Abstract
Adolescents with Type 1 diabetes mellitus (T1DM) are at risk for hyperfiltration and elevated urinary albumin-to-creatinine ratio (ACR), which are early indicators of diabetic nephropathy. Adolescents with T1DM also develop early changes in blood pressure, cardiovascular structure, and function. Our aims were to define the relationships between hyperfiltration, ACR, and 24-h ambulatory blood pressure over time in adolescents with T1DM. Normotensive, normoalbuminuric adolescents (n = 98) with T1DM underwent baseline and 2-yr 24-h ambulatory blood pressure monitoring, glomerular filtration rate (eGFR) estimated by cystatin C (Larsson equation), and ACR measurements. Linear regression models adjusted for diabetes duration, sex, and HbA1c were used to determine associations. Hyperfiltration (eGFR ≥ 133 ml/min) was present in 31% at baseline and 21% at 2-yr follow-up. Hyperfiltration was associated with greater odds of rapid GFR decline (>3 ml·min−1·yr−1) [OR: 5.33, 95%; CI: 1.87–15.17; P = 0.002] over 2 yr. Natural log of ACR at baseline was associated with greater odds of hyperfiltration (OR: 1.71, 95% CI: 1.00–2.92; P = 0.049) and 2-yr follow-up (OR: 2.14, 95%; CI: 1.09–4.19; P = 0.03). One SD increase in eGFR, but not ln ACR, at 2-yr follow-up conferred greater odds of nighttime nondipping pattern (OR: 1.96, 95% CI: 1.06–3.63; P = 0.03). Hyperfiltration was prevalent at baseline and at 2-yr follow-up, predicted rapid decline in GFR, and was related to ACR. Elevated GFR at 2-yr follow-up was associated with nighttime nondipping pattern. More work is needed to better understand early relationships between renal hemodynamic and systemic hemodynamic changes in adolescents with T1DM to reduce future cardiorenal complications.
Keywords: albumin-to-creatinine ratio, 24-h ambulatory blood pressure, glomerular filtration rate, nephropathy, Type 1 diabetes
INTRODUCTION
Diabetic nephropathy (DN) is the leading cause of end-stage renal disease in the developed world (28). Characteristic early renal functional changes of DN include hyperfiltration and elevated albuminuria (3). Large prospective randomized controlled studies such as The Diabetes Control and Complications Trial reported that renal functional decline can occur before the onset of albuminuria in some adults with Type 1 diabetes mellitus (T1DM), potentially reflecting underlying renal injury that is clinically silent. In smaller clinical studies in normotensive normoalbuminuric adult T1DM patients, glomerular hyperfiltration is associated with higher nocturnal diastolic blood pressure and blunted nocturnal decrease in diastolic blood pressure (19). In adolescents with T1DM, renal structural changes on kidney biopsy can be detected before the onset of albuminuria, and are also associated with elevated diastolic blood pressure (7). In patients with T1DM who have already developed microalbuminuria, blood pressure, using ambulatory blood pressure monitoring (ABPM) is elevated compared with normoalbuminuric patients with T1DM (12, 22). Early blood pressure changes are of importance in adolescents with T1DM due to the high risk of atherosclerosis and early-onset clinical cardiovascular disease (9, 11), which are also associated with albuminuria (14).
In children with short-duration T1DM, however, the relationship(s) between hyperfiltration, albuminuria excretion, and changes in the cardiovascular system have not been fully elucidated. As part of our focus on early cardiorenal changes in adolescents with TIDM, we have followed two similar research cohorts in which 24-h ABPM, estimated glomerular filtration rate (eGFR), and ACR were measured over time. This has provided us with an opportunity to extend previous studies by examining how these three important variables relate to each other and vary over time in adolescents with T1DM during puberty.
Accordingly, our aim was to examine the associations(s) between hyperfiltration, systemic hemodynamics (24-h ABPM), and urinary albumin-to-creatinine ratio (ACR) in TIDM adolescents with short-duration disease at baseline and after two years of follow-up in order to learn more about increased future vascular risk. We hypothesized that in this study cohort, hyperfiltration would be associated with albuminuria and with ABPM profiles and that these relationships would persist over time (21, 31).
MATERIALS AND METHODS
Study population.
A total of 98 adolescent participants were included in these analyses. Fifty-five adolescents with T1DM in puberty were enrolled in the Long-term Puberty Follow-Up (LPF) observational cohort and were followed at the Hospital for Sick Children (Toronto, ON) between 1997 and 2001 (26). Inclusion criteria for the LPF cohort included duration of diabetes >5 yr, Tanner Stage II-III puberty, normoalbuminuria (AER <20 μg/min on 2/3 overnight urine collections), normal clinic blood pressure (within pediatric reference values), and the absence of chronic illness (other than hypothyroidism or mild asthma), and a willingness to complete at least two 24-h ABPM. More recently (December 2011 to January 2014), an additional, separate cohort, consisting of 43 adolescents with T1DM were enrolled and followed at the Hospital for Sick Children as part of an ancillary observational study to the AdDIT (Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial, NCT01581476) clinical trial (AdDIT ancillary). These study participants declined participation in the interventional aspects of the AdDIT trial but consented to participation in noninterventional, observational follow-up designated as the AdDIT ancillary observational cohort. These study participants met entry criteria for the AdDIT trial, which included the following: age (10–16 yr old), minimum duration of T1DM of at least 1 yr, and a standardized log ACR (residual) greater than 1.2 mg/mmol; therefore, they were considered “high risk” for the eventual development of microalbuminuria. Study participants taking part in AdDIT ancillary did not receive ACE inhibitors or statins. These two separate cohorts had very similar baseline characteristics (except for diabetes duration), entry criteria, and data collected on renal function and 24-h ABPM using similar methods in youth with T1DM over the same longitudinal follow-up period. Accordingly, to better understand the relationship(s) between early renal hemodynamic and systemic hemodynamic changes, the data from these two highly similar data sets were merged to form the combined study cohort, totaling 98 youth with T1DM.
Study protocol.
We recruited study participants followed for their routine diabetes care at the Hospital for Sick Children. At study entry (baseline), 24-h ABPM profiles, blood samples for HbA1c, LDL, serum creatinine, and three first morning urine collections for the determination of ACR were obtained from each subject. Thereafter, study participants continued to attend the clinic every 3 or 4 mo, ABPM was performed approximately every 6 mo [every 6 mo (LPF population), baseline and 2 yr (AdDIT ancillary)], and subjects remained in the study for a minimum of two years or more. All participants younger than 18 yr provided either assent or consent, and parents signed informed consent documents. This study was approved by the Hospital for Sick Children Research and Ethics Board.
