Abstract
Maternal obesity in pregnancy has been linked to a spectrum of adverse developmental changes. Involvement of eCBs in obesity is well characterized. However, information regarding eCB physiology in obesity associated with pregnancy is sparse. This study evaluated fetomaternal hepatic, systemic, and placental eCB molecular changes in response to maternal consumption of a HFD. From ≥9 mo before conception, nonpregnant baboons (Papio spp.) were fed a diet of either 45 (HFD; n = 11) or 12% fat or a control diet (CTR; n = 11), and dietary intervention continued through pregnancy. Maternal and fetal venous plasma samples were evaluated using liquid chromatography-mass spectrometry to quantify AEA and 2-AG. Placental, maternal and fetal hepatic tissues were analyzed using RT-PCR, Western blot, and immunohistochemistry. mRNA and protein expression of endocannabinoid receptors (CB1R and CB2R), FAAH, DAGL, MAGL, and COX-2 were determined. Statistical analyses were performed with the nonparametric Scheirer-Ray-Hare extension of the Kruskal-Wallis test to analyze the effects of diet (HFD vs. CTR), fetal sex (male vs. female), and the diet × sex interaction. Fetal weight was influenced by fetal sex but not by maternal diet. The increase in maternal weight in animals fed the HFD vs. the CTR diet approached significance (P = 0.055). Maternal circulating 2-AG concentrations increased, and fetal circulating concentrations decreased in the HFD group, independently of fetal sex. CB1R receptor expression was detected in syncytiotrophoblasts (HFD) and the fetal endothelium (CTR and HFD). Placental CB2R protein expression was higher in males and lower in female fetuses in the HFD group. Fetal hepatic CB2R, FAAH, COX-2 (for both fetal sexes), and DAGLα (in male fetuses) protein expression decreased in the HFD group compared with the CTR group. We conclude that consumption of a HFD during pregnancy results in fetal systemic 2-AG and hepatic eCB deficiency.
Keywords: endogenous cannabinoids, high-fat diet, liver, placenta, pregnancy
INTRODUCTION
Maternal obesity (MO) affects fetal development, which in turn influences each individual’s lifetime health trajectory. Specifically, MO has been linked to offspring autism spectrum disorders, inflammatory bowel syndrome, fibromyalgia, asthma, nonalcoholic fatty liver disease (NAFLD), diabetes, and obesity (5, 22, 81). Several suggested mechanisms have linked obesity to offspring health, such as oxidative stress (10), inflammation (4), fatty acid transport (51), and stress-related pathways (25). The endogenous cannabinoid (eCB) system (ECS) is involved in appetite regulation and lipogenesis (20). Although the spectrum of adverse offspring health conditions programmed by MO is likely to be broad, it includes a spectrum of conditions that are described as clinical endocannabinoid deficiency syndrome (69). The ECS is the family of biologically active lipids, derivatives of ω-3 fatty acids, that regulate vascular tone, metabolic rate, neurogenesis, inflammation, and stress responses, which are all hallmarks of MO. In addition, circulating eCB concentrations are increased in obese individuals. We have shown that fetal circulating eCB concentrations are reduced in MO in human pregnancy (70), and placental eCB content is decreased in a baboon MO model of overfeeding (13). Many different patterns of MO exist, including prepregnancy obesity vs. pregnancy-related weight gain and overeating vs. high-fat diet (HFD), which makes human epidemiological studies of the mechanisms of developmental programming by MO challenging. Animal models represent an opportunity to dissect specific mechanisms related to the various dietary patterns that result in MO and provide important data for the development of interventional strategies in humans. This study describes the influence of maternal HFD and fetal sex on systemic, hepatic, and placental eCB systems and tests the hypothesis that maternal consumption of a HFD leads to fetal eCB deprivation.
MATERIALS AND METHODS
Care of Animals
To produce obesity, normal weight female baboons (Papio spp.) were fed a diet of 45% fat (HFD; n = 11) ad libitum, whereas controls (CTR; n = 11) ate 12% fat for ≥9 mo before conception and during pregnancy. The dietary composition is described in detail elsewhere (53, 57). Animals were housed in a controlled outdoor environment in social cages of 14–16 females and one male with full physical and social activity (33). Cesarean sections were performed under general anesthesia at 0.9 days gestation (165 dGA), as previously described (57). Fetal, placental, and maternal tissues were collected and preserved by either formalin fixation (for immunohistochemistry) or by freezing in liquid nitrogen and storage at −80°C for protein/RNA quantification. All procedures were approved by the Institutional Animal Care and Use Committee at the Southwest National Primate Research Center (protocol no. 1244PC).
Estimation of 2-Arachidonoyl Glycerol and Anandamide Concentrations in Fetal and Maternal Serum Using Liquid Chromatography-Mass Spectrometry/Mass Spectrometry
Both 2-arachidonoyl glycerol (2-AG) and anandamide (AEA) in fetal and maternal serum were quantified as previously described (13). Briefly, AEA, arachidonoyl ethanolamide, internal standard d4-AEA, 2-AG, and internal standard d5-2-AG were purchased from Sigma-Aldrich. All chemicals and solvents (liquid chromatography-mass spectrometry grade) were obtained from Fisher Scientific Chemicals. Aliquots (50 µl) of standards and study samples were extracted using toluene (1 ml) (84) containing a mixture of 100 ng/ml d4-AEA and d5-2AG as standards. Upon solvent removal, the obtained residue was dissolved in reconstitution solution (120 μl, 80:20 vol/vol of acetonitrile and water with 0.1% FA) and subjected to analysis. AEA and 2-AG were eluted using a CORTECs C18 column (2.1 × 50 mm, 1.6 µM; Waters Technologies) with a mobile phase consisting of 0.1% formic acid in water-acetonitrile (linear gradient of 40–90% mobile phase, 2 min) and a flow rate of 0.5 ml/min. The UHPLC system was connected to an API-5500 QTRAP mass spectrometer (AB Sciex; Singapore). Briefly, we used a multiple-reaction monitoring mode with an electrospray ionization source in positive ion mode. The following transition pairs were monitored: m/z 348.9/62.1 for AEA, 352.9/66.1 for d4-AEA, 379.1/298.0 for 2-AG, and 384.2/287.0 for d5-2-AG. The identification and quantification of analytes were confirmed by comparison of precursors and product ion m/z values and LC retention times with standards and by comparing the results with previously reported data (36). The peaks of analytes were integrated and quantified using Analyst 1.6.2 software (AB Sciex).
Reverse Transcription Real-Time Quantitative PCR
The TRIzol method was used to isolate RNA from tissue samples (cat. no. 15596026 Life Technologies), and cDNA was synthesized according to the manufacturer’s instructions (Applied Biosystems; Roche). Quantitative PCR was performed using FastStart Essential DNA Probe Master Mix (cat. no. 06402682001; Roche) and TaqMan Gene Expression Assay Probes (Life Technologies) for quantification of endocannabinoid receptors (CB1R; Hs01038522), fatty acid amide hydrolase-1 (FAAH-1; HS01038664), and diacylglyceride lipase A (DAGL-A; Hs01023067). The housekeeping gene used for relative quantifications of monoacylglycerol lipase (MAGL), CB1R, and FAAH was 18S either as probe set (Hs99999901) or primer set with 5′-GTAACCCGTTGAACCCCATT (forward) and 3′-CCATCCAATCGGTAGTAGCG (reverse) primers. The CB2R, cyclooxygenase2 (COX-2), and MAGL transcripts were detected using FastStart Essential DNA Green Master Mix (cat. no. 06402712001; Roche) with 5′ (forward) and 3′ (reverse) primers: CBR2 with 5′-TCCTGGGAGAGGACAGAAAA and 3′-CAGGCTGTCTTCCACAGGCTGTCTTCCA; COX2 with 5′ GTTCCACCCGCAGTACAGAA (forward) and 3′-AGGGCTTCAGCATAAAGCGT (reverse); and MAGL with 5′ ATGCAAACGCCAGCACATA (forward) and 3′ CATCCCCCAGACCATGCG (reverse). β-Actin was used for detection of the mRNA of the housekeeping gene (5′-CCAGAGGCGTACAGGGATAG and 3′-CCAACCGCGAGAAGATGA).
Immunohistochemistry
Immunohistochemistry (IHC) was performed using a CB1R monoclonal primary antibody (cat. no. Img-CB1R-mab001; Immunogenes, Budakeszi, Hungary), a CB2R monoclonal primary antibody (cat. no. H00001269-M01; Novus Biologicals, Littleton, CO), and an anti-mouse secondary antibody (Vectastain ABC Kit anti-mouse IgG, cat. no. PK-4002; Vector Laboratories, Burlingame, CA). The tissue (5 µm thick) was deparaffinized in xylene (cat. no. X3S3; Fisher, Houston, TX) twice and subsequently rehydrated using an EtOH series of 100, 95, 70, and 50% for 5 min each, all at room temperature (RT). Slides were boiled twice in antigen-retrieval solution (10 mM sodium citrate, 0.05% Tween-20, pH 6.0) using the 900-W microwave setting for 6 min each. After incubation at RT for 5 min, the slides were washed in PBST [1× PBS (cat. no. PB399500) + 0.1% Tween-20 (cat. no. P1379, 100 ml; Sigma-Aldrich, St. Louis, MO)] for 5 min, placed into 1.5% peroxide solution (cat. no. H341-500; Fisher) in PBS for 20 min, and subsequently washed four times for 5 min with PBST. The slides were then incubated for 50 min in the blocking serum provided in the Vectastain ABC Kit. After blocking, the slides were incubated with either CB1R (1:200) or CB2R (1:500) antibodies at 4°C in a humidified tray. Subsequently, the slides were washed four times for 5 min with PBST, incubated for 30 min in the Vectastain ABC reagent, and washed four times for 5 min with PBST. DAB (3,3′-diaminobenzidine tetrahydrochloride) Enhanced Liquid Substrate (cat. no. D3939; Sigma-Aldrich) was prepared and applied according to manufacturer’s instructions, and the reaction was stopped after 45”-3′ (depending on signal intensity) with high-purity water (cat. no. 23-249-590; Fisher). The exposure time was the same for the given tissue type and experimental group. The slides were covered with fluorescence mounting medium (cat. no. S3023; Dako, Carpinteria, CA) and scanned using the NanoZoomer SQ (Hamamatsu, Middlesex, NJ); quantification was performed with ImageScope version 11.1.2.752 by Aperio (Leica Biosystems, Buffalo Grove, IL), as previously described (13). Briefly, a quantification algorithm was developed for each tissue type, and the staining intensities of CB1R and CB2R were estimated using positive pixel counts. The staining intensity is expressed as a histoscore, as previously described (13, 56).
