days of either homotypic or heterotypic stress loading, we applied the α2-adrenoceptor agonist, UK14304, either by in vitro brainstem perfusion to examine its ability to modulate GABAergic synaptic inputs to vagal motoneurons or in vivo brainstem microinjection to observe actions to modulate antral tone and motility. In neurons from naïve rats, GABAergic currents were unresponsive to exogenous application of UK14304. In contrast, GABAergic currents were inhibited by UK14304 in all neurons from homotypic and, in a subpopulation of neurons, heterotypic stressed rats. In control rats, UK14304 microinjection inhibited gastric tone and motility via withdrawal of vagal cholinergic tone; in heterotypic stressed rats, the larger inhibition of antrum tone was due to a concomitant activation of peripheral nonadrenergic, noncholinergic pathways. These data suggest that stress induces plasticity in brainstem vagal neurocircuits, leading to an upregulation of α2-mediated responses.
NEW & NOTEWORTHY Catecholaminergic neurons of the A2 area play a relevant role in stress-related dysfunction of the gastric antrum. Brief periods of chronic stress load induce plastic changes in the actions of adrenoceptors on vagal brainstem neurocircuits.
Keywords: catecholamines, neurogastroenterology, stomach, stress, vagus
INTRODUCTION
A rapid response to either an acute internal or external undesirable threat is a reflexive protective mechanism that allows for relatively brief, but necessary, adaptations to maintain physiological homeostasis. Conversely, prolonged stress represents a more serious challenge and requires more sustained modifications. A lack of resilience, or adaptability, to adverse events frequently induces dysfunctions of gastric (delayed emptying) and colonic (accelerated) motility (2, 42, 49, 52, 63). Indeed functional gastrointestinal (GI) disorders, such as functional dyspepsia (FD) and irritable bowel syndrome (IBS), are correlated positively with stress (18, 24, 28, 35, 36), and stressful situations exacerbate GI symptoms in susceptible individuals (50).
Acute stress events affect the brain-gut axis (49, 50) and induce neuroplasticity within the brainstem vagal neurocircuits that control the upper GI tract (56). Gastric tone and motility are modulated by the pacemaker neurons of the dorsal motor nucleus of the vagus (DMV), whose activity is regulated by glutamatergic, catecholaminergic, and mainly GABAergic synaptic inputs originating, for the most part, from the adjacent nucleus tractus solitarius (NTS) (1, 48, 56, 57). The vagal motor output modulates gastric tone and motility via activation of either cholinergic excitatory postganglionic neurons or nonadrenergic, noncholinergic (NANC), mainly nitrergic, but also VIPergic inhibitory postganglionic neurons (29, 56). A vagally mediated gastric relaxation, therefore, may be evoked by withdrawal of cholinergic tone or activation of the NANC pathway.
Our group has shown that, when microinjected in the DMV of naïve rats, the prototypical antistress neuromodulator, oxytocin, induces gastric relaxation via activation of a NANC pathway. Furthermore, we showed that, in the same animals, oxytocin does not modulate the GABAergic synapse between NTS and DMV neurons (11). Following pretreatment with either acute stress or the prototypical stress hormone, corticotropin-releasing factor (CRF), however, the gastric response to oxytocin microinjection in the DMV was reduced significantly (11). Most importantly, following CRF pretreatment, the mechanism of action of oxytocin included effects on VIPergic enteric neurons as well as on the previously unresponsive GABAergic synapse (11). These data suggest that acute stress causes plastic rearrangements in brainstem vagal neurocircuits.
Central catecholamines are known to play a prominent role in the stress-related pathophysiology, and robust catecholaminergic responses to stress are mediated via neurons of the A2 and A6 (locus coeruleus) areas, which provide inputs to hypothalamic neurons involved in the HPA axis response (32, 41, 60, 61). In particular, the A2 area, embedded in the caudal brainstem comprising neurons of both NTS and DMV (30, 34, 59), is located strategically to provide a modulation of the neurocircuits that control vagal sensory-motor reflexes, including vagal motor outputs to the upper GI tract. Indeed, brainstem microinjections of catecholaminergic agents increase compliance of the gastric fundus (33) and contribute to the receptive-relaxation reflex (46), likely via activation of α2-adrenoceptors. Furthermore, electrophysiological studies have shown profound effects of norepinephrine on DMV neurons, including both a marked α2-adrenoceptor-mediated inhibition of the neurons themselves (23, 38) as well as a reduction of evoked glutamatergic synaptic inputs (8). The response to α2-adrenoceptor stimulation of brainstem vagal neurocircuits and gastric tone and motility, however, has not been investigated in animals following periods of chronic stress.
The aim of the present study, therefore, was to test the hypothesis that different types of chronic stress influence gastric functions by inducing neuronal plasticity in the response of vagal neurocircuits to an α2-adrenoceptor agonist.
MATERIAL AND METHODS
Male Sprague-Dawley rats (180–260 g) were housed in an AAALAC accredited Animal Care Facility maintained at 24°C on a 12-h:12-h light/dark cycle with food and water provided ad libitum. All procedures were conducted in accordance with the National Institutes of Health guidelines, with the approval of the Penn State University College of Medicine Institutional Animal Care and Use Committee and according to the policies and regulations of journal policy on animal experimentation.
Stress paradigms.
Animals were divided randomly in three groups: 1) control, 2) chronic homotypic stress (CHo), and 3) chronic heterotypic stress (CHe). The experimental procedures (62) were conducted for five consecutive days between 9:00am and 12:00pm. Rats in the control group were handled each day, but no stressor was loaded; rats in the CHo group underwent 2 h of restraint stress every day, whereas rats in the CHe group underwent one different type of stress (restraint, forced swim, water avoidance, cold, and restraint) each day throughout the experimental protocol.
During the restraint stress, rats were placed for 2 h in a cylinder that did not allow movement. During the forced swim experiment, rats were placed for 20 min in a container (36 × 26 × 24 cm) filled with water at room temperature, such that their hind paws could not touch the bottom of the container. During the water avoidance stress paradigm, rats were placed for 40 min on a round platform (6-cm diameter) in a container with room temperature water at a level just below the top of the platform. Rats undergoing the cold stress experiment were placed for 2 h in a cage maintained at 4°C. For each rat, the number of fecal pellets excreted during the period of stress loading was counted at the end of the session.
In vitro electrophysiological recordings.
Immediately after the final stress load, rats were anesthetized with isoflurane (5% with air), and the brainstem was removed rapidly and immersed in ice-cold Krebs solution. Coronal brain slices containing the dorsal vagal complex (DVC) were cut at 300-µm thickness using a vibratome; slices were incubated in oxygenated Krebs solution at 30°C for at least 90 min before use. A single brainstem slice was transferred in a perfusion chamber, held in place with a nylon mesh on the stage of a microscope (Nikon E600FN), and maintained at 32 ± 1°C with continuous perfusion with Krebs solution.
Whole-cell patch-clamp recordings were made from medial, i.e., gastric-projecting (56), DMV neurons using glass pipettes with 2- to 5-MΩ tip resistance when filled with potassium gluconate-based internal solution. Recordings were conducted using a single-electrode voltage clamp amplifier (Axopatch 200A; Molecular Devices, Union City, CA). Data were filtered at 2 kHz, digitized via a Digidata 1440A interface, and analyzed using pClamp10 software (Molecular Devices). Only recordings with a series, i.e., access + pipette, resistance <20 MΩ, were used.
Basic properties of DMV neurons.
Neurons were current clamped at −60 mV and injected with a 16-ms-long pulse of direct current (DC) sufficient to evoke a single action potential at its offset. For a neuronal recording to be accepted, the membrane had to be stable at the holding potential, the action potential evoked following injection of DC had to have an amplitude of at least 60 mV, and the membrane had to return to baseline at the end of the afterhyperpolarization (AHP).
Electrophysiological properties measured included the membrane input resistance (measured from the current deflection obtained by stepping the membrane from −50 to −60 mV), the duration of the action potential measured at the threshold, the amplitude and duration of the AHP following the firing of a single action potential, the frequency of action potential firing in response to depolarizing DC pulses of 400-ms duration, and intensities ranging from 30 pA to 150 pA in step increments.
Evoked and miniature inhibitory postsynaptic currents.
A bipolar stimulating electrode (~125-μm tip separation; World Precision Instruments, Sarasota, FL) was placed in the subnucleus centralis or medialis of the NTS and used to evoke inhibitory postsynaptic currents (eIPSCs). eIPSCs were recorded in neurons voltage clamped at −50 mV, and the perfusing Krebs solution was supplemented with the nonselective ionotropic glutamate antagonist kynurenic acid (1 mM). Electrical stimuli (100–500 μA, 0.05–1.00 ms) were applied every 20 s throughout the recording period.