ABPM assessments.
Twenty-four-hour ABPM was performed (Spacelabs 90207, ABP monitor, Spacelabs Healthcare, Arlington, VA) using two different cuff sizes (upper arm circumferences 17–26 cm and 24–32 cm) at baseline and after 2 yr. Blood pressure was recorded automatically every 20 min during the daytime (0800 to 2000) and every 50 min during the night (0000 to 0600). The monitor was programmed with automatic edit criteria (maximum systolic and diastolic blood pressures of 240 and 150 mmHg, respectively, and minimum systolic and diastolic blood pressures of 70 and 40 mmHg, respectively) that allowed for the deletion of extraneous blood pressure readings. Recordings were excluded that contained <27–45 daytime readings and 5–8 nighttime readings. The monitor was also programmed to retry unsuccessful attempts, and if it failed a second time, then the reading was considered to be invalid. For direct blood pressure measures, the mean of the daytime and nighttime systolic and diastolic 24-h ABPM readings was calculated. Dipping status was dichotomized by the presence or absence of nocturnal blood pressure dipping, as defined by <10% drop (nondipper) or >10% drop (dipper) in mean nighttime (sleep) blood pressure compared with mean daytime (awake) blood pressure. Statistical analyses of 24-h ABPM data were completed on measured values, and separately on data that were converted to standardized deviation scores (SDS) using normative pediatric reference values consisting of established percentiles normalized for the non-Gaussian distribution of 24-h blood pressure in children according to age and sex (using the least mean squares method) (32). No differences were observed between measured 24-h ABPM values or normalized SDS values (data not shown) in the overall statistical analysis.
Serum analyses and eGFR calculations.
Urinary albumin concentration was determined by radioimmunoassay. Urinary ACR was calculated from spot urine collections. HbA1c was measured by a highly specific high-performance liquid chromatography method incorporating a step for removal of the labile fraction (27). A single operator measured serum cystatin C by immunoassay (Siemens, Newark, DE) on a BN Prospect system nephelometer. GFR was calculated in milliliters per minute from cystatin C measurements according to equations by Larsson and colleagues [eGFR = 77.24 × ([serum cystatin C]−1.2623)], as previously described (13). GFR estimates by the Larsson equation have demonstrated strong agreement with measured GFR in adolescents (2). We defined hyperfiltration a priori as eGFR >133 ml·min−1·1.73 m−2 for the Larsson equation, which represents eGFR at the 95th percentile for healthy adolescents (12–17 yr of age) in the National Health and Nutrition Examination Survey (NHANES) (8, 15) based on the same equation. Rapid GFR decline was defined as an annual GFR loss >3 ml/min (4). As sensitivity analyses, we also included GFR estimated by Zappitelli (eGFR [ml·min−1·1.73 m−2] = 75.94 × [cystatin C]−1.17).
Statistical analysis.
Analyses were performed in SAS (version 9.4 or higher; SAS Institute, Cary, NC). The heterogeneity of the two cohorts (LPF and AdDIT) was evaluated with histograms and QQplots for key variables to ensure it was meaningful to combine the cohorts for analyses. Demographic and clinical characteristics among girls and boys with T1DM were compared using Studentʼs t-test for normally distributed continuous variables, Wilcoxon for non-normally distributed continuous variables (e.g., ACR). and χ2 for categorical variables. Generalized linear regression models were employed to evaluate the associations between ACR, eGFR, and blood pressure measures, unadjusted and adjusted for sex, diabetes duration, and HbA1c. Univariable and multivariable logistic regression models were also used to examine the relationships with hyperfiltration and nighttime blood pressure dipping patterns. Finally, ACR was examined across tertiles (low: <0.46, mid: 0.46–0.81, and high: ≥0.81 mg/mmol). In the generalized linear regression and logistic regression models, ACR was natural log-transformed due to positively skewed distribution. Significance was based on an α-level of 0.05. Analyses were considered exploratory, and hypotheses generating a priori and adjustments for multiple comparisons were not applied.
RESULTS
Characteristics of participants at baseline and 2-yr follow-up.
At baseline, the mean age of participants was 13.3 ± 2.1 yr with 54 boys and 45 girls (Table 1). Mean duration of T1DM was 6.8 ± 2.8 yr with mean HbA1c of 8.6 ± 1.3%. The mean serum cystatin C and creatinine at baseline (cystatin C: 0.67 mg/l; creatinine: 53.33 mmol/l) and 2-yr follow-up (cystatin C: 0.68; creatinine: 57.30 mmol/l) were lower than the mean values reported for nondiabetic adolescents from NHANES (cystatin C: 0.83 mg/l; and creatinine: 61.89 mmol/l). Thirty-one percent of the entire cohort displayed hyperfiltration at baseline, and 22% at 2-yr follow-up.
Table 1.