Western Blotting
Placenta and liver samples were crushed in liquid nitrogen and homogenized in RIPA buffer (cat. no. R0287; Sigma-Aldrich) five times for intervals of 30 s each. Homogenates were centrifuged for 30 min at 3,500 rpm at 4°C. The supernatants were placed in new centrifuge tubes and stored at −20°C. The protein concentration per sample was quantified using a spectrophotometer (BioTek). The samples were warmed at 70°C for 10 min. Forty micrograms of protein was loaded and separated in 9–12% SDS-PAGE gels under reducing conditions and then blotted to PVDF membranes. Membranes were blocked with 5% fat-free milk (cat. no. 170-6404; Bio-Rad) for 1 h. Membranes were probed with primary antibodies to detect CB1R (1:1,000, cat. no. IMG-CB1R-mAb001; Immunogenes), CB2R (1:1,000, cat. no. H00001269-M01; Abnova), FAAH-1 (1:1,000, cat. no. ab54615; Abcam, Cambridge, MA), DAGLα (1:1,000, cat no. SC-390409; Santa Cruz Biotechnology), MAGL (1:1,000, cat. no. 100035; Cayman Chemical, Ann Arbor, MI), and COX-2 (1:1,000, cat. no. ab62331; Abcam) in 5% BSA (cat. no. A4503; Sigma-Aldrich) in TBS-T for 3–12 h. After washing in fresh TBS-T (3 times for a total of 30 min), membranes were incubated with peroxidase-conjugated anti-mouse secondary antibody (cat. no. 715-035-150; Jackson ImmunoResearch Laboratories) at 1:10,000 in 5% BSA in TBS-T for 1 h. Membranes were washed in fresh TBS-T (3 times for a total of 30 min), and the bands were detected using clarity Western ECL substrate (cat. no. 170-5060; Bio-Rad) and a ChemiDoc-IT TS3 815 Imager (Ultra Violet Products). Band intensities were quantified using ImageJ software (National Institutes of Health).
Statistical Analysis
All data are summarized as means ± SE. Shapiro-Wilk and Levene’s tests were used to assess normality and equality of variance, respectively. Because multiple variables did not meet the criteria for a 2 × 2 analysis of variance due to nonnormal distributions and very small sample sizes, the nonparametric Scheirer-Ray-Hare extension of the Kruskal-Wallis test was used to analyze the effect of diet (HFD vs. CTR), fetal sex (male vs. female), and the diet × sex interaction. CB1R and CB2R data from IHC were analyzed using the same approach, but the effects of fetal sex and the diet × sex interaction were not calculated because of the IHC experimental setup. The significance was set at 0.05.
RESULTS
Fetal and Maternal Morphometry
Fetal weight was influenced by fetal sex but not by maternal dietary pattern (Table 1). The increase in maternal weight in animals fed the HFD approached significance (P = 0.055).
Table 1.
Maternal and fetal weights in CTR and HFD groups
| Female Fetuses |
Male Fetuses |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| CTR | HFD | CTR | HFD | Diet | Sex | Diet × sex | |
| Fetal wt, g | 712.8 ± 64.62 (n = 7) | 767.4 ± 31.32 (n = 4) | 798.8 ± 28.66 (n = 4) | 853.4 ± 16.24 (n = 6) | 0.220 | 0.045 | 0.195 |
| Maternal CS wt, kg | 17.5 ± 1.27 (n = 6) | 20.7 ± 0.88 (n = 4) | 17.7 ± 0.56 (n = 4) | 20.2 ± 0.88 (n = 6) | 0.055 | 0.744 | 0.156 |
| Placental wt, g | 171.8 ± 5.03 (n = 6) | 202.6 ± 14.06 (n = 4) | 159.3 ± 4.75 (n = 2) | 229.4 ± 6.36 (n = 5) | |||
| Liver, g | 20.8 ± 2.56 (n = 3) | 26.0 ± 1 (n = 4) | 24.4 ± 0 (n = 1) | 24.9 ± 0.97 (n = 5) | |||
Values are means ± SE. CTR, control; HFD, high-fat diet; CS, Cesarean section. Note: HFD animals were fed a diet of 45% fat, and CTR were animals fed a diet of 12% fat. Because placental and liver weights presented missing values, only descriptive data are presented for these variables. Mat CS weight is maternal weight at the time of the Cesarean section.
Circulating Concentrations of 2-AG and AEA in Dams and Their Fetuses
Maternal 2-AG concentrations were increased and fetal concentrations decreased in the HFD group independent of fetal sex (Table 2).
Table 2.
AEA and 2-AG concentrations by diet and fetal sex
| Female Fetuses |
Male Fetuses |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| CTR | HFD | CTR | HFD | Diet | Sex | Diet × sex | |
| Fetus | |||||||
| AEA, nM/l | 0.71 ± 0.17 (n = 4) | 0.89 ± 0.22 (n = 5) | 1.10 ± 0.13 (n = 5) | 0.65 ± 0.05 (n = 6) | 0.21 | 0.53 | 0.06 |
| 2-AG, nM/l | 53.99 ± 12.82 (n = 4) | 22.94 ± 3.22 (n = 5) | 59.40 ± 26.38 (n = 5) | 22.79 ± 3.66 (n = 6) | 0.01 | 0.51 | 0.58 |
| Mother | |||||||
| AEA, nM/l | 1.82 ± 0.19 (n = 3) | 1.81 ± 0.17 (n = 5) | 1.46 ± 0.28 (n = 5) | 1.83 ± 0.17 (n = 6) | 0.6 | 0.44 | 0.4 |
| 2-AG, nM/l | 86.26 ± 11.86 (n = 3) | 109.96 ± 29.08 (n = 5) | 82.21 ± 11.76 (n = 5) | 194.27 ± 30.61 (n = 6) | 0.05 | 0.25 | 0.17 |
| Maternal/fetal ratio | |||||||
| AEA | 3.58 ± 0.84 (n = 3) | 2.37 ± 0.42 (n = 5) | 1.39 ± 0.30 (n = 5) | 2.91 ± 0.39 (n = 6) | 0.5 | 0.16 | 0.03 |
| 2-AG | 2.27 ± 0.73 (n = 3) | 4.84 ± 1.00 (n = 5) | 3.89 ± 2.30 (n = 5) | 9.36 ± 1.64 (n = 6) | 0.02 | 0.28 | 0.53 |
Values are means ± SE. AEA, anandamide; 2-AG, 2-arachidonoyl glycerol; CTR, control; HFD, high-fat diet.
Endocannabinoid System Expression in Placenta and Maternal and Fetal Liver
Maternal and fetal hepatic endocannabinoid system.
immunohistochemistry.
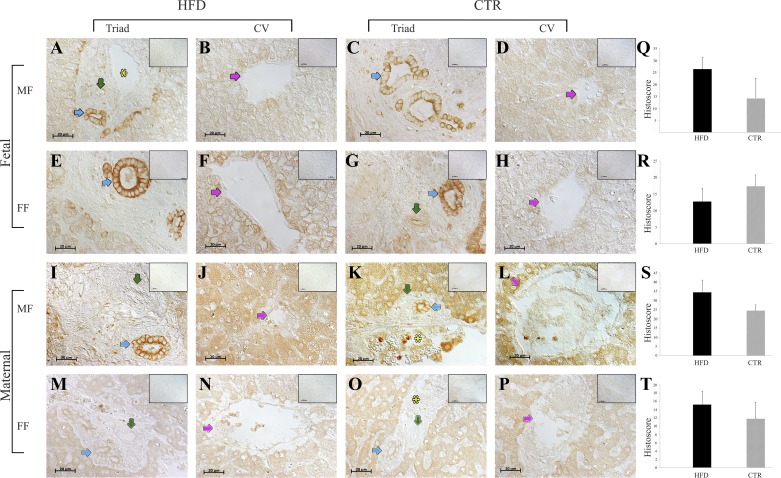
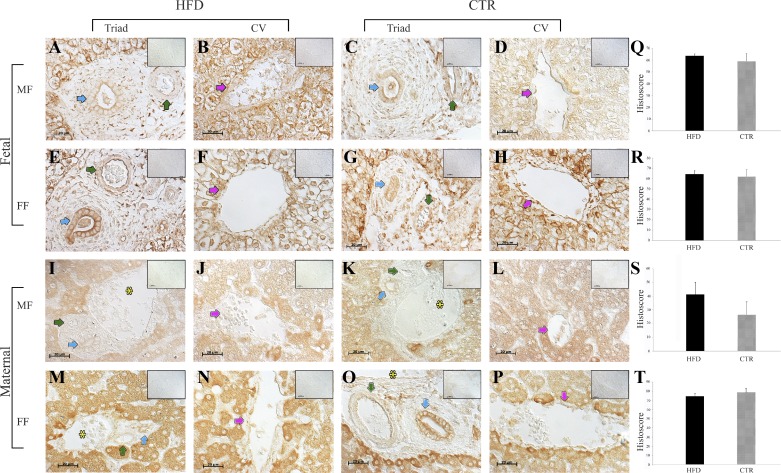
CB1R was expressed in the cytoplasm (dams) and cell membranes (fetuses) of the periportal hepatocytes that surround the central vein and the bile duct epithelium (Fig. 1). Histoscores were not different between the HFD and CTR groups. CB2R expression was evident in the fibroblasts of the connective tissues of the triad, bile ducts, pericentral hepatocytes, and arterial walls (Fig. 2), and histoscores were not different between the HFD and CTR groups.
