Abstract
Hyperandrogenemia and hyperinsulinemia are believed to play prominent roles in polycystic ovarian syndrome (PCOS). We explored the effects of low-dose dihydrotestosterone (DHT), a model of PCOS, on insulin signaling in metabolic and reproductive tissues in a female mouse model. Insulin resistance in the energy storage tissues is associated with type 2 diabetes. Insulin signaling in the ovaries and pituitary either directly or indirectly stimulates androgen production. Energy storage and reproductive tissues were isolated and molecular assays were performed. Livers and white adipose tissue (WAT) from DHT mice displayed lower mRNA and protein expression of insulin signaling intermediates. However, ovaries and pituitaries of DHT mice exhibited higher expression levels of insulin signaling genes/proteins. Insulin-stimulated p-AKT levels were blunted in the livers and WAT of the DHT mice but increased or remained the same in the ovaries and pituitaries compared with controls. Glucose uptake decreased in liver and WAT but was unchanged in pituitary and ovary of DHT mice. Plasma membrane GLUTs were decreased in liver and WAT but increased in ovary and pituitary of DHT mice. Skeletal muscle insulin-signaling genes were not lowered in DHT mice compared with control. DHT mice did not display skeletal muscle insulin resistance. Insulin-stimulated glucose transport increased in skeletal muscles of DHT mice compared with controls. DHT mice were hyperinsulinemic. However, the differential mRNA and protein expression pattern was independent of hyperinsulinemia in cultured hepatocytes and pituitary cells. These findings demonstrate a differential effect of DHT on the insulin-signaling pathway in energy storage vs. reproductive tissues independent of hyperinsulinemia.
Keywords: DHT, glucose transport, hyperandrogenemia or androgen excess, insulin signaling, reproductive endocrinology
INTRODUCTION
Androgen excess or hyperandrogenemia (HA) occurs in roughly 8% of women (22) and is the most common endocrine disorder of reproductive aged women. HA in females is a diagnosing characteristic of polycystic ovary syndrome (PCOS) and is associated with metabolic dysfunction and insulin resistance in ~70% of women with HA (21). Studies have suggested that the insulin resistance is selective to certain tissues (8, 28). Some long-standing studies have shown that skeletal muscles, cultured myocytes (6), and white adipose tissue (WAT) (11) from PCOS women displayed intrinsic insulin resistance. However, other studies have shown that cultured adipocytes (9) and cultured myocytes (8) from PCOS women did not display insulin resistance once removed from the in vivo environment, suggesting that an extrinsic effect (possibly high androgens) was the cause of the insulin resistance. Due to as lack of noninvasive techniques, studies of metabolic function and molecular insulin action in the livers of PCOS women is lacking.
Diet-induced obese mice were partially fertile and hyperandrogenic. These mice were insulin resistant in energy storage tissues but maintained insulin action in the reproductive tissues (36). This difference in insulin action may have been due to the obesity or HA. Thus we recently developed a low-dose dihydrotestosterone (DHT) mouse model that displayed pathogenic levels of androgen (HA) independent of obesity (1). Low-dose DHT mice displayed metabolic and reproductive dysfunction similar to women with PCOS (1). The goal of this study was to consider the effects of DHT on insulin-signaling intermediates in energy storage compared with reproductive tissues.
Many studies have examined insulin signaling in PCOS (9, 10, 12, 22, 28). All three primary energy storage tissues [liver (1), WAT (9), and skeletal muscle (14)] have been shown to be insulin resistant in varying conditions and models. The primary cause of this insulin resistance is still not very well understood. Both intrinsic (defects in intracellular insulin signaling) (9) and in vivo environmental effects (hyperinsulinemia) (12) have been thought to play a role. In addition to investigating androgen effects on insulin signaling in different tissues, we additionally sought to examine whether androgen excess independent of hyperinsulinemia influences insulin signaling genes in cultured liver cells compared with cultured pituitary cell lines.
The rationale and significance for examining insulin signaling in the ovaries and pituitaries was that maintained insulin action in the reproductive tissues stimulated the pathogenic production of excess androgens. Insulin resistance in the liver (1) and WAT (18) contributes to whole body glucose metabolism dysfunction and hyperinsulinemia (16). Insulin signaling in reproductive tissues plays a role in PCOS pathogenesis by insulin serving as a co-gonadotropin, helping to increase theca cell testosterone synthesis (27, 29, 37).
Using the obesity-independent low-dose DHT mouse model, we found that mRNA and proteins in the insulin-signaling pathway are differentially affected by low-dose DHT in a tissue-specific manner. Insulin-signaling intermediates (mRNA and proteins) were lowered in energy storage tissues of DHT mice but remained the same or increased in reproductive tissues and this was independent of hyperinsulinemia in liver and pituitary cells. Additionally, this differential effect was also seen at the basal and insulin-stimulated glucose transport level, where glucose transport was decreased in liver WAT but increased in ovaries or remained the same in the pituitaries of DHT mice. These tissue-specific differences may be of value for tissue-specific targeting of therapeutic compounds.
MATERIALS AND METHODS
Animals.
Mice were maintained in a mixed background (CD1/129SvJ/C57BL6). Female mice were used for all experimental studies and were maintained with food and water ad libitum under a 14-h:10-h light-dark cycle. At 2 mo of age, lean female mice were inserted with a 4-mm DHT pellet (DHT mice) or an empty pellet (control mice) (1). Over the course of 3 mo, metabolic and reproductive tests were performed, and at the end of the 3 mo, the mice were euthanized and several tissues were harvested for further molecular and biochemical analysis. DHT mice displayed a twofold increase in serum DHT levels compared with control mice (1). The body mass of control and DHT mice were the same at the time of death (Con: 29 ± 1; DHT: 30 ± 2).
In vivo insulin administration.
At 3 mo post-insertion, the control and DHT mice were fasted overnight and administered 0.5 U/kg body wt (BW) insulin via intraperitoneal (ip) injection (Eli Lilly, Indianapolis, IN) or saline. Ten minutes after ip injection, the mice were euthanized via cervical dislocation, and liver tissues, WAT, pituitaries, and ovaries were extracted. Insulin release is biphasic, with the peak occurring between 10 and 15 min (15). Equal amounts of the designated tissues were collected from the specified location from each mouse: the left liver lobe, WAT from the abdominal mesenteric depots, and gastrocnemius skeletal muscles. Procedures and protocols performed were approved by the Johns Hopkins Animal Care and Use Committee.
qRT-PCR.
Liver tissues, WAT, pituitaries, and ovaries from fed (control and DHT) mice were harvested, and RNA was isolated using TRIzol (Bio-Rad), The RNA was reverse transcribed via an iScript cDNA synthesis kit (Bio-Rad) and real-time quantitative PCR (qRT-PCR) was performed using iQ SYBR Green reagent (Bio-Rad) and an iCycler iQ5 Q-PCR machine (Bio-Rad). Primers for the genes tested are listed in Table 1.
Table 1.
