Abstract
Pudendal nerve stimulation is a promising treatment approach for lower urinary tract dysfunction, including symptoms of overactive bladder. Despite some promising clinical studies, there remain many unknowns as to how best to stimulate the pudendal nerve to maximize therapeutic efficacy. We quantified changes in bladder capacity and voiding efficiency during single-fill cystometry in response to electrical stimulation of the sensory branch of the pudendal nerve in urethane-anesthetized female Wistar rats. Increases in bladder capacity were dependent on both stimulation amplitude and rate. Stimulation that produced increases in bladder capacity also led to reductions in voiding efficiency. Also, there was a stimulation carryover effect, and increases in bladder capacity persisted during several nonstimulated trials following stimulated trials. Intravesically administered PGE2 reduced bladder capacity, producing a model of overactive bladder (OAB), and sensory pudendal nerve stimulation again increased bladder capacity but also reduced voiding efficiency. This study serves as a basis for future studies that seek to maximize the therapeutic efficacy of sensory pudendal nerve stimulation for the symptoms of OAB.
INTRODUCTION
Overactive bladder (OAB) symptoms, including urinary urgency, frequency, urgency incontinence, and nocturia often fail to improve following pharmacological treatment (2). Furthermore, the success of other therapies for OAB, including sacral neuromodulation, posterior tibial nerve stimulation, and intravesical onabotulinumtoxinA injections, is limited (19, 30), and there remains a need for improved therapeutic options.
Pudendal nerve stimulation is a promising therapeutic approach for the treatment of the symptoms of OAB (7, 10, 20), which may work by activating a larger proportion of afferent fibers that reflexively contribute to bladder inhibition (24) than unilateral stimulation of the S3 sacral nerve (13, 28). Despite promising initial clinical feasibility demonstrations, it remains unclear how to optimally stimulate the pudendal nerve to reduce OAB symptoms. Most experimental work on pudendal nerve stimulation to treat OAB symptoms has been done in cats, particularly, with a focus on the neurochemical mechanisms underlying bladder inhibition by pudendal nerve stimulation (e.g., 3, 18, 21). Although the impact of different stimulus parameters on bladder inhibition has been explored (3, 31, 37), this has been largely conducted under isovolumetric conditions, and there was little parametric exploration during filling cystometry. Also, considerably less work on the effects of pudendal nerve stimulation on OAB symptoms has been conducted in rats (33).
Furthermore, most invasive stimulation of the pudendal nerve has occurred at the proximal compound nerve before branching (3, 25, 26, 36). However, stimulation at a more distal location along the nerve may enable selective targeting of sensory nerve fibers, thus allowing higher stimulus intensities and possibly greater therapeutic efficacy without unwanted motor activation.
In this study, we stimulated the sensory branch of the pudendal nerve (22). The sensory branch contains nerve fibers that innervate the urethra, as well as nerve fibers that comprise the dorsal genital nerve and innervate the genitals and perigenital skin (4, 23). This may be analogous to selective stimulation of the dorsal genital nerve in humans, although it is unclear whether the dorsal genital nerve in humans also innervates the proximal or midurethra.
Stimulation of the sensory branch of the pudendal nerve may confer greater therapeutic benefit than more proximal pudendal nerve stimulation. However, we are aware of only two studies of pudendal nerve stimulation in the rat that focus on increasing bladder capacity (5, 33), and these studies did not target the sensory branch of the pudendal nerve. Thus, the goal of this study was to quantify how stimulation parameters, specifically amplitude and rate, impacted bladder capacity and voiding efficiency. Stimulation amplitude and pulse repetition frequency were varied systematically during single-fill cystometry in urethane-anesthetized female Wistar rats. The primary experiments focused on characterizing the effects of stimulation amplitude and frequency using intravesical saline. Secondary experiments using intravesical PGE2 were also conducted to determine whether sensory pudendal stimulation increased bladder capacity in this model of OAB (17). These results serve to inform parameter optimization for sensory pudendal nerve stimulation to treat OAB.
METHODS
All animal care and experimental procedures were reviewed and approved by the Duke University Institutional Animal Care and Use Committee.
Surgical preparation and equipment setup.
Female Wistar rats (n = 26), weighing between 237 and 296 g, were anesthetized with urethane (1.2 g/kg sc, supplemented as necessary). Body temperature was monitored using an esophageal temperature probe and maintained at 36–38°C with a water blanket. Heart rate and arterial blood oxygen saturation levels were monitored using a pulse oximeter (Nonin Medical, 2500A VET).
For cystometrogram (CMG) measurements, the bladder was exposed via a midline abdominal incision. A PE-90 catheter, the tip of which was heated to create a collar, was inserted into the bladder lumen through a small incision in the apex of the bladder dome and secured with a 6-0 silk suture. The abdominal wall was closed in layers with 3-0 silk suture. The bladder catheter was connected via a three-way stopcock to an infusion pump (Braintree Scientific, BS-8000 or Harvard Apparatus PHD 4400) and to a pressure transducer (ArgoTrans; ArgonMedical Devices, Plano, TX) connected to a bridge amplifier and filter (13-6615-50; Gould Instruments, Valley View, OH) for measuring intravesical pressure. Data were sampled at 1 kHz using a PowerLab system (AD Instruments, Colorado Springs, CO).
External urethral sphincter (EUS) EMG was measured using two different approaches (Fig. 1A). Experiments with intravesical PGE2 used two PFA-coated platinum-iridium wires (140-µm diameter, A-M Systems, Sequim, WA). These wires were inserted percutaneously using a needle to pierce the skin, one on each side of the urinary meatus (Fig. 1B). Experiments with saline infusion used two platinum contacts bonded to a silicone backing with wires welded to each contact (Fig. 1C). This sheet-electrode was placed between the urethra and the pubic symphysis using an intra-abdominal approach. EUS EMG leads were connected through a preamplifier (HIP5, Grass Products, Warwick, RI) to an amplifier (P511, Grass Products), and a subcutaneous needle served as a ground. Signals were filtered (3 Hz–3 kHz) and were sampled at 20 kHz.