Fig. 1.
Endocannabinoid receptor 1 (CB1R) protein expression in the maternal and fetal livers of high-fat diet (HFD; n = 11) and control (CTR; n = 10) animals. A–D: representative images of fetal livers from male fetuses (MF) in the CTR (n = 3) and HFD (n = 6) groups. E–H: the fetal livers from female fetuses (FF) in the CTR (n = 6) and HFD (n = 3) groups. I–L: maternal livers of dams with MF in the CTR (n = 4) and HFD (n = 5) groups. M–P: maternal liver sections from dams carrying FF in the CTR (n = 5) and HFD (n = 4) groups. Q–T: quantitative analyses. Blue arrows point to bile ducts, green arrows point to arteries, pink arrows point to central veins (CV), and yellow asterisks indicate the portal vein. Negative controls (omission of primary antibodies) are shown in A–P, insets (top right). Scale bars, 20 μm. The data are presented as means ± SE. The portal triad is defined by the portal vein, bile duct, and hepatic artery.
Fig. 2.
Endocannabinoid receptor 2 (CB2R) protein expression in the maternal and fetal livers of high-fat diet (HFD; n = 11) and control (CTR; n = 10) animals. A–D: representative images of fetal livers from male fetuses (MF) in the CTR (n = 3) and HFD (n = 6) diet groups. E–H: the fetal livers from female fetuses (FF) in the CTR (n = 4) and HFD (n = 4) groups. I–L: maternal livers of dams with MF in the CTR (n = 3) and HFD (n = 5) groups. M–P: maternal liver sections from dams carrying FF in the CTR (n = 5) and HFD (n = 4) groups. Q–T: quantitative analyses. Blue arrows point to bile ducts, green arrows point to arteries, pink arrows point to central veins (CV), and yellow asterisks indicate the portal vein. Negative controls (omission of primary antibodies) are shown in A–P, insets (top right). Scale bars, 20 μm. The data are presented as means ± SE.
rt-pcr and western blot analyses.
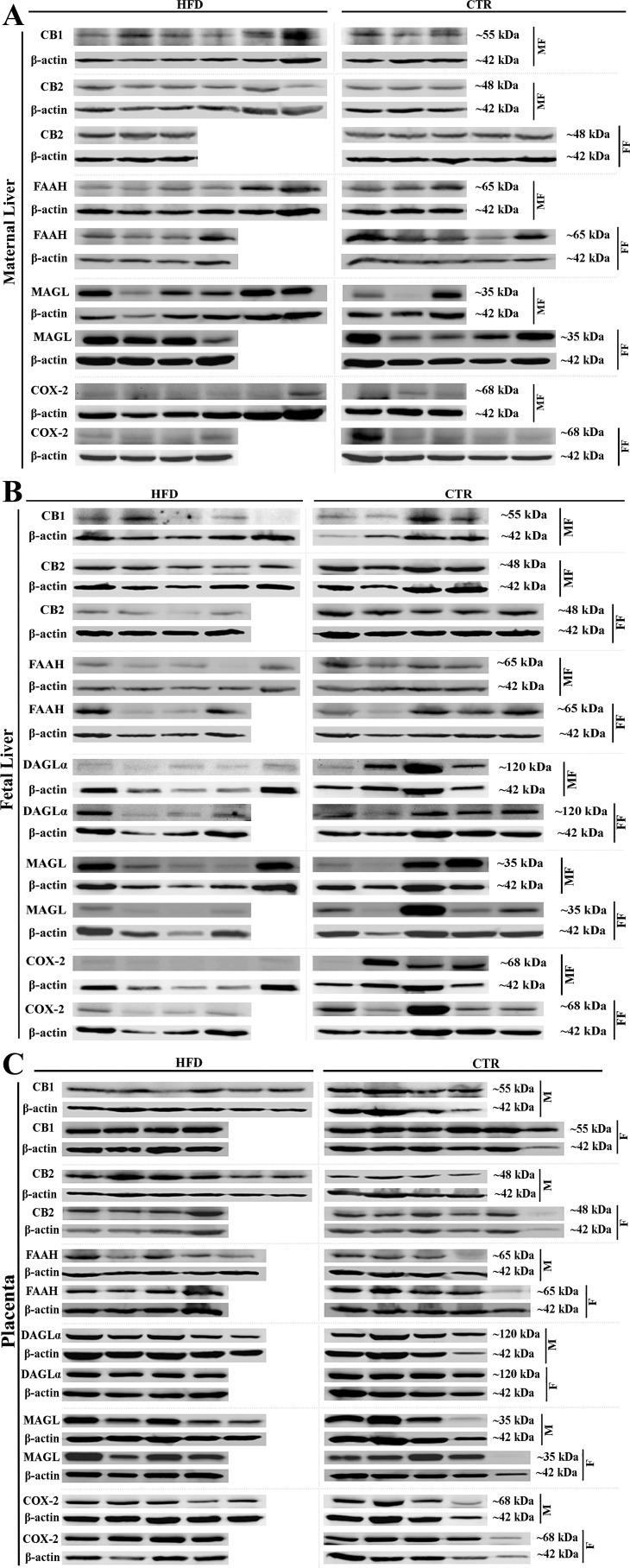
Gene product expression was similar between all groups (Table 3). Western blot analyses showed, that compared with the control diet, CB1R expression (full-length receptor, 55 kDa) was increased in HFD dams carrying male fetuses by 40% and decreased in their fetuses by 75% (Table 3). The expression of long isoforms of CB1R was low and below the detection limit in the HFD dams and their female fetuses. Maternal hepatic CB2R, FAAH-1, MAGL, and COX-2 protein expression was similar between groups. Fetal expression of CB2R, FAAH, and COX-2 was decreased in both male and female fetuses, and the expression of DAGLα was decreased in the male fetuses of the HFD group compared with that in fetuses of the CTR group. Hepatic fetal MAGL protein expression did not differ between the HFD and control groups (Fig. 3 and Table 3).
Table 3.
Fetal and maternal hepatic expression of main endocannabinoid receptors and metabolizing enzymes in male and female fetuses near term of HFD and CTR mothers
| Female Fetuses |
Male Fetuses |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| CTR | HFD | CTR | HFD | Diet | Sex | Diet × sex | |
| CB1R | |||||||
| CB1R protein (fetal) | 1.33 ± 0.50 (n = 4) | 0.52 ± 0.20 (n = 5) | 0.086 | ||||
| CB1R protein (maternal) | 0.95 ± 0.23 (n = 3) | 1.41 ± 0.45 (n = 4) | 0.289 | ||||
| CB1R transcript (fetal) | 12.74 ± 5.99 (n = 6) | 6.33 ± 5.15 (n = 5) | 10.34 ± 8.61 (n = 5) | 0.59 ± 0.36 (n = 5) | 0.171 | 0.356 | 0.523 |
| CB1R transcript (maternal) | 0.02 ± 0.01 (n = 3) | 1.94 ± 1.22 (n = 5) | 13.01 ± 9.35 (n = 4) | 1.64 ± 0.90 (n = 6) | 0.111 | 0.087 | 0.056 |
| CB2 | |||||||
| CB2R protein (fetal) | 1.06 ± 0.06 (n = 5) | 0.45 ± 0.08 (n = 4) | 1.08 ± 0.15 (n = 4) | 0.80 ± 0.09 (n = 5) | 0.003 | 0.324 | 0.214 |
| CB2 protein (maternal) | 1.14 ± 0.08 (n = 4) | 1.23 ± 0.05 (n = 4) | 1.23 ± 0.22 (n = 3) | 1.48 ± 0.23 (n = 6) | 0.146 | 0.355 | 0.391 |
| CB2R transcript (fetal) | 3.61 ± 1.08 (n = 6) | 3.57 ± 2.18 (n = 5) | 7.43 ± 5.14 (n = 5) | 14.59 ± 7.42 (n = 6) | 0.768 | 0.467 | 0.292 |
| CB2R transcript (maternal) | 0.40 ± 0.15 (n = 4) | 2.91 ± 1.45 (n = 5) | 2.11 ± 1.02 (n = 3) | 1.07 ± 0.23 (n = 4) | 0.412 | 0.298 | 0.205 |
| FAAH | |||||||
| FAAH protein (fetal) | 1.08 ± 0.18 (n = 5) | 0.51 ± 0.14 (n = 4) | 1.15 ± 0.15 (n = 4) | 0.43 ± 0.09 (n = 5) | 0.001 | 0.938 | 0.508 |
| FAAH protein (maternal) | 0.97 ± 0.25 (n = 4) | 0.95 ± 0.10 (n = 4) | 0.87 ± 0.08 (n = 4) | 0.84 ± 0.13 (n = 6) | 0.885 | 0.594 | 0.809 |
| FAAH transcript (fetal) | 2.20 ± 0.74 (n = 6) | 1.01 ± 0.43 (n = 5) | 1.09 ± 0.33 (n = 5) | 3.27 ± 1.85 (n = 6) | 0.657 | 0.839 | 0.356 |
| FAAH transcript (maternal) | 0.06 ± 0.02 (n = 4) | 1.59 ± 0.63 (n = 5) | 3.08 ± 2.69 (n = 3) | 1.46 ± 0.56 (n = 6) | 0.061 | 0.086 | 0.121 |
| DAGLA | |||||||
| DAGL-α protein (fetal) | 0.39 ± 0.06 (n = 5) | 0.35 ± 0.08 (n = 4) | 0.67 ± 0.22 (n = 4) | 0.17 ± 0.05 (n = 5) | 0.036 | 0.608 | 0.076 |
| DAGL-α transcript (fetal) | 1.27 ± 0.32 (n = 6) | 0.95 ± 0.11 (n = 5) | 1.29 ± 0.57 (n = 4) | 0.49 ± 0.04 (n = 4) | 0.175 | 0.073 | 0.350 |
| DAGLA transcript (maternal) | 3.32 ± 2.89 (n = 5) | 1.91 ± 0.95 | 0.32 ± 0.20 (n = 3) | 1.30 ± 0.37 (n = 6) | 0.275 | 0.965 | 0.975 |
| MAGL | |||||||
| MAGL protein (fetal) | 0.75 ± 0.31 (n = 5) | 0.17 ± 0.05 (n = 4) | 0.57 ± 0.30 (n = 4) | 0.53 ± 0.16 (n = 5) | 0.240 | 0.483 | 0.062 |
| MAGL protein (maternal) | 1.05 ± 0.14 (n = 5) | 1.34 ± 0.28 (n = 4) | 0.85 ± 0.30 (n = 4) | 1.38 ± 0.20 (n = 6) | 0.156 | 0.612 | 0.995 |
| MAGL transcript (fetal) | 6.58 ± 6.27 (n = 5) | 7.79 ± 7.44 (n = 4) | 5.63 ± 4.19 (n = 5) | 9.22 ± 8.52 (n = 5) | 0.330 | 0.756 | 0.382 |