Primers used in this study for qRT-PCR
| qRT-PCR Primers | Sense (5′ to 3′) | Antisense (5′ to 3′) | Function |
|---|---|---|---|
| Insulin receptor (Ir) | GACTTACAGATGGTTGGGCA | AAGACCAACTGTCCTGCCAC | Insulin signaling (+) |
| Insulin receptor substrate 1 (Irs1) | TGTCACCCAGTGGTAGTTGCTC | CTCTCAACAGGAGGTTTGGCATG | Insulin signaling (+) |
| Insulin receptor substrate 2 (Irs2) | CCAGTAAACGGAGGTGGCTACA | CCATAGACAGCTTGGAGCCACA | Insulin signaling (+) |
| Phosphoinositide 3-kinase catalytic domain (Pi3kcd) | ACCATCAGTGGCTCTGCGGTTT | GTGGTCTTCTGGGAACTCACCT | Insulin signaling (+) |
| Protein kinase B (Akt2) | CCAACACCTTTGTCATACGCTGC | GCTTCAGACTGTTGGCGACCAT | Insulin signaling (+) |
| Glucose transporter 1 (Glut1) | GCTTCTCCAACTGGACCTCAAAC | ACGAGGAGCACCGTGAAGATGA | Glucose uptake (+) |
| Glucose transporter 2 (Glut2) | GTTGGAAGAGGAAGTCAGGGCA | ATCACGGAGACCTTCTGCTCAG | Glucose uptake (+) |
| Glucose transporter 4 (Glut 4) | AAAAGTGCCTGAAACCAGAG | TCACCTCCTGCTCTAAAAGG | Glucose uptake (+) |
Plasma membrane isolation.
Following the protocol of Luiken et al. (23, 35), liver tissues, WAT, pituitaries, and ovaries from fed (control and DHT) mice were extracted, incubated for 30 min in a cold high-salt solution (2 M NaCl, 20 mM HEPES, 5 mM NaN3, pH 7.4) and then centrifuged for 5 min at 1,000 g. The tissues were homogenized in TES buffer (20 mM Tris, 250 mM sucrose, 1 mM EDTA, pH 7.4) supplemented with protease inhibitors. The homogenates then underwent sequential centrifugations at 4°C: 1) homogenate: 10 min at 100 g = pellet P1; 2) supernatant: 10 min at 5,000 g = pellet P2 (plasma membrane).
The pellets were resuspended in TES buffer with protease inhibitors, and protein concentration was determined via BCA assay. GAPDH was used as a plasma membrane loading control. IR and ER were also used to validate enrichment of plasma membrane proteins in the P2 pellets compared with the lysate (data not shown). Lysate from tissues was prepared as described immediately below.
Western blotting.
Liver tissues, WAT, pituitaries, and ovaries from fed and overnight fasted (control and DHT) mice were harvested, homogenized in 1× lysis buffer (Bio-Rad), centrifuged at high speed (15,000 g) (34), and then equal amounts of protein in Laemmli sample buffer were separated via SDS-PAGE (Thermo Scientific, Waltham, MA) and transferred to nitrocellulose membranes. Each blot contained equal amounts of protein between the conditions (control, DHT, insulin, basal, etc.). For liver, skeletal muscle, and WAT, roughly 20–40 g of protein were loaded. For ovaries and pituitary, roughly 10–15 g were loaded. The membranes were blocked, incubated in primary antibodies (p-AKT, AKT, IRb, IRS1, IRS2, GLUT1, GLUT2, GLUT4, and actin; see Table 2 for antibody details, concentrations, manufacturers, and validation [PMID] citations), then incubated with secondary antibodies (goat anti-mouse or goat anti-rabbit, Bio-Rad), and detected using enhanced chemiluminescence (PerkinElmer Life Sciences, Boston, MA). The Western procedure was performed as previously described (3). Values were expressed as phospho protein over total protein or actin.
Table 2.
Antibodies used in this study
| Peptide/Protein Target | Antibody ID (RRID) | Name of Antibody | Manufacturer, Catalog no., and/or Name of Individual Providing the Antibody | Species Raised In; Monoclonal or Polyclonal | Dilution Used | Citation |
|---|---|---|---|---|---|---|
| phospho-AKT | AB_329824 | p-AKT (Ser473) | Cell Signaling Technology #9270 | Rabbit; polyclonal | 1:1,000 | PMID:28650797 |
| AKT | AB_329825 | AKT | Cell Signaling Technology #9272 | Rabbit; polyclonal | 1:1,000 | PMID:25057190 |
| Insulin receptor β | AB_784101 | IRb | Santa Cruz BioTechnology #57341 | Mouse; monoclonal | 1:1,000 | PMID:28438486 |
| Insulin receptor substrate 1 | AB_330333 | IRS1 | Cell Signaling Technology #2382S | Rabbit; polyclonal | 1:200 | PMID:27967242 |
| Insulin receptor substrate 2 | AB_2125771 | IRS2 (L1326) | Cell Signaling Technology #3089S | Rabbit; polyclonal | 1:200 | PMID:27967242 |
| Glucose transporter 1 | AB_2571629 | GLUT1 | Millipore #MABS132 | Mouse; polyclonal | 1:1,000 | PMID:26238734 |
| Glucose transporter 2 | AB_10859605 | GLUT2 | Abcam #ab95256 | Rabbit; polyclonal | 1:1,000 | PMID:28324019 |
| Glucose transporter 4 | AB_823508 | GLUT4 | Cell Signaling Technology #2213 | Mouse; monoclonal | 1:1,000 | PMID:27689415 |
| β-Actin | AB_626632 | b-Actin | Santa Cruz BioTechnology #47778 | Mouse; monoclonal | 1:2,000 | PMID:25488013 |
| GAPDH | AB_627678 | GAPDH | Santa Cruz BioTechnology #47724 | Mouse; polyclonal | 1:2,000 | PMID:27476657 |
Luminex assay: phospho protein measurements.
At 3 mo post-insertion, the livers, WAT, pituitaries, and ovaries of fed and 16-h-fasted control and DHT mice were harvested, homogenized in Bio-Rad Cell Lysis buffer (Bio-Rad Laboratories, Hercules, CA), and analyzed to determine phospho AKT and AKT protein levels using a Milliplex Map 2-Plex Total/Phospho AKT Kit on a Luminex 200IS platform.
Glucose uptake assays.
Glucose uptake assays were performed as previously described (3) with slight modifications. Overnight-fasted animals were euthanized, tissues were extracted, incubated in HEPES buffered saline (HBS; plus 32 mM mannitol, 8 mM glucose, and 0.1% radioimmunoassay grade BSA) for 1 h. To perform the glucose uptake assays, the tissues were incubated in 2DG transport media (HBS with 4 mM 2DG and 2 μi/ml 3H-2DG) for 10 min at 30°C, washed twice with a 4 mM 2DG rinse (HBS with no radiolabel) for 5 min on ice (to remove extracellular 3H-2DG), and stored at −80°C for further analysis. For the insulin-stimulated glucose uptake assays, the tissues were incubated in 12 nM insulin for 20 min at 30°C before incubation in the transport media. One hundred milliliters of homogenized tissue lysates were added to 5 ml of scintillation fluid (PerkinElmer) and counted on a LS6500 liquid scintillation counter (Beckman Coulter, Fullerton, CA) to determine disintegrations per minute (dpm). DPM were normalized to total protein content.
Hepatocyte and pituitary cell culture.