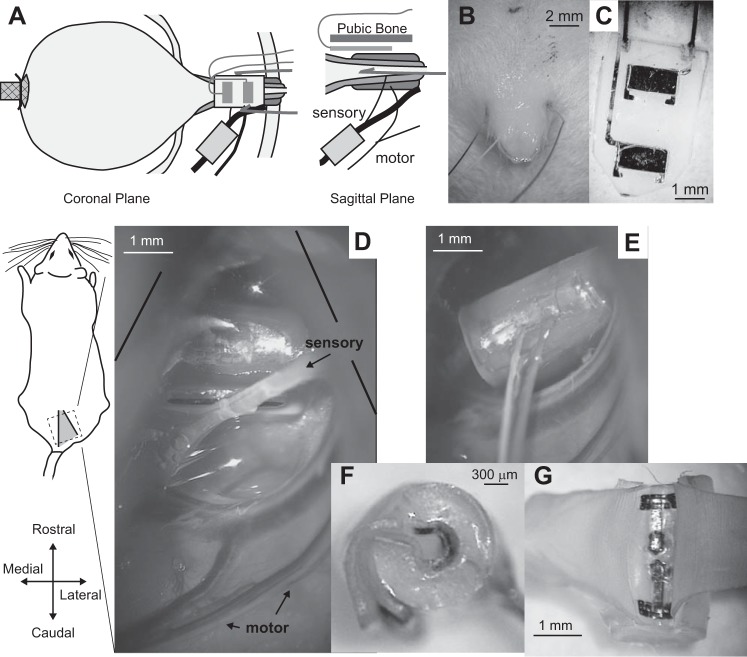
Fig. 1.
Experimental setup. A: placement of the bladder catheter, nerve cuff, and electrodes for recording external urethral sphincter (EUS) electromyogram (EMG). Two different types of EMG electrodes were used (in different experiments), percutaneous wires (B) and flat metal contacts embedded in a silicone substrate (C) that was placed underneath the pubic bone. D: view through the surgical microscope of the isolated sensory pudendal nerve in the ischiorectal fossa. E: view showing placement of the nerve cuff on the sensory pudendal nerve. F: side view of a nerve cuff showing the orientation of the incoming wires, as well as the tabs that are used to open the cuff. G: view of the inside of the nerve cuff after pulling the cuff open at the tabs. The exposed portion of the contacts are near the outside of the cuff.
After placement of the bladder catheter and EUS EMG electrodes, the animal was carefully turned to a prone position. After resecting of the gluteal muscles at the midline, the ischium was spread apart from the sacrum to expose the ischiorectal fossa, and the sensory branch of the pudendal nerve was isolated from connective tissue (Fig. 1D). For PGE2 experiments, custom bipolar nerve cuffs were placed around the sensory branch and held in place with the use of Kwik-Cast (World Precision Instruments, Sarasota, FL). The custom cuffs consisted of 10 stranded stainless-steel wires (AS631; Cooner Wire, Chatsworth, CA) placed ~1 mm apart through 2-mm long, 500-µm inner-diameter silicone tubing. The exposed wires were insulated with silicone (MED-1137; NuSil, Carpinteria, CA), and a slit was cut along the length of the tubing to allow placement of the nerve into the cuff. For saline-only experiments, the same custom nerve cuff was used in one experiment. For other saline-only experiments, nerve cuffs that were 300-µm inner diameter with 3-mm length (n = 4) were initially used (Fig. 1, F and G) (CorTec, Freiburg, Germany). With improved dissection of the sensory branch from connective tissue, it became possible to use cuffs that had only a 200-µm inner diameter (2 mm in length, n = 4). These were easier to implant as they required less dissection of the nerve. The results were qualitatively the same with both electrode sizes (see discussion). Following nerve cuff placement, the incision was sutured closed in layers with 3-0 silk suture. The animal was then carefully turned back into a supine position for cystometric testing.
Preliminary testing indicated that 100 µM of PGE2 consistently reduced bladder capacity as a model of OAB (12, 17). PGE2 (Sigma-Aldrich) was dissolved in ethanol to 10 mM concentration and stored in a −20°C freezer. On days of the experiments, this stock solution was diluted in saline to 100 μM.
Experimental procedures.
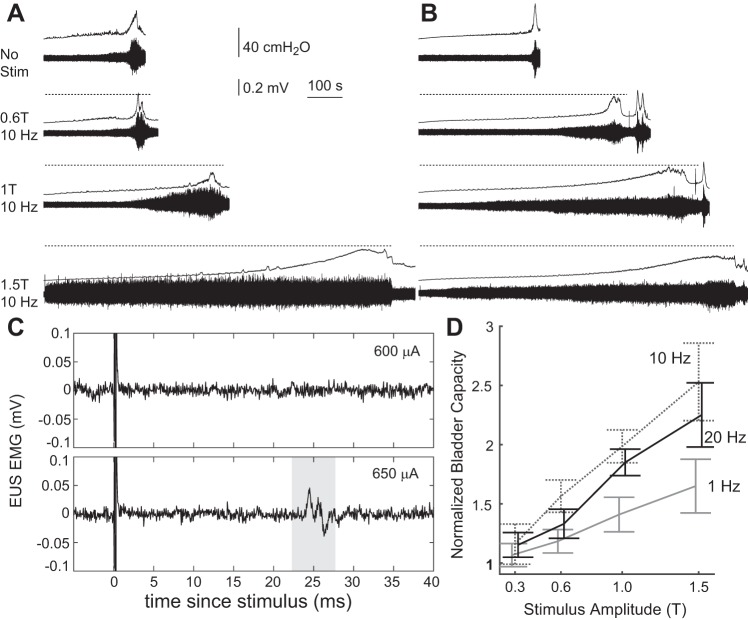
The stimulus amplitude required to evoke a reflex response in the EUS was determined. With the bladder empty, stimulation pulses were delivered at 0.1 to 1 Hz to the sensory pudendal nerve, while evoked EUS EMG activity was monitored on an oscilloscope. The minimum amplitude required to evoke consistently (more than 50% of the time) reflex activation of the EUS was considered the stimulus threshold or 1T (1 times threshold) and was verified to within 10% accuracy (absence of consistent reflex activation at 0.9T). Experiments with percutaneous EMG electrodes (PGE2 experiments) did not show consistent reflex activation of the sphincter. In these experiments, a 200-µA stimulus was used primarily (n = 6), based on preliminary testing with intravesical saline.