| MAGL transcript (maternal) | 0.30 ± 0.09 (n = 3) | 2.99 ± 2.51 (n = 4) | 1.01 ± 0.15 (n = 2) | 9.52 ± 8.76 (n = 6) | 0.582 | 0.147 | 0.250 |
| COX-2 | |||||||
| COX-2 protein (fetal) | 0.47 ± 0.12 (n = 5) | 0.16 ± 0.02 (n = 4) | 0.50 ± 0.24 (n = 4) | 0.03 ± 0.00 (n = 5) | 0.005 | 0.221 | 0.477 |
| COX-2 protein (maternal) | 0.05 ± 0.02 (n = 5) | 0.06 ± 0.03 (n = 4) | 0.05 ± 0.03 (n = 3) | 0.03 ± 0.01 (n = 6) | 0.586 | 0.501 | 0.873 |
| COX-2 transcript (fetal) | 2.43 ± 1.59 (n = 6) | 6.09 ± 4.20 (n = 4) | 5.77 ± 4.43 (n = 5) | 3.54 ± 2.68 (n = 5) | 0.765 | 0.822 | 0.231 |
| COX-2 transcript (maternal) | 3.81 ± 3.09 (n = 4) | 5.84 ± 3.93 (n = 4) | 0.94 ± 0.23 (n = 4) | 1.72 ± 0.68 (n = 6) | 0.941 | 0.890 | 0.667 |
Values are means ± SE. CTR, control; HFD, high-fat diet; CB1R, endocannabinoid receptor 1; CB2R, endocannabinoid receptor 2; FAAH, fatty acid amide hydrolase-1; DAGLA, diacylglyceride lipase A; MAGL, monoacylglycerol lipase; COX-2, cyclooxygenase-2. All P values were calculated using the Scheirer-Ray-Hare extension of the Kruskal-Wallis test with 1degree of freedom. Protein expressions are evaluated by Western blot analyses, transcripts by quantitative RT-PCR.
Fig. 3.
Protein expression of CB1R, CB2R, fatty acid amide hydrolase-1 (FAAH), diacylglyceride lipase A (DAGLα), monoacylglycerol lipase (MAGL), and cyclooxygenase-2 (COX-2) in the liver and placenta. A: image of Western blot of CB1R, CB2R, FAAH, MAGL, and COX-2 in the maternal liver (n = 3–6). B: image of Western blot of CB1R, CB2R, FAAH, DAGLα, MAGL, and COX-2 in the fetal liver (n = 4–6). C: image of Western blot analysis (CB1R, CB2R, FAAH, DAGL, MAGL, and COX-2) in the placenta (n = 4–6). CTR, control; HFD, high-fat diet; MF, male fetus; FF, female fetus; M, male; F, female.
Placental endocannabinoid system expression.
immunohistochemistry.
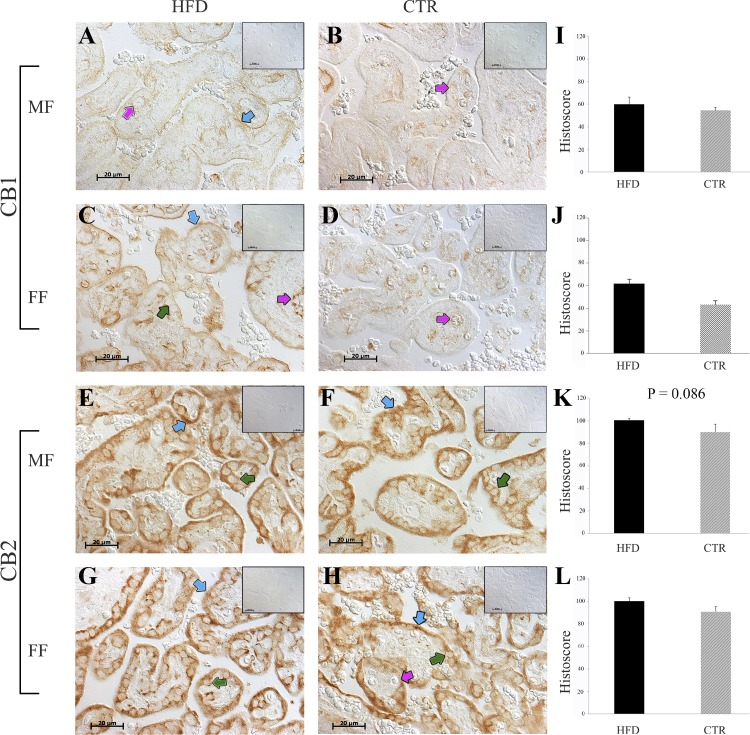
CB1R was expressed in cytotrophoblasts (CTs) and the villous core and fetal capillaries (FC) of the CTR group and in the syncytiotrophoblast (ST), CTs, and fetal capillaries of the HFD group. CB2R was expressed in the STs and CTs of both groups (Fig. 4). Histoscores for the HFD and CTR groups were similar.
Fig. 4.
CB1R and CB2R protein expression in the placenta from high-fat diet (HFD; n = 10) and control (CTR; n = 11) animals. A, B, E, and F: placental expression of CB1R in male fetuses (MF). C, D, G, and H: representative images of the placental sections from female fetuses (FF). Blue arrows point to syncytiotrophoblasts, green arrows point to cytotrophoblasts, and pink arrows point to fetal capillaries. The negative controls (omission of the primary antibodies) are shown as insets (A–H, top right). Scale bar, 20 μm. I–L: immunohistochemical quantifications for the HFD and CTR groups. Data are presented as means ± SE.
rt-pcr and western blot analyses.
Gene expression did not differ between the two groups. CB2R protein expression was increased in male fetuses and decreased in the female fetuses from HFD dams (Table 4).
Table 4.
Placental expression of main endocannabinoid receptors and metabolizing enzymes in placentas of male and female fetuses near term of HFD and CTR mothers
| Female Fetuses |
Male Fetuses |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| CTR | HFD | CTR | HFD | Diet | Sex | Diet × sex | |
| CB1R | |||||||
| CB1R protein | 1.16 ± 0.21 (n = 5) | 1.10 ± 0.11 (n = 4) | 1.38 ± 0.43 (n = 4) | 2.21 ± 0.63 (n = 6) | 0.529 | 0.312 | 0.675 |
| CB1R transcript | 1.80 ± 0.99 (n = 6) | 7.77 ± 7.14 (n = 5) | 20.45 ± 19.79 (n = 5) | 101.42 ± 68.06 (n = 6) | 0.521 | 0.619 | 0.947 |
| CB2 | |||||||
| CB2R protein | 1.22 ± 0.25 (n = 5) | 0.85 ± 0.10 (n = 4) | 0.80 ± 0.14 (n = 4) | 1.54 ± 0.09 (n = 6) | 0.452 | 0.452 | 0.006 |
| CB2R transcript | 2.19 ± 1.04 (n = 6) | 1.57 ± 0.73 (n = 4) | 4.72 ± 2.95 (n = 5) | 0.13 ± 0.07 (n = 4) | 0.187 | 0.130 | 0.261 |
| FAAH-1 | |||||||
| FAAH protein | 0.81 ± 0.18 (n = 4) | 0.77 ± 0.08 (n = 4) | 1.28 ± 0.32 (n = 4) | 0.74 ± 0.09 (n = 6) | 0.613 | 0.388 | 0.275 |
| FAAH-1 transcript | 1.47 ± 0.46 (n = 6) | 1.69 ± 0.85 (n = 5) | 2.82 ± 1.09 (n = 5) | 1.05 ± 0.17 (n = 6) | 0.675 | 0.952 | 0.429 |
| DAGL-α | |||||||
| DAGL-α protein | 0.66 ± 0.07 (n = 4) | 0.68 ± 0.06 (n = 4) | 0.92 ± 0.35 (n = 4) | 0.52 ± 0.03 (n = 5) | 0.597 | 0.180 | 0.215 |
| DAGLA transcript | 1.36 ± 1.14 (n = 5) | 1.24 ± 0.42 (n = 5) | 4.09 ± 3.9 (n = 4) | 2.11 ± 1.00 (n = 6) | 0.291 | 0.641 | 0.624 |
| MAGL | |||||||
| MAGL protein | 1.13 ± 0.29 (n = 5) | 1.79 ± 0.24 (n = 4) | 1.08 ± 0.25 (n = 4) | 1.08 ± 0.14 (n = 5) | 0.348 | 0.084 | 0.183 |
| MAGL transcript | 1.69 ± 1.09 (n = 4) | 0.30 ± 0.09 (n = 4) | 1.02 ± 0.19 (n = 2) | 2.01 ± 1.54 (n = 3) | 0.080 | 0.128 | 0.535 |
| COX-2 | |||||||
| Cox2 protein | 1.00 ± 0.21 (n = 5) | 1.33 ± 0.25 (n = 4) | 0.41 ± 0.07 (n = 4) | 0.52 ± 0.06 (n = 5) | 0.199 | 0.001 | 1.000 |
| Cox2 transcript | 1.7 ± 1.10 (n = 4) | 0.20 ± 0.09 (n = 4) | 1.02 ± 0.19 (n = 2) | 2.01 ± 1.53 (n = 3) | 0.078 | 0.092 | 0.426 |
Values are means ± SE. CTR, control; HFD, high-fat diet; CB1R, endocannabinoid receptor 1; CB2R, endocannabinoid receptor 2; FAAH, fatty acid amide hydrolase-1; DAGLA, diacylglyceride lipase A; MAGL, monoacylglycerol lipase; COX-2, cyclooxygenase-2. All P values were calculated using the Scheirer-Ray-Hare extension of the Kruskal-Wallis test with 1 degree of freedom. Protein expressions are evaluated by Western blot analyses, transcripts by quantitative RT-PCR.