We used 1 nM DHT as this is the concentration found in our DHT mice in vivo (1). The H2.35 female mouse hepatocyte and αT3 female mouse pituitary cell lines were used. Cells were cultured as previously described (1, 2). Briefly, cells were cultured in DMEM with 4.5 g/l glucose, L-glutamine, and sodium pyruvate. H2.35 cells were transfected using Lipofectamine 2000 (Thermo Fisher) with an AR-pBabe-Puro vector to overexpress WT AR as previously described (1). The cells were then incubated in fresh media with or without 1 nM DHT for 24 h. The experiments were performed in serum-starved and non-serum-starved conditions. The nonstarved data are presented in the graphs. Serum-starved insulin levels would be similar to fasted state insulin levels in vivo, thus relatively low. Non-serum-starved insulin levels would be similar to fed state in vivo insulin levels, thus relatively normal. Cells were harvested using TRIzol and processed for qRT-PCR analysis or lysed using Bio-Rad lysis buffer and processed via Western blot analysis.
Metabolic testing: glucose tolerance test and insulin tolerance test.
Mice fasted overnight (16 h) received ip injections of 2 g/kg BW glucose (glucose tolerance test; GTT) or 0.3 U/kg BW insulin (insulin tolerance test; ITT; Lilly), and tail blood was obtained to determine blood glucose at time points between 0 and 120 min using a One Touch Ultra glucometer (Life Scan, Milpitas, CA).
Estrous cyclicity and ovarian morphology.
The estrus cycle was tested by obtaining vaginal smears for 14 consecutive days and analyzing the cytology of the smears as previously described (25). At 3 mo post-insertion of DHT, ovaries of control and DHT mice were obtained, sectioned at 5 mm, and morphological analyses were performed. The number of atretic follicles (AF) and corpora lutea (CL) were determined as previously described (25).
Statistical analysis.
All data were analyzed by unpaired two-tailed t-tests using Prism software (GraphPad) between the two groups indicated by the line above the two groups. The one exception is Fig. 2, E–H. For Fig. 2, E–H, unpaired two-tailed t-tests were used comparing control with the tested groups and insulin only to the insulin plus DHT group. Different letters indicate a statistically significant difference. The same letters indicate no statistically significant difference. All results were expressed as means ± SE. A value of P < 0.05 was defined as statistically significant.
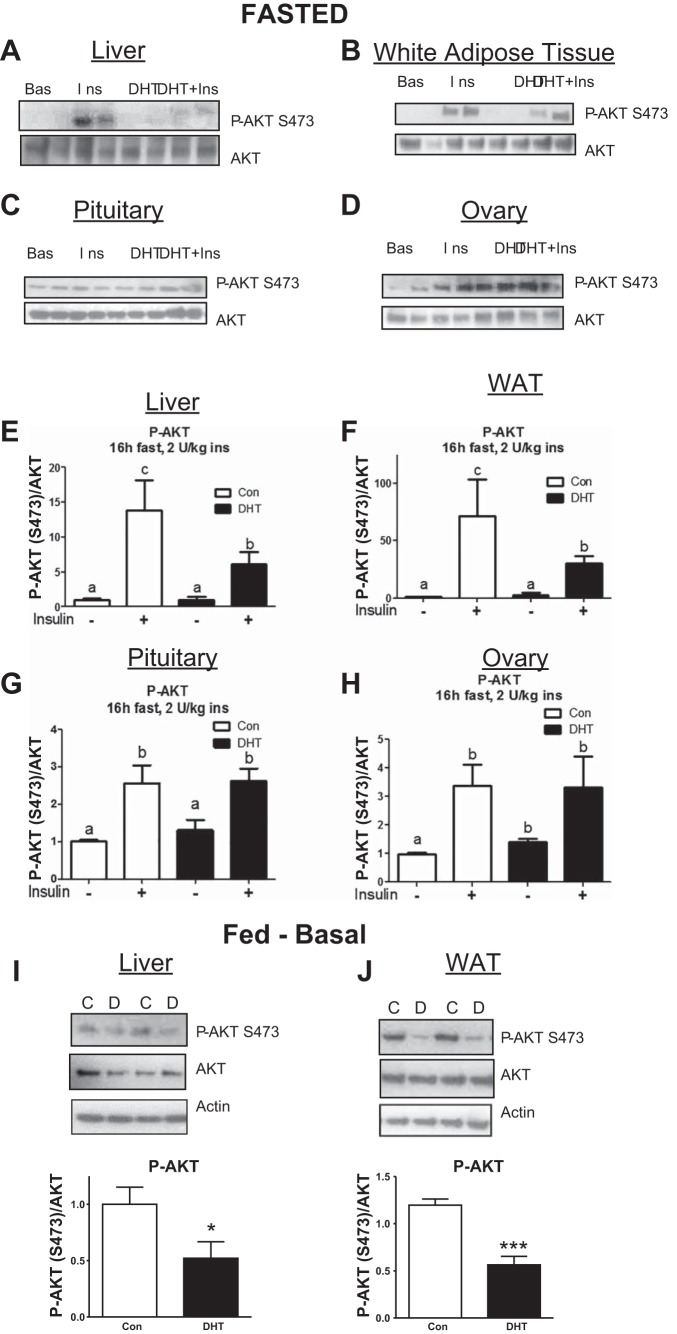
Fig. 2.
Low-dose DHT caused insulin resistance in the liver and WAT but not in the pituitaries and ovaries of fasted mice. At 3 mo post-insertion, control and DHT mice were fasted for 16 h and then injected with saline or 2 U/kg insulin. After 10 min, livers, WAT, pituitaries, and ovaries were harvested and subjected to Western blot analysis A–D) and multiplex Luminex assay analysis E–H) of p-AKT S473 and AKT. Livers (I) and WAT (J) were harvested from fed control and DHT mice and processed for Western blot analysis using antibodies against p-AKT, AKT, and/or actin; n = 4–7/group. Unpaired 2-tailed t-tests were used comparing control with the tested groups and insulin only to the insulin plus DHT group. Different letters indicate a statistically significant difference. Same letters indicate no statistically significant difference. *P < 0.05.
RESULTS
Differential tissue-specific effects of low-dose DHT on insulin signaling mRNA.