Miniature IPSCs (mIPSCs) were recorded using a KCl intracellular solution, Krebs solution supplemented with 1 mM kynurenic acid and tetrodotoxin (0.3 µM), in neurons at a holding potential of −50 mV.
Drug application and data analysis.
All drugs were dissolved in the perfusing Krebs solution at concentrations described previously as being effective (11, 38). Agonists were applied for a period of time sufficient for the response to reach plateau; neurons were allowed to recover for at least 15 min before drug reapplication. eIPSCs and mIPSCs were analyzed offline with Clampfit (Molecular Devices) or MiniAnalysis software (Synaptosoft, Leonia, NJ). Each neuron served as its own control; that is, the response of any neuron was assessed before and after drug application using a paired Student’s t-test; intergroup comparisons were analyzed using either ANOVA followed by a post hoc Tukey’s comparison or the χ2 test. A minimum variation of ±20% in eIPSC amplitude or mIPSC frequency or amplitude was taken as indication of a response. Only responding neurons are included in the statistical analyses; results are expressed as means ± SE with significance defined as P < 0.05.
In vivo gastric recordings.
Rats were fasted overnight (water ad libitum) and anesthetized with thiobutabarbital (Inactin; 100–150 mg/kg ip). The anesthesia level was monitored continuously, and, once a deep plan of anesthesia was achieved (absence of palpebral reflex), rats were intubated with a tracheal catheter and a midline laparotomy was performed to expose the anterior gastric wall. An encapsulated miniature strain gauge (6 × 8 mm; AT Engineering, Hershey, PA) was aligned with the gastric circular smooth muscle and sutured to the anterior antrum, and the laparotomy was closed with a 5-0 suture. The signals of the strain gauge were amplified (EXP CLSG-2; QuantaMetrics, Newton, PA), filtered (low pass cut off = 0.1 Hz, AT Engineering), digitized via a Digidata 1320 interface, and recorded using AxoScope software (Molecular Devices). Rats were then placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA), core temperature was kept at 37°C with a heating pad, and the lower medulla was exposed via blunt dissection. The meningeal membranes above the vagal trigone were dissected, and the exposed lower medulla was covered with a gauze soaked in prewarmed saline for at least 1 h to stabilize.
A glass micropipette (20- to 30-µm tip diameter) was directed into the DVC (in mm: +0.4–0.6 from calamus scriptorius, 0.1–0.3 left from midline, −0.6–0.65 from the brainstem dorsal surface). Drugs were microinjected in 60-nl volumes via a picospritzer or were applied to the surface of the fourth ventricle (2 µl). All drugs were dissolved in isotonic PBS. Fluorescent microspheres were included in the injectate for post hoc verification of the injection site. Gastric tone and motility were monitored for 5 min before drug application and for at least 15 min following the microinjection.
Gastric tone was measured as absolute tone variation (in mg) from baseline. Gastric motility was calculated using the following formula, as described previously (10): motility index = (N1 × 1 + N2 × 2 + N3 × 4 + N4 × 8)/t × 100, where N equals the number of peaks in a particular force range (N1 = 25–50 mg, N2 = 51–100 mg, N3 = 101–200 mg, N 4 > 201 mg) and t equals the time interval over which the gastric motility was measured. The effect of drugs on gastric motility was measured relative to the averaged value of gastric motility before microinjection (baseline = 100%).
The NO-synthase inhibitor nitro-l-arginine methyl ester (l-NAME) (10 mg/kg), the VIP antagonist (50 µg/kg), or the muscarinic agonist bethanechol (50 µg/kg) were administered intravenously before the second microinjection of UK14304.
At the conclusion of the experiment, rats were fixed via transcardial perfusion with 0.1 M PBS followed by paraformaldehyde (PFA, 4%) in 0.1 M PBS. Brainstems were extracted and postfixed in 4% PFA overnight and then transferred to 0.1 M PBS with 20% sucrose for 2 days. The brainstem was frozen, and coronal sections (40-µm thickness) throughout the rostrocaudal extent of the DVC were cut using a microtome. Every fourth slice was mounted immediately to identify the injection site using a Nikon E400 microscope; the other slices were stored in long-term storage buffer at −80°C until processing for immunohistochemistry.
Immunohistochemistry.
All immunohistochemical procedures were carried out at room temperature on a shaking platform. The primary antibodies used included the following: mouse anti-tyrosine hydroxylase 1:10,000 (TH; Immunostar, Hudson, WI) and mouse anti-dopamine-β-hydroxylase 1:30,000 (DβH; Millipore, Bedford, MA). The secondary antibody was biotinylated donkey anti-mouse immunoglobulins (IgG, from Jackson ImmunoResearch, West Grove, PA) diluted at 1:500. The detection complex was ExtrAvidin-horseradish peroxidase (Sigma-Aldrich, St. Louis, MO) diluted at 1:1,500.
One-color immunoperoxidase labeling was used to localize DβH-immunoreactive (IR) with a dark blue reaction product and TH-IR with a brown reaction. Every fourth brainstem section was rinsed with three 10-min 0.1 M PBS rinses and treated with 30% methanol in hydrogen peroxide for 30 min. The sections were permeabilized by three 10-min washes in Tris-buffered PBS containing 0.3% TritonX-100 and 0.05% thimerosal (TPBS). The sections were then exposed to TPBS with 10% normal horse serum for 1 h and then incubated in mouse anti-TH or mouse anti-DβH primary antibody diluted in TPBS with 10% normal horse serum for 3 days. The sections were then rinsed with TPBS three times for 10 min each and incubated with TPBS containing 1% normal horse serum, followed by secondary antibody, which was diluted in TPBS containing 1% normal horse serum for overnight incubation. After being rinsed with TPBS three times for 10 min each, the sections were incubated in ExtrAvidin-horseradish peroxidase for 4 h. Immunoreactive neurons were visualized with an imidazole-intensified diaminobenzidine reaction (TH), or a Vector SG (Vector, Burlingame, CA) reaction (DβH) with peroxide generated by glucose oxidase produced a dark blue reaction. Sections were washed three time for 10 min each in TPBS, mounted on subbed slides, dehydrated, and coverslipped with Cytoseal (Thermo Fisher Scientific, Waltham, MA).
Data were evaluated by comparing the change in response between pre- and posttreatment values within each group by ANOVA or paired t-test (SPSS, Chicago, IL) and are reported as means ± SE. In all instances, significance was set at P < 0.05.
Drugs and solutions.
Krebs solution was composed of the following (in mM): 126.00 NaCl, 25.00 NaHCO3, 2.50 KCl, 1.20 MgCl2, 2.40 CaCl2, 1.20 NaH2PO4, and 11.00 dextrose, maintained at pH 7.4 by bubbling with 95% O2-5% CO2. Potassium gluconate intracellular solution was composed of the following (in mM): 128.00 K-gluconate, 10.00 KCl, 0.30 CaCl2, 1.00 MgCl2, 10.00 HEPES, 1.00 EGTA, 2.00 Na2ATP, and 0.25 NaGTP, adjusted to pH 7.36 with KOH. Potassium chloride intracellular solution was composed of the following (in mM): 140.00 KCl, 1.00 CaCl2, 10.00 HEPES, 10.00 EGTA, 2.00 Na2ATP, and 0.25 NaGTP, adjusted to pH 7.36 with HCl. PBS was composed of the following (in mM): 124.00 NaCl, 26.00 NaHCO3, and 2.00 KH2PO4, pH 7.4. Long-term storage buffer was PBS 0.1 M, sucrose 30%, and ethylene glycol 30%.
Tetrodotoxin was purchased from Alomone (Jerusalem, Israel); UK14304 was purchased from Tocris Bioscience (Ellisville, MO); all other chemicals were purchased from Sigma-Aldrich.
RESULTS
Rats in the homotypic group showed adaptation to stress.