DISCUSSION
Maternal Systemic and Hepatic ECS Responses to HFD
The effect of a HFD on AEA/2-AG concentrations in systemic circulation is controversial. In a nonpregnant canine model and in lean and obese human subjects, a HFD did not affect AEA but did decrease 2-AG concentrations (27, 79). In contrast, 2-AG concentrations were increased in obesity and hyperphagia associated with Prader-Willi syndrome (45), in obese postmenopausal women with insulin resistance (1), and in women with polycystic ovarian syndrome (40). These controversial effects could be explained in part by the differences in insulin sensitivity in the different obesity- and HFD-related phenotypes; e.g., decreased insulin sensitivity is associated with increased 2-AG levels (1). Obesity in pregnancy is associated with decreased insulin sensitivity (26), and 2-AG concentrations increase with advanced gestation. Differences in Maternal body weight between HFD and CTR animals at the time of cesarean section approached significance (P = 0.055), as described in a study reporting our model of HFD feeding (57). In agreement with this observation, Higgins et al. (38) demonstrated a significant increase in percentage of body fat despite the absence of differences in the absolute weight in Papio spp. after 8 wk of HFD-HS intervention. In the present study, animals underwent 9 mo of dietary intervention before pregnancy, and our previous work demonstrated an increased percentage of body fat in the HFD group (18, 57). The mean weight of baboons in the HFD group in our study was 20 kg, which was within the higher percentile for this population (18) and correlated with 20% body fat (18) in these species. For comparison, adult lean baboons in the colony have 5−10% body fat. Increases in maternal 2-AG concentrations in the HFD group in our study are in agreement with our published data in a pregnant baboon model of natural obesity associated with overeating (12). The increased circulating level of 2-AG was reported in the HFD induced NAFLD in nonpregnant animals and in humans and was associated with increased hepatic CB1R expression (50, 77, 82). In our study, none of the hepatic maternal endocannabinoid receptors or degrading enzymes were affected by consumption of a HFD. In some conditions, such as viral hepatitis C, the increase in systemic 2-AG concentrations was not associated with differences in hepatic MAGL activity (63), which is explained by the general response of stellate cells to injury via increased synthesis of 2-AG (76). Finally, the absence of an effect of a HFD on maternal hepatic FAAH expression could be attributed to the fact that we investigated FAAH-1 but not FAAH-2, which is associated with lipid droplet formation (42). Our data contrast with those of another publication that reported decreased hepatic FAAH activity (61) and increased hepatic AEA concentrations in the HFD group in nonpregnant adult mice. The HFD intervention in the rodent study was performed over 14 wk of age, which is equivalent to the human lifespan between 14 and 34 yr of age, and the intervention in our study was performed during 6 mo of pregnancy and 9 mo postpregnancy, which is equivalent to approximately 3 yr of life in humans. One of the weaknesses of our study was that we were not able to measure hepatic concentrations of eCB.
Plasma AEA concentrations have recently been identified as markers of NAFLD in the human population (44). However, selection of the patients in the human population was made based on the histological NAFLD criteria, whereas experimental selection in our study was based on HFD exposure. In our animal model (Papio spp.), exposure of adult nonpregnant animals to 7–14 wk of a HFD was associated with hyperlipidemia and inflammation (48, 72), which are features of the metabolic syndrome in humans that ultimately could lead to NAFLD (2). In general, in the human population, morbid obesity was associated with liver steatosis in 88% of the studied group, with an average age of 43.1 yr (73).
Placental ECS response to a HFD
The decreased 2AG concentrations in fetal circulation could be associated with the diminished mechanisms of placental transport or changes in fetal metabolism in maternal obesity. The maternal BMI influences fetal fat accumulation through the regulation of placental weight and placental fatty acid transporters (32). The increase in placental weight, however, was not associated with the increased volume of the terminal villi, which is the main location of transport, but rather with the expansion of stem villi in maternal obesity (70). Interestingly, eCBs are regulated by leptin (54), but evidence suggests that the placenta in obesity in human and nonhuman primate pregnancies is leptin resistant (28, 29). Although the existence of specific endocannabinoid membrane transporters has been proposed, eCBs could also be transported through the cytosol using fatty acid-binding proteins (FABP), heat shock protein 70, albumin, adiposomes, caveolar-related endocytosis, and hydrolysis-driven, FAAH-dependent facilitated diffusion (for AEA). In contrast, the 2-AG hydrolysis does not affect 2-AG uptake (30). The reports regarding placental FABP expression in maternal obesity are controversial (83). Our data are in line with a recently published study regarding decreased FABP expression in the placentas of obese women (71), which might be associated with decreased eCB transport. The decrease in fetal circulating concentration of 2-AG could not be explained by increased enzymatic degradation since placental expressions of key molecules of eCB degradations, FAAH, MAGL, and COX-2 described in the placenta (19, 23) were not different in our study. Long-chain polyunsaturated fatty acids (LC-PUFA) are precursors of eCB synthesis; the systemic eCB deficiency could not be attributed to placental LC-PUFA deficiency because placental uptake of LC-PUFA is not affected by a HFD in macaques (59). Another molecule upstream of eCB synthesis is brain-derived neurotrophic factor (80), and its expression is decreased in MO (65) in a fetal sex-dependent manner. The placental changes associated with maternal obesity in the framework of the study reported here are presented in Fig. 5.
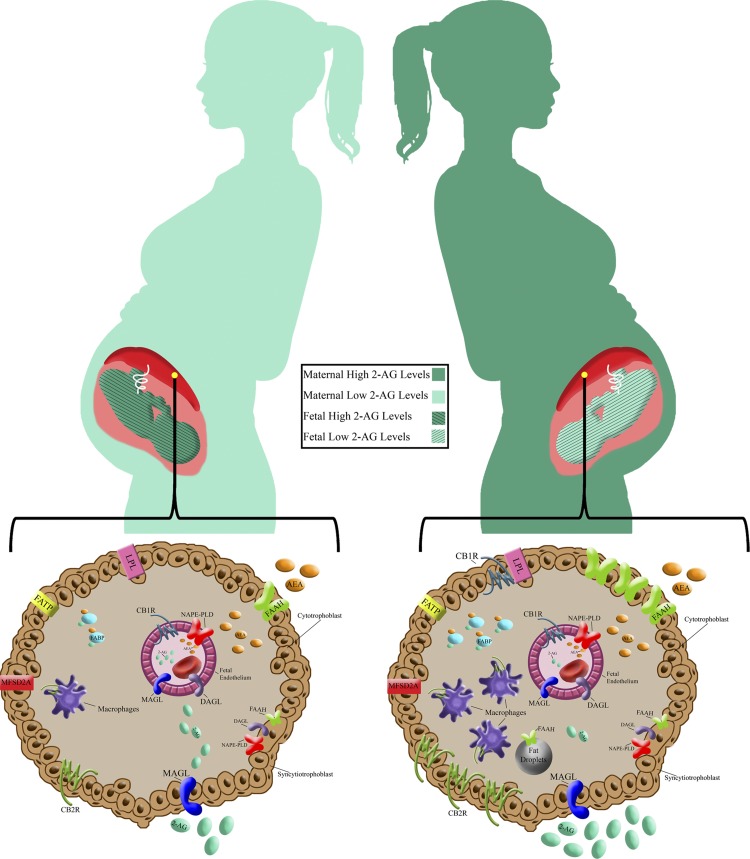
Fig. 5.
Graphic summary of presented findings in a framework of selected reported obesity-related placental changes, which are relevant to endocannabinoid transport and metabolism. Top: increased maternal and decreased fetal systemic 2-arachidonoyl glycerol (2-AG) concentrations in maternal high-fat diet (HFD) consumption. Placental 2-AG concentration is decreased in maternal obesity (MO) (13). Bottom: cross-sections of placental terminal villi. CB1R is expressed in the fetal endothelium of control (CTR) animals and additionally in the syncytiotrophoblast (ST) of the HFD group. Macrophages infiltration (17, 28), fatty acid-binding protein (FABP) and fatty acid transport protein (FATP) expression (24), lipoprotein lipase (LPL) activity (66), and lipid droplet (LD) formation (39) were increased, and docosahexaenoic acid (DHA) was reduced (16) in placentas in MO and in the HFD group. MFSD2A is expressed in placental ST and is a transporter of DHA (58, 64), which in turn is a precursor of endogenous cannabinoid (eCB) (31). Associations with eCB metabolism: LDs are functional sides of AEA inactivation (42). CB2R is expressed in the cytotrophoblast (CT) and macrophages (52), FABP is involved in eCB transport (43), and LPL activation is CB1R mediated (78). The fetal endothelium expresses monoacylglycerol lipase (MAGL), diacylglycerol lipase-α (DAGLα), N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD), and CB1R; CT expresses CB2R, DAGL, Fatty Acid Amide Hydrolase 1 (FAAH-1), NAPE-PLD, and MAGL, and ST expresses CB1R, MAGL, and FAAH-1.