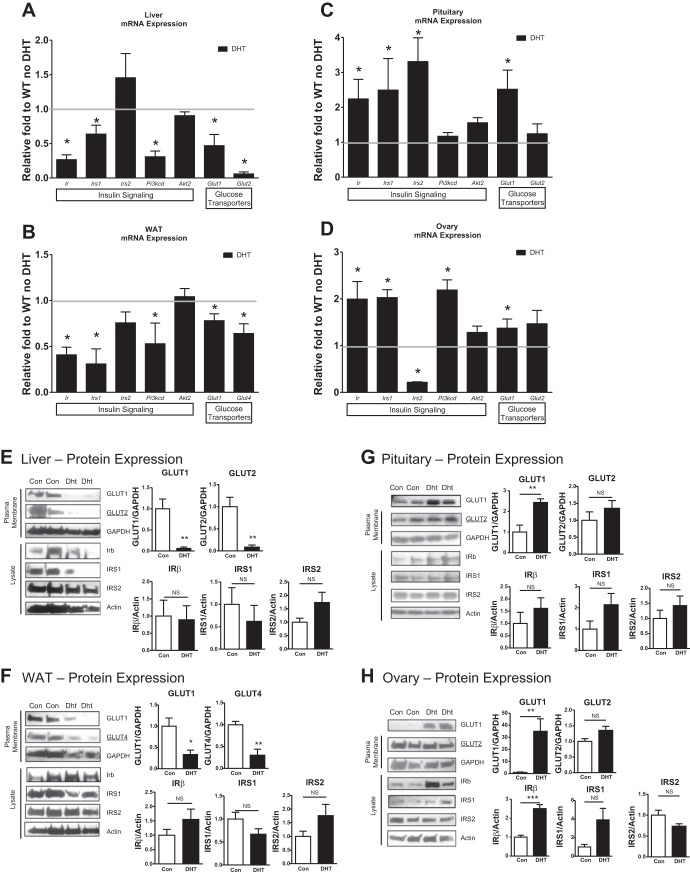
Studies in our laboratory have shown that diet-induced obese mice were partially fertile, hyperandrogenic, and insulin resistant in energy storage tissues but maintained insulin action in the reproductive tissues (36). We sought to determine whether this differential tissue-specific effect on insulin action could be induced by hyperandrogenemia. Livers from fed mice with DHT displayed lowered basal mRNA levels of genes involved in insulin signaling compared with livers of control mice: insulin receptor (Ir), insulin receptor substrate (Irs1), and phosphoinositide 3-kinase catalytic domain (Pi3kcd) (Fig. 1A). WAT of mice receiving DHT displayed decreases in genes involved in insulin signaling (Fig. 1B) as well. However, in contrast to the liver and WAT, pituitaries and ovaries of DHT mice displayed a different response to DHT. Ir and Irs1/2 mRNA levels were significantly increased in pituitaries of DHT mice compared with control mice (Fig. 1C). Ir, Irs1, and Pi3kcd mRNA levels displayed a twofold increase in the ovaries of DHT mice compared with controls (Fig. 1D). The glucose transporters, GLUT1 and GLUT2 (in the livers) and GLUT1 and GLUT4 (in the WAT), were decreased in DHT mice, but GLUT1 and GLUT2 were not decreased in the pituitaries and ovaries compared with controls (Fig. 1, A–D). DHT mice displayed similar Irs2 mRNA in the livers and WAT; however, Irs2 mRNA levels were significantly decreased in the ovaries of DHT mice compared with controls (Fig. 1, A–B and D). These findings suggest that DHT differentially alters mRNA expression levels of insulin signaling and glucose transporter genes in energy storage compared with reproductive tissues.
Fig. 1.
Mice receiving low-dose dihydrotestosterone (DHT) display differential altered insulin signaling mRNA expression levels in reproductive vs. energy storage tissues. C57BL6 were used in this study. At 3 mo post-original insertion, the livers (A), white adipose tissue (WAT; B), pituitaries (C), and ovaries (D) of control and DHT mice were harvested in the fed state and processed for qRT-PCR analysis using TRIzol for RNA isolation or processed for Western blot analysis after cell lysis: livers (E), WAT (F), pituitaries (G), and ovaries (H). See Table 1 for a list of the abbreviations and functions of qRT-PCR primers and Table 2 for information on antibodies used for Western blotting; n = 10/group for qRT-PCR and n = 4/group for Western blotting. *P < 0.05, **P < 0.01, ***P < 0.001.
Differential tissue-specific effects of low-dose DHT on insulin signaling protein levels.
To determine whether low-dose DHT differentially regulated insulin protein levels similar to its regulation of mRNA expression, we examined the total protein levels of several key insulin signaling and glucose metabolism proteins. Low-dose DHT mice displayed lowered GLUTs (GLUT1 and GLUT2, liver; GLUT1 and GLUT4, WAT) protein levels but no change in IRβ, IRS1, and IRS2 in liver (Fig. 1E) and WAT (Fig. 1F) compared with control mice. In contrast, pituitaries of low-dose DHT mice displayed increased GLUT1 but no change in GLUT2, IRb, IRS1, or IRS2 (Fig. 1G) compared with controls. Low-dose DHT mice exhibited increased GLUT1 and IRβ protein levels but no change in GLUT2, IRS1, and IRS2 in the ovary (Fig. 1H) compared with control mice. These data suggest that DHT differentially alters protein levels of insulin signaling and glucose transporter proteins in energy storage compared with reproductive tissues.
Low-dose DHT differentially regulated insulin action in energy storage tissues compared with reproductive tissues of fasted mice.
Lowered insulin stimulated phosphorylation of AKT (p-AKT) in the energy storage tissues is a hallmark of insulin resistance. Western blot analysis and Luminex multiple ligand assays were used to determine whether low-dose DHT differentially alters insulin-stimulated phospho-AKT in energy storage tissues compared with reproductive tissues. Insulin increased p-AKT in the livers and the WAT of control mice compared with basal control mice (Fig. 2, A and B). This insulin-stimulated increase in p-AKT was blunted in the DHT mice (Fig. 2, A and B), indicating that low-dose DHT can cause insulin resistance in the liver. As shown previously in our laboratory (7), insulin increased p-AKT 1.5 to 2-fold in the ovaries and pituitaries, respectively, compared with saline-injected control mice (Fig. 2, C and D). In contrast to the liver and WAT, this insulin-stimulated increase is increased in the pituitaries and maintained in the ovaries of mice receiving low-dose DHT compared with saline-injected control mice (Fig. 2, C and D). These findings were confirmed with Luminex multi-ligand assays for P-AKT and AKT (Fig. 2, E–H).
Mice receiving low dose DHT exhibited lowered p-AKT in liver and WAT in the fed state at basal levels compared with control mice (Fig. 2I–J). These finding suggest that the insulin signaling pathway is dysregulated by low dose DHT in the fed state as well.
Low-dose DHT differentially regulated basal glucose uptake, plasma membrane GLUT1, and insulin-stimulated glucose uptake in energy storage tissues compared with reproductive tissues of fasted mice.
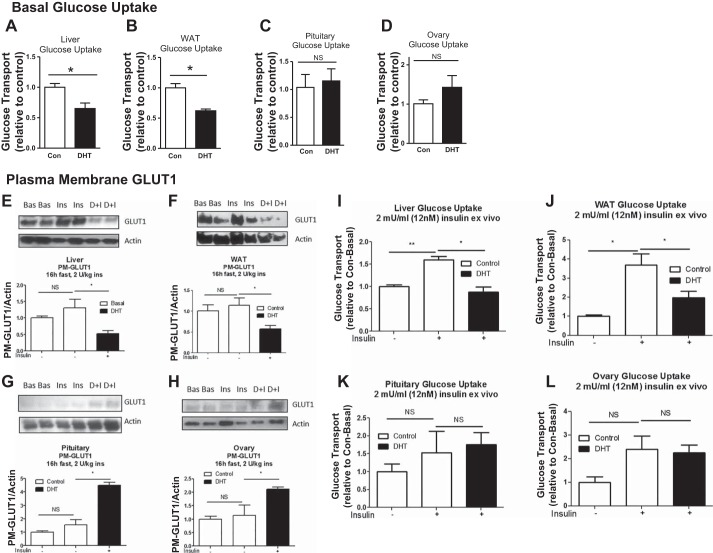
We next sought to determine whether basal glucose uptake in energy storage tissues and reproductive tissues is differentially altered in our model. DHT mice displayed lowered basal glucose uptake in the livers and WAT compared with control mice (Fig. 3, A and B). In contrast, glucose uptake was unaltered in the pituitaries and ovaries of control and DHT mice (Fig. 3, C and D). In conjunction with the basal glucose transport findings, plasma membrane GLUT1 levels were lowered in the livers and WAT of DHT mice (Fig. 3, E and F) but increased in the ovaries and pituitaries of DHT mice (Fig. 3, G and H). Insulin-stimulated glucose uptake was lowered in liver and WAT (Fig. 3, I and J) but unaltered in the pituitary and ovary (Fig. 3, K and L) of DHT mice compared with controls. These data further suggest that low-dose DHT causes a differential tissue-specific response on glucose metabolism in female mice.