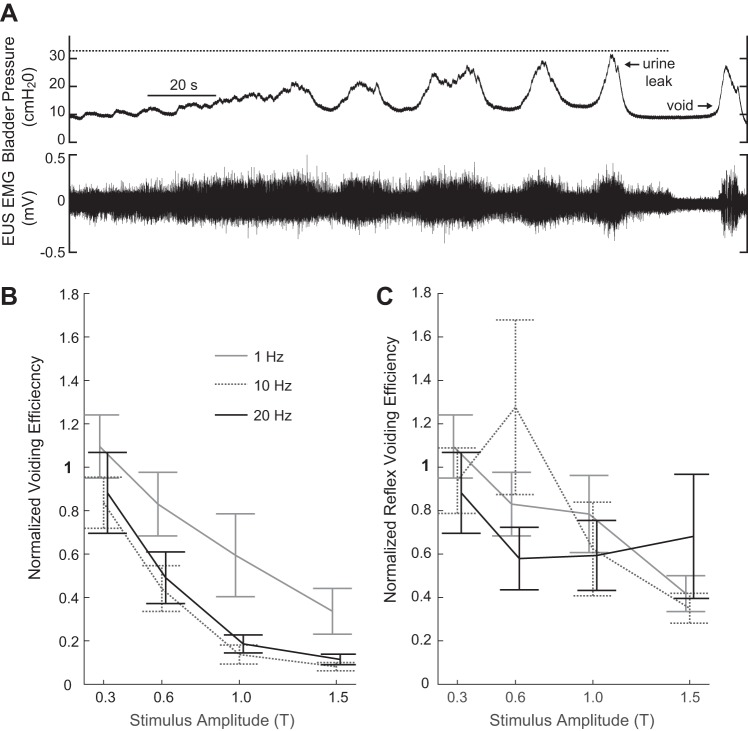
The bladder was filled continuously with physiological saline at room temperature (2–8 ml/h) using an infusion pump with an open urethra for at least 45 min during postsurgical recovery. The bladder was subsequently emptied, and CMGs were recorded. For each CMG, the bladder was filled until a micturition event was observed, at which time the infusion pump was turned off (single-fill cystometry). Using single-fill cystometry, we were able to measure both bladder capacity and voiding efficiency, rather than just functional bladder capacity. For PGE2 experiments, ~1 min after the bladder pressure returned to baseline, the bladder was emptied via the catheter using a syringe. For nonstimulated trials in saline-only experiments, the bladder was emptied immediately following bladder pressure return to baseline. For stimulation in saline-only experiments, two approaches were used. In the first two experiments, the bladder was emptied upon pressure return to baseline, the same as during nonstimulated trials. In seven experiments, the stimulus was terminated following the first micturition event, and the bladder was not emptied immediately to determine whether withdrawal of the inhibitory stimulus was sufficient to promote or enable a “reflexive” voiding contraction that would efficiently empty the bladder. On the basis of preliminary experiments, these reflexive voids, when present, tended to occur relatively quickly (within 20 s) after stimulus termination. In these seven experiments, if after ~20 s, no micturition event had occurred, then the bladder was emptied. However, if another micturition event occurred, the bladder was emptied after the bladder pressure returned to baseline (Fig. 3A). Voided and residual volumes were recorded and used to calculate bladder capacity and voiding efficiency, where bladder capacity was calculated as the sum of the voided volume(s) and residual volume, and voiding efficiency was calculated as the ratio of the voided volume to the bladder capacity. For saline-only experiments, in which a second micturition event occurred before bladder emptying, the voided volume from the first and second micturition events was collected separately. For these trials, voiding efficiency was calculated as the first voided volume divided by the bladder capacity, and “reflex” voiding efficiency was calculated as the sum of the two voided volumes divided by the bladder capacity.
Fig. 3.
Impact of sensory pudendal nerve stimulation on voiding efficiency. A: portion of a trial with stimulation at 1.5 T, 20 Hz illustrating stimulus termination following the first expulsion of urine. The expelled urine was collected and measured. If a second void occurred within 20 s of stimulus termination, the urine was collected separately and measured (n = 7 experiments). The volume from the first micturition event contributed to the normalized voiding efficiency. Values were normalized relative to nonstimulation control trials. Volumes from both the first micturition event and the second micturition event (if present) contributed to the normalized “reflex” voiding efficiency. B: summary data showing changes in voiding efficiency, relative to nonstimulation control trials, as a function of stimulus amplitude and pulse repetition frequency (means ± SE). Voiding efficiency varied as a function of amplitude and frequency (P < 0.001 for both amplitude and rate, two-way ANOVA) with no statistically significant interaction term (n = 7 for 0.3T to 1T, n = 6 for 1.5T, 20 Hz, and n = 5 for 1.5T, 1 and 10 Hz). Increasing stimulation amplitude decreased normalized voiding efficiency. This decrease was larger at 10 and 20 Hz than 1 Hz. C: waiting up to 20 s following stimulus termination elicited subsequent bladder contractions that resulted in the expulsion of additional fluid. Despite the increase in expelled fluid, voiding efficiency remained reduced for 1 Hz (P = 0.002) and 10 Hz (P < 0.001) at 1.5 T (the amplitude that led to the greatest increase in bladder capacity) relative to control levels.
For PGE2 experiments, following control CMGs with saline, the bladder was infused continuously with 100 µM PGE2 solution for 1 h. The bladder was subsequently emptied, and CMGs were recorded while filling the bladder with 100 µM PGE2. Following baseline PGE2 trials, trials with sensory pudendal stimulation were interleaved with PGE2 trials without stimulation.
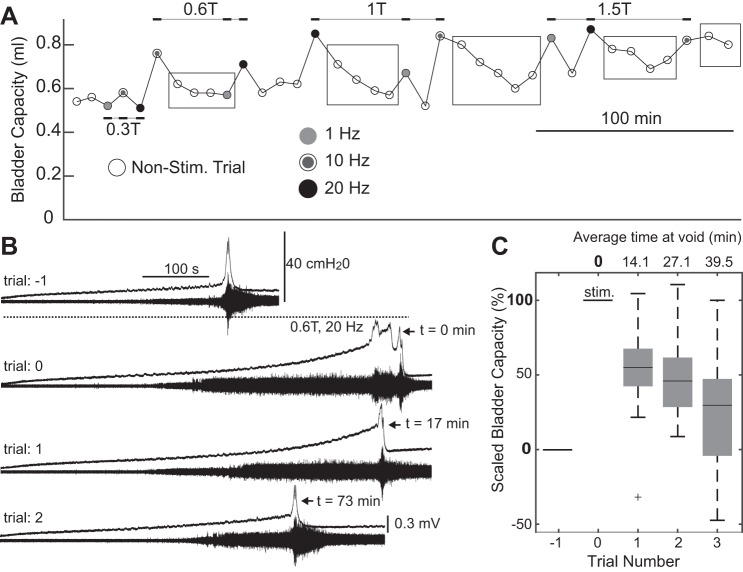
For saline-only experiments, sensory pudendal stimulation trials followed baseline control trials. The following trials in which stimulation increased bladder capacity (approximately greater than 20%), nonstimulation trials were run until the bladder capacity returned to a steady state (Fig. 4A). Stimulation amplitudes (0.3T, 0.6T, and 1T, n = 9, and 1.5T, n = 6 for 1 Hz, 10 Hz and n = 7 for 20 Hz) were tested from lowest to highest amplitude due to concerns of higher-amplitude stimuli producing protracted changes in bladder capacity. At each amplitude, three stimulus rates were presented (1, 10, and 20 Hz) in randomized order. Stimuli were biphasic, 100 µs per phase pulses delivered via a voltage to current convertor (model 2200, A-M Systems) controlled via an analog output channel on the PowerLab system. Electrical stimulation started at the start of the pump and stopped at the first micturition event (n = 9, PGE2 and n = 7, saline-only) or continued throughout the entire trial (n = 2, saline-only).