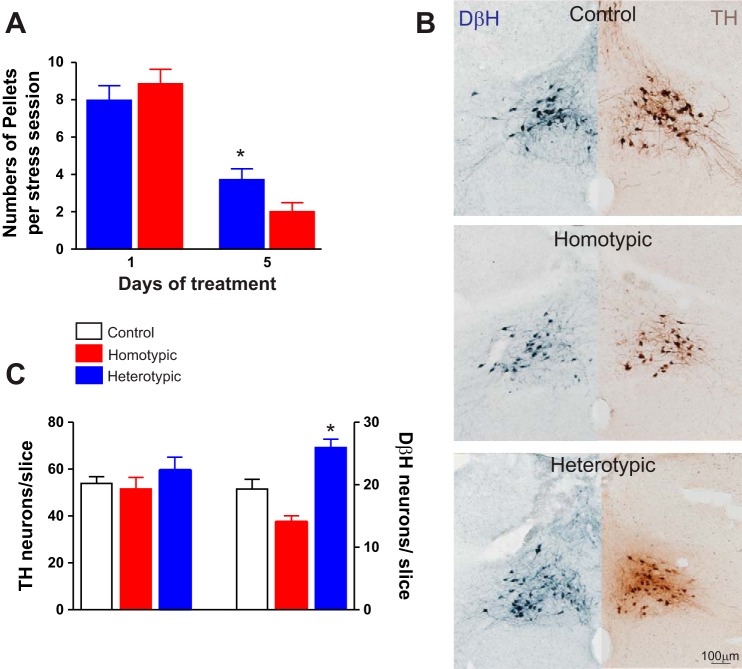
The number of fecal pellets excreted on the first day of stress loading was not significantly different between rats from the homotypic (CHo), 8.8 ± 0.8 (N = 27), or from the heterotypic (CHe), 8.0 ± 0.8 (N = 32), stress groups. At the end of the fifth day, however, the number of fecal pellets expelled by rats from the CHo stress group, 2.0 ± 0.5, was significantly lower than the number of pellets expelled by rats from the CHe group, 4.2 ± 0.6 (P < 0.05 vs. homotypic; P < 0.05 vs. day 1), suggesting a greater degree of stress adaptation in rats in the CHo stress group (Fig. 1).
Fig. 1.
Characterization of the fecal pellet output and immunocytochemistry of the A2 area. A: graphical summary illustrating the number of fecal pellets expelled during the periods of stress loading. Note that the number of fecal pellets is decreased over time in both chronic homotypic stress (CHo) (N = 27) and chronic heterotypic stress (CHe) (N = 32) rats; however, at day 5 CHe rats had a significantly higher number of pellets. *P < 0.05 vs. CHo. B: representative micrographs showing dopamine-β-hydroxylase-immunoreactive (DβH-IR) (left, blue) and tyrosine hydroxylase-immunoreactive (TH-IR) (right, brown) neurons in area A2 in control (top), CHo (middle), and CHe (bottom) stress rats. All slices were obtained at similar brainstem levels. C: summary graphic showing the number of DβH-IR and TH-IR neurons in A2 area of control (N = 6 slices/5 rats; open bar), CHo (6 slices/4 rats; red bar), and CHe (6 slices/5 rats; blue bar) rats. Note the increased DβH-IR in rats that underwent CHe, but not CHo, stress. Data were analyzed with 1-way ANOVA followed by Tukey’s test. *P < 0.05 vs. control and CHo.
Rats in the heterotypic group showed an increased number of noradrenergic neurons in the brainstem A2 area.
The number of TH-IR neurons in the A2 area, at the level of the intermediate DVC (i.e., DMV, NTS, and area postrema), were similar among control (54.0 ± 2.8 neurons/slice; N = 6 slices per rat, N = 5 rats), CHo stress (52.0 ± 4.8 neurons/slice; N = 6 slices per rat, N = 4 rats), and CHe stress rats (60.0 ± 5.8 neurons/slice; N = 6 slices per rat, N = 5 rats; P > 0.05 for each). In contrast, there was a decrease in DβH-IR following CHo stress (19.0 ± 1.5 neurons/slice; N = 6 slices per control rats, N = 5 rats and 14.0 ± 1.3 neurons/slice; N = 6 slices per CHo rats, N = 4 rats, respectively; P < 0.05). Conversely, an increased number of DβH-IR neurons was found in the CHe stress group (26.0 ± 1.3 neurons/slice; N = 6 slices per rat, N = 5 rats, P < 0.05 vs. control and CHo group rats; Fig. 1).
These data indicate that in stressed rats there is an alteration the number of noradrenergic neurons in the A2 area.
Basic electrophysiological properties.
Whole-cell recordings were made from 24 gastric-projecting DMV neurons from 12 rats in the control group, 24 gastric-projecting DMV neurons from 11 rats in CHo stress group, and 26 gastric-projecting DMV neurons from 14 rats in CHe stress group.
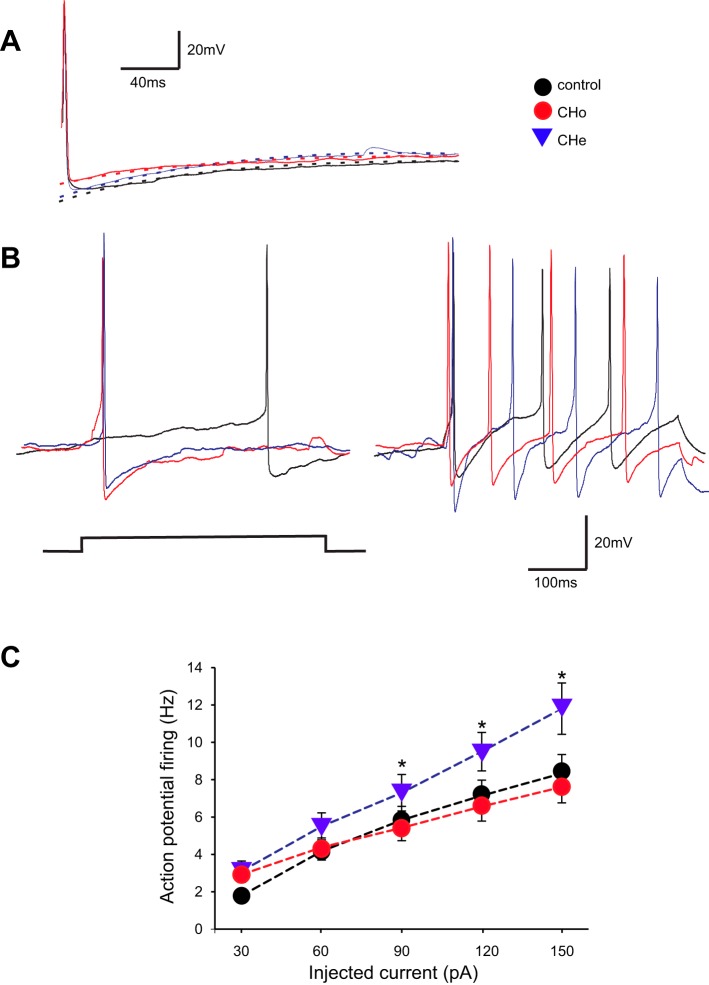
The basic electrophysiological properties of DMV neurons in the control, CHo, and CHe groups are summarized in Table 1. Overall, the electrophysiological properties were similar among the groups, apart from the higher input resistance and higher frequency response to injected current in neurons of the CHe group (30–150 pA; P < 0.05 vs. control and CH groups; Fig. 2).
Table 1.
Basic properties of DMV neurons
| Input Resistance, MOhm | Action Potential Duration, ms | AHP Amplitude, mV | AHP Decay Constant, ms | mIPSC Frequency, events/s | mIPSC Amplitude, pA | UK14304-Induced Current, pA | |
|---|---|---|---|---|---|---|---|
| Control | 384 ± 42 | 2.8 ± 0.2 | 18.7 ± 1.1 | 159.0 ± 31.5 | 1.0 ± 0.2 | 89.0 ± 7.3 | 56 ± 12 |
| Homotypic stress | 277 ± 19* | 2.4 ± 0.1 | 18.3 ± 1.1 | 111.0 ± 26.8 | 1.7 ± 0.2 | 89.0 ± 9.2 | 80 ± 15 |
| Heterotypic stress | 483 ± 44 | 2.7 ± 0.1 | 18.4 ± 0.9 | 151.0 ± 42.7 | 1.5 ± 0.3 | 90.0 ± 6.1 | 62 ± 12 |
Values are means ± SE. DMV, dorsal motor nucleus of the vagus; AHP, afterhyperpolarization; mIPSC, miniature inhibitory postsynaptic current.
P < 0.05 vs. heterotypic stress.
Fig. 2.
Dorsal motor nucleus of the vagus (DMV) neurons from the chronic heterotypic stress (CHe) group are more excitable. A: representative traces of single action potentials evoked in DMV neurons from control, chronic homotypic stress (CHo), and CHe groups. Neurons were current clamped at −60 mV before injection of a short (16 ms) depolarizing current pulse of intensity sufficient to evoke a single action potential at its offset. Note that the amplitude and duration of the action potential and the amplitude and time constant of decay of the afterhyperpolarization (AHP) were similar among groups. B: representative traces showing the response of DMV neurons from control, CHo, and CHe rats following injection of 30 (left) and 150 pA (right) DC (400 ms long). C: frequency-response curves for DMV neurons from control (N = 24 neurons from 12 rats), CHo (24 neurons from 11 rats), and CHe (26 neurons from 14 rats) stress groups. Note that neurons from the CHe stress group are more excitable and fire more action potentials than neurons from the other groups. Holding potential = −60 mV. Data were analyzed with 1-way ANOVA followed by Tukey’s test. *P < 0.05.