Baseline demographics and 2-yr follow-up of T1DM adolescents
| Variable | Baseline (n = 98) | Two-Year Follow-Up (n = 98) |
|---|---|---|
| Age, yr | 13.3 ± 2.1 (8.2–16.9) | 15.4 ± 2.0 (10.6–19.1) |
| Girls, % | 45 | 45 |
| Body mass index, kg/m2 | 21 ± 3 (15–29) | 23 ± 3 (16–32) |
| Height, cm | 157 ± 13 (123–181) | 165 ± 11 (135–187) |
| Weight, kg | 53 ± 14 (26.2–83.5) | 62 ± 13 (33.8–92.3) |
| HbA1c, % | 8.6 ± 1.3 (4.9–13.4) | 8.8 ± 1.4 (6.6–14.1) |
| Diabetes duration, yr | 6.8 ± 2.7 (1.2–13.9) | 8.9 ± 2.7 (3.7–16.2) |
| Triglycerides, mmol/l | 0.87 ± 0.41 (0.31–3.20) | 1.04 ± 0.90 (0.37–8.22) |
| Total cholesterol, mmol/l | 4.25 ± 0.74 (2.46–6.67) | 4.20 ± 1.40 (2.53–14.44) |
| HDL, mmol/l | 1.53 ± 0.37 (0.10–2.41) | 1.45 ± 0.3 (0.78–2.47) |
| LDL, mmol/l | 2.26 ± 0.57 (1.03–3.54) | 2.21 ± 0.59 (1.00–4.07) |
| Creatinine, mmol/l | 53.3 ± 10.0 (35.0–75.0) | 57.3 ± 12.0 (33.0–85.0) |
| Cystatin C, mg/l | 0.67 ± 0.12 (0.40–1.12) | 0.68 ± 0.10 (0.35–0.89) |
| GFR ml·min−1·1.73 m−2 (Larsson) | 125 ± 23 (74–199) | 121 ± 20 (92–227) |
| ACR, mg/mmol | 0.61 (0.40–0.90) (0.00–13.30) | 0.80 (0.43–1.33) (0.00–11.60) |
| 24-h SBP, mmHg | 114 ± 8 (98–137) | 116 ± 7 (98–134) |
| 24-h DBP, mmHg | 67 ± 6 (51–88) | 67 ± 5 (56–79) |
| 24-h MAP, mmHg | 84 ± 6 (71–102) | 84 ± 5 (75–99) |
| 24-h HR, bpm | 81.0 ± 9 (53–103) | 80 ± 9 (55–97) |
| Day SBP, mmHg | 117 ± 8 (100–140) | 120 ± 8 (101–140) |
| Day DBP, mmHg | 70 ± 6 (53–88) | 70 ± 6 (57–83) |
| Day MAP, mmHg | 86 ± 6 (72–102) | 88 ± 6 (75–103) |
| Day HR, bpm | 84 ± 9 (53–105) | 83 ± 10 (56–105) |
| Night SBP, mmHg | 104 ± 6 (90–130) | 107 ± 8 (90–125) |
| Night DBP, mmHg | 57 ± 5 (46–71) | 58 ± 5 (48–74) |
| Night MAP, mmHg | 75 ± 5 (65–93) | 76 ± 5 (75–103) |
| Night HR, bpm | 71 ± 10 (48–97) | 70 ± 9 (49–90) |
| Nondipper (n/total) | 38/95 | 34/89 |
Data are expressed as means ± SD for normally distributed variables, median, P25–P75 for positively skewed variables and % for categorical variables. T1DM, Type 1 diabetes mellitus; HDL, high-density lipoprotein; LDL, low-density lipoprotein; GFR, glomerular filtration rate; ACR, albumin-to-creatinine ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate. Range (min–max) are included for all continuous variables.
Characteristics of participants stratified by hyperfiltration.
Participants with and without hyperfiltration at baseline were of similar age, body mass index (BMI), HbA1c, and lipid profiles (Table 2). There were more girls than boys with hyperfiltration, but this did not reach statistical significance (Table 2). Compared with those with normofiltration, ACR was significantly higher in participants with hyperfiltration (ACR). Compared with those with normofiltration, those with hyperfiltration had lower systolic blood pressure (24-h, daytime) at baseline and at 2-yr follow-up (24-h, daytime, nighttime). At baseline, heart rate (HR; 24-h, daytime and nighttime) was not different between those with normofiltration compared with those with hyperfiltration; however, at 2-yr follow-up, HR was significantly higher in those with hyperfitration.
Table 2.
Baseline demographics stratified by hyperfiltration in T1DM adolescents
| Hyperfiltration at Baseline |
|||
|---|---|---|---|
| Variable | Yes (n = 29) | No (n = 66) | P Value |
| Age, yr | 13 ± 2 | 13 ± 2 | 0.28 |
| Sex (girls) | 55% | 38% | 0.12 |
| Body mass index, kg/m2 | 21.0 ± 3.8 | 21.7 ± 3.0 | 0.53 |
| Height, cm | 152.4 ± 14.5 | 159.7 ± 12.1 | 0.012 |
| Weight, kg | 49 ± 14 | 55 ± 13 | 0.04 |
| HbA1c, % | 8.8 ± 1.5 | 8.5 ± 1.1 | 0.33 |
| Diabetes duration, yr | 6.6 ± 2.6 | 7.0 ± 2.8 | 0.53 |
| Triglycerides, mmol/l | 0.82 (0.63–0.99) | 0.77 (0.64–0.95) | 0.85 |
| Total cholesterol, mmol/l | 4.35 ± 0.65 | 4.21 ± 0.77 | 0.40 |
| HDL, mmol/l | 1.64 ± 0.32 | 1.47 ± 0.38 | 0.045 |
| LDL, mmol/l | 2.26 ± 0.54 | 2.26 ± 0.58 | 0.99 |
| Creatinine, mmol/l | 49.3 ± 10.1 | 55.7 ± 9.4 | 0.04 |
| Cystatin C, mg/l | 0.53 ± 0.05 | 0.73 ± 0.09 | <0.0001 |
| GFR ml/min (Larsson) | 152 ± 17 | 113 ± 12 | <0.0001 |
| GFR ml·min−1·1.73 m−2 (Zappitelli) | 161 ± 21 | 112 ± 15 | <0.0001 |
| Rapid GFR decline, % | 68% (19/28) | 33% (21/63) | 0.002 |
| ACR, mg/mmol | 0.77 (0.55–1.37) | 0.54 (0.39–0.87) | 0.02 |
| 24-h SBP, mmHg | 111 ± 7 | 115 ± 8 | 0.03 |
| 24-h DBP, mmHg | 66 ± 7 | 67 ± 6 | 0.46 |
| 24-h MAP, mmHg | 82 ± 5 | 84 ± 6 | 0.08 |
| 24-h HR, bpm | 80 ± 8 | 83 ± 10 | 0.12 |
| Day SBP, mmHg | 114 ± 6 | 118 ± 8 | 0.02 |
| Day DBP, mmHg | 69 ± 7 | 70 ± 6 | 0.34 |
| Day MAP, mmHg | 84 ± 6 | 87 ± 6 | 0.02 |
| Day HR, bpm | 86 ± 10 | 83 ± 9 | 0.24 |
| Night SBP, mmHg | 103 ± 6 | 104 ± 7 | 0.42 |
| Night DBP, mmHg | 57 ± 5 | 58 ± 6 | 0.31 |
| Night MAP, mmHg | 75 ± 5 | 75 ± 5 | 0.93 |
| Night HR, bpm | 73 ± 12 | 70 ± 9 | 0.17 |
| Nondipper (n/total) | 55% (16/29) | 37% (24/65) | 0.10 |
Data are expressed as means ± SD for normally distributed variables, median, P25–P75 for positively skewed variables, and % for categorical variables.