Fig. 4.
Stimulation of the sensory pudendal nerve often led to increases in bladder capacity on subsequent nonstimulated trials (stimulation carryover effect). A: bladder capacities for each trial in an experiment. Circles indicate the start of each trial. Black rectangles were added to highlight trials that show a stimulus carryover effect. B: sequential trials collected during a single experiment. Stimulation at 0.6 T, 20 Hz (trial 0) increased bladder capacity and initially disrupted coordinated voiding. On the subsequent trial (trial 1) the bladder capacity remained increased. On the subsequent and final trial of the series and the experiment (trial 2) the bladder capacity decreased, but remained elevated compared with the control trial (trial −1) conducted before stimulation. C: distributions of scaled bladder capacity on the three trials following stimulation at 1 or 1.5 T and 10 or 20 Hz. Bladder capacities were scaled such that 0% is the bladder capacity of the trial preceding stimulation, and 100% is the bladder capacity of the stimulation trial. Only groups of trials in which stimulation increased bladder capacity by at least 20% relative to the preceding trial and in which at least three nonstimulation trials followed were included in this analysis (n = 15, 48% of trials). Box plots were created using MATLAB’s boxplot() command, and the center bars represent the median value, box edges are the 25th and 75th percentiles, and the whiskers extend to the most extreme data points, which are not considered to be outliers. Outliers are indicated by the plus sign (+).
For PGE2 experiments, stimulation testing started at 100 µA (n = 1), 200 µA (n = 6), or 400 µA (n = 2), all at 20 Hz. For cases in which the stimulation did not clearly increase bladder capacity (n = 2), the stimulus amplitude was increased by a factor of two until an increase in bladder capacity was evident. In one experiment, this occurred after one increase (two times), and in the other experiment, two increases were necessary (four times initial amplitude).
Data analysis.
All signals were collected using a PowerLab/16SP acquisition unit (AD Instruments) in conjunction with LabChart Pro for visualization (versions 7 and 8, AD Instruments). All analysis was performed using MATLAB (Mathworks, Natick, MA).
For display, pressure traces were low-pass filtered using a first-order zero-phase Butterworth filter with a 5-Hz cutoff, and EUS EMG was high-pass filtered at 70 Hz using a first-order zero-phase Butterworth filter.
Summary data are presented as either means ± SE or as boxplots using MATLAB’s boxplot() function. All repeated trials within an experiment were averaged together to create a single value per experiment. Voiding efficiency and bladder capacity values were normalized to nonstimulation saline control trials. For saline-only experiments, the normalization was made relative to nonstimulation trial data collected before any stimulation. Normalized data were included in two-way ANOVA (anovan(), MATLAB). Paired t-tests were used for comparison of different conditions, with the exception of comparison to control values where a t-test compared whether or not a condition’s mean was different than 1 (t-test (x, y) and t-test (x, 1), MATLAB). Tests with P ≤ 0.05 were considered to be statistically significant.
RESULTS
Results presented are from n = 18 rats, half for saline-only experiments and half for PGE2 experiments. An additional eight experiments were conducted that were not analyzed due to experimental failures. Six PGE2 experiments were excluded: three in which 100 µM PGE2 caused urine leaking at low bladder pressures, one in which 100 µM PGE2 did not decrease bladder capacity, and two in which the self-manufactured nerve cuffs broke during placement. Two saline-only experiments were excluded. One was excluded because of a lack of a stimulus-evoked EUS reflex, and the other was excluded because of a bladder that became acontractile after replacing the bladder catheter due to air in the line. No experiments were excluded on the basis of stimulation results.
Sensory pudendal nerve stimulation during single-fill cystometry increased bladder capacity. The increase in bladder capacity was dependent upon stimulation amplitude and pulse repetition frequency (two-way ANOVA, P < 0.001 for both amplitude and frequency, n = 9 for 0.3T to 1T, n = 7 for 1.5T, 20 Hz, and n = 6 for 1.5T, 1 and 10 Hz). Stimulation at 10 Hz led to the largest increases in bladder capacity, but the relative increases in bladder capacity were not different between 10 and 20 Hz except at 0.6 T (P < 0.001) (Fig. 2C). Increasing stimulation amplitude from 1T to 1.5T resulted in an increase in bladder capacity for 1 Hz and 20 Hz stimulation (P = 0.01 and P = 0.03, respectively), but not for 10 Hz. The increase in bladder capacity from 1T to 1.5T (for 1 and 20 Hz, and sometimes at 10 Hz; see Fig. 2A) suggests a lack of saturation of effect at 1T.
Fig. 2.
Sensory pudendal nerve stimulation increased bladder capacity. A and B: example traces of bladder pressure and EUS EMG from two experiments showing increases in bladder capacity with increasing stimulation amplitude at 10 Hz. Dashed lines above the pressure traces indicate periods of stimulation. C: reflex EMG responses to stimulus pulses at amplitudes of 600 and 650 µA. A reflex response at 25 ms is present at 650 µA, but not at 600 µA. Other amplitudes are reported as fractions of the stimulus reflex threshold (or 1T, or 1 times threshold). D: summary data showing changes in bladder capacity, relative to nonstimulation control trials, as a function of stimulus amplitude and pulse repetition frequency (means ± SE). Bladder capacity varied as a function of amplitude and frequency (P < 0.001 for both amplitude and rate, two-way ANOVA) with no statistically significant interaction term (n = 9 for 0.3 T to 1T, n = 7 for 1.5T, 20 Hz, and n = 6 for 1.5T, 1 and 10 Hz). Increasing stimulus amplitude led to increased bladder capacities. Statistically significant increases from 1T to 1.5T stimulation were observed for 1 and 20 Hz (P = 0.01 and P = 0.03, paired t-tests), but not for 10 Hz. The average normalized bladder capacity during 10 Hz stimulation was greater than during 20-Hz stimulation only at 0.6 T (P < 0.001).