In the presence of tetrodotoxin (0.3 µM) and kynurenic acid (1 mM), perfusion with the α2-adrenoceptor agonist UK14304 (1 µM) induced an outward current of similar proportion and magnitude in all groups: control 42 ± 12 pA in 9 of 13 neurons, CHo 65 ± 11 pA in 11 of 22 neurons, and CHe 55 ± 8 pA in 11 of 19 neurons (P > 0.05; data not shown). These data suggest that the postsynaptic effects of UK14304 were similar among the groups.
UK14304 modulates inhibitory neurotransmission to gastric-projecting DMV neurons from stressed but not from control rats.
To assess the pre- vs. postsynaptic effects of UK14304, a series of experiments were conducted on miniature and evoked inhibitory currents.
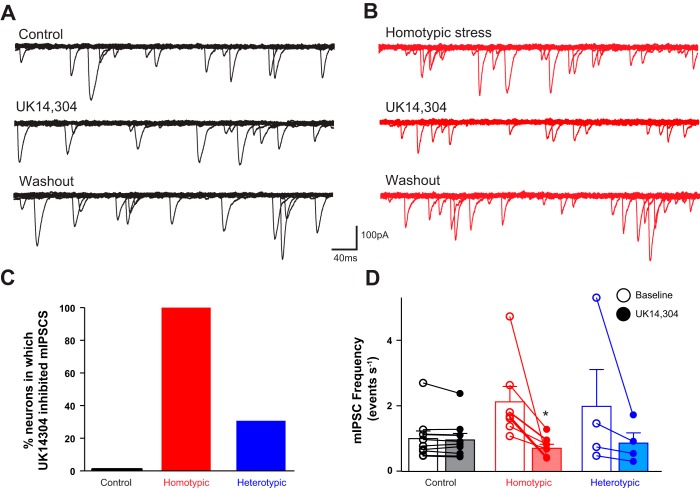
In nine neurons from control rats, perfusion of brainstem slices with the α2-adrenoceptor agonist UK14304 (1 μM) did not alter mIPSCs frequency (from 1.01 ± 0.23 events/s to 0.96 ± 0.20 events/s, P > 0.05) or amplitude (from 107.0 ± 11.1 to 99.0 ± 9.9 pA, P > 0.05) on any DMV neurons tested. In five neurons in which UK14304 failed to modulate mIPSC frequency (from 0.96 ± 0.10 to 0.97 ± 0.10 events/s), the ability of CRF to modulate the response to UK14304 was assessed. Following washout and recovery from initial application of UK14304, perfusion of 100 nM CRF did not change mIPSC frequency (from 0.78 ± 0.10 to 0.77 ± 0.10 events/s, P > 0.05) or amplitude (from 106.0 ± 11.1 to 99.0 ± 9.9 pA, P > 0.05). Upon washout of CRF, reapplication of UK14304 reduced mIPSC frequency in all five neurons (from 0.8 ± 0.1 to 0.5 ± 0.1 events/s, i.e., 68.0 ± 6.3% of baseline, P < 0.05), without affecting the amplitude (from 90.0 ± 11.1 to 81.0 ± 6.9 pA, P > 0.05), suggesting a presynaptic site of action (Fig. 3).
Fig. 3.
Stress uncovers the α2-adrenoceptor-mediated decrease in miniature inhibitory postsynaptic current (mIPSC) frequency. A: representative traces of mIPSCs recorded in a gastric-projecting dorsal motor nucleus of the vagus (DMV) neuron from a control rat. Perfusion with the α2-adrenoceptor agonist UK14304 (1 µM) did not change the frequency or amplitude of mIPSCs. Holding potential = −50 mV. B: representative traces of mIPSCs recorded in a gastric-projecting DMV neuron from a chronic homotypic stress (CHo) rat. Perfusion of the slice with UK14304 reduced the frequency of mIPSCs. Holding potential = −50 mV. C: graphic representation of the percentage of neurons in control (solid bar; N = 0/9), CHo stress (red bar; N = 7/7), or chronic heterotypic stress (CHe) stress (blue bar; N = 4/13) in which perfusion with UK14304 decreased mIPSC frequency. D: graphic representation of the UK14304-induced modulation of the mIPSC frequency in DMV neurons from rats in control, CHo, and CHe stress load groups. In the CHo and CHe groups, only responsive neurons are depicted. Data were analyzed with a χ2 test. *P < 0.05 vs. baseline.
In contrast, in 4 out of 13 neurons (i.e., 31%) from CHe rats, perfusion with UK14304 decreased mIPSC frequency from 1.99 ± 1.12 to 0.87 ± 0.31 events/s (i.e., 58.1 ± 8.9% of baseline, P < 0.05) without affecting the amplitude (from 87.00 ± 12.09 to 81.00 ± 6.58 pA). In the remaining nine neurons, UK14304 had no effect on either frequency (from 0.83 ± 0.12 to 0.76 ± 0.10 events/s) or amplitude (from 82.0 ± 5.2 to 83.0 ± 9.7 pA; Fig. 3).
In five of the nine neurons from CHe rats in which initial UK14304 application failed to change mIPSC frequency (0.96 ± 0.20 events/s at baseline vs. 0.85 ± 0.20 events/s in UK14304), CRF application following UK14304 washout and recovery did not change mIPSC frequency or amplitude. After CRF washout, reapplication of UK14304 significantly decreased mIPSC frequency (from 0.78 ± 0.10 events/s to 0.51 ± 0.10 events/s, P < 0.05, 65.0 ± 11.2% of baseline) without altering the amplitude (from 98.0 ± 1.6 to 102.0 ± 9.2 pA; data not shown).
In all seven gastric-projecting DMV neurons tested from CHo rats, perfusion with UK14304 decreased mIPSC frequency from 2.13 ± 0.47 to 0.71 ± 0.12 events/s (40.5 ± 8.0% of baseline, P < 0.05) without altering the amplitude (from 89.00 ± 9.17 to 85.00 ± 7.47 pA, P > 0.05; Fig. 3).
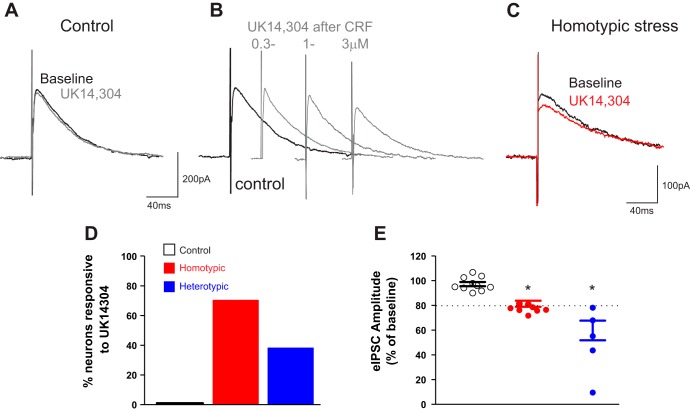
Similar to mIPSCs, application of UK14304 in control rats did not change the amplitude of eIPSCs (from 274.00 ± 51.70 to 269.00 ± 54.38 pA, P > 0.05) in any of 15 gastric-projecting neurons tested. In three neurons in which UK14304 (1 µM) failed to change amplitude of eIPSC (from 280.0 ± 125.0 pA to 256.0 ± 113.3 pA, 92.3 ± 1.5% of baseline), we also tested the ability of the adenylate cyclase activator, forskolin, to modulate eIPSC amplitude. Following washout and recovery of initial UK application, the brainstem slices were perfused with forskolin (10 μM), which itself did not change eIPSC amplitude (from 273.0 ± 134.7 to 288.0 ± 138.4 pA, P > 0.05). Reapplication of UK after forskolin decreased the eIPSC amplitude in three of three neurons by 76.0 ± 6.8% (from 322.0 ± 162.2 to 259.0 ± 138.8 pA, P < 0.05). In eight neurons in which UK14304 application failed to alter the amplitude of eIPSC (from 188.0 ± 26.0 to 184.0 ± 25.8 pA), following washout and recovery of initial UK14304 application, perfusion of CRF (100 nM) did not change eIPSC amplitude. Reapplication of UK14304 after CRF washout reduced eIPSC amplitude in four out of eight neurons (from 251.0 ± 25.3 to 184.0 ± 31.7 pA, 73.4 ± 9.6% of control; Fig. 4).
Fig. 4.