At 2-yr follow-up, there were significantly more girls than boys with hyperfiltration (75% vs. 32%, P = 0.0005). HbA1c, triglyceride, and ACR were greater in those with hyperfiltration; however, there were no differences in age, BMI, LDL-C, HDL-C, and total cholesterol between participants with and without hyperfiltration, (Table 3).
Table 3.
Two-year follow-up demographics stratified by hyperfiltration in T1DM adolescents
| Hyperfiltration at Two-Year Follow-Up |
|||
|---|---|---|---|
| Varaible | Yes (n = 20) | No (n = 72) | P Value |
| Age (years) | 15.7 ± 2.3 | 15.3 ± 1.9 | 0.53 |
| Sex (girls) | 75% | 32% | 0.0005 |
| Body mass index, kg/m2 | 22.4 ± 3.0 | 22.4 ± 3.1 | 0.95 |
| Height, cm | 163 ± 13 | 166 ± 10 | 0.30 |
| Weight, kg | 60 ± 13 | 62 ± 12 | 0.50 |
| HbA1c, % | 9.7 ± 1.9 | 8.6 ± 1.2 | 0.02 |
| Diabetes duration, yr | 8.8 ± 4.8 | 8.9 ± 4.7 | 0.89 |
| Triglycerides, mmol/l | 0.89 (0.77–1.33) | 0.70 (0.61–0.93) | 0.02 |
| Total cholesterol, mmol/l | 4.14 ± 1.46 | 4.43 ± 0.80 | 0.39 |
| HDL, mmol/l | 1.41 ± 0.29 | 1.54 ± 0.31 | 0.10 |
| LDL, mmol/l | 2.38 ± 0.59 | 2.17 ± 0.58 | 0.19 |
| Creatinine, mmol/l | 60.1 ± 11.74 | 50.9 ± 10.7 | 0.02 |
| Cystatin C, mg/l | 0.54 ± 0.05 | 0.72 ± 0.07 | <0.0001 |
| GFR, ml·min−1·1.73 m−2 (Larsson) | 149 ± 20 | 113 ± 10 | <0.0001 |
| GFR ml·min−1·1.73 m−2 (Zappitelli) | 158 ± 26 | 113 ± 13 | <0.0001 |
| ACR, mg/mmol | 0.86 (0.63–1.48) | 0.56 (0.37–0.87) | 0.001 |
| 24-h SBP, mmHg | 111 ± 6 | 117 ± 7 | 0.0006 |
| 24-h DBP, mmHg | 67 ± 5 | 67 ± 5 | 0.94 |
| 24-h MAP, mmHg | 83 ± 5 | 85 ± 5 | 0.14 |
| 24-h HR | 83 ± 6 | 78 ± 9 | 0.06 |
| Day SBP, mmHg | 115 ± 8 | 121 ± 7 | 0.001 |
| Day DBP, mmHg | 70 ± 7 | 70 ± 6 | 0.88 |
| Day MAP, mmHg | 86 ± 6 | 88 ± 5 | 0.29 |
| Day HR | 87 ± 8 | 82 ± 11 | 0.09 |
| Night SBP, mmHg | 104 ± 6 | 108 ± 8 | 0.04 |
| Night DBP, mmHg | 59 ± 5 | 58 ± 6 | 0.37 |
| Night MAP, mmHg | 76 ± 5 | 77 ± 5 | 0.67 |
| Night HR | 72 ± 6 | 68 ± 10 | 0.07 |
| Nondipper (n/total) | 58% (11/19) | 36% (25/69) | 0.09 |
Data are expressed as means ± SD for normally distributed variables, median, P25–P75 for positively skewed variables, and % for categorical variables.
Characteristics of participants stratified by sex.
Compared with boys, girls of similar age and diabetes duration, had higher triglycerides, total cholesterol, LDL, and 24-h, daytime and nighttime HR at baseline (Supplemental Table S1; all supplemental material for this article is available on the journal web site). Conversely, boys had higher 24-h and daytime SBP than girls at baseline (Supplemental Table S1). There was a greater prevalence of hyperfiltration in girls compared with boys (39% vs. 24%) at baseline, but this did not reach statistical significance. No significant differences were observed in the prevalence of nighttime nondipping pattern between boys and girls at baseline (Supplemental Table S1).
At 2-yr follow-up, girls had significantly higher HbA1c, total cholesterol, HDL, LDL, and 24-h, daytime and nighttime HR compared with boys. Furthermore, girls had higher eGFR (131 ± 22 vs. 114 ± 15 ml/min/1.73 m2, P = 0.0002) and ACR (0.94 [0.62–1.73] vs. 0.67 [0.37–1.20] mg/mmol, P = 0.02) than boys. Hyperfiltration was also more prevalent in girls at 2-yr follow-up (40% vs. 9%, P = 0.005). Similar to observations at baseline, boys had higher 24-h and daytime SBP at 2-yr follow-up, and girls had higher 24-h, daytime and nighttime HR (Supplemental Table S2).
Relationship between ACR and eGFR at baseline and 2-yr follow-up.
When stratifying participants by tertiles of baseline ACR, the participants in the high tertile (≥0.81 mg/mmol) had higher eGFR (LSM ± SE: 133 ± 4 ml/min) than those in the low tertile (<0.46 mg/mmol) (LSM ± SE: 119 ± 4 ml/min, P = 0.009) after adjusting for sex, diabetes duration, and HbA1c. Similar relationships were observed when stratifying ACR at 2-yr follow-up, with participants in the high ACR tertile (≥1.13 mg/mmol) having higher eGFR (LSM ± SE: 130 ± 4 ml/min) than those in the low ACR tertile (<0.55 mg/mmol) (LSM ± SE: 120 ± 4 ml/min, P = 0.046) after multivariable adjustments (Fig. 1). In fact, compared with participants in the low ACR tertile, those in the mid- and high-ACR tertiles had a greater portion of hyperfiltration at both baseline (39% vs. 39% vs. 14%, P = 0.03 for high, mid, low ACR tertiles) and 2-yr follow-up (46% vs. 15% vs. 8%, P = 0.002, for high, mid, and low ACR tertiles).
Fig. 1.