Increasing stimulation amplitude also decreased voiding efficiency. Changes in voiding efficiency were dependent on stimulation amplitude and pulse repetition frequency (two-way ANOVA, P < 0.001 for both amplitude and frequency, n = 7 for 0.3T to 1T, n = 6 for 1.5T, 20 Hz, and n = 5 for 1.5T, 1 and 10 Hz) (Fig. 3B). On average, 10-Hz stimulation led to the largest decreases in voiding efficiency. At higher amplitudes, micturition events followed termination of the stimulus. However, at the amplitude that yielded the largest increases in bladder capacity, “reflex” voiding efficiency was still less than control values for 1 or 10 Hz (P = 0.002 and P < 0.001, respectively). The reflex voiding efficiency was not statistically different from control values for 1.5T, 20 Hz, although these data were highly variable.
Stimulation of the sensory pudendal nerve often produced a carry-over effect, and bladder capacity was increased on subsequent nonstimulation trials (Fig. 4, A and B). Because of high variability in the magnitude of this effect, bladder capacity values from trials that followed 1 or 1.5T and 10 or 20 Hz were combined in summary data (Fig. 4C). For analysis of carry-over effects, only groups of trials in which stimulation increased bladder capacity by at least 20% relative to the preceding control trial and in which at least three nonstimulated trials followed the stimulated trial were included (n = 15, 48% of trials). For the first trial following a stimulation trial, bladder capacity remained elevated by 55% (median) relative to baseline. For the third nonstimulation trial, with a void at an average of 39.5 min following termination of the stimulus, the bladder capacity remained elevated by 29%.
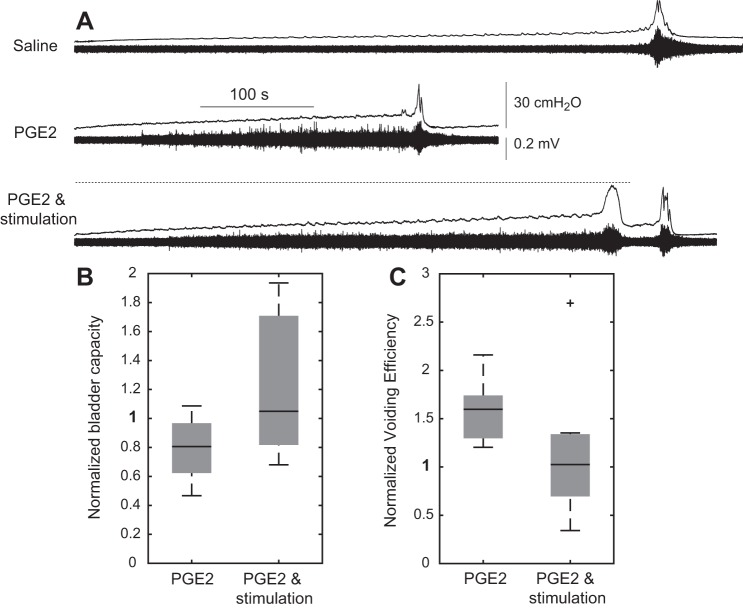
Intravesical PGE2 decreased bladder capacity (P = 0.02) and increased voiding efficiency (P < 0.001, n = 9) (Fig. 5). Sensory pudendal nerve stimulation at 20 Hz increased bladder capacity relative to PGE2 (P = 0.004). Similar to the results with saline, an increase in bladder capacity from stimulation also resulted in a decrease in voiding efficiency relative to the PGE2 condition.
Fig. 5.
Sensory pudendal nerve stimulation increases bladder capacity in an intravesical PGE2 model (100 μM) of overactive bladder. A: example traces showing a decrease in bladder capacity with intravesical PGE2 and increase in bladder capacity with sensory pudendal stimulation. B: bladder capacity decreased with PGE2 (P = 0.02) and increased (relative to PGE2) with stimulation (P = 0.004) (n = 9). C: voiding efficiency increased with PGE2 (P < 0.001) and decreased (relative to PGE2) with stimulation [P = 0.05; P = 0.01 with removal of the outlier (+)]. All stimulation was at 20 Hz with amplitudes ranging from 100 to 800 µA (mean 330 ± 63 µA SE). Voided volume (to compute the voiding efficiency) included volume expelled during the first micturition event through 1 min following stimulus termination.
DISCUSSION
Stimulation of the sensory pudendal nerve led to large increases in bladder capacity (Fig. 2D). The largest increase was at 1.5T at 10 Hz, in which the average bladder capacity was 253% of the nonstimulated control trials. There was evidence of saturation at 10 Hz, as the increase in bladder capacity was not statistically different between 1T and 1.5T. This was dependent on the experiment, as can be seen by the differences in bladder capacity between 1T and 1.5T in Fig. 2A and Fig. 2B. Further testing is required to identify a stimulus amplitude beyond which further increases in amplitude do not increase bladder capacity (i.e., an amplitude in which the therapeutic benefit is saturated).
Of the three stimulus rates tested, 10 Hz led to the largest increases in bladder capacity. The difference between 10 and 20 Hz was not statistically different, except at 0.6T. At 0.6T, the bladder capacity at 10 Hz was larger than at 20 Hz in every experiment (n = 9). This suggests that the system might be more sensitive to rate at low-stimulus amplitudes and that this sensitivity may be reduced at higher-stimulus amplitudes. In testing of stimulation to inhibit isovolumetric bladder contraction in cats, 5-Hz stimulation produced similar levels of inhibition as 10 Hz (31, 37). However, this must be weighed against the tolerability of low rate stimulation, as it has been suggested that stimulation rates less than 10 Hz are unpleasant (6).