Fig. 3.
Low-dose DHT differential effects on basal and insulin-stimulated glucose uptake in the liver and WAT compared with the pituitary and ovary. At 3 mo post-insertion, control and DHT mice were fasted for 16 h, and livers, WAT, pituitaries, and ovaries were harvested and subjected to ex vivo 3H-2-deoxy-glucose basal glucose transport assays (A–D); n = 4–7/group. E–H: a subset of samples were analyzed via Western blot analysis with antibodies against GLUT1 and actin; n = 4–7/group. I–L: another subset of samples were subjected to ex vivo 3H-2-deoxy-glucose insulin-stimulated glucose transport assays; n = 4–7/group. *P < 0.05.
Skeletal muscles did not display low-dose DHT-induced insulin resistance.
In contrast to liver and WAT, low-dose DHT increased fed basal and fasted insulin-stimulated p-AKT (Fig. 4, A and B) and had no change in ex vivo basal glucose transport in skeletal muscles (Fig. 4C). However, DHT mice displayed increased insulin-stimulated glucose transport compared with control mice (Fig. 4D). Low-dose DHT did not decrease insulin signaling mRNA levels in skeletal muscle as in liver and WAT (Fig. 4E). Instead, low-dose DHT resulted in increased Ir and GLUT4 mRNA expression (Fig. 4E). In addition, low-dose DHT mice exhibited increased GLUT1, GLUT4, IRb, IRS1, and IRS2 protein levels in skeletal muscle compared with control mice (Fig. 4F).
Fig. 4.
Low-dose DHT did not result in insulin resistance in skeletal muscles. Skeletal muscles extracted from fed control and DHT mice (A) and from control and DHT mice that were fasted for 16 h then injected with saline or 2 U/kg insulin (B) were harvested and subjected to Western blot analysis for p-AKT S473 and AKT. Basal (C) and insulin-stimulated (D) ex vivo 3H-2-deoxy-glucose transport assays were performed in extracted skeletal muscles. E: skeletal muscles of control and DHT mice were harvested and processed for qRT-PCR analysis using TRIzol for RNA isolation. F: skeletal muscles from control and DHT mice were harvested in the fed state and processed for Western blot analysis after cell lysis. See Table 1 for a list of the abbreviations and functions of qRT-PCR primers and Table 2 for information on antibodies used for Western blotting; n = 10/group for qRT-PCR and n = 4/group for Western blotting; *P < 0.05, **P < 0.01, ***P < 0.001. Bas, basal; Ins, insulin; C, control; D, DHT, dihydrotestosterone; n = 4–7/group; different letters indicate significance.
DHT differentially regulated insulin signaling mRNA and protein expression independent of hyperinsulinemia.
Low-dose DHT resulted in fed and fasted hyperinsulinemia (Fig. 5A). To determine whether androgen excess differentially regulates insulin signaling mRNA and protein independent of hyperinsulinemia, mRNA and protein expression were assessed in a DHT cell model not displaying high insulin levels. DHT-treated cells resulted in a similar pattern of differential regulation of energy storage compared with reproductive tissues as seen above. DHT lowered mRNA expression of several insulin signaling intermediates (Ir, Pik3ca, and Glut1) in H2.35 female mouse hepatocytes (Fig. 5B), whereas DHT increased mRNA expression of insulin signaling intermediates (Ir, Irs1, Irs2, Glut1, and Glut2) in αT3 female mouse pituitary cells (Fig. 5C). DHT lowered the protein levels of GLUT1 and GLUT2 in H2.35 cultured hepatocytes; but increased the protein levels of GLUT1 and IRS1 in αT3 cultured pituitary cells compared with controls (Fig. 5, D and E). These findings suggest that androgen differential regulation of insulin signaling intermediates acts independent of hyperinsulinemia in cultured liver and pituitary cell lines.
Fig. 5.
Low-dose DHT differentially regulates insulin signaling mRNA expression independent of hyperinsulinemia. A: 3 wk after DHT insertion, fed and fasted, basal and glucose-stimulated insulin levels were determined from serum of tail blood samples of control and DHT mice using an ELISA. B and C: a low-dose DHT cell model was developed to remove the aspect of hyperinsulinemia. The experiments were performed in serum starved and non-serum-starved conditions. The nonstarved data are presented in the graphs. H2.35 female mouse hepatocytes (B) and αT3 female mouse pituitary cell lines (C) were used. mRNA expression was determined via qRT-PCR analysis, and protein expression was determined via Western blot analysis [H2.35 (D) and αT3 (E); n = 6/group. Same letters indicate no statistically significant difference. *P < 0.05, **P < 0.01, ***P < 0.001.
Low dose DHT causes whole body reproductive and metabolic dysfunction.