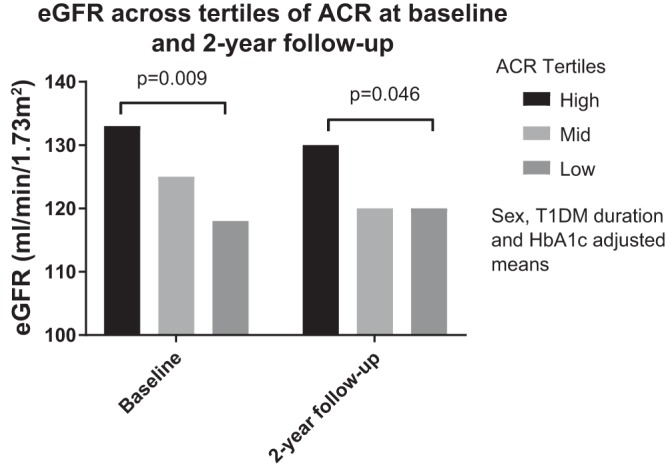
The mean eGFR values are provided at baseline and at 2-yr follow-up for ACR characterized into low, middle, and high tertiles for the study cohort.
After adjusting for sex, diabetes duration, and HbA1c, baseline ACR (expressed as natural logarithm of ACR, [ln ACR]) was associated with baseline eGFR (β ± SE: 5.66 ± 2.38, P = 0.02). Conversely, the association between ln ACR and eGFR at 2-yr follow-up did not reach statistical significance after multivariable adjustments (β ± SE: 2.96 ± 2.12, P = 0.17). One SD increase in ln ACR at baseline was associated with greater odds of hyperfiltration at baseline (OR: 1.71, 95% CI: 1.00–2.92, P = 0.049) and 2-yr follow-up (OR: 2.14, 95% CI: 1.09–4.19, P = 0.03) after multivariable adjustments (Fig. 2).
Fig. 2.
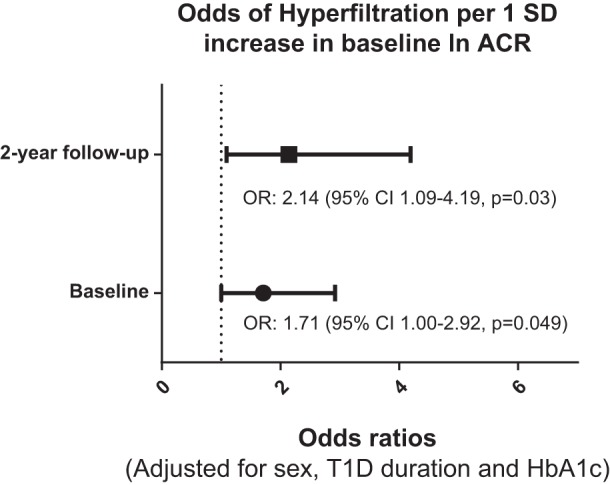
The odds ratios (adjusted for sex, duration of diabetes, and HbA1c) of hyperfiltration per 1 SD in ln ACR are presented at baseline and at 2-yr follow-up. Baseline ACR tertiles: <0.46, 0.46–0.81, and ≥0.81 mg/mmol; 2-yr follow-up ACR tertiles: <0.55, 0.55–1.13, and ≥1.13 mg/mmol.
Compared with participants with normofiltration, those with hyperfiltration experienced a significantly greater decline in eGFR over the 2-yr follow-up (18 ± 23 vs. −2 ± 17 ml/min, P < 0.0001). Similarly, hyperfiltration conferred greater odds of rapid GFR decline (OR: 5.33, 95% CI: 1.87–15.17, P = 0.002) after adjusting for diabetes duration, sex, and HbA1c.
Relationship between ACR, eGFR, and blood pressure at baseline and 2-yr follow-up.
In univariable analyses, eGFR inversely associated with 24-h and daytime SBP (Supplemental Table S3) at baseline and 2-yr follow-up. These associations were attenuated after multivariable adjustments, likely due to the sex-related differences in SBP. Similarly, eGFR positively associated with 24-h and daytime HR at 2-yr follow-up (Supplemental Table S3), but lost statistical significance after multivariable adjustments (again likely due to sex-related differences in HR). The only association that remained statistically significant in an adjusted model was between eGFR and nighttime DBP at 2-yr follow-up (β ± SE: 0.07 ± 0.03, P = 0.02). One SD increase in eGFR at 2-yr follow-up conferred greater odds of nighttime nondipping pattern (OR: 1.96, 95% CI: 1.06–3.63, P = 0.03) after adjusting for sex, diabetes duration, and HbA1c. This association lost statistical significance for baseline eGFR after multivariable adjustments (OR: 1.23, 95% CI: 0.79–1.92, P = 0.35).
In univariable analyses, ln ACR did not associate with any blood pressure measures at either baseline or 2-yr follow-up (Supplemental Table S4). In multivariable models, there was an inverse relationship between ln ACR and daytime HR (β ± SE: −2.39 ± 1.18, P = 0.047). In contrast to eGFR, ln ACR was not associated with greater odds of nighttime nondipping pattern at baseline or at 2-yr follow-up (data not shown).
In sensitivity analyses, we stratified the baseline cohorts into participants who had both hyperfiltration and high tertile of ACR (n = 13), hyperfiltration and low tertile of ACR (n = 4), normofiltration and high tertile of ACR (n = 20), and normofiltration and low tertile of ACR (n = 27) (Supplemental Table S5). While there were no significant changes in systemic hemodynamic measures (24-h SBP, DBP, mean arterial pressure, HR, and percentage dipping) over 2-yr follow-up across the groups, there were important differences in ACR and eGFR. Participants in the hyperfiltration and high-ACR tertile, and hyperfiltration and low-ACR tertile groups experienced a greater decline in eGFR compared with participants in the other two groups (Supplemental Table S5).
DISCUSSION
Youth with T1DM are at risk for cardiovascular and renal complications that may present up to 20–30 yr after diagnosis. Complex hormonal and physiological changes occur during puberty and contribute both to deteriorating glycemic control and an increased risk for cardiorenal complications (1). Therefore, studying perturbations in cardiovascular and renal characteristics early in the natural history of T1DM, particularly during puberty, may help identify key pathophysiological factors that contribute to the development and progression of early renal and cardiovascular dysfunction in T1DM.
In this longitudinal study of adolescents with T1DM, hyperfiltration was prevalent at both baseline and 2-yr follow-up, was associated with greater GFR decline overall, and predicted rapid decline in GFR. The drop in hyperfiltration prevalence over 2 yr was consistent with rapid GFR decline. In addition, albumin excretion was related to hyperfiltration, and adolescents in the higher ACR tertile had higher eGFR. Finally, elevated eGFR at 2-yr follow-up was associated with greater odds of nighttime nondipping pattern.