Stimulation-evoked increases in bladder capacity also led to decreases in voiding efficiency (Fig. 3B). At the highest stimulation amplitudes, it was not uncommon for leaking (overflow incontinence) to occur at high bladder pressures, rather than a coordinated detrusor contraction. Termination of the stimulus allowed subsequent voids to occur, but voiding efficiency was still reduced relative to nonstimulated trials. These results suggest that it is possible to so strongly promote urine storage, through detrusor inhibition and sphincter activation (e.g., see Fig. 3A). that subsequent voiding may be impaired. In humans, high-amplitude intra-anal electrical stimulation for 5 min, placed to target the pudendal nerve, prevented volitional voiding (1). However, further testing of this observation is warranted in nonanesthetized animals, as well as in other species that exhibit sphincter relaxation during voiding (e.g., cats, humans), unlike rats, which exhibit phasic bursting of the sphincter during voiding. Additionally, there has been no mention of impaired voiding during pudendal nerve stimulation in humans (10, 11, 25), and this may reflect differences between human and rat physiology, differences in stimulation parameters, notably stimulus amplitude and electrode location, or a lack of sensitivity to changes in voiding efficiency in the clinical studies.
In addition to increasing bladder capacity during a stimulation trial, sensory pudendal nerve stimulation increased bladder capacities on subsequent nonstimulated trials (stimulus carryover effect, Fig. 4). The time to return back to a steady-state bladder capacity varied, but tended to occur within 1 h. The utility of this observation is unclear, especially given that the maximum level of inhibition (maximum bladder capacity) occurred during stimulation, not after, but it may suggest that intermittent stimulation will be sufficient to treat the symptoms of OAB. Jiang and Lindström (16) also observed this stimulus carryover phenomenon from vaginal, anal, and in one experiment, dorsal clitoral nerve stimulation. In their experiments, stimulation was delivered with an empty bladder for 5 min, but they still observed increases in bladder capacity from anovaginal stimulation that persisted for ~40 min following stimulus termination. In previous work by Jiang (15), electrical stimulation of the bladder produced prolonged decreases in bladder capacity. This phenomenon was inhibited by a N-methyl-d-aspartate (NMDA) antagonist CPPene, and Jiang and Lindström hypothesized that the inhibition carryover effect is a form of NMDA-mediated synaptic plasticity, although this hypothesis has yet to be formally tested.
As is evident in Fig. 4, A and B, the steady-state bladder capacity following multiple unstimulated trials after a stimulated trial was elevated relative to the bladder capacity before stimulation. However, we are not able to determine whether these increases are stimulation- or time-related. There is prior evidence from human studies that short durations of stimulation (e.g., 20 min) applied via intravaginal or intra-anal electrodes can lead to improvements in the symptoms of OAB over multiple days (9, 27). Indeed, this same approach is currently used clinically for tibial nerve stimulation (30 min, once a week). However, further testing is needed to verify this observation with sensory pudendal nerve stimulation.
Intravesical PGE2 reduces bladder capacity in rats (12, 14) and causes “a strong urgency sensation” when given to healthy women, also leading to reduced bladder capacities (29). Consistent with these observations, intravesical PGE2 reduced bladder capacity. Sensory pudendal nerve stimulation at 20 Hz subsequently increased bladder capacity relative to the PGE2-only condition. Similar to the testing with intravesical saline, the increase in bladder capacity was accompanied by a decrease in voiding efficiency. These results suggest that sensory pudendal nerve stimulation may work under a variety of conditions.
All PGE2 experiments were conducted before saline-only experiments. The impact of intravesical PGE2 on bladder capacity appeared to change over time. By comparison, intravesical saline-only experiments appeared to result in more consistent bladder capacities over time. Coupled with the unexpected presence of stimulation carryover effects, which meant single testing blocks could take 2–3 h, saline-only experiments were judged to be better suited for quantifying the effects of stimulus parameter variations.
These results were all based on experiments involving sensory pudendal nerve (22) stimulation. It is unknown how these results relate to stimulation of the dorsal genital nerve, or both the motor and sensory branch in the ischiorectal fossa, or the compound pudendal nerve at the lumbosacral plexus (just distal to the branching of the pelvic nerve). Although researchers may refer to all of these locations as pudendal nerve stimulation, they contain different neural components, and specificity should be used wherever possible to clarify the location of the stimulus.
Different nerve cuff designs and sizes were used during the saline-only stimulation experiments. The goal was not to make inferences regarding the impact of different nerve cuff designs. Rather, the changes in nerve cuff design made the experiments easier to conduct. Importantly, all major observations were qualitatively the same between the different nerve cuff sizes. Specifically, for both the 200- and 300-μm diameter cuffs, we observed: 1) an increase in bladder capacity as a function of increasing amplitude up to 1.5T, with 10 Hz generally eliciting larger bladder capacities than 20 Hz and 1 Hz; 2) increasing stimulus amplitudes that also led to decreases in voiding efficiency; and 3) increased bladder capacity in the trials that followed a stimulus trial (stimulus carryover). Statistical comparisons were not made due to the low experiment count with each nerve cuff type and the lack of order-randomization (smaller cuffs were used after larger cuffs). The impact of nerve cuff design on stimulation outcomes, including nerve cuff diameter, electrode spacing, and electrode size is currently unknown and should be addressed in future studies.
Pudendal nerve stimulation in humans reduces urgency/frequency, as well as the number of incontinent episodes (10, 11, 25). These human studies targeted the compound pudendal nerve (11, 25) or the dorsal genital nerve, a distal branch of the pudendal nerve (10). However, it is not clear from these studies how best to stimulate the pudendal nerve to achieve therapeutic efficacy, as these studies focused on evaluation of pudendal nerve stimulation as a treatment option, rather than on optimizing the treatment. Modification of stimulus rate, amplitude, and location may improve therapeutic outcomes. In this study, we present results from two rat models (intravesical saline and PGE2), in which we quantified the effects of stimulation of the sensory branch of the pudendal nerve.
Our results suggest that increasing stimulus amplitude above the bulbocavernosus reflex threshold is beneficial, but it is unclear whether or not stimulation at an amplitude above sensory/motor threshold would increase therapeutic efficacy in humans. Reflexively driven increases in EUS activity may reflexively inhibit bladder contractions [guarding reflexes (8), reafference (40)], leading to the increases in bladder capacity and loss of coordinated voiding contractions.
Consistent with prior studies of optimal stimulation, rates for inhibiting isovolumetric bladder contractions (31, 34), our results show an effect of stimulus rate, with the largest increases in bladder capacity at 10 Hz (vs. 1 and 20 Hz), but the optimal rate to relieve the symptoms of OAB in humans is unclear. Similarly, the optimal stimulus timing and duration are also unclear. Our results show prolonged increases in bladder capacity following stimulation, suggesting that continuous stimulation may not be needed.