Stress and corticotropin-releasing factor (CRF) uncover the α2-adrenoceptor-mediated decrease of evoked inhibitory postsynaptic current (eIPSC) amplitude. A: representative traces of eIPSCs recorded in a dorsal motor nucleus of the vagus (DMV) neuron from control rat following electrical stimulation of nucleus tractus solitarius (NTS). Note that perfusion with UK14304 (1 µM) did not affect the amplitude of eIPSCs. Holding potential = −50 mV. B: representative traces of eIPSCs recorded in a DMV neuron from a control rat. Note that following perfusion with CRF and recovery to baseline amplitude, a second perfusion with UK14304 reduced the eIPSC amplitude in a concentration-dependent manner. Holding potential = −50 mV. C: representative traces of eIPSCs recorded in a DMV neuron from a chronic homotypic stress (CHo) rat. Perfusion of the slice with UK14304 reduced eIPSC amplitude even without pretreatment of the slice with CRF. Holding potential = −50 mV. D: graphic representation of the percentage of neurons in control (solid bar; N = 0/10), CHo stress (red bar; N = 12/17), or chronic heterotypic stress (CHe) stress (blue bar; N = 5/13) in which perfusion with UK14304 decreased eIPSC amplitude. E: graphic representation of the UK14304-induced modulation of the eIPSC amplitude in control, CHo, and CHe stress groups. In the CHo and CHe groups, only responsive neurons are depicted. Dashed line represents the “threshold” change to classify the neurons as responsive. Data were analyzed with 1-way ANOVA followed by Tukey’s test. P < 0.05 vs. baseline.
In CHo rats, perfusion of UK14304 (1 µM) decreased the amplitude of eIPSCs in 12 of 17 neurons, i.e., 71% of neurons were responsive to UK14304 perfusion, from 222.0 ± 17.8 to 174.0 ± 17.0 pA (77.8 ± 1.4% of control; Fig. 4). The inhibition of the eIPSC amplitude by perfusion with UK14304 was concentration dependent: 0.3 µM 90.0 ± 1.3% (N = 3), 1.0 µM 77.0 ± 1.3% (N = 8), and 3.0 µM 53.0 ± 8.9% (N = 6) of baseline. In four of these neurons in which UK14304 decreased the amplitude of the eIPSC, exposure to CRF (100 nM) did not augment the inhibitory effects of UK14304 further, i.e., 75.0 ± 1.4% and 80.0 ± 3.0% of baseline before and after CRF, respectively (P > 0.05). Similarly, in four neurons in which UK14304 perfusion had no effect on eIPSC amplitude, perfusion with CRF did not uncover the inhibitory effects of UK14304, i.e., 103.0 ± 1.9% and 96.0 ± 0.2% of baseline before and after CRF, respectively (P > 0.05; data not shown).
In CHe rats, perfusion of UK14304 (1 µM) decreased the amplitude of eIPSC in 5 of 13 neurons (38%, from 96.0 ± 9.7 to 48.0 ± 12.0 pA, 51.0 ± 11.0% of control; P < 0.05 vs. control; Fig. 4). The inhibition of the eIPSC amplitude by perfusion with UK14304 was concentration dependent: 0.3 µM 75.0 ± 2.8% (N = 5), 1.0 µM 51.0 ± 11.8% (N = 5), and 3.0 µM 54.0 ± 7.0% (N = 3) of baseline.
In a group of six neurons from CHe stress rats in which UK14304 had no effect on eIPSC amplitude (from 337 ± 48 to 317 ± 46 pA), following UK14304 washout, application of CRF did not change the eIPSC amplitude (from 319 ± 39 to 313 ± 36 pA). Reapplication of UK14304 following CRF washout decreased the eIPSC amplitude (from 364.0 ± 7.5 to 241.0 ± 24.0 pA, 66.6 ± 8.1% of control) in two of these neurons (data not shown).
These results suggested that CHo and CHe stress uncovers modulation of the GABAergic synapse by α2-adrenoceptor agonists, in a manner similar to that observed after elevation of cAMP levels or exposure to CRF in control conditions.
In vivo gastric recordings.
We then conducted a series of in vivo experiments to investigate whether the response of antrum tone and motility to UK application was altered in stressed animals.
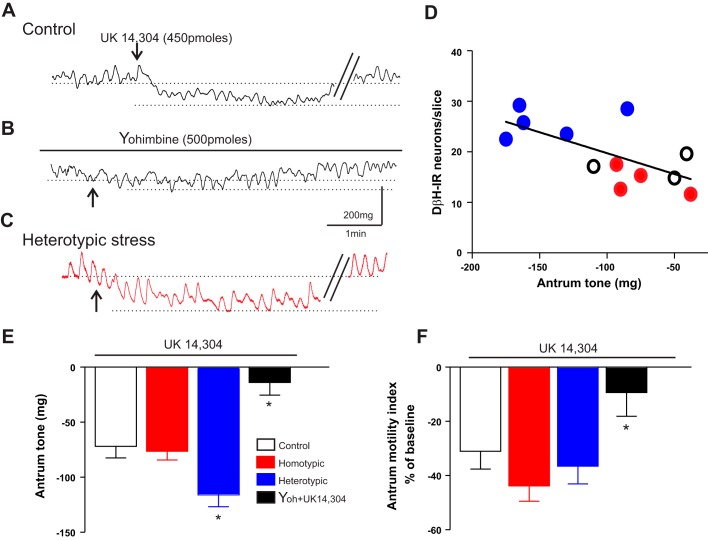
In eight control rats, microinjection of UK14304 (450 pmol/60 nl) in the DVC induced a −71.0 ± 10.5 mg decrease in antrum tone, with a −30.0 ± 6.6% change in motility. Pretreatment with the α2-adrenoceptor antagonist yohimbine (500 pmol) did not itself change antrum tone or motility (10.0 ± 3.9 mg and −12.0 ± 8.2%; N = 7). In the presence of yohimbine, however, the response to a repeated microinjection of UK14304 on antrum tone (−13.0 ± 11.4 mg) and motility (−9.1 ± 8.7%) was attenuated significantly (P < 0.05 vs. UK14304 alone; Fig. 5).
Fig. 5.
Microinjection of UK14304 in the dorsal vagal complex (DVC) induced a larger decrease in antrum tone of chronic heterotypic stress (CHe) rats. A: representative recording from the antrum of a control rat showing the decrease in tone upon microinjection of UK14304 (450 pmol/60 nl) in the DVC. B: pretreatment with the α2-adrenoceptor antagonist, yohimbine (500 pmol/2 µl on the floor of the 4th ventricle), prevented the decrease in tone and motility in response to a second microinjection of UK14304. C: representative trace from the antrum of a CHe rat. Note that the UK14304-induced decrease in gastric tone is larger than the decrease obtained in control animals. D: graphic representation showing the positive correlation between the number of dopamine-β-hydroxylase-immunoreactive (DβH-IR) neurons in the brainstem A2 area and the decrease in antrum tone obtained upon microinjection of UK14304 in the DVC (N = 12). E: summary graph representing the effects of UK14304 microinjection in the DVC on antrum tone in control (N = 8), chronic homotypic stress (CHo) (N = 10), and CHe (N = 11) rats. Note the larger inhibition of tone induced by UK14304 in CHe rats. Pretreatment with the α2-adrenoceptor antagonist, yohimbine, antagonized the UK14304-induced decrease in antrum tone. Data were analyzed with 1-way ANOVA followed by Tukey’s test. *P < 0.05 vs. control. F: summary graph representing the effects of UK14304 microinjection in the DVC on antrum motility in control rats (N = 8), CHo (N = 11), and CHe (N = 10) rats. No significant variations in antrum motility were observed among the groups. Pretreatment with the α2-adrenoceptor antagonist, yohimbine, antagonized the UK14304-induced decrease in antrum motility (N = 8). Data were analyzed with 1-way ANOVA followed by Tukey’s test. *P < 0.05 vs. control.
We then conducted a series of experiments to investigate the mechanism by which UK14304 induces antral relaxation.
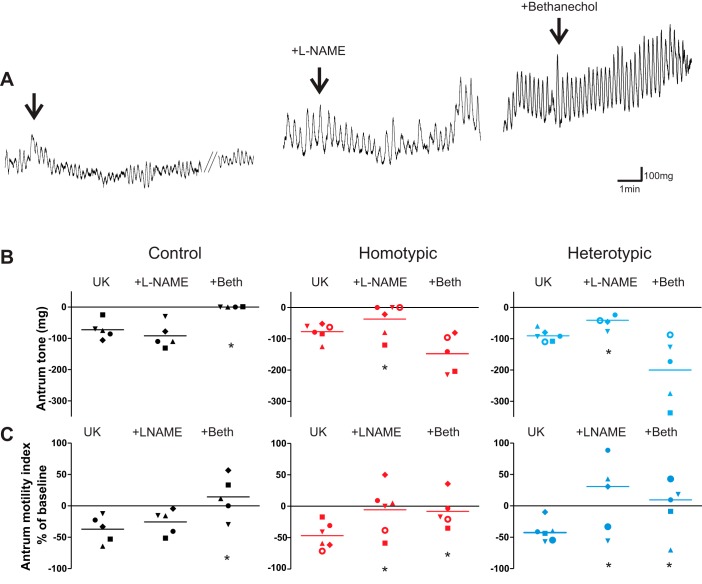
In four control rats, microinjection of UK14304 in the DVC induced a −64.0 ± 13.4 mg and a −37.1 ± 9.5% decrease in antrum tone and motility. Bethanechol infusion increased tone by 265.0 ± 81.4 mg and motility by 276.4 ± 66.0%. As demonstrated previously (11, 37), if UK14304 microinjection did not reduce antrum tone and motility during exogenous stimulation by supramaximal doses of the muscarinic cholinergic agonist, bethanechol, then one may infer that the effects of UK14304 are mediated by a postganglionic cholinergic pathway to the stomach. Indeed, in the presence of bethanechol, the subsequent microinjection of UK14304 had no effect on antral tone and motility (0 ± 0 mg and 14.3 ± 14.7% reduction of tone and motility, respectively; P < 0.05 vs. UK14304 alone; Fig. 6).