Studies in the AdDIT trial have shown that adolescents with T1DM with higher ACR values within the normal range exhibit early, asymptomatic evidence of renal and cardiovascular risk compared with those with lower ACR values (16), including early atherosclerotic risk markers assessed by biochemical and vascular ultrasound techniques (14). Adolescents with T1DM and the higher ACR values also exhibit sympathetic nervous system activation compared with lower risk ACR individuals (6). In the current analysis, we have extended these studies by examining the relationships between ACR and important variables such as 24-h ABPM and eGFR during puberty over a 2-yr follow-up period, with the goal of developing a more comprehensive understanding of these key pathophysiological factors during early disease.
Previous studies have reported that adolescents with T1DM and hyperfiltration have higher ACR (5). In the current analysis, we calculated eGFR by serum cystatin C. Cystatin C is generally considered to more accurately reflect measured GFR compared with creatinine-based estimates (2, 25). Therefore, the current analysis confirms and extends previous findings regarding the relationships in adolescents with T1DM by using cystatin C-calculated eGFR, and also explores potential relationships between ACR and GFR with 24-h ABPM cross-sectionally and longitudinally during a 2-yr follow-up period during puberty. Taken together, these data further expand our understanding of the complexity of early cardiorenal changes in adolescents with T1DM.
While hyperfiltration has been reported to precede albuminuria and to predispose to cardiovascular risk and development of DN (4), the relationship(s) between hyperfiltration and blood pressure profiles in adolescents with T1DM during puberty are less clear, and furthermore, how these relationships vary over time is not known. In a previous acute physiological study in adolescents with uncomplicated T1DM during clamped hyperglycemia, those with hyperfiltration had higher measured SBP and HR compared with those without hyperfiltration (and also compared with healthy controls at baseline); however, the temporal aspects of these relationships were not examined (33). In a small cross-sectional study of 38 normotensive, normoalbuminuric participants with T1DM, higher nocturnal diastolic blood pressure and a blunted decrease in diastolic blood pressure was associated with hyperfiltration; however, longitudinal assessment of these observations was not examined (19). Similarly, in a large observational study in Japanese patients with prediabetes and prehypertension, the prevalence of hyperfiltration was proportional to increases in blood glucose (17) and to increases in blood pressure (18).
In the present study, at the 2-yr follow-up, after adjusting for sex, duration of diabetes, and HbA1c, we observed significant associations between eGFR and nocturnal DBP and between one SD increase in eGFR and nighttime blood pressure nondipping status. Nighttime nondipping is an important prognostic risk factor for end-organ damage, cardiovascular disease, and mortality in adults with and without diabetes mellitus, risks that likely extend to adolescents with T1DM (20, 21, 31). Deterioration in renal function is associated with nighttime nondipping status and elevated nocturnal diastolic blood pressure (10, 29). In a cross-sectional population-based study of 560 healthy middle-aged adults (40–62 yr), it was observed that subjects with the lowest (eGFR 43.2–74.4 ml·min−1·1.73 m−2) and middle tertiles (74.5–87.7 ml·min−1·1.73 m−2) of eGFR had the highest independent risk of nondipping status compared with those in the highest eGFR tertiles (88.1–152.5 ml·min−1·1.73 m−2) (10). Nondipping status has also been associated with renal injury in T1DM. In a 10-yr longitudinal study of 40 normoalbuminuric adolescents (mean age 17.7 yr) and young adults with T1DM of short duration, nondipping status was related with more extensive renal morphological changes (thicker basement membrane and enlarged mesangial matrix volume fraction per glomerulus) and with long-term hyperfiltration, with no differences observed based on sex (30). The current set of observations is consistent with these study results and extends these findings to T1DM adolescents of a younger age category (13.3 yr) during puberty, at a time of complex hormonal physiological changes. In this younger cohort, we did not find any statistically significant associations with elevated HR or SBP with eGFR; however, a significant association was observed for eGFR and nocturnal DBP and nondipping status. Mechanistically, it is not known how blunted nocturnal blood pressure dipping is linked to the risk of nephropathy and renal hemodynamic functional changes in T1DM. Torbjörnsdotter et al. (29), who previously demonstrated that elevated nocturnal HR (reflecting autonomic neuropathy) and nocturnal blood pressure are related to glomerulopathy in normoalbuminuric adolescent T1DM patients, have suggested that a disturbance in sympathovagal balance due to increased activation of the systemic nervous system could be pathognomonic. Soltysiak et al. (27) have demonstrated an association with elevated blood pressure and urinary angiotensinogen and decreased renal sodium excretion in children with T1DM, suggesting that early activation of the renin-angiotensin-aldosterone pathway may contribute to hypertension and early renal injury.
A previous small longitudinal cohort study (23) in normotensive normoalbuminuric T1DM patients was consistent with transition from normoalbuminuria to microalbuminuria over a 3-yr period being associated with less nocturnal dipping. In contrast, we did not observe any statistically significant associations between ACR and 24-h blood pressure parameters (including dipping patterns) or HR. The lack of an association between ACR and 24-h ABPM data is consistent with prior observations made in nested case-control studies of children with T1DM. For example, in the Oxford Regional Prospective study, there were no differences in SBP or DBP between microalbuminuric and normoalbuminuric T1DM patients before the onset of microalbuminuria (24). Additional work is required to confirm and better understand the impact of urinary albumin excretion and hyperfiltration on systemic blood pressure profiles and HR changes in adolescents with T1DM.
Over the course of the 2-yr follow-up period in our cohort, participants with hyperfiltration at baseline experienced a significantly greater decline in eGFR compared with participants with normofiltration at baseline. This rapid GFR decline may be reflective of early renal injury. While rapid GFR decline is known to succeed hyperfiltration and precede impaired GFR in adults with T1DM (4), it is unclear whether this intermediate phenotype of DN exists in youth with T1DM. Elevated ACR at baseline also predicted hyperfiltration at a 2-yr follow-up. Overall, these observations may suggest that early hemodynamic functional abnormalities in the kidney, such as hyperfiltration and urinary albumin excretion, are related and predate changes in systemic hemodynamic measures such as blood pressure.