In in vitro cell culture models, steatosis induced by oleic acid was associated with downregulation of the CB2R gene (21). Interestingly, in our study, we observed fetal sex-dependent differences in the effect of a HFD on placental CB2R expression; it was upregulated in males and downregulated in female fetuses. In agreement with our data, uptake of oleic acid was 43% lower in male offspring and 73% higher in female offspring of obese women than of nonobese women (11). CB2R was expressed in ST and CT in placenta, which play an important role in fatty acid metabolism (46).
Endothelial dysfunction is the hallmark of pregnancies complicated by MO (62, 74). The location of CB1R in the fetal placental endothelium is in agreement with previously published data in the baboon (13) and human placenta (34). In the present study, CB1R expression was located mostly in the fetal vascular endothelium of CTR animals, and maternal feeding with the HFD shifted this expressionn toward the STs. In the endothelium, CB1R is involved in vascular relaxation (75). The shift in CB1R expression to the outer layer of the STs in the HFD group might be associated with the increased lipoprotein lipase (LPL) activity in the STs in MO (66) because LPL activity is regulated via a CB1R-related mechanism (20). Thus, it is possible that in a HFD, the eCB effect could be shifted from vascular to metabolic responses. Another explanation of the CB1R shift might be associated with increased lipopolysaccharide (LPS) concentrations described in obesity (49). In the gut, for example, CB1R expression is controlled by the gut bacterial content (55), and LPS upregulates CB1R expression (9).
Fetal Hepatic eCBs and Maternal Consumption of HFD
In the fetal liver, CB1R was expressed predominantly on the membranes of hepatocytes, whereas in the maternal liver, CB1R was localized intracellularly in hepatocytes and on the membrane of the biliary epithelium. These differences in the cellular location might be attributed to the differences in the maternal and fetal hepatic CB1R isoforms CB1 (55 kDa), CB1b (49 kDa), and CB1a (46 kDa) (8). The short isoforms are expressed readily on the cell surface, whereas having a long NH2 terminus diminishes CB1R translocation to the membrane (7). These isoforms play different roles in tissue metabolism and have different affinities for CB1R blockers (37). Specifically, the CB1b is the most abundant isoform in human hepatocytes and exceeds the expression of CB1R and CB1a by 10–15 times (35, 37). BMI and CB1b expression in hepatocytes had a direct, positive correlation (37). Decreased fetal hepatic CB2R expression in our study is not in agreement with the increased hepatic CB2R expression in the offspring of a rat model of maternal HFD (67). These differences could be explained by differences in the developmental stage: fetal in the present study vs. adulthood in rodents. The decreased CB2R might be a contributing factor for developmental programming and higher susceptibility to liver damage in the offspring of obese mothers. In mice, the lack of CB2R enhanced liver fibrosis (41) and impaired hepatocyte proliferation (15), leading to greater weight gain with age (3). However, this increase in body weight was not accompanied by an increase in inflammatory responses. Hepatic Cnr1 expression was not affected, whereas hepatic faah mRNA expression was decreased in the female offspring that were exposed prenatally to a HFD in a rodent model (67). In agreement with these data, FAAH protein expression was decreased in the fetal liver of HFD dams in our study. Both MAGL and FAAH play a role in the enzymatic degradation of 2-AG (23). However, expression of MAGL was unchanged by maternal HFD in our experimental setting, in line with the finding in the rodent model of maternal HFD. The expression of DAGLα was decreased in the liver of male fetuses in our HFD group. The pharmacological inhibition of DAGL decreased 2-AG and prostaglandin concentrations in the brain (60). The formation of prostaglandin H(2) glycerol ester [PGH (2)-G] from 2-AG occurs through the alternative degradation pathway of COX-2-mediated oxidation (6, 47, 68).
In summary, to our knowledge, this study is the first report of decreased concentrations of fetal systemic 2-AG in response to a HFD. A HFD-stimulated maternal ECS is associated with fetal hepatic and placental changes in key ECS receptors and metabolic enzymes.
GRANTS
This study was supported by National Institutes of Health Grant HD-21350 to P. Nathanielsz and Texas Tech University Health Sciences Center startup funds to N. Schlabritz-Loutsevitch.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.G., C.L., N.G., C.S., M. Carrillo, R.R.K., S.M., M. Chuecos, and N.S.-L. performed experiments; K.G., P.N., and N.S.-L. interpreted results of experiments; K.G., C.L., N.G., C.S., R.R.K., E.L., M. Chuecos, G.V., P.N., and N.S.-L. drafted manuscript; K.G., C.L., N.G., C.S., R.R.K., E.L., M. Chuecos, G.V., P.N., and N.S.-L. edited and revised manuscript; K.G., C.L., N.G., C.S., M. Carrillo, R.R.K., E.L., S.M., M. Chuecos, G.V., P.N., and N.S.-L. approved final version of manuscript; M. Carrillo and N.S.-L. prepared figures; E.L. and N.S.-L. analyzed data; P.N. and N.S.-L. conceived and designed research.
ACKNOWLEDGMENTS
We acknowledge the help of E. Arzate with images and Melissa Waggoner with manuscript editing. We acknowledge the generosity Dr. Charles Burns (Pathology, MCH) and his team for continuous support. We thank Dr. Raul Martinez-Zaguilan (TTUHSC), who provided essential study equipment. We acknowledge help of Saloni Cholia (MISD, high school student) with the image analyses.
REFERENCES
- 1.Abdulnour J, Yasari S, Rabasa-Lhoret R, Faraj M, Petrosino S, Piscitelli F, Prud’ Homme D, Di Marzo V. Circulating endocannabinoids in insulin sensitive vs. insulin resistant obese postmenopausal women. A MONET group study. Obesity (Silver Spring) 22: 211–216, 2014. doi: 10.1002/oby.20498. [DOI] [PubMed] [Google Scholar]
- 2.Adolph TE, Grander C, Grabherr F, Tilg H. Adipokines and Non-Alcoholic Fatty Liver Disease: Multiple Interactions. Int J Mol Sci 18: 18, 2017. doi: 10.3390/ijms18081649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agudo J, Martin M, Roca C, Molas M, Bura AS, Zimmer A, Bosch F, Maldonado R. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 53: 2629–2640, 2010. doi: 10.1007/s00125-010-1894-6. [DOI] [PubMed] [Google Scholar]
- 4.Alfaradhi MZ, Kusinski LC, Fernandez-Twinn DS, Pantaleão LC, Carr SK, Ferland-McCollough D, Yeo GS, Bushell M, Ozanne SE. Maternal Obesity in Pregnancy Developmentally Programs Adipose Tissue Inflammation in Young, Lean Male Mice Offspring. Endocrinology 157: 4246–4256, 2016. doi: 10.1210/en.2016-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet 2: 27, 2011. doi: 10.3389/fgene.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci 35: 284–292, 2014. doi: 10.1016/j.tips.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Andersson H, D’Antona AM, Kendall DA, Von Heijne G, Chin CN. Membrane assembly of the cannabinoid receptor 1: impact of a long N-terminal tail. Mol Pharmacol 64: 570–577, 2003. doi: 10.1124/mol.64.3.570. [DOI] [PubMed] [Google Scholar]
- 8.Bagher AM, Laprairie RB, Kelly ME, Denovan-Wright EM. Co-expression of the human cannabinoid receptor coding region splice variants (hCB1) affects the function of hCB1 receptor complexes. Eur J Pharmacol 721: 341–354, 2013. doi: 10.1016/j.ejphar.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Bariani MV, Domínguez Rubio AP, Cella M, Burdet J, Franchi AM, Aisemberg J. Role of the endocannabinoid system in the mechanisms involved in the LPS-induced preterm labor. Reproduction 150: 463–472, 2015. doi: 10.1530/REP-15-0211. [DOI] [PubMed] [Google Scholar]
- 10.Bautista CJ, Rodríguez-González GL, Morales A, Lomas-Soria C, Cruz-Pérez F, Reyes-Castro LA, Zambrano E. Maternal obesity in the rat impairs male offspring aging of the testicular antioxidant defence system. Reprod Fertil Dev 29: 1950–1957, 2017. doi: 10.1071/RD16277. [DOI] [PubMed] [Google Scholar]
- 11.Brass E, Hanson E, O’Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta 34: 503–509, 2013. doi: 10.1016/j.placenta.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brocato B, Janjetovich Z, Slominski A, Giancarlo M, Hubbard G, Dick E, Schlabritz-Loutsevitch N. Critical enzymes for endocannabinoid metabolism in a baboon model of maternal obesity (vol 208, S93, 2013). Am J Obstet Gynecol 208: 407, 2013. doi: 10.1016/j.ajog.2012.10.360. [DOI] [Google Scholar]
- 13.Brocato B, Zoerner AA, Janjetovic Z, Skobowiat C, Gupta S, Moore BM II, Slominski A, Zhang J, Schenone M, Phinehas R, Ferry RJ Jr, Dick E Jr, Hubbard GB, Mari G, Schlabritz-Loutsevitch N. Endocannabinoid crosstalk between placenta and maternal fat in a baboon model (Papio spp.) of obesity. Placenta 34: 983–989, 2013. doi: 10.1016/j.placenta.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buckley NE. The peripheral cannabinoid receptor knockout mice: an update. Br J Pharmacol 153: 309–318, 2008. doi: 10.1038/sj.bjp.0707527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerf ME, Herrera E. High fat diet administration during specific periods of pregnancy alters maternal fatty acid profiles in the near-term rat. Nutrients 8: 25, 2016. doi: 10.3390/nu8010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Challier JC, Basu S, Bintein T, Minium J, Hotmire K, Catalano PM, Hauguel-de Mouzon S. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta 29: 274–281, 2008. doi: 10.1016/j.placenta.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comuzzie AG, Cole SA, Martin L, Carey KD, Mahaney MC, Blangero J, VandeBerg JL. The baboon as a nonhuman primate model for the study of the genetics of obesity. Obes Res 11: 75–80, 2003. doi: 10.1038/oby.2003.12. [DOI] [PubMed] [Google Scholar]