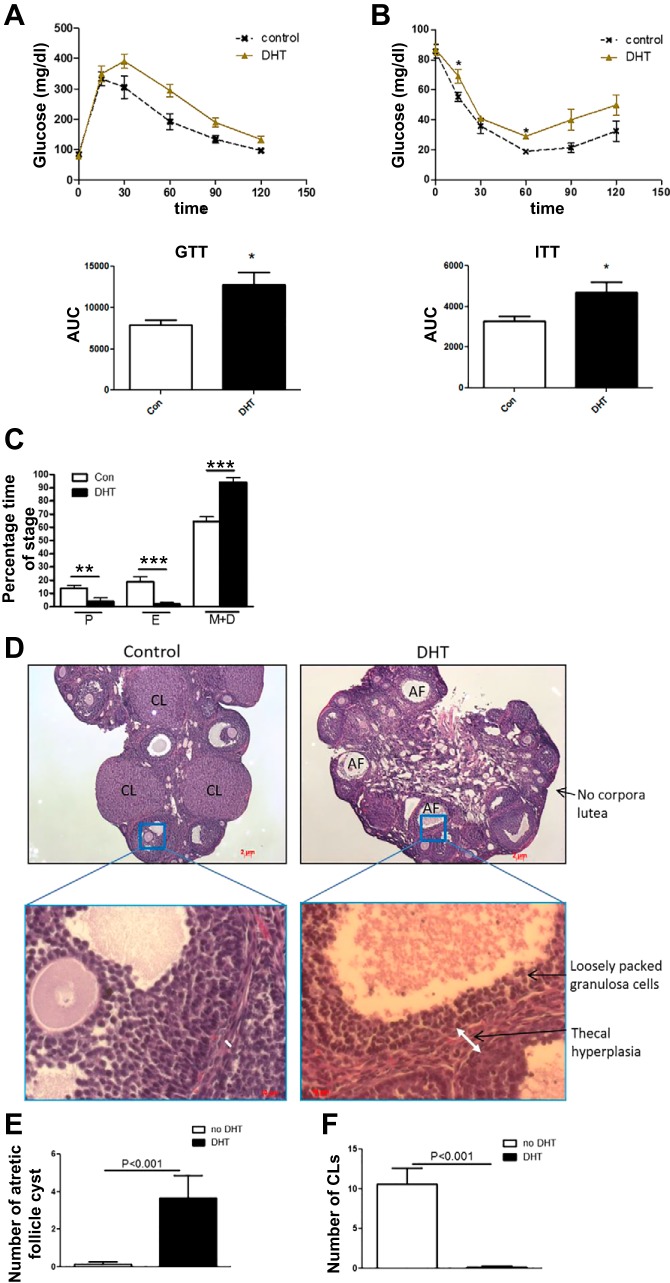
As seen previously (1), low-dose DHT mice displayed impaired glucose tolerance and impaired insulin sensitivity compared with control mice (Fig. 6, A and B). Additionally, low-dose DHT mice displayed increased time in the metestrus/diestrus phase of the estrus cycle compared with control mice, indicating that the DHT mice were acyclic or not cycling properly through the estrus cycle (Fig. 6C). Low-dose DHT mice displayed loosely packed granulosa cells, thecal cell hyperplasia, increased atretic follicles, and decreased corpora lutea compared with control mice (Fig. 6, D–F), suggesting decreased fertility as was seen previously (25) using this low-dose DHT model.
Fig. 6.
DHT mice displayed impaired glucose tolerance, impaired insulin sensitivity, and reproductive dysfunction seen as reduced corpora lutea, increased atretic follicles, and acyclity. A and B: control and low-dose DHT mice fasted for 16 h received intraperitoneal injections of 2 g/kg body wt glucose (GTT) or 0.3 U/kg body wt insulin (ITT; Lilly, Indianapolis, IN), and tail blood was obtained to determine blood glucose at time points between 0 and 120 min using a One Touch Ultra glucometer and One Touch Ultra test strips (Life Scan, Milpitas, CA). C: the estrus cycle was tested by obtaining vaginal smears for 14 consecutive days and analyzing the cytology of the smears. D: at 3 mo post-insertion of DHT, ovaries of control and DHT mice were obtained, sectioned at 5 mm, and morphological analyses were performed. Number of atretic follicles (AF; E) and corpora lutea (CL; F) were determined as previously described (25); n = 6–8/group. *P< 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
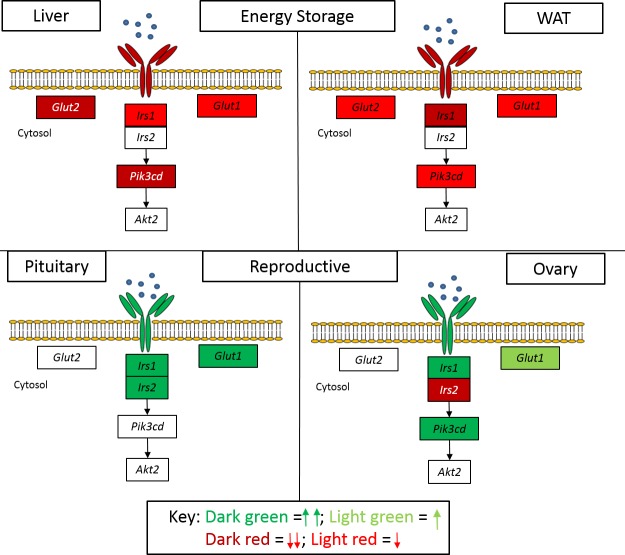
Here, we show that low-dose DHT resulted in the differential regulation of insulin signaling mRNA intermediates and protein levels in energy storage compared with reproductive tissues (Fig. 7). This differential regulation was also seen at the level of insulin action as determined by p-AKT levels and at the level of glucose metabolic function as measured by basal and insulin-stimulated glucose uptake. This androgen excess-mediated effect was independent of high insulin levels in liver and pituitary cell lines. In summary, low-dose DHT caused a lowering of the components of insulin signaling in energy storage tissues but increased the components of insulin signaling in reproductive tissues. This is depicted graphically in our proposed model in Fig. 7. The low-dose DHT mouse model had previously been used by our group to assess hepatic insulin signaling (1) and androgen receptor function in ovarian theca cells (25). We showed that our low-dose DHT model resulted in reproductive and ovarian dysfunction (25), which was partially rescued by the deletion of AR in the ovary thecal cells.
Fig. 7.
Model depicting the effects of DHT on insulin signaling mRNA expression in energy storage compared to reproductive tissues. Hyperandrogenemia results in differential tissue-specific effects on insulin action that may define metabolic and reproductive dysfunction. DHT lowered several insulin signaling genes in the liver and WAT, but increased several insulin signaling genes in the pituitary and ovary. Dark red = decrease, P < 0.01; light red = decrease, P < 0.05; dark green = increase, P < 0.01; light green = increase, P < 0.05.
Studies have reported decreases in p-AKT and glucose transport in skeletal muscle biopsies of PCOS women compared with non-PCOS women (8, 14). Other groups have shown that decreased insulin action at the level of p-AKT and glucose transport is not conserved in cultured myotubes from PCOS women compared with control women (12); claiming that the muscle insulin resistance reported in the other studies was an extrinsic effect, not an intrinsic defect. The adaptation (or extrinsic effect) is proposed to be hyperinsulinemia. Our findings show that low-dose DHT increased insulin-stimulated p-AKT and had no change in ex vivo basal glucose transport but increased insulin-stimulated glucose uptake in skeletal muscles (Fig. 3). GLUT4 is roughly 2- to 10-fold more abundant than GLUT1 in skeletal muscles (5, 19), but the majority of GLUT4 resides intracellularly and is inactive until stimulated by insulin. Here, increased GLUT4 mRNA and plasma membrane protein levels had no effect on basal glucose transport but were correlated with increased insulin-stimulated glucose uptake. Our findings fall in line with the Dunaif et al. studies (6, 11), which proposed that skeletal muscle insulin resistance is an intrinsic (or genetic) defect. If skeletal muscle insulin resistance is an intrinsic (or genetic) defect in PCOS woman, this may explain why in our model (postpubertal, low-dose DHT) we did not observe skeletal muscle insulin resistance. DHT-induced increased insulin-stimulated p-AKT in skeletal muscles may be a compensatory mechanism to alleviate the insulin resistance in the WAT and liver. Yet another possibility is that obesity may contribute to muscle insulin resistance whereas in lean conditions hyperandrogenemia contributes only to liver and WAT dysfunction.