The present study has several limitations. First, the relatively small sample size may have limited our ability to detect changes in 24-h ABPM over the 2-yr follow-up. Second, although heterogeneity tests did not reveal significant differences in key variables, participants in this study were included from two separate T1DM cohorts, which may have introduced residual confounding. Third, the large age range (10–16 yr of age) may have introduced unfavorable between-patient heterogeneity in terms of hormonal changes in adolescence and puberty. To limit the impact of age fluctuations and variable pubertal stages, future prospective studies should aim to recruit adolescents with a lower maximum age to better control the uniformity of the baseline cardiovascular and renal physiologies within the cohort. Fourth, our study presented data from baseline and a 2-yr follow-up, with no intermediate periods of study. Although we adjusted for a variety of important confounding variables, we cannot rule out the presence of unknown risk factors that may have biased the present analyses. Fifth, comparisons between groups (hyperfiltration and normofiltration) were not adjusted for sex; however, we noted a numerically higher yet nonstatistically significant percentage of girls in the hyperfiltration group. This imbalance may explain lower blood pressure levels in the hyperfiltration group as well as higher ACR. Finally, our analyses were considered exploratory and hypothesis generating, and adjustments for multiple comparisons were not employed. The strengths of our study include providing longitudinal ABPM, ACR, and cystatin C data in adolescents with T1DM.
In conclusion, hyperfiltration was prevalent in adolescents with T1DM and was associated with albumin excretion and rapid GFR decline. Greater eGFR at 2-yr follow-up was associated with greater odds of nighttime nondipping pattern. Conversely, ACR was not significantly associated with 24-h ABPM changes over 2 yr. Even in young children with a short duration of disease, early detection of circadian blood pressure changes may be informative in a subset of adolescents with T1DM for risk of renal functional decline and hyperfiltration.
GRANTS
Funding was provided by the Juvenile Diabetes Research Foundation-Canadian Clinical Trial Network, the Diabetes Canada (formerly the Canadian Diabetes Association), the Heart and Stroke Foundation of Canada, Physician Services Incorporated Foundation, the SickKids Labatt Family Heart Center Innovation fund and National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases T32 DK-063687. D. Z. I. Cherney was also supported by an Award from the Canadian Institutes of Health Research (CIHR). J. W. Scholey was supported by the CIHR-AMGEN Canada Incorporated Chair in Kidney Research, and support was, in part, provided by the CIHR.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.A.L., M.S., P.B., R.M., and E.B.S. analyzed data; J.A.L., M.S., P.B., D.Z.I.C., and E.B.S. interpreted results of experiments; J.A.L., M.S., and P.B. prepared figures; J.A.L. and M.S. drafted manuscript; J.A.L., P.B., D. Daneman, D. Dunger, F.H.M., J.W.S., D.Z.I.C., and E.B.S. edited and revised manuscript; J.A.L., M.S., P.B., R.M., D. Daneman, D. Dunger, H.N.R., F.H.M., J.W.S., D.Z.I.C., and E.B.S. approved final version of manuscript; D. Daneman and E.B.S. conceived and designed research; E.B.S. performed experiments.
Supplemental Data
ACKNOWLEDGMENTS
The authors are grateful to the study participants whose time and effort are critical to the success of our research program. We also thank the study coordinators and, in particular, Laura Motran who compiled the final database.
REFERENCES
- 1.Acerini CL, Williams RM, Dunger DB. Metabolic impact of puberty on the course of type 1 diabetes. Diabetes Metab 27: S19–S25, 2001. [PubMed] [Google Scholar]
- 2.Bacchetta J, Cochat P, Rognant N, Ranchin B, Hadj-Aissa A, Dubourg L. Which creatinine and cystatin C equations can be reliably used in children? Clin J Am Soc Nephrol 6: 552–560, 2011. doi: 10.2215/CJN.04180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjornstad P, Cherney DZ, Maahs DM, Nadeau KJ. Diabetic kidney disease in adolescents with Type 2 diabetes: new insights and potential therapies. Curr Diab Rep 16: 11, 2016. doi: 10.1007/s11892-015-0708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjornstad P, Cherney DZ, Snell-Bergeon JK, Pyle L, Rewers M, Johnson RJ, Maahs DM. Rapid GFR decline is associated with renal hyperfiltration and impaired GFR in adults with Type 1 diabetes. Nephrol Dial Transplant 30: 1706–1711, 2015. doi: 10.1093/ndt/gfv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjornstad P, Roncal C, Milagres T, Pyle L, Lanaspa MA, Bishop FK, Snell-Bergeon JK, Johnson RJ, Wadwa RP, Maahs DM. Hyperfiltration and uricosuria in adolescents with type 1 diabetes. Pediatr Nephrol 31: 787–793, 2016. doi: 10.1007/s00467-015-3299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YH, Craig ME, Davis EA, Cotterill AM, Couper JJ, Cameron FJ, Benitez-Aguirre PZ, Dalton RN, Dunger DB, Jones TW, Donaghue KC; Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial . Cardiac autonomic dysfunction is associated with high-risk albumin-to-creatinine ratio in young adolescents with type 1 diabetes in AdDIT (adolescent type 1 diabetes cardio-renal interventional trial). Diabetes Care 38: 676–681, 2015. doi: 10.2337/dc14-1848. [DOI] [PubMed] [Google Scholar]
- 7.Drummond K, Mauer M; International Diabetic Nephropathy Study Group . The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 51: 1580–1587, 2002. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 8.Fadrowski JJ, Neu AM, Schwartz GJ, Furth SL. Pediatric GFR estimating equations applied to adolescents in the general population. Clin J Am Soc Nephrol 6: 1427–1435, 2011. doi: 10.2215/CJN.06460710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jørgensen ME, Almdal TP, Carstensen B. Time trends in mortality rates in type 1 diabetes from 2002 to 2011. Diabetologia 56: 2401–2404, 2013. doi: 10.1007/s00125-013-3025-7. [DOI] [PubMed] [Google Scholar]
- 10.Kastarinen H, Vasunta RL, Ukkola O, Kesäniemi YA. Glomerular filtration rate is related to dipping pattern in ambulatory blood pressure monitoring—a cross-sectional population-based study. J Hum Hypertens 24: 247–253, 2010. doi: 10.1038/jhh.2009.66. [DOI] [PubMed] [Google Scholar]