- 19.Costa MA. The endocannabinoid system: A novel player in human placentation. Reprod Toxicol 61: 58–67, 2016. doi: 10.1016/j.reprotox.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S, Tomassoni F, Cervino C, Nisoli E, Linthorst AC, Pasquali R, Lutz B, Stalla GK, Pagotto U. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112: 423–431, 2003. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Gottardi A, Spahr L, Ravier-Dall’Antonia F, Hadengue A. Cannabinoid receptor 1 and 2 agonists increase lipid accumulation in hepatocytes. Liver Int 30: 1482–1489, 2010. doi: 10.1111/j.1478-3231.2010.02298.x. [DOI] [PubMed] [Google Scholar]
- 22.Dearden L, Ozanne SE. Early life origins of metabolic disease: Developmental programming of hypothalamic pathways controlling energy homeostasis. Front Neuroendocrinol 39: 3–16, 2015. doi: 10.1016/j.yfrne.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Di Marzo V. Endocannabinoids: synthesis and degradation. Rev Physiol Biochem Pharmacol 160: 1–24, 2008. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- 24.Díaz P, Harris J, Rosario FJ, Powell TL, Jansson T. Increased placental fatty acid transporter 6 and binding protein 3 expression and fetal liver lipid accumulation in a mouse model of obesity in pregnancy. Am J Physiol Regul Integr Comp Physiol 309: R1569–R1577, 2015. doi: 10.1152/ajpregu.00385.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn 37: 95–110, 2017. doi: 10.1002/pd.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endo S, Maeda K, Suto M, Kaji T, Morine M, Kinoshita T, Yasui T, Irahara M. Differences in insulin sensitivity in pregnant women with overweight and gestational diabetes mellitus. Gynecol Endocrinol 22: 343–349, 2006. doi: 10.1080/09513590600724836. [DOI] [PubMed] [Google Scholar]
- 27.Engeli S, Lehmann AC, Kaminski J, Haas V, Janke J, Zoerner AA, Luft FC, Tsikas D, Jordan J. Influence of dietary fat intake on the endocannabinoid system in lean and obese subjects. Obesity (Silver Spring) 22: E70–E76, 2014. doi: 10.1002/oby.20728. [DOI] [PubMed] [Google Scholar]
- 28.Farley D, Tejero ME, Comuzzie AG, Higgins PB, Cox L, Werner SL, Jenkins SL, Li C, Choi J, Dick EJ Jr, Hubbard GB, Frost P, Dudley DJ, Ballesteros B, Wu G, Nathanielsz PW, Schlabritz-Loutsevitch NE. Feto-placental adaptations to maternal obesity in the baboon. Placenta 30: 752–760, 2009. doi: 10.1016/j.placenta.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farley DM, Choi J, Dudley DJ, Li C, Jenkins SL, Myatt L, Nathanielsz PW. Placental amino acid transport and placental leptin resistance in pregnancies complicated by maternal obesity. Placenta 31: 718–724, 2010. doi: 10.1016/j.placenta.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Fowler CJ, Ghafouri N. Does the hydrolysis of 2-arachidonoylglycerol regulate its cellular uptake? Pharmacol Res 58: 72–76, 2008. doi: 10.1016/j.phrs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Freitas HR, Isaac AR, Malcher-Lopes R, Diaz BL, Trevenzoli IH, De Melo Reis RA. Polyunsaturated fatty acids and endocannabinoids in health and disease. Nutr Neurosci: 1–20, 2017. doi: 10.1080/1028415X.2017.1347373. [DOI] [PubMed] [Google Scholar]
- 32.Friis CM, Qvigstad E, Paasche Roland MC, Godang K, Voldner N, Bollerslev J, Henriksen T. Newborn body fat: associations with maternal metabolic state and placental size. PLoS One 8: e57467, 2013. doi: 10.1371/journal.pone.0057467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frost PA, Hubbard GB, Dammann MJ, Snider CL, Moore CM, Hodara VL, Giavedoni LD, Rohwer R, Mahaney MC, Butler TM, Cummins LB, McDonald TJ, Nathanielsz PW, Schlabritz-Loutsevitch NE. White monkey syndrome in infant baboons (Papio species). J Med Primatol 33: 197–213, 2004. doi: 10.1111/j.1600-0684.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 34.Fügedi G, Molnár M, Rigó J Jr, Schönléber J, Kovalszky I, Molvarec A. Increased placental expression of cannabinoid receptor 1 in preeclampsia: an observational study. BMC Pregnancy Childbirth 14: 395, 2014. doi: 10.1186/s12884-014-0395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosh S, Gonzalez-Mariscal I, Egan JM, Moaddel R. Targeted proteomics of cannabinoid receptor CB1 and the CB1b isoform. J Pharm Biomed Anal 144: 154–158, 2017. doi: 10.1016/j.jpba.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong Y, Li X, Kang L, Xie Y, Rong Z, Wang H, Qi H, Chen H. Simultaneous determination of endocannabinoids in murine plasma and brain substructures by surrogate-based LC-MS/MS: Application in tumor-bearing mice. J Pharm Biomed Anal 111: 57–63, 2015. doi: 10.1016/j.jpba.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 37.González-Mariscal I, Krzysik-Walker SM, Doyle ME, Liu QR, Cimbro R, Santa-Cruz Calvo S, Ghosh S, Cieśla Ł, Moaddel R, Carlson OD, Witek RP, O’Connell JF, Egan JM. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci Rep 6: 33302, 2016. doi: 10.1038/srep33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins PB, Bastarrachea RA, Lopez-Alvarenga JC, Garcia-Forey M, Proffitt JM, Voruganti VS, Tejero ME, Mattern V, Haack K, Shade RE, Cole SA, Comuzzie AG. Eight week exposure to a high sugar high fat diet results in adiposity gain and alterations in metabolic biomarkers in baboons (Papio hamadryas sp.). Cardiovasc Diabetol 9: 71, 2010. doi: 10.1186/1475-2840-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirschmugl B, Desoye G, Catalano P, Klymiuk I, Scharnagl H, Payr S, Kitzinger E, Schliefsteiner C, Lang U, Wadsack C, Hauguel-de Mouzon S. Maternal obesity modulates intracellular lipid turnover in the human term placenta. Int J Obes 41: 317–323, 2017. doi: 10.1038/ijo.2016.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juan CC, Chen KH, Wang PH, Hwang JL, Seow KM. Endocannabinoid system activation may be associated with insulin resistance in women with polycystic ovary syndrome. Fertil Steril 104: 200–206, 2015. doi: 10.1016/j.fertnstert.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Julien B, Grenard P, Teixeira-Clerc F, Van Nhieu JT, Li L, Karsak M, Zimmer A, Mallat A, Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 128: 742–755, 2005. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 42.Kaczocha M, Glaser ST, Chae J, Brown DA, Deutsch DG. Lipid droplets are novel sites of N-acylethanolamine inactivation by fatty acid amide hydrolase-2. J Biol Chem 285: 2796–2806, 2010. doi: 10.1074/jbc.M109.058461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaczocha M, Glaser ST, Deutsch DG. Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci USA 106: 6375–6380, 2009. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimberly WT, O’Sullivan JF, Nath AK, Keyes M, Shi X, Larson MG, Yang Q, Long MT, Vasan R, Peterson RT, Wang TJ, Corey KE, Gerszten RE. Metabolite profiling identifies anandamide as a biomarker of nonalcoholic steatohepatitis. JCI Insight 2: e92989, 2017. doi: 10.1172/jci.insight.92989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knani I, Earley BJ, Udi S, Nemirovski A, Hadar R, Gammal A, Cinar R, Hirsch HJ, Pollak Y, Gross I, Eldar-Geva T, Reyes-Capo DP, Han JC, Haqq AM, Gross-Tsur V, Wevrick R, Tam J. Targeting the endocannabinoid/CB1 receptor system for treating obesity in Prader-Willi syndrome. Mol Metab 5: 1187–1199, 2016. doi: 10.1016/j.molmet.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolahi K, Louey S, Varlamov O, Thornburg K. Real-time tracking of BODIPY-C12 long-chain fatty acid in human term placenta reveals unique lipid dynamics in cytotrophoblast cells. PLoS One 11: e0153522, 2016. doi: 10.1371/journal.pone.0153522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem 275: 33744–33749, 2000. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- 48.Kushwaha RS, Reardon CA, Getz GS, Lewis DS, Rice KS, Carey KD, McGill HC Jr. Metabolic mechanisms for responses to dietary cholesterol and fat in high and low LDL responding baboons (Papio sp.). J Lipid Res 35: 633–643, 1994. [PubMed] [Google Scholar]
- 49.Lassenius MI, Ahola AJ, Harjutsalo V, Forsblom C, Groop PH, Lehto M. Endotoxins are associated with visceral fat mass in type 1 diabetes. Sci Rep 6: 38887, 2016. doi: 10.1038/srep38887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Zhou L, Xiong K, Godlewski G, Mukhopadhyay B, Tam J, Yin S, Gao P, Shan X, Pickel J, Bataller R, O’Hare J, Scherer T, Buettner C, Kunos G. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 142: 1218–1228.e1, 2012. doi: 10.1053/j.gastro.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Long NM, Rule DC, Zhu MJ, Nathanielsz PW, Ford SP. Maternal obesity upregulates fatty acid and glucose transporters and increases expression of enzymes mediating fatty acid biosynthesis in fetal adipose tissue depots. J Anim Sci 90: 2201–2210, 2012. doi: 10.2527/jas.2011-4343. [DOI] [PubMed] [Google Scholar]