Fig. 6.
Following stress load the UK14304 (UK)-induced effects on antrum tone and motility are mediated by withdrawal of cholinergic tone and activation of nonadrenergic, noncholinergic nitric oxide (NANC-NO) pathway. A: representative traces from a control animal showing that microinjection of 450 pmol/60 nl of UK in the dorsal vagal complex (DVC) decreases antrum tone and motility (left). Upon recovery from the UK-induced relaxation, following intravenous administration of nitro-l-arginine methyl ester (l-NAME) (10 mg/kg), a second microinjection of UK induced a similar inhibition as in naïve rats (middle). Upon recovery and following intravenous administration of bethanechol (50 µg/kg), the inhibitory effects of UK microinjection were prevented (right). Arrows indicate UK microinjection. B: data points showing the antrum tone response of individual animals to microinjection of UK alone (left column), in the presence of l-NAME (middle column), and in the presence of bethanechol (Beth) (right column) for control (left, black; N = 5), CHo (middle, red; N = 6), and CHe (right, blue; N = 6). Bars represent the mean response; data were analyzed with 1-way ANOVA followed by Tukey’s test. *P < 0.05 vs. UK alone. C: data points showing the antrum motility response of individual animals to microinjection of UK alone (left column), in the presence of l-NAME (middle column), and in the presence of bethanechol (right column) for control (left, black; N = 5), CHo (middle, red; N = 6), and CHe (right, blue; N = 6). Bars represent the mean response; data were analyzed with 1-way ANOVA followed by Tukey’s test. *P < 0.05 vs. UK alone.
In five rats, microinjection of UK14304 induced a −72.4 ± 13.4 mg and a −37.1 ± 9.5% decrease in antrum tone and motility, respectively. After a 45- to 60-min recovery period, intravenous infusion of the NO synthase inhibitor, l-NAME, increased antrum tone and motility by 121.0 ± 42.0 mg and 78.0 ± 53.6%, respectively. In the presence of l-NAME, reapplication of UK14304 induced a similar decrease in antrum tone and motility as in naïve rats, i.e., −92.0 ± 17.6 mg and −26.0 ± 8.8%, respectively; P > 0.05 vs. UK14304 alone (Fig. 6).
These data indicate that in naïve rats the inhibitory effects of UK14304 microinjection in the DVC on antrum tone and motility occur exclusively via withdrawal of cholinergic pathways.
In rats that received CHo stress, microinjection of UK14304 into the DVC induced a comparable decrease in antrum tone and motility (−83.0 ± 7.8 mg and −43.0 ± 5.7%, respectively, N = 10; P > 0.05 vs. control). Conversely, in rats that received CHe stress, microinjection of UK into the DVC induced a significantly larger decrease in antrum tone (−131.0 ± 11.5 mg, N = 11; P < 0.05 vs. control) but had a similar effect on antrum motility (−36.0 ± 6.5%; P > 0.05 vs. control; Fig. 5).
In both CHo and CHe stress rats, perfusion with yohimbine increased antrum tone significantly (53.0 ± 13.8 and 37.0 ± 7.5 mg, N = 6 and 8 in CHo and CHe, respectively; P < 0.05 vs. control), but not motility (−6.5 ± 6.3 and 2.3 ± 8.6% N = 4 and 5 in CHo and CHe respectively; P > 0.05 vs. control; data not shown).
These data suggest that, in stressed rats, antrum tone is decreased due to the activation of a noradrenergic input and tonic activation of α2-adrenoceptors on vagal motoneurons and, furthermore, that CHe stress also increases the response to exogenous application of α2-adrenoceptor agonists.
In five CHo rats, microinjection of UK in the DVC induced a −44.0 ± 9.9% decrease in antrum motility. In four rats out of five, bethanechol infusion increased motility by 519.0 ± 158.2%. In the presence of bethanechol, the second microinjection of UK14304 induced no effect on motility (−1.0 ± 12.9%; P < 0.05 vs. UK14304 alone; Fig. 6). In the remaining rat, the motility response to UK14304 microinjection in the presence of bethanechol was unchanged.
In the same five CHo rats, microinjection of UK14304 in the DVC induced a −68.0 ± 6.0 mg decrease in antrum tone. In all rats, bethanechol infusion increased tone by 302.0 ± 39.0 mg but was ineffective in preventing the decrease in tone induced by UK14304 microinjection (Fig. 6).
These data suggest that the noradrenergic control of antrum tone and motility involves different pathways. Furthermore, these data indicate that, in contrast to control rats, the inhibitory effects of UK microinjection in the DVC were not due exclusively to modulation of the cholinergic pathway, but also another pathway was involved.
In six CHo rats, intravenous infusion of l-NAME increased antrum tone and motility by 115.0 ± 32.4 mg and 88.7 ± 42.4%, respectively. In the presence of l-NAME, the response to reapplication of UK14304 was significantly attenuated (−37.0 ± 20.0 mg and −5.5 ± 15.7%, respectively; P < 0.05 vs. UK14304 alone; Fig. 6).
In all of the six rats tested, pretreatment with VIP antagonist, which itself had no effect on tone and motility, decreased the antrum tone or motility response to UK14304 microinjection, i.e., −77.0 ± 10.7 vs. −68.0 ± 21.0 mg, −47.0 ± 8.4 vs. −38.0 ± 14.9% in UK14304 alone and after VIP antagonist (P > 0.05; data not shown).
Similar results were obtained in CHe rats. The data are summarized in Fig. 6.
These data indicate that, following stress, the UK14304-mediated inhibition of antrum motility involves activation of the NANC pathway in addition to withdrawal of the cholinergic pathway. In contrast, in stressed rats the UK14304-induced decrease in antrum tone is mediated by NO only.
DISCUSSION
In the present study, we have shown that the response of vagal neurocircuits to the α2-adrenoceptor agonist, UK14304, varies according to the type of chronic stress. In particular, we have shown that 1) stress alters DβH-IR, but not TH-IR, in neurons of the A2 area; 2) perfusion with UK14304 does not modulate GABAergic currents in DMV neurons from control rats, unless the brainstem slices are pretreated with CRF or forskolin; 3) perfusion with UK14304 decreases GABAergic currents via presynaptic actions in DMV neurons from stressed rats, even before exposure to CRF; 4) microinjection of UK14304 induces a larger decrease in the antrum tone of heterotypic stressed rats; 5) in control rats, the UK14304-induced gastroinhibition is mediated exclusively by withdrawal of cholinergic pathways; and 6) in stressed rats, the UK14304-mediated gastroinhibition is mediated by a combined action on withdrawal of cholinergic and activation of NANC pathways.
Taken together, our data indicate that brief periods of chronic stress induce plasticity in brainstem vagal neurocircuits.
Rodents loaded with CHo stress have impaired GI motility, i.e., delayed gastric emptying and accelerated colonic transit, which is restored to prestress levels within 4–5 days of stress load. Conversely, GI motility is still dysfunctional in rodents that undergo CHe stress (2, 5, 6, 62). Our data indicate that CHo rats showed a greater degree of adaptation to stress as seen by the reduction in stress-induced fecal pellet count by day 5. These data provide further confirmation of the many studies showing that Sprague-Dawley rats adapt to CHo stress, as demonstrated by the reduction in HPA axis activation (26, 31), the attenuation of behavioral response to stress (27), as well as the prevention of adrenal hypertrophy, among other behavioral outcomes (22, 53).