Glucose uptake was measured in several different tissue types and was used to assess the DHT-induced effect on glucose metabolism. The rationale for measurement of basal glucose transport was that these tissues undergo basal glucose transport primarily using GLUT1 (19). The insight offered by measurement of basal glucose uptake is that this is a fundamentally valid comparison. We found that DHT lowered basal and insulin-stimulated glucose uptake in the liver and WAT (coinciding with the IGT) but had no effect on basal or insulin-stimulated glucose uptake in reproductive tissues. GLUT1 is expressed in all tissues and accounts for basal glucose uptake (3, 17). GLUT2 is the primary GLUT expressed in the liver, kidney, and pancreas and has a high Km (or low affinity for its substrate) for glucose uptake. The majority of insulin-stimulated glucose uptake is mediated by GLUT4-expressing tissues [skeletal muscle (80%)], and the remainder is mediated by cardiac muscle and adipose tissue (30); little is known of insulin-stimulated glucose uptake in ovaries (31) and pituitary. GLUT4 is not present in hepatocytes (17). Thus liver glucose uptake is primarily non-insulin dependent. However, the liver accounts for 33% of glucose uptake (via GLUT1 and GLUT2) after a meal, thus making it a valid target of assessing glucose uptake (33). In our model, DHT lowered hepatic glucose uptake presumably by decreased GLUT1 and GLUT2 mRNA expression (Fig. 1A) and lowered plasma membrane GLUT1 and GLUT2 (Fig. 1, E and F) but decreased WAT glucose transport presumably by lowered GLUT4 mRNA expression (Fig. 1B), lowered insulin-stimulated p-AKT (Fig. 2B), and lowered plasma membrane GLUT1 and GLUT4 (Fig. 1F). Hence, DHT displayed different mechanisms in different tissues.
Previous studies have shown differential effects of androgen excess on insulin target tissues (28). However, this is to our knowledge the first account showing direct differential effects of androgen excess on energy storage tissues compared with reproductive tissues. The significance of this is that both accounts (lowered insulin action in the energy storage tissues and maintained insulin action in the reproductive tissues) perpetuate the pathogenic condition of hyperandrogenemia. Insulin resistance in the liver (1) and WAT (18) contribute to whole body glucose metabolism dysfunction and hyperinsulinemia (16). Insulin signaling in reproductive tissues plays a role in PCOS pathogenesis by insulin serving as a co-gonadotropin, helping to increase theca cell testosterone synthesis (27, 29, 37). Some studies propose that hyperinsulinemia prolongs this cycle (20). Our findings suggest that this differential effect on energy storage compared with reproductive tissues may be due to androgen excess independent of high insulin levels (Fig. 5, B and C). Our low-dose DHT, normal-insulin cell culture model (H2.35 hepatocytes and αT3 pituitary cells) displayed a similar trend seen in vivo, indicating that it is likely androgen excess and not hyperinsulinemia impacts the mRNA expression in liver and pituitary cells.
The rationale for measuring mRNA and protein expression in H2.35 and αT3 (liver and pituitary cell lines, respectively) was to determine whether the differential effect on expression seen in vivo was influenced by hyperinsulinemia. Thus we only measured total expression. However, we have shown recently that low-dose DHT decreased insulin-stimulated p-AKT in H2.35 cultured hepatocytes similarly to what is seen in our low-dose DHT mice (1).
One cannot be certain that changes in mRNA expression would be reflected in the respective protein levels (13). Thus we examined the protein levels of several insulin signaling and glucose metabolism intermediates in energy storage compared with reproductive tissues. Insulin receptor β and insulin receptor ½ were selected to be examined because they were altered in all tissues. GLUT1, GLUT2, and GLUT4 were examined respectively as they are key components of glucose uptake and metabolism. Interestingly, IRβ, IRS1, and IRS2 protein levels were not altered in liver and WAT despite their mRNA levels being decreased (Fig. 1). However, GLUT levels were decreased in liver and WAT. This falls in line with our previous findings that insulin signaling is disrupted at the level of phosphoinositide 3-kinase in the liver in our low-dose DHT lean hyperandrogenic model (1), leading to hepatic insulin resistance and increased hepatic glucose production. The mechanism for WAT low-dose DHT-induced decreased insulin-stimulated p-AKT is yet to be determined, but our findings suggest it is not due to lowered IRβ, IRS1, or IRS2. Interestingly, in obesity-linked hyperandrogenemia (4) and obesity-linked type 2 diabetes (32), the dysfunction in insulin signaling is reported to begin at the level of the insulin receptor. This suggest that there is a mechanistic difference between obesity- and non-obesity-linked insulin resistance.
Studies on insulin response in WAT exposed to androgen excess have revealed interesting findings. In vivo WAT from women with PCOS displayed insulin resistance compared with WAT from non-PCOS women (8, 26); however, cultured adipocytes from PCOS women displayed no change in insulin sensitivity (9, 24). These findings suggest that WAT insulin resistance was due to in vivo environmental conditions, not an intrinsic defect as in skeletal muscle. Our DHT model displayed lowered insulin action in the WAT (Fig. 2).
It is known that insulin potentiates ovarian androgen production (10). Thus androgen excess having the ability to increase insulin signaling genes in the ovary will lead to increased insulin-induced production of ovarian androgens. In addition, the ability of androgen excess to lower insulin signaling genes in the liver and WAT will result in increased insulin production, which will lead to further insulin-induced ovarian androgen production. This is the vicious potentiating cycle of PCOS. Finding therapeutics to lower the effects of androgen excess at the level of specific tissues will be imperative in the next generation of PCOS therapies.
In conclusion, the findings presented in this study advance the field and present a new conceptual idea for therapeutic targeting for women with PCOS. We showed that androgen excess via DHT and likely independent of hyperinsulinemia in key tissues such as the liver and ovary resulted in differential effects on insulin signaling in energy storage tissues compared with reproductive tissues.
GRANTS
This work was supported by the National Institutes of Health (Grants R00-HD068130 04 to S.Wu). Technical support was provided by the Integrated Physiology Core of the Baltimore Diabetes Research Training Center (pilot and feasibility award P30DK079637).
AUTHOR CONTRIBUTIONS
S.A. and S.W. conceived and designed research; S.A., K.B., P.X., and S.W. performed experiments; S.A., K.B., P.X., and S.W. analyzed data; S.A. and S.W. interpreted results of experiments; S.A. prepared figures; S.A. drafted manuscript; S.A. and S.W. edited and revised manuscript; S.A., K.B., P.X., and S.W. approved final version of manuscript.