- 52.Malfitano AM, Basu S, Maresz K, Bifulco M, Dittel BN. What we know and do not know about the cannabinoid receptor 2 (CB2). Semin Immunol 26: 369–379, 2014. doi: 10.1016/j.smim.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maloyan A, Muralimanoharan S, Huffman S, Cox LA, Nathanielsz PW, Myatt L, Nijland MJ. Identification and comparative analyses of myocardial miRNAs involved in the fetal response to maternal obesity. Physiol Genomics 45: 889–900, 2013. doi: 10.1152/physiolgenomics.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Monteleone P, Maj M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: beyond the homeostatic control of food intake. Psychoneuroendocrinology 38: 312–330, 2013. doi: 10.1016/j.psyneuen.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 55.Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 6: 392, 2010. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nassar A, Cohen C, Agersborg SS, Zhou W, Lynch KA, Heyman ER, Olson A, Lange H, Siddiqui MT. A new immunohistochemical ER/PR image analysis system: a multisite performance study. Appl Immunohistochem Mol Morphol 19: 195–202, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Nathanielsz PW, Yan J, Green R, Nijland M, Miller JW, Wu G, McDonald TJ, Caudill MA. Maternal obesity disrupts the methionine cycle in baboon pregnancy. Physiol Rep 3: e12564, 2015. doi: 10.14814/phy2.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL, Silver DL. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509: 503–506, 2014. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 59.O’Tierney-Ginn P, Roberts V, Gillingham M, Walker J, Glazebrook PA, Thornburg KL, Grove K, Frias AE. Influence of high fat diet and resveratrol supplementation on placental fatty acid uptake in the Japanese macaque. Placenta 36: 903–910, 2015. doi: 10.1016/j.placenta.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ogasawara D, Deng H, Viader A, Baggelaar MP, Breman A, den Dulk H, van den Nieuwendijk AM, Soethoudt M, van der Wel T, Zhou J, Overkleeft HS, Sanchez-Alavez M, Mori S, Nguyen W, Conti B, Liu X, Chen Y, Liu QS, Cravatt BF, van der Stelt M. Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition. Proc Natl Acad Sci USA 113: 26–33, 2016. [Correction: Proc Natl Acad Sci USA 113: E664, 2016. doi: 10.1073/pnas.1525714113. ] doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, Harvey-White J, Mackie K, Offertáler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 115: 1298–1305, 2005. doi: 10.1172/JCI200523057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pardo F, Silva L, Sáez T, Salsoso R, Gutiérrez J, Sanhueza C, Leiva A, Sobrevia L. Human supraphysiological gestational weight gain and fetoplacental vascular dysfunction. Int J Obes 39: 1264–1273, 2015. doi: 10.1038/ijo.2015.57. [DOI] [PubMed] [Google Scholar]
- 63.Patsenker E, Sachse P, Chicca A, Gachet MS, Schneider V, Mattsson J, Lanz C, Worni M, de Gottardi A, Semmo M, Hampe J, Schafmayer C, Brenneisen R, Gertsch J, Stickel F, Semmo N. Elevated levels of endocannabinoids in chronic hepatitis C may modulate cellular immune response and hepatic stellate cell activation. Int J Mol Sci 16: 7057–7076, 2015. doi: 10.3390/ijms16047057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prieto-Sánchez MT, Ruiz-Palacios M, Blanco-Carnero JE, Pagan A, Hellmuth C, Uhl O, Peissner W, Ruiz-Alcaraz AJ, Parrilla JJ, Koletzko B, Larqué E. Placental MFSD2a transporter is related to decreased DHA in cord blood of women with treated gestational diabetes. Clin Nutr 36: 513–521, 2017. doi: 10.1016/j.clnu.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 65.Prince CS, Maloyan A, Myatt L. Maternal obesity alters brain derived neurotrophic factor (BDNF) signaling in the placenta in a sexually dimorphic manner. Placenta 49: 55–63, 2017. doi: 10.1016/j.placenta.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiao L, Guo Z, Bosco C, Guidotti S, Wang Y, Wang M, Parast M, Schaack J, Hay WW Jr, Moore TR, Shao J. Maternal High-Fat Feeding Increases Placental Lipoprotein Lipase Activity by Reducing SIRT1 Expression in Mice. Diabetes 64: 3111–3120, 2015. doi: 10.2337/db14-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramírez-López MT, Arco R, Decara J, Vázquez M, Noemí Blanco R, Alén F, Suárez J, Gómez de Heras R, Rodríguez de Fonseca F. Exposure to a highly caloric palatable diet during the perinatal period affects the expression of the endogenous cannabinoid system in the brain, liver and adipose tissue of adult rat offspring. PLoS One 11: e0165432, 2016. [Erratum: PLoS One 12: e0173653, 2017. doi: 10.1371/journal.pone.0173653. ] doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rouzer CA, Marnett LJ. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem Rev 111: 5899–5921, 2011. doi: 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Russo EB. Clinical endocannabinoid deficiency (CECD): can this concept explain therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol Lett 25: 31–39, 2004. [PubMed] [Google Scholar]
- 70.Schlabritz-Loutsevitch N, German N, Ventolini G, Larumbe E, Samson J. Fetal syndrome of endocannabinoid deficiency (FSECD) in maternal obesity. Med Hypotheses 96: 35–38, 2016. doi: 10.1016/j.mehy.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 71.Segura MT, Demmelmair H, Krauss-Etschmann S, Nathan P, Dehmel S, Padilla MC, Rueda R, Koletzko B, Campoy C. Maternal BMI and gestational diabetes alter placental lipid transporters and fatty acid composition. Placenta 57: 144–151, 2017. doi: 10.1016/j.placenta.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Shi Q, Hornsby PJ, Meng Q, Vandeberg JF, Vandeberg JL. Longitudinal analysis of short-term high-fat diet on endothelial senescence in baboons. Am J Cardiovasc Dis 3: 107–119, 2013. [PMC free article] [PubMed] [Google Scholar]
- 73.Solga SF, Clark JM, Alkhuraishi AR, Torbenson M, Tabesh A, Schweitzer M, Diehl AM, Magnuson TH. Race and comorbid factors predict nonalcoholic fatty liver disease histopathology in severely obese patients. Surg Obes Relat Dis 1: 6–11, 2005. doi: 10.1016/j.soard.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Spradley FT, Palei AC, Granger JP. Increased risk for the development of preeclampsia in obese pregnancies: weighing in on the mechanisms. Am J Physiol Regul Integr Comp Physiol 309: R1326–R1343, 2015. doi: 10.1152/ajpregu.00178.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stanley CP, Hind WH, Tufarelli C, O’Sullivan SE. The endocannabinoid anandamide causes endothelium-dependent vasorelaxation in human mesenteric arteries. Pharmacol Res 113, Pt A: 356–363, 2016. doi: 10.1016/j.phrs.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun LJ, Yu JW, Wan L, Zhang XY, Shi YG, Chen MY. Endocannabinoid system activation contributes to glucose metabolism disorders of hepatocytes and promotes hepatitis C virus replication. Int J Infect Dis 23: 75–81, 2014. doi: 10.1016/j.ijid.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 77.Tam J, Liu J, Mukhopadhyay B, Cinar R, Godlewski G, Kunos G. Endocannabinoids in liver disease. Hepatology 53: 346–355, 2011. doi: 10.1002/hep.24077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vettor R, Pagano C. The role of the endocannabinoid system in lipogenesis and fatty acid metabolism. Best Pract Res Clin Endocrinol Metab 23: 51–63, 2009. doi: 10.1016/j.beem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Woolcott OO, Richey JM, Kabir M, Chow RH, Iyer MS, Kirkman EL, Stefanovski D, Lottati M, Kim SP, Harrison LN, Ionut V, Zheng D, Hsu IR, Catalano KJ, Chiu JD, Bradshaw H, Wu Q, Bergman RN. High-fat diet-induced insulin resistance does not increase plasma anandamide levels or potentiate anandamide insulinotropic effect in isolated canine islets. PLoS One 10: e0123558, 2015.[Erratum: PLoS One 10: e0131033, 2015. doi: 10.1371/journal.pone.0131033. ] doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeh ML, Selvam R, Levine ES. BDNF-induced endocannabinoid release modulates neocortical glutamatergic neurotransmission. Synapse 71: e21962, 2017. doi: 10.1002/syn.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zambrano E, Nathanielsz PW. Mechanisms by which maternal obesity programs offspring for obesity: evidence from animal studies. Nutr Rev 71, Suppl 1: S42–S54, 2013. doi: 10.1111/nure.12068. [DOI] [PubMed] [Google Scholar]
- 82.Zelber-Sagi S, Azar S, Nemirovski A, Webb M, Halpern Z, Shibolet O, Tam J. Serum levels of endocannabinoids are independently associated with nonalcoholic fatty liver disease. Obesity (Silver Spring) 25: 94–101, 2017. doi: 10.1002/oby.21687. [DOI] [PubMed] [Google Scholar]
- 83.Zhu MJ, Ma Y, Long NM, Du M, Ford SP. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol 299: R1224–R1231, 2010. doi: 10.1152/ajpregu.00309.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zoerner AA, Batkai S, Suchy MT, Gutzki FM, Engeli S, Jordan J, Tsikas D. Simultaneous UPLC-MS/MS quantification of the endocannabinoids 2-arachidonoyl glycerol (2AG), 1-arachidonoyl glycerol (1AG), and anandamide in human plasma: minimization of matrix-effects, 2AG/1AG isomerization and degradation by toluene solvent extraction. J Chromatogr B Analyt Technol Biomed Life Sci 883-884: 161–171, 2012. doi: 10.1016/j.jchromb.2011.06.025. [DOI] [PubMed] [Google Scholar]