In this study, stimulation of the sensory branch of the pudendal nerve increased bladder capacity during saline and PGE2 cystometry, but the choice of what part (or parts) of the pudendal nerve to stimulate is unclear. Future work is needed to clarify the effects of sensory pudendal nerve stimulation on voiding efficiency. Stimulation of the compound pudendal nerve may have positive impacts on sexual dysfunction (39), interstitial cystitis (26), pelvic pain, constipation (32), and fecal incontinence (35). It is unclear how stimulation of a more distal component of the pudendal nerve may affect these other potential therapeutic targets, although dorsal genital nerve stimulation reduces fecal incontinence in humans (38).
Conclusions.
Sensory pudendal nerve stimulation increased bladder capacity in the anesthetized female Wistar rat during intravesical saline and PGE2 installation. Ten-hertz stimulation at 1.5T led to the largest increase in bladder capacity. Stimulation that produced increases in bladder capacity also led to decreases in voiding efficiency, suggesting a link between promotion of bladder filling and inhibition of voiding, rather than a complete separation between filling and voiding phases. Further work should verify this in awake animals, as well as other species. This work serves as the basis for future work aimed at optimizing the benefit of pudendal nerve stimulation for the symptoms of OAB.
GRANTS
Research described herein was funded by the GlaxoSmithKline/Galvani Bioelectronics Research Program and in part by National Institutes of Health Grant K12 DK-100024.
DISCLOSURES
J. A. Hokanson, C. L. Langdale and W. M. Grill are listed as inventors on provisional patent applications with Duke University on peripheral nerve stimulation to treat bladder dysfunction. J. A. Hokanson, C. L. Langdale and W. M. Grill have rights to future compensation through a patent licensing arrangement. A. Sridhar works for Galvani Bioelectronics, which has an interest in the field of peripheral nerve stimulation to treat bladder dysfunction.
AUTHOR CONTRIBUTIONS
J.A.H., C.L.L., A.S., and W.M.G. conceived and designed research; J.A.H. performed experiments; J.A.H. and C.L.L. analyzed data; J.A.H., C.L.L., and W.M.G. interpreted results of experiments; J.A.H. prepared figures; J.A.H. drafted manuscript; J.A.H., C.L.L., and W.M.G. edited and revised manuscript; J.A.H., C.L.L., A.S., and W.M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Gilda Mills and Danielle Degoski for assistance during these experiments.
REFERENCES
- 1.Brindley GS, Rushton DN, Craggs MD. The pressure exerted by the external sphincter of the urethra when its motor nerve fibres are stimulated electrically. Br J Urol 46: 453–462, 1974. doi: 10.1111/j.1464-410X.1974.tb10184.x. [DOI] [PubMed] [Google Scholar]
- 2.Chancellor MB, Migliaccio-Walle K, Bramley TJ, Chaudhari SL, Corbell C, Globe D. Long-term patterns of use and treatment failure with anticholinergic agents for overactive bladder. Clin Ther 35: 1744–1751, 2013. doi: 10.1016/j.clinthera.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 3.Chen ML, Shen B, Wang J, Liu H, Roppolo JR, de Groat WC, Tai C. Influence of naloxone on inhibitory pudendal-to-bladder reflex in cats. Exp Neurol 224: 282–291, 2010. doi: 10.1016/j.expneurol.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danziger ZC, Grill WM. Dynamics of the sensory response to urethral flow over multiple time scales in rat. J Physiol 593: 3351–3371, 2015. doi: 10.1113/JP270911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dieter AA, Degoski DJ, Dolber PC, Fraser MO. The effects of bilateral bipolar sacral neurostimulation on urinary bladder activity during filling before and after irritation in a rat model. Neurourol Urodyn 34: 387–391, 2015. doi: 10.1002/nau.22556. [DOI] [PubMed] [Google Scholar]
- 6.Erlandson BE, Fall M, Carlsson CA. The effect of intravaginal electrical stimulation on the feline urethra and urinary bladder. Electrical parameters. Scand J Urol Nephrol Suppl 7: 5–18, 1977. [PubMed] [Google Scholar]
- 7.Farag FF, Martens FMJ, Rijkhoff NJM, Heesakkers JPFA. Dorsal genital nerve stimulation in patients with detrusor overactivity: a systematic review. Curr Urol Rep 13: 385–388, 2012. doi: 10.1007/s11934-012-0273-x. [DOI] [PubMed] [Google Scholar]
- 8.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geirsson G, Fall M. Maximal functional electrical stimulation in routine practice. Neurourol Urodyn 16: 559–565, 1997. doi:. [DOI] [PubMed] [Google Scholar]
- 10.Goldman HB, Amundsen CL, Mangel J, Grill J, Bennett M, Gustafson KJ, Grill WM. Dorsal genital nerve stimulation for the treatment of overactive bladder symptoms. Neurourol Urodyn 27: 499–503, 2008. doi: 10.1002/nau.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groen J, Amiel C, Bosch JLHR. Chronic pudendal nerve neuromodulation in women with idiopathic refractory detrusor overactivity incontinence: results of a pilot study with a novel minimally invasive implantable mini-stimulator. Neurourol Urodyn 24: 226–230, 2005. doi: 10.1002/nau.20131. [DOI] [PubMed] [Google Scholar]
- 12.Hokanson JA, Langdale CL, Sridhar A, Grill WM. OAB without an overactive bladder in the acute prostaglandin E2 rat model. Am J Physiol Renal Physiol 313: F1169–F1177, 2017. doi: 10.1152/ajprenal.00270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang JC, Deletis V, Vodušek DB, Abbott R. Preservation of pudendal afferents in sacral rhizotomies. Neurosurgery 41: 411–415, 1997. doi: 10.1097/00006123-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Ishizuka O, Mattiasson A, Andersson K-E. Prostaglandin E2-induced bladder hyperactivity in normal, conscious rats: involvement of tachykinins? J Urol 153: 2034–2038, 1995. doi: 10.1016/S0022-5347(01)67397-X. [DOI] [PubMed] [Google Scholar]