In rats, stressful stimuli induce an upregulation of central catecholamine levels in areas such as the locus coeruleus (25, 60) and the neurons of the A2 group (41, 44). Stress-mediated activation of the locus coeruleus, however, increases colonic motility but does not appear to modulate gastric functions (40, 51, 55, 58). The A2 area provides major inputs to the medial parvocellular neurons in the paraventricular nucleus of the hypothalamus to induce CRF synthesis and release in response to stress (21, 44), possibly via activation of α1-adrenoceptors (43). In addition to this indirect pathway, catecholaminergic neurons of the A2 area, which may represent a subgroup distinct from those that project to the hypothalamus, also have a direct effect on gastric-related brainstem vagal circuits (33, 38, 46). Our results indicate the number of DβH-IR A2 area neurons in which DβH is increased in CHe rats, suggestive of increased norepinephrine signaling in local vagal neurocircuits. The resulting increase in norepinephrine release activates α2-adrenoceptors to inhibit DMV neuronal excitability, via both direct actions on the membrane of the DMV neurons as well as indirect effects to decrease the NTS to DMV GABAergic transmission, hence the vagal control of gastric tone and motility. Because the amplitude of the outward current induced in DMV neurons following UK14304 perfusion was similar in all experimental groups, the larger tone decrease observed in CHe is likely to be due to an effect of UK14304 on GABAergic inputs impinging onto DMV neurons, rather than on DMV neurons themselves.
The functions of the upper GI tract, including gastric tone and motility, are finely tuned by the activity of vagal preganglionic DMV neurons (13, 56). The pacemaking DMV motoneurons are influenced heavily by tonic GABAergic inputs (1, 47), originating, in large part, from NTS neurons (57). Many neuroactive substances that control gastric motility via central vagal mechanisms do not, however, appear to modulate this GABA input in basal conditions (56).
Increasing levels of cAMP in the GABAergic terminal either by pharmacological (e.g., forskolin, TRH, CRF, or agents that increase adenylate cyclase activity) (9, 16), physiological, pathophysiological (e.g., feeding or stress) (7, 13, 19, 20, 39, 54), or surgical (e.g., vagal deafferentation) (14) means, however, results in receptor insertion onto the presynaptic terminal membrane such that the previous unresponsive GABAergic synaptic inputs can be modulated. Our data suggest that the α2-adrenoceptor-mediated NTS to DMV GABAergic synaptic transmission is modulated in a similar manner. In fact, in naive DMV neurons from control rats, perfusion with UK14304 failed to alter GABAergic neurotransmission unless the cAMP levels were elevated, either by forskolin or CRF.
In a subpopulation of DMV neurons from CHe rats, however, α2-adrenoceptor activation is capable of inhibiting GABAergic neurotransmission, even in absence of pharmacological manipulations with CRF, suggesting that, in these neurons, the cAMP levels were sufficiently elevated to allow inhibitory neurotransmission to be modulated. Conversely, in all DMV neurons from the CHo stress group, α2-adrenoceptor activation was able to modulate GABAergic neurotransmission. Because the modulation of GABAergic synapses as a result of temporary elevations in cAMP levels appear to be relatively brief (11, 12, 15), it is unclear whether this persistent GABAergic modulation reflects longer-term increases in cAMP levels or whether other, as yet unidentified, longer-lasting mechanisms are involved. In this regard, it is clear that stress during periods of early life development can alter brainstem neurocircuitry permanently (17, 45). It remains to be determined whether the neuroplasticity observed following chronic stress is permanent or, if temporary, over what time period normal neuronal functions are restored.
In a series of experiments in which the effects of acute, CHo, and CHe stress loads were tested, Takahashi’s group (2–4, 6, 62, 63) showed that the increased levels of CRF in the hypothalamus of acute and CHe stressed rodents were correlated linearly with a reduction in gastric, but an increase in colonic, motility. These same studies also showed that, in CHo stressed rodents, however, the levels of hypothalamic oxytocin increased in relation to stress adaptation. Our group showed recently that, both in acute stress, as well as following CRF administration, the ability of oxytocin to modulate GABAergic transmission in vagal neurocircuits underwent plastic rearrangements (11) in a manner similar to those observed in the present study. We thus put forward the hypothesis that the α2-adrenoceptor-mediated tonic inhibition of GABAergic transmission observed as a consequence of CHo stress load in the present study may serve as a gastroprotective mechanism to prevent further dysfunctions of gastric motility induced by prolonged periods of stress. This potentially protective mechanism, however, is observed to a lesser extent in a small subpopulation of vagal motoneurons from CHe stress rats, who also responded with a more profound gastroinhibition upon α2-adrenoceptor activation. It is of interest to note, in this regard, that CHe also showed some degree of adaptation, albeit to a lesser extent than CHo. It is thus possible that, although the final measurable, static outcome, whether analyzed with electrophysiological or in vivo methods, is qualitatively similar in CHo and CHe groups, although quantitatively larger in CHe, which show a lesser degree of adaptation, the pathophysiological significance of the neurocircuit is different, i.e., CHo may engage a protective mechanism that limits further gastric dysmotility; conversely, CHe may worsen the dysmotility by further enhancing the gastroinhibition induced by α2-adrenoceptor activation.
The question arises, then, as to what further type of plasticity occurs to distinguish the CHo from CHe stress to result in adaptation or maladaptation, respectively. It is possible that the increased CRF synthesis and release observed in CHe rats (62) engages a vagal neurocircuit that is less sensitive and/or less open to neuroplasticity. Consequently, this neurocircuit is differentially responsive to α2-adrenoceptor activation than that engaged during CHo stress. Indeed, our previous studies have shown that exposure to CRF triggers the involvement of a different vagal efferent pathway; as a result, both the in vitro as well as the in vivo gastric response to the prototypical antistress peptide, oxytocin, are altered radically (11).
Thus it is possible that in CHo the pre- and postsynaptic α2-adrenoceptor responses to noradrenaline attenuate the adverse gastric responses to stress and restore basal gastric functions. Conversely, in CHe, the upregulation of noradrenergic A2 area neurons in combination with the increased sensitivity of vagal neurocircuits to α2-adrenoceptor activation prevents the physiological adaptation to stress and results in an exaggerated and adverse, gastric response.
At the moment, however, it is unclear why both gastric tone and motility responses to microinjection of UK14304 occur exclusively via withdrawal of cholinergic pathways in control rats. Conversely, in stressed rats, the UK14304 -mediated decrease in antrum motility is mediated by a combined effect that includes cholinergic withdrawal and increased release of NO by the NANC pathway, and the gastroinhibitory effects on tone are mediated exclusively by NANC pathways. This observation also suggests that the adrenergic control of antrum tone and motility involves different pathways.
In conclusion, these results indicate that chronic stress involves the rearrangement of vagal neurocircuits that control gastric functions. Although the pathophysiological changes that occur in the dorsal vagal complex that result in either adaptation or maladaptation of the upper GI tract are yet to be uncovered completely, the present study provides new insights, not only into the plasticity of these neural circuits, but also in the role played by catecholamines in the hindbrain following a brief period of chronic stress.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK 55530, DK 78364, and DK 99350.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.J., K.N.B., L.T., and R.A.T. conceived and designed research; Y.J., K.N.B., L.T., and R.A.T. performed experiments; Y.J., K.N.B., L.T., and R.A.T. analyzed data; Y.J., K.N.B., L.T., and R.A.T. interpreted results of experiments; Y.J., K.N.B., L.T., and R.A.T. prepared figures; Y.J., K.N.B., L.T., and R.A.T. drafted manuscript; Y.J., K.N.B., L.T., and R.A.T. edited and revised manuscript; Y.J., K.N.B., L.T., and R.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Cesare M. and Zoraide Travagli and W. Nairn Browning for support and encouragement.