References
- 1.Andrisse S, Childress S, Ma Y, Billings K, Chen Y, Xue P, Stewart A, Sonko ML, Wolfe A, Wu S. Low-dose dihydrotestosterone drives metabolic dysfunction via cytosolic and nuclear hepatic androgen receptor mechanisms. Endocrinology 158: 531–544, 2017. doi: 10.1210/en.2016-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrisse S, Koehler RM, Chen JE, Patel GD, Vallurupalli VR, Ratliff BA, Warren DE, Fisher JS. Role of GLUT1 in regulation of reactive oxygen species. Redox Biol 2: 764–771, 2014. doi: 10.1016/j.redox.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrisse S, Patel GD, Chen JE, Webber AM, Spears LD, Koehler RM, Robinson-Hill RM, Ching JK, Jeong I, Fisher JS. ATM and GLUT1-S490 phosphorylation regulate GLUT1 mediated transport in skeletal muscle. PLoS One 8: e66027, 2013. doi: 10.1371/journal.pone.0066027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycystic ovary syndrome. Nat Rev Dis Primers 2: 16057, 2016. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 5.Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Annu Rev Biochem 81: 507–532, 2012. doi: 10.1146/annurev-biochem-060109-094246. [DOI] [PubMed] [Google Scholar]
- 6.Book CB, Dunaif A. Selective insulin resistance in the polycystic ovary syndrome. J Clin Endocrinol Metab 84: 3110–3116, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab 12: 295–305, 2010. doi: 10.1016/j.cmet.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciaraldi TP, Aroda V, Mudaliar S, Chang RJ, Henry RR. Polycystic ovary syndrome is associated with tissue-specific differences in insulin resistance. J Clin Endocrinol Metab 94: 157–163, 2009. doi: 10.1210/jc.2008-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbould A, Dunaif A. The adipose cell lineage is not intrinsically insulin resistant in polycystic ovary syndrome. Metabolism 56: 716–722, 2007. doi: 10.1016/j.metabol.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamanti-Kandarakis E, Argyrakopoulou G, Economou F, Kandaraki E, Koutsilieris M. Defects in insulin signaling pathways in ovarian steroidogenesis and other tissues in polycystic ovary syndrome (PCOS). J Steroid Biochem Mol Biol 109: 242–246, 2008. doi: 10.1016/j.jsbmb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 41: 1257–1266, 1992. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 12.Eriksen M, Pørneki AD, Skov V, Burns JS, Beck-Nielsen H, Glintborg D, Gaster M. Insulin resistance is not conserved in myotubes established from women with PCOS. PLoS One 5: e14469, 2010. doi: 10.1371/journal.pone.0014469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 4: 117, 2003. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Højlund K, Glintborg D, Andersen NR, Birk JB, Treebak JT, Frøsig C, Beck-Nielsen H, Wojtaszewski JF. Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes 57: 357–366, 2008. doi: 10.2337/db07-0706. [DOI] [PubMed] [Google Scholar]
- 15.Jenssen T, Hartmann A. Emerging treatments for post-transplantation diabetes mellitus. Nat Rev Nephrol 11: 465–477, 2015. doi: 10.1038/nrneph.2015.59. [DOI] [PubMed] [Google Scholar]
- 16.Kanuri BN, Kanshana JS, Rebello SC, Pathak P, Gupta AP, Gayen JR, Jagavelu K, Dikshit M. Altered glucose and lipid homeostasis in liver and adipose tissue pre-dispose inducible NOS knockout mice to insulin resistance. Sci Rep 7: 41009, 2017. doi: 10.1038/srep41009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karim S, Adams DH, Lalor PF. Hepatic expression and cellular distribution of the glucose transporter family. World J Gastroenterol 18: 6771–6781, 2012. doi: 10.3748/wjg.v18.i46.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol 25: 2498–2510, 2005. doi: 10.1128/MCB.25.6.2498-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacombe VA. Expression and regulation of facilitative glucose transporters in equine insulin-sensitive tissue: from physiology to pathology. ISRN Vet Sci 2014: 409547, 2014. doi: 10.1155/2014/409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landay M, Huang A, Azziz R. Degree of hyperinsulinemia, independent of androgen levels, is an important determinant of the severity of hirsutism in PCOS. Fertil Steril 92: 643–647, 2009. doi: 10.1016/j.fertnstert.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84: 165–169, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Lizneva D, Gavrilova-Jordan L, Walker W, Azziz R. Androgen excess: Investigations and management. Best Pract Res Clin Obstet Gynaecol 37: 98–118, 2016. doi: 10.1016/j.bpobgyn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JF. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of FAT/CD36. Diabetes 51: 3113–3119, 2002. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- 24.Lystedt E, Westergren H, Brynhildsen J, Lindh-Astrand L, Gustavsson J, Nystrom FH, Hammar M, Strålfors P. Subcutaneous adipocytes from obese hyperinsulinemic women with polycystic ovary syndrome exhibit normal insulin sensitivity but reduced maximal insulin responsiveness. Eur J Endocrinol 153: 831–835, 2005. doi: 10.1530/eje.1.02027. [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Andrisse S, Chen Y, Childress S, Xue P, Wang Z, Jones D, Ko C, Divall S, Wu S. Androgen receptor in the ovary theca cells plays a critical role in androgen-induced reproductive dysfunction. Endocrinology 158: 98–108, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannerås-Holm L, Leonhardt H, Kullberg J, Jennische E, Odén A, Holm G, Hellström M, Lönn L, Olivecrona G, Stener-Victorin E, Lönn M. Adipose tissue has aberrant morphology and function in PCOS: enlarged adipocytes and low serum adiponectin, but not circulating sex steroids, are strongly associated with insulin resistance. J Clin Endocrinol Metab 96: E304–E311, 2011. doi: 10.1210/jc.2010-1290. [DOI] [PubMed] [Google Scholar]
- 27.Munir I, Yen HW, Geller DH, Torbati D, Bierden RM, Weitsman SR, Agarwal SK, Magoffin DA. Insulin augmentation of 17alpha-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology 145: 175–183, 2004. doi: 10.1210/en.2003-0329. [DOI] [PubMed] [Google Scholar]
- 28.Nada SE, Thompson RC, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues. Endocrinology 151: 5165–5173, 2010. doi: 10.1210/en.2010-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 83: 2001–2005, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Olefsky JM. Insulin-stimulated glucose transport minireview series. J Biol Chem 274: 1863, 1999. doi: 10.1074/jbc.274.4.1863. [DOI] [PubMed] [Google Scholar]
- 31.Purcell SH, Chi MM, Lanzendorf S, Moley KH. Insulin-stimulated glucose uptake occurs in specialized cells within the cumulus oocyte complex. Endocrinology 153: 2444–2454, 2012. doi: 10.1210/en.2011-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 126: 12–22, 2016. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharabi K, Tavares CD, Rines AK, Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med 46: 21–33, 2015. doi: 10.1016/j.mam.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suski JM, Lebiedzinska M, Wojtala A, Duszynski J, Giorgi C, Pinton P, Wieckowski MR. Isolation of plasma membrane-associated membranes from rat liver. Nat Protoc 9: 312–322, 2014. doi: 10.1038/nprot.2014.016. [DOI] [PubMed] [Google Scholar]
- 35.Tepavčević S, Vojnović Milutinović D, Macut D, Žakula Z, Nikolić M, Božić-Antić I, Romić S, Bjekić-Macut J, Matić G, Korićanac G. Dihydrotestosterone deteriorates cardiac insulin signaling and glucose transport in the rat model of polycystic ovary syndrome. J Steroid Biochem Mol Biol 141: 71–76, 2014. doi: 10.1016/j.jsbmb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Wu S, Divall S, Wondisford F, Wolfe A. Reproductive tissues maintain insulin sensitivity in diet-induced obesity. Diabetes 61: 114–123, 2012. doi: 10.2337/db11-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou QH, Chen X, Leng L, Zhang JS, Tang NJ. [Effects of dibutyl phthalate and monobutyl phthalate on testosterone secretion and insulin-like factor 3 expression of Leydig tumor cells in mice]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 31: 83–87, 2013. [PubMed] [Google Scholar]