- 15.Jiang CH. Modulation of the micturition reflex pathway by intravesical electrical stimulation: an experimental study in the rat. Neurourol Urodyn 17: 543–553, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Jiang CH, Lindström S. Prolonged increase in micturition threshold volume by anogenital afferent stimulation in the rat. Br J Urol 82: 398–403, 1998. doi: 10.1046/j.1464-410X.1998.00682.x. [DOI] [PubMed] [Google Scholar]
- 17.Langdale CL, Hokanson JA, Sridhar A, Grill WM. Stimulation of the pelvic nerve increases bladder capacity in the prostaglandin E2 rat model of overactive bladder. Am J Physiol Renal Physiol 313: F657–F665, 2017. doi: 10.1152/ajprenal.00116.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson JA, Ogagan PD, Chen G, Shen B, Wang J, Roppolo JR, de Groat WC, Tai C. Involvement of metabotropic glutamate receptor 5 in pudendal inhibition of nociceptive bladder activity in cats. J Physiol 589: 5833–5843, 2011. doi: 10.1113/jphysiol.2011.215657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangera A, Apostolidis A, Andersson K-E, Dasgupta P, Giannantoni A, Roehrborn C, Novara G, Chapple C. An updated systematic review and statistical comparison of standardised mean outcomes for the use of botulinum toxin in the management of lower urinary tract disorders. Eur Urol 65: 981–990, 2014. doi: 10.1016/j.eururo.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Mashni JW, Peters KM. Potential use of pudendal nerve stimulation for voiding dysfunction. Curr Bladder Dysfunct Rep 5: 177–182, 2010. doi: 10.1007/s11884-010-0064-5. [DOI] [Google Scholar]
- 21.McGee MJ, Danziger ZC, Bamford JA, Grill WM. A spinal GABAergic mechanism is necessary for bladder inhibition by pudendal afferent stimulation. Am J Physiol Renal Physiol 307: F921–F930, 2014. doi: 10.1152/ajprenal.00330.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol 248: 532–549, 1986. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- 23.Pastelín CF, Juárez R, Damaser MS, Cruz Y. Neural pathways of somatic and visceral reflexes of the external urethral sphincter in female rats. J Comp Neurol 520: 3120–3134, 2012. doi: 10.1002/cne.23079. [DOI] [PubMed] [Google Scholar]
- 24.Peters KM. Alternative approaches to sacral nerve stimulation. Int Urogynecol J Pelvic Floor Dysfunct 21: 1559–1563, 2010. doi: 10.1007/s00192-010-1282-2. [DOI] [PubMed] [Google Scholar]
- 25.Peters KM, Feber KM, Bennett RC. Sacral versus pudendal nerve stimulation for voiding dysfunction: a prospective, single-blinded, randomized, crossover trial. Neurourol Urodyn 24: 643–647, 2005. doi: 10.1002/nau.20174. [DOI] [PubMed] [Google Scholar]
- 26.Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int 100: 835–839, 2007. doi: 10.1111/j.1464-410X.2007.07082.x. [DOI] [PubMed] [Google Scholar]
- 27.Plevnik S, Janez J. Maximal electrical stimulation for urinary incontinence: report of 98 cases. Urology 14: 638–645, 1979. doi: 10.1016/0090-4295(79)90545-4. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt RA. Applications of neurostimulation in urology. Neurourol Urodyn 7: 585–592, 1988. doi: 10.1002/nau.1930070607. [DOI] [Google Scholar]
- 29.Schüssler B. Comparison of the mode of action of prostaglandin E2 (PGE2) and sulprostone, a PGE2-derivative, on the lower urinary tract in healthy women. A urodynamic study. Urol Res 18: 349–352, 1990. doi: 10.1007/BF00300786. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui NY, Wu JM, Amundsen CL. Efficacy and adverse events of sacral nerve stimulation for overactive bladder: a systematic review. Neurourol Urodyn 29, Suppl 1: S18–S23, 2010. doi: 10.1002/nau.20786. [DOI] [PubMed] [Google Scholar]
- 31.Snellings AE, Grill WM. Effects of stimulation site and stimulation parameters on bladder inhibition by electrical nerve stimulation. BJU Int 110: 136–143, 2012. doi: 10.1111/j.1464-410X.2011.10789.x. [DOI] [PubMed] [Google Scholar]
- 32.Spinelli M, Malaguti S, Giardiello G, Lazzeri M, Tarantola J, Van Den Hombergh U. A new minimally invasive procedure for pudendal nerve stimulation to treat neurogenic bladder: description of the method and preliminary data. Neurourol Urodyn 24: 305–309, 2005. doi: 10.1002/nau.20118. [DOI] [PubMed] [Google Scholar]
- 33.Su X, Nickles A, Nelson DE. Comparison of neural targets for neuromodulation of bladder micturition reflex in the rat. Am J Physiol Renal Physiol 303: F1196–F1206, 2012. doi: 10.1152/ajprenal.00343.2012. [DOI] [PubMed] [Google Scholar]
- 34.Tai C, Smerin SE, de Groat WC, Roppolo JR. Pudendal-to-bladder reflex in chronic spinal-cord-injured cats. Exp Neurol 197: 225–234, 2006. doi: 10.1016/j.expneurol.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Thomas GP, George AT, Dudding TC, Nicholls RJ, Vaizey CJ. A pilot study of chronic pudendal nerve stimulation for faecal incontinence for those who have failed sacral nerve stimulation. Tech Coloproctol 18: 731–737, 2014. doi: 10.1007/s10151-014-1174-4. [DOI] [PubMed] [Google Scholar]
- 36.Wenzel BJ, Boggs JW, Gustafson KJ, Grill WM. Closed loop electrical control of urinary continence. J Urol 175: 1559–1563, 2006. doi: 10.1016/S0022-5347(05)00657-9. [DOI] [PubMed] [Google Scholar]
- 37.Woock JP, Yoo PB, Grill WM. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol 294: R1880–R1889, 2008. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Worsøe J, Fynne L, Laurberg S, Krogh K, Rijkhoff NJM. Electrical stimulation of the dorsal clitoral nerve reduces incontinence episodes in idiopathic faecal incontinent patients: a pilot study. Colorectal Dis 14: 349–355, 2012. doi: 10.1111/j.1463-1318.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 39.Yih JM, Killinger KA, Boura JA, Peters KM. Changes in sexual functioning in women after neuromodulation for voiding dysfunction. J Sex Med 10: 2477–2483, 2013. doi: 10.1111/jsm.12085. [DOI] [PubMed] [Google Scholar]
- 40.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol 212: 218–225, 2008. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]