REFERENCES
- 1.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 300: G21–G32, 2011. doi: 10.1152/ajpgi.00363.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Sustained acceleration of colonic transit following chronic homotypic stress in oxytocin knockout mice. Neurosci Lett 495: 77–81, 2011. doi: 10.1016/j.neulet.2011.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Babygirija R, Bülbül M, Yoshimoto S, Ludwig K, Takahashi T. Central and peripheral release of oxytocin following chronic homotypic stress in rats. Auton Neurosci 167: 56–60, 2012. doi: 10.1016/j.autneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Babygirija R, Yoshimoto S, Gribovskaja-Rupp I, Bülbül M, Ludwig K, Takahashi T. Social interaction attenuates stress responses following chronic stress in maternally separated rats. Brain Res 1469: 54–62, 2012. doi: 10.1016/j.brainres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Babygirija R, Zheng J, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Sustained delayed gastric emptying during repeated restraint stress in oxytocin knockout mice. J Neuroendocrinol 22: 1181–1186, 2010. doi: 10.1111/j.1365-2826.2010.02069.x. [DOI] [PubMed] [Google Scholar]
- 6.Babygirija R, Zheng J, Ludwig K, Takahashi T. Central oxytocin is involved in restoring impaired gastric motility following chronic repeated stress in mice. Am J Physiol Regul Integr Comp Physiol 298: R157–R165, 2010. doi: 10.1152/ajpregu.00328.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthoud HR, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav 89: 517–524, 2006. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Bertolino M, Vicini S, Gillis R, Travagli A. Presynaptic alpha2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol 272: G654–G661, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Blake CB, Smith BN. cAMP-dependent insulin modulation of synaptic inhibition in neurons of the dorsal motor nucleus of the vagus is altered in diabetic mice. Am J Physiol Regul Integr Comp Physiol 307: R711–R720, 2014. doi: 10.1152/ajpregu.00138.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Browning KN, Babic T, Holmes GM, Swartz E, Travagli RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol 591: 1563–1580, 2013. doi: 10.1113/jphysiol.2012.246827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browning KN, Babic T, Toti L, Holmes GM, Coleman FH, Travagli RA. Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor. J Physiol 592: 4591–4605, 2014. doi: 10.1113/jphysiol.2014.278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browning KN, Kalyuzhny AE, Travagli RA. Mu-opioid receptor trafficking on inhibitory synapses in the rat brainstem. J Neurosci 24: 7344–7352, 2004. doi: 10.1523/JNEUROSCI.1676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol 4: 1339–1368, 2014. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browning KN, Travagli RA. Functional organization of presynaptic metabotropic glutamate receptors in vagal brainstem circuits. J Neurosci 27: 8979–8988, 2007. doi: 10.1523/JNEUROSCI.1105-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Browning KN, Travagli RA. Modulation of inhibitory neurotransmission in brainstem vagal circuits by NPY and PYY is controlled by cAMP levels. Neurogastroenterol Motil 21: 1309–e126, 2009. doi: 10.1111/j.1365-2982.2009.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastric function. Neurogastroenterol Motil 22: 1154–1163, 2010. doi: 10.1111/j.1365-2982.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Card JP, Levitt P, Gluhovsky M, Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J Neurosci 25: 9102–9111, 2005. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology S0016-5085(16)00223-7, 2016. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Drucker DJ. The biology of incretin hormones. Cell Metab 3: 153–165, 2006. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev 86: 805–847, 2006. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 21.Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann NY Acad Sci 1018: 25–34, 2004. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- 22.Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur J Neurosci 36: 2547–2555, 2012. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol 393: 213–231, 1987. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukudo S. IBS: Autonomic dysregulation in IBS. Nat Rev Gastroenterol Hepatol 10: 569–571, 2013. doi: 10.1038/nrgastro.2013.166. [DOI] [PubMed] [Google Scholar]
- 25.George SA, Knox D, Curtis AL, Aldridge JW, Valentino RJ, Liberzon I. Altered locus coeruleus-norepinephrine function following single prolonged stress. Eur J Neurosci 37: 901–909, 2013. doi: 10.1111/ejn.12095. [DOI] [PubMed] [Google Scholar]
- 26.Grissom N, Bhatnagar S. Habituation to repeated stress: get used to it. Neurobiol Learn Mem 92: 215–224, 2009. doi: 10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grissom N, Kerr W, Bhatnagar S. Struggling behavior during restraint is regulated by stress experience. Behav Brain Res 191: 219–226, 2008. doi: 10.1016/j.bbr.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grover M, Drossman DA. Functional abdominal pain. Curr Gastroenterol Rep 12: 391–398, 2010. doi: 10.1007/s11894-010-0125-0. [DOI] [PubMed] [Google Scholar]
- 29.Grundy D, Brookes S, editors. Neural Control of Gastrointestinal Function. Williston, VT: Morgan & Claypool Life Sciences, 2012. [Google Scholar]
- 30.Guo JJ, Browning KN, Rogers RC, Travagli RA. Catecholaminergic neurons in rat dorsal motor nucleus of vagus project selectively to gastric corpus. Am J Physiol Gastrointest Liver Physiol 280: G361–G367, 2001. doi: 10.1152/ajpgi.2001.280.3.G361. [DOI] [PubMed] [Google Scholar]
- 31.Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci 7: 61, 2013. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman JP, McKlveen JM, Ghosal S, Kopp B, Wulsin A, Makinson R, Scheimann J, Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol 6: 603–621, 2016. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman MA, Niedringhaus M, Alayan A, Verbalis JG, Sahibzada N, Gillis RA. Characterization of noradrenergic transmission at the dorsal motor nucleus of the vagus involved in reflex control of fundus tone. Am J Physiol Regul Integr Comp Physiol 294: R720–R729, 2008. doi: 10.1152/ajpregu.00630.2007. [DOI] [PubMed] [Google Scholar]
- 34.Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. II. Dopaminergic, noradrenergic (A1 and A2) and adrenergic neurons, nerve fibers, and presumptive terminal processes. J Comp Neurol 233: 308–332, 1985. doi: 10.1002/cne.902330303. [DOI] [PubMed] [Google Scholar]
- 35.Khoo J, Rayner CK, Feinle-Bisset C, Jones KL, Horowitz M. Gastrointestinal hormonal dysfunction in gastroparesis and functional dyspepsia. Neurogastroenterol Motil 22: 1270–1278, 2010. doi: 10.1111/j.1365-2982.2010.01609.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim SE, Chang L. Overlap between functional GI disorders and other functional syndromes: what are the underlying mechanisms? Neurogastroenterol Motil 24: 895–913, 2012. doi: 10.1111/j.1365-2982.2012.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol 543: 135–146, 2002. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez-Peña y Valenzuela I, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol 286: G333–G339, 2004. doi: 10.1152/ajpgi.00289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, Drucker DJ. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev 55: 167–194, 2003. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 40.Mönnikes H, Schmidt BG, Tebbe J, Bauer C, Taché Y. Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus nuclei stimulates colonic motor function in rats. Brain Res 644: 101–108, 1994. doi: 10.1016/0006-8993(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 41.Myers B, Scheimann JR, Franco-Villanueva A, Herman JP. Ascending mechanisms of stress integration: implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neurosci Biobehav Rev 74: 366–375, 2017. doi: 10.1016/j.neubiorev.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakade Y, Fukuda H, Iwa M, Tsukamoto K, Yanagi H, Yamamura T, Mantyh C, Pappas TN, Takahashi T. Restraint stress stimulates colonic motility via central corticotropin-releasing factor and peripheral 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol 292: G1037–G1044, 2007. doi: 10.1152/ajpgi.00419.2006. [DOI] [PubMed] [Google Scholar]
- 43.Plotsky PM, Cunningham ET Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev 10: 437–458, 1989. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- 44.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol 300: R222–R235, 2011. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinaman L, Banihashemi L, Koehnle TJ. Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiol Behav 104: 632–640, 2011. doi: 10.1016/j.physbeh.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol 285: R479–R489, 2003. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterol Motil 10: 305–313, 1998. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 48.Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol 512: 149–162, 1998. doi: 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stengel A, Taché Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp Biol Med (Maywood) 235: 1168–1178, 2010. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stengel A, Taché Y. Neuroendocrine control of the gut during stress: corticotropin-releasing factor signaling pathways in the spotlight. Annu Rev Physiol 71: 219–239, 2009. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 117: 33–40, 2007. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep 11: 270–277, 2009. doi: 10.1007/s11894-009-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taché Y, Maeda-Hagiwara M, Turkelson CM. Central nervous system action of corticotropin-releasing factor to inhibit gastric emptying in rats. Am J Physiol 253: G241–G245, 1987. [DOI] [PubMed] [Google Scholar]
- 54.Tache Y, Million M. Central corticotropin-releasing factor and the hypothalamic-pituitary-adrenal axis in gastrointestinal physiology. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. Cambridge, MA: Academic, 2006, p. 791–816. [Google Scholar]
- 55.Tache Y, Monnikes H, Bonaz B, Rivier J. Role of CRF in stress-mediated alterations of gastric and colonic motor function. Ann NY Acad Sci 697: 233–243, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol 13: 389–401, 2016. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol 260: G531–G536, 1991. [DOI] [PubMed] [Google Scholar]
- 58.Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583: 194–203, 2008. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol 272: R59–R67, 1997. [DOI] [PubMed] [Google Scholar]
- 60.Wood SK, Valentino RJ. The brain norepinephrine system, stress and cardiovascular vulnerability. Neurosci Biobehav Rev 74, Pt B: 393–400, 2017. doi: 10.1016/j.neubiorev.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Z, Wang L, Gao W, Hu F, Zhang J, Ren Y, Lin R, Feng Q, Cheng M, Ju D, Chi Q, Wang D, Song S, Luo M, Zhan C. A central catecholaminergic circuit controls blood glucose levels during stress. Neuron 95: 138–152.e5, 2017. doi: 10.1016/j.neuron.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 62.Zheng J, Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol 299: G946–G953, 2010. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng J, Dobner A, Babygirija R, Ludwig K, Takahashi T. Effects of repeated restraint stress on gastric motility in rats. Am J Physiol Regul Integr Comp Physiol 296: R1358–R1365, 2009. doi: 10.1152/ajpregu.90928.2008. [DOI] [PubMed] [Google Scholar]