- 11.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J; American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity and Metabolism; American Heart Association Council on High Blood Pressure Research; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on the Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and Outcomes Research . Cardiovascular risk reduction in high-risk pediatric patients. Circulation 114: 2710–2738, 2006. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 12.Lafferty AR, Werther GA, Clarke CF. Ambulatory blood pressure, microalbuminuria, and autonomic neuropathy in adolescents with type 1 diabetes. Diabetes Care 23: 533–538, 2000. doi: 10.2337/diacare.23.4.533. [DOI] [PubMed] [Google Scholar]
- 13.Larsson A, Malm J, Grubb A, Hansson LO. Calculation of glomerular filtration rate expressed in ml/min from plasma cystatin C values in mg/l. Scand J Clin Lab Invest 64: 25–30, 2004. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 14.Maftei O, Pena AS, Sullivan T, Jones TW, Donaghue KC, Cameron FJ, Davis E, Cotterill A, Craig ME, Gent R, Dalton N, Daneman D, Dunger D, Deanfield J, Couper JJ. Early atherosclerosis relates to urinary albumin excretion and cardiovascular risk factors in adolescents with type 1 diabetes: Adolescent type 1 Diabetes cardio-renal Intervention Trial (AdDIT). Diabetes Care 37: 3069–3075, 2014. doi: 10.2337/dc14-0700. [DOI] [PubMed] [Google Scholar]
- 15.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 16.Marcovecchio ML, Woodside J, Jones T, Daneman D, Neil A, Prevost T, Dalton RN, Deanfield J, Dunger DB; AdDIT Investigators . Adolescent Type 1 diabetes cardio-renal intervention trial (AdDIT): urinary screening and baseline biochemical and cardiovascular assessments. Diabetes Care 37: 805–813, 2014. doi: 10.2337/dc13-1634. [DOI] [PubMed] [Google Scholar]
- 17.Okada R, Wakai K, Naito M, Morita E, Kawai S, Yin G, Ozawa N, Furuta M, Koyama E, Tsuchiya R, Kouno N, Hamajima N. Renal hyperfiltration in prediabetes confirmed by fasting plasma glucose and hemoglobin A1c. Ren Fail 34: 1084–1090, 2012. doi: 10.3109/0886022X.2012.717516. [DOI] [PubMed] [Google Scholar]
- 18.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant 27: 1821–1825, 2012. doi: 10.1093/ndt/gfr651. [DOI] [PubMed] [Google Scholar]
- 19.Pecis M, Azevedo MJ, Gross JL. Glomerular hyperfiltration is associated with blood pressure abnormalities in normotensive normoalbuminuric IDDM patients. Diabetes Care 20: 1329–1333, 1997. doi: 10.2337/diacare.20.8.1329. [DOI] [PubMed] [Google Scholar]
- 20.Pickering TG, Kario K. Nocturnal non-dipping: what does it augur? Curr Opin Nephrol Hypertens 10: 611–616, 2001. doi: 10.1097/00041552-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Pietrzak I, Fendler W, Drozdz I, Mianowska B, Mlynarski W, Szadkowska A. Arterial stiffness, BMI, dipping status and ACE D/I polymorphism in Type 1 diabetic children. Exp Clin Endocrinol Diabetes. 124: 283–287, 2016. doi: 10.1055/s-0042-101243. . [DOI] [PubMed] [Google Scholar]
- 22.Poulsen PL, Ebbehøj E, Hansen KW, Mogensen CE. 24-h Blood pressure and autonomic function is related to albumin excretion within the normoalbuminuric range in IDDM patients. Diabetologia 40: 718–725, 1997. doi: 10.1007/s001250050739. [DOI] [PubMed] [Google Scholar]
- 23.Poulsen PL, Hansen KW, Mogensen CE. Ambulatory blood pressure in the transition from normo- to microalbuminuria. A longitudinal study in IDDM patients. Diabetes 43: 1248–1253, 1994. doi: 10.2337/diab.43.10.1248. [DOI] [PubMed] [Google Scholar]
- 24.Schultz CJ, Dalton RN, Neil HA, Konopelska-Bahu T, Dunger DB; Oxford Regional Prospective Study Group . Markers of renal tubular dysfunction measured annually do not predict risk of microalbuminuria in the first few years after diagnosis of Type I diabetes. Diabetologia 44: 224–229, 2001. doi: 10.1007/s001250051603. [DOI] [PubMed] [Google Scholar]
- 25.Sharma AP, Yasin A, Garg AX, Filler G. Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clin J Am Soc Nephrol 6: 1599–1608, 2011. doi: 10.2215/CJN.10161110. [DOI] [PubMed] [Google Scholar]
- 26.Sochett EB, Daneman D.. Abnormal night/day ratio may not be a predictive marker for microalbuminuria in adolescents with Type 1 diabetes [Abstract]. In: Abstracts of the 63rd Scientific Sessions of the American Diabetes Association June 13–17, New Orleans, Louisiana, USA. Washington, DC: American Diabetes Association; 2003. [Google Scholar]
- 27.Soltysiak J, Skowronska B, Fichna P, Ostalska-Nowicka D, Stankiewicz W, Lewandowska-Stachowiak M, Lipkowska K, Zachwieja J. Urinary angiotensinogen and urinary sodium are associated with blood pressure in normoalbuminuric children with diabetes. Pediatr Nephrol 29: 2373–2378, 2014. doi: 10.1007/s00467-014-2861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.System USRD. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institutes of Diabetes and Digestive and Kidney Diseases, 2012. [Google Scholar]
- 29.Torbjörnsdotter TB, Jaremko GA, Berg UB. Ambulatory blood pressure and heart rate in relation to kidney structure and metabolic control in adolescents with Type I diabetes. Diabetologia 44: 865–873, 2001. doi: 10.1007/s001250100528. [DOI] [PubMed] [Google Scholar]
- 30.Torbjörnsdotter TB, Jaremko GA, Berg UB. Nondipping and its relation to glomerulopathy and hyperfiltration in adolescents with type 1 diabetes. Diabetes Care 27: 510–516, 2004. doi: 10.2337/diacare.27.2.510. [DOI] [PubMed] [Google Scholar]
- 31.Viera AJ, Lin F-C, Hinderliter AL, Shimbo D, Person SD, Pletcher MJ, Jacobs DR Jr. Nighttime blood pressure dipping in young adults and coronary artery calcium 10–15 years later: the coronary artery risk development in young adults study. Hypertension 59: 1157–1163, 2012. doi: 10.1161/HYPERTENSIONAHA.112.191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wühl E, Witte K, Soergel M, Mehls O, Schaefer F; German Working Group on Pediatric Hypertension . Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 20: 1995–2007, 2002. doi: 10.1097/00004872-200210000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Yang GK, Maahs DM, Perkins BA, Cherney DZI. Renal hyperfiltration and systemic blood pressure in patients with uncomplicated type 1 diabetes mellitus. PLoS One 8: e68908, 2013. doi: 10.1371/journal.pone.